-
乙烯(C2H4)是一种自然界中广泛存在的内源性植物激素,它能促进色素、香气等物质在果蔬中的积累,改善果蔬的色泽、风味和口感[1],因此可作为催熟剂应用于果蔬的生产和种植中[2]. 但同时,由于乙烯可以促进呼吸酶的氧化作用[3],即使在低温下,微量乙烯也会导致储藏室和冰箱中新鲜的产品逐渐成熟和变质[4 − 5],使得果蔬的贮藏保鲜期缩短. 因此,清除低温储存环境中的乙烯可有效维持水果和蔬菜的品质,延长货架期,具有很大的商业价值.
目前已开发出的乙烯脱除方法,主要可分为物理吸附[6 − 8]、化学氧化[9 − 10]和催化氧化[6,11 − 13]等. 催化氧化法与其他方法相比具有运行成本低、重复使用性高、转化效率高等优势[14],而低温催化氧化又因反应条件温和,适用于产品保鲜条件下的乙烯脱除,因此意义重大[15]. 研究发现Pt基催化剂相比于Pd、Au和Ag等金属催化剂拥有更高的乙烯催化氧化活性,并且在较长的反应时间和循环使用中表现出优异的耐久性[16],因此被选为本次研究的活性金属. 同时SBA-15[17]具有出色的热稳定性和机械稳定性,其限域作用会使得金属前驱体盐在煅烧分解和还原过程中不易团聚,得到分散性较好的金属颗粒[18],在乙烯催化氧化中表现出高活性[19],因此被选为本研究的载体.
鉴于催化剂表面水分子的物理吸附是导致催化剂失活的关键因素[20],因而调节催化剂表面的疏水性是十分必要的. 目前对催化材料的表面进行改性以提高其疏水性的策略已经被开发并证明是有效的[21 − 22],但其中多数研究都存在操作复杂、回收率低、需要使用大量有机试剂等诸多问题[23],因此仍需要开发一种简便、高效的技术提高催化剂表面的疏水性以改善催化剂低温催化氧化乙烯的活性.
本文采用浸渍法将1% Pt负载到SBA-15上,并使用硅烷偶联剂(三甲基氯硅烷,TMCS)对催化剂进行甲基硅烷化改性,以获得疏水型低温乙烯催化氧化剂. 对催化剂在0 oC下氧化痕量乙烯(0.005%)的效率进行了研究,并通过调整TMCS用量以确定最佳甲基硅烷化剂量,以期提升催化剂低温催化氧化乙烯效果,为构建高效疏水催化剂提供支撑.
-
本文所用试剂有三甲基氯硅烷(TMCS, GC, 阿拉丁试剂)、EO20PO70EO20 (P123, AR, Aldrich公司)、正硅酸四乙酯(TEOS, AR, 国药集团化学试剂有限公司)、氯铂酸六水合物(H2PtCl6·6H2O, AR, 上海阿拉丁生化科技股份有限公司)和盐酸(HCl, AR, 南京化学试剂有限公司).
-
介孔SBA-15的合成[24]:以三嵌段共聚物P123为结构导向剂,TEOS为硅源. 具体步骤为,在烧杯中加入8 g P123和300 mL HCl水溶液(1.6 mol·L−1),在40 oC下搅拌4 h直到P123完全溶解,溶液呈均匀的乳白色. 继续滴加18 g TEOS,在40 oC搅拌24 h. 然后将反应液转移至自生压反应釜,在100 oC下反应48 h. 冷却后过滤,以去离子水洗至中性,在80 oC下烘干,并在550 oC焙烧6 h(升温速率1 oC·min−1),以去除模版剂P123,所得样品记为SBA-15.
Pt/SBA-15的制备:采用普通浸渍法制备Pt/SBA-15. 称取0.3 g SBA-15载体,加入20 mL去离子水和0.797 mL H2PtCl6 (1 g·mL−1)溶液,在90 oC水浴条件下搅拌并蒸干. 将得到的固体在充分研磨后置于300 oC管式炉中焙烧2 h (升温速率为5 ℃·min−1),冷却后置于20 mL·min−1的H2氛围中以200 oC还原2 h,所得样品记为1% Pt/SBA-15.
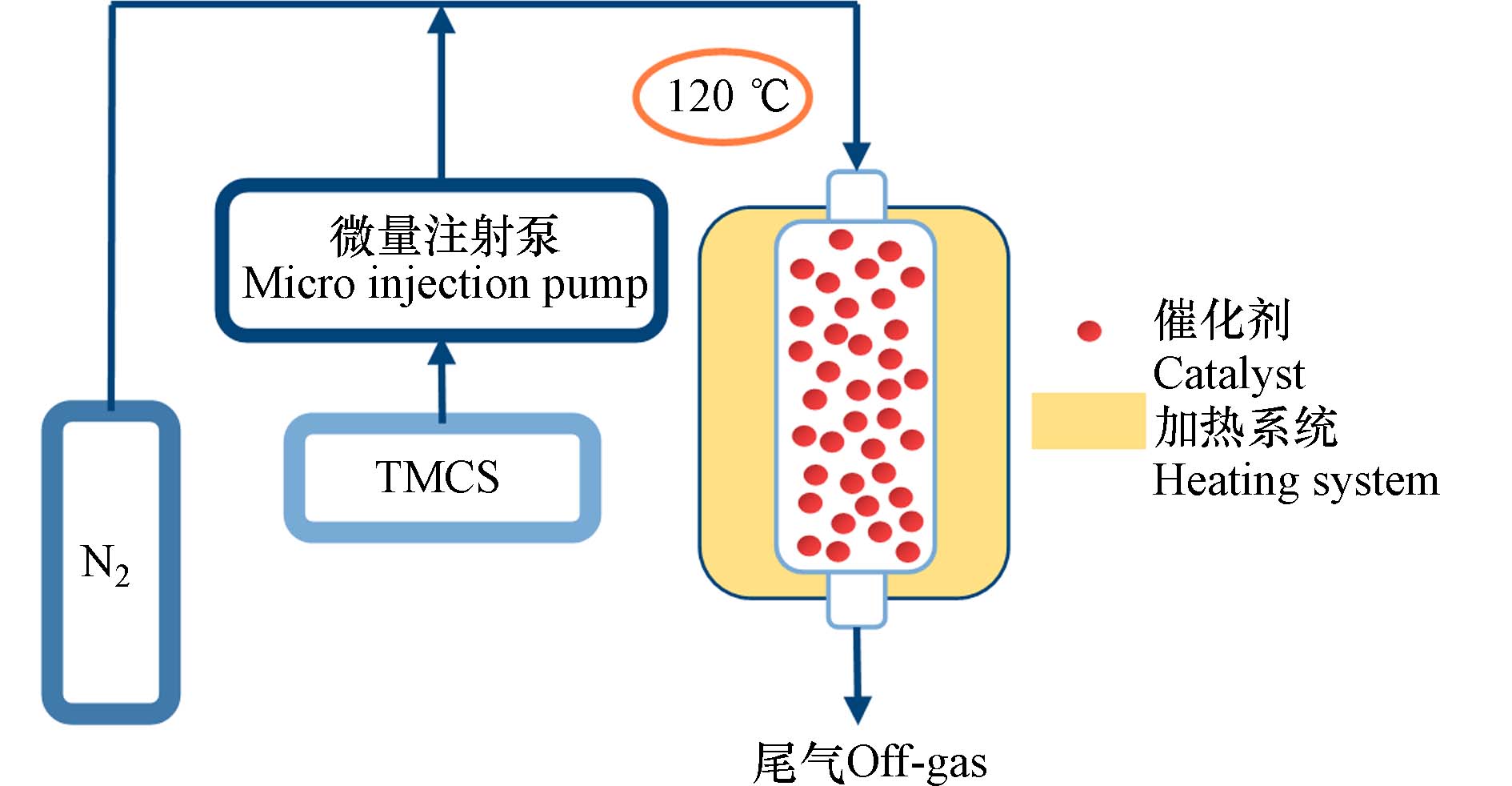
Pt/SBA-15-TMCS的制备:TMCS硅烷化反应采用实验室自主搭建的反应装置(图1). 在120 oC连续流动N2 (20 mL·min−1)氛围下,微量注射泵泵入并气化的TMCS,与催化剂反应. 连续进样12 h,所得硅烷化产物(Pt/SBA-15-TMCS). 实验中TMCS用量与Pt/SBA-15用量比例分别为1:12、1:6、1:3 (mL·g−1).
-
使用X射线衍射仪(XRD, 日本理学株式会社, D/max-RA)对催化剂进行物相分析;采用ICP-AES(美国PerkinElmer公司, Avio500)以获得催化剂表面具体负载的Pt含量;使用透射电子显微镜(TEM, 日本电子株式会社, JEM-200CX)观察催化剂的微观形貌特征;通过比表面积分析仪(BET, 美国MICROMERITICS公司, ASAP2020)测定N2吸附-脱附等温线和孔径分析以确定催化剂的比表面积和孔径大小;采用傅里叶红外光谱仪(FT-IR, 美国赛默飞世尔公司, Nicolet IS10)对催化剂进行CO吸附原位漫反射光谱分析;通过测量催化剂与液态水的接触角(Static contact angle measurements, 美国迪飞公司, OCA30)表征催化剂的疏水性.
-
样品的CO吸附原位红外光谱是通过美国赛默飞世尔公司的Nicolet IS10分析得到的. 扫描的波数范围为
1700 —2300 cm−1, 分辨率为4 cm−1, 扫描次数为32次. 具体地,将样品粉末平铺于红外样品池中,并以5 oC·min−1的升温速率升到150 oC在N2 (20 mL·min−1)氛围下预处理2 h. 降至室温后,在N2氛围下取背景. 随后将N2切换为CO/N2 (20 mL·min−1)以吸附CO,待吸附30 min后再将CO/N2切换回N2吹扫30 min,最后用红外光谱扫描获得CO在样品表面化学吸附的谱图. -
乙烯的催化氧化反应在内径约为4 mm的固定床流动反应器中进行. 具体地,将0.4 g催化剂(40—60目)装入U型反应管中,并在150 oC连续流动N2(20 mL·min−1)下对催化剂进行预处理2 h. 冷却至室温后,将U型管置于0 oC的低温水浴恒温槽中稳定30 min. 然后将混合气体(C2H4, 0.005%; O2, 20%; N2, 5%; He, 平衡)在0 oC下以
1500 mL·h−1·g−1的空间速度(SV)加入催化剂床. 出口气体组分通过在线气相色谱法(北分瑞利3420A, 配备氢火焰离子化检测器和与Porapak-Q柱连接的Ni催化转换器)进行检测. 出口气体与气相色谱通过六通阀连接,气体组分每5 min记录1次. C2H4转化率通过([C2H4]in-[C2H4]out)×100/[C2H4]out获得,其中[C2H4]in和[C2H4]out分别对应于C2H4的初始浓度和C2H4出口浓度. 单次实验结束后,将U型管中催化剂取出并回收以备重复测试. -
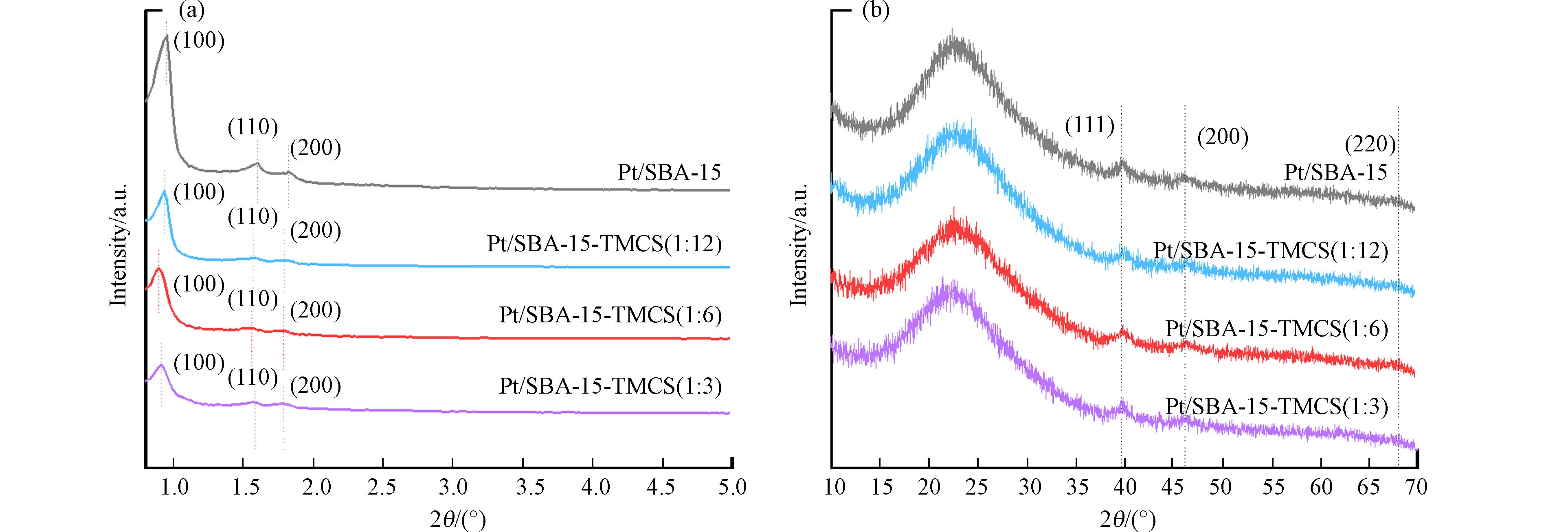
从Pt/SBA-15和Pt/SBA-15-TMCS的结构表征来看,XRD图谱显示,4种催化剂均检测出了SBA-15和Pt纳米粒子的特征衍射峰(图2). 其中,0.95o、1.57o、1.79o的特征峰位置分别可以对应于SBA-15的(100)、(110)、(200)晶面;39.7o、46.2o、67.9o处的特征峰位置则分别对应于Pt 纳米粒子的(111)、(200)和(220)晶面. 15o—30o处归因于SBA-15的特征鼓包峰. 结果证明了SBA-15的成功合成,并且Pt成功负载到了SBA-15载体上. 值得注意的是,TMCS修饰后各催化剂仍然能够保持SBA-15的骨架六方有序结构,同时SBA-15的(100)晶面处峰强明显减弱,这意味着TMCS的改性不会影响SBA-15的整体结构,但可能会造成SBA-15的晶格失配,这也从侧面说明了Pt/SBA-15-TMCS的成功合成. 此外,各催化剂的XRD图谱中,Pt纳米粒子的衍射峰强度基本保持一致,证明了本次实验4种催化剂的Pt纳米粒子负载量基本一致,同时,TMCS的修饰不会对Pt纳米粒子的负载产生影响. ICP-AES测试结果显示,4种催化剂的Pt纳米粒子负载量质量分数分别为1.03%, 0.98%, 1.05%以及1.02%.
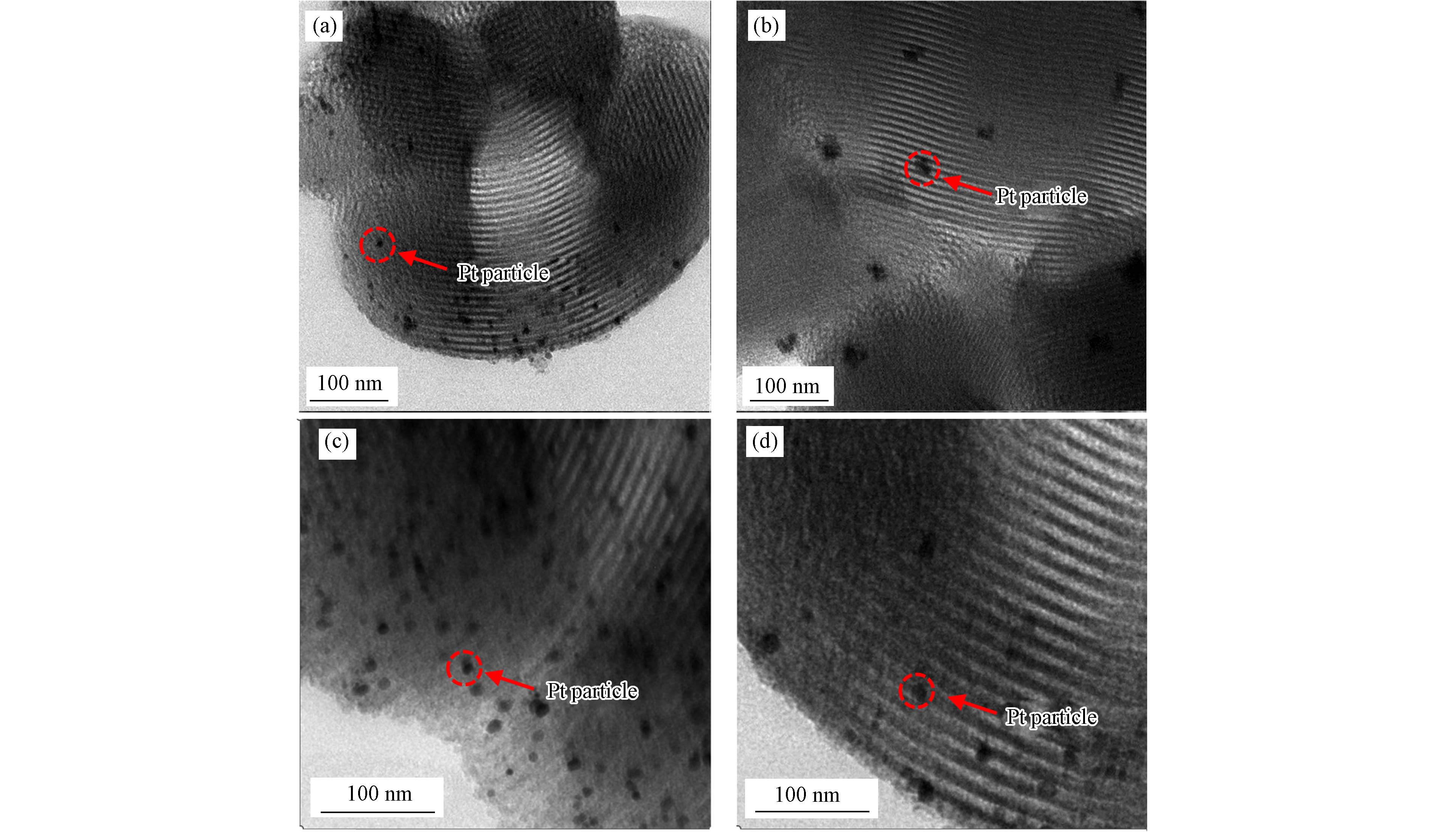
TEM照片展示了4种催化剂更为清晰的微观结构(图3). 在垂直于孔道方向可以看到相互平行的直通孔道,清晰的微观孔道证实了SBA-15的成功合成,这与XRD表征结果相一致. 无论是Pt/SBA-15,还是Pt/SBA-15-TMCS,Pt纳米粒子都能够均匀地分散在SBA-15的载体孔道内,这说明我们这种先浸渍负载Pt纳米粒子,再使用TMCS修饰调节催化剂疏水性的合成方法不仅能够使得Pt被充分限域在SBA-15的介孔孔道内,而且TMCS的修饰过程不会影响Pt纳米粒子的分散. 预计这种方法制备得到的疏水型高分散Pt基催化剂将会完美适用于乙烯的低温催化氧化反应.
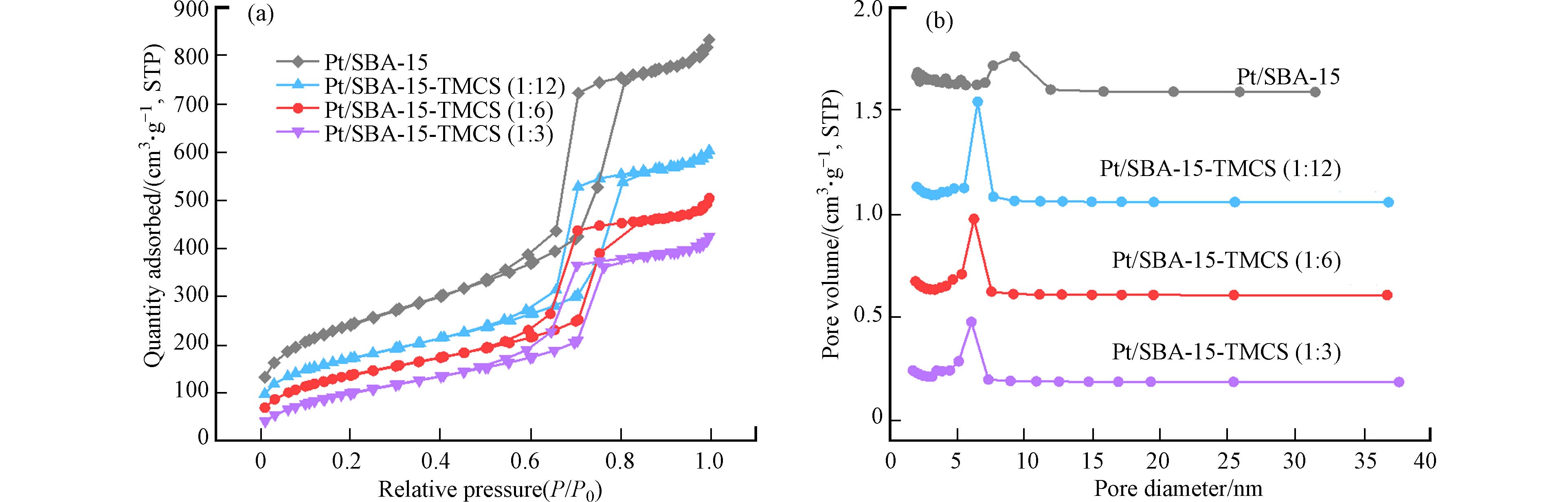
为了探究TMCS修饰对Pt/SBA-15催化剂孔隙结构的影响,对各催化剂进行了比表面积分析以及孔径分布的测定. 如图4所示,所有样品均为在相对压力0.6—0.8处具有H1型滞后环的Type Ⅳ型等温线,表明所有样品均有典型的有序中孔结构[25]. 结果证明,TMCS修饰后,SBA-15的介孔结构仍然保持良好. 然而,N2吸脱附等温线显示,随着TMCS修饰量的逐渐增加,N2吸附量逐渐降低;同时,孔径分布图显示,催化剂在TMCS接枝后,孔径尺寸略微降低,并且随着TMCS用量的不断增加,介孔体积逐渐减小. 这样的测试结果证明,TMCS会接枝在SBA-15的内孔道,过量的TMCS修饰可能会造成催化剂部分介孔孔道堵塞,从而影响乙烯的低温催化氧化反应.
表1总结了Pt/SBA-15以及Pt/SBA-15-TMCS样品的孔隙结构参数. 具体来看,Pt/SBA-15拥有最大的比表面积、孔体积以及孔径尺寸,分别为832 m2·g−1、1.33 cm3·g−1和5.89 nm. 随着TMCS修饰量的增加,比表面积、孔体积以及孔径尺寸都在逐渐减小,这与前面的观察结果均是一致. 这是因为TMCS不仅会接枝在SBA-15的外表面,也会在催化剂内孔道团聚形成较大的颗粒[26],覆盖在孔壁表面,从而影响介孔结构.
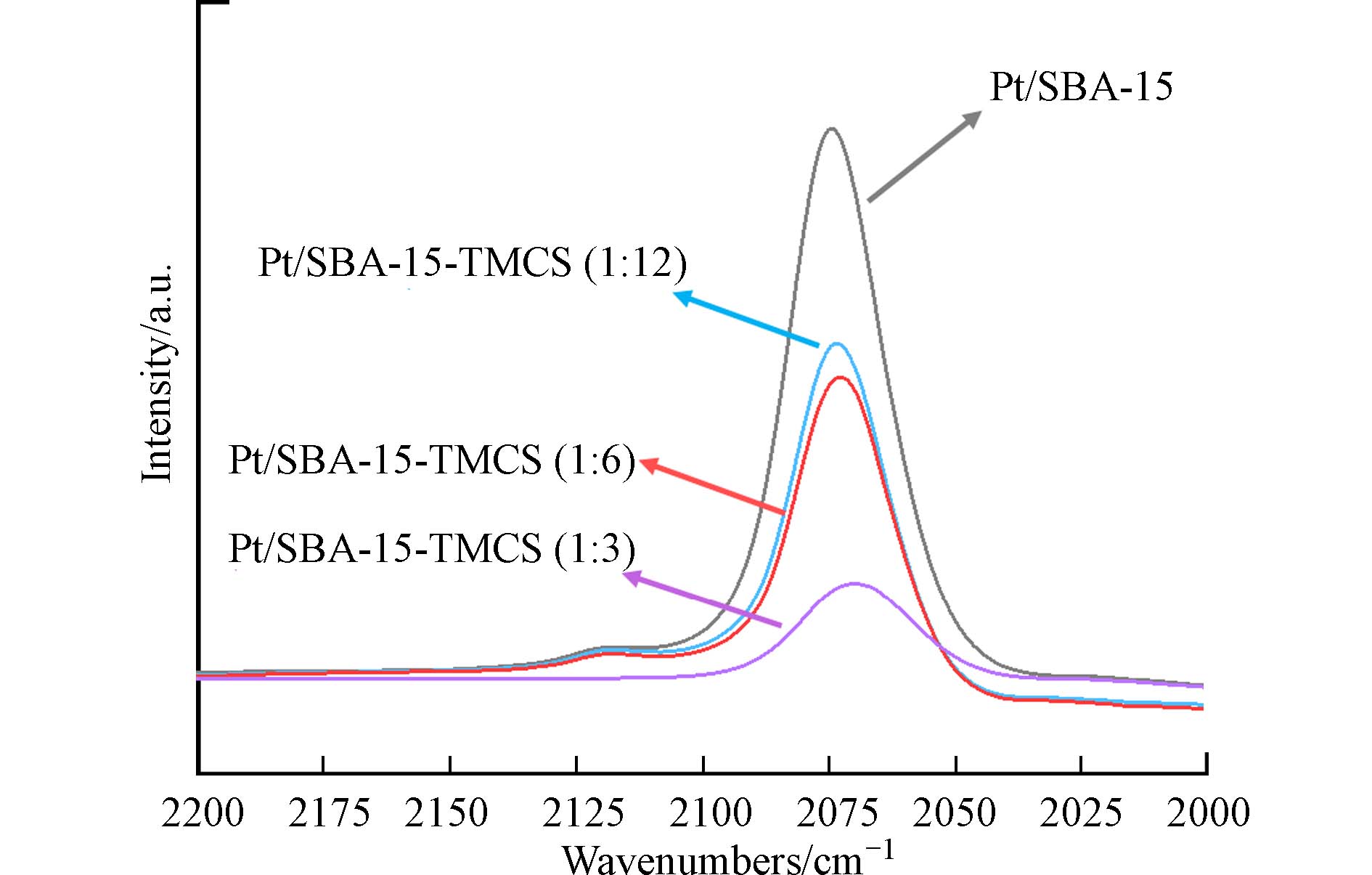
为了验证TMCS在SBA-15内孔道团聚对乙烯低温催化氧化反应的影响,对这4种催化剂进行了CO吸附原位漫反射红外光谱分析. 如图5所示,在TMCS修饰前,Pt/SBA-15拥有最高的CO吸附强度,说明对于拥有1.33 cm3·g−1孔体积的Pt/SBA-15,CO能够轻松进入SBA-15的内孔道并且被Pt纳米粒子所吸附. 而随着TMCS修饰量的增加,CO吸附强度逐渐减弱,同时,CO的吸附峰位置还发生了一些轻微的红移现象. 这个结果很好地证明了TMCS的修饰会阻碍CO与Pt纳米粒子的接触,从而影响CO在金属Pt上的吸附. 根据观察结果可以推测,适量的TMCS接枝可能会改善催化剂整体的疏水环境,而过量的TMCS修饰则会堵塞催化剂的载体孔道,从而不利于催化剂的催化效果.
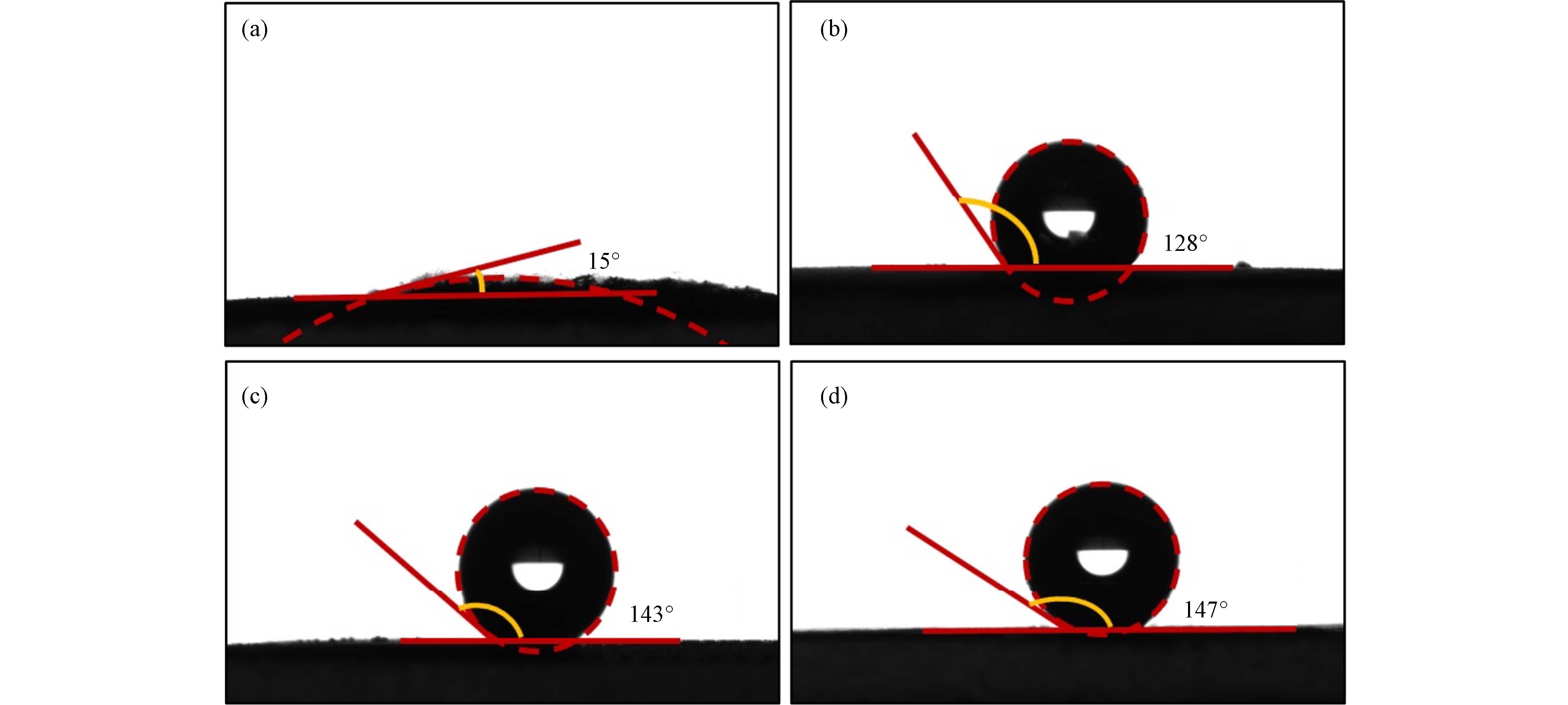
由于乙烯催化氧化过程中催化剂表面水分子的物理吸附是导致催化剂失活的关键因素,因此本实验进行了各催化剂与液态水接触角的测定,结果如图6所示. 一般来说,具有较高疏水性的催化剂对应有较大的接触角. SBA-15作为一种介孔分子筛,对于水的亲和力较好,因此Pt/SBA-15的水接触角仅为15o. 然而,在TMCS对催化剂进行修饰之后,催化剂的疏水性大大提高,Pt/SBA-15-TMCS (1:12),Pt/SBA-15-TMCS (1:6)和Pt/SBA-15-TMCS (1:3)的接触角分别增加到128o、143o和147o. 结果说明,TMCS能够很容易地与SBA-15表面的羟基基团发生反应[27],使得较为疏水的甲基硅烷接枝到SBA-15表面,从而有效提高催化剂表面的疏水性.
-
本次研究使用固定床流动反应器在0 oC下评价各催化剂对乙烯的催化氧化活性,反应过程中每隔5 min对出口气体进行一次采集,检测出口气体组成,计算各气体组成浓度,测试结果如图7a所示.
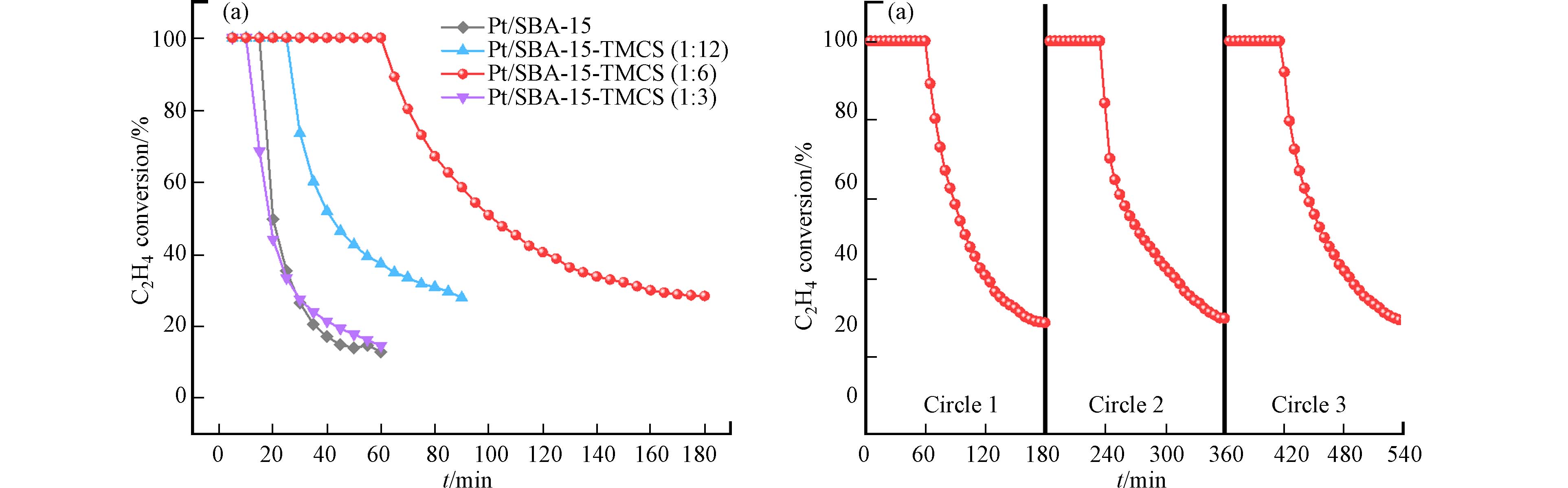
从结果来看,Pt/SBA-15与Pt/SBA-15-TMCS (1:3)仅能在最初始阶段完全转化痕量乙烯,而后对乙烯的转化效率迅速降低,并在1 h后仅剩15%左右的乙烯转化率. 而Pt/SBA-15-TMCS (1:12)和Pt/SBA-15-TMCS (1:6)对乙烯的氧化效果有明显提升,TMCS改性显著提高了他们的催化活性. 特别是Pt/SBA-15-TMCS (1:6)催化剂,能够保持接近1 h对痕量乙烯的100%完全转化的催化活性,并且在3 h后仍然能够保持29%的乙烯转化率. 这样明显的催化活性的改善可以归因于TMCS改性大大提高了材料的疏水性,减弱了催化剂对于水的亲和能力[28],减少了乙烯氧化过程中形成的水分子对于催化剂的吸附影响,从而使得催化效果得以提升. 而Pt/SBA-15-TMCS (1:3)催化效果并未得到显著提升的原因则可能是过量的TMCS接枝并且团聚在SBA-15的内孔道,堵塞了部分介孔孔道,使得乙烯分子难以与Pt纳米粒子接触,活性位点大大减少. 因此尽管催化剂表面的疏水性增加,但有效活性位点的损失还是显著影响了催化剂在乙烯氧化中的催化活性. 此外,将回收后的Pt/SBA-15-TMCS (1:6)催化剂再一次置于150度N2气氛下预处理2 h,催化剂催化性能又得以恢复. 重复利用3次,催化剂的催化性能没有明显降低,稳定性良好(图7b).
-
利用适量TMCS对催化剂进行改性的方法,不仅能够使得SBA-15保持良好的介孔结构,而且有效提升了催化剂表面的疏水性,Pt/SBA-15-TMCS(1:6)与Pt/SBA-15-TMCS(1:12)在低温下均表现出相比于Pt/SBA-15更好的催化氧化乙烯的效果. 其中,Pt/SBA-15-TMCS (1:6)在0 oC时可将Pt/SBA-15对痕量乙烯100%的催化氧化活性从不到20 min延长至近1 h,并且3 h后仍然能够保持29%的乙烯转化率,显著改善了Pt/SBA-15催化氧化乙烯的活性. 从催化剂的表征结果来看,这种催化剂性能上的改善是良好的介孔结构和较强的疏水性共同作用的结果,既能够保证Pt纳米粒子均匀地分散在SBA-15载体孔道内,又大大减弱了反应过程中所形成的水分子所导致的表面活性Pt位点的失活. 这项工作的发现突出表明,TMCS修饰是构建疏水性SBA-15表面的一种高效方法,可以扩展到构建用于低温催化氧化乙烯的高效疏水催化剂.
甲基硅烷化改性显著促进乙烯在Pt/SBA-15上的低温催化氧化
Enhanced low-temperature catalytic oxidation of ethylene on Pt/SBA-15 by methylsilylation modification
-
摘要: 以介孔分子筛SBA-15为载体,采用浸渍法制备Pt基催化剂,通过三甲基氯硅烷(TMCS)对催化剂进行表面改性以提高其疏水性. 表征结果表明甲基硅烷化显著促进了催化剂的表面疏水性. 乙烯的低温催化氧化结果显示,随着TMCS接枝量的增加,催化剂的反应活性呈现火山型变化规律. Pt/SBA-15-TMCS (1:6)的催化活性最高,能够保持约1 h对痕量乙烯100%的催化氧化效果,3 h后仍有29%的乙烯转化率,相比于Pt/SBA-15有显著提升. 适量TMCS的改性带来的催化剂疏水性的改善能够有效抑制反应过程中水分子吸附对催化剂的不利影响,而过量TMCS的修饰则易团聚形成大颗粒堵塞介孔孔道,减少乙烯分子与活性位点的接触,降低催化效果.Abstract: We prepared Pt based catalysts by the impregnation method using SBA-15 as the support, and the catalysts were surface modified by trimethylchlorosilane to improve their hydrophobicity. The characterization results indicated that surface modification remarkably enhanced the surface hydrophobicity of the catalysts. The results of low-temperature catalytic oxidation of ethylene showed that with the increase of TMCS dosage, the catalytic activity of the catalyst displayed a volcano-type variation. Among the test catalysts, Pt/SBA-15-TMCS (1:6) showed the highest catalytic activity, maintaining 100% conversion of trace ethylene for approximately 1 h and 29% ethylene conversion even after 3 h, which was significantly improved as compared with Pt/SBA-15. The improvement in hydrophobicity of the catalyst from the modification by a moderate amount of TMCS can effectively inhibit the adverse effect of water molecule adsorption on the catalyst during the reaction, whereas the modification of excessive TMCS tends to agglomerate and form large particles that block the mesoporous pores, reducing the contact between ethylene and the active sites, giving rise to low catalytic activity.
-
Key words:
- low temperature catalytic oxidation /
- ethylene /
- Pt/SBA-15 /
- surface modification.
-
乙烯(C2H4)是一种自然界中广泛存在的内源性植物激素,它能促进色素、香气等物质在果蔬中的积累,改善果蔬的色泽、风味和口感[1],因此可作为催熟剂应用于果蔬的生产和种植中[2]. 但同时,由于乙烯可以促进呼吸酶的氧化作用[3],即使在低温下,微量乙烯也会导致储藏室和冰箱中新鲜的产品逐渐成熟和变质[4 − 5],使得果蔬的贮藏保鲜期缩短. 因此,清除低温储存环境中的乙烯可有效维持水果和蔬菜的品质,延长货架期,具有很大的商业价值.
目前已开发出的乙烯脱除方法,主要可分为物理吸附[6 − 8]、化学氧化[9 − 10]和催化氧化[6,11 − 13]等. 催化氧化法与其他方法相比具有运行成本低、重复使用性高、转化效率高等优势[14],而低温催化氧化又因反应条件温和,适用于产品保鲜条件下的乙烯脱除,因此意义重大[15]. 研究发现Pt基催化剂相比于Pd、Au和Ag等金属催化剂拥有更高的乙烯催化氧化活性,并且在较长的反应时间和循环使用中表现出优异的耐久性[16],因此被选为本次研究的活性金属. 同时SBA-15[17]具有出色的热稳定性和机械稳定性,其限域作用会使得金属前驱体盐在煅烧分解和还原过程中不易团聚,得到分散性较好的金属颗粒[18],在乙烯催化氧化中表现出高活性[19],因此被选为本研究的载体.
鉴于催化剂表面水分子的物理吸附是导致催化剂失活的关键因素[20],因而调节催化剂表面的疏水性是十分必要的. 目前对催化材料的表面进行改性以提高其疏水性的策略已经被开发并证明是有效的[21 − 22],但其中多数研究都存在操作复杂、回收率低、需要使用大量有机试剂等诸多问题[23],因此仍需要开发一种简便、高效的技术提高催化剂表面的疏水性以改善催化剂低温催化氧化乙烯的活性.
本文采用浸渍法将1% Pt负载到SBA-15上,并使用硅烷偶联剂(三甲基氯硅烷,TMCS)对催化剂进行甲基硅烷化改性,以获得疏水型低温乙烯催化氧化剂. 对催化剂在0 oC下氧化痕量乙烯(0.005%)的效率进行了研究,并通过调整TMCS用量以确定最佳甲基硅烷化剂量,以期提升催化剂低温催化氧化乙烯效果,为构建高效疏水催化剂提供支撑.
1. 材料与方法(Materials and methods)
1.1 实验材料
本文所用试剂有三甲基氯硅烷(TMCS, GC, 阿拉丁试剂)、EO20PO70EO20 (P123, AR, Aldrich公司)、正硅酸四乙酯(TEOS, AR, 国药集团化学试剂有限公司)、氯铂酸六水合物(H2PtCl6·6H2O, AR, 上海阿拉丁生化科技股份有限公司)和盐酸(HCl, AR, 南京化学试剂有限公司).
1.2 催化剂的制备
介孔SBA-15的合成[24]:以三嵌段共聚物P123为结构导向剂,TEOS为硅源. 具体步骤为,在烧杯中加入8 g P123和300 mL HCl水溶液(1.6 mol·L−1),在40 oC下搅拌4 h直到P123完全溶解,溶液呈均匀的乳白色. 继续滴加18 g TEOS,在40 oC搅拌24 h. 然后将反应液转移至自生压反应釜,在100 oC下反应48 h. 冷却后过滤,以去离子水洗至中性,在80 oC下烘干,并在550 oC焙烧6 h(升温速率1 oC·min−1),以去除模版剂P123,所得样品记为SBA-15.
Pt/SBA-15的制备:采用普通浸渍法制备Pt/SBA-15. 称取0.3 g SBA-15载体,加入20 mL去离子水和0.797 mL H2PtCl6 (1 g·mL−1)溶液,在90 oC水浴条件下搅拌并蒸干. 将得到的固体在充分研磨后置于300 oC管式炉中焙烧2 h (升温速率为5 ℃·min−1),冷却后置于20 mL·min−1的H2氛围中以200 oC还原2 h,所得样品记为1% Pt/SBA-15.
Pt/SBA-15-TMCS的制备:TMCS硅烷化反应采用实验室自主搭建的反应装置(图1). 在120 oC连续流动N2 (20 mL·min−1)氛围下,微量注射泵泵入并气化的TMCS,与催化剂反应. 连续进样12 h,所得硅烷化产物(Pt/SBA-15-TMCS). 实验中TMCS用量与Pt/SBA-15用量比例分别为1:12、1:6、1:3 (mL·g−1).
1.3 催化剂的表征
使用X射线衍射仪(XRD, 日本理学株式会社, D/max-RA)对催化剂进行物相分析;采用ICP-AES(美国PerkinElmer公司, Avio500)以获得催化剂表面具体负载的Pt含量;使用透射电子显微镜(TEM, 日本电子株式会社, JEM-200CX)观察催化剂的微观形貌特征;通过比表面积分析仪(BET, 美国MICROMERITICS公司, ASAP2020)测定N2吸附-脱附等温线和孔径分析以确定催化剂的比表面积和孔径大小;采用傅里叶红外光谱仪(FT-IR, 美国赛默飞世尔公司, Nicolet IS10)对催化剂进行CO吸附原位漫反射光谱分析;通过测量催化剂与液态水的接触角(Static contact angle measurements, 美国迪飞公司, OCA30)表征催化剂的疏水性.
1.4 CO吸附原位红外漫反射光谱测定
样品的CO吸附原位红外光谱是通过美国赛默飞世尔公司的Nicolet IS10分析得到的. 扫描的波数范围为
1700 —2300 cm−1, 分辨率为4 cm−1, 扫描次数为32次. 具体地,将样品粉末平铺于红外样品池中,并以5 oC·min−1的升温速率升到150 oC在N2 (20 mL·min−1)氛围下预处理2 h. 降至室温后,在N2氛围下取背景. 随后将N2切换为CO/N2 (20 mL·min−1)以吸附CO,待吸附30 min后再将CO/N2切换回N2吹扫30 min,最后用红外光谱扫描获得CO在样品表面化学吸附的谱图.1.5 催化性能测定
乙烯的催化氧化反应在内径约为4 mm的固定床流动反应器中进行. 具体地,将0.4 g催化剂(40—60目)装入U型反应管中,并在150 oC连续流动N2(20 mL·min−1)下对催化剂进行预处理2 h. 冷却至室温后,将U型管置于0 oC的低温水浴恒温槽中稳定30 min. 然后将混合气体(C2H4, 0.005%; O2, 20%; N2, 5%; He, 平衡)在0 oC下以
1500 mL·h−1·g−1的空间速度(SV)加入催化剂床. 出口气体组分通过在线气相色谱法(北分瑞利3420A, 配备氢火焰离子化检测器和与Porapak-Q柱连接的Ni催化转换器)进行检测. 出口气体与气相色谱通过六通阀连接,气体组分每5 min记录1次. C2H4转化率通过([C2H4]in-[C2H4]out)×100/[C2H4]out获得,其中[C2H4]in和[C2H4]out分别对应于C2H4的初始浓度和C2H4出口浓度. 单次实验结束后,将U型管中催化剂取出并回收以备重复测试.2. 结果与讨论(Results and discussion)
2.1 催化剂表征结果
从Pt/SBA-15和Pt/SBA-15-TMCS的结构表征来看,XRD图谱显示,4种催化剂均检测出了SBA-15和Pt纳米粒子的特征衍射峰(图2). 其中,0.95o、1.57o、1.79o的特征峰位置分别可以对应于SBA-15的(100)、(110)、(200)晶面;39.7o、46.2o、67.9o处的特征峰位置则分别对应于Pt 纳米粒子的(111)、(200)和(220)晶面. 15o—30o处归因于SBA-15的特征鼓包峰. 结果证明了SBA-15的成功合成,并且Pt成功负载到了SBA-15载体上. 值得注意的是,TMCS修饰后各催化剂仍然能够保持SBA-15的骨架六方有序结构,同时SBA-15的(100)晶面处峰强明显减弱,这意味着TMCS的改性不会影响SBA-15的整体结构,但可能会造成SBA-15的晶格失配,这也从侧面说明了Pt/SBA-15-TMCS的成功合成. 此外,各催化剂的XRD图谱中,Pt纳米粒子的衍射峰强度基本保持一致,证明了本次实验4种催化剂的Pt纳米粒子负载量基本一致,同时,TMCS的修饰不会对Pt纳米粒子的负载产生影响. ICP-AES测试结果显示,4种催化剂的Pt纳米粒子负载量质量分数分别为1.03%, 0.98%, 1.05%以及1.02%.
TEM照片展示了4种催化剂更为清晰的微观结构(图3). 在垂直于孔道方向可以看到相互平行的直通孔道,清晰的微观孔道证实了SBA-15的成功合成,这与XRD表征结果相一致. 无论是Pt/SBA-15,还是Pt/SBA-15-TMCS,Pt纳米粒子都能够均匀地分散在SBA-15的载体孔道内,这说明我们这种先浸渍负载Pt纳米粒子,再使用TMCS修饰调节催化剂疏水性的合成方法不仅能够使得Pt被充分限域在SBA-15的介孔孔道内,而且TMCS的修饰过程不会影响Pt纳米粒子的分散. 预计这种方法制备得到的疏水型高分散Pt基催化剂将会完美适用于乙烯的低温催化氧化反应.
为了探究TMCS修饰对Pt/SBA-15催化剂孔隙结构的影响,对各催化剂进行了比表面积分析以及孔径分布的测定. 如图4所示,所有样品均为在相对压力0.6—0.8处具有H1型滞后环的Type Ⅳ型等温线,表明所有样品均有典型的有序中孔结构[25]. 结果证明,TMCS修饰后,SBA-15的介孔结构仍然保持良好. 然而,N2吸脱附等温线显示,随着TMCS修饰量的逐渐增加,N2吸附量逐渐降低;同时,孔径分布图显示,催化剂在TMCS接枝后,孔径尺寸略微降低,并且随着TMCS用量的不断增加,介孔体积逐渐减小. 这样的测试结果证明,TMCS会接枝在SBA-15的内孔道,过量的TMCS修饰可能会造成催化剂部分介孔孔道堵塞,从而影响乙烯的低温催化氧化反应.
表1总结了Pt/SBA-15以及Pt/SBA-15-TMCS样品的孔隙结构参数. 具体来看,Pt/SBA-15拥有最大的比表面积、孔体积以及孔径尺寸,分别为832 m2·g−1、1.33 cm3·g−1和5.89 nm. 随着TMCS修饰量的增加,比表面积、孔体积以及孔径尺寸都在逐渐减小,这与前面的观察结果均是一致. 这是因为TMCS不仅会接枝在SBA-15的外表面,也会在催化剂内孔道团聚形成较大的颗粒[26],覆盖在孔壁表面,从而影响介孔结构.
表 1 催化剂的结构性质Table 1. Structure properties of the catalysts催化剂Catalyst SBET/(m2·g−1) Vmeso/(cm3·g−1) dmeso/nm Pt/SBA-15 832 1.33 5.89 Pt/SBA-15-TMCS (1:12) 587 0.96 5.84 Pt/SBA-15-TMCS (1:6) 484 0.80 5.67 Pt/SBA-15-TMCS (1:3) 381 0.67 5.29 为了验证TMCS在SBA-15内孔道团聚对乙烯低温催化氧化反应的影响,对这4种催化剂进行了CO吸附原位漫反射红外光谱分析. 如图5所示,在TMCS修饰前,Pt/SBA-15拥有最高的CO吸附强度,说明对于拥有1.33 cm3·g−1孔体积的Pt/SBA-15,CO能够轻松进入SBA-15的内孔道并且被Pt纳米粒子所吸附. 而随着TMCS修饰量的增加,CO吸附强度逐渐减弱,同时,CO的吸附峰位置还发生了一些轻微的红移现象. 这个结果很好地证明了TMCS的修饰会阻碍CO与Pt纳米粒子的接触,从而影响CO在金属Pt上的吸附. 根据观察结果可以推测,适量的TMCS接枝可能会改善催化剂整体的疏水环境,而过量的TMCS修饰则会堵塞催化剂的载体孔道,从而不利于催化剂的催化效果.
由于乙烯催化氧化过程中催化剂表面水分子的物理吸附是导致催化剂失活的关键因素,因此本实验进行了各催化剂与液态水接触角的测定,结果如图6所示. 一般来说,具有较高疏水性的催化剂对应有较大的接触角. SBA-15作为一种介孔分子筛,对于水的亲和力较好,因此Pt/SBA-15的水接触角仅为15o. 然而,在TMCS对催化剂进行修饰之后,催化剂的疏水性大大提高,Pt/SBA-15-TMCS (1:12),Pt/SBA-15-TMCS (1:6)和Pt/SBA-15-TMCS (1:3)的接触角分别增加到128o、143o和147o. 结果说明,TMCS能够很容易地与SBA-15表面的羟基基团发生反应[27],使得较为疏水的甲基硅烷接枝到SBA-15表面,从而有效提高催化剂表面的疏水性.
2.2 催化剂对乙烯的低温催化氧化
本次研究使用固定床流动反应器在0 oC下评价各催化剂对乙烯的催化氧化活性,反应过程中每隔5 min对出口气体进行一次采集,检测出口气体组成,计算各气体组成浓度,测试结果如图7a所示.
从结果来看,Pt/SBA-15与Pt/SBA-15-TMCS (1:3)仅能在最初始阶段完全转化痕量乙烯,而后对乙烯的转化效率迅速降低,并在1 h后仅剩15%左右的乙烯转化率. 而Pt/SBA-15-TMCS (1:12)和Pt/SBA-15-TMCS (1:6)对乙烯的氧化效果有明显提升,TMCS改性显著提高了他们的催化活性. 特别是Pt/SBA-15-TMCS (1:6)催化剂,能够保持接近1 h对痕量乙烯的100%完全转化的催化活性,并且在3 h后仍然能够保持29%的乙烯转化率. 这样明显的催化活性的改善可以归因于TMCS改性大大提高了材料的疏水性,减弱了催化剂对于水的亲和能力[28],减少了乙烯氧化过程中形成的水分子对于催化剂的吸附影响,从而使得催化效果得以提升. 而Pt/SBA-15-TMCS (1:3)催化效果并未得到显著提升的原因则可能是过量的TMCS接枝并且团聚在SBA-15的内孔道,堵塞了部分介孔孔道,使得乙烯分子难以与Pt纳米粒子接触,活性位点大大减少. 因此尽管催化剂表面的疏水性增加,但有效活性位点的损失还是显著影响了催化剂在乙烯氧化中的催化活性. 此外,将回收后的Pt/SBA-15-TMCS (1:6)催化剂再一次置于150度N2气氛下预处理2 h,催化剂催化性能又得以恢复. 重复利用3次,催化剂的催化性能没有明显降低,稳定性良好(图7b).
3. 结论(Conclusion)
利用适量TMCS对催化剂进行改性的方法,不仅能够使得SBA-15保持良好的介孔结构,而且有效提升了催化剂表面的疏水性,Pt/SBA-15-TMCS(1:6)与Pt/SBA-15-TMCS(1:12)在低温下均表现出相比于Pt/SBA-15更好的催化氧化乙烯的效果. 其中,Pt/SBA-15-TMCS (1:6)在0 oC时可将Pt/SBA-15对痕量乙烯100%的催化氧化活性从不到20 min延长至近1 h,并且3 h后仍然能够保持29%的乙烯转化率,显著改善了Pt/SBA-15催化氧化乙烯的活性. 从催化剂的表征结果来看,这种催化剂性能上的改善是良好的介孔结构和较强的疏水性共同作用的结果,既能够保证Pt纳米粒子均匀地分散在SBA-15载体孔道内,又大大减弱了反应过程中所形成的水分子所导致的表面活性Pt位点的失活. 这项工作的发现突出表明,TMCS修饰是构建疏水性SBA-15表面的一种高效方法,可以扩展到构建用于低温催化氧化乙烯的高效疏水催化剂.
-
表 1 催化剂的结构性质
Table 1. Structure properties of the catalysts
催化剂Catalyst SBET/(m2·g−1) Vmeso/(cm3·g−1) dmeso/nm Pt/SBA-15 832 1.33 5.89 Pt/SBA-15-TMCS (1:12) 587 0.96 5.84 Pt/SBA-15-TMCS (1:6) 484 0.80 5.67 Pt/SBA-15-TMCS (1:3) 381 0.67 5.29 -
[1] 魏军亚, 耿沙, 刘跃威, 等. 乙烯利处理对采后宝岛蕉果实后熟期品质的影响[J]. 热带农业科学, 2021, 41(1): 68-73. WEI J Y, GENG S, LIU Y W, et al. Effects of ethephon treatment on the quality of postharvest banana fruits in Baodao[J]. Chinese Journal of Tropical Agriculture, 2021, 41(1): 68-73 (in Chinese).
[2] WANG L K, ZHANG F, QIAO H. Chromatin regulation in the response of ethylene: Nuclear events in ethylene signaling[J]. Small Methods, 2020, 4(8): 1900288. doi: 10.1002/smtd.201900288 [3] HAYAMA H, TATSUKI M, ITO A, et al. Ethylene and fruit softening in the stony hard mutation in peach[J]. Postharvest Biology and Technology, 2006, 41(1): 16-21. doi: 10.1016/j.postharvbio.2006.03.006 [4] ROMANAZZI G, FELIZIANI E, BAÑOS S B, et al. Shelf life extension of fresh fruit and vegetables by chitosan treatment[J]. Critical Reviews in Food Science and Nutrition, 2017, 57(3): 579-601. doi: 10.1080/10408398.2014.900474 [5] KUMAR D, KALITA P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries[J]. Foods, 2017, 6(1): 8. doi: 10.3390/foods6010008 [6] RAJE L, SHERLEKAR S, RAMAKRISHNAN K, et al. Post harvest preservation of mangoes by controlled chemical release agents and adsorbent[J]. Acta Horticulturae, 1997(455): 622-628. doi: 10.17660/ActaHortic.1997.455.80 [7] KELLER N, DUCAMP M N, ROBERT D, et al. Ethylene removal and fresh product storage: A challenge at the frontiers of chemistry. toward an approach by photocatalytic oxidation[J]. Chemical Reviews, 2013, 113(7): 5029-5070. doi: 10.1021/cr900398v [8] JANJARASSKUL T, SUPPAKUL P. Active and intelligent packaging: The indication of quality and safety[J]. Critical Reviews in Food Science and Nutrition, 2018, 58(5): 808-831. doi: 10.1080/10408398.2016.1225278 [9] 黄邦彦, 李为为, 吴立, 等. 乙烯吸收剂延长香蕉贮运寿命的研究与应用[J]. 热带作物学报, 1988, 9(2): 69-74. HUANG B Y, LI W W, WU L, et al. Studies on the use of ethylene absorbents to prolong storage life of bananas[J]. Chinese Journal of Tropical Crops, 1988, 9(2): 69-74 (in Chinese).
[10] 陈存坤, 高芙蓉, 薛文通, 等. 臭氧处理对新疆厚皮甜瓜贮藏品质和生理特性的影响[J]. 食品科学, 2016, 37(20): 215-220. doi: 10.7506/spkx1002-6630-201620037 CHEN C K, GAO F R, XUE W T, et al. Effects of ozone treatment on storage quality and physiological characteristics of Xinjiang thick-skinned melon[J]. Food Science, 2016, 37(20): 215-220 (in Chinese). doi: 10.7506/spkx1002-6630-201620037
[11] MARTÍNEZ-ROMERO D, BAILÉN G, SERRANO M, et al. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: A review[J]. Critical Reviews in Food Science and Nutrition, 2007, 47(6): 543-560. doi: 10.1080/10408390600846390 [12] 张桦, 曹美秋, 许哲生, 等. 常温下CO氧化催化剂的研究[J]. 环境化学, 1984, 3(6): 42-48. ZHANG H, CAO M Q, XU Z S, et al. Study on CO oxidation catalyst at room temperature[J]. Environmental Chemistry, 1984, 3(6): 42-48 (in Chinese).
[13] 劳彩娴, 卢立新. TiO2/ACF的制备及其光催化降解乙烯的性能研究[J]. 现代化工, 2020, 40(1): 185-188. LAO C X, LU L X. Preparation of TiO2/ACF and study on its photo-catalytic performance in degradation of ethylene[J]. Modern Chemical Industry, 2020, 40(1): 185-188 (in Chinese).
[14] WEI H Y, SEIDI F, ZHANG T W, et al. Ethylene scavengers for the preservation of fruits and vegetables: A review[J]. Food Chemistry, 2021, 337: 127750. doi: 10.1016/j.foodchem.2020.127750 [15] QI Y, LI C L, LI H, et al. Elimination or removal of ethylene for fruit and vegetable storage via low-temperature catalytic oxidation[J]. Journal of Agricultural and Food Chemistry, 2021, 69(36): 10419-10439. doi: 10.1021/acs.jafc.1c02868 [16] JIANG C X, HARA K, FUKUOKA A. Low-temperature oxidation of ethylene over platinum nanoparticles supported on mesoporous silica[J]. Angewandte Chemie International Edition, 2013, 52(24): 6265-6268. doi: 10.1002/anie.201300496 [17] ZHAO D, FENG J, HUO Q, et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores[J]. Science, 1998, 279(5350): 548-552. doi: 10.1126/science.279.5350.548 [18] 杨梦婷, 肖笑傲, 施宜春, 等. 用于甲烷二氧化碳重整的Ni/SBA-15催化剂的研究进展[J]. 应用化工, 2020, 49(增刊2): 227-233. YANG M T, XIAO X A, SHI Y C, et al. Research progress of Ni/SBA-15 catalyst for methane carbon dioxide reforming[J]. Applied Chemical Industry, 2020, 49(Sup 2): 227-233 (in Chinese).
[19] SATTER S S, HIRAYAMA J, KOBAYASHI H, et al. Water-resistant Pt sites in hydrophobic mesopores effective for low-temperature ethylene oxidation[J]. ACS Catalysis, 2020, 10(22): 13257-13268. doi: 10.1021/acscatal.0c02816 [20] SATTER S S, YOKOYA T, HIRAYAMA J, et al. Oxidation of trace ethylene at 0 ℃ over platinum nanoparticles supported on silica[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 11480-11486. [21] RUDDY D A, TILLEY T D. Highly selective olefin epoxidation with aqueous H2O2 over surface-modified TaSBA15 prepared via the TMP method[J]. Chemical Communications, 2007(32): 3350-3352. doi: 10.1039/b706443h [22] SATTER S S, HIRAYAMA J, NAKAJIMA K, et al. Low temperature oxidation of trace ethylene over Pt nanoparticles supported on hydrophobic mesoporous silica[J]. Chemistry Letters, 2018, 47(8): 1000-1002. doi: 10.1246/cl.180364 [23] 蒋平, 马丽, 潘剑, 等. 化学气相沉积三甲基氯硅烷制备两亲性HZSM-5沸石[J]. 催化学报, 2009, 30(6): 503-508. doi: 10.3321/j.issn:0253-9837.2009.06.006 JIANG P, MA L, PAN J, et al. Preparation of amphiphilic HZSM-5 zeolite by chemical vapor deposition of trimethylchlorosilane[J]. Chinese Journal of Catalysis, 2009, 30(6): 503-508 (in Chinese). doi: 10.3321/j.issn:0253-9837.2009.06.006
[24] ZHAO D Y, HUO Q S, FENG J L, et al. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures[J]. Journal of the American Chemical Society, 1998, 120(24): 6024-6036. doi: 10.1021/ja974025i [25] ZHANG L X, SHI J L, YU J, et al. A new In-situ reduction route for the synthesis of Pt nanoclusters in the channels of mesoporous silica SBA-15[J]. Advanced Materials, 2002, 14(20): 1510-1513. doi: 10.1002/1521-4095(20021016)14:20<1510::AID-ADMA1510>3.0.CO;2-W [26] HARA K, AKAHANE S, WIENCH J W, et al. Selective and efficient silylation of mesoporous silica: A quantitative assessment of synthetic strategies by solid-state NMR[J]. The Journal of Physical Chemistry C, 2012, 116(12): 7083-7090. doi: 10.1021/jp300580f [27] LU Z Q, LI J M, FU X L, et al. Superhydrophobic Pt@SBA-15 catalyst for hydrogen water isotope exchange reactions[J]. International Journal of Hydrogen Energy, 2022, 47(41): 18080-18087. doi: 10.1016/j.ijhydene.2022.03.279 [28] JARONIEC C P, KRUK M, JARONIEC M, et al. Tailoring surface and structural properties of MCM-41 silicas by bonding organosilanes[J]. The Journal of Physical Chemistry B, 1998, 102(28): 5503-5510. doi: 10.1021/jp981304z 期刊类型引用(0)
其他类型引用(1)
-






 下载:
下载: