-
新污染物是指新近被发现或关注,对生态安全或人类健康存在风险,尚未被纳入管理或现有管理措施不足以有效控制其风险的污染物. 2022年5月24日,国务院办公厅发布的《新污染物治理行动方案》指出,目前国内外广泛关注的新污染物主要包括国际公约管控的持久性有机污染物、内分泌干扰物和抗生素等. 近年来,新污染物在世界各地的水环境中被频繁检出,虽然它们在水中浓度非常低(通常在ng·L–1 — μg·L–1级别),但它们通常具有环境持久性和生物累积性等特征,严重威胁生态环境安全和人类健康[1-7]. 高级氧化技术(AOPs)是去除水中新污染物的重要方法之一[8-12],常见的AOPs包括以羟基自由基(HO•)和硫酸根自由基(SO4•−)为核心的氧化技术. HO•(E0 = 1.8—2.7 V,NHE)和SO4•−(E0 = 2.5—3.1 V,NHE)均具有很强的氧化能力[13]. 然而,相较于HO•(t1/2 = 1 μs),SO4•−(t1/2 = 30–40 μs)具有更长的半衰期[14]且氧化污染物的选择性更强[15]. 因此,近年来,基于SO4•−的AOPs在水污染控制领域受到越来越多的关注[16-21].
SO4•−主要通过活化过一硫酸盐(PMS)或过二硫酸盐(PDS)产生,常见的活化方法包括能量活化[22-27]和过渡金属活化[28-30]等. 然而,PMS和PDS成本较高且具有一定的毒性,限制了其在水处理领域的应用[31]. 相较于PMS和PDS,亚硫酸盐(S(Ⅳ))价格低廉且毒性更低[32-33]. 因此,活化S(Ⅳ)(S(Ⅳ)-AOPs)作为替代活化过硫酸盐产生SO4•−的方法受到研究者们的广泛关注. 目前,多种方法和化学物质已被用于活化S(Ⅳ)产生SO4•−,包括紫外辐射[31]、Fe(Ⅱ)和Cu(Ⅱ)等低价态过渡金属[34-36]、高锰酸钾和高铁酸钾等氧化剂[37-42]以及零价铁等非均相材料[43-45]. 这些S(Ⅳ)-AOPs中SO4•−的产生机制主要包括:S(Ⅳ)通过单电子转移反应生成亚硫酸根自由基(SO3•−),生成的SO3•−在有氧条件下会通过一系列反应生成SO4•−. 显然,S(Ⅳ)-AOPs比PDS/PMS-AOPs所涉及的自由基转化反应更为复杂,使得同一种S(Ⅳ)-AOP的反应机制常常存在争议.
然而,目前研究者们主要围绕开发新的S(Ⅳ)-AOPs开展研究,鲜有研究对现有的S(Ⅳ)-AOPs及其反应机制进行较为详细的分类与总结. 因此,本文系统总结了S(Ⅳ)的理化性质及S(Ⅳ)-AOPs中含硫自由基的转化机制;梳理了各类S(Ⅳ)-AOPs的反应机制;阐述了pH、S(Ⅳ)投量、溶解氧(DO)和背景基质等因素对S(Ⅳ)-AOPs氧化水中污染物效能的影响;以期为S(Ⅳ)-AOPs在水污染控制领域的应用提供参考.
-
S(Ⅳ)是一种强还原剂,广泛应用于食品、饮料及医药行业[33]. S(Ⅳ)常作为各种食物和果汁饮料产品的添加剂,与其他食品添加剂(如,姜黄素、苯甲酸盐和富马酸等)相比,S(Ⅳ)同时具有漂白、防腐、抗菌、脱色和抗氧化功能,即集多种功能于一身,且价格低廉、易于保存. 在葡萄酒和啤酒中加入适量的S(Ⅳ),可使葡萄酒色泽稳定,消除啤酒中的异味,使其风味纯正[33].
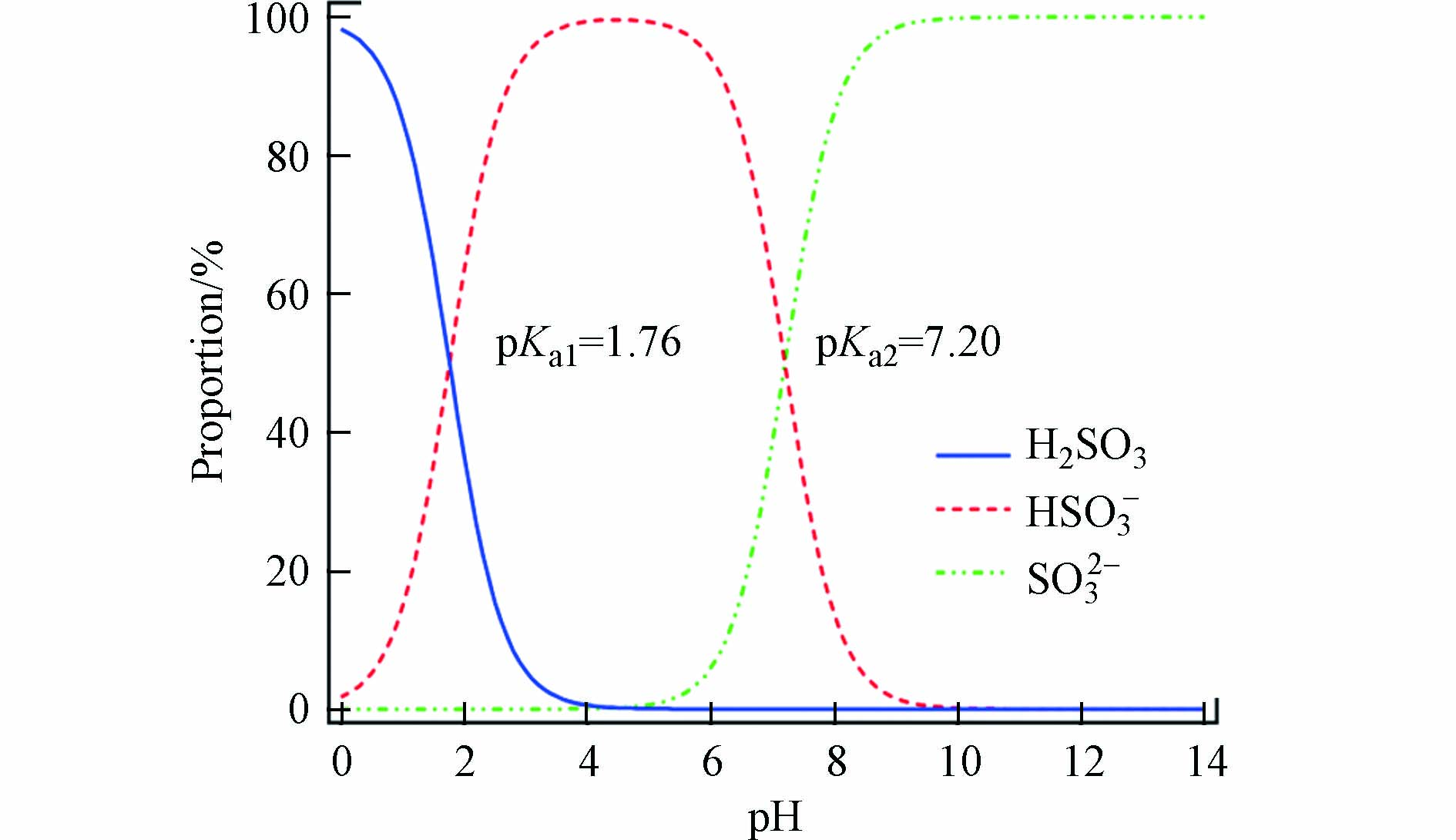
S(Ⅳ)是一种含氧酸盐,其母体酸-亚硫酸(H2SO3)有两个pKa,即pKa1 = 1.76,pKa2 = 7.20. 不同pH条件下,亚硫酸在水中的形态分布如图1所示,可见S(Ⅳ)在pH 1.76–7.2范围内主要是以亚硫酸氢根(HSO3−)的形式存在,当pH>7.2时则主要以亚硫酸根(SO32−)的形式存在. 当pH<4.0时,随着pH的降低,HSO3−逐渐转化为H2SO3并有二氧化硫(SO2)气体从溶液中逸出[46].
常见的亚硫酸盐包括亚硫酸钠(Na2SO3)和亚硫酸钙(CaSO3). Na2SO3易溶于水且溶液呈碱性,其溶解度随温度升高而增大(0 ℃时,125.4 g·L–1;18 ℃时,678.0 g·L–1);CaSO3微溶于水(25 ℃时Ksp = [Ca2+][SO32−] = (3.1 ± 1.5) × 10–7)且溶解度随pH降低而升高[47]. 下文如无特别说明,则研究中所使用的S(Ⅳ)为Na2SO3.
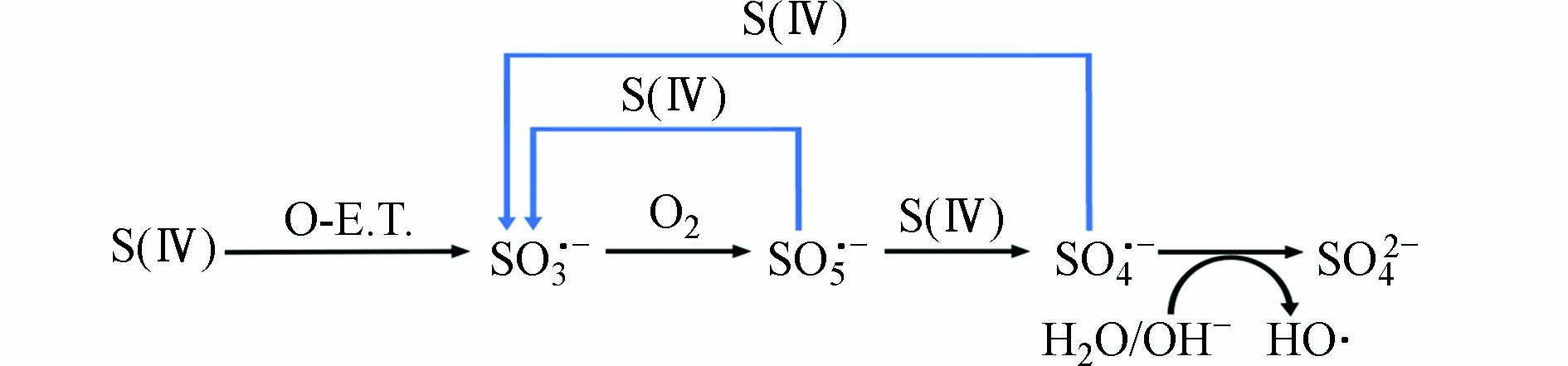
在S(Ⅳ)-AOPs中,含硫自由基的转化机制如图2所示,其中所涉及的反应方程及相应的反应速率常数如表1所示. S(Ⅳ)首先通过单电子转移反应生成SO3•−,SO3•−会以接近扩散的速率与氧气(O2)发生反应生成过一硫酸根自由基(SO5•−). 所生成的SO5•−会与S(Ⅳ)通过两种途径发生反应,一种是生成过一硫酸(氢)根(SO52–、HSO5–)和SO3•−,另一种是生成SO4•−和硫酸根(SO42−). 生成的SO4•−可与水或氢氧根(OH−)发生反应生成HO•. 此外,SO4•−也可与S(Ⅳ)发生反应生成SO3•−.
-
目前,已开发出了多种活化S(Ⅳ)的方法,本文根据S(Ⅳ)活化方法将S(Ⅳ)-AOPs分为以下五类:低价态过渡金属活化S(Ⅳ)技术[36, 51-67]、过渡金属材料活化S(Ⅳ)技术[43-45, 68-85]、氧化剂活化S(Ⅳ)技术[37-40, 42, 86-102]、外加能量活化S(Ⅳ)技术[103-118]以及多种方式联合活化S(Ⅳ)技术[17, 31, 83, 85, 108-119].
-
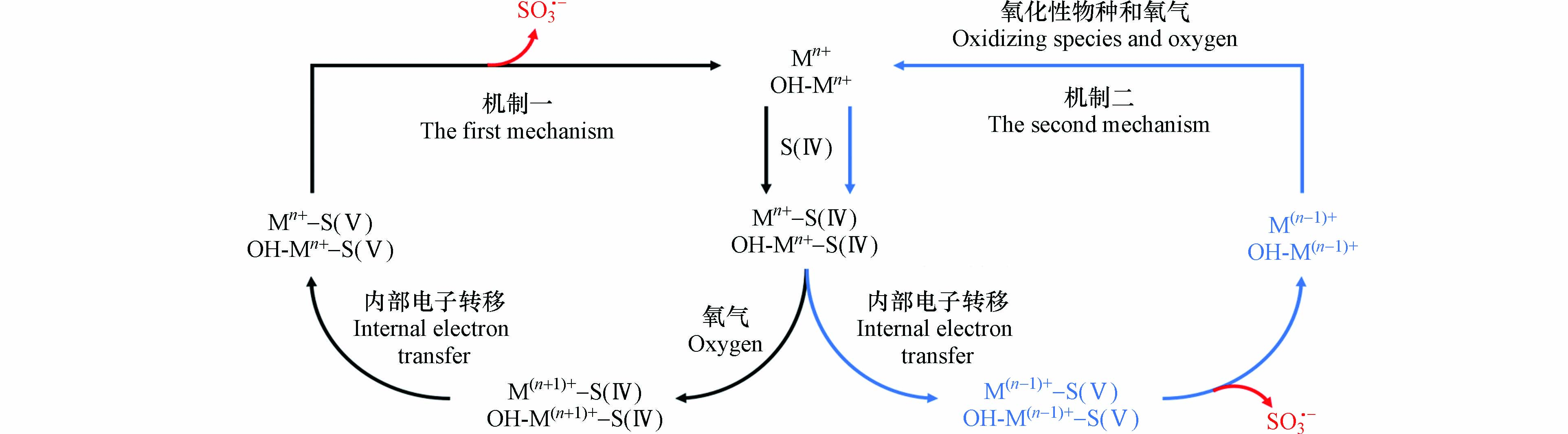
可活化S(Ⅳ)的低价态过渡金属包括Mn(Ⅱ)、Fe(Ⅱ)、Fe(Ⅲ)、Cu(Ⅱ)和Co(Ⅱ)等. 这些低价态过渡金属首先与S(Ⅳ)发生单电子转移反应生成SO3•−[36, 51-64],属于均相反应,所涉及的反应机制可分为两种,如图3所示. 低价态过渡金属离子(Mn+)与S(Ⅳ)首先会通过球内络合作用生成Mn+-S(Ⅳ)中间体. Mn+-S(Ⅳ)中间体在有氧条件下可与O2发生反应生成M(n+1)+-S(Ⅳ)中间体,所生成的M(n+1)+-S(Ⅳ)中间体发生内部电子转移反应生成Mn+-S(Ⅴ)中间体并快速分解生成Mn+和SO3•−. 此外,Mn+-S(Ⅳ)中间体也可直接发生内部电子转移反应生成M(n-1)+-S(Ⅴ)中间体并快速分解生成M(n-1)+和SO3•−,生成的M(n-1)+可被体系中的氧化性物种(如SO5•−和SO4•−)和O2氧化为Mn+.
在有氧条件下,生成的SO3•−可通过链式反应进一步转化为SO5•−、SO4•−和HO•,因此在低价态过渡金属活化S(Ⅳ)技术中,SO5•−、SO4•−和HO•通常被认为是氧化水中污染物的活性氧化剂[54, 57, 60, 64, 66]. 但是,非自由基活性氧化剂也可能在低价态过渡金属活化S(Ⅳ)技术中产生并对水中污染物的降解做出贡献. 例如,Zhang等[65]发现,Mn(Ⅲ)是Fe(Ⅱ)/Mn(Ⅱ)/S(Ⅳ)体系氧化阿特拉津的主要活性氧化剂. 然而,研究者们并未进一步详细阐述非自由基活性氧化剂在低价态过渡金属活化S(Ⅳ)技术中的生成情况、转化机制及其对水中污染物降解的贡献.
低价态过渡金属活化S(Ⅳ)技术氧化水中污染物效能的总结如表2所示. 其中,Fe(Ⅱ)/S(Ⅳ)、Fe(Ⅲ)/S(Ⅳ)和Mn(Ⅱ)/S(Ⅳ)体系在酸性条件下可高效降解水中污染物,但在中性或碱性条件下,对水中污染物的降解效率较低或几乎无降解作用[54, 56, 59-60, 65]. 例如,在初始pH(pHini)分别为5.0和7.0条件下,反应时间为20 min时,Fe(Ⅱ)/S(Ⅳ)体系对卡马西平的去除率分别为87%以上和20%左右[60]. 而Cu(Ⅱ)/S(Ⅳ)和Co(Ⅱ)/S(Ⅳ)体系在碱性条件下可高效降解水中污染物,但在酸性或中性条件下,对水中污染物的降解效率较低或几乎无降解作用[53, 57, 58, 66]. 例如,在pHini 6.0和8.0条件下,反应时间为20 min时,Co(Ⅱ)/S(Ⅳ)体系对碘海醇的去除率分别小于20%和高达100%[53]. 通常情况下,Fe(Ⅱ)/S(Ⅳ)和Fe(Ⅲ)/S(Ⅳ)体系氧化水中污染物的最佳pH适用范围为3.0–6.0[54, 56, 59-60, 64],Mn(Ⅱ)/S(Ⅳ)体系氧化水中污染物的最佳pH适用范围不高于4.0[54, 56, 67],而Co(Ⅱ)/S(Ⅳ)和Cu(Ⅱ)/S(Ⅳ)体系氧化水中污染物的最佳pH适用范围分别为8.0–10.0[53, 57, 66]和8.0–11.0[57- 58].
综上所述,低价态过渡金属活化S(Ⅳ)技术反应条件温和,操作简单,可快速高效降解水中多种污染物. 然而,低价态过渡金属活化S(Ⅳ)技术存在如下不足:最佳pH适用范围较窄;在连续流反应器中,低价态过渡金属的回收较为困难,重复利用性差;过渡金属离子排入水体可能会造成二次污染;过渡金属络合物或沉淀物仍需进一步处理等.
-
为了克服低价态过渡金属活化S(Ⅳ)技术存在的诸多不足,现有研究开发出了多种过渡金属材料替代低价态过渡金属活化S(Ⅳ)以氧化水中污染物. 这些过渡金属材料包括零价铁及改性零价铁[43-45]、过渡金属氧化物[69, 76, 84]、负载过渡金属的硅基或硅铝酸盐材料[73, 77-78]、含过渡金属的金属-有机框架(MOF)材料[72, 79]和过渡金属硫化物[75, 80-81]等.
过渡金属材料活化S(Ⅳ)过程的反应机制本质上与低价态过渡金属活化S(Ⅳ)过程的反应机制相同,即溶液中或材料上的低价态过渡金属首先与S(Ⅳ)发生单电子转移反应生成SO3•−,属于非均相反应. 例如,硫化亚铁、零价铁及改性零价铁等过渡金属材料可通过多种方式(溶解或腐蚀)向溶液释放Fe(Ⅱ),Fe(Ⅱ)与S(Ⅳ)反应首先生成SO3•−的机制如图3所示[43-45, 75];氧化铜和硫化钴等过渡金属材料主要通过材料表面的活性位点(即低价态过渡金属位点)与S(Ⅳ)结合并活化S(Ⅳ)首先生成SO3•−,且O2使得材料上的低价态过渡金属实现循环[72, 73, 76-81]. 与低价态过渡金属活化S(Ⅳ)技术类似,SO5•−、SO4•−和HO•亦被认为是过渡金属材料活化S(Ⅳ)技术氧化水中污染物的活性氧化剂[43-45, 72-73, 75-81].
表2总结了过渡金属材料活化S(Ⅳ)技术氧化水中污染物的效能. 过渡金属材料活化S(Ⅳ)技术氧化水中污染物的最佳pH适用范围与材料中的过渡金属类型有关. 当过渡金属材料为硫化亚铁、零价铁及改性零价铁等含铁材料时,过渡金属活化S(Ⅳ)技术氧化水中污染物的最佳pH适用范围通常为6.0–7.0. 例如,反应时间为20 min时,在pHini 6.0和7.0的条件下,FeS/S(Ⅳ)体系对普萘洛尔的去除率分别为95%以上和90%左右;但在pHini 8.0和9.0条件下,FeS/S(Ⅳ)体系对普萘洛尔几乎无降解作用[75]. 而当过渡金属材料为氧化铜和硫化钴等含铜或钴材料时,过渡金属材料活化S(Ⅳ)技术氧化水中污染物的最佳pH适用范围通常为pH 7.0–8.0. 例如,反应时间为30 min时,在pHini 7.0和8.0条件下,CoS/S(Ⅳ)体系对碘海醇的去除率为78%左右和90%以上;但在pHini 6.0条件下,CoS/S(Ⅳ)体系对碘海醇的去除率低于40%[81].
过渡金属材料活化S(Ⅳ)技术操作简单,反应条件温和,最佳pH适用范围通常为6.0–8.0. 相较于低价态过渡金属,大部分过渡金属材料(除硫化亚铁、零价铁及改性零价铁外)具有回收简单、重复利用性高和反应后金属泥量少的优点. 然而,过渡金属材料活化S(Ⅳ)技术的使用往往需要投加大量过渡金属材料(不低于10 mg·L–1,多数时候在100 mg·L–1以上);材料的制作或购买会增加水污染控制的成本;部分材料可能会出现金属浸出的问题,容易造成二次污染.
-
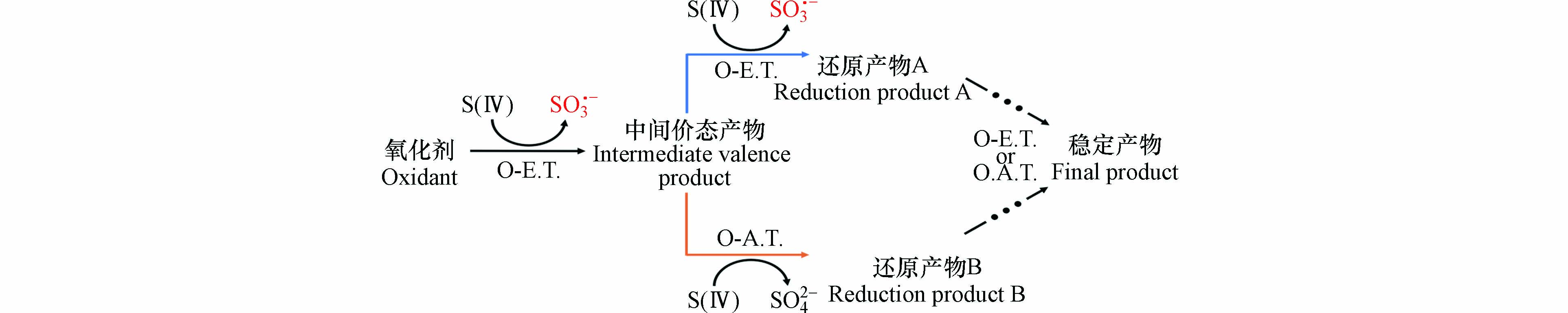
高锰酸盐(Mn(Ⅶ))、高铁酸盐(Fe(Ⅵ))、二氧化氯(ClO2)、PDS和四价铈(Ce(Ⅳ))等氧化剂可活化S(Ⅳ)氧化水中污染物[38, 93, 97-99, 102]. 此外,有研究[88, 90, 92]表明,S(Ⅳ)在还原六价铬(Cr(Ⅵ))和溴酸盐(BrO3−)等无机污染物的过程中也可产生活性氧化剂,从而同步去除共存的有机污染物. 这些氧化剂与S(Ⅳ)反应的机制如图4所示.
这些氧化剂首先与S(Ⅳ)发生单电子转移反应生成中间价态产物和SO3•−,属于均相反应. 生成的中间价态产物通常具有一定的氧化性,可继续与S(Ⅳ)发生单电子转移反应生成还原产物A和SO3•−,也可与S(Ⅳ)发生氧原子转移反应生成还原产物B和SO42−. 生成的还原产物A和B如果不是稳定的还原产物,还会继续与S(Ⅳ)发生一系列单电子(或氧原子)转移反应生成SO3•−(或SO42−),直到转化为稳定产物. Mn(Ⅶ)、Fe(Ⅵ)、Cr(Ⅵ)、Ce(Ⅳ)、ClO2、PDS和BrO3−与S(Ⅳ)的反应均遵循上述反应机制. 此外,ClO2也会与S(Ⅳ)首先发生氧原子转移反应生成ClO•和SO42−(图4未标出),生成的ClO•可与S(Ⅳ)发生单电子转移反应生成ClO−和SO3•−,所生成的ClO−会与S(Ⅳ)发生氧原子转移反应生成Cl−和SO42−[120].
已被研究的氧化剂可分为非金属氧化剂(如,ClO2、PDS和BrO3−)和高价态金属氧化剂(如,Mn(Ⅶ)、Fe(Ⅵ)、Ce(Ⅳ)和Cr(Ⅵ)). 当非金属氧化剂活化S(Ⅳ)时,SO5•−、SO4•−和HO•通常被认为是氧化剂活化S(Ⅳ)技术氧化水中污染物的主要活性氧化剂[88, 93, 97- 98]. 其中,ClO2会与S(Ⅳ)发生氧原子转移反应生成ClO•和SO42−,因此ClO•也被认为是ClO2/S(Ⅳ)体系氧化水中污染物的活性氧化剂[93, 97].
然而,当高价态金属氧化剂活化S(Ⅳ)时,活性氧化剂的种类较为复杂,这主要是因为高价态金属氧化剂与S(Ⅳ)发生反应不仅会产生自由基物种,而且还会产生中间价态活性金属. 例如,Mn(Ⅶ)与S(Ⅳ)发生反应生成SO3•−的同时,自身会被S(Ⅳ)依次还原为Mn(Ⅵ)、Mn(Ⅴ)、Mn(Ⅳ)、Mn(Ⅲ)和Mn(Ⅱ)[38-40, 96, 100]. 其中,Mn(Ⅳ)和Mn(Ⅲ)的氧化能力较弱,Mn(Ⅶ)/S(Ⅳ)体系氧化水中污染物过程中它们的贡献基本可被忽略. 因此,Mn(Ⅶ)/S(Ⅳ)体系氧化水中污染物过程中的活性氧化剂包括强氧化性的自由基(SO4•−和HO•)和活性锰(Mn(Ⅵ)和Mn(Ⅴ)). Mn(Ⅶ)/S(Ⅳ)体系氧化水中污染物的过程中活性氧化剂的贡献与污染物结构、S(Ⅳ)与Mn(Ⅶ)初始浓度摩尔比及pH有关[100]. 类似地,Fe(Ⅵ)活化S(Ⅳ)过程中的活性氧化剂有SO4•−、HO•、Fe(Ⅴ)和Fe(Ⅳ)[37, 87, 94-95, 101],Cr(Ⅵ)活化S(Ⅳ)过程中的主要活性氧化剂是SO4•−和Cr(Ⅴ)[90, 92]. Ce(Ⅳ)具有一定的特殊性,由于Ce(Ⅳ)被S(Ⅳ)还原为Ce(Ⅲ)只需一步单电子转移反应,因此Ce(Ⅳ)活化S(Ⅳ)过程中的主要活性氧化剂是SO4•−和HO•,不涉及中间价态活性金属.
氧化剂活化S(Ⅳ)技术可高效降解水中污染物(见表2). 其中,Mn(Ⅶ)/S(Ⅳ)体系可在较宽的pH范围内超快速降解水中多种污染物. 例如,在pHini 5.0的条件下,Mn(Ⅶ)/S(Ⅳ)体系可在0.15 s内对苯酚、环丙沙星和甲基蓝的去除率达100%[40]. Fe(Ⅵ)/S(Ⅳ)体系通常在碱性条件下可高效降解水中多种污染物. 例如,在pH 9.0的条件下,反应时间为30 s时,Fe(Ⅵ)/S(Ⅳ)体系对苯酚、环丙沙星、甲基蓝、罗丹明B和甲基橙的去除率为95%以上[101]. ClO2/S(Ⅳ)体系可在较宽的pH范围内高效降解水中污染物. 例如,在pH 6.0—11.0条件下,反应时间为10 s时,ClO2/S(Ⅳ)体系对卡马西平的去除率达55%以上[97];在pH 5.0–9.0条件下,反应时间为3 min时,ClO2/S(Ⅳ)体系对阿特拉津的去除率为80%以上[93]. PDS/S(Ⅳ)体系在pH 3.0条件下高效降解水中污染物. 例如,在pHini 3.0条件下,反应时间为50 min时,PDS/S(Ⅳ)体系对甲基橙的去除率达85%以上[98]. S(Ⅳ)在pH≤5.0条件下可高效还原Cr(Ⅵ)或BrO3–,且可高效降解共存的有机污染物. 例如,在pHini 4.0条件下,S(Ⅳ)不仅可在5 min内高效还原Cr(Ⅵ)且可有效降解共存的对氯苯酚、对氯苯甲酸、阿莫西林、雌二醇和布洛芬[90];在pHini 4.0条件下,S(Ⅳ)可在30 min内有效还原BrO3–,且此过程中共存的扑热息痛、双酚A、卡马西平、诺氟沙星和阿特拉津的去除率达80%以上[88].
氧化剂活化S(Ⅳ)技术具有操作简单和快速、高效降解水中污染物的优势,但该技术对混合条件要求较高,例如,Mn(Ⅶ)/S(Ⅳ)体系通常需要快速混合方可高效去除水中污染物. 此外,部分技术对pH条件要求较高,例如,Cr(Ⅵ)(或BrO3–)/S(Ⅳ)体系通常需要在pH≤5.0的条件下方可对共存有机污染物达到高效的同步去除作用.
-
外加能量(如超声波、电、可见光和紫外光等)也可活化S(Ⅳ)产生活性氧化剂以降解水中污染物. S(Ⅳ)在外加能量的作用下可发生单电子转移反应生成SO3•−[83, 103-117],反应机制如图5所示. 其中,路径1是指能量直接将S(Ⅳ)分解为SO3•−和电子(e−),e−会与水反应生成水合电子(eaq−),eaq−可与O2发生反应生成超氧自由基(HO2•/O2•−);而路径2主要是指能量借助一些半导体材料或感光物质间接活化S(Ⅳ). 例如,能量作用于半导体材料产生电子空穴(h+)和e−,h+活化S(Ⅳ)生成SO3•−,e−则依次转化为eaq−和HO2•/O2•−. 此外,能量也可作用于感光物质生成相应的激发态物质,这些激发态物质也能与S(Ⅳ)发生单电子转移反应生成SO3•−(图5未标出).
超声波[109]和电[118]是通过路径1直接活化S(Ⅳ)生成SO3•−,可见光[110, 116-117]是通过路径2间接活化S(Ⅳ)生成SO3•−,紫外光既能通过路径1直接活化S(Ⅳ)生成SO3•−[104, 114-115],也能通过路径2间接活化S(Ⅳ)生成SO3•−[106, 111-113]. 因此,SO4•−、HO•、O2•−和HO2•通常被认为是外加能量活化S(Ⅳ)技术氧化水中污染物的活性氧化剂. 但是,当使用含铁的半导体材料作为感光剂时,中间价态活性铁也可能是可见光活化S(Ⅳ)氧化水中污染物的活性氧化剂. 例如,在pH 10.0条件下,活性铁(Fe(Ⅳ)或Fe(Ⅴ))和自由基均是可见光/Fe2O3/S(Ⅳ)体系[116]和可见光/ZnFe2O4@PAIN/S(Ⅳ)体系[117]氧化水中污染物的活性氧化剂,但在酸性和中性条件下,SO4•−和HO•等自由基才是这两个体系氧化水中污染物的活性氧化剂.
关于超声波或电活化S(Ⅳ)技术,目前仅有其氧化As(Ⅲ)的研究报道. 如表2所示,在pH 7.0条件下,反应时间为30–40 min时,超声波/S(Ⅳ)和电/S(Ⅳ)体系均对As(Ⅲ)的去除率达100%[109, 118]. 可见光活化S(Ⅳ)技术的最佳pH适用范围与感光剂或半导体材料的性质有关. 例如,当ZnFe2O4@PAIN作为感光材料时,反应时间为30 min时,可见光/S(Ⅳ)体系在pH 5.0的条件下可完全氧化As(Ⅲ)[117];当Fe2O3作为感光材料时,反应时间为50 min时,可见光/S(Ⅳ)体系在pH 10.0条件下对硝酚砷酸的去除率达100%[116];当亚甲基蓝作为感光剂时,反应时间为30 min时,可见光/S(Ⅳ)体系在pH 7.3条件下可同步去除As(Ⅲ)和亚甲基蓝,去除率分别为100%和80% [110]. 紫外光/S(Ⅳ)体系的最佳pH适用范围与活化路径有关. 当紫外光通过路径1活化S(Ⅳ)时,紫外光/S(Ⅳ)体系通常在中性和碱性条件下(pH 7.0—10.0)高效降解水中污染物[104, 114-115];而当紫外光通过路径2活化S(Ⅳ)时,紫外光/S(Ⅳ)体系的最佳pH适用范围与感光剂的性质有关. 例如,当ZnO作为感光剂时,紫外光/S(Ⅳ)体系氧化水中污染物的最佳pH为12.0[113];而当TiO2作为感光剂时,紫外光/S(Ⅳ)体系氧化水中污染物的最佳pH为7.0[106].
外加能量活化S(Ⅳ)技术通常具有高效、简便、二次污染小和pH适用范围广的优势. 但外加超声波、电和紫外光等能量需要额外的设备和能耗,使用可见光也需要配套的设备和材料,均会增加水处理成本.
-
基于上述四类S(Ⅳ)-AOPs的优缺点,研究者们开发出多种方式联合活化S(Ⅳ)技术. 多种方式联合活化S(Ⅳ)技术可有效弥补单一方式活化S(Ⅳ)技术的不足,且对水中污染物的去除效能更高. 例如,Fe(Ⅲ)/S(Ⅳ)体系和Fe(Ⅱ)/S(Ⅳ)体系具有环境友好的优点,但Fe(Ⅲ)-S(Ⅳ)中间体内部的电子转移步骤过于缓慢,是体系反应的限速步骤,而紫外光(甚至是可见光)可以加速中间体内部的电子转移. 因此,Guo等[17]和Yu等[119]分别将紫外光引入Fe(Ⅲ)/S(Ⅳ)体系和Fe(Ⅱ)/S(Ⅳ)体系,构成的紫外光/Fe(Ⅲ)/S(Ⅳ)体系和紫外光/Fe(Ⅱ)/S(Ⅳ)体系氧化水中污染物的效能要高于单独的紫外光/S(Ⅳ)体系、Fe(Ⅲ)/S(Ⅳ)体系和Fe(Ⅱ)/S(Ⅳ)体系. Xiang等[83]将可见光引入电/S(Ⅳ)体系,使用MoS2作为光阳极,利用光电化学产生e−和h+,e−在外加电位的作用下进行移动从而形成电流. 仅在阳极施加0.6 V的电位,可见光/电/S(Ⅳ)体系对水中污染物的去除率可达80%,远高于单独的电/S(Ⅳ)体系对水中污染物的氧化效能(不足30%). Jia等[108]将电引入Mn(Ⅱ)/S(Ⅳ)体系,在pH 6.0条件下,构成的电/Mn(Ⅱ)/S(Ⅳ)体系对水中污染物的去除率达94%以上,而电/S(Ⅳ)体系和Mn(Ⅱ)/S(Ⅳ)体系对水中污染物的去除率均低于50%.
多种方式联合活化S(Ⅳ)技术也存在一些问题,例如,多种活化方式的联合使用会增加工艺的复杂性,需要额外的材料和设备,增加工艺的运行成本.
-
pH是影响S(Ⅳ)-AOPs氧化有机污染物效能的关键因素之一. 首先,pH会影响活化剂与S(Ⅳ)的存在形态,从而影响活化剂与S(Ⅳ)的反应特性,进而影响S(Ⅳ)-AOPs氧化水中污染物的效能. 例如,由于SO32−与超声波、电和紫外光的反应性要高于HSO3−,因此当pH由5.0升至9.0时,超声波、电和紫外光活化S(Ⅳ)技术氧化水中污染物的表观速率常数也随之升高[109, 114, 118].
其次,对于含有多种活性氧化剂的体系(如,Mn(Ⅶ)/S(Ⅳ)体系、可见光/Fe2O3/S(Ⅳ)体系和可见光/ZnFe2O4@PAIN/S(Ⅳ)体系等),pH还会影响活性氧化剂的产生及其对水中污染物降解的贡献. 例如,随着pHini的升高,Mn(Ⅶ)/S(Ⅳ)体系氧化水中污染物过程中SO4•−和HO•的贡献逐渐升高,而活性锰的贡献逐渐降低[100],这主要是由于活性锰的活性随着pH值的升高而逐渐降低.
最后,对于一些非均相材料/S(Ⅳ)体系(如过渡金属材料/S(Ⅳ)体系、可见光/材料/S(Ⅳ)体系和紫外光/材料/S(Ⅳ)体系等),pH还会影响材料基底的性质,从而影响材料与S(Ⅳ)的反应特性,进而影响体系氧化水中污染物的效能. 例如,由于酸性条件不利于硅基材料的表面羟基化,而表面羟基化是促使材料表面的Co(Ⅱ)或Cu(Ⅱ)与S(Ⅳ)结合的重要条件,因此掺杂钴或铜的硅基材料/S(Ⅳ)体系在pH<7.0条件下氧化水中污染物的效能低于该体系在pH>7.0条件下氧化水中污染物的效能[73, 77].
-
当固定活化剂投量时,随着S(Ⅳ)投量的增加,S(Ⅳ)-AOPs氧化水中污染物的效能先升高再降低,说明S(Ⅳ)投量过高或过低均不利于S(Ⅳ)-AOPs对污染物的降解. 这是因为当S(Ⅳ)投量过低时,活化剂活化S(Ⅳ)产生SO4•−和HO•等活性氧化剂的量过低;当S(Ⅳ)投量过高时,过量的S(Ⅳ)会快速淬灭体系中产生的活性氧化剂,与水中污染物竞争活性氧化剂,从而使得S(Ⅳ)-AOPs对污染物的去除效能较低. 例如,在pHini 5.0条件下,固定Mn(Ⅶ)初始浓度为50 μmol·L–1,当S(Ⅳ)初始浓度由0增至0.25 mmol·L–1时,Mn(Ⅶ)/S(Ⅳ)体系对苯酚的去除率不断增加,而当S(Ⅳ)初始浓度由0.25 mmol·L–1增至2.0 mmol·L–1时,该体系对苯酚的去除率不断降低[42].
-
S(Ⅳ)的种类对S(Ⅳ)-AOPs氧化水中污染物的效能具有显著影响. 研究表明,当一次性投加大量Na2SO3时,S(Ⅳ)-AOPs氧化水中污染物的效能显著低于将等量Na2SO3进行分次投加的S(Ⅳ)-AOPs,因此提出使用缓释型S(Ⅳ)(CaSO3)替代Na2SO3以提高S(Ⅳ)-AOPs去除污染物的效能. S(Ⅳ)不仅是S(Ⅳ)-AOPs中活性氧化剂的前驱体,也可与污染物竞争活性氧化剂. CaSO3的缓释作用可削弱S(Ⅳ)对活性氧化剂的淬灭作用进而提高S(Ⅳ)和活性氧化剂的利用率. 例如,Rao等[38]发现,在pH 8.0条件下,Mn(Ⅶ)/CaSO3体系对磺胺甲恶唑、硝基苯、咖啡因、苯甲酸和对氯苯甲酸的去除量比Mn(Ⅶ)/Na2SO3体系高2–7倍. 类似地,Shao等[95]发现,在pH 8.0条件下,反应时间为2 s时,Fe(Ⅵ)/CaSO3体系和Fe(Ⅵ)/Na2SO3体系对磺胺甲恶唑的去除率分别为100%和不足60%.
-
DO对S(Ⅳ)-AOPs氧化污染物效能具有重要影响. DO会影响SO4•−和HO•等自由基的产生,但不会影响中间价态活性金属和ClO•等的产生. 因此,DO对S(Ⅳ)-AOPs氧化污染物效能的影响与活性氧化剂的贡献有关. 例如,在pHini 5.0条件下,当Mn(Ⅶ)和S(Ⅳ)的初始浓度分别为50 μmol·L–1和250 μmol·L–1时,SO4•−和HO•是Mn(Ⅶ)/S(Ⅳ)体系降解苯酚的活性氧化剂,当DO浓度由0 mg·L–1增至4.0 mg·L–1时,Mn(Ⅶ)/S(Ⅳ)体系对苯酚的去除量由不足1.0 μmol·L–1提高至20 μmol·L–1[42];而当Mn(Ⅶ)和S(Ⅳ)的初始浓度分别为50 μmol·L–1和25 μmol·L–1时,中间价态活性锰是Mn(Ⅶ)/S(Ⅳ)体系氧化苯酚的活性氧化剂,当DO浓度由0 mg·L–1增加至16.0 mg·L–1时,Mn(Ⅶ)/S(Ⅳ)体系对苯酚的去除量几乎不变[42].
-
背景基质(共存阴离子、腐殖酸(HA)和天然有机物(NOM)等)对S(Ⅳ)-AOPs氧化水中污染物效能具有抑制作用. 这主要是因为共存阴离子、HA和NOM等会与水中污染物竞争活性氧化剂,降低体系氧化水中污染物的效能. 例如,在pH 7.0条件下,当氯离子(Cl–)浓度为5 mmol·L–1时,ClO2/S(Ⅳ)体系对阿特拉津的去除率由84%下降至55.3%;当HA浓度为5 mg·L–1时,该体系对阿特拉津的去除率由84%下降至73.19%[93]. 常见背景基质与活性氧化剂(如SO4•−和HO•)的反应速率常数如表3所示.
此外,共存阴离子、HA和NOM等还可影响活化剂与S(Ⅳ)的反应,进而抑制S(Ⅳ)-AOPs对水中污染物的降解. 共存阴离子、HA和NOM等可能会与低价态过渡金属发生络合、沉淀或螯合反应,阻碍低价态过渡金属与S(Ⅳ)的结合,从而抑制低价态过渡金属与S(Ⅳ)的反应性;HA和NOM等可能会吸收外加能量,降低外加能量的利用率,进而降低外加能量活化S(Ⅳ)技术对污染物的降解.
-
S(Ⅳ)-AOPs可高效去除水中多种污染物(如药物及个人护理品、抗生素和有机染料等),在水污染控制领域具有十分广阔的应用前景. 但当前大部分研究仍停留在实验室阶段,其实际应用前景尚需通过中试及生产试验进行验证. 由于背景基质会对S(Ⅳ)-AOPs去除污染物的效能具有一定的抑制作用,因此仍需进一步研究以推动S(Ⅳ)-AOPs在实际水污染控制中的应用. 目前关于S(Ⅳ)-AOPs的研究主要有以下几个问题:
(1)以往的研究通常使用单一的手段鉴定S(Ⅳ)-AOPs中活性氧化剂的产生和作用. 研究者们往往仅采用淬灭实验、ESR实验或氧化探针化合物实验中的一种方法确定体系中活性氧化剂的种类. 例如,淬灭实验是最常用的鉴定活性氧化剂(如SO4•−和HO•)的方法之一,研究者们常对比淬灭剂加入前后S(Ⅳ)-AOPs对污染物的去除情况,从而判定体系中是否存在某种活性氧化剂. 但是,淬灭剂的加入可能会改变体系的反应路径. 因此,不能仅靠淬灭实验来确定活性氧化剂的种类. 此外,由于探针化合物往往可以被多种活性氧化剂氧化,因此不能仅靠体系氧化探针化合物实验确定活性氧化剂的种类. 例如,研究者们常用对二甲氧基苯(DMOB)作为ClO•的探针化合物,但DMOB还会与HO•、SO4•−和Cl•反应. 因此,有必要采用多种鉴定手段鉴定体系中的活性氧化剂.
(2)亟需开发更为绿色安全的S(Ⅳ)活化方法. 尽管目前研究者们已开发出多种活化S(Ⅳ)的方法,但这些方法仍有一定的局限性. 例如,低价态过渡金属活化S(Ⅳ)技术的pH适用条件较为极端(pH≤6.0或pH≥8.0);过渡金属材料活化S(Ⅳ)技术中的过渡金属材料投加量较高(通常不低于100 mg·L–1),增加水处理成本;氧化剂活化S(Ⅳ)技术可能生成有害产物,如ClO2活化S(Ⅳ)后会产生ClO2−和ClO3−,二者均为消毒副产物且浓度高时会危害人体健康;外加能量活化S(Ⅳ)技术则需要额外的设备和能源. 因此,有必要开发出更为绿色安全的S(Ⅳ)活化方法,同时兼顾经济、高效和操作简单等特点.
(3)在S(Ⅳ)-AOPs中,S(Ⅳ)既是自由基的前驱体,又会消耗产生的活性氧化剂. 因此后续的研究需要探究提升S(Ⅳ)-AOPs电子效率的方法,以期提高S(Ⅳ)-AOPs处理污染物的效能.
基于亚硫酸盐的高级氧化技术及其反应机制研究进展
Recent advances of sulfite-based advanced oxidation processes and their reaction mechanisms
-
摘要: 近年来,基于亚硫酸盐的高级氧化技术(S(Ⅳ)-AOPs)受到广泛关注,但活化S(Ⅳ)过程中涉及各种含硫自由基和非自由基物种的转化,使得S(Ⅳ)-AOPs的反应机制较为复杂,同一种S(Ⅳ)-AOP的反应机制常常存在争议. 然而,鲜有研究对现有的S(Ⅳ)-AOPs及其反应机制进行较为详细的分类与总结. 基于此,总结了S(Ⅳ)的理化性质以及S(Ⅳ)-AOPs中含硫自由基的转化机制,综述了各类活化S(Ⅳ)的方法,系统阐述了S(Ⅳ)-AOPs的反应机制、活性氧化剂的产生及贡献,解析了影响S(Ⅳ)-AOPs氧化水中污染物效能的因素. 针对目前S(Ⅳ)-AOPs的研究现状和需求进行展望,以期为S(Ⅳ)-AOPs在水污染控制领域的应用提供参考.Abstract: Sulfite-based advanced oxidation processes (S(Ⅳ)-AOPs) have received increasing attention in recent years. The S(Ⅳ) activation process involves the transformation of various sulfur-containing radicals and non-radical species, which usually makes the mechanism of a S(Ⅳ)-AOP complicated and controversial. However, the existing S(Ⅳ)-AOPs and their reaction mechanisms were rarely classified and summarized in detail. Therefore, this study concluded the physicochemical properties of S(Ⅳ) and the transformation mechanism of sulfur-containing radicals, reviewed various methods for activating S(Ⅳ), systematically illustrated reaction mechanisms in S(Ⅳ)-AOPs as well as the generation and contribution of active oxidants, and analyzed factors influencing the efficacy of contaminants degradation by S(Ⅳ)-AOPs. Finally, the future advances were proposed based on the current research status and needs about SⅣ)-AOPs to lay the foundation for S(Ⅳ)-AOPs application in the control of water pollution.
-
Key words:
- advanced oxidation processes /
- sulfite /
- reaction mechanisms /
- factors /
- the control of water pollution.
-

-
表 1 S(Ⅳ)链式反应方程及相应的反应速率常数
Table 1. Equation and corresponding rate constant of S(Ⅳ) chain reactions
序号
Sequence number反应方程
Equations速率常数k /(L·mol–1·s–1)
Rate constants参考文献
References1 $ {\text{SO}}_{\text{3}}^{\cdot-}\text{+}{\text{O}}_{\text{2}}\text{}\to \text{}{\text{SO}}_{\text{5}}^{\cdot-} $ 2.5×109 [48] 2 $ {\text{SO}}_{\text{5}}^{\cdot-}\text{+}{\text{HSO}}_{\text{3}}^{-}\text{}\to \text{}{\text{HSO}}_{5}^{-}\text{+}{\text{SO}}_{\text{3}}^{\cdot-} $ 8.6×103—3.0×105 [49] 3 $ {\text{SO}}_{\text{5}}^{\cdot-}\text{+}{\text{SO}}_{\text{3}}^{2-}\text{}\to {\text{SO}}_{5}^{2-}\text{+}{\text{SO}}_{\text{3}}^{\cdot-} $ 2.5×104—3.8×106 [49] 4 $ {\text{SO}}_{\text{5}}^{\cdot-}\text{+}{\text{HSO}}_{\text{3}}^{-}\text{}\to \text{}{\text{HSO}}_{\text{4}}^{-}\text{+}{\text{SO}}_{\text{4}}^{\cdot-} $ 3.6×102—3.0×105 [49] 5 $ {\text{SO}}_{\text{5}}^{\cdot-}\text{+}{\text{SO}}_{\text{3}}^{2-}\text{}\to \text{}{\text{SO}}_{4}^{2-}\text{+}{\text{SO}}_{\text{4}}^{\cdot-} $ 7.5×104—1.0×107 [49] 6 $ {\text{SO}}_{\text{5}}^{\cdot-}\text{+}{\text{SO}}_{\text{5}}^{\cdot-}\text{}\to {\text{2}\text{SO}}_{\text{4}}^{\cdot-}\text{+}{\text{O}}_{\text{2}} $ 5.2×106—6.0×108 [49] 7 $ {\text{HSO}}_{\text{5}}^{-}\text{+}{\text{HSO}}_{\text{3}}^{-}\text{}\to {\text{2HSO}}_{\text{4}}^{-} $ 1.0×103 [50] 8 $ {\text{SO}}_{5}^{2-}\text{+}{\text{SO}}_{3}^{2-}\text{}\to \text{}{\text{2SO}}_{4}^{2-} $ — — 9 $ {\text{SO}}_{\text{4}}^{\cdot-}\text{+}{\text{HSO}}_{\text{3}}^{-}\text{}\to \text{}{\text{HSO}}_{\text{4}}^{-}\text{+}{\text{SO}}_{\text{3}}^{\cdot-} $ >2.0×109 [13] 10 $ {\text{SO}}_{\text{4}}^{\cdot-}\text{+}{\text{SO}}_{3}^{2-}\text{}\to {\text{SO}}_{4}^{2-}\text{+}{\text{SO}}_{\text{3}}^{\cdot-} $ >2.0×109 [13] 11 $ {\text{SO}}_{\text{4}}^{\cdot-}\text{+}{\text{H}}_{\text{2}}\text{O}\to {\text{SO}}_{4}^{2-}\text{+}{\text{HO}}^{\cdot}\text{+}{\text{H}}^{\text{+}} $ <60.0 [13] 12 $ {\text{SO}}_{\text{4}}^{\cdot-}\text{+}{\text{OH}}^{-}\text{}\to {\text{SO}}_{4}^{2-}\text{+}{\text{HO}}^{\cdot} $ 4.6×107—8.3×107 [13] - 未有研究报道(The data has not been reported) 表 2 不同S(IV)-AOPs去除水中污染物的效能总结
Table 2. The summary of contaminants degradation efficacy by various S(Ⅳ)-AOPs
S(Ⅳ)-AOPs类型
Types of
S(Ⅳ)-AOPs活化剂
Activator目标污染物
Target
contaminants最佳反应条件及污染物降解效能
Optimum reaction conditions
and contaminants
degradation efficacy活性氧化剂
Active
oxidizing
specie影响因素
Influencing
factors低价态过渡金属活化S(Ⅳ)技术 Fe(Ⅱ) 卡马西平[60]
(CBZ)pHini = 5.0,[Fe(Ⅱ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
0.5 mmol·L−1,[CBZ]0 = 5 μmol·L−1;反应20 min,CBZ的去除率达到87.3%.SO4•−、SO5•−和HO• pHini、Fe(Ⅱ)投量和S(Ⅳ)投量. 橙II[59]
(Orange II)pHini = 4.0,[Fe(Ⅱ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[橙II]0 = 10 mg·L−1;反应60 min,橙II的去除率达到约80%.SO4•−、SO5•−和HO• pHini、Fe(Ⅱ)投量、S(Ⅳ)投量和卤素离子. Fe(Ⅲ) 卡马西平[54]
(CBZ)pHini = 3.0,[Fe(Ⅲ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
0.5 mmol·L−1,[CBZ]0 = 5 μmol·L−1;反应20 min,CBZ的去除率达到78.3%.SO4•−、SO5•−和HO• pHini、Fe(Ⅲ)投量、S(Ⅳ)投量和腐殖酸. 苯胺[56](Aniline) pHini = 4.0,[Fe(Ⅲ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[苯胺]0 = 10 μmol·L−1;反应60 min,苯胺的去除率达到70%.SO4•−、SO5•−和HO• pHini、Fe(Ⅲ)投量和S(Ⅳ)投量. 橙Ⅱ[59]
(Orange Ⅱ)pHini = 4.0,[Fe(Ⅲ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[橙 II]0 = 10 mg·L−1;反应60 min,橙 II的去除率达到约80%.SO4•−、SO5•−和HO• pHini、Fe(Ⅲ)投量、S(Ⅳ)投量和卤素离子. 四溴双酚A[64]
(TBBPA)pHini = 4.0,[Fe(Ⅲ)]0 = 0.04 mmol·L−1,[Na2SO3]0 =
2.0 mmol·L−1,[TBBPA]0 = 10 μmol·L−1;反应30 min,TBBPA的去除率达到73%.SO4•−和HO• pHini、Fe(Ⅲ)投量、S(Ⅳ)投量、HCO3−和腐殖酸. 低价态过渡金属活化S(Ⅳ)技术 Cu(Ⅱ) 草甘膦[58]
(Glyphosate)pHini = 11.0,[Cu(Ⅱ)]0 = 0.025 mmol·L−1,[Na2SO3]0 = 0.25 mmol·L−1,[草甘膦]0 = 6 μmol·L−1;反应30 min,草甘膦的去除率达到93%. SO4•−和HO• pHini、Cu(Ⅱ)投量和S(Ⅳ)投量. 碘海醇[53]
(Iohexol)pHini = 8.0,[Cu(Ⅱ)]0 = 0.01 mmol·L−1,[Na2SO3]0 =
0.5 mmol·L−1,[碘海醇]0 = 10 μmol·L−1;反应40 min,碘海醇的去除率达到100%.SO4•−和HO• pHini、Cu(Ⅱ)投量、S(Ⅳ)投量、HCO3−和Cl−. 对乙酰氨基酚[57]
(PARA)pHini = 10.0,[Cu(Ⅱ)]0 = 0.01 mmol·L−1,[Na2SO3]0 = 1.0 mmol·L−1,[PARA]0 = 10 μmol·L−1;反应60 min,PARA的去除率达到93%. SO4•−、SO5•−和HO• pHini、Cu(Ⅱ)投量和S(Ⅳ)投量. Mn(Ⅱ)/
Fe(Ⅱ)阿特拉津[65]
(ATZ)pHini = 6.0,[Fe2+]0 = [Mn2+]0 = 25 μmol·L−1,
[Na2SO3]0 = 1.0 mmol·L−1,[ATZ]0 = 5μmol·L−1;
反应60 s,ATZ的去除率达到100%.Mn(Ⅲ) pHini、Fe2+投量、Mn2+投量、腐殖酸、HCO3−和磷酸根 Co(Ⅱ) 碘海醇[53]
(Iohexol)pHini = 8.0,[Co(Ⅱ)]0 = 0.01 mmol·L−1,[Na2SO3]0 =
0.5 mmol·L−1,[碘海醇]0 = 10 μmol·L−1;反应20 min,碘海醇的去除率达到100%.SO4•−和HO• pHini、Co(Ⅱ)投量、S(Ⅳ)投量、HCO3−和Cl−. 对乙酰氨基酚[66]
(PARA)pHini = 9.0,[Co(Ⅱ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[PARA]0 = 10 μmol·L−1;反应30 min,PARA的去除率达到83%.SO4•−和SO5•− pHini、Co(Ⅱ)投量和S(Ⅳ)投量. 过渡金属材料活化S(Ⅳ)技术 CoNSi 酸性橙[73](AO7) pHini = 7.0,[CoNSi]0 = 0.25 g·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[AO7]0 = 20 μmol·L−1;反应20 min,[Co2+] = 0.17 μmol·L−1,AO7的去除率达到79.4%.SO5•−、SO4•−和HO• pHini、温度、S(Ⅳ)投量、催化剂投量、Cl−、 HCO3−和腐殖酸. CuCo2S4 四环素[80](TTC) pHini = 10.0,[CuCo2S4]0 = 0.01 g·L−1,[Na2SO3]0 =
0.25 mmol·L−1,[TTC]0 = 10 μmol·L−1;反应1 min,TTC的去除率达到100%.SO4•−和HO• pHini. CuNSi 三价砷[77]
(As(III))pHini = 8.0,[CuNSi]0 = 0.1 g·L−1,[Na2SO3]0 =
0.1 mmol·L−1,[As(III)]0 = 5 μmol·L−1;反应60 min,[Co2+] < 1.3 μmol·L−1,As(III)的去除率达到90%.SO5•−、SO4•−和HO• pHini、温度、S(Ⅳ)投量和 HCO3−. Co-SBA-15 对乙酰氨基酚[78]
(APAP)pHini = 7.0,[Co-SBA-15]0 = 0.2 mmol·L−1,[Na2SO3]0 = 0.1 mmol·L−1,[APAP]0 = 10 μmol·L−1,T=45 ℃;反应30 min,APAP的去除率达到93%以上. SO5•−、SO4•−和HO• Co-SBA-15和S(Ⅳ)的投量比、pHini和温度. Co-MOF 甲基橙[72]
(MO)pHini = 8.9,[Co-MOF]0 = 0.4 g·L−1,[Na2SO3]0 =
5.0 mmol·L−1,[MO]0 = 20 mg·L−1;反应35 min,[Co2+] = 20 μmol·L−1,MO的去除率达到74%.SO4•− - Co-TiO2 甲硝唑[85]
(MNZ)pH = 7.0,[Co-TiO2]0 = 0.5 g·L−1,[Na2SO3]0 =
5.0 mmol·L−1,[MNZ]0 = 50 μmol·L−1;反应18 min,MNZ的去除率达到100%.SO4•−、HO•和O2•− 磷酸盐、Co-TiO2投量、pHini、S(Ⅳ)投量、Cl−、Br−和NO2−. Fe0-C 活性艳红X-3B[45]
(X-3B)pHini = 7.0,[Fe-C]0 = 0.3 g·L−1,[Na2SO3]0 =
5.0 mmol·L−1,[X-3B]0 = 20 mg·L−1;反应30 min,
X-3B的去除率达到90%以上.SO4•−和HO• pHini、Fe-C投量和S(Ⅳ)投量. CoS 碘海醇[81]
(Iohexol)pH = 8.0,[CoS]0 = 0.05 g·L−1, [Na2SO3]0 =
0.5 mmol·L−1,[碘海醇]0 = 10 μmol·L−1;反应30 min,碘海醇的去除率达到90%以上.SO4•−和HO• pH、CoS投量、S(Ⅳ)投量、磷酸盐、腐殖酸、Cl−和HCO3−. FeS 普萘洛尔[75]
(PRO)pHini = 6.0,[FeS]0 = 20 mg·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[PRO]0 = 10 μmol·L−1;反应20 min,[Fe2+] = 0.375 mg·L−1,PRO的去除率达到95%以上.SO4•−和HO• pHini、FeS投量、S(Ⅳ)投量、Cl−和HCO3−. Fe0 活性艳红
X-3B[44](X-3B)pHini = 6.0,[Fe0]0 = 0.5 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[X-3B]0 = 20 mg·L−1;反应90 min,
X-3B的去除率达到78%.SO5•−、SO4•−和HO• pHini、Fe0投量、S(Ⅳ)投量、I−和 HCO3−. Fe0-Cu0 磺胺甲基嘧啶[43]
(SMT)pHini = 6.0,[Fe-Cu]0 = 80 mg·L−1,Cu含量为40%,[Na2SO3]0 = 1.0 mmol·L−1,[SMT]0 = 5 mg·L−1;反应
10 min,SMT的去除率达到87%.SO4•−和HO• Cu含量、Fe-Cu投量、pHini和S(Ⅳ)投量. CuO 碘海醇[76]
(Iohexol)pHini = 8.0,[CuO]0 = 0.5 g·L−1, [Na2SO3]0 =
0.5 mmol·L−1,[碘海醇]0 = 10 μmol·L−1;反应10 min,碘海醇的去除率达到95%.SO4•−和HO• pHini、CuO投量、S(Ⅳ)投量、 Cl−、腐殖酸和 HCO3−. 氧化剂活化S(Ⅳ)技术 Mn(Ⅶ) 布洛芬[38](IBU) pH = 5.0,[Mn(Ⅶ)]0 = 0.05 mmol·L−1,[IBU]0 =
0.5 μmol·L−1;[Na2SO3]0 = 0.5 mmol·L−1,反应10 s,IBU的去除率达到100%;[CaSO3]0 = 0.5 mmol·L−1,
反应80 s,IBU的去除率达到100%.Mn(Ⅴ)、Mn(Ⅵ)、SO4•−和HO• pH、S(Ⅳ)种类和S(Ⅳ)投加速率. 对氨基苯胂酸[39]
(ASA)pH = 5.0,[Mn(Ⅶ)]0 = 0.05 mmol·L−1,[Na2SO3]0 = 0.25 mmol·L−1,[ASA]0 = 5 μmol·L−1;反应15 s,
ASA的去除率达到71%.Mn(Ⅴ)、Mn(Ⅵ)、SO4•−和HO• pH、S(Ⅳ)和Mn(Ⅶ)的摩尔比、EDTA、腐殖酸、卤素离子和共存阳离子. 苯酚、环丙沙星和甲基蓝[40] pHini = 5.0,[Mn(Ⅶ)]0 = 0.05 mmol·L−1,[Na2SO3]0 = 0.25 mmol·L−1,[污染物]0 = 5 μmol·L−1;反应0.15 s,污染物的去除率达到100%. Mn(Ⅲ) pHini和焦磷酸盐. 安赛蜜和
卡马西平[96]pHini = 5.0,[Mn(Ⅶ)]0 = 0.05 mmol·L−1,[Na2SO3]0 = 0.25 mmol·L−1,[污染物]0 = 5 μmol·L−1;反应15 s,污染物的去除率达到80%以上. Mn(Ⅲ) pHini、腐殖酸、NO2−、Cl−、Ca2+和Mn2+. 苯并三唑和
咖啡因[100]pHini = 3.0,[Mn(Ⅶ)]0 = 0.05 mmol·L−1,[Na2SO3]0 = 0.25 mmol·L−1,[污染物]0 = 5 μmol·L−1;反应10 s,污染物的去除率达到100%. Mn(Ⅲ)、Mn(Ⅴ)、Mn(Ⅵ)、SO4•−和HO• pHini、污染物种类及S(Ⅳ)和Mn(Ⅶ)的摩尔比. Fe(Ⅵ) 磺胺甲恶唑、苯并三唑、苯酚、环丙沙星、甲基蓝、罗丹明B和甲基橙[101] pH = 9.0,[Fe(Ⅵ)]0 = 0.05 mmol·L−1,[Na2SO3]0 =
0.25 mmol·L−1,[污染物]0 = 5 μmol·L−1;反应30 s,磺胺甲恶唑的去除率达到68%,苯并三唑的去除率达到85%,其他污染物去除率达到95%以上.SO4•−和HO• pH和污染物种类. N,N-二乙基-3-甲酰胺[37](DEET) pH = 8.0,[Fe(Ⅵ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
0.4 mmol·L−1,[DEET]0 = 4 μmol·L−1;反应10 s,DEET的去除率达到100%.SO4•− pH、S(Ⅳ)投量、Fe(Ⅵ)投量、腐殖酸、Cl−和CO32−. 阿特拉津[87]
(ATZ)pH = 8.0,[Fe(Ⅵ)]0 = 0.05 mmol·L−1,[Na2SO3]0 =
0.2 mmol·L−1,[ATZ]0 = 5 μmol·L−1;反应10 s,ATZ的去除率达到74.4%.SO4•−和HO• pH、S(Ⅳ)和Fe(Ⅵ)的摩尔比、腐殖酸、Cl−和CO32−. 磺胺甲恶唑、恩诺沙星、卡马西平、双氯酚酸钠、阿特拉津和
布洛芬[95]pH = 8.0,[Fe(Ⅵ)]0 = 0.05 mmol·L−1,[CaSO3]0 =
0.15 mmol·L−1,[前四种污染物]0 = 5 μmol·L−1,[后两种污染物]0 = 2.5 μmol·L−1;反应2 min,阿特拉津和布洛芬的去除率达到70%,其余污染物去除率达到100%.Fe(Ⅴ)和Fe(Ⅳ) 磷酸盐和污染物种类. 碘化造影剂[94]
(IPM、DTZ)pH = 8.0,[Fe(Ⅵ)]0 = 0.05 mmol·L−1,[Na2SO3]0 =
0.2 mmol·L−1,[污染物]0 = 0.5 μmol·L−1;反应10 s,污染物的去除率达到87.7%.SO4•−和HO• pH、S(Ⅳ)投量、Fe(Ⅵ)投量、腐殖酸、Cl−和CO32−. ClO2 阿特拉津、双酚A、磺胺甲恶唑和卡马西平[93] pH = 7.0,[ClO2]0 = 0.1 mmol·L−1,[Na2SO3]0 =
0.2 mmol·L−1,[污染物]0 = 1 μmol·L−1;反应3 min,
污染物的去除率达到84%以上.ClO•、SO4•−和HO• pH、温度、ClO2投量、S(Ⅳ)投量、Cl−、Br−、CO32−和腐殖酸 卡马西平[97]
(CBZ)pH = 9.0,[ClO2]0 = 0.03 mmol·L−1,[Na2SO3]0 =
0.03 mmol·L−1,[CBZ]0 = 2 μmol·L−1;反应10 s,CBZ的去除率达到80%.ClO•、SO4•−和Cl2O3 pH、S(Ⅳ)投量、Cl−、CO32−和天然有机物. PDS 甲基橙[98](MO) pHini = 3.0,[PDS]0 = 20.0 mmol·L−1,[Na2SO3]0 =
20.0 mmol·L−1,[MO]0 = 10 mg·L−1;反应50 min,污染物的去除率达到85%以上.SO4•−和HO• pHini、S(Ⅳ)投量、PDS投量和温度. Cr(Ⅵ) 三价砷[92]
(As(Ⅲ))pHini = 3.5,[Cr(Ⅵ)]0 = 0.05 mmol·L−1,[Na2SO3]0 = 0.4 mmol·L−1,[As(III)]0 = 50 μmol·L−1;反应60 min,Cr(Ⅵ)的去除率达到100%,As(III)的去除率达到60%. SO4•−和HO• pHini、Cr(Ⅵ)投量、S(Ⅳ)投量和共存阳离子. 酸性橙[91](AO7) pHini = 3.0,[Cr(Ⅵ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
0.5 mmol·L−1,[AO7]0 = 50 μmol·L−1;反应60 min,Cr(Ⅵ)的去除率达到82%,AO7的去除率达到86.1%.SO4•−和HO• pHini、Cr(Ⅵ)投量、S(Ⅳ)投量和Cl−. 氧化剂活化S(Ⅳ)技术 Cr(Ⅵ) 对氯苯酚、对氯苯甲酸、阿莫西林、雌二醇和布洛芬[90] pHini = 4.0,[Cr(Ⅵ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[污染物]0 = 5 μmol·L−1;反应5 min,Cr(Ⅵ)和污染物的去除率达到100%.Cr(Ⅴ)和SO4•− pHini和污染物种类. BrO3− 扑热息痛、苯酚、双酚A、卡马西平、诺氟沙星和阿特拉津[88] pHini = 4.0,[BrO3−]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[污染物]0 = 10 μmol·L−1;反应30 min,污染物的去除率达到80%以上.SO4•−和HO• pHini和水基质. 外加能量活化S(Ⅳ)技术 超声波 三价砷[109]
(As(Ⅲ))pH = 7.0,650 W超声器,[Na2SO3]0 = 1.0 mmol·L−1,[As(Ⅲ)]0 = 5 μmol·L−1;反应30 min,As(Ⅲ)的去除率达到100%. SO4•−和HO• pH、S(Ⅳ)投量、超声器功率和腐殖酸. 紫外光 碘帕醇[104](IPM) pH = 7.0,光强度 = 0.17 mW·cm−2,[Na2SO3]0 =
0.5 mmol·L−1,[IPM]0 = 2 μmol·L−1;反应30 min,IPM的去除率达到80%.SO5•−和SO4•− pH、Cl−、I−和天然有机物. 磺胺甲恶唑[115]
(SMX)pHini = 6.9,光照强度 = 0.24 mW·cm−2,[Na2SO3]0 = 0.1 mmol·L−1,[SMX]0 = 1 μmol·L−1;反应30 min,SMX的去除率达到95%. SO5•−、SO4•−和HO• pHini、S(Ⅳ)投量、DO浓度、CO32−和天然有机物. 邻苯二甲酸二乙酯[114](DEP) pH = 10.11,I0 = 4.88 × 10−7 Einstein·s−1,L = 4.04 cm,[Na2SO3]0 = 8.0 mmol·L−1,[DEP]0 = 10 μmol·L−1;反应30 min,DEP的去除率达到100%. SO5•−、SO4•−和HO• pH. 氧氟沙星[113]
(Floxin)pHini = 12.0,光强度 = 0.087 mW·cm−2,感光剂ZnO,[Na2SO3]/[ZnO] = 1:3,[氧氟沙星]0 = 1 mg·L−1;反应
5 min,氧氟沙星的去除率达到100%.SO4•−和HO• pHini、S(Ⅳ)和ZnO的摩尔比、Cl−、NO3−、SO42−和CO32−. 甲硝唑[112](MTX) pHini = 12.0,光强度 = 0.087 mW·cm−2,感光剂ZnO,[Na2SO3]/[ZnO] = 1:3,[MTX]0 = 1 mg·L−1;反应
5 min,MTX的去除率达到90%以上.SO4•−和HO• pHini、S(Ⅳ)和ZnO的摩尔比、Cl−、NO3−、SO42−和CO32−. 环丙沙星[111]
(CFX)pHini = 12.0,光强度 = 0.087 mW·cm−2,感光剂ZnO,[Na2SO3]/[ZnO] = 1:3,[MTX]0 = 1 mg·L−1;反应
60 min,MTX的去除率达到98%.HO• pHini、S(Ⅳ)和ZnO的摩尔比、Cl−、NO3−、SO42−和CO32−. 多氯联苯[106]
(PCBs)pHini = 7.0,感光剂TiO2,[Na2SO3]/[TiO2] = 1:1,[PCBs]0 = 1 mg·L−1;反应60 min,PCBs的去除率达到98.5%. HO•和O2•− pHini、S(Ⅳ)和TiO2的摩尔比、NO3−和NO2−. 电 三价砷[118]
(As(Ⅲ))pH = 7.0,石墨电极,电压 = 2 V,[Na2SO3]0 =
1.0 mmol·L−1,[As(Ⅲ)]0 = 5 μmol·L−1;反应40 min,As(Ⅲ)的去除率达到100%.SO5•−、SO4•−、HO•和HO2• 离子强度、pH、S(Ⅳ)投量、电压、Cl−、NO3−、HCO3−和天然有机物. 可见光 三价砷[110]
(As(Ⅲ))和亚甲基蓝(MB+)pH = 7.3, [Na2SO3]0 = 0.05 mmol·L−1,[MB+]0 =
0.27 μmol·L−1,MB+为感光剂,[As(Ⅲ)]0 = 5 μmol·L−1;反应30 min,As(Ⅲ)的去除率达到100%,MB+的去除率达到80%.SO5•−、SO4•−和HO• pH、MB+投量和天然有机物. 硝酚胂酸[116]
(roxarsone)pH = 10.0, [Na2SO3]0 = 2.0 mmol·L−1,[赤铁矿]0 =
0.03 g·L−1,赤铁矿为感光剂,[硝酚胂酸]0 =
19 μmol·L−1;反应50 min,硝酚胂酸的去除率达到100%.Fe(Ⅳ)和Fe(Ⅴ) - 三价砷[117]
(As(Ⅲ))pHini = 5.0,[ZnFe2O4@PANI]0 = 10 mg·L−1,
[Na2SO3]0 = 4.0 mmol·L−1,[As(Ⅲ)]0 = 50 mg·L−1;反应30 min,As(Ⅲ)的去除率达到100%.SO4•−、HO•和Fe(Ⅳ) pHini、S(Ⅳ)投量和PANI浓度. 多种方式联合活化S(Ⅳ)技术 紫外光+
过渡金属2,4,6-三氯苯酚[17]
(2,4,6-TCP)pHini = 4.0,[Fe(Ⅲ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[2,4,6-TCP]0 = 10 mg·L−1;反应180 min,2,4,6-TCP的去除率达到95%.SO4•−和HO• Fe(Ⅲ)络合剂. 双酚A[119](BPA) pHini = 6.0,[Fe(Ⅱ)]0 = 0.1 mmol·L−1,[Na2SO3]0 =
1.0 mmol·L−1,[BPA]0 = 1 mg·L−1;反应60 min,BPA的去除率达到90%.SO5•−、SO4•−和HO• S(Ⅳ)投量和光照强度. 布洛芬[31](IBU) pH = 7.0,[Mn(Ⅱ)]0 = 0.036 mmol·L−1,[Na2SO3]0 = 0.25 mmol·L−1,[IBU]0 = 2 μmol·L−1;反应400 s,IBU的去除率达到100%. SO4•−和HO• - 多种方式联合活化S(Ⅳ)技术 可见光+电 氨氮[83]
(NH3-N)pH = 12.0, [Na2SO3]0 = 0.1 mmol·L−1,[MoS2]0 =
3.0 mg,MoS2为感光剂,其面积1.0 cm2,[NH3-N]0 = 10 mg·L−1;反应6 h,NH3-N的去除率达到80%.SO5•−和SO4•− pH和MoS2投量. 电+过渡
金属双酚A[108](BPA) Hini = 6.0,100 mA电流,[Mn2+]0 = 0.2 mmol·L−1,[Na2SO3]0 = 2.0 mmol·L−1,[BPA]0 = 10 μmol·L−1;反应40 min,BPA的去除率达到94.2%. SO4•− pHini、S(Ⅳ)投量和电流大小. - 文献未提及(The data has not been reported) 表 3 常见背景基质与SO4•−和HO•的反应速率常数
Table 3. Equation and corresponding rate constant of water matrix reacting with SO4•− and HO•
序号
Sequence number反应方程
Equations速率常数k/(L·mol−1·s−1)
Rate constants参考文献
Reference1 $ {\mathrm{H}\mathrm{O}}^{\cdot}+{\mathrm{C}\mathrm{l}}^{-}\to {\mathrm{C}\mathrm{l}}^{\cdot}+{\mathrm{O}\mathrm{H}}^{-} $ 1.10 × 109 [121] 2 $ {\mathrm{H}\mathrm{O}}^{\cdot}+{\mathrm{C}\mathrm{O}}_{3}^{2-}\to {\mathrm{C}\mathrm{O}}_{3}^{\cdot-}+{\mathrm{O}\mathrm{H}}^{-} $ 3.90 × 108 [122] 3 $ {{\mathrm{H}\mathrm{O}}^{\cdot}+\mathrm{H}\mathrm{C}\mathrm{O}}_{3}^{-}\to {\mathrm{C}\mathrm{O}}_{3}^{\cdot-}+{\mathrm{H}}_{2}\mathrm{O} $ 8.60 × 106 [123] 4 $ {\mathrm{H}\mathrm{O}}^{\cdot}+\mathrm{H}\mathrm{A}\to \mathrm{P}\mathrm{r}\mathrm{o}\mathrm{d}\mathrm{u}\mathrm{c}\mathrm{t}\mathrm{s} $ 2.50 × 104 [124] 5 $ {\text{SO}}_{\text{4}}^{\cdot-}+{\mathrm{C}\mathrm{l}}^{-}\to {\mathrm{C}\mathrm{l}}^{\cdot}+{\text{SO}}_{\text{4}}^{2-} $ 2.50 × 108 [125] 6 $ {\text{SO}}_{\text{4}}^{\cdot-}+{\mathrm{C}\mathrm{O}}_{3}^{2-}\to {\mathrm{C}\mathrm{O}}_{3}^{\cdot-}+{\text{SO}}_{\text{4}}^{2-} $ 4.10 × 106 [125] 7 $ {{\text{SO}}_{\text{4}}^{\cdot-}+\mathrm{H}\mathrm{C}\mathrm{O}}_{3}^{-}\to {\mathrm{C}\mathrm{O}}_{3}^{\cdot-}+{\text{SO}}_{\text{4}}^{2-} $ 9.20 × 106 [126] 8 $ {\text{SO}}_{\text{4}}^{\cdot-}+\mathrm{H}\mathrm{A}\to \mathrm{P}\mathrm{r}\mathrm{o}\mathrm{d}\mathrm{u}\mathrm{c}\mathrm{t}\mathrm{s} $ 5.10 × 103 [127] -
[1] SCHWARZENBACH R P, ESCHER B I, FENNER K, et al. The challenge of micropollutants in aquatic systems [J]. Science, 2006, 313(5790): 1072-1077. doi: 10.1126/science.1127291 [2] STUART M, LAPWORTH D, CRANE E, et al. Review of risk from potential emerging contaminants in UK groundwater [J]. Sci Total Environ, 2012, 416: 1-21. doi: 10.1016/j.scitotenv.2011.11.072 [3] PEREIRA L C, de SOUZA A O, FRANCO BERNARDES M F, et al. A perspective on the potential risks of emerging contaminants to human and environmental health [J]. Environ Sci Pollut Res Int, 2015, 22(18): 13800-13823. doi: 10.1007/s11356-015-4896-6 [4] PINTADO-HERRERA M G, WANG C C, LU J T, et al. Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China[J]. J Hazard Mater, 2017, 323(Pt A): 128-138. [5] SENGAR A, VIJAYANANDAN A. Human health and ecological risk assessment of 98 pharmaceuticals and personal care products (PPCPs) detected in Indian surface and wastewaters[J]. Sci Total Environ, 2022, 807(Pt 1): 150677. [6] 刘远. 北方污水厂出水和再生处理中新兴有机污染物的分布特征[D]. 天津: 天津大学, 2018. LIU Y. Distribution characteristics of emerging organic pollutants in effluents and reclaimed treatment process of wastewater treatment plants in North China[D]. Tianjin: Tianjin University, 2018(in Chinese).
[7] 陈鹏. 新兴有机污染物在三条典型河流中的存在、组成分布与来源[D]. 广州: 广东工业大学, 2021. CHEN P. The occurrence, distribution and source of emerging organic contaminants in three typical rivers[D]. Guangzhou: Guangdong University of Technology, 2021(in Chinese).
[8] 朱欢欢, 孙韶华, 冯桂学, 等. 紫外联用高级氧化技术处理饮用水应用进展 [J]. 水处理技术, 2019, 45(3): 1-7,13. ZHU H H, SUN S H, FENG G X, et al. Research progress of ultraviolet combined advanced oxidation technology for drinking water treatment [J]. Technology of Water Treatment, 2019, 45(3): 1-7,13(in Chinese).
[9] DHANGAR K, KUMAR M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: A review [J]. Sci Total Environ, 2020, 738: 140320. doi: 10.1016/j.scitotenv.2020.140320 [10] Faheem, DU J K, KIM S H, et al. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role [J]. Environ Sci Pollut Res Int, 2020, 27(30): 37286-37312. doi: 10.1007/s11356-020-07612-y [11] 袁敏, 邓文勇, 刘倩, 等. 高级氧化技术处理有机染料废水的研究进展 [J]. 广州化工, 2021, 49(23): 5-7. YUAN M, DENG W Y, LIU Q, et al. Research progresson treatment of organic dye wastewater by advanced oxidation technology [J]. Guangzhou Chemical Industry, 2021, 49(23): 5-7(in Chinese).
[12] TIMMERS P H A, SLOOTWEG T, KNEZEV A, et al. Improved drinking water quality after adding advanced oxidation for organic micropollutant removal to pretreatment of river water undergoing dune infiltration near The Hague, Netherlands [J]. J Hazard Mater, 2022, 429: 128346. doi: 10.1016/j.jhazmat.2022.128346 [13] NETA P, HUIE R E, ROSS A B. Rate constants for reactions of inorganic radicals in aqueous solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. doi: 10.1063/1.555808 [14] HOELDERICH W F, KOLLMER F. Oxidation reactions in the synthesis of fine and intermediate chemicals using environmentally benign oxidants and the right reactor system [J]. Pure And Applied Chemistry, 2000, 72(7): 1273-1287. doi: 10.1351/pac200072071273 [15] YANG Y, PIGNATELLO J J, MA J, et al. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs) [J]. Environ Sci Technol, 2014, 48(4): 2344-2351. doi: 10.1021/es404118q [16] MEZYK S P, RICKMAN K A, MCKAY G, et al. Remediation of chemically-contaminated waters using sulfate radical reactions: Kinetic studies[M]. Aquatic Redox Chemistry. American Chemistry Society. 2011: 247-263. [17] GUO Y G, LOU X Y, FANG C L, et al. Novel photo-sulfite system: Toward simultaneous transformations of inorganic and organic pollutants [J]. Environ Sci Technol, 2013, 47(19): 11174-11181. doi: 10.1021/es403199p [18] KWON M, KIM S, YOON Y, et al. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82- processes for wastewater treatment [J]. Chemical Engineering Journal, 2015, 269: 379-390. doi: 10.1016/j.cej.2015.01.125 [19] LI D W, CHEN D Z, YAO Y Y, et al. Strong enhancement of dye removal through addition of sulfite to persulfate activated by a supported ferric citrate catalyst [J]. Chemical Engineering Journal, 2016, 288: 806-812. doi: 10.1016/j.cej.2015.12.008 [20] LIAN L S, YAO B, HOU S D, et al. Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents [J]. Environ Sci Technol, 2017, 51(5): 2954-2962. doi: 10.1021/acs.est.6b05536 [21] SONG W, LI J, FU C X, et al. Establishment of sulfate radical advanced oxidation process based on Fe2+/O2/dithionite for organic contaminants degradation[J]. Chemical Engineering Journal, 2021, 410. [22] ANTONIOU M G, de la CRUZ A A, DIONYSIOU D D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e– transfer mechanisms [J]. Applied Catalysis B:Environmental, 2010, 96(3-4): 290-298. doi: 10.1016/j.apcatb.2010.02.013 [23] 林匡飞, 张雨, 张猛, 等. 原位热活化过硫酸盐降解VOCs的温度模拟与试验研究 [J]. 安全与环境学报, 2022, 22(1): 420-426. LIN K F, ZHANG Y, ZHANG M, et al. Temperature simulation and experimental study on degradation of VOCs by in situ thermal activated persulfate [J]. Journal of Safety and Environment, 2022, 22(1): 420-426(in Chinese).
[24] SHUKLA P R, WANG S B, ANG H M, et al. Photocatalytic oxidation of phenolic compounds using zinc oxide and sulphate radicals under artificial solar light [J]. Separation and Purification Technology, 2010, 70(3): 338-344. doi: 10.1016/j.seppur.2009.10.018 [25] 薛洪海, 高斯屿, 付依, 等. 紫外活化过硫酸盐技术去除水中人工甜味剂的研究进展 [J]. 科学技术与工程, 2019, 19(32): 17-23. XUE H H, GAO S Y, FU Y, et al. Review on degradation of artificial sweeteners in aqueous solution by ultraviolet activated persulfate technology [J]. Science Technology and Engineering, 2019, 19(32): 17-23(in Chinese).
[26] 温学, 韦新东, 薛洪海, 等. 紫外/过硫酸盐降解水中氧氟沙星的动力学和机理 [J]. 科学技术与工程, 2018, 18(1): 342-347. WEN X, WEI X D, XUE H H, et al. Degradation kinetics and mechanisms of ofloxacin in water by peroxydisulfate/ultraviolet [J]. Science Technology and Engineering, 2018, 18(1): 342-347(in Chinese).
[27] 张恒, 吴琳琳, 陈力可, 等. UV-254 nm活化过硫酸盐降解麻黄碱的影响因素和机理 [J]. 环境化学, 2020, 39(6): 1607-1616. doi: 10.7524/j.issn.0254-6108.2019120501 ZHANG H, WU L L, CHEN L K, et al. Influencing factors and mechanisms of ephedrine degradation by UV-254 nm activated persulfate [J]. Environmental Chemistry, 2020, 39(6): 1607-1616(in Chinese). doi: 10.7524/j.issn.0254-6108.2019120501
[28] WANG J Q, HASAER B, YANG M, et al. Anaerobically-digested sludge disintegration by transition metal ions-activated peroxymonosulfate (PMS): Comparison between Co2+, Cu2+, Fe2+ and Mn2 [J]. Sci Total Environ, 2020, 713: 136530. doi: 10.1016/j.scitotenv.2020.136530 [29] 朱睿, 谭烨, 李春全, 等. 基于过渡金属活化的过硫酸盐高级氧化技术研究进展 [J]. 化工矿物与加工, 2022, 51(1): 49-55. ZHU R, TAN Y, LI C Q, et al. Research progress of advanced persulfate oxidation technology based on activation by transition metals [J]. Industrial Minerals & Processing, 2022, 51(1): 49-55(in Chinese).
[30] 田婷婷, 李朝阳, 王召东, 等. 过渡金属活化过硫酸盐降解有机废水技术研究进展 [J]. 化工进展, 2021, 40(6): 3480-3488. TIAN T T, LI C Y, WANG S D, et al. Research progress of transition metal activated persulfate to degrade organic wastewater [J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3480-3488(in Chinese).
[31] RAO D D, DONG H Y, LIAN L S, et al. New mechanistic insights into the transformation of reactive oxidizing species in an ultraviolet/sulfite system under aerobic conditions: Modeling and the impact of Mn(Ⅱ) [J]. ACS ES& T WATER, 2021, 1(8): 1785-1795. [32] MACCREHAN W A, JENSEN J S, HELZ G R. Detection of sewage organic chlorination products that are resistant to dechlorination with sulfite [J]. Environmental Science & Technology, 1998, 32(22): 3640-3645. [33] DALTON-BUNNOW M F. Review of sulfite sensitivity [J]. Am J Hosp Pharm, 1985, 42(10): 2220-2226. [34] KULKARNI U S, DIXIT S G. Destruction of phenol from wastewater by oxidation with sulfite-oxygen [J]. Industrial & Engineering Chemistry Research, 1991, 30(8): 1916-1920. [35] ZHANG L, CHEN L, XIAO M, et al. Enhanced decolorization of orange Ⅱ solutions by the Fe(Ⅱ)–sulfite system under xenon lamp irradiation [J]. INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, 2013, 52(30): 10089-10094. [36] YU Y T, DING W, ZHANG L, et al. Decolorization of orange II in water induced by ferrous/sulfite system at near neutral pH values [J]. Advanced Materials Research, 2013, 821/822: 484-487. doi: 10.4028/www.scientific.net/AMR.821-822.484 [37] SUN S F, PANG S Y, JIANG J, et al. The combination of ferrate(VI) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants [J]. Chemical Engineering Journal, 2018, 333: 11-19. doi: 10.1016/j.cej.2017.09.082 [38] RAO D D, CHEN J, DONG H Y, et al. Enhanced oxidation of organic contaminants by Mn(Ⅶ)/CaSO3 under environmentally relevant conditions: Performance and mechanisms [J]. Water Res, 2021, 188: 116481. doi: 10.1016/j.watres.2020.116481 [39] SHI Z Y, JIN C, ZHANG J, et al. Insight into mechanism of arsanilic acid degradation in permanganate-sulfite system: Role of reactive species [J]. Chemical Engineering Journal, 2019, 359: 1463-1471. doi: 10.1016/j.cej.2018.11.030 [40] SUN B, GUAN X H, FANG J Y, et al. Activation of manganese oxidants with bisulfite for enhanced oxidation of organic contaminants: The involvement of Mn(Ⅲ) [J]. Environ Sci Technol, 2015, 49(20): 12414-12421. doi: 10.1021/acs.est.5b03111 [41] SUN B, DONG H Y, HE D, et al. Modeling the kinetics of contaminants oxidation and the generation of manganese(Ⅲ) in the permanganate/bisulfite process [J]. Environmental Science & Technology, 2016, 50(3): 1473-1482. [42] SUN B, BAO Q Q, GUAN X H. Critical role of oxygen for rapid degradation of organic contaminants in permanganate/bisulfite process [J]. J Hazard Mater, 2018, 352: 157-164. doi: 10.1016/j.jhazmat.2018.03.024 [43] DONG Q X, DONG H R, LI Y J, et al. Degradation of sulfamethazine in water by sulfite activated with zero-valent Fe-Cu bimetallic nanoparticles [J]. J Hazard Mater, 2022, 431: 128601. doi: 10.1016/j.jhazmat.2022.128601 [44] XIE P C, GUO Y Z, CHEN Y Q, et al. Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants [J]. Chemical Engineering Journal, 2017, 314: 240-248. doi: 10.1016/j.cej.2016.12.094 [45] XU J, WANG X R, PAN F, et al. Synthesis of the mesoporous carbon-nano-zero-valent iron composite and activation of sulfite for removal of organic pollutants [J]. Chemical Engineering Journal, 2018, 353: 542-549. doi: 10.1016/j.cej.2018.07.030 [46] TARTAR H V, GARRETSON H H. The thermodynamic ionization constants of sulfurous acid at 25°1 [J]. Journal of the American Chemical Society, 1941, 63(3): 808-816. doi: 10.1021/ja01848a049 [47] PASIUK-BRONIKOWSKA W, BRONIKOWSKI T, ULEJCZYK M. Mechanism and kinetics of autoxidation of calcium sulfite slurries [J]. Environmental Science & Technology, 1992, 26(10): 1976-1981. [48] BRANDT C, van ELDIK R. Transition metal-catalyzed oxidation of sulfur(Ⅳ) oxides. atmospheric-relevant processes and mechanisms [J]. Chemical Reviews, 1995, 95(1): 119-190. doi: 10.1021/cr00033a006 [49] DAS T N. Reactivity and role of SO5·- radical in aqueous medium chain oxidation of sulfite to sulfate and atmospheric sulfuric acid generation [J]. Journal of Physical Chemistry A, 2001, 105(40): 9142-9155. doi: 10.1021/jp011255h [50] FISCHER M, WARNECK P. Photodecomposition and photooxidation of hydrogen sulfite in aqueous solution [J]. Journal of Physical Chemistry, 1996, 100(37): 15111-15117. doi: 10.1021/jp953236b [51] CHEN L, TANG M, CHEN C, et al. Efficient bacterial inactivation by transition metal catalyzed auto-oxidation of sulfite [J]. Environ Sci Technol, 2017, 51(21): 12663-12671. doi: 10.1021/acs.est.7b03705 [52] 张立. Fe(Ⅲ)/S(Ⅳ)体系降解四溴双酚A效能及机理研究[D]. 武汉: 华中科技大学, 2019. ZHANG L. Study on the degradation of tetrabromobisphenol A by Fe(Ⅲ)/S(Ⅳ) system[D]. Wuhan: Huazhong University of Science and Technology, 2019(in Chinese).
[53] ZHAO X D, WU W J, YAN Y G. Efficient abatement of an iodinated X-ray contrast media iohexol by Co(Ⅱ) or Cu(Ⅱ) activated sulfite autoxidation process [J]. Environ Sci Pollut Res Int, 2019, 26(24): 24707-24719. doi: 10.1007/s11356-019-05601-4 [54] DONG H Y, WEI G F, YIN D Q, et al. Mechanistic insight into the generation of reactive oxygen species in sulfite activation with Fe(III) for contaminants degradation [J]. J Hazard Mater, 2020, 384: 121497. doi: 10.1016/j.jhazmat.2019.121497 [55] ZHOU D N, CHEN L, LI J J, et al. Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: A state-of-the-art minireview [J]. Chemical Engineering Journal, 2018, 346: 726-738. doi: 10.1016/j.cej.2018.04.016 [56] YUAN Y N, LUO T, XU J, et al. Enhanced oxidation of aniline using Fe(Ⅲ)-S(Ⅳ) system: Role of different oxysulfur radicals [J]. Chemical Engineering Journal, 2019, 362: 183-189. doi: 10.1016/j.cej.2019.01.010 [57] LUO T, YUAN Y N, ZHOU D N, et al. The catalytic role of nascent Cu(OH)2 particles in the sulfite-induced oxidation of organic contaminants [J]. Chemical Engineering Journal, 2019, 363: 329-336. doi: 10.1016/j.cej.2019.01.114 [58] CHEN L, HUANG X Y, TANG M, et al. Rapid dephosphorylation of glyphosate by Cu-catalyzed sulfite oxidation involving sulfate and hydroxyl radicals [J]. Environmental Chemistry Letters, 2018, 16(4): 1507-1511. doi: 10.1007/s10311-018-0767-y [59] CHEN L, PENG X Z, LIU J H, et al. Decolorization of orange Ⅱ in aqueous solution by an Fe(Ⅱ)/sulfite system: Replacement of persulfate [J]. INDUSTRIAL & ENGINEERING CHEMISTRY RESEARCH, 2012, 51(42): 13632-13638. [60] 李阳, 关小红, 董红钰. Fe(Ⅱ)活化亚硫酸盐降解卡马西平的动力学及机制研究 [J]. 土木与环境工程学报(中英文), 2021, 43(6): 165-171. LI Y, GUAN X H, DONG H Y. Kinetics and mechanism of carbamazepine degradation through activating sulfite by Fe(Ⅱ) [J]. Journal of Civil and Environmental Engineering, 2021, 43(6): 165-171(in Chinese).
[61] REDDY K B, van ELDIK R. Kinetics and mechanism of the sulfite-induced autoxidation of Fe(Ⅱ) in acidic aqueous solution [J]. Atmospheric Environment. Part A. General Topics, 1992, 26(4): 661-665. doi: 10.1016/0960-1686(92)90177-M [62] KARATZA D, PRISCIANDARO M, LANCIA A, et al. Calcium bisulfite oxidation in the flue gas desulfurization process catalyzed by iron and manganese ions [J]. Industrial & Engineering Chemistry Research, 2004, 43(16): 4876-4882. [63] KARATZA D, PRISCIANDARO M, LANCIA A, et al. Sulfite oxidation catalyzed by cobalt ions in flue gas desulfurization processes [J]. J Air Waste Manag Assoc, 2010, 60(6): 675-680. doi: 10.3155/1047-3289.60.6.675 [64] XIE P C, ZHANG L, WANG J W, et al. Transformation of tetrabromobisphenol a in the iron ions-catalyzed auto-oxidation of HSO32-/SO32- process [J]. Separation and Purification Technology, 2020, 235: 116197. doi: 10.1016/j.seppur.2019.116197 [65] ZHANG J M, MA J, SONG H R, et al. Organic contaminants degradation from the S(Ⅳ) autoxidation process catalyzed by ferrous-manganous ions: A noticeable Mn(III) oxidation process [J]. Water Res, 2018, 133: 227-235. doi: 10.1016/j.watres.2018.01.039 [66] YUAN Y N, ZHAO D, LI J J, et al. Rapid oxidation of paracetamol by cobalt(Ⅱ) catalyzed sulfite at alkaline pH [J]. Catalysis Today, 2018, 313: 155-160. doi: 10.1016/j.cattod.2017.12.004 [67] BERGLUND J, FRONAEUS S, ELDING L I. Kinetics and mechanism for manganese-catalyzed oxidation of sulfur(Ⅳ) by oxygen in aqueous solution [J]. Inorganic Chemistry, 1993, 32(21): 4527-4538. doi: 10.1021/ic00073a011 [68] LI G, WANG C, YAN Y P, et al. Highly enhanced degradation of organic pollutants in hematite/sulfite/photo system [J]. Chemical Engineering Journal, 2020, 386: 124007. doi: 10.1016/j.cej.2019.124007 [69] LEI Y, HAO Y X, CHENG H, et al. Degradation of orange Ⅱ by Fe2O3 and CeO2 nanocomposite when assisted by NaHSO3 [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 628: 127315. doi: 10.1016/j.colsurfa.2021.127315 [70] 武文敬. 铜氧化物活化亚硫酸盐降解碘海醇效能与反应机制[D]. 泉州: 华侨大学, 2020. WU W J. Degradation efficacy and reaction mechanism of iohexol by activation of sulfite with copper oxides[D]. Quanzhou: Huaqiao University, 2020(in Chinese).
[71] HUANG L Z, WEI X L, GAO E L, et al. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: A biomimetic approach towards efficient radical generation [J]. Applied Catalysis B:Environmental, 2020, 268: 118459. doi: 10.1016/j.apcatb.2019.118459 [72] ZHANG W Y, YIN C K, JIN Y Z, et al. Co-MOF as a highly efficient catalyst for contaminants degradation via sulfite activation [J]. Inorganic Chemistry Communications, 2021, 126: 108498. doi: 10.1016/j.inoche.2021.108498 [73] DING W, XIAO W L, HUANG W X, et al. Sulfite activation on a silica-supported well-dispersed cobalt catalyst via an electron transfer complex path [J]. Journal of Cleaner Production, 2020, 257: 120457. doi: 10.1016/j.jclepro.2020.120457 [74] PRESCOTT B D, JR. “Scombroid poisoning” and bluefish: The Connecticut connection [J]. Conn Med, 1984, 48(2): 105-110. [75] CHEN Y Q, TONG Y, XUE Y W, et al. Degradation of the β-blocker propranolol by sulfite activation using FeS [J]. Chemical Engineering Journal, 2020, 385: 123884. doi: 10.1016/j.cej.2019.123884 [76] WU W J, ZHAO X D, JING G H, et al. Efficient activation of sulfite autoxidation process with copper oxides for iohexol degradation under mild conditions [J]. Sci Total Environ, 2019, 695: 133836. doi: 10.1016/j.scitotenv.2019.133836 [77] DING W, HUANG X Y, ZHANG W D, et al. Sulfite activation by a low-leaching silica-supported copper catalyst for oxidation of As(III) in water at circumneutral pH [J]. Chemical Engineering Journal, 2019, 359: 1518-1526. doi: 10.1016/j.cej.2018.11.020 [78] 权晓琪, 许佩瑶, 杨帆, 等. 分子筛催化剂-亚硫酸盐体系降解水中对乙酰氨基苯酚 [J]. 分子催化, 2019, 33(6): 561-569. doi: 10.16084/j.cnki.issn1001-3555.2019.06.008 QUAN X Q, XU P Y, YANG F, et al. Degradation of acetaminophen in water by molecular sieve catalyst-sulfite system [J]. Journal of Molecular Catalysis(China), 2019, 33(6): 561-569(in Chinese). doi: 10.16084/j.cnki.issn1001-3555.2019.06.008
[79] ZHAO X D, WU W J, JING G H, et al. Activation of sulfite autoxidation with CuFe2O4 prepared by MOF-templated method for abatement of organic contaminants [J]. Environ Pollut, 2020, 260: 114038. doi: 10.1016/j.envpol.2020.114038 [80] WU Y, SHAO S J, ZHAO X D. CuCo2S4/sulfite reaction for efficient removal of tetracycline in water [J]. Environmental Chemistry Letters, 2022, 20(3): 1589-1594. doi: 10.1007/s10311-022-01412-1 [81] WU Y F, XING Y Y, ZHAO X D, et al. Mechanistic insights into rapid sulfite activation with cobalt sulfide towards iohexol abatement: Contribution of sulfur conversion [J]. Chemical Engineering Journal, 2022, 429: 132404. doi: 10.1016/j.cej.2021.132404 [82] GUO B Y, MA J F, SHI Y C, et al. Co3O4/CoO ceramic catalyst: Bisulfite assisted catalytic degradation of methylene blue [J]. CERAMICS INTERNATIONAL, 2021, 47(19): 27617-27623. doi: 10.1016/j.ceramint.2021.06.186 [83] FAN X, ZHOU Y R, ZHANG G, et al. In situ photoelectrochemical activation of sulfite by MoS2 photoanode for enhanced removal of ammonium nitrogen from wastewater [J]. Applied Catalysis B:Environmental, 2019, 244: 396-406. doi: 10.1016/j.apcatb.2018.11.061 [84] MEI Y, ZENG J C, SUN M Y, et al. A novel Fenton-like system of Fe2O3 and NaHSO3 for Orange II degradation [J]. Separation and Purification Technology, 2020, 230: 115866. doi: 10.1016/j.seppur.2019.115866 [85] ZHANG Y L, CHU W. Enhanced degradation of metronidazole by cobalt doped TiO2/sulfite process under visible light [J]. Separation and Purification Technology, 2022, 291: 120900. doi: 10.1016/j.seppur.2022.120900 [86] 王亿承. 高铁酸钾/亚硫酸钠体系降解水中二氯芬酸钠规律的研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. WANG Y C. Research on the degradation of diclofenac sodium in water by potassium ferrate(Ⅵ)/sodium sulfite system[D]. Harbin: Harbin Institute of Technology, 2019(in Chinese).
[87] 孙绍芳, 李佳龙, 邱琪, 等. Fe(Ⅵ)/Na2SO3体系降解阿特拉津效能 [J]. 中国环境科学, 2021, 41(1): 192-198. SUN S F, LI J L, QIU Q, et al. Degradation efficiency of atrazine by Fe(Ⅵ)/Na2SO3 system [J]. China Environmental Science, 2021, 41(1): 192-198(in Chinese).
[88] QIAO J L, FENG L Y, DONG H Y, et al. Overlooked role of sulfur-centered radicals during bromate reduction by sulfite [J]. Environ Sci Technol, 2019, 53(17): 10320-10328. doi: 10.1021/acs.est.9b01783 [89] SHAO B B, DONG H Y, FENG L Y, et al. Influence of [sulfite]/[Fe(Ⅵ)] molar ratio on the active oxidants generation in Fe(VI)/sulfite process [J]. J Hazard Mater, 2020, 384: 121303. doi: 10.1016/j.jhazmat.2019.121303 [90] DONG H Y, WEI G F, CAO T C, et al. Insights into the oxidation of organic cocontaminants during Cr(Ⅵ) reduction by sulfite: The overlooked significance of Cr(V) [J]. Environ Sci Technol, 2020, 54(2): 1157-1166. doi: 10.1021/acs.est.9b03356 [91] 刘庆泽, 黄颖, 王兆慧. 高盐环境下Cr(Ⅵ)/亚硫酸盐体系氧化降解效能研究 [J]. 水生态学杂志, 2019, 40(3): 71-77. doi: 10.15928/j.1674-3075.2019.03.010 LIU Q Z, HUANG Y, WANG Z H. Potential of chromium(Ⅵ)/sulfite for treating highly saline wastewater [J]. Journal of Hydroecology, 2019, 40(3): 71-77(in Chinese). doi: 10.15928/j.1674-3075.2019.03.010
[92] JIANG B, LIU Y K, ZHENG J T, et al. Synergetic transformations of multiple pollutants driven by Cr(Ⅵ)-sulfite reactions [J]. Environ Sci Technol, 2015, 49(20): 12363-12371. doi: 10.1021/acs.est.5b03275 [93] WANG Z Y, LI J, SONG W, et al. Rapid degradation of atrazine by a novel advanced oxidation process of bisulfite/chlorine dioxide: Efficiency, mechanism, pathway [J]. Chemical Engineering Journal, 2022, 445: 136558. doi: 10.1016/j.cej.2022.136558 [94] YANG T, MA J, WU S S, et al. Activation of ferrate(Ⅵ) by sulfite for effectively degrading iodinated contrast media and synchronously controlling I-DBPs formation [J]. Chemical Engineering Journal, 2022, 442: 136011. doi: 10.1016/j.cej.2022.136011 [95] SHAO B B, DONG H Y, SUN B, et al. Role of ferrate(Ⅳ) and ferrate(V) in activating ferrate(Ⅵ) by calcium sulfite for enhanced oxidation of organic contaminants [J]. Environ Sci Technol, 2019, 53(2): 894-902. doi: 10.1021/acs.est.8b04990 [96] CHOW C H, SZE-YIN LEUNG K. Transformations of organic micropollutants undergoing permanganate/bisulfite treatment: Kinetics, pathways and toxicity [J]. Chemosphere, 2019, 237: 124524. doi: 10.1016/j.chemosphere.2019.124524 [97] LI J, CASSOL G S, ZHAO J, et al. Superfast degradation of micropollutants in water by reactive species generated from the reaction between chlorine dioxide and sulfite [J]. Water Res, 2022, 222: 118886. doi: 10.1016/j.watres.2022.118886 [98] 唐海, 张昊楠, 段升飞, 等. SO32−活化S2O82−降解偶氮染料废水的机制研究 [J]. 中国环境科学, 2018, 38(3): 959-967. TANG H, ZHANG H N, DUAN S F, et al. Mechanism research for degradation of azo dying wastewater based on persulfate activated by sulphite [J]. China Environmental Science, 2018, 38(3): 959-967(in Chinese).
[99] 袁光明, 皮若冰, 吴钊成, 等. 高铁酸盐-亚硫酸盐体系氧化降解水中污染物阿特拉津 [J]. 化工进展, 2020, 39(9): 3794-3800. doi: 10.16085/j.issn.1000-6613.2019-1989 YUAN G M, PI R B, WU Z C, et al. Oxidative degradation of atrazine in water by ferrate-sulfite system [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3794-3800(in Chinese). doi: 10.16085/j.issn.1000-6613.2019-1989
[100] CHEN J, RAO D D, DONG H Y, et al. The role of active manganese species and free radicals in permanganate/bisulfite process [J]. J Hazard Mater, 2020, 388: 121735. doi: 10.1016/j.jhazmat.2019.121735 [101] ZHANG J, ZHU L, SHI Z Y, et al. Rapid removal of organic pollutants by activation sulfite with ferrate [J]. Chemosphere, 2017, 186: 576-579. doi: 10.1016/j.chemosphere.2017.07.102 [102] KEREZSI I, LENTE G, FÁBIÁN I. Highly efficient photoinitiation in the cerium(Ⅲ)-catalyzed aqueous autoxidation of sulfur(Ⅳ). An example of comprehensive evaluation of photoinduced chain reactions [J]. J Am Chem Soc, 2005, 127(13): 4785-4793. doi: 10.1021/ja0439120 [103] CAO Y, QIU W, LI J, et al. Review on UV/sulfite process for water and wastewater treatments in the presence or absence of O2 [J]. Sci Total Environ, 2021, 765: 142762. doi: 10.1016/j.scitotenv.2020.142762 [104] CAO Y, QIU W, LI J, et al. Sulfite enhanced transformation of iopamidol by UV photolysis in the presence of oxygen: Role of oxysulfur radicals [J]. Water Res, 2021, 189: 116625. doi: 10.1016/j.watres.2020.116625 [105] MILH H, YU X Y, CABOOTER D, et al. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes [J]. Sci Total Environ, 2021, 764: 144510. doi: 10.1016/j.scitotenv.2020.144510 [106] ENTEZARI M, GODINI H, SHEIKHMOHAMMADI A, et al. Enhanced degradation of polychlorinated biphenyls with simultaneous usage of reductive and oxidative agents over UV/sulfite/TiO2 process as a new approach of advanced oxidation/reduction processes [J]. Journal of Water Process Engineering, 2019, 32: 100983. doi: 10.1016/j.jwpe.2019.100983 [107] 罗涛. 能量辅助活化亚硫酸盐氧化水中As(Ⅲ)[D]. 武汉: 武汉大学, 2020. LUO T. Energy-assisted sulfite activation for As(Ⅲ) oxidation in water[D]. Wuhan: Wuhan University, 2020(in Chinese).
[108] JIA L X, PEI X W, YANG F. Electrolysis-assisted Mn(II)/sulfite process for organic contaminant degradation at near-neutral pH [J]. Water, 2019, 11(8): 1608. doi: 10.3390/w11081608 [109] LUO T, XU J, LI J J, et al. Strengthening arsenite oxidation in water using metal-free ultrasonic activation of sulfite [J]. Chemosphere, 2021, 281: 130860. doi: 10.1016/j.chemosphere.2021.130860 [110] LUO T, WANG H, CHEN L, et al. Visible light-driven oxidation of arsenite, sulfite and thiazine dyes: A new strategy for using waste to treat waste [J]. Journal of Cleaner Production, 2021, 280: 124374. doi: 10.1016/j.jclepro.2020.124374 [111] SHEIKHMOHAMMADI A, ASGARI E, HASHEMZADEH B. Photo-catalytic degradation of ciprofloxacin by UV/ZnO/SO3 process: Performance, kinetic, degradation pathway, energy consumption and total cost of system[J]. International Journal of Environmental Analytical Chemistry, 2023, 103(17): 1-15. [112] RASOULZADEH H, SHEIKHMOHAMMADI A, ASGARI E. Efficient destruction of metronidazole and ofloxacin antibiotics in the aqueous solutions by a new advanced oxidation process based on sulphite[J]. International Journal of Environmental Analytical Chemistry, 2023,103(18): 1-20. [113] RASOULZADEH H, ALINEJAD A, SHEIKHMOHAMMADI A. Improvement of Floxin photocatalytic degradability in the presence of sulfite: Performance, kinetic, degradation pathway, energy consumption and total cost of system[J]. International Journal of Environmental Health Research, 2022,32(12): 1-17. [114] CHU Y Y, XU L J, GAN L, et al. Efficient destruction of emerging contaminants in water by UV/S(Ⅳ) process with natural reoxygenation: Effect of pH on reactive species [J]. Water Res, 2021, 198: 117143. doi: 10.1016/j.watres.2021.117143 [115] LIU S L, FU Y S, WANG G S, et al. Degradation of sulfamethoxazole by UV/sulfite in presence of oxygen: Efficiency, influence factors and mechanism [J]. Separation and Purification Technology, 2021, 268: 118709. doi: 10.1016/j.seppur.2021.118709 [116] LI G, JIN Y X, YAN Y P, et al. The alkaline photo-sulfite system triggers Fe(Ⅳ/Ⅴ) generation at hematite surfaces [J]. Chemical Engineering Journal, 2020, 401: 126124. doi: 10.1016/j.cej.2020.126124 [117] LEI D S, XUE J Q, BI Q, et al. Visible-light activation of sulfite by ZnFe2O4@PANI photocatalyst for As(Ⅲ) removal: The role of radicals and Fe(IV) [J]. Applied Surface Science, 2022, 578: 151940. doi: 10.1016/j.apsusc.2021.151940 [118] LUO T, PENG Y, CHEN L, et al. Metal-free electro-activated sulfite process for As(Ⅲ) oxidation in water using graphite electrodes [J]. Environ Sci Technol, 2020, 54(16): 10261-10269. doi: 10.1021/acs.est.9b07078 [119] YU Y T, LI S Q, PENG X Z, et al. Efficient oxidation of bisphenol A with oxysulfur radicals generated by iron-catalyzed autoxidation of sulfite at circumneutral pH under UV irradiation [J]. Environmental Chemistry Letters, 2016, 14(4): 527-532. doi: 10.1007/s10311-016-0573-3 [120] PAN C W, GAO Q Y, STANBURY D M. Kinetics of the benzaldehyde-inhibited oxidation of sulfite by chlorine dioxide [J]. Inorg Chem, 2016, 55(1): 366-370. doi: 10.1021/acs.inorgchem.5b02770 [121] BULMAN D M, MEZYK S P, REMUCAL C K. The impact of pH and irradiation wavelength on the production of reactive oxidants during chlorine photolysis [J]. Environ Sci Technol, 2019, 53(8): 4450-4459. doi: 10.1021/acs.est.8b07225 [122] GUO K H, WU Z H, SHANG C, et al. Radical chemistry and structural relationships of PPCP degradation by UV/chlorine treatment in simulated drinking water [J]. Environ Sci Technol, 2017, 51(18): 10431-10439. doi: 10.1021/acs.est.7b02059 [123] SUN P Z, TYREE C, HUANG C H. Inactivation of Escherichia coli, Bacteriophage MS2, and Bacillus spores under UV/H2O2 and UV/peroxydisulfate advanced disinfection conditions [J]. Environ Sci Technol, 2016, 50(8): 4448-4458. doi: 10.1021/acs.est.5b06097 [124] LUTZE H V, BIRCHER S, RAPP I, et al. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter [J]. Environ Sci Technol, 2015, 49(3): 1673-1680. doi: 10.1021/es503496u [125] LUTZE H V, KERLIN N, SCHMIDT T C. Sulfate radical-based water treatment in presence of chloride: Formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate [J]. Water Res, 2015, 72: 349-360. doi: 10.1016/j.watres.2014.10.006 [126] DOGLIOTTI L, HAYON E. Flash photolysis of per[oxydi]sulfate ions in aqueous solutions. The sulfate and ozonide radical anions [J]. The Journal of Physical Chemistry, 1967, 71(8): 2511-2516. doi: 10.1021/j100867a019 [127] YANG Y, JIANG J, LU X L, et al. Production of sulfate radical and hydroxyl radical by reaction of ozone with peroxymonosulfate: A novel advanced oxidation process [J]. Environ Sci Technol, 2015, 49(12): 7330-7339. doi: 10.1021/es506362e -



 下载:
下载: