-
由于氨氮具有水生生物毒性、导致水体富营养化和水生生态平衡破坏等特点,其去除一直是废水处理中的主要挑战[1-2]。目前,地下水氨氮污染也引起了人们的关注,2019年发布的《中国生态环境状况公报》[3]指出全国10168个国家级地下水水质监测70%以上的水体氨氮含量超标。
目前,氨氮污染去除技术主要分为生物处理法、物理处理法和化学处理法[4]。物理法主要包括反渗透法[5]、离子交换法[6-7]和吹脱法等[8];化学法主要包括折点氯化法[9]、化学沉淀法[10]和电化学法[11];生物法主要为硝化-反硝化技术[12]、厌氧氨氧化和各类新型生物脱氮技术。近年来,电化学催化氧化已成为一种有前景的除氨方法,相比于其他高级氧化技术,其优点包括二次污染物的产生量最少[13],不需要另加氧化还原剂,易于自动化等;但也有耗电量大、成本高等缺点。据报道,电化学催化氧化可有效处理来自发电厂[14],市政[15]、垃圾填埋场[16]和焦化厂[17]等含氨氮的废水。
本研究以含氯含氨氮地下水为研究对象,将吹脱除氨作为减轻后续处理负荷及减少水力停留时间的预处理单元,在电化学催化氧化的基础上设计适合实际工程应用的连续流反应器,采用吹脱-电化学催化氧化组合的方式使氨氮指标达到排放限值。对比了光/电协同氧化法(电化学法与光催化法联合)和单独电化学氧化法降解氨氮的效果、转化产物,具体分析了氨氮氧化机理,为电化学催化氧化组合工艺处理氨氮污染地下水提供了新思路。
-
N,N-二乙基-1,4-苯二胺 (DPD) 游离氯试剂购于北京松原科创科技有限公司,氯化钌水合物(RuCl3·xH2O)、纳氏试剂(Hg-KI-NaOH)、酒石酸钾钠(NaKC4H4O6)、氯化钠(NaCl)、氢氧化钠(NaOH)、5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)均购于上海阿拉丁生化科技股份有限公司,丙酮(C3H6O)、异丙醇(C3H8O)、乙醇(C2H5OH)购于国药集团化学试剂有限公司。本实验及药剂配置用水为Milli-Q(美国Millipore公司)制备的超纯水。紫外灯灯管购于海宁市硖石万华电光源厂(254 nm,4~6 W),钌铱涂层泡沫钛电极和泡沫钛电极购于陕西环亚赛纳氢有限公司,圆筒形钛网购于苏州舒尔泰工业科技有限公司。
钛钌圆筒型电极制备方法:以圆筒形钛网用作电极基底材料,先用800目和2 000目砂纸打磨抛光,再将其置于质量分数为1%的氢氟酸溶液中化学抛光,清洗后烘干。将电极置于马弗炉以450 ℃恒温煅烧2 h制得二氧化钛保护层。采用浸渍提拉法将0.2 mol·L−1 RuCl3-异丙醇聚合前驱体溶胶涂覆电极,并置于马弗炉在450 ℃下处理10 min,不断重复次此步骤直至达到所需涂覆量(16次)后,450 ℃煅烧2 h得到圆筒型钛钌阳极。
实验水样为上海某钢铁工业区原固废堆场区域的地下水(简称地下水)。其氨氮质量浓度为49~54 mg·L−1,总氮为51.7~58.3 mg·L−1,硝态氮为0.1~0.6 mg·L−1,氯离子为471~485 mg·L−1,pH为9.5~10.6,电导率为1 512~1 708 μS·cm−1。以下实验如未经特殊说明均为上述水质条件。
-
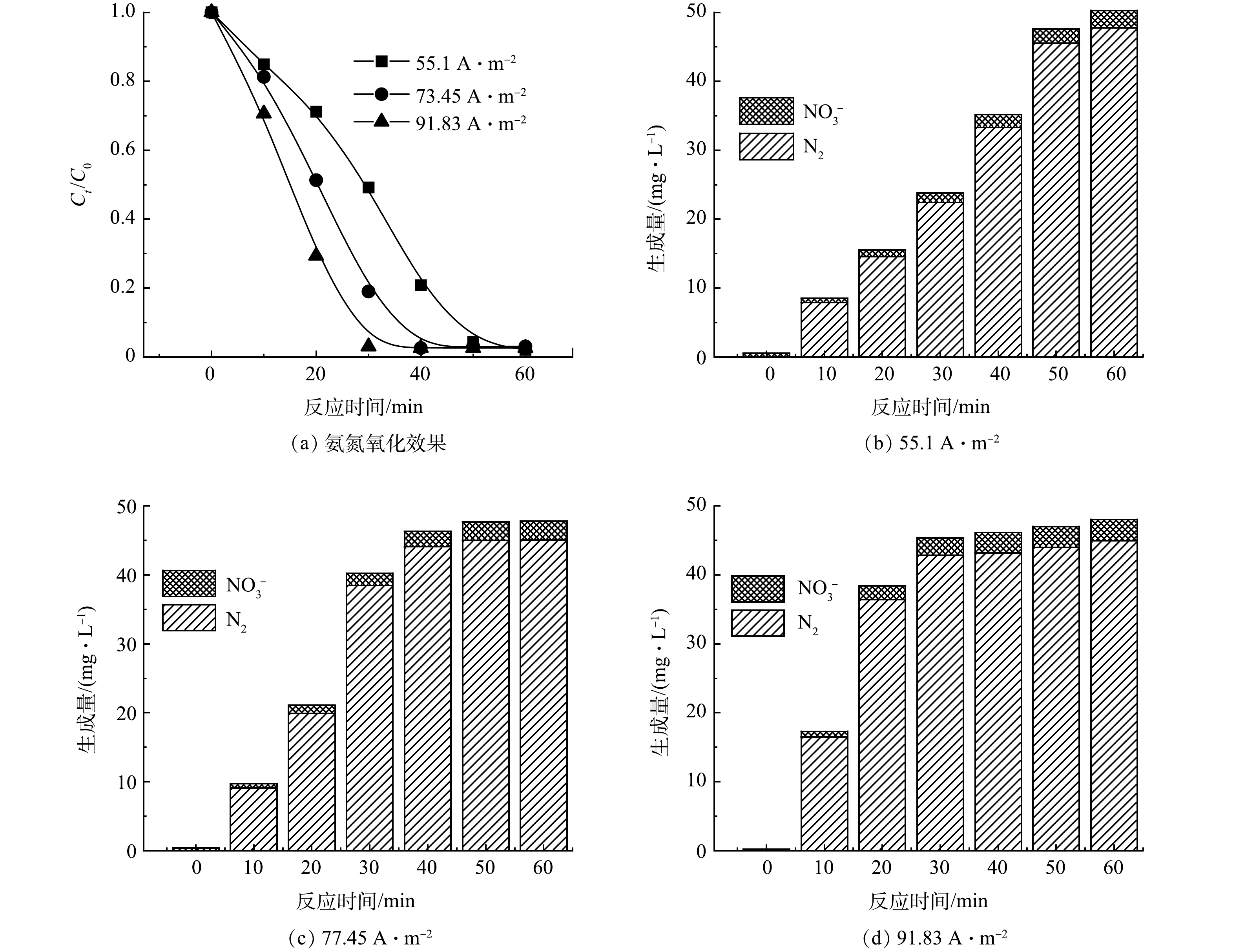
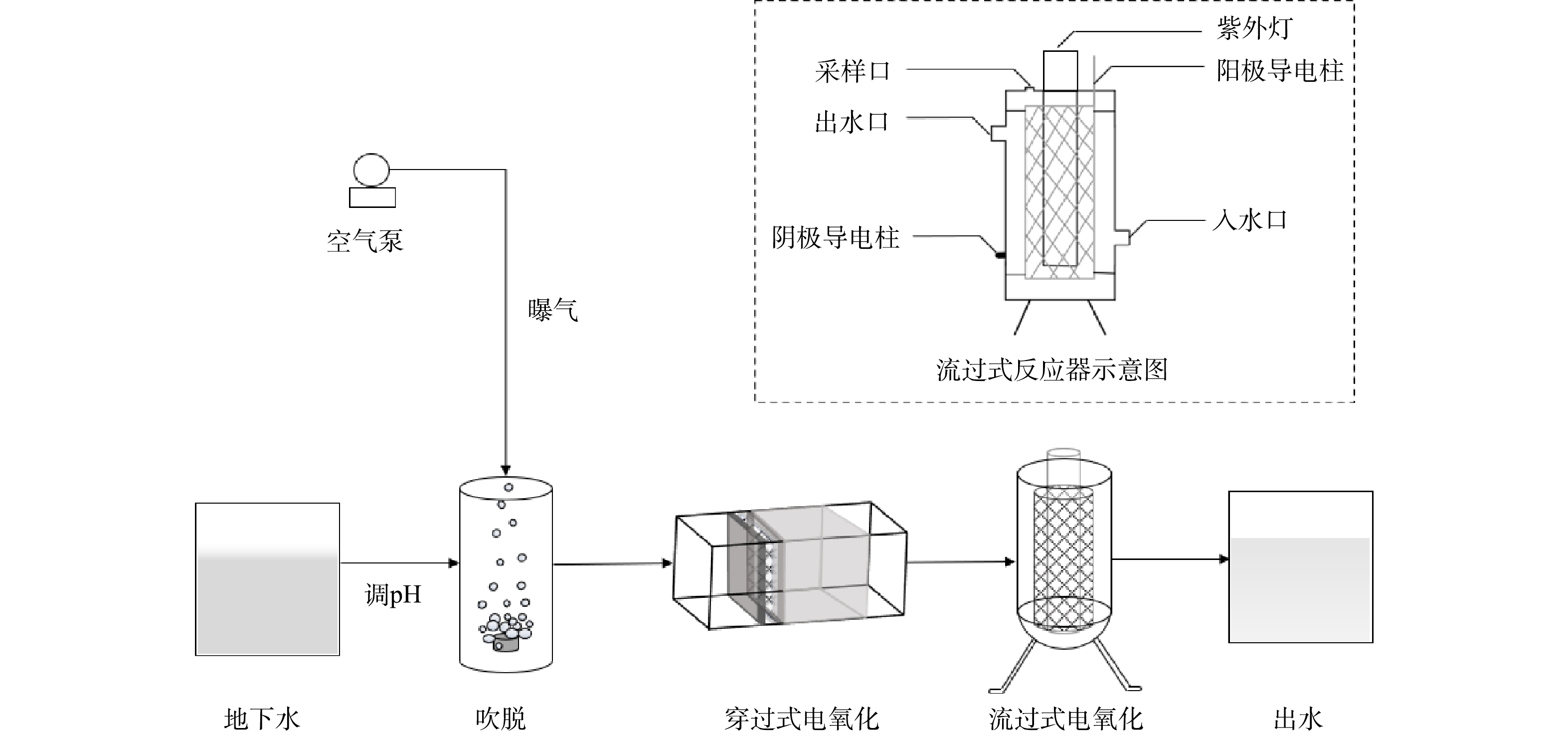
单独电化学实验装置采用流过式反应器(图1),反应器的不锈钢内壁作为阴极,钛钌圆筒形电极为阳极,极间距为2 mm,内置紫外灯之后电解反应池有效容积为140 mL,将进水口与出水口以泵管连接并用蠕动泵控制循环流量。在无紫外光照射的情况下,考察了电流密度分别为55.1、73.46、91.83 A·m−2时的氨氮氧化效果。
光/电协同氧化法实验采用图1所示流过式反应器,在单独电化学体系的基础上增加紫外线辐照。吹脱-电化学催化氧化组合实验包括吹脱、穿过式电氧化、流过式电氧化(图1)。穿过式电氧化反应器以钌铱涂层泡沫钛为阳极(极板大小50 mm×50 mm,厚度2 mm,孔径50~100 μm)和泡沫钛板为阴极,阴阳极间放置塑料介电网隔开。流过式电氧化部分采用如图1所示的流过式反应器。调节地下水pH为12后,在1 000 mL容量的量筒中装入800 mL水样,以8 L·min−1的空气流量吹脱8 h。经吹脱预处理的地下水随后进入电化学连续流装置,穿过式电氧化反应器电流密度为106 A·m−2,水力停留时间为10 min;流过式反应器电流密度为73.45 A·m−2,水力停留时间为5 min。
-
总氮(TN)的测试方法为燃烧法,使用仪器为总有机碳/总氮分析仪(TOC-L);NH4+-N采用纳氏试剂法(HJ 535-2009),通过紫外分光光度计(U-3900)进行测定;NO2−-N、NO3−-N、Cl−使用离子色谱(ICS-5000)进行测定;活性氯质量浓度采用N,N-二乙基-1,4-苯二胺分光光度法(HJ 586-2010),使用仪器为哈希测定仪(DR 3900);活性自由基的产生情况以DMPO为捕获剂,通过电子顺磁共振波谱仪(Bruker E500)测试。
-
如图2(a)所示,随着电流密度的升高,氨氮去除率呈升高趋势。在电流密度为73.46 A·m−2的条件下电解40 min时,氨氮的去除率已超过95%。采用零级反应动力学拟合反应过程中氨氮质量浓度曲线,所有k值的R2均>0.97,表明氨氮降解遵循零级反应动力学。图2(b)~(d)反映了在单独电化学氧化降解氨氮过程中NO3−和N2的生成量(即TN减少的量),其氧化产物大部分转化为N2,少部分转化为副产物NO3−,体系中NO2−的质量浓度低于检测限。随着电解时间的增加,NO3−生成量逐渐提高。在电流密度为73.46 A·m−2时,电解60 min后,N2生和NO3−的生成量分别为45.09 mg·L−1和2.73 mg·L−1,TN去除率为82.58%。高电流密度虽然能够提高反应效率,但会增加能耗,出于经济和高效的考虑,选择73.46 A·m−2进行后续研究。
-
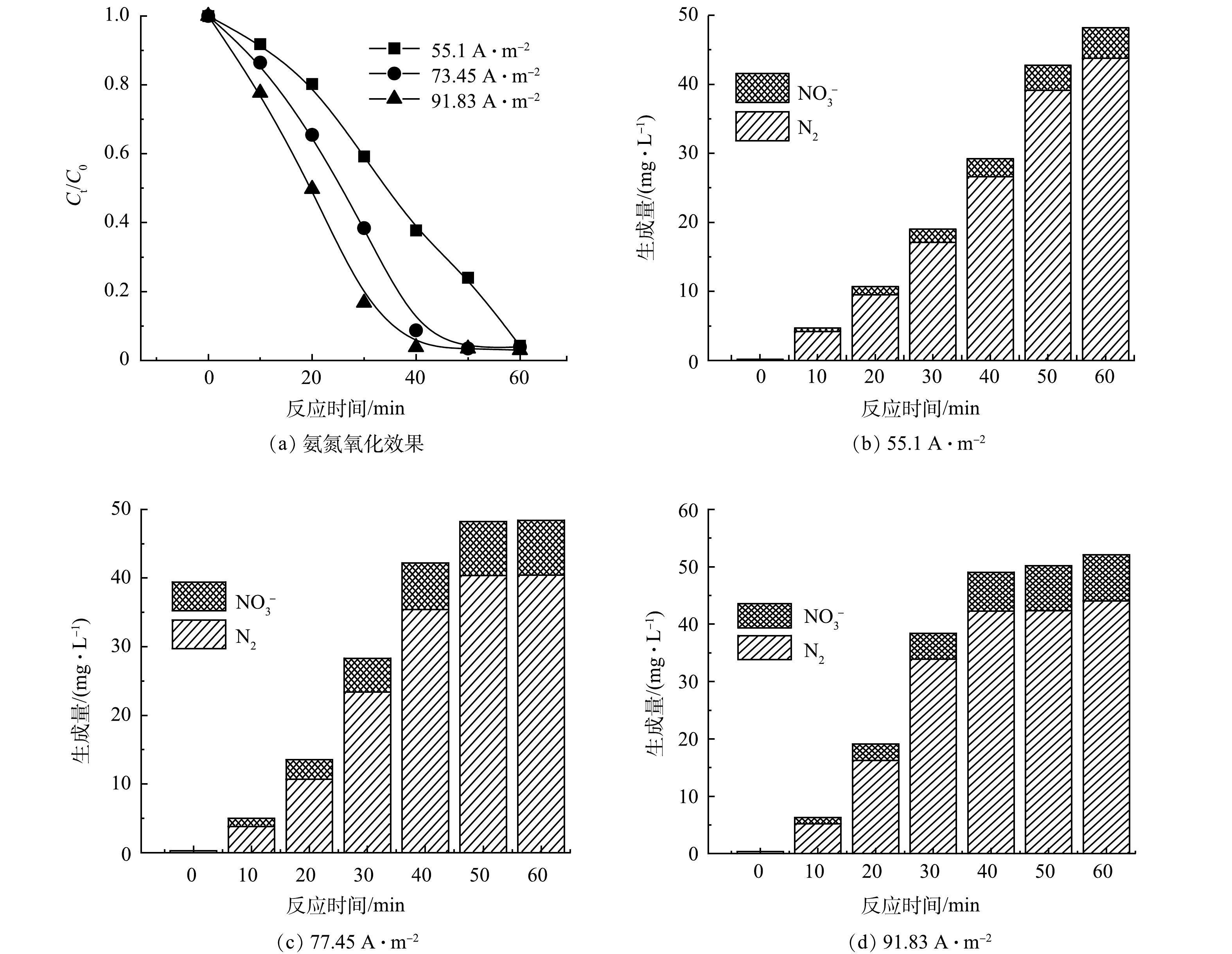
由图3(a)可知,光/电协同氧化法降解氨氮的效果随电流密度的升高而提高,但氨氮去除率低于单独电化学氧化法。相较于单独电化学氧化法,在电流密度分别为55.1、73.46、91.83 A·m−2的条件下光电催化反应30 min后,氨氮的去除率分别低10.01%、19.43%、13.67%。图3(b)~(d)反映了不同电流密度下NO3−和N2的生成量。随着电解反应的进行,NO3−和N2的生成量不断上升,且电流密度的升高促进了NO3−的形成。在3种电流密度条件下电解60 min后,NO3−的生成量分别达到4.419、8.033、8.061 mg·L−1,相较于单独电化学氧化法,光/电协同氧化法产生了更多硝酸盐。
-
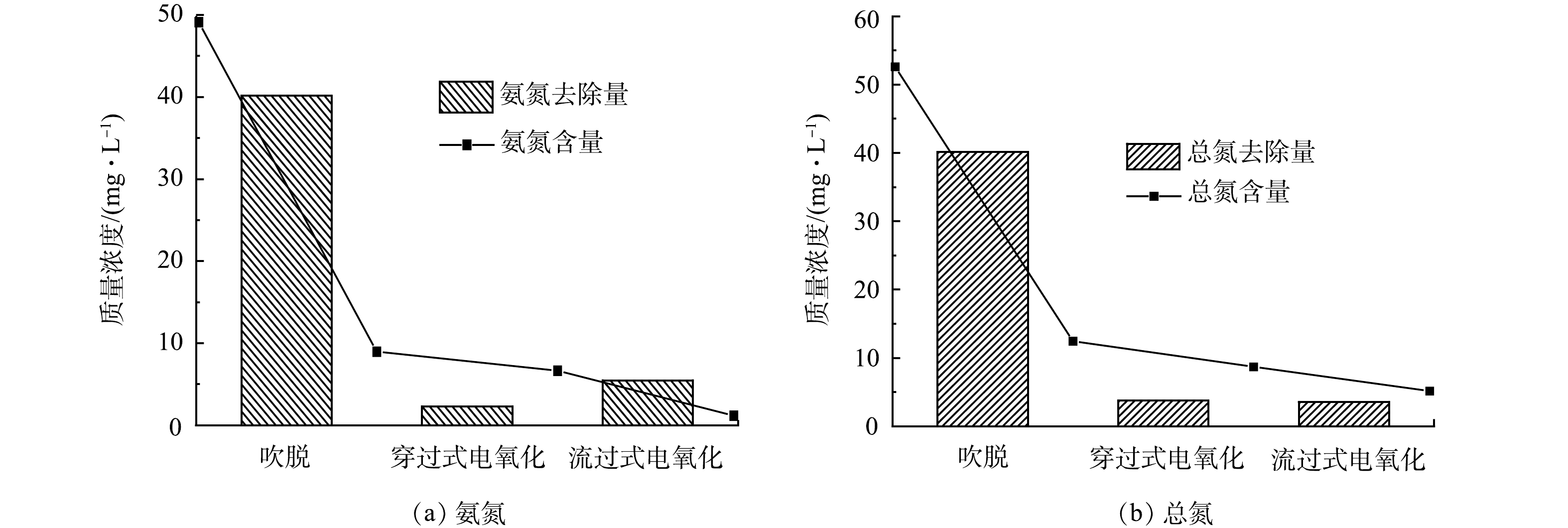
地下水的pH为9.5~10.6满足吹脱除氨的条件,但由于曝气过程中引入的CO2会导致溶液pH下降[18],因此加入NaOH将地下水调至pH=12以保证吹脱除氨效率。由图4可知,氨氮污染地下水经过吹脱-电催化氧化组合工艺处理,最终TN和NH4+-N出水质量浓度分别为5.15 mg·L−1和1.17 mg·L−1,去除率为90.23%和97.62%。单独电化学氧化法、光/电协同氧化法和吹脱-电化学催化氧化组合法均能将出水的氨氮质量浓度控制在1.5 mg·L−1以下,组合工艺的TN去除率较单独电催化氧化法和光/电协同氧化法分别高7.66%和12.18%。3种工艺降解氨氮的单位能耗从低到高分别是:吹脱-电化学催化氧化,单独电化学氧化,光/电协同氧化。吹脱法和电催化氧化法的耦合有效利用了地下水的碱度和氯化物,同时,两个电化学单元的组合拓展了该工艺的适用范围,提升了出水水质,利于废水处理设备朝着小型化的方向发展。
-
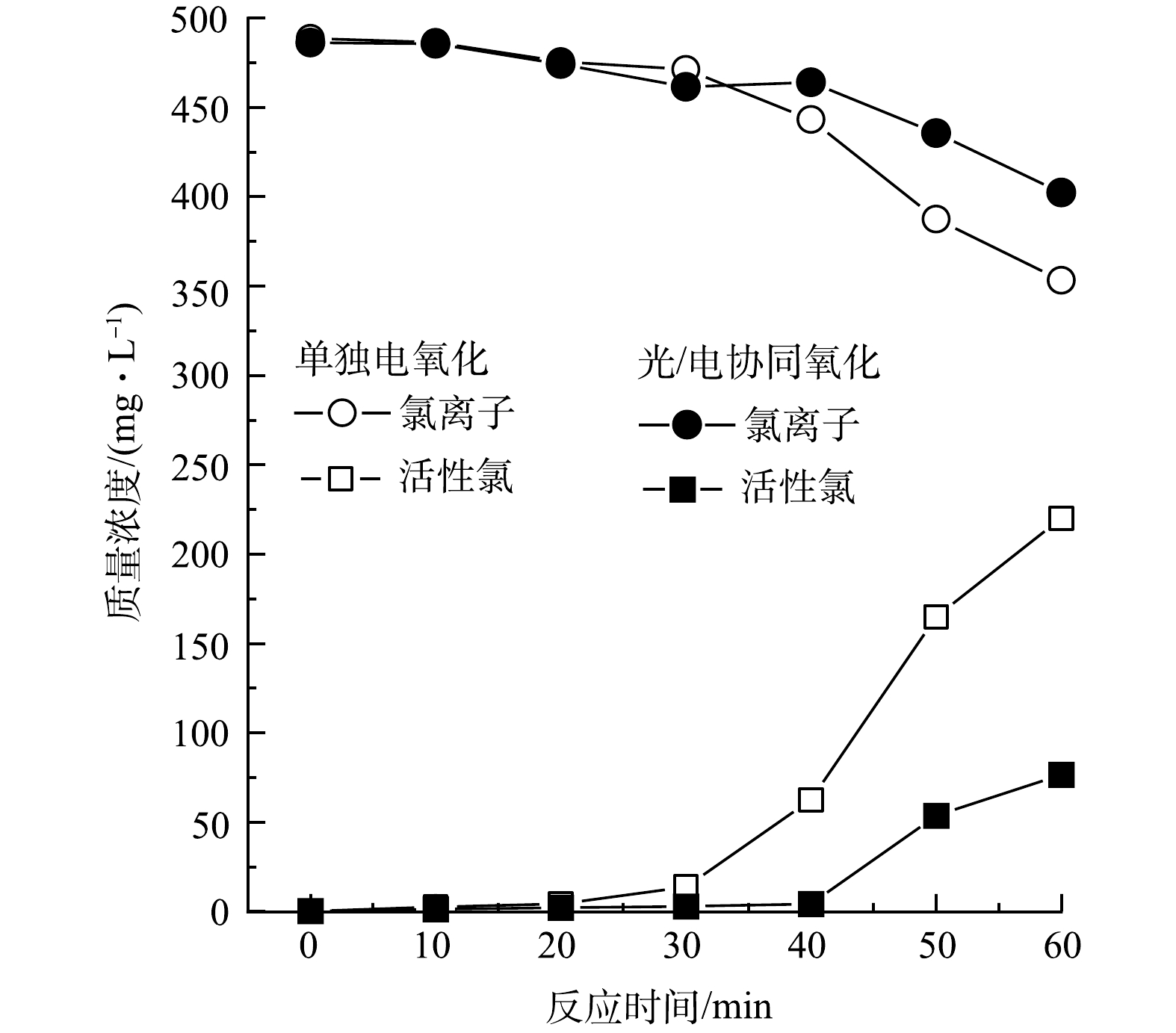
1)活性氯介导的氨氮氧化机理。电化学反应中会产生的活性氯、HO·等强氧化性物质。活性氯是通过Cl−在阳极表面原位生成的Cl2水解形生成。阳极生成的活性氯是氨氮转化为氮气的重要因素,类似氨氮的折点氯化。但研究表明,电化学催化氧化发生的机制不同于传统折点加氯反应中先生成一氯胺、二氯胺、三氯胺,最后在活性氯/氨氮摩尔比达到1.5时生成氮气[19-20];在电化学催化氧化过程中,氨氮转化为氮气发生在电解过程的最开始,远在活性氯/氨氮摩尔比达到1.5之前[21-22]。
图5反映了Cl−随时间的变化以及氨氮氧化过程中活性氯的质量浓度。在电解时间0~20 min内,Cl−质量浓度逐渐降低,但活性氯质量浓度并无明显升高。这是由于溶液中的Cl−在阳极表面转化形成的活性氯随即与水中所存氨氮发生反应生成氯胺,活性氯在间接氧化中被消耗,氨氮完全降解后,活性氯质量浓度增至相对较高的质量浓度。在相同的电解条件下,添加紫外光之后,活性氯增加的量更少,说明在紫外线的照射下,部分活性氯通过光解反应转化生成·Cl和ClO·。
2)自由基介导的氨氮氧化机理。HO·和·Cl通过水的电解和Cl−的氧化生成于阳极表面,或是在紫外光的体系中,通过光解反应生成。有研究[23]表明,HO·倾向于将次氯酸或者次氯酸根转化为ClO·,而非Cl·。ClO·是一种选择性强氧化剂,易与富电子基团发生反应(氨氮),其氧化还原电位为1.5~1.8 V(vs. NHE)[24],低于HO·的氧化还原电位(2.8 V)。在紫外光的催化作用下,HO·、Cl·通过自由基链式反应转化为ClO·[25-26],自由基氧化氨氮生成N2[24,27-28]和NO3−[24]。紫外光对氯胺的光解作用以及HO·、ClO·氧化氨氮过程中生成的含氮自由基(·NH2),易进一步被氧化生成硝态氮[29],导致光/电协同氧化体系中NH4+-N的转化率低于单独电化学氧化体系。
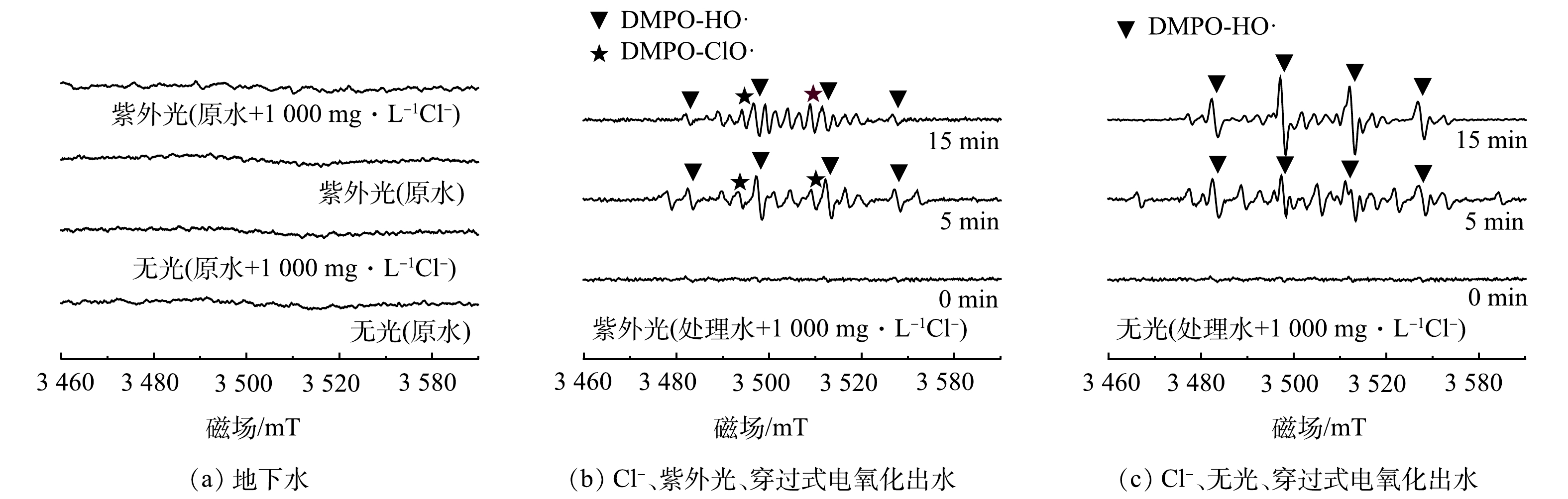
如图6(a)所示,用流过式电氧化反应器处理地下水的实验表明,无论是否添加紫外光和Cl−都未检测到自由基的相关信号,说明地下水中复杂的污染物是自由基的潜在淬灭剂。而吹脱-电化学催化氧化组合法处理的出水后,在添加1 000 mg·L−1 Cl−的情况下出现了明显的自由基信号峰,表明在此期间大部分污染物被去除。
光/电协同氧化体系中(图6(b)),5min时出现了信号比为1:2:2:1的DMPO-HO·特征峰以及与杂峰叠加的DMPO-ClO·信号峰。反应进行至15min时,随着DMPO-HO·信号变弱,DMPO-ClO·的加合信号逐渐清晰,其位置与其他研究[30-31]的结果高度一致,表明溶液中同时存在HO·和ClO·。在电催化体系中(图6(c)),DMPO-HO·的信号随着时间的增加而增强,未发现明显的ClO·和·Cl的特征峰。这说明在无紫外光的条件下,阳极表面生成的强氧化性的HO·几乎未将HClO和ClO−转化为ClO·和·Cl。
-
1)采用吹脱-电化学催化氧化组合工艺处理含氨氮地下水,在实验操作条件下,氨氮、总氮的去除率分别为97.62%、90.23%。
2)单独电化学氧化法处理含氨氮地下水的效果优于光/电协同氧化法。在73.46 A·m−2的电流密度下电解30 min,与光/电协同氧化法相比,单独电化学氧化法中氨氮的去除率高了19.43%,副产物NO3−的生成量少了5.299 mg·L−1,活性氯的生成量多了10.8 mg·L−1。体系中活性氯质量浓度变化和自由基的检测结果显示,ClO·仅在紫外光的催化下由HO·和活性氯转化产生,HO·和活性氯等强氧化性物质的消耗导致了加光体系中氨氮去除率的降低。
吹脱-电化学催化氧化组合工艺去除地下水中氨氮
Removal of ammonia nitrogen from groundwater by combined stripping-electrochemical oxidation process
-
摘要: 以上海某钢铁工业区固废堆场氨氮污染的地下水为研究对象,研究了单独电化学氧化法、光/电协同氧化法、吹脱-电化学催化氧化组合法对氨氮的去除效果,提出能高效除氨氮且适合工程实际应用的吹脱-电催化氧化组合工艺。结果表明,相较单独电化学氧化,光/电协同氧化体系中的强氧化活性物质(HO·、HOCl、ClO−等)通过自由基链式反应和光解反应生成氧化性更弱的ClO·,反而削弱氨氮去除效果。而吹脱-电催化氧化组合工艺下氨氮、总氮的去除率分别为97.62%、90.23%,出水满足《地下水质量标准》(GB/T 14818-2017)IV类标准。电子顺磁共振(EPR)和活性氯生成实验证实HO·和活性氯(HOCl、ClO−、Cl2)在氨氮转化过程中的重要作用。本实验为进一步研究和设计基于电化学法处理废水中的氨氮技术和组合工艺提供了理论依据。Abstract: In this work, three approaches, including electrochemical, photoelectrocatalytic, and combined stripping-electrochemical oxidation methods, were firstly compared in treating the polluted groundwater containing ammonia nitrogen caused by solid waste in a steel industrial zone of Shanghai. The combined stripping-electrochemical oxidation process with the greatest ammonia nitrogen removal efficiency which is suitable for engineering practical application was proposed. Compared with electrochemical oxidation, the strong oxidizing active substances (HO·, HOCl, ClO−) in the photo-electric oxidation system could generate weaker ClO· through free radical chain reaction, thus reducing the ammonia nitrogen removal efficiency. For the combined stripping-electrochemical oxidation, the removal rates of ammonia nitrogen and total nitrogen could reach 97.62% and 90.23%, respectively, the corresponding effluent could meet the Class IV standard of Groundwater Quality Standard (GB/T 14818-2017). Through electron paramagnetic resonance (EPR) and active chlorine formation experiments, we demonstrated that HO· and active chlorine (HOCl, ClO−, Cl2)) played crucial roles in the ammonia removal. Our study provides a theoretical basis for designing electrochemical oxidation degradation of ammonia nitrogen technology and combination process.
-
Key words:
- ammonia nitrogen /
- stripping /
- electrocatalytic oxidation /
- photoelectrocatalytic /
- free radical
-

-
-
[1] XU W, DU D, LAN R, et al. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia[J]. Applied Catalysis B:Environmental, 2016, 237: 1101-1109. [2] ZHANG X, WU Y, LIU X, et al. Ammonia emissions may be substantially underestimated in China[J]. Environmental Science and Technology, 2017, 51(21): 12089-12096. doi: 10.1021/acs.est.7b02171 [3] 生态环境部. 2019中国生态环境状况公报[J]. 中国能源, 2020, 42(7): 1. [4] 王琳, 牟春霞, 王丽. 高氨氮含量废水的处理方法及研究现状[J]. 水处理技术, 2021, 47(5): 1-5. doi: 10.16796/j.cnki.1000-3770.2021.05.001 [5] MASSE L, MASSé D, PELLERIN Y. The effect of pH on the separation of manure nutrients with reverse osmosis membranes[J]. Journal of Membrane Science, 2008, 325(2): 914-919. doi: 10.1016/j.memsci.2008.09.017 [6] JORGENSEN T C, WEATHERLEY L R. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants[J]. Water Research, 2003, 37(8): 1723-1728. doi: 10.1016/S0043-1354(02)00571-7 [7] AMINI N, PAPINEAU I, STORCK V, et al. Long-term performance of biological ion exchange for the removal of natural organic matter and ammonia from surface waters[J]. Water Research, 2018, 146(12): 1-9. [8] BONMATı́ A, FLOTATS X. Air stripping of ammonia from pig slurry: characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion[J]. Waste Management, 2003, 23(3): 261-272. doi: 10.1016/S0956-053X(02)00144-7 [9] 方建章, 黄少斌. 化学沉淀法去除水中氨氮的试验研究[J]. 环境科学与技术, 2002, 25(5): 34-35. doi: 10.19672/j.cnki.1003-6504.2002.05.015 [10] 张胜利, 刘丹, 曹臣. 次氯酸钠氧化脱除废水中氨氮的研究[J]. 工业用水与废水, 2009, 40(3): 23-26. [11] DENG Y, ENGLEHARDT J D. Electrochemical oxidation for landfill leachate treatment[J]. Waste Management, 2007, 27(3): 380-388. doi: 10.1016/j.wasman.2006.02.004 [12] KALYUZHNYI S, GLADCHENKO M, MULDER A, et al. DEAMOX-new biological nitrogen removal process based on anaerobic ammonia oxidation coupled to sulphide-driven conversion of nitrate into nitrite[J]. Water Research, 2006, 40(19): 3637-3645. doi: 10.1016/j.watres.2006.06.010 [13] PéREZ G, IBáñEZ R, URTIAGA A M, et al. Kinetic study of the simultaneous electrochemical removal of aqueous nitrogen compounds using BDD electrodes[J]. Chemical Engineering Journal, 2012, 197: 475-482. doi: 10.1016/j.cej.2012.05.062 [14] VANLANGENDONCK Y, CORBISIER D, VAN LIERDE A. Influence of operating conditions on the ammonia electro-oxidation rate in wastewaters from power plants (ELONITA technique)[J]. Water Research, 2005, 39(13): 3028-3034. doi: 10.1016/j.watres.2005.05.013 [15] LI L, LIU Y. Ammonia removal in electrochemical oxidation: mechanism and pseudo-kinetics[J]. Journal of Hazardous Materials, 2009, 161(2/3): 1010-1016. [16] YE Z, ZHANG H, ZHANG X, et al. Treatment of landfill leachate using electrochemically assisted UV/chlorine process: Effect of operating conditions, molecular weight distribution and fluorescence EEM-PARAFAC analysis[J]. Chemical Engineering Journal, 2016, 286: 508-516. doi: 10.1016/j.cej.2015.10.017 [17] ZHENG W, ZHU L, LIANG S, et al. Discovering the importance of ClO(*) in a coupled electrochemical system for the simultaneous removal of carbon and nitrogen from secondary coking wastewater effluent[J]. Environmental Science and Technology, 2020, 54(14): 9015-9024. doi: 10.1021/acs.est.9b07704 [18] BOUSEK J, SCROCCARO D, SIMA J, et al. Influence of the gas composition on the efficiency of ammonia stripping of biogas digestate[J]. Bioresource Technology, 2016, 203: 259-66. doi: 10.1016/j.biortech.2015.12.046 [19] MARGERUM D W, GRAY E T, HUFFMAN R P. Chlorination and the Formation of N-Chloro Compounds in Water Treatment[M]. Fourth International Congress of Pesticide Chemistry, Zurich, 1979. [20] QIANG Z, ADAMS C D J E S, TECHNOLOGY. Determination of monochloramine formation rate constants with stopped-flow spectrophotometry[J]. Environmental Science and Technology, 2004, 38(5): 1435-1444. doi: 10.1021/es0347484 [21] KIM K W, KIM Y J, KIM I T, et al. Electrochemical conversion characteristics of ammonia to nitrogen[J]. Water Research, 2006, 40(7): 1431-1441. doi: 10.1016/j.watres.2006.01.042 [22] ZHANG C, HE D, MA J, et al. Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications[J]. Water Research, 2018, 145: 220-230. doi: 10.1016/j.watres.2018.08.025 [23] ZHANG Y, LI J, BAI J, et al. Extremely efficient decomposition of ammonia N to N2 using ClO(*) from reactions of HO(*) and HOCl generated in situ on a novel bifacial photoelectroanode[J]. Environmental Science and Technology, 2019, 53(12): 6945-6953. doi: 10.1021/acs.est.9b00959 [24] HUANG X, ZHANG Y, BAI J, et al. Efficient degradation of N-containing organic wastewater via chlorine oxide radical generated by a photoelectrochemical system[J]. Chemical Engineering Journal, 2020, 392: 1-10. [25] FANG J, FU Y, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system[J]. Environmental Science and Technology, 2014, 48(3): 1859-1868. doi: 10.1021/es4036094 [26] WANG Y, COUET M, GUTIERREZ L, et al. Impact of DOM source and character on the degradation of primidone by UV/chlorine: Reaction kinetics and disinfection by-product formation[J]. Water Research, 2020, 172: 1-10. [27] KISHIMOTO N, KATAYAMA Y, KATO M, et al. Technical feasibility of UV/electro-chlorine advanced oxidation process and pH response[J]. Chemical Engineering Journal, 2018, 334: 2363-2372. doi: 10.1016/j.cej.2017.11.108 [28] SIMIC M, HAYON E. Intermediated produced from the one-electron oxidation and reduction of hydroxylamines. Acid-base properties of the amino, hydroxyamino, and methoxyamino radicals[J]. Journal of the American Chemical Society, 1971, 93(23): 159-163. [29] POZOS N, SCOW K, WUERTZ S, et al. UV disinfection in a model distribution system[J]. Water Research, 2004, 38(13): 3083-3091. doi: 10.1016/j.watres.2004.04.011 [30] HAO R, MAO X, QIAN Z, et al. Simultaneous removal of SO2 and NO using a novel method of ultraviolet irradiating chlorite-ammonia complex[J]. Environmental Science and Technology, 2019, 53(15): 9014-9023. doi: 10.1021/acs.est.8b06950 [31] HAO R, MAO X, WANG Z, et al. A novel method of ultraviolet/NaClO2-NH4OH for NO removal: Mechanism and kinetics[J]. Journal of Hazardous Materials, 2019, 368: 234-242. doi: 10.1016/j.jhazmat.2019.01.042 -



 下载:
下载: