-
有机气溶胶(organic aerosol,OA)是大气细颗粒物的重要成分,在灰霾的形成过程中起重要作用,是影响我国灰霾污染频发的关键内因之一[1 − 3]. 在我国,OA对大气气溶胶的贡献在10%—70%,某些地区可达 80%[4 − 5]. 有机气溶胶来源和成分复杂,对空气质量、大气化学和气候强迫有着深远的影响[6 − 7]. 其中最广为人知的吸收光碳质气溶胶是黑碳(blank carbon, BC)和棕碳(brown carbon,BrC), 棕碳是一类能够在可见光区和近紫外光区(200—550 nm之间)对太阳辐射产生较强吸收的有机物,该类物质兼有散射和吸光能力、大多显棕黄色或棕褐色. 棕碳对地区辐射强迫、区域气候效应有重要的影响,最新的模式研究表明,棕碳对全球辐射强迫的贡献约为黑碳的27%—70%[8],IPCC(政府间气候变化委员会)在第五次评估报告中首次把棕碳对气候变化的影响作为着重关注和讨论的议题 [9]. 目前,棕碳已成为国际大气环境领域的研究热点之一[10 − 12].
BrC来源复杂,有一次源和二次源之分. 其一次源主要是生物质燃料或化石燃料的不完全燃烧的排放[13],而二次源主要是在大气气溶胶老化过程中形成二级吸光化合物,例如在高氮氧化物(NOx)水平下产生芳香族二次有机气溶胶,或者通过各种水相反应,如木质素氧化[14],以及通过反应性摄取异戊二烯形成光吸收低聚物等[15]. 目前对于BrC的光学性质研究最为广泛,而其中最为常用的方法就是溶剂萃取法,即将采集的样品用溶剂进行萃取,随后再对萃取液进行分析的一种研究方法[16]. 该方法既可以有效地分析BrC的化学组成,还可以避免其他物质(如黑碳)对BrC光学性质的影响. 近年来一些工作探讨了不同溶剂萃取BrC效果的差异,其主要集中在乙腈、甲醇、正己烷等混合溶剂与水[17],这些结果表明溶剂的选择对BrC光学性质有很大影响.
我国城市大气复合污染严重,2013年1月以来,大尺度区域能见度降低、灰霾现象频繁发生,其中致使多地的大气颗粒物浓度增大,能见度显著下降的主要原因之一就是大气中有机物浓度的变化. 灰霾发生期间大气颗粒物中有机物的浓度显著增加,有机气溶胶对消光的贡献越来越大. 如德州颗粒物中有机物对大气总消光的贡献可达75.5%[18],珠江三角洲秋冬季有机气溶胶是对消光系数贡献最大的组分,占总消光的(45.9 ± 1.6)%[19];在北京地区,有机气溶胶对消光的贡献可达到57%[20]. 为了更好地治理我国城市地区的大气环境污染问题,生态环境等部门共同展开调研,关于京津冀以及其周边28个城市和汾渭平原11个城市共39个城市的大气污染情况的研究. 运城市是我国能源重化工基地,化工行业尤其是煤化工企业较多。PM2.5是运城市大气中的主要污染物,在汾渭平原处于比较高的水平。分析该市PM2.5中化学组分,确定其污染特征对科学治理雾霾,提高空气质量具有重要意义. 本研究采集运城市冬季大气PM2.5样品,分析化学组成和BrC光学性质的特征,获取了冬季污染阶段运城市大气PM2.5化学组成、吸光特性及其影响因素,以期为运城市有效控制PM2.5污染提供理论基础.
-
样品采集位于运城市技工学院教学楼顶(35°02'11″ N,110°96'86″E),距离地面20 m左右。站点位于运城市盐湖区,周边以交通源为主,为典型的工业城市站点. 采样时间为2019年10月15日至2020年3月1日(共64 d),每日10: 00 至次日09: 00. 采样器为四通道大气颗粒物采样仪( 武汉天虹,TH-16A) ,采样流量为 16. 67 L·min−1,采用石英滤膜和聚四氟乙烯膜.
-
分别用超纯水(电导率 18.2 MΩ cm)和甲醇(HPLC级)对PM2.5中的具有吸光作用的有机组分进行提取和测量,测量方法如下所示.
水提取:取1/2滤膜(5.965 cm2)在20 mL超纯水中超声萃取30 min,用孔径为0.45 μm的聚四氟乙烯亲水滤头进行过滤. 利用紫外可见分光光度计(UV-2550,岛津,日本)对提取液的吸光度进行测定. 采用低速扫描模式测定200—700 nm波长范围的水溶性有机碳(water-soluble organic carbon,WSOC)的吸收值,采样间隔为0.5 nm. 利用阴离子色谱仪(ICS-1100,戴安,美国)和阳离子色谱仪(DX-80,戴安,美国)对样品中水溶性无机离子组分(F-、Cl-、NO2-、NO3-、SO42-、Na+、NH4+、K+、Mg2+、Ca2+)进行分析和测定. 利用TOC分析仪(TOC-L CPN,岛津,日本)测定样品溶液中的WSOC. 利用热光碳分析仪(DRI Model 2001A,沙漠研究所,美国)测定EC、OC的浓度,Turpin与Huntzicker的研究[21]认为可通过(OC /EC) min估计二次有机碳(SOC,μg·m −3)和一次有机碳(POC,μg·m −3)的浓度.
甲醇提取:取1.124 cm2的滤膜在5 mL甲醇中超声萃取30 min并用疏水性滤头进行过滤,利用与WSOC同样的方法和步骤测定甲醇提取液( methanol extractable organic carbon,MEOC)的吸光度.
-
吸光系数Abs(Mm−1)是用来表征物质在大气浓度下吸光特性的参数,提取液中的吸光度(Aλ,w )可通过公式(1)转换为吸光系数数( Absλ,w,Mm−1 ) [22]:
式中,Aλ,w为不同波长下的吸光度,一般选取A700为作为基线漂移,Vl(mL)为定容体积(20 mL),Va(m3)为采样标况体积,L(m)为比色皿光程(0.01 m). 本研究中分别用Absλ,W和Absλ,M代表水提取液和甲醇提取液的吸光系数.
质量吸收效率(MAE,m2·g−1 )表征物质吸光组分吸收效率的参数,可通过公式(2)计算得到:
Ångström吸收指数AAE是用来表征BrC吸光作用对波长的依赖程度,计算公式见(3). 其中Aλ,w为不同波长下的吸光度值,K为常数,将吸光度值与波长进行指数拟合,即可得到WSOC的AAEW值.
本研究中分别用AAEW和AAEM代表水提取液和甲醇提取液的Ångström吸收指数. 本研究主要对300、330、365、400、450、500、550 nm下水和甲醇提取液的吸光特性进行研究,并且由于以往的研究表明365 nm时棕碳有显著的吸光性,并且在该波长下非棕碳类物质的吸光性几乎没有干扰,所以将365 nm作为代表吸收波长进行着重分析.
-
正式采集样品前,所有的滤膜在450 ℃马弗炉中灼烧4 h ,去除残留的有机物后,将两种滤膜放入恒温恒湿箱 ( SPX-250C)中平衡24 h,用十万分之一的电子天平进行称重。在采样结束后,同样条件下再次进行称重,最后放入冰箱保存,以便后续分析. 采样时所用的镊子和膜托等均用二氯甲烷∶甲醇 (V∶V=1∶1) 的溶剂抽提过的脱脂棉擦拭干净以防止引入污染, 减小实验误差.
水溶性无机离子的质量控制和质量保证:在其他实验条件不变的情况下,用空白膜做同样的分析处理,进行对比,并且以相对空白膜3倍的标准偏差作为样品的检出限,结果发现,文中检测的10种离子的检出限均小于0.005符合要求,同时所有离子的标准曲线的R2均达到0.99及以上,表明线性良好,最后采用基质的加标法进行回收率的验证,结果表明,所有水溶性离子的回收率均达到94%及以上,表明仪器稳定,准确性良好.
WSOC质量控制和质量保证:在正式进行样品分析之前,用邻苯二甲酸氢钾溶液进行测试,确保仪器的准确性,并在过程中扣除样品的空白值.
EC/OC的质量控制和质量保证:在正式进行样品分析之前,首先烘烤仪器30 min 确保仪器内部的残留物均已去除,而后放入空白样品,进行空白运行检测,空白值低于0.5 μg ·cm−2C后方可进行下一步,在前两步符合标准的前提下,采用CH4/CO2标准气体对仪器最后进行矫正,符合标准后,方可正式进行样品分析,分析结束后,再次采用CH4/CO2标准气体校准仪器,确保数据的准确性.
-
采样期站点PM2.5的平均浓度为(105±56.5) μg·m−3,浓度范围为6.21—325 μg·m−3,最大值出现在1月23日,浓度为325 μg·m−3,为国家二级标准的4.3倍. 按照环境空气质量标准(GB 3095—2012)规定的PM2.5的二级24 h平均浓度限值(75 μg·m−3)计算,采样期间约有54.3%的日均浓度值超过该标准,表明运城市秋冬季大气污染比较严重.
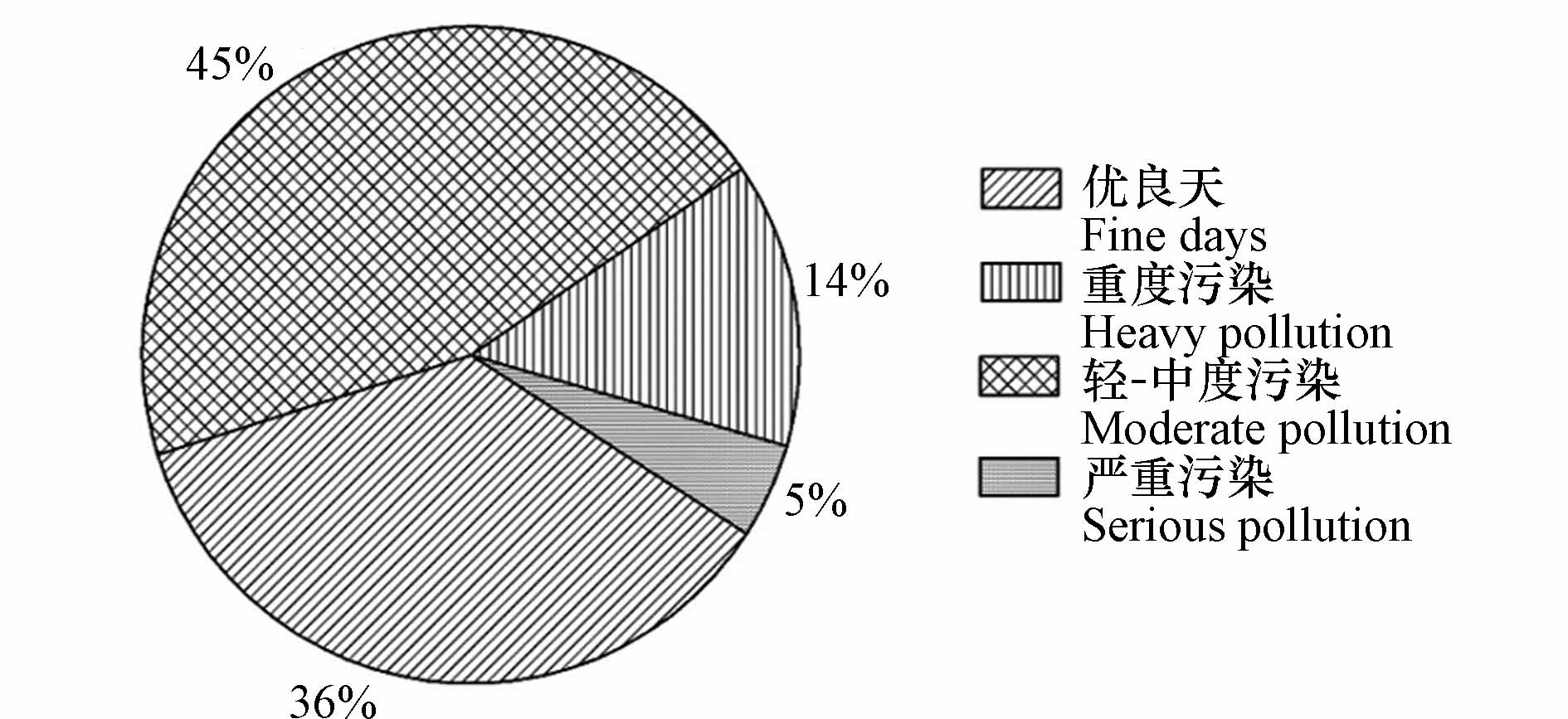
根据《环境空气质量指数(AQI)技术规定(试行)》(HJ 633—2012)规定将观测期间运城市大气污染状况进行分类(图1),可以看出采样期间运城市大气以优良天(AQI≤100)和轻-中度污染(100<AQI≤200)为主,分别为23 d和29 d,占总采样天数的36%和45%,重度污染(200<AQI≤300)和严重污染(AQI>300)分别有9 d和3 d,占总采样天数的14%和5%.
4个时期的PM2.5日均浓度分别为(67.6±25.7) μg·m−3(优良天)、(120±36.3) μg·m−3(轻-中度污染)、(159±55.7) μg·m−3(重度污染)、(193±112) μg·m−3(严重污染),其中,轻-中度污染、重度污染和严重污染的浓度是国家空气质量二级标准的1.6倍、2.12倍和2.57倍. 轻-中度污染期间 PM2.5质量浓度比优良天增加了77.5%,重度污染和严重污染期间PM2.5质量浓度比优良天分别增加了135%和186%. 从图2可见,重度-严重污染期间,运城市大气具有较高的相对湿度和较低的风速,利于污染物的二次转化和积累,导致PM2.5浓度的猛增。同时,由于部分采样时间与运城市供暖时间(2019年11月1日至2020年3月31日)相重合,因此大量的燃烧排放可能与两个时期PM2.5浓度激增有关.
-
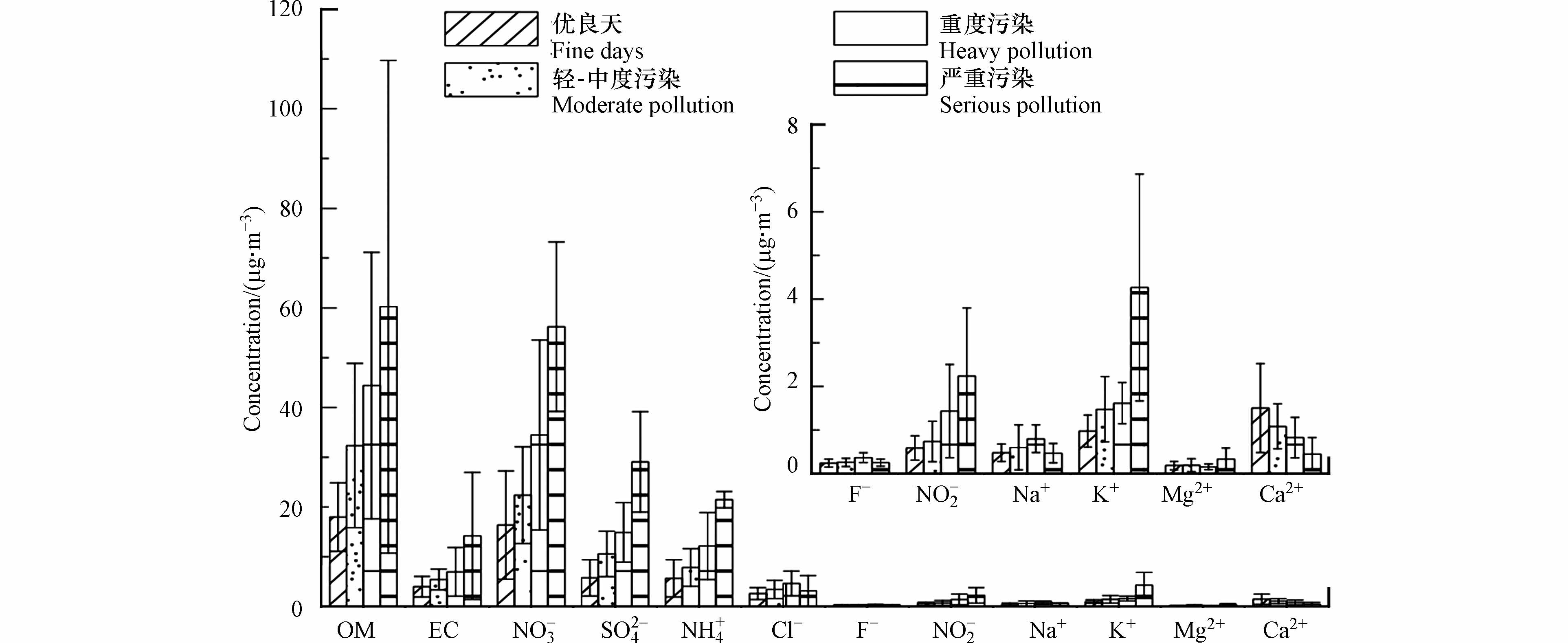
图3比较了不同污染条件下运城市PM2.5化学成分的差异,从中可以看出二次无机离子(NH4+、NO3-、SO42-)和有机质(OM=1.6×OC)[23]是PM2.5的主要成分,二者分别占PM2.5的29.0%和43.0%,总计共占PM2.5总浓度的72.0%,表明运城市冬季二次污染较为严重,水溶性无机离子为主要污染物. 从图3也可以看出,大部分成分随着污染的加剧,浓度逐渐增加,但是,来源于扬尘的Ca2+呈现降低的趋势,也表明扬尘不是造成运城市秋冬季PM2.5污染的因素.
通过计算不同污染时段各化学成分在PM2.5中的占比发现,二次无机离子的占比从39.6%(优良天)和38.9%(轻-中度污染),上升为40.4%(重度污染)和46.9%(严重污染),与此同时有机物的占比从29.7%(优良天)、30.8%(轻-中度污染)和29.1%(重度污染),下降到26.5%(严重污染),表明有机物的排放比较稳定.
在10种水溶性离子中,NO3 -、NH4 +和 SO 42-是最主要的成分,其中NO3-的浓度最大,在4个时段平均浓度逐渐增加,分别为(12.4±10.9)μg·m−3、(22.4±9.7)μg·m−3、(34.5±19.1)μg·m−3和(56.2±17.0)μg·m−3,说明运城地区硝酸盐污染较为严重. 之前的研究表明可以通过比较颗粒物中NO3-与SO42-质量浓度的方法来判断移动源(如机动车)和固定源(如燃煤)对大气中氮和硫的贡献量[24],若比值较高则说明SO2和NOx主要来源于机动车排放,反之则说明固定源的贡献量更大[25]. 本研究中,NO3-/ SO42-的比值在优良天、轻-中度污染、重度污染和严重污染的比值分别为2.15、2.11、2.31、1.93,均大于1,远远高于北京(1.05)[26],杭州市(0.63)[27]和沈阳市(0.98)[28]等其他城市,表明交通等流动源对运城市大气污染的影响更大. 轻-中度污染和重度-严重污染期间低风速高湿的气象条件有助于二次酸性气溶胶硫酸盐和硝酸盐的形成. 本研究中重度-严重污染期Cl-质量浓度和占比均最高,而KCl是植物秸秆的主要成分存在于生物质燃烧排放污染物中,表明生物质燃烧提高了重度-严重污染期间Cl-浓度变化. Ca2+、Mg2+离子是扬尘源的示踪物,本研究中Ca2+、Mg2+质量浓度在重度-严重污染期间最大,但占比最低,表明重度-严重污染期较低的风速利于扬尘源的积累,但扬尘源并不是重度-严重污染期间主要污染源.
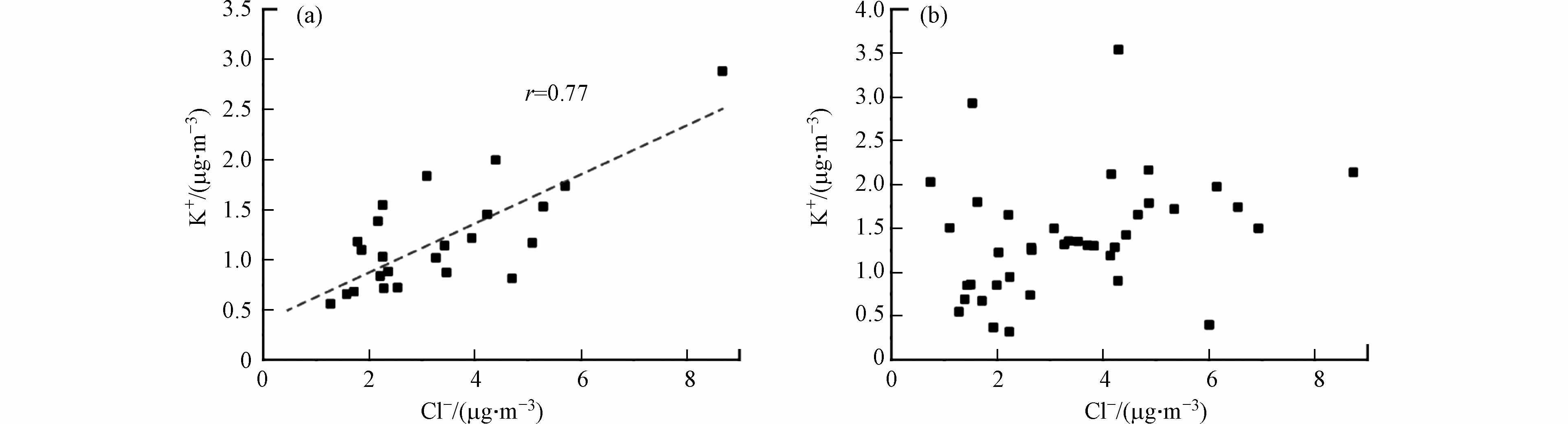
K+通常作为生物质燃烧源的指示离子[29],而大气中的Cl-主要来源于生物质和化石化石燃料燃烧. 相关性分析发现,在12月份之前的样品中,Cl-与K+具有较高的相关性[r= 0.77,见图4(a)],而随着温度降低,采暖期间燃煤源的增加,导致化石燃料排放增加,进而使样品中Cl-浓度增加,而Cl-与K+之间相关性消失(见图4(b)),表明与采暖前相比采暖期间运城市大气中PM2.5污染源发生变化,除了受生物质燃烧的影响外,燃煤排放的影响也逐渐增加.
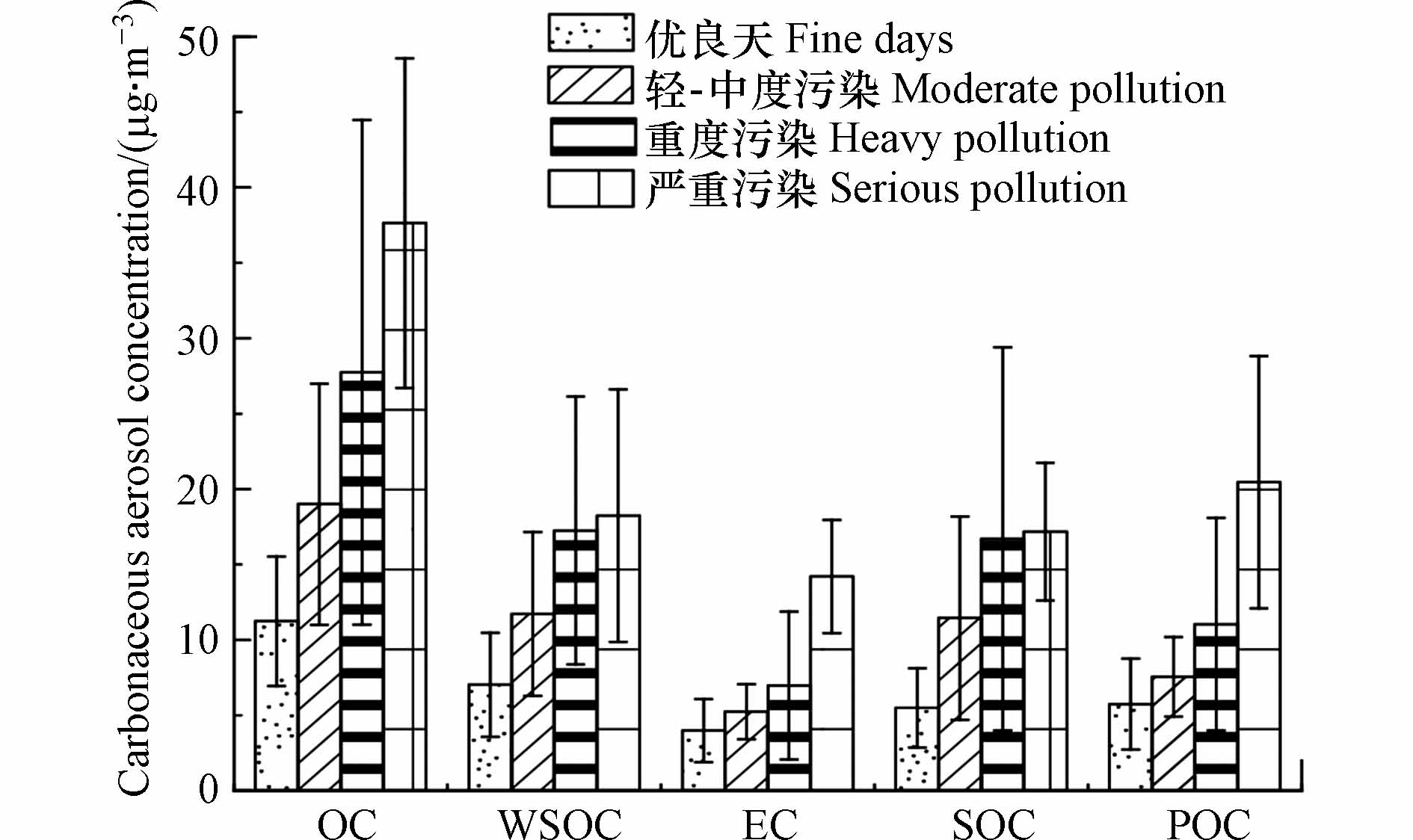
图5为不同污染状况下有机碳(OC)、元素碳(EC)、水溶性有机碳(WSOC)、二次有机碳(SOC)和一次有机碳(POC)的浓度特征,其中OC、WSOC和POC浓度波动较大,范围分别为6.14—59.5 μg·m−3,3.24—33.6 μg·m−3,2.05 —50.9 μg·m−3. 其中OC和EC在不同时期具有相似的趋势特征,随着污染的加剧,OC、EC和POC浓度依次升高,严重污染阶段,浓度分别为(37.1±15.8)μg·m−3、(14.21±5.84)μg·m−3和(20.45±9.36)μg·m−3,分别为优良天的3.35倍、3.56倍和3.56倍. WSOC和SOC有相似的变化趋势,在重污染和严重污染阶段变化不大,表明二者有相似的组成或来源.
-
吸光系数Abs(Mm−1)是用来表征物质在大气浓度下吸光特性的参数,Abs越小,则表明物质的吸光性越弱. 图6显示了甲醇提取(MSBrC)与水提取(WSBrC)Abs在300 nm至550 nm波长范围内的变化. 随着波长的降低,MSBrC和WSBrC的Abs值均逐渐增大,并且与可见光波段相比紫外波段的Abs值明显更高,表明波长越短即越靠近紫外区MSBrC和WSBrC的吸光性越强. 与WSBrC相比MSBrC在各个波长上均表现出更大的Abs值,其中Abs365,M与Abs365,W在优良天,轻-中度污染和重度-严重污染天的平均比值分别为2.0,2.6和2.8(表1),表明甲醇提取液中含有更多的吸光性棕碳物质,且随着污染的加剧,棕碳类物质的吸光性能逐渐增加,这与Chen等[17]在2010年和Liang等[30]在2020年观察到的结果类似. 由于甲醇与水的极性不同,所以在其他条件一定的情况下,两种溶剂所提取的物质的极性也不同,造成了吸光性的差异. 与水相比甲醇的极性更大,更可能提取出一些水无法提取出的强吸光性有机化合物.
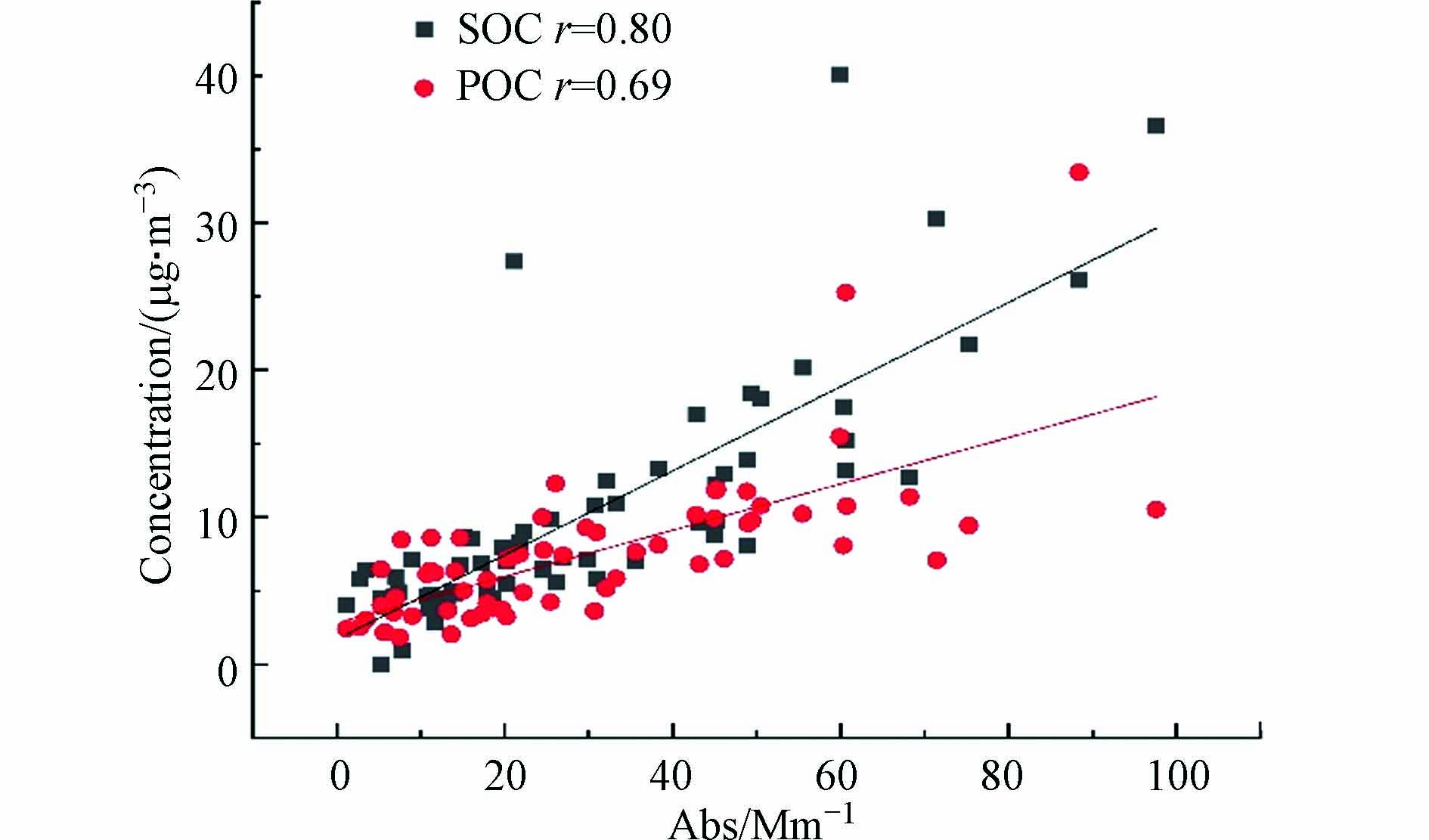
图7显示了本研究所有样品PM2.5中MSBrC的Abs365,M值与SOC、POC的相关性. 可以看出,采样期间Abs365,M与POC和SOC均具有较强的相关性,相关性系数分别为r=0.69和r=0.80,表明采样期间一次来源和二次来源均对棕碳吸光度有重要影响,这也与前文所述相吻合,进一步比较可发现与SOC相比POC的相关性稍弱,说明采样期间二次来源对棕碳吸光度的影响更大.
质量吸收效率MAE(m2·g−1)是判断物质吸光能力的参数,即MAE越大表明单位质量的吸光能力越强,MAE的大小与有机碳的来源、大小等因素有关与含量无关. 如表1所示,在365 nm下,不同时期甲醇提取棕碳的MAEM均大于水提取棕碳的MAEW,表明单位质量下甲醇提取的棕碳吸光能力更强.
本研究MAEM平均值为(1.57±0.61)m2·g−1与洛杉矶夏季的MSBrC的MAE值相近(1.56 m2·g−1),略高于2011年北京冬季((1.45±0.26) m2·g−1)和2014年冬季西安供暖期间MAE值((1.5±0.2) m2·g−1),说明以发展工业为主的运城市,冬季供暖时期产生的MSBrC的具有非常强的吸光能力.
Ångström吸收指数AAE是用来表征棕碳吸光特性与波长之间关系的指数,即反映了棕碳的吸光作用对波长的依赖性,AAE值越大,表明其对波长的依赖性越强即吸光作用对波长的变化更敏感. 如表1所示,重度-严重污染AAE值最高,其次是轻-中度污染和优良天,说明重度-严重污染期间BrC的吸光效应对波长的依赖性最强,优良天最弱,同时3个时期,AAEM均大于AAEW,从而可以看出相比于WSOC,MEOC的吸光作用对波长变化的依赖更明显.
已有研究表明,生物质燃烧产生的BrC的AAE值大于6[31 − 32];而经过二次转化形成的新鲜的二次有机气溶胶AAE值约为7[33]. 本研究AAEM的平均值分别为(7.26±1.10)和(4.64—9.86). 其中,大部分AAEM值在7—9之间. 重度-严重污染AAEM的平均值最高但变化幅度最小,平均值为(7.67±1.23)接近于7,变化范围为6.49—9.86,表明重度-严重污染有机碳来源相对单一,燃烧源可能是主要来源,与之相比轻-中度污染的AAEM变化幅度最大,变化范围为5.40—9.38,表明轻-中度污染期的有机碳吸光性对波长依赖性变化范围更大,表明其来源更多样.
-
(1) 运城冬季PM2.5的平均浓度为(105±56.5)μg·m−3,浓度范围为6.21—325 μg·m−3最大值为国家二级标准的4.3倍,污染天占总采样天数的64%,说明运城地区冬季污染严重.
(2) 运城冬季大气中二次无机离子和有机质是PM2.5的主要成分,二者之和占PM2.5总浓度的72%,其中NO3-的浓度的最大,并且随着污染程度的加深其浓度呈上升趋势,表明运城地区以硝酸盐污染严重,且机动车尾气是污染的主要来源. 不同污染等级下,随着污染程度加重OC、WSOC和POC浓度波动较大且浓度呈上升趋势,但在重度-严重污染期间WSOC和SOC变化趋势相似,表明二者有相似的来源或组成.
(3) 运城冬季的MSBrC和WSBrC在300 nm至550 nm范围内均有显著吸收,且MSBrC在各个波长上均表现出更大的Abs值,表明甲醇作为溶剂对BrC的提取更充分.
(4) Abs365,M值与SOC(r=0.80)、POC(r=0.69)的相关性分析表明,运城冬季整体的MSBrC主要来自二次形成,一次排放为次要来源. AAEM值大部分在7—9之间,表明燃烧源为大气PM2.5主要来源且二次污染现象严重.
运城冬季细颗粒物化学组成及棕碳吸光特性
Chemical composition and light absorption characteristics of brown carbon in Yuncheng in Winter
-
摘要: 为了更好地探究我国城市地区大气污染问题,2019年10月15—2020年3月1日在山西省运城市采用四通道大气颗粒物采样仪每23 h 进行1次细颗粒物(PM2.5)样品采集,分析了样品中有机碳(OC)、元素碳(EC)、水溶性有机碳(WSOC)、水溶性离子的浓度,并对比分析了甲醇提取液和水提取液的紫外-可见吸光特性. 结果显示,采样期间PM2.5质量浓度变化范围为6.21—325 μg·m−3,其中有41 d达到《环境空气质量指数(AQI)技术规定(试行)》(HJ 633—2012)规定轻度污染及以上的标准,占总天数的64%,说明运城市冬季污染严重. 其中,二次无机水溶性离子和有机质为PM2.5的主要组成成分,分别占PM2.5质量浓度的39.6%、29.7%(优良天),38.9%、30.8%(轻-中度污染),40.4%、29.1%(重度污染),38.9%、26.5%(严重污染). NO3−是含量最高的水溶性离子,并且4个时期NO3−/ SO42−的比值分别为2.15、2.11、2.31和1.93,说明机动车尾气排放的NOx是污染的主要来源. 对运城市水溶性棕碳 (WSBrC) 和甲醇溶性棕碳(MSBrC) 在365 nm下不同时期Abs、AAE、MAE进行分析,发现所有时期甲醇提取液的有机组分光吸收效率均高于水提取液. 对MSBrC与SOC和POC进行线性拟合,结果显示Abs365,M 与SOC (r= 0.80) 和POC (r=0.69)都具有较强相关性,表明其二次光化学反应为BrC主要来源.Abstract: In order to better explore the problem of air pollution in urban areas in China, a four-channel sampler was used to collect fine particulate matter (PM2.5) samples daily in Yuncheng City, Shanxi Province from October 15, 2019 to March 1, 2020. The concentrations of organic carbon (OC), elemental carbon (EC), water-soluble organic carbon (WSOC) and water-soluble ions in the samples were analyzed, and the light absorption of methanol extract and water extract were compared. The results showed that the concentration of PM2.5 varied from 6.21 μg·m−3 to 325 μg·m−3 during the sampling period. 41 d reached the standard of mild pollution or above stipulate in the Technical Provisions of Ambient Air Quality Index (AQI) (HJ 633—2012), accounting for 64% of the total days, indicating that air pollution in Yuncheng was very serious in winter. Secondary inorganic water-soluble ions and organic matter were the main components of PM2.5, accounting for 39.6% and 29.7% (fine days), 38.9% and 30.8% (moderate pollution), 40.4% and 29.1% (heavy pollution), 38.9% and 26.5% (serious pollution) of PM2.5 mass concentration, respectively. NO3− was the highest species, and the ratios of NO3−/ SO42− in the four periods were 2.15, 2.11, 2.31 and 1.93, respectively, indicating that NOx from motor vehicle exhaust was the main source of NO3−. The optical parameters, Abs, AAE and MAE of water-soluble brown carbon (WSBrC) and methanol extractable brown carbon (MSBrC) at 365 nm showed that the absorption efficiency of the unit extracted by methanol was higher than that of the water extract at all stages. The linear fit between MSBrC and SOC showed Abs365,M, SOC (r= 0.80) and POC (r=0.69), indicating that secondary photochemical reactions were the main source of BrC.
-
Key words:
- fine particle /
- chemical composition /
- brown carbon /
- light absorption properties /
- source.
-

-
表 1 光学参数
Table 1. Optical parameters
优良天
Fine days轻-中度污染
Moderate pollution重度-严重污染
Heavy-serious pollutionAV±SD Range AV±SD Range AV±SD Range Abs365,W/(Mm−1) 8.46±3.94 1.78—17.62 13.76±4.63 5.04—27.62 16.89±7.70 6.31—30.25 Abs365,M/(Mm−1) 17.61±11.70 1.18—46.17 34.73±19.40 7.03—71.48 46.92±27.22 9.04—97.61 MAEw/(m2·g−1) 1.27±0.60 0.37—1.66 1.18±0.40 0.5—2.10 0.97±0.24 0.62—1.40 MAEM/(m2·g−1) 1.40±0.65 0.19—2.44 1.77±0.60 0.61—2.84 1.60±0.38 0.87—2.13 AAEW 4.65±1.05 2.89—6.15 5.72±1.44 3.84—9.64 6.10±1.32 4.44—8.28 AAEM 6.75±1.03 4.64—8.79 7.46±1.11 5.40—9.38 7.67±1.23 6.49—9.86 -
[1] LI X R, JIANG L, BAI Y, et al. Wintertime aerosol chemistry in Beijing during haze period: Significant contribution from secondary formation and biomass burning emission[J]. Atmospheric Research, 2019, 218: 25-33. doi: 10.1016/j.atmosres.2018.10.010 [2] GUO S, HU M, GUO Q F, et al. Primary sources and secondary formation of organic aerosols in Beijing, China[J]. Environmental Science & Technology, 2012, 46(18): 9846-9853. [3] PONGPIACHAN S, CHOOCHUAY C, CHALACHOL J, et al. Chemical characterisation of organic functional group compositions in PM2.5 collected at nine administrative provinces in northern Thailand during the Haze Episode in 2013[J]. Asian Pacific Journal of Cancer Prevention, 2013, 14(6): 3653-3661. doi: 10.7314/APJCP.2013.14.6.3653 [4] JI D S, ZHANG J K, HE J, et al. Characteristics of atmospheric organic and elemental carbon aerosols in urban Beijing, China[J]. Atmospheric Environment, 2016, 125: 293-306. doi: 10.1016/j.atmosenv.2015.11.020 [5] WANG Q Q, HUANG X H H, ZHANG T, et al. Organic tracer-based source analysis of PM2.5 organic and elemental carbon: A case study at Dongguan in the Pearl River Delta, China[J]. Atmospheric Environment, 2015, 118: 164-175. doi: 10.1016/j.atmosenv.2015.07.033 [6] LASKIN A, LASKIN J, NIZKORODOV S A. Chemistry of atmospheric brown carbon[J]. Chemical Reviews, 2015, 115(10): 4335-4382. doi: 10.1021/cr5006167 [7] ZHANG Q, SHEN Z X, ZHANG T, et al. Spatial distribution and sources of winter black carbon and brown carbon in six Chinese megacities[J]. Science of the Total Environment, 2021, 762: 143075. doi: 10.1016/j.scitotenv.2020.143075 [8] LIN G X, PENNER J E, FLANNER M G, et al. Radiative forcing of organic aerosol in the atmosphere and on snow: Effects of SOA and brown carbon[J]. Journal of Geophysical Research: Atmospheres, 2014, 119(12): 7453-7476. doi: 10.1002/2013JD021186 [9] IPCC. Climate Change 2013: The Physical Science Basis. Contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change[R]. Cambridge: Cambridge University Press, 2013. [10] LIN P, BLUVSHTEIN N, RUDICH Y, et al. Molecular chemistry of atmospheric brown carbon inferred from a nationwide biomass burning event[J]. Environmental Science & Technology, 2017, 51(20): 11561-11570. [11] WASHENFELDER R A, AZZARELLO L, BALL K, et al. Complexity in the evolution, composition, and spectroscopy of brown carbon in aircraft measurements of wildfire plumes[J]. Geophysical Research Letters, 2022, 49(9): e2022GL098951. [12] YAN J P, WANG X P, GONG P, et al. Review of brown carbon aerosols: Recent progress and perspectives[J]. Science of the Total Environment, 2018, 634: 1475-1485. doi: 10.1016/j.scitotenv.2018.04.083 [13] ANDREAE M O, GELENCSÉR A. Black carbon or brown carbon?The nature of light-absorbing carbonaceous aerosols[J]. Atmospheric Chemistry and Physics, 2006, 6(10): 3131-3148. doi: 10.5194/acp-6-3131-2006 [14] HOFFER A, GELENCSÉR A, GUYON P, et al. Optical properties of humic-like substances (HULIS) in biomass-burning aerosols[J]. Atmospheric Chemistry and Physics, 2006, 6(11): 3563-3570. doi: 10.5194/acp-6-3563-2006 [15] LIN Y H, BUDISULISTIORINI S H, CHU K, et al. Light-absorbing oligomer formation in secondary organic aerosol from reactive uptake of isoprene epoxydiols[J]. Environmental Science & Technology, 2014, 48(20): 12012-12021. [16] 赵美玲, 黄汝锦, 杨露. 甲醇和有机混合溶剂提取棕碳气溶胶光学性质的差异及原因探讨[J]. 地球环境学报, 2021, 12(6): 666-676. doi: 10.7515/JEE212017 ZHAO M L, HUANG R J, YANG L. Effects of extraction solvents on the optical properties of brown carbon aerosols[J]. Journal of Earth Environment, 2021, 12(6): 666-676(in Chinese). doi: 10.7515/JEE212017
[17] CHEN Y, BOND T C. Light absorption by organic carbon from wood combustion[J]. Atmospheric Chemistry and Physics, 2010, 10(4): 1773-1787. doi: 10.5194/acp-10-1773-2010 [18] 徐伟召, 朱雯斐, 王甜甜, 等. 冬季德州市大气颗粒物消光与化学组成关系研究[J]. 环境科学学报, 2019, 39(4): 1057-1065. doi: 10.13671/j.hjkxxb.2019.0051 XU W Z, ZHU W F, WANG T T, et al. Relationship between the aerosol light extinction and chemical composition in winter of Dezhou City[J]. Acta Scientiae Circumstantiae, 2019, 39(4): 1057-1065(in Chinese). doi: 10.13671/j.hjkxxb.2019.0051
[19] 付晓辛. 珠江三角洲地区PM2.5浓度组成变化及其对粒子酸度和消光的影响[D]. 广州: 中国科学院研究生院(广州地球化学研究所) 2015. FU X X. Influence of PM2.5 major components on aerosol acidity and light extinction in the Pearl River Delta region[D]. Guangzhou: Chinese Academy of Sciences (Guangzhou Institute of Geochemistry) 2015(in Chinese).
[20] WANG Y H, LIU Z R, ZHANG J K, et al. Aerosol physicochemical properties and implications for visibility during an intense haze episode during winter in Beijing[J]. Atmospheric Chemistry and Physics, 2015, 15(6): 3205-3215. doi: 10.5194/acp-15-3205-2015 [21] TURPIN B J, HUNTZICKER J J. Identification of secondary organic aerosol episodes and quantitation of primary and secondary organic aerosol concentrations during SCAQS[J]. Atmospheric Environment, 1995, 29(23): 3527-3544. doi: 10.1016/1352-2310(94)00276-Q [22] XIE M J, CHEN X, HAYS M D, et al. Light absorption of secondary organic aerosol: Composition and contribution of nitroaromatic compounds[J]. Environmental Science & Technology, 2017, 51(20): 11607-11616. [23] VECCHI R, CHIARI M, D’ALESSANDRO A, et al. A mass closure and PMF source apportionment study on the sub-micron sized aerosol fraction at urban sites in Italy[J]. Atmospheric Environment, 2008, 42(9): 2240-2253. doi: 10.1016/j.atmosenv.2007.11.039 [24] WANG Y, ZHUANG G S, ZHANG X Y, et al. The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai[J]. Atmospheric Environment, 2006, 40(16): 2935-2952. doi: 10.1016/j.atmosenv.2005.12.051 [25] 狄一安, 杨勇杰, 周瑞, 等. 北京春季城区与远郊区不同大气粒径颗粒物中水溶性离子的分布特征[J]. 环境化学, 2013, 32(9): 1604-1610. doi: 10.7524/j.issn.0254-6108.2013.09.002 DI Y A, YANG Y J, ZHOU R, et al. Size distributions of water-soluble inorganic ions at urban and rural sites in Beijing during spring[J]. Environmental Chemistry, 2013, 32(9): 1604-1610(in Chinese). doi: 10.7524/j.issn.0254-6108.2013.09.002
[26] 刘保献, 张大伟, 陈添, 等. 北京市PM2.5主要化学组分浓度水平研究与特征分析[J]. 环境科学学报, 2015, 35(12): 4053-4060. LIU B X, ZHANG D W, CHEN T, et al. Characteristics and major chemical compositions of PM2.5 in Beijing[J]. Acta Scientiae Circumstantiae, 2015, 35(12): 4053-4060(in Chinese).
[27] 吴丹, 蔺少龙, 杨焕强, 等. 杭州市PM2.5中水溶性离子的污染特征及其消光贡献[J]. 环境科学, 2017, 38(7): 2656-2666. WU D, LIN S L, YANG H Q, et al. Pollution characteristics and light extinction contribution of water-soluble ions of PM2.5 in Hangzhou[J]. Environmental Science, 2017, 38(7): 2656-2666(in Chinese).
[28] 张显, 田莎莎, 刘盈盈, 等. 沈阳市采暖期与非采暖期空气PM2.5污染特征及来源分析[J]. 环境科学, 2019, 40(3): 1062-1070. ZHANG X, TIAN S S, LIU Y Y, et al. Pollution characteristics and source apportionment of PM2.5 in heating and non-heating periods in Shenyang[J]. Environmental Science, 2019, 40(3): 1062-1070(in Chinese).
[29] ANDREAE M O. Soot carbon and excess fine potassium: Long-range transport of combustion-derived aerosols[J]. Science, 1983, 220(4602): 1148-1151. doi: 10.1126/science.220.4602.1148 [30] LIANG Y H, WANG X F, DONG S W, et al. Size distributions of nitrated phenols in winter at a coastal site in North China and the impacts from primary sources and secondary formation[J]. Chemosphere, 2020, 250: 126256. doi: 10.1016/j.chemosphere.2020.126256 [31] PARK R J, KIM M J, JEONG J I, et al. A contribution of brown carbon aerosol to the aerosol light absorption and its radiative forcing in East Asia[J]. Atmospheric Environment, 2010, 44(11): 1414-1421. doi: 10.1016/j.atmosenv.2010.01.042 [32] HECOBIAN A, ZHANG X, ZHENG M, et al. Water-Soluble Organic Aerosol material and the light-absorption characteristics of aqueous extracts measured over the Southeastern United States[J]. Atmospheric Chemistry and Physics, 2010, 10(13): 5965-5977. doi: 10.5194/acp-10-5965-2010 [33] BONES D L, HENRICKSEN D K, MANG S A, et al. Appearance of strong absorbers and fluorophores in limonene-O3 secondary organic aerosol due to NH4+-mediated chemical aging over long time scales[J]. Journal of Geophysical Research: Atmospheres, 2010, 115(D5): D05203. -



 下载:
下载: