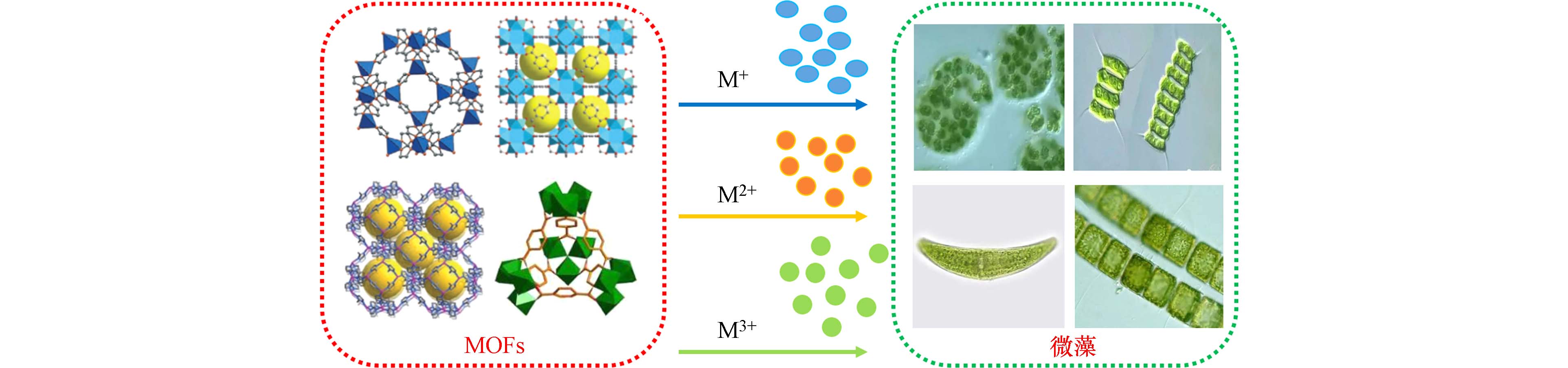
-
随着工业化进程的快速发展,全球温室效应不断加剧且氮磷营养物质排放日益增加,由此引发的水体富营养化日趋严重,消除有害蓝藻和控制水体富营养化成为全球共同面临的世界难题之一[1]. 针对水体富营养化有害蓝藻,国内外普遍采用传统的物理(超声、曝气、打捞等)[2]、化学(絮凝剂、强氧化剂、纳米材料等)[3 − 6]及生物(溶藻微生物、化感物质、水生生物等)[7 − 11]方法,鉴于化学方法成本低、见效快,其在有害蓝藻治理方面展现出广阔的应用前景[5, 12].
金属有机框架材料(metal organic frameworks,MOFs)是由金属离子或金属团簇与有机配体结合形成的纳米多晶材料[13],其具有孔隙率高、比表面积大、吸附性能高、反应活性佳、孔径形状可调及功能设计性强等优点[14],被广泛应用于吸附[15]、分离[16]、催化[17 − 18]、监测[19]和抑菌[20]等环境治理和检测领域. 研究表明,MOFs材料可通过静电引力、配位作用等方式附着于藻细胞表面[21],也可以通过释放金属离子的方式进入藻细胞[22],同时还可以在光照作用下生成活性自由基团[18, 23],上述途径均可导致藻细胞生长受到抑制或死亡.
近期楚弘宇等[24]对MOFs材料光催化除藻机制进行了总结,但关于离子释放及絮凝沉淀等方面的除藻性能和机理尚未阐述;此外,MOFs材料除藻性能的影响因素及MOFs材料对藻类有机物和藻毒素等分泌物的作用效果还需进一步明确[9]. 因此,本文基于MOFs材料的除藻性能,综述了MOFs材料对典型绿藻(小球藻、莱茵衣藻)和水华/赤潮藻属(铜绿微囊藻和米氏凯伦藻)的毒理效应,重点分析了MOFs材料抑藻的主要影响因素,阐述了MOFs材料对藻类的致毒作用机理,并对MOFs材料除藻的研究方向进行了展望,以期为MOFs材料的潜在应用提供借鉴和参考.
-
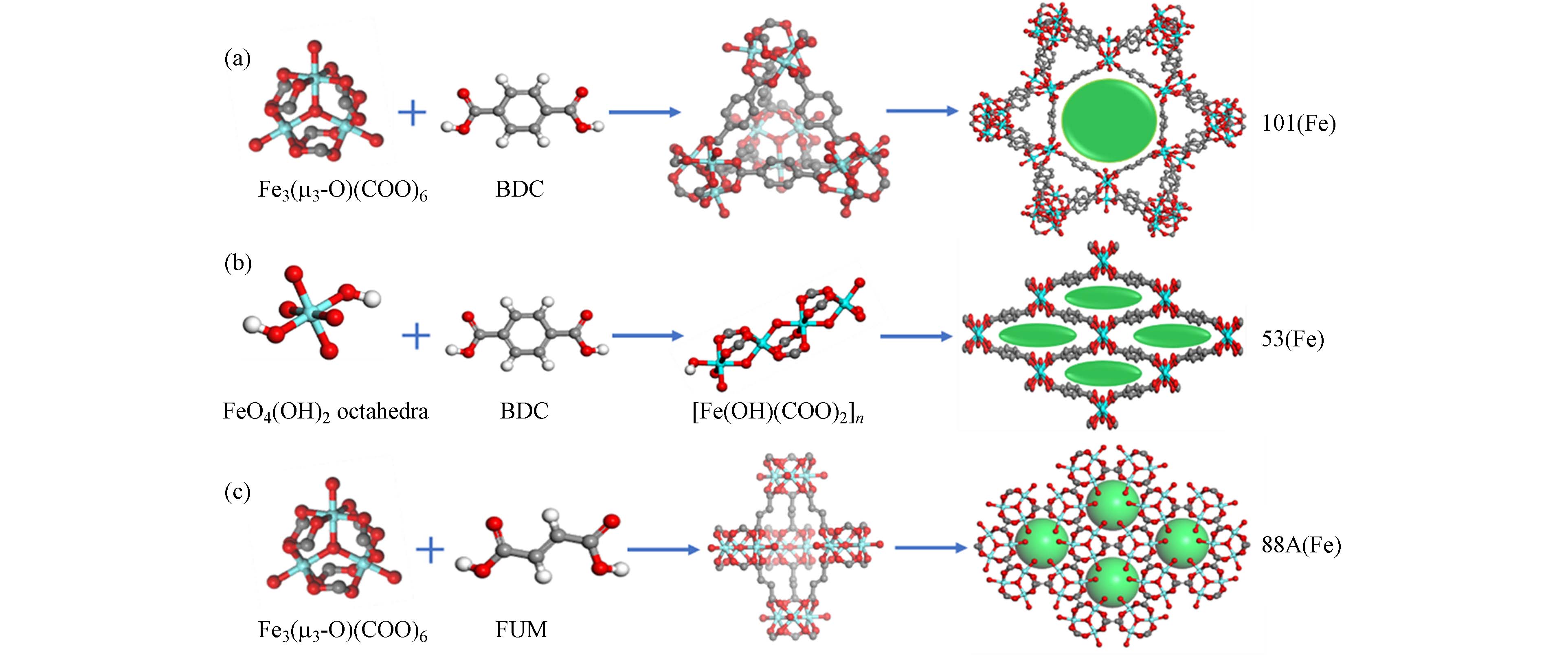
MOFs因特殊结构和可调功能,其对环境污染物的吸附去除[14 − 15]和催化氧化[17 − 18]表现出优异性能. 该材料主要分为单一MOFs和复合MOFs,常见的单一MOFs系列包括多孔协调网络材料(Porous coordination networks,PCNs)[25]、莱瓦希尔材料(Materials of Institut Lavoisier,MILs)[26]、挪威奥斯陆大学材料(University of Oslo,UIOs)[23]、类沸石咪唑酯骨架材料(Zeolitic imidazolate frameworks,ZIFs)[27]和网状金属有机框架材料(Isoreticular metal-organic frameworks,IRMOFs)[28]等5种类型. 单一MOFs的合成方法主要有水热/溶剂合成法、微波加热法、超声法、机械化学法及电化学法等[29 − 31]. 四川大学Zhang等[27]以Zn (CH3COO)2和甲基咪唑为原材料,利用机械化学合成法制备了ZIF-8;此外,该课题组还使用水热合成法制备了不同拓扑结构MIL-101(Fe)、MIL-53(Fe)和MIL-88A(Fe)系列[26](图1). 通过对单一MOFs进行修饰、改性或负载,可合成复合MOFs[23, 32 − 34],其中,基于Cu-MOFs、ZIF-8、UIO-66及MIL-101等制备的复合MOFs被广泛应用于抑藻研究[23, 34 − 36].
-
不同MOFs材料对典型藻属的抑制效果如表1所示. 由表1可知,MOFs材料对不同藻属的去除率范围为10.0%—99.9%,其中,Ag/AgCl@g-C3N4@UIO-66(NH2)抑藻性能最佳[23].
-
具有抑藻功能的单一MOFs材料主要包括Cu-MOFs[36 − 37, 43]、Zr-MOFs[23]、ZIFs[22, 35]及MOF-535[35]. Martín-Betancor等[22]比较了3种ZIFs材料对微藻的毒性效应,研究表明,处理48 h后3种材料对聚球藻(Synechococcus sp.)的叶绿素a抑制率均高于80%,其中Ag-ZIF的抑制率高达85%;对于鱼腥藻(Anabaena sp.)而言,Zn-ZIF和Ag-ZIF材料的抑藻效果明显高于Co-ZIF. Ag-ZIF对莱茵衣藻(Chlamydomonas reinhardtii)的抑制率高达90%,Zn-ZIF和Co-ZIF对Chlamydomonas sp.的抑藻率仅为30%和40%. Hu等[17]和Wang等[36]发现,Cu-MOF对藻类也有明显的抑制作用,处理6 h后米氏凯伦藻(Karenia mikimotoi)和铜绿微囊藻(Microcystis aeruginosa)的抑制率分别为77.5%和89.7%,不同的抑藻效应可能与藻细胞初始浓度及藻属类别有关. 另有研究发现,不同单一MOFs材料对M. aeruginosa的抑制效果为Cu-MOF-74 > ZIF-8 > Zn-MOF-74 > MIL-125(Ti)[44]. 单一MOFs材料具有纳米结构,可在光的激发下产生电子和空穴对氧化除藻,单一MOFs材料主要是通过释放金属离子抑藻.
-
单一MOFs材料对藻类的抑藻效果普遍低于90%,基于MOFs材料的可调控性和可修饰性,部分研究团队通过负载、修饰和改性等途径合成复合MOFs材料,以此提高其除藻性能[18, 23, 34]. Hu等[17]通过水热合成法将SNP-TiO2负载于Cu-MOF,发现SNP-TiO2@Cu-MOF对M. aeruginosa的抑制效果由77.5%提高到93.8%;同样,ZIF-8经负载获得的Ag/AgCl@ZIF-8对M. aeruginosa的抑制效果由10.0%提高到93.1%[35, 41]. 此外,Li等[21]将MIL-101(Cr)改性得到NH2-MIL-101(Cr),结果显示NH2-MIL-101(Cr)对M. aeruginosa的絮凝效果更佳,当NH2-MIL-101(Cr)浓度为30 mg·L−1时,处理3 h后藻类去除率高于95%. 综上,构建复合MOFs材料使抑藻性能得到显著提升.
复合MOFs材料除了对藻类叶绿素a有去除效果外,还对藻蓝蛋白(Phycocyanin,PC)、异藻蓝蛋白(Allophycocyanin,APC)和藻红素(Phycoerythrin,PE)等光合色素及藻细胞结构产生影响. Bi2O3@Cu-MOF对米氏凯伦藻(Karenia mikimotoi)细胞中的PC、APC和PE等光合色素含量有显著抑制作用[42];同样,Ag/AgCl@g-C3N4@UIO-66(NH2)(AGU)光催化降解M. aeruginosa过程中PC、APC和PE含量均呈快速下降趋势,同时藻细胞膜和内部结构被破坏,导致藻类死亡[23].
-
MOFs材料对M. aeruginosa 的抑制效果与MOFs浓度和处理时间呈正相关. 当Cu-MOF-74初始浓度为1、5、10 mg·L−1时,处理120 h后叶绿素a去除率分别为56%、59%和57%[44];NH2-MIL-101(Cr)初始浓度为10、20、50 mg·L−1时,处理0.5 h后抑藻率分别为40%、72%和90%[21];以上结果表明,高剂量的MOFs材料可提供更多的活性位点,从而产生絮凝效果并通过沉淀去除藻细胞. 另有研究发现,Cu-MOF浓度为1—100 mg·L−1时对M. aeruginosa的抑藻率为15.3%—89.7%,而浓度高于100 mg·L−1时抑藻率并无明显变化,其原因可能是由于Cu-MOF在高浓度时发生的团聚现象降低了Cu-MOF的活性位点[36];此外,不同浓度AGU(5—100 mg·L−1)在光照条件下均可抑制M. aeruginosa,其中30 mg·L−1剂量抑制效果最佳(3h可达99.9%),而高于该浓度时抑藻率反而有所降低,说明催化剂用量过大可能导致团聚或沉淀,从而降低光催化效率[23]. 由此可见,MOFs材料浓度越大,其对藻类抑制效果越明显,然而浓度过高时MOFs材料可能发生团聚.
-
反应体系pH值会对MOFs材料的吸附能力、表面尺寸和能带结构产生影响,同时也对MOFs材料的光催化活性起着重要作用. 当pH介于4.0—8.0时,NH2-MIL-101(Cr)对M. aeruginosa的抑藻率为94%—98%,高于8.0时抑藻率急剧下降,其原因可能是Cr-MOFs在碱性条件下被反应导致抑藻效果变差[21];与之不同的是,AGU催化剂在pH < 6.6时带正电荷而藻细胞表面带负电荷,光照条件下该MOFs材料对藻类的抑制效果随着pH值的增加而减小,因为酸性环境下的静电引力更有利于其吸附藻细胞进而通过光催化去除藻[23]. 综上,MOFs 材料在不同 pH 环境下呈现出不同电荷状态,其对藻类的去除机制也可能不同.
-
当MOFs浓度为10 mg·L−1时,在黑暗环境下处理6 h后Cu-MOF和Ag/AgCl@ZIF-8对M. aeruginosa抑藻率仅为8.5%和12.6%,而光照条件下分别为27.1%和93.1%,表明光照可以促进MOFs抑藻[35 − 36],其原因是由于光照引发MOFs产生自由基发生催化反应. MOFs材料在光照条件不仅可以发生光催化反应抑制藻类生长,还可以进一步降解藻类有机物及藻毒素等分泌物. Zhao等[18]对比分析了不同光照条件下PCN-224和NZVI@PCN-224对小球藻(Chlorella vulgaris)的影响效果,发现在黑暗条件下,两种MOFs材料对藻细胞和藻类有机物无明显影响,而光照条件下两种MOFs材料均发生了光催化反应,导致C. vulgaris可变荧光(Fv)与最大荧光(Fm)的比值(Fv/Fm)显著降低,且藻类有机物中类蛋白(峰A,Ex/Em = 225/350 nm) 和类腐殖酸类物质 (峰B,Ex/Em = 220/404 nm和峰C,Ex/Em = 225/440 nm)亦被降解;此外,蓝藻M. aeruginosa释放出的黄腐酸类物质和腐殖酸类物质在Ag/AgCl@ZIF-8光催化处理下同样被降解[41].
-
藻类生长代谢过程中分泌的藻类有机物(Algal organic matter,AOM)极易与水体中的微塑料[45 − 46]、纳米材料[47 − 48]等污染物结合,影响其迁移转化;此外,AOM还会与污染物争夺活性位点[49],抑制MOFs材料催化产生自由基. 为考察AOM对MOFs材料抑藻效果的影响,Fan等在分别添加富里酸(Fulvic acid,FA)和腐殖酸(Humic acid,HA)条件下比较了AGU的抑藻性能,发现FA和HA均可削减AGU的光催化抑藻效果[23],该削减作用主要是由于有机质对AGU产生的活性氧物种(Reactive oxygen species,ROS)有猝灭作用,有机质的消光反应也可能导致AGU光催化活性降低.
-
鉴于MOFs材料由金属离子和有机配体构成,其与藻类共同存在时可释放金属离子,并通过金属离子导致微藻生长受到抑制或死亡[39]. 研究表明,Co-ZIF、Zn-ZIF、Ag-ZIF及Al-PMOF和Al-PMOF (Cu)等单一MOFs材料均通过释放金属离子进行抑藻[22, 39],抑藻机理如图2所示. Fan等[44]同样发现Cu-MOF-74是通过释放Cu2+抑藻,扫描电镜分析结果显示,经1.0 mg·L−1 Cu-MOF-74处理48 h后,铜绿微囊藻藻细胞表面出现凹坑,同时藻细胞形态发生了变化,表明藻细胞受损;进一步对比Cu-MOF-74和Zn-MOF-74、Ag/AgCl@ZIF-8和ZIF-8的抑藻作用,发现MOFs材料释放Cu2+和Ag +对藻类细胞的破坏能力明显强于Zn2+[44]. 然而MOFs材料释放金属离子的浓度不一样,且不同金属离子对不同藻属的毒性也有所差异,因此,抑藻效果差异显著.
-
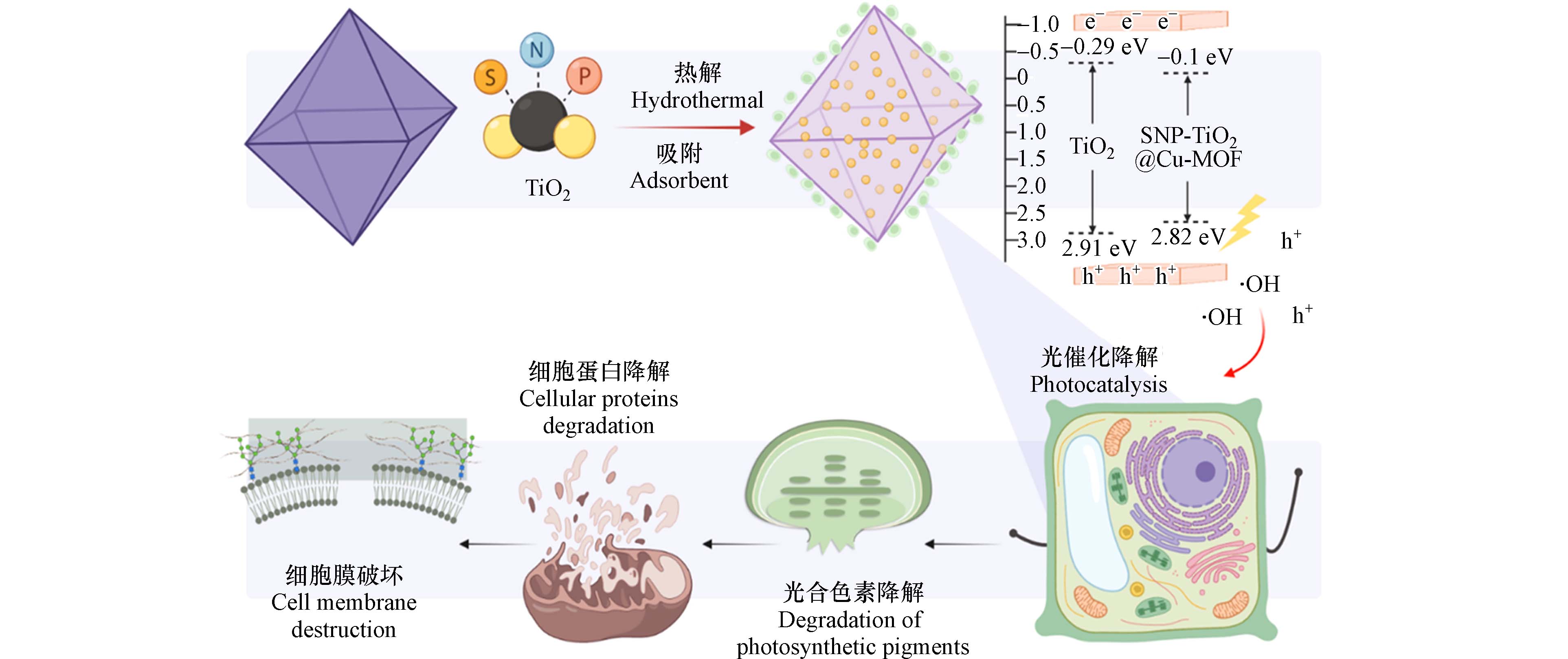
MOFs材料具有丰富的活性位点,因此,可以催化过氧化物、过硫酸盐等用于高级氧化降解环境中有机污染物. 研究表明,MOFs材料主要是通过光催化、类芬顿及硫酸根自由基介导三种途径[50]. 针对现有MOFs材料催化去除藻类的文献均为光催化降解机理,如Ag/AgCl@ZIF-8 coating[35]、Bi2O3@Cu-MOF[42]、SNP-TiO2@Cu-MOF[17]、AGU[23]和g-C3N4/Cu-MOF[36]等MOFs材料在光照条件下生成超氧自由基(O2-·)和羟基自由基(HO·)等活性自由基团,进一步导致藻细胞抗氧化酶活系统受损和藻细胞壁破裂[42]. 其中,SNP-TiO2@Cu-MOF光催化降解K. mikimotoi过程如图3所示:SNP-TiO2@Cu-MOF在可见光的激发下通过电子跃迁到导带(Conduction band,CB)上并在价带(Valence band,VB)上光生成空穴,随后释放出h+和HO·等活性氧自由基破坏藻细胞壁并降解光合色素.
-
常规MOFs材料多为纳米颗粒结构,而藻细胞则以微米级漂浮于水面或悬浮于水体中. 由此可见,MOFs材料可附着于藻细胞表面,通过遮光作用导致藻细胞死亡,或者通过集聚絮凝作用将藻细胞沉淀. Li等[39]研究表明,NH2-MIL-125(Ti)通过絮凝沉淀可抑制C. reinhardtii生长;该课题组同时比较分析了MIL-101(Cr)和NH2-MIL-101(Cr)对M. aeruginosa的抑藻性能,发现NH2-MIL-101(Cr)远高于MIL-101(Cr),其原因可能是有机连接剂中的氨基通过桥接和捕获作用来增强絮凝除藻性能[21]. 综上,采用氨基基团对MOFs材料进行修饰有利于改善微藻细胞与MOFs材料之间的絮凝效果.
-
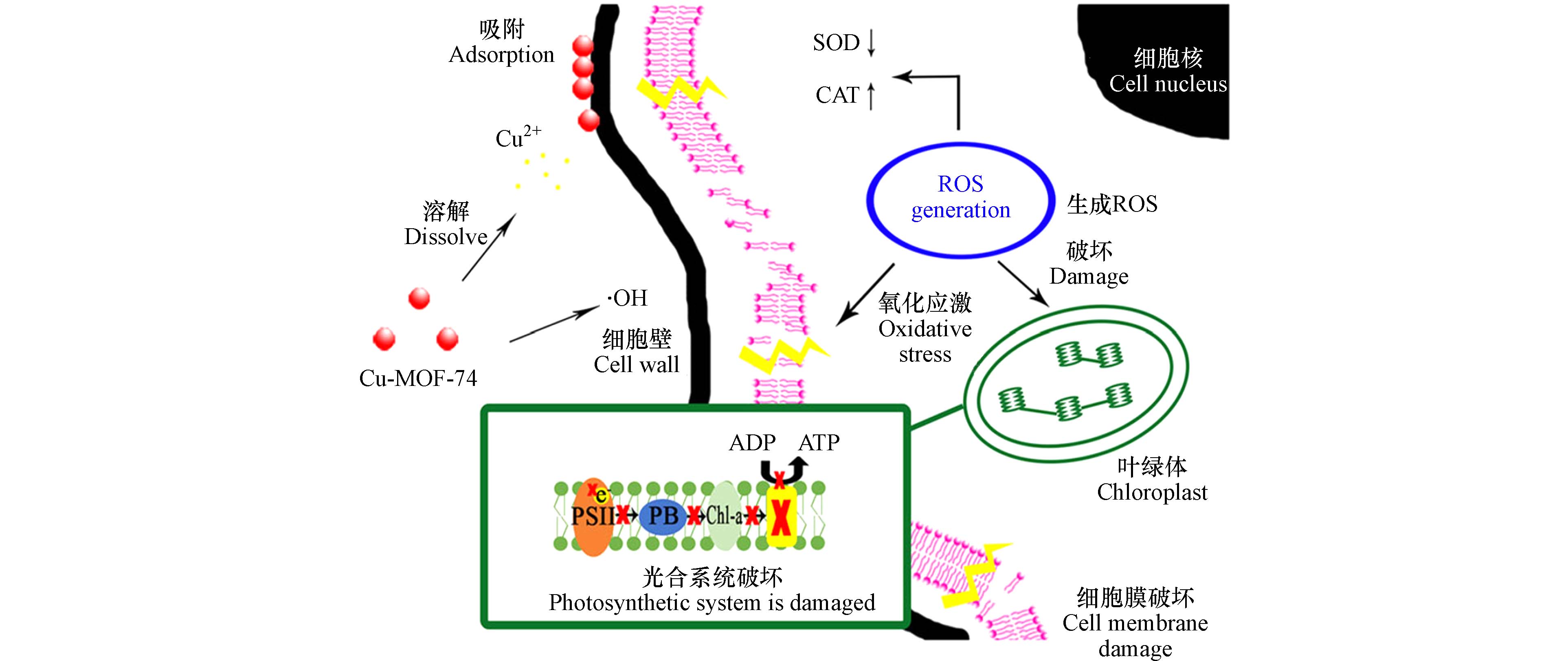
氧化损伤是指藻细胞在环境胁迫下氧化系统和抗氧化系统的失衡,导致藻细胞组织受损或破坏的现象. MOFs材料可诱导藻细胞产生O2-·、HO·、单线态氧(1O2)等ROS,并诱导藻细胞内发生氧化应激而失衡. Wang等[40]研究了Zn-MOF-FA对铜绿微囊藻的抑制机理,发现藻类在Zn-MOF-FA胁迫96 h后丙二醛(Malondialdehyde,MDA)含量有所增加,但超氧化物歧化酶(Superoxide dismutase,SOD)和过氧化氢酶(Catalase,CAT)活性均降低,光合作用PSII的最大量子产量(The maximum quantum yield of PSII,Fv/Fm)及最大相对电子传递效率(The maximum relative electron transfer rate,rETRmax)同样也明显降低,表明藻类抗氧化酶活系统破坏,光合作用减弱;Cu-MOF-74[37, 51]和Ag/AgCl@ZIF-8[41]等对藻类的胁迫作用机理也有报道,暴露于MOFs材料后藻类生长明显被抑制,且藻细胞内SOD 活性降低、CAT活性增加,其中,Cu-MOF-74材料对M. aeruginosa的氧化损伤潜在机制如图4所示,Cu-MOF-74材料释放Cu2+并产生HO·胁迫M. aeruginosa,随后藻细胞抗氧化酶活系统破坏导致藻细胞破裂凋亡.
-
MOFs材料抑藻可能多种机理共存. Fan等认为Cu-MOF-74释放的Cu2+并非抑制铜绿微囊藻生长的主要因素,而氧化损伤才是抑藻的主要原因,同时MOFs材料的絮凝作用也加剧了抑藻效果[38];Ag/AgCl@ZIF-8对铜绿微囊藻的抑制机理亦涉及催化降解和氧化损伤[41]. 此外,研究发现纳米银颗粒可能导致与藻细胞光合作用或生理代谢相关的部分基因表达上调或下调,进而影响藻细胞的生长及繁殖[52 − 53]. 然而现有MOFs材料抑藻机制并未涉及藻细胞基因变化,因此,研究暴露于MOFs材料后藻细胞的关键基因表达将有利于进一步明晰MOFs材料抑藻机制.
-
随着工业废水大量排放及温室效应日趋加剧,全球水体富营养化频发,因此,有害蓝藻的处理和治理技术迫在眉睫. 目前使用MOFs材料抑藻仅局限于使用绿藻和蓝藻作为目标污染物,尚无对甲藻、硅藻和褐藻生态毒性方面的研究;另外,MOFs材料应用于富营养化水体后其迁移转化及生物毒性尚不明确. 因此,MOFs材料在水体富营养化控藻方面的研究还有待进一步开展,主要发展方向和趋势如下:
(1) MOFs材料的特异结构使其在吸附和催化降解污染物方面展现出广阔的应用前景,然而现有研究仅关注于藻类的去除性能及机制,后续有待针对导致水体富营养化的氮/磷营养盐、藻类有机物及藻毒素等分泌物开发、合成新型MOFs材料,实现多种污染物质的同步高效去除;
(2) 现有MOFs材料抑藻试验研究多为实验室模拟环境,研究体系仅考察了纯培养条件下MOFs材料对单一藻属生态毒性的影响,而真实环境条件下富营养化水体有害藻属种类繁多,且水体中涉及其他水生生物和多种污染物共存. 因此,应用MOFs材料抑藻前,有待进一步开展MOFs材料对其他水生生物的毒理评估及多种污染物共存环境下MOFs材料的抑藻性能及机制;
(3) 现有MOFs材料抑藻方面的研究仅限于小球藻、莱茵衣藻、铜绿微囊藻和米氏凯伦藻等几类,此外,MOFs材料胁迫条件下藻类的蛋白质组学及基因组学调控机制尚无报道,因此,亟待开展MOFs材料对不同藻属的生态毒性和机理等研究,并结合分子生物学技术揭示其抑藻机制.
金属有机框架材料抑藻性能及机理研究进展
Advanced research in the inhibition and mechanisms of metal organic frameworks for microalgae
-
摘要: 全球温室效应不断加剧和氮磷营养物质排放,由此引发的水体富营养化日趋严重,寻求经济、高效的有害蓝藻控制和治理技术迫在眉睫. 金属有机框架材料因具有孔隙率高、比表面积大、吸附性能高、反应活性佳、孔径形状可调及功能设计性强等优点,在有害蓝藻治理方面展现出广阔的应用前景. 本文综述了MOFs材料对典型绿藻和水华/赤潮藻属的毒理效应,重点分析了MOFs材料抑藻的主要影响因素,阐述了MOFs材料对藻类的致毒作用机理,并对MOFs材料除藻的研究方向进行了展望,以期为MOFs材料的潜在应用提供借鉴和参考.Abstract: In consideration of the increasingly serious eutrophication caused by the intensifying global greenhouse effect and the discharge of nitrogen and phosphorus nutrients, it is urgent to explore economic and efficient control and treatment technologies for harmful cyanobacteria. Due to the advantages of high porosity, large specific surface area, high adsorption performance, good reactivity, adjustable pore shape and strong functional design, etc., metal organic framework materials (MOFs) show broad application prospects in controlling harmful cyanobacteria. In this review, the toxicological effects of MOFs on typical green algae and harmful bloom/red tide cyanobacteria are summarized, and the main influencing factors of MOFs for microalgae inhibition are analyzed; in addition, the toxic mechanisms of MOFs on microalgae are expounded, and the research directions of MOFs on microalgae removal are also prospected. It is hoped to provide reference and consulting for the potential application of MOFs.
-
Key words:
- MOFs /
- eutrophication /
- harmful cyanobacteria /
- catalytic degradation /
- inhibition mechanisms.
-

-
表 1 MOFs材料抑藻性能及机理
Table 1. Inhibition and mechanism of MOFs for microalgae
金属有机
框架材料
MOFsMOFs初始
浓度/(mg·L−1)
Initial MOFs
concentration微藻
Microalgae藻细胞数/
(cells·mL−1)
Cell number处理时间/h
Treatment
time去除率/%
Removal
rate去除机理
Removal
mechanisms参考文献
ReferenceZIF-8 10 M. aeruginosa 3.6×106 6 10.0 NA [35] MOF-535 24.5 Cu‐MOF‐74 1.0 M. aeruginosa 6.01×106 120 75.5 絮凝沉淀
离子释放
氧化损伤[37 − 38] UIO-66-NH2 30 M. aeruginosa 3.450a 3 15 NA [23] Al-PMOF
Al-PMOF (Cu)
Al-PMOF (Ni)10 C. reinhardtii 5.0×105 72 71 ± 9
85 ± 1
33 ± 7离子释放 [39] Co-ZIF 50 Anabaena sp. NA 24 50 离子释放 [22] C. reinhardtii NA 48 40 Zn-ZIF 50 Anabaena sp. NA 24 80 C. reinhardtii NA 48 30 Ag-ZIF 50 Anabaena sp. NA 24 80 Synechococcus sp. NA 48 > 85 C. reinhardtii NA 24 90 Cu-MOF 100 K. mikimotoi 1.0×105 6 77.5 NA [17] NH2- MIL-101(Cr) 20 M. aeruginosa 3.0×106 3 > 95 絮凝沉淀 [21] NH2-MIL-125(Ti) 10 C. reinhardtii 5.0×105 24 34 絮凝沉淀 [39] Zn-MOF-FA 4(8) M. aeruginosa 0.745 a 96 > 75(83) 氧化损伤 [40] Ag/AgCl@ZIF-8 10 M. aeruginosa 3.6×106 6 93.1 催化降解
氧化损伤[41] Ag/AgCl@ZIF-8 coating NA M. aeruginosa 6.48×106 4 98.5 催化降解 [35] Ag/AgCl@g-C3N4@UIO-66(NH2) 30 M. aeruginosa 3.450a 3 99.9 催化降解 [23] SNP-TiO2@Cu-MOF 100 K. mikimotoi 1.0×105 6 93.8 催化降解 [17] Cu-MOF 100 M. aeruginosa 1.0×106 6 89.7 催化降解 [36] g-C3N4/Cu-MOF 50 81.5 Bi2O3@Cu-MOF 60 K. mikimotoi 8.0×104 4 96.4 催化降解 [42] Cu-BDC-MOF 1.0 M. aeruginosa 5.0×105 120 NA 离子释放 [43] 注:“NA”表示未提及;“a”表示藻细胞叶绿素a浓度,单位为mg·L−1.
Notes: “NA” means the date is not available;“a” means the Chlorophyll a concentration of the microalgae, mg·L−1. -
[1] ZHAO M M, QU D, SHEN W D, et al. Effects of dissolved organic matter from different sources on Microcystis aeruginosa growth and physiological characteristics[J]. Ecotoxicology and Environmental Safety, 2019, 176: 125-131. doi: 10.1016/j.ecoenv.2019.03.085 [2] PAERL H W, GARDNER W S, HAVENS K E, et al. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients[J]. Harmful Algae, 2016, 54: 213-222. doi: 10.1016/j.hal.2015.09.009 [3] KIM W, KIM M, HONG M, et al. Killing effect of deinoxanthins on cyanobloom-forming Microcystis aeruginosa: Eco-friendly production and specific activity of deinoxanthins[J]. Environmental Research, 2021, 200: 111455. doi: 10.1016/j.envres.2021.111455 [4] SANO D, ISHIFUJI S, SATO Y, et al. Identification and characterization of coagulation inhibitor proteins derived from Cyanobacterium Microcystis aeruginosa[J]. Chemosphere, 2011, 82(8): 1096-1102. doi: 10.1016/j.chemosphere.2010.12.005 [5] SUN S Q, TANG Q X, XU H, et al. A comprehensive review on the photocatalytic inactivation of Microcystis aeruginosa: Performance, development, and mechanisms[J]. Chemosphere, 2023, 312: 137239. doi: 10.1016/j.chemosphere.2022.137239 [6] XIN H J, YANG S, TANG Y L, et al. Mechanisms and performance of calcium peroxide-enhanced Fe(ii) coagulation for treatment of Microcystis aeruginosa-laden water[J]. Environmental Science:Water Research & Technology, 2020, 6(5): 1272-1285. [7] KO S R, LEE Y K, SRIVASTAVA A, et al. The selective inhibitory activity of a fusaricidin derivative on a bloom-forming Cyanobacterium, Microcystis sp[J]. Journal of Microbiology and Biotechnology, 2019, 29(1): 59-65. doi: 10.4014/jmb.1804.04031 [8] KONG Y, ZOU P, YANG Q, et al. Physiological responses of Microcystis aeruginosa under the stress of antialgal actinomycetes[J]. Journal of Hazardous Materials, 2013, 262: 274-280. doi: 10.1016/j.jhazmat.2013.08.032 [9] LIU H L, GUO X L, LIU L, et al. Simultaneous microcystin degradation and Microcystis aeruginosa inhibition with the single enzyme microcystinase A[J]. Environmental Science & Technology, 2020, 54(14): 8811-8820. [10] MEYER N, BIGALKE A, KAULFUß A, et al. Strategies and ecological roles of algicidal bacteria[J]. FEMS Microbiology Reviews, 2017, 41(6): 880-899. doi: 10.1093/femsre/fux029 [11] SUN S Q, HU S S, ZHANG B, et al. Allelopathic effects and potential allelochemical of Sargassum fusiforme on red tide microalgae Heterosigma akashiwo[J]. Marine Pollution Bulletin, 2021, 170: 112673. doi: 10.1016/j.marpolbul.2021.112673 [12] MATTHIJS H C P, JANČULA D, VISSER P M, et al. Existing and emerging cyanocidal compounds: New perspectives for cyanobacterial bloom mitigation[J]. Aquatic Ecology, 2016, 50(3): 443-460. doi: 10.1007/s10452-016-9577-0 [13] ZHANG X L, HU X G, WU H, et al. Persistence and recovery of ZIF-8 and ZIF-67 phytotoxicity[J]. Environmental Science & Technology, 2021, 55(22): 15301-15312. [14] RASULI L, DEHGHANI M H, ALIMOHAMMADI M, et al. Mesoporous metal organic frameworks functionalized with the amino acids as advanced sorbents for the removal of bacterial endotoxins from water: Optimization, regression and kinetic models[J]. Journal of Molecular Liquids, 2021, 339: 116801. doi: 10.1016/j.molliq.2021.116801 [15] YANG W X, HAN Y, LI C H, et al. Shapeable three-dimensional CMC aerogels decorated with Ni/Co-MOF for rapid and highly efficient tetracycline hydrochloride removal[J]. Chemical Engineering Journal, 2019, 375: 122076. doi: 10.1016/j.cej.2019.122076 [16] GAO Q A, WEI Y Y, WANG L L, et al. Three novel Co(ii)-based MOFs: Syntheses, structural diversity, and adsorption properties[J]. CrystEngComm, 2022, 24(39): 6854-6864. doi: 10.1039/D2CE01085B [17] HU L J, CHEN J F, WEI Y S, et al. Photocatalytic degradation effect and mechanism of Karenia mikimotoi by non-noble metal modified TiO2 loading onto copper metal organic framework (SNP-TiO2@Cu-MOF) under visible light[J]. Journal of Hazardous Materials, 2023, 442: 130059. doi: 10.1016/j.jhazmat.2022.130059 [18] ZHAO J, LYU C Y, ZHANG R, et al. Self-cleaning and regenerable nano zero-valent iron modified PCN-224 heterojunction for photo-enhanced radioactive waste reduction[J]. Journal of Hazardous Materials, 2023, 442: 130018. doi: 10.1016/j.jhazmat.2022.130018 [19] RANA L K, KAUR P, MARIS T, et al. An insight into sensitive detection of metal ions using a novel cobalt MOF: Single crystal, photoluminescence, and theoretical studies[J]. CrystEngComm, 2022, 24(30): 5460-5473. doi: 10.1039/D2CE00385F [20] CAI C J, FAN G D, DU B H, et al. Metal-organic-framework-based photocatalysts for microorganism inactivation: A review[J]. Catalysis Science & Technology, 2022, 12(12): 3767-3777. [21] LI Y L, XU Z T, WANG W X. Effective flocculation of harmful algae Microcystis aeruginosa by nanoscale metal-organic framework NH2-MIL-101(Cr)[J]. Chemical Engineering Journal, 2022, 433: 134584. doi: 10.1016/j.cej.2022.134584 [22] MARTÍN-BETANCOR K, AGUADO S, RODEA-PALOMARES I, et al. Co, Zn and Ag-MOFs evaluation as biocidal materials towards photosynthetic organisms[J]. Science of the Total Environment, 2017, 595: 547-555. doi: 10.1016/j.scitotenv.2017.03.250 [23] FAN G D, ZHAN J J, LUO J, et al. Fabrication of heterostructured Ag/AgCl@g-C3N4@UIO-66(NH2) nanocomposite for efficient photocatalytic inactivation of Microcystis aeruginosa under visible light[J]. Journal of Hazardous Materials, 2021, 404: 124062. doi: 10.1016/j.jhazmat.2020.124062 [24] 楚弘宇, 王崇臣, 刘昂. MOFs基材料光催化除藻研究进展[J]. 稀有金属, 2023, 47(1): 116-123. CHU H Y, WANG C C, LIU A. Algae removal via photocatalysis over MOFs-based materials: A mini review[J]. Chinese Journal of Rare Metals, 2023, 47(1): 116-123 (in Chinese).
[25] JIN P X, WANG L, MA X L, et al. Construction of hierarchical ZnIn2S4@PCN-224 heterojunction for boosting photocatalytic performance in hydrogen production and degradation of tetracycline hydrochloride[J]. Applied Catalysis B:Environmental, 2021, 284: 119762. doi: 10.1016/j.apcatb.2020.119762 [26] ZHANG Z, CHEN Y, WANG Z, et al. Effective and structure-controlled adsorption of tetracycline hydrochloride from aqueous solution by using Fe-based metal-organic frameworks[J]. Applied Surface Science, 2021, 542: 148662. doi: 10.1016/j.apsusc.2020.148662 [27] ZHANG Z, CHEN Y, HU C Y, et al. Efficient removal of tetracycline by a hierarchically porous ZIF-8 metal organic framework[J]. Environmental Research, 2021, 198: 111254. doi: 10.1016/j.envres.2021.111254 [28] MIRSOLEIMANI-AZIZI S M, SETOODEH P, ZEINALI S, et al. Tetracycline antibiotic removal from aqueous solutions by MOF-5: Adsorption isotherm, kinetic and thermodynamic studies[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 6118-6130. doi: 10.1016/j.jece.2018.09.017 [29] SHETA S, SALEM S R, EL-SHEIKH S. A novel Iron(III)-based MOF: Synthesis, characterization, biological, and antimicrobial activity study[J]. Journal of Materials Research, 2022, 37: 2356-2367. doi: 10.1557/s43578-022-00644-9 [30] ZERAATI M, MOGHADDAM-MANESH M, KHODAMORADI S, et al. Ultrasonic assisted reverse micelle synthesis of a novel Zn-metal organic framework as an efficient candidate for antimicrobial activities[J]. Journal of Molecular Structure, 2022, 1247: 131315. doi: 10.1016/j.molstruc.2021.131315 [31] ZHENG H A, WANG D R, SUN X, et al. Surface modified by green synthetic of Cu-MOF-74 to improve the anti-biofouling properties of PVDF membranes[J]. Chemical Engineering Journal, 2021, 411: 128524. doi: 10.1016/j.cej.2021.128524 [32] ZENG S, LIU Y S, WANG Y M, et al. Photo-Fenton self-cleaning carbon fibers membrane supported with Zr-MOF@Fe2O3 for effective phosphate removal from algae-rich water[J]. Chemosphere, 2023, 323: 138175. doi: 10.1016/j.chemosphere.2023.138175 [33] LI Z J, GONG C C, HUO P P, et al. Synthesis of magnetic core-shell Fe3O4@PDA@Cu-MOFs composites for enrichment of microcystin-LR by MALDI-TOF MS analysis[J]. RSC Advances, 2020, 10(49): 29061-29067. doi: 10.1039/D0RA04125D [34] LI Y L, WANG W X. Internalization of the metal–organic framework MIL-101(Cr)-NH2 by a freshwater alga and transfer to zooplankton[J]. Environmental Science & Technology, 2023, 57(1): 118-127. [35] FAN G D, CHEN Z, WANG B, et al. Photocatalytic removal of harmful algae in natural waters by Ag/AgCl@ZIF-8 coating under sunlight[J]. Catalysts, 2019, 9(8): 698. doi: 10.3390/catal9080698 [36] WANG Z Y, XU Y A, WANG C X, et al. Photocatalytic inactivation of harmful algae Microcystis aeruginosa and degradation of microcystin by g-C3N4/Cu-MOF nanocomposite under visible light[J]. Separation and Purification Technology, 2023, 313: 123515. doi: 10.1016/j.seppur.2023.123515 [37] FAN G D, ZHOU J J, ZHENG X M, et al. Growth inhibition of Microcystis aeruginosa by copper-based MOFs: Performance and physiological effect on algal cells[J]. Applied Organometallic Chemistry, 2018, 32(12): e4600. doi: 10.1002/aoc.4600 [38] FAN G D, BAO M C, ZHENG X M, et al. Growth inhibition of harmful cyanobacteria by nanocrystalline Cu-MOF-74: Efficiency and its mechanisms[J]. Journal of Hazardous Materials, 2019, 367: 529-538. doi: 10.1016/j.jhazmat.2018.12.070 [39] LI Y L, SHANG S S, SHANG J, et al. Toxicity assessment and underlying mechanisms of multiple metal organic frameworks using the green algae Chlamydomonas reinhardtii model[J]. Environmental Pollution, 2021, 291: 118199. doi: 10.1016/j.envpol.2021.118199 [40] WANG X X, HUANG K W, GAO J S, et al. Effects on photosynthetic and antioxidant systems of harmful cyanobacteria by nanocrystalline Zn-MOF-FA[J]. Science of the Total Environment, 2021, 792: 148247. doi: 10.1016/j.scitotenv.2021.148247 [41] FAN G D, YOU Y F, WANG B, et al. Inactivation of harmful cyanobacteria by Ag/AgCl@ZIF-8 coating under visible light: Efficiency and its mechanisms[J]. Applied Catalysis B:Environmental, 2019, 256: 117866. doi: 10.1016/j.apcatb.2019.117866 [42] WANG M J, CHEN J F, HU L J, et al. Heterogeneous interfacial photocatalysis for the inactivation of Karenia mikimotoi by Bi2O3 loaded onto a copper metal organic framework (Bi2O3@Cu-MOF) under visible light[J]. Chemical Engineering Journal, 2023, 456: 141154. doi: 10.1016/j.cej.2022.141154 [43] KIM Y, KALIMUTHU P, NAM G, et al. Cyanobacteria control using Cu-based metal organic frameworks derived from waste PET bottles[J]. Environmental Research, 2023, 224: 115532. doi: 10.1016/j.envres.2023.115532 [44] FAN G D, HONG L, ZHENG X M, et al. Growth inhibition of Microcystic aeruginosa by metal-organic frameworks: Effect of variety, metal ion and organic ligand[J]. RSC Advances, 2018, 8(61): 35314-35326. doi: 10.1039/C8RA05608K [45] CAI Y, MU W J, JIA K, et al. Effects of three nanomaterials on growth, photosynthetic characteristics and production of reactive oxygen species of diatom Nitzschia palea[J]. Chemistry and Ecology, 2022, 38(2): 145-161. doi: 10.1080/02757540.2021.2023508 [46] DALY G, GHINI V, ADESSI A, et al. Towards a mechanistic understanding of microalgae-bacteria interactions: Integration of metabolomic analysis and computational models[J]. FEMS Microbiology Reviews, 2022, 46(5): fuac020. doi: 10.1093/femsre/fuac020 [47] QIAN H F, ZHU K, LU H P, et al. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses[J]. Science of the Total Environment, 2016, 572: 1213-1221. doi: 10.1016/j.scitotenv.2016.08.039 [48] GARG S, WANG K, WAITE T D. Impact of Microcystis aeruginosa exudate on the formation and reactivity of iron oxide particles following Fe(II) and Fe(III) addition[J]. Environmental Science & Technology, 2017, 51(10): 5500-5510. [49] JIA P L, ZHOU Y P, ZHANG X F, et al. Cyanobacterium removal and control of algal organic matter (AOM) release by UV/H2O2 pre-oxidation enhanced Fe(II) coagulation[J]. Water Research, 2018, 131: 122-130. doi: 10.1016/j.watres.2017.12.020 [50] YANG Y, CHEN H, LU J F. Inactivation of algae by visible-light-driven modified photocatalysts: A review[J]. Science of the Total Environment, 2023, 858: 159640. doi: 10.1016/j.scitotenv.2022.159640 [51] FAN G D, ZHOU J J, ZHENG X M, et al. Fast photocatalytic inactivation of Microcystis aeruginosa by metal-organic frameworks under visible light[J]. Chemosphere, 2020, 239: 124721. doi: 10.1016/j.chemosphere.2019.124721 [52] CAO M M, HUANG X T, WANG F, et al. Transcriptomics and metabolomics revealed the biological response of Chlorella pyrenoidesa to single and repeated exposures of AgNPs at different concentrations[J]. Environmental Science & Technology, 2021, 55(23): 15776-15787. [53] TSIOLA A, TONCELLI C, FODELIANAKIS S, et al. Low-dose addition of silver nanoparticles stresses marine plankton communities[J]. Environmental Science:Nano, 2018, 5(8): 1965-1980. doi: 10.1039/C8EN00195B -



 下载:
下载: