-
抗生素是由微生物或高等动植物在生活过程中所产生的能杀灭其他微生物或具有抗病原体的代谢次级产物,主要的作用是抑菌或杀菌[1]. 常见的四环素类抗生素(tetracycline antibiotics, TCs),包括四环素(tetracycline, TC)、土霉素(oxytetracycline, OTC)、金霉素(chlortetracycline, CTC)、强力霉素(doxycycline, DC)等,是由放线菌产生的可抑制细菌蛋白合成的一类抗生素、具有广谱性,其名称源于它们内部结构均含并四苯骨架,对非典型生物如革兰氏阳性和革兰氏阴性细菌、衣原体以及支原体发挥治疗作用[2]. 然而TCs具有较长的半衰期、在生态环境中长期累积、威胁环境安全,即使低浓度也可能通过食物链进入人体体内,引发肝脏损伤,增大人群患病风险[3 − 4]. 因此,去除环境中的TCs意义深远.
目前已有几种较为成熟的方法去除TCs,如吸附法、离子交换法、光降解法、高级氧化降解法、生物降解法等. 与其他方法相比,吸附法因成本低、操作简便和效率高被广泛用于TCs的去除[5];而光降解和氧化降解因能降解持久性的污染物,在抗生素的去除研究中也引起了学术界广泛关注,尤其是高级氧化法中的类Fenton催化氧化法和硫酸根基氧化法,是近年来降解抗生素的研究热点[6].
传统的吸附材料如活性炭、氧化石墨烯、沸石复合材料等存在吸附能力低、再生性和稳定性不理想的缺点,另外,类Fenton法和过硫酸盐法中的催化剂在降解过程中易呈现粉末状而导致回收困难且成本昂贵等劣势,催促了磁性碳基纳米材料(magnetic carbon-based composite nanomaterials, MCCN)的产生并得到广泛应用[7]. MCCN实质上是对碳基纳米材料的磁性改性. 碳基纳米材料如生物多孔炭、金属有机骨架化合物(metal organic frameworks, MOFs)衍生生物炭以及石墨烯氮化碳g-C3N4等,源于其丰富的孔容和高比表面积,在吸附工艺中对抗生素表现出较好的吸附性能,在高级氧化降解过程中可运载催化剂,并激活H2O2或过硫酸盐产生活性物种,实现降解抗生素. 对新型碳基纳米材料进行磁性改性后,利于碳基纳米材料的磁性分离和回收利用. MCCN通过吸附和降解两种方法去除TCs见图1.
-
基于吸附法具备很多优势如成本低廉、适用广泛和操作简便等,被各学术界用以探究处理抗生素,各种生物炭、MOFs、石墨烯等单独作为吸附剂存在吸附能力低,稳定性差、孔隙率小的缺陷. 故发展具有高比表面积、大孔容量和丰富孔结构等优点的新型多孔碳吸附剂,是去除抗生素的理想选择,但仍面临难分离、不易回收等困境. 由此,借助磁性材料外磁场分离和磁回收的优势,构筑MCCN用作吸附剂吸附去除TCs[8 − 11]. 文献报道各类吸附剂对TCs的吸附如表1所示.
-
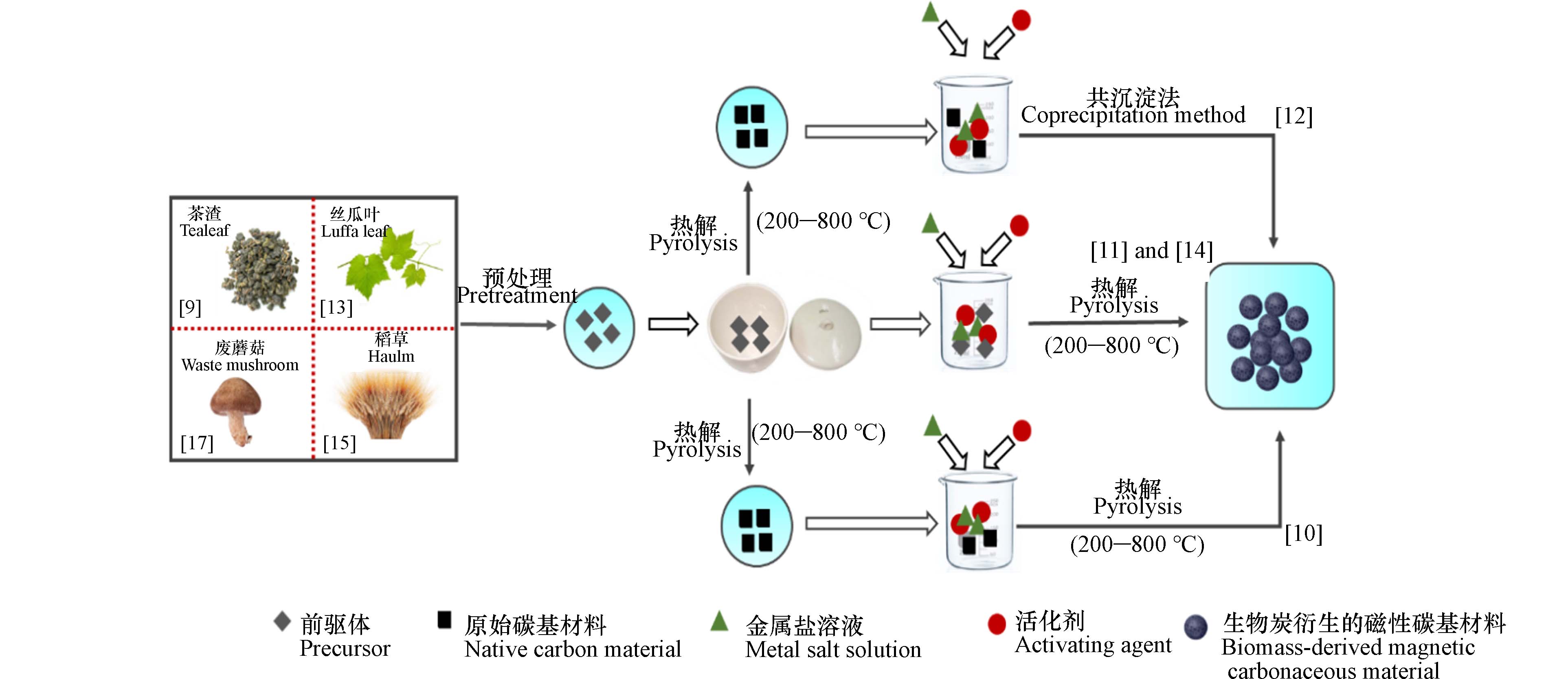
生物质衍生的碳基纳米材料主要由如茶渣[9 − 11]、松树皮[12]、丝瓜叶[13]、酒糟[14]等天然物质在隔绝氧气条件下燃烧合成. 该碳基材料与常见改性活性炭不同的是,对其进行磁性改性、高温炭化改性和加入一些碱活化剂(如KHCO3[10]、K2FeO4[11]、K2CO3[15])合成孔隙结构更丰富的碳基材料,从而提高对TCs的吸附性能. Zhang等[16]以杨木为碳源,加入KOH活化和磁性Fe3O4修饰后获得磁性生物炭(poplar wood biochar modified with Fe3O4, MPBC),通过TEM、SEM、FT-IR和XPS等手段对MPBC进行充分表征,以研究其理化性质. 还考察了其对TC和喹诺酮类抗生素吸附实验,着重探讨实验机理,吸附实验表明;MPBC与抗生素之间存在孔隙填充、π-π相互作用、表面络合、氢键和静电作用等,而TC的吸附效果优于喹诺酮类抗生素(四环素
7028 —89.58 mg·g−1、喹诺酮35.54—60.31 mg·g−1),这是MPBC与两类抗生素之间氢键作用引起的差异.生物质衍生的磁性碳基材料对TCs产生吸附差异主要源于两方面,一方面是原料的选择;生物质原料选择木质纤维素前体主要包括农业废渣[9]和林业废木[12]等. 木质纤维素原料的主要成分有纤维素、半纤维素、木质素以及少量的无机矿物质,在热解过程中,木质素高度芳香化结构增加,同时纤维素挥发促进了生物炭孔隙结构的形成[10]. 另一方面是合成方法和活化改性;合成方法和活化改性是导致MCCN的吸附性能差异关键因素. MCCN的制备主要有3条途径,每条途径均在制备过程中加入活化剂进行改性,对相关文献总结MCCN制备过程汇总如图2所示. 途径1先通过前驱体高温热解合成相应的原始碳基材料,再加入活化剂二次高温热解制备[10];途径2直接在原料中加入活化剂,并通过高温热解合成[11,14];途径3在获得原始碳基材料基础上,加入活化剂合成(无需二次高温热解)[12].
不同的合成方法和改性对碳基材料的理化性质影响很大. Gao等[17]分别通过共沉淀法和热解浸渍法制备两种磁性生物炭:MBC-1和MBC-2,二者对TC的吸附量对应为42.31 mg·g−1和24.31 mg·g−1,产生吸附差异主要源于共沉淀法合成的MBC-1中含氧化铁成分多,具有较多的含氧官能团为吸附提供更多的反应位点. 高温热解合成时,不同热解温度使碳基材料分孔结构、比表面积和孔隙结构产生明显差异. Li等[9]探究了热解温度500—800 ℃对碳基材料的影响,结果发现热解温度800 ℃时碳基材料比表面积更大,孔隙结构更丰富. 加入不同的活化剂对碳基材料的影响更显著. 碱活化剂改性的碳基材料比表面积增量大、表面官能团增多;磁性活化剂改性的碳基材料使其表面和中空结构负载了磁源,既能增大复合碳基材料的比表面积,也便于磁分离和回收.
-
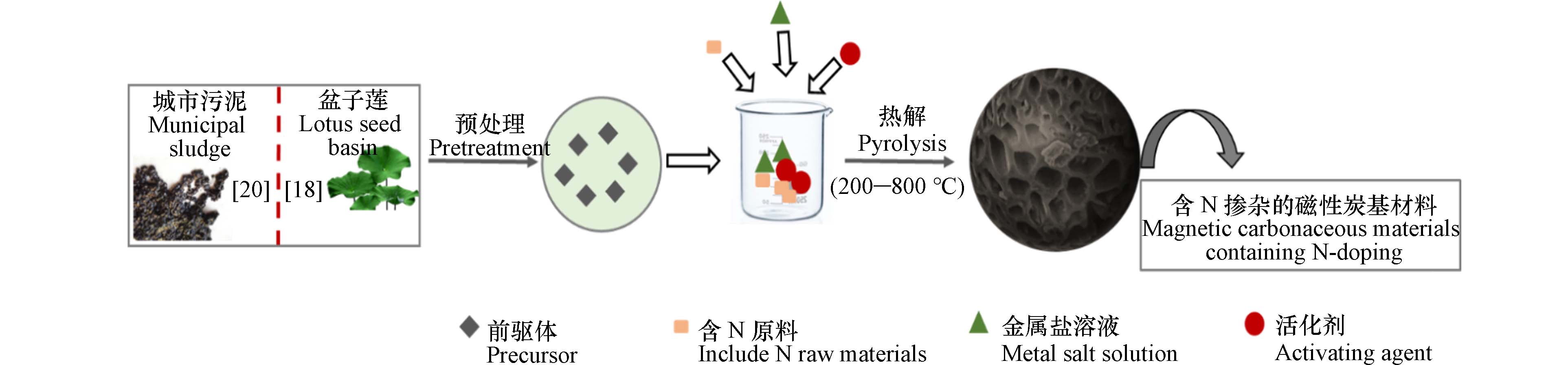
在生物质碳基材料的基础上,掺杂杂原子是提高碳基材料吸附性能的另一种策略. 由于杂原子如N原子上存在孤对电子,引入到碳基材料表面的电荷密度增加,引起杂原子与C原子之间的电负性差异,导致电荷重新分布,以加速电子转移从而形成更多的活性位点. N-掺杂生物质碳基材料中石墨-N、吡啶-N和吡咯-N是主要的吸附位点,对TCs表现出良好的吸附性能. N-掺杂改性的生物炭被广泛应用于酸性气体、重金属和有机污染物的去除,但也存在降低生物炭的比表面积和孔体积等问题. 故协同磁性原子(如Fe)掺杂,使整个MCCN孔隙更发达,对TCs呈现更优异的吸附效果[18 − 22].
含N-掺杂的MCCN一般选择含氮量较高的原料如非木质素纤维材料,包括城市污泥、禽畜粪便等,通常采用一锅法[20 − 22]制备,将文献制备流程汇总如图3所示. 热解温度对合成含N-掺杂的MCCN的影响与生物质衍生的MCCN一致,这里主要采用金属盐溶液和含氮原料(如尿素)对碳基材料进行改性. Mei[22]等以水稻秸秆为前驱体,同时加入FeCl3·H2O和尿素,先通过一步法混合搅拌24 h,再于700 ℃下高温热解获得含N-掺杂磁性生物炭材料Fe-N-RSBC,对照条件下制备了相应的RSBC、N-RSBC、Fe-RSBC,将四者分别通过SEM、XRD、XPS和FT-IR等多个方法表征后表明;N原子改性使生物炭的形貌结构、晶体结构、光电能谱及其官能团发生明显改变,而Fe原子改性不但改变碳基材料的Zeta电位,还降低了其微孔体积,更利于碳基材料形成介孔结构. 以四者为吸附剂对四环素作探究吸附实验,分析得出Fe-N-RSBC对TC吸附效果最佳,最大饱和吸附量为156 mg·g−1,吸附机理以孔隙填充为主,还存在氢键、π-π作用和静电作用等. Fe-N-RSBC对TC吸附最佳是因为Fe和N原子共改性改变了含碳、氧、铁官能团结构、扩大了生物炭的表面积,促使生物炭的孔隙结构更发达;此外,Fe-N共修饰提高了磁性碳基材料的石墨化程度,增大了碳基材料的亲水性和极性.
-
以MOFs为前驱体衍生的磁性碳基材料是去除TCs的优异吸附剂. Hu等[23]使用MIL-88B(Fe)涂覆的Zn-MOF前驱体直接在700 ℃热解后合成含核-壳石墨结构的吸附剂Fein/C-700,用于去除水体中的金霉素,并对金霉素的吸附机理进行探讨. 结果表明该吸附动力学和等温吸附模型符合准二级动力学和Langmuir模型,吸附机理以静电相互作用、表面络合、氢键、π-π电子供体-受体(π-πEDA)相互作用为主,对实验室金霉素最大吸附量为546.5 mg·g−1,对实际水体中金霉素平均吸附量为380 mg·g−1.
-
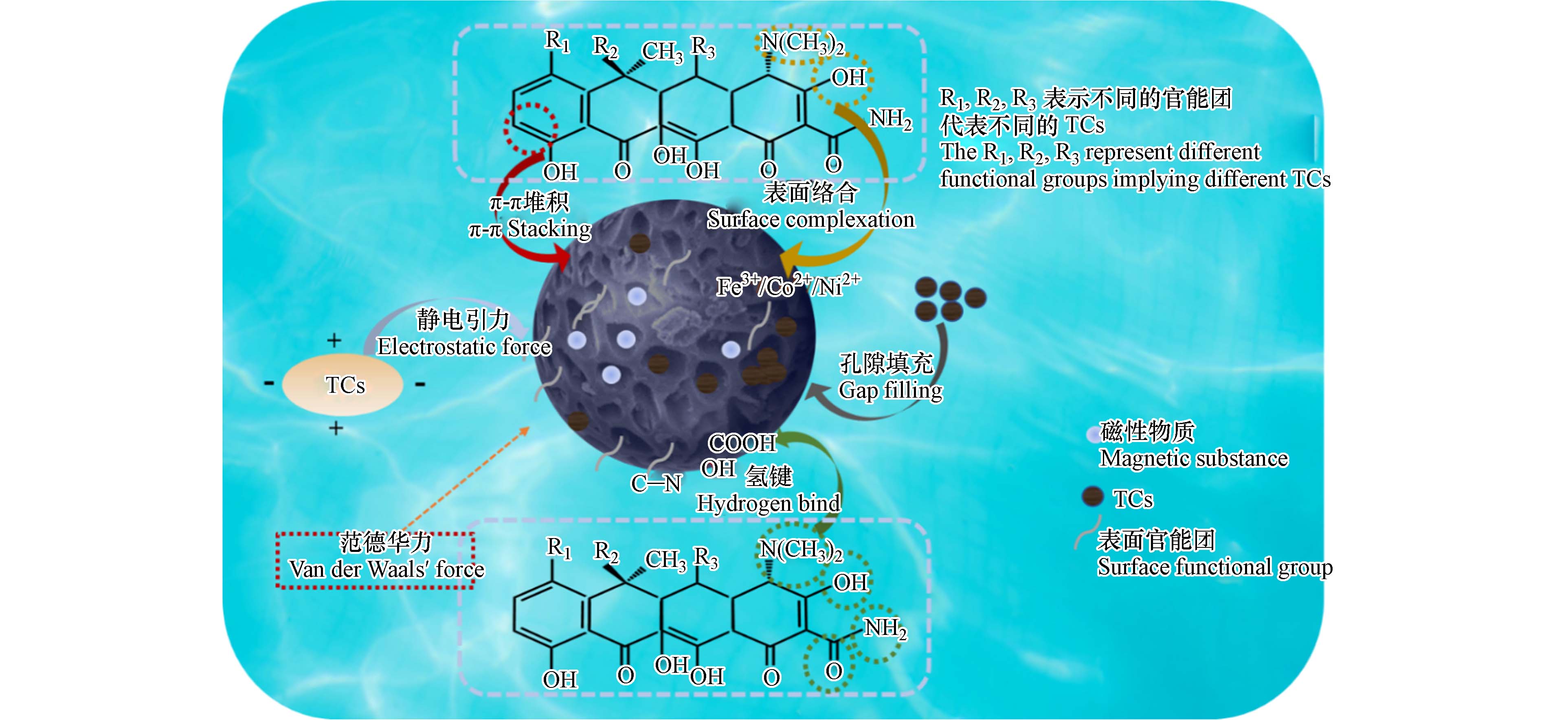
MCCN通过物理化学改性后,其比表面积、孔隙结构、形貌结构等物理性质发生显著变化,表面官能团、离子和极性等化学性质大幅度改变,理化性质改性增大了MCCN的吸附性. 对此,本文进一步总结了各类MCCN运用于四环素类抗生素的吸附去除机理总结如图4所示,图中MCCN表面附着TCs分子,且一些的孔洞被TCs填充,这表明MCCN的物理性质通过范德华力和孔隙填充作用吸附TCs;而其表面官能团(—COOH、—C=O、—OH)和表面离子则通过π-π作用、氢键、表面络合、静电作用等机制吸附TCs,这些作用力均是化学性的. 因此在吸附过程中,MCCN的理化性质共同影响TCs的吸附,且以MCCN的化学吸附机理为主.
在吸附过程中背景溶液的酸碱度、阴阳离子以及其他抗生素对TCs的吸附影响不可忽略. 溶液的酸碱度影响MCCN和TCs的分子分布形态,若MCCN所处溶液的pH值对应的Zeta电位大于零点电荷(pHpzc)电位,MCCN呈正电性,对阴离子且带负电荷的TCs的静电排斥作用力较弱;而当pH值的改变,相应的Zeta电位小于零点电荷(pHpzc)电位时,MCCN的负电性增大,对TCs静电排斥力增强,吸附能力下降. 常见的阳离子K+、Na+、Ca2+、Mg2+中K+和Na+对TCs的吸附均有影响,而Ca2+、Mg2+由于电荷较高,占据吸附位点体积较大,导致MCCN对TCs的吸附能力明显下降. 阴离子PO42−、SO42−、CO32−对TCs的吸附影响显著,尤其PO43−和CO32−水解产生HCO3−、HPO42−、H2PO4−使背景溶液的碱性增强,削弱MCCN的吸附能力. 其他种类的抗生素在不同pH范围中分布形态不同,可通过静电作用和π-π堆积作用与TCs竞争吸附位点,从而抑制TCs的吸附.
-
目前,高级氧化法(advanced oxidation processes, AOPs),尤其类Fenton法和过硫酸根基法,因产生的活性物种可将污染物降解成无毒或者低毒的小分子化合物,被认为是降解有机污染物(如抗生素)最有效的方法[24]. 生物质碳基材料如石墨烯、生物炭和碳气凝胶,作为过硫酸盐基AOPs的催化剂已被广泛研究. MCCN在高级氧化法中发挥两方面作用,一方面将过硫酸盐或H2O2激活产生活性物种,与其他催化剂协同降解TCs,另一方面对TCs进行磁分离,便于循环使用[25-26]. 应用各类MCCN高级氧化法降解TCs文献见表2所示.
-
生物质碳基材料具有成本低、环境友好、易广泛获得的优点而被广泛应用,其丰富的含碳量、高比面积和结构缺陷位点促使对TCS的吸附性和催化性很强. 该材料可分散金属,克服抗生素催化降解过程中金属的浸出率问题,同时常与过渡金属(Fe、Co、Ni)或过渡金属氧化物协同刺激过硫酸盐(PS)和H2O2产生活性物种(SO4−·、1O2、·OH),以增强MCCN的催化性能,此时过渡金属相当于提供磁源,在降解过程中易进行磁分离和回收,也高效参与催化降解抗生素[27 − 30].
MCCN的合成方法和改性相较于吸附过程,MCCN的理化性质在高级氧化降解过程中发挥多元作用,MCCN的物理性质如比表面积和孔隙结构主要提供吸附作用,将TCs吸附到吸附剂表面. 而化学性质如表面官能团COOH、OH、C=O主要活化H2O2和PDS产生对应的·OH和SO4−·[29-30],活化PMS直接产生SO4−·和OH[27]. 含Fe物质或表面缺陷激活PMS和PDS产生SO4−·和·OH,若含铁物质为Fe3O4,存在Fe2+和Fe3+的氧化还原反应,促进电子转移. 此外,C=O还可活化PMS和PDS产生单线态氧1O2以降解TCs[28].
Ni等[28]以海藻酸钠为碳前体,KOH为造孔剂,掺杂Fe、Co、Ni 3种金属分别合成相应的磁性碳气凝胶(SA-Fe、SA-Co、SA-Ni),三者的比表面积分别高达
2760 m2·g−1、2988 m2·g−1和2420 m2·g−1,其中SA-Fe对TC的去除能力达2000 mg·g−1,0.1 g·L−1的SA-Fe对200 mg·L−1的TC去除率为100%,重复使用5次后去除率仅下降4%,这都归因于不同价态的金属颗粒(Fe2+、Fe3+)在降解过程中发生氧化还原反应以及碳基材料中的酮基C=O接受电子生成单线态氧1O2激活PMS发挥降解作用. -
在碳基纳米材料已广泛用于PS以及H2O2的活化基础上,从自由基类型和石墨化程度角度考虑掺杂原子可提高生物质碳基材料的催化性能,其中,N杂原子由于其极大的电负性被众多研究者关注. N原子的掺杂可增加碳表面的碱度、增强与PMS或PS的亲和力,被N原子改性的生物质碳基材料电子结构会发生改变,电子转移速率增大,从而提高生物质碳基材料的催化活性[31 − 34]. 此类文献报道较多的是Fe和Co与N掺杂的生物质碳基材料,如磁性N-掺杂碳纳米纤维(Fe/Fe3C@NCNF-800)[35]、含FeC和FeN磁性生物炭(Fe-N@FMC)[36]、含Co的N-掺杂纳米碳(C-Co-TN)[37]等,结构中Fe和石墨N协同作用诱导PMS产生非自由基1O2降解TCs,去除率在80%—100%的范围. 含N-掺杂的MCCN的合成方法、改性以及理化性质与生物质衍生的MCCN在降解过程中相似,其降解优势在于N原子掺杂改性使整个复合材料结构中石墨N、吡啶N、吡咯N增加,激活更多的PMS产生SO4−·降解TCs.
Zeng等[31]以绿色、经济的生物质碳基材料为出发点,收集地下水处理厂、梧桐叶和尿素中的含铁污泥为原料,通过一步热解法制备磁性N掺杂的铁污泥生物炭(N-BCFe),并于过硫酸盐PS作用下降解TC,在最佳实验条件下,在120 min内对10 mg·L−1的TC去除率为86.56%. 结合EPR、自由基猝灭实验和XPS光谱,证实了对TC的降解机理为自由基SO4−·、·OH和非自由基1O2发挥活性作用. 此外,N-掺杂的生物质碳基材料可降低光型半导体(如TiO2、CeO2)的带隙,防止光载流子的复合,从而增加催化剂活性[38].
-
石墨烯氮化碳g-C3N4是一种新型的生物质碳基材料,属于含有聚合物结构的n型半导体. g-C3N4具有良好的电子带隙(约2.7 eV)、较宽的吸收光谱带和较高的稳定性,它独特的网络孔结构常被用作铁基的支撑材料,其分子骨架可吸附含铁离子,防止铁离子扩散至溶液中,避免抗生素催化降解中铁的沥出[39-40]. 然而g-C3N4在光照条件下产生的空穴-电子对易复合且比表面积较小,故将g-C3N4与其他光型半导体如CeO2、BiOX组成复合物,或在其表面掺杂贵金属,可捕获光生电子,以防止空穴-电子对复合[41 − 48].
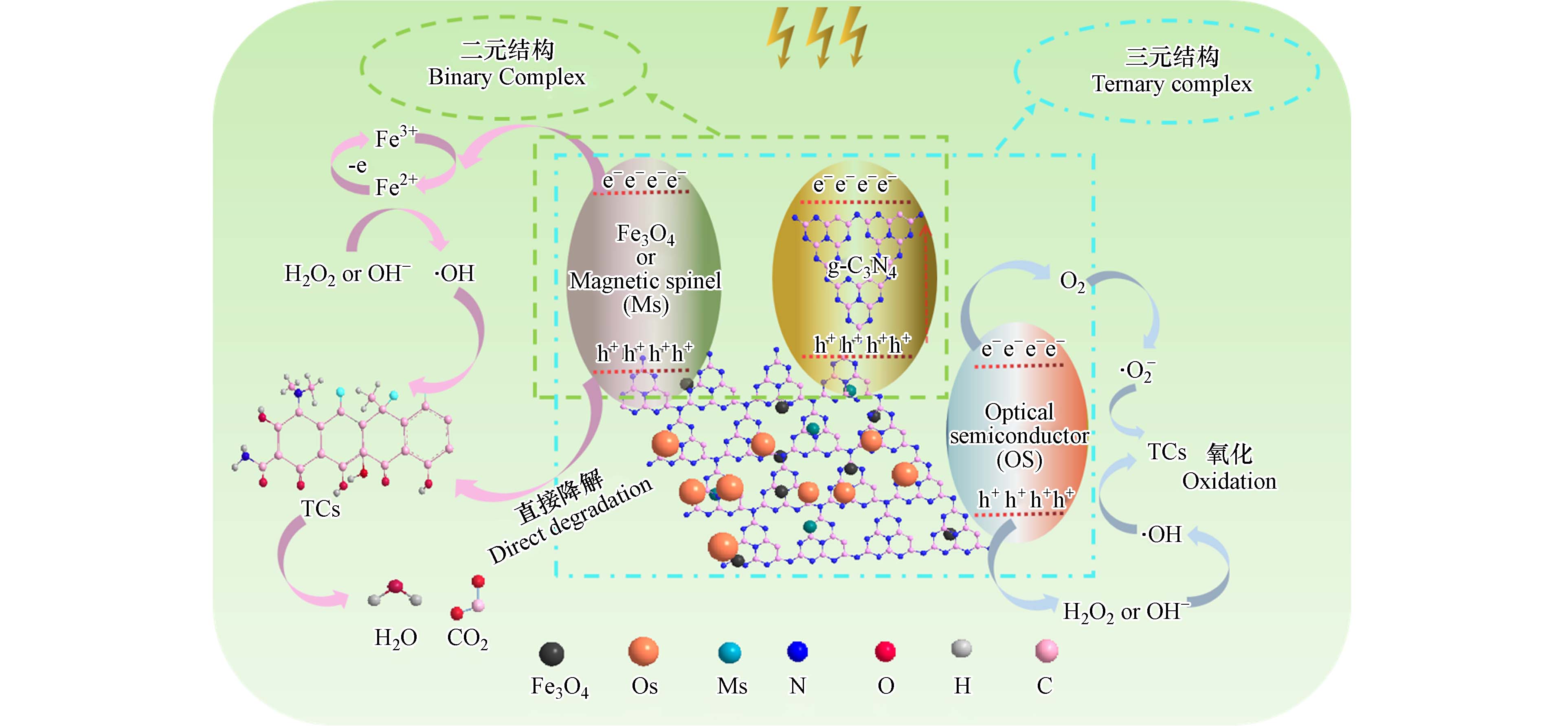
磁性g-C3N4碳基材料采用共沉淀法[41]、水热法[43]、共聚法[44]等方法合成二元或三元复合材料,其理化性质取决于与g-C3N4复合的单元,当只有磁性物质与g-C3N4组成二元复合物时,二者可构成异质结构产生活性物种降解TCs,若除了磁性物种还有其他光型半导体参与组成三元复合材料,则含Fe磁性物质中的Fe3+和Fe2+的发生氧化还原反应,促进电子转移,增强g-C3N4与其他光型半导体异质结构中电子和空穴的转移[45].
此前已报道的磁性g-C3N4碳基材料主要通过H2O2参与的类芬顿反应降解TCs. 本研究总结了MCCN参与类芬顿反应的降解机理如图5所示;复合物中的磁性物质导带上的电子e−被Fe3+捕获后,使Fe3+被还原成Fe2+促进H2O2或OH−产生活性物种·OH降解TCs,价带上的空穴h+可将TCs直接降解. 复合物中的任一单元,其导带上的电子e−均可被O2捕获后产生·O2−,价带上的空穴h+又可使H2O2或OH−产生活性物种·OH从而降解TCs.
H2O2的用量和背景溶液的pH是影响磁性g-C3N4碳基材料对TCs类芬顿降解的重要因素. H2O2可以与Fe3+反应循环再生Fe2+并参与Fenton反应,当H2O2剂量较高时,会产生更多的·OH,但H2O2剂量过大时,会导致·OH猝灭,使TCs的降解受阻. 背景溶液的pH对TCs过程的影响与其他的MCCN的讨论一致,即与磁性碳基材料和TCs的表面电荷分布密切相关.
-
生物质衍生和含N-掺杂的MCCN主要通过过硫酸盐法降解TCs,本文作者通过总结将高级氧化降解机理途径分为自由基途径和非自由基途径见表3,MCCN表面的官能团(-COOH、-OH、C=O等)、磁性离子以及缺陷等激活PMS、PDS和H2O2产生SO4−·或·OH,含N-掺杂的MCCN结构中由于存在石墨N、吡啶N、吡咯N可活化PMS产生SO4−·,SO4−·和·OH两种自由基共同作用去除TCs. 而官能团C=O激活PMS产生的单线态1O2、MCCN表面的电子和空穴通过非自由基方式降解TCs. 降解机理示意图的绘制如图6所示.
水质的pH值、阴离子是影响TCs降解的两大因子,pH通过影响MCCN的TCs表面电荷分布从而影响对PMS和PDS的活化,在较高pH值范围内,由于MCCN显负电荷,与PDS/PMS(S2O82−/HSO5−)产生静电斥力,阻碍两者之间的接触,从而阻碍活化. 在强碱条件下,PMS不稳定分解为SO42−和O2,PDS转化为活性更低的SO52−,这使产生活性物种的数量减少,最终降低了MCCN对TCs的降解. 阴离子诸如Cl−、HCO3−、NO3−、H2PO4−等对TCs降解的影响较大,归因于这些阴离子猝灭SO4−·、·OH后产生氧化能力较弱的活性物种,以致于降低对TCs的去除率. 如Cl−、HCO3−、NO3−、H2PO4四种离子猝灭SO4−·或·OH后产生的活性物种分别为·Cl2−、·CO3−、NO3·、·HPO4−,这些活性物种降解TCs的能力远比SO4−·、·OH弱,进一步抑制TCs的降解.
-
本文综述了近3年各类MCCN用于吸附和高级氧化降解去除TCs的研究.
(1)在作为吸附剂去除TCs的研究中,MCCN通过改性和活化后,比表面积和孔隙率增大,增强了对TCs的吸附性,尤其是杂原子N-掺杂改性的MCCN对TCs表现更高的吸附活性,是一类有效吸附TCs的磁性生物质碳吸附剂.
(2)在作为催化剂降解TCs的研究中,生物质衍生和N-掺杂改性的MCCN主要作载体以协同其他催化剂降解TCs,同时还激活过硫酸盐或H2O2产生活性物种,高效降解TCs. 此外,磁性石墨烯氮化碳g-C3N4与其他催化剂构造异质结构,使TCs快速降解,可作降解去除TCs的优异材料.
(3)MCCN的合成原料取自木质类天然物质、动物粪便、城市污泥、农业/食品废弃物等,原料的选取节约成本,方便对固废物的再回收利用,促进水体抗生素的修复工作.
在TCs水处理工艺中,MCCN通过吸附和降解去除TCs表现出较强的应用潜力,但在今后研究中仍面临着许多挑战.
(1)发展新型且性能更佳的MCCN
丰富的天然植物原料,如木本、禾本、草本、藻类、蕨类、苔藓等提供多样化的碳基材料,可发展成新型绿色的MCCN. 若将MCCN运用于吸附去除TCs,可考虑先将各种天然植物原料通过碳化后得到形态、结构差异的碳基材料,再将不同的碳基材料通过改变合成方法、加入活性剂、引入不同官能团和增加碳量子点等进行修饰,以获得吸附性能更加优异的磁性碳基材料;若将MCCN运用于降解去除TCs,则需要考虑磁源与碳质材料中异质结构策略、催化剂中导带和价带的电子转移速率以及光响应区域等.
(2)MCCN二次污染的问题
在MCCN参与吸附去除或高级氧化法降解TCs时,应最大限度地减少处理技术带来的环境足迹,如铁磁源易发生团聚导致吸附性和催化性降低,且可能存在含铁离子或其他半导型金属离子(Ti2+、Ce2+)溶出等问题,对环境造成二次污染,故考虑将碳基材料发生偶联反应后并作模板,可更大程度分散铁磁源和防止金属浸出.
(3)发展低成本MCCN
MCCN的种类主要源自碳基材料的活化和物理改性,磁性N-掺杂改性或石墨烯氮化碳g-C3N4材料虽能有效去除TCs,但制备成本较高,寻求合成方法简单、试剂价格低廉、性能优异的MCCN是未来研究的重要考量因素.
(4)增加MCCN实用性
目前文献报道磁性生物质碳基材料主要运用于有机污染物和重金属的去除、杀菌消毒、CO2的还原等领域,但关于MCCN运用于去除TCs的研究尚处于实验室模拟阶段,而实际环境复杂复杂多变,影响四环素类抗生素的因素众多,如环境酸碱度、共存阴阳离子以及其他持久性污染物等,因此需要重视磁性生物质碳基材料的实用性、稳定性以及商业性,以便应用到实际环境中.
(5)深入探究MCCN对TCs吸附去除/降解机理
尽管已有相关文献初步探究磁性碳基材料的吸附机理和光催化机理,但仍需进一步确定这些材料表面官能团、表界面效应、杂原子等化学结构在吸附机制中所起的作用,以及在光催化降解过程中光载流子的产生、重组、分离和转移所引起的作用,因此需要更为充足的表征技术手段和辅以理论计算进一步探究机理.
磁性碳基复合纳米材料对四环素类抗生素的吸附和降解研究进展
Research progress on adsorption and degradation of tetracycline antibiotics by magnetic carbon-based composite nanomaterials
-
摘要: 近年来,磁性碳基复合纳米材料(magnetic carbon-based composite nanomaterials,MCCN)因其具有比表面积大,方便磁分离及回收循环利用等优点而引起众多科研工作者关注. 鉴于四环素类抗生素(tetracycline antibiotics,TCs)在环境中耐性强、不易分解,长期累积对人体健康造成潜在的威胁. 由此,本文综述了近3年来发展的各类新型MCCN作为优良吸附剂和催化剂应用于TCs的去除,并在吸附和降解两方面对去除TCs的机理和影响因子作出总结. 重点阐述了磁性碳基复合纳米材料作吸附剂时,其碳前驱体被炭化、活化、磁化以及杂原子改性后获得的磁性碳基材料,通过氢键、π-π作用、静电作用等机制吸附去除TCs;作为催化剂时,磁性碳基材料主要作为基底,负载功能化催化剂,通过激活一些过硫酸盐,促使催化过程中产生活性物种如·O2−、·OH、1O2等,将TCs降解为CO2、H2O或其他小分子物质. 最后,对磁性碳基纳米复合材料未来的研究方向提出一些建议.
-
关键词:
- 磁性碳基复合纳米材料 /
- 四环素类抗生素 /
- 吸附 /
- 降解.
Abstract: In recent years, magnetic carbon-based composite nanomaterials (MCCN) had attracted the attention of many researchers because of their large specific surface area, convenient magnetic separation and recycling. In view of Tetracycline antibiotics (TCs) had strong tolerance and were not easy to decompose in the environment, and long-term accumulation posed a potential threat to human health. Therefore, the application of various new MCCN developed in recent three years was summarized in this study, magnetic carbon composite nano-materials were used as excellent adsorbents and catalysts to remove TCs, and summarized the mechanism and influencing factors of TCs removal in both adsorption and degradation. It was emphasized that when MCCN were used as adsorbents, the carbon precursors are carbonized, activated, magnetized and modified by heteroatoms, and TCs is removed by hydrogen bonding, π-π interaction and electrostatic interaction. As a catalyst, magnetic carbon based materials mainly serve as substrates, loading functionalized catalysts, and activating some persulfates to promote the production of active species during the catalytic process, such as O2−,·OH, 1O2 in the catalytic process, to degrade TCs into CO2 , H2O or other small molecules. Finally, some suggestions on the future research direction of magnetic carbon-based composite nanomaterials were put forward. -

-
表 1 MCCN吸附去除TCs
Table 1. Removal of TCs by adsorption of MCCN
吸附剂
Adsorbent原料
Raw materials吸附容量 /(mg·g−1)Adsorption
capacity热解温度/℃ Pyrolysis
temperature比表面积/(m2·g−1) Specific
surface area平均孔径/ nm Average pore
size吸附机理
Adsorption mechanism磁性多孔生物炭(MPBC-700) 茶渣[9] 333.22 700 1066.00 — abcd 磁性茶渣生物炭(MKHBC) 茶渣[10] 236.93 700 1035.11 2.51 ab 磁性多孔生物炭(H-Fe-BC) 茶渣[11] 229.30 700 852.00 2.65 abcde 磁性松树皮生物炭(M-PBB) 松树皮[12] 293.32 600 — — e 磁性生物炭(LLB-MB) 丝瓜叶[13] 1755.9 — 2565.4 2.28 abce 磁性酒糟生物炭(V-MFB-MCs-800) 酒糟[14] 323 800 161.174 — abcf 磁性生物炭(HAs/KMBC) 稻草[15] 1743 600 — — bc 磁性杨木生物炭(MPBC) 杨木[16] 89.58 — 122.12 12.24 abcde 磁性生物炭(MBC) 废木耳[17] 42.31 700 333.4 5.50 cd 磁性分级多孔碳(LPC-NC) 莲子盆[18] 506.6 800 1621.3 — bf 磁性多孔碳(N-MPC) 阴离子树脂[19] 603.4 850 781.1 2.86 abc 改性磁性污泥碳(MNSBC) 城市污泥[20] 197.3 700 243 3.56 abe 磁性多孔碳(M-PLAC) 木质素[21] 1306.0 800 252.21 2.99 abce 铁氮共改性生物炭(N-RSBC) 稻草[22] 156.0 700 606.22 2.207 abcde MOF衍生磁性石墨碳(Fein/C-700) Zn-MOF[23] 546.5 700 244.87 — abce 注:a. 孔隙填充; b. π-π作用; c. 氢键; d. 络合作用; e. 静电作用; f. 阳离子-π作用. —. 无数据.
Note: a. Pore filling; b.π-π interaction; c. Hydrogen bonding; d. Complexation; e. Electrostatic action; f. Cation-π interaction.表 2 MCCN高级氧化法降解去除TCs
Table 2. Degradation and removal of TCs by advanced oxidation of MCCN
催化剂
Catalyst
(Abbreviviation)激活对象
Activation
object去除率/%
Removal rate活性物种
Active
species降解产物
Degradation
product降解途径
Degradation
pathway参考文献
Reference铁钴双金属生物炭(BC@CFC) PMS 96.63 SO4−·、·OH CO2、H2O、SO42− 自由基 [27] 磁性气凝胶(SA-Fe) PMS 100.0 1O2 CO2、H2O 非自由基 [28] 磁性介孔碳(MC) PDS 92.9 SO4−·、·O2−、1O2 CO2、H2O 自由基 [29] 磁性石墨生物炭(FeOx@g-BC) H2O2 100.0 1O2、·OH CO2、H2O 自由基和非自由基 [30] 磁性N掺杂铁污泥碳材料(N-BCFe) PMS 86.56 SO4−·、·OH、1O2 CO2、H2O 自由基和非自由基 [31] Co/N共掺杂生物炭(Co-N/KC-900) PMS 99.0 1O2 CO2、H2O 非自由基 [32] N、S共掺杂磁性碳(Fe@NS-C) PMS 91.07 1O2、电子转移 CO2、H2O、NH4+ 非自由基 [33] 磁性生物炭(MBC) PDS 87.2 1O2 — 非自由基 [34] 磁性碳纳米纤维(Fe/Fe3C@NCNF) PMS 100.0 ·O2-、1O2 CO2、H2O 自由基和非自由基 [35] 磁性生物炭(Fe-N@FMC) PDS 90.5 SO4−·、·OH CO2、H2O 自由基 [36] 磁性N掺杂纳米碳(C-Co-TN) PMS 94.0 1O2 CO2、H2O 非自由基 [37] N掺杂磁性碳纳米纤维(MCMs@TiO) — 98.95 ·O2−、·OH CO2、H2O 自由基 [38] Fe3O4/CeO2/g-C3N4(FCG) H2O2 96.63 ·OH CO2、H2O 自由基 [39] g-C3N4/NiFe2O4(CN/NFO) H2O2 78.0 ·O2−、·OH — 自由基 [40] CuFe2O4/g-C3N4(CFO/CN) — 94.0 ·O2−、·OH CO2、H2O 自由基 [41] Fe3O4/g-C3N4/rGO(无) — 86.7 ·O2−、·OH — 自由基 [42] Pd/g-C3N4/Fe3O4(无) — 97.0 h+、·O2− CO2、H2O 自由基和非自由基 [43] MLD/g-C3N4/Fe3O4(无) H2O2 95.8 ·OH CO2、H2O 自由基 [44] g-C3N4/BiOBr/Fe3O4(无) — 100.0 h+、·OH、·O2− CO2、H2O 自由基和非自由基 [45] 注:激活对象中,PMS:过一硫酸盐;PDS:过二硫酸盐.
Note: Among the activated objects, PMS: Persulfate; PDS: Persulphate.表 3 MCCN对TCs的降解途径
Table 3. Degradation pathway of TCs by MCCN
项目
Project激活对象
Activation object自由基
Radical非自由基
Nonradical
官能团及磁性离子H2O2
PMS
PDS·OH
SO4−·和·OH
SO4−·1O2 缺陷 PMS SO4−· — e− — — 直接降解 h+ — — 直接降解 石墨-N、吡啶-N、吡咯-N PMS SO4−· — -
[1] YANG Q L, GAO Y, KE J, et al. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods[J]. Bioengineered, 2021, 12(1): 7376-7416. doi: 10.1080/21655979.2021.1974657 [2] MARKLEY J L, WENCEWICZ T A. Tetracycline-inactivating enzymes[J]. Frontiers in Microbiology, 2018, 9: 1058 doi: 10.3389/fmicb.2018.01058 [3] CAO Y Y, WANG X R, BAI H J, et al. Fluorescent detection of tetracycline in foods based on carbon dots derived from natural red beet pigment[J]. LWT-Food Science and Technology, 2022, 157: 113100. doi: 10.1016/j.lwt.2022.113100 [4] SHENG D H, YING X T, LI R, et al. Polydopamine-mediated modification of ZIF-8 onto magnetic nanoparticles for enhanced tetracycline adsorption from wastewater[J]. Chemosphere, 2022, 308: 136249. doi: 10.1016/j.chemosphere.2022.136249 [5] 龙星宇, 王志敏, 张江群, 等. Fe3O4/GO/ZnO材料的制备及其对盐酸土霉素吸附研究[J]. 贵州师范大学学报(自然科学版), 2023, 41(1): 80-86. LONG X Y, WANG Z M, ZHANG J Q, et al. Preparation of Fe3O4/GO/ZnO materials and their adsorption to oxytetracycline hydrochloride[J]. Journal of Guizhou Normal University (Natural Sciences), 2023, 41(1): 80-86(in Chinese)
[6] LIU Z M, GAO Z M, WU Q. Activation of persulfate by magnetic zirconium-doped manganese ferrite for efficient degradation of tetracycline[J]. Chemical Engineering Journal, 2021, 423: 130283. doi: 10.1016/j.cej.2021.130283 [7] LUO X W, SHEN M X, LIU J H, et al. Resource utilization of piggery sludge to prepare recyclable magnetic biochar for highly efficient degradation of tetracycline through peroxymonosulfate activation[J]. Journal of Cleaner Production, 2021, 294: 126372. doi: 10.1016/j.jclepro.2021.126372 [8] JIANG Y C, LUO M F, NIU Z N, et al. In-situ growth of bimetallic FeCo-MOF on magnetic biochar for enhanced clearance of tetracycline and fruit preservation[J]. Chemical Engineering Journal, 2023, 451: 138804. doi: 10.1016/j.cej.2022.138804 [9] LI B, ZHANG Y, XU J, et al. Facile preparation of magnetic porous biochars from tea waste for the removal of tetracycline from aqueous solutions: Effect of pyrolysis temperature[J]. Chemosphere, 2022, 291: 132713. doi: 10.1016/j.chemosphere.2021.132713 [10] LI B, ZHANG Y, XU J, et al. Simultaneous carbonization, activation, and magnetization for producing tea waste biochar and its application in tetracycline removal from the aquatic environment[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105324. doi: 10.1016/j.jece.2021.105324 [11] ZHANG Y, HUANG Z J, FANG X, et al. Preparation of magnetic porous biochar through hydrothermal pretreatment combined with K2FeO4 activation to improve tetracycline removal[J]. Microporous and Mesoporous Materials, 2022, 343: 112188. doi: 10.1016/j.micromeso.2022.112188 [12] RYENCHINDORJ U, ZAIB Q, PUTRA A S, et al. Production and characterization of cost-effective magnetic pine bark biochar and its application to remove tetracycline from water[J]. Environmental Science and Pollution Research International, 2022, 29(41): 62382-62392. doi: 10.1007/s11356-022-19866-9 [13] SU Y J, ZHENG Y Y, FENG M Q, et al. Magnetic Luffa-leaf-derived hierarchical porous biochar for efficient removal of rhodamine B and tetracycline hydrochloride[J]. International Journal of Molecular Sciences, 2022, 23(24): 15703. doi: 10.3390/ijms232415703 [14] XIANG Y J, ZHOU Y Z, YAO B, et al. Vinasse-based biochar magnetic composites: Adsorptive removal of tetracycline in aqueous solutions[J]. Environmental Science and Pollution Research International, 2023, 30(4): 8916-8927. [15] LI M F, WANG P, HUANG C X, et al. Effect of dissolved humic acids and coated humic acids on tetracycline adsorption by K2CO3-activated magnetic biochar[J]. Scientific Reports, 2022, 12: 18966. doi: 10.1038/s41598-022-22830-9 [16] ZHANG X Z, ZHEN D W, LIU F M, et al. An achieved strategy for magnetic biochar for removal of tetracyclines and fluoroquinolones: Adsorption and mechanism studies[J]. Bioresource Technology, 2023, 369: 128440. doi: 10.1016/j.biortech.2022.128440 [17] GAO F, XU Z X, DAI Y J. Removal of tetracycline from wastewater using magnetic biochar: A comparative study of performance based on the preparation method[J]. Environmental Technology & Innovation, 2021, 24: 101916. [18] WANG T, XUE L, LIU Y H, et al. N self-doped hierarchically porous carbon derived from biomass as an efficient adsorbent for the removal of tetracycline antibiotics[J]. Science of the Total Environment, 2022, 822: 153567. doi: 10.1016/j.scitotenv.2022.153567 [19] ZHU Y T, SHEN J, GUO M L, et al. Nitrogen-doped magnetic porous carbon material from low-cost anion-exchange resin as an efficient adsorbent for tetracyclines in water[J]. Environmental Science and Pollution Research International, 2023, 30(10): 27315-27327. [20] MA Y F, LU T M, TANG J Y, et al. One-pot hydrothermal synthesis of magnetic N-doped sludge biochar for efficient removal of tetracycline from various environmental waters[J]. Separation and Purification Technology, 2022, 297: 121426. doi: 10.1016/j.seppur.2022.121426 [21] TIAN Y X, YIN Y B, JIA Z Y, et al. One-pot preparation of magnetic nitrogen-doped porous carbon from lignin for efficient and selective adsorption of organic pollutants[J]. Environmental Science and Pollution Research International, 2023, 30(6): 14943-14958. [22] MEI Y L, XU J, ZHANG Y, et al. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution[J]. Bioresource Technology, 2021, 325: 124732. doi: 10.1016/j.biortech.2021.124732 [23] HU X, XIE Y J, HE R N, et al. Nano-iron wrapped by graphitic carbon in the carbonaceous matrix for efficient removal of chlortetracycline[J]. Separation and Purification Technology, 2021, 279: 119693. doi: 10.1016/j.seppur.2021.119693 [24] FAN B B, TAN Y, WANG J X, et al. Application of magnetic composites in removal of tetracycline through adsorption and advanced oxidation processes (AOPs): A review[J]. Processes, 2021, 9(9): 1644. doi: 10.3390/pr9091644 [25] 权衡, 牛琳, 时迪, 等. 负载纳米零价铁的铁碳材料制备及其降解抗生素性能研究[J]. 环境科学研究, 2022, 35(12): 2732-2747. QUAN H, NIU L, SHI D, et al. Preparation of iron-carbon materials loaded with nano zero-valent iron and their performance of degrading antibiotics[J]. Research of Environmental Sciences, 2022, 35(12): 2732-2747(in Chinese).
[26] 王婧, 何文宇, 何欣阳, 等. 氧化钴纳米球与低载银的相互作用及其对炭烟氧化的影响[J]. 陕西师范大学学报(自然科学版), 2022, 50(2): 29-37,2. WANG J, HE W Y, HE X Y, et al. The interaction between cobalt oxide nanospheres and low-loaded silver and its effects on soot oxidation[J]. Journal of Shaanxi Normal University (Natural Science Edition), 2022, 50(2): 29-37,2(in Chinese).
[27] YANG W H, JING L Y, WANG T, et al. Multi-level porous layered biochar modified cobalt-iron composite as a reusable synergistic activator of peroxymonosulfate for enhanced tetracycline degradation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2022, 132: 104209. doi: 10.1016/j.jtice.2022.104209 [28] NI J X, GAO Y, SUN Y R, et al. High-efficiency removal of antibiotic pollutants by magnetic carbon aerogel: Inherent roles of adsorption synergistic catalysis[J]. Journal of Cleaner Production, 2022, 375: 134105. doi: 10.1016/j.jclepro.2022.134105 [29] ZHANG L B, WANG Y Q, SHI Y H, et al. Heterogeneous catalytic oxidation of tetracycline hydrochloride based on persulfate activated by Fe3O4/MC composite[J]. Chemical Engineering Journal, 2022, 447: 137406. doi: 10.1016/j.cej.2022.137406 [30] ZHANG X, WU H Y, CHEN M, et al. Graphitic biochar with in situ confined magnetic iron oxides via synchronous pyrolysis of lignin as an effective H2O2 activator for fast degradation of organic pollutants[J]. International Journal of Biological Macromolecules, 2022, 205: 329-340. doi: 10.1016/j.ijbiomac.2022.02.076 [31] ZENG H P, LI J X, XU J X, et al. Preparation of magnetic N-doped iron sludge based biochar and its potential for persulfate activation and tetracycline degradation[J]. Journal of Cleaner Production, 2022, 378: 134519. doi: 10.1016/j.jclepro.2022.134519 [32] ZHU H, GUO A, WANG S M, et al. Efficient tetracycline degradation via peroxymonosulfate activation by magnetic Co/N Co-doped biochar: Emphasizing the important role of biochar graphitization[J]. Chemical Engineering Journal, 2022, 450: 138428. doi: 10.1016/j.cej.2022.138428 [33] HE D D, ZHU K, HUANG J, et al. N, S co-doped magnetic mesoporous carbon nanosheets for activating peroxymonosulfate to rapidly degrade tetracycline: Synergistic effect and mechanism[J]. Journal of Hazardous Materials, 2022, 424(Pt C): 127569. [34] XIONG S, DENG Y C, GONG D X, et al. Magnetically modified in situ N-doped Enteromorpha prolifera derived biochar for peroxydisulfate activation: Electron transfer induced singlet oxygen non-radical pathway[J]. Chemosphere, 2021, 284: 131404. doi: 10.1016/j.chemosphere.2021.131404 [35] ZHU K, XIA W, HE D D, et al. Facile fabrication of Fe/Fe3C embedded in N-doped carbon nanofiber for efficient degradation of tetracycline via peroxymonosulfate activation: Role of superoxide radical and singlet oxygen[J]. Journal of Colloid and Interface Science, 2022, 609: 86-101. doi: 10.1016/j.jcis.2021.11.178 [36] ZHUO S N, SUN H, WANG Z Y, et al. A magnetic biochar catalyst with dual active sites of Fe3C and Fe4N derived from floc: The activation mechanism for persulfate on degrading organic pollutant[J]. Chemical Engineering Journal, 2023, 455: 140702. doi: 10.1016/j.cej.2022.140702 [37] LI Q Q, LIU J D, REN Z J, et al. Catalytic degradation of antibiotic by Co nanoparticles encapsulated in nitrogen-doped nanocarbon derived from Co-MOF for promoted peroxymonosulfate activation[J]. Chemical Engineering Journal, 2022, 429: 132269. doi: 10.1016/j.cej.2021.132269 [38] ZHOU Y S, CAI T M, LIU S, et al. N-doped magnetic three-dimensional carbon microspheres@TiO2 with a porous architecture for enhanced degradation of tetracycline and methyl orange via adsorption/photocatalysis synergy[J]. Chemical Engineering Journal, 2021, 411: 128615. doi: 10.1016/j.cej.2021.128615 [39] WANG S, LONG J R, JIANG T, et al. Magnetic Fe3O4/CeO2/g-C3N4 composites with a visible-light response as a high efficiency Fenton photocatalyst to synergistically degrade tetracycline[J]. Separation and Purification Technology, 2021, 278: 119609. doi: 10.1016/j.seppur.2021.119609 [40] LU C Y, WANG J, CAO D L, et al. Synthesis of magnetically recyclable g-C3N4/NiFe2O4 S-scheme heterojunction photocatalyst with promoted visible-light-response photo-Fenton degradation of tetracycline[J]. Materials Research Bulletin, 2023, 158: 112064. doi: 10.1016/j.materresbull.2022.112064 [41] SUN X Y, HUANG L K, WANG G Z, et al. Efficient degradation of tetracycline under the conditions of high-salt and coexisting substances by magnetic CuFe2O4/g-C3N4 photo-Fenton process[J]. Chemosphere, 2022, 308(1): 136204. [42] SHAN J Y, WU X L, LI C F, et al. Photocatalytic degradation of tetracycline hydrochloride by a Fe3O4/g-C3N4/rGO magnetic nanocomposite mechanism: Modeling and optimization[J]. Environmental Science and Pollution Research International, 2023, 30(3): 8098-8109. doi: 10.1007/s11356-022-22770-x [43] XU Q, LIU L, WEI J L, et al. The magnetically separable Pd/C3N4/Fe3O4 nanocomposite as a bifunctional photocatalyst for tetracycline degradation and hydrogen evolution[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2022, 641: 128404. doi: 10.1016/j.colsurfa.2022.128404 [44] ZHANG X, REN B, LI X, et al. High-efficiency removal of tetracycline by carbon-bridge-doped g-C3N4/Fe3O4 magnetic heterogeneous catalyst through photo-Fenton process[J]. Journal of Hazardous Materials, 2021, 418: 126333. doi: 10.1016/j.jhazmat.2021.126333 [45] PREEYANGHAA M, DHILEEPAN M D, MADHAVAN J, et al. Revealing the charge transfer mechanism in magnetically recyclable ternary g-C3N4/BiOBr/Fe3O4 nanocomposite for efficient photocatalytic degradation of tetracycline antibiotics[J]. Chemosphere, 2022, 303(2): 135070. [46] 王廷媛, 尚越, 龙星宇. Fe3O4@SiO2@CeO2应用于废水中亚甲基蓝的吸附研究[J]. 贵州师范大学学报(自然科学版), 2021, 39(5): 22-28. WANG T Y, SHANG Y, LONG X Y. Study on the adsorption of Methylene blue from wastewater by Fe3O4@SiO2@CeO2[J]. Journal of Guizhou Normal University (Natural Sciences), 2021, 39(5): 22-28(in Chinese).
[47] 孔婷, 张金牛, 姜紫赫, 等. BiOI光催化性能的调控机制[J]. 陕西师范大学学报(自然科学版), 2022, 50(1): 43-52. KONG T, ZHANG J N, JIANG Z H, et al. Regulation mechanism of BiOI photocatalytic performance[J]. Journal of Shaanxi Normal University (Natural Science Edition), 2022, 50(1): 43-52(in Chinese).
[48] ZHAI W J, HE J F, HU S T, et al. Enhanced photocatalytic degradation of tetracycline over magnetic La0.7Sr0.3MnO3/g-C3N4 p-n heterojunction arising from the synergistic effects of oxygen vacancy defects and high-potential photogenerated electrons[J]. Journal of Alloys and Compounds, 2022, 918: 165699. doi: 10.1016/j.jallcom.2022.165699 -




 下载:
下载: