-
近年来,随着工业化进程的不断加快,镉(Cd)、汞(Hg)和铅(Pb)等重金属的污染问题愈加严重[1]。其中,水体镉污染尤为严峻,我国部分江河湖泊受到不同程度的镉污染[2]。例如,龙江河发生的严重镉污染事件,水体的镉含量超标80倍[3]。安宁河镉含量最高曾达0.6 mg·L−1,远远超过国家地表水Ⅴ类标准(GB 3838-2002)[4]。海河总镉含量也曾超过地表水Ⅱ类标准的16.7%~83.9%[5]。水体中的镉不仅能直接通过饮用水进入人体,还会通过富集于水生动植物中再经食物链累积,最终通过饮食被摄入人体。进入人体的镉会沉积在骨骼、皮肤和肾脏等组织中,进而造成组织器官的损伤甚至癌变,严重影响人体健康[6-9]。因此,水体镉污染的治理至关重要。
去除水体重金属的方法常见的有物理方法、化学方法和生物方法[10]。与物理、化学方法相比,生物修复方法具有低成本、无二次污染、高效率等优点[11]。其中,植物修复技术通过超富集植物从污染水体中摄取重金属并将其富集在体内,从而实现对污染水体的修复[12],具有巨大发展潜力。决定植物修复技术修复重金属污染效果和应用价值的最关键之处,是找到合适的重金属超富集植物[13]。通常,重金属超富集植物是指富集后重金属含量能超过1 000 mg·kg−1(干质量)的植物,而重金属含量仅需超过100 mg·kg−1(干质量)则可被定义为镉超富集植物[14]。
浮萍,由于生长速度快、污染物去除效率高,是一种理想的污染水体修复材料和研究污染物胁迫理论的模式植物[15]。浮萍共有5属36个种,生长速度快,其生物量每16~24 h增加1倍,有利于前期的育种扩繁[16-17]。浮萍是形态最简单的开花水生植物,仅有叶状体和根,可以避免离子的长距离运输,离子转运效率极高[18]。浮萍能在温度为10~40 ℃、pH为5.0~9.0条件下生长,环境适应能力强[19-20]。浮萍对Cu、Zn、Cd、Pb等重金属都有一定富集和耐受能力[21-22],并在采集收获、加工应用等方面表现出较大优势,已受到广泛关注[23]。
然而,有关浮萍在重金属耐受和富集方面的研究目前都还主要集中在少数品种上,缺乏系统的研究[21,24-29]。多根紫萍(Spirodela polyrrhiza)对镉非常敏感,其相对生长速率和光合色素含量在1 μmol·L−1的镉胁迫7 d后显著降低,被认为是一种具有开发应用价值的重金属指示物[24-25]。而少根紫萍(Landoltia punctata)在3 mg·L−1 的镉处理10 d后,富集系数和对水体中镉的去除率分别可达到为770和72.43%,具有明显的高富集优势[26]。由此可见,由于基因型不同,不同种属的浮萍对重金属镉的富集效果差异较大。因此,比较不同浮萍种属间镉富集效果的差异,筛选最适合的浮萍品种,对进一步应用植物修复技术具有现实意义。
本研究为筛选出最适于治理水体镉污染的浮萍品种,通过30 mg·L−1 镉处理7 d初筛90株浮萍株系,以叶片颜色变化为判断标准,获得7株优势浮萍株系。对该7株浮萍株系进行了种属鉴定和进一步复筛,在0.5 mg·L−1(低质量浓度)和10 mg·L−1(高质量浓度)镉浓度下处理7 d,比较了7株浮萍株系的生长速率、叶绿素含量、对镉的富集效果和去除率,以期筛选出对镉富集效果最佳的浮萍品种,为今后利用最佳浮萍品种治理水体镉污染提供参考。
-
实验所用90株浮萍材料来源于贵州大学生命科学学院浮萍种质资源库。实验处理前,采用Hoagland培养液(含1.5%蔗糖)对资源库保存的浮萍株系进行预培养[30]。培养条件为温度25 ℃、光暗比16 h∶8 h、湿度75%、光照强度为5 000 lx,培养时间为7 d。预培养后,挑选出生长状态良好的浮萍进行后续的实验处理。表1为初筛获得的7个优势浮萍株系材料的基本信息。
-
试剂:CdCl2标准溶液(北京北方伟业,北京),巯基乙醇(Aladdin,上海),CTAB(Aladdin,上海),95%乙醇,浓硝酸,核酸提取液(Acmec,上海),异丙醇(Aladdin,上海),Taq酶(Trans Taq DNA Polymerase High Fidelity,北京),培养基组分参见Hoagland培养说明[30]。
仪器:人工气候培养箱(Thermo GXZ-80,美国),超净台(孚夏SW-CJ-1D,浙江),灭菌锅(ZEALWAY DR60DA;美国),pH 仪(雷磁pHS-3C,上海),离心机(平凡TGL-205,湖南),PCR仪(Thermo T100,美国),水平电泳仪(JUNYI JY1600C,北京),火焰原子分光光度计(Analytik Jena AG NovaAA 400P,德国),控温式远红外消煮炉(四平 LWY84B,吉林)。
-
1)浮萍初筛。将90株浮萍在25 ℃、光暗比为16 h∶8 h、湿度75%、光照强度5 000 lx的条件下用30 mg·L−1 CdCl2处理7 d,以浮萍叶片颜色变化为筛选指标进行初筛,叶片颜色变化较小的浮萍为优势株系。
2)浮萍复筛实验处理。将预培养得到的浮萍材料按相同质量接种至含有0.5 mg·L−1和10 mg·L−1CdCl2的1/5 Hoagland培养基进行培养,以不含镉的1/5Hoagland培养基为对照组。培养时间为7 d,7 d后收集样品进行后续分析。
3)样品收集。7 d后,取各组培养液50 mL,以3 500 r·min−1离心10 min,取上清液置4 ℃冰箱待测;用流动的自来水、超纯水依次冲洗浮萍,滤纸吸干水分后记录鲜质量,于60 ℃烘箱中过夜烘干磨粉。称取0.1 g浮萍粉末于消解管中,加入2 mL浓硝酸过夜,再加入4 mL浓硝酸充分混匀,于280 ℃消解4 h。每次消解均设置空白对照,以消除此过程产生的误差。消解完毕后,用去离子水将消解管中剩余冷却至室温的消解液全部洗出,定容至50 mL待分析;称取0.5 g新鲜浮萍样品于15 mL离心管中,于−20 ℃冷冻1 h,加入 10 mL预热至50 ℃的乙醇(浓度为95%),充分混匀后暗置3 h,收集上清液用于测定叶绿素含量。
-
1)分子生物学鉴定种属。CTAB法提取浮萍DNA[31],叶绿体 atpF-atpH间隔序列由引物atpF(5’-ACTCGCACACACTCCCTTTCC-3’)和引物atpH(5’-GCTTTTATGGAAGCTTAAACAAT-3’)扩增,rpS16内含子序列由引物rpS16F(5’-AAACGATGTGGTARAAAGCAAC-3’)和引物rpS16R(5’-AACATCWATTGCAASGATTCGATA-3’)扩增,PCR所用DNA聚合酶为高保真Taq酶,反应程序为:95 ℃预热5 min,94 ℃变性30 s,50 ℃退火30 s,72 ℃延伸45 s,30个循环。引物合成和测序由北京擎科公司完成。通过NCBI Blast比对和MegaX构建系统发育树鉴定浮萍株系的种属。
2)相关指标的计算方法。植物生长速率根据式(1)计算;使用火焰原子分光光度计测定浮萍及培养液中重金属镉含量[26],再根据式(2)计算镉含量;浮萍对水体中镉的去除率根据式(3)计算;生物富集系数(bioconcentration factors,BCF)是植物组织与水环境中重金属的浓度比,反映了浮萍对重金属镉的富集能力,是常用的超富集植物评价指标[32],生物富集系数根据式(4)计算;叶绿素a含量和叶绿素b含量分别根据式(5)和式(6)计算。
式中:
v 为植株生长速率,g·d−1;Δm 为浮萍培养前后鲜质量变化,g;t为浮萍培养周期,d[33]。式中:
w 为单位重量样品中镉含量,mg·kg−1;C1 、C2 分别为样品消解液中镉含量和空白消解液镉质量浓度,mg·L−1;V 为样品消解液总体积,mL;m 为消解时称取干粉总质量,g。式中:
C0 、Ct 分别为初始镉质量浓度及处理结束后残留镉质量浓度,mg·L−1。式中:
RBCF 为生物富集系数;Cp 为植物中镉的质量浓度,mg·kg−1;Cw 为培养溶液中镉的质量浓度,mg·L−1。式中:
Cchla 、Cchlb 分别为叶绿素a、叶绿素b的质量浓度,mg·L−1;A1 和A2 分别为叶绿素溶液在663 nm和645 nm处的吸光度,根据提取液中的叶绿素浓度,换算成每克鲜叶中叶绿素含量,mg·g−1。 -
采用
Excel 和GraphPad Prism 6.0 软件进行数据处理,采用Multiple t tests法进行分析比较。数据均为平均值±SD,重复3次。与对照组比较,*表示在P<0.05 水平下差异显著,**表示在P<0.01 水平下差异显著。 -
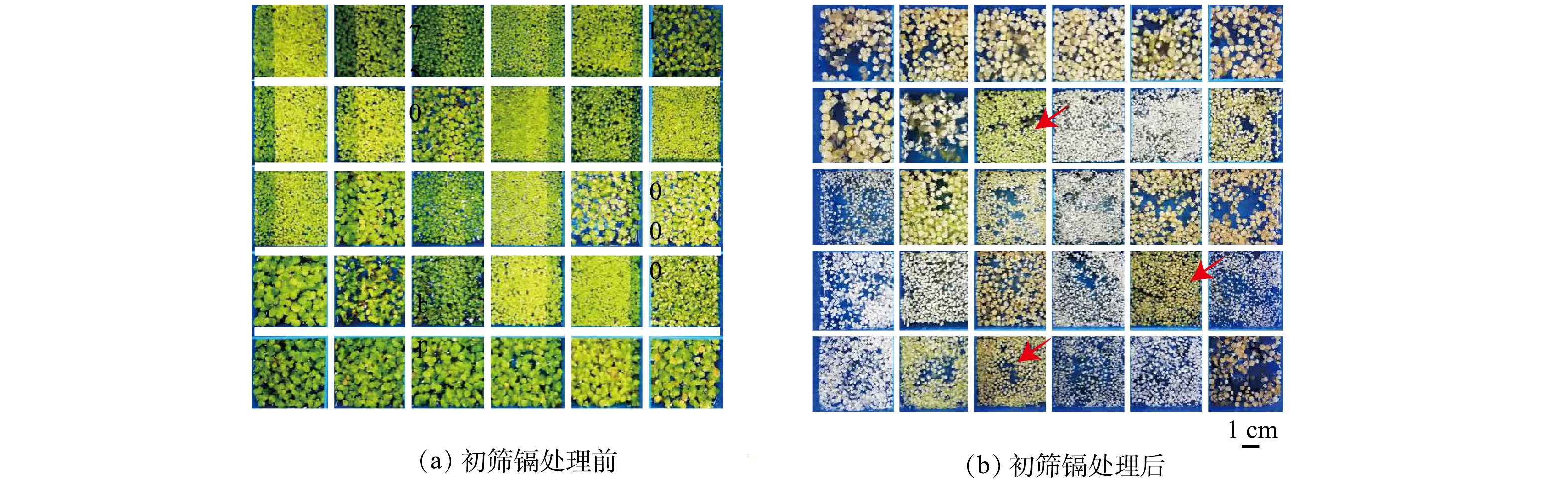
90个浮萍株系经过30 mg·L−1镉处理7 d后,有7个株系叶片颜色变化较小,仍然保持绿色,生长状况受镉影响较小。因此,初步确定以上7个浮萍株系为镉耐受优势株系,整体的浮萍株系耐受率为7.78%。图1为部分筛选结果,红色箭头所指的浮萍株系为经过镉处理前后叶片仍然保持绿色的株系,为初筛的优势株系。
-
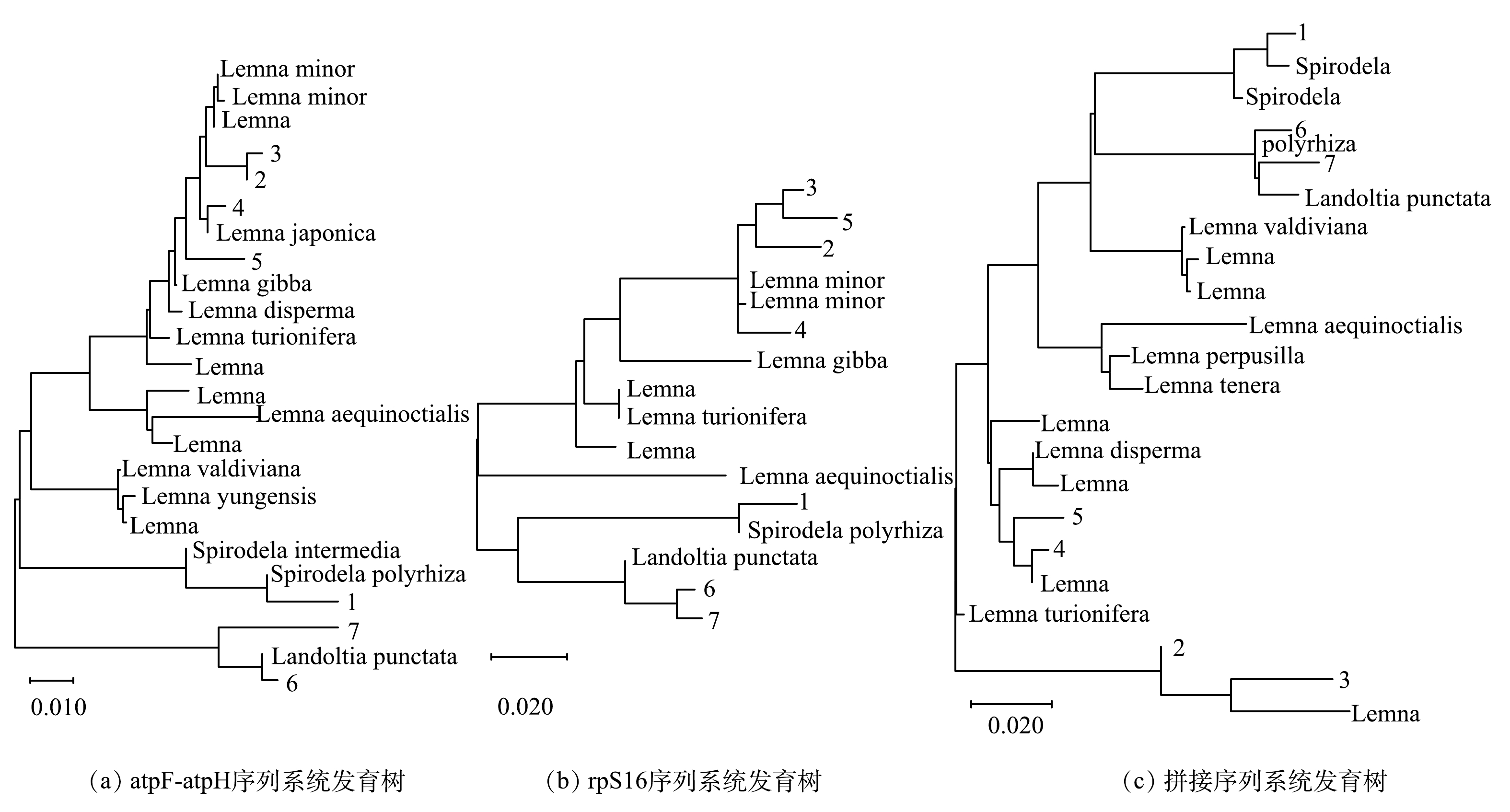
图2为PCR扩增的凝胶电泳图,atpF-atpH 间隔序列长度在 600~700 bp,rpS16内含子序列长度在1 000~1 100 bp。根据NCBI Blast比对(表2),确定了7个优化浮萍的种属,包括3个属4个种。7个浮萍中,1号株系为Spirodela polyrhiza,2号和3号株系为Lemna minor,4号和5号株系为Lemna japonica,6号和7号株系为Landoltia punctata。根据atpF-atpH 序列比对结果,最高相似性达到了99.70%,为4号株系;最低相似性达到了97.25%,为7号株系。根据rpS16序列比对结果,最高相似性达到了99.70%,为6号株系;最低相似性达到了94.80%,为4号和5号株系。7个浮萍株系与参考序列Blast比对整体相似性较高。
根据atpF-atpH 间隔序列、rpS16序列和拼接序列通过Mega-X构建系统发育树(图3),1号株系与S. polyrhiza聚为一类;2号、3号株系与L. minor聚为一类;根据拼接序列系统发育树,4号、5号株系与L. japonica聚为一类;6号、7号株系与L. punctata聚为一类。系统发育树的聚类结果与Blast比对结果一致。
-
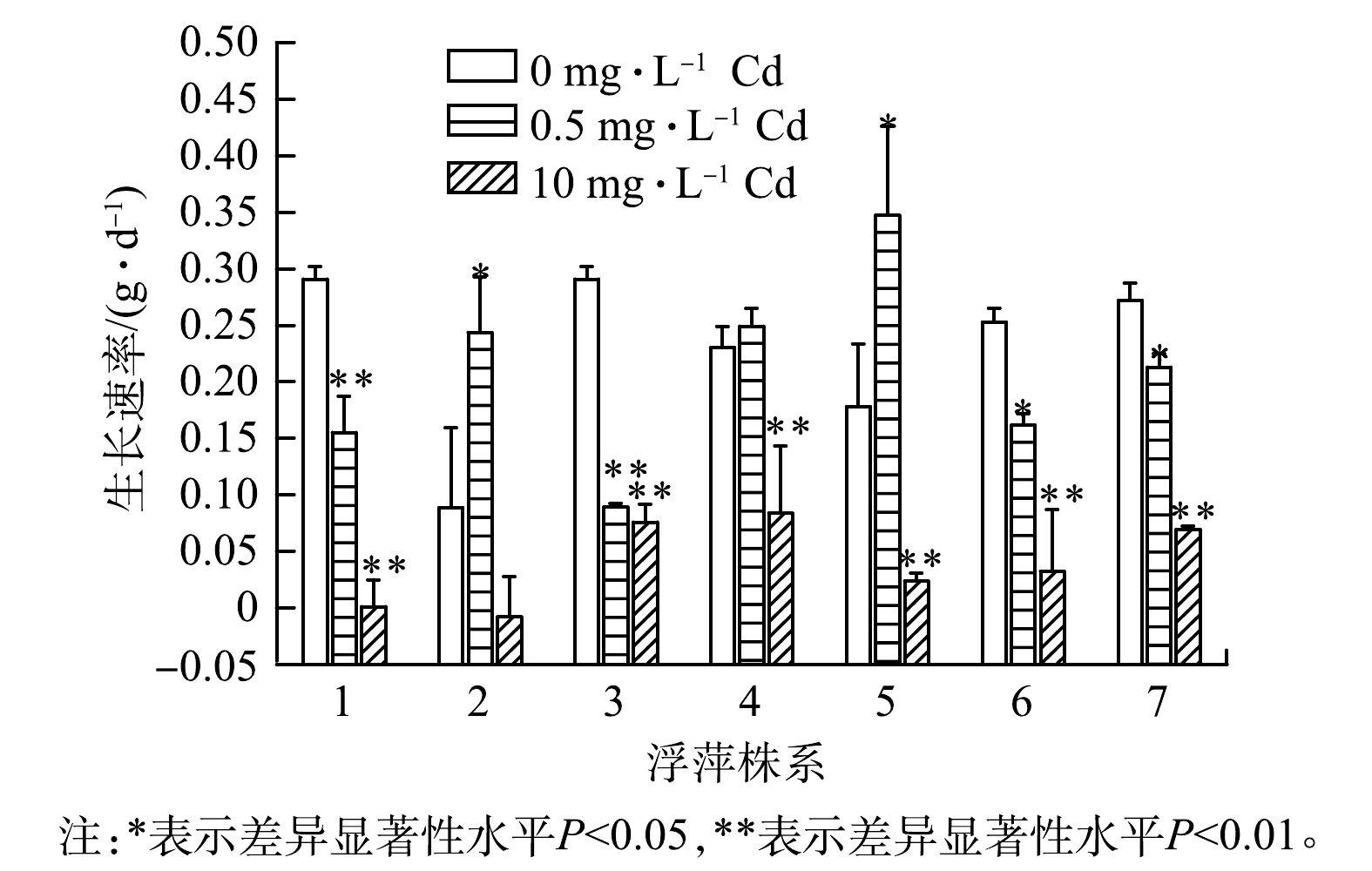
由图4可以看出,在低浓度镉处理后,7个浮萍株系均可正常生长。其中,2号和5号株系的平均生长速率甚至高于对照组。这可能与植物内生菌能促进植物生长和提高植物解毒能力有关[34-35]。在高浓度镉处理后,2号株系生长受到抑制,而其余6个浮萍株系仍能继续生长,但生长速率均降低。这与KHAN等[27]的研究结果一致,即0.5 mg·L−1是浮萍耐受镉的最大质量浓度,超过后浮萍生长会受到抑制。唐利萍等[26]的研究表明,浮萍的镉最适生长质量浓度为0~0.5 mg·L−1,在0.5~10 mg·L−1镉胁迫下造成的氧化损伤超过了浮萍自身解毒能力,导致浮萍的生长受到抑制,这也支持了本研究的结果。
-
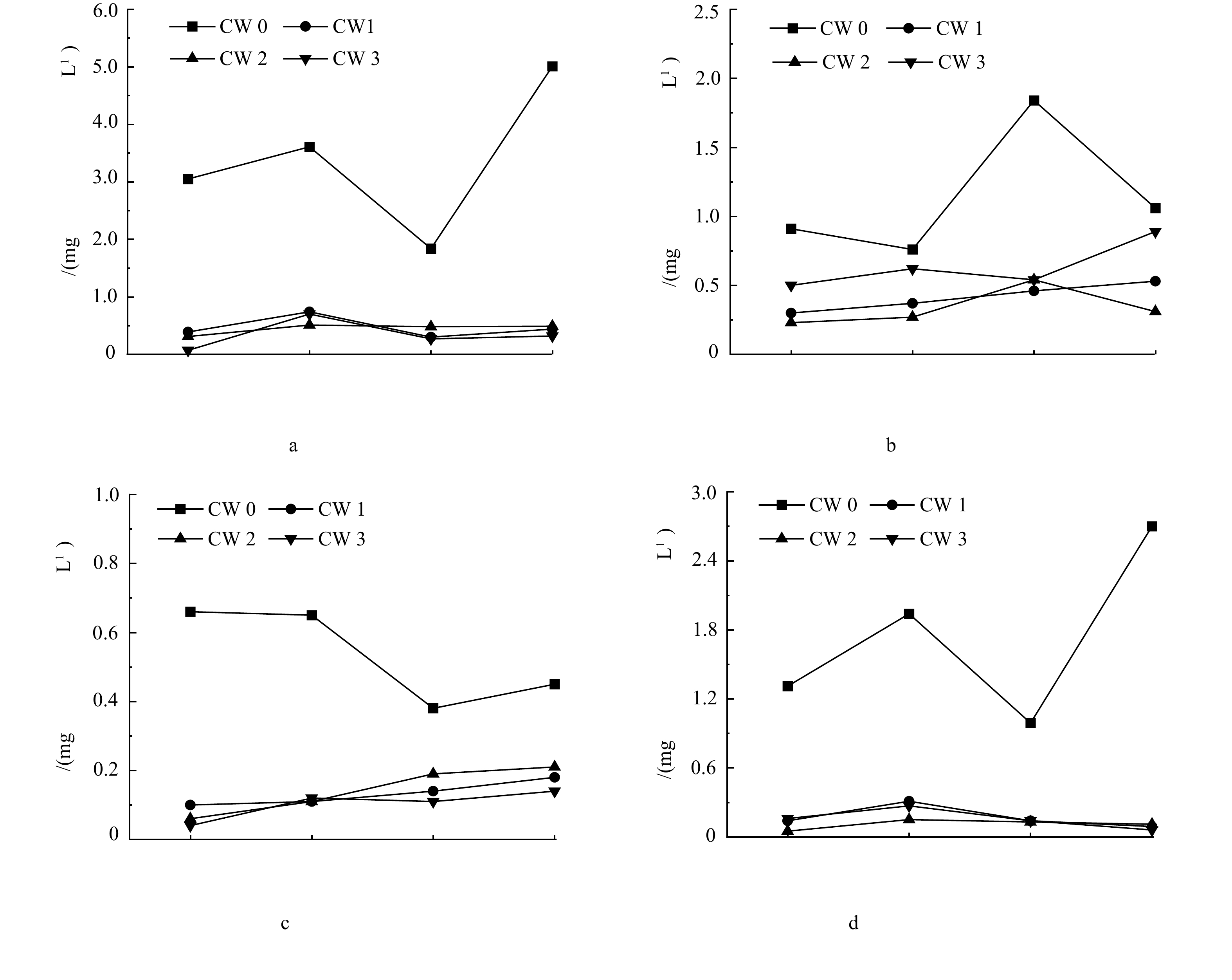
在低浓度镉处理7 d后,除了3号和6号株系,其他株系叶绿素a含量未出现明显下降。在高浓度镉处理7 d后,除7号株系外,其他株系叶绿素a含量均降低,其中3号株系下降最多,降低了97.70%(P<0.01)(图5(a)。在低浓度镉处理后,包括1号、4号和7号在内的3个株系叶绿素b含量未出现明显下降。在高浓度镉处理后,7个株系叶绿素b含量全部降低(图5(b))。在低浓度镉处理后,2号株系叶绿素a含量与叶绿素b含量的比值升高。在高浓度镉处理后,2号、4号、5号和7号株系叶绿素a含量与叶绿素b含量的比值升高,表明此4个株系叶绿素a降幅小于叶绿素b,叶绿素b含量受镉胁迫影响更大(图5(c))。在低浓度镉处理后,包括1号、4号和7号在内的3个株系总叶绿素含量未明显下降。在高浓度镉处理后,7个株系总叶绿素含量都呈现下降的趋势。3号株系与对照组相比总叶绿素含量下降最多,降低了96.99%(P<0.001)(图5(d))。
镉对浮萍叶绿素含量的影响结果表明:在0.5 mg·L−1镉胁迫下,7个浮萍株系中叶绿素含量变化不大;在10 mg·L−1镉胁迫下,叶绿素含量随生长速率一同下降,表明0.5~10 mg·L−1镉不仅能抑制浮萍的生长,也能降低浮萍叶绿素含量。有研究[36]表明,镉作为非生物胁迫因素,能刺激植物产生氧化应激作用。随着镉浓度升高,植物体内积累的活性氧自由基不断增多,诱导膜脂质过氧化,从而破坏叶绿素膜结构,这可能是导致本研究高浓度镉处理后叶绿素含量下降的原因[37-38]。值得注意的是,在7个优势株系中,4号株系(L. japonica)和7号株系(L. punctata)浮萍在低浓度镉和高浓度镉胁迫下仍保持较高的叶绿素含量且较为稳定,在一定程度上能维持自身正常生理生化活动,有耐受镉胁迫的巨大潜力。
-
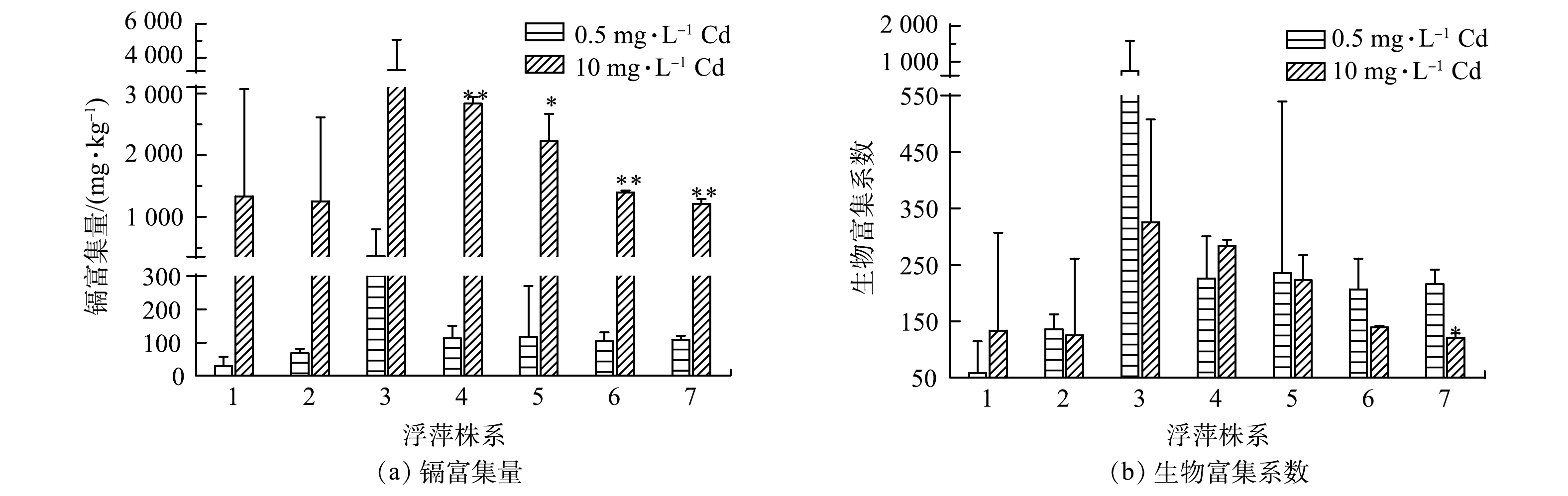
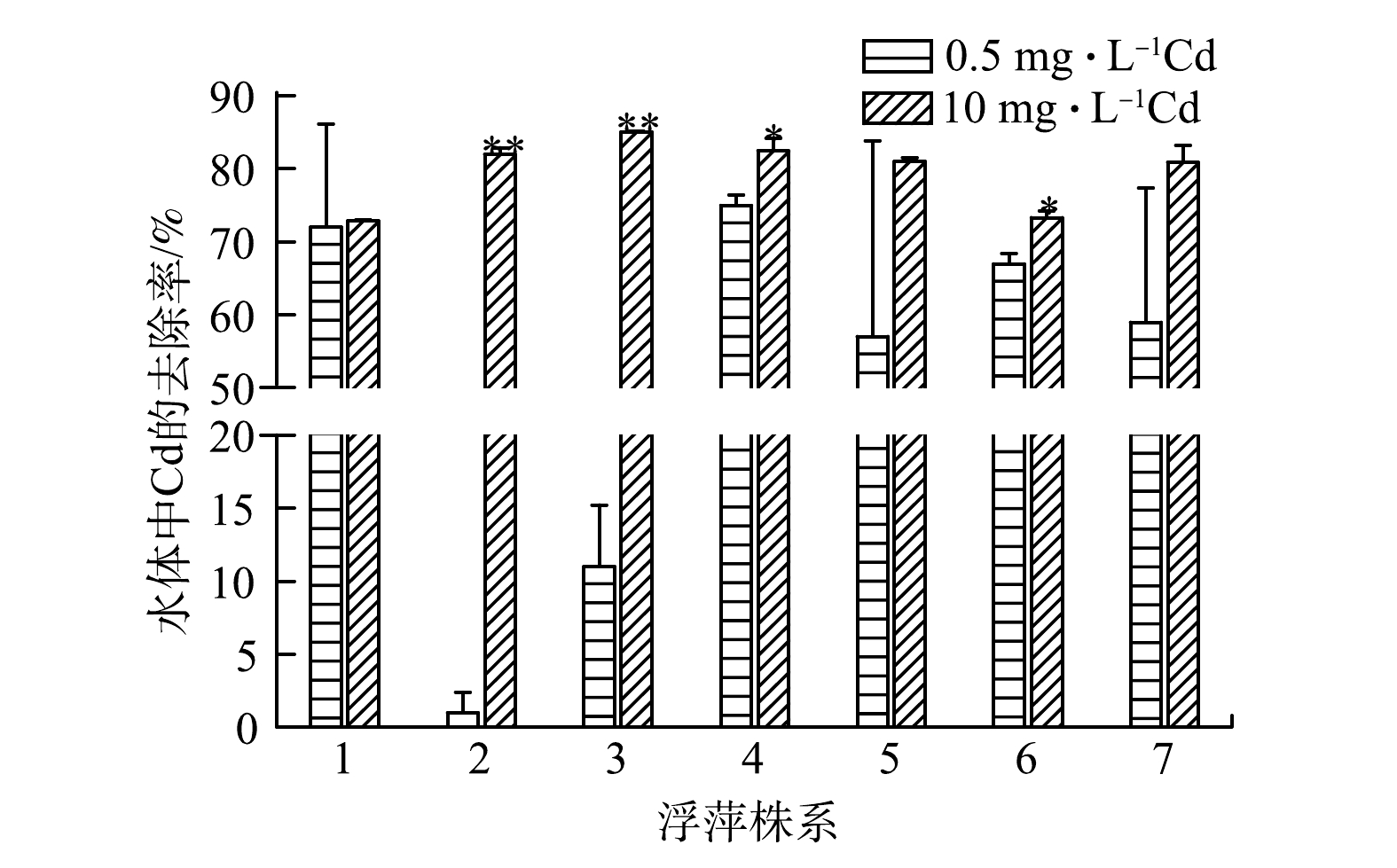
如图6 (a)所示,在低浓度镉处理7 d后,3号、4号、5号、6号和7号株系镉富集量均大于100 mg·kg−1,达到了镉超富集植物的标准[13];在高浓度镉处理后,7个株系的镉富集量均超过1 200 mg·kg−1,均远远超过超富集植物马缨丹(Lantana camara L.)的最大富集量(423 mg·kg−1)[39],也超过了水生超富集植物有翅星蕨(Microsorum pteropus)的最大富集量(1160.72 mg·kg−1)[40]。目前,已发表文献[26]中的植物最高镉富集量为2 544.5 mg·kg−1。本研究中的3号和4号株系分别可达3 259.55 mg·kg−1和2 834.30 mg·kg−1,均超过目前已知植物的最高镉富集量。
生物富集系数是用来衡量植物富集能力的重要指标,也是鉴定超富集植物的标准之一[32]。一般来说,BCF大于1表明植物能从环境中吸收并富集重金属,可使环境中的重金属浓度低于植物体内重金属浓度[14,41]。由图6(b)可看出,7个浮萍株系在低浓度镉和高浓度镉处理后的BCF均大于50,远远高于超富集植物马缨丹(L. camara)[39]、多裂翅果菊(Pterocypsela laciniata)[42]和忍冬(Lonicera japonica Thunb)[43]的最大BCF(3.14、4.55和25.41)。其中,4号株系BCF变化不大,维持在200~300,在低浓度镉和高浓度镉胁迫下富集能力仍然保持稳定。综上所述,7个浮萍株系在高浓度镉处理后富集量大于100 mg·kg−1,表明浮萍是一种优良的镉超富集植物。此外,4号株系(L. japonica)受镉影响较小,在低浓度镉和高浓度镉胁迫下仍能维持较大的BCF,在镉污染水体修复方面具有更广泛的实际应用价值。
-
由图7可以看出,在低浓度镉处理后,4号株系对水体中镉的去除率最高,为75.00%;在高浓度镉处理后,7个浮萍株系对水体中镉的去除率均高于70%。其中,3号和4号株系对水体中镉的去除率分别为85.05%和82.50%,去除效果较好。在其他水生植物对水体中镉的去除研究中,茭白(Zinania latifolia Turcz.)在处理6 d后仅能去除水体中50%的镉[44],超富集植物有翅星蕨(M. pteropus)在10 mg·L−1镉处理7 d后对水体中镉的去除率为39.12%~53.99%[45]。在相同处理时长下,大叶珍珠草(Micranthemum umbrosum S.F Blake)对水体中镉的去除率最高为70.4%[46],低于本研究结果。在另一项有关浮萍去除镉的研究中,经3 mg·L−1镉处理同样时长,浮萍对水体中镉的去除率最高为72.43%[26],低于本研究中10 mg·L−1镉处理后7个株系对水体中镉的去除率。以上结果说明,在相同处理条件或处理时长下,浮萍与其他水生植物相比,镉耐受和富集能力更为突出[29],表明浮萍是一种可用于去除水体重金属镉污染且效果良好的水生植物。在国内外的研究中,浮萍中的绿萍属(Lemna)和少根紫萍属(Landoltia)对水体中镉的最高去除率均在70%以上[27,40]。本实验中的7个浮萍株系在高浓度镉胁迫后对水体中镉的去除率也保持在了70%以上,3号和4号株系对水体中镉的去除率分别达到了85.05%和82.50%,最高富集量和最大BCF分别为3 259.55 mg·kg−1和727.05。这表明初筛、品种鉴定和复筛的筛选系统与普通的少数品种筛选相比具有明显的优势。
目前国内外关于L. japonica的研究较少,有关其在水体重金属富集与去除方面的研究更是鲜有报道。4号和5号株系作为L. japonica,在高浓度镉胁迫下,镉富集量分别为2 834.30 mg·kg−1和2 226.06 mg·kg−1,对水体中镉的去除率分别达到了82.50%和81.00%,具有优良的镉耐受和富集效果,为L. japonica在应用于重金属污染水体修复方面提供了基础。在本研究中,4号株系 (L. japonica)在镉胁迫下不仅能维持自身正常生长,还拥有优良的镉富集能力,在去除水体镉方面效果显著,是本研究筛选出的最优株系。
-
1)经过0.5 mg·L−1镉处理7 d后,3号、4号、5号、6号和7号5个浮萍株系的镉富集量均大于100 mg·kg−1,生物富集系数均超过50;在10 mg·L−1镉处理7 d后,7个株系的镉富集量均超过1 200 mg·kg−1,对水体中镉的去除率均高于70%。上述结果表明浮萍是一种镉超富集植物。
2)在经10 mg·L−1镉处理后,L. japonica品种的镉富集量超过了2 200 mg·kg−1,对水体中镉的去除率超过了80.00%,表明其具有优良的镉耐受和富集特性。
3)4号株系在经过0.5 mg·L−1和10 mg·L−1镉处理后,其生物富集系数分别为226.15和283.43,对水体中镉的去除率分别为75.00%和82.50%;在10 mg·L−1镉胁迫后,镉富集量达2 834.30 mg·kg−1,是本研究筛选出的用于修复水体镉污染的最优株系。
耐镉浮萍筛选、鉴定及其对镉的富集效果
Screening, identification and enrichment effects of cadmium-tolerant duckweeds
-
摘要: 近年来,水体镉污染日益严峻,筛选超富集植物用于其治理具有重要意义。本研究以90个浮萍株系为实验材料,采用30 mg·L−1 的镉处理7 d,获得7个镉耐受优势株系。通过Blast比对和构建系统发育树鉴定该7个浮萍株系的种属,确定为Spirodela polyrhiza、Lemna japonica、Lemna minor和Landoltia punctata。通过低质量浓度(0.5 mg·L−1)和高质量浓度(10 mg·L−1)镉处理7 d,进一步比较和研究了7个优势株系对镉的富集效果。结果表明,低浓度镉处理后,5个株系的镉富集量超过100 mg·kg−1;高浓度镉处理后,7个浮萍株系的镉富集量超过1 200 mg·kg−1,生物富集系数大于120,对水体中镉的去除率高于70%。其中,4号株系(L. japonica)为本次筛选出的最佳株系,其镉富集量、生物富集系数和对水体中镉的去除率分别达到2 834.30 mg·kg−1、283.43和82.50%。Abstract: In recent years, the cadmium pollution in water has become severe, and it is of great significance to screen hyperaccumulators for its treatment. In this study, 90 duckweed strains were taken as experimental materials and treated with 30 mg·L−1 cadmium for 7 days, then 7 cadmium-tolerant dominant strains were obtained. Through Blast comparison and construction of phylogenetic trees, the species of these 7 duckweed strains were identified as Spirodela polyrhiza, Lemna japonica, Lemna minor and Landoltia punctata. After treatment with low concentration (0.5 mg·L−1) and high concentration (10 mg·L−1) cadmium for 7 days, the enrichment effects of 7 dominant strains were further studied and compared. Under low concentration cadmium treatment, the cadmium concentration of 5 duckweed strains exceeded 100 mg·kg−1. Under high concentration cadmium treatment, the cadmium concentration of 7 duckweed strains exceeded 1 200 mg·kg−1. The bioconcentration factors were higher than 120, and the water cadmium removal rates were higher than 70%. Among them, 4 duckweed strain (L. japonica) was the best strain selected in this study, and its cadmium concentration, bioconcentration factor and water cadmium removal rate reached 2834.30 mg·kg−1, 283.43 and 82.50, respectively.
-
Key words:
- duckweed /
- heavy metal cadmium /
- species identification /
- hyperaccumulators /
- water pollution
-
近年来,我国水产养殖业快速发展,随之带来的环境问题也日益凸显。剩余饵料、养殖对象排泄物等的排放导致尾水中氮磷普遍超标。同时,为了预防和控制疾病,大量的抗生素被广泛应用于水产养殖中[1]。养殖环境中抗生素污染问题已不容忽视,四环素类、喹诺酮类、磺胺类、氯霉素类[2-3]等在养殖尾水或养殖水域中广泛存在。残留在水中的抗生素不仅直接威胁鱼虾的生存,还会加剧环境中耐药菌和耐药基因问题[4]。目前,国家正在大力发展绿色健康养殖业,养殖尾水治理力度不断增加,研发绿色、高效、低成本的抗生素去除技术对于降低抗生素排放、缓解水环境污染具有非常重要的价值。
人工湿地具有处理成本低、操作简单、不会形成二次污染等特点被广泛应用于水产养殖尾水的处理,主要通过基质吸附、微生物降解和植物吸收等过程去除废水中的污染物[5]。然而已有研究表明,不同设计参数与系统结构,如流态、基质类型和组成结构、植物类型与组成结构、水力停留时间和水力负荷、pH和季节因素(如气温、光照等)等均会导致营养盐和抗生素的去除效率存在明显差异[6-7]。同时,抗生素的存在可能会影响人工湿地系统中营养盐的去除。但是,部分研究结果和结论尚不完全一致。有研究表明,废水中抗生素的存在会降低氮磷的去除效率[8]。另一些研究表明,抗生素的存在反而提高氨氮的去除率[9]。此外,也有研究表明,2 mg·L−1的土霉素不会影响人工湿地系统对氮、磷的去除[10]。抗生素的存在对人工湿地系统去除氮磷等营养盐的影响机制尚不清晰,相关研究有待深入探讨。
本研究以3套不同基质和植物条件的上行垂直潜流人工湿地小试系统为研究对象,探究不同季节、不同基质和是否种植耐盐植物海马齿(Sesuvium portulacastrum)情况下,上行垂直潜流人工湿地系统对养殖尾水中4种典型抗生素的去除效果及抗生素的存在是否影响人工湿地系统对营养盐的去除效率。本研究结果有望为进一步改进人工湿地系统设计参数,提高污染物去除效率,促进人工湿地在淡水和海水养殖尾水治理中的应用。
1. 材料与方法
1.1 人工湿地设计
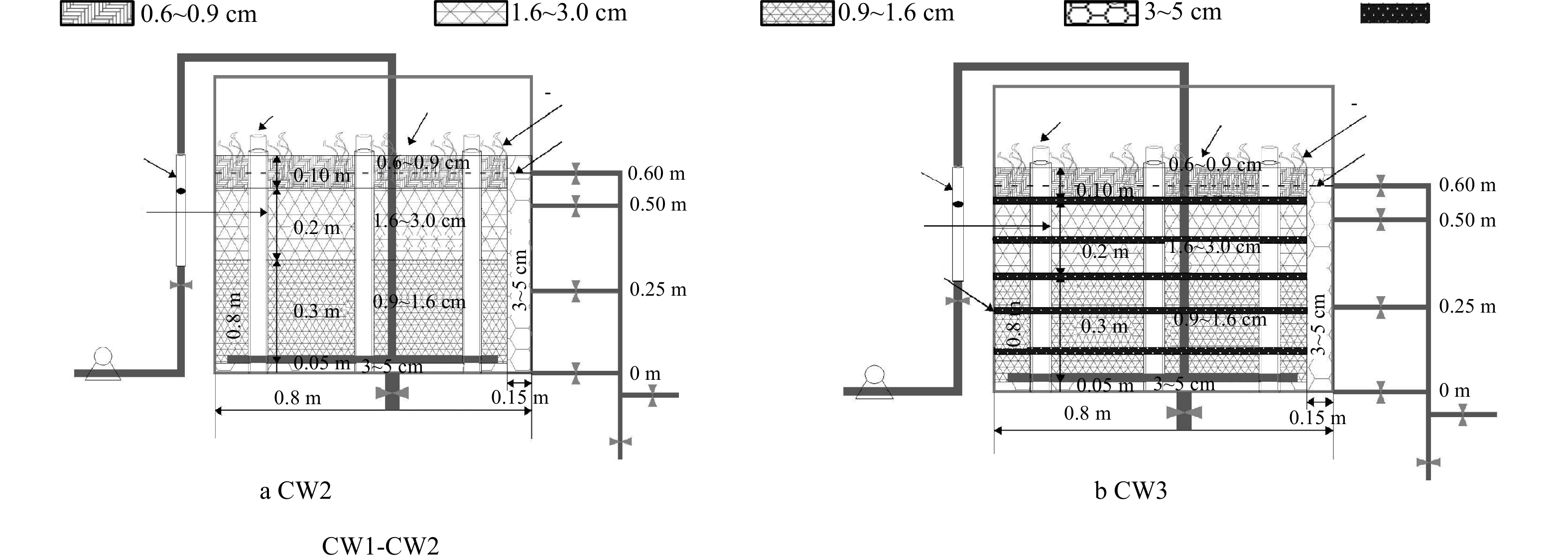
本研究构建了3种不同基质或植物条件的垂直潜流人工湿地小试系统(CW1、CW2和CW3),结构示意图如图1所示。系统由圆柱体玻璃钢制成,高80 cm、半径为40 cm,水位控制在60 cm,侧面设置3个不同高度的采样口,本研究统一从最底部采样口采样。系统中种植的植物为多年生且生命力顽强耐干旱的草本盐生植物—海马齿,选取高度在15~20 cm,生长良好的植株移栽至系统中,种植密度为40株·m−2。CW1的基质组成结构为底部铺设5 cm左右的砾石,中下层铺设30 cm高小粒径沸石,中上层铺设20 cm高中粒径沸石,上层铺设10 cm高麦饭石。CW2在CW1的基础上种植海马齿(图1(a))。CW3在CW2的基础上用生物炭层替换相同体积的沸石层(图1(b)),使用2.5 kg生物炭分5层铺设替换共0.05 m3沸石。本实验中使用的生物炭是经过500 ℃高温裂解的玉米秸秆生物炭。
1.2 实验设计
实验时间为2021年3月(春季)—2021年6月(夏季)。其中,春季的实验时间为3月10日—4月22日;夏季的实验时间为5月12日—6月25日。受试用的水产养殖尾水均来自福建省淡水水产研究所科研中试基地。本实验选用了养殖水体中常被检出的4种抗生素(氟苯尼考、土霉素、氧氟沙星和磺胺甲恶唑),设计添加质量浓度为250 µg·L−1。春、夏季实验开始前均通过向系统中输入未添加抗生素的养殖尾水进行挂膜,持续20 d。春季实验结束后,通过清水对系统进行清洗,持续20 d。系统采用间歇流(快速进水)运行方式,包括进水-反应-排水-排空闲置4个阶段,每个周期时长为4 d。其中进水时间0.5 h,反应时间为89 h,排水时间为0.5 h,排空闲置6 h。系统每个周期的进水水量约为8 000 L,水力停留时间(HRT)设置为0 (进水)和3 d。春季和夏季,分别采集HRT为0 (进水)和3 d的抗生素和营养盐水样进行分析。
1.3 营养盐、抗生素水样前处理及测定方法
待营养盐样品采集完成后,将其装入1 000 mL聚乙烯塑料瓶中,在现场用WTW便携式水质测定仪(Multi 3630)测定水温(T)、电导率(EC)、溶解氧(DO)和pH后,将样品贮存于4 ℃采样箱中,立即带回实验室。用0.45 μm的玻璃纤维滤膜过滤500 mL水样,用于硝态氮(NO3−-N)、氨氮(NH4+-N)的测定,未过滤的水样用于总氮(TN)和总磷(TP)的测定,48 h内完成样品的分析,测定方法参照相关文献[11]。
抗生素水样采集完成后装入100 mL棕色玻璃瓶中并立即运回实验室,用0.22 μm的玻璃纤维滤膜过滤样品,取1 mL注入液相进样小瓶。植物体内抗生素提取方法主要参照陈军[8]并根据多次实验结果进行相应改进。抗生素水样和植物抗生素样品均用高效液相色谱串联质谱仪(LC-MS)测定,其具体参数为:色谱柱为XTERRA MS C18(3.0 mm×100 mm,5 μm)。色谱柱柱温为35 ℃,进样量为5 µL。流动相流速为0.4 mL·min−1,流动相A是体积分数为0.1%的甲酸-水溶液。流动相B为甲醇,洗脱梯度程序设置如下:0~0.5 min,5%B;0.5~3 min,5%~40%B;3~4 min,40%B;4~5 min,40%~95%B;5~7.5 min,95%B;7.5~7.51 min,95%~5%B;7.51~9 min,5%B。
1.4 数据分析
本研究应用One-Way ANOVA单因素方差分析研究不同组别去除率总体分布是否有显著性差异;相关统计分析应用SPSS 26软件进行;图件制作应用Origin软件完成;数据预处理采用Excel软件完成。抗生素和营养盐的去除率按照公式(1)计算。
η=ci−ceci×100% (1) 式中:
η为去除率,%;ci ce 2. 结果与讨论
2.1 进、出水水质变化
春季和夏季进、出水水质状况见表1和表2。总体而言,夏季进、出水平均水温比春季平均水温分别高5.65 ℃和7.20 ℃;夏季进、出水pH平均值比春季分别低0.21和0.26;夏季进、出水电导率平均值比春季分别高5.73 μs·cm−1和7.42 μs·cm−1;夏季进、出水溶解氧平均值比春季分别低0.81 mg·L−1和3.97 mg·L−1。春季和夏季,进水pH均呈弱酸性,经过系统处理后pH呈弱碱性;与进水相比,出水电导率均有所上升,而溶解氧含量下降。
表 1 春季无抗生素和抗生素存在条件下进、出水水质状况Table 1. Characteristics of inlet and outlet water before and after antibiotic exposure in spring实验条件 系统 温度/ ℃ pH 电导率/(μs·cm−1) 溶解氧/(mg·L−1) 无抗生素 CW0 21.33±0.79 6.88±0.12 69.33±9.60 7.25±0.93 CW1 22.30±0.37 7.87±0.05 136.2±9.43 5.40±0.93 CW2 22.53±0.33 7.75±0.02 141.7±9.80 5.32±0.26 CW3 22.43±0.37 7.71±0.06 154.0±11.0 5.56±0.78 有抗生素 CW0 26.63±1.78 6.82±0.07 53.50±3.21 7.25±0.27 CW1 26.70±2.24 7.90±0.17 124.8±1.32 5.21±0.25 CW2 26.90±2.30 7.77±0.12 132.43±3.74 5.09±0.32 CW3 26.83±2.24 7.77±0.10 142.13±3.56 5.26±0.38 注:CW0为进水。 表 2 夏季无抗生素和抗生素存在条件下进、出水水质状况Table 2. Characteristics of inlet and outlet water before and after antibiotic exposure in summer实验条件 系统 温度/ ℃ pH 电导率/(μs·cm−1) 溶解氧/(mg·L−1) 无抗生素 CW0 29.63±0.85 6.89±0.10 57.93±0.37 6.28±0.34 CW1 29.40±0.65 7.84±0.10 129.47±5.20 1.44±0.01 CW2 29.57±0.74 7.69±0.10 140.83±7.24 1.15±0.07 CW3 29.70±0.86 7.64±0.10 151.33±8.01 1.28±0.04 有抗生素 CW0 29.33±0.86 6.29±0.06 83.37±11.64 6.60±0.61 CW1 28.60±1.23 7.52±0.10 142.47±7.90 1.27±0.08 CW2 28.87±1.35 7.46±0.07 149.30±7.68 1.36±0.20 CW3 28.80±1.31 7.38±0.08 162.43±2.80 1.54±0.12 注:CW0为进水。 2.2 人工湿地设计参数对养殖尾水中抗生素去除效率的影响
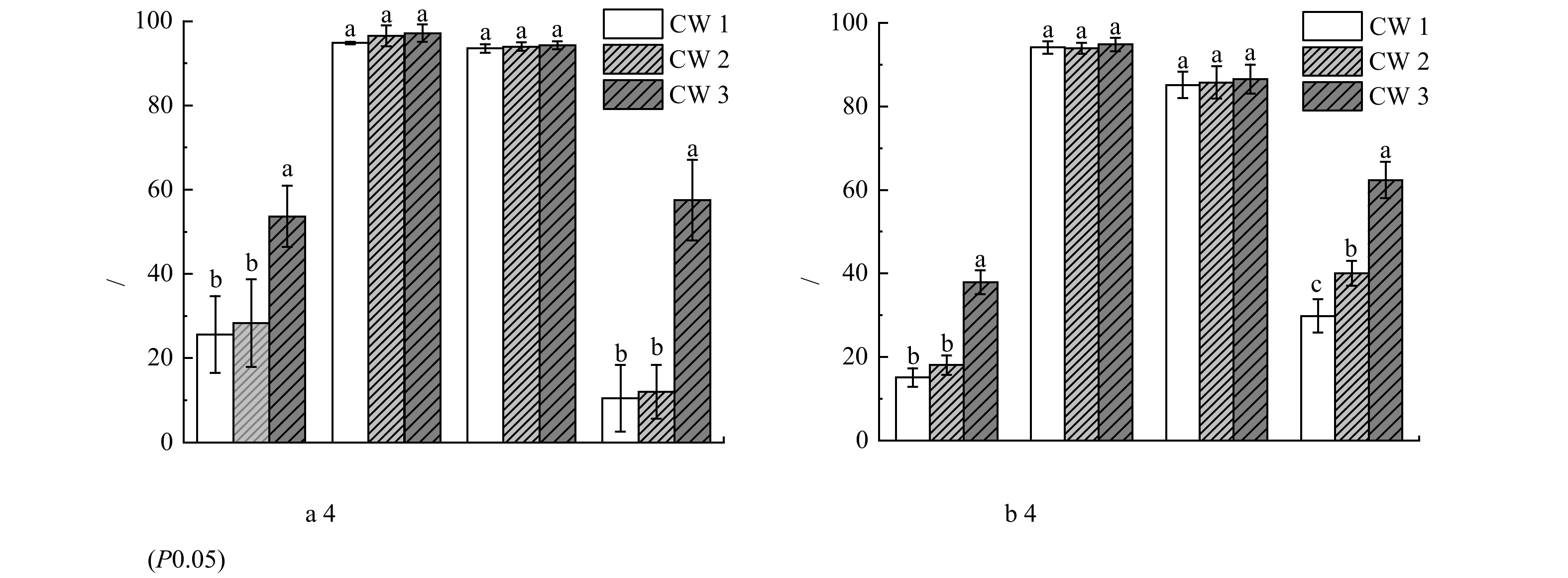
春季和夏季,3套系统进、出水中氟苯尼考、土霉素、氧氟沙星和磺胺甲恶唑质量浓度见表3;CW1、CW2和CW3对4种抗生素的去除率见图2。无论春季或夏季,3套系统对土霉素和氧氟沙星的去除率均无明显差异,且均具有良好的去除能力。春季,与CW1相比,CW2对氟苯尼考和磺胺甲恶唑的去除率分别提升了2.71%和1.59%;与CW2相比,CW3对氟苯尼考和磺胺甲恶唑的去除率分别提升了25.34%和45.48%。夏季,与CW1相比,CW2对氟苯尼考和磺胺甲恶唑的去除率分别提升了2.97%和10.16%;与CW2相比,CW3对氟苯尼考和磺胺甲恶唑的平均去除率分别提高19.87%和22.36%。结果表明,种植海马齿可以在一定程度上提升以沸石为主人工湿地系统对氟苯尼考和磺胺甲恶唑的去除效果,添加生物炭可以显著提高(p≤0.05)这两种抗生素的去除率。
表 3 春季和夏季3套系统进、出水中抗生素的质量浓度Table 3. Antibiotics concentrations in the inlet and outlet water of three systems in spring and summer μg·L-1季节 系统 氟苯尼考 土霉素 氧氟沙星 磺胺甲恶唑 春季 CW0 257.5±20.85 237.03±15.36 309.91±15.38 271.16±9.38 CW1 193.11±36.01 12.35±0.23 20.42±2.11 244.92±11.88 CW2 186.12±37.31 8.14±5.77 19.00±2.34 243.29±8.73 CW3 120.41±25.50 10.47±1.03 18.10±2.05 136.45±38.87 夏季 CW0 238.78±8.09 250.04±19.43 255.80±16.31 250.33±10.00 CW1 202.65±6.14 14.87±4.92 37.84±7.97 175.76±13.73 CW2 195.48±1.34 15.34±4.41 36.29±9.84 150.50±13.55 CW3 148.00±2.86 13.12±5.14 34.31±8.88 94.58±14.43 注:CW0为进水。 人工湿地系统去除抗生素的主要途径包括基质吸附截留、植物吸收、光降解和生物降解等过程,对于垂直潜流人工湿地系统而言,光解作用可以忽略[12]。本研究中3套垂直潜流湿地系统对土霉素和氧氟沙星均有良好的净化效果,而磺胺甲恶唑和氟苯尼考的去除率明显低于土霉素和氧氟沙星,这与其他研究结论一致[13]。土霉素和氧氟沙星分别属于四环素类和氟喹诺酮类抗生素。基质吸附作用是废水中喹诺酮类和四环素类抗生素的主要去除途径[14],一旦暴露在环境中可以快速光解或者被基质吸附,这可能是3套系统均表现出对土霉素和氧氟沙星有良好净化能力的主要原因。氟苯尼考属于甲砜霉素的单氟衍生物,具有氟、氯多个卤代基团和苯环结构,性质较为稳定,不易发生光解和水解,在常规的人工湿地中难以被去除[15]。磺胺甲恶唑属于磺胺类抗生素,在环境中降解速度缓慢,生物降解是磺胺类的抗生素的最主要去除路径[16]。已有研究发现好氧微生物降解、部分厌氧微生物或者兼性厌氧微生物均可促进磺胺甲恶唑降解[17-18]。由于不同研究的进水水质、基质、植物等存在较大差异,系统内微生物群落结构和多样性也不同,磺胺甲恶唑去除率存在较大的差异[19-20]。与一些研究相比[21],本研究中CW1和CW2对磺胺甲恶唑的去除率偏低。这可能是因为水产养殖尾水C/N较低,碳源不足时,不利于磺胺甲恶唑的降解[13]。水体中微生物的种群、数量和活性都与水体中有机质的含量成正相关[22],寡营养态水体中磺胺甲恶唑的降解效率普遍偏低[19]。此外,不同研究中除了植物和基质对抗生素吸收和吸附能力存在差异外,磺胺甲恶唑初始质量浓度的差异,导致其对系统微生物群落结构和多样性的影响也不同,这可能是影响人工湿地系统对其净化能力存在差异的另一重要因素[16]。部分研究也发现,以沸石为主的人工湿地对低浓度的磺胺甲恶唑的去除效果不佳[5,19]。
除夏季土霉素外,植物组(CW2)对4种抗生素的去除率均优于无植物组(CW1)。植物主要通过根系的吸收作用、根际的吸附和截留、根系分泌物和氧气释放增强微生物活性等方式直接或间接去除废水中的抗生素[23]。虽然大部分研究认为植物吸收过程不是人工湿地去除磺胺甲恶唑和氟苯尼考的主要途径,但是适当种植植物可以提高抗生素的去除率[24]。本研究中检测到海马齿中土霉素的质量浓度在851.55~1691.48 µg·L−1,磺胺甲恶唑的质量浓度在125.07~291.67 µg·L−1,表明海马齿能够通过吸收过程移除水体中土霉素和磺胺甲恶唑。结合植物组和无植物组系统对4种抗生素的去除率,说明海马齿对这4种抗生素去除起到一定的促进作用。相比春季,夏季促进效果更明显,可能因为夏季植物生长更旺盛,有利于海马齿对污染物的吸收。
基质是人工湿地的重要组成部分之一,除了对污染物具有吸附作用外,还可为微生物和植物提供基本的生长环境和营养物质[12]。因此,基质的物理化学特征直接影响人工湿地系统对抗生素的净化能力。春季和夏季,添加生物炭均可显著提高垂直潜流人工湿地对氟苯尼考和磺胺甲恶唑的净化能力。相比沸石,生物炭孔隙结构明显、比表面积大,且具有亲水、疏水和酸碱性等性质[25],对抗生素的吸附速度更快,尤其在酸性环境[26]。人工湿地对抗生素的净化能力与基质的类型、组成、微生物群落结构等关系密切,添加生物炭后会改变系统内pH、营养盐和氧气的流动、植物生长状况等,影响系统内其他基质表面的微生物群落结构,进而提升系统对抗生素的净化能力[27]。pH是影响抗生素去除效率的关键因素之一,在酸性条件下磺胺类抗生素以阳离子形式存在。CW3的pH低于CW1和CW2,更有利于生物炭对磺胺甲恶唑和氟苯尼考的吸附。
2.3 抗生素对营养盐去除的影响
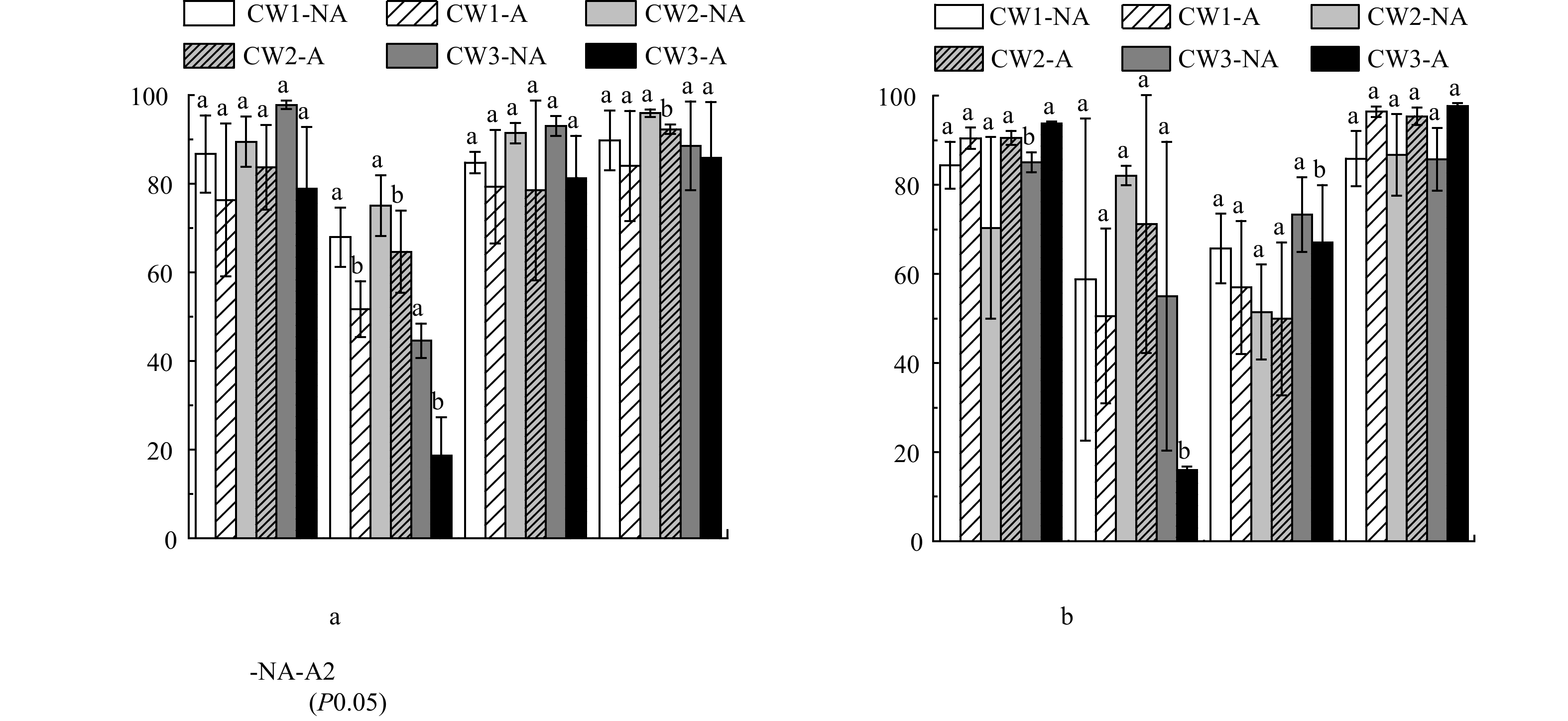
春季和夏季抗生素添加前后3套系统进、出水中总氮、总磷、氨氮和硝态氮的质量浓度见图3。春季,抗生素存在条件下,3种不同条件系统的总氮、总磷、氨氮和硝态氮平均去除率分别下降了11.69%、17.53%、10.04%和4.07%,且3套系统均显著降低了其对总磷的去除效率(P≤0.05)(图4(a))。夏季,抗生素存在条件下,总磷和氨氮的平均去除率分别下降了19.41%和5.53%,而总氮和硝态氮平均去除率分别提高了11.66%和10.42%(图4(b))。
本研究结果表明,春季和夏季抗生素存在对人工湿地去除总氮和硝氮的影响截然不同,而对总磷和氨氮的去除均表现为抑制作用,且在春季3套系统均可以显著降低对总磷的去除效率。其他研究[28]也发现,添加质量浓度为50~100 ng·L−1的氟喹诺酮类抗生素后,TP的去除率下降。基质吸附、植物吸收和聚磷菌(PAOs)的吸收是人工湿地系统中磷的主要去除路径。基质中吸附的土霉素(四环素类)和氧氟沙星(喹诺酮类)可能会与磷争夺吸附位点,导致磷的去除效率下降[9]。抗生素的存在也可能影响聚磷菌的丰度,削弱微生物对磷的吸收过程。YI等[8]发现添加2 mg·L−1的环丙沙星后会降低系统中聚磷菌的丰度,减少细菌对磷的吸收。夏季系统中溶解氧的浓度显著低于春季(表1和表2),厌氧或缺氧条件下,聚磷菌会释放磷,在一定程度上抑制尾水中总磷的去除,这可能是夏季总磷去除率比春季下降更明显的原因之一。
硝化、反硝化、厌氧氨氧化等微生物转化过程是人工湿地系统去除废水中无机氮的主要机制。课题组前期研究发现,抗生素添加前尾水中的细菌群落主要为α-变形菌(α-Proteobacteria)、γ-变形菌(γ-Proteobacteria)、拟杆菌(Bacteroidia)和放线菌(Actinomycete),这些微生物在人工湿地系统去除氮的过程中起着非常关键的作用[29]。好氧条件下,硝化细菌[30](如假单胞菌(Pseudomonas))将系统中NH4+氧化成NO3−的过程是人工湿地系统去除氨氮的重要途径。抗生素的添加可能会影响微生物群落的结构和多样性,从而影响氮的转化过程。YAN等[31]研究发现人工湿地系统中微生物多样性和丰度指数与磺胺甲恶唑、氧氟沙星、罗红霉素等抗生素呈负相关关系,多种抗生素的存在会降低水体中变形菌门(Proteobacteria)的丰度,导致系统对氨氮的去除效率下降,这与本研究的结论一致。夏季,抗生素添加后氨氮去除率下降幅度比春季小,可能因为夏季水温更高,促进微生物硝化作用,提高氨氮去除率,在一定程度上抵消抗生素存在对氨氮去除的不利影响。反硝化过程是指在厌氧条件下,硝酸盐和亚硝酸盐被反硝化细菌异化还原为N2的过程,是系统中硝态氮去除的重要形式。YI等[8]研究发现添加2 mg·L−1的环丙沙星会降低系统对硝态氮的去除。本研究中抗生素添加对硝态氮去除的影响由春季的抑制作用转为夏季的促进作用,可能是因为夏季尾水中溶解氧(<2 mg·L−1)远低于春季(5.09~5.26 mg·L−1),低氧或厌氧条件下有利于拟杆菌和放线菌等反硝化菌群繁殖,促进反硝化过程的发生,提高硝氮的去除效率。硝态氮在进水中占总氮的比重在27.70%~53.89%,是无机氮的主要赋存形态,尤其是夏季,这可能是抗生素添加对总氮和硝态氮去除的影响一致的原因。CHEN等[32]也发现2 mg·L−1的四环素影响了系统内反硝化菌的丰度,系统内硝态氮浓度增加,降低了对总氮的去除率。
在已有的研究中,人工湿地系统基质的组成结构和类型、运行方式、流式、植物类型等不尽相同,且添加的抗生素种类和质量浓度也存在差异,大部分研究结论尚不一致。CHEN[32]等研究发现系统中四环素的质量浓度为2 mg·L−1时不会影响除磷效果,但会降低系统对总氮的去除率;TONG等[33]研究发现添加质量浓度为0.1、10和1 000 µg·L−1氧氟沙星后,人工湿地系统中氨氮的去除率由72.60%提高至80.70%~82.10%;KUYPERS等[34]研究发现在磺胺甲嘧啶质量浓度为100 µg·L−1时,处理组的氨氮去除率略高于对照组。本研究仅考虑4种不同类型抗生素复合对营养盐去除的影响,将来研究中会进一步分析抗生素添加前后微生物群落结构的变化,为阐明本研究试验抗生素添加浓度条件下对营养盐去除影响的机理提供理论依据。
3. 结 论
1)本研究所构建的3种不同条件垂直潜流人工湿地系统对氧氟沙星和土霉素在春季和夏季均表现出良好的去除效果,去除率均在85%以上。与氧氟沙星和土霉素相比,系统对磺胺甲恶唑和氟苯尼考的去除效率相对较低,春季,系统对氟苯尼考和磺胺甲恶唑的去除率分别为17.23%~67.50%和8.37%~67.87%;夏季,系统对氟苯尼考和磺胺甲恶唑的去除率分别为12.01%~41.29%和19.28%~67.04%。
2)春季和夏季,种植海马齿整体上可以提高系统对4种抗生素的去除效率,但均不具有显著性差异;春季和夏季,添加生物炭均可提高系统对4种抗生素的去除效率,且会显著提高系统对磺胺甲恶唑和氟苯尼考的去除效率。
3)无论是春季还是夏季,4种抗生素添加均对总磷和氨氮的去除产生一定的负面影响,但不同季节对总氮和硝态氮去除效率的影响存在差异。在抗生素存在的条件下,春季,3种不同条件的人工湿地系统对总氮、总磷、氨氮和硝态氮平均去除率分别下降了11.69%、17.53%、10.04%和4.07%;夏季,总磷和氨氮的平均去除率分别下降了19.41%和5.53%,而总氮和硝态氮的平均去除率分别提高了11.67%和10.42%。
-
表 1 浮萍株系编号和采集信息
Table 1. Duckweed species number and collection place
株系编号 采集地 经纬度 1 北京市昌平区 E116°42'47'' N40°10'92'' 2 贵阳市花溪区 E106°39'23'' N26°26'59'' 3 贵阳市花溪区 E106°39'57'' N26°26'49'' 4 北京市昌平区 E116°42'47" N40°10'92" 5 重庆市大足区 E105°51′1″ N29°27′48″ 6 重庆市大足区 E105°45′28″ N29°27′57″ 7 重庆市大足区 E105°45′23″ N29°27′55″ 表 2 7个浮萍Blast种属鉴定结果
Table 2. Species identification of 7 duckweed strains by Blast
株系编号 atpF-atpH比对序列 atpF-atpH序列相似性/% rpS16比对序列 rpS16 序列相似性/% 种属 1 MN419335 99.28 KJ503285 99.69% Spirodela polyrhiza 2 KX212888 99.00 KX212891 99.38% Lemna minor 3 KX212888 98.49 KX212891 99.69% Lemna minor 4 KJ921747 99.70 EU568887 94.80% Lemna japonica 5 KJ921747 99.11 EU568887 94.80% Lemna japonica 6 KJ630555 99.30 KJ503327 99.70% Landoltia punctata 7 KJ630555 97.25 KJ503327 99.60% Landoltia punctata -
[1] 周建军, 周桔, 冯仁国. 我国土壤重金属污染现状及治理战略[J]. 中国科学院院刊, 2014, 29(3): 315-320. [2] 毛智勇, 李大勇, 龙迪勇, 等. 重金属污染与生态修复问题研究——以江西省新余市为例[J]. 鄱阳湖学刊, 2013, 3: 5-15. [3] 王俊能, 赵学敏, 胡国成, 等. 广西龙江鱼类镉含量分布特征及生物积累特性分析[J]. 环境科学, 2019, 40(1): 488-495. [4] 朱泊丞, 施泽明, 王新宇, 等. 安宁河水体中重金属空间分布特征及来源识别[J]. 四川冶金, 2018, 40(4): 24-31. doi: 10.3969/j.issn.1001-5108.2018.04.006 [5] 朱映川, 刘雯, 周遗品, 等. 水体重金属污染现状及其治理方法研究进展[J]. 广东农业科学, 2008, 8: 143-146. doi: 10.3969/j.issn.1004-874X.2008.06.054 [6] CHENG C H, MA H L, DENG Y Q, et al. Oxidative stress, cell cycle arrest, DNA damage and apoptosis in the mud crab (Scylla paramamosain) induced by cadmium exposure[J]. Chemosphere, 2021, 263: 128277. doi: 10.1016/j.chemosphere.2020.128277 [7] PAITHANKAR J G, SAINI S, DWIVEDI S, et al. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction[J]. Chemosphere, 2021, 262: 128350. doi: 10.1016/j.chemosphere.2020.128350 [8] PINHEIRO J E G, MORAES P Z, RODRIGUEZ M D, et al. Cadmium exposure activates NADPH oxidase, renin-angiotensin system and cyclooxygenase 2 pathways in arteries, inducing hypertension and vascular damage[J]. Toxicology letters, 2020, 333: 80-89. doi: 10.1016/j.toxlet.2020.07.027 [9] THÉVENOD F, CHAKRABORTY P K. The role of wnt/beta-catenin signaling in renal carcinogenesis: Lessons from cadmium toxicity studies[J]. Current Molecular Medicine, 2010, 10(4): 387-404. doi: 10.2174/156652410791316986 [10] 张坤, 罗书. 水体重金属污染治理技术研究进展[J]. 中国环境管理干部学院学报, 2010, 20(3): 62-64. doi: 10.3969/j.issn.1008-813X.2010.03.018 [11] 魏欢欢. 重金属污染水体生物修复治理技术[J]. 化工管理, 2020, 30: 100-101. doi: 10.3969/j.issn.1008-4800.2020.33.049 [12] SALT D E, BLAYLOCK M, KUMAR N P, et al. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants[J]. Biotechnology (N Y), 1995, 13(5): 468-474. [13] LI J T, GURAJALA H K, WU L H, et al. Hyperaccumulator plants from China: A synthesis of the current state of knowledge[J]. Environmental Science & Technology, 2018, 52(21): 11980-11994. [14] KÜPPER H, LEITENMAIER B. Cadmium-accumulating plants[J]. Metal Ions in Life Sciences, 2013, 11: 373-393. [15] EKPERUSI A O, SIKOKI F D, NWACHUKWU E O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective[J]. Chemosphere, 2019, 223: 285-309. doi: 10.1016/j.chemosphere.2019.02.025 [16] BAEK G, SAEED M, CHOI H K. Duckweeds: Their utilization, metabolites and cultivation[J]. Applied Biological Chemistry, 2021, 64(1): 73. doi: 10.1186/s13765-021-00644-z [17] YANG G L, FENG D, LIU Y T, et al. Research progress of a potential bioreactor: Duckweed[J]. Biomolecules, 2021, 11(1). [18] DUFF R B. The occurrence of apiose in Lemna (duckweed) and other angiosperms[J]. Biochemical Journal, 1965, 94(3): 768-772. doi: 10.1042/bj0940768 [19] 王兴利, 吴晓晨, 王晨野, 等. 水生植物生态修复重金属污染水体研究进展[J]. 环境污染与防治, 2020, 42(1): 107-112. [20] 种云霄, 胡洪营, 钱易. pH及无机氮化合物对细脉浮萍生长的影响[J]. 生态学报, 2003, 11: 2293-2298. doi: 10.3321/j.issn:1000-0933.2003.11.012 [21] 李菲菲. 重金属元素铅(Pb)和镉(Cd)对浮水植物紫背浮萍(Spirodela polyrrhiza)的毒理学效应研究[D]. 南京: 南京师范大学, 2016. [22] 李玥. 镉、铜、锌对四种水生植物的毒性效应[D]. 长春: 东北师范大学, 2007. [23] 崔姜伟, 崔卫华, 郝春博. 浮萍在环境保护领域的应用研究进展[J]. 环境工程, 2015, 33(S1): 306-309. [24] CHEN D, ZHANG H, WANG Q, et al. Intraspecific variations in cadmium tolerance and phytoaccumulation in giant duckweed (Spirodela polyrhiza)[J]. Journal of hazardous materials, 2020, 395: 122672. doi: 10.1016/j.jhazmat.2020.122672 [25] ROLLI N M, SUVARNAKHANDI S S, MULGUND G S, et al. Biochemical responses and accumulation of cadmium in Spirodela polyrhiza[J]. Journal of Environmental Biology, 2010, 31(4): 529-532. [26] 唐利萍, 方扬, 靳艳玲, 等. 重金属镉超富集浮萍品种筛选及其对水体中镉的去除效果[J]. 应用与环境生物学报, 2015, 21(5): 830-836. [27] KHAN M A, WANI G A, MAJID H, et al. Differential bioaccumulation of select heavy metals from wastewater by Lemna minor[J]. Bulletin of Environmental Contamination and Toxicology, 2020, 105(5): 777-783. doi: 10.1007/s00128-020-03016-3 [28] WANG X, ZHANG B, WU D, et al. Chemical forms governing Cd tolerance and detoxification in duckweed (Landoltia punctata)[J]. Ecotoxicology and Environmental Safety, 2021, 207: 111553. doi: 10.1016/j.ecoenv.2020.111553 [29] CHAUDHURI D, MAJUMDER A, MISRA A K, et al. Cadmium removal by Lemna minor and Spirodela polyrhiza[J]. International Journal of Phytoremediation, 2014, 16(7-12): 1119-1132. [30] HOAGLAND D R, Davis A R. The composition of the cell sap of the plant in relation to the absorption of ions[J]. Journal of General Physiology, 1923, 5(5): 629-646. doi: 10.1085/jgp.5.5.629 [31] 李荣华, 夏岩石, 刘顺枝, 等. 改进的CTAB提取植物DNA方法[J]. 实验室研究与探索, 2009, 28(9): 14-16. doi: 10.3969/j.issn.1006-7167.2009.09.005 [32] DOUCETTE W J, SHUNTHIRASINGHAM C, DETTENMAIER E M, et al. A review of measured bioaccumulation data on terrestrial plants for organic chemicals: Metrics, variability, and the need for standardized measurement protocols[J]. Environmental Toxicology and Chemistry, 2018, 37(1): 21-33. doi: 10.1002/etc.3992 [33] NAUMANN B, EBERIUS M, APPENROTH K J. Growth rate based dose-response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St[J]. Journal of Plant Physiology, 2007, 164(12): 1656-1664. doi: 10.1016/j.jplph.2006.10.011 [34] KHAN AR, ULLAH I, WAQAS M, et al. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi[J]. Ecotoxicology and Environmental Safety, 2017, 136: 180-188. doi: 10.1016/j.ecoenv.2016.03.014 [35] HALIM M A, RAHMAN M M, MEGHARAJ M, et al. Cadmium immobilization in the rhizosphere and plant cellular detoxification: role of plant-growth-promoting rhizobacteria as a sustainable solution[J]. Journal of Agricultural and Food Chemistry, 2020, 68(47): 13497-13529. doi: 10.1021/acs.jafc.0c04579 [36] YANG G L, ZHENG M M, TAN A J, et al. Research on the mechanisms of plant enrichment and detoxification of cadmium[J]. Biology (Basel), 2021, 10(6). [37] 宇克莉, 孟庆敏, 邹金华. 镉对玉米幼苗生长、叶绿素含量及细胞超微结构的影响[J]. 华北农学报, 2010, 25(3): 118-123. doi: 10.7668/hbnxb.2010.03.026 [38] 朱志勇, 郝玉芬, 李友军, 等. 镉对小麦旗叶叶绿素含量及籽粒产量的影响[J]. 核农学报, 2011, 25(5): 1010-1016. [39] LIU S, ALI S, YANG R, et al. A newly discovered Cd-hyperaccumulator Lantana camara L[J]. Journal of Hazardous Materials, 2019, 371: 233-242. doi: 10.1016/j.jhazmat.2019.03.016 [40] LAN X Y, YAN Y Y, YANG B, et al. Subcellular distribution of cadmium in a novel potential aquatic hyperaccumulator - Microsorum pteropus[J]. Environmental Pollution, 2019, 248: 1020-1027. doi: 10.1016/j.envpol.2019.01.123 [41] ZHANG C, ZHANG P, MO C, et al. Cadmium uptake, chemical forms, subcellular distribution, and accumulation in Echinodorus osiris Rataj[J]. Environmental Science Processes & Impacts, 2013, 15(7): 1459-1465. [42] ZHONG L, LIN L, LIAO M, et al. Phytoremediation potential of Pterocypsela laciniata as a cadmium hyperaccumulator[J]. Environmental Science and Pollution Research International, 2019, 26(13): 13311-13319. doi: 10.1007/s11356-019-04702-4 [43] LIU Z, HE X, CHEN W, et al. Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator-Lonicera japonica Thunb[J]. Journal of Hazardous Materials, 2009, 169(1-3): 170-175. doi: 10.1016/j.jhazmat.2009.03.090 [44] 单丹, 黄宝成, 冯华军, 等. 两种水生植物对镉净化潜能研究[J]. 科技通报, 2012, 28(7): 173-175. doi: 10.3969/j.issn.1001-7119.2012.07.040 [45] 兰心宇, 王军军, 阎蕴运, 等. 水生植物有翅星蕨(Microsorum pteropus)对镉的超富集能力及抗性生理研究[J]. 中国科学:生命科学, 2017, 47(10): 1113-1123. [46] ISLAM M S, UENO Y, SIKDER M T, et al. Phytofiltration of arsenic and cadmium from the water environment using Micranthemum umbrosum (J. F. Gmel) S. F Blake as a hyperaccumulator[J]. International Journal of Phytoremediation, 2013, 15(10): 1010-1021. doi: 10.1080/15226514.2012.751356 期刊类型引用(7)
1. 隋雪晴,左尚武,周磊,张雪琦,王月圆,卢烨彬,杨扬,成水平. 部分饱和垂直流人工湿地抗生素去除性能及规律研究. 环境科学学报. 2025(01): 177-187 .  百度学术
百度学术
2. 侯天元,汤冬梅,张丽萍,周巧红,吴振斌,武俊梅. 抗生素对人工湿地处理水产养殖尾水的影响及其缓解途径. 水生生物学报. 2025(04): 61-71 .  百度学术
百度学术
3. 张美,王家宏,白杨. 铁碳强化潮汐流-潜流复合人工湿地处理模拟养殖尾水的启动运行效果. 环境科学研究. 2024(04): 800-811 .  百度学术
百度学术
4. 郑仕夫,徐慧敏,陈曦,裘丽萍,宋超,范立民,李丹丹,孟顺龙,徐跑. 水产养殖尾水处理技术的研究现状和发展趋势. 中国农学通报. 2024(12): 159-164 .  百度学术
百度学术
5. 王梦婷,郑于聪,郝梦晴,程晓阳,王晓昌,陈荣,DZAKPASU Mawuli. 多种类抗生素对垂直流人工湿地净化作用的影响机制. 环境工程学报. 2024(05): 1365-1372 .  本站查看
本站查看
6. 樊祥科,邹宏海. 人工智能在池塘养殖生产中的应用前景探析. 黑龙江水产. 2024(05): 618-624 .  百度学术
百度学术
7. 王飞儿,林杉,张秀玲,沈瑶瑶,张水清,江艳云,胡荣桂. 微塑料输入与秸秆添加对潮土和黄棕壤氮淋溶的影响. 农业工程学报. 2023(23): 94-102 .  百度学术
百度学术
其他类型引用(4)
-






 下载:
下载: