-
采选冶废水中通常含有大量高浓度的重金属,如铅(Pb)、镉(Cd)等,这些重金属通过下渗扩散作用进入地下水中,造成地下水的污染[1]. 据统计,全国约有90%的地下水遭受不同程度的重金属污染[2]. 磁黄铁矿(FeS)对Pb、Cd等重金属均有显著的吸附性能[3 − 5],因此,这类有色金属矿山作业的副产物被作为吸附填料逐渐用在重金属污染的地下水的修复中,并显示出巨大的应用潜力[6 − 7]. 但FeS易团聚[3],也易被地下水中高价活性金属,如铁锰氧化物[8- 9]氧化而失活. 采用海藻酸钠、羧甲基纤维素钠等[3, 10 − 11]等包埋材料能够有效提升FeS的分散性和抗氧化性[12],改善其反应活性,增强其对重金属的吸附性能[3].
然而,目前对FeS的报道多集中于对单一重金属的吸附性能[10, 13],少有研究关注FeS对多金属的同步吸附. 以全球公认三大污染行业之一的电镀行业废水为例,通常含有大量Pb、Cd等重金属[6- 7]. 在FeS同步吸附多金属的过程中,由于重金属的电负性、吸附亲和力等差异,会竞争吸附点位,从而影响吸附效率[14]. 另外,在地下水环境中,MnO2等金属氧化物的广泛存在也会显著影响FeS的吸附性能[8, 15]. 因此,深入研究FeS同步吸附多金属的效率、机制以及MnO2的影响能为优化FeS组分和结构提供数据支撑和理论依据.
本研究选取环境友好且廉价易得的海藻酸钠(SA)[13],采用交联技术制备FeS-SA,提升其稳定性和吸附活性,研究FeS-SA对Pb和Cd的同步吸附性能以及吸附机制,探讨了Pb和Cd的竞争吸附以及MnO2对同步吸附的影响,以期为FeS-SA修复多金属污染地下水提供数据支撑和理论依据.
-
FeS-SA和FeS的制备及表征:采用交联法制备FeS-SA[16],用注射器以12 滴·min−1的速度向190 mL含0.556 g的FeSO4·7H2O溶液中滴加10 mL Na2S/SA混合溶液(0.480 g Na2S·9H2O和0.100 g SA),再加入2.00 g CaCl2与SA产生交联. 制备时探究了SA为0.050 g、0.100 g和0.200 g时对Pb和Cd吸附量的影响,其中0.100 g SA添加量制备出的FeS-SA表现出较好的吸附性能. FeS的制备过程与FeS-SA相似,无SA和CaCl2的添加. 制备过程保持无氧环境,去离子水均先通入高纯氮气30 min. 将FeS-SA和FeS悬浊液离心(
6000 r·min−1,5 min),弃去上清液,用去离子水清洗沉淀物3次以去除残留的Na2S、FeSO4和CaCl2,沉淀物冷冻干燥备用. 用X射线光电子能谱(XPS,Thermo esca lab 250Xi)分析FeS-SA表面的Fe和S形态. 用X射线衍射仪(XRD,XRD-7000 )表征样品的物相组成.FeS-SA(或FeS)中Fe含量测定:取0.100 g FeS-SA(或FeS),加入2 mL的浓HCl和浓HNO3,充分溶解后过0.22 μm聚砜滤膜,用电感耦合等离子体发射光谱仪(ICP-OES,Optima
8300 )测定总Fe的含量.分散性能测试:取0.010 g FeS-SA(或FeS),加入8 mL去离子水,超声5 min使颗粒均匀分散于去离子水中,用纳米粒度仪(ZS90)测试其的水合粒径分布. 取45 mL FeS-SA(或FeS)悬浊液,分别在第0、5、10、20、40 min拍照记录悬浊液沉降情况,测量沉降距离.
抗MnO2氧化性能测定:取0.020 g FeS-SA或0.012 g FeS(FeS含量一致),分别加入0.042 g MnO2和200 mL去离子水使得溶液中初始Mn:Fe物质的量比为4:1,MnO2相对于FeS过量存在,溶液初始pH值为6.5(未调节),水浴恒温振荡器(25 ℃,200 r·min−1),分时段取1 mL溶液过0.22 μm聚砜滤膜,用ICP-OES测定溶液中Mn2+的含量.
-
吸附动力学实验:单一体系中,取200 mL 0.6 mmol·L−1的Pb(NO3)2(或Cd(NO3)2)溶液,加入0.020 g FeS-SA(或0.012 g FeS)使得溶液中初始Fe:(Pb+Cd)物质的量比为1. 研究中考察了溶液中初始Fe:(Pb+Cd)物质的量比为0.5—2.0时Pb和Cd吸附量的变化,结果表明物质的量比为1,即0.020 g FeS-SA(或0.012 g FeS)具有较高的吸附量. 混合体系中,取200 mL 0.3 mmol·L−1的Pb(NO3)2和Cd(NO3)2混合溶液,加入0.020 g FeS-SA(或0.012 g FeS). 溶液初始pH值为6.5(未调节),水浴恒温振荡(25 ℃,200 r·min−1),分时段取1 mL溶液过0.22 μm聚砜滤膜,用ICP-OES分析Fe、Pb和Cd的含量. 收集反应后的FeS-SA,浸泡于pH=5,0.4 mol·L−1的柠檬酸24 h(200 r·min−1)后用去离子水清洗3次,将干燥后的FeS-SA放入Pb或(Cd)溶液中进行吸附反应,探究FeS-SA的可重复利用性.
等温吸附实验:取200 mL初始浓度为0.06、0.12、0.18、0.24、0.30、0.36、0.48、0.60 mmol·L−1的Pb(NO3)2(或Cd(NO3)2)溶液,分别加入0.020 g FeS-SA(或0.012 g FeS),水浴恒温振荡24 h(预实验表明24 h已达到吸附平衡),反应条件与动力学实验相同.
MnO2对FeS-SA同步吸附Pb和Cd的影响:取200 mL浓度均为0.3 mmol·L−1的Pb(NO3)2和Cd(NO3)2混合溶液,分别加入0.02 g FeS-SA(或0.012 g FeS)和0.042 g MnO2. 反应条件与动力学实验相同.
-
应用准二级动力学模型模拟FeS-SA(或FeS)对Pb和Cd的吸附动力学[17]:
式中,qt和qe分别为t时刻和反应平衡时溶液Pb和Cd的吸附量(mg·g−1);k2为准二级反应速率常数(g·mg−1·min−1);t为反应时间(min).
用Langmuir等温吸附模型模拟FeS-SA(或FeS)对Pb和Cd的吸附行为[17]:
式中,Qe和Qm分别为吸附平衡量和饱和吸附量(mg·g−1);Ce为平衡浓度(mg·L−1);KL为吸附常数(L·mg−1),KL值越大,表示吸附亲和力越强.
-
样品分析采用试剂空白和3个平行样进行质量控制,Pb、Cd、Mn、Fe元素的标准曲线可决系数均大于0.999,回收率为95.0%—105%.
-
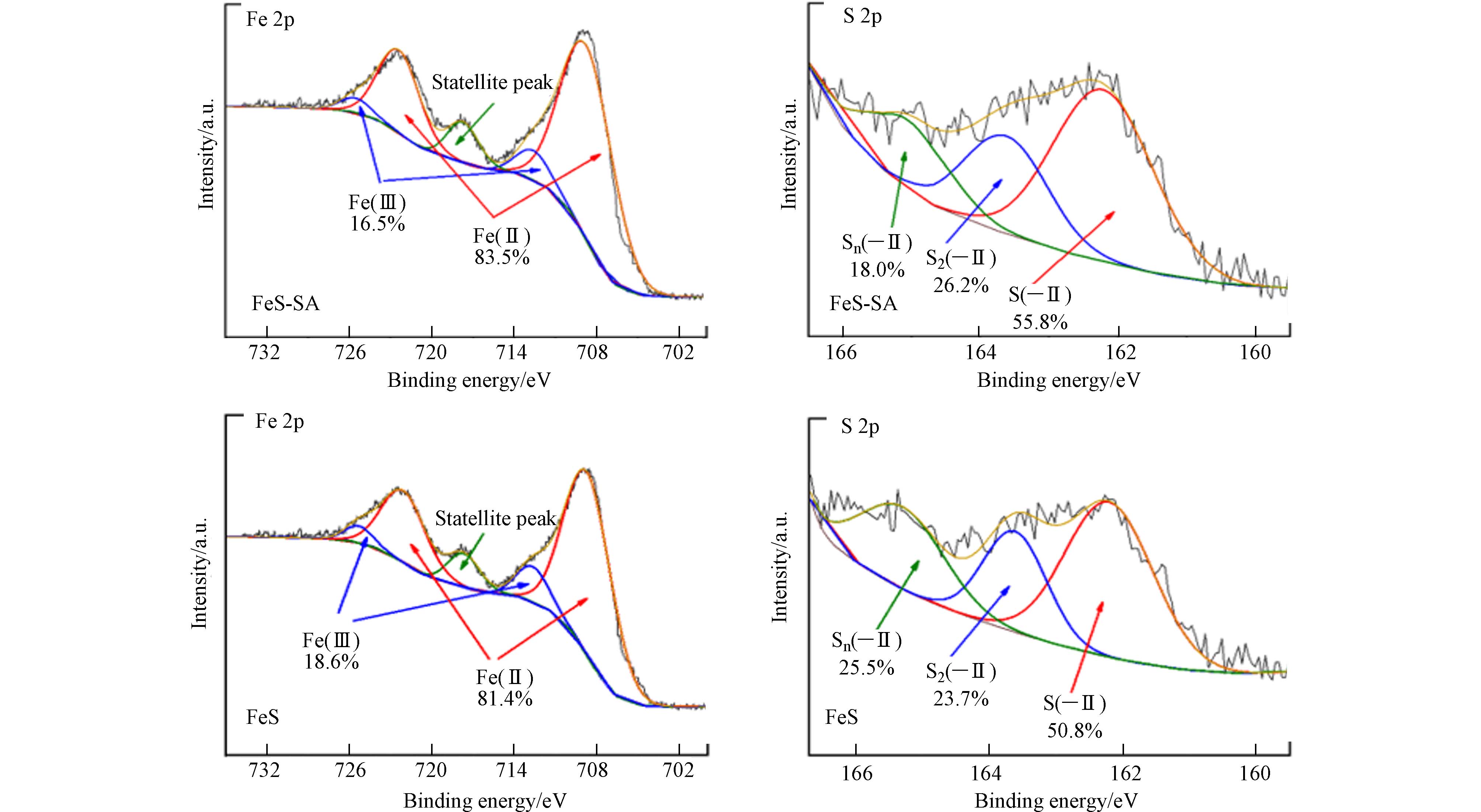
XPS分析表明,FeS-SA和FeS中Fe和S元素主要以Fe(Ⅱ)和S(−Ⅱ)的价态存在(图1),Fe(Ⅱ)的占比分别为83.5%和81.4%,S2−的占比分别为55.8%和50.8%. 同时还检测到Fe(Ⅲ)、S2(−Ⅱ)和Sn(−Ⅱ),S2(−Ⅱ)的存在说明合成过程中形成了部分FeS2[18],而Fe(Ⅲ)和Sn(−Ⅱ)的存在归因于样品处理过程中的表面氧化[19]. 研究表明,即使是新鲜合成隔氧保存的FeS表面仍能检测到Fe(Ⅲ)和Sn(−Ⅱ)[20],这是因为样品处理过程中存在少量的Fe(Ⅱ)和S(−Ⅱ)表面氧化. 结合1.1小节中FeS-SA和FeS的制备均是在无氧条件下操作,并且FeS-SA和FeS中Fe和S的形态未有显著差异. 综上,FeS-SA和FeS均未发生明显氧化,Fe和S主要以Fe(Ⅱ)和S(−Ⅱ)的价态存在.
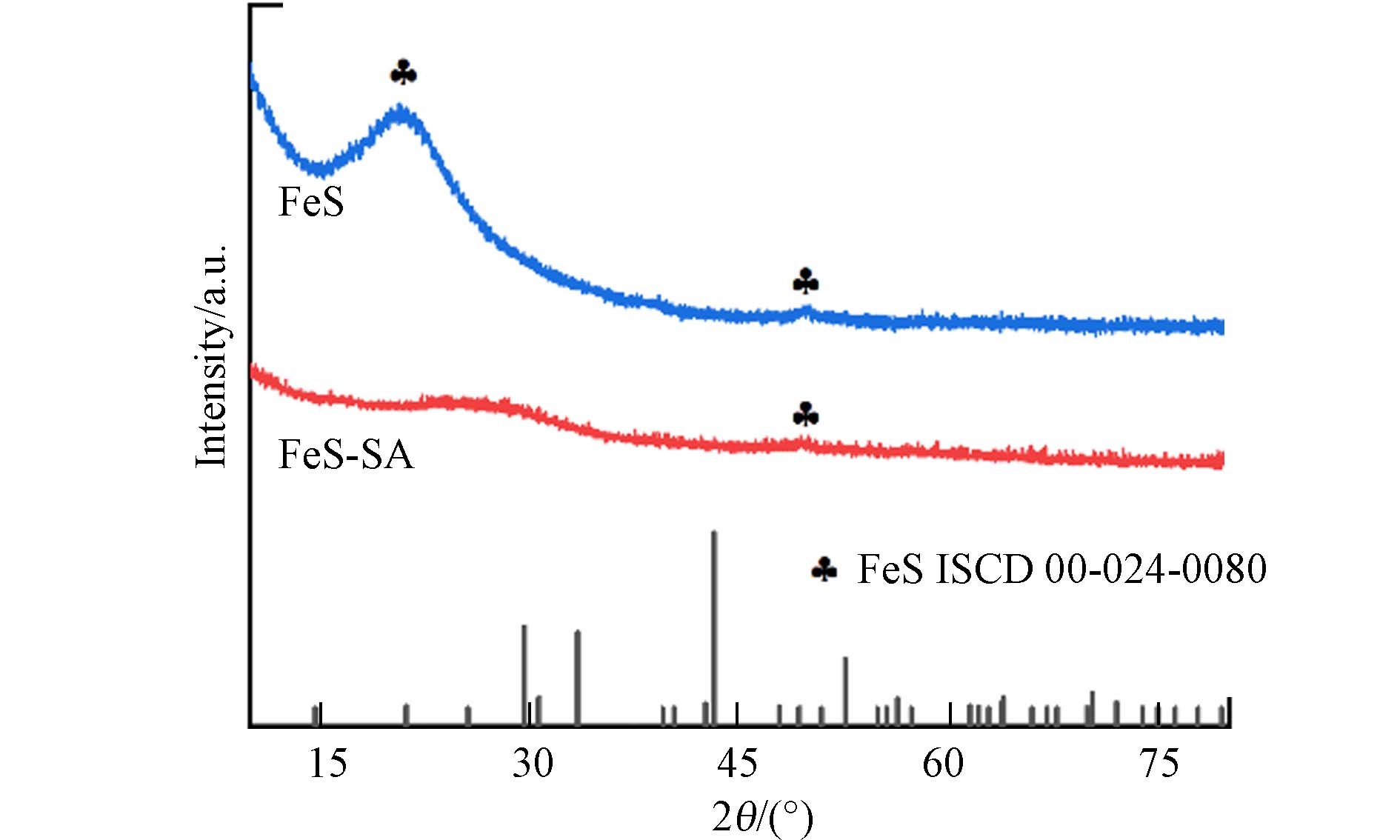
通过XRD进一步分析样品的结晶度,FeS-SA中FeS结晶度较差,在21.1°和49.8°处的峰说明了合成的是无定形FeS(图2). FeS-SA中FeS结晶度相对于未改性的FeS显著降低,这是因为SA与CaCl2交联形成的凝胶层抑制了溶液的局部对流和紊乱,减缓了离子扩散速率[21 − 22],使得FeS晶核形成及生长速率受限,从而结晶度减弱. 结合XPS和XRD分析,FeS-SA中FeS主要以Fe(Ⅱ)和S(−Ⅱ)价态存在,结晶度较差.
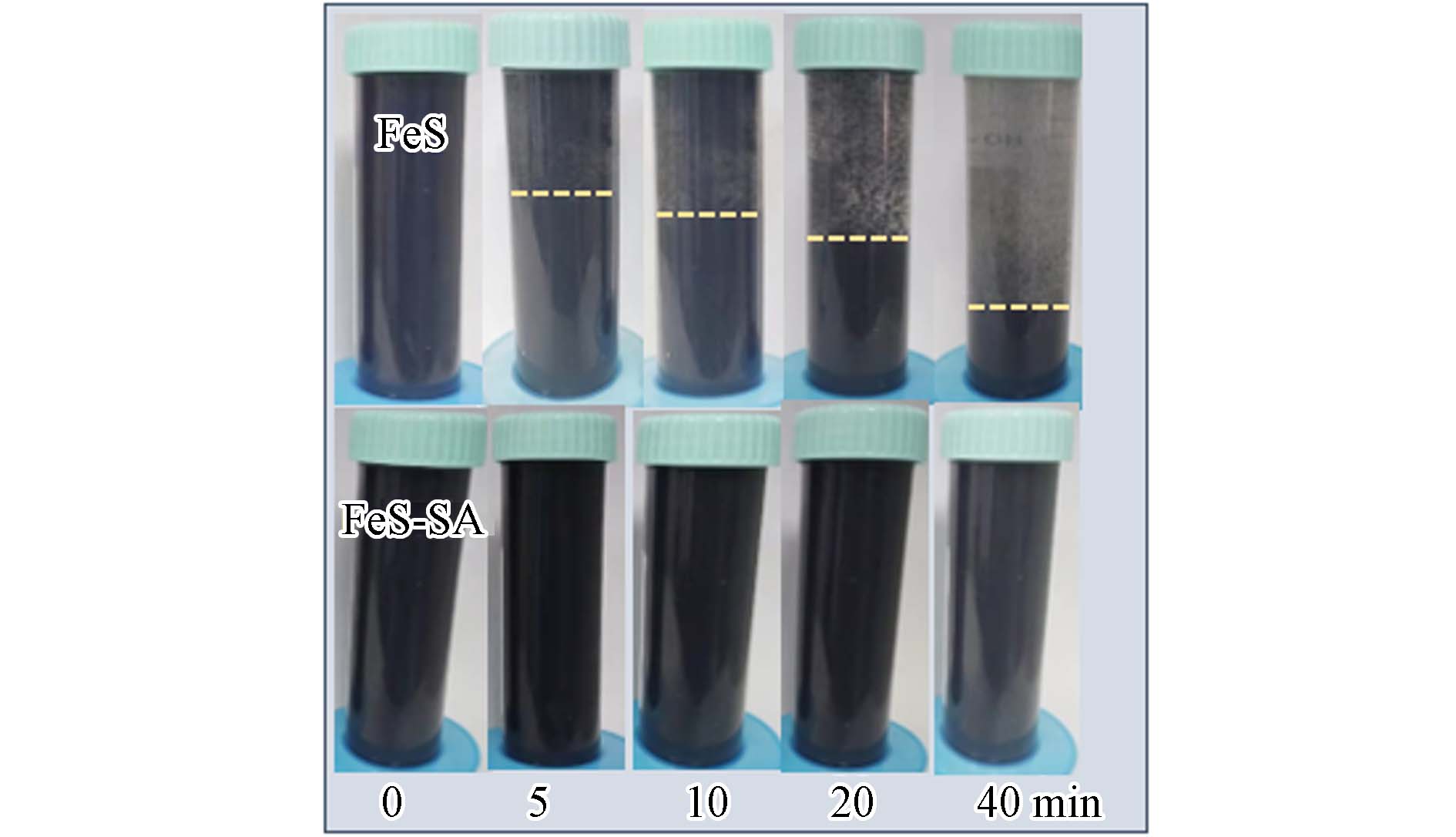
与FeS相比,FeS-SA在溶液中的分散性得到了显著提升. 沉降实验表明,静置5 min,未改性的FeS颗粒出现明显的液固分界层,静置20 min,分界层下降了45%,40 min时,分界层下降幅度达到了75%,而FeS-SA颗粒在静置40 min后仍未出现明显沉降(图3),说明SA显著提升了FeS-SA的分散性能. 这是因为SA含有丰富的羧基和羟基[23],羧基的双齿桥接作用与FeS颗粒相结合[16],有利于将FeS均匀分散于SA与Ca2+交联形成的孔隙结构中[24]. 另外,SA中的羟基也能通过氢键与FeS连接,进一步加强FeS的双层结构[10],提升了FeS之间的空间位阻[17]和静电斥力[12],使得FeS-SA具有更好的分散性.
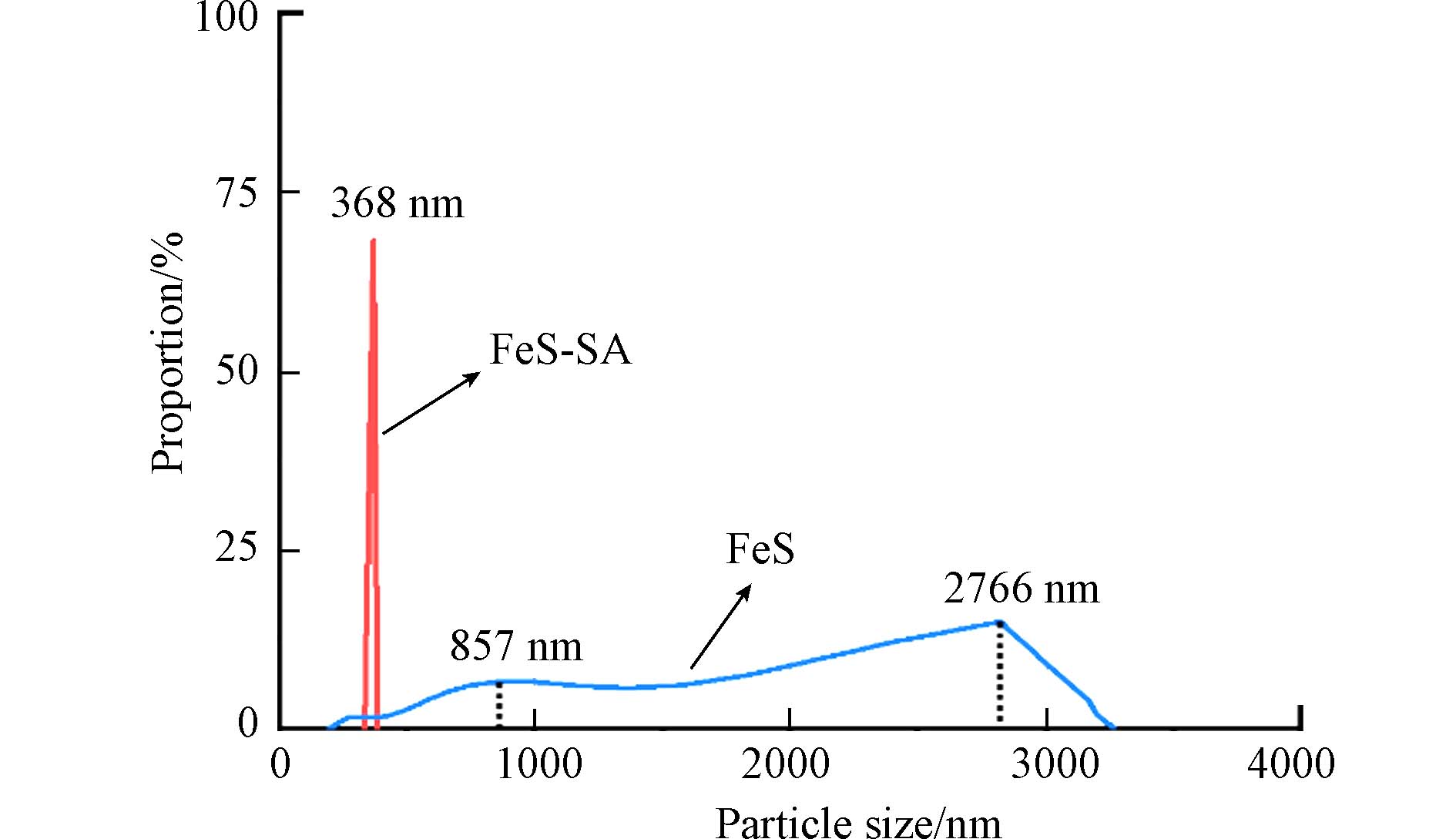
SA不仅提高了FeS-SA的分散性,也使得FeS-SA颗粒尺寸更加均一. 纳米粒度仪分析表明,FeS的水合粒径分布在857—
2766 nm之间,而FeS-SA的水合粒径显著降低,集中分布在368 nm(图4). SA与Ca2+发生离子交换反应[18],产生交联[25],形成具有孔隙的凝胶层[16]. SA上的羧基和羟基通过络合作用将Fe2+有效地分散在凝胶层的孔隙中[12],FeS颗粒生长速率得以保持一致,从而使得FeS颗粒尺寸更加均一. -
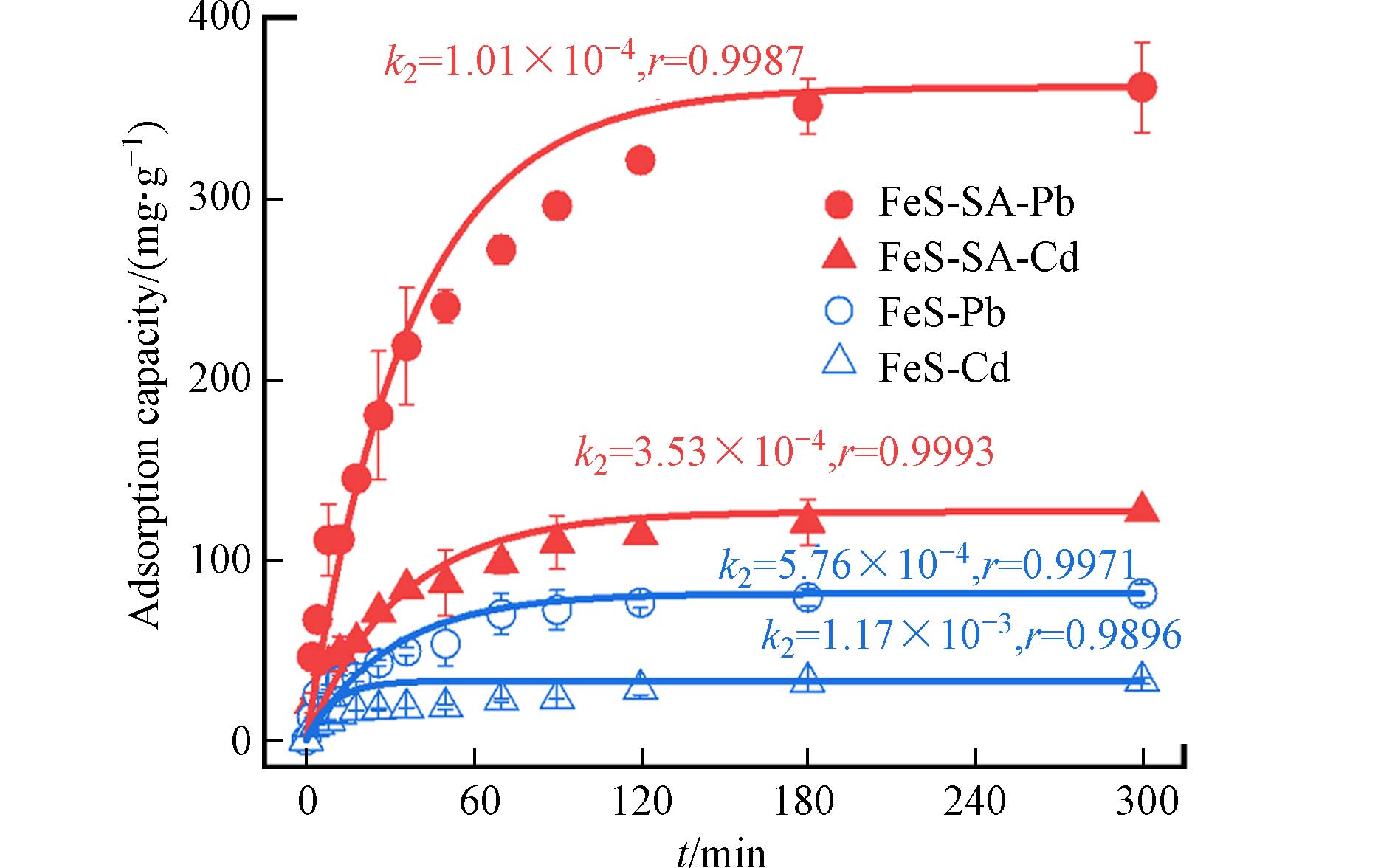
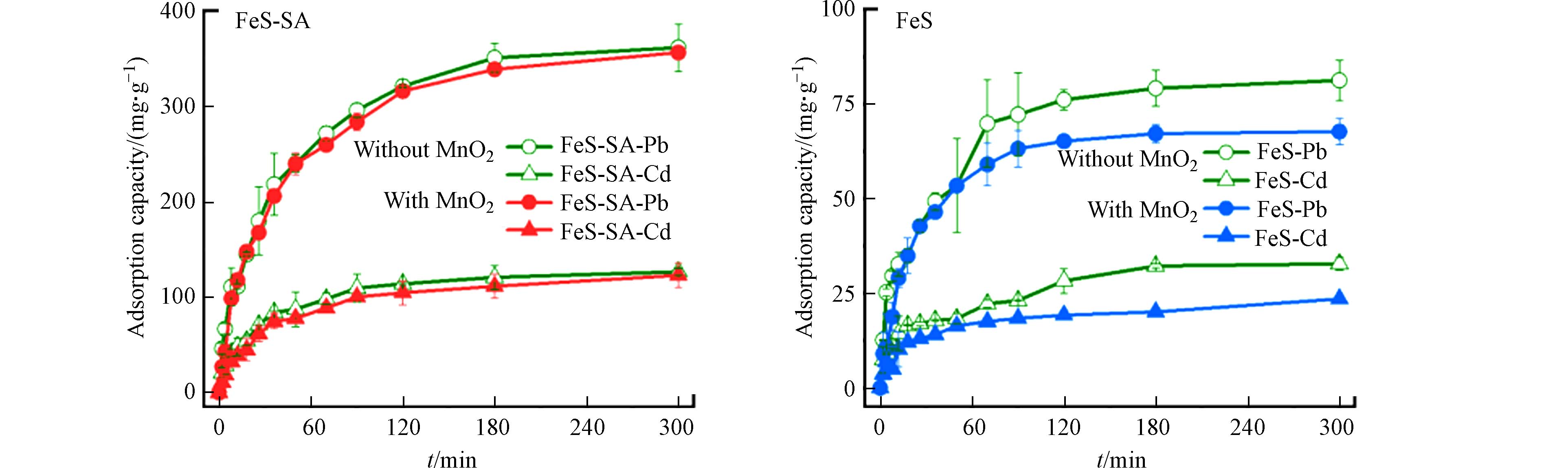
与FeS相比,FeS-SA对Pb和Cd的吸附性能显著提升,吸附容量分别提高了90.6%和114%,饱和吸附量达到了637 mg·g−1和334 mg·g−1,并且单位FeS对Pb和Cd的饱和吸附量也得到了显著提升. 以Pb为例,FeS对Pb的饱和吸附量为333 mg·g−1,而FeS-SA对Pb的吸附量则增加了180%,达到936 mg·g−1(扣减SA对吸附量的贡献,并归一到单位FeS)(图5). 吸附过程分为快速吸附和缓慢吸附两个阶段,96.4%的Pb在120 min内被快速吸附在FeS-SA上,而剩下的3.6%在另外180 min完成吸附. 准二级动力学模型能有效拟合吸附过程(r>0.99),Pb在FeS-SA和FeS上的吸附速率常数分别为5.80×10−5 g·mg−1·min−1和9.31×10−5 g·mg−1·min−1. 相较于FeS,FeS-SA对Pb表现出更大的吸附容量和更快的吸附速率,同时,FeS-SA具有良好的可重复利用性,重复使用3次后对Pb和Cd的吸附量保持在75%. 这与FeS-SA良好的分散性以及更小更均一的颗粒尺寸有关[12, 17],SA不仅有效分散了FeS,还提高了FeS的稳定性[26],增强了FeS的反应活性[27, 28].
近中性环境下,FeS表面以≡S−基团占主导,Pb和Cd则主要以Pb2+和Cd2+离子的形式存在[25]. FeS上的≡S−基团是一种路易斯碱,与路易斯酸Pb2+和Cd2+有很强的亲和力[29, 30],通过表面络合作用吸附Pb2+和Cd2+[12, 31 − 32]:
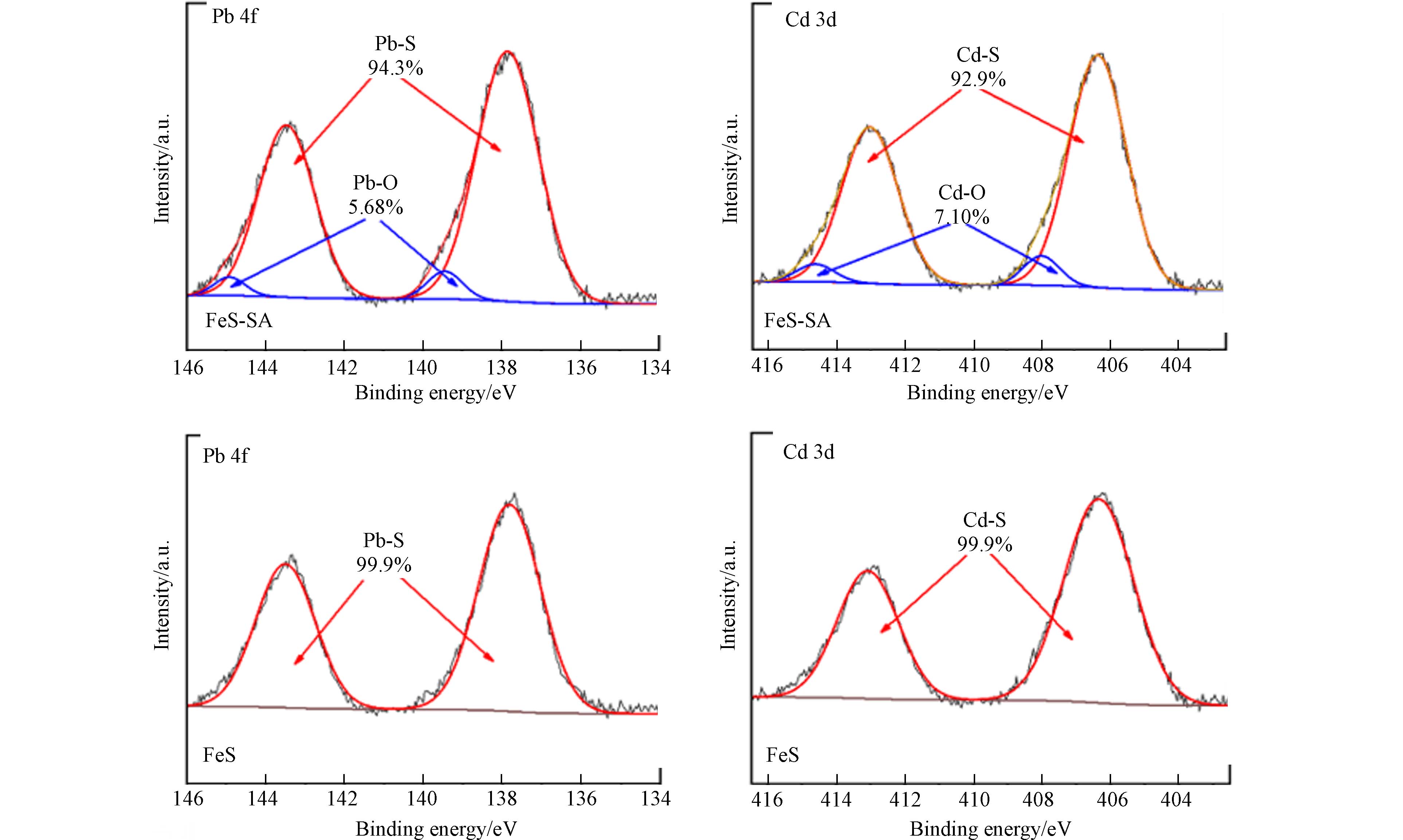
XPS分析表明,反应后的样品表面检测到Pb和Cd元素(图6). FeS反应后表面的Pb和Cd主要与S元素结合;而在FeS-SA中,94.3%的Pb和92.9%的Cd与S元素结合,这是≡S−的络合作用所致;5.68%的Pb和7.10%的Cd与O元素结合,这可能是FeS-SA表面SA上的羧基络合以及≡FeOH的络合所致.
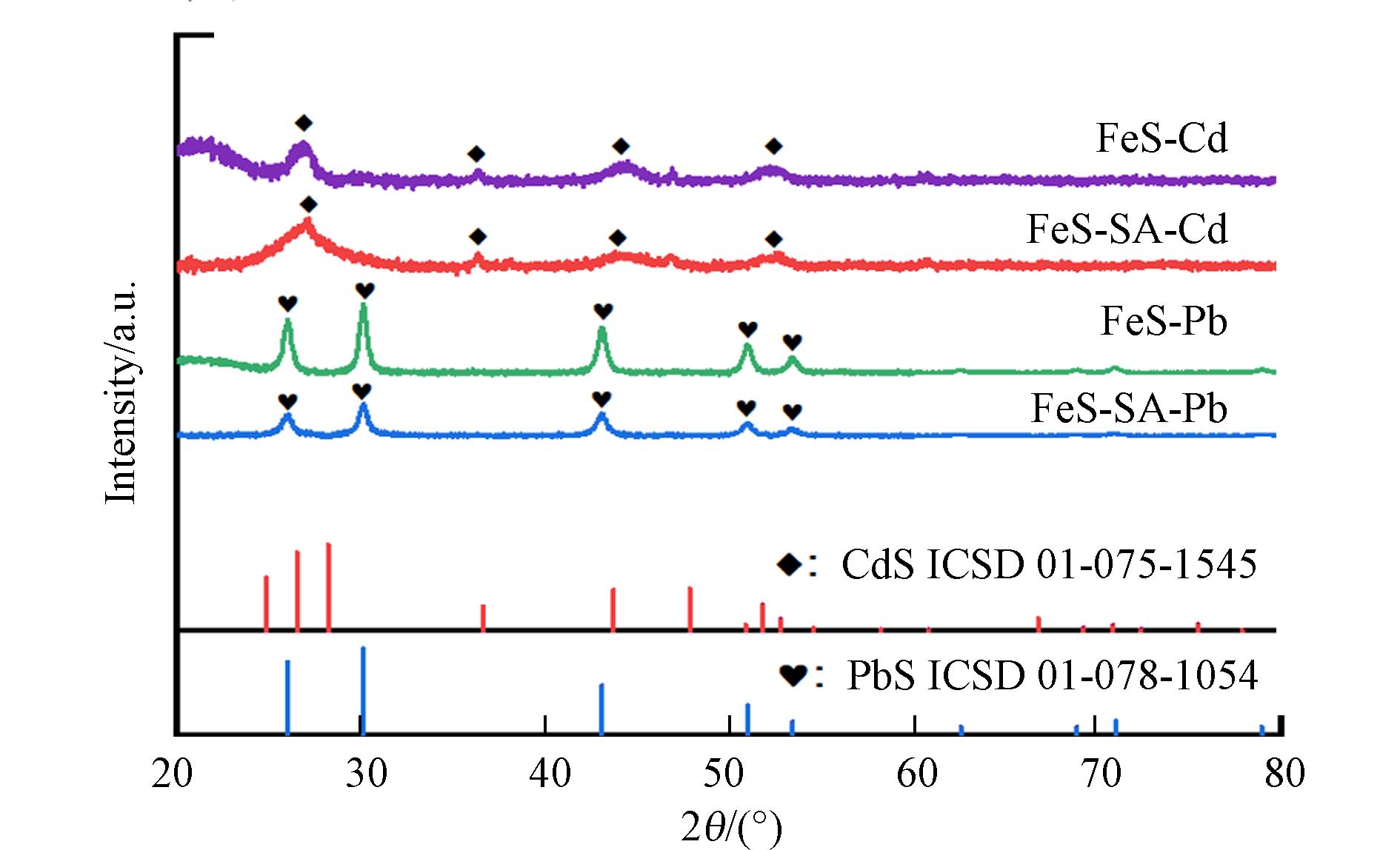
XRD分析吸附反应结束后的沉淀物,发现了晶型PbS和CdS. 在26.0°、30.2°、43.1°、51.0°和53.5°出现了PbS的特征峰(ICSD#01-078-
1054 ),而在26.5°、36.1°、43.9°和52.0°则观察到CdS的特征峰(ICSD#01-075-1545 )(图7).另一方面,PbS和CdS的生成也可能是离子交换所致. FeS在中性环境中溶解度很低(7.94×10−19)[33],在含有Pb2+和Cd2+的溶液中发生溶解,生成溶解度更低的PbS(1×10−28)和CdS(8×10−27)[28]:
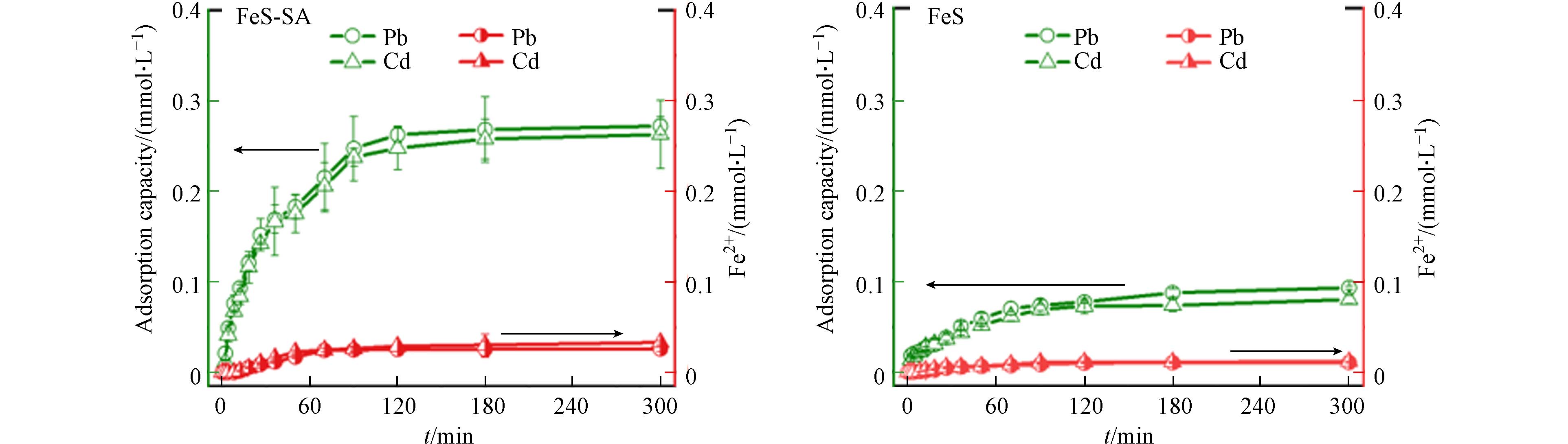
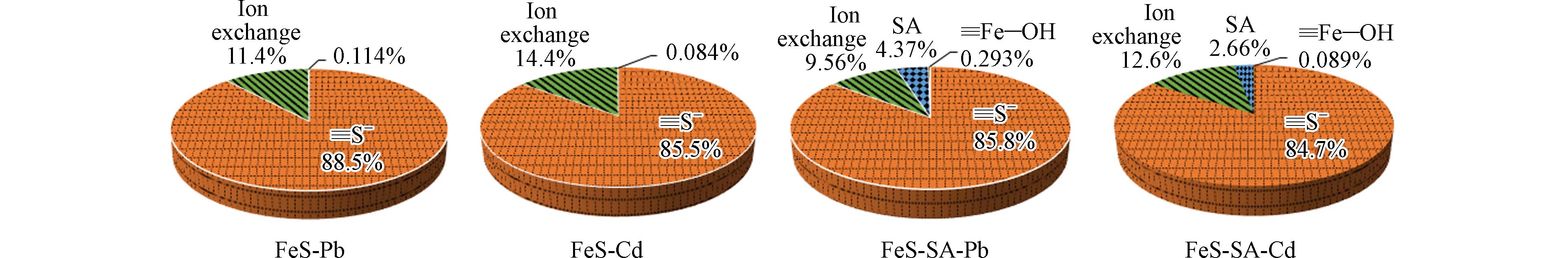
反应8 min,溶液中检测到Fe2+离子,Fe2+的释放在120 min达到平衡(图8). FeS-SA吸附Pb和Cd后,溶液中Fe2+含量分别为0.026 mmol·L−1和0.033 mmol·L−1,占到了Pb和Cd总吸附量的9.56%和12.6%,说明只有9.56%的Pb和12.6%的Cd是以离子交换的形式吸附到FeS-SA.
FeS表面小部分羟基化≡Fe−OH也能通过络合作用吸附Pb2+和Cd2+[5, 27]:
反应后溶液pH分别下降了0.54和0.25,计算Pb2+和Cd2+吸附量分别为1.87 mg·g−1和0.30 mg·g−1,占到总吸附量的0.29%和0.09%,说明≡Fe−OH表面络合吸附并非主吸附路径. 总结以上分析,吸附路径主要包括4种:≡S−和≡Fe−OH表面络合、离子交换以及SA吸附,其中≡S−表面络合作用占主导,对吸附量的贡献约为85%,离子交换约占10%,SA吸附贡献不足5%,而≡Fe−OH表面络合不足0.5%(图9).
-
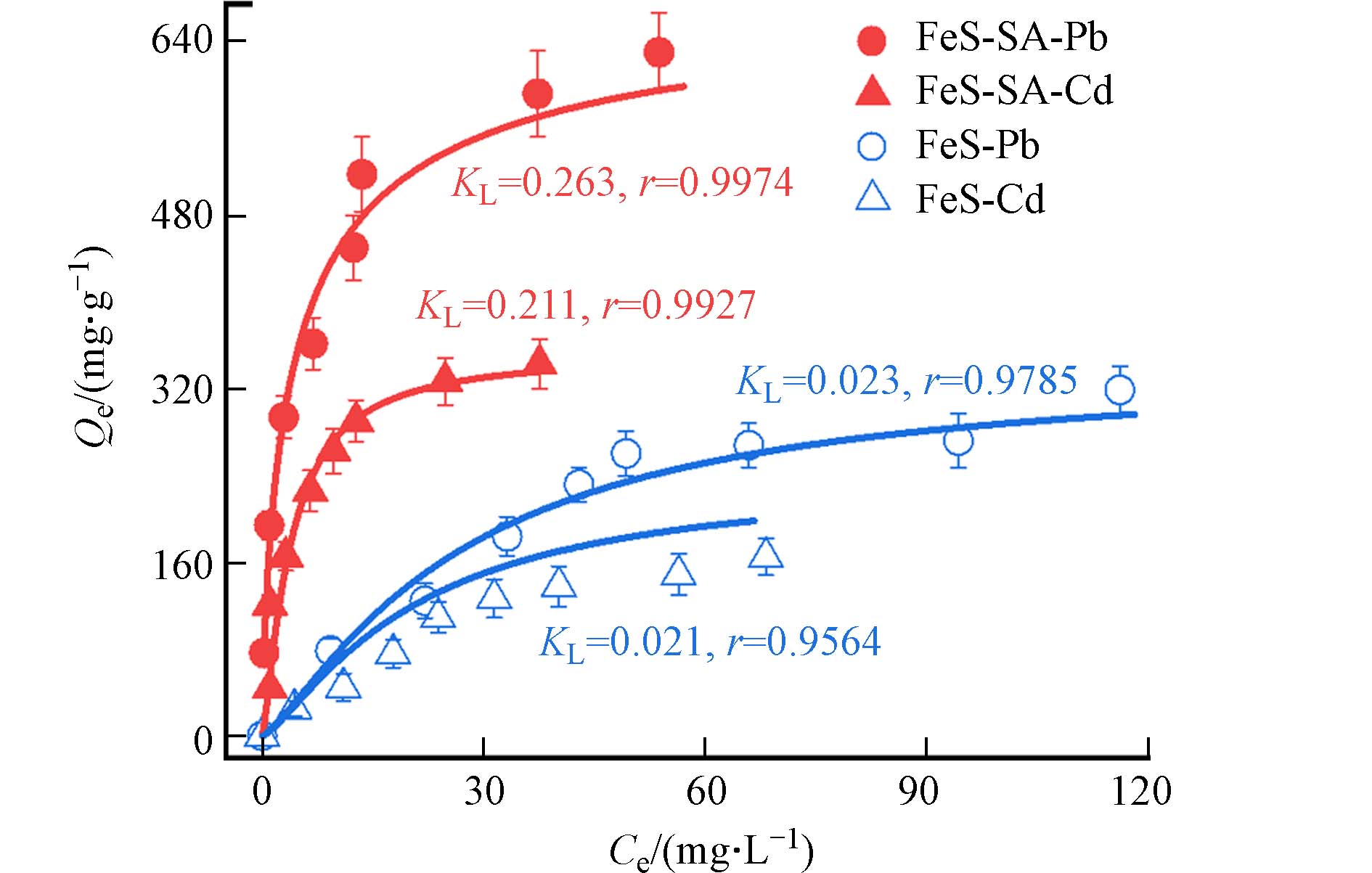
在Pb和Cd混合体系中,FeS-SA对Pb和Cd的吸附量分别为361 mg·g−1和127 mg·g−1(图10). 用摩尔量归一化后,FeS-SA对混合体系中Pb和Cd的吸附量分别为1.74 mmol·g−1和1.13 mmol·g−1,较单一体系的吸附量分别下降了43.3%和62.1%. 与Pb相比,Cd在混合体系中的吸附量降低幅度更大,说明Pb在一定程度上抑制了FeS-SA对Cd的吸附. 这与FeS-SA对Pb具有较高的吸附亲和力有关[12].
从Langmuir等温吸附模型的拟合结果可知,在单一体系中FeS-SA对Pb的吸附常数KL值为 0.263 L·mg−1,比Cd的吸附常数高出24.8%(图11),表明FeS-SA对Pb具有更高的吸附亲和力. 动力学实验也表明了Pb比Cd的吸附速率更快(图5),说明Pb能快速地占据FeS-SA上的吸附点位,相较于Cd,FeS-SA对Pb显示出吸附优势. 从前面的吸附机制探讨可知,FeS-SA主要是通过≡S−表面络合作用吸附Pb和Cd,贡献了近85%的吸附量. Pb的电负性是Cd的2.38倍[34],亲电性更强,更易被路易斯碱≡S−吸附. 因此,在混合体系中,Pb优先占据FeS-SA的吸附点位,展示出比Cd更高的吸附容量和更快的吸附速率.
当混合体系中存在MnO2时,FeS-SA对Pb和Cd的吸附量分别为356 mg·g−1和123 mg·g−1(图12),比无MnO2的混合体系分别降低了1.42%和3.25%,但MnO2对FeS吸附Pb和Cd的抑制率分别为16.7%和28.2%,这与SA有效抑制MnO2对FeS的氧化有关,保证了FeS上吸附点位的反应活性[9].
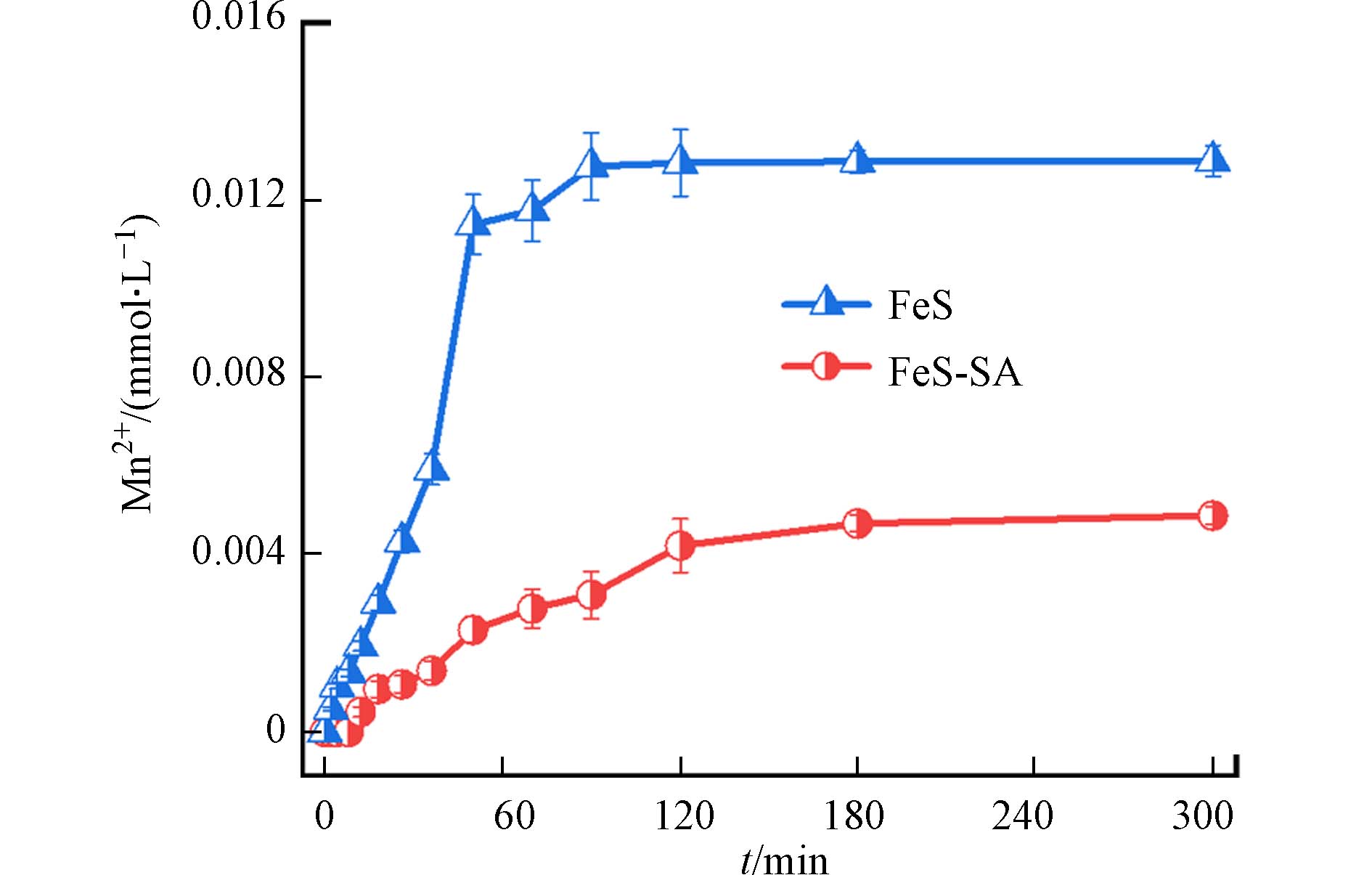
与FeS相比,FeS-SA抗MnO2氧化性能显著提升. 分析溶液中Mn2+浓度发现,FeS-SA体系中Mn2+的生成量(
0.0049 mmol·L−1)比FeS体系(0.0129 mmol·L−1)低62.0%(图13),说明未包裹SA的FeS更易被MnO2氧化[8, 9]:包裹SA后,FeS的氧化率从2.15%降低到0.813%,使得更多活性点位得以保留[17],从而显著提升了FeS的吸附性能. 另外,FeS-SA体系中,Mn2+的生成速率符合一级动力学,速率常数为1.63×10−5 mmol·L−1·min−1,比FeS体系下降了62.1%,说明FeS-SA的氧化速率显著降低,反应活性点位的寿命得以延长,提供了更充分的吸附时间[35],从而有助于FeS-SA对Pb和Cd的吸附. 综上,SA不仅显著降低了FeS的氧化率,还延缓了FeS的氧化速率,为FeS吸附Pb和Cd提供了更多的活性点位和吸附时间.
-
相对于FeS,FeS-SA颗粒尺寸更小更均一,且具有更高的分散性. SA增加了空间位阻和静电斥力,FeS-SA的分散性显著提升. 分散性的提升,显著增强了FeS-SA的吸附性能. FeS-SA对Pb和Cd的饱和吸附量为637 mg·g−1和334 mg·g−1,比FeS分别提升了180%和220%(归一到单位FeS),对Pb和Cd的吸附亲和力提高了1个数量级. 机制分析表明,FeS-SA对Pb和Cd存在≡S−和≡Fe−OH络合、离子交换和SA吸附4种吸附路径. 其中,≡S−络合贡献了近85%的吸附量,是主吸附路径. 混合体系中,FeS-SA对Pb和Cd的吸附量分别达到361 mg·g−1和127 mg·g−1,比单一体系分别下降了43.3%和62.1%,Pb在混合体系中显示出竞争吸附优势. SA抑制了活性FeS与MnO2的直接接触,增强了其对MnO2抗氧化性,因此MnO2对FeS-SA同步吸附Pb和Cd无显著影响(吸附量下降率<4%). FeS-SA吸附性能和抗MnO2氧化性能的显著提升,以及吸附机制的多样性,显示出FeS-SA在地下水重金属复合污染修复中的巨大应用潜力.
海藻酸钠改性硫化亚铁对Pb和Cd的同步吸附性能和机制
Simultaneous adsorption performance and mechanism of ferrous sulfide modified with sodium alginate (FeS-SA) towards Pb and Cd
-
摘要: 采选冶废水下渗造成地下水Pb和Cd复合污染. 本文通过交联技术制备了海藻酸钠改性硫化亚铁(FeS-SA),研究了FeS-SA对水中Pb和Cd的同步吸附动力学、吸附容量及MnO2的影响,探讨了吸附机制及Pb的竞争吸附优势. 研究表明,相对于FeS,FeS-SA具有更高的分散性和稳定性. FeS-SA颗粒尺寸更小更均一,吸附性能得到显著提升. 相较于FeS,FeS-SA对Pb和Cd的吸附容量为637 mg·g−1和334 mg·g−1,分别提升了90.6%和114%,对Pb和Cd的吸附亲和力也提升了1个数量级. FeS-SA对Pb和Cd的吸附机制主要包括≡S−表面络合、离子交换、海藻酸钠(SA)吸附和≡Fe−OH表面络合吸附4种,其中≡S−表面络合贡献了近85%的吸附量. 混合体系中FeS-SA对Pb和Cd的吸附量为361 mg·g−1和127 mg·g−1. 与Pb相比(43.3%),Cd的吸附量较之单一体系下降幅度更大,为62.1%,这与FeS-SA对Pb具有更高的吸附亲和力和更快的吸附速率有关,因此,Pb在同步吸附中占据优势. 由于SA有效阻隔了MnO2对FeS的氧化,MnO2对FeS-SA同步吸附Pb和Cd无显著影响(<4%). FeS-SA吸附性能和抗环境干扰性能的显著提升以及吸附机制的多样化表明其在环境修复中具有很大的应用潜力,本研究为FeS-SA在修复重金属复合污染地下水中的应用提供了数据支持和理论依据.
-
关键词:
- 海藻酸钠改性硫化亚铁 /
- 同步吸附 /
- 铅 /
- 镉 /
- 吸附机制.
Abstract: Multi-polluted groundwater from lead (Pb) and cadmium (Cd) was caused by the infiltration of wastewater from mining, dressing and smelting industries. Ferrous sulfide modified with sodium alginate (FeS-SA) was prepared via crosslinking technology. The simultaneous adsorption kinetics and adsorption capacity of FeS-SA towards Pb and Cd in aqueous solution and the effects of MnO2 on adsorption were studied. The adsorption mechanism and competitive adsorption advantage of Pb were discussed. Generally, higher dispersion and stability were observed for FeS-SA than that of in non-modified FeS. Smaller and more homogeneous particle size was obtained for FeS-SA, and hence, the adsorption performance was significantly improved. The adsorption capacity of FeS-SA towards Pb and Cd were 637 mg·g−1 and 334 mg·g−1, increased by 90.6% and 114%, respectively compared with FeS. Moreover, the adsorption affinity of FeS-SA towards Pb and Cd was increased by one order of magnitude. Four adsorption mechanisms, including ≡S− surface complexation, ion exchange, sodium alginate adsorption, and ≡Fe−OH surface complexation contributed in adsorption process. In particular, ≡S− surface complexation dominated in adsorption, accounting for 85%. Furthermore, the adsorption capacity of FeS-SA towards Pb and Cd in multi-polluted system were 361 mg·g−1 and 127 mg·g−1, respectively. It was noteworthy that the adsorption capacity of Cd decreased by 62.1% more than that of Pb (43.3%) compared with single system. Lead was found to be dominant in simultaneous adsorption due to higher adsorption affinity and faster adsorption rate with FeS-SA. More importantly, MnO2 had no significant effect (<4%) on the simultaneous adsorption of Pb and Cd due to the effective inhibition by sodium alginate to FeS oxidation by MnO2. Overall, the significant improvement in adsorption performance and resistance to environmental interference, as well as the diversification of adsorption mechanism, indicating that FeS-SA had great potential in environmental remediation. This study provided the data support and theoretical basis for the application of FeS-SA in the remediation of groundwater contaminated by heavy metals. -

-
-
[1] 钱建平, 李伟, 张力, 等. 地下水中重金属污染来源及研究方法综析[J]. 地球与环境, 2018, 46(6): 613-620. QIAN J P, LI W, ZHANG L, et al. Source and research status of heavy metal pollution in groundwater: A review[J]. Earth and Environment, 2018, 46(6): 613-620 (in Chinese).
[2] HAN D M, CURRELL M J, CAO G L. Deep challenges for China’s war on water pollution[J]. Environmental Pollution, 2016, 218: 1222-1233. doi: 10.1016/j.envpol.2016.08.078 [3] SUN Y, LIU Y L, LOU Z M, et al. Enhanced performance for Hg(Ⅱ) removal using biomaterial (CMC/gelatin/starch) stabilized FeS nanoparticles: Stabilization effects and removal mechanism[J]. Chemical Engineering Journal, 2018, 344: 616-624. doi: 10.1016/j.cej.2018.03.126 [4] ZHAO Y, TIAN S T, GONG Y Y, et al. Efficient removal of lead from water using stabilized iron sulfide nanoparticles: Effectiveness and effects of stabilizer [J]. Water, Air, & Soil Pollution, 2019, 230(6): 1-14. [5] PARK M, LEE K S, RYU J, et al. Investigation of Cd(Ⅱ) sorption by mackinawite (FeS) under anoxic conditions[J]. Journal of Analytical Science and Technology, 2022, 13(1): 1-11. doi: 10.1186/s40543-021-00310-5 [6] 苗立永, 员玉良, 王铮. FeS流化床处理电镀废水中重金属离子的试验研究[J]. 工业水处理, 2008, 28(9): 21-24. doi: 10.3969/j.issn.1005-829X.2008.09.006 MIAO L Y, YUN Y L, WANG Z. Experimental research of the treatment of heavy metal ions in electroplating wastewater with FeS fluid bed[J]. Industrial Water Treatment, 2008, 28(9): 21-24 (in Chinese). doi: 10.3969/j.issn.1005-829X.2008.09.006
[7] 张越. FeS流化床处理电镀废水的试验研究[J]. 山西化工, 2016, 36(5): 133-136. ZHANG Y. FeS processing galvanization waste water fluid bed craft research[J]. Shanxi Chemical Industry, 2016, 36(5): 133-136 (in Chinese).
[8] SCHIPPERS A, JØRGENSEN B B. Biogeochemistry of pyrite and iron sulfide oxidation in marine sediments[J]. Geochimica et Cosmochimica Acta, 2002, 66(1): 85-92. doi: 10.1016/S0016-7037(01)00745-1 [9] SCHIPPERS A, JØRGENSEN B B. Oxidation of pyrite and iron sulfide by manganese dioxide in marine sediments[J]. Geochimica et Cosmochimica Acta, 2001, 65(6): 915-922. doi: 10.1016/S0016-7037(00)00589-5 [10] GONG Y Y, TANG J C, ZHAO D Y. Application of iron sulfide particles for groundwater and soil remediation: A review[J]. Water Research, 2016, 89: 309-320. doi: 10.1016/j.watres.2015.11.063 [11] CHEN Y N, LIANG W Y, LI Y P, et al. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review[J]. Chemical Engineering Journal, 2019, 362: 144-159. doi: 10.1016/j.cej.2018.12.175 [12] DUAN J, JI H D, ZHAO X, et al. Immobilization of U(Ⅵ) by stabilized iron sulfide nanoparticles: Water chemistry effects, mechanisms, and long-term stability[J]. Chemical Engineering Journal, 2020, 393: 124692. doi: 10.1016/j.cej.2020.124692 [13] 凌海波, 全森, 侯松, 等. 海藻酸钠改性硫化亚铁对水中Cr(Ⅵ)去除性能研究[J]. 环境科学与技术, 2022, 45(4): 54-60. LING H B, QUAN S, HOU S, et al. Sodium alginate modified ferrous sulfide for the removal of Cr(Ⅵ)from aqueous system[J]. Environmental Science & Technology, 2022, 45(4): 54-60 (in Chinese).
[14] 王琼杰, 张勇, 张阳阳, 等. 老化微塑料对水体中重金属铜和锌的吸附行为研究[J]. 环境科学学报, 2021, 41(7): 2712-2726. WANG Q J, ZHANG Y, ZHANG Y Y, et al. Adsorption of heavy metal ions Cu2+ and Zn2+ onto UV-aged microplastics in aquatic system[J]. Acta Scientiae Circumstantiae, 2021, 41(7): 2712-2726 (in Chinese).
[15] 余东, 周金龙, 张杰, 等. 新疆喀什地区地下水铁锰水文地球化学及演化规律[J]. 环境科学学报, 2021, 41(6): 2169-2181. YU D, ZHOU J L, ZHANG J, et al. Hydrogeochemistry and evolution of iron and manganese in groundwater in Kashgar, Xinjiang[J]. Acta Scientiae Circumstantiae, 2021, 41(6): 2169-2181 (in Chinese).
[16] 邓智瀚. 海藻酸钠改性FeS纳米颗粒处理Cr(Ⅵ)污染土壤的机理及性能研究 [D]. 成都: 成都理工大学, 2019. DENG Z H. Study on mechanism and properties of repair Cr(Ⅵ) in soil using FeS nanoparticles modified sodium alginate [D]. Chengdu: Chengdu University of Technology, 2019(in Chinese).
[17] SUN M Y, CHENG G H, GE X L, et al. Aqueous Hg(Ⅱ) immobilization by chitosan stabilized magnetic iron sulfide nanoparticles[J]. Science of the Total Environment, 2018, 621: 1074-1083. doi: 10.1016/j.scitotenv.2017.10.119 [18] FENG D M, ZHANG X, SUN Y, et al. Surface-defective FeS2 for electrochemical NH3 production under ambient conditions[J]. Nano Materials Science, 2020, 2(2): 132-139. doi: 10.1016/j.nanoms.2019.07.002 [19] WANG Y X, YANG J P, CHOU S L, et al. Uniform yolk-shell iron sulfide-carbon nanospheres for superior sodium-iron sulfide batteries[J]. Nature Communications, 2015, 6: 8689. doi: 10.1038/ncomms9689 [20] MATAMOROS V A, CESPEDES O, JOHNSON B R G, et al. A highly reactive precursor in the iron sulfide system[J]. Nature Communications, 2018, 9: 3125. doi: 10.1038/s41467-018-05493-x [21] LI H, ZHAO T L, QIAN F J, et al. A model of extracellular polymeric substances on crystal growth and morphogenesis of struvite: Effects of sodium alginate[J]. Powder Technology, 2021, 380: 80-88. doi: 10.1016/j.powtec.2020.11.037 [22] WEI L, HONG T Q, LIU H B, et al. The effect of sodium alginate on struvite crystallization in aqueous solution: A kinetics study[J]. Journal of Crystal Growth, 2017, 473: 60-65. doi: 10.1016/j.jcrysgro.2017.03.039 [23] GAO X P, GUO C, HAO J J, et al. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives[J]. International Journal of Biological Macromolecules, 2020, 164: 4423-4434. doi: 10.1016/j.ijbiomac.2020.09.046 [24] 杨晓武, 李志刚, 李培枝, 等. 海藻酸钠凝胶海绵体的制备及其对Pb2+和Cu2+的吸附[J]. 精细化工, 2021, 38(1): 162-168. YANG X W, LI Z G, LI P Z, et al. Preparation of sodium alginate gel sponge and its adsorption for Pb2+ and Cu2+[J]. Fine Chemicals, 2021, 38(1): 162-168 (in Chinese).
[25] CÓRDOVA B M, JACINTO C R, ALARCÓN H, et al. Chemical modification of sodium alginate with thiosemicarbazide for the removal of Pb(II) and Cd(II) from aqueous solutions[J]. International Journal of Biological Macromolecules, 2018, 120: 2259-2270. doi: 10.1016/j.ijbiomac.2018.08.095 [26] MOHAMED ANWAR P, MURUGANANTHAM S, KARUNANITHY M, et al. Optical, structural and electrical properties of AgSbO3 nanotips prepared by thermal evaporation technique for thermoelectric effect applications[J]. Materials Today:Proceedings, 2021, 36: 492-498. doi: 10.1016/j.matpr.2020.05.148 [27] GONG Y Y, LIU Y Y, XIONG Z, et al. Immobilization of mercury by carboxymethyl cellulose stabilized iron sulfide nanoparticles: Reaction mechanisms and effects of stabilizer and water chemistry[J]. Environmental Science & Technology, 2014, 48(7): 3986-3994. [28] ZOU Q R, WANG W Y, ZHANG T, et al. Simultaneous removal of Cr(Ⅵ), Cd, and Pb from aqueous solution by iron sulfide nanoparticles: Influencing factors and interactions of metals[J]. Chinese Journal of Chemical Engineering, 2021, 40: 245-255. doi: 10.1016/j.cjche.2020.10.021 [29] DU H H, CHEN W L, CAI P, et al. Competitive adsorption of Pb and Cd on bacteria-montmorillonite composite[J]. Environmental Pollution, 2016, 218: 168-175. doi: 10.1016/j.envpol.2016.08.022 [30] ZHANG M J, ZHU L Y, HE C H, et al. Adsorption performance and mechanisms of Pb(Ⅱ), Cd(Ⅱ), and Mn(Ⅱ) removal by a β-cyclodextrin derivative[J]. Environmental Science and Pollution Research International, 2019, 26(5): 5094-5110. doi: 10.1007/s11356-018-3989-4 [31] HYUN S P, KIM B A, SON S, et al. Cadmium(Ⅱ) removal by mackinawite under anoxic conditions[J]. ACS Earth and Space Chemistry, 2021, 5(6): 1306-1315. doi: 10.1021/acsearthspacechem.0c00276 [32] TAN K L, HAMEED B H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 74: 25-48. doi: 10.1016/j.jtice.2017.01.024 [33] COLES C A, RAO S R, YONG R N. Lead and cadmium interactions with mackinawite: retention mechanisms and the role of pH[J]. Environmental Science & Technology, 2000, 34(6): 996-1000. [34] GE Q L, TIAN Q, WANG S F, et al. Highly efficient removal of lead/cadmium by phosphoric acid-modified hydrochar prepared from fresh banana peels: Adsorption mechanisms and environmental application[J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2022, 38(49): 15394-15403. doi: 10.1021/acs.langmuir.2c02693 [35] WU J, WANG X B, ZENG R J. Reactivity enhancement of iron sulfide nanoparticles stabilized by sodium alginate: Taking Cr (Ⅵ) removal as an example[J]. Journal of Hazardous Materials, 2017, 333: 275-284. doi: 10.1016/j.jhazmat.2017.03.023 -



 下载:
下载: