-
亚硝胺是使用氯化化学品对含有有机胺的水进行消毒以及药品、橡胶和塑料生产过程中产生的有害副产物,其对水源的污染引起了各个国家的广泛重视[1 − 2]. 其中N-二甲基亚硝胺(NDMA)是许多国家饮用水系统中检出最多的亚硝胺,对饮用水安全造成了较大的隐患[3 − 4]. NDMA前体可来自城市废水排放的有机物、富营养化水体中的藻类有机物、药品和个人护理产品(PPCPs)以及胺基水处理聚合物. 加利福尼亚州的NDMA排放限值为 10 ng·L−1,公共卫生目标为 3 ng·L−1[5],突出了识别 NDMA 前体以及降低NDMA排放率的必要性.
含氮消毒副产物(N-DBPs)具有比碳基消毒副产物(C-DBPs)更高的遗传毒性和细胞毒性,可对生物体的消化系统、神经系统造成损害[6 − 7]. 鉴于其危害,NDMA等亚硝胺已被广泛研究. 目前,NDMA的去除方法主要有活性炭吸附法、微生物法、微滤(MF)和反渗透(RO)膜法,高级氧化法等[7 − 11]. 催化加氢还原技术是一种简单高效、可操作性强的处理消毒副产物的方法,该技术主要是在催化剂作用下,氢气等氢源被活化为氢物种,污染物与氢物种发生氧化还原反应,使得污染物被还原或碳卤键断裂释放卤素原子的技术[12 − 13],具有绿色高效的特点. 常用催化剂一般为负载型催化剂,其中贵金属主要Pt、Pd、Ru等,载体有Al2O3、SiO2、SBA-15等,现已被应用于NO3−[14]、Cr(Ⅵ)[15]等高价无机盐离子的还原,氯乙酸[16]、四溴双酚A[17]的脱卤反应等.
载体的性质是决定负载型金属催化剂催化活性的关键因素. 载体会影响表面活性金属的电子状态,或参与反应以实现更多的动力学途径[18 − 19]. 氧化铈是一种重要的稀土氧化物,CeO2基催化剂较其他碳载体及非金属氧化物载体制备催化剂有着优异的氧化还原性能和电荷转移效应,在多相催化等领域得到了广泛的应用. 不同晶面的CeO2会显著影响催化剂的性能,如表面稳定性,氧空位形成能,以及与负载贵金属之间的相互作用[20 − 21]. 尽管对CeO2形态-反应活性关系的研究已经取得了很大进展,但所研究的催化反应主要是氧化反应[22 − 23],而CeO2形态对NDMA的液相加氢还原反应等其他反应的研究还未见报道.
本文通过调变水热温度合成了纳米棒(NR)、纳米立方体(NC)、纳米八面体(NO)的CeO2载体,采用沉淀沉积法制备了不同形貌载体负载的Ru基催化剂,将其应用于水中NDMA的液相催化加氢还原,探究了不同形貌载体在不同反应条件下对催化剂活性的影响以及催化剂稳定性的变化. 尝试通过寻找具有更强金属-载体相互作用的催化剂,研究催化剂结构性质与催化活性之间的关系,对于高效去除水中污染物具有重要意义.
-
六水合硝酸铈(分析纯)、十二水合磷酸钠(分析纯)采购于国药化学试剂有限公司,氢氧化钠采购于南京化学试剂公司,N-亚硝基二甲胺购于USA Sigma-Aldrich公司,无水三氯化钌(RuCl3,>98.0%)购于Aladdin公司. 氮气(99.99%)和氢气(99.99%)来自南京天泽气体公司.
-
本研究中纳米立方体(c-CeO2)、纳米八面体(o-CeO2)以及纳米棒(r-CeO2)等3种形貌CeO2均采用水热法合成 :
纳米棒CeO2(r-CeO2):将1.736 g Ce(NO)3·6H2O溶于30 mL水中,充分搅拌后加入70 mL包含19.2 g NaOH的溶液,继续搅拌0.5 h后倒入反应釜中,在100 ℃温度下反应24 h. 反应釜降至室温后,用去离子水将溶液洗至中性置于60 ℃烘箱过夜干燥,取出后于马弗炉用500 ℃焙烧3 h,最终得到纳米棒CeO2(r-CeO2).
纳米立方体CeO2(c-CeO2):将1 g Ce(NO)3·6H2O溶于30 mL水中,充分搅拌后加入10 mL含有8 g NaOH的溶液,继续搅拌10 min后倒入反应釜中,在200 ℃温度下反应24 h,去离子水洗至中性、干燥后于马弗炉中350 ℃焙烧4 h,最终得到纳米立方体CeO2(c-CeO2).
纳米八面体CeO2(o-CeO2):将0.858 g Ce(NO)3·6H2O溶于10 mL水中,在搅拌状态下加入70 mL含有
0.0076 g Na3PO4·12H2O的溶液,继续搅拌0.5 h后倒入50 mL反应釜中,在170 ℃温度下反应10 h,洗涤、干燥后放至马弗炉500 ℃焙烧3 h,最终得到纳米八面体CeO2(o-CeO2).采用沉淀沉积法制备负载型Ru基催化剂,具体制备方法如下:首先,称取一定量的载体加入含有三氯化钌溶液的去离子水中,充分搅拌0.5 h后,向其中缓慢滴加NaOH 溶液,调节溶液pH值大于8,室温下继续搅拌3—4 h使其充分反应沉淀,抽滤处理所得溶液并用去离子水清洗至中性,得到的沉淀物置于烘箱过夜干燥,烘干后的材料在马弗炉中300 ℃焙烧4 h,降至室温后在200 ℃条件下H2还原2 h(流速20 mL·min−1),最终得到所需催化剂,分别记为Ru/c-CeO2、Ru/o-CeO2、Ru/r-CeO2,Ru理论负载量质量分数为1.0%.
-
使用透射电子显微镜(TEM,JEM-200CX)观察催化剂的形貌及其表面的金属颗粒分布;使用X射线衍射仪(XRD,D/max-RA)对催化剂进行晶相分析;使用X射线光电子能谱仪(XPS, ESCALAB250)分析催化剂表面Ru、Ce等的化学形态;使用激光拉曼光谱仪(Raman,Lab RAM Aramis)测试不同载体Ru基催化剂表面氧空位; 使用原位红外吸附红外光谱(in-situ CO-IR,Nexus 870)对催化剂表面Ru贵金属结构性质进行测定.
-
常温状态下将含有适量催化剂的200 mL去离子水加入容量为250 mL的四颈圆底烧瓶中,调节至所需pH,在反应开始前先通入H2(流速为100 mL·min−1)对催化剂进行预活化处理,30 min后以100 mL·min−1流速通入N2以排除溶液中溶解的残余气体,期间加入一定量的NDMA储备液进行充分搅拌,一段时间后将气阀切换至H2 并开始计时. 取样时样品由1mL注射器提取再经过0.22 μm滤膜过滤后待测. 样品检测使用高效液相色谱(
1200 Series,美国Agilent公司),紫外检测器波长为238 nm,色谱柱型号为Zorbax Eclipse XDB-C18(4.6 mm×250 mm,5 μm,安捷伦),流动相为甲醇:水=20:80(V/V),流速1.0 mL·min−1,柱温25 ℃,保留时间3.5 min.选用反应初活性(r0)对催化剂活性进行评价,表示当去除率低于20%的反应阶段,单位时间内单位质量催化剂降解的NDMA浓度,单位为mmol·L−1·g−1· h−1,反应初活性约在反应进行6 min时获得.
-
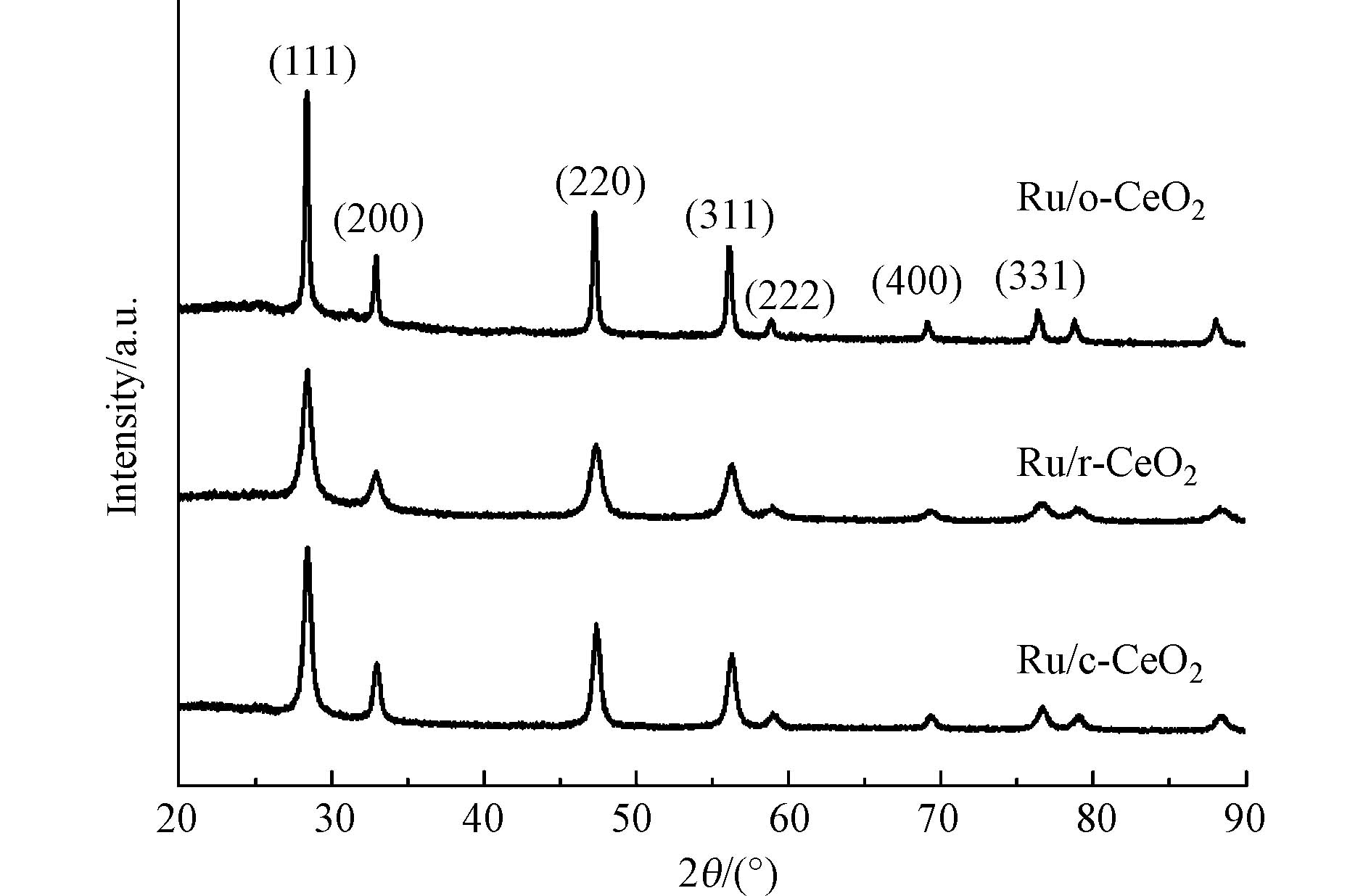
催化剂的X射线衍射图谱如图1所示,3种样品在2θ=28.5°、33.1°、47.4°、56.3°、59.1°、69.4°、76.7°处均有特征峰,分别对应于CeO2典型结构的(111)、(200)、(220)、(311)、(222)、(400)、(331)晶面[22],表明所有CeO2载体均为立方萤石型结构. XRD谱图中没有与Ru物种相关的峰,可能由于Ru的负载量低或Ru物种进入CeO2晶格所致. 八面体形貌的CeO2特征衍射峰更强且更窄,说明Ru/o-CeO2的晶粒结晶性好,这可能归因于较高的水热温度利于晶核的形成和颗粒的生长,从而形成的CeO2具有较高的结晶度和较大的微晶尺寸[23].
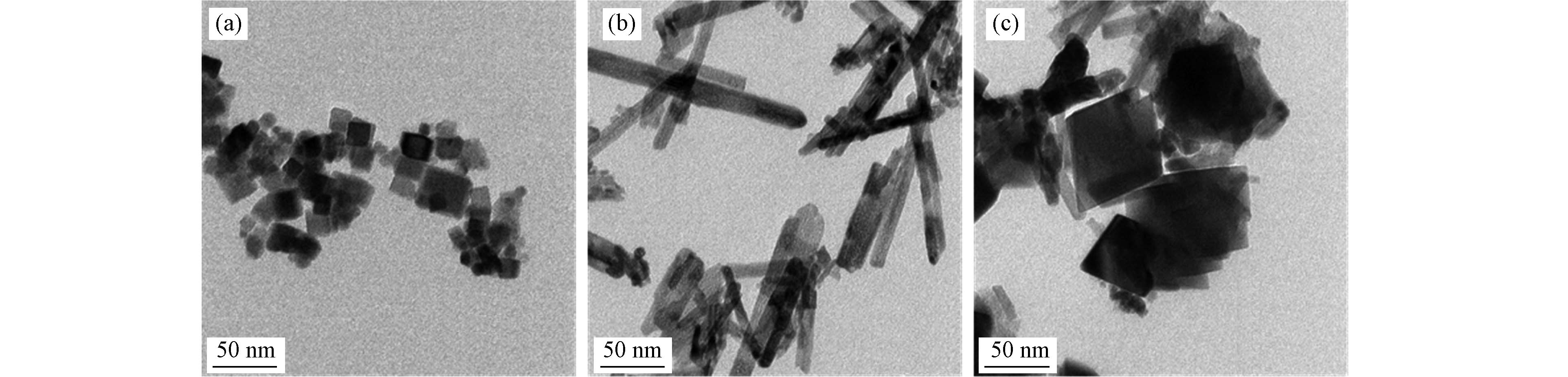
图2展示了3种Ru/CeO2催化剂透射电子显微镜图像,分别证实了纳米立方体纳米棒和八面体3种形态氧化铈的形成. Ru/c-CeO2纳米颗粒均匀,其边缘长度大多在30—40 nm之间;Ru/r-CeO2纳米粒子的直径分布较窄(约12 nm),长度分布较宽(60—150 nm);Ru/o-CeO2的显微镜图片中八面体纳米颗粒高度在60—90 nm.
用X射线光电子能谱研究了催化剂中Ru、Ce和O的化学状态,所有的XPS能谱都用284.6 eV处的C1s峰进行了校正,相关数据见表1.
图3(a)为催化剂样品Ru 3d轨道的XPS图谱,结果表明,催化剂在280.1 eV、281.5 eV、283.2 eV附近有3个Ru 3d5/2的峰,可分别归属于Ru0、Ru4+、Ru6+[18]. 在催化剂中以带正电的Ru物种为主,如表1所示,Ru/o-CeO2、Ru/r-CeO2与Ru/c-CeO2的Ru0、Run+各不相同说明金属状态受载体性质的影响很大. 此外,Run+的含量顺序为:Ru/c-CeO2>Ru/r-CeO2>Ru/o-CeO2,Run+具有插入CeO2表面晶格的能力,可以诱导CeO2纳米结构调节氧空位浓度. Ru/c-CeO2表面含有较多的Run+,表明Ru与CeO2之间的强的金属-载体相互作用.
图3(b)为催化剂样品Ce 3d轨道的XPS谱图,通过去卷积方法可以识别出由自旋轨道对产生的峰共10个,图中用u和v标识出两组自旋轨道线,分别代表Ce 3d3/2与Ce 3d5/2自旋轨道组分特征峰,其中v(u)1、v(u)3、v(u)4所代表的6个峰来自Ce4+的贡献,v(u)0、v(u)2所代表的4个峰来自Ce3+的贡献[24]. 如表1所示,Ru/c-CeO2、Ru/r-CeO2和Ru/o-CeO2样品中Ce3+/Ce4+比值分别为0.38、0.38、0.45,其中Ru/o-CeO2中Ce3+/Ce4+原子比最高,与OV/OL的结果一致,说明Ce3+含量与氧空位浓度具有相关性. Ce3+可诱导材料中氧空位的形成,这些缺陷位对催化加氢反应中的H2与反应物的吸附很关键[21, 25].
图3(c)为催化剂的O1s谱图,显示表面氧存在两种状态. 结合能为529.3—529.6 eV的峰表示为OL,代表体相晶格氧,在531.1—531.3 eV处的峰代表表面化学吸附氧,表示为OV,归属于氧化物缺陷或表面氧离子[20]. OV/OL比率是衡量CeO2纳米颗粒表面氧空位浓度的指标,通过比较表1中不同催化剂的OV/OL比值数据能够看出,CeO2纳米颗粒表面氧物种的含量与形状/晶面有关, Ru/o-CeO2的OV/OL比值高于Ru/c-CeO2、Ru/r-CeO2,这一现象表明Ru/o-CeO2表现出更高的化学吸附氧浓度,可能是由于Ce3+在八面体形貌氧化铈中的比例较高而产生更多的氧空位[26].
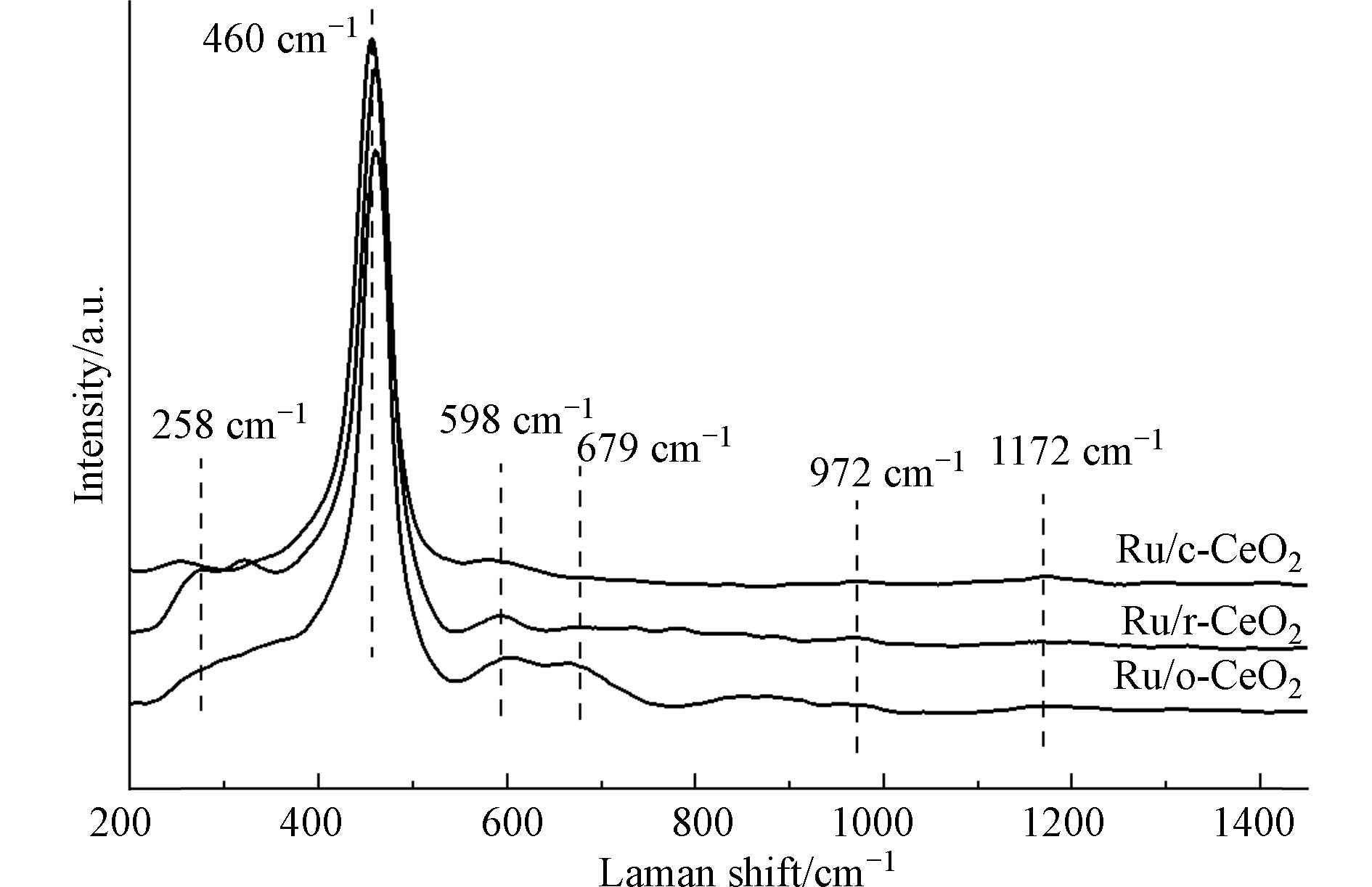
如图4展示了在532 nm激光激发下的Ru/CeO2材料拉曼光谱. 所有的样品在460 cm−1处都有一个尖锐的峰,该峰属于CeO2萤石相的F2g模式,在258、598、
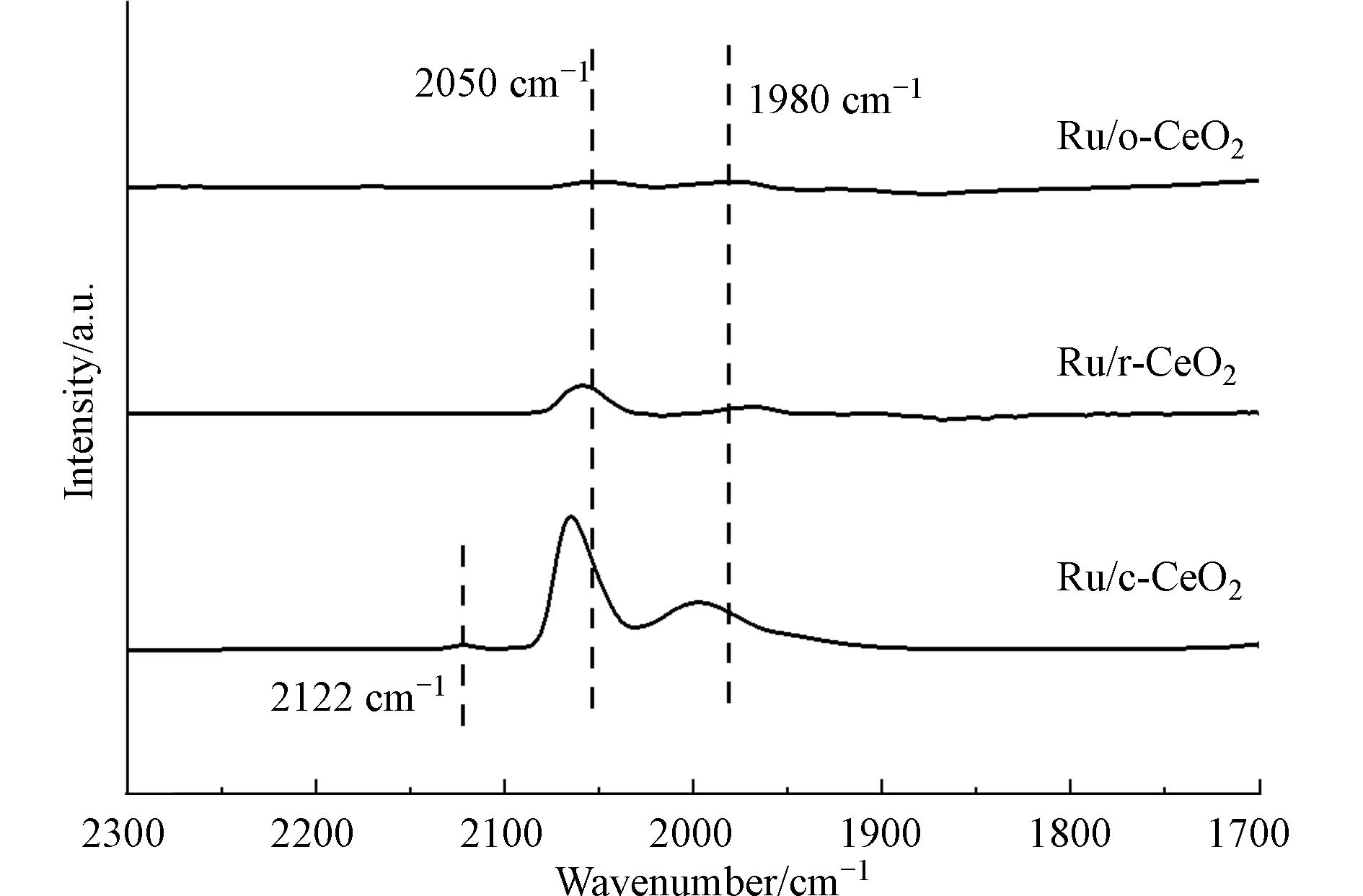
1172 cm−1处的3个弱峰,分别属于二阶横声波(2TA)模式、缺陷诱导(D)模式和二阶纵向光学(2LO)模式[18]. 与文献对比后上述四个峰为CeO2特征峰,在679 cm−1与972 cm−1处的峰为CeO2经Ru负载后产生的新峰,可归属于金属−载体相互作用形成的Ru—O—Ce键[21]. D峰与F2g峰的相对强度之比(D/F2g)可表示CeO2表面氧空位浓度的大小,3种材料的D/F2g相对强度之比为Ru/o-CeO2(0.114)>Ru/r-CeO2(0.072)>Ru/c-CeO2(0.055),表明Ru/o-CeO2具有相对多的本征缺陷位和丰富的氧空位.为了进一步探究催化剂表面Ru物种的结构信息,使用原位CO吸附红外光谱对3种催化剂进行分析. CO原位DRIFT能够检测CO吸附过程中的活性物种,进而探究负载在催化剂表面的贵金属结构性质. 从图5中可以看到,所有催化剂在2050 cm−1均有吸收峰,它归属于CO在金属态Ru上的线式峰,1980 cm−1处的峰表示在Ru-CeO2界面上与Ru桥式键合的CO[27],桥式吸收峰极弱,表明Ru主要是以分离或较小的Ru集合形式存在. Ru/c-CeO2的吸收峰发生红移是由线式吸附态CO物种之间偶极-偶极作用增强造成的[28],进一步说明该催化剂中的Ru主要以纳米团簇形式存在,分散性较好. Ru/c-CeO2相较于Ru/o-CeO2与Ru/o-CeO2在经过N2吹扫后在
2122 cm−1附近仍有吸收峰,可能归属于CO在CeO2上的弱吸附峰[29]. 同样,图中观察Ru/o-CeO2与Ru/r-CeO2的CO吸收峰明显弱于Ru/c-CeO2的吸收峰,表明Ru/c-CeO2的CO吸附位点多,具有大量的Ru活性位点. -
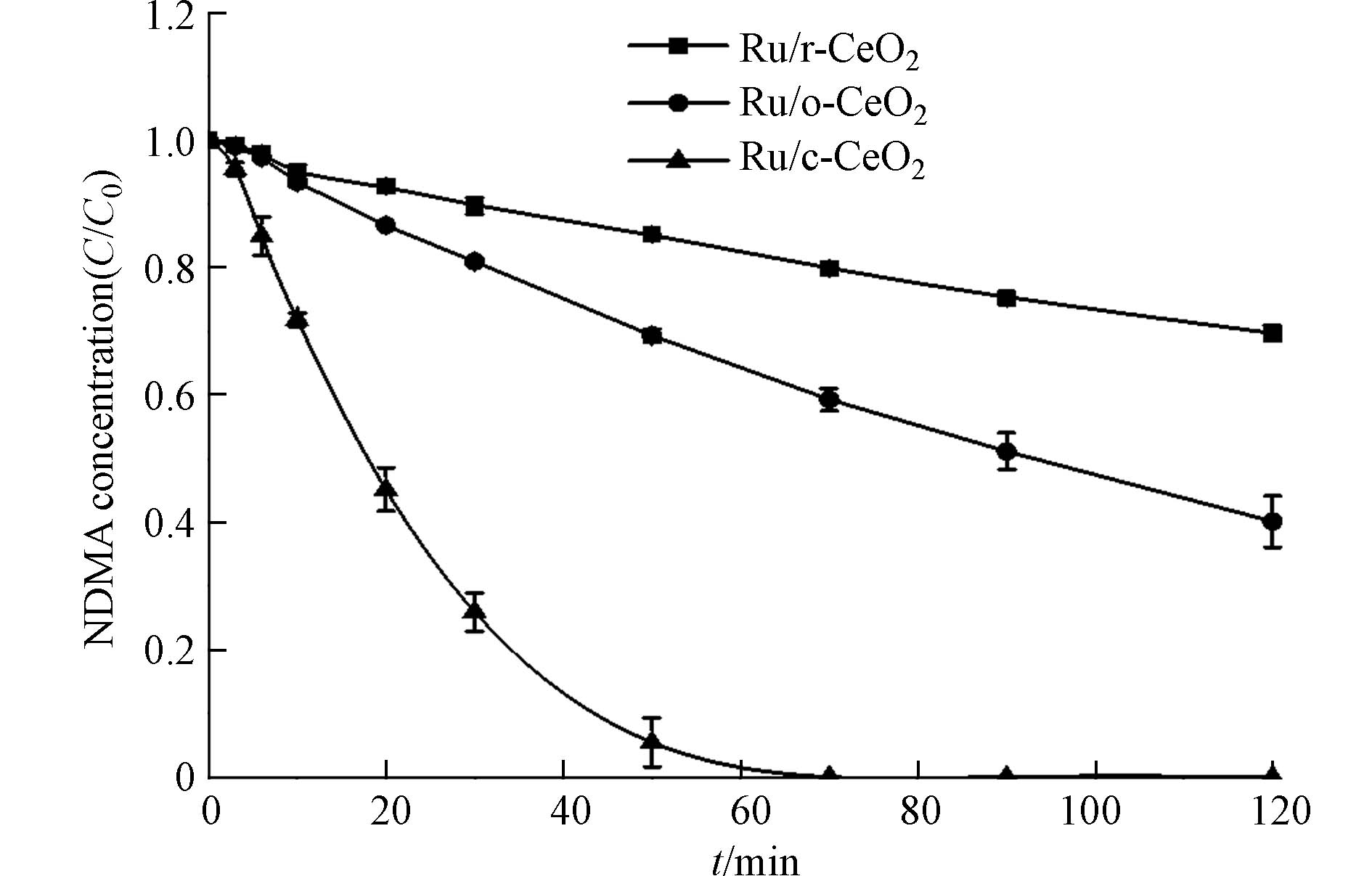
为探究不同形貌载体负载Ru基催化剂的载体对NDMA催化还原反应活性的影响,选择初始浓度约为0.02 mmol·L−1、pH=6,投加0.100 g·L−1的Ru/c-CeO2、Ru/o-CeO2、Ru/r-CeO2催化剂,在常温常压条件下进行加氢催化还原反应.
催化剂对NDMA的催化还原效果如图6所示,Ru/c-CeO2对NDMA实现完全加氢还原可以在70 min内完成,而Ru/o-CeO2与Ru/r-CeO2在120 min时仅有59.9%和30.3%的NDMA被降解去除,此结果说明Ru/c-CeO2比于Ru/o-CeO2和Ru/r-CeO2对NDMA具有更高的加氢还原活性,具体降解效果为Ru/c-CeO2>Ru/o-CeO2>Ru/r-CeO2. 因为3种催化剂负载量较一致,反应活性的差异与催化剂载体形貌的不同有关.
对于液相催化加氢反应而言,NDMA主要通过与活性H*进行有效反应来还原降解,Ru0和Run+都是催化还原反应所必需的活性位点. 其中,Ru0用于活化氢气产生活性H*,Run+用于活化污染物便于进行还原反应. XPS表征的结果显示,3种催化剂的Run+含量为Ru/c-CeO2>Ru/r-CeO2>Ru/o-CeO2,Ru/c-CeO2上的Run+占比最高,为94.65%. 这表明Ru和c-CeO2之间具有强烈的金属-载体相互作用,更多的Run+意味着对NDMA还原反应催化活性更强. XPS和拉曼的表征结果显示,Ru/o-CeO2催化剂相比于Ru/c-CeO2与Ru/r-CeO2有着更高的OV/OL比,这表明Ru/o-CeO2表面的氧空位更多,有着大量的缺陷位作为反应活性位点与吸附位点,增加了Ru在载体表面的分散度. 这两种因素的共同作用导致了3种催化剂的NDMA还原活性按照Ru/c-CeO2>Ru/o-CeO2>Ru/r-CeO2的顺序排列. 可以看出,金属-载体相互作用强的Ru/c-CeO2催化还原NDMA效果要显著优于氧空位较多的Ru/o-CeO2. 综上,在不同形貌CeO2为载体的负载型催化剂催化还原NDMA反应中,具有强金属-载体相互作用的催化剂更有利于NDMA的还原降解.
-
为探究NDMA催化加氢反应中传质阻力对反应活性的影响,选择初始浓度约为0.02 mmol·L−1、pH=6,分别投加0.050、0.075、0.100、0.125 g·L−1的Ru/c-CeO2催化剂,在常温常压条件下进行反应.
从图7可以看出,催化剂投加量与NDMA还原的速率成正比,投加量的增加使得反应物的转化效率明显提高,然而经质量标化后的催化剂初活性基本上保持稳定,结果说明NDMA污染物在本实验条件下的催化加氢还原反应受到传质阻力的影响可忽略不计. 利用一级吸附动力学方程(见式1)对催化剂投加量还原NDMA的反应数据进行拟合,速率常数分别为
0.0099 、0.0376 、0.0602 、0.1259 min−1,随着催化剂剂量的增加,吸附速率常数在不断增加.其中,c0表示NDMA初始浓度(mmol·L−1),c表示NDMA反应浓度(mmol·L−1),kA表示反应速率常数(min−1),t表示反应时间(min).
-
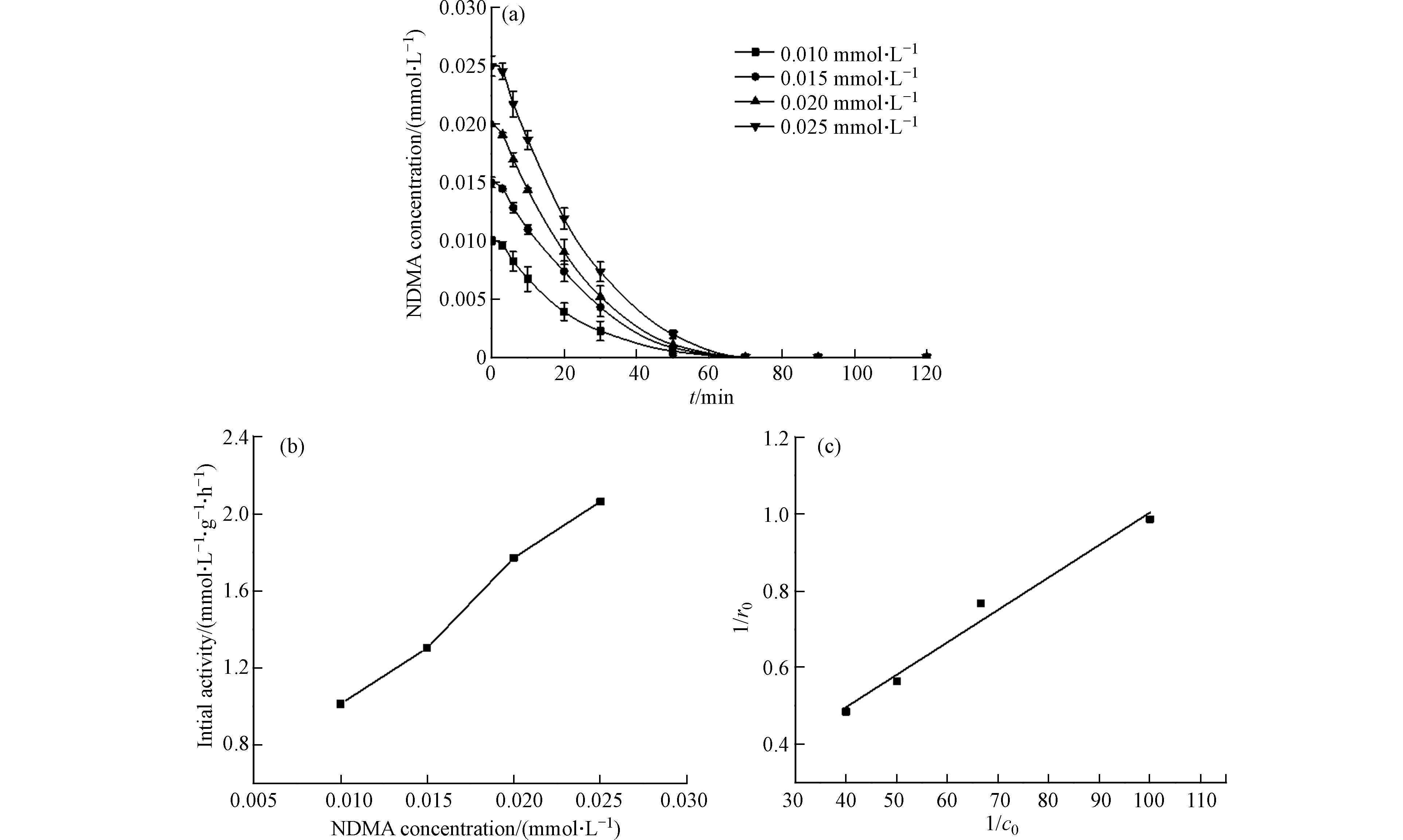
对于非均相的催化反应,吸附在催化剂表面反应物的浓度会影响催化剂的催化反应活性[30]. 使用Ru/c-CeO2催化剂对不同初始浓度的NDMA进行液相催化加氢还原反应. 催化降解效果和反应初活性随着NDMA初始浓度变化如图8所示,初活性随着反应物初始浓度的增大而增大表明Ru/c-CeO2对NDMA加氢还原的初活性与NDMA初始浓度呈正相关. 随着NDMA初始浓度的增加(从0.01 mmol·L−1到0.025 mmol·L−1),Ru/c-CeO2的初活性从1.014 mmol·L−1·g−1·h−1升至2.062 mmol·L−1·g−1·h−1,表明该反应是吸附促进机制. 采用Langmuir-Hinshelwood模型来拟合实验结果(见式2、3). 对1/c0与1/r0的关系采取线性拟合后结果如图8(c)所示,NDMA反应中1/c0与1/r0的线性相关系数R2为0.982,说明在负载型Ru基催化剂上催化加氢的NDMA受吸附作用控制,NDMA在催化剂表面的吸附作用是反应速率控制步骤[31].
其中,r0表示催化还原反应初始反应速率(mmol·L−1·g−1·h−1),c0表示NDMA初始浓度(mmol·L−1), k表示反应速率常数(h−1),b表示吸附平衡常数(L·mmol−1).
-
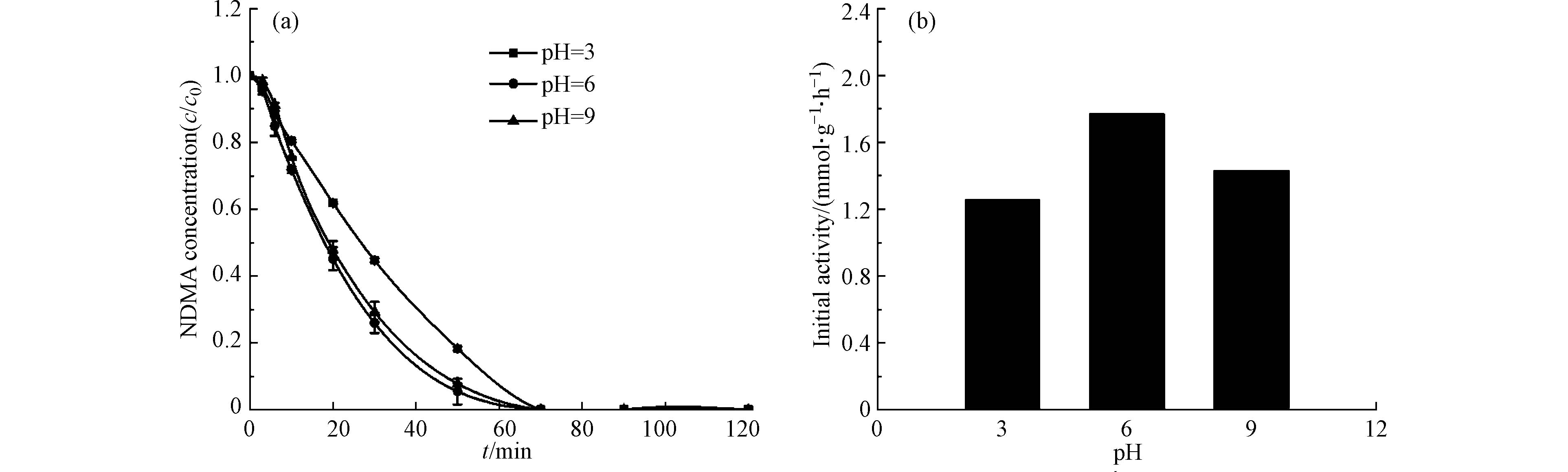
为探究溶液pH对NDMA催化加氢反应的影响,选择初始浓度约为0.02 mmol·L−1、pH=3、6、9,投加0.100 g·L−1的Ru/c-CeO2催化剂,在常温常压条件下进行催化还原反应.
NDMA的反应浓度变化随时间变化的曲线见图9. 从图9可以看出,溶液pH的变化对NDMA的加氢还原速率基本上没有影响,初始溶液pH为酸性、中性与碱性的条件下反应物均能够在70 min内反应完全,pH=6和pH=9相比于pH=3时,初始反应速率更快. CeO2载体的等电点在5—6之间,在酸性环境下催化剂表面带正电荷,碱性环境下表面带负电荷[32]. pH=3时NDMA主要以(CH3)2N-N-OH+的质子化形式存在[33],与带正电荷的催化剂有一定的排斥,使得初活性较低,当反应pH值由6提高至9,催化剂表面带负电荷可能会与碱性条件下NDMA的形态产生一定的斥力,使得初活性从中性条件下的较好吸附状态逐步下降,结果表明反应物在催化剂表面的吸附对于催化还原降解反应起着重要作用.
-
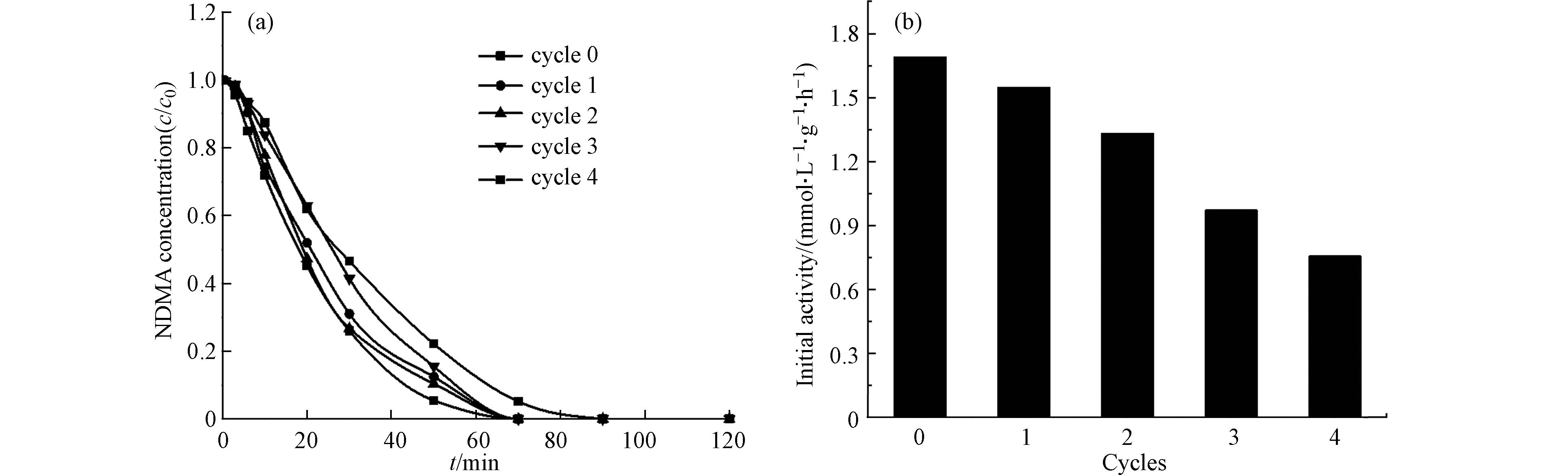
为了评价催化剂的稳定性,在每次反应后用去离子水对溶液进行抽滤清洗,将收集到的材料干燥后回收,以初始pH=6,0.02 mmol·L−1的NDMA初始浓度, 0.100 g·L−1的催化剂Ru/c-CeO2用量为反应条件,对NDMA在Ru/c-CeO2上进行了多次催化加氢还原反应,结果如图10所示. 经过5个连续的反应循环,初始活性损失了55%,但多次循环后Ru/c-CeO2仍能在90 min完全降解NDMA,表明催化还原效果良好,具有较好的实际应用价值. Ru/c-CeO2催化剂逐步失活的原因可能是由于Ru颗粒极易被空气氧化,导致RuOx的生成降低了催化活性,也可能是因为在反应容器内的强烈搅拌,使材料在还原反应过程中失去了活性的Ru颗粒[34].
-
1)3种Ru基催化剂按Run+含量多少排序为:Ru/c-CeO2>Ru/r-CeO2>Ru/o-CeO2,立方体形貌氧化铈的负载型催化剂具有较强的金属-载体相互作用.
2)对于NDMA的液相加氢还原反应,三种Ru基催化剂的催化活性顺序为:Ru/c-CeO2>Ru/o-CeO2>Ru/r-CeO2. 进一步分析得知,金属-载体相互作用与氧空位的分散作用共同影响了催化反应,其中金属-载体相互作用占主导地位.
3)NDMA液相催化加氢还原反应符合Langmuir-Hinshelwood模型,随着初始反应pH的升高,催化剂对NDMA催化还原效果呈倒火山型变化.
4)催化剂经过5次循环利用,对NDMA仍具有良好的催化还原效果.
CeO2形貌对Ru/CeO2液相催化还原N-二甲基亚硝胺的影响
Effect of CeO2 morphology on Ru/CeO2 for liquid phase catalytic reduction of N-nitrosodimethylamine
-
摘要: 采用3种不同形貌的氧化铈(立方体c-CeO2、棒状r-CeO2与八面体o-CeO2)为载体,采用沉淀沉积法制备了Ru/CeO2催化剂,并研究了水中N-二甲基亚硝胺(NDMA)的催化加氢还原反应. 结果表明,3种催化剂的NDMA还原活性顺序为Ru/c-CeO2>Ru/o-CeO2>Ru/r-CeO2. XPS、拉曼等表征结果显示,Ru/c-CeO2具有较高的Run+和适量的氧空位含量,其金属-载体相互作用最强,具有最高的还原活性. NDMA液相催化还原反应符合朗格缪尔-欣谢尔伍德模型,NDMA在催化表面的吸附是反应的控制步骤. 催化剂对NDMA催化还原效率随着pH的升高呈倒火山型变化. 催化剂经过5次循环利用后,仍有较好的催化活性.
-
关键词:
- 液相催化加氢 /
- N-二甲基亚硝胺(NDMA) /
- Ru/CeO2 /
- 金属-载体相互作用.
Abstract: Ru/CeO2 catalysts were prepared by precipitation deposition method using three ceria oxides with different morphologies (cubic-CeO2, rod-CeO2 and octahedral-CeO2) as supports, and the catalytic hydrogenation reduction of N-dimethylnitrosamine (NDMA) was studied in water. The results show that the removal efficiency of NDMA on the three catalysts follows Ru/c-CeO2 > Ru/o-CeO2 > Ru/r-CeO2. The characterization results of XPS and Raman show that Ru/c-CeO2 has higher Run+ and appropriate oxygen vacancy content, which has stronger metal support interaction and higher reduction activity than other catalysts. The NDMA liquid phase catalytic reduction reaction conforms to the Langmuir-Hinshelwood model, and the conversion of adsorbed NDMA on the catalytic surface is the rate-determining step. The liquid phase catalytic reduction of N-nitrosodimethylamine on Ru/CeO2 shows an inverted volcanic change with the increase of pH. The catalyst still has good catalytic activity after five cycles. -

-
表 1 催化剂中Ru、Ce含量占比及氧空位比值
Table 1. The content of Ru,Ce in catalysts and Ce3+/Ce4+, OV/OL
催化剂
CatalystRu0/% Ru4+/% Ru6+/% Ce3+/% Ce3+/Ce4+ OV/OL Ru/c-CeO2 5.35 40.47 54.18 27.4 0.38 0.99 Ru/r-CeO2 13.14 46.84 40.02 27.7 0.38 0.97 Ru/o-CeO2 49.47 22.90 27.63 30.8 0.45 5.52 注:含量计算在同元素间进行. -
[1] SGROI M, ROCCARO P, OELKER G, et al. N-nitrosodimethylamine (NDMA) formation during ozonation of wastewater and water treatment polymers[J]. Chemosphere, 2016, 144: 1618-1623. doi: 10.1016/j.chemosphere.2015.10.023 [2] 蔡宏铨, 裴赛峰, 张昀, 等. 我国城市饮用水中N-亚硝基二甲胺分布水平与健康风险评估[J]. 环境与职业医学, 2021, 38(11): 1231-1236,1243. CAI H Q, PEI S F, ZHANG Y, et al. Distribution and health risk assessment of N-nitrosodimethylamine in urban drinking water in China[J]. Journal of Environmental and Occupational Medicine, 2021, 38(11): 1231-1236,1243 (in Chinese).
[3] WHO. IARC monographs on the evaluation of carcinogenic risks to humans; proceedings of the Conference of the IARC monographs on the evaluation of carcinogenic risks to humans, Lyon, FRANCE, F Oct 10-17, 2006 [C]. World Health Organization: GENEVA, 2010. [4] SGROI M, VAGLIASINDI F G A, SNYDER S A, et al. N-Nitrosodimethylamine (NDMA) and its precursors in water and wastewater: A review on formation and removal[J]. Chemosphere, 2018, 191: 685-703. doi: 10.1016/j.chemosphere.2017.10.089 [5] CHEN W H, WANG C Y, HUANG T H. Formation and fates of nitrosamines and their formation potentials from a surface water source to drinking water treatment plants in Southern[J]. Chemosphere, 2016, 161: 546-554. doi: 10.1016/j.chemosphere.2016.07.027 [6] KIM D, AMY G L, KARANFIL T. Disinfection by-product formation during seawater desalination: A review[J]. Water Research, 2015, 81: 343-355. doi: 10.1016/j.watres.2015.05.040 [7] WEBSTER T S, CONDEE C, HATZINGER P B. Ex situ treatment of N-nitrosodimethylamine (NDMA) in groundwater using a fluidized bed reactor[J]. Water Research, 2013, 47(2): 811-820. doi: 10.1016/j.watres.2012.11.011 [8] DAI X D, ZOU L D, YAN Z F, et al. Adsorption characteristics of N-nitrosodimethylamine from aqueous solution on surface-modified activated carbons[J]. Journal of Hazardous Materials, 2009, 168(1): 51-56. doi: 10.1016/j.jhazmat.2009.01.119 [9] SGROI M, ROCCARO P, OELKER G L, et al. N-nitrosodimethylamine (NDMA) formation at an indirect potable reuse facility[J]. Water Research, 2015, 70: 174-183. doi: 10.1016/j.watres.2014.11.051 [10] WANG X F, YANG H W, ZHOU B H, et al. Effect of oxidation on amine-based pharmaceutical degradation and N-Nitrosodimethylamine formation[J]. Water Research, 2015, 87: 403-411. doi: 10.1016/j.watres.2015.07.045 [11] SZCZUKA A, HUANG N, MacDONALD J A, et al. N-nitrosodimethylamine formation during UV/hydrogen peroxide and UV/chlorine advanced oxidation process treatment following reverse osmosis for potable reuse[J]. Environmental Science & Technology, 2020, 54(23): 15465-15475. [12] ALONSO F, BELETSKAYA I P, YUS M. Metal-mediated reductive hydrodehalogenation of organic halides[J]. Chemical Reviews, 2002, 102(11): 4009-4092. doi: 10.1021/cr0102967 [13] LI M H, HE J, TANG Y Q, et al. Liquid phase catalytic hydrogenation reduction of Cr(VI) using highly stable and active Pd/CNT catalysts coated by N-doped carbon[J]. Chemosphere, 2019, 217: 742-753. doi: 10.1016/j.chemosphere.2018.11.007 [14] YU L, LI D, XU Z Y, et al. Polyaniline coated Pt/CNT as highly stable and active catalyst for catalytic hydrogenation reduction of Cr(VI)[J]. Chemosphere, 2023, 310: 136685. doi: 10.1016/j.chemosphere.2022.136685 [15] STRUKUL G, GAVAGNIN R, PINNA F, et al. Use of palladium based catalysts in the hydrogenation of nitrates in drinking water: From powders to membranes[J]. Catalysis Today, 2000, 55(1/2): 139-149. [16] ZHOU J, HAN Y X, WANG W J, et al. Reductive removal of chloroacetic acids by catalytic hydrodechlorination over Pd/ZrO2 catalysts[J]. Applied Catalysis B:Environmental, 2013, 134/135: 222-230. doi: 10.1016/j.apcatb.2013.01.005 [17] WU K, ZHENG M J, HAN Y X, et al. Liquid phase catalytic hydrodebromination of tetrabromobisphenol A on supported Pd catalysts[J]. Applied Surface Science, 2016, 376: 113-120. doi: 10.1016/j.apsusc.2016.03.101 [18] ZHENG C L, MAO D J, XU Z Y, et al. Strong Ru-CeO2 interaction boosts catalytic activity and stability of Ru supported on CeO2 nanocube for soot oxidation[J]. Journal of Catalysis, 2022, 411: 122-134. doi: 10.1016/j.jcat.2022.04.030 [19] RO I, RESASCO J, CHRISTOPHER P. Approaches for understanding and controlling interfacial effects in oxide-supported metal catalysts[J]. ACS Catalysis, 2018, 8(8): 7368-7387. doi: 10.1021/acscatal.8b02071 [20] WANG Z, HUANG Z P, BROSNAHAN J T, et al. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion[J]. Environmental Science & Technology, 2019, 53(9): 5349-5358. [21] HUANG H, DAI Q G, WANG X Y. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene[J]. Applied Catalysis B:Environmental, 2014, 158/159: 96-105. doi: 10.1016/j.apcatb.2014.01.062 [22] TAN H Y, WANG J, YU S Z, et al. Support morphology-dependent catalytic activity of Pd/CeO2 for formaldehyde oxidation[J]. Environmental Science & Technology, 2015, 49(14): 8675-8682. [23] DONG F, MENG Y, HAN W L, et al. Morphology effects on surface chemical properties and lattice defects of Cu/CeO2 catalysts applied for low-temperature CO oxidation[J]. Scientific Reports, 2019, 9: 12056. doi: 10.1038/s41598-019-48606-2 [24] GAO X Q, ZHU S H, DONG M, et al. Ru/CeO2 catalyst with optimized CeO2 morphology and surface facet for efficient hydrogenation of ethyl levulinate to γ-valerolactone[J]. Journal of Catalysis, 2020, 389: 60-70. doi: 10.1016/j.jcat.2020.05.012 [25] TAN L, LI T, ZHOU J, et al. Liquid-phase hydrogenation of N-nitrosodimethylamine over Pd-Ni supported on CeO2-TiO2: The role of oxygen vacancies[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2018, 558: 211-218. [26] LIU P C, NIU R Y, LI W, et al. Morphology effect of ceria on the ammonia synthesis activity of Ru/CeO2 catalysts[J]. Catalysis Letters, 2019, 149(4): 1007-1016. doi: 10.1007/s10562-019-02674-1 [27] ASSMANN J, NARKHEDE V, KHODEIR L, et al. On the nature of the active state of supported ruthenium catalysts used for the oxidation of carbon monoxide: steady-state and transient kinetics combined with in situ infrared spectroscopy[J]. The Journal of Physical Chemistry B, 2004, 108(38): 14634-14642. doi: 10.1021/jp0401675 [28] YEE N, CHOTTINER G S, SCHERSON D A. Carbon monoxide adsorption on Ru-modified Pt surfaces: time-resolved infrared reflection absorption studies in ultrahigh vacuum[J]. The Journal of Physical Chemistry B, 2005, 109(12): 5707-5712. doi: 10.1021/jp044641i [29] FORCE C, BELZUNEGUI J P, SANZ J, et al. Influence of precursor salt on metal particle formation in Rh/CeO2 catalysts[J]. Journal of Catalysis, 2001, 197(1): 192-199. doi: 10.1006/jcat.2000.3067 [30] DONG Z P, LE X, DONG C X, et al. Ni@Pd core-shell nanoparticles modified fibrous silica nanospheres as highly efficient and recoverable catalyst for reduction of 4-nitrophenol and hydrodechlorination of 4-chlorophenol[J]. Applied Catalysis B:Environmental, 2015, 162: 372-380. doi: 10.1016/j.apcatb.2014.07.009 [31] LIU H, LONG L, XU Z Y, et al. Pd-NCQD composite confined in SBA-15 as highly active catalyst for aqueous phase catalytic hydrodechlorination of 2, 4-dichlorophenoxyacetic acid[J]. Chemical Engineering Journal, 2020, 400: 125987. doi: 10.1016/j.cej.2020.125987 [32] 周娟, 陈欢, 李晓璐, 等. Pd/CeO2催化水中溴酸盐的加氢还原研究[J]. 中国环境科学, 2011, 31(8): 1274-1279. ZHOU J, CHEN H, LI X L, et al. Study on liquid phase catalytic hydrogenation of bromate over Pd/CeO2 catalyst[J]. China Environmental Science, 2011, 31(8): 1274-1279 (in Chinese).
[33] SUN Y H, SUN S, WU T Y, et al. Highly effective electrocatalytic reduction of N-nitrosodimethylamine on Ru/CNT catalyst[J]. Chemosphere, 2022, 305: 135414. doi: 10.1016/j.chemosphere.2022.135414 [34] LI M H, SUN Y H, TANG Y Q, et al. Efficient removal and recovery of copper by liquid phase catalytic hydrogenation using highly active and stable carbon-coated Pt catalyst supported on carbon nanotube[J]. Journal of Hazardous Materials, 2020, 388: 121745. doi: 10.1016/j.jhazmat.2019.121745 -



 下载:
下载: