-
高铁酸盐(Fe(Ⅵ))是一种环境友好的氧化剂[1],与含有富电子基团的有机微污染物(例如胺类[2-3]、硫醇硫酯类[4]、酚类[5])具有较高的反应活性. 与二氧化氯、高锰酸盐、臭氧和次氯酸盐等常见氧化剂相比[6],Fe(Ⅵ)在酸性pH值下具有最高的氧化还原电位,且会减少溴代和氯代消毒副产物的生成[7]. 然而,Fe(Ⅵ)生产成本较高、在水溶液中的稳定性较低,这阻碍了其在水处理中的广泛应用. 因此,通过催化Fe(Ⅵ)氧化,提高Fe(Ⅵ)的利用效率具有重要意义.
在各种催化剂中,钌(Ru)因其多功能性而占有特殊的地位. ZSM-5(一种具有规整结构的微孔晶态硅铝酸盐分子筛)、TiO2和CeO2作为钌基催化剂的载体,在高锰酸盐氧化体系中性能稳定,钌能很好地附着到载体表面. 另外,ZSM-5、TiO2和CeO2作为载体,本身不会对催化效果产生影响. 因此本文选择ZSM-5、TiO2和CeO2作为载体. 已有研究证明在常见水处理pH范围内,催化剂Ru/ZSM-5、Ru/TiO2或Ru/CeO2能显著提高高锰酸盐氧化速率[8-9]. 此外,Ru/CeO2和Ru/TiO2具有良好的稳定性,在连续6次重复试验中其催化活性基本保持不变[8];但在连续10次重复试验中,Ru/ZSM-5的催化效能迅速下降,其稳定性比Ru/CeO2和Ru/TiO2差[9]. Ru作为催化剂在高铁酸盐氧化中的应用却鲜有报道. 因此,本研究以CeO2、TiO2和 ZSM-5为载体的非均相钌催化剂,并考察了其在不同pH条件下催化Fe(Ⅵ)氧化苯酚的性能,提出了Ru催化Fe(Ⅵ)氧化中的机理并评估了催化剂的稳定性.
-
水合三氯化钌(RuCl3·xH2O, 37% Ru)购自J&K化学公司;CeO2、TiO2、苯酚以及甲醇、甲酸等有机溶剂购自国药化学试剂有限公司;咖啡因购自Sigma公司;ZSM-5购自南开大学催化剂厂. 实验中所有溶液均使用超纯水配制.
采用湿式化学合成法[10]制备固体高铁酸钾(K2FeO4,纯度>95%). 将固体K2FeO4溶解在超纯水中制备K2FeO4储备溶液,然后用0.45 μm亲水性滤头过滤,通过紫外分光光度计在510 nm测量浓度,并在30 min内使用,以减少其自分解的影响.
-
将装有200 mL一定浓度有机物和一定量催化剂的溶液调节至指定pH值,投加高铁酸钾储备液后,反应开始计时,定时取样,采用硫代硫酸钠或者盐酸羟胺淬灭,水样经醋酸纤维膜(孔径0.22 μm)过滤后定量分析.
-
将RuCl3的干固体溶于pH 2.0的盐酸溶液中,配置成浓度为1 mol·L−1的RuCl3酸性溶液,弱酸环境可以有效防止三价钌被空气氧化为四价钌. 然后在室温条件下(约20 ℃)向RuCl3酸溶液投加载体,在磁力搅拌器上进行剧烈搅拌,使之达到均匀混合的状态,搅拌过程持续4 h. 然后通过高速离心机将固体从溶液中分离开来,用超纯水对催化剂进行反复冲洗,直至冲洗液的pH约为近中性,再将最终离心的固体置于温度为40 ℃的真空干燥箱中进行干燥. 待催化剂彻底干燥以后,即可进行实验. 利用场发射扫描电子显微镜(SEM,型号HITACHIS-4800)观察催化剂的表面形貌. 采用高分辨透射电子显微镜(TEM,型号为JEM-2100)和能量色散X射线光谱仪(EDAX)对催化剂表面的钌氢氧化物的颗粒形貌进行观察,同时对选定区域进行元素分析. 采用 Coulter SA 3100 吸附分析仪进行氮吸附-解吸,测定催化剂的比表面积和孔体积. 新合成的催化剂和使用后的催化剂分别经硝酸微波消解,采用电感耦合等离子体发射光谱法(ICP-AES)测定其Ru含量.
-
采用超高效液相色谱仪(UPLC,型号H-Class)测定苯酚和咖啡因的浓度. 采用BEH C18柱子,柱温控制在35 ℃;测定苯酚时流动相为水:甲醇=65:35,测定咖啡因时流动相为水(0.1%甲酸):甲醇=70:30;检测波长是254 nm(苯酚)和273 nm(咖啡因).
-
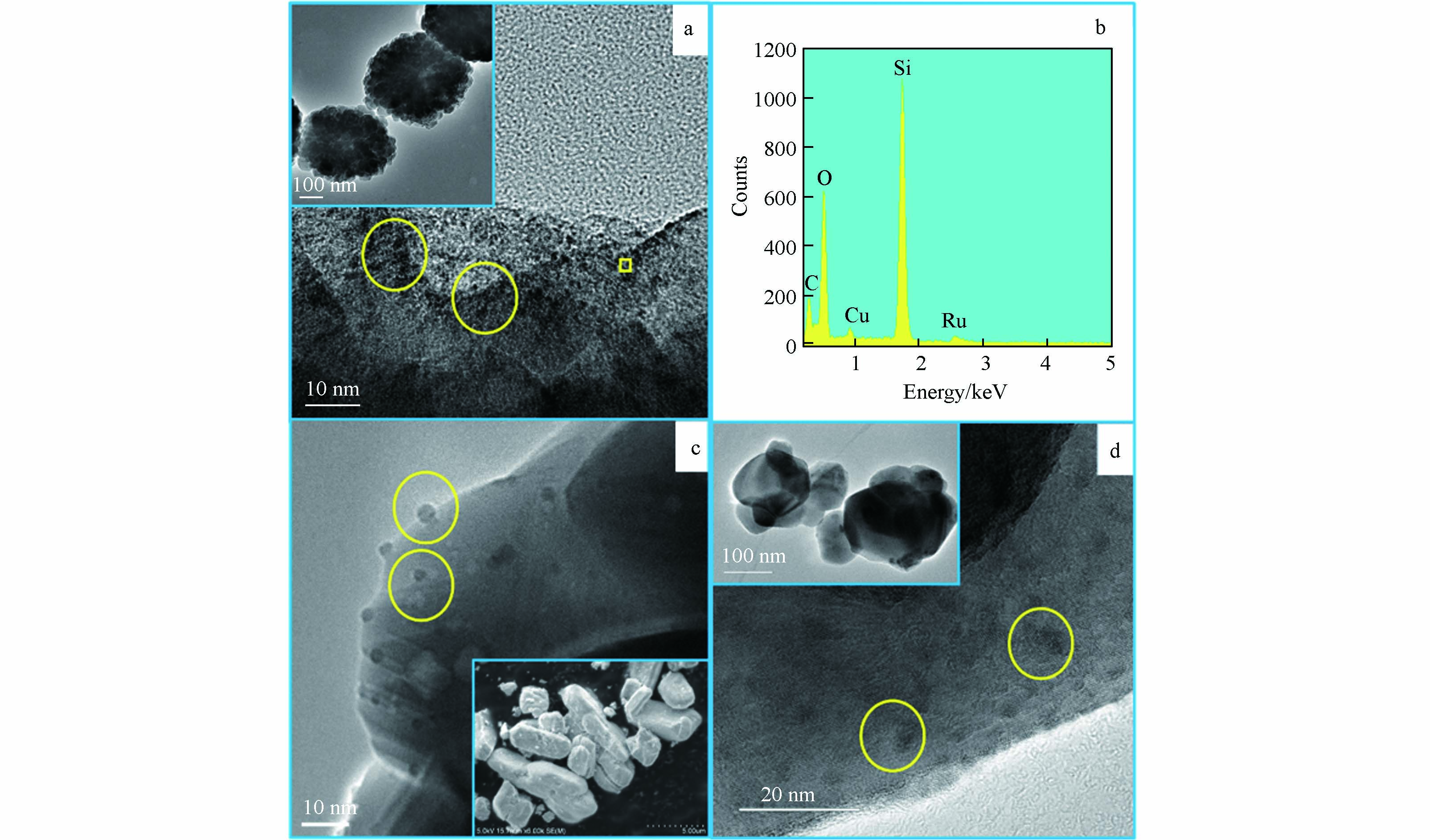
新合成的Ru/ZSM-5、Ru/CeO2和Ru/TiO2催化剂中Ru的含量分别为0.69‰、0.80‰和0.94‰. SEM、TEM和EDAX的结果均证明载体上存在Ru颗粒(图1). 图1表面ZSM-5和TiO2为球形,CeO2为立方体形状. TEM结果表明,在Ru/ZSM-5、Ru/CeO2和Ru/TiO2中,Ru以粒径约为2.5 nm的颗粒存在于ZSM-5、TiO2和CeO2的表面. 制备过程中载体与RuCl3酸溶液反应结束后,通过离心将固体从溶液中分离,然后用超纯水对催化剂进行反复冲洗,因此在冲洗过程中RuCl3可能会发生水解,生成相应的氢氧化钌,推测载体表面的纳米颗粒为氢氧化钌的颗粒 [8]. 在Ru/ZSM-5中,ZSM-5的比表面积较高,通过TEM观测到Ru在ZSM-5表面的分散性也更好(表1). Ru/ZSM-5的区域EDAX分析表明Ru被成功负载(图1b).
-
已有研究证明Fe(Ⅵ)氧化有机微污染物符合二级反应动力学,因此本研究假设在有催化剂或没有催化剂的情况下,Fe(Ⅵ)氧化苯酚的反应在初始阶段遵循二级反应. 苯酚降解可能发生在溶液中(即非催化反应)和/或催化剂表面(即催化反应). 反应动力学方程1为简化的动力学模型,该模型同时考虑了催化反应和非催化反应.
式中,ku(L·mol−1·min−1)为非催化反应的二级速率常数,kc(L·mol−1·min−1)为催化反应的二级速率常数;kT(L·mol−1·min−1)是整个反应的表观二级速率常数;kobs(min−1)等于kT[Fe(Ⅵ)]. 在以前的研究中,Fe(Ⅵ)浓度随时间的变化是通过紫外分光光度计在510 nm测量的,但在本研究中,由于固体催化剂的干扰,无法测定溶液中Fe(Ⅵ)的浓度.
如图2所示,Ru/ZSM-5、Ru/CeO2和Ru/TiO2催化剂加入后,kobs随催化剂用量的增加呈线性增加. 当Ru/ZSM-5的投加量从0增加到0.5 g·L−1时,kobs几乎增加了10倍. Ru/ZSM-5的Ru载量最低(0.69‰),但其催化效能与另外两种催化剂相当。由于ZSM-5比表面积远高于TiO2和CeO2,且钌纳米颗粒在ZSM-5载体上有良好分散性. 已有研究证明CeO2、TiO2和 ZSM-5作为载体,本身不会对钌催化效果产生影响[11]. 虽然载体本身对非均相钌催化剂的性能影响不大,但决定了钌纳米颗粒的分散性[8],而钌的分散性对其催化性能起着决定性作用。
-
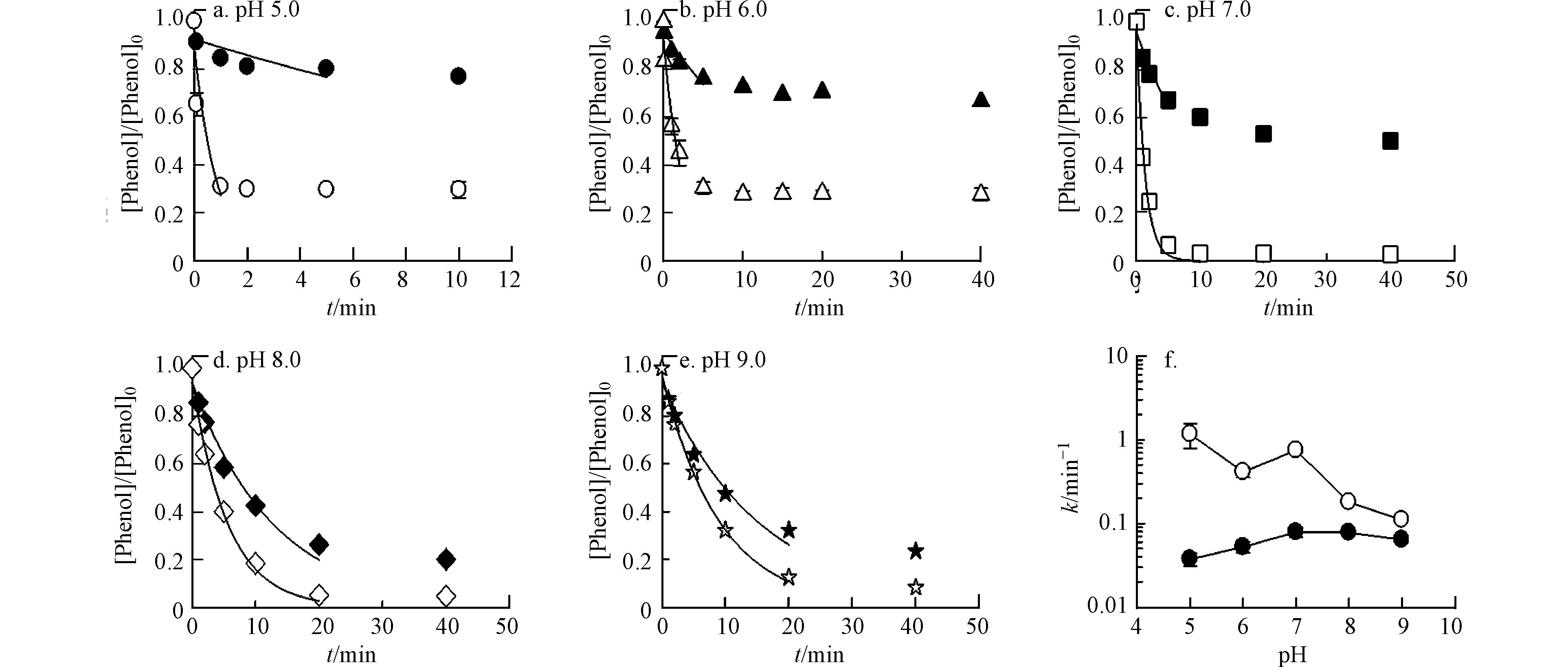
不同pH值下,Fe(Ⅵ)存在不同酸碱形态(pKa1 = 3.5,pKa2 = 7.3)[12],且不同形态的Fe(Ⅵ)活性不同. 苯酚(PhOH)也存在分子态和电离态(pKa = 10),在pH 5.0—9.0范围内,苯酚主要以分子态PhOH存在,因此可以忽略其电离态. 假设不同形态的Fe(Ⅵ)通过平行反应对PhOH进行氧化(反应 2—4):
在不投加催化剂的情况下,当pH从5.0升高到7.0时,kobs从3.74×10−2 min−1增加到7.89×10−2 min−1,随着pH进一步升高到9.0,kobs从7.89×10−2 min−1下降到6.40×10−2 min−1(图3). 以往的研究证明HFeO4−比FeO42−的反应活性更高,但在pH 5.0和6.0下kobs相对较低,这可能是由于在低pH条件下Fe(Ⅵ)会快速自分解[12].
在3种催化剂中,选择Ru/ZSM-5作为模型催化剂来评估pH对Fe(Ⅵ)氧化苯酚性能的影响. 如图3所示,Ru/ZSM-5催化Fe(Ⅵ) 氧化苯酚的动力学也表现出很强的pH依赖性. 在pH 5.0—9.0范围内,Ru/ZSM-5极大地提高了苯酚的去除速率. 除pH 7.0外,Ru/ZSM-5催化Fe(Ⅵ)氧化苯酚的kobs (min−1)随pH升高而降低. 这是由于随着pH的升高,Fe(Ⅵ)氧化电位逐渐降低,因此生成的Rux+的量也就相应逐渐下降,最终使得催化效果下降. 在pH 7.0时,Ru/ZSM-5催化效能升高,这可能是因为在pH 7.0时,钌的催化活性较高[13].
-
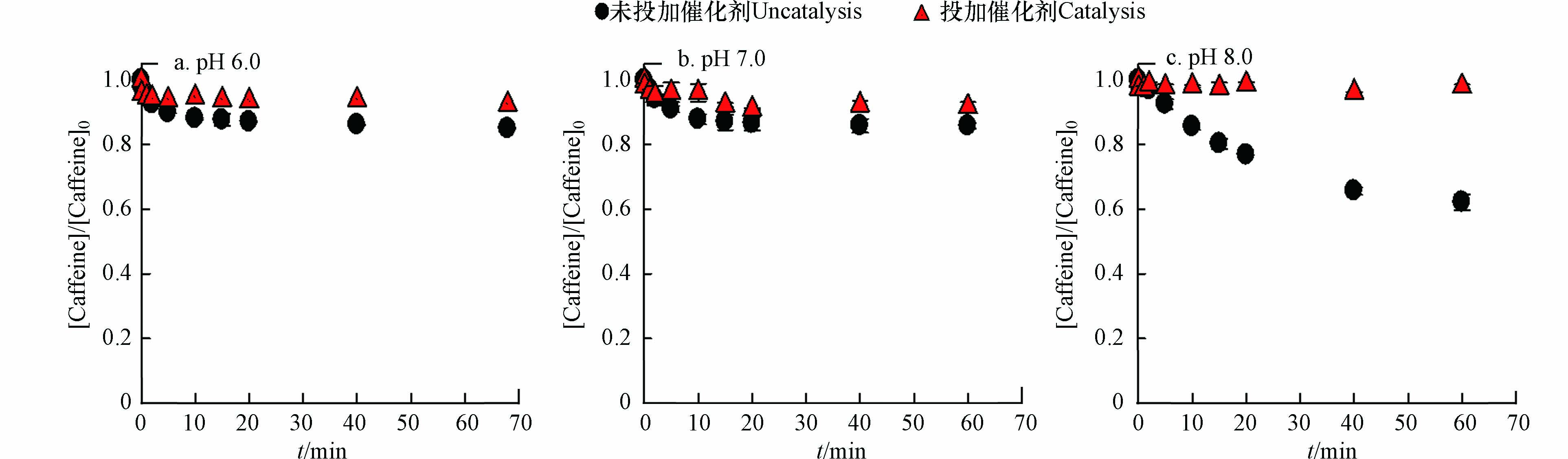
研究表明,在Fe(Ⅵ)氧化过程中,可能有中间态Fe(Ⅳ)、Fe(Ⅴ)和羟基自由基(HO·)等反应活性中间体[14]. 高铁酸盐氧化咖啡因过程中,主要是中间价态铁Fe(Ⅴ)和Fe(Ⅳ)在发挥氧化效能,而Fe(Ⅵ)不能降解咖啡因[15]. 因此,本文选择咖啡因作为探针化合物,检测和评估Fe(Ⅴ)和Fe(Ⅳ)对Fe(Ⅵ)-Ru/ZSM-5体系的贡献. 如图4所示,由于Ru/ZSM-5的存在,Fe(Ⅵ)对咖啡因的降解被完全抑制. 因此,可以排除 Fe(Ⅵ)-Ru/ZSM-5体系中Fe(Ⅴ)和Fe(Ⅳ)的贡献. 已有研究证明[16],Fe(Ⅵ)氧化过程中产生的HO·对有机物的降解作用可以忽略不计. 因此,排除了HO·对苯酚降解的贡献.
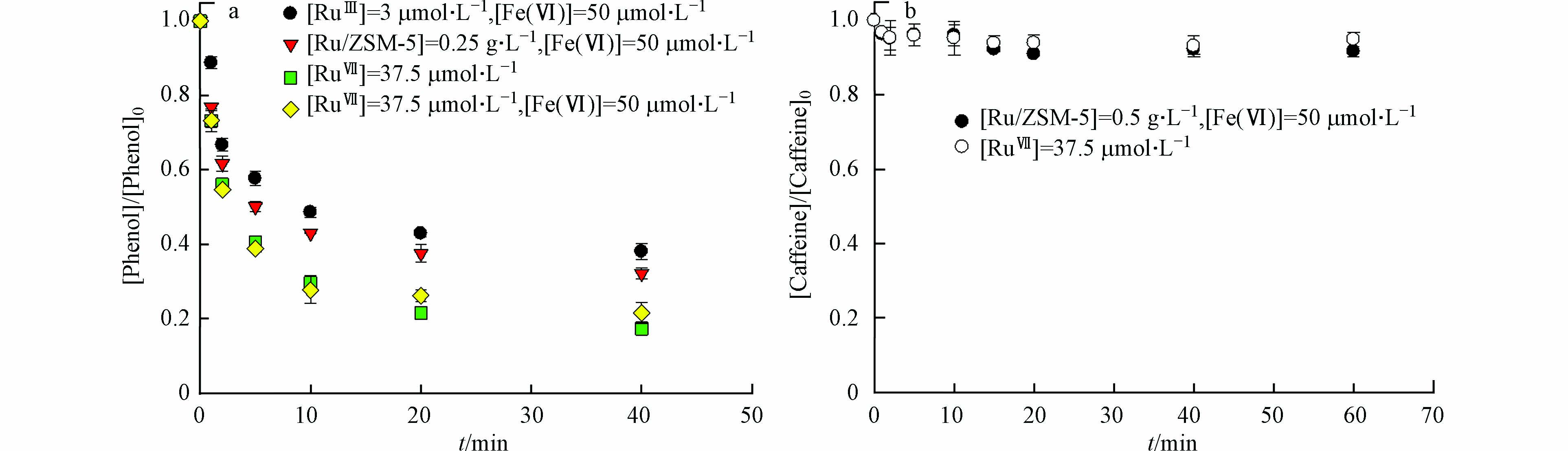
在高锰酸钾氧化的过程中,钌纳米颗粒具有电子穿梭的作用[8]. 简而言之,RuⅢ被高锰酸盐氧化成更高的氧化态RuⅦ,其作为氧化物种被有机微污染物还原到其初始状态RuⅢ. 因此,本研究假设Ru催化Fe(Ⅵ)氧化过程中的活性物种也是RuⅦ. 本文通过投加KRuO4,评估了RuⅦ对Fe(Ⅵ)氧化苯酚的影响. 在pH 7.0下,单独RuⅦ降解苯酚速率比Fe(Ⅵ)更高,但其对咖啡因的降解几乎没有影响(图5). Fe(Ⅵ)/RuⅢ降解苯酚的动力学规律与Fe(Ⅵ)/RuⅦ相同,但Fe(Ⅵ)/RuⅦ的反应速率常数更大. 这是因为Fe(Ⅵ)/RuⅦ中的Fe(Ⅵ)和RuⅦ都参与苯酚降解,而Fe(Ⅵ)/RuⅢ中部分Fe(Ⅵ)用于转化RuⅢ为中间态RuⅦ. 可以确定RuⅦ是Ru催化Fe(Ⅵ)氧化的主要活性物种,也是降解有机微污染物的选择性氧化剂[12].
-
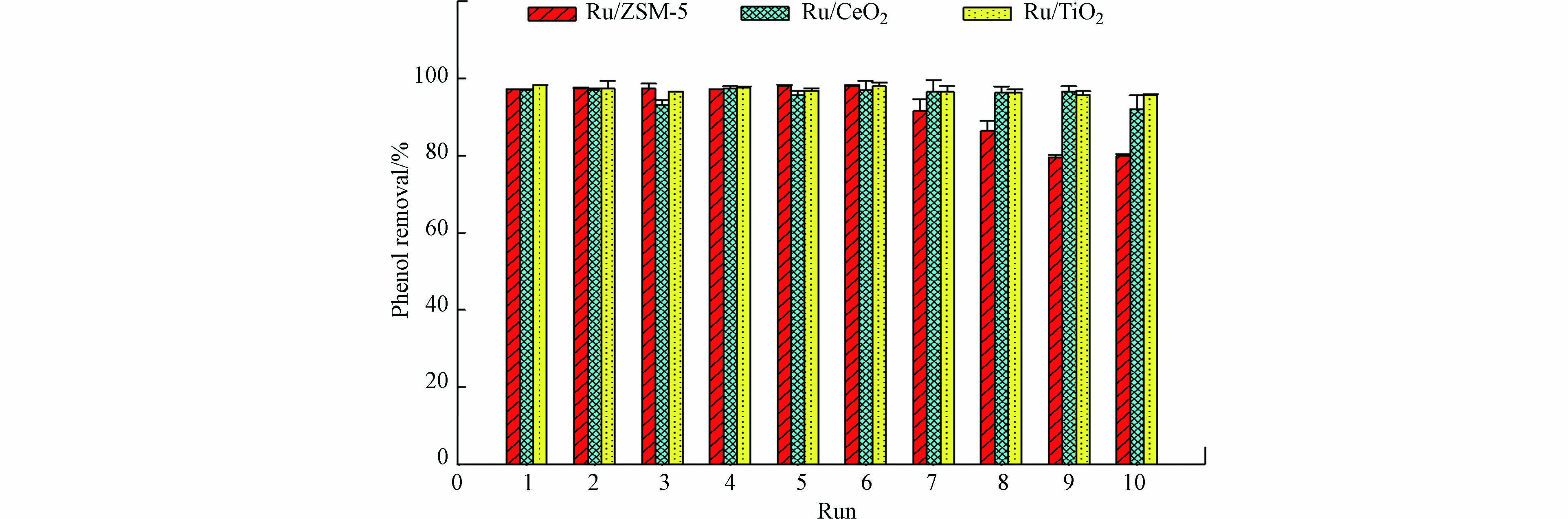
在催化反应和工业应用中,催化剂的可重复使用性和稳定性至关重要. 因此本文评估了Ru/CeO2、 Ru/TiO2和Ru/ZSM-5连续使用10次的稳定性(图6). 在Ru/CeO2和Ru/TiO2催化体系中,苯酚的去除率始终高于92%;而当催化剂为Ru/ZSM-5时,在前6次实验中苯酚的去除率保持不变,但从第6次到第10次循环实验中,苯酚的去除率从98.1%逐渐下降到80.0%. 经过10次循环使用后,Ru/ZSM-5、Ru/CeO2和Ru/TiO2中Ru负载量分别从0.69‰降至0.65‰、0.80‰至0.77‰、0.94‰至0.84‰. 因此,Ru的泄露并不是Ru/ZSM-5性能下降的主要原因. Fe(Ⅵ)的主要还原产物是Fe(Ⅲ),即氢氧化铁沉淀物,这些沉淀物可能吸附在催化剂表面,导致催化剂部分活性位点被覆盖. ZSM-5是一种中孔沸石,具有较高的表面积,因此对氢氧化铁的吸附能力更高(表1). ICP结果证实在使用后的Ru/ZSM-5上存在Fe元素,质量占比为0.04‰,而Ru/CeO2和Ru/TiO2上的Fe可以忽略不计. 因此,Ru/ZSM-5 性能下降可能主要与沉积的氢氧化铁掩盖了Ru/ZSM-5表面的活性位点有关. Ru/TiO2和Ru/CeO2稳定性好,有利于其工程或中试应用,但应尽量避免氢氧化铁在催化剂表面沉积.
-
Ru/ZSM-5、Ru/CeO2和Ru/TiO2可以有效催化Fe(Ⅵ)氧化降解苯酚,且催化效能随催化剂用量线性增加. 钌纳米颗粒的良好分散性是其获得较好催化性能的关键,因此,在未来研究中,单原子钌基催化剂具有广阔前景. Ru催化Fe(Ⅵ)氧化降解苯酚的动力学表现出很强的pH依赖性. Ru催化Fe(Ⅵ)氧化降解苯酚过程中的主要活性物质为RuⅦ和Fe(Ⅵ). Ru/TiO2和Ru/CeO2的稳定性优于Ru/ZSM-5,有利于其中试或工程实践中的应用.
非均相钌催化高铁酸盐氧化降解苯酚
Enhanced ferrate oxidation by ruthenium nanoparticles for degradation of phenol
-
摘要: 本研究开发了以ZSM-5、TiO2和CeO2为载体的非均相钌(Ru)催化剂,并检验了其催化高铁酸盐(Fe(Ⅵ))氧化苯酚的性能. 随着Ru/ZSM-5、Ru/TiO2或Ru/CeO2催化剂投加量的增加,Fe(Ⅵ)氧化苯酚的伪一级反应速率常数(kobs,min-1)呈线性增长趋势. 催化剂的效能主要取决于载体上钌纳米颗粒的分散情况,受钌负载量的影响不明显. 虽然Ru/ZSM-5的Ru载量最低,但ZSM-5的比表面积较高,且Ru在ZSM-5表面的分散性好,使其催化效能与另外两种催化剂相当. 非均相钌催化性能受pH影响很大,在pH 5.0—9.0时显著提高了苯酚的去除率. Ru的催化机理为RuIII被Fe(Ⅵ)氧化为RuⅦ中间体,RuⅦ具有较高活性和氧化能力,可以快速氧化苯酚,从而促进Fe(Ⅵ)氧化苯酚的速率. 研究通过连续10次重复使用实验考察了3种催化剂的稳定性,证明Ru/CeO2和Ru/TiO2的稳定性优于Ru/ZSM-5,Ru/ZSM-5稳定性下降的主要原因是ZSM-5比表面积较大,更容易吸附Fe(Ⅵ)的还原产物——氢氧化铁,从而使得Ru活性位点被氢氧化铁掩盖.Abstract: In this study, we developed ruthenium-based (Ru-based) catalysts with ZSM-5, TiO2 and CeO2 as supports, and examined their performance for catalyzing ferrate (Fe(Ⅵ)) oxidation of caffeine or phenol. With the introduction of Ru/ZSM-5, Ru/TiO2, and Ru/CeO2, the pseudo-first-order reaction rate constants (kobs, min-1) of Fe(Ⅵ) oxidation of phenol increase linearly with the dosage of each catalyst. The enhancement from each catalyst depends more on the dispersion of ruthenium nanoparticles on the supports than the loading of ruthenium. Hence, we observed comparable catalytic performance for these three catalysts with Ru/ZSM-5 having the highest dispersion, but the lowest loading of ruthenium nanoparticles. Ruthenium nanoparticles significantly increased the removal of phenol at pH 5.0—9.0, while its catalytic performance was greatly affected by pH. Ru intermediate with higher oxidation state, RuⅦ, was proved to be the major active species in ruthenium catalyzed Fe(Ⅵ) oxidation. To investigate the stability of catalysts, we used Ru/CeO2, Ru/TiO2 and Ru/ZSM-5 for ten consecutive times under same reaction conditions. It was found that the stability of Ru/CeO2 and Ru/TiO2 are better than Ru/ZSM-5, which would favor their application in pilot or engineering practice. The depressed performance of Ru/ZSM-5 may be mainly associated with masking active sites by the deposited ferric hydroxide.
-

-
图 1 (a) Ru/ZSM-5的TEM图,(b)Ru/ZSM-5表面黄色方框内的EDAX图,(c) Ru/CeO2的TEM图,(d) Ru/TiO2的TEM图;载体的SEM图像显示在(a)、(c)和(d)的插图中.
Figure 1. (a) TEM image of Ru/ZSM-5, (b) EDAX image of Ru/ZSM-5 surface in the yellow box, (c) TEM image of Ru/CeO2, and (d) TEM image of Ru/TiO2. The SEM images of supports were shown in the insets of (a), (c) and (d).
表 1 催化剂及其载体的性质
Table 1. Characteristics of synthesized catalysts and supports
平均粒径/μm
Average
diameter比表面积/(m2·g−1)
BET Surface
area微孔表面积/(m2·g−1)
Micropore surface
area外比表面积/(m2·g−1)
External surface
area孔径/nm
Pore size孔体积/
(m3·g−1)
Pore volume微孔体积/(m3·g−1)
Micropore
volumeZSM-5 0.5 461 323 138 0.5 0.386 0.132 Ru/ZSM-5 0.5 407 280 127 0.5 0.272 0.088 TiO2 0.15 4.87 — — — — — Ru/TiO2 0.15 4.70 — — — — — CeO2 30 4.53 — — — — — Ru/CeO2 30 4.87 — — — — — -
[1] ZHENG L, DENG Y. Settleability and characteristics of ferrate(Ⅵ)-induced particles in advanced wastewater treatment [J]. Water Research, 2016, 93: 172-178. doi: 10.1016/j.watres.2016.02.015 [2] SUN S F, LIU Y L, MA J, et al. Transformation of substituted anilines by ferrate(Ⅵ): Kinetics, pathways, and effect of dissolved organic matter [J]. Chemical Engineering Journal, 2018, 332: 245-252. doi: 10.1016/j.cej.2017.08.116 [3] JOHNSON M D, HORNSTEIN B J. Oxidation of aniline by ferrate(Ⅵ): Formation of an iron-imido species [J]. Abstracts of Papers of the American Chemical Society, 2000, 219: U843-U843. [4] SHARMA V K, CHEN L, ZBORIL R. Review on high valent FeVI (ferrate): A sustainable green oxidant in organic chemistry and transformation of pharmaceuticals [J]. ACS Sustainable Chemistry & Engineering, 2016, 4(1): 18-34. [5] LEE Y, YOON J, von GUNTEN U. Kinetics of the oxidation of phenols and phenolic endocrine disruptors during water treatment with ferrate (Fe(Ⅵ)) [J]. Environmental Science & Technology, 2005, 39(22): 8978-8984. [6] 张静, 张宏龙, 王定祥, 等. 强化高锰酸钾氧化体系中自由基的产生与利用研究进展 [J]. 环境化学, 2021, 40(2): 487-496. doi: 10.1002/etc.4944 ZHANG J, ZHANG H L, WANG D X, et al. Generation and utilization of radicals during enhanced permanganate oxidation: A review [J]. Environmental Chemistry, 2021, 40(2): 487-496(in Chinese). doi: 10.1002/etc.4944
[7] LI C, LUO F, DONG F L, et al. Chlorine decay and trihalomethane formation following ferrate(Ⅵ) preoxidation and chlorination of drinking water [J]. Chemosphere, 2017, 187: 413-420. doi: 10.1016/j.chemosphere.2017.08.084 [8] ZHANG J, SUN B, GUAN X H, et al. Ruthenium nanoparticles supported on CeO2 for catalytic permanganate oxidation of butylparaben [J]. Environmental Science & Technology, 2013, 47(22): 13011-13019. [9] ZHANG J, SUN B, HUANG Y Y, et al. Catalyzing the oxidation of sulfamethoxazole by permanganate using molecular sieves supported ruthenium nanoparticles [J]. Chemosphere, 2015, 141: 154-161. doi: 10.1016/j.chemosphere.2015.06.088 [10] THOMPSON G W, OCKERMAN L T, SCHREYER J M. Preparation and purification of potassium ferrate( Ⅵ) [J]. Journal of the American Chemical Society, 1951, 73(3): 1379-1381. [11] 张静. RuⅢ催化KMnO4氧化去除水中新兴微污染物的效能与机理[D]. 哈尔滨: 哈尔滨工业大学, 2014. ZHANG J. Performance and mechanism of Ruⅲ-catalyzed permanganate oxidation of emerging micropollutants in water[D]. Harbin: Harbin Institute of Technology, 2014(in Chinese).
[12] HU L H, MARTIN H M, ARCE-BULTED O, et al. Oxidation of carbamazepine by Mn(VII) and Fe(Ⅵ): Reaction kinetics and mechanism [J]. Environmental Science & Technology, 2009, 43(2): 509-515. [13] OUNKHAM W L, WEEDEN J A, FROST B J. Aqueous-phase nitrile hydration catalyzed by an in situ generated air-stable ruthenium catalyst [J]. Chemistry - A European Journal, 2019, 25(42): 10013-10020. doi: 10.1002/chem.201902224 [14] LEE Y, KISSNER R, von GUNTEN U. Reaction of ferrate(Ⅵ) with ABTS and self-decay of ferrate(Ⅵ): Kinetics and mechanisms [J]. Environmental Science & Technology, 2014, 48(9): 5154-5162. [15] ZHU J H, YU F L, MENG J R, et al. Overlooked role of Fe(Ⅳ) and Fe(Ⅴ) in organic contaminant oxidation by Fe(Ⅵ) [J]. Environmental Science & Technology, 2020, 54(15): 9702-9710. [16] WANG D X, LIANG J L, ZHANG H L, et al. Reinvestigation of ferrate(Ⅵ) oxidation of bisphenol A over a wide pH range [J]. ACS ES& T Water, 2022, 2(1): 156-164. -



 下载:
下载: