-
近年来,内分泌干扰化合物(endocrine disrupting chemicals,EDCs)对人类健康和环境安全的负面影响受到极大关注[1-3]。双酚A(bisphenol A,BPA)作为一种具有代表性的环境内分泌干扰物质,即使以痕量水平进入水体,也可通过生物积累对人体造成危害[4]。由于传统的物理吸附、生物降解等废水处理方法难以对BPA实现高效降解,因此,迫切需要寻求一种高效且无二次污染的方法去除BPA,光催化技术则具有这种潜在优势。
光催化氧化技术的关键是光催化剂。在众多催化剂中,g-C3N4因其禁带宽度窄(2.7 eV)、稳定性高、成本低廉和毒性低成为国内外研究者高度重视的材料[5]。但g-C3N4比表面积小、光生电子和空穴复合效率高,严重影响了其光催化活性,对其进行改性或与其他半导体复合构建异质结是提高光催化性能的有效方式[6]。SINGH等[7]制备了SnO2/g-C3N4复合材料,光反应1.5 h后对罗丹明B的去除率可达98.73%。WANG等[8]合成了ZnO/g-C3N4异质结,促进了光生电荷的产生和分离,在可见光下降解四氯苯酚的光催化活性增强。BI等[9]制备了g-C3N4/TNTs复合材料,在可见光下降解罗丹明B的性能得到大幅提升,这归因于构建了直接Z型异质结。磷酸银因具有较高的量子产量(高达90%),表现出优异的光催化氧化能力,但其实际应用受到光腐蚀和溶解度低的影响[10]。基于g-C3N4的高稳定性以及Ag3PO4的高量子产率,将Ag3PO4与g-C3N4复合构筑异质结,既能提高光催化效率,又可以解决Ag3PO4容易光腐蚀的问题。RAEISI-KALLIABADI等[11]研究了Ag3PO4/g-C3N4光催化降解利福平;HU等[12]研究了Ag3PO4/g-C3N4光催化降解甲基橙和四环素;WANG等[13]研究了Ag3PO4/g-C3N4光催化降解罗丹明B。这些研究结果表明,将Ag3PO4与g-C3N4 复合,可增加对可见光的吸收并可产生更多的活性位点,从而促进光生电子-空穴对的分离和传输,还可增强对可见光的吸收,使得Ag3PO4/g-C3N4的光催化活性优于单一的g-C3N4。有研究者发现,在光催化剂中加入小尺寸的石墨相氮化碳量子点(CNQDs)可以提高光催化性能[14-16]。因此,本研究利用CNQDs进一步修饰Ag3PO4/g-C3N4复合材料,采用溶剂热法和原位沉淀法制备了一系列不同质量比的CNQDs/Ag3PO4/g-C3N4复合材料;通过XRD、SEM、FT-IR、XPS、TEM、BET等分析手段表征了材料的晶型结构、微观形貌及其化学价态;此外,考察了这些复合材料降解BPA的光催化活性和稳定性、主要活性物种和关键中间产物,以期为开发新型光催化剂和建立双酚A降解方法提供参考。
-
试剂:三聚氰胺、硝酸银、磷酸氢二钠(国药控股化学试剂有限公司);硝酸和硫酸(湖南惠宏试剂有限公司);无水乙醇、对苯醌、乙二胺四乙酸二钠、叔丁醇(天津光复科技发展有限公司);无水甲醇、氢氧化钠(天津市科密欧化学试剂有限公司);双酚A(阿拉丁试剂有限公司)。上述实验药品均为分析纯,实验用水为超纯水。
仪器:X射线衍射仪(D/Max 2500,日本理学公司);X射线光电子能谱(K-Alpha,美国赛默飞公司);场发射电子显微镜(JSM-6610LV,日本电子株式会社);气相色谱-质谱联用仪(7980B-5977,美国安捷伦科技有限公司);高分辨透射电子显微镜(Tecnai G2F20,美国FEI公司);傅里叶变换红外光谱仪(Nicolet 380,美国Thermo公司);比表面积-孔径分析仪(NOVA-2200c,美国Quantachrome公司);高效液相色谱(AJL-1260,美国安捷伦科技有限公司)。
-
采用二次煅烧法制备g-C3N4:将三聚氰胺放入马弗炉煅烧,以5 ℃·min−1的速度升温至520 ℃,保温4 h;继续升温至550 ℃,保温3 h;研磨过筛,得到淡黄色固体即为g-C3N4,标记为CN。取2 g制备好的块状g-C3N4,加入硝酸和硫酸酸化,超声剥离20 h后,洗涤数次以去除酸,干燥得到超薄g-C3N4纳米片。取g-C3N4纳米片1 g于70 mL超纯水中,超声分散1 h后转移至高压反应釜,200 ℃保温10 h,得到CNQDs溶液。
采用原位沉积法和溶剂热法制备CNQDs/Ag3PO4/g-C3N4:将g-C3N4分散在超纯水中超声60 min,加入硝酸银和磷酸氢二钠溶液,搅拌3 h后离心干燥,得到二元复合光催化剂Ag3PO4/g-C3N4,标记为ACN。取1 g的ACN,加入30 mL CNQDs和40 mL无水乙醇,置于高压反应釜中180 ℃下反应24 h,干燥后制得三元复合光催化剂CNQDs/Ag3PO4/g-C3N4,标记为CACN。通过改变Ag3PO4和g-C3N4的质量百分比制备了一系列复合光催化剂,标记为CACN-x(其中x=10、20、30、40、50)。
-
取50 mL 10 mg·L−1 BPA溶液于比色管中。然后加入制备的不同光催化材料(0.5 g·L−1),放置光化学反应仪中,先暗反应30 min,再开启氙灯光反应120 min。实验过程中在每个设定的固定时间节点用5 mL注射器取2 mL溶液样品,经过0.22 μm水系滤膜过滤后得到上清液,通过高效液相色谱测定BPA的残留浓度。光反应结束后将剩余溶液收集起来,洗涤离心后烘干,用于后续重复实验。
-
1) XRD分析。图1(a)表明,g-C3N4有位于12.8°和27.3°的2个特征峰,分别对应纯氮化碳的(100)和(002)晶面(PDF 78-1747)[17-18]。图1(d)为纯Ag3PO4的XRD图。其中所有衍射峰的位置和相对强度都可以归属为立方相磷酸银的晶面特征峰(PDF 70-0702)[19]。在图1(b)和图1(c)中的ACN和CACN中均出现了g-C3N4和Ag3PO4的衍射峰,峰的位置几乎没有变化,此外,没有出现其他物质的衍射峰。这说明Ag3PO4成功地负载在g-C3N4上面,且CNQDs的加入没有导致材料的各组分之间发生化学反应生成其他物质,故样品的晶体结构保持不变。
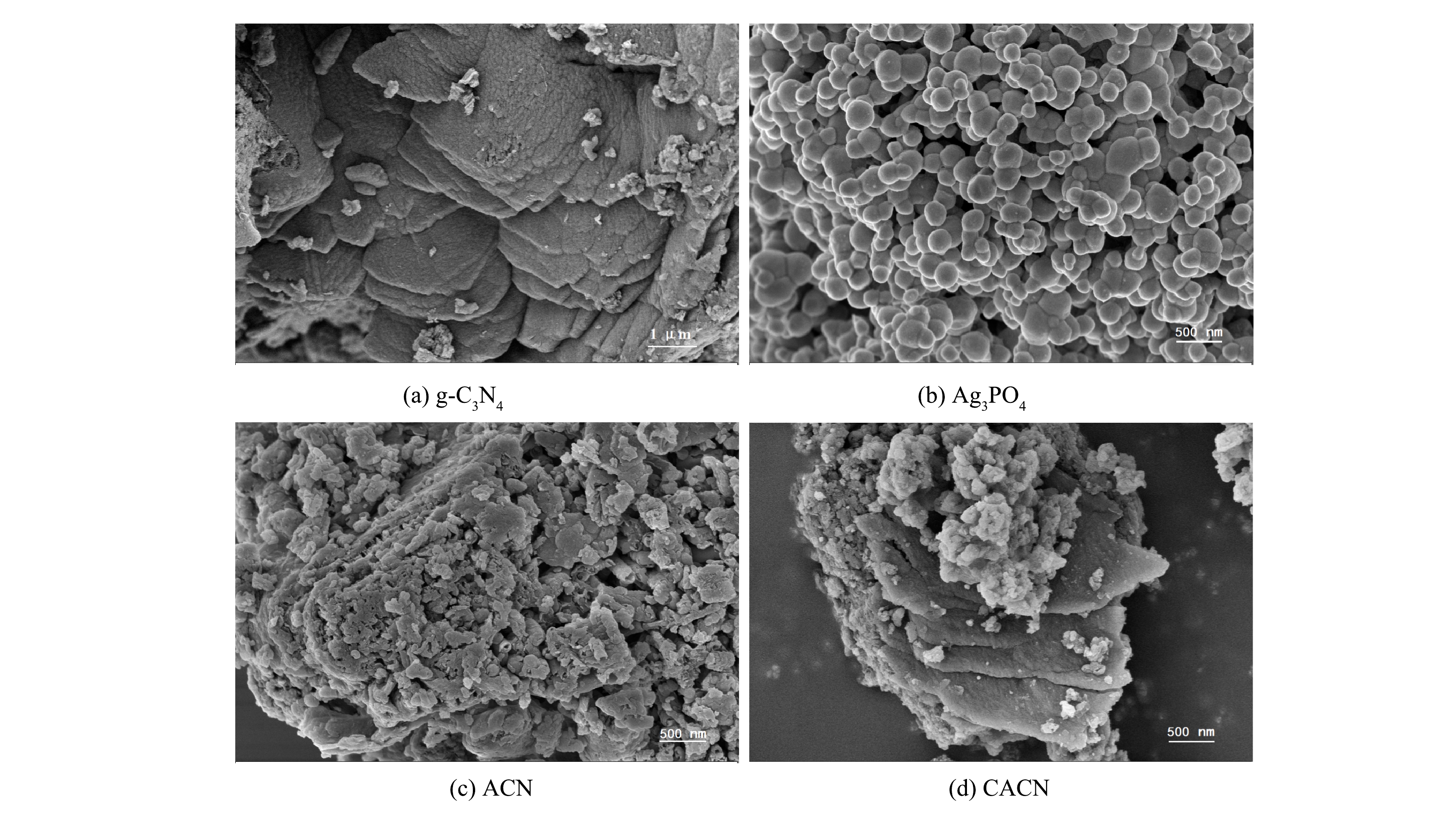
2) SEM分析。由图2(a)和图2(b)可知,g-C3N4表现为典型的片层状紧密堆积结构;Ag3PO4呈现出不规则球状颗粒结构,直径约为100~500 nm,颗粒间有明显的粘连现象。由图2(c)可以看出,磷酸银颗粒已经均匀覆盖在氮化碳的片层结构上,且磷酸银的直径远小于氮化碳,磷酸银颗粒填充在氮化碳表面以及空隙中。由于CNQDs尺寸极小,故在TEM图中才能观察到。
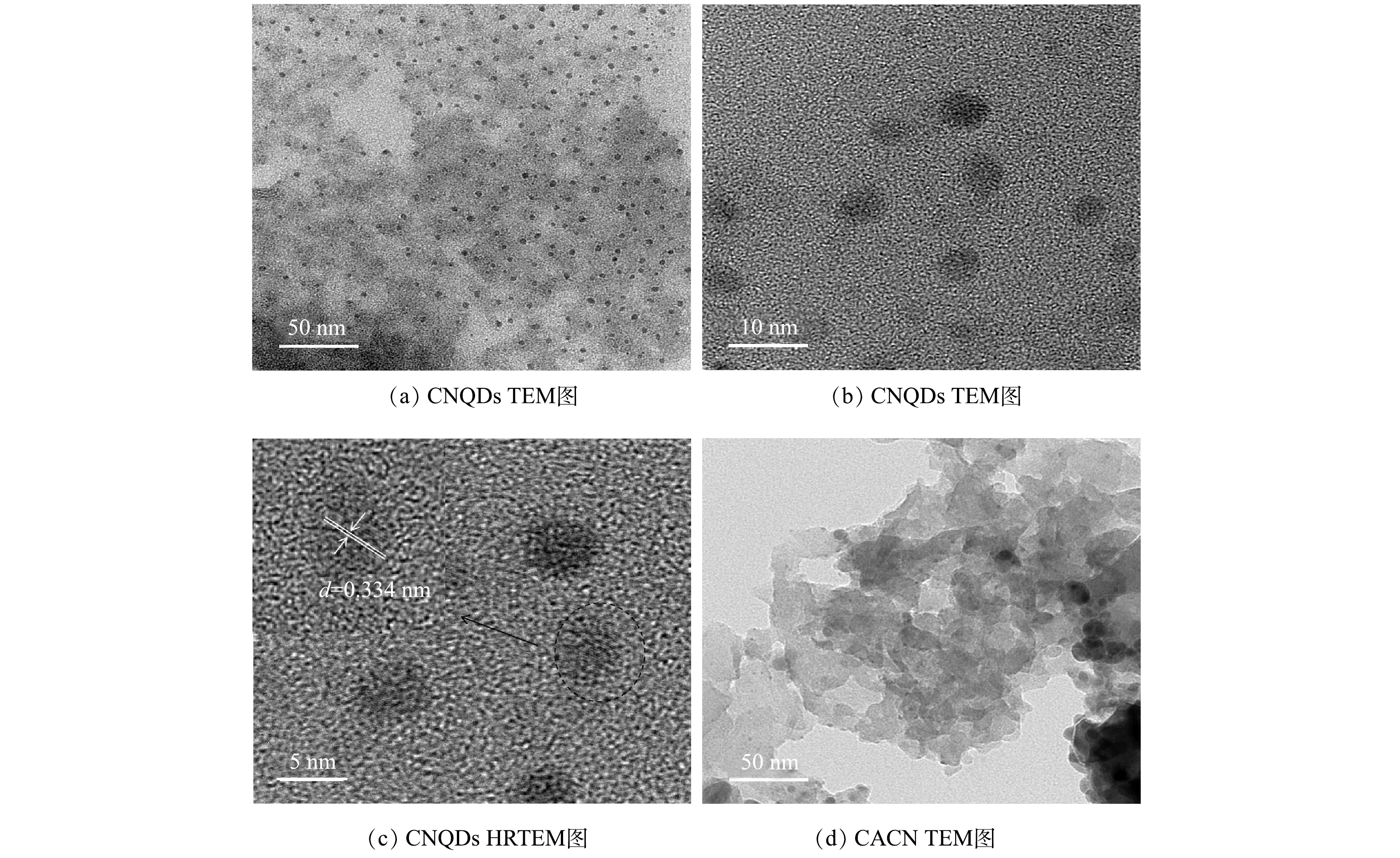
3) TEM分析。从图3可以看出,CNQDs直径为3~7 nm,且在溶液中分布较均匀,CNQDs的HRTEM图中显示量子点内的晶格条纹呈平行状,其晶格间距为0.334 nm,与氮化碳量子点的(002)平面一致(JCPDS87-1526)[14,20]。由CACN的TEM图可以看到CNQDs和Ag3PO4已均匀负载在g-C3N4上。
4) FTIR分析。由图4可知,位于558 cm−1和1 015 cm−1处的2个特征峰,主要与P-O键的伸缩振动有关[21]。在808 cm−1处的尖吸收峰来源于g-C3N4中s-三嗪环的呼吸振动,位于1 241 cm−1处的吸收峰来自于C—N单键的伸缩振动,在1 321、1 461和1 574 cm−1处的吸收峰来源于C—N—C的伸缩振动,在1 638 cm−1处的特征峰来自C=N双键的伸缩振动[22-23]。以上这些结果均证明,CACN复合材料已成功制备。
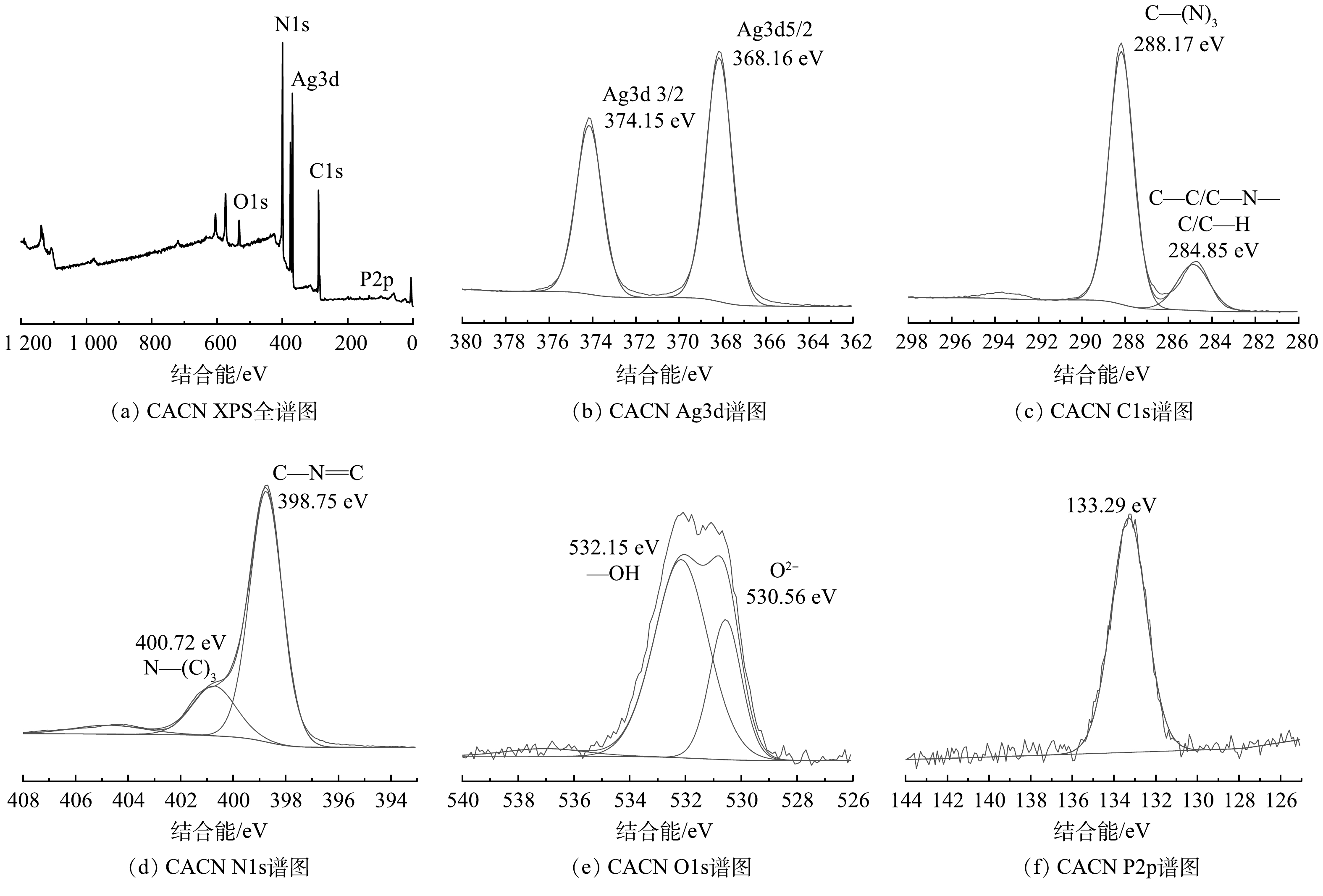
5) XPS分析。图5(a)中含有Ag、C、N、O和P等元素,并且尚未检测到其他元素的存在。图5(b)中Ag元素有2个特征峰Ag3d5/2和Ag3d3/2,结合能分别为368.16 eV和374.15 eV,表明Ag元素是以Ag+形式存在于复合材料中[24]。由图5(c)中的C1s图谱可见,结合能位于284.85 eV和288.17 eV的2个主峰分别与C—C/C—N—C/C—H以及C—(N)3的特征峰吻合[25]。由图5(d)可知,N1s的结合能为398.75 eV的特征峰可以归因于C—N=C官能团的存在,结合能位于400.72 eV的特征峰则可能与N—(C)3的存在有关[23]。如图5(e)所示,O1s的2个分峰,一处结合能位于530.56 eV的峰代表了Ag3PO4中的O2−的峰,另外一处结合能为532.15 eV的峰代表了—OH官能团,证明CNQDs中存在羧酸根基团[26]。图5(f)中P2p的电子轨道区域图谱中的特征峰只有1个,其结合能在133.29 eV,与文献中记载PO43−中P5+的特征峰的位置相吻合[27]。以上XPS表征结果表明,CACN复合材料已成功合成。
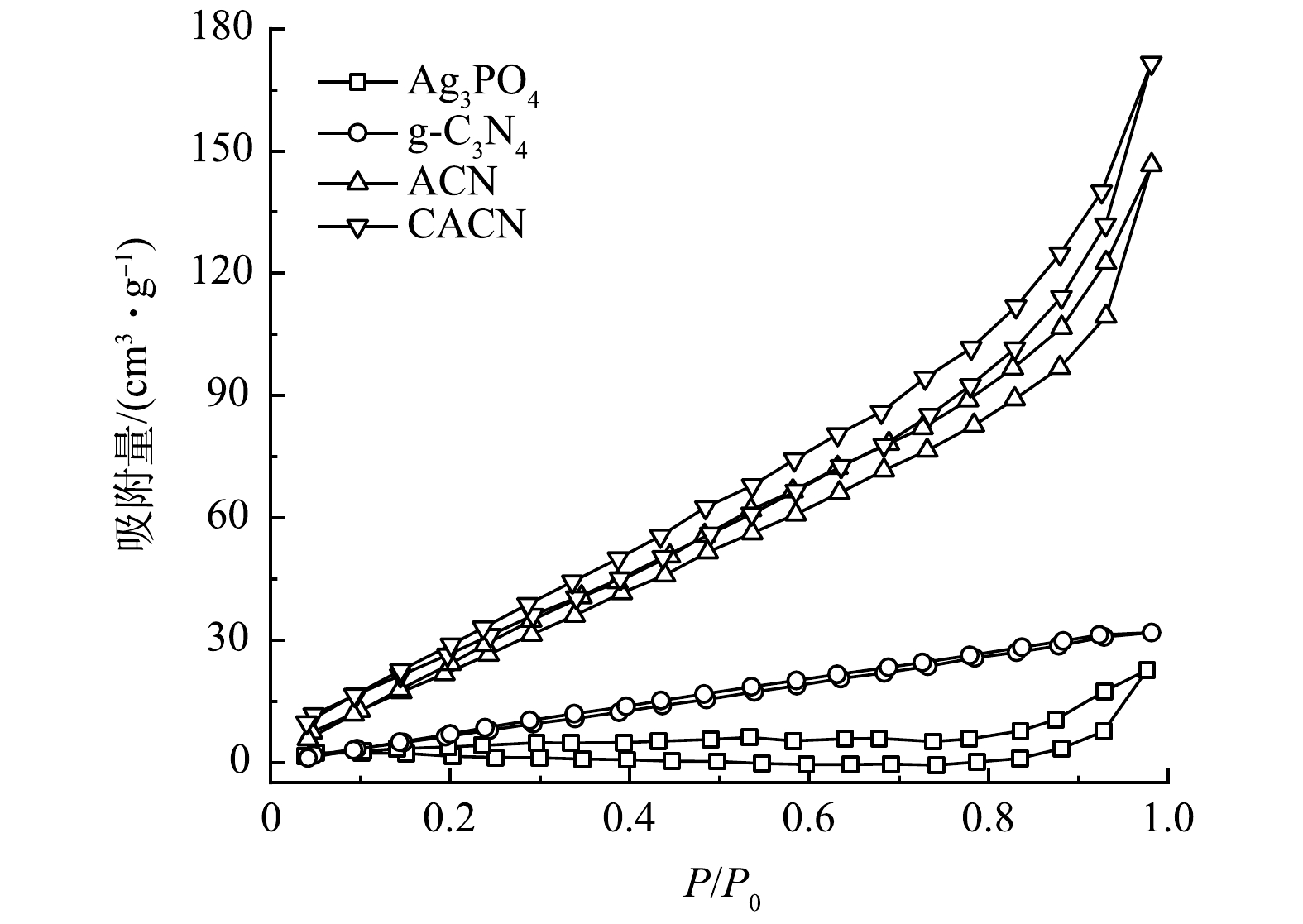
6) BET分析。由图6可以看出,图中曲线的趋势符合Ⅳ型等温线,这与中孔材料的特性一致。由表1可见,g-C3N4、Ag3PO4、ACN和CACN的比表面积分别为28.301、9.641、105.190、117.759 m2·g−1。纯g-C3N4的比表面积较小,磷酸银的加入在很大程度上增加了氮化碳材料的比表面积。而引入CNQDs后,相比于ACN,CACN的比表面积增加了1.2倍,但孔径略微有所减小,这可能是由于CNQDs的加入引起了阻塞效应。比表面积的增加,能提供的活性位点也会随之增加,进而可提高复合材料的光催化活性。
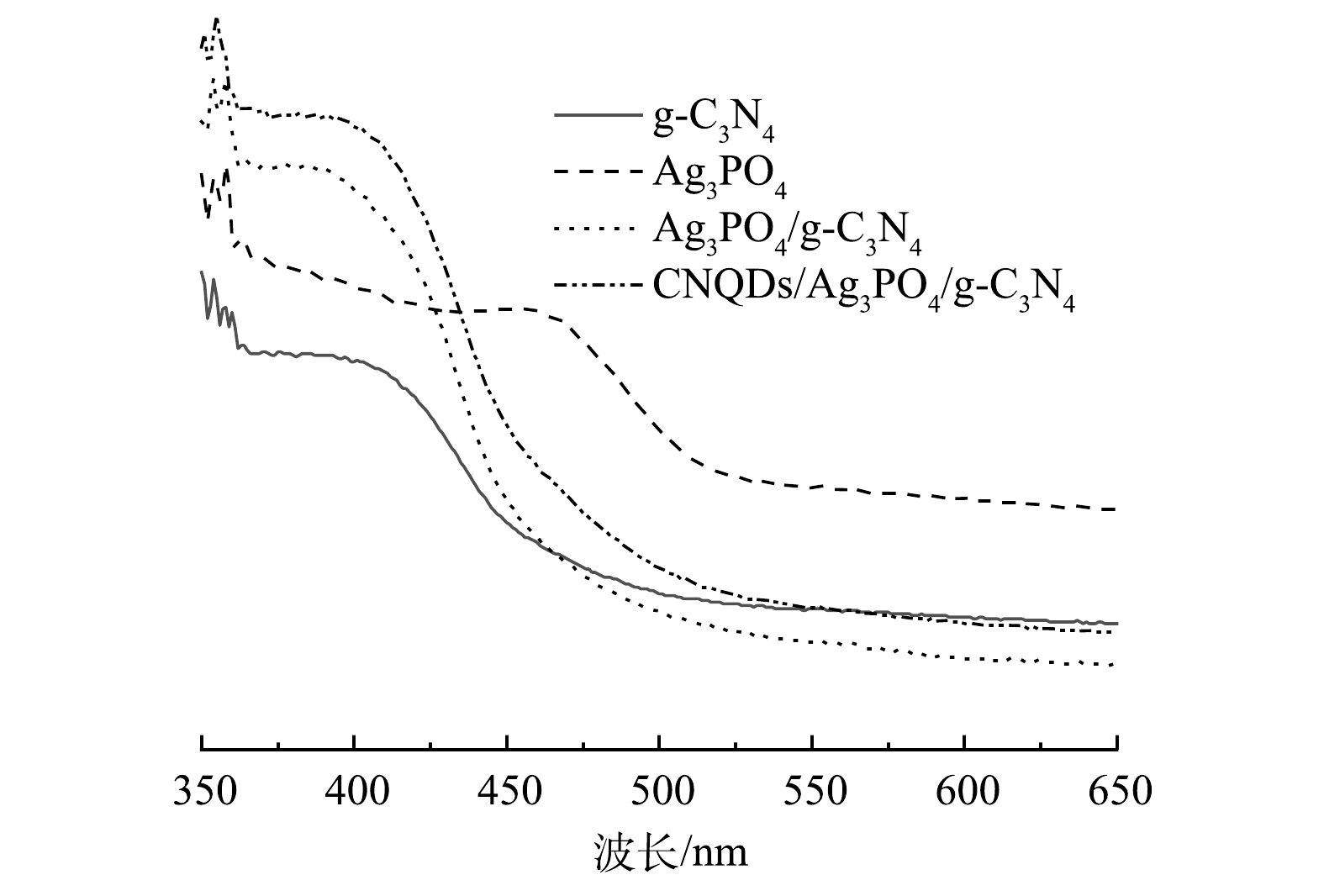
7)光吸收特性分析。由图7可知,不同光催化材料在可见光和紫外区域都表现出良好的吸光性能,g-C3N4的吸收边缘在470 nm处,Ag3PO4的吸收边缘在540 nm处。相比于纯g-C3N4和ACN,CACN的光吸收范围更宽,峰强度更高。
-
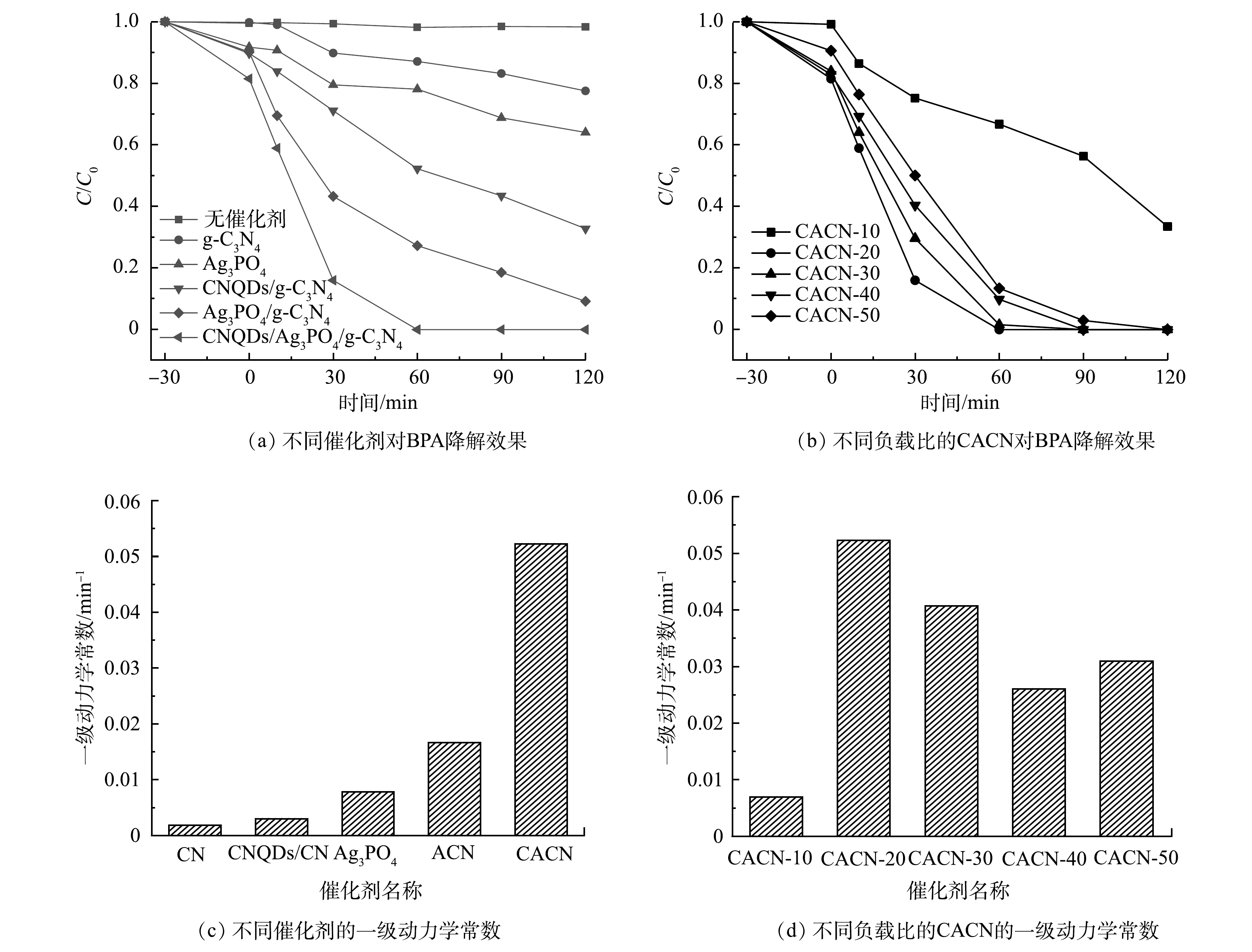
由图8可知,如果不添加光催化剂,在可见光照射下,BPA浓度几乎不变化,其自分解效应可以忽略。由图8(a)可知,加入了CNQDs之后,60 min内CACN对BPA的去除率可达到100%。同一时间内g-C3N4、Ag3PO4和ACN对BPA降解率分别为12.91%、47.84%和72.84%。由图8(b)可知,除CACN-10外,三元复合催化剂CACN-x(x=20、30、40、50)60 min内对BPA的降解效果分别为100%、98.55%、90.30%、86.71%,反应120 min后降解效果均达到100%,说明CNQDs的加入可以为反应提供更多活性位点,提高体系的光催化活性。
-
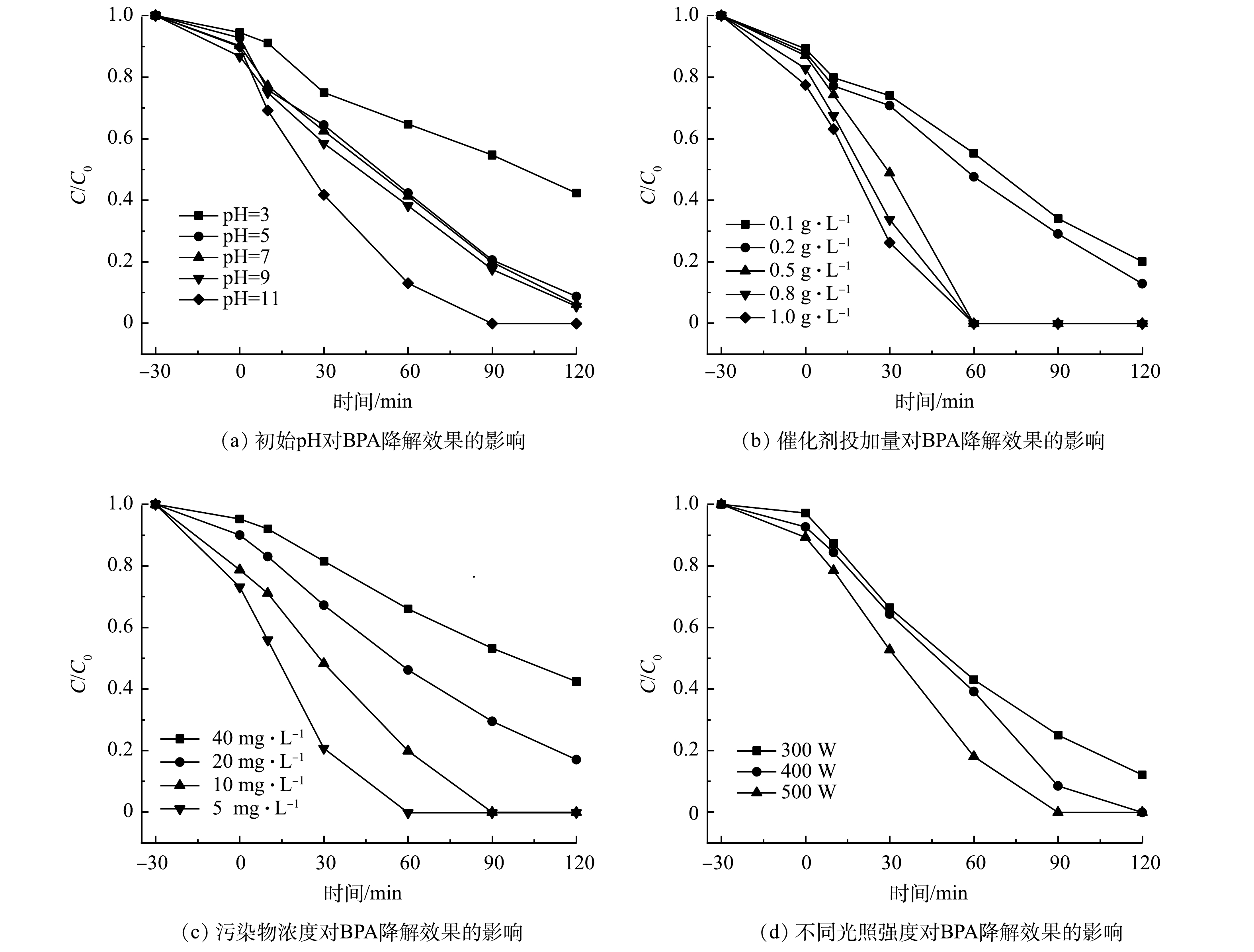
从图9(a)可以看出,当pH为3时,光照120 min后BPA的去除率为57.63%;随着pH逐渐升高,催化体系对BPA的降解效果不断增强,当pH为11时降解效果最好,90 min达到100%。这可能是因为,在碱性条件下,电子-空穴对更容易捕获OH−的电子而生成·OH。从图9(b)可以看出,当催化剂投加量由0.2 g·L−1增加至0.5 g·L−1时,对BPA的去除率由0.5 g·L−1增加到1.0 g·L−1,对BPA去除率均可达到100%。当催化剂投加量较低时,无法为BPA降解提供足够的反应活性位点;随着催化剂的增加,活性位点越来越多,光催化效果也逐渐增强。由图9(c)可以看出,当BPA初始质量浓度分别为5、10、20、40 mg·L−1时,反应120 min后,BPA降解率分别为100%、100%、81.02%和55.46%。BPA初始质量浓度越低,光催化降解越彻底。由图9(d)可以看出,当光照强度分别为300、400和500 W时,光反应90 min后,对BPA降解率分别为75.02%、91.54%和100%。这是因为光照强度越高,复合光催化剂表面的电子-空穴对越多,降解效率越高,光催化降解越彻底。
-
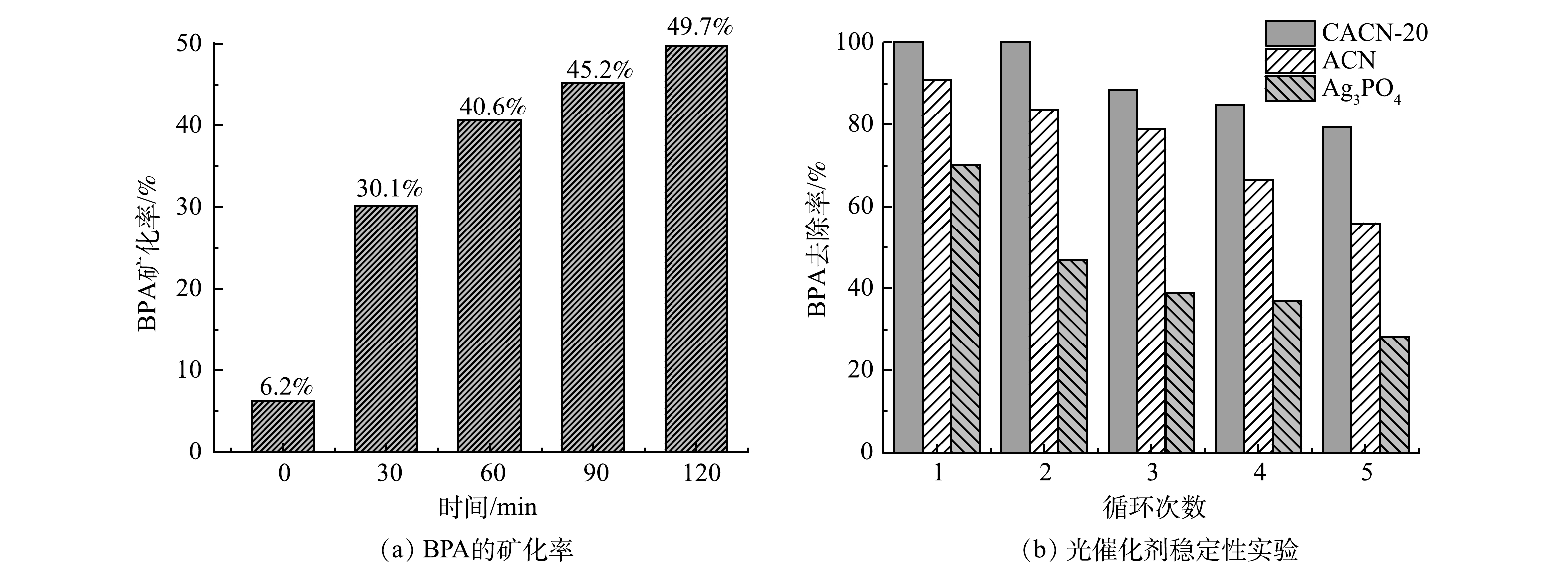
在实际应用中,光催化剂的矿化率以及稳定性至关重要。从图10(a)可以看出,在经过了30 min的暗反应和120 min的光反应之后,BPA的矿化率可以达到49.7%。这表明有49.7%的BPA被完全转化为二氧化碳和水。此外,对纯磷酸银、ACN和CACN-20进行光催化循环实验(图10(b))。经过5次循环后,CACN-20复合光催化材料对BPA的降解率仍然保持在79.86%(第1次降解率为100%);ACN变成55.7%(第2次降解率为90.9%);纯Ag3PO4下降到28.05%(第1次降解率为67.32%)。以上结果表明,CACN-20复合材料有较强的光稳定性和可重复利用性。
-
反应活性物种在有机污染物的光催化降解中起着极其重要的作用。分别选择对苯醌(BZQ)、叔丁醇(t-BuOH)和乙二胺四乙酸二钠(EDTA-2Na)作为羟基自由基(·OH)、超氧化物自由基(·O2−)和空穴(h+)的淬灭剂,淬灭剂的浓度均为5 mmol·L−1。加入不同自由基淬灭剂对BPA降解效果的影响如图11所示。由图11可知,不添加淬灭剂时,BPA的去除率可以达到100%;加入t-BuOH后BPA去除率为80.57%,表明t-BuOH在一定程度上抑制了BPA的光降解过程;添加BZQ和EDTA-2Na时,BPA的去除率仅有7.50%和18.36%,这表明·O2−、h+都是主要的光反应活性物种。以上结果表明,在光催化降解BPA过程中活性物种的贡献顺序为·O2−>h+>·OH。
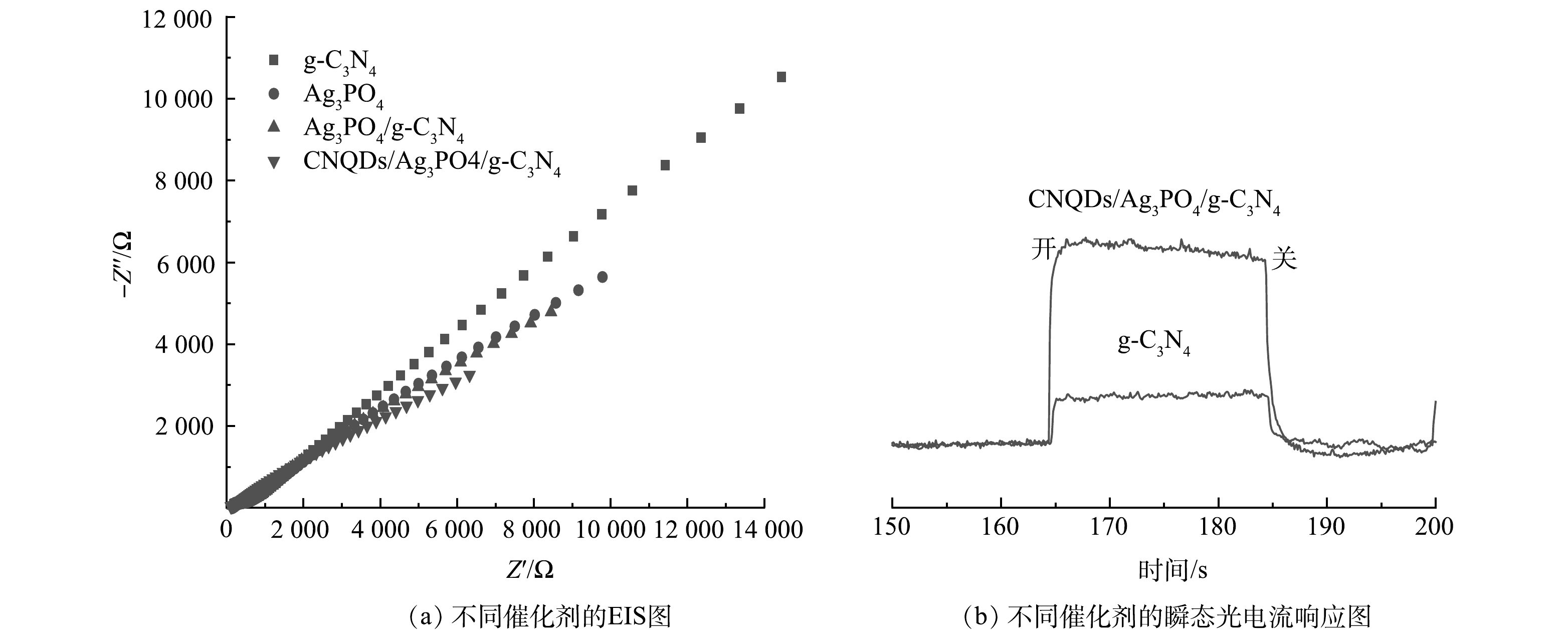
为了分析各光催化材料电子-空穴对的分离情况,在0.5 mol·L−1的硫酸钠溶液中进行了EIS测试和瞬态光电流响应测试,结果如图12所示。EIS的电弧半径越小,意味着催化剂表面电荷转移受到的的阻力越小,电子转移效率更高。由图12(a)可以看出,相比其他3种材料,CNQDs/Ag3PO4/g-C3N4的电阻更弱,意味着其光催化性能更好。这与光催化降解效果一致。由图12(b)可以看出,催化剂CNQDs/Ag3PO4/ g-C3N4相比于纯g-C3N4具有更强的光电流密度,说明CNQDs/Ag3PO4/g-C3N4光生电子-空穴对的分离效率明显提高。
采用气相色谱-质谱联用仪(GC-MS)对光催化降解BPA过程的中间产物进行定性分析,通过与NIST 17.1谱库对比,确定了几种可能的中间产物(表2)。根据GC-MS测定结果以及自由基淬灭实验结果,提出在光催化反应过程中BPA可能的降解路径(图13):其一,BPA被吸附到光催化剂表面,·OH和 h+可以攻击BPA中的C-C键生成4-异丙基苯酚,然后,中间产物通过·O2-氧化生成羟基二甲基苯酚,再氧化成4-羟基苯二酸,最终转化为二氧化碳和水;其二,活性物种还可以直接将BPA开环,再氧化成简单烷烃,最终转化成二氧化碳和水。
-
1)引入CNQDs不仅没有改变g-C3N4和Ag3PO4的物化性质,且具有较大比表面积,能为反应提供更多活性位点,能有效提高光生载流子的分离效率。
2)三元复合光催化剂CNQDs/Ag3PO4/g-C3N4对BPA的去除率在60 min内可达到100%,分别为g-C3N4的7.74倍、Ag3PO4的2.09倍及Ag3PO4/g-C3N4的1.38倍,矿化率达到49.7%,且循环使用5次后对BPA的去除率仍有79.86%。
3) CNQDs/Ag3PO4/g-C3N4光催化降解BPA的最佳反应条件为:pH为11、污染物质量浓度为5 mg·L−1、催化剂投加量为1 g·L−1、光照强度为500 W。光催化降解过程中主要的活性物种为·O2−和h+。推测有2种可能的BPA降解路径:一是BPA先转化为单环有机物,再转化为二氧化碳和水;二是BPA经活性物种直接开环形成简单烷烃,最后氧化成二氧化碳和水。
CNQDs/Ag3PO4/g-C3N4复合光催化材料的制备及其对双酚A的降解性能
Preparation of CNQDs/Ag3PO4/g-C3N4 composite photocatalyst and its degradation performance of bisphenol A
-
摘要: 采用溶剂热法和原位沉淀法制备了CNQDs/Ag3PO4/g-C3N4三元复合光催化剂,通过SEM、TEM、XRD、FT-IR、XPS、BET等手段对其进行了表征;以BPA为目标污染物,考察了pH、BPA初始浓度、催化剂投加量及光照强度等因素对BPA去除效果的影响;同时,对该三元复合催化剂光催化降解BPA的机制进行了探讨。结果表明,CNQDs/Ag3PO4/g-C3N4 在反应60 min时对BPA的去除率可以达到100%,分别为g-C3N4、Ag3PO4、Ag3PO4/g-C3N4的7.74、2.09和1.38倍;光催化降解BPA的最佳反应条件为:pH为11、污染物质量浓度为5 mg·L−1、催化剂投加量为1 g·L−1、光照强度为500 W;在光催化降解BPA的过程中,主要反应活性物种为·O2−和h+。基于GC-MS对反应过程中间产物的检测结果,推测出BPA可能的2种降解路径:一是BPA先转化为单环有机物,再转化为二氧化碳和水;二是BPA经活性物种直接开环形成简单烷烃,最后氧化成二氧化碳和水。
-
关键词:
- CNQDs/Ag3PO4/g-C3N4 /
- 光催化 /
- 双酚A /
- 废水处理
Abstract: The CNQDs/Ag3PO4/g-C3N4 ternary composite photocatalyst was prepared by solvothermal method and in-situ precipitation method, and it was characterized by SEM, TEM, XRD, FT-IR, XPS, BET and other ways; BPA was the target pollutant, the effects of factors such as pH, BPA concentration, catalyst dosage and light intensity on BPA removal were investigated; at the same time, the mechanism of photocatalytic degradation of BPA by the ternary composite catalyst was discussed. The results showed that 100% removal rate of BPA occurred in 60 minutes after CNQDs/Ag3PO4/g-C3N4 photocatalytic degradation, which was 7.74 times of g-C3N4, 2.09 times of Ag3PO4 and 1.38 times of Ag3PO4/g-C3N4, respectively. The optimal reaction conditions for photocatalytic degradation of BPA were following: pH 11, BPA concentration of 5 mg·L−1, catalyst dosage of 1 g·L−1, and light intensity of 500 W. In the process of photocatalytic degradation of BPA, the main reactive species are ·O2− and h+. Based on the detection results of intermediate products in the reaction process by GC-MS, two possible degradation paths of BPA were inferred, one was that BPA first transformed into monocyclic organic matter, then into CO2 and H2O, the other one was BPA transformed into simple alkane through direct ring opening reaction, then were oxidized into CO2 and H2O.-
Key words:
- CNQDs/Ag3PO4/g-C3N4 /
- photocatalytsis /
- bisphenol A /
- wastewater treatment
-

-
表 1 不同材料的比表面积、孔径、孔容
Table 1. Specific surface area, pore size and capacity of different materials
样品 比表面积/(m2·g−1) 孔径/nm 孔体积/(cm3·g−1) g-C3N4 28.301 16.694 0.042 Ag3PO4 9.641 20.852 0.036 ACN 105.190 18.637 0.211 CACN 117.759 18.446 0.247 表 2 BPA降解中间产物
Table 2. BPA degradation intermediates
编号 名称 化学式 m/z 1 4-异丙基苯酚 C9H10O 134 2 羟基-2-甲基苯酚 C8H8O2 135 3 2,3,5-三甲基己烷 C9H20 207 4 异丙苯 C9H12 120 5 十一烷 C11H24 85 6 4-羟基苯二酸 C7H7O3 206 7 5,7-二甲基十一烷 C13H28 86 -
[1] ARIS A Z, MOHD HIR Z A, RAZAK M R. Metal-organic frameworks (MOFs) for the adsorptive removal of selected endocrine disrupting compounds (EDCs) from aqueous solution: A review[J]. Applied Materials Today, 2020, 21: 100796. doi: 10.1016/j.apmt.2020.100796 [2] WANG R, MA X, LIU T, et al. Degradation aspects of endocrine disrupting chemicals: A review on photocatalytic processes and photocatalysts[J]. Applied Catalysis A:General, 2020, 597: 117547. doi: 10.1016/j.apcata.2020.117547 [3] WANG S, ZHU Z, HE J, et al. Steroidal and phenolic endocrine disrupting chemicals (EDCs) in surface water of Bahe River, China: Distribution, bioaccumulation, risk assessment and estrogenic effect on Hemiculter leucisculus[J]. Environmental Pollution, 2018, 243: 103-114. doi: 10.1016/j.envpol.2018.08.063 [4] 胡文兰, 厉志玉, 刘健毅, 等. 杭州市场罐头类食品的双酚A污染调查及其膳食风险评估[J]. 中国食物与营养, 2013, 19(10): 13-16. doi: 10.3969/j.issn.1006-9577.2013.10.003 [5] LI F, TANG M, LI T, et al. Two-dimensional graphene/g-C3N4 in-plane hybrid heterostructure for enhanced photocatalytic activity with surface-adsorbed pollutants assistant[J]. Applied Catalysis B:Environmental, 2020, 268: 118397. doi: 10.1016/j.apcatb.2019.118397 [6] LUO W, CHEN X, WEI Z, et al. Three-dimensional network structure assembled by g-C3N4 nanorods for improving visible-light photocatalytic performance[J]. Applied Catalysis B:Environmental, 2019, 255: 117761. doi: 10.1016/j.apcatb.2019.117761 [7] SINGH J, KUMARI P, BASU S. Degradation of toxic industrial dyes using SnO2/g-C3N4 nanocomposites: Role of mass ratio on photocatalytic activity[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2019, 371: 136-143. doi: 10.1016/j.jphotochem.2018.11.014 [8] WANG J, XIA Y, ZHAO H, et al. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution[J]. Applied Catalysis B:Environmental, 2017, 206: 406-416. doi: 10.1016/j.apcatb.2017.01.067 [9] BI X, YU S, LIU E, et al. Construction of g-C3N4/TiO2 nanotube arrays Z-scheme heterojunction to improve visible light catalytic activity[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2020, 603: 125193. doi: 10.1016/j.colsurfa.2020.125193 [10] CHEN X, YU C, ZHU R, et al. Photocatalytic performance and mechanism of Z-scheme CuBi2O4/Ag3PO4 in the degradation of diclofenac sodium under visible light irradiation: Effects of pH, H2O2, and S2O82−[J]. Science of the Total Environment, 2020, 711: 134643. doi: 10.1016/j.scitotenv.2019.134643 [11] RAEISI-KHEIRABADI N, NEZAMZADEH-EJHIEH A. A Z-scheme g-C3N4/Ag3PO4 nanocomposite: Its photocatalytic activity and capability for water splitting[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33381-33395. doi: 10.1016/j.ijhydene.2020.09.028 [12] HU Z, LYU J, GE M. Role of reactive oxygen species in the photocatalytic degradation of methyl orange and tetracycline by Ag3PO4 polyhedron modified with g-C3N4[J]. Materials Science in Semiconductor Processing, 2020, 105: 104731. doi: 10.1016/j.mssp.2019.104731 [13] WANG H, LEI Z, LI L, et al. Holey g-C3N4 nanosheet wrapped Ag3PO4 photocatalyst and its visible-light photocatalytic performance[J]. Solar Energy, 2019, 191: 70-77. doi: 10.1016/j.solener.2019.08.071 [14] ZHOU J, CAI W, DING J, et al. 0D/1D Z-scheme g-C3N4 quantum dot/WO3 composite for efficient Cr (VI) photoreduction under visible light[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105292. doi: 10.1016/j.jece.2021.105292 [15] YANG S, LIU C, WANG J, et al. Enhanced photocatalytic activity of g-C3N4 quantum dots/Bi3.64Mo0.36O6.55 nanospheres composites[J]. Journal of Solid State Chemistry, 2020, 287: 121347. doi: 10.1016/j.jssc.2020.121347 [16] LIN X, XU D, ZHAO R, et al. Highly efficient photocatalytic activity of g-C3N4 quantum dots (CNQDs)/Ag/Bi2MoO6 nanoheterostructure under visible light[J]. Separation and Purification Technology, 2017, 178: 163-168. doi: 10.1016/j.seppur.2017.01.020 [17] XU J, YU H, GUO H. Synthesis and behaviors of g-C3N4 coupled with LaxCo3-xO4 nanocomposite for improved photocatalytic activeity and stability under visible light[J]. Materials Research Bulletin, 2018, 105: 342-348. doi: 10.1016/j.materresbull.2018.04.006 [18] CHANG F, ZHENG J, WANG X, et al. Heterojuncted non-metal binary composites silicon carbide/g-C3N4 with enhanced photocatalytic performance[J]. Materials Science in Semiconductor Processing, 2018, 75: 183-192. doi: 10.1016/j.mssp.2017.11.043 [19] GUO J, DDI Y, CHEN X, et al. Synthesis and characterization of Ag3PO4/LaCoO3 nanocomposite with superior mineralization potential for bisphenol A degradation under visible light[J]. Journal of Alloys and Compounds, 2017, 696: 226-233. doi: 10.1016/j.jallcom.2016.11.251 [20] ZHOU L, TIAN Y, LEI J, et al. Self-modification of g-C3N4 with its quantum dots for enhanced photocatalytic activity[J]. Catalysis Science & Technology, 2018, 8(10): 2617-2623. [21] EL MASAOUDI H, BENABDALLAH I, JABER B, et al. Enhanced visible light photocatalytic activity of Cu2+-doped Ag3PO4 nanoparticles[J]. Chemical Physics, 2021, 545: 111133. doi: 10.1016/j.chemphys.2021.111133 [22] RAJALAKSHMI N, BARATHI D, MEYVEL S, et al. S-scheme Ag2CrO4/g-C3N4 photocatalyst for effective degradation of organic pollutants under visible light[J]. Inorganic Chemistry Communications, 2021, 132: 108849. doi: 10.1016/j.inoche.2021.108849 [23] NIU P, LIU G, CHENG H M. Nitrogen vacancy-promoted photocatalytic activity of graphitic carbon nitride[J]. Journal of Physical Chemistry C, 2012, 116(20): 11013-11018. doi: 10.1021/jp301026y [24] SHI H, YANG S, HAN C, et al. Fabrication of Ag/Ag3PO4/WO3 ternary nanoparticles as superior photocatalyst for phenol degradation under visible light irradiation[J]. Solid State Sciences, 2019, 96: 105967. doi: 10.1016/j.solidstatesciences.2019.105967 [25] CUI Y, ZHANG X, GUO R, et al. Construction of Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance and mechanism[J]. Separation and Purification Technology, 2018, 203: 301-309. doi: 10.1016/j.seppur.2018.04.061 [26] CHEN X, LIU Q, WU Q, et al. Incorporating graphitic carbon nitride (g-C3N4) quantum dots into bulk-heterojunction polymer solar cells leads to efficiency enhancement[J]. Advanced Functional Materials, 2016, 26(11): 1719-1728. doi: 10.1002/adfm.201505321 [27] MA Z, HUANG X, Xu N, et al. An effective strategy for boosting photoinduced charge separation of Ag3PO4 by BiVO4 with enhanced visible light photodegradation efficiency for levofloxacin and methylene blue[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2020, 229: 117986. doi: 10.1016/j.saa.2019.117986 -




 下载:
下载: