-
目前,由于在医疗、畜牧和水产养殖等领域的广泛应用[1],过量的抗生素以原型或代谢产物形式被排入水体中,对水生生态系统和人类健康造成了不可估量的危害。目前,抗生素废水处理方法主要有高级氧化法(advanced oxidation processes, AOPs)[2-3]、吸附法[4]、生物法[5]等。其中,作为高级氧化法之一的光催化氧化法因其处理成本低,降解效率高和无二次污染等优点,具有很好的应用前景。
研发高效稳定且成本低廉的光催化剂是目前光催化技术应用的关键。近年来,非金属半导体石墨相氮化碳(g-C3N4)因其原料廉价易得、物理化学性质稳定、能带结构合适与可见光响应活性强等特点[6-7],被认为是最具潜力的新型光催化剂。然而,块体g-C3N4的表面积小、电荷重组快、可见光利用率低,使其在实际应用中受到极大限制。目前,通过耦合其他半导体构建异质结是提高g-C3N4光催化活性的有效方法,2个半导体间密切接触可以形成较大的界面面积,并产生激子解离界面和空间带电载流子迁移通道,加速光生载流子分离,从而提升g-C3N4的光催化活性[8]。例如,SUN等[9]通过静电自组装制备了SnO2/g-C3N4异质结,在可见光照射50 min后,对罗丹明B的降解率达到96.9%;ZHANG等[8]采用聚合偶联共沉淀方法制备了增强分子氧活化的FeOOH QDs/CQDs/g-C3N4异质结,在可见光照射下可以降解85.5%的土霉素。以上结果表明,通过构建异质结可以增强g-C3N4的催化活性,但仍有巨大的提升空间。
基于SO4·−的AOPs因其氧化性能强、氧化剂稳定性好、环保无害等优点被广泛应用于降解有机污染物[10-11]。SO4·−通常可以通过光、微波、超声波、加热、过渡金属离子、金属和无金属催化剂等活化过一硫酸盐(Peroxymonosulfate, PMS, HSO5−)和过二硫酸盐(Peroxodisulfate, PS, S2O82−)产生[12-15]。其中,过渡金属离子易活化,无需额外能量输入,已被广泛研究[16]。而在基于过渡金属的体系中,铁基催化剂/过硫酸盐由于铁的化学状态不同(Fe0、Fe2+、Fe3+),是一个高度灵活的体系。因此,将铁基催化剂/过硫酸盐体系与光催化耦合,构建多反应耦合型高级氧化体系用于高效降解水中有机污染物是目前研究的热点。
在铁基催化剂中,FeOOH由于环境友好性、自然丰度、相对稳定性、可见光响应、耐腐蚀性强和低成本而引起广泛关注。因此,本研究采用原位沉淀法将FeOOH与g-C3N4复合,成功制备出FeOOH/g-C3N4异质光催化剂,并通过耦合过一硫酸盐(PMS)构建多反应耦合型高级氧化体系(光催化+PMS活化),研究了其降解环丙沙星(Ciprofloxacin, CIP)的性能,考察了各影响因素对CIP催化降解效果的影响,评价了体系的稳定性并提出了可能的催化降解机理。
-
CIP (C17H18FN3O3)、PMS (2KHSO5·KHSO4·K2SO4)购于上海麦克林生化科技有限公司;三聚氰胺(C3H6N6)、六水合氯化铁(FeCl3·6H2O)、碳酸氢铵(NH4HCO3)、无水乙醇购于天津市科密欧化学试剂有限公司,所有试剂均为分析纯,实验用水为超纯水。
-
以三聚氰胺作为原料,采用热缩聚法[17]制备g-C3N4。将一定量的g-C3N4分散在40 mL无水乙醇中,然后加入1 mmol的FeCl3·6H2O,搅拌至溶解完全后再加入3 mmol的NH4HCO3,在室温下搅拌8 h,待反应完全后离心,用无水乙醇和去离子水洗涤数次后,真空冷冻干燥10 h即可得到样品。在FeOOH/g-C3N4中,FeOOH的理论质量百分比分别为3%、5%和10%。
-
采用Rigaku Ultima IV型X射线衍射仪(X-ray diffraction, XRD)对光催化剂的晶体结构进行表征。采用JEM-2100F型高分辨透射电镜(Transmission electron microscope, TEM)对催化剂的微观形貌进行表征。催化剂的X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)采用单色铝辐射的K-Alpha光谱仪测定,并以284.8 eV处的C1s峰作为参考校正结合能。采用Perkinelmer Lambda 1050型UV/Vis分光光度计测定了催化剂的紫外-可见漫反射光谱(UV-vis diffuse reflection spectra, UV-vis DRS)。
-
称取40 mg FeOOH/g-C3N4分散于100 mL浓度为10 mg·L−1的环丙沙星溶液中,并加入10 mg的PMS,先在黑暗下搅拌30 min,然后以氙灯为可见光源(λ>400 nm,300 W)进行光催化反应。每隔15 min从光催化反应器中抽取3 mL的悬浮液,微孔过滤(孔径为0.45 μm)后通过紫外可见分光光度计(TU-1900, Pgeneral)测定样品浓度。将反应后的催化剂离心洗涤,真空干燥后按照上述步骤重复4次,考察催化剂的稳定性和可重复性。
-
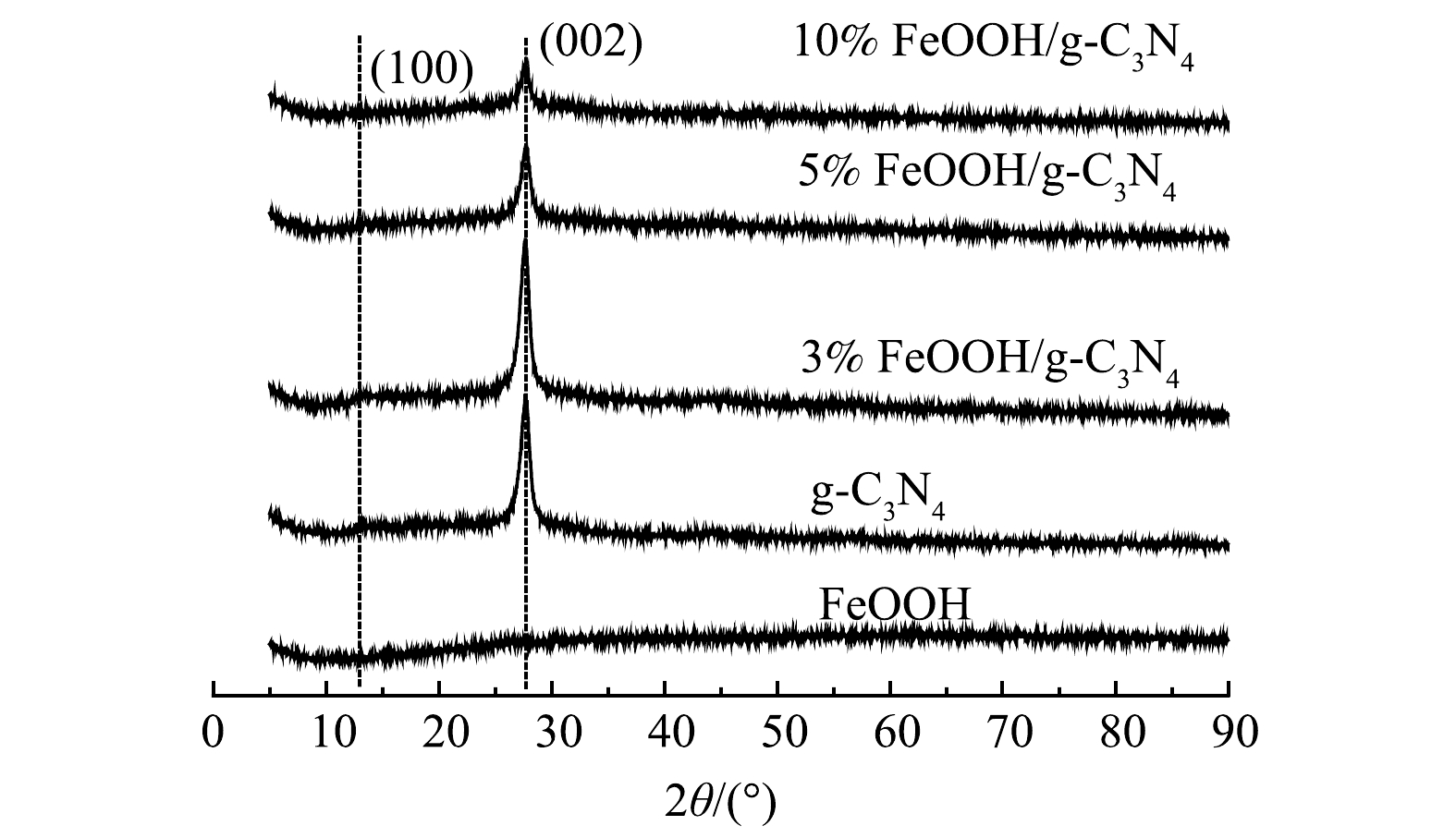
1)XRD表征。如图1所示,FeOOH的谱图中未检测到衍射峰。这表明其为无定型结构。g-C3N4的谱图在13.1°和27.6°处有2个典型的衍射峰,分别属于g-C3N4的(100)和(002)晶面[18]。FeOOH/g-C3N4系列材料中均出现g-C3N4的特征峰。随着FeOOH负载量的增加,g-C3N4各晶面的衍射峰强度逐渐减弱。这可能是由于FeOOH与g-C3N4的强耦合影响了其结晶度[19]。
2) TEM表征。如图2(a)~(b)所示,g-C3N4呈现片层堆积态,而FeOOH呈现聚集态;由图2(c)~(d)可见,与g-C3N4和FeOOH的TEM图像比较,可以观察到在FeOOH/g-C3N4复合物的片层中出现聚集体,这表明FeOOH已被成功负载。
3) XPS表征。在全谱图中(图3(a))观察到了C、N、O和Fe 4种元素,表明FeOOH已经成功负载到g-C3N4表面。C1s、N1s、O1s和Fe2p的高清分谱如图3(b)~(e)所示。在C1s谱图(图3(b))中,288.25 eV和284.8 eV的2个峰分别归因于—N—C=N—和C—C键[20]。在N1s光谱(图3(c))中可以观察到以399.21、400.77、401.71和404.67 eV为中心的4个峰,分别可归因于C=N—C、N—(C)3、C—N—H 和π激发或充电效应[21]。在O1s光谱(图3(d))中,在529.75、531.18和532.69 eV处存在3个峰,其中529.75 eV和531.18 eV的2个峰都与Fe—O—Fe键有关[22],532.69 eV处的峰可归因于催化剂表面的吸附水[23]。图3(e)显示了5% FeOOH/g-C3N4的Fe2p光谱。可以观察到在709.90、712.91、719.90、723.83和732.45 eV处有特征峰。其中,在709.90、712.91和723.83 eV处的结合能峰分别属于Fe2p3/2和Fe2p1/2光谱,在Fe2p3/2中,709.90 eV处的峰表示Fe2+,712.91 eV的峰表示Fe3+。此外,在719.90和732.45 eV处分别观测到Fe2p3/2和Fe2p1/2的振动卫星峰[17]。可以观察到,在C1s谱(图3(b))中,使用后的5% FeOOH/g-C3N4的结合能峰有些微红移;在N1s、O1s和Fe2p的谱图(图3(c)~(e))中,使用后的5% FeOOH/g-C3N4的结合能峰均有些微蓝移。这表明,催化反应已引发元素化学环境发生改变。在Fe2p3/2中,709.90 eV处的Fe2+和712.91 eV的Fe3+峰面积占总铁的比例为14.21%和85.79%,经降解CIP反应后,两者占总铁的比例调整为39.23%和60.77%。Fe3+的比例降低了25.02%。表明部分Fe3+利用电子被还原为Fe2+。
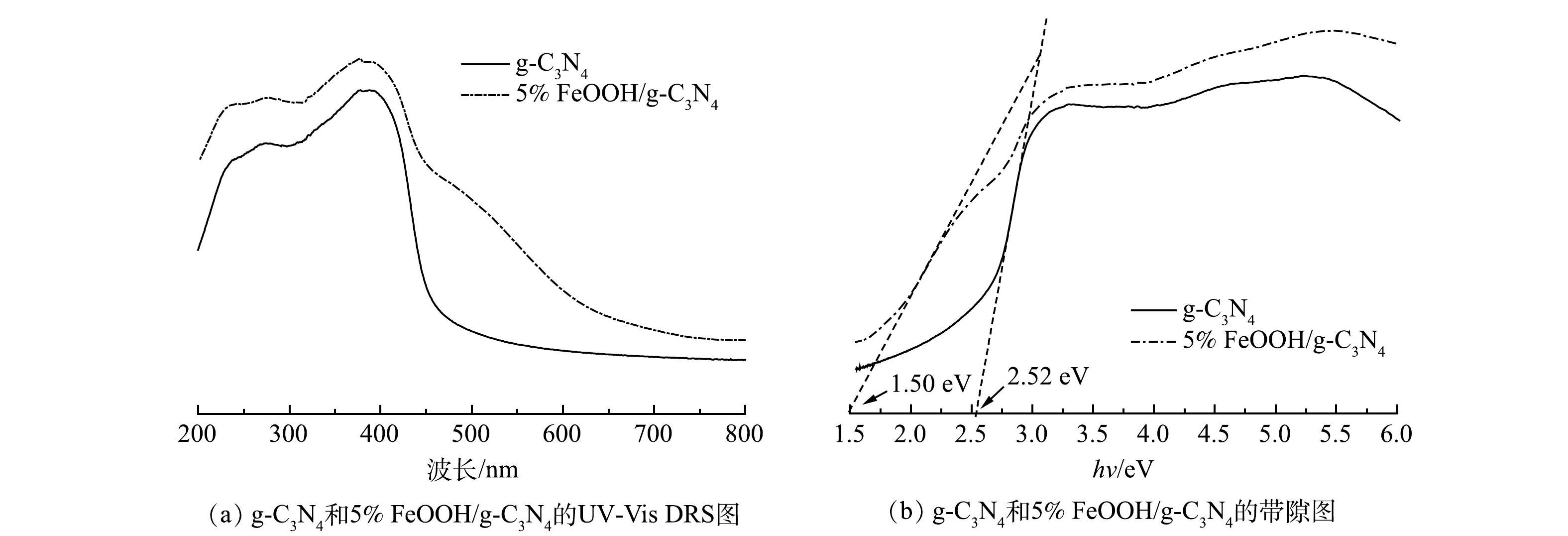
4) UV-vis分析。如图4(a)所示,复合FeOOH后,FeOOH/g-C3N4的光吸收能力大幅度提升,光吸收边缘显著扩展。这是由于FeOOH在几乎整个UV-Vis区域都表现出光吸收能力[24],其与g-C3N4之间的界面电荷转移效应使得FeOOH/g-C3N4复合催化剂在可见光区域得到明显增强的光吸收能力。此外,根据Tauc曲线,通过Kubelka-Munk方程计算了g-C3N4和FeOOH/g-C3N4的带隙值。如图4(b)所示,g-C3N4和FeOOH/g-C3N4的带隙值分别为2.52 eV和1.50 eV。带隙的变窄意味着e−从价带(valence band, VB)跃迁至导带(conduction band, CB)所需能量减少,有利于光吸收。由此可见,与FeOOH的复合大大扩展了g-C3N4的可见光吸收,提高了其光吸收能力,从而提高其光催化活性。
-
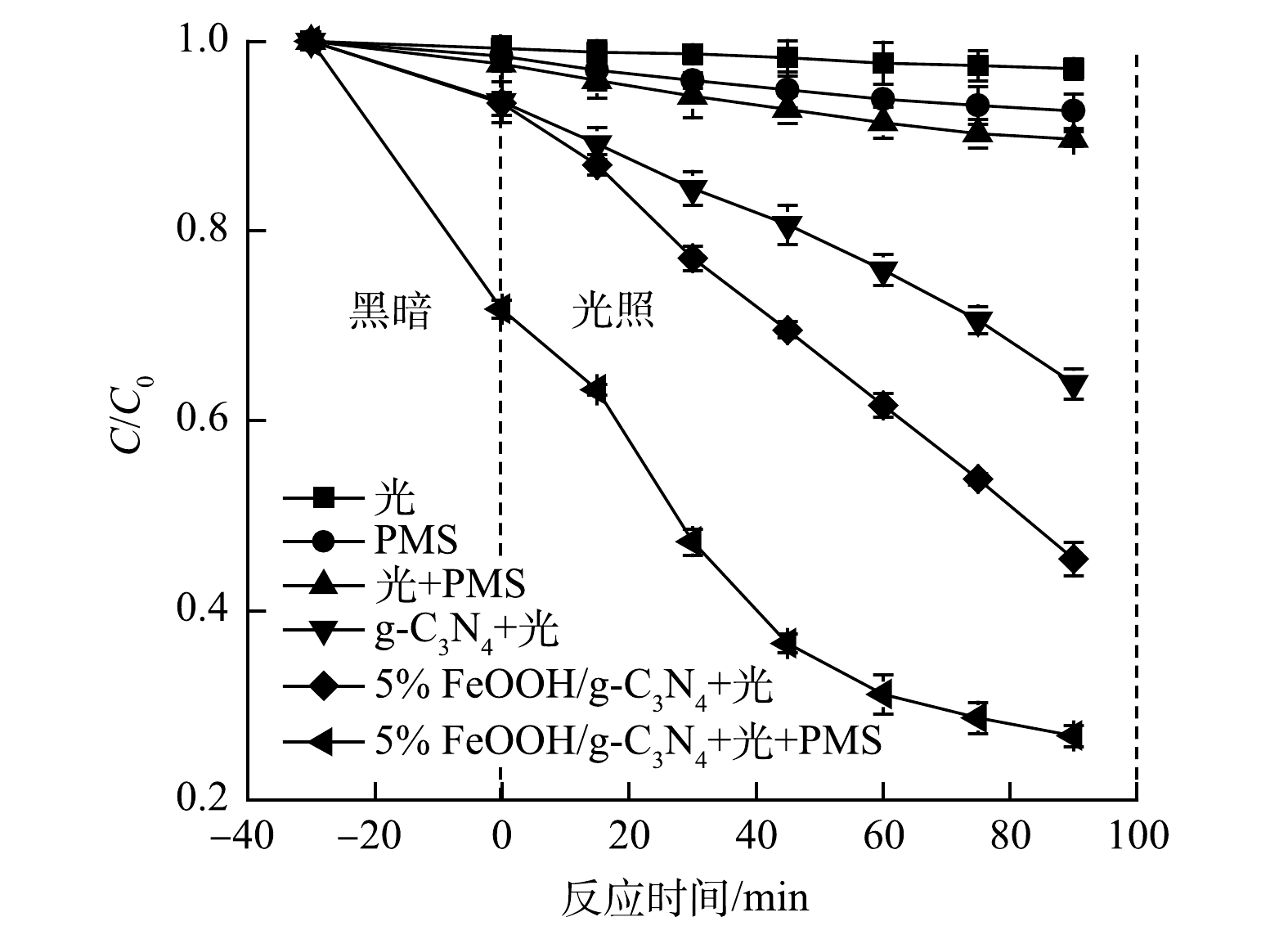
如图5所示,在可见光下,CIP几乎不会被降解,单独的PMS对CIP的降解率仅为7.35%;而在vis/PMS体系中,CIP的降解率为10.30%。这表明单独的PMS在光照下对CIP的氧化能力有限。在vis/催化剂体系中,在黑暗条件下,g-C3N4和FeOOH/g-C3N4在30 min后分别吸附了6.39%和6.56%的CIP;光照90 min后,g-C3N4对CIP的降解率为36.10%,而FeOOH/g-C3N4对CIP的降解率为54.59%。这表明FeOOH与g-C3N4的复合提高了g-C3N4的光催化活性。而在vis/催化剂/PMS体系中,可以看到,在黑暗条件下,30 min后FeOOH/g-C3N4对CIP的降解率达到28.19%,相较于vis/催化剂体系有大幅度的提升。这表明,除了少量吸附外,FeOOH/g-C3N4激活了PMS产生·OH和SO4·−并对CIP进行降解。之后,在光照90 min后,FeOOH/g-C3N4对CIP的降解率达到73.24%。这表明光催化+PMS活化体系可以加速污染物的氧化过程,从而显著提升催化剂对污染物的降解率。
-
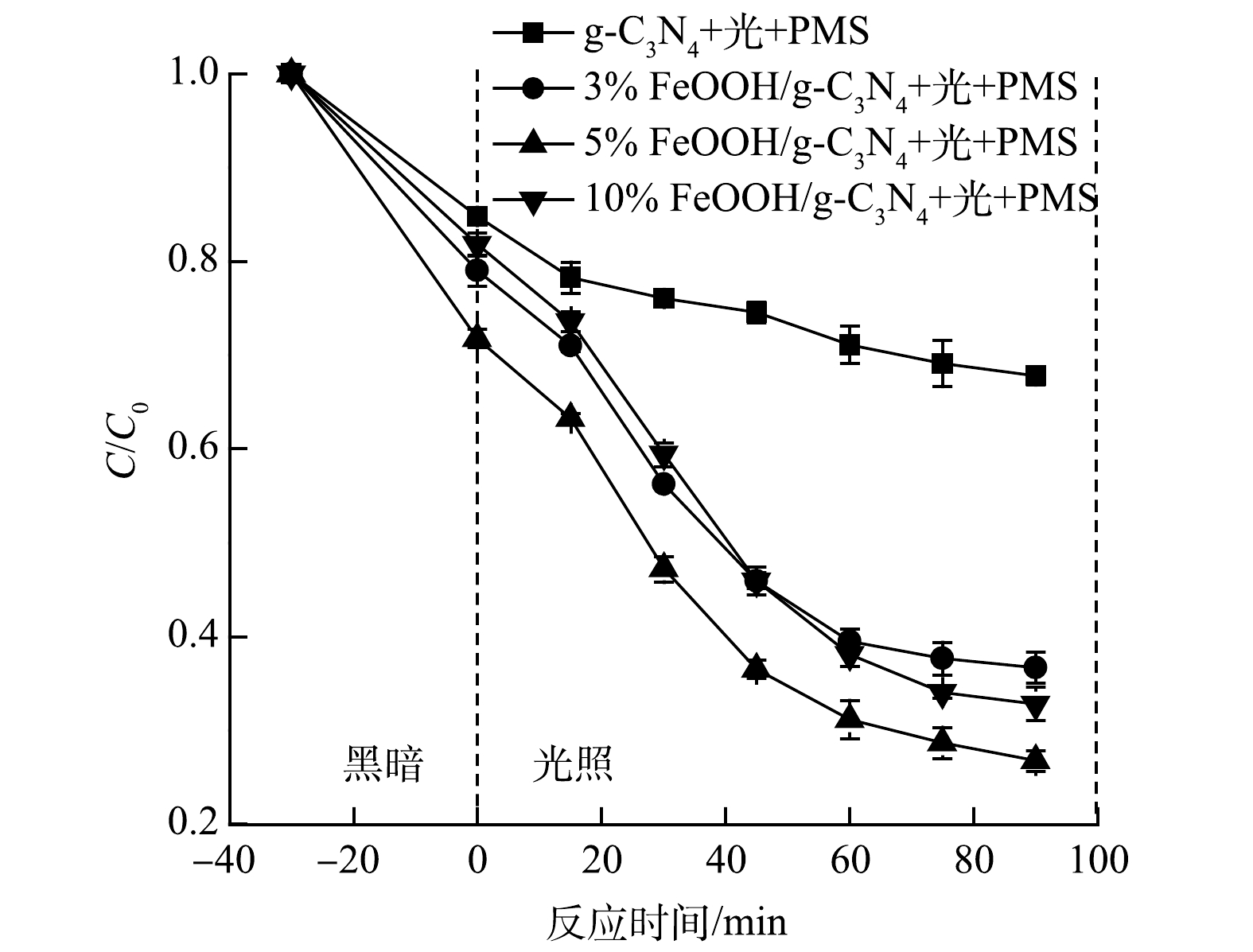
1)FeOOH负载量对CIP降解的影响。FeOOH的负载量对催化剂性能的影响结果见图6。复合FeOOH后,在黑暗条件下,催化剂对CIP的降解率由15.18%提升至28.20%。这表明复合FeOOH后催化剂的吸附性能有所增强,且FeOOH促进了PMS活化生成·OH和SO4·−。当FeOOH的负载量由3%提升至5%时,CIP的降解率由63.33%提升至73.24%。当FeOOH的负载量进一步提升至10%时,CIP的降解率反而下降至67.17%。这表明,与FeOOH的复合可以提高g-C3N4的光催化活性,但过量的FeOOH可以作为载流子的重组位点,从而降低光催化效率[25]。当FeOOH负载量为5%时,对CIP降解效果最佳,故后续实验均使用此负载量的光催化剂。
2)催化剂投加量对CIP降解的影响。由图7可见,当5% FeOOH/g-C3N4的投加量由0.1 g·L−1增加至0.4 g·L−1时,CIP的降解效率由60.47%增加至73.24%。这是因为:在一定范围内,增加催化剂投加量可以增加反应活性位点,从而产生更多的活性自由基,进而提高对污染物的降解效率。然而,当投加量增至0.6 g·L−1时,CIP的降解率反而下降至62.16%。这可能是由于过多的催化剂导致光穿透受阻,减少了催化剂暴露在光照下的表面积[26]。
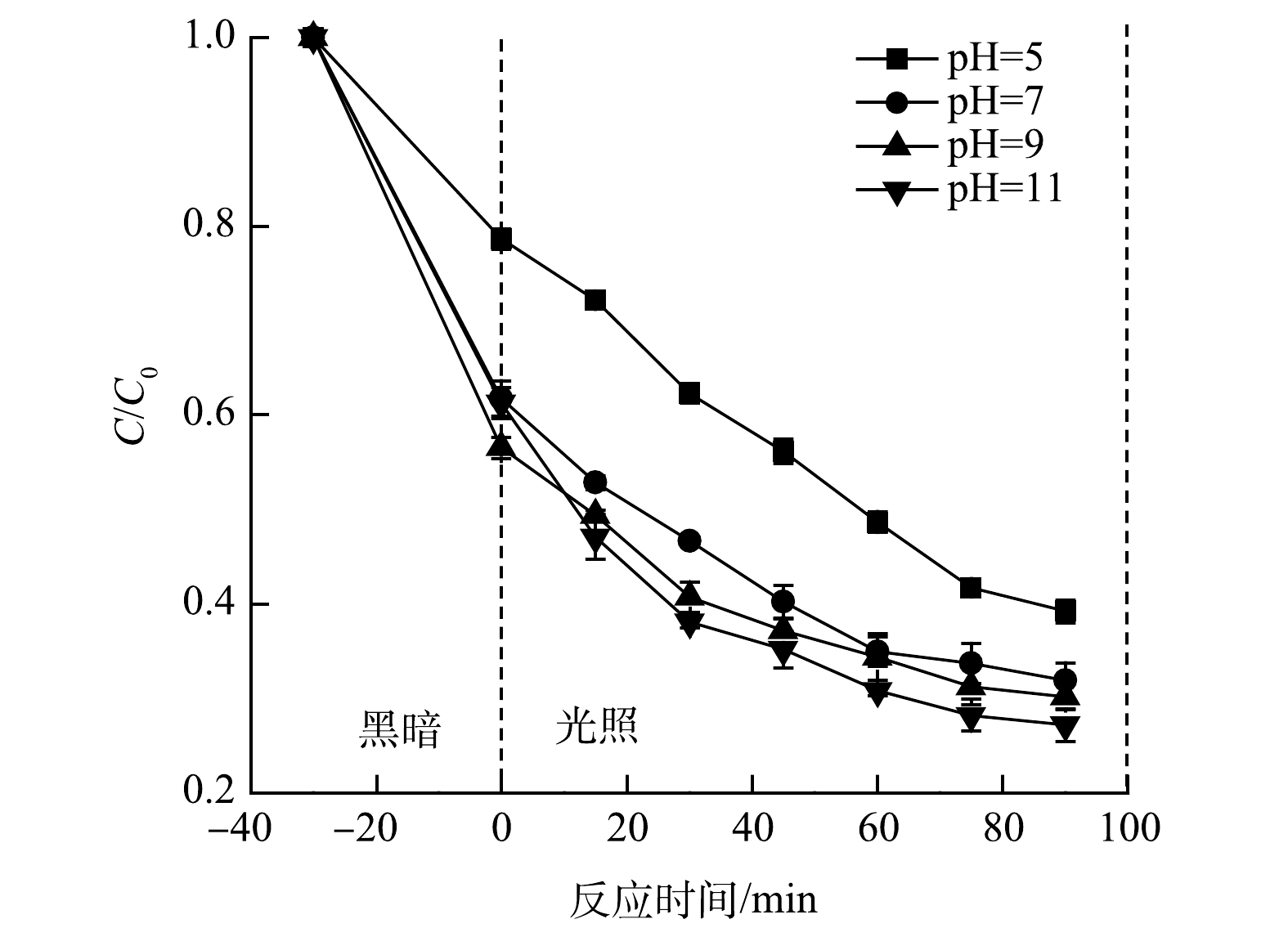
3) pH对CIP降解的影响。初始pH对降解CIP的影响结果见图8。当溶液初始pH为5.0、7.0、9.0和11.0时,CIP的降解率分别为60.78%、68.14%、69.86%和72.80%。在碱性条件下CIP的降解率更高。这可能是由于PMS的pKa为9.4,当溶液的pH低于9.4时,PMS主要产生SO52−,从而PMS激活被抑制[27]。因此,PMS在碱性条件下可以更好地分解为活性自由基,从而提高了CIP的降解效率。
-
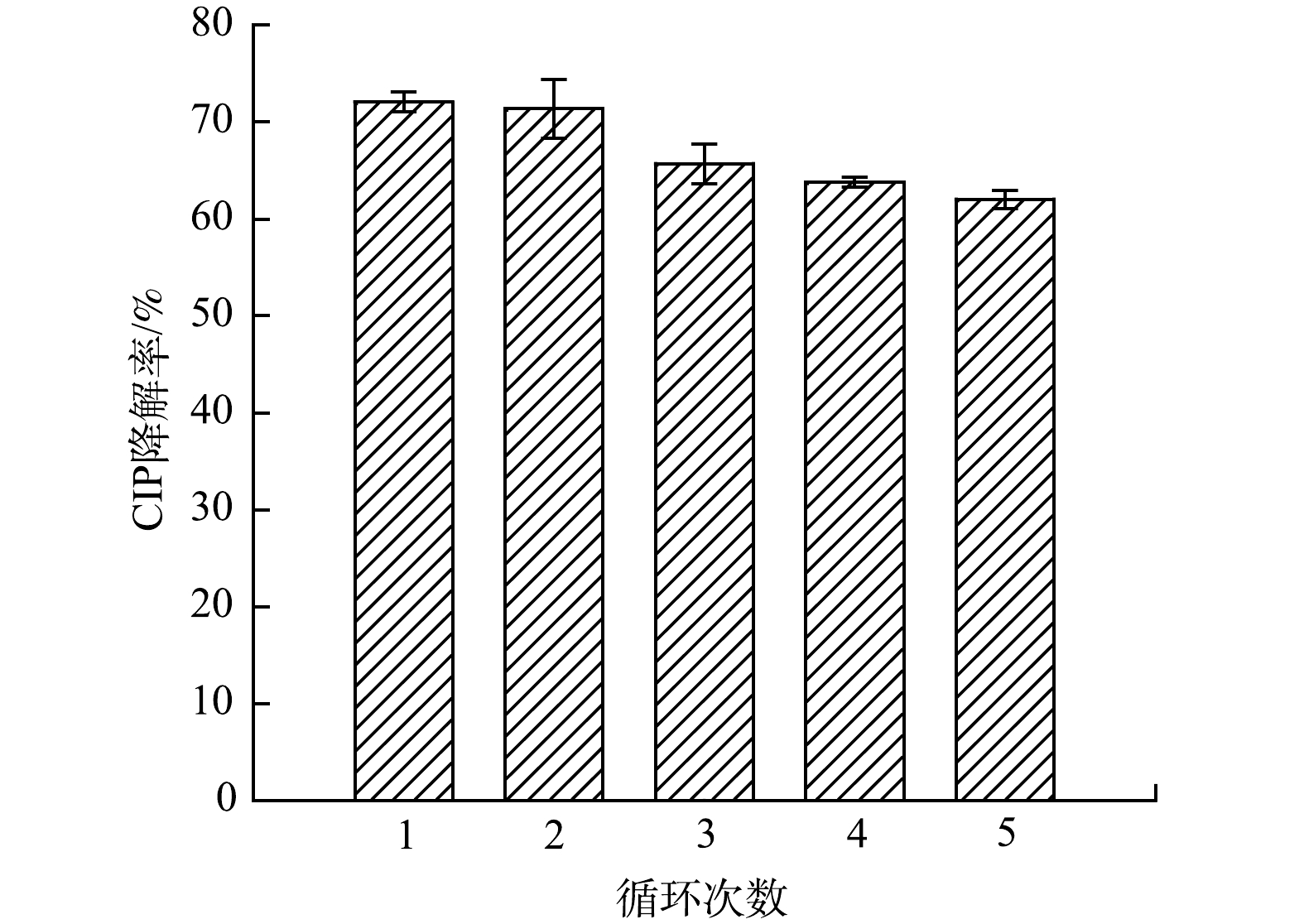
通过循环实验研究了5% FeOOH/g-C3N4的可重复利用性。如图9所示,在5次循环降解实验中,5% FeOOH/g-C3N4对CIP的降解率分别为73.24%、71.36%、65.69%、63.79%和62.02%。经过5次循环后,5% FeOOH/g-C3N4对CIP的降解率仅下降了11.22%。这可能是由于光催化剂的团聚和损失,以及CIP降解中间体对活性位点的阻断[28]。总体而言,所制备的催化剂重复性能较好。
-
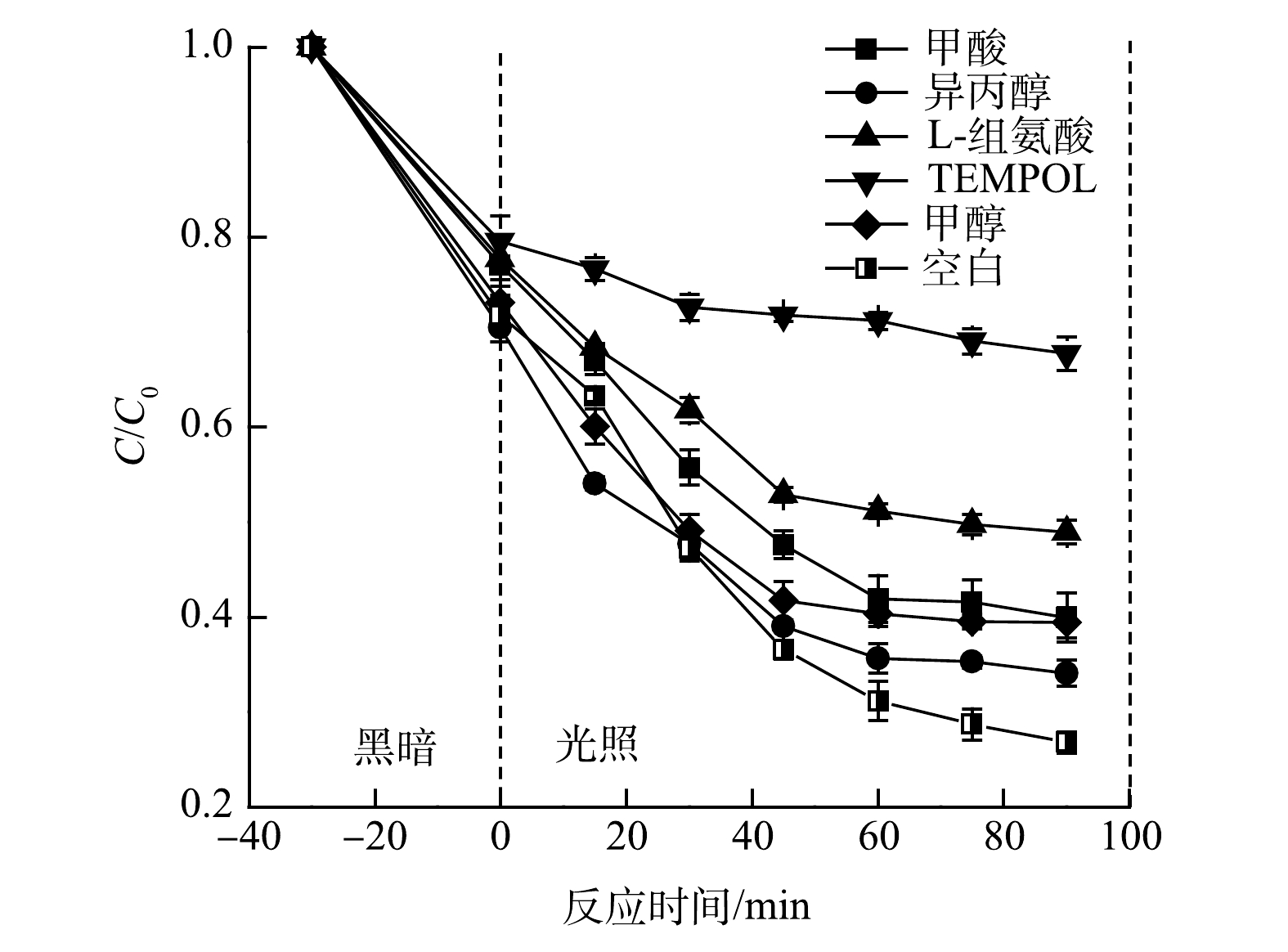
为了确定vis/FeOOH/g-C3N4/PMS体系降解CIP过程中主要的活性物种,采用多种捕获剂进行了捕获实验。其中,采用甲酸、L-组氨酸、TEMPOL和异丙醇作为h+、1O2、·O2−和·OH的捕获剂,采用甲醇对·OH和SO4·−进行捕获,捕获剂的浓度均为5 mmol·L−1。如图10所示,在甲酸、L-组氨酸、TEMPOL、异丙醇和甲醇存在下,CIP的降解效率分别为60.08%、51.06%、32.26%、65.93%和60.64%,其中SO4·−对CIP降解的抑制率为5.29%。上述结果表明,h+、1O2、·O2−、·OH和SO4·−均参与了CIP的光催化反应,而L-组氨酸和TEMPOL对CIP的降解抑制效果更强,表明在该反应体系中1O2和·O2−起主要作用。
基于以上结果可推测, FeOOH/g-C3N4/PMS/Vis体系对CIP的催化降解机理如下。首先,在黑暗状态下,FeOOH/g-C3N4可以激活PMS产生·OH和SO4·−自由基[28](式(1)~式(2)),产生的自由基进一步降解CIP。其次,在可见光照射下,FeOOH/g-C3N4被激发产生e−和h+,基于界面耦合效应,g-C3N4的CB上的e−迁移至FeOOH,FeOOH的VB上的h+迁移至g-C3N4。e−可以激活O2产生·O2—(式(3)),也可以被PMS捕获产生SO4·−自由基(式(1)),然后·O2—和SO4·−通过一系列反应转化为H2O2、·OH和1O2(式(4)~式(7));同时,部分电子通过直接界面电荷转移途径直接从g-C3N4的CB迁移到FeOOH中,促进Fe3+/Fe2+的循环(式(8)~式(9))。此外,从FeOOH转移至g-C3N4中的h+可以与PMS反应形成SO4·−。最后,·O2−、1O2、·OH、h+、SO4·−参与反应并有效降解CIP。
-
1)通过原位沉淀法制备了FeOOH/g-C3N4复合光催化剂。在pH为9.0、催化剂投加量为0.4 g·L−1时,光照90 min后,该FeOOH/g-C3N4/PMS/Vis体系对CIP的降解效率可达73.24%。
2)在vis/PMS反应条件下,相较于g-C3N4,5% FeOOH/g-C3N4表现出最佳催化活性。这可能是由于FeOOH与g-C3N4的复合增强了催化剂的光吸收能力,提升了其载流子分离效率。此外,5% FeOOH/g-C3N4在5个循环实验后对CIP降解效率仅下降10%左右,表现出满意的可重复性能。
3)自由基捕获实验结果表明,该FeOOH/g-C3N4/PMS/Vis体系中,h+、1O2、·O2−、·OH和SO4·−均参与了CIP的降解,其中1O2和·O2−起主要作用。
FeOOH/g-C3N4异质光催化剂耦合过硫酸盐降解环丙沙星
Catalytic degradation of ciprofloxacin by FeOOH/g-C3N4 heterogeneous photocatalyst activating peroxymonosulfate system
-
摘要: 采用原位沉淀法制备了FeOOH/g-C3N4异质光催化剂,并通过耦合过一硫酸盐(PMS)建立了多反应耦合型高级氧化体系,在此体系下考察了其对环丙沙星(CIP)的去除性能。在催化剂/可见光/PMS反应体系下,相对于g-C3N4,FeOOH/g-C3N4的催化性能明显提高,其中5% FeOOH/g-C3N4表现出最优异的催化性能,表明FeOOH的耦合提高了g-C3N4的光催化活性,且光催化+PMS活化体系加速了污染物的降解。此外,分别考察了FeOOH负载量、催化剂投加量、pH等因素对CIP降解性能的影响。结果表明:在催化剂投加量为0.4 g·L−1、pH为9.0时,5% FeOOH/g-C3N4对CIP的降解率达到72.34%。使用XRD、TEM、XPS、UV-vis DRS等分析方法对催化剂进行了表征, FeOOH的复合显著增强了g-C3N4的光吸收能力,提高了其光催化活性。最后,基于实验数据与活性物种捕获实验,初步提出了FeOOH/g-C3N4对CIP催化降解的可能机理。
-
关键词:
- FeOOH/g-C3N4 /
- 光催化 /
- 过一硫酸盐 /
- 环丙沙星
Abstract: The FeOOH/g-C3N4 heterogeneous photocatalyst was prepared by in-situ precipitation method, and the multi-reaction coupling advanced oxidation technology was further established by coupling the photocatalysis with peroxymonosulfate (PMS) activation, and its removal performance for ciprofloxacin (CIP) was investigated. Under vis/PMS conditions, the photocatalytic activity of FeOOH/g-C3N4 significantly increased compared to that of g-C3N4. Among them, 5% FeOOH/g-C3N4 showed the most excellent photocatalytic performance, indicating that FeOOH coupling could improve the photocatalytic activity of g-C3N4, and photocatalysis + PMS activation system accelerated the degradation of pollutants. The effects of various factors (i.e., FeOOH loading, photocatalyst dosage and initial pH) on CIP degradation efficiency were studied. The results showed that when the photocatalyst dosage was 0.4 g·L−1, and pH was 9.0, the best degradation efficiency of 5% FeOOH/g-C3N4 for CIP was 72.34%. The photocatalysts were characterized by XRD, TEM, XPS, and DRS, and the results indicated FeOOH coupling could greatly enhance the light absorption capacity of g-C3N4 and further improve its photocatalytic activity. Based on experimental data and active species capture experiment, CIP possible degradation mechanism by FeOOH/g-C3N4 photocatalyst was proposed.-
Key words:
- FeOOH/g-C3N4 /
- photocatalysis /
- peroxymonosulfate /
- ciprofloxacin
-
目前,由于在医疗、畜牧和水产养殖等领域的广泛应用[1],过量的抗生素以原型或代谢产物形式被排入水体中,对水生生态系统和人类健康造成了不可估量的危害。目前,抗生素废水处理方法主要有高级氧化法(advanced oxidation processes, AOPs)[2-3]、吸附法[4]、生物法[5]等。其中,作为高级氧化法之一的光催化氧化法因其处理成本低,降解效率高和无二次污染等优点,具有很好的应用前景。
研发高效稳定且成本低廉的光催化剂是目前光催化技术应用的关键。近年来,非金属半导体石墨相氮化碳(g-C3N4)因其原料廉价易得、物理化学性质稳定、能带结构合适与可见光响应活性强等特点[6-7],被认为是最具潜力的新型光催化剂。然而,块体g-C3N4的表面积小、电荷重组快、可见光利用率低,使其在实际应用中受到极大限制。目前,通过耦合其他半导体构建异质结是提高g-C3N4光催化活性的有效方法,2个半导体间密切接触可以形成较大的界面面积,并产生激子解离界面和空间带电载流子迁移通道,加速光生载流子分离,从而提升g-C3N4的光催化活性[8]。例如,SUN等[9]通过静电自组装制备了SnO2/g-C3N4异质结,在可见光照射50 min后,对罗丹明B的降解率达到96.9%;ZHANG等[8]采用聚合偶联共沉淀方法制备了增强分子氧活化的FeOOH QDs/CQDs/g-C3N4异质结,在可见光照射下可以降解85.5%的土霉素。以上结果表明,通过构建异质结可以增强g-C3N4的催化活性,但仍有巨大的提升空间。
基于SO4·−的AOPs因其氧化性能强、氧化剂稳定性好、环保无害等优点被广泛应用于降解有机污染物[10-11]。SO4·−通常可以通过光、微波、超声波、加热、过渡金属离子、金属和无金属催化剂等活化过一硫酸盐(Peroxymonosulfate, PMS, HSO5−)和过二硫酸盐(Peroxodisulfate, PS, S2O82−)产生[12-15]。其中,过渡金属离子易活化,无需额外能量输入,已被广泛研究[16]。而在基于过渡金属的体系中,铁基催化剂/过硫酸盐由于铁的化学状态不同(Fe0、Fe2+、Fe3+),是一个高度灵活的体系。因此,将铁基催化剂/过硫酸盐体系与光催化耦合,构建多反应耦合型高级氧化体系用于高效降解水中有机污染物是目前研究的热点。
在铁基催化剂中,FeOOH由于环境友好性、自然丰度、相对稳定性、可见光响应、耐腐蚀性强和低成本而引起广泛关注。因此,本研究采用原位沉淀法将FeOOH与g-C3N4复合,成功制备出FeOOH/g-C3N4异质光催化剂,并通过耦合过一硫酸盐(PMS)构建多反应耦合型高级氧化体系(光催化+PMS活化),研究了其降解环丙沙星(Ciprofloxacin, CIP)的性能,考察了各影响因素对CIP催化降解效果的影响,评价了体系的稳定性并提出了可能的催化降解机理。
1. 材料与方法
1.1 实验材料与试剂
CIP (C17H18FN3O3)、PMS (2KHSO5·KHSO4·K2SO4)购于上海麦克林生化科技有限公司;三聚氰胺(C3H6N6)、六水合氯化铁(FeCl3·6H2O)、碳酸氢铵(NH4HCO3)、无水乙醇购于天津市科密欧化学试剂有限公司,所有试剂均为分析纯,实验用水为超纯水。
1.2 g-C3N4和FeOOH/g-C3N4的制备
以三聚氰胺作为原料,采用热缩聚法[17]制备g-C3N4。将一定量的g-C3N4分散在40 mL无水乙醇中,然后加入1 mmol的FeCl3·6H2O,搅拌至溶解完全后再加入3 mmol的NH4HCO3,在室温下搅拌8 h,待反应完全后离心,用无水乙醇和去离子水洗涤数次后,真空冷冻干燥10 h即可得到样品。在FeOOH/g-C3N4中,FeOOH的理论质量百分比分别为3%、5%和10%。
1.3 分析方法
采用Rigaku Ultima IV型X射线衍射仪(X-ray diffraction, XRD)对光催化剂的晶体结构进行表征。采用JEM-2100F型高分辨透射电镜(Transmission electron microscope, TEM)对催化剂的微观形貌进行表征。催化剂的X射线光电子能谱(X-ray photoelectron spectroscopy, XPS)采用单色铝辐射的K-Alpha光谱仪测定,并以284.8 eV处的C1s峰作为参考校正结合能。采用Perkinelmer Lambda 1050型UV/Vis分光光度计测定了催化剂的紫外-可见漫反射光谱(UV-vis diffuse reflection spectra, UV-vis DRS)。
1.4 实验方法
称取40 mg FeOOH/g-C3N4分散于100 mL浓度为10 mg·L−1的环丙沙星溶液中,并加入10 mg的PMS,先在黑暗下搅拌30 min,然后以氙灯为可见光源(λ>400 nm,300 W)进行光催化反应。每隔15 min从光催化反应器中抽取3 mL的悬浮液,微孔过滤(孔径为0.45 μm)后通过紫外可见分光光度计(TU-1900, Pgeneral)测定样品浓度。将反应后的催化剂离心洗涤,真空干燥后按照上述步骤重复4次,考察催化剂的稳定性和可重复性。
2. 结果与讨论
2.1 催化剂的表征
1)XRD表征。如图1所示,FeOOH的谱图中未检测到衍射峰。这表明其为无定型结构。g-C3N4的谱图在13.1°和27.6°处有2个典型的衍射峰,分别属于g-C3N4的(100)和(002)晶面[18]。FeOOH/g-C3N4系列材料中均出现g-C3N4的特征峰。随着FeOOH负载量的增加,g-C3N4各晶面的衍射峰强度逐渐减弱。这可能是由于FeOOH与g-C3N4的强耦合影响了其结晶度[19]。
2) TEM表征。如图2(a)~(b)所示,g-C3N4呈现片层堆积态,而FeOOH呈现聚集态;由图2(c)~(d)可见,与g-C3N4和FeOOH的TEM图像比较,可以观察到在FeOOH/g-C3N4复合物的片层中出现聚集体,这表明FeOOH已被成功负载。
3) XPS表征。在全谱图中(图3(a))观察到了C、N、O和Fe 4种元素,表明FeOOH已经成功负载到g-C3N4表面。C1s、N1s、O1s和Fe2p的高清分谱如图3(b)~(e)所示。在C1s谱图(图3(b))中,288.25 eV和284.8 eV的2个峰分别归因于—N—C=N—和C—C键[20]。在N1s光谱(图3(c))中可以观察到以399.21、400.77、401.71和404.67 eV为中心的4个峰,分别可归因于C=N—C、N—(C)3、C—N—H 和π激发或充电效应[21]。在O1s光谱(图3(d))中,在529.75、531.18和532.69 eV处存在3个峰,其中529.75 eV和531.18 eV的2个峰都与Fe—O—Fe键有关[22],532.69 eV处的峰可归因于催化剂表面的吸附水[23]。图3(e)显示了5% FeOOH/g-C3N4的Fe2p光谱。可以观察到在709.90、712.91、719.90、723.83和732.45 eV处有特征峰。其中,在709.90、712.91和723.83 eV处的结合能峰分别属于Fe2p3/2和Fe2p1/2光谱,在Fe2p3/2中,709.90 eV处的峰表示Fe2+,712.91 eV的峰表示Fe3+。此外,在719.90和732.45 eV处分别观测到Fe2p3/2和Fe2p1/2的振动卫星峰[17]。可以观察到,在C1s谱(图3(b))中,使用后的5% FeOOH/g-C3N4的结合能峰有些微红移;在N1s、O1s和Fe2p的谱图(图3(c)~(e))中,使用后的5% FeOOH/g-C3N4的结合能峰均有些微蓝移。这表明,催化反应已引发元素化学环境发生改变。在Fe2p3/2中,709.90 eV处的Fe2+和712.91 eV的Fe3+峰面积占总铁的比例为14.21%和85.79%,经降解CIP反应后,两者占总铁的比例调整为39.23%和60.77%。Fe3+的比例降低了25.02%。表明部分Fe3+利用电子被还原为Fe2+。
4) UV-vis分析。如图4(a)所示,复合FeOOH后,FeOOH/g-C3N4的光吸收能力大幅度提升,光吸收边缘显著扩展。这是由于FeOOH在几乎整个UV-Vis区域都表现出光吸收能力[24],其与g-C3N4之间的界面电荷转移效应使得FeOOH/g-C3N4复合催化剂在可见光区域得到明显增强的光吸收能力。此外,根据Tauc曲线,通过Kubelka-Munk方程计算了g-C3N4和FeOOH/g-C3N4的带隙值。如图4(b)所示,g-C3N4和FeOOH/g-C3N4的带隙值分别为2.52 eV和1.50 eV。带隙的变窄意味着e−从价带(valence band, VB)跃迁至导带(conduction band, CB)所需能量减少,有利于光吸收。由此可见,与FeOOH的复合大大扩展了g-C3N4的可见光吸收,提高了其光吸收能力,从而提高其光催化活性。
2.2 FeOOH/g-C3N4的催化性能
如图5所示,在可见光下,CIP几乎不会被降解,单独的PMS对CIP的降解率仅为7.35%;而在vis/PMS体系中,CIP的降解率为10.30%。这表明单独的PMS在光照下对CIP的氧化能力有限。在vis/催化剂体系中,在黑暗条件下,g-C3N4和FeOOH/g-C3N4在30 min后分别吸附了6.39%和6.56%的CIP;光照90 min后,g-C3N4对CIP的降解率为36.10%,而FeOOH/g-C3N4对CIP的降解率为54.59%。这表明FeOOH与g-C3N4的复合提高了g-C3N4的光催化活性。而在vis/催化剂/PMS体系中,可以看到,在黑暗条件下,30 min后FeOOH/g-C3N4对CIP的降解率达到28.19%,相较于vis/催化剂体系有大幅度的提升。这表明,除了少量吸附外,FeOOH/g-C3N4激活了PMS产生·OH和SO4·−并对CIP进行降解。之后,在光照90 min后,FeOOH/g-C3N4对CIP的降解率达到73.24%。这表明光催化+PMS活化体系可以加速污染物的氧化过程,从而显著提升催化剂对污染物的降解率。
2.3 各因素对FeOOH/g-C3N4催化降解CIP效果的影响
1)FeOOH负载量对CIP降解的影响。FeOOH的负载量对催化剂性能的影响结果见图6。复合FeOOH后,在黑暗条件下,催化剂对CIP的降解率由15.18%提升至28.20%。这表明复合FeOOH后催化剂的吸附性能有所增强,且FeOOH促进了PMS活化生成·OH和SO4·−。当FeOOH的负载量由3%提升至5%时,CIP的降解率由63.33%提升至73.24%。当FeOOH的负载量进一步提升至10%时,CIP的降解率反而下降至67.17%。这表明,与FeOOH的复合可以提高g-C3N4的光催化活性,但过量的FeOOH可以作为载流子的重组位点,从而降低光催化效率[25]。当FeOOH负载量为5%时,对CIP降解效果最佳,故后续实验均使用此负载量的光催化剂。
2)催化剂投加量对CIP降解的影响。由图7可见,当5% FeOOH/g-C3N4的投加量由0.1 g·L−1增加至0.4 g·L−1时,CIP的降解效率由60.47%增加至73.24%。这是因为:在一定范围内,增加催化剂投加量可以增加反应活性位点,从而产生更多的活性自由基,进而提高对污染物的降解效率。然而,当投加量增至0.6 g·L−1时,CIP的降解率反而下降至62.16%。这可能是由于过多的催化剂导致光穿透受阻,减少了催化剂暴露在光照下的表面积[26]。
3) pH对CIP降解的影响。初始pH对降解CIP的影响结果见图8。当溶液初始pH为5.0、7.0、9.0和11.0时,CIP的降解率分别为60.78%、68.14%、69.86%和72.80%。在碱性条件下CIP的降解率更高。这可能是由于PMS的pKa为9.4,当溶液的pH低于9.4时,PMS主要产生SO52−,从而PMS激活被抑制[27]。因此,PMS在碱性条件下可以更好地分解为活性自由基,从而提高了CIP的降解效率。
2.4 FeOOH/g-C3N4的重复利用性
通过循环实验研究了5% FeOOH/g-C3N4的可重复利用性。如图9所示,在5次循环降解实验中,5% FeOOH/g-C3N4对CIP的降解率分别为73.24%、71.36%、65.69%、63.79%和62.02%。经过5次循环后,5% FeOOH/g-C3N4对CIP的降解率仅下降了11.22%。这可能是由于光催化剂的团聚和损失,以及CIP降解中间体对活性位点的阻断[28]。总体而言,所制备的催化剂重复性能较好。
2.5 CIP的降解机理
为了确定vis/FeOOH/g-C3N4/PMS体系降解CIP过程中主要的活性物种,采用多种捕获剂进行了捕获实验。其中,采用甲酸、L-组氨酸、TEMPOL和异丙醇作为h+、1O2、·O2−和·OH的捕获剂,采用甲醇对·OH和SO4·−进行捕获,捕获剂的浓度均为5 mmol·L−1。如图10所示,在甲酸、L-组氨酸、TEMPOL、异丙醇和甲醇存在下,CIP的降解效率分别为60.08%、51.06%、32.26%、65.93%和60.64%,其中SO4·−对CIP降解的抑制率为5.29%。上述结果表明,h+、1O2、·O2−、·OH和SO4·−均参与了CIP的光催化反应,而L-组氨酸和TEMPOL对CIP的降解抑制效果更强,表明在该反应体系中1O2和·O2−起主要作用。
基于以上结果可推测, FeOOH/g-C3N4/PMS/Vis体系对CIP的催化降解机理如下。首先,在黑暗状态下,FeOOH/g-C3N4可以激活PMS产生·OH和SO4·−自由基[28](式(1)~式(2)),产生的自由基进一步降解CIP。其次,在可见光照射下,FeOOH/g-C3N4被激发产生e−和h+,基于界面耦合效应,g-C3N4的CB上的e−迁移至FeOOH,FeOOH的VB上的h+迁移至g-C3N4。e−可以激活O2产生·O2—(式(3)),也可以被PMS捕获产生SO4·−自由基(式(1)),然后·O2—和SO4·−通过一系列反应转化为H2O2、·OH和1O2(式(4)~式(7));同时,部分电子通过直接界面电荷转移途径直接从g-C3N4的CB迁移到FeOOH中,促进Fe3+/Fe2+的循环(式(8)~式(9))。此外,从FeOOH转移至g-C3N4中的h+可以与PMS反应形成SO4·−。最后,·O2−、1O2、·OH、h+、SO4·−参与反应并有效降解CIP。
HSO−5+e−→SO⋅−4+OH− (1) HSO−5+e−→SO2−4+⋅OH (2) e−+O2→⋅O−2 (3) ⋅O−2+2H++e−→H2O2 (4) H2O2+e−→⋅OH+OH− (5) SO⋅−4+OH−→SO2−4+⋅OH (6) ⋅OH+⋅O−2→1O2+OH− (7) Fe3++e−→Fe2+ (8) Fe2++H2O2→Fe3++⋅OH+OH− (9) 3. 结论
1)通过原位沉淀法制备了FeOOH/g-C3N4复合光催化剂。在pH为9.0、催化剂投加量为0.4 g·L−1时,光照90 min后,该FeOOH/g-C3N4/PMS/Vis体系对CIP的降解效率可达73.24%。
2)在vis/PMS反应条件下,相较于g-C3N4,5% FeOOH/g-C3N4表现出最佳催化活性。这可能是由于FeOOH与g-C3N4的复合增强了催化剂的光吸收能力,提升了其载流子分离效率。此外,5% FeOOH/g-C3N4在5个循环实验后对CIP降解效率仅下降10%左右,表现出满意的可重复性能。
3)自由基捕获实验结果表明,该FeOOH/g-C3N4/PMS/Vis体系中,h+、1O2、·O2−、·OH和SO4·−均参与了CIP的降解,其中1O2和·O2−起主要作用。
-
-
[1] SHI Y, WAN D, HUANG J, et al. Stable LBL self-assembly coating porous membrane with 3D heterostructure for enhanced water treatment under visible light irradiation[J]. Chemosphere, 2020, 252: 126581. doi: 10.1016/j.chemosphere.2020.126581 [2] 杨婷婷, 陈星, 陈长斌, 等. CeO2/g-C3N4光催化-芬顿高效降解盐酸强力霉素[J]. 环境工程学报, 2021, 15(8): 2576-2587. [3] WANG H, YE Z, LIU C, et al. Visible light driven Ag/Ag3PO4/AC photocatalyst with highly enhanced photodegradation of tetracycline antibiotics[J]. Applied Surface Science, 2015, 353: 391-399. doi: 10.1016/j.apsusc.2015.06.125 [4] LI R, WANG Z, ZHAO X, et al. Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water[J]. Environmental Science and Pollution Research, 2018, 25(31): 31136-31148. doi: 10.1007/s11356-018-3064-1 [5] 陈小洁, 李凤玉, 郝雅宾. 两种水生植物对抗生素污染水体的修复作用[J]. 亚热带植物科学, 2012, 41(4): 1-7. [6] AN X, WU S, TANG Q, et al. Strongly coupled polyoxometalates/oxygen doped g-C3N4 nanocomposites as Fenton-like catalysts for efficient photodegradation of sulfosalicylic acid[J]. Catalysis Communications, 2018, 112: 63-67. doi: 10.1016/j.catcom.2018.03.013 [7] HU J, ZHANG P, AN W, et al. In-situ Fe-doped g-C3N4 heterogeneous catalyst via photocatalysis-Fenton reaction with enriched photocatalytic performance for removal of complex wastewater[J]. Applied Catalysis B:Environmental, 2019, 245: 130-142. doi: 10.1016/j.apcatb.2018.12.029 [8] ZHANG M M, LAI C, LI B S, et al. Unravelling the role of dual quantum dots cocatalyst in 0D/2D heterojunction photocatalyst for promoting photocatalytic organic pollutant degradation[J]. Chemical Engineering Journal, 2020, 396: 1-13. [9] SUN C Y, YANG J K, ZHU Y, et al. Synthesis of 0D SnO2 nanoparticles/2D g-C3N4 nanosheets heterojunction: improved charge transfer and separation for visible-light photocatalytic performance[J]. Journal of Alloys and Compounds, 2021,871: 1-14. [10] LIN K Y A, ZHANG Z Y. Degradation of bisphenol a using peroxymonosulfate activated by one-step prepared sulfur-doped carbon nitride as a metal-free heterogeneous catalyst[J]. Chemical Engineering Journal, 2017, 313: 1320-1327. doi: 10.1016/j.cej.2016.11.025 [11] LIU J, ZHOU J, DING Z, et al. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye[J]. Ultrasonics Sonochemistry, 2017, 34: 953-959. doi: 10.1016/j.ultsonch.2016.08.005 [12] GUAN Y H, MA J, LI X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system[J]. Environmental Science & Technology, 2011, 45(21): 9308-9314. [13] CAI C, ZHANG H, ZHONG X, et al. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of orange II in water[J]. Journal of Hazardous Materials, 2015, 283: 70-79. doi: 10.1016/j.jhazmat.2014.08.053 [14] JI Y, DONG C, KONG D, et al. Heat-activated persulfate oxidation of atrazine: Implications for remediation of groundwater contaminated by herbicides[J]. Chemical Engineering Journal, 2015, 263: 45-54. doi: 10.1016/j.cej.2014.10.097 [15] WACŁAWEK S, LUTZE H V, GRüBEL K, et al. Chemistry of persulfates in water and wastewater treatment: A review[J]. Chemical Engineering Journal, 2017, 330: 44-62. doi: 10.1016/j.cej.2017.07.132 [16] ZOU, CHEN, LW, et al. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine[J]. Environmental Science & Technology, 2013, 47(20): 11685-11691. [17] YANG H, ZHANG S, CAO R, et al. Constructing the novel ultrafine amorphous iron oxyhydroxide/g-C3N4 nanosheets heterojunctions for highly improved photocatalytic performance[J]. Scientific Reports, 2017, 7(1): 8686. doi: 10.1038/s41598-017-09283-1 [18] WU Y Z, WARD-BOND J, LI D L, et al. g-C3N4@alpha-Fe2O3/C photocatalysts: Synergistically intensified charge generation and charge transfer for NADH regeneration[J]. ACS Catalysis, 2018, 8(7): 5664-5674. doi: 10.1021/acscatal.8b00070 [19] LI C, DU Y, WANG D, et al. Unique P—Co—N surface bonding states constructed on g-C3N4 nanosheets for drastically enhanced photocatalytic activity of H2 evolution[J]. Advanced Functional Materials, 2017, 27(4): 1604328. doi: 10.1002/adfm.201604328 [20] WANG K, LI Y, LI J, et al. Boosting interfacial charge separation of Ba5Nb4O15/g-C3N4 photocatalysts by 2D/2D nanojunction towards efficient visible-light driven H2 generation[J]. Applied Catalysis B:Environmental, 2020, 263: 1-10. [21] BICALHO H A, RIOS R D F, BINATTI I, et al. Efficient activation of peroxymonosulfate by composites containing iron mining waste and graphitic carbon nitride for the degradation of acetaminophen[J]. Journal of Hazardous Materials, 2020, 400: 1-10. [22] GUO T, WANG K, ZHANG G K, et al. A novel alpha-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance[J]. Applied Surface Science, 2019, 469: 331-339. doi: 10.1016/j.apsusc.2018.10.183 [23] KATSUMATA H, SAKAI T, SUZUKI T, et al. Highly efficient photocatalytic activity of g-C3N4/Ag3PO4 hybrid photocatalysts through Z-scheme photocatalytic mechanism under visible light[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 8018-8025. [24] ZHAO H, TIAN C, MEI J, et al. Synergistic effect and mechanism of catalytic degradation toward antibiotic contaminants by amorphous goethite nanoparticles decorated graphitic carbon nitride[J]. Chemical Engineering Journal, 2020, 390: 1-12. [25] BANIC N, ABRAMOVIC B, KRSTIC J, et al. Photodegradation of thiacloprid using Fe/TiO2 as a heterogeneous photo-Fenton catalyst[J]. Applied Catalysis B:Environmental, 2011, 107(3/4): 363-371. doi: 10.1016/j.apcatb.2011.07.037 [26] DANGWANG DIKDIM J M, GONG Y, NOUMI G B, et al. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation[J]. Chemosphere, 2019, 217: 833-842. doi: 10.1016/j.chemosphere.2018.10.177 [27] REN H T, JIA S Y, WU Y, et al. Improved photochemical reactivities of Ag2O/g-C3N4 in phenol degradation under UV and visible light[J]. Industrial & Engineering Chemistry Research, 2014, 53(45): 17645-17653. [28] SHI Y H, LI J S, WAN D J, et al. Peroxymonosulfate-enhanced photocatalysis by carbonyl-modified g-C3N4 for effective degradation of the tetracycline hydrochloride[J]. Science of the Total Environment, 2020, 749: 1-14. -






 下载:
下载: