-
如果未达标处理的生活废水直接排入河流和湖泊中,其中所含的过量悬浮物、氨氮和磷等污染物会引起河流水体富营养化,从而导致水体耗氧与供氧失衡,自净能力减弱,水质逐渐恶坏,最终形成季节性或终年黑臭水体[1]。黑臭水体不仅使水体失去了原有的使用价值和生态服务功能,也直接威胁到居民的身心健康。
目前,治理黑臭水体的主要方法包括底泥疏浚[2]、人工曝气[3]、生物修复[4]等。底泥疏浚能够有效去除水体中的内源污染物,但其工程量大、成本高,也会对河道底栖生物产生一定负面影响。人工曝气可增加水体中空气或氧气含量,但不适用于重度黑臭水体;同时,此方法还会消耗大量电能。生物修复具有无害、稳定的优点,但对水体中pH和温度等因素要求较高,这极大地限制了其在黑臭水体处理中的应用。因此,寻求一种能够实现同步去除黑臭水体中内源污染物且成本低廉、反应条件温和的治理技术具有重要意义。
絮凝技术在水处理工艺中应用广泛,是去除胶体悬浮物最经济有效的方法。聚合硫酸铁(PFS)作为一种常见的絮凝剂,不仅能通过电荷中和作用使胶体失稳沉降,还能与磷产生沉淀,形成微絮体[5]。而PFS与助凝剂聚丙烯酰胺(PAM)配合使用后,有利于微絮体“桥连”而形成粗大絮体,加快絮体的沉降速度,减少絮凝剂的使用量。但传统絮凝过程仍存在絮凝沉降时间长的缺陷,导致其适用范围受限。磁絮凝技术是指通过向絮体中加入磁粉,然后借助外磁场力作用以实现絮体快速沉降的一项技术[6]。因此,本文通过将一种常用磁粉(MPs,主要成分为Fe3O4)与上述PFS+PAM絮凝剂进行复配,以克服传统絮凝剂沉降时间长的缺点。然而,单纯的絮凝技术难以实现对黑臭水体中氨氮的去除[7],因此,需要联用吸附技术。
壳聚糖是一种天然的高分子材料[8],可用作吸附剂,其分子结构含有大量的—NH2和—OH等活性集团,性质活泼,可以对其进行烷基化、酰化、接枝、质子化等多种改性以增强其吸附性能[9]。但壳聚糖机械强度较低,在使用过程中容易流失。因此,选择一种合适的载体来负载壳聚糖,对提高其稳定性至关重要。众所周知,沸石是一种分布广泛、具有多孔道、高比表面积的硅铝酸盐矿物,其基本结构为硅氧四面体和铝氧四面体。同时,沸石不仅是一种良好的载体,还对水体中NH4+等阳离子具有较好的吸附去除效果[10]。因此,本研究拟将壳聚糖质化改性后与沸石结合,制备成球状吸附剂,以期增加壳聚糖的机械强度和稳定性,用以实现同步去除氨氮和总磷(TP)。
综上所述,本文采用聚合硫酸铁(PFS)、磁粉(MPs)和聚丙烯酰胺(PAM)作为磁复配絮凝剂,质化壳聚糖-沸石(PCZ)作为吸附剂,考察了磁絮凝-吸附技术同步去除黑臭水体中浊度、氨氮和TP的效果,分析了PFS、MPs和PAM的复配使用对浊度和TP的去除机理以及PCZ对氨氮和TP的去除机理,以期为实现黑臭水体中浊度、氨氮和TP的同步去除提供参考。
-
实验所用聚合硫酸铁(PFS)、商用磁粉(MPs,Fe3O4> 99%)、聚丙烯酰胺(PAM)、壳聚糖、人造沸石、盐酸、醋酸、氢氧化钠、氯化铵、磷酸二氢钾均为分析纯。
-
称取10.00 g壳聚糖于0.01 mol·L−1盐酸溶液中静置8 h,分离后用超纯水将固体物质洗涤至中性,干燥、研磨、过筛备用,得到质化壳聚糖(PC)。随后将PC与沸石按1∶1的质量比加入至体积分数为5%的醋酸溶液中,搅拌4 h,形成均匀米白色悬浮液,然后将悬浮液逐滴滴入250 mL 2.00 mol·L−1的NaOH溶液中形成沉淀,将上述沉淀分离并用超纯水洗涤3次,所得产物于1.77 mol·L−1的NaOH溶液中静置8 h后过滤并洗涤至中性,冷冻干燥12 h,获得最终产物质化壳聚糖-沸石(PCZ)。
-
模拟水样:以适量的高岭土、氯化铵和磷酸二氢钾储备液为原料配制成pH=7、浊度为240.00 NTU、氨氮为10.75 mg·L−1和总磷为1.12 mg·L−1的模拟水样。实际水样:在中国湖南省湘潭市排查的24条黑臭水体中,根据地理位置、取样难易程度及污染程度等因素,选择左干渠、和平公园、爱劳渠3个黑臭水体的水样进行实际处理效果研究。
-
1)絮凝实验。取1 L黑臭水样置于ZR4-6型六联混凝搅拌器上,在室温下分别向水样中投加一定剂量的絮凝材料,设置程序为:转速300 r·min−1快速搅拌1 min,再以140 r·min−1中速搅拌2 min,然后以70 r·min−1慢速搅拌10 min,最后在磁铁(100 mm×100 mm×20 mm,0.5 T)的作用下,沉降一定时间(0.5、1、3 、5、10 、20、30 min),取2~3 cm深度处的上清液测定黑臭水体中的浊度和TP。
优化PFS、MPs和PAM的投加量和投配顺序。一段式中PFS+MPs+PAM同时在快速阶段投加;两段式中先在快速阶段投加PFS+MPs(PAM+MPs),再在慢速阶段投加PAM(PFS);三段式中PFS、MPs和PAM分别在快、中、慢速阶段投加。在3中投配方案中,三者浓度一致。
2)絮凝-吸附实验。在室温条件下,取1 L絮凝后的水样,加入一定剂量的吸附剂,设置转速为200 r·min−1,吸附7 h后在上清液2~3 cm深度处取样,样品过0.45 μm滤头,然后测定黑臭水体中污染物的指标。
3)水体指标测试及材料表征。浊度采用SGZ-A浊度计测定;氨氮按照HJ 535-2009《水质氨氮的测定纳氏试剂分光光度法》的方法进行测定;TP按照GB 11893-89《水质总磷的测定钼酸铵分光光度法》的方法测量。采用扫描电镜(FEI Quanta 250 FEG)连接X射线能谱对材料的表面性能进行研究;Zeta电位采用Zetasizer Nano S90型Zeta电位仪进行测量;利用激光粒度仪(Mastersizer 2000)对样品粒径大小进行测试;运用傅里叶红外光谱仪(NICOLET 380)和X射线光电子能谱仪(Thermo Scientific Escalab 250Xi)确定物质结构及成分;采用热重分析仪(model TA Q-6000)探究物质热稳定性;样品的比表面积、直径、体积和孔隙分布特征通过麦克ASAP2020型物理吸附分析仪进行分析。
-
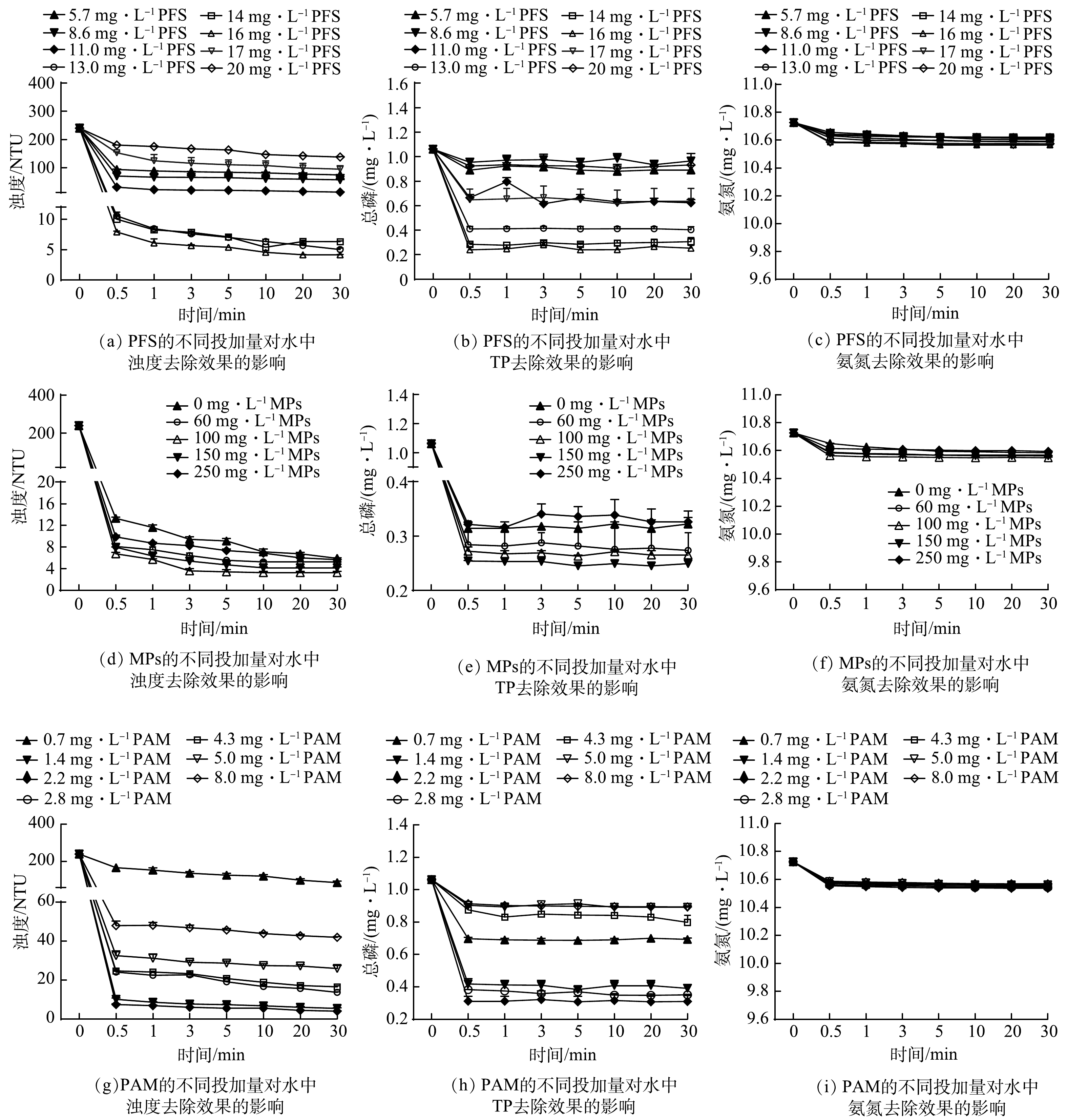
1)单一絮凝材料的投加量对水体中浊度、TP和氨氮去除效果的影响。首先通过絮凝实验探究了PFS (5.70~20.00 mg·L−1)、MPs (0~250.00 mg·L−1)和PAM (0.70~8.00 mg·L−1)的投加量对水体中浊度、TP和氨氮去除效果的影响,结果如图1所示。PFS、MPs、PAM投加量的变化对氨氮的去除效果影响较小。这是因为单独的磁絮凝技术难以实现对黑臭水体中氨氮的去除,在絮凝阶段水体中的氨氮浓度基本没有变化,在其他磁絮凝技术相关文献中也有类似的结果[11-12]。因此,后续磁絮凝实验将着重讨论絮凝剂用量对浊度和总磷去除效果的影响,而氨氮将在后文中通过联合吸附技术加以去除。
由图1(a)~(b)可见,随着PFS投加量的增加,水体中浊度和TP的剩余量呈现先减少后增加的趋势。当PFS投加量为16.00 mg·L−1时,浊度和TP的最低剩余量分别达到4.20 NTU和0.28 mg·L−1,去除率分别为98.25%和73.48%。这可能是由于PFS具有较大的比表面积,在水体中带正电,能够与带负电的污染物结合,从而发挥电荷中和作用使水体失稳;此外,当PFS投加量过少时,胶体表面的絮凝分子过少,难以形成絮体;而过量的PFS会使水体中胶体表面的电荷发生逆转,导致已失稳的絮凝体重新稳定[13]。此外,磷和PFS在废水中会发生一系列复杂的化学反应[14],当废水为中性或弱碱性时,其中所含的磷会以H2PO4−1和HPO42−形式存在,而PFS的水解产物以带正电荷的高聚络合物为主。因此,废水中的磷与PFS的水解产物可以结合生成难溶性的金属磷酸盐络合物,并在网捕卷扫和吸附架桥作用下被去除[13]。而当PFS的投加量过多时,未与磷反应的过量阳离子会在水体中产生电荷排斥作用,从而导致水体中剩余TP含量有所增加。
同样,随着磁粉投加量的增加,浊度和TP的剩余量也呈现先减少后增加的趋势。如图1(d)~(e)所示,当磁粉投加量增至100.00 mg·L−1时,浊度和TP的最低剩余量分别降至3.50 NTU和0.27 mg·L−1,去除率分别为98.54%和74.43%。磁粉的投加,增加了水体中悬浮粒子的数量,使水体中颗粒间的碰撞概率增大,同时形成了以MPs为核心,逐层包裹污染物的磁絮凝体,可增大絮体的比重,加快沉降速度。但当磁粉投加量超过100.00 mg·L−1时,过多的磁粉不仅增加了水体浊度,还会破坏已经形成的絮体[6,15],导致絮凝效果变差。
图1(g)和图1(h)表明,随着PAM投加量的增加,浊度和TP的剩余量同样呈现先减少后增加的趋势。当PAM的投加量增至2.20 mg·L−1后,水体中浊度和TP的剩余量开始逐渐增加。这是因为,适量的PAM可通过其链状分子的桥联絮凝和网捕作用使微絮体尺寸增大,絮凝效果增强。但由于PAM呈现负电性,过多的PAM会使水体中的胶体出现再稳现象,导致浊度和TP的去除率反而降低。因此,本实验确定的PFS、MPs和PAM的最佳投加量分别为16.00、100.00和2.20 mg·L−1。
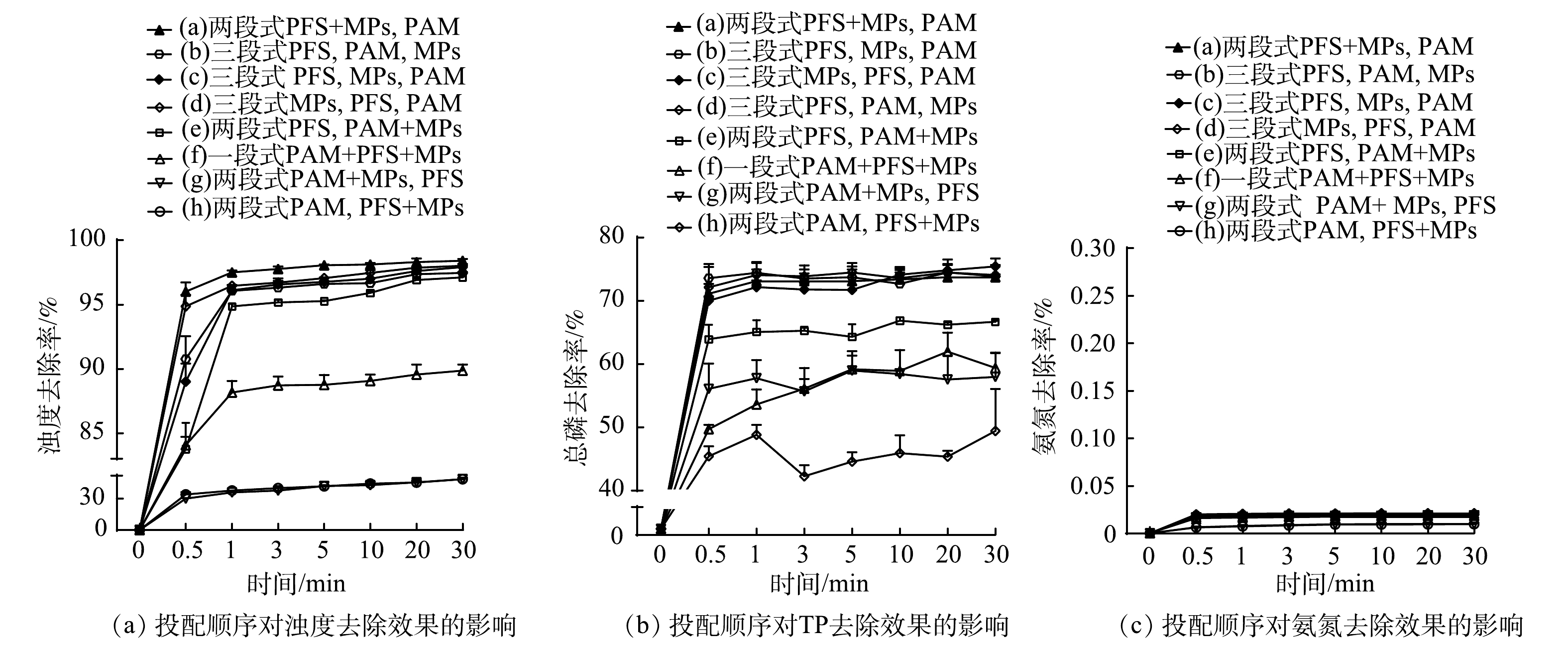
2)联合投加多种絮凝剂时,絮凝剂投配顺序对水体中浊度、TP和氨氮去除效果的影响。当投加多种絮凝剂时,不同的投配顺序可能会使絮凝效果产生明显差异。由图2可见,在几种三段式投配方式中,MPs的投加越早,浊度和TP的去除率也越高。这可能是因为,磁粉的优先投加会使其与絮体的结合更紧密,从而增强对浊度和TP的去除。而越往后添加MPs,会导致其只能附着在絮体的边缘,很难形成以磁核为中心的絮体,在外磁场的作用下,MPs易与絮体分离,从而会降低对浊度和TP的去除效果[6]。另外,一段式投配方式中浊度和TP的去除率也不高。这是因为,絮凝剂的同时投加会使搅拌速率难以确定,影响絮体形成;且PAM投加过早,其高分子结构容易在快速阶段遭到破坏。在两段式投加方式中,当MPs与PAM共同投加时,PAM发挥吸附卷扫作用与水体中的悬浮物发生交联,磁粉也被当作悬浮物桥连团聚,导致磁粉的利用率降低,进而影响磁絮凝效果。而在所有投配方式中,在快速阶段投加PFS+MPs后再在慢速阶段投加PAM 的两段式投配方式的絮凝效果最好,其对水体浊度和TP的去除率分别为98.58%和78.10%。其原因是,PFS投入水体能形成带正电性的水解产物,其可在MPs表面形成静电屏障,有助于MPs的嵌入及均匀分布,从而有利于其与水体中带负电的污染物产生电荷中和,强化絮凝过程。而慢速搅拌阶段加入的PAM作为一种助凝剂可促使微磁絮体通过吸附架桥作用而变大,从而在重力与外磁场的共同作用下实现加速沉降。
-
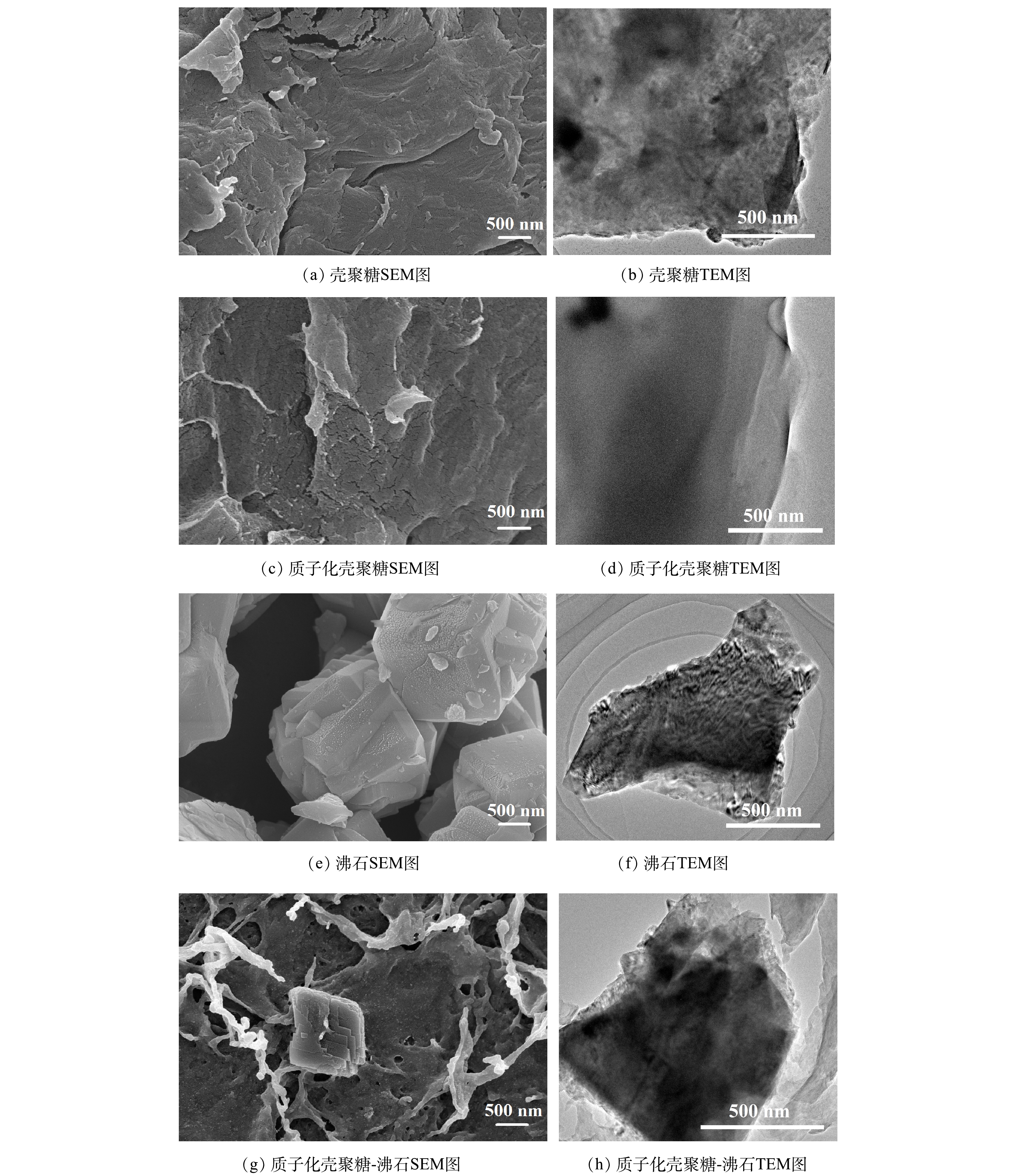
1)质化壳聚糖-沸石(PCZ)吸附剂的表征。由图3(a)~(b)可知,壳聚糖呈板状且表面有部分褶皱。当其经过质子化处理后(图3(c)~(d)),表面并无明显的变化。说明质子化反应对壳聚糖的结构影响很小。图3(e)和图3(f)分别为沸石的SEM图和TEM图。沸石表面的形貌较为规则,且呈均匀分散的块状结构,颗粒尺寸在0.5~2 μm。当质化壳聚糖与沸石结合后(图3(g)~(h)),沸石表面变得更为粗糙。这表明质化壳聚糖已成功负载在沸石上[16]。
质化壳聚糖、沸石和质化壳聚糖-沸石的比表面积、平均孔径和总孔容积结果如表1所示。质化壳聚糖-沸石的比表面积、平均孔径和总孔容积均介于质化壳聚糖与沸石之间。这是因为质化壳聚糖由于桥连机制与沸石表面的羟基相互作用而形成了复合材料[17]。
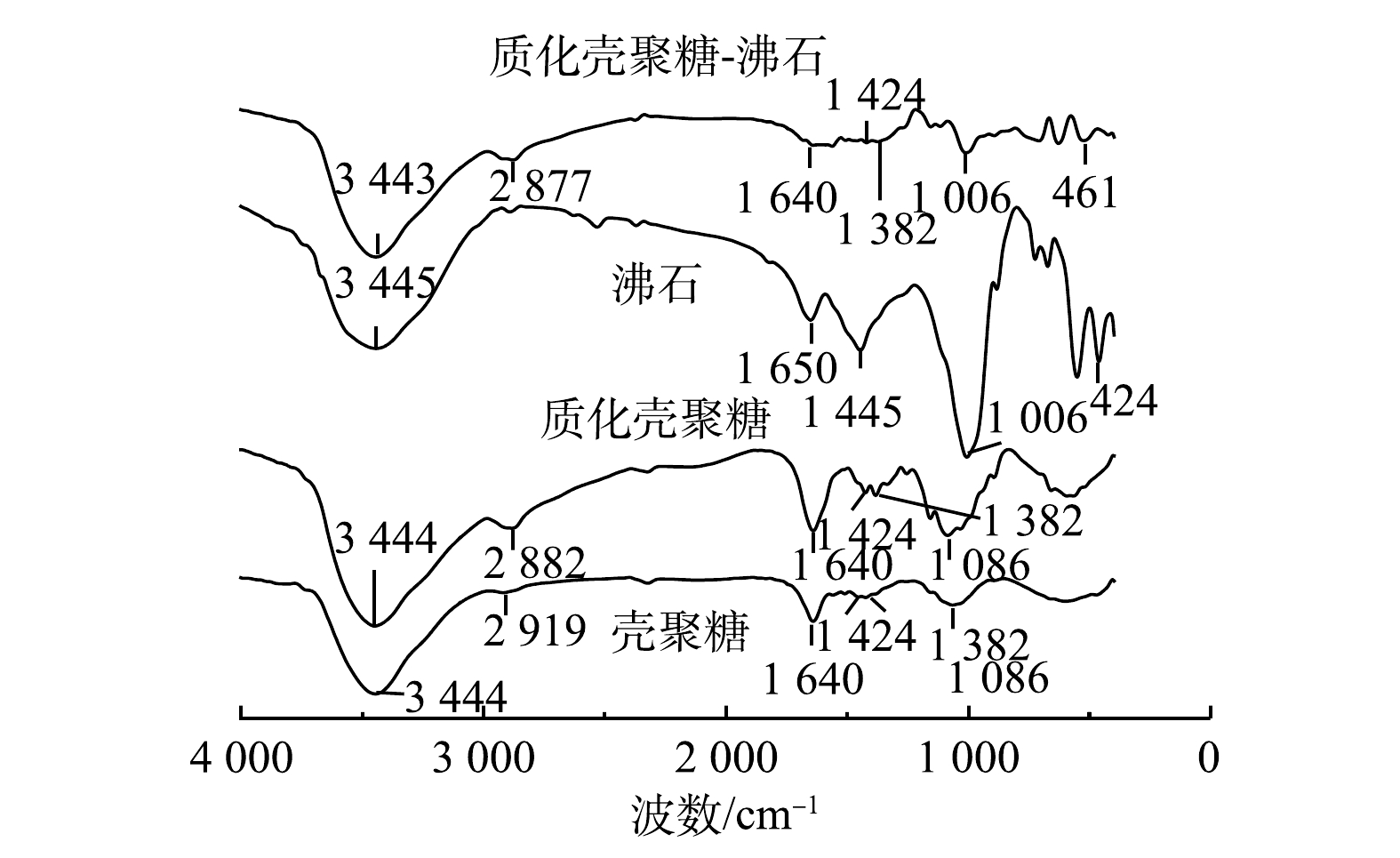
为进一步验证质化壳聚糖已成功负载在沸石上,考察了壳聚糖、质化壳聚糖、沸石和质化壳聚糖-沸石的红外光谱(图4)。壳聚糖在3 444 cm−1处出现了—OH和—NH2的伸缩振动峰,而在2 919 cm−1处的吸收峰为—CH3中的—CH伸缩振动峰,1 640 cm−1处的吸收峰是由—NH2的弯曲振动而引起的。另外,—CN和—COH的伸缩振动峰分别出现在1 424 cm−1和1 086 cm−1处。与壳聚糖相比,质化壳聚糖在1 640、1 424和1 086 cm−1处的吸收峰强度更高。这主要是由壳聚糖在质子化过程中形成的—NH3+基团发生对称变形振动所引起的。该结果表明壳聚糖已成功质化[18]。而人造沸石在3 445 cm−1处的吸收峰是由沸石Al—OH—Si结构中的—OH伸缩振动引起的。1 650 cm−1为H—O—H的弯曲振动峰,400~1 100 cm−1处的吸收峰是由于Si—O(Al—O)的伸缩振动、Si—O—Si的骨架振动、硅氧四面体和铝氧四面体内部的Si—O—Si、Al—O—Si的振动而产生[19]。在质化壳聚糖-沸石的红外光谱中,出现了—NH3+的吸收峰,且在1 006 cm−1和424 cm−1处出现了Si—O(或Al—O)和Si—O—Si、Al—O—Si的伸缩振动带[19]。上述结果进一步证实质化壳聚糖已成功负载在沸石上。
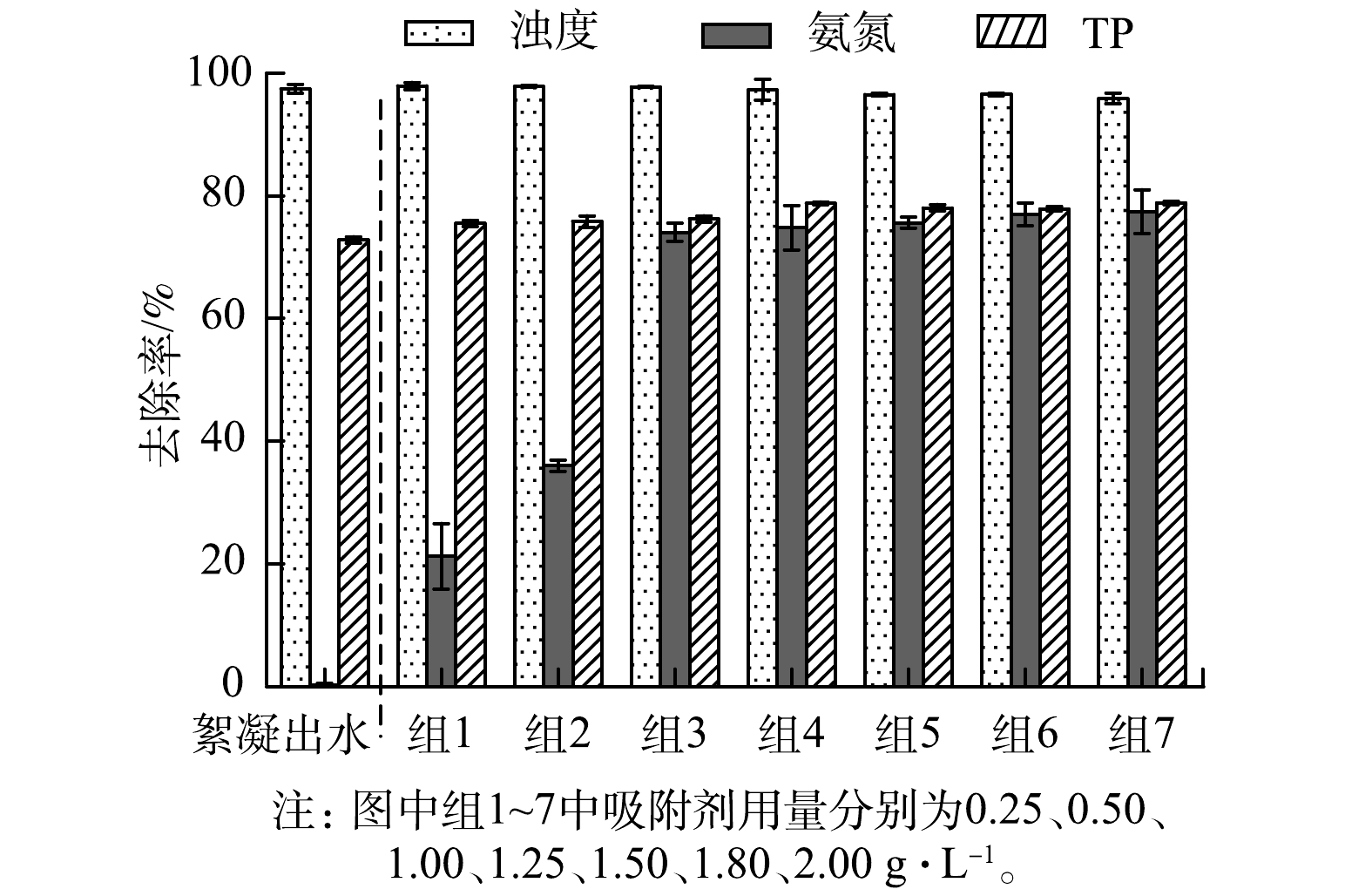
2)吸附剂用量对水体中浊度、氨氮和TP去除效果的影响。在室温条件下,取1 L在上述最优条件下絮凝处理后的出水,分别加入不同质量的PZC,考察其对水体中浊度、氨氮和TP的吸附效果。如图5所示,水体中浊度、氨氮和TP的去除率均随PZC的增加而升高。这主要是因为,随着吸附剂用量的增加,其表面的吸附位点也会随之增加,从而有利于其对污染物的吸附去除。但随着吸附达到平衡,PZC用量由1.25 g增加到2.00 g时,水体中浊度、氨氮和TP的去除率增加并不明显。考虑到经济成本、后期处理等因素,本文选择1.25 g为PCZ的最佳投加量,而此时水样中浊度、氨氮和TP的去除率分别为98.00%、76.30%和77.50%。
-
1)PFS、MPs和PAM的复配使用对水体中浊度和TP的去除机理。为了探究磁粉的引入对絮体结构的影响及其成分的变化,将传统絮凝(只用PAM和PFS为絮凝剂)和PFS、MPs以及PAM三者复配絮凝处理黑臭水体后产生的絮体进行真空干燥,研磨过筛后,进行SEM和FTIR表征分析。SEM结果(图6)表明,磁粉的引入能够增加絮体中悬浮颗粒的浓度,增强颗粒间的有效碰撞,使得结构松散的絮体转变为密实的磁絮体,粘聚效果明显增强,有利于絮体在重力与外磁场力的共同作用下快速分离。
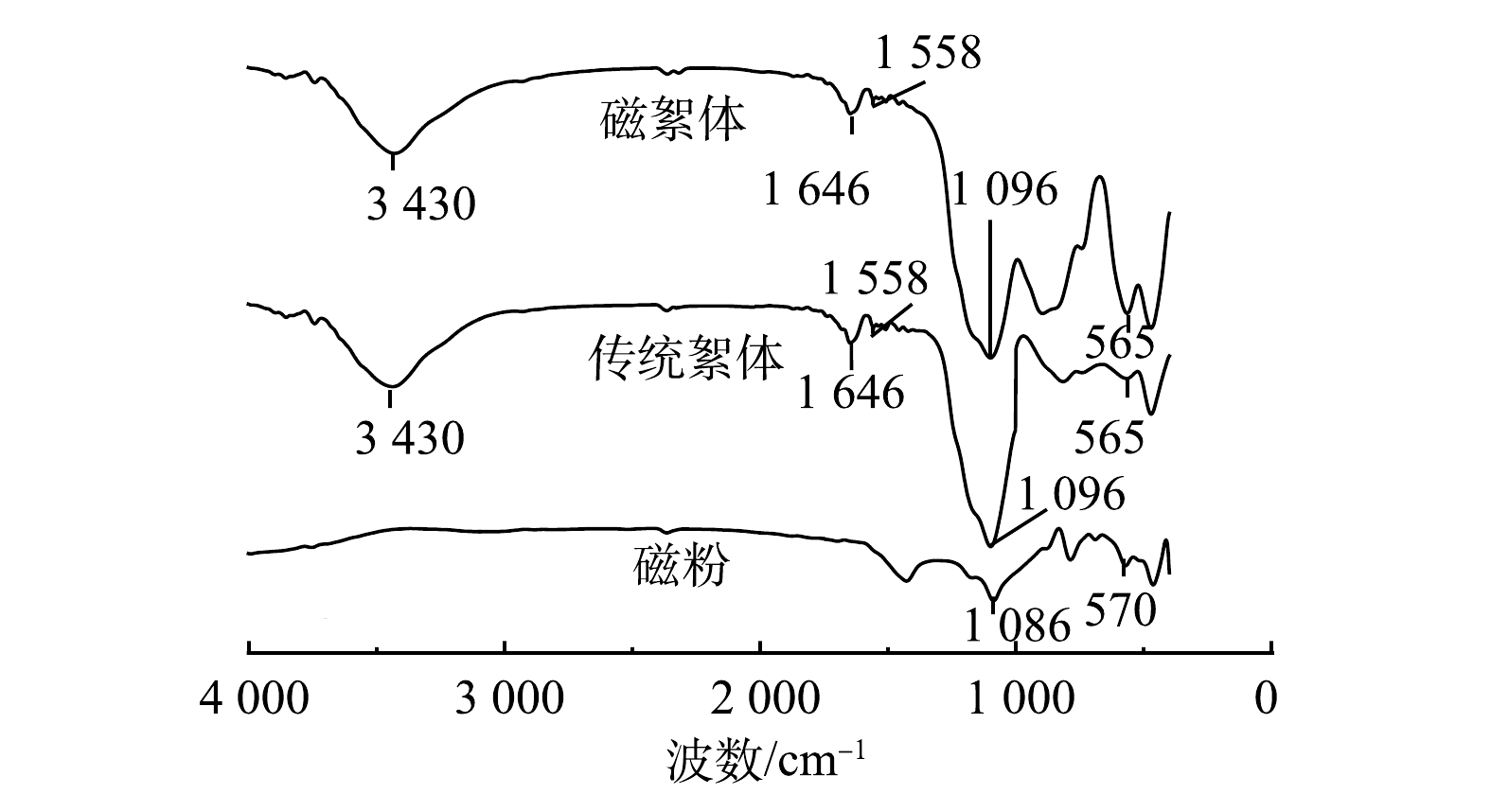
如图7所示,传统絮体和磁絮体在3 430 cm−1处的宽吸收峰是形成氢键的羟基缔合峰,表明其具有明显的羟基配合物结构特征;1 646和1558 cm−1处的吸收峰是絮体吸附或结合水的振动峰,是由PAM的特征性官能团(酰胺键)所产生的[20];1 096 cm−1处的吸收峰主要是由磷酸根中P—O的反对称伸缩振动与Fe—O—H的弯曲振动[21]共同引起;与传统絮体相比,磁絮体在565 cm−1处的吸收峰更强,这主要是由磁粉中Fe—O键弯曲振动和伸缩振动所造成[22]。以上结果表明,磁粉的引入能够增加表面吸附位点,有利于通过化学吸附沉淀实现对磷的去除。
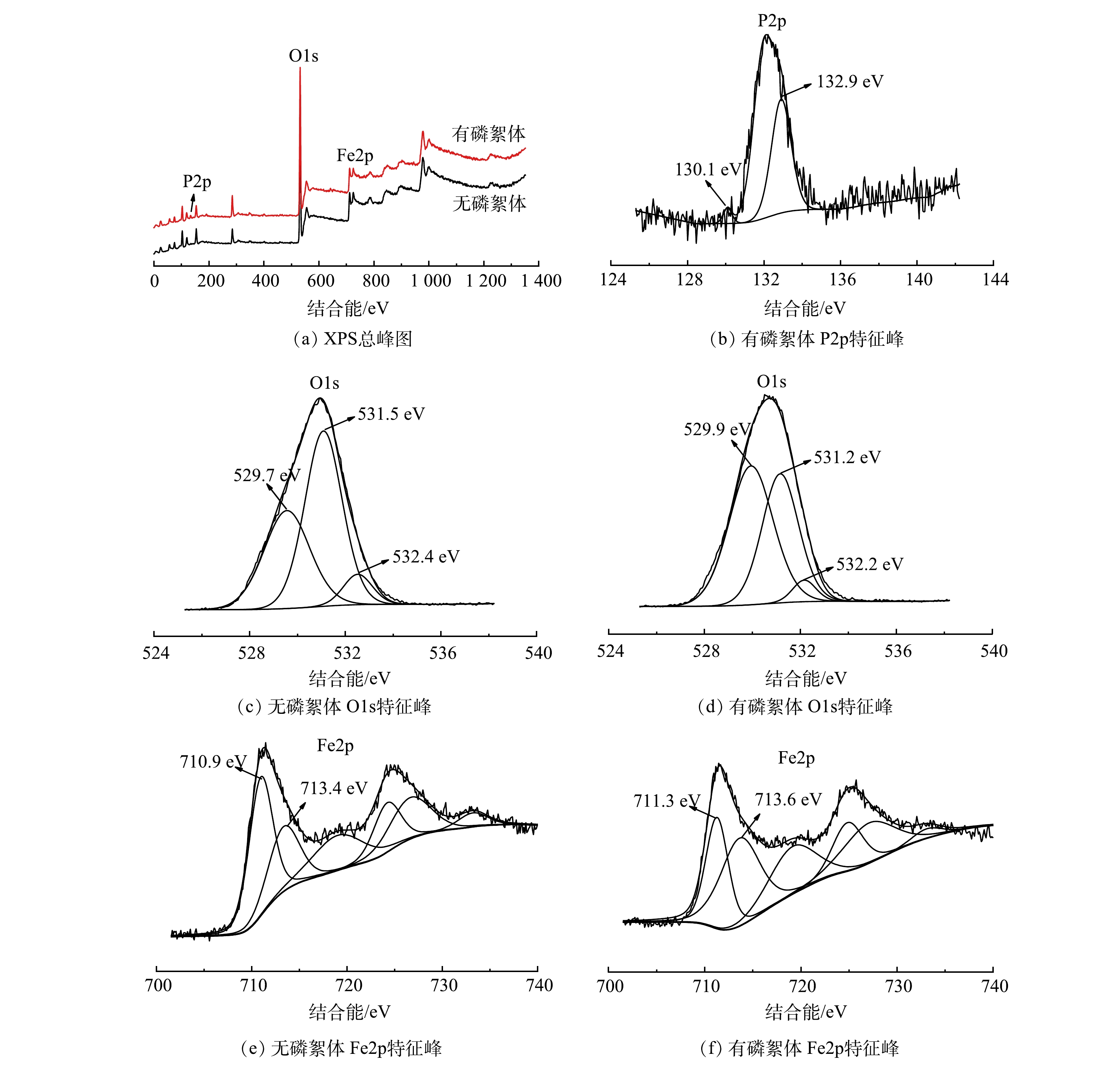
为了进一步了解磁絮凝作用对水体中磷的去除机理,分别对含磷和不含磷的模拟废水(皆含高岭土)进行磁絮凝沉降处理,并将形成的絮体真空干燥后进行XPS分析,结果如图8所示。与无磷絮体相比,有磷絮体上出现了P2p特征峰(图8(a)),证明磷污染物成功絮凝在絮体中。由P2p的XPS精细谱图(图8(b))可知,结合能位于130.1 eV和132.9 eV处的谱峰分别归属于磷化物和Fe—O—P [21,23]。这证实磁絮凝过程中产生了磷酸铁沉淀物。在无磷絮体O1s的XPS图谱中,结合能为529.7、531.5和532.4 eV处的峰分别对应无磷絮体中的Fe—O—H和P—O—H键[24]。相比于无磷絮体,在有磷絮体O1s图谱中,其Fe—O—Fe键的峰向右偏移了0.2 eV,而Fe—O—H键和P—O—H键的峰向左偏移了0.3 eV。这说明絮凝作用中磷酸盐污染物与铁氧体发生了配体交换,形成了铁氧体-磷配合键[21,24]。该结果与FITR的结果一致。相比于无磷絮体的Fe2p XPS图谱,有磷絮体的Fe2p特征峰整体向右微移。这说明在Fe的价带中可能发生了电子转移,且形成了以Fe—O—P为结合方式的络合物[24]。
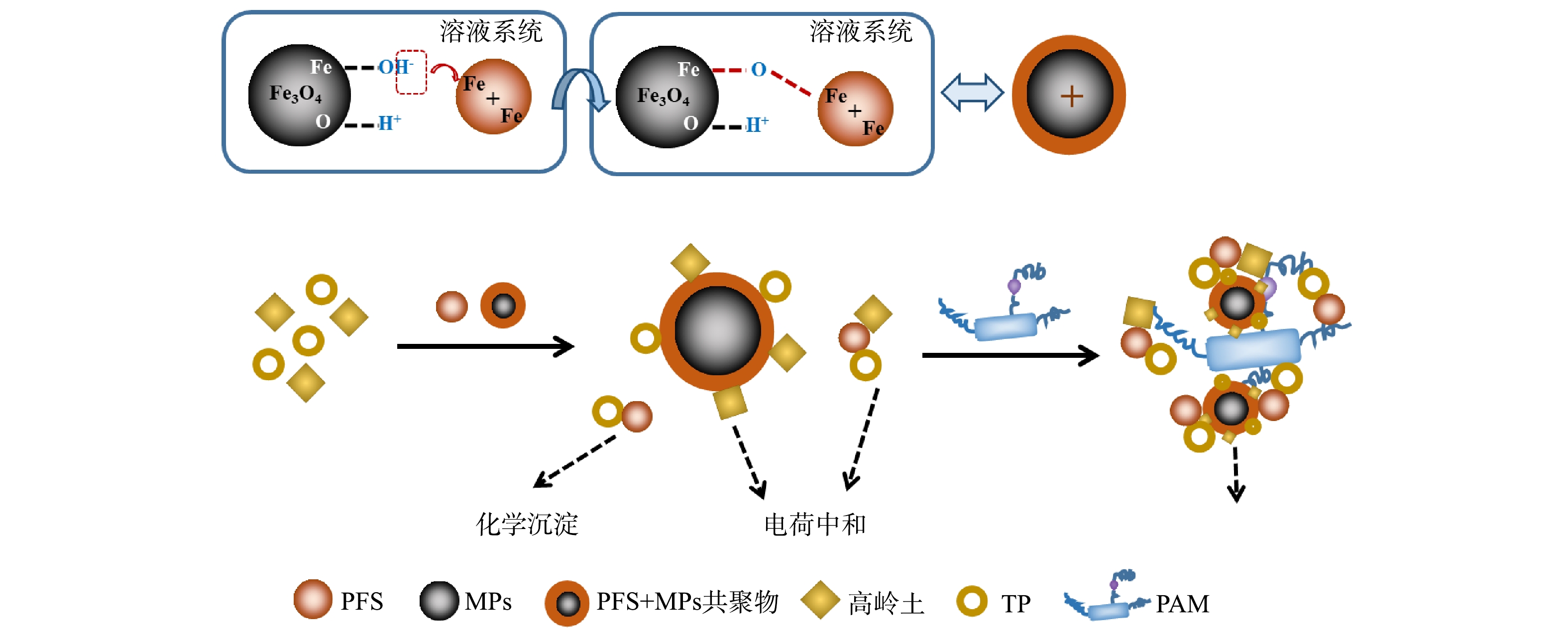
图9为PFS、MPs和PAM复配使用去除浊度和TP的简要机理图。投入PFS后,产生了Fe(OH)2+等水解产物,这些带正电荷的水解产物通过静电吸引环绕在带负电荷的磁粉周围,形成PFS+MPs共聚物,黑臭水体中的悬浮颗粒物通过静电作用吸附在这些共聚物上。而复配絮凝剂对TP的去除不仅因为电荷中和作用,其还会与TP发生一系列复杂的化学反应,形成FePO4沉淀物,从而在PAM作用下形成微磁絮体,并通过吸附架桥作用变大。因此,可在重力和外磁场力的共同作用下快速实现固液分离。
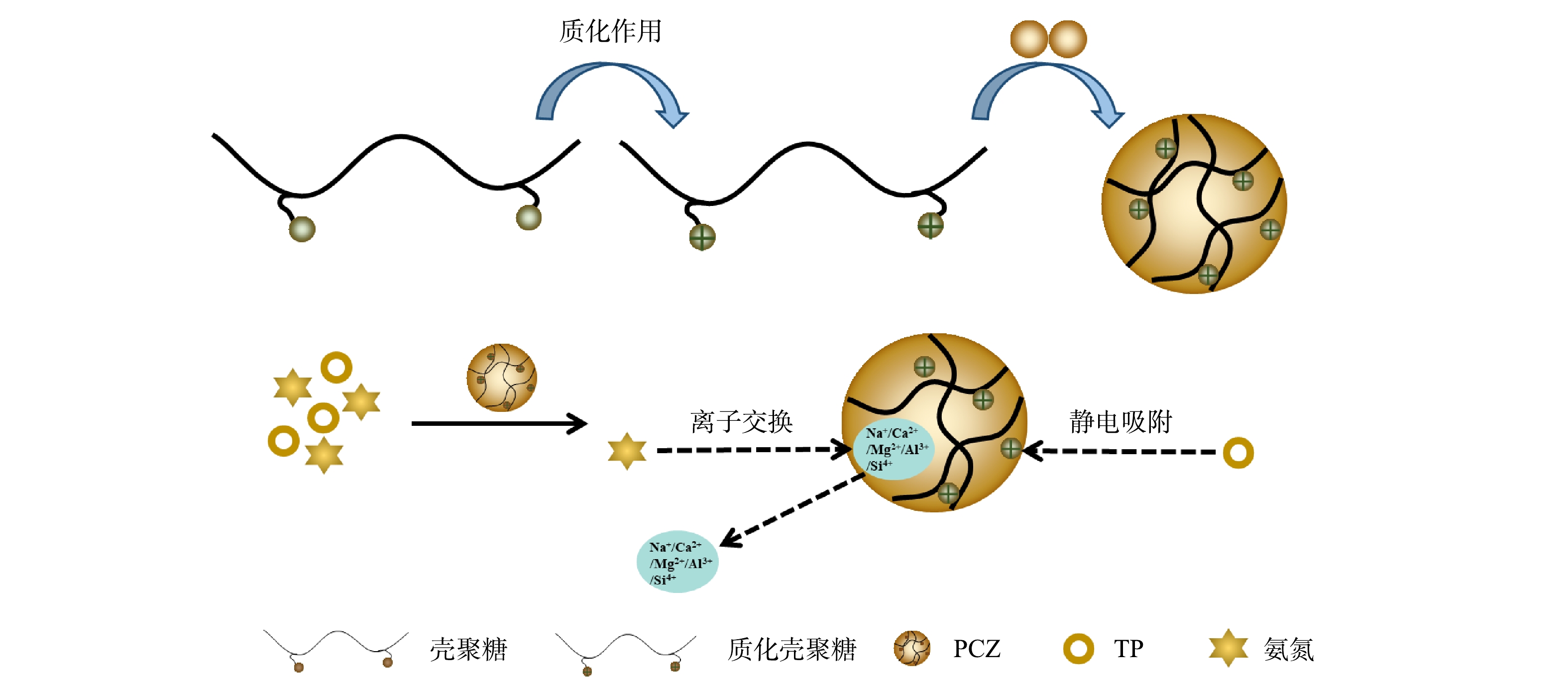
2)质化壳聚糖−沸石吸附剂对氨氮和TP的去除机理。为了解PCZ吸附氨氮和TP前后的结构与成分的变化,将吸附平衡后的吸附剂烘干,研磨过筛后,进行SEM-EDS、FTIR、Zeta测试。由SEM表征结果(图10(a)~(b))可知,质化壳聚糖类似一个黏结剂将沸石连接起来,呈现疏松多孔表面,在吸附氨氮和TP之后,PCZ部分孔隙被堵塞,孔道减少,表面变得更为光滑。PCZ对氨氮和TP吸附前后的EDS(图10(c)~(d))及元素含量(表2)分析表明,PCZ吸附污染物后N元素含量增加了3.09%。这里的N元素主要来源于水体中的氨氮,说明氨氮已经被成功吸附到吸附剂上。同时,吸附污染物后,吸附剂中Na、Mg、Al、Si、Ca的元素含量均有不同程度的降低。这可能是因为这些元素跟水体中的氨氮发生了阳离子交换[25]。而因水体中的磷在经过磁絮凝处理后大部分都得到了去除,所以在吸附段磷含量很低,故而在吸附剂中未能检出。
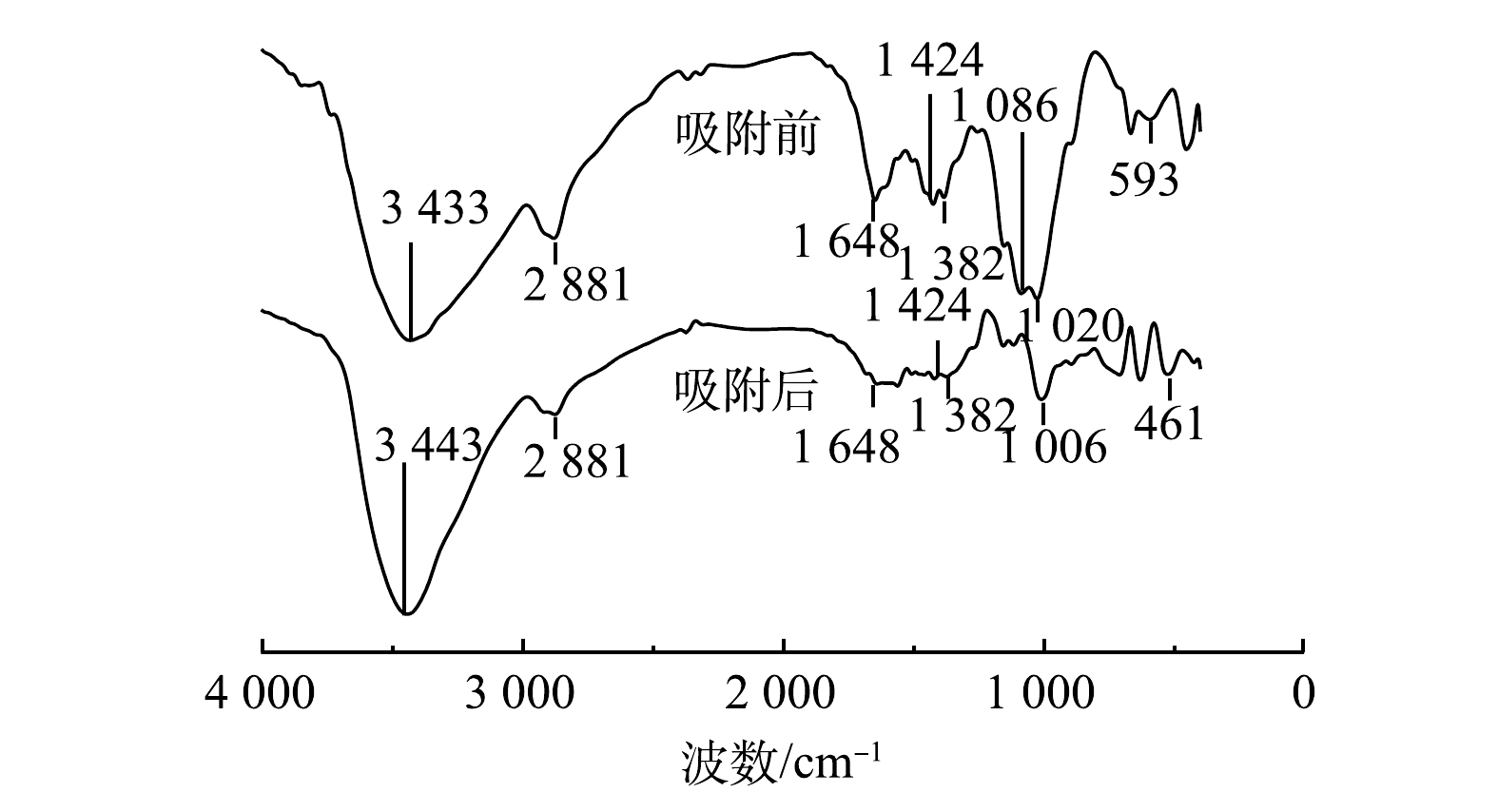
PCZ吸附氨氮和磷酸盐前后的FTIR图谱如图11所示。在3 433 cm−1与3 443 cm−1处的吸收带为—OH的伸缩振动带,此振动带是由吸附剂表面的吸附态水分子或吸附剂结构空腔中阳离子的结合态水分子中的—OH伸缩振动所引起的。当PCZ吸附污染物后,在2 881、1 684、1 424和1 382 cm−1处的吸收峰显著增强,其中在1 424 cm−1处的吸收峰对应于N—H键的对称弯曲振动峰,其峰值的增强说明溶液中更多铵根离子吸附到PCZ上,从而导致废水中氨氮浓度的降低。另外,当PCZ吸附污染物后,在1 020 cm−1和593 cm−1处出现了2个新的吸收峰,分别代表PO的反对称伸缩振动峰和PO2的反对称弯曲振动峰。该结果表明,吸附的磷酸基团可以通过配体交换取代吸附剂表面的羟基[26],同时PCZ中壳聚糖因质子化呈现正电性,这也能会对磷酸根产生静电吸附作用。
由此可见,PCZ吸附去除氨氮和TP的机理可总结如下(图12):壳聚糖通过质子化作用形成质化壳聚糖,与沸石结合后生成吸附剂PCZ。因为PCZ中的各种阳离子能够与水体中的氨根离子发生离子交换,从而可实现对氨氮的去除。同时,PCZ因NH3+的引入而具有正电性,而且随着PCZ对氨氮吸附量地不断增加,在沸石的层间与外表面形成了正电层,因此,磷酸根可通过静电吸附作用单层吸附于PCZ之上,从而实现废水中TP的去除。
-
为了进一步验证本研究中磁絮凝-吸附技术在实际水体中的应用效果,取中国湖南省湘潭市排查的24条黑臭水体中的左干渠、和平公园、爱劳渠水样进行实验。实验水质及处理效果如表3所示。在最优条件下,浊度的去除效果能达到城镇污水处理厂污染物排放一级标准(≤10.00 NTU),TP和氨氮的去除效果能分别满足地表水环境质量Ⅲ类标准(≤0.20 mg·L−1)和Ⅴ类标准(≤2.00 mg·L−1)要求,说明本研究中絮凝−吸附技术对黑臭水体具有较好的实际处理效果。
-
1)在联合投药的前提下,PFS、MPs和PAM的最佳投加量分别为16.00、100.00、2.20 mg·L−1,投配顺序为先投加PFS+MPs,再投加PAM;在最佳磁絮凝条件下,浊度和TP的去除率分别为98.58%和78.10%。吸附剂质化壳聚糖-沸石(PCZ)的最佳投加量为1.25 g·L−1时,在此投加量下絮凝出水中浊度、氨氮和TP的去除率分别可达98.00%、76.30%和77.50%。
2) PFS、MPs和PAM的复配使用对浊度的去除主要通过电荷中和作用,而对TP的去除不仅由于电荷的中和作用,还与TP发生了一系列复杂的化学反应,形成磷酸铁沉淀物;PCZ主要通过离子交换作用去除氨氮,通过静电吸附作用去除总磷。
3)在最佳操作条件下,磁絮凝-吸附联用技术对实际黑臭水体中浊度的去除效果能达到城镇污水处理厂污染物排放一级标准(≤10.00 NTU),对TP和氨氮的去除效果分别能满足地表水环境质量Ⅲ类标准(≤0.20 mg·L−1)和Ⅴ类标准(≤2.00 mg·L−1)。
磁絮凝-吸附技术对黑臭水体中浊度、氨氮和总磷的去除效果及机理
Performance and mechanism of turbidity, ammonia nitrogen and total phosphorus removal from black and odorous water by magnetic flocculation-adsorption technology
-
摘要: 采用磁絮凝-吸附技术开展了同步去除黑臭水体浊度、氨氮和总磷(TP)实验。在磁絮凝阶段,通过聚合硫酸铁(PFS)、磁粉(MPs)和聚丙烯酰胺(PAM)复配使用,利用电荷中和作用去除浊度和TP;同时,利用化学吸附沉淀去除TP;在此阶段中,当PFS、MPs、PAM的投加量分别为16.00、100.00、2.20 mg·L−1且以PFS+MPs在快速阶段先投加,PAM在慢速阶段后投加的顺序投配时,絮凝效果达到最佳。在吸附阶段,吸附剂质化壳聚糖-沸石(PCZ)主要通过离子交换作用去除氨氮以及通过静电吸附作用去除TP;当PCZ的投加量为1.25 g·L−1时吸附效果达到最佳。利用所研究的磁絮凝-吸附技术对实际黑臭水体进行处理,其出水浊度能达到城镇污水处理厂污染物排放一级标准(≤10.00 NTU),TP和氨氮也分别能满足地表水环境质量Ⅲ类标准(≤0.20 mg·L−1)和Ⅴ类标准(≤2.00 mg·L−1)要求。Abstract: In this paper, a magnetic flocculation−adsorption technology was studied for simultaneous removal of turbidity, ammonia nitrogen and total phosphorus (TP) in black and odorous water. At the magnetic flocculation stage, polyferric sulfate (PFS), magnetic powder (MPs) and polyacrylamide (PAM) were jointly used to remove turbidity and TP through charge neutralization, especially to remove TP through chemical adsorption precipitation. In the process of magnetic flocculating, the optimal dosages of PFS, MPs and PAM were 16.00, 100.00 and 2.20 mg·L−1, respectively. Both PFS and MPs were added first at the rapid mixing stage, then PAM was added later at the slow mixing stage, the best flocculation effect occurred. At the adsorption stage, protonated chitosan combined with zeolite (PCZ) was used to remove ammonia nitrogen and TP by ion exchange and electrostatic adsorption. At PCZ dosage of 1.25 g·L−1, the best adsorption performance occurred. When this magnetic flocculation-adsorption technology was used to treat the actual black and odorous water, the turbidity of the treated wastewater could meet the class I standard of pollutant discharge of urban sewage treatment plant (≤10.00 NTU), and TP and ammonia nitrogen could also meet the class III standard (≤0.20 mg·L−1) and class V standard (≤2.00 mg·L−1) of surface water environmental quality, respectively.
-

-
表 1 3种材料的比表面积与孔性质
Table 1. Surface areas and pore properties of three materials
材料 比表面积/(m2·g−1) 平均孔径/nm 总孔容积
/(10−3 cm3·g−1)质化壳聚糖 1.34 17.02 5.70 沸石 2.46 15.01 9.50 质化壳聚糖-沸石 2.24 15.19 8.40 表 2 PCZ对氨氮和TP吸附前后的EDS能谱分析结果
Table 2. EDS analyses of PCZ before and after NH4+ and TP adsorption
元素 吸附前 吸附后 质量分数/% 原子分数/% 质量分数/% 原子分数/% C 23.37 30.24 28.70 34.78 N 10.06 11.16 13.15 13.67 O 51.03 49.56 54.45 49.53 Na 4.98 3.37 0.19 0.12 Mg 2.55 1.63 1.24 0.74 Al 1.87 1.08 0.89 0.48 Si 3.57 1.97 1.14 0.59 Ca 2.57 1.00 0.24 0.09 表 3 实际水体处理前后水质情况
Table 3. Quality of actual water before and after treatment
实际水体 pH 浊度
/NTU正磷盐
/(mg·L−1)总磷
/(mg·L−1)氨氮
/(mg·L−1)左干渠原水
(轻度黑臭)7.50 10.10 0.46 0.49 7.19 左干渠原水
絮凝−吸附后7.62 2.90 0.10 0.10 1.45 和平公园原水
(轻度黑臭)7.20 18.00 0.12 0.14 1.55 和平公园原水
絮凝−吸附后7.34 3.00 0.06 0.09 0.55 爱劳渠原水
(重度黑臭)7.02 54.00 0.38 0.53 13.03 爱劳渠原水
絮凝−吸附后7.26 3.00 0.06 0.09 2.02 -
[1] WANG X, WANG Y G, SUN C H, et al. Formation mechanism and assessment method for urban black and odorous water body: A review[J]. Chinese Journal of Applied Ecology, 2016, 27(4): 1331-1340. [2] 曹承进, 陈振楼, 王军, 等. 城市黑臭河道底泥生态疏浚技术进展[J]. 华东师范大学学报(自然科学版), 2011(1): 32-42. [3] 王海. 城市污水处理厂曝气系统节能降耗影响因素及控制模式研究[D]. 青岛: 青岛理工大学, 2010. [4] ROMANTSCHUK M, SARAND I, PET N T, et al. Means to improve the effect of in situ bioremediation of contaminated soil: An overview of novel approaches[J]. Environmental Pollution, 2000, 107(2): 179-185. doi: 10.1016/S0269-7491(99)00136-0 [5] AGBOVI H K, WILSON L D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater[J]. Carbohydrate Polymers, 2018, 189: 360-370. doi: 10.1016/j.carbpol.2018.02.024 [6] LV M, ZHANG Z H, ZENG J Y, et al. Roles of magnetic particles in magnetic seeding coagulation-flocculation process for surface water treatment[J]. Separation and Purification Technology, 2019, 212: 337-343. doi: 10.1016/j.seppur.2018.11.011 [7] ZHENG H, ZHU S, JIANG T, et al. Investigations of coagulation-flocculation process by performance optimization, model prediction and fractal structure of flocs[J]. Desalination, 2011, 269: 148-156. doi: 10.1016/j.desal.2010.10.054 [8] BHATNAGAR A, SILLANPAA M. Application of chitin and chitosan derivatives for the detoxification of water and wastewater: A short review[J]. Advances in Colloid & Interface Science, 2009, 152: 26. [9] BRATSKAYA S Y, AZAROVA Y A, MATOCHKINA E G, et al. N-(2-(2-pyridyl) ethyl) chitosan: Synthesis, characterization and sorption properties[J]. Carbohydrate Polymers, 2012, 87(1): 869-875. doi: 10.1016/j.carbpol.2011.08.081 [10] LI S, YUAN Z, GONG W, et al. The mechanism study of trace Cr(Ⅵ) removal from water using Fe0, nanorous modified with chitosan in porous anodic alumina[J]. Applied Surface Science, 2015, 328: 606-613. doi: 10.1016/j.apsusc.2014.12.094 [11] 段志辉, 李彦, 李光柱, 等. 磁絮凝深度处理生活污水[J]. 中国农村水利水电, 2019(7): 110-113. doi: 10.3969/j.issn.1007-2284.2019.07.021 [12] 陈瑜, 李军, 陈旭娈, 等. 磁絮凝强化污水处理的试验研究[J]. 中国给水排水, 2011, 27(17): 78-81. [13] 蔡吉祥, 翟露露, 薛江鹏, 等. 聚合硫酸铁对某纺织服装城废水除磷效果的影响[J]. 轻纺工业与技术, 2020, 49(7): 17-18. doi: 10.3969/j.issn.2095-0101.2020.07.007 [14] AHMAD A L, SUMATHI S, HAMEED B H. Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC[J]. Chemical Engineering Journal, 2006, 118: 99-105. doi: 10.1016/j.cej.2006.02.001 [15] AMBASHTA R D, SILLANPää M. Water purification using magnetic assistance: A review[J]. Journal of Hazardous Materials, 2010, 180: 38-49. doi: 10.1016/j.jhazmat.2010.04.105 [16] 柳富杰, 吴海玲, 韦巧艳, 等. 壳聚糖改性沸石对蔗糖溶液中酚酸的吸附性能研究[J]. 现代食品科技, 2021, 37(5): 180-187. [17] FUTALAN C M, KAN C C, DALIDA M L, et al. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite[J]. Carbohydrate Polymers, 2011, 83: 528-536. doi: 10.1016/j.carbpol.2010.08.013 [18] 仉春华, 王文君, 安晓雯等. 质子化壳聚糖的除磷性能[J]. 环境工程学报, 2013, 2: 568-572. [19] JAVAD K, VAHID J. Alginate beads impregnated with magnetic Chitosan@Zeolite nanocomposite for cationic methylene blue dye removal from aqueous solution[J]. International Journal of Biological Macromolecules, 2020, 154: 1426-1437. doi: 10.1016/j.ijbiomac.2019.11.024 [20] LIU H Y, YANG X G, ZHANG Y, et al. Flocculation characteristics of polyacrylamide grafted cellulose from Phyllostachys heterocycla: An efficient and eco-friendly flocculant[J]. Water Research, 2014, 59: 165-171. doi: 10.1016/j.watres.2014.04.022 [21] LI S J, JIANG F, LEI T, et al. Phosphorus removal by in situ sprayed ferric chloride in Dianchi Lake: Efficiency, stability, and mechanism[J]. Process Safety and Environmental Protection, 2019, 131: 320-328. doi: 10.1016/j.psep.2019.09.021 [22] SRASRI K, THONGROJ M, CHAIJIRAAREE P, et al. Recovery potential of cellulose fiber from newspaper waste: An approach on magnetic cellulose aerogel for dye adsorption material[J]. International Journal of Biological Macromolecules, 2018, 119: 662-668. doi: 10.1016/j.ijbiomac.2018.07.123 [23] YANG W Y, ZHANG L, CHEN Y, et al. Improved electrochemical performances and magnetic properties of lithium iron phosphate with in situ Fe2P surface modification by the control of the reductive gas flow rate[J]. Applied Surface Science, 2020, 521: 146-389. [24] SUN D N, HONG X T, WU K M, et al. Simultaneous removal of ammonia and phosphate by electro-oxidation and electrocoagulation using RuO2–IrO2/Ti and microscale zero-valent iron composite electrode[J]. Water Research, 2020, 169: 115-239. [25] YANG K, ZHANG X, CHAO C, et al. In-situ preparation of NaA zeolite/chitosan porous hybrid beads for removal of ammonium from aqueous solution[J]. Carbohydrate Polymers, 2014, 107: 103-109. doi: 10.1016/j.carbpol.2014.02.001 [26] HE Y H, LIN H, DONG Y B, et al. Simultaneous removal of ammonium and phosphate by alkaline-activated and lanthanum-impregnated zeolite[J]. Chemosphere, 2016, 164: 387-395. doi: 10.1016/j.chemosphere.2016.08.110 -



 下载:
下载: