-
水体氟污染被公认为是世界范围内严重的环境问题之一[1]. 目前,全球约三分之一的地区、超过35个国家存在氟污染,尤其是发展中国家的农村地区氟中毒情况较严重[2]. 在多数情况下,碱性水体、矿物含氟多、地下水闭流的地区氟污染比较严重. 工业“三废”大量排放也是造成很多地区氟污染的重要原因[3]. 水体中除少量镁-氟化物复合物(MgF+)以外,超过95%以上以F−形态存在[4]. 而氟是人体和生态必需的元素之一,少量氟化物有利于骨骼生长发育和预防蛀牙,但摄取过多轻者出现氟斑牙、氟骨病等疾病,重者则会产生骨骼变脆、骨质疏松、神经损伤等病变[5]. 因此,保障氟污染水体的高效处理具有重要现实意义. 目前常用的除氟方法有膜分离法、离子交换法、沉淀法、电凝聚法、吸附法等[6 − 15],其中吸附法由于其吸附效率高、成本低、操作便捷的优点而得到广泛应用.
近期研究表明,稀土元素具有较低的离子电位和较高的碱度,如氧化镧、氧化铈等,它们具有很强的将表面羟基(−OH)解离为氢氧根离子(OH−)的能力[16],对水中F−具有极佳的吸附性能[17-18]. 常用的商用除氟剂如活性氧化铝(AA)和氧化铁,使用时受溶液酸碱性影响较大,金属离子容易溶出[19]. 相比之下,水合氧化铈(HCO)稳定性良好,具备优良的抗酸碱溶出性能,比表面积大,且除氟活性高[20]. 此外,铈氧化物对氟具有高选择性,在各种阴离子共存时,HCO的选择性顺序为:F−>HPO42−>SO42−≥Br−[21]. 因此,HCO在水体除氟领域具备良好的应用前景.
然而,HCO的小尺寸特性使其在实际应用中分离困难、颗粒易流失及难回收利用[22-23],若将其负载于活性炭、介孔硅、生物质或离子交换树脂等大尺寸多孔载体内部则能有效地解决以上问题[24 − 27]. 其中,离子交换树脂D201为均匀的大颗粒球体结构,尺寸介于0.5—0.7 mm之间,具有良好的机械强度和水力学特性;且耐酸碱性能好,易于表面改性;其应用于固定床操作系统中,过水流量均匀,压头损失小,且操作简便,易脱附再生. 因此,D201可作为一种优良的载体材料,近期受到越来越多的关注[28]. 此外,D201表面修饰的季铵基团携带大量强正电荷,可与F−产生静电吸附作用,能够显著提高材料的除氟能力[29].
本研究中以D201为载体,将高活性HCO纳米颗粒嵌入其内,制备得到高性能铈基复合纳米材料HCO@D201. 考察溶液pH、反应温度、反应时间、竞争离子等因素对其除氟性能的影响规律,探究复合材料深度除氟的基本性能与作用机制,评价复合材料循环吸附再生性能,为复合纳米材料在水体高效除氟中的实际应用提供理论依据.
-
硫酸铈(四水合物)购买自国药集团化学试剂有限公司,分析纯. 无水乙醇、氯化钠、氟化钠、硝酸钠、硫酸钠、氢氧化钠、二水合柠檬酸钠等药品均购买自上海阿拉丁生化科技股份有限公司,分析纯. 实验用水为超纯水.
-
用电子天平分别称取6.74、33.7、67.4 g的CeS2O8·4H2O在含20% (V/V)乙醇的水溶液中充分混合,调节pH=2. 分别在上述铈盐溶液中加入5 g D201载体,采用加热板控制反应温度为55 ℃. 不断搅拌反应,使溶液充分浓缩后,将D201从浓缩液中滤出,在298 K下通风干燥,获得负载Ce(Ⅳ)的D201. 称取5 g NaOH配制500 mL NaOH溶液,加入材料,控制温度30℃下持续搅拌12 h,将Ce(Ⅳ)以Ce(Ⅳ)氢氧化物沉淀到D201的孔道中. 将反应后的材料滤出,用去离子水洗至中性,将材料放入250 mL 5%的NaCl溶液搅拌30 min并重复3次,将材料中残留的OH−转为Cl−,随后将材料洗至中性. 用纯乙醇漂洗材料半分钟后放入60 ℃烘箱中干燥24 h,即可得到3种固载量的纳米氧化铈复合材料 HCO@D201.
-
使用扫描电子显微镜(SEM)观察HCO@D201的形貌特征;X射线衍射(XRD)表征载入纳米HCO的晶体结构;材料在用硝酸-高氯酸溶液进行酸消化后,用电感耦合等离子体发射光谱仪(ICP)测定HCO@D201中的铈含量;透射电子显微镜(TEM)得到载入的纳米HCO颗粒的形貌结构和大小;低温氮气吸附-吸附等温线(BET)来测定比表面积;光电子能谱(XPS)测定元素的结合能,以C 1s标准峰(284.8 eV)为参照.
-
使用氟化钠配制浓度为100 mg·L–1 的F−标准储备液. 配制pH=5—6的总离子强度缓冲溶液(TISAB I:0.2 mol·L–1柠檬酸钠和1 mol·L–1硝酸钠,pH=5—6)辅助F−浓度的测定. 采用F−选择电极法,按国家水质监测标准方法(GB
7484 -87)测定F−含量[30],根据质量守恒计算F−吸附量. -
分别称取6份0.025 g HCO@D201置于干燥的150 mL聚乙烯试剂瓶中. 分别配制F−浓度分别为5、10、20、40、60、80 mg·L–1的溶液,调节溶液pH=4,分别取50 mL不同氟浓度的溶液加入瓶中,在298 K、180 r·min–1 的摇床中持续反应24 h. 根据F−初始浓度由低到高分别取15、15、10、5、5、5 mL平衡液,分别加入5 mL TISAB I后定容至25 mL,测量溶液中剩余F−浓度,利用氟标准曲线计算吸附量.
-
分别称取10份0.025 g HCO@D201置于干燥的150 mL聚乙烯试剂瓶中. 分别加入30 mg·L–1的氟溶液,分别控制氟溶液的pH为2、3、4、5、6、7、8、9、10、11,在298 K、180 r·min–1的摇床中反应24 h. 各取15mL平衡液,加入5 mL TISAB I后定容至25 mL,测量溶液中剩余F−浓度,利用氟标准曲线计算吸附量.
-
称取0.5 g HCO@D201置于干燥的1L聚乙烯试剂瓶中. 配制30 mg·L–1的氟溶液,将pH调为4,取1 L加入烧瓶. 在恒温298 K、180 r·min–1下,间隔0、1、2、4、10、15、20、40、60、80、100、120、150、180、240、300、360、420、480、600、720 min移取1 mL反应溶液,加入5 mL TISAB I后定容至25 mL. 测量溶液中剩余F−浓度,利用氟标准曲线计算吸附量.
-
称取0.025 g HCO@D201置于干燥的150 mL聚乙烯试剂瓶中. 配制含有不同竞争离子(Cl−、SO42−、HCO3−)的氟溶液,设定初始溶液中F−浓度为30 mg·L–1,竞争离子与F−的摩尔比分别为0、5、10、20、40、60. 控制pH=4,每个瓶子分别移取50 mL所配溶液,在恒温298 K、180 r·min–1的摇床中反应24 h. 各取15 mL平衡液,加入5 mL TISAB I后定容至25 mL,测量溶液中剩余F−浓度,利用氟标准曲线计算吸附量.
-
称取6份0.025 g HCO@D201置于干燥的150 mL聚乙烯试剂瓶中. 配制30 mg·L–1的氟溶液,控制pH=4,分别取50 mL氟溶液于瓶中,在恒温298 K、180 r·min–1的摇床中吸附12 h. 分离吸附剂,取15 mL平衡液,测量溶液中剩余的F−浓度,利用氟标准曲线计算吸附量. 混合所有材料,用去离子水洗去表面多余的F−. 称取5 g NaOH配制100 mL氢氧化钠溶液,加入材料在恒温298 K、180 r·min–1的摇床中脱附12 h,取5 mL反应后溶液,用HCl将溶液调至中性,加入5 mL TISAB I后定容至25 mL,测量溶液中剩余的F−浓度,利用氟标准曲线计算脱附量. 用去离子水充分清洗吸附剂至中性. 称取5 g NaCl配制100 mL氯化钠溶液,加入材料. 在恒温298 K、180 r·min–1的摇床中反应12 h后分离材料,充分清洗后放入60℃烘箱中干燥24 h. 重复上述操作5次,考察材料的再生性能.
-
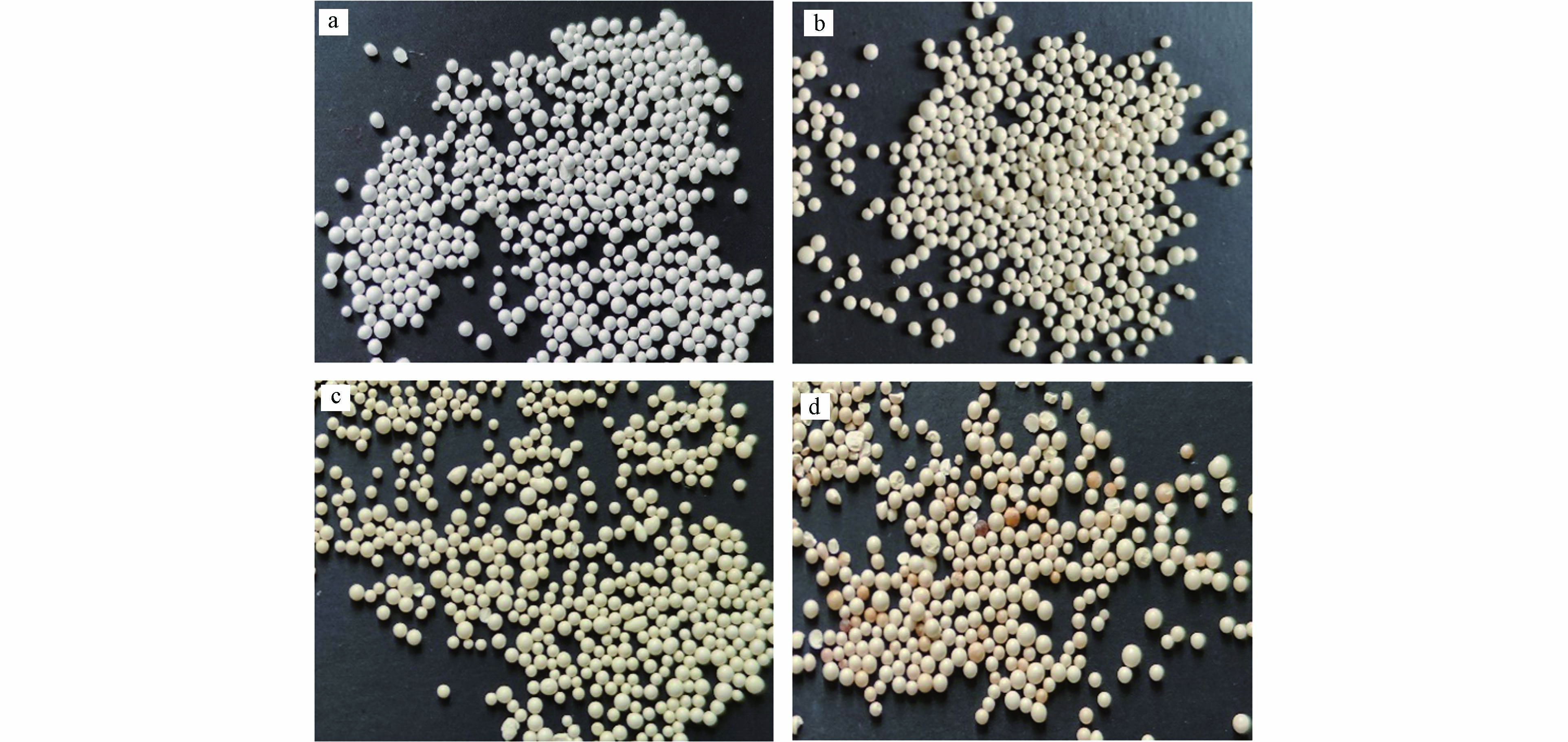
为了获得不同铈含量的复合纳米材料,配制不同浓度铈盐作为铈前驱体溶液. 由ICP分析可得,复合材料铈含量由低到高分别为3.1%、8.3%、11.7%. 为方便区分,将3种材料分别记为HCO@D201-3.1、HCO@D201-8.3、HCO@D201-11.7. 如图1所示,复合材料与D201形状相似,均为球形. D201本身呈米白色,复合材料呈现淡黄色,有一定金属光泽,且铈固载量越高,复合材料的颜色越深.
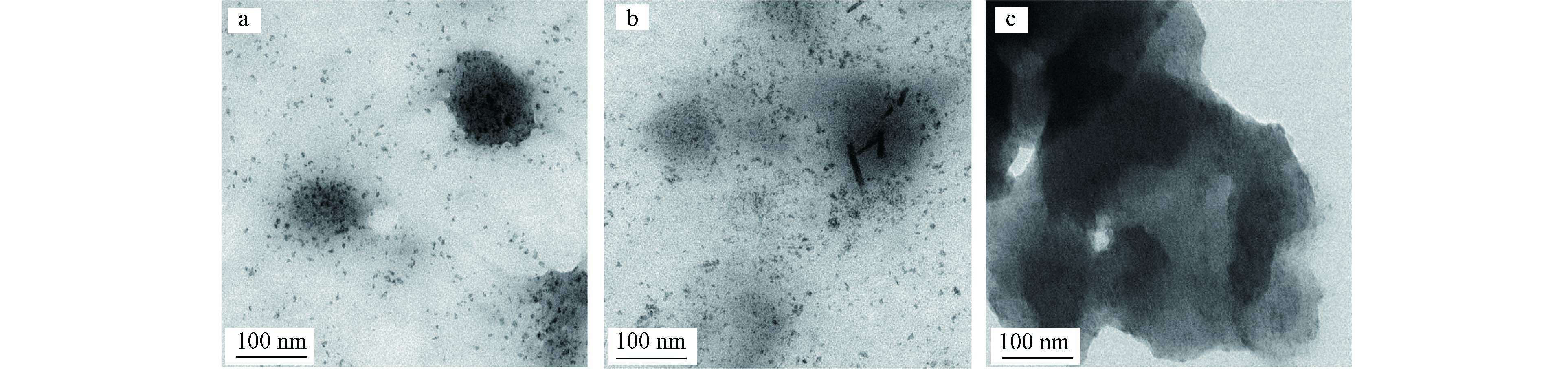
复合纳米材料HCO@D201-3.1、HCO@D201-8.3、HCO@D201-11.7的比表面积分别为18.1、34.8、51.2 m2·g–1,D201的比表面积仅为2.1 m2·g–1,复合材料的比表面积得到显著增加. 这是由于载入的纳米级HCO粒子具有较大的比表面积,能够暴露出大量活性位点. 图2显示了3种不同铈基复合纳米材料的TEM表征结果. 铈固载量较低的HCO@D201-3.1与HCO@D201-8.3内部嵌入的HCO以均匀分散的纳米颗粒形态存在,粒径约为10 nm. 相比之下,HCO@D201-11.7所含HCO则分散性欠佳,存在较为明显的团聚现象. 可见,较高的固载量可能会拥堵载体的纳米孔道,影响HCO在载体内部的均匀分散.
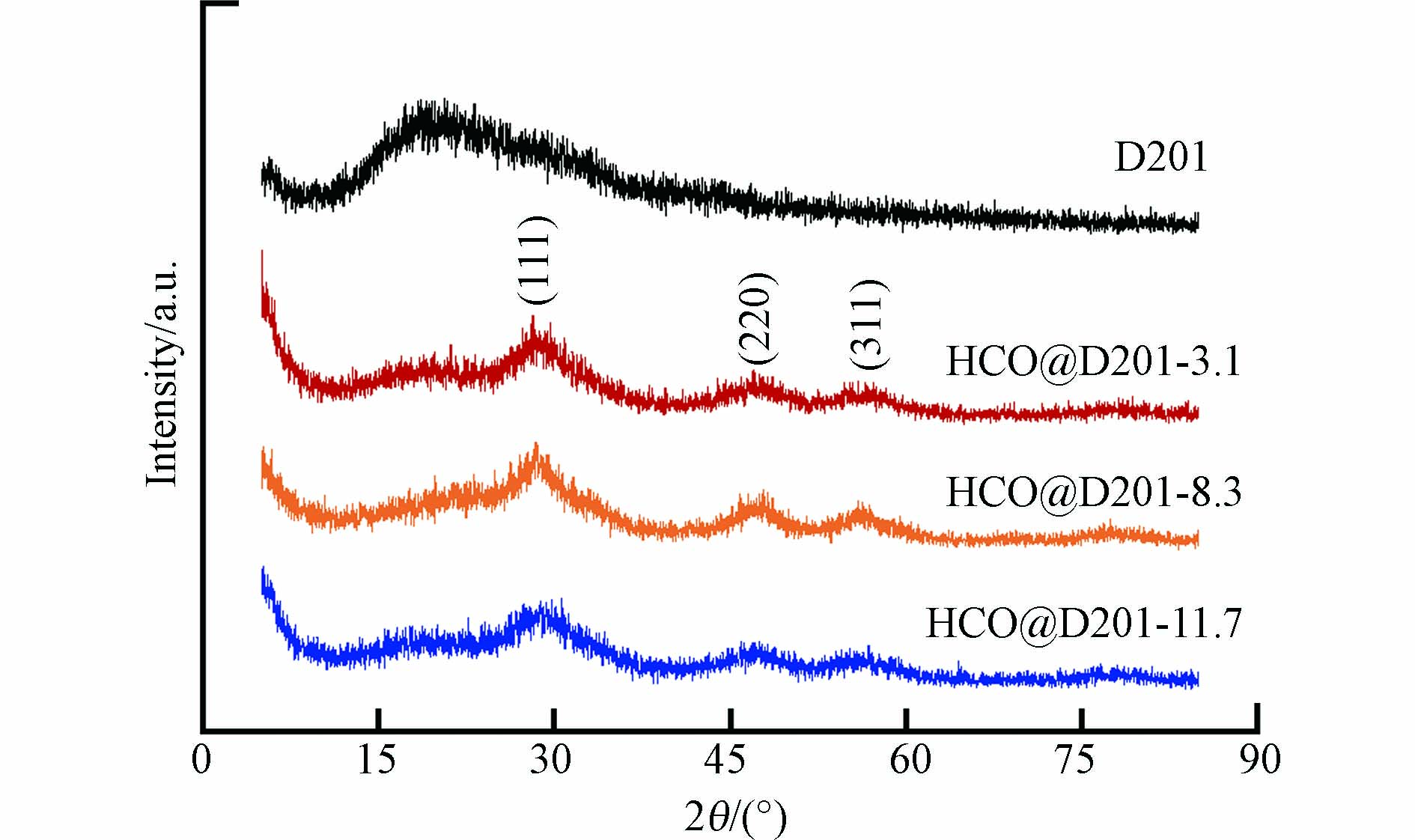
利用X-ray衍射仪来研究复合纳米材料中纳米颗粒的晶体结构,如图3所示. D201在结合能为15°—25°左右出现宽峰,并无明显特征峰[31]. 不同铈含量的复合材料分别在28.5°、47.4°、56.2°结合能处出现明显的衍射特征峰,与文献中报告的HCO的XRD衍射峰相似[31],这说明载入的活性物质为HCO粒子,但其特征峰不够尖锐,可能是受到D201基体结构的影响. 此外,2.7部分的XPS能谱分析发现在拟合出的10个谱峰中分为6个Ce(Ⅳ)、4个Ce(Ⅲ) 的特征峰. 结合能在904.2、898.4、885.6、882.1 eV处的谱峰为Ce(Ⅲ),表明复合材料中存在Ce(Ⅳ)/Ce(Ⅲ)混合价态,这可能是由于材料制备时部分HCO在固载过程中产生了氧空位[16]. 以上结果表明,纳米HCO粒子已经成功固载到D201基体中,并得到3种不同固载量的铈基复合纳米材料HCO@D201.
-
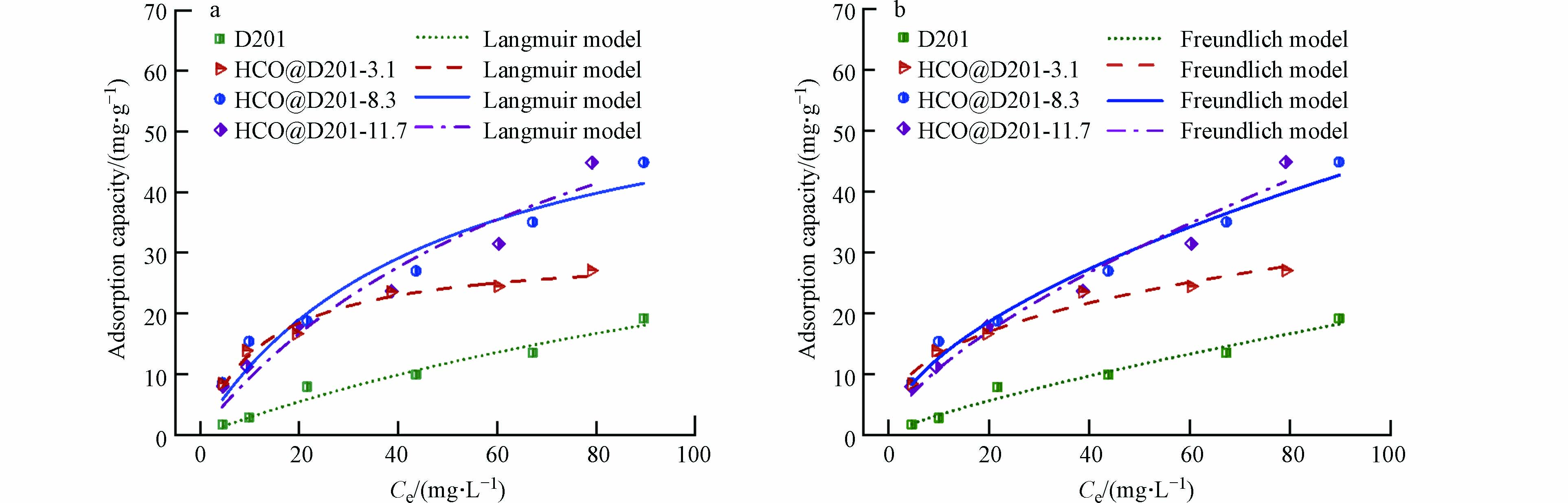
为探究不同固载量的铈基复合纳米材料去除水中氟的饱和吸附量的差异,本文以基体材料D201为对比,测定25 ℃条件下复合材料对水中F−的吸附等温线,如图4所示. 结果表明,复合纳米材料对氟的吸附能力远高于D201,且铈负载量较高时除氟性能更好.
采用Langmuir (图4a)和Freundlich (图4b)等温模型对实验数据进行拟合,具体公式如下:
Langmuir等温吸附方程:
Freundlich等温吸附方程:
式中,Qe (mg·g–1)是复合材料对F−的平衡吸附量;Qm (mg·g–1)是复合材料的最大单层吸附量;Ce (mg·L–1)表示F−的平衡浓度;KL(L·mg–1)、KF [(mg∙g–1)·(L·mg–1)1/n]是平衡吸附常数;1/n为Freundlich模型温度相关常数,表示吸附反应进行的难易程度. Langmuir拟合吸附过程中主要为化学反应的单层吸附. Freundlich是以大量实验数据为基础,总结得出吸附剂主要以多层物理吸附为主的方程.
经计算,两种模型的拟合结果R2均大于0.93,说明Langmuir与Freundlich等温模型对复合材料除氟均具有较好的拟合结果,且Freundlich拟合度略微高一些,说明复合材料除氟并非单一的单层吸附过程. 如表1所示,经计算得到D201对F−最大吸附量为16.3 mg·g–1; 而HCO@D201-3.1最大吸附量为70.9 mg·g–1,吸附性能得到显著升高;HCO@D201-8.3和HCO@D201-11.7 的最大吸附量分别为84.2 mg·g–1 和85.1 mg·g–1,较铈负载量为3.1%时有明显提升. 这是因为铈固载量较高时,能提供更多的活性吸附位点所致. 但当负载量从8.3%继续升高至11.7%,饱和吸附量变化不大,这可能是较多的HCO颗粒堵塞了载体部分孔隙,影响了F−在HCO活性位点上的扩散与吸附,导致吸附容量未出现明显增加. 因此,复合纳米材料的吸附容量远高于商用D201,表明了铈基复合纳米材料对水中F−存在较高的吸附性能. 根据以上不同负载量材料吸附容量的对比结果,并综合考虑材料制备成本,本研究将主要采用HCO@D201-8.3进行后续吸附性能实验探究.
-
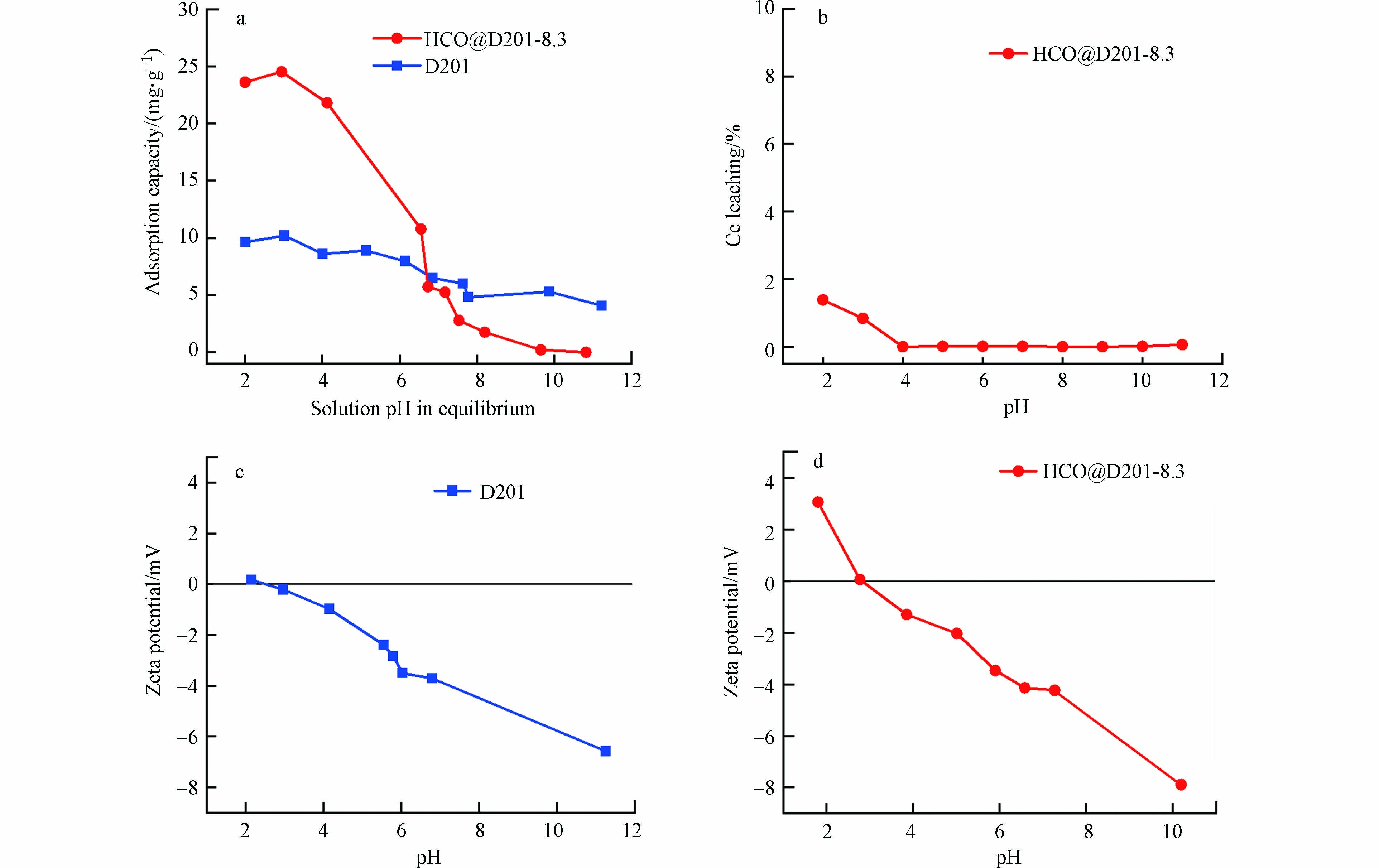
溶液pH对复合材料除氟影响如图5a所示. 初始浓度为30 mg·L–1时,在pH<6.0的酸性溶液中,HCO@D201-8.3对F−的吸附量远超D201,在pH=2.9时最高可达24.5 mg·g–1. 相比之下,D201除F−性能在溶液pH=3.0时达到顶峰,最大吸附容量仅为10.2 mg·g–1. 随着pH不断升高(pH>6.0),HCO@D201-8.3的除氟能力呈明显下降趋势. 此外,HCO@D201-8.3的 pH稳定性如图5b所示. 当pH≥4.0时,Ce溶出为0,此条件下复合材料内氧化物可稳定存在;随着pH持续下降,Ce元素逐渐开始浸出,当pH降到2.0时其对应Ce溶出率仅为1.39%. 因此,复合材料HCO@D201-8.3在使用中耐酸碱性能好,具有良好的pH稳定性. Zeta电位结果显示(图5c和d),复合材料等电点pHzpc为2.82,在此pH区间附近的氟吸附量明显高于其他区间. 此时HCO@D201-8.3表面所带正电荷量较高,复合材料与F−之间的静电吸引和配体交换作用大大增强,因此除氟能力更强. 随着溶液碱性增强,HCO表面会因去质子化而带负电,与F−产生静电排斥,同时OH−会与F−间会产生竞争,从而吸附量呈逐渐下降趋势. 当pH>9.7时,复合材料对氟吸附量几乎为0,可通过碱溶液将吸附饱和的复合材料进行脱附,实现材料的重复使用. 相比D201,HCO@D201-8.3在pH为2.0—6.0范围内有良好的除氟性能.
-
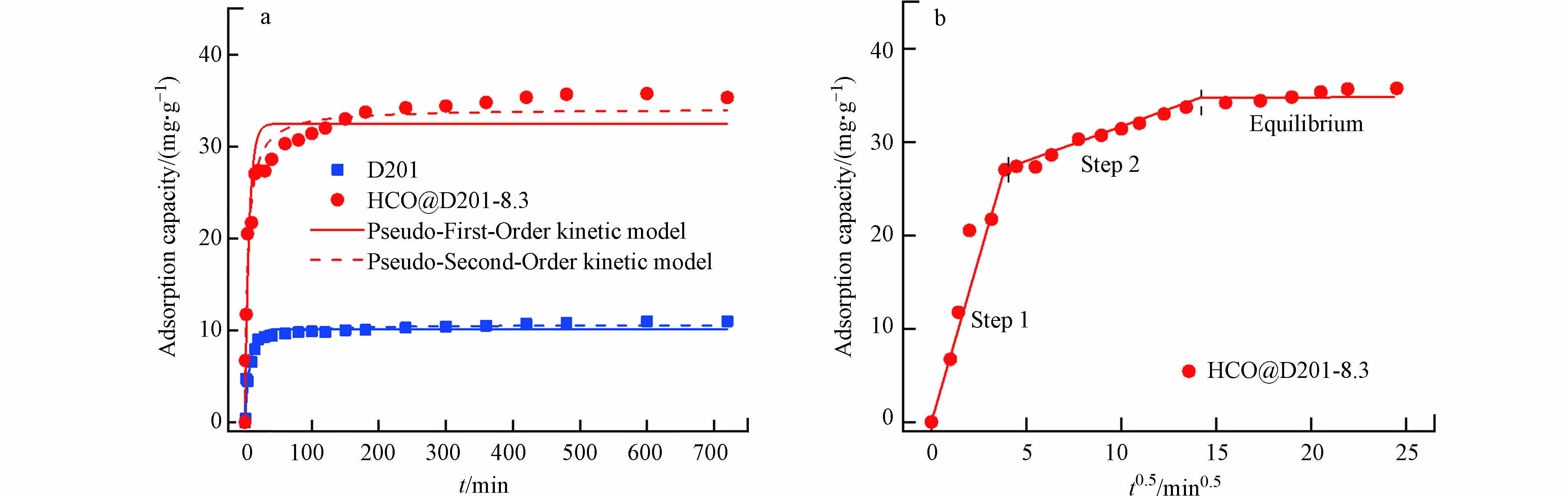
复合材料HCO@D201-8.3与D201的氟吸附动力学曲线如图6a所示. HCO@D201-8.3在前20 min对氟的吸附量急速上升,随后曲线上升趋势放缓,逐渐达到吸附饱和. 在298 K温度下, HCO@D201-8.3在30 min内吸附量可达饱和吸附量的80%,且在120 min内可达吸附平衡. 复合材料良好的动力学行为可能与材料表面的化学结构有关,载体表面与高电荷三甲胺基团结合,因此有大量固定的正电荷(N+),大大加强了F−在基体孔道内的传质扩散作用,因此可显著促进复合材料对F−的吸附动力学速率和HCO纳米粒子利用率. 本文采用伪一级、伪二级动力学模型及颗粒内扩散模型对数据进行拟合,具体公式如下:
式中,Qe (mg·g–1)为复合材料对F−的平衡吸附容量;Qt (mg·g–1)为时间t时复合材料的吸附量;k1 (min–1)、k2 (g·mg–1·min–1))为系数;t (min)为反应时间;k3(g·mg–1·min–0.5)为动力学速率常数.
图6a 为伪一级动力学和伪二级动力学模型对F−的时间依赖性吸附数据的非线性拟合. 结果表明,复合材料的吸附过程更符合伪二级动力学模型,相关系数R2 > 0.97(见表2),进一步表明涉及价态力的化学吸附是限制速度的关键过程. 图6b为颗粒内扩散模型的拟合结构,可分为3个阶段. 具体来说,第一阶段是由膜扩散控制的外表面吸附,F−主要吸附在HCO@D201-8.3的外表面,因初始阶段吸附速率较快. 第二部分为缓慢吸附阶段,其中颗粒内扩散是控制速率的步骤. 当颗粒外表面吸附达到饱和时,F−进入孔内区域,被复合材料内部活性位点所吸附. 当F−在颗粒孔内扩散时,扩散阻力增大,导致扩散速率下降. 最后,复合材料在120 min达到吸附平衡阶段,此时F−的吸附和解吸速率基本相同.
-
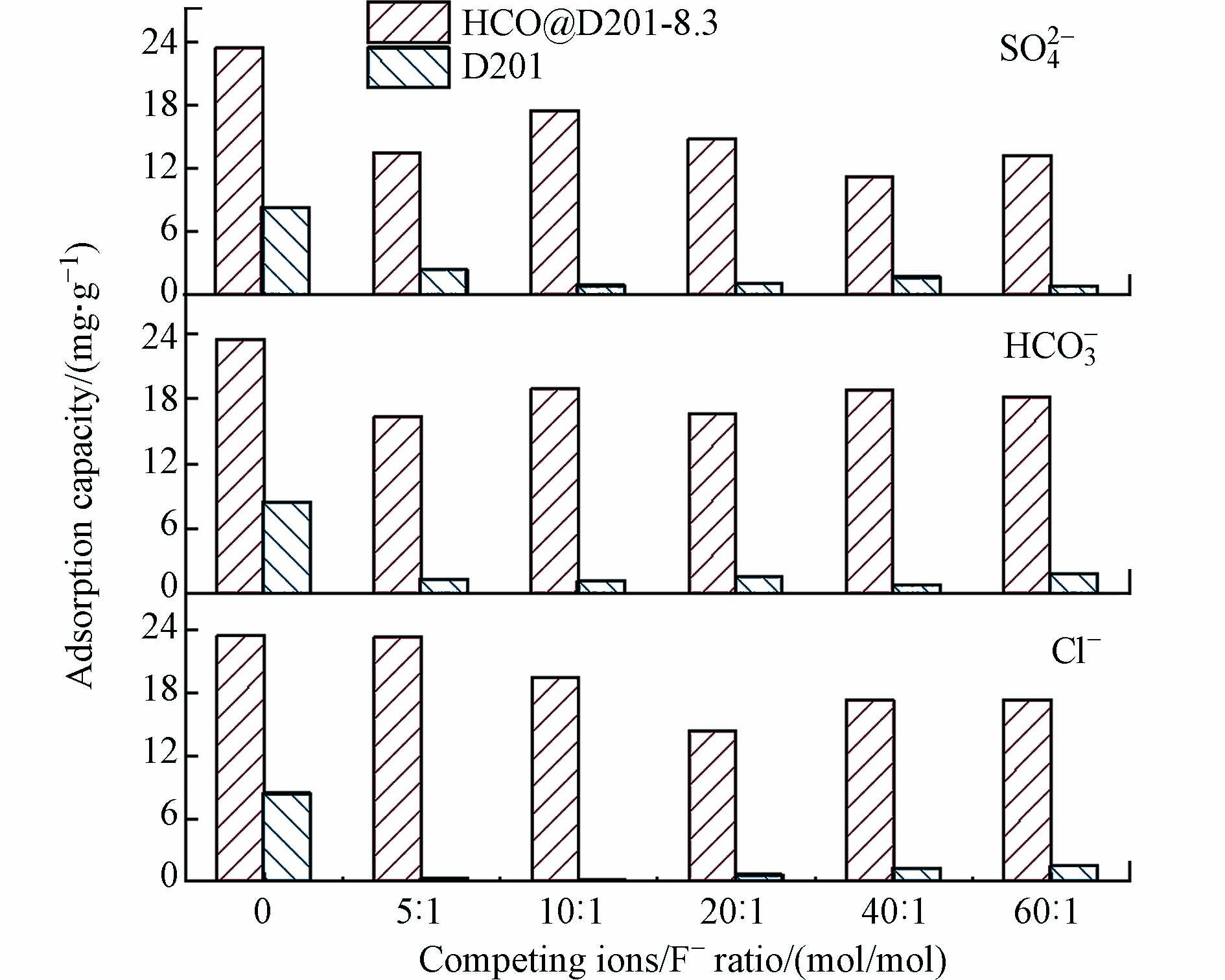
天然水或废水中含有一些常见的共存阴离子,它们可能会不同程度地与F−竞争吸附剂的活性位点. 分别以SO42−、Cl−和HCO3−作为竞争离子,研究不同浓度的竞争离子对复合材料除氟的影响,并以载体材料D201作为对比. 由图7可知,竞争离子与F−摩尔比为5:1时,SO42−参与竞争时吸附量较无竞争时减半,HCO3−参与竞争时除氟效果降低了约40%,而相同摩尔比下Cl−参与竞争时复合材料的除氟性能几乎没有变化. 因此,3种竞争离子对HCO@D201-8.3的影响程度大小为SO42−> HCO3−>Cl−. 然而,D201在竞争离子的摩尔比仅为5:1时,SO42−、HCO3−、Cl− 3种离子都严重影响了D201的除氟效果. 这是因为D201主要依靠表面带正电季铵基团的静电作用吸附F−,当竞争离子浓度增加时,与F−间竞争作用逐渐增强,最终导致D201对氟的吸附量趋于零. 而复合材料对F−的吸附量虽有所波动,但仍保持在原吸附量的50%以上. 此时,复合材料中的纳米HCO与F−的特异性配位作用成为主导. 由此可见,相较于D201,HCO@D201-8.3对F−表现出了较高的吸附选择性.
-
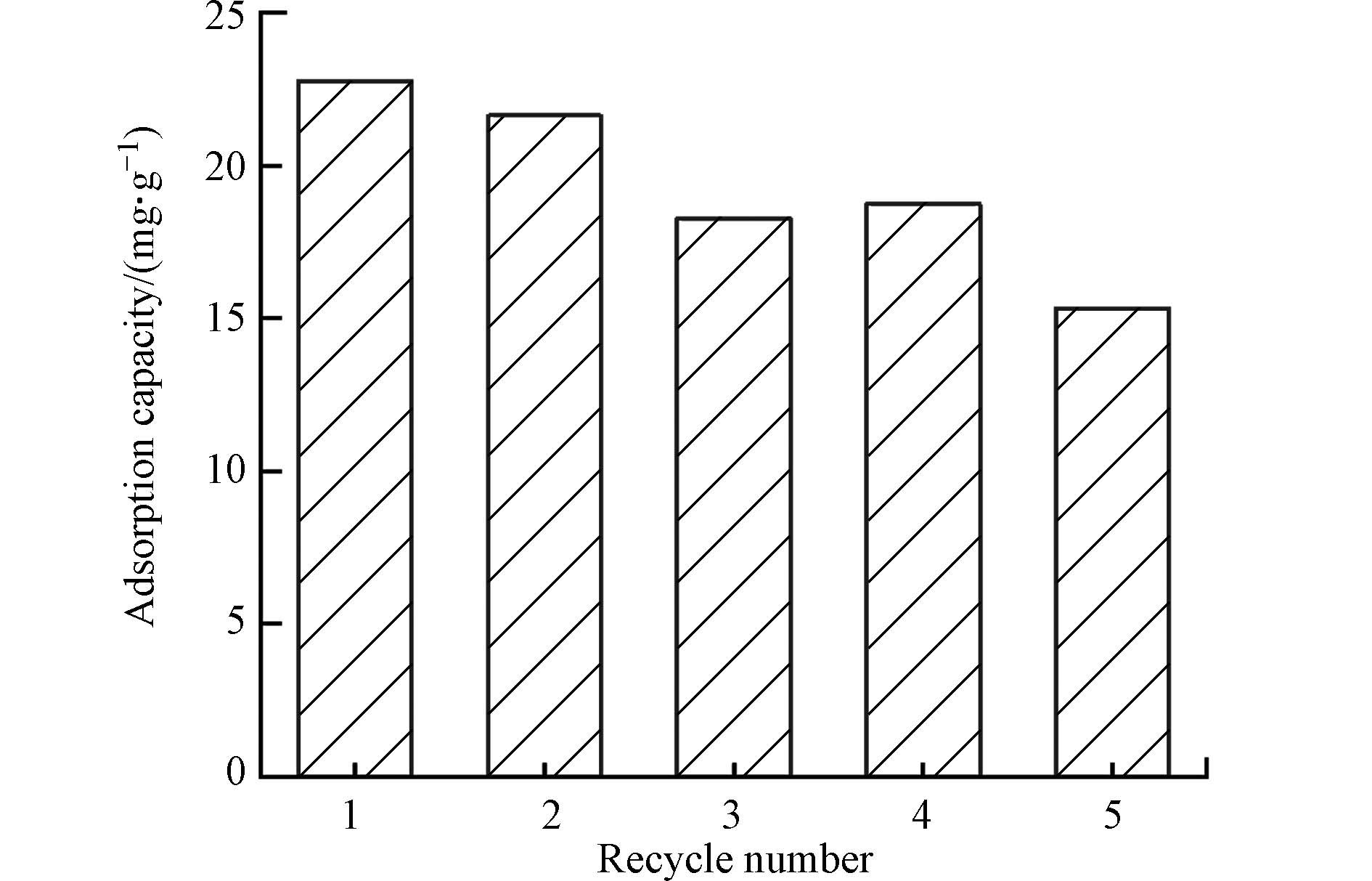
以质量分数5% 的NaOH溶液为脱附剂,对复合纳米材料HCO@D201-8.3进行循环吸附-脱附实验,如图8所示. 可以看出,第1、2次循环后复合材料的除氟能力变化不大,分别为22.8 mg·g–1和21.6 mg·g–1,均在20.0 mg·g–1以上. 在3、4次循环后,吸附量略低于20.0 mg·g–1,分别为18.3 mg·g–1和18.7 mg·g–1. 经5次脱附循环后,吸附量有所降低,仍保持在原吸附量的60%以上,有良好的除氟效果. 结果表明,铈基复合纳米材料具有良好的再生性能,在实际水体除氟领域具有良好的应用前景.
-
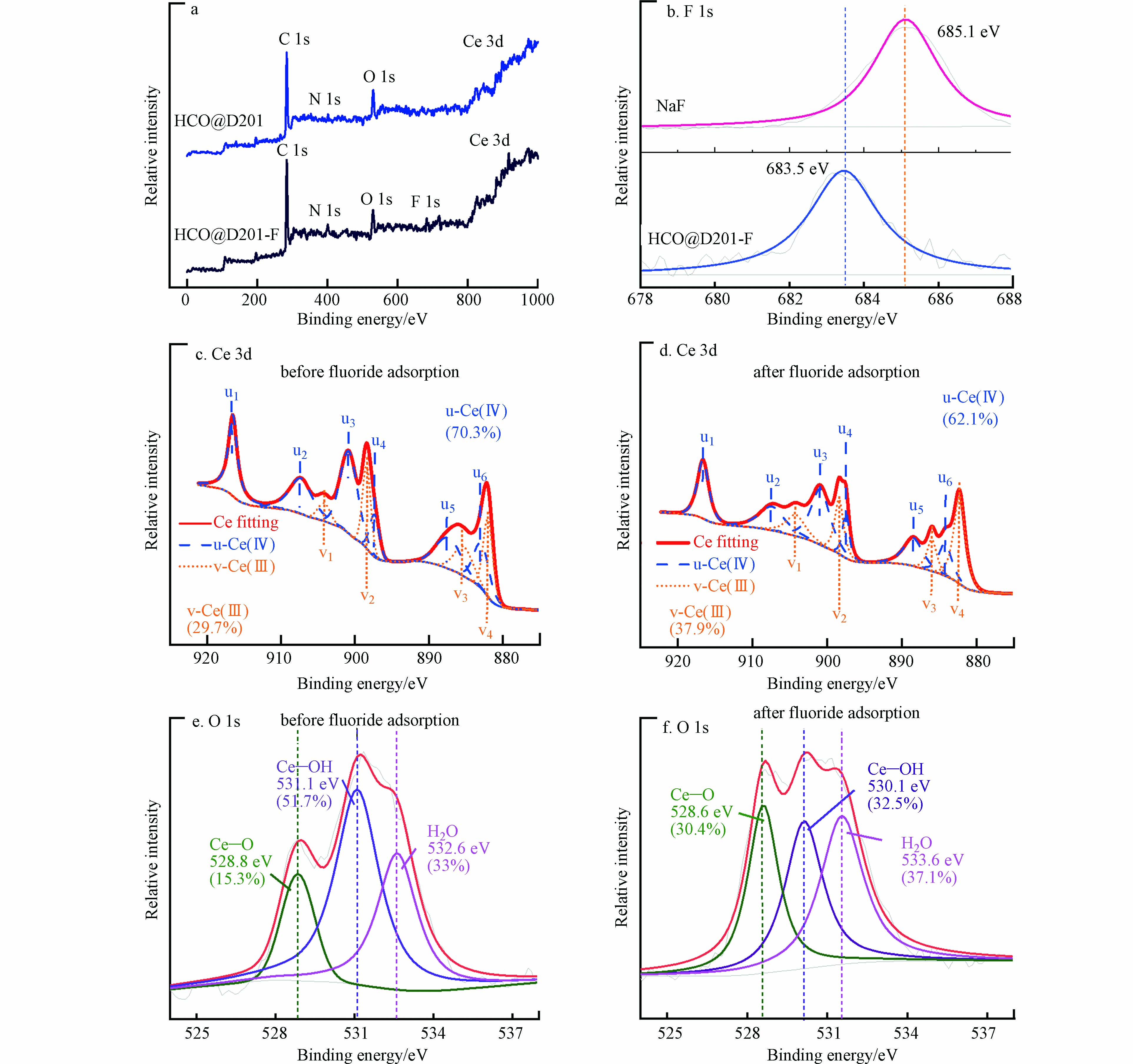
为探究复合纳米材料的除氟机理,对吸附前后的材料进行了XPS能谱表征,并对F 1s、O 1s和Ce 3d的结合能峰进行分析对比. 由图9a可知,在吸附氟后复合材料的全谱中出现了F 1s的结合能峰,说明F−已经被吸附到HCO@D201-8.3内部. 而这一新出现的F 1s峰的结合能为683.5 eV,对比氟化钠的标准F 1s结合能(685.1 eV)降低了1.6 eV(图9b). 这是因为HCO与F−间存在强配位作用[12],当F−被吸附到HCO@D201上,由于氟周围环境与结合方式的变化,F 1s峰结合能的特征峰位置会出现明显的偏移. 图9c为吸附前Ce 3d的拟合分峰结果,图中的划线、短点线分别表示Ce(Ⅳ)和Ce(Ⅲ)特征峰. 由图可知,HCO@D201中同时存在Ce(Ⅳ)和Ce(Ⅲ),这可能是因为HCO在负载过程中产生了氧空位[16].
此外,对比图9c和图9d可知,Ce(Ⅳ)和Ce(Ⅲ)在吸附前的峰面积分别占比分别为70.3%和29.7%,在吸附氟后Ce(Ⅳ)的比例减少了8.2%,这可能是因为有一部分Ce(Ⅳ)在吸附氟的过程中受到F高电负性的影响,转变成了Ce(Ⅲ)[32]. 由图9e和图9f可知吸附前Ce−O、Ce−OH与H2O的峰面积分别占比15.3%、51.7%和33.0%. 吸附F−后,材料中Ce−O与H2O的峰面积占比分别提高了15.1%和4.1%,而Ce−OH的峰面积占比却降低了19.2%. 这可能是由于Ce−OH中的羟基在吸附过程中被氟替换,形成了Ce−F. XPS结果表明,HCO@D201-8.31是通过表面羟基基团与F−的配体交换作用实现了对水中氟的特异性吸附.
-
表3为不同除氟剂的性能对比结果. 相比现有材料,本研究所制备的复合材料HCO@D201-8.3表现出较为优异的除氟性能,且耐酸碱好,易脱附再生,实用性强,具有较好的应用前景.
-
1)根据XRD、TEM等分析可知,HCO已成功固载在D201内部. 当固载量较低时,HCO呈均匀分散状态,粒径约为10 nm;当固载量较高时,HCO在载体内部呈现出一定的团聚状态.
2)BET结果显示,铈固载量为3.1%、8.3%、11.7%时复合材料的比表面积分别为18.1、34.8、51.2 m2·g−1,远大于D201比表面积. 由于材料合成时氧空位的出现,复合材料中铈元素为Ce(Ⅳ)和Ce(Ⅲ)混合价态.
3)除氟性能实验表明,铈固载量由小到大时,复合材料的最大饱和吸附量依次为70.9 mg·g–1、84.2 mg·g–1、85.1 mg·g–1,远高于D201. 综合考虑制备成本与材料性能,选取铈固载量为8.3%的HCO@D201-8.3作为研究对象.
4)HCO@D201-8.3的最适pH应用范围为2.0—6.0,在120 min内可达到吸附平衡,且受竞争离子的影响较小. 5次循环再生实验表明材料具有良好的应用稳定性.
5)XPS分析结果表明,HCO@D201-8.3对F−的特异性吸附主要通过表面羟基与F−之间的配体交换作用而实现.
铈基复合纳米材料的研制及其除氟特性
Preparation of cerium-based composite nanomaterials using for defluorination from water
-
摘要: 通过“前驱体导入-原位沉淀”法将纳米水合氧化铈(HCO)固载到大孔阴离子交换树脂载体D201内部. 通过改变铈盐的用量,制备出3种不同HCO负载量(3.1%、8.3%、11.7%)的铈基复合纳米材料,系统探究其对水中F−的吸附行为及机制. XRD、TEM及BET分析表明,HCO纳米颗粒已成功固载在D201基体内部,且复合材料具有良好的晶型结构和较大的比表面积. 静态吸附实验显示,HCO负载量增加有利于提高除氟性能. 综合考虑成本与性能,选择HCO固载量8.3%为最佳复合材料,吸附量可达84.2 mg·g–1,符合Langmuir吸附等温模型;最佳吸附pH区间为2.0—6.0,且在120 min内能够达到吸附平衡;竞争离子/F−比值高达60时吸附容量仍保持在原吸附量50%以上,吸附选择性明显优于商用D201;经5次吸附-再生除氟性能仍高于原吸附量60%,表现出良好的再生能力. XPS分析结果显示,复合材料表面羟基与F−之间的配体交换作用是特异性除氟的主要原因.Abstract: Nano-sized hydrated cerium oxide (HCO) was immobilized within a commercial porous polystyrene anion exchanger D201 though “precursor leading/ in-situ precipitation”. Three kinds of Ce-based nanocomposites with different HCO loading (3.1%, 8.3%, 11.7%) were prepared by changing the concentration of cerium salt, and the adsorption behavior and mechanism on defluorination from water were systematically investigated. Analysis of XRD, TEM and BET indicated that HCO nanoparticles have been successfully supported in D201 matrix, and the nanocomposites have good crystalline structure and large specific surface area. Batch experiments showed that the increase of HCO loading was favorable to the improvement of defluorination performance. Upon evaluating the balance between cost and performance, the nanocomposite with 8.3% HCO loading was selected as the best adsorbent, and the maximum adsorption capacity can reach 84.2 mg·g–1, which is in line with the Langmuir adsorption isothermal model. The optimal adsorption pH range is 2.0—6.0, and the adsorption equilibrium can be reached within 120 min. When the competitive ion /F− ratio is as high as 60, the adsorption capacity is still more than 50% of the original adsorption capacity, and the adsorption selectivity is obviously better than commercial D201. After 5 times of adsorption and regeneration, the defluorination performance is still 60% higher than the original adsorption capacity, showing a good regeneration ability. The results of XPS analysis showed that the ligand exchange between F− and the hydroxyl groups on the surface of nanocomposite was the main reason for specific defluorination.
-
Key words:
- hydrated cerium oxide /
- nanocomposite /
- fluorine /
- adsorption.
-

-
表 1 HCO@D201吸附氟的等温拟合参数
Table 1. Adsorption isotherm fitting parameters of HCO@D201
材料
MaterialsLangmuir Freundlich Qe/(mg·g–1) KL/(L·g–1) R2 1/n KF/
(mg∙g–1)·(L·mg–1)1/nR2 D201 16.3 0.0186 0.9666 0.782 0.54 0.9731 HCO@D201-3.1 70.9 0.0328 0.9780 0.359 5.75 0.9669 HCO@D201-8.3 84.2 0.0158 0.9379 0.553 3.55 0.9808 HCO@D201-11.7 85.1 0.0121 0.9337 0.650 2.39 0.9659 表 2 材料对氟的吸附动力学拟合参数
Table 2. Adsorption kinetics fitting parameters of HCO@D201-8.3 and D201
材料
Materials伪一级动力学
Pseudo first order kinetics伪二级动力学
Pseudo second order kineticsQe /(mg·g–1) R2 k1 /(min–1) Qe / (mg·g–1) R2 k2/
(g ·mg–1·min–1)D201 10.2 0.9460 0.1235 10.6 0.9708 0.0189 HCO@D201-8.3 32.5 0.9142 0.1611 34.1 0.9728 0.0067 表 3 材料除氟性能对比
Table 3. Comparison of Different Materials
吸附材料
The adsorption materialpH T /K 吸附量/(mg·g−1)
The adsorption quantity参考文献
ReferenceFe2 (SO4)3-MGAA 5.0 298 16.8 [33] 纳米氧化铝 6.2 298 14.0 [34] [Eu3(L2)2(OH)(DMF)0.22(H2O)5.78] 7.1 298 57.0 [35] 改性纳米氧化铝 6.2 2.98 5.7 [36] 聚吡咯/四氧化三铁 6.5 298 17.6 [37] HZO-201 6.8 298 24.2 [38] MIL-53 (Fe) 6.8 298 17.0 [39] HAP@D201 7.0 298 21.4 [40] LIBONs 3—10 298 14.5 [41] 氧化铝负载镧170℃烘焙产物 3—8 308 44.9 [42] SAE 7.0 298 21.7 [43] 氧化钙改性氧化铝 5.5 298 96.3 [44] D201 4.0 298 16.3 本研究 HCO@D201-3.1 4.0 298 70.9 本研究 HCO@D201-8.3 4.0 298 84.2 本研究 HCO@D201-11.7 4.0 298 85.1 本研究 -
[1] AMINI M, ABBASPOUR K C, BERG M, et al. Statistical modeling of global geogenic arsenic contamination in groundwater[J]. Environmental Science & Technology, 2008, 42(10): 3669-3675. [2] 周振, 马文, 方小军, 等. 砷、氟污染地下水净化技术进展[J]. 净水技术, 2021, 40(10): 7-19. ZHOU Z, MA W, FANG X J, et al. Advances in purification technology for groundwater polluted by arsenic and fluorine[J]. Water Purification Technology, 2021, 40(10): 7-19 (in Chinese).
[3] 张晓丽. 聚硅酸金属盐类絮凝剂的制备及除氟性能研究[D]. 济南: 山东建筑大学, 2022. ZHANG X L. Study on preparation and fluoride removal performance of polysilicate metal salt flocculant[D]. Jinan: Shandong Jianzhu University, 2022 (in Chinese).
[4] 李凤嫣, 蒋天宇, 余涛, 等. 环境中氟的来源及健康风险评估研究进展[J]. 岩矿测试, 2021, 40(6): 793-807. doi: 10.3969/j.issn.0254-5357.2021.6.ykcs202106001 LI F Y, JIANG T Y, YU T, et al. Review on sources of fluorine in the environment and health risk assessment[J]. Rock and Mineral Analysis, 2021, 40(6): 793-807 (in Chinese). doi: 10.3969/j.issn.0254-5357.2021.6.ykcs202106001
[5] 鲁涵, 曾妍妍, 周金龙, 等. 巴楚县浅层地下水中氟的分布特征及影响因素分析[J]. 环境化学, 2021, 40(11): 3455-3463. doi: 10.7524/j.issn.0254-6108.2020071602 LU H, ZENG Y Y, ZHOU J L, et al. Distribution characteristics and influencing factors of fluorine in shallow groundwater of Bachu County[J]. Environmental Chemistry, 2021, 40(11): 3455-3463 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020071602
[6] KAGNE S, JAGTAP S, THAKARE D, et al. Bleaching powder: A versatile adsorbent for the removal of fluoride from aqueous solution[J]. Desalination, 2009, 243(1/2/3): 22-31. [7] 张强英, 陶金帅, 李伟, 等. 废弃茶叶吸附剂去除水中的氟离子[J]. 环境化学, 2022, 41(4): 1303-1311. doi: 10.7524/j.issn.0254-6108.2020122002 ZHANG Q Y, TAO J S, LI W, et al. Waste tea-leaves as adsorbent for the removal of fluoride from water solution[J]. Environmental Chemistry, 2022, 41(4): 1303-1311 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020122002
[8] HE J S, SIAH T S, CHEN J P. Performance of an optimized Zr-based nanoparticle-embedded PSF blend hollow fiber membrane in treatment of fluoride contaminated water[J]. Water Research, 2014, 56: 88-97. doi: 10.1016/j.watres.2014.02.030 [9] TANG W W, HE D, ZHANG C Y, et al. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes[J]. Water Research, 2017, 120: 229-237. doi: 10.1016/j.watres.2017.05.009 [10] 郑国河, 李剑超, 卢堂俊, 等. 镧掺杂纳米材料合成及其高氟选择性吸附特性[J]. 环境化学, 2009, 28(6): 823-828. ZHENG G H, LI J C, LU T J, et al. Synthesis of la doped nano materials used in high-selective defluorination[J]. Environmental Chemistry, 2009, 28(6): 823-828 (in Chinese).
[11] SHEN J J, SCHÄFER A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review[J]. Chemosphere, 2014, 117: 679-691. doi: 10.1016/j.chemosphere.2014.09.090 [12] DAMTIE M M, WOO Y C, KIM B, et al. Removal of fluoride in membrane-based water and wastewater treatment technologies: Performance review[J]. Journal of Environmental Management, 2019, 251: 109524. doi: 10.1016/j.jenvman.2019.109524 [13] LÓPEZ-GUZMÁN M, ALARCÓN-HERRERA M T, IRIGOYEN-CAMPUZANO J R, et al. Simultaneous removal of fluoride and arsenic from well water by electrocoagulation[J]. The Science of the Total Environment, 2019, 678: 181-187. doi: 10.1016/j.scitotenv.2019.04.400 [14] YADAV K K, KUMAR S, PHAM Q B, et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review[J]. Ecotoxicology and Environmental Safety, 2019, 182: 109362. doi: 10.1016/j.ecoenv.2019.06.045 [15] ZHANG Y Y, QIAN Y, LI W, et al. Fluoride uptake by three lanthanum based nanomaterials: Behavior and mechanism dependent upon lanthanum species[J]. The Science of the Total Environment, 2019, 683: 609-616. doi: 10.1016/j.scitotenv.2019.05.185 [16] ZHANG Y, YANG M, DOU X M, et al. Arsenate adsorption on an Fe-Ce bimetal oxide adsorbent: Role of surface properties[J]. Environmental Science & Technology, 2005, 39(18): 7246-7253. [17] 纪现凯. 纳米磷酸钛及树脂负载磷酸钛复合材料高效净化水中氟离子的性能研究[D]. 秦皇岛: 燕山大学, 2015: 5-7. JI X K. Titanium phosphate and resin encapsulated titanium phosphate for the efficient removal of fluoride ions from water[D]. Qinhuangdao: Yanshan University, 2015: 5-7 (in Chinese).
[18] DOU X M, MOHAN D, PITTMAN C U, et al. Remediating fluoride from water using hydrous zirconium oxide[J]. Chemical Engineering Journal, 2012, 198/199: 236-245. doi: 10.1016/j.cej.2012.05.084 [19] YADAV K K, GUPTA N, KUMAR V, et al. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability[J]. Environment International, 2018, 111: 80-108. doi: 10.1016/j.envint.2017.11.014 [20] 杨鑫波. 石灰石/READ-F反应器处理含氟废水的研究[D]. 重庆: 重庆大学, 2008: 29-30. YANG X B. Study on removal of fluoride from wastewater by limestone/READ-F reactor[D]. Chongqing: Chongqing University, 2008: 29-30 (in Chinese).
[21] 李晓云, 宋宽秀, 颜秀茹, 等. 稀土化合物在水体除氟技术中应用研究的进展[J]. 化学工业与工程, 1999, 16(5): 286-291. LI X Y, SONG K X, YAN X R, et al. Development of deflourination from water by rare earth compound[J]. Chemical Industry and Engineering, 1999, 16(5): 286-291 (in Chinese).
[22] 王帆. 合成多孔硅基氧化铈微球及除氟性能的研究[D]. 南宁: 广西大学, 2018: 9-10. WANG F. The synthetic of porous silicon base cerium oxide microspheres and its application in removal of fluoride[D]. Nanning: Guangxi University, 2018: 9-10 (in Chinese).
[23] 丁鸿. 氧化铈及其磁性纳米复合材料对水中磷酸盐吸附性能的研究[D]. 太原: 太原理工大学, 2017: 12-13. DING H. Study on adsorption performance for phosphate in aqueous solution by cerium oxide and its magnetic nanocomposites[D]. Taiyuan: Taiyuan University of Technology, 2017: 12-13 (in Chinese).
[24] TENG J E, ZHANG Q R, YANG Q G, et al. New route to the charged functional assisted nano-lanthanum hydroxide composite with superior lead sorption capacities[J]. Science of Advanced Materials, 2015, 7(9): 1722-1729. doi: 10.1166/sam.2015.2393 [25] LAI L, XIE Q, CHI L N, et al. Adsorption of phosphate from water by easily separable Fe3O4@SiO2 core/shell magnetic nanoparticles functionalized with hydrous lanthanum oxide[J]. Journal of Colloid and Interface Science, 2016, 465: 76-82. doi: 10.1016/j.jcis.2015.11.043 [26] ZHOU Y M, YU C X, SHAN Y. Adsorption of fluoride from aqueous solution on La3+-impregnated cross-linked gelatin[J]. Separation and Purification Technology, 2004, 36(2): 89-94. doi: 10.1016/S1383-5866(03)00167-9 [27] KAMBLE S P, JAGTAP S, LABHSETWAR N K, et al. Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan[J]. Chemical Engineering Journal, 2007, 129(1/2/3): 173-180. [28] 黄蓉. 废PET乙二醇醇解单体BHET的离子交换树脂脱色研究[D]. 北京: 中国科学院大学, 2021. HUANG R. Decolorization of waste PET glycol alcoholysis monomer BHET by ion exchange resin[D]. Beijing: University of Chinese Academy of Sciences, 2021 (in Chinese).
[29] CHEN L, ZHAO X, PAN B C, et al. Preferable removal of phosphate from water using hydrous zirconium oxide-based nanocomposite of high stability[J]. Journal of Hazardous Materials, 2015, 284: 35-42. doi: 10.1016/j.jhazmat.2014.10.048 [30] GB 7484-87, 水质氟化物的测定离子选择电极法[S]. 北京: 中国标准出版社, 1987. GB 7484-87, Water quality-determination of fluoride-ion selective electrode method[S]. Beijing: Standards Press of China, 1987 (in Chinese).
[31] CHU S L, LI X, ROBERTSON A W, et al. Electrocatalytic CO2 reduction to ethylene over CeO2-supported Cu nanoparticles: Effect of exposed facets of CeO2[J]. Acta Physico Chimica Sinica, 2020: 2009023. [32] CHEN L, ZHANG K S, HE J Y, et al. Performance and mechanism of hierarchically porous Ce-Zr oxide nanospheres encapsulated calcium alginate beads for fluoride removal from water[J]. RSC Advances, 2016, 66(43): 36296-36306. [33] 徐雷, 马培根, 丁文明. 硫酸铁改性活性氧化铝除氟性能及机理探究[J]. 北京化工大学学报(自然科学版), 2017, 44(6): 18-24. XU L, MA P G, DING W M. Defluorination performance of activated alumina modified by ferric sulfate[J]. Journal of Beijing University of Chemical Technology (Natural Science Edition), 2017, 44(6): 18-24 (in Chinese).
[34] MA A Q, KE F, JIANG J, et al. Two lanthanide-based metal-organic frameworks for highly efficient adsorption and removal of fluoride ions from water[J]. CrystEngComm, 2017, 19(16): 2172-2177. doi: 10.1039/C7CE00291B [35] SHIVAPRASAD P, SINGH P K, SAHARAN V K, et al. Synthesis of nano alumina for defluoridation of drinking water[J]. Nano-Structures & Nano-Objects, 2018, 13: 109-120. [36] KUMAR E, BHATNAGAR A, KUMAR U, et al. Defluoridation from aqueous solutions by nano-alumina: Characterization and sorption studies[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1042-1049. [37] BHAUMIK M, LESWIFI T Y, MAITY A, et al. Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic nanocomposite[J]. Journal of Hazardous Materials, 2011, 186(1): 150-159. doi: 10.1016/j.jhazmat.2010.10.098 [38] PAN B C, XU J S, WU B, et al. Enhanced removal of fluoride by polystyrene anion exchanger supported hydrous zirconium oxide nanoparticles[J]. Environmental Science & Technology, 2013, 47(16): 9347-9354. [39] ZHAO X D, LIU D H, HUANG H L, et al. The stability and defluoridation performance of MOFs in fluoride solutions[J]. Microporous and Mesoporous Materials, 2014, 185: 72-78. doi: 10.1016/j.micromeso.2013.11.002 [40] QIU H, YE M C, ZHANG M D, et al. Nano-hydroxyapatite encapsulated inside an anion exchanger for efficient defluoridation of neutral and weakly alkaline water[J]. ACS ES& T Engineering, 2021, 1(1): 46-54. [41] 张钰卿, 刘佳, 许兵, 等. 含氟废水处理中的除氟吸附技术研究进展[J]. 净水技术, 2022, 41(5): 23-29,61. ZHANG Y Q, LIU J, XU B, et al. Research progress of adsorption technology for defluorination in fluoride-containing wastewater treatment[J]. Water Purification Technology, 2022, 41(5): 23-29,61 (in Chinese).
[42] 顾浩, 许昭怡, 李丽媛, 等. 氧化铝负载镧去除水中F–的研究[J]. 环境科学学报, 2009, 29(3): 589-593. GU H, XU Z Y, LI L Y, et al. Removal of fluoride from water using lanthanum oxide-coated alumina[J]. Acta Scientiae Circumstantiae, 2009, 29(3): 589-593 (in Chinese).
[43] 宋明珊, 李舒舒, 叶长青. 有氧热解改性木屑及其对氟离子的吸附研究[J]. 广州化学, 2023, 48(2): 36-45. SONG M S, LI S S, YE C Q. Study on modification of sawdust by aerobic pyrolysis and its adsorption of fluoride ions[J]. Guangzhou Chemistry, 2023, 48(2): 36-45 (in Chinese).
[44] CAMACHO L M, TORRES A, SAHA D, et al. Adsorption equilibrium and kinetics of fluoride on sol-gel-derived activated alumina adsorbents[J]. Journal of Colloid and Interface Science, 2010, 349(1): 307-313. doi: 10.1016/j.jcis.2010.05.066 -



 下载:
下载: