-
氧化水处理是水处理中的一种通用处理单元,可用于消毒、污染物降解和改善其他处理方案[1]. 消毒是水处理过程中不可或缺的一个单元. 目前,在实际水处理过程中,应用较多的消毒剂为次氯酸钠、二氧化氯、液氯、氯胺等,大多为含氯消毒剂. 不同种类的含氯消毒剂所生成的氯代消毒副产物(CDBPs)也存在差异[2 − 5],其中次氯酸钠是我国常用的饮用水消毒剂之一[6].
溶解有机物(DOM)是一种复杂的异质化学结构混合物. 它普遍存在于地表水源中[7 − 11]. DOM主要包括腐殖质、蛋白质和其他芳香族或脂肪族有机化合物的复杂混合物[12],其中DOM的富电子部分(如酚类化合物、烯烃和有机胺),被公认为是饮用水和废水处理中通过多种途径消耗化学氧化剂的主要消耗者[13 − 15]. 例如,Sharma等 [13]对Fe(Ⅵ) 和Fe(Ⅴ) 氧化多种有机化合物(胺类、酚类、醇类和烃类等)的动力学和机理进行了评估,结果表明Fe(Ⅵ) 通常通过电子转移来完成对有机化合物的氧化. 其电子转移步骤为FeⅥ →FeⅤ→FeⅢ和FeⅥ→FeⅣ→FeⅡ.
大部分消毒副产物(DBPs)有潜在的致癌、致畸、致突变的“三致”毒性[16],其中致癌性较强的是卤乙酸(HAAs)、三卤甲烷(THMs)和溴酸盐,致畸和致突变性较强的是卤代乙腈(HANs)、卤化硝基甲烷(HNMs)和卤化氰[17]. 由于潜在的健康风险和饮用水中DBPs的广泛存在,许多国家和组织对饮用水中的DBPs进行了管理,以减轻包括THMs、HAAs在内的多种DBPs造成的健康风险. 2006年,中国颁布了《中国饮用水水质标准》(GB/T5749-2006), 2012年开始实施,共规范了106种污染物,其中DBPs 14种. 从管制的DBP化合物的数量来看,中国饮用水中的DBPs规定比美国更为严格. 地表水作为饮用水供给链的前端,是保证居民用水安全的重要一环. 因此,有必要探究地表水经过不同的氧化消毒工艺之后其DBPs的生成水平.
DBPs的形成一般是通过混凝、絮凝、沉淀和过滤等物理处理单元去除DOM前体来缓解的[18]. 在一些公用事业中,化学氧化剂的预氧化也被用于提高混凝效率以及去除新出现的令人担忧的污染物[19 − 20]. 大多数氧化剂可以选择性地与DOM中的富电子部分(如酚类化合物、烯烃和有机胺)反应[13 − 15],并可改变天然有机物(NOM)的物理/化学性质,甚至使其矿化. 其中,我国使用最广泛的是高锰酸盐(Mn(Ⅶ),酸性条件下氧化还原电位为1.51 V,碱性条件下氧化还原电位为0.56 V )[21],Mn(Ⅶ)可以与水中DOM发生直接和间接的作用,以有效提高对地表水中DOM的去除;高铁酸钾(Fe(Ⅵ),酸性条件下氧化还原电位为2.20 V,碱性条件下氧化还原电位为0.72 V )预氧化是近几年国内外研究热点[13,22],由于Fe(Ⅵ)中六价铁的存在,使得Fe(Ⅵ)具有较强的氧化性,在使用Fe(Ⅵ)预氧化时,不会引入任何有毒有害物质[21].
本研究以浙江东部某水源水(下文简称水源水)为研究对象,旨在从水源水的回用角度出发,探究Mn(Ⅶ)与Fe(Ⅵ)预氧化对水源水中DOM的转化及氯化后对DBPs生成势的影响情况. 主要研究内容包括:(1)不同氧化剂(Fe(Ⅵ)与Mn(Ⅶ))对DOM的荧光转化;(2)Mn(Ⅶ)与Fe(Ⅵ)预氧化对氯化后DBPs生成势的变化,为该水源水的水环境健康构建提供理论依据.
-
本研究采集水源水5 L,分5瓶装入容量为1 L的塑料瓶中,运送入冷库保存,7日内进行实验操作和样品分析. 高锰酸钾(KMnO4)、次氯酸钠(NaClO)、抗坏血酸(Ascorbic acid)均为分析纯,购自国药集团化学试剂有限公司;高铁酸钾(K2FeO4)购自北京伊诺凯科技有限公司;1,2-二溴丙烷及各种DBPs标准品为色谱纯,购自阿法埃莎(中国)化学有限公司;甲基叔丁基醚(MTBE)为色谱纯,购自赛默飞世尔科技(中国)有限公司.
-
氧化消毒实验:分别取50 mL水样于50 mL离心管中,加入标定后的不同浓度梯度的氧化剂(其中Mn(Ⅶ)浓度梯度为:0、1、2、5、10、20 mg·L−1;Fe(Ⅵ)浓度梯度为:0、1.25、2.5、6.25、12.5、25 mg·L−1)氧化30 min后,加入标定后游离氯的浓度为10 mg·L−1的次氯酸钠溶液消毒24 h后用抗坏血酸进行猝灭,反应在常温下进行,避光. 游离氯(FC)使用哈希试剂包在DR 6000分光光度计进行标定.
三维荧光光谱的测定:取10 mL水样经孔径为0.45 μm的有机滤膜过滤于10 mL一次性离心管中,装入润洗过的1 cm 四面通光石英比色皿. 采用美国Cary Eclipse型荧光分光光度计,在设置光电倍增电压为700 V的情况下,将激发和发射狭缝宽度均设置为5 nm,以24000 nm·min−1 的扫描速度进行三维荧光光谱扫描,扫描光谱进行仪器自动校正. 同时,三维荧光光谱扫描范围设置为激发波长(Ex)为200—420 nm,扫描间隔5 nm;发射波长(Em)280—550 nm,扫描间隔5 nm. 后续三维荧光光谱分析需将水样三维荧光光谱扣除超纯水的三维荧光光谱[23],以消除拉曼散射的影响. 应用含有drEEM工具包的MATLAB软件进行三维荧光数据分析.
DBPs的测定:取20 mL水样于40 mL萃取瓶中,加入6 g无水硫酸钠,再加入2 mL含有200 μg·L−1 1,2-二溴丙烷内标物的MTBE萃取液,后将萃取瓶置于涡旋振荡器,以2500 r·min−1的转速震荡10 min,结束后静置5 min左右,待有机相与水层分界明显时,将上层液体移取约1 mL经孔径为0.45 μm的有机滤膜过滤于2 mL棕色进样瓶中,确保瓶中无分层,通过气相色谱电子捕获检测器检测样品中DBPs的浓度. 仪器条件设置为进样量1 μL,进样口温度200 ℃,氮气流速2.4318 mL·min−1,分流比为7:1,升温程序:35 ℃保持15 min,以25 ℃·min−1的速度上升至145 ℃,保持3 min,再以35 ℃·min−1的速度上升至240 ℃,保持5 min,检测器温度300 ℃,运行时间2 min.
总有机碳(TOC)的测定:取40 mL水样过0.45 μm的有机滤膜过滤于TOC进样瓶中,采用TOC-VCPH分析仪 (Shimadzu, Japan)进行测定.
-
平行因子分析(PARAFAC)基于最小方差理论,PARAFAC分析将由样本数、激发光谱和发射光谱组成的三维荧光光谱数据集X(I×J×K)分解成3个载荷矩阵,即激发光谱矩阵A(J×N)、发射光谱矩阵B(K×N)和相对浓度矩阵C(I×N),以及一个残差矩阵E(I×J×K)来反映各样品原三维荧光光谱,具体公式见式1. 其中,Xijk、ain、bjn、ckn和eijk分别代表三维矩阵、激发光谱矩阵、发射光谱矩阵、相对浓度矩阵和残差矩阵中元素;N表示独立的荧光组分数. 其中,PARAFAC分析出Fmax值可表示组分含量的相对多少[24 − 26].
区域荧光积分分析(FRI)通过固定激发波长和发射波长范围划分三维荧光光谱[27],将不同范围内荧光强度积分用于量化分析DOM组成和含量变化,具体公式见式2.
Qi代表第i个组分区域荧光强度积分值,l(λExλEm)代表相应波长处荧光强度,ΔλEx和ΔλEm分别代表激发波长和发射波长的间隔.
为了评估各DBPs所产生的合成毒性变化,采用了细胞毒性指数(CTI)进行计算[28 − 29].
LC50 值是根据回归分析计算的 DBP s浓度,其诱导的细胞密度为阴性对照的 50%,CX表示每一种DBPs的浓度.
-
运用Matlab R2018a软件中的drEEM工具箱处理三维荧光数据并进行区域积分法和平行因子分析. Origin 2021用于数据分析和绘图. 荧光特征参数荧光指数(FI)、自生源指标 (BIX)、腐殖化指数(HIX)的计算如表1 所示.
-
水源水和不同种类的氧化剂氧化后的10个水样中 DOM 的三维荧光特征光谱图和各区域 DOM 标准荧光积分体积如图1、图2所示. 可以看出氧化后的水样 DOM 的荧光光谱特性(图1)和荧光区域标准化积分体积具有明显的变化(图2).
其中水源水水样中DOM 的类蛋白质组分(区域Ⅰ,区域Ⅱ)和富里酸类(区域Ⅲ)的荧光积分体积相对较高,积分比例达到了12.22%、32.32%和36.66%,而氧化后的10个水样中DOM的区域Ⅰ、区域Ⅱ和区域Ⅲ的荧光积分体积几乎被完全降解. 区域Ⅳ多为水体中水生植物及浮游微生物代谢等活动产生,一定程度上可以表征水体生物(微生物、藻类等)的代谢,Mn(Ⅶ)氧化后该区域的平均荧光积分体积降低了72.60%,总荧光积分体积降低了63.78%,而Fe(Ⅵ)氧化后该区域的平均荧光积分体积降低了76.74%,总荧光积分体积降低了68.55%,且随着Fe(Ⅵ)浓度的升高,腐殖质类(区域Ⅴ)的荧光积分体积呈现出下降的趋势,表明Fe(Ⅵ)作为氧化剂氧化降解水中的DOM时,其氧化效果要强于Mn(Ⅶ),且二者对DOM均有较好的降解效果.
-
11个水样中 DOM 的 FI、BIX、HIX指标如图3所示,对于水源水水样其FI、BIX指标分别为2.23和0.78,表明水样DOM的来源主要为陆源与内源混合,从各指标数值大小可以判断该流域水体自生源特征较明显,这主要是由于该流域水体生态环境相对稳定,DOM受水体微生物活动因素影响较明显;另外HIX指标为3.94,表明水样有一定的腐殖质化程度但相对较弱. 其中Mn(Ⅶ)氧化后水样FI指标的范围位于1.54—2.31(均值为1.96),表明氧化后虽对FI有所降低,但水样仍具有一定的自生源特性;BIX的范围是0.70—0.93(均值为0.80),相较于氧化前水样,平均陆源输入特性有所降低,但个别浓度下陆源输入的特性明显增强,表明Mn(Ⅶ)对水体的陆源DOM降解效果不明显;HIX的范围是1.00—1.89(均值为1.37),其平均降解率为65.23%,表明Mn(Ⅶ)对腐殖质类DOM具有较好的降解效果. 另外Fe(Ⅵ)氧化后水体FI指标的范围位于1.87—2.47(均值为2.09),这一结果跟Mn(Ⅶ)氧化后的结果类似;BIX的范围是0.70—0.83(均值为0.73),相较于氧化前水样,陆源输入特性反而有所增强;HIX的范围是1.15—2.43(均值为1.32),其平均降解率为66.50%,这一结果与Mn(Ⅶ)预氧化相比无明显变化,表明Fe(Ⅵ)对腐殖质类DOM也具有较好的降解效果.
-
已有的研究将DOM划分为6类[10,35],分别为类富里酸、类腐殖酸、低激发色氨酸类、高激发色氨酸类、低激发酪氨酸类、高激发酪氨酸类,这些DOM广泛存在于河流和湖泊水体中. 基于PARAFAC模型对永宁江江口地表水以及Mn(Ⅶ)和Fe(Ⅵ)分别氧化后的水样DOM的三维荧光图谱进行分析,得到3种荧光组分. 其中,C1(Ex/Em=345,450 nm)(图4a)类陆生腐殖质成分,C2(Ex/Em=225,425 nm)(图4c)类富里酸,C3(Ex/Em=320,400 nm)(图4e)海洋腐殖酸. 组分C1为类陆生腐殖质,该荧光组分具有潜在的陆源特征,如来自土壤、森林溪流和湿地的溶解有机质输入来源[36]. 组分C2为类富里酸物质,类富里酸物质属于芳香氨基酸腐殖物质,分子量较大,反映来自外源输入的腐殖酸和富里酸所形成的荧光峰,与腐殖质中羟基及羧基有关[37 − 38]. 组分C3类似于紫外区陆地类腐殖酸荧光峰A(<250 nm,290—325 nm/370—430 nm)[39],其特点是分子量低,常见于与生物活动相关的海洋环境,在废水、湿地和农业环境中也有发现[40];与刘笑菡等[41]研究中UVC类腐殖质荧光组分(<250 nm,305 nm/412—420 nm)相似,这些相似的荧光组分被证实具有外源指示意义.
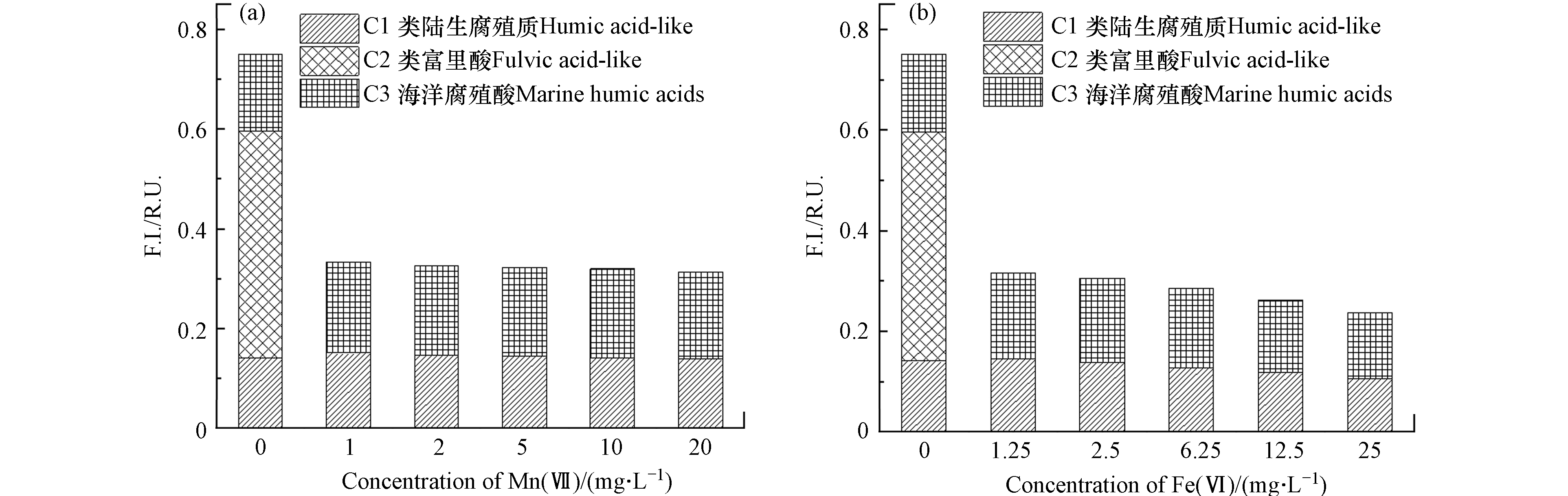
解析出荧光组分类陆生腐殖质C1组分、类富里酸C2组分、海洋腐殖酸C3组分后,根据荧光组分最大荧光强度(Fmax)绘制出不同浓度Mn(Ⅶ)和Fe(Ⅵ)氧化后各组分荧光强度变化图(图5),对于水源水水样的C1、C2和C3荧光强度分别为0.14、0.45、0.15 R.U.,另外可以明显看出,在两种不同的氧化剂作用下C2组分即类富里酸均被完全降解, 降解率达到100%;其中Mn(Ⅶ)氧化后C1组分的荧光强度无明显变化,而C3组分的荧光强度反而有些微增强(图5a),相较于氧化前水样平均荧光强度升高了15.59%. 而Fe(Ⅵ)氧化后,C1组分的荧光强度有所降低(图5b),平均降解率为9.72%,降解效果不明显;对于C3组分,其在低浓度Fe(Ⅵ)作用下荧光强度有所升高,而随着Fe(Ⅵ)浓度的进一步增加,荧光强度呈现出下降的趋势. 对于C3组分在两种氧化剂的作用下其荧光强度有轻微升高的现象可能是由于水样在氧化剂的作用下,部分大分子类富里酸DOM被氧化为陆源DOM,进而造成C3组分荧光强度“不减反增”的现象,C3组分这一结果跟“2.1.2”中BIX的变化情况是一致的.
-
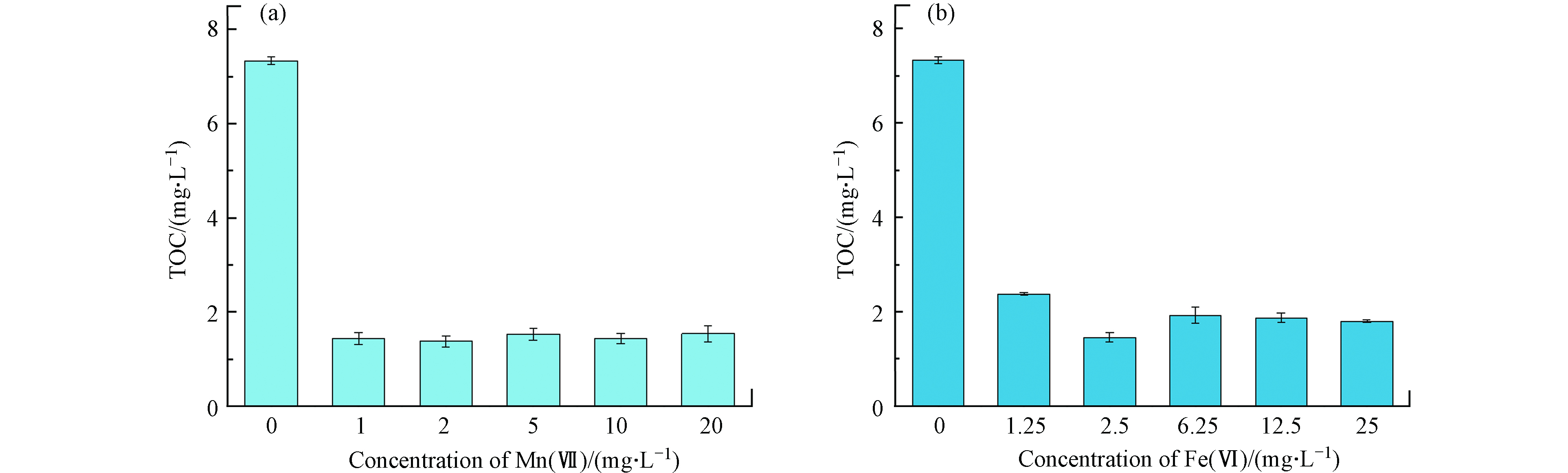
不同浓度Mn(Ⅶ)和Fe(Ⅵ)预氧化对水样TOC的变化情况如图6所示. 图6a表明,随着氧化剂浓度的升高,Mn(Ⅶ)预氧化在初始氧化剂浓度为1.0 mg·L−1下TOC的浓度明显下降,降解率为80.34%,之后趋于稳定,表明Mn(Ⅶ)预氧化对DOM的官能团的破坏效果比较显著. 图6b表明,Fe(Ⅵ)预氧化虽然在初始浓度为1.25 mg·L−1下对TOC的降解效果不如Mn(Ⅶ)预氧化显著,但其在浓度为2.5 mg·L−1时对TOC的降解率仍达到了80.11%,且随着氧化剂浓度的进一步升高,水样TOC的浓度呈现出上升的现象并趋于稳定,可能是由于Fe(Ⅵ)预氧化除了对DOM的官能团的破坏效果显著之外,还具有对非可溶性的有机物具有氧化降解的作用.
-
在对水样进行Mn(Ⅶ)和Fe(Ⅵ)两种氧化剂的预氧化并氯化后,主要检出了三氯甲烷(TCM)、二氯一溴甲烷(DCBM)、二溴一氯甲烷(DBCM)、三氯丙酮(1,1,1-TCP)和三溴甲烷(TBM)等5种C-DBPs;另外也检测出了二氯乙腈(DCAN)、溴氯乙腈(BCAN)和二溴乙腈(DBAN)等3种N-DBPs. 图7和图8中数据为两组平行样的均值,从11个水样的数据中可以看出含碳消毒副产物(C-DBPs)的总生成量均高于含氮消毒副产物(N-DBPs)的总生成量,且C-DBPs中TBM的含量较低,平均占比为0.41%;而N-DBPs中DBAN的含量也比较低,平均占比为3.85%.
-
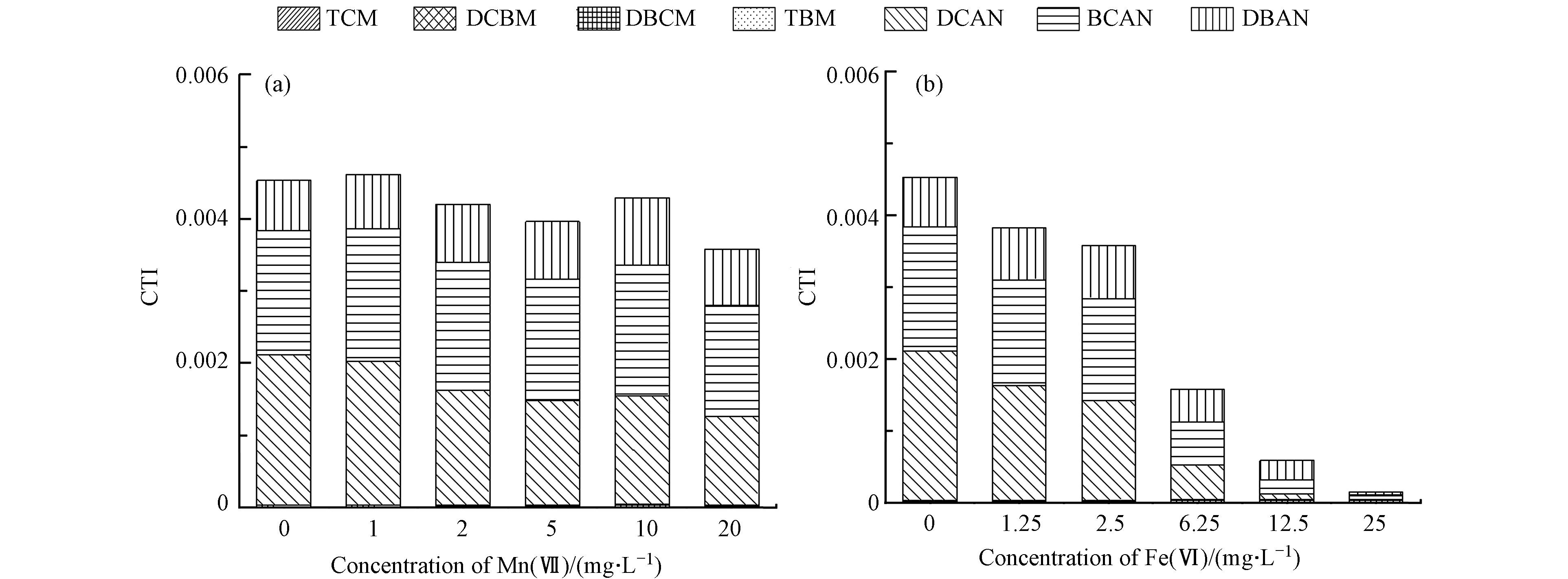
图7a表明,当不同浓度的Mn(Ⅶ)对水样进行预氧化并氯化后,起主要变化的C-DBPs为TCM,另外4种C-DBPs(DCBM、DBCM、1,1,1-TCP和TBM)无明显变化. 对于溴代THMs,可能是由于其前驱物的稳定性较强,Mn(Ⅶ)预氧化不足以将其官能团进行有效破坏. 随着Mn(Ⅶ)浓度的升高,TCM的浓度呈现出先升高再降低的变化趋势,可能是由于随着Mn(Ⅶ)浓度的升高,水中一部分DOM的官能团被氧化为TCM前驱物的官能团,进而在游离氯的作用下被转化为TCM,因此TCM呈现出浓度升高的现象,而随着Mn(Ⅶ)浓度的进一步升高(达到20 mg·L−1时),小分子状态的DOM被氧化降解,最终使得TCM呈现出急剧下降的现象,降低率为33.60%,表明高浓度的Mn(Ⅶ)对TCM的前驱物具有一定的降解作用. 图7b表明, 当不同浓度的Fe(Ⅵ)对水样进行预氧化并氯化后,5种C-DBPs均有不同程度和规律的变化;随着Fe(Ⅵ)浓度的升高,1,1,1-TCP呈现出逐渐下降的趋势,最终降解率达到了99.46%,表明Fe(Ⅵ)作为氧化剂时,对1,1,1-TCP的前驱物具有较好的降解作用;而另外3种C-DBPs(DCBM、DBCM和TBM)均呈现出先升高后降低的趋势,当Fe(Ⅵ)浓度为25 mg·L−1时,DCBM、DBCM和TBM的降解率分别为22.34%、35.28%和1.38%,这一结果跟不同浓度的Mn(Ⅶ)先氧化再氯化后TCM的变化情况有类似之处;而TCM的浓度则呈现出只增不减的现象,可能是由于部分DOM的官能团在Fe(Ⅵ)的预氧化作用下被转化为TCM的前驱物. 而从Fe(Ⅵ)预氧化下TOC的浓度变化规律考虑,可能是由于部分非溶解性的有机物在Fe(Ⅵ)预氧化作用下被转化为溶解性的TCM的前驱物,进而在游离氯的作用下被转化为TCM,且Fe(Ⅵ)对TCM的前驱物的降解效果不显著.
综上所述,从C-DBPs的总量的变化情况来看,Fe(Ⅵ)预氧化对DOM含碳官能团的破坏作用要优于Mn(Ⅶ)预氧化;从各个C-DBPs浓度的变化情况来看,Mn(Ⅶ)预氧化仅对TCM前驱物的官能团具有一定的破坏作用,对其他4种C-DBPs(DCBM、DBCM、1,1,1-TCP和TBM)前驱物的官能团的破坏作用较差;而Fe(Ⅵ)预氧化对3种C-DBPs(DCBM、DBCM和TBM)前驱物均有一定的破坏作用,但对TCM前驱物的官能团的破坏作用不明显. 因此在C-DBPs的生成势方面,随着氧化剂浓度的升高,Mn(Ⅶ)预氧化对TCM生成势的变化呈现出先增强后减弱的现象,对另外4种C-DBPs(DCBM、DBCM、1,1,1-TCP和TBM)的生成势无明显作用;而Fe(Ⅵ)预氧化对1,1,1-TCP的生成势具有明显的降低作用,对其它3种C-DBPs(DCBM、DBCM和TBM)生成势的变化呈现出先增强后减弱的现象,而对TCM生成势的变化不明显.
-
图8a表明,当不同浓度的Mn(Ⅶ)对水样进行预氧化并氯化后,N-DBPs中起主要变化的物质为DCAN,其浓度变化范围为7.76—12.55 μg·L−1(平均浓度为9.78 μg·L−1),平均降解率为25.40%. 而BCAN和DBAN几乎无明显变化. 可能是由于溴代HANs前驱物的稳定性较强,Mn(Ⅶ)预氧化不足以将其官能团进行有效破坏. N-DBPs的总浓度变化不明显,其浓度变化范围为10.21—15.38 μg·L−1(平均浓度为12.51 μg·L−1),平均降解率为20.62%. 图8b表明,当不同浓度的Fe(Ⅵ)对水样进行预氧化并氯化后,随着Fe(Ⅵ)浓度的升高,3种N-DBPs(DCAN、BCAN、DBAN)的浓度均有不同程度的降低,且N-DBPs的总浓度呈现出逐渐下降的趋势,当Fe(Ⅵ)的浓度为25 mg·L−1时,相比不加氧化剂的水样,N-DBPs的降解率达到了99.09%.
综上所述,从N-DBPs的总量的变化情况来看,Fe(Ⅵ)预氧化对DOM含氮官能团的破坏作用要明显优于Mn(Ⅶ)预氧化;从各个N-DBPs浓度的变化情况来看,Mn(Ⅶ)预氧化仅对DCAN前驱物的官能团具有一定的破坏作用;而Fe(Ⅵ)预氧化对3种N-DBPs前驱物均具有非常明显的破坏作用. 因此在N-DBPs的生成势方面,随着氧化剂浓度的升高,Mn(Ⅶ)预氧化仅对DCAN的生成势具有一定的减弱作用,而对另外两种N-DBPs(BCAN和DBAN)的生成势无明显作用;而Fe(Ⅵ)预氧化对3种N-DBPs(DCAN、BCAN和DBAN)的生成势均具有明显的降低作用.
-
Wagner等[28]对各种DBPs对CHO细胞细胞毒性和基因毒性进行了完整的综述,Richardson等[29]通过计算CTI评估了13种酰胺类的N-DBPs的细胞毒性. 基于以上理论依据,本研究通过对Mn(Ⅶ)和Fe(Ⅵ)两种预氧化并氯化后所产生的C-DBPs和N-DBPs对CHO细胞进行细胞毒性的评估并计算其CTI如图9所示.
从CTI的计算结果可以看出,相比3种主要N-DBPs的CTI,4种主要的C-DBPs所占的CTI值几乎可以忽略不计. 另外,虽然Fe(Ⅵ)预氧化在C-DBPs的生成量方面要略高于Mn(Ⅶ)预氧化,但由于C-DBPs所占的CTI值相比N-DBPs的CTI值平均相差了2至4个数量级,且Fe(Ⅵ)预氧化对N-DBPs的生成势有明显的抑制作用,因此随着氧化剂浓度的升高Fe(Ⅵ)预氧化对CHO细胞的CTI值明显降低. 单从细胞毒性方面考虑,Fe(Ⅵ)预氧化对CHO细胞的毒害效果要明显低于Mn(Ⅶ)预氧化.
-
(1)水源水水样DOM的来源主要为陆源与内源混合且具有一定的腐殖质化程度;Mn(Ⅶ)和Fe(Ⅵ)预氧化对HIX的指标具有较好的降解效果. 基于PARAFAC模型识别出氧化后的水样DOM主要由3个荧光组分组成,分别为2种类腐殖质荧光组分C1(Ex/Em=345、450 nm)、C3(Ex/Em=320,400 nm)和1种类富里酸荧光组分 C2(Ex/Em=225、425 nm),并且氧化后类富里酸C2组分被完全降解,降解率达到100%.
(2)水样TOC的浓度变化规律表明Mn(Ⅶ)和Fe(Ⅵ)作为氧化剂均具有较好的氧化性,另外Fe(Ⅵ)预氧化除了对DOM的官能团的破坏效果显著之外,可能还具有对非可溶性的有机物具有氧化降解的作用.
(3)对水样进行Mn(Ⅶ)和Fe(Ⅵ)两种氧化剂的预氧化并氯化后,在C-DBPs的生成势方面,Mn(Ⅶ)预氧化对TCM生成势的变化呈现出先增强后减弱的现象,而Fe(Ⅵ)预氧化对3种C-DBPs(DCBM、DBCM和TBM)生成势的变化均呈现出先增强后减弱的现象,但对TCM生成势的变化不明显. 在N-DBPs的生成势方面,Mn(Ⅶ)预氧化仅对DCAN的生成势具有一定的减弱作用,而Fe(Ⅵ)预氧化对3种N-DBPs(DCAN、BCAN和DBAN)的生成势均具有明显的降低作用. 且Fe(Ⅵ)预氧化对CHO细胞的毒害效果要明显低于Mn(Ⅶ)预氧化.
(4)对比Mn(Ⅶ)和Fe(Ⅵ)两种预氧化工艺,可以看出Fe(Ⅵ)不仅在氧化能力上优于Mn(Ⅶ)预氧化,在N-DBPs的生成量和细胞毒性方面所产生的毒害作用也明显低于Mn(Ⅶ)预氧化,且Fe(Ⅵ)氧化作用后生成的三价Fe具有絮凝的作用,可有效降低水体中悬浮物和胶体的浓度. 对饮用水质量及N-DBPs浓度要求较高的处理单位可考虑该工艺. 但其缺点是Fe(Ⅵ)不稳定、易失活,在实际应用中有一定的限制作用.
水源水Fe(Ⅵ)与Mn(Ⅶ)预氧化对天然有机物的组成特征及消毒副产物生成势的影响
Effect of Fe(Ⅵ) and Mn(Ⅶ) pre-oxidation of water source water on the composition characteristics of natural organic matter and disinfection by-product generation potential
-
摘要: 溶解有机物(DOM)普遍存在于地表水源中,是饮用水消毒副产物的重要前驱物. 本研究考察了Fe(Ⅵ)与Mn(Ⅶ)预氧化对浙江东部某水源水中DOM的组成特征及消毒副产物生成势的影响. 运用三维荧光光谱(3D-EEMs)分析了两种氧化剂在不同的浓度梯度下对水样进行预氧化后,水样DOM的荧光光谱特性、荧光特征参数和荧光组分等的变化情况,及氯化后消毒副产物(DBPs)的生成情况. 结果表明,水源水水样DOM的来源主要为陆源与内源混合且具有一定的腐殖质化程度,Mn(Ⅶ)和Fe(Ⅵ)预氧化不仅对荧光DOM具有较好的降解效果,对腐殖质类DOM也具有一定的降解作用. 在DBPs的生成势方面,Mn(Ⅶ)预氧化对三氯甲烷(TCM)生成势的变化呈现出先增强后减弱的现象,对二氯乙腈(DCAN)的生成势具有一定的减弱作用. 而Fe(Ⅵ)预氧化对三氯丙酮(1,1,1-TCP)、溴氯乙腈(BCAN)、二溴乙腈(DBAN)和DCAN的生成势均具有明显的降低作用,对二氯一溴甲烷(DCBM)、二溴一氯甲烷(DBCM)和三溴甲烷(TBM)生成势的变化呈现出先增强后减弱的现象. 研究结果推动了水源水氧化消毒工艺的发展并为相关研究提供理论指导.Abstract: Dissolved organic matter (DOM) is commonly found in surface water sources and is an important precursor of drinking water disinfection by-products. In this study, the effects of Fe(Ⅵ) and Mn(Ⅶ) preoxidation on the compositional characteristics and disinfection by-product generation potential of DOM in a water source in eastern Zhejiang were investigated. Three-dimensional fluorescence spectroscopy (3D-EEMs) was used to analyze the changes of fluorescence spectral characteristics, fluorescence characteristic parameters and fluorescence components of DOM in water samples after pre-oxidation of the two oxidants at different concentration gradients, and the generation of disinfection by-products (DBPs) after chlorination. The results showed that the source of DOM in the water samples was mainly mixed with endogenous sources and had a certain degree of humicification, and the pre-oxidation of Mn(Ⅶ) and Fe(Ⅵ) not only had a good degradation effect on fluorescent DOM, but also had a certain degradation effect on humic DOM. As for the generation potential of DBPs, the changes of Mn(Ⅶ) preoxidation on trichloromethane(TCM) generation potential showed an enhancement and then weakening, and the generation potential of dichloroacetonitrile(DCAN) had a certain weakening effect. The Fe(Ⅵ) pre-oxidation had a significant reduction in the generation potential of trichloroacetone(1,1,1-TCP), bromochloro-acetonitrile(BCAN), dibromoacetonitrile (DBAN) and DCAN, and an enhancement and then weakening in the generation potential of dichlorobromomethane (DCBM), dibromochloromethane (DBCM) and tribromomethane (TBM). The results of the study promote the development of oxidative disinfection process for water sources and provide theoretical guidance for related research.
-
氧化水处理是水处理中的一种通用处理单元,可用于消毒、污染物降解和改善其他处理方案[1]. 消毒是水处理过程中不可或缺的一个单元. 目前,在实际水处理过程中,应用较多的消毒剂为次氯酸钠、二氧化氯、液氯、氯胺等,大多为含氯消毒剂. 不同种类的含氯消毒剂所生成的氯代消毒副产物(CDBPs)也存在差异[2 − 5],其中次氯酸钠是我国常用的饮用水消毒剂之一[6].
溶解有机物(DOM)是一种复杂的异质化学结构混合物. 它普遍存在于地表水源中[7 − 11]. DOM主要包括腐殖质、蛋白质和其他芳香族或脂肪族有机化合物的复杂混合物[12],其中DOM的富电子部分(如酚类化合物、烯烃和有机胺),被公认为是饮用水和废水处理中通过多种途径消耗化学氧化剂的主要消耗者[13 − 15]. 例如,Sharma等 [13]对Fe(Ⅵ) 和Fe(Ⅴ) 氧化多种有机化合物(胺类、酚类、醇类和烃类等)的动力学和机理进行了评估,结果表明Fe(Ⅵ) 通常通过电子转移来完成对有机化合物的氧化. 其电子转移步骤为FeⅥ →FeⅤ→FeⅢ和FeⅥ→FeⅣ→FeⅡ.
大部分消毒副产物(DBPs)有潜在的致癌、致畸、致突变的“三致”毒性[16],其中致癌性较强的是卤乙酸(HAAs)、三卤甲烷(THMs)和溴酸盐,致畸和致突变性较强的是卤代乙腈(HANs)、卤化硝基甲烷(HNMs)和卤化氰[17]. 由于潜在的健康风险和饮用水中DBPs的广泛存在,许多国家和组织对饮用水中的DBPs进行了管理,以减轻包括THMs、HAAs在内的多种DBPs造成的健康风险. 2006年,中国颁布了《中国饮用水水质标准》(GB/T5749-2006), 2012年开始实施,共规范了106种污染物,其中DBPs 14种. 从管制的DBP化合物的数量来看,中国饮用水中的DBPs规定比美国更为严格. 地表水作为饮用水供给链的前端,是保证居民用水安全的重要一环. 因此,有必要探究地表水经过不同的氧化消毒工艺之后其DBPs的生成水平.
DBPs的形成一般是通过混凝、絮凝、沉淀和过滤等物理处理单元去除DOM前体来缓解的[18]. 在一些公用事业中,化学氧化剂的预氧化也被用于提高混凝效率以及去除新出现的令人担忧的污染物[19 − 20]. 大多数氧化剂可以选择性地与DOM中的富电子部分(如酚类化合物、烯烃和有机胺)反应[13 − 15],并可改变天然有机物(NOM)的物理/化学性质,甚至使其矿化. 其中,我国使用最广泛的是高锰酸盐(Mn(Ⅶ),酸性条件下氧化还原电位为1.51 V,碱性条件下氧化还原电位为0.56 V )[21],Mn(Ⅶ)可以与水中DOM发生直接和间接的作用,以有效提高对地表水中DOM的去除;高铁酸钾(Fe(Ⅵ),酸性条件下氧化还原电位为2.20 V,碱性条件下氧化还原电位为0.72 V )预氧化是近几年国内外研究热点[13,22],由于Fe(Ⅵ)中六价铁的存在,使得Fe(Ⅵ)具有较强的氧化性,在使用Fe(Ⅵ)预氧化时,不会引入任何有毒有害物质[21].
本研究以浙江东部某水源水(下文简称水源水)为研究对象,旨在从水源水的回用角度出发,探究Mn(Ⅶ)与Fe(Ⅵ)预氧化对水源水中DOM的转化及氯化后对DBPs生成势的影响情况. 主要研究内容包括:(1)不同氧化剂(Fe(Ⅵ)与Mn(Ⅶ))对DOM的荧光转化;(2)Mn(Ⅶ)与Fe(Ⅵ)预氧化对氯化后DBPs生成势的变化,为该水源水的水环境健康构建提供理论依据.
1. 材料与方法(Materials and methods)
1.1 实验材料和主要试剂
本研究采集水源水5 L,分5瓶装入容量为1 L的塑料瓶中,运送入冷库保存,7日内进行实验操作和样品分析. 高锰酸钾(KMnO4)、次氯酸钠(NaClO)、抗坏血酸(Ascorbic acid)均为分析纯,购自国药集团化学试剂有限公司;高铁酸钾(K2FeO4)购自北京伊诺凯科技有限公司;1,2-二溴丙烷及各种DBPs标准品为色谱纯,购自阿法埃莎(中国)化学有限公司;甲基叔丁基醚(MTBE)为色谱纯,购自赛默飞世尔科技(中国)有限公司.
1.2 实验步骤
氧化消毒实验:分别取50 mL水样于50 mL离心管中,加入标定后的不同浓度梯度的氧化剂(其中Mn(Ⅶ)浓度梯度为:0、1、2、5、10、20 mg·L−1;Fe(Ⅵ)浓度梯度为:0、1.25、2.5、6.25、12.5、25 mg·L−1)氧化30 min后,加入标定后游离氯的浓度为10 mg·L−1的次氯酸钠溶液消毒24 h后用抗坏血酸进行猝灭,反应在常温下进行,避光. 游离氯(FC)使用哈希试剂包在DR 6000分光光度计进行标定.
三维荧光光谱的测定:取10 mL水样经孔径为0.45 μm的有机滤膜过滤于10 mL一次性离心管中,装入润洗过的1 cm 四面通光石英比色皿. 采用美国Cary Eclipse型荧光分光光度计,在设置光电倍增电压为700 V的情况下,将激发和发射狭缝宽度均设置为5 nm,以24000 nm·min−1 的扫描速度进行三维荧光光谱扫描,扫描光谱进行仪器自动校正. 同时,三维荧光光谱扫描范围设置为激发波长(Ex)为200—420 nm,扫描间隔5 nm;发射波长(Em)280—550 nm,扫描间隔5 nm. 后续三维荧光光谱分析需将水样三维荧光光谱扣除超纯水的三维荧光光谱[23],以消除拉曼散射的影响. 应用含有drEEM工具包的MATLAB软件进行三维荧光数据分析.
DBPs的测定:取20 mL水样于40 mL萃取瓶中,加入6 g无水硫酸钠,再加入2 mL含有200 μg·L−1 1,2-二溴丙烷内标物的MTBE萃取液,后将萃取瓶置于涡旋振荡器,以2500 r·min−1的转速震荡10 min,结束后静置5 min左右,待有机相与水层分界明显时,将上层液体移取约1 mL经孔径为0.45 μm的有机滤膜过滤于2 mL棕色进样瓶中,确保瓶中无分层,通过气相色谱电子捕获检测器检测样品中DBPs的浓度. 仪器条件设置为进样量1 μL,进样口温度200 ℃,氮气流速2.4318 mL·min−1,分流比为7:1,升温程序:35 ℃保持15 min,以25 ℃·min−1的速度上升至145 ℃,保持3 min,再以35 ℃·min−1的速度上升至240 ℃,保持5 min,检测器温度300 ℃,运行时间2 min.
总有机碳(TOC)的测定:取40 mL水样过0.45 μm的有机滤膜过滤于TOC进样瓶中,采用TOC-VCPH分析仪 (Shimadzu, Japan)进行测定.
1.3 分析方法
平行因子分析(PARAFAC)基于最小方差理论,PARAFAC分析将由样本数、激发光谱和发射光谱组成的三维荧光光谱数据集X(I×J×K)分解成3个载荷矩阵,即激发光谱矩阵A(J×N)、发射光谱矩阵B(K×N)和相对浓度矩阵C(I×N),以及一个残差矩阵E(I×J×K)来反映各样品原三维荧光光谱,具体公式见式1. 其中,Xijk、ain、bjn、ckn和eijk分别代表三维矩阵、激发光谱矩阵、发射光谱矩阵、相对浓度矩阵和残差矩阵中元素;N表示独立的荧光组分数. 其中,PARAFAC分析出Fmax值可表示组分含量的相对多少[24 − 26].
stringUtils.convertMath(!{formula.content}) (1) 区域荧光积分分析(FRI)通过固定激发波长和发射波长范围划分三维荧光光谱[27],将不同范围内荧光强度积分用于量化分析DOM组成和含量变化,具体公式见式2.
stringUtils.convertMath(!{formula.content}) (2) Qi代表第i个组分区域荧光强度积分值,l(λExλEm)代表相应波长处荧光强度,ΔλEx和ΔλEm分别代表激发波长和发射波长的间隔.
为了评估各DBPs所产生的合成毒性变化,采用了细胞毒性指数(CTI)进行计算[28 − 29].
stringUtils.convertMath(!{formula.content}) (3) LC50 值是根据回归分析计算的 DBP s浓度,其诱导的细胞密度为阴性对照的 50%,CX表示每一种DBPs的浓度.
1.4 数据处理
运用Matlab R2018a软件中的drEEM工具箱处理三维荧光数据并进行区域积分法和平行因子分析. Origin 2021用于数据分析和绘图. 荧光特征参数荧光指数(FI)、自生源指标 (BIX)、腐殖化指数(HIX)的计算如表1 所示.
表 1 荧光特征参数的计算及光谱参数描述Table 1. Description of fluorescence analysis methods and spectrum parameters荧光Fluorescence 计算方法Calculation method 指示参数Index parameters FI Ex=370 nm 时,Em 在470 nm 处和520 nm处的荧光强度比值 FI<1.4时,DOM主要为陆源输入;当1.4<FI<1.9时,DOM具有内源释放与外源输入的双重特征;当FI>1.9时,DOM主要来内源输入[30 − 31] BIX Ex=310 nm 时,Em 在380 nm与430 nm 处荧光强度的比值 BIX>1.0时,表示DOM主要为藻类或细菌等自生来源(内源)且有机质为新近产生;当0.8<BIX<1.0时,说明DOM具有较强的自生源特征;BIX<0.8时,主要为陆源输入[32] HIX Ex=254 nm 时,Em 在435—480 nm和300—345 nm区间最大荧光强度之比[33] 当HIX<3时,表示腐殖化程度弱且有新近自生源,数值越高表明腐殖化程度越高[34] 2. 结果与讨论(Results and discussion)
2.1 三维荧光光谱分析
2.1.1 氧化后DOM 的荧光光谱特性及区域积分
水源水和不同种类的氧化剂氧化后的10个水样中 DOM 的三维荧光特征光谱图和各区域 DOM 标准荧光积分体积如图1、图2所示. 可以看出氧化后的水样 DOM 的荧光光谱特性(图1)和荧光区域标准化积分体积具有明显的变化(图2).
其中水源水水样中DOM 的类蛋白质组分(区域Ⅰ,区域Ⅱ)和富里酸类(区域Ⅲ)的荧光积分体积相对较高,积分比例达到了12.22%、32.32%和36.66%,而氧化后的10个水样中DOM的区域Ⅰ、区域Ⅱ和区域Ⅲ的荧光积分体积几乎被完全降解. 区域Ⅳ多为水体中水生植物及浮游微生物代谢等活动产生,一定程度上可以表征水体生物(微生物、藻类等)的代谢,Mn(Ⅶ)氧化后该区域的平均荧光积分体积降低了72.60%,总荧光积分体积降低了63.78%,而Fe(Ⅵ)氧化后该区域的平均荧光积分体积降低了76.74%,总荧光积分体积降低了68.55%,且随着Fe(Ⅵ)浓度的升高,腐殖质类(区域Ⅴ)的荧光积分体积呈现出下降的趋势,表明Fe(Ⅵ)作为氧化剂氧化降解水中的DOM时,其氧化效果要强于Mn(Ⅶ),且二者对DOM均有较好的降解效果.
2.1.2 氧化后DOM 的荧光特征参数分析
11个水样中 DOM 的 FI、BIX、HIX指标如图3所示,对于水源水水样其FI、BIX指标分别为2.23和0.78,表明水样DOM的来源主要为陆源与内源混合,从各指标数值大小可以判断该流域水体自生源特征较明显,这主要是由于该流域水体生态环境相对稳定,DOM受水体微生物活动因素影响较明显;另外HIX指标为3.94,表明水样有一定的腐殖质化程度但相对较弱. 其中Mn(Ⅶ)氧化后水样FI指标的范围位于1.54—2.31(均值为1.96),表明氧化后虽对FI有所降低,但水样仍具有一定的自生源特性;BIX的范围是0.70—0.93(均值为0.80),相较于氧化前水样,平均陆源输入特性有所降低,但个别浓度下陆源输入的特性明显增强,表明Mn(Ⅶ)对水体的陆源DOM降解效果不明显;HIX的范围是1.00—1.89(均值为1.37),其平均降解率为65.23%,表明Mn(Ⅶ)对腐殖质类DOM具有较好的降解效果. 另外Fe(Ⅵ)氧化后水体FI指标的范围位于1.87—2.47(均值为2.09),这一结果跟Mn(Ⅶ)氧化后的结果类似;BIX的范围是0.70—0.83(均值为0.73),相较于氧化前水样,陆源输入特性反而有所增强;HIX的范围是1.15—2.43(均值为1.32),其平均降解率为66.50%,这一结果与Mn(Ⅶ)预氧化相比无明显变化,表明Fe(Ⅵ)对腐殖质类DOM也具有较好的降解效果.
2.1.3 氧化对DOM荧光组分的影响
已有的研究将DOM划分为6类[10,35],分别为类富里酸、类腐殖酸、低激发色氨酸类、高激发色氨酸类、低激发酪氨酸类、高激发酪氨酸类,这些DOM广泛存在于河流和湖泊水体中. 基于PARAFAC模型对永宁江江口地表水以及Mn(Ⅶ)和Fe(Ⅵ)分别氧化后的水样DOM的三维荧光图谱进行分析,得到3种荧光组分. 其中,C1(Ex/Em=345,450 nm)(图4a)类陆生腐殖质成分,C2(Ex/Em=225,425 nm)(图4c)类富里酸,C3(Ex/Em=320,400 nm)(图4e)海洋腐殖酸. 组分C1为类陆生腐殖质,该荧光组分具有潜在的陆源特征,如来自土壤、森林溪流和湿地的溶解有机质输入来源[36]. 组分C2为类富里酸物质,类富里酸物质属于芳香氨基酸腐殖物质,分子量较大,反映来自外源输入的腐殖酸和富里酸所形成的荧光峰,与腐殖质中羟基及羧基有关[37 − 38]. 组分C3类似于紫外区陆地类腐殖酸荧光峰A(<250 nm,290—325 nm/370—430 nm)[39],其特点是分子量低,常见于与生物活动相关的海洋环境,在废水、湿地和农业环境中也有发现[40];与刘笑菡等[41]研究中UVC类腐殖质荧光组分(<250 nm,305 nm/412—420 nm)相似,这些相似的荧光组分被证实具有外源指示意义.
 图 4 不同浓度Mn(Ⅶ)和Fe(Ⅵ)氧化后水样DOM三维荧光图谱及最大激发/发射波长分布(a)-(b)C1组分及最大Ex/Em;(c)-(d)C2组分及最大Ex/Em;(e)-(f)C3组分及最大Ex/Em;Figure 4. 3D-EEM spectrum and maximum excitation/emission wavelength distribution of DOM sfter oxidized by different concentrations of Mn(Ⅶ) and Fe(Ⅵ) (a)-(b) C1 component and maximum Ex/Em; (c)-(d) C2 component and maximum Ex/Em; (e)-(f) C3 component and maximum Ex/Em
图 4 不同浓度Mn(Ⅶ)和Fe(Ⅵ)氧化后水样DOM三维荧光图谱及最大激发/发射波长分布(a)-(b)C1组分及最大Ex/Em;(c)-(d)C2组分及最大Ex/Em;(e)-(f)C3组分及最大Ex/Em;Figure 4. 3D-EEM spectrum and maximum excitation/emission wavelength distribution of DOM sfter oxidized by different concentrations of Mn(Ⅶ) and Fe(Ⅵ) (a)-(b) C1 component and maximum Ex/Em; (c)-(d) C2 component and maximum Ex/Em; (e)-(f) C3 component and maximum Ex/Em解析出荧光组分类陆生腐殖质C1组分、类富里酸C2组分、海洋腐殖酸C3组分后,根据荧光组分最大荧光强度(Fmax)绘制出不同浓度Mn(Ⅶ)和Fe(Ⅵ)氧化后各组分荧光强度变化图(图5),对于水源水水样的C1、C2和C3荧光强度分别为0.14、0.45、0.15 R.U.,另外可以明显看出,在两种不同的氧化剂作用下C2组分即类富里酸均被完全降解, 降解率达到100%;其中Mn(Ⅶ)氧化后C1组分的荧光强度无明显变化,而C3组分的荧光强度反而有些微增强(图5a),相较于氧化前水样平均荧光强度升高了15.59%. 而Fe(Ⅵ)氧化后,C1组分的荧光强度有所降低(图5b),平均降解率为9.72%,降解效果不明显;对于C3组分,其在低浓度Fe(Ⅵ)作用下荧光强度有所升高,而随着Fe(Ⅵ)浓度的进一步增加,荧光强度呈现出下降的趋势. 对于C3组分在两种氧化剂的作用下其荧光强度有轻微升高的现象可能是由于水样在氧化剂的作用下,部分大分子类富里酸DOM被氧化为陆源DOM,进而造成C3组分荧光强度“不减反增”的现象,C3组分这一结果跟“2.1.2”中BIX的变化情况是一致的.
2.2 预氧化对TOC的变化
不同浓度Mn(Ⅶ)和Fe(Ⅵ)预氧化对水样TOC的变化情况如图6所示. 图6a表明,随着氧化剂浓度的升高,Mn(Ⅶ)预氧化在初始氧化剂浓度为1.0 mg·L−1下TOC的浓度明显下降,降解率为80.34%,之后趋于稳定,表明Mn(Ⅶ)预氧化对DOM的官能团的破坏效果比较显著. 图6b表明,Fe(Ⅵ)预氧化虽然在初始浓度为1.25 mg·L−1下对TOC的降解效果不如Mn(Ⅶ)预氧化显著,但其在浓度为2.5 mg·L−1时对TOC的降解率仍达到了80.11%,且随着氧化剂浓度的进一步升高,水样TOC的浓度呈现出上升的现象并趋于稳定,可能是由于Fe(Ⅵ)预氧化除了对DOM的官能团的破坏效果显著之外,还具有对非可溶性的有机物具有氧化降解的作用.
2.3 预氧化对DOM的DBPs生成势的影响
在对水样进行Mn(Ⅶ)和Fe(Ⅵ)两种氧化剂的预氧化并氯化后,主要检出了三氯甲烷(TCM)、二氯一溴甲烷(DCBM)、二溴一氯甲烷(DBCM)、三氯丙酮(1,1,1-TCP)和三溴甲烷(TBM)等5种C-DBPs;另外也检测出了二氯乙腈(DCAN)、溴氯乙腈(BCAN)和二溴乙腈(DBAN)等3种N-DBPs. 图7和图8中数据为两组平行样的均值,从11个水样的数据中可以看出含碳消毒副产物(C-DBPs)的总生成量均高于含氮消毒副产物(N-DBPs)的总生成量,且C-DBPs中TBM的含量较低,平均占比为0.41%;而N-DBPs中DBAN的含量也比较低,平均占比为3.85%.
2.3.1 C-DBPs
图7a表明,当不同浓度的Mn(Ⅶ)对水样进行预氧化并氯化后,起主要变化的C-DBPs为TCM,另外4种C-DBPs(DCBM、DBCM、1,1,1-TCP和TBM)无明显变化. 对于溴代THMs,可能是由于其前驱物的稳定性较强,Mn(Ⅶ)预氧化不足以将其官能团进行有效破坏. 随着Mn(Ⅶ)浓度的升高,TCM的浓度呈现出先升高再降低的变化趋势,可能是由于随着Mn(Ⅶ)浓度的升高,水中一部分DOM的官能团被氧化为TCM前驱物的官能团,进而在游离氯的作用下被转化为TCM,因此TCM呈现出浓度升高的现象,而随着Mn(Ⅶ)浓度的进一步升高(达到20 mg·L−1时),小分子状态的DOM被氧化降解,最终使得TCM呈现出急剧下降的现象,降低率为33.60%,表明高浓度的Mn(Ⅶ)对TCM的前驱物具有一定的降解作用. 图7b表明, 当不同浓度的Fe(Ⅵ)对水样进行预氧化并氯化后,5种C-DBPs均有不同程度和规律的变化;随着Fe(Ⅵ)浓度的升高,1,1,1-TCP呈现出逐渐下降的趋势,最终降解率达到了99.46%,表明Fe(Ⅵ)作为氧化剂时,对1,1,1-TCP的前驱物具有较好的降解作用;而另外3种C-DBPs(DCBM、DBCM和TBM)均呈现出先升高后降低的趋势,当Fe(Ⅵ)浓度为25 mg·L−1时,DCBM、DBCM和TBM的降解率分别为22.34%、35.28%和1.38%,这一结果跟不同浓度的Mn(Ⅶ)先氧化再氯化后TCM的变化情况有类似之处;而TCM的浓度则呈现出只增不减的现象,可能是由于部分DOM的官能团在Fe(Ⅵ)的预氧化作用下被转化为TCM的前驱物. 而从Fe(Ⅵ)预氧化下TOC的浓度变化规律考虑,可能是由于部分非溶解性的有机物在Fe(Ⅵ)预氧化作用下被转化为溶解性的TCM的前驱物,进而在游离氯的作用下被转化为TCM,且Fe(Ⅵ)对TCM的前驱物的降解效果不显著.
综上所述,从C-DBPs的总量的变化情况来看,Fe(Ⅵ)预氧化对DOM含碳官能团的破坏作用要优于Mn(Ⅶ)预氧化;从各个C-DBPs浓度的变化情况来看,Mn(Ⅶ)预氧化仅对TCM前驱物的官能团具有一定的破坏作用,对其他4种C-DBPs(DCBM、DBCM、1,1,1-TCP和TBM)前驱物的官能团的破坏作用较差;而Fe(Ⅵ)预氧化对3种C-DBPs(DCBM、DBCM和TBM)前驱物均有一定的破坏作用,但对TCM前驱物的官能团的破坏作用不明显. 因此在C-DBPs的生成势方面,随着氧化剂浓度的升高,Mn(Ⅶ)预氧化对TCM生成势的变化呈现出先增强后减弱的现象,对另外4种C-DBPs(DCBM、DBCM、1,1,1-TCP和TBM)的生成势无明显作用;而Fe(Ⅵ)预氧化对1,1,1-TCP的生成势具有明显的降低作用,对其它3种C-DBPs(DCBM、DBCM和TBM)生成势的变化呈现出先增强后减弱的现象,而对TCM生成势的变化不明显.
2.3.2 N-DBPs
图8a表明,当不同浓度的Mn(Ⅶ)对水样进行预氧化并氯化后,N-DBPs中起主要变化的物质为DCAN,其浓度变化范围为7.76—12.55 μg·L−1(平均浓度为9.78 μg·L−1),平均降解率为25.40%. 而BCAN和DBAN几乎无明显变化. 可能是由于溴代HANs前驱物的稳定性较强,Mn(Ⅶ)预氧化不足以将其官能团进行有效破坏. N-DBPs的总浓度变化不明显,其浓度变化范围为10.21—15.38 μg·L−1(平均浓度为12.51 μg·L−1),平均降解率为20.62%. 图8b表明,当不同浓度的Fe(Ⅵ)对水样进行预氧化并氯化后,随着Fe(Ⅵ)浓度的升高,3种N-DBPs(DCAN、BCAN、DBAN)的浓度均有不同程度的降低,且N-DBPs的总浓度呈现出逐渐下降的趋势,当Fe(Ⅵ)的浓度为25 mg·L−1时,相比不加氧化剂的水样,N-DBPs的降解率达到了99.09%.
综上所述,从N-DBPs的总量的变化情况来看,Fe(Ⅵ)预氧化对DOM含氮官能团的破坏作用要明显优于Mn(Ⅶ)预氧化;从各个N-DBPs浓度的变化情况来看,Mn(Ⅶ)预氧化仅对DCAN前驱物的官能团具有一定的破坏作用;而Fe(Ⅵ)预氧化对3种N-DBPs前驱物均具有非常明显的破坏作用. 因此在N-DBPs的生成势方面,随着氧化剂浓度的升高,Mn(Ⅶ)预氧化仅对DCAN的生成势具有一定的减弱作用,而对另外两种N-DBPs(BCAN和DBAN)的生成势无明显作用;而Fe(Ⅵ)预氧化对3种N-DBPs(DCAN、BCAN和DBAN)的生成势均具有明显的降低作用.
2.3.3 细胞毒性
Wagner等[28]对各种DBPs对CHO细胞细胞毒性和基因毒性进行了完整的综述,Richardson等[29]通过计算CTI评估了13种酰胺类的N-DBPs的细胞毒性. 基于以上理论依据,本研究通过对Mn(Ⅶ)和Fe(Ⅵ)两种预氧化并氯化后所产生的C-DBPs和N-DBPs对CHO细胞进行细胞毒性的评估并计算其CTI如图9所示.
从CTI的计算结果可以看出,相比3种主要N-DBPs的CTI,4种主要的C-DBPs所占的CTI值几乎可以忽略不计. 另外,虽然Fe(Ⅵ)预氧化在C-DBPs的生成量方面要略高于Mn(Ⅶ)预氧化,但由于C-DBPs所占的CTI值相比N-DBPs的CTI值平均相差了2至4个数量级,且Fe(Ⅵ)预氧化对N-DBPs的生成势有明显的抑制作用,因此随着氧化剂浓度的升高Fe(Ⅵ)预氧化对CHO细胞的CTI值明显降低. 单从细胞毒性方面考虑,Fe(Ⅵ)预氧化对CHO细胞的毒害效果要明显低于Mn(Ⅶ)预氧化.
3. 结论(Conclusions)
(1)水源水水样DOM的来源主要为陆源与内源混合且具有一定的腐殖质化程度;Mn(Ⅶ)和Fe(Ⅵ)预氧化对HIX的指标具有较好的降解效果. 基于PARAFAC模型识别出氧化后的水样DOM主要由3个荧光组分组成,分别为2种类腐殖质荧光组分C1(Ex/Em=345、450 nm)、C3(Ex/Em=320,400 nm)和1种类富里酸荧光组分 C2(Ex/Em=225、425 nm),并且氧化后类富里酸C2组分被完全降解,降解率达到100%.
(2)水样TOC的浓度变化规律表明Mn(Ⅶ)和Fe(Ⅵ)作为氧化剂均具有较好的氧化性,另外Fe(Ⅵ)预氧化除了对DOM的官能团的破坏效果显著之外,可能还具有对非可溶性的有机物具有氧化降解的作用.
(3)对水样进行Mn(Ⅶ)和Fe(Ⅵ)两种氧化剂的预氧化并氯化后,在C-DBPs的生成势方面,Mn(Ⅶ)预氧化对TCM生成势的变化呈现出先增强后减弱的现象,而Fe(Ⅵ)预氧化对3种C-DBPs(DCBM、DBCM和TBM)生成势的变化均呈现出先增强后减弱的现象,但对TCM生成势的变化不明显. 在N-DBPs的生成势方面,Mn(Ⅶ)预氧化仅对DCAN的生成势具有一定的减弱作用,而Fe(Ⅵ)预氧化对3种N-DBPs(DCAN、BCAN和DBAN)的生成势均具有明显的降低作用. 且Fe(Ⅵ)预氧化对CHO细胞的毒害效果要明显低于Mn(Ⅶ)预氧化.
(4)对比Mn(Ⅶ)和Fe(Ⅵ)两种预氧化工艺,可以看出Fe(Ⅵ)不仅在氧化能力上优于Mn(Ⅶ)预氧化,在N-DBPs的生成量和细胞毒性方面所产生的毒害作用也明显低于Mn(Ⅶ)预氧化,且Fe(Ⅵ)氧化作用后生成的三价Fe具有絮凝的作用,可有效降低水体中悬浮物和胶体的浓度. 对饮用水质量及N-DBPs浓度要求较高的处理单位可考虑该工艺. 但其缺点是Fe(Ⅵ)不稳定、易失活,在实际应用中有一定的限制作用.
-
图 4 不同浓度Mn(Ⅶ)和Fe(Ⅵ)氧化后水样DOM三维荧光图谱及最大激发/发射波长分布(a)-(b)C1组分及最大Ex/Em;(c)-(d)C2组分及最大Ex/Em;(e)-(f)C3组分及最大Ex/Em;
Figure 4. 3D-EEM spectrum and maximum excitation/emission wavelength distribution of DOM sfter oxidized by different concentrations of Mn(Ⅶ) and Fe(Ⅵ) (a)-(b) C1 component and maximum Ex/Em; (c)-(d) C2 component and maximum Ex/Em; (e)-(f) C3 component and maximum Ex/Em
表 1 荧光特征参数的计算及光谱参数描述
Table 1. Description of fluorescence analysis methods and spectrum parameters
荧光Fluorescence 计算方法Calculation method 指示参数Index parameters FI Ex=370 nm 时,Em 在470 nm 处和520 nm处的荧光强度比值 FI<1.4时,DOM主要为陆源输入;当1.4<FI<1.9时,DOM具有内源释放与外源输入的双重特征;当FI>1.9时,DOM主要来内源输入[30 − 31] BIX Ex=310 nm 时,Em 在380 nm与430 nm 处荧光强度的比值 BIX>1.0时,表示DOM主要为藻类或细菌等自生来源(内源)且有机质为新近产生;当0.8<BIX<1.0时,说明DOM具有较强的自生源特征;BIX<0.8时,主要为陆源输入[32] HIX Ex=254 nm 时,Em 在435—480 nm和300—345 nm区间最大荧光强度之比[33] 当HIX<3时,表示腐殖化程度弱且有新近自生源,数值越高表明腐殖化程度越高[34] -
[1] LEE Y, von GUNTEN U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical)[J]. Water Research, 2010, 44(2): 555-566. doi: 10.1016/j.watres.2009.11.045 [2] GRÜNWALD A, ŠŤASTNÝ B, SLAVÍČKOVÁ K, et al. Formation of haloforms during chlorination of natural waters[J]. Acta Polytechnica, 2002, 42(2): 234-243. [3] RICHARDSON S D. The role of GC-MS and LC-MS in the discovery of drinking water disinfection by-products[J]. Journal of Environmental Monitoring: JEM, 2002, 4(1): 1-9. doi: 10.1039/b105578j [4] 张盛军, 张大钰, 董燕, 等. 二氧化氯消毒副产物的生成规律研究[J]. 中国给水排水, 2013, 29(9): 70-71, 76. ZHANG S J, ZHANG D Y, DONG Y, et al. Formation rule of chlorine dioxide disinfection by-products[J]. China Water & Wastewater, 2013, 29(9): 70-71, 76 (in Chinese).
[5] 朱红霞, 薛荔栋, 刘进斌, 等. 含氯消毒副产物的种类、危害与地表水污染现状[J]. 环境科学研究, 2020, 33(7): 1640-1648. doi: 10.13198/j.issn.1001-6929.2020.06.14 ZHU H X, XUE L D, LIU J B, et al. Types, hazards and pollution status of chlorinated disinfection by-products in surface water[J]. Research of Environmental Sciences, 2020, 33(7): 1640-1648 (in Chinese). doi: 10.13198/j.issn.1001-6929.2020.06.14
[6] 许春凤, 马铃, 周智勇, 等. 次氯酸钠在饮用水消毒方面的应用[J]. 西南给排水, 2015, 37(2): 3. XU C F, MA L, ZHOU Z Y, et al. Application of sodium hypochlorite in drinking water disinfection [J]. Southwest Water Supply and Drainage, 2015, 37(2): 3(in Chinese).
[7] PERNET-COUDRIER B, CLOUZOT L, VARRAULT G, et al. Dissolved organic matter from treated effluent of a major wastewater treatment plant: Characterization and influence on copper toxicity[J]. Chemosphere, 2008, 73(4): 593-599. doi: 10.1016/j.chemosphere.2008.05.064 [8] YAO X, ZHANG Y L, ZHU G W, et al. Resolving the variability of CDOM fluorescence to differentiate the sources and fate of DOM in Lake Taihu and its tributaries[J]. Chemosphere, 2011, 82(2): 145-155. doi: 10.1016/j.chemosphere.2010.10.049 [9] TANG J, SHI T Z, WU X W, et al. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment[J]. Chemosphere, 2015, 122: 154-161. doi: 10.1016/j.chemosphere.2014.11.032 [10] 何伟, 白泽琳, 李一龙, 等. 溶解性有机质特性分析与来源解析的研究进展[J]. 环境科学学报, 2016, 36(2): 359-372. doi: 10.13671/j.hjkxxb.2015.0117 HE W, BAI Z L, LI Y L, et al. Advances in the characteristics analysis and source identification of the dissolved organic matter[J]. Acta Scientiae Circumstantiae, 2016, 36(2): 359-372 (in Chinese). doi: 10.13671/j.hjkxxb.2015.0117
[11] KAMJUNKE N, von TÜMPLING W, HERTKORN N, et al. A new approach for evaluating transformations of dissolved organic matter (DOM) via high-resolution mass spectrometry and relating it to bacterial activity[J]. Water Research, 2017, 123: 513-523. doi: 10.1016/j.watres.2017.07.008 [12] 任志敏, 李雅馨月, 林子琛, 等. 光谱法在城市污水溶解性有机物处理中的应用[J]. 科技创新与应用, 2021, 11(25): 180-182. REN Z M, LI Y, LIN Z C, et al. Application of spectroscopy in the treatment of dissolved organic matter in municipal sewage[J]. Technology Innovation and Application, 2021, 11(25): 180-182 (in Chinese).
[13] RICHARDSON S D, PLEWA M J, WAGNER E D, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research[J]. Mutation Research/Reviews in Mutation Research, 2007, 636(1/2/3): 178-242. [14] KUNDU B, RICHARDSON S D, SWARTZ P D, et al. Mutagenicity in Salmonella of halonitromethanes: A recently recognized class of disinfection by-products in drinking water[J]. Mutation Research, 2004, 562(1/2): 39-65. [15] LIU H J, LIU R P, TIAN C, et al. Removal of natural organic matter for controlling disinfection by-products formation by enhanced coagulation: A case study[J]. Separation and Purification Technology, 2012, 84: 41-45. doi: 10.1016/j.seppur.2011.07.009 [16] DONG H Y, ZHANG H F, WANG Y, et al. Disinfection by-product (DBP) research in China: Are we on the track?[J]. Journal of Environmental Sciences, 2021, 110: 99-110. doi: 10.1016/j.jes.2021.03.023 [17] von GUNTEN U. Oxidation processes in water treatment: Are we on track?[J]. Environmental Science & Technology, 2018, 52(9): 5062-5075. [18] SHARMA V K. Ferrate(Ⅵ) and ferrate(V) oxidation of organic compounds: Kinetics and mechanism[J]. Coordination Chemistry Reviews, 2013, 257((2): ): 495-510. doi: 10.1016/j.ccr.2012.04.014 [19] GAN W H, GE Y X, ZHONG Y, et al. The reactions of chlorine dioxide with inorganic and organic compounds in water treatment: Kinetics and mechanisms[J]. Environmental Science: Water Research & Technology, 2020, 6(9): 2287-2312. [20] LI J, PANG S Y, WANG Z, et al. Oxidative transformation of emerging organic contaminants by aqueous permanganate: Kinetics, products, toxicity changes, and effects of Manganese products[J]. Water Research, 2021, 203: 117513. doi: 10.1016/j.watres.2021.117513 [21] SHARMA V K, TRIANTIS T M, ANTONIOU M G, et al. Destruction of microcystins by conventional and advanced oxidation processes: A review[J]. Separation and Purification Technology, 2012, 91: 3-17. doi: 10.1016/j.seppur.2012.02.018 [22] 李昂, 林英姿, 朱洋, 等. 饮用水处理中常用预氧化剂的种类及特点[J]. 辽宁化工, 2020, 49(1): 57-59, 61. doi: 10.14029/j.cnki.issn1004-0935.2020.01.013 LI A, LIN Y Z, ZHU Y, et al. Types and characteristics of common preoxidants in drinking water treatment[J]. Liaoning Chemical Industry, 2020, 49(1): 57-59, 61 (in Chinese). doi: 10.14029/j.cnki.issn1004-0935.2020.01.013
[23] HASHIMOTO K, YAMASHITA M. Seasonal variation in quality and chemical composition of the muscles of the spotted mackerel Scomber australasicus and Pacific mackerel S. japonicus[J]. Fisheries Science, 2019, 85(4): 767-775. doi: 10.1007/s12562-019-01324-0 [24] HE X S, XI B D, CUI D Y, et al. Influence of chemical and structural evolution of dissolved organic matter on electron transfer capacity during composting[J]. Journal of Hazardous Materials, 2014, 268: 256-263. doi: 10.1016/j.jhazmat.2014.01.030 [25] XIAO X, XI B D, HE X S, et al. Redox properties and dechlorination capacities of landfill-derived humic-like acids[J]. Environmental Pollution, 2019, 253: 488-496. doi: 10.1016/j.envpol.2019.07.044 [26] 董永成. 武进区地表水水质分布特性及其荧光溶解性有机物来源解析[D]. 上海: 华东理工大学, 2020: 95. DONG Y C. Characteristics of water quality and source analysis of fluorescent dissolved organic material in Wujin district surface water[D]. Shanghai: East China University of Science and Technology, 2020: 95 (in Chinese)
[27] SHANG Y X, SONG K S, JACINTHE P A, et al. Characterization of CDOM in reservoirs and its linkage to trophic status assessment across China using spectroscopic analysis[J]. Journal of Hydrology, 2019, 576: 1-11. doi: 10.1016/j.jhydrol.2019.06.028 [28] WAGNER E D, PLEWA M J. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review[J]. Journal of Environmental Sciences, 2017, 58: 64-76. doi: 10.1016/j.jes.2017.04.021 [29] RICHARDSON S D, FASANO F, ELLINGTON J J, et al. Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water[J]. Environmental Science & Technology, 2008, 42(22): 8330-8338. [30] McKNIGHT D M, BOYER E W, WESTERHOFF P K, et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity[J]. Limnology and Oceanography, 2001, 46(1): 38-48. doi: 10.4319/lo.2001.46.1.0038 [31] 白璐, 徐雄, 刘权震, 等. 武汉市不同类型天然水体中溶解性有机质的三维荧光光谱特征[J]. 光谱学与光谱分析, 2022, 42(5): 1642-1647. BAI L, XU X, LIU Q Z, et al. Characterization and analysis of dissolved organic matter in different types of natural water in Wuhan by three-dimensional fluorescence spectra[J]. Spectroscopy and Spectral Analysis, 2022, 42(5): 1642-1647 (in Chinese).
[32] JAFFÉ R, BOYER J N, LU X, et al. Source characterization of dissolved organic matter in a subtropical mangrove-dominated estuary by fluorescence analysis[J]. Marine Chemistry, 2004, 84(3/4): 195-210. [33] 张洪. 毛乌素沙地地表水、地下水和矿井水的可溶性有机碳光谱特征[D]. 西安: 西安建筑科技大学, 2021: 74. HANG H. Spectral characteristics of DOC in surface water, groundwater and mine water of maowusu Shamo[D]. Xi'an: Xi'an University of Architecture and Technology, 2021: 74 (in Chinese).
[34] 何杰, 李学艳, 林欣, 等. 光谱特征法辨识不同污染景观河道中溶解性有机物的组分与来源[J]. 环境科学学报, 2021, 41(3): 1000-1010. HE J, LI X Y, LIN X, et al. Spectral feature method was used to identify the components and sources of dissolved organic matter in different polluted landscape channels[J]. Acta Scientiae Circumstantiae, 2021, 41(3): 1000-1010 (in Chinese).
[35] 杨欣, 吴支行, 叶寅, 等. 店埠河农业小流域水体溶解性有机质三维荧光光谱的平行因子分析[J]. 光谱学与光谱分析, 2022, 42(3): 978-983. YANG X, WU Z H, YE Y, et al. Parallel factor analysis of fluorescence excitation emission matrix spectroscopy of DOM in waters of agricultural watershed of dianbu river[J]. Spectroscopy and Spectral Analysis, 2022, 42(3): 978-983 (in Chinese).
[36] HE W, HUR J. Conservative behavior of fluorescence EEM-PARAFAC components in resin fractionation processes and its applicability for characterizing dissolved organic matter[J]. Water Research, 2015, 83: 217-226. doi: 10.1016/j.watres.2015.06.044 [37] 周石磊, 孙悦, 黄廷林, 等. 周村水库大气湿沉降氮磷及溶解性有机物特征[J]. 水资源保护, 2020, 36(3): 52-59. ZHOU S L, SUN Y, HUANG T L, et al. Characteristics of nitrogen, phosphorus and dissolved organic matter in atmospheric wet deposition of Zhoucun Reservoir[J]. Water Resources Protection, 2020, 36(3): 52-59 (in Chinese).
[38] 刘堰杨, 秦纪洪, 刘琛, 等. 基于三维荧光及平行因子分析的川西高原河流水体CDOM特征[J]. 环境科学, 2018, 39(2): 720-728. doi: 10.13227/j.hjkx.201708208 LIU Y Y, QIN J H, LIU C, et al. Characteristics of chromophoric dissolved organic matter(CDOM) in rivers of western Sichuan Plateau based on EEM-PARAFAC analysis[J]. Environmental Science, 2018, 39(2): 720-728 (in Chinese). doi: 10.13227/j.hjkx.201708208
[39] 简正军, 徐健. 基于三维荧光光谱的鄱阳湖湿地水体有色可溶性有机物组成特征和来源[J]. 环境科学学报, 2022, 42(2): 213-223. doi: 10.13671/j.hjkxxb.2021.0458 JIAN Z J, XU J. Composition characteristics and source of chromophoric dissolved organic matter in Poyang Lake wetland based on three-dimensional fluorescence excitation-emission matrix spectroscopy[J]. Acta Scientiae Circumstantiae, 2022, 42(2): 213-223 (in Chinese). doi: 10.13671/j.hjkxxb.2021.0458
[40] FELLMAN J B, HOOD E, SPENCER R G M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review[J]. Limnology and Oceanography, 2010, 55(6): 2452-2462. doi: 10.4319/lo.2010.55.6.2452 [41] 刘笑菡, 张运林, 殷燕, 等. 三维荧光光谱及平行因子分析法在CDOM研究中的应用[J]. 海洋湖沼通报, 2012(3): 133-145. doi: 10.13984/j.cnki.cn37-1141.2012.03.015 LIU X H, ZHANG Y L, YIN Y, et al. Application of three-dimensional fluorescence spectroscopy and parallel factor analysis in cdom study[J]. Transactions of Oceanology and Limnology, 2012(3): 133-145 (in Chinese). doi: 10.13984/j.cnki.cn37-1141.2012.03.015
期刊类型引用(1)
1. 付蔚,梁建奎,吴胜念,李珏纯,季闻翔,董慧峪,于建伟,张洪刚,张颖,苗钦奎,强志民. 长距离调水水源中溶解性有机物特征及消毒副产物生成势的变化解析. 环境工程学报. 2024(06): 1550-1558 .  百度学术
百度学术
其他类型引用(0)
-






 DownLoad:
DownLoad: