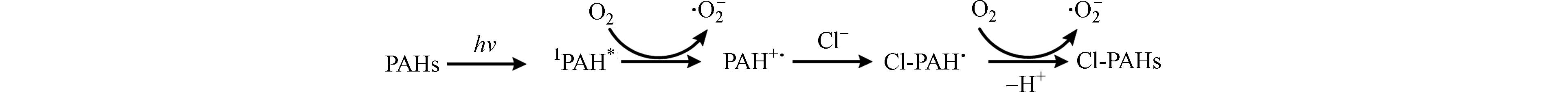-
氯代多环芳烃(chlorinated polycyclic aromatic hydrocarbons, Cl-PAHs)是多环芳烃(polycyclic aromatic hydrocarbons, PAHs)苯环上的一个或多个氢原子被氯原子取代的PAHs衍生物[1]. 工业的热处理[2]、金属二次冶炼[3 − 4]、自来水消毒[5 − 6]和垃圾的排放[7]都会产生Cl-PAHs. 一些Cl-PAHs比母体PAHs显示出更强的致癌、致畸毒性,作为新型持久性污染物长期存在于人类生活环境中,对人类的生活健康有潜在威胁[8]. PAHs光化学过程被认为是水体环境中Cl-PAHs的主要来源之一. 氯离子(Cl−)浓度较高的潮间带、河流和氯碱厂附近沼泽的沉积物中,Cl-PAHs含量丰富,其中6-氯苯并[a]芘、1-氯芘、9,10-二氯蒽、9-氯菲、2-氯芴和9-氯芴等Cl-PAHs含量高达24.1 ng·g-1[9 − 12]. 有学者在模拟潮间带反应体系中加入6种PAHs(萘、菲、蒽、荧蒽、芘、苯并[a]芘),经光照后均检测到了其氯代产物[13].
天然水中Cl-PAHs的光化学生成(光致氯代)主要包括两种机制:直接光转化和间接光转化[14]. 直接光转化是PAHs分子吸收紫外或可见光区域内的光子能量,跃迁到激发态,通过激发态或其进一步转化生成的自由基与Cl−发生反应生成Cl-PAHs. 间接光转化是由另外一个化合物(敏化剂)吸收光子,产生活性自由基,诱导Cl−向氯自由基(Cl·)转化,从而引发PAHs向Cl-PAHs转化.
光转化是水体中Cl-PAHs生成的主要途径之一,研究PAHs的光致氯代过程和机理,有利于更好地了解其在环境中的迁移转化规律. 本文针对Cl-PAHs污染现状与来源、毒性、光稳定性、光化学产生机理和光致氯代影响因素等几个方面进行综述.
-
城市化和工业活动产生的废水具有高浓度Cl-PAHs[2,7],而自然产生的Cl-PAHs主要源于PAHs的光致氯代反应[9]. Cl-PAHs的高光稳定性造成了其在水环境中的持久性,同时由于Cl-PAHs的毒性作用,人类患癌的风险大幅度增加. 因此下文对水环境中Cl-PAHs的污染现状与来源、毒性、光稳定性进行了总结,为 Cl-PAHs的健康风险评估提供一定参考.
-
自然水环境中大多数 Cl-PAHs 因水溶性较低,主要被吸附在悬浮颗粒物和沉积物上. 近年来,诸多学者就水环境中沉积物及悬浮性颗粒物所吸附的Cl-PAHs进行探究,进而阐述水污染状况,详见表1 . Ohura等[17]对中国黄海、斯里兰卡康提湖和尼甘布泻湖(受工业化影响区域)中水体沉积物进行探究,发现约20 种Cl-PAHs存在于水体沉积物中,主要以 6-氯苯并芘(平均浓度为0.24 ng·g−1)、1-氯芘(平均浓度0.16 ng·g−1)、8-氯荧蒽(平均浓度为0.065 ng·g−1)为主. Wang等[18]在中国毛威海湾的沉积物上检测到18种Cl-PAHs,其中9-氯菲(平均浓度为1.12 ng·g−1)含量最高. Yuan等[12]在中国珠江水域的悬浮性颗粒物上检测到8种Cl-PAHs,以9-氯菲(平均浓度107.6 ng·g−1)、蒽的一氯代产物(2-氯蒽和9-氯蒽(平均浓度为72.4 ng·g−1))、2,7-二氯芴(平均浓度为63.9 ng·g−1)为主. 目前,随着城市化和工业活动增加,水环境中Cl-PAHs污染程度不断加深,如废物焚烧会产生PAHs二氯衍生物[19]、二次铅冶炼厂烟囱气体包含菲的氯代产物[20] ,上述污染源的Cl-PAHs会在河流、海洋和地下水中富集,进而危害人类健康. 使用次氯酸对自来水消毒会产生Cl-PAHs已被研究报道. Shiraishi等[15]在自来水中检测到萘、菲、芴和荧蒽的氯代衍生物(Cl-PAHs总浓度为10−1—10−2 ng·L−1 ). Wang等[16]在中国河南自来水样中检测到9,9-二氯芴(平均浓度为0.9 ng·L−1)、9-氯菲(平均浓度为0.9 ng·L−1)、2-氯蒽(平均浓度为0.9 ng·L−1)、9,10-二氯蒽(平均浓度为0.9 ng·L−1)、1-氯芘(平均浓度为0.9 ng·L−1)等5种Cl-PAHs. Sankoda等[9]研究了阿奥罗滩涂、菊池川河和白川河内Cl-PAHs的分布,发现上述地区水环境中存在多种Cl-PAHs,主要以2-氯蒽(平均浓度为63.77 ng·g−1)、9,10-二氯蒽(平均浓度为604.2 ng·g−1)为主,这主要源于PAHs在海水中的光致氯代反应.
-
由于Cl-PAHs与PAHs在环境中的生物积累性和难降解性,暴露于这种受控物质会增加人类患癌症的风险,有文献[8, 21]报道了基于酵母细胞检测的芳基碳水化合物受体(AhR)介导的毒性潜能(公式①),能够有效预测分析环境Cl-PAHs与PAHs的过量毒性和毒性当量(TEQs).
其中,Ci表示每种Cl-PAHs和PAHs的浓度;REPBapi表示每种Cl-PAHs和PAHs单体基于酵母实验系统里获得的相对于BaP相对毒性潜力[22]. 一些典型母体PAHs及其Cl-PAHs相对毒性潜力如表2 所示. 一般来说,Cl-PAHs相对毒性潜力主要取决于化合物结构尺寸及氯原子在PAHs分子上的取代数量[7],如低分子量PAHs的氯代产物(蒽、菲、芴的氯代产物)呈现出比母体更强的相对毒性潜力,其相对毒性潜力随着氯代程度而增加. 而高分子量Cl-PAHs(芘、䓛、苯并[a]蒽的氯代产物)并未呈现上述规律. 除上述规律外,氯原子在PAHs分子上的取代位点的不同也会影响其相对毒性,例如3,9-二氯菲的毒性(REPBapi=0.32)强于1,9-二氯菲(REPBapi=0.12)和9,10-二氯菲(REPBapi=0.16),9-氯蒽(REPBapi=0.03)和1-氯蒽(REPBapi=0.03)的毒性弱于2-氯蒽(REPBapi=0.1).
-
了解PAHs和Cl-PAHs在环境中的光稳定性对于阐明其环境行为具有重要意义. 有研究以450 W高压汞灯为光源,在恒温水浴(25℃)的转盘光反应器中,研究了PAHs和Cl-PAHs在正己烷中的光降解,反应符合一级反应动力学. PAHs和Cl-PAHs的光降解速率常数、半衰期(光稳定性)和分子极性(偶极矩)如表3所示[23 − 24]. Cl-PAHs呈现出比对应母体PAHs更大的分子极性,有理论认为化合物的极性影响其在乙腈/水混合极性溶液中的光稳定性,但Cl-PAHs光解速率常数和半衰期与分子极性之间没有显著相关性[24]. 菲的氯代产物(9-氯菲、3,9-二氯菲、9,10-二氯菲、3,9,10-三氯菲)、多氯二苯并对二噁英(PCDD)和多氯二苯并呋喃(PCDF)的光降解实验表明,光稳定性随着氯代程度的增加而增强[25]. 将Cl-PAHs的光稳定性与母体PAHs的光稳定性进行比较,发现半衰期的增加顺序如下: 苯并[a]芘 <苯并[a]蒽 < 芘< 菲 < 芴. 这一趋势与相应的Cl-PAHs的光稳定性基本一致,由此推论,Cl-PAHs的光稳定性可能强烈依赖于母体PAHs的光稳定性[24]. 然而,菲的氯代产物(9-氯菲、3,9-二氯菲、9,10-二氯菲、3,9,10-三氯菲)和1-氯芘的降解速率均高于相对应的母体PAHs,而7-氯苯并[a]蒽和6-氯苯并[a]芘在辐射光的照射下则比各自的母体PAHs更稳定.
-
水环境中PAHs的间接和直接光转化都会导致Cl-PAHs的生成. 已有研究表明,PAHs的间接光致氯代(Cl·与PAHs发生亲电反应)是形成Cl-PAHs的重要途径[19]. 而Cl-PAHs还可以通过直接光转化生成. 在光敏化过程中,PAHs吸收太阳光后,自身被激发到单线态(1PAH*),1PAH*可以通过与氧气系统间能量交换生成三线态多环芳烃(3PAH*)或通过光电离生成多环芳烃自由基阳离子(PAH+·),同时伴随着单线态氧(1O2)、超氧自由基(
$ {\cdot\rm{O}}_{\text{2}}^{-} $ )和$ \text{HO}\cdot $ 等活性氧物种的生成[26 − 28]. 由PAHs的不同激发态、自由基中间体等与Cl−进一步反应生成Cl-PAHs. -
有机物的间接光致氯代反应已有一些报道,一般认为是通过间接光解形成的Cl·与有机物反应生成[29 − 34]. 含氯有机物(多氯联苯和多氯苯酚等)在光降解或光催化过程中会进行脱氯反应生成Cl·[35, 36]. Cl·还可以通过半导体光催化剂受光激发产生的空穴(
$ {\text{h}}_{\text{VB}}^{\text{+}} $ )氧化Cl−生成,例如AgCl半导体在光照下生成Cl·(方程式1)[37]. 此外,Fe(Ⅲ)经光照生成的羟基自由基($ \text{HO·} $ )和Cl−反应(方程式2-4)或Fe(Ⅲ)和Cl−的络合物经光照也会生成Cl·(方程式5)[34]. 方程式4中的$ {\text{C}\text{l}\text{OH}}^{\text{-}\text{·}} $ 自由基,在酸性条件下解离平衡利于向生成Cl·的方向进行,而方程式5中 Fe(Ⅲ)和Cl−的配位形式也在酸性条件下占主导地位(碱性条件下Fe(Ⅲ) 则主要和OH-形成配合物),因此Cl·主要在酸性条件下生成. 当体系的pH>7.5,Cl·几乎不生成[38, 39]. 方程式6 中Cl·与Cl-进一步反应生成二氯阴离子自由基($ {\text{C}\text{l}}_{\text{2}}^{\text{-}}\text{·} $ ),Cl·与$ {\text{C}\text{l}}_{\text{2}}^{\text{-}}\text{·} $ 攻击PAHs生成Cl-PAHs(方程式7),且Cl-PAHs的生成量随着pH值的降低而增加[26]. 但实际上PAHs在酸性盐水中的光致氯代产率极低,理论上Cl-PAHs的生成量是实际观测到的50-1000 倍. 这是由于PAHs在发生光致氯代反应的同时,PAHs和Cl-PAHs自身也发生了光降解,因此在反应过程中氯代产物的生成量呈现先增加后减少的动态趋势[26].根据理论计算,自由基对芳香环上特定碳的反应优先性可通过前沿电子密度(FED)理论预测[40]. 除了对称和能量相同的位点,不同芳香环位点上的氢被
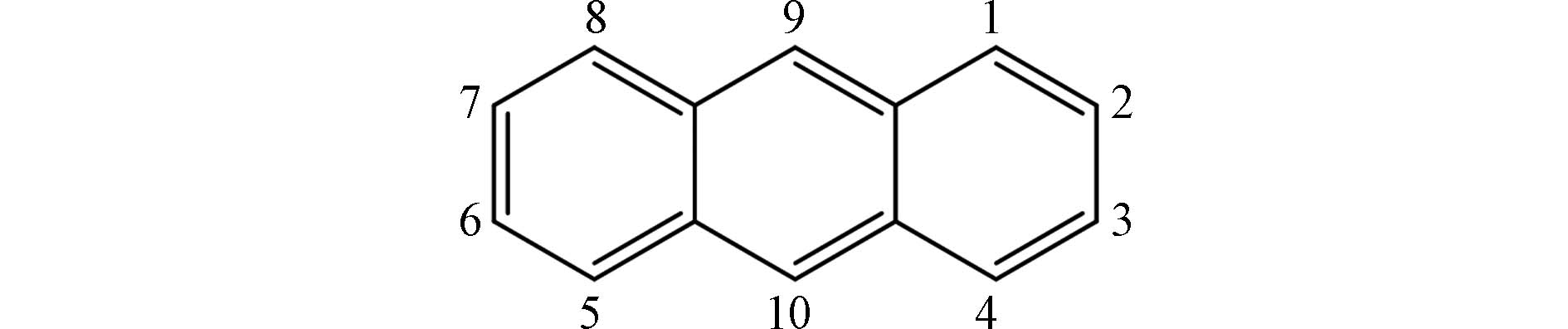
$ \text{Cl} \cdot$ 取代,可以生成不同取代程度与取代位点的PAHs氯代衍生物[19]. 例如蒽(Ant)(图1)反应活性最强的位点在9号位和10号位,其次是1号和2号位. 因此9号位和10号位被Cl·/$ {\text{Cl}}_{\text{2}}^{{-}}\cdot $ 攻击的概率最高,Cl·取代9号位的氢,生成9-氯蒽,而后在10号位进行二次氯化反应,生成9,10-二氯蒽[19]. 这跟实验中蒽经过光致氯代反应生成9-氯蒽、9,10-二氯蒽等氯代产物得到的结果相一致[10, 40]. -
Yang等[41]发现在不存在其他光敏物质,含有氯化钠的乙腈:水为1:9的溶液中,苯并[a]芘(BaP)经模拟太阳光照射后检测到有6-氯苯并[a]芘(6-ClBaP)产生. 这是由BaP受光激发,生成的激发态络合物和Cl-反应生成的. PAHs在经光激发后生成1PAH*,1PAH*首先与氧气发生能量转移,1PAH*转变为3PAH*,随后3PAH*与氧气形成络合物,然后转化为PAHs和1O2的络合物中间体,最终与Cl−反应生成Cl-PAHs(图2). 3PAH*的能量转移在碱性介质中比酸性介质中更有效,促进PAHs与氧气激发态络合物的形成[42, 43],因此该途径在碱性水体中更易发生.
-
Cl-PAHs的形成也可能由光激发单线态多环芳烃的单电子氧化引起,这种机制也强烈依靠氧气的存在. 这是由于PAHs在有机极性介质中很容易发生电荷转移,从而产生PAH+·[44]. PAHs的单线态失去电子产生PAH+·,氧气得到电子产生
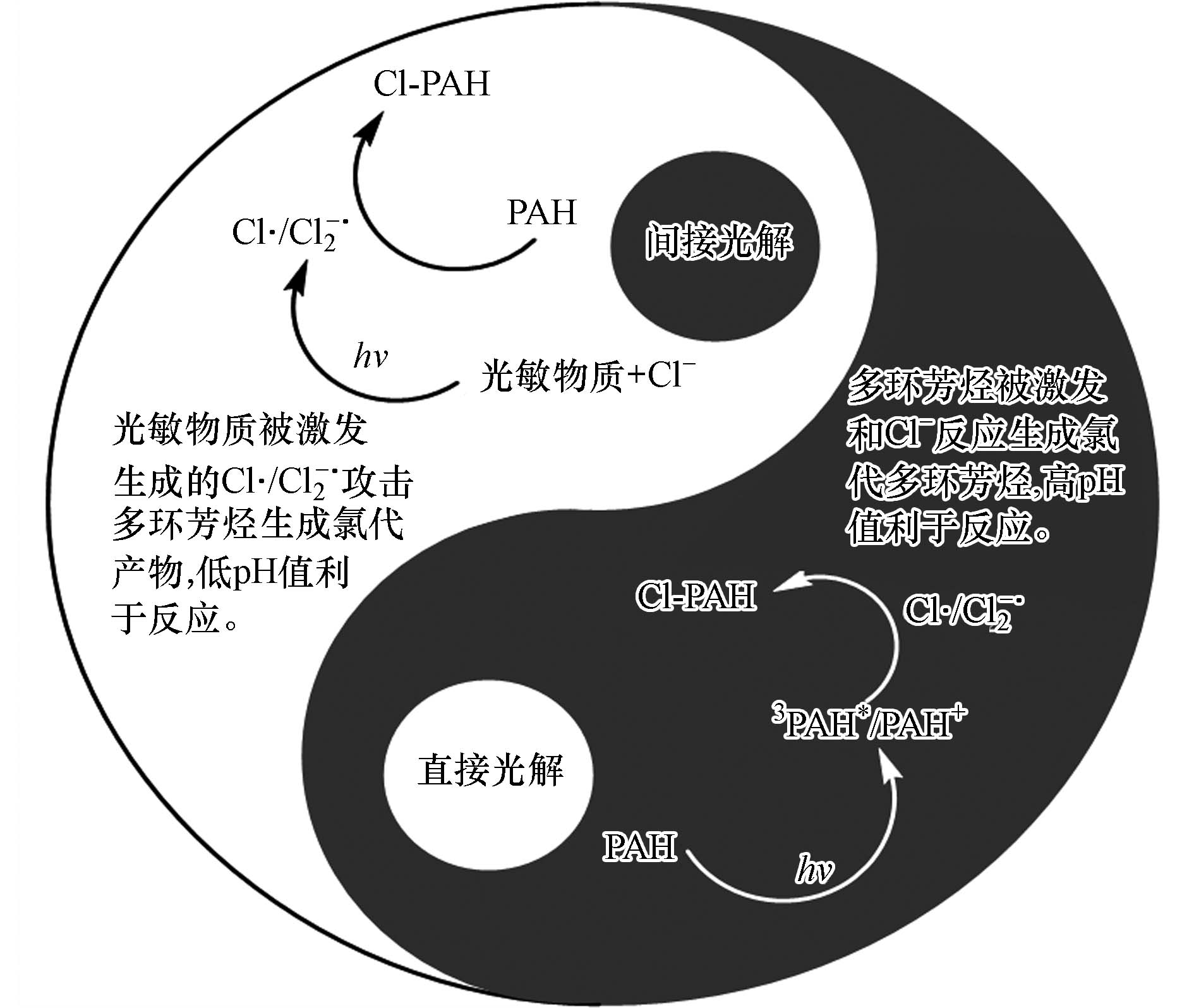
$ {\cdot{{\mathrm{O}}}}_{\text{2}}^{{-}} $ . 下一步,Cl-作为弱亲核试剂,攻击PAH+·中的离域碳正离子. 随后,经氧气氧化和脱质子,得到最终产物Cl-PAHs(图3)[28]. PAHs吸收能量或者电子转移产生的活性中间体(3PAH*、PAH+·)和Cl−反应是直接光转化生成Cl-PAHs的主要途径,同时由于PAHs活性中间体反应活性的差异性,导致PAHs的反应途径不同.水中PAHs氯代反应的间接光转化途径是其它光敏物质被激发,产生Cl·,引发PAHs的氯代,低pH值有利于Cl·的生成及进一步的氯代反应. 水体组成复杂,天然水体通常为中性或弱碱性,含高浓度Cl−的海水及盐湖水偏弱碱性,印度的洛纳尔湖pH值甚至达到10.5[45]. 以Cl·的途径形成的Cl-PAHs比较困难,而且Cl·很容易被水中的其它物质淬灭,因此Cl·能够有效进攻低浓度目标有机污染物的几率较低. 中性及弱碱性条件下有机物自身受光激发,同时与水中的氧气进行电子和能量转移,通过生成的活性中间体(3PAH*、PAH+·)和Cl−反应生成氯代产物的几率则会大幅度增加. 由此可以推测:水体环境中Cl-PAHs是主要通过直接和间接光转化两种途径生成的,这两种途径相互竞争、相互补充,共同影响自然水体环境Cl-PAHs的光化学形成 (如图4所示).
-
自然水体是一个复杂的多组分体系,无机离子、溶解性有机物(DOM)以及悬浮性颗粒物等环境物质,能够产生活性自由基或淬灭PAHs激发态,从而抑制或促进PAHs的光致氯代反应[46 − 48]. 下文将会具体综述不同环境物质对PAHs光致氯代反应的影响.
-
除人为向自然水体环境中排放的有机物外,自然水域中存在着许多的DOM,例如富里酸(FA)和腐殖酸(HA). 它们具有强光敏化能力,可以吸收太阳光,同时含有羧基、羰基、醇羟基、甲氧基、醌基等多种功能基团,能够产生许多活性自由基物种,攻击环境中的化学物质并引发其降解[49-50]. FA和HA作为DOM对Cl-PAHs的光转化有着不可忽视的作用. 一些研究认为DOM会促进PAHs的光转化,这是由于HA和FA等DOM具有强光敏性和许多功能性基团. 首先DOM吸收光子能量生成为单线态溶解性有机物(1DOM*)和三线态溶解性有机物(3DOM*)(方程式8),然后与氧气发生能量或电子转移生成1O2、
$ {\cdot{{\mathrm{O}}}}_{\text{2}}^{{-}} $ 等活性氧物种(方程式9-12),增强了PAHs的在水体环境中的间接光降解[46, 51 − 53]. 另外DOM能够影响卤素离子的存在形式,例如3DOM* 与Cl−反应生成Cl·(方程式13)[54],生成的Cl·会引发PAHs的间接光致氯代反应[26].另外有研究得出相反的结论,PAHs自身也能够吸收一定波长的光进行直接光解[55],而DOM对PAHs的光吸收有竞争作用,PAHs吸收的光子数减少,最终抑制PAHs的直接光降解. 公式②—⑤可以计算光屏蔽因子θ,从而得到DOM在PAHs光转化过程中的屏蔽影响程度. 若θ小于1,则说明还有其他效应抑制PAHs的光降解[49-50],例如PAHs与DOM的结合可以将其激发单线态或三线态淬灭为基态,从而降低PAHs的光降解速率.
相对分子质量较大的芘和FA具有更高的结合常数,随着FA的浓度增加,芘的速率常数会呈现出较大程度的降低[56],这种结合效应可能会影响PAHs的直接光致氯代反应[41]. 同时由于相对分子质量较大的PAHs具有更大摩尔吸光系数和吸收波长范围,光屏蔽效应对相对分子质量较大的PAHs光转化过程影响更大[57].
S为实际光屏蔽因子;
$ {{k}}_{\text{PAH}\text{s}\text{+}{\text{Cl}}^{-}+\text{DOM}} $ 为在含有DOM的氯化钠溶液中PAHs的光降解速率常数;$ {{k}}_{\text{PAHs+}{\text{Cl}}^{-}} $ 为PAHs在氯化钠溶液中的光降解速率常数.α为DOM水溶液在特定波长下的吸收系数 cm−1;
$ \text{A} $ 为DOM水溶液在特定波长下的吸光度;b为光程长度 cm;c为DOM溶液的浓度 mol·L−1.Sλ为理论光屏蔽因子;l为光程长度 cm.
θ为光屏蔽因子.
-
自然水域中含有众多的无机离子,其中一些具有反应活性的离子在PAHs的光转化过程具有重要的影响. 海水中常见的CO32−、 HCO3−、Br−和I−可以与
$ \text{HO}\cdot $ 发生反应生成相应的自由基(方程式14—16)[47, 49],这就意味着无机离子会与PAHs竞争$ \text{HO}\cdot $ 等活性氧自由基,降低PAHs的光降解速率. 同时由于无机离子对$ \text{HO}\cdot $ 的竞争,水体中Cl·的含量减少,降低了PAHs与Cl·的碰撞机率,从而会影响PAHs的间接光致氯代反应.水体环境中硝酸盐(NO3-)和亚硝酸盐(NO2-)具有光敏性,能够吸收300—355 nm波长下的光,产生
$ \text{HO}\cdot $ 、$ {\cdot{{\mathrm{O}}}}_{\text{2}}^{-} $ 等活性氧自由基(方程式17—19).$ \text{HO}\cdot $ 被Cl−淬灭,生成Cl·,可能引发PAHs的间接光致氯代反应. 另一方面,NO3-和NO2-会竞争性吸收光,PAHs吸收的光子变少,抑制了PAHs光降解[58]. -
二氧化硅(SiO2)、氧化铝(Al2O3)、二氧化钛(TiO2)等作为天然的悬浮性颗粒物在地表水中普遍存在. PAHs具有疏水性,倾向于吸附在尺寸较小、比表面积大的悬浮性颗粒物上,同时悬浮性颗粒物具有光敏性,能有效诱导PAHs的光降解[12]. 例如锐钛矿相二氧化钛的导带(CB)和价带(VB)之间的带隙为3.2 eV,在紫外光(315—400 nm)照射下VB电子被激发并移动到CB生成导带电子(
$ {\text{e}}_{\text{CB}}^{\text{-}} $ ),在VB生成空穴$ {\text{h}}_{\text{VB}}^{\text{+}} $ (方程式20 )[59].$ {\text{h}}_{\text{VB}}^{\text{+}} $ 具有氧化性,能够将PAHs的激发态3PAH*氧化为PAH+·(方程式21—22),PAH+. 的形成增强了PAHs的光降解速率 [41, 44].$ {\text{h}}_{\text{VB}}^{\text{+}} $ 还会氧化水分子和Cl−生成氧化性较弱的$ \text{HO}\cdot $ 和Cl·,$ {\text{e}}_{\text{CB}}^{\text{-}} $ 能够与氧气反应生成$ {\cdot{{\mathrm{O}}}}_{\text{2}}^{{-}} $ (方程式23—24),光照射下颗粒物表面还可以产生1O2,这些活性物种在颗粒物表面的积累增强了PAHs的光降解[48]. 在含有悬浮性颗粒物的体系中,由于颗粒物自身和活性氧物种的催化氧化作用,增强了PAHs的光降解,3种悬浮性颗粒物上PAHs光降解速率按以下顺序递增:SiO2 < Al2O3 < TiO2[44]. 同时,在研究中发现BaP在悬浮性颗粒表面也生成了6-ClBaP, 6-ClBaP在颗粒物表面的生成量按以下顺序依次递减:SiO2 > Al2O3 > TiO2,PAHs在颗粒物表面的光降解率增加,氯代产物的含量却降低,因此可以推测:PAHs的光解途径与氯代途径彼此竞争、共同诱导着PAHs的光转化[41]. -
综上所述,Cl-PAHs作为一种新型持久性污染物具有毒性和光稳定性,会威胁环境及人体健康,因此深入探究Cl-PAHs在水环境中的光化学生成机理及环境因素对光致氯代反应的影响具有重要意义. 但实际水体是极其复杂的系统,各种环境因素综合影响下,实际水体中Cl-PAHs的主要光致氯代生成途径是目前研究的难点. 总结相关研究现状及进展,今后研究应对以下几个方面进一步拓展和深入:(a)完善水中各种环境因素对PAHs光致氯代的影响并结合实际水体进行探究;(b)探究水中PAHs直接和间接光转化对光致氯代反应的贡献度;(c)研究水中PAHs直接光转化过程中活性中间体(3PAH*、PAH+·)对于光致氯代反应的贡献度;(d)进行水中高分子量Cl-PAHs光化学生成的研究;(e)深入研究水中多氯取代PAHs的直接光反应生成机制. 望本文能引起未来对Cl-PAHs的认识和关注,进一步阐明Cl-PAHs在水环境中的光化学生成机制及迁移转化规律,为环境污染风险评估提供更多的理论依据,以便对Cl-PAHs进行污染防治.
水环境中氯代多环芳烃的光化学生成及影响因素研究进展
Research advances on photochemical generation and influencing factors of chlorinated polycyclic aromatic hydrocarbons in water environment
-
摘要: 氯代多环芳烃(chlorinated polycyclic aromatic hydrocarbons,Cl-PAHs)是多环芳烃(polycyclic aromatic hydrocarbons,PAHs)的一种衍生物,广泛存在于各种环境介质中. 一些Cl-PAHs显示出与二噁英相似的毒性特性,甚至更高的毒性当量(TEQs). 了解Cl-PAHs的来源和形成机制对于控制其排放和降低人类暴露于这些有机污染物的风险有重要意义. 然而,目前关于Cl-PAHs的来源、形成机制和环境特征仍缺乏全面的了解. 该综述旨在总结Cl-PAHs在水环境中的污染现状与来源、毒性、光化学形成机理以及PAHs光致氯代影响因素,并对今后的PAHs光致氯代研究方向进行了展望,进而为该类化合物的环境污染及生态风险评估提供相应参考.Abstract: Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs), the derivatives of polycyclic aromatic hydrocarbons (PAHs), are widely present in various environmental media. Some Cl-PAHs display similar toxic properties and even higher toxic equivalent quantities (TEQs) than dioxins. Understanding the sources and formation mechanisms of Cl-PAHs is important for controlling their emissions and reducing the risk of human exposure to these organic pollutants. Nevertheless, comprehensive knowledge on the sources, formation mechanisms, and environmental characteristics of Cl-PAHs is lacking. Here, this review summarized the current pollution status, sources, toxicity, photochemical formation mechanisms of Cl-PAHs, and photochlorination influencing factors of PAHs in water environment. Furthermore, this review recommended future research directions for PAHs photochlorination and then provided corresponding references for the environmental pollution and ecological risk assessment of this class of compounds.
-
Key words:
- PAHs /
- Cl-PAHs /
- photochlorination /
- chlorination mechanism.
-

-
表 1 水环境中Cl-PAHs污染现状
Table 1. Current pollution status of Cl-PAHs in water environment
时间
Year基质类型
Type of matrix地点
Location主要Cl-PAHs
Main PAHs参考文献
Reference1985 自来水 日本 筑波 萘、菲、芴和荧蒽的氯代衍生物 [15] 2015 自来水 中国 河南 9,9-二氯芴、9-氯菲、2-氯蒽、9,10-二氯蒽,1-氯芘 [16] 2004 沉积物 中国 黄海
斯里兰卡 康提湖
斯里兰卡 尼甘布泻湖6-氯苯并芘、1-氯芘、8-氯荧蒽 [17] 2014 沉积物 中国 毛威海 9-氯菲 [18] 2012 沉积物 亚洲 阿奥罗滩涂
亚洲 菊池川河
亚洲 白川河2-氯蒽、9,10-二氯蒽 [9] 2016 悬浮性颗粒物 中国 珠江 9-氯菲、2-氯蒽、9-氯蒽、2,7-二氯芴 [12] 表 2 PAHs 和 Cl-PAHs相对毒性潜力
Table 2. Relative toxicity potential of PAHs and Cl-PAHs
化合物
Compounds缩写
AbbreviationREPBapi[19] 1-氯蒽 1-ClAnt 0.03 2-氯蒽 2-ClAnt 0.1 9-氯蒽 9-ClAnt 0.03 9,10-二氯蒽 9,10-Cl2Ant 0.2 9-氯菲 9-ClPhe 0.03 1,9-二氯菲 1,9-Cl2Phe 0.12 3,9-二氯菲 3,9-Cl2Phe 0.32 9,10-二氯菲 9,10-Cl2Phe 0.16 3,9,10-三氯菲 3,9,10-Cl3Phe 0.77 3-氯芴 3-ClFluor 0.17 8-氯芴 8-ClFluor 0.18 3,8-二氯芴 3,8-Cl2Fluor 5.7 6-氯䓛 6-ClChry 2.1 6,12-氯䓛 6,12-Cl2Chry 0.03 1-氯芘 1-ClPyr 0.1 7-氯苯并[a]蒽 7-ClBaA 0.83 7,12-二氯苯并[a]蒽 7,12-Cl2BaA 0.1 6-氯苯并[a]芘 6-ClBaP 0.09 蒽 Ant 0.01 菲 Phe 0.004 芴 Flu 0.01 芘 Pyr 0.05 苯并[a]蒽 BaA 1.4 䓛 Chr 2.5 苯并[a]芘 BaP 1 表 3 PAHs和Cl-PAHs的光降解速率常数、半衰期和偶极矩
Table 3. Photodegradation rate constants, half-lives, and dipole moments of PAHs and Cl-PAHs
化合物
Compounds光降解速率常数/h-1[23]
Photodegradation rate constants半衰期/h[23]
Half-lives偶极矩/ C·m[24]
Dipole moments9-氯菲 0.089 7.8 2.531 3,9-二氯菲 0.082 8.5 0.652 9,10-二氯菲 0.049 14.2 3.844 3,9,10-三氯菲 0.048 14.4 2.391 3-氯芴 0.004 158 2.188 8-氯芴 0.018 38.1 3.231 3,8-二氯芴 0.0035 198 0.802 1-氯芘 0.315 2.2 2.829 7-氯苯并[a]蒽 0.104 6.7 2.584 6-氯苯并[a]芘 0.243 2.9 2.861 菲 0.035 19.7 0.060 芴 0.031 22.4 0.414 芘 0.203 3.4 0.003 苯并[a]蒽 0.221 3.1 0.124 苯并[a]芘 1.67 0.4 0.054 -
[1] ZHAO X Q, CHENG P F, BORCH T, et al. Humidity induces the formation of radicals and enhances photodegradation of chlorinated-PAHs on Fe(Ⅲ)-montmorillonite[J]. Journal of Hazardous Materials, 2022, 423: 127210. doi: 10.1016/j.jhazmat.2021.127210 [2] VUONG Q T, THANG P Q, OHURA T, et al. Chlorinated and brominated polycyclic aromatic hydrocarbons in ambient air: Seasonal variation, profiles, potential sources, and size distribution[J]. Reviews in Environmental Science and Bio/Technology, 2020, 19(2): 259-273. doi: 10.1007/s11157-020-09535-z [3] LIN B C, YANG Y P, YANG L L, et al. Congener profiles and process distributions of polychlorinated biphenyls, polychlorinated naphthalenes and chlorinated polycyclic aromatic hydrocarbons from secondary copper smelting[J]. Journal of Hazardous Materials, 2022, 423: 127125. doi: 10.1016/j.jhazmat.2021.127125 [4] JIN R, LIU G R, ZHENG M H, et al. Secondary copper smelters as sources of chlorinated and brominated polycyclic aromatic hydrocarbons[J]. Environmental Science & Technology, 2017, 51(14): 7945-7953. [5] XU X, XIAO R Y, DIONYSIOU D D, et al. Kinetics and mechanisms of the formation of chlorinated and oxygenated polycyclic aromatic hydrocarbons during chlorination[J]. Chemical Engineering Journal, 2018, 351: 248-257. doi: 10.1016/j.cej.2018.06.075 [6] LIU Q Z, XU X, FU J J, et al. Role of hypobromous acid in the transformation of polycyclic aromatic hydrocarbons during chlorination[J]. Water Research, 2021, 207: 117787. doi: 10.1016/j.watres.2021.117787 [7] SUN J L, ZENG H, NI H G. Halogenated polycyclic aromatic hydrocarbons in the environment[J]. Chemosphere, 2013, 90(6): 1751-1759. doi: 10.1016/j.chemosphere.2012.10.094 [8] WANG P C, QI A N, HUANG Q, et al. Spatial and temporal variation, source identification, and toxicity evaluation of brominated/chlorinated/nitrated/oxygenated-PAHs at a heavily industrialized area in Eastern China[J]. Science of the Total Environment, 2022, 822: 153542. doi: 10.1016/j.scitotenv.2022.153542 [9] SANKODA K, KURIBAYASHI T, NOMIYAMA K, et al. Occurrence and source of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) in tidal flats of the ariake bay, Japan[J]. Environmental Science & Technology, 2013, 47(13): 7037-7044. [10] SANKODA K, NOMIYAMA K, YONEHARA T, et al. Evidence for in situ production of chlorinated polycyclic aromatic hydrocarbons on tidal flats: Environmental monitoring and laboratory scale experiment[J]. Chemosphere, 2012, 88(5): 542-547. doi: 10.1016/j.chemosphere.2012.03.017 [11] HORII Y, OHURA T, YAMASHITA N, et al. Chlorinated polycyclic aromatic hydrocarbons in sediments from industrial areas in Japan and the United States[J]. Archives of Environmental Contamination and Toxicology, 2009, 57(4): 651-660. doi: 10.1007/s00244-009-9372-1 [12] YUAN K, QING Q, WANG Y R, et al. Characteristics of chlorinated and brominated polycyclic aromatic hydrocarbons in the Pearl River Estuary[J]. Science of the Total Environment, 2020, 739: 139774. doi: 10.1016/j.scitotenv.2020.139774 [13] SANKODA K, NOMIYAMA K, KURIBAYASHI T, et al. Halogenation of polycyclic aromatic hydrocarbons by photochemical reaction under simulated tidal flat conditions[J]. Polycyclic Aromatic Compounds, 2013, 33(3): 236-253. doi: 10.1080/10406638.2013.770406 [14] SHANKAR R, JUNG J H, LOH A, et al. Environmental significance of lubricant oil: A systematic study of photooxidation and its consequences[J]. Water Research, 2020, 168: 115183. doi: 10.1016/j.watres.2019.115183 [15] SHIRAISHI H, PILKINGTON N H, OTSUKI A, et al. Occurrence of chlorinated polynuclear aromatic hydrocarbons in tap water[J]. Environmental Science & Technology, 1985, 19(7): 585-590. [16] WANG X L, KANG H Y, WU J F. Determination of chlorinated polycyclic aromatic hydrocarbons in water by solid-phase extraction coupled with gas chromatography and mass spectrometry[J]. Journal of Separation Science, 2016, 39(9): 1742-1748. doi: 10.1002/jssc.201501286 [17] OHURA T, SAKAKIBARA H, WATANABE I, et al. Spatial and vertical distributions of sedimentary halogenated polycyclic aromatic hydrocarbons in moderately polluted areas of Asia[J]. Environmental Pollution, 2015, 196: 331-340. doi: 10.1016/j.envpol.2014.10.028 [18] WANG Y J, LIAO R Q, LIU W L, et al. Chlorinated polycyclic aromatic hydrocarbons in surface sediment from Maowei Sea, Guangxi, China: Occurrence, distribution, and source apportionment[J]. Environmental Science and Pollution Research, 2017, 24(19): 16241-16252. doi: 10.1007/s11356-017-9193-0 [19] JIN R, ZHENG M H, LAMMEL G, et al. Chlorinated and brominated polycyclic aromatic hydrocarbons: Sources, formation mechanisms, and occurrence in the environment[J]. Progress in Energy and Combustion Science, 2020, 76: 100803. doi: 10.1016/j.pecs.2019.100803 [20] XU Y, YANG L L, ZHENG M H, et al. Chlorinated and brominated polycyclic aromatic hydrocarbons from metallurgical plants[J]. Environmental Science & Technology, 2018, 52(13): 7334-7342. [21] HUANG C, XU X, WANG D H, et al. The aryl hydrocarbon receptor (AhR) activity and DNA-damaging effects of chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs)[J]. Chemosphere, 2018, 211: 640-647. doi: 10.1016/j.chemosphere.2018.07.087 [22] LIU Q Z, XU X, LIN L H, et al. Occurrence, health risk assessment and regional impact of parent, halogenated and oxygenated polycyclic aromatic hydrocarbons in tap water[J]. Journal of Hazardous Materials, 2021, 413: 125360. doi: 10.1016/j.jhazmat.2021.125360 [23] OHURA T, AMAGAI T, MAKINO M. Behavior and prediction of photochemical degradation of chlorinated polycyclic aromatic hydrocarbons in cyclohexane[J]. Chemosphere, 2008, 70(11): 2110-2117. doi: 10.1016/j.chemosphere.2007.08.064 [24] OHURA T, KITAZAWA A, AMAGAI T, et al. Occurrence, profiles, and photostabilities of chlorinated polycyclic aromatic hydrocarbons associated with particulates in urban air[J]. Environmental Science & Technology, 2005, 39(1): 85-91. [25] CHOUDHRY G G, BARRIE WEBSTER G R. Environmental photochemistry of PCDDs. 2. Quantum yields of the direct phototransformation of 1, 2, 3, 7-tetra-, 1, 3, 6, 8-tetra-, 1, 2, 3, 4, 6, 7, 8-hepta-, and 1, 2, 3, 4, 6, 7, 8, 9-octachlorodibenzo-p-dioxin in aqueous acetonitrile and their sunlight half-lives[J]. Journal of Agricultural and Food Chemistry, 1989, 37(1): 254-261. doi: 10.1021/jf00085a059 [26] OHURA T, MIWA M. Photochlorination of polycyclic aromatic hydrocarbons in acidic brine solution[J]. Bulletin of Environmental Contamination and Toxicology, 2016, 96(4): 524-529. doi: 10.1007/s00128-015-1723-1 [27] WU S P, SCHWAB J, YANG B Y, et al. Effect of phenolic compounds on photodegradation of anthracene and benzo[a]anthracene in media of different polarity[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2015, 309: 55-64. doi: 10.1016/j.jphotochem.2015.05.004 [28] ERICKSON, PAUL R. Transformation Mechanisms of Organic Micropollutants via Direct and Indirect Photochemistry[D]. Switzerland, Swiss Federal Institute of Technology Zurich, 2014: 103-107. [29] LI W, LI Y Q, XU K. Facile, electrochemical chlorination of graphene from an aqueous NaCl solution[J]. Nano Letters, 2021, 21(2): 1150-1155. doi: 10.1021/acs.nanolett.0c04641 [30] WU Y X, YANG Y, LIU Y Z, et al. Modelling study on the effects of chloride on the degradation of bezafibrate and carbamazepine in sulfate radical-based advanced oxidation processes: Conversion of reactive radicals[J]. Chemical Engineering Journal, 2019, 358: 1332-1341. doi: 10.1016/j.cej.2018.10.125 [31] LIU H, ZHAO H M, QUAN X E, et al. Formation of chlorinated intermediate from bisphenol A in surface saline water under simulated solar light irradiation[J]. Environmental Science & Technology, 2009, 43(20): 7712-7717. [32] 胡学锋, 吴蕾, 骆永明. 苯胺在含富里酸/Fe(Ⅲ)高盐水体中的光氯化[J]. 环境化学, 2014, 33(4): 611-616. doi: 10.7524/j.issn.0254-6108.2014.04.003 HU X F, WU L, LUO Y M. Photochlorination of aniline in Fe(Ⅲ)/fulvic acid-containing saline water under simulated solar light irradiation[J]. Environmental Chemistry, 2014, 33(4): 611-616 (in Chinese). doi: 10.7524/j.issn.0254-6108.2014.04.003
[33] REMUCAL C K, MANLEY D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment[J]. Environmental Science:Water Research & Technology, 2016, 2(4): 565-579. [34] WU L, HU X F. Photochlorination of aniline in Fe3+-containing saline water under simulated solar light irradiation[J]. Environmental Chemistry, 2012, 9(6): 558. doi: 10.1071/EN12143 [35] LIN Y J, TENG L S, LEE A, et al. Effect of photosensitizer diethylamine on the photodegradation of polychlorinated biphenyls[J]. Chemosphere, 2004, 55(6): 879-884. doi: 10.1016/j.chemosphere.2003.11.059 [36] JI H H, CHANG F, HU X F, et al. Photocatalytic degradation of 2, 4, 6-trichlorophenol over g-C3N4 under visible light irradiation[J]. Chemical Engineering Journal, 2013, 218: 183-190. doi: 10.1016/j.cej.2012.12.033 [37] HU X F, WANG X W, DONG L L, et al. Aniline chlorination by in situ formed Ag–Cl complexes under simulated solar light irradiation[J]. Water Science and Technology, 2015, 71(11): 1679-1685. doi: 10.2166/wst.2015.149 [38] JING L, CHEN B, ZHANG B Y, et al. Naphthalene degradation in seawater by UV irradiation: The effects of fluence rate, salinity, temperature and initial concentration[J]. Marine Pollution Bulletin, 2014, 81(1): 149-156. doi: 10.1016/j.marpolbul.2014.02.003 [39] CHIRON S, MINERO C, VIONE D. Photodegradation processes of the antiepileptic drug carbamazepine, relevant to estuarine waters[J]. Environmental Science & Technology, 2006, 40(19): 5977-5983. [40] TU Z N, QI Y M, TANG X S, et al. Photochemical transformation of anthracene (ANT) in surface soil: Chlorination and hydroxylation[J]. Journal of Hazardous Materials, 2023, 452: 131252. doi: 10.1016/j.jhazmat.2023.131252 [41] YANG M N, ZHANG H J, CHANG F, et al. Self-sensitized photochlorination of benzo[a]pyrene in saline water under simulated solar light irradiation[J]. Journal of Hazardous Materials, 2021, 408: 124445. doi: 10.1016/j.jhazmat.2020.124445 [42] FASNACHT M P, BLOUGH N V. Kinetic analysis of the photodegradation of polycyclic aromatic hydrocarbons in aqueous solution[J]. Aquatic Sciences, 2003, 65(4): 352-358. doi: 10.1007/s00027-003-0680-7 [43] FASNACHT M P, BLOUGH N V. Aqueous photodegradation of polycyclic aromatic hydrocarbons[J]. Environmental Science & Technology, 2002, 36(20): 4364-4369. [44] KARGAR N, AMANI-GHADIM A R, MATIN A A, et al. Abatement efficiency and fate of EPA-Listed PAHs in aqueous medium under simulated solar and UV-C irradiations, and combined process with TiO2 and H2O2[J]. Ege Journal of Fisheries and Aquatic Sciences, 2020, 37(1): 15-27. doi: 10.12714/egejfas.37.1.03 [45] NIMONKAR Y S, GODAMBE T, KULKARNI A, et al. Oligotrophy vs. copiotrophy in an alkaline and saline habitat of Lonar Lake[J]. Frontiers in Microbiology, 2022, 13: 939984. doi: 10.3389/fmicb.2022.939984 [46] LUO L J, XIAO Z Y, CHEN B W, et al. Natural porphyrins accelerating the phototransformation of benzo[a]pyrene in water[J]. Environmental Science & Technology, 2018, 52(6): 3634-3641. [47] DONG Y X, PENG W Y, LIU Y J, et al. Photochemical origin of reactive radicals and halogenated organic substances in natural waters: A review[J]. Journal of Hazardous Materials, 2021, 401: 123884. doi: 10.1016/j.jhazmat.2020.123884 [48] LI X T, ZHAO H X, QU B C, et al. Photoformation of environmentally persistent free radicals on particulate organic matter in aqueous solution: Role of anthracene and formation mechanism[J]. Chemosphere, 2022, 291: 132815. doi: 10.1016/j.chemosphere.2021.132815 [49] FAN J L, SUN X B, LIU Y D, et al. New insight into environmental photochemistry of PAHs induced by dissolved organic matters: A model of naphthalene in seawater[J]. Process Safety and Environmental Protection, 2022, 161: 325-333. doi: 10.1016/j.psep.2022.03.017 [50] ZHAO S Y, XUE S, ZHANG J M, et al. Dissolved organic matter-mediated photodegradation of anthracene and pyrene in water[J]. Scientific Reports, 2020, 10: 3413. doi: 10.1038/s41598-020-60326-6 [51] SUN X K, BAI J E, DONG D B. Influence factors of enhanced photosensitized degradation of PAHs on soil surface using humic acid under UV irradiation[J]. Polycyclic Aromatic Compounds, 2021, 41(8): 1739-1748. doi: 10.1080/10406638.2019.1695218 [52] WANG J Q, CHEN J W, QIAO X L, et al. DOM from mariculture ponds exhibits higher reactivity on photodegradation of sulfonamide antibiotics than from offshore seawaters[J]. Water Research, 2018, 144: 365-372. doi: 10.1016/j.watres.2018.07.043 [53] ZHOU C Z, XIE Q, WANG J Q, et al. Effects of dissolved organic matter derived from freshwater and seawater on photodegradation of three antiviral drugs[J]. Environmental Pollution, 2020, 258: 113700. doi: 10.1016/j.envpol.2019.113700 [54] ZHANG K, PARKER K M. Halogen radical oxidants in natural and engineered aquatic systems[J]. Environmental Science & Technology, 2018, 52(17): 9579-9594. [55] CARENA L, PUSCASU C G, COMIS S, et al. Environmental photodegradation of emerging contaminants: A re-examination of the importance of triplet-sensitised processes, based on the use of 4-carboxybenzophenone as proxy for the chromophoric dissolved organic matter[J]. Chemosphere, 2019, 237: 124476. doi: 10.1016/j.chemosphere.2019.124476 [56] XIA X H, LI G C, YANG Z F, et al. Effects of fulvic acid concentration and origin on photodegradation of polycyclic aromatic hydrocarbons in aqueous solution: Importance of active oxygen[J]. Environmental Pollution, 2009, 157(4): 1352-1359. doi: 10.1016/j.envpol.2008.11.039 [57] JIA Z, WANG Z C, LU K J. in situ investigation of N deposit effects on polycyclic aromatic hydrocarbons (PAHs) photolysis in snow[J]. Atmospheric Research, 2023, 286: 106676. doi: 10.1016/j.atmosres.2023.106676 [58] RICKER H M, LEONARDI A, NAVEA J G. Reduction and photoreduction of NO2 in humic acid films as a source of HONO, ClNO, N2O, NO X, and organic nitrogen[J]. ACS Earth and Space Chemistry, 2022, 6(12): 3066-3077. doi: 10.1021/acsearthspacechem.2c00282 [59] LI L Z. The reaction mechanism of photoelectrocatalysis on the surface of TiO2 nanotube array electrode[J]. Asia-Pacific Journal of Chemical Engineering, 2020, 15: e2511. doi: 10.1002/apj.2511 -



 下载:
下载: