-
全氟烷基酸(perfluoroalkyl acids,PFAAs)是由完全氟化的烷基链和酸性官能团组成的化合物,因其兼具疏水性和疏油性等化学特性,常作为工业助剂和表面活性剂而应用于工业和消费品生产领域,如金属电镀、半导体、家具和烹饪器具等[1]。在2009年,PFAAs被列入斯德哥尔摩公约的新型持久性有机污染物。PFAAs易在生物体内聚集,能够长距离迁移、沉积和附着,一旦进入人体后很难通过代谢降解方式排出体外,可对肝脏、内分泌、神经系统和免疫性能等方面产生毒性危害[2]。PFAAs家族成员PFOA和 PFOS因其稳定性,应用最为广泛,且作为全氟化合物前体的最终降解物质,在自然环境中检测频率最高[3]。随着氟化工产业的快速发展,中国逐渐成为氟化产品生产和使用大国。秦交友等[4]的研究表明,我国东南地区主要河流表层水体中PFAAs质量浓度为0.90~231.52 ng·L−1,部分河段PFOA和PFOS的生态风险熵大于1,主要排放源为工业污水排放。LI等[5]研究表明,小清河流域道PFAAs总质量浓度高达325.280 µg·L−1。
由于PFAAs在水环境中广泛检出,故有关PFAAs的去除方法备受学者们关注。目前的去除方法集中在微波降解法、膜处理法、高级氧化法、吸附法和植物富集等。其中化学方法大多数条件复杂、能耗高,比较经济实用的方法主要为吸附法和植物吸收法[6-7]。用于处理含氟水体的吸附剂主要包括金属氧化物、离子交换树脂、沉积物、碳材料和矿物材料等,在pH、温度、吸附时间和离子强度等因素的干扰下,各吸附剂对PFAAs的吸附容量有较大波动[8]。植物修复已作为富营养化水体的常用修复技术。有研究[9]表明,除了氮磷等营养盐外,一些具有耐盐和耐毒性植物还可以直接吸收水体中的有机污染物和重金属并将其富集于体内。YIN等[10]的研究表明,芦苇床对水体中PFAAs的去除率最高可达42%~49%;孔潇潇等[11]发现,金鱼藻对PFOS的去除率可达90%以上;在实际河岸湿地植物调查中发现,凤眼莲对PFOA的生物浓缩系数最高,并且具有纤维生根系统的植物对PFOA积累量更高[12]。
已有研究表明,水生植物对单一的营养盐或全氟烷基酸污染的水体具有一定的修复效果,但关于其对于含有这些污染物复合污染水体的研究较少。基于此,本研究针对营养盐和全氟烷基酸复合污染水体的处理问题,选取4种水生植物进行室内静态水培实验,对比研究了不同水生植物对复合污染水体的修复效果,以期为水生植物在水体治理修复工程中的应用提供参考。
-
根据富营养化河道修复技术常用的植物类型,结合植物对全氟化合物富集作用的已有研究,如表1所示,选择鸢尾(Iris tectorum)、芦苇(Phragmites australis)、金鱼藻(Phragmites australis)和眼子菜(Potamogetonpusillus) 4种水生植物进行研究。植物购自江苏南京某花卉市场,实验中对所有植物进行5 d的预培养,以恢复植物的根系活性。
实验用水取自南京某工业园区附近的河道,该河道长期接纳城市污水和工业废水。水样中污染物质量浓度分别为总氮7.13 mg·L−1,总磷0.68 mg·L−1,水体为劣V类水。向水体中人为添加PFOA、PFOS标液,初始质量浓度按60 µg·L−1计算,以模拟氮磷和全氟烷基酸的复合污染水体。
-
选取生长状况良好的植株,鸢尾和芦苇株高修剪至30 cm,金鱼藻和眼子菜株高修剪至20 cm,清洗干净后放置于聚乙烯桶中进行水培实验。设计4个植物组和1个对照组,每组设3个重复,植物组每个桶中放置3棵植株,加入3 L模拟污染水体,挺水植物只有根部与水体接触,沉水植物完全位于水面下。
实验在25 ℃植物光照培养室内进行,实验周期为29 d,分别在第1、2、4、7、11、16、22、29 天上午9:00采集水样,以确定水生植物对氮磷及全氟烷基酸的去除效果。29 d后,采取植物样品,用去离子水冲洗干净,干燥后用粉碎机粉碎,过100目筛后分类存储,以测定植物中污染物含量。
-
1)水样处理。水体中氮磷的测定参照《水和废水监测分析方法(第4版)》,TN 质量浓度的测定用碱性过硫酸钾法,TP 质量浓度的测定用钼锑抗分光光度法[13];水体中PFOA、PFOS的测定参照WANG[14]的方法,水样先采用OasisRWAX固相萃取柱进行萃取,然后用醋酸铵溶液(25 mmol·L−1,pH = 4)冲洗、甲醇和氨水甲醇液(0.10% )洗脱,高纯氮出之后用0.2 µm滤膜过滤,上机测定待测物质。
2)植物处理。实验过程中,观察植物的生长情况,实验结束后测定植物生物量、植株高度等生长指标以及植物不同部位全氟烷基酸的含量,并根据式(1)和式(2)计算植物对全氟烷基酸的富集和转移系数。植物中PFOA、PFOS的测定参照FELIZETER[15]的方法,用MTBE溶液提取出植物样品中的全氟烷基酸后氮吹浓缩,经过Florisil SPE柱进行萃取,用甲醇-MTBE溶液(体积比为30∶70)洗脱,高纯氮吹之后经过ENVI -Carb净化再氮吹,最后经过滤膜过滤,收集样液上机测定待测物质。
式中:Tf 为转移系数; C1为植物水上部分PFAAs富集含量, µg·g−1; C2为植物水下部分PFAAs富集含量,µg·g−1。
式中:Bf 为富集系数; Cp为植物中PFAAs富集含量, µg·g−1; Cw为水体中PFAAs富集含量, µg·g−1。
采用excel 2003和SPSS18对数据进行处理和分析,采用origin 2018进行图表绘制。
-
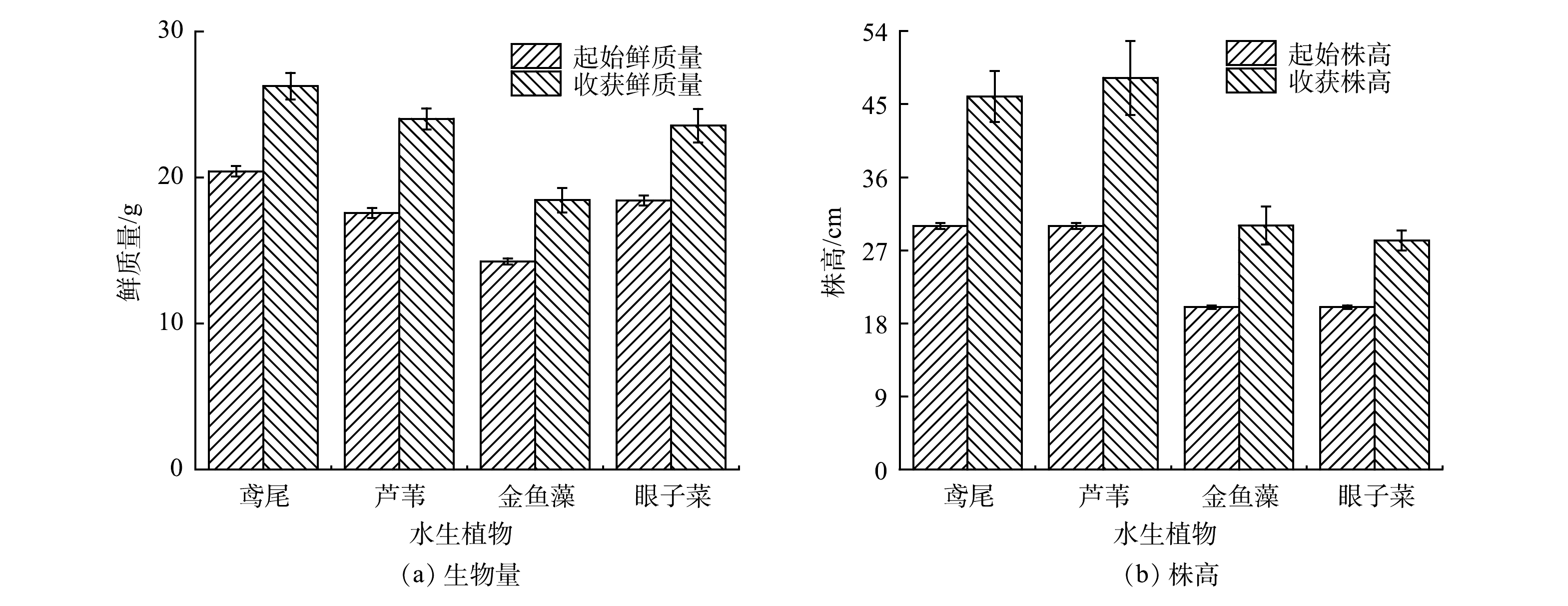
预培养5 d后,4种水生植物均生长良好,在相同的污染水体中培养29 d后,各植物的生长状态呈现差异性,其生物量和株高的变化如图1所示。所有植物的生长量和植株高均有所增加。这表明该水体的污染程度在所选水生植物的耐受能力范围内。其中,芦苇的生长状况最好,生物量增加了(6.45±0.72) g,增长率为(36.71±1.06)%;其余3种水生植物生物量变化相差不大,增长率为27.78%~29.43%。除眼子菜外,各水生植物的株高增高均大于10 cm,表明其对该复合污染水体的耐受能力较强。
-
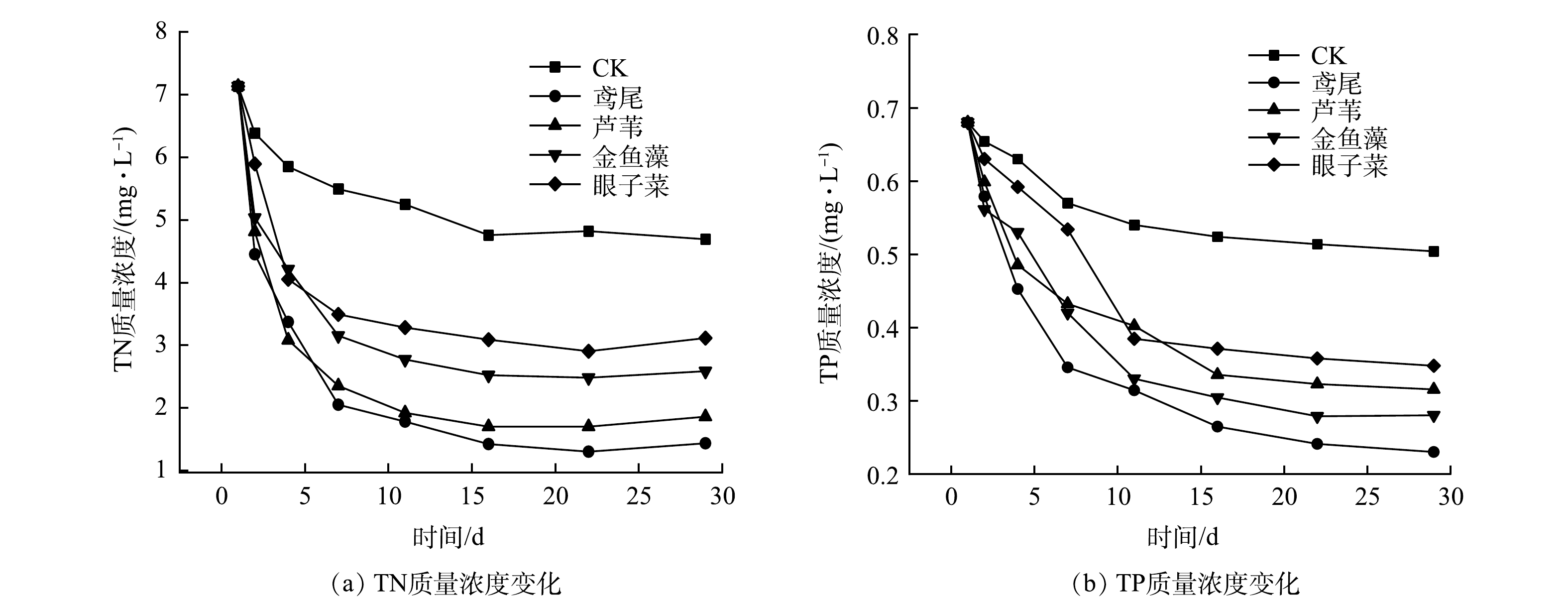
实验过程中,各植物处理组水体TN质量浓度变化如图2(a)所示。随着处理时间的延长,水体中TN质量浓度呈现先快速下降后趋于稳定的趋势,实验开始16 d后TN质量浓度基本稳定,下降幅度不明显。其中,鸢尾对TN的去除效果最好,水体最终TN质量浓度为1.43 mg·L−1;芦苇去除效果次之,TN质量浓度由7.13 mg·L−1降至1.86 mg·L−1,均达到地表水环境质量标准中的V类水标准(GB 3838-2002);金鱼藻和眼子菜处理后的水体中TN质量浓度略高,但TN去除率均能达到50%。在此复合污染水体中,4种水生植物对TN的去除效果表现为挺水植物优于沉水植物,其中鸢尾对水体中TN的去除率最高,为(79.90±4.79)%,比芦苇、金鱼藻、眼子菜的TN去除率分别高出5.95%、16.15%、23.54%。
各植物处理组水体中TP质量浓度变化与TN类似,但下降趋势较为平缓(图2(b))。在前16 d,水体中TP质量浓度变化幅度较大,不同水生植物对TP的去除效果有所不同。对照组中TN质量浓度的下降可能是因为原水中微生物的氨化、硝化、反硝化作用, TP质量浓度的下降可能是因为水体中颗粒态磷的沉降作用。相比之下,眼子菜和芦苇处理的水体中TP 质量浓度较高,但低于对照组,表明其对TP具有一定的去除能力。水体中TP质量浓度最低的是鸢尾处理组,金鱼藻处理组次之,表明鸢尾对TP的去除效果较好。在本研究的复合污染水体中,4种水生植物对TP的去除效果表现为鸢尾>金鱼藻>芦苇>眼子菜(表2)。
-
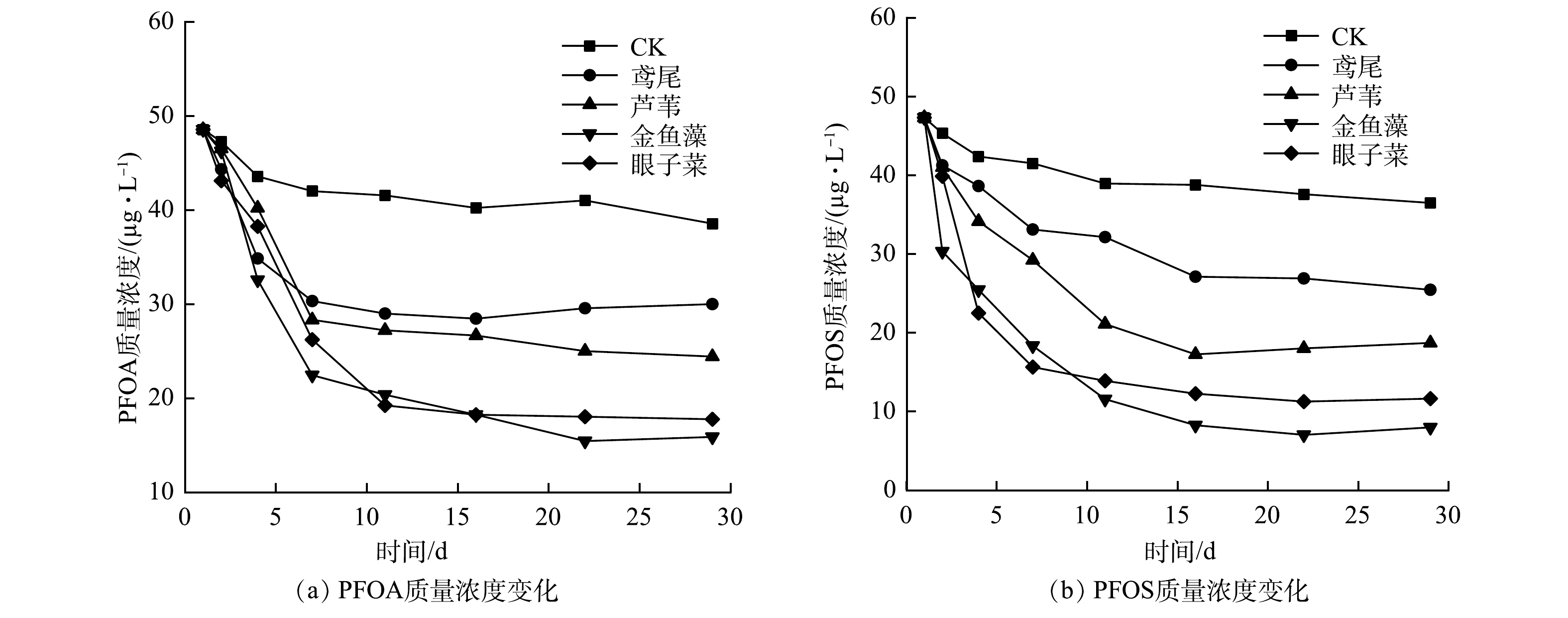
1)水体中PFOA、PFOS质量浓度的变化。实验开始时PFOA、PFOS的加标质量浓度为60 µg·L−1,初始污染水体中PFOA/PFOS的测定平均值为48.56 µg·L−1和47.32 µg·L−1,平均加标回收率为80.93%和78.86%。实验过程中PFOA、PFOS的质量浓度变化如图3所示。各植物处理组水体中PFOA、PFOS质量浓度均呈现下降趋势,实验结束后,各植物处理组的PFOA、PFOS质量浓度均显著小于对照组。实验开始后,各植物处理组水体中PFOA质量浓度在前16 d缓慢下降,16 d后趋于稳定,其中鸢尾处理组水体中PFOA质量浓度较高。各植物处理组水体中PFOS质量浓度的下降速度先快后慢,尤其是金鱼藻处理组,经过7 d的处理,水体中PFOS质量浓度从47.32 µg·L−1降至18.33 µg·L−1,但在7~29 d其变化幅度则较小,仅为10.35 µg·L−1。相较于其他几种植物,金鱼藻处理组水体中PFOS的质量浓度最低。
由表3可以看出,4种水生植物对PFOA、PFOS具有一定的富集效果,且相互之间具有差异性。各植物处理组对PFOA的去除率为(38.25±3.25)%~(67.33±5.58)%,显著高于CK处理组的(15.54±0.59)%。各植物处理组对PFOA的去除率高低排序依次为:金鱼藻>眼子菜>芦苇>鸢尾。各植物处理组对PFOS的去除率高低趋势与PFOA相同,去除率从高到低依次为(83.14±5.49)%、(75.43±5.34)%、(60.47±4.29)%和(46.23±3.93)%。不同植物组之间的差异性整体表现为沉水植物对PFOA、PFOS的富集效果强于挺水植物,沉水植物对PFOA、PFOS的去除率分别比挺水植物高13.73%~29.03%和14.96%~36.91%。
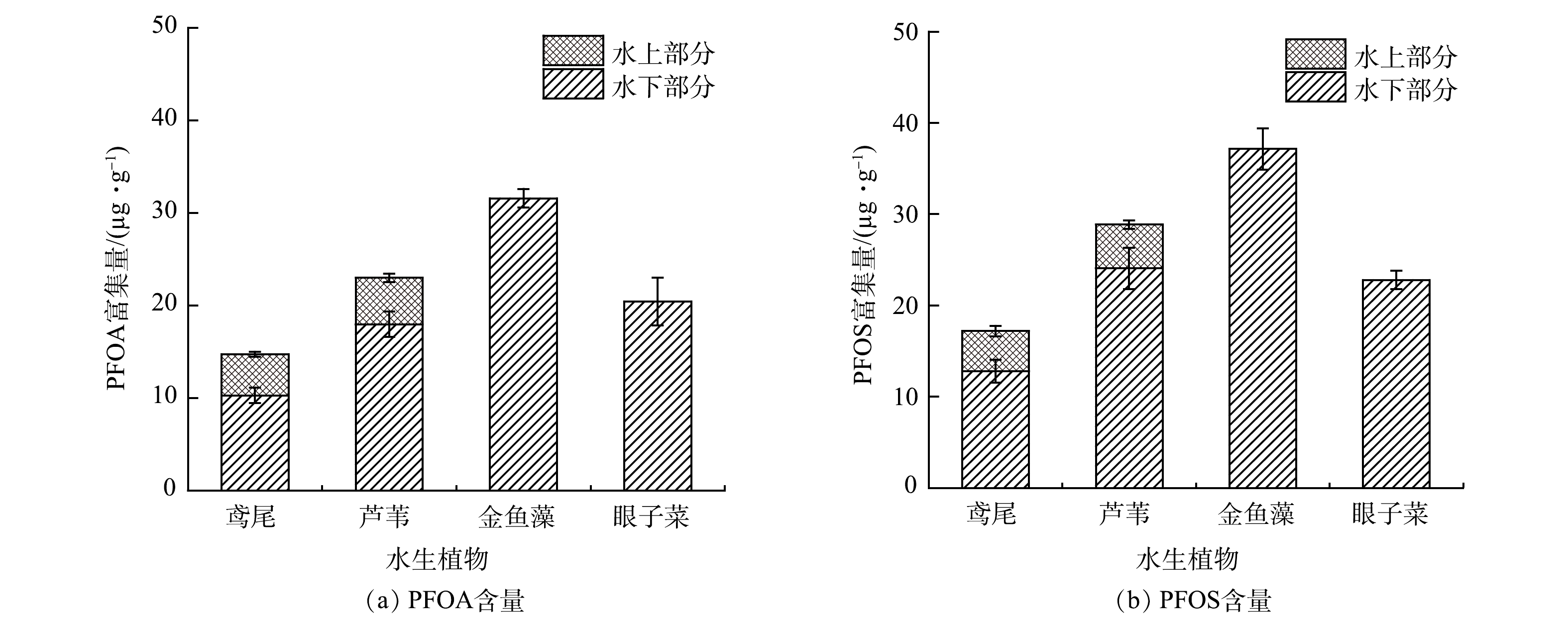
2)供试植物中PFOA、PFOS含量的变化。供试植株由采购市场通过营养液人工培养,实验前植物体内未检测出全氟烷基酸,实验结束后,植物体内PFOA、PFOS的积累量如图4所示。同一水体经过相同培养时间后,植物体内PFOA、PFOS的富集量各不相同。同一植物对不同毒性物质的耐受上限有所差异,不同植物对相同毒性物质的吸收能力也是不同的。当水体中同时存在多种污染物时,营养物质与毒性物质会发生协同效应,这些都会导致植物体内PFOA、PFOS积累量的差异。
由图4(a)可以看出,在相同培养条件下,单棵水生植株对PFOA的积累量表现为金鱼藻>眼子菜>芦苇>鸢尾,其体内PFOA的含量差异与去除率相对应。金鱼藻对PFOA的富集含量高达(31.56±1.01) µg·g−1,约是鸢尾富集的5倍多。由图4(b)可以看出,不同植物体内PFOS的富集含量差异与PFOA相似,鸢尾的富集能力相对较差,其体内PFOS富集质量浓度为(17.19±1.06) µg·g−1,金鱼藻、眼子菜、芦苇对PFOS的富集含量分别比鸢尾高出(30.65±2.25)、(16.28±1.02)和(2.16±0.53) µg·g−1。
由图4可知,各挺水植物水下部分对PFOA、PFOS的富集浓度显著大于其水上部分。其中,鸢尾水下部分PFOA富集含量为(10.27±0.85) µg·g−1,是水上部分的2倍多;芦苇水上部分与水下部分PFOA含量差异大于鸢尾,其水下部分PFOA富集含量为(17.95±1.38) µg·g−1,是水上部分的3.56倍。2种挺水植物水上部分与水下部分PFOS的富集浓度差异趋势与PFOA相似。其中,芦苇水下部分PFOS富集含量较高,为(24.05±2.26) µg·g−1,其水上部分含量仅为(4.81±0.45) µg·g−1。本实验中,植物对PFOA、PFOS富集能力变现为芦苇水下部分>鸢尾水下部分>金鱼藻>眼子菜>芦苇水上部分>鸢尾水上部分。
4种水生植物对PFOA、PFOS的富集系数和转移系数如表4所示。整体看来,沉水植物对PFOA、PFOS的富集系数均大于1,表明其具有较好的污染物富集能力。在挺水植物中,水上部分的富集系数很小,均小于1;水下部分富集系数大于水上部分,其中,芦苇根部对PFOS的富集系数为1.29。相应地,鸢尾和芦苇对PFOA、PFOS的转移系数均低于1,且对PFOS的转移系数小于PFOA。
-
氮磷是植物的营养元素,但若两者含量过高,也会对植物的生长产生胁迫作用,甚至会导致植物的衰亡和消退。本研究的用水为高富营养化水体,实验结束时各植物生物量均有所增加。这表明该水体中各污染物浓度未超过所选植物的耐受浓度。挺水植物中,芦苇生物量的增长大于鸢尾,与胡世琴等[16]研究结果相反。这可能是因为,本研究中植物的生长受到PFOA、PFOS的胁迫作用,而芦苇对PFOA、PFOS的耐受能力更强。沉水植物中,金鱼藻生物量的增长大于眼子菜,与樊恒亮等[17]研究结果一致。这是因为,在高富营养化水体中,金鱼藻比眼子菜更具生长优势。
本研究结果表明,选用的水生植物对复合污染水体中的营养盐去除率较高,但由于对氮磷的去除机制不同,供试植物对氮磷的去除效果存在一定的差异性。不同植物对氮的去除率要高于磷,并且生物量较小的鸢尾和眼子菜对氮的去除效果较好。这是因为,在植物修复水体过程中,虽然N的去除包括植物的吸收作用,但主要去除机制还是硝化、反硝化等微生物作用[18],N的去除更受植物根区环境和根系微生物生长情况的影响。而金鱼藻和鸢尾在对磷的去除上表现出一定的优势,因为磷的去除主要通过沉淀吸附及植物的吸收作用,生物量和竞争吸附质则是本实验中除磷的主要影响因素。本研究中,植物对氮磷的去除率比金树权等[19]的研究结果低,这可能是因为水体中多种污染物的竞争作用。
本研究结果表明,所选择的水生植物均对PFOA和PFOS具有一定的富集作用,但由于植物种类不同,各植物对PFOA、PFOS的富集效果存在一定的差异。如表4所示,金鱼藻对PFOA、PFOS的富集系数为1.07~4.05,鸢尾和芦苇的富集系数为0.27~0.74,沉水植物对PFOA、PFOS的积累能力强于挺水植物。这主要是由于沉水植物对污染物的吸收不仅包括根系的吸附转运作用,还包括叶子表面的直接交换作用。该研究结果与蒋曌泽等[20]检测河道原位植物体内全氟化合物含量的结果相一致,表明沉水植物在净化含氟水体时更具有优势。金鱼藻在高富营养化条件下生长较快,生物量大于眼子菜,从而能增强其对PFOA、PFOS的吸收能力。孔潇潇等[11]的研究表明,在含单一全氟化合物的水体中,PFAAs在金鱼藻中的积累量显著高于眼子菜,并且金鱼藻在去除长链PFAAs时比眼子菜更有优势。
挺水植物不同部位对PFOA、PFOS的富集能力存在明显差异。由图4可以看出,PFOA、PFOS在挺水植物体内的分布趋向于在根部积累。这表明挺水植物主要通过根系从污染水体中吸附全氟烷基酸,与ZHANG等[21]的研究结果相符。长链PFASs对植物根系的吸附亲和力更强,而短链PFASs更容易在植物茎叶中积累。由表4可以看出,鸢尾和芦苇的根部对PFOA、PFOS的富集系数为0.34~1.29,显著大于茎叶部分的富集系数0.14~0.26,并且转移系数都很小。这表明,富集在根部的PFOA、PFOS不易向茎叶转移,采用植物去除污染水体中的长链全氟烷基酸时,需定期清理植物根部。芦苇对PFOA、PFOS的富集能力要强于鸢尾。这是因为PFOA、PFOS作为长链化合物,对植物的富集能力与其根部性质密切相关,而芦苇根须长、通气组织发达,有助于增加根的孔隙度、氧转移和光合速率,故可使其在污水中能够较好地吸附污染物。
本研究中的4种水生植物对PFOS的去除率均高于其对PFOA的去除率,这与WANG等[14]的研究结果相一致。全氟碳链长度、疏水性值(logkow)和阴离子官能团类型是影响全氟化合物在植物体内积累的3个主要理化性质。PFOA与PFOS链长相同,但PFOS的疏水性(logkow=5.02)更高,且磺酸官能团具有较好的亲脂性,使其能够通过植物细胞膜的脂质双层积累在植物体内。从芦苇和鸢尾根部PFOA、PFOS的含量可以看出,植物根部对PFOS的富集能力远大于其对PFOA的富集能力,这可能是由2种物质在植物根部的运输机制不同所致。WEN等[22]的研究表明,PFOA和PFOS均是通过阴离子通道进入玉米根部但途径不同,二者非竞争性效应,PFOA通过慢阴离子通道输运而PFOS通过快阴离子通道运输。不仅水生植物对PFOS的富集效果优于对PFOA的富集效果,WANG等[23]的研究表明,莴苣、玉米、番茄、卷心菜等陆生蔬菜也表现出相同的趋势。
-
1)在营养盐和全氟烷基酸复合污染水体中,4种水生植物对营养盐的去除效果显著,且不同植物之间有所差异,其中鸢尾对营养盐物质的去除表现出较好的优势。
2)在营养盐和全氟烷基酸复合污染水体中,4种水生植物可以有效富集水体中的PFOA和PFOS,其中沉水植物金鱼藻和眼子菜对水体中全氟烷基酸具有较好的去除效果。
3) 4种水生植物对PFOA和PFOS的富集能力有所差异。金鱼藻的富集能力最强,挺水植物中PFOA和PFOS主要积累在植株根部,且PFOS比PFOA更难向植株上方转移。
4种水生植物对复合污染水体中营养盐和全氟烷基酸的去除效果
Removal effect of nutrient salts and perfluoroalkyl acids in waterbody with combined pollutants by 4 aquatic plants
-
摘要: 近年来,水体中全氟烷基酸的暴露、来源和去除受到国内外关注,关于植物对全氟烷基酸单一污染水体净化的研究较多,但对于水体营养盐和全氟烷基酸等复合污染治理的研究较为缺乏。为此,选取鸢尾(Iris tectorum)、芦苇(Phragmites australis)、金鱼藻(Phragmites australis)和眼子菜(Potamogetonpusillus)为研究对象,通过室内水培实验研究了4种水生植物对复合污染水体中营养盐和全氟烷基酸(PFAAs)的去除效果。结果表明,4种水生植物对复合污染水体中营养盐和全氟烷基酸(PFAAs)均具有较好的去除能力,且不同植物对各污染物的去除效果有所差异。各植物对TN、TP、全氟辛酸(PFOA)和全氟辛烷磺酸(PFOS)的去除率分别为(56.36±4.83)%~(79.90±4.79)%、(47.36±2.18)%~(64.52±4.78)%、(38.25±3.25)%~(67.33±5.58)%和(46.23±3.93)%~(83.14±5.49)%。鸢尾对营养盐的去除效果最好,对TN和TP的去除率分别为(79.90±4.79)%和(64.52±4.78)%,但对PFOA和PFOS的去除率仅为(38.25±3.25)%和(46.23±3.93)%;金鱼藻对全氟烷基酸的去除效果显著,其植株体内PFOA和PFOS的富集含量分别可达(31.56±1.01) µg·g−1和(37.15±1.54) µg·g−1。所有植物对PFOS的富集效果优于PFOA,且在挺水植物中,PFOS比PFOA更倾向于在植物根部积累。Abstract: In recent years, the exposure, source identification and removal of perfluoroalkyl acids have received extensive attentions. The existing studies mainly focused on the purification effect of perfluoroalkyl acid polluted waterbody by plants, however, few studies paid attention to the nutrient salts and perfluoroalkyl acids polluted waterbody. In this study, Tectorum, Phragmites australis, Phragmites australis and Potamogetonpusillus was selected as research objects. Laboratory hydroponic experiments were conducted to study the removal effect of nitrogen, phosphorus and perfluoroalkyl acids(PFAAs) by four plants. The results showed that all four plants had good purification effect on nutrients and perfluoroalkyl acids (PFAAs) in waterbody with combined pollutants, and different plants presented different removal effects. The removal rates of TN, TP, PFOA and PFOS were (56.36±4.83)%~(79.90±4.79)%, (47.36±2.18)%~(64.52±4.78)%, (38.25±3.25)%~(67.33±5.58)% and (46.23±3.93)%~(83.14±5.49)%, respectively. Iris tectorum showed the best removal effect of TN and TP, and the corresponding removal rates were (79.90±4.79)% and (64.52±4.78)%, respectively, but the removal rates of Iris tectorum on PFOA and PFOS were only (38.25±3.25)% and (46.23±3.93)%, respectively. Phragmites australis showed the best removal effect of perfluoroalkyl acids, the PFOA and PFOS enrichment in the plants were up to (31.56±1.01) µg·g−1 and (37.15±1.54) µg·g−1, respectively. The enrichment effect of PFOS in all plants was better than that of PFOA, and in emergent plants, PFOS was more inclined to accumulate in plant roots than PFOA.
-
Key words:
- phytoremediation /
- polluted waterbody /
- nutrients salts /
- perfluoroalkyl acids
-

-
表 1 实验水生植物种类
Table 1. Species of aquatic plants selected in the experiment
植物名称 科 生活类型 鸢尾 鸢尾科 挺水草本 芦苇 禾本科 挺水草本 篦齿眼子菜 眼子菜科 沉水草本 金鱼藻 金鱼藻科 沉水草本 表 2 不同水生植物对营养盐物质的去除率
Table 2. Removal rate of nutrients bydifferent aquatic plants
% 污染物 CK 鸢尾 芦苇 金鱼藻 眼子菜 TN 29.85±1.79 79.90±4.79 73.95±2.21 63.75±6.05 56.36±4.83 TP 24.44±1.46 64.52±4.78 52.52±3.25 58.99±2.44 47.36±2.18 表 3 不同水生植物对全氟烷基酸的去除率
Table 3. Removal rate of perfluoroalkyl acids by different aquatic plants
% 污染物 CK 鸢尾 芦苇 金鱼藻 眼子菜 PFOA 15.54±0.59 38.25±3.25 49.71±3.18 67.33±5.58 63.44±4.79 PFOS 20.56±1.22 46.23±3.93 60.47±4.29 83.14±5.49 75.43±5.34 表 4 不同植物对PFOA、PFOS的富集系数和转移系数
Table 4. Enrichment and transfer coefficients of PFOA and PFOS in different plants
植物 部位 富集系数 转移系数 BFPFOA BFPFOS TFPFOA TFPFOS 鸢尾 水下部分 0.34 0.50 0.44 0.34 水上部分 0.15 0.17 芦苇 水下部分 0.74 1.29 0.27 0.20 水上部分 0.20 0.26 金鱼藻 水下部分 1.98 4.66 眼子菜 水下部分 1.15 1.96 -
[1] 史亚利, 潘媛媛, 王杰明, 等. 全氟化合物的环境问题[J]. 化学进展, 2009, 21(2): 369-376. [2] 任肖敏, 张连营, 郭良宏. 多溴联苯醚和全氟烷基酸的分子毒理机制研究[J]. 环境化学, 2014, 33(10): 1662-1671. doi: 10.7524/j.issn.0254-6108.2014.10.012 [3] FU J, GAO Y, CUI L, et al. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China[J]. Science Report, 2016, 6: 38039. doi: 10.1038/srep38039 [4] 秦文友, 周云桥, 张梦, 等. 中国东南主要河流表层水中全氟烷基酸的赋存特征及风险评价[J]. 环境化学, 2021, 40(6): 1749-1762. [5] LI Q, ZHANG Y, LU Y, et al. Risk ranking of environmental contaminants in Xiaoqing River, a heavily polluted river along urbanizing Bohai Rim[J]. Chemosphere, 2018, 204: 28-35. doi: 10.1016/j.chemosphere.2018.04.030 [6] 刘洋, 胡筱敏, 赵研, 等. 全氟化合物及其替代品的处理技术[J]. 环境化学, 2018, 37(8): 1860-1868. doi: 10.7524/j.issn.0254-6108.2017122904 [7] LI P, ZHI D, ZHANG X, et al. Research progress on the removal of hazardous perfluorochemicals: A review[J]. Journal of Environmental Management, 2019, 250: 109488. doi: 10.1016/j.jenvman.2019.109488 [8] 洪雷, 丁倩云, 亓祥坤, 等. 吸附法去除水中全氟化合物的研究进展[J]. 环境化学, 2021, 40(7): 2193-2203. doi: 10.7524/j.issn.0254-6108.2020031303 [9] 唐宇力, 钱萍, 张海珍, 等. 8种观赏水湿生植物对重金属Cd和Pb的吸收固定能力[J]. 环境工程学报, 2017, 11(9): 5313-5319. doi: 10.12030/j.cjee.201701060 [10] YIN T, TRAN N H, et al. Biotransformation of polyfluoroalkyl substances by microbial consortia from constructed wetlands under aerobic and anoxic conditions[J]. Chemosphere, 2019, 233: 101-109. doi: 10.1016/j.chemosphere.2019.05.227 [11] 孔潇潇, 王铁宇, 张晓军, 等. 全氟化合物对水生植物的生态效应研究Ⅱ: 金鱼藻对水中PFOS的生物富集及生理响应[J]. 生态毒理学报, 2015, 10(2): 445-453. [12] MUDUMBI J B N, NTWAMPE S K O, MUGANZA M, et al. Susceptibility of Riparian wetland plants to perfluorooctanoic acid (PFOA) accumulation[J]. International journal of phytoremediation, 2014, 16(9): 926-936. doi: 10.1080/15226514.2013.810574 [13] 国家环境保护总局. 水和废水监测分析方法(第四版) [M]. 北京: 中国环境科学出版社, 2002. [14] WANG P, LU Y, WANG T, et al. Occurrence and transport of 17 perfluoroalkyl acids in 12 coastal rivers in south Bohai coastal region of China with concentrated fluoropolymer facilities[J]. Environmental Pollution, 2014, 190: 115-122. doi: 10.1016/j.envpol.2014.03.030 [15] FELIZETER S, MCLACHLAN M S, et al. Uptake of perfluorinated alkyl acids by hydroponically grown lettuce (Lactuca sativa)[J]. Environmental Science & Technology, 2012, 46(21): 11735-11743. [16] 胡世琴. 人工湿地不同植被净化污水效果及其氮磷累积研究[J]. 水土保持研究, 2017, 24(1): 200-206. [17] 樊恒亮, 谢丽强, 宋晓梅, 等. 沉水植物对水体营养的响应及氮磷积累特征[J]. 环境科学与技术, 2017, 40(3): 42-48. [18] LEE C, FLETCHER T D, SUN G. Nitrogen removal in constructed wetland systems[J]. Engineering in Life Sciences, 2009, 9(1): 11-22. doi: 10.1002/elsc.200800049 [19] 金树权, 周金波, 包薇红, 等. 5种沉水植物的氮、磷吸收和水质净化能力比较[J]. 环境科学, 2017, 38(1): 156-161. [20] 蒋曌泽, 王铁宇, 张晓军, 等. 全氟化合物对水生植物的生态效应研究Ⅰ——典型城市河道全氟化合物的暴露水平及植物富集特征[J]. 生态毒理学报, 2015, 10(2): 435-444. [21] ZHANG D, ZHANG W, LIANG Y. Distribution of eight perfluoroalkyl acids in plant-soil-water systems and their effect on the soil microbial community[J]. Science of the Total Environment, 2019, 697: 134146. doi: 10.1016/j.scitotenv.2019.134146 [22] WEN B, LI L, LIU Y, et al. Mechanistic studies of perfluorooctane sulfonate, perfluorooctanoic acid uptake by maize[J]. Plant and Soil, 2013, 370(1-2): 345-354. doi: 10.1007/s11104-013-1637-9 [23] WANG T, YING G, SHI W, et al. Uptake and translocation of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) by wetland plants: Tissue- and cell-level distribution visualization with desorption electrospray ionization mass spectrometry (DESI-MS) and transmission electron microscopy equipped with energy-dispersive spectroscopy (TEM-EDS)[J]. Environmental Science & Technology, 2020, 54(10): 6009-6020. -



 下载:
下载: