-
甲醛是一种无色、有刺激性气味的气体,被国际癌症研究机构定义为一类致癌物质[1]。甲醛污染具有致癌风险大、污染源存在范围广[2]、释放周期长[3-4]等特点。调研表明,我国存在较普遍的住宅甲醛超标现象[5]。目前,针对甲醛的空气净化方案多以吸附技术为主。甲醛具有相对较高的蒸汽压 (25 ℃时为517 kPa) 和较低的沸点 (-19.5 ℃) ,因此吸附法对甲醛的净化效果远不如其他可挥发性有机化合物 (volatile organic compounds,VOCs) [6]。光催化技术的原理是利用一定波长的光线激发半导体催化剂,原位产生羟基自由基等强氧化还原性物种参与化学反应,是一种安全、高效、反应条件温和的绿色技术,在新能源合成和污染物降解领域具有广阔应用前景。
TiO2是研究最广泛的半导体光催化剂之一,具有较好的结构稳定性、储量丰富、无毒等特点。但传统TiO2由于量子效率低,在实际的室内空气净化过程中,存在催化剂易失活[7]和产生有害中间副产物[8]的问题。带隙调控是一种常见的改性思路,通过在晶格中掺杂金属或非金属元素,提高可见光响应能力和光催化性能。但掺杂过程通常比较困难[9],且掺杂元素可能会成为载流子的复合中心[10],有研究者通过引入氧空位来提高量子效率。CHEN等[11]最早通过高压氢化得到表面无序、带有氧空位的黑色TiO2,其能出现带尾状态,表现出较好的可见光响应能力和催化性能,同时研究了黑色TiO2的合成和结构特征。
制备黑色TiO2的方法众多,包括氢热处理、化学还原 、电化学还原、金属还原等[12]。常规的黑色TiO2合成需要在高压、还原性气氛条件下进行,操作复杂且成本高。为简化合成方法,KATAL等[13]直接在真空环境下退火合成黑色TiO2,并表现出与氢气煅烧相当的催化性能。本研究将采用溶胶-凝胶法在氩气下煅烧得到黑色TiO2,通过TEM、XRD、DRS、XPS等技术表征其结构特征,并测试其对甲醛的光催化净化效率。为进一步提高材料的表面传质效率,拟将黑色TiO2与活性炭复合,探究吸附-催化协同降解机制。最后,在相同工况下对比黑色TiO2/活性炭复合结构与传统净化材料的甲醛净化性能,为黑色TiO2/活性炭的长期甲醛净化效果提供参考。
-
钛酸四丁酯 (C16H36O4Ti) 购于上海麦克林生化科技有限公司;冰醋酸 (CH3COOH) 、无水乙醇 (CH3CH2OH) 、盐酸 (HCl) 、硝酸 (HNO3) 均为分析纯并购于天津市元立化工有限公司;活性炭为椰壳活性炭,购于长葛市炭尔诺催化技术有限公司;高锰酸钠浸渍活性氧化铝 (NaMnO4@Al2O3) 购于美国Purafil公司,高锰酸钠负载量为12%。
-
1) 黑色TiO2的制备。将14 mL无水乙醇与13 mL去离子水混合,以1 mol·L−1盐酸调节混合液pH至2~3,制得A液。将14 mL的钛酸四丁酯、9 mL冰醋酸、28 mL无水乙醇溶液混合,搅拌均匀,制得B液。在强力搅拌下,将A液以每秒1 滴的速度加入到B液。滴加完毕后,恒温继续搅拌100 min,室温静置12 h凝胶,老化24 h,于105 ℃下干燥24 h,研磨,分别在空气、氩气氛围下以15 ℃·min−1先升温至200 ℃,恒温1 h,再以相同的速度升温至500 ℃,恒温煅烧2 h,分别得到白色和黑色TiO2,记为W-TiO2和B-TiO2。
2) 黑色TiO2/活性炭复合材料制备。活性炭研磨后,用0.25 mol·L−1硝酸浸渍处理1.5 h后,用蒸馏水清洗至中性。在上述B液中加入一定量活性炭,重复上述步骤,分别制得含有3%、7%、10%、15%、25%、40%质量分数活性炭的黑色TiO2,记为B-TiO2-3AC、B-TiO2-7AC、B-TiO2-10AC、B-TiO2-15AC、B-TiO2-25AC、B-TiO2-40AC。
-
使用日本Rigaku Smartlab SE型衍射仪,利用Cu靶以2 °·min−1扫描10°~80°,得到X射线衍射图 (XRD) 。采用透射电子显微镜 (TEM,日本JEOL JEM 2100F) 在纳米尺度下观察黑色TiO2的形貌特征。扫描电子显微镜 (SEM,捷克TESCAN MIRA LMS) 用于观察活性炭与TiO2的复合结构特征。X射线光电子能谱 (XPS,美国Thermo Scientific K-Alpha) 用于分析TiO2表层元素的化学状态,键能参考值为C1s 284.8 eV。通过紫外-可见漫反射吸收光谱 (UV-Vis DRS,Hitachi U4150) 得到光催化剂在200~800 nm的吸光度,并通过Tauc曲线计算确定光催化剂的带隙宽度。采用电子顺磁共振技术 (EPR,德国Bruker EMXplus-6/1) 获取未成对电子的运动状态,用于佐证氧空位的存在。
-
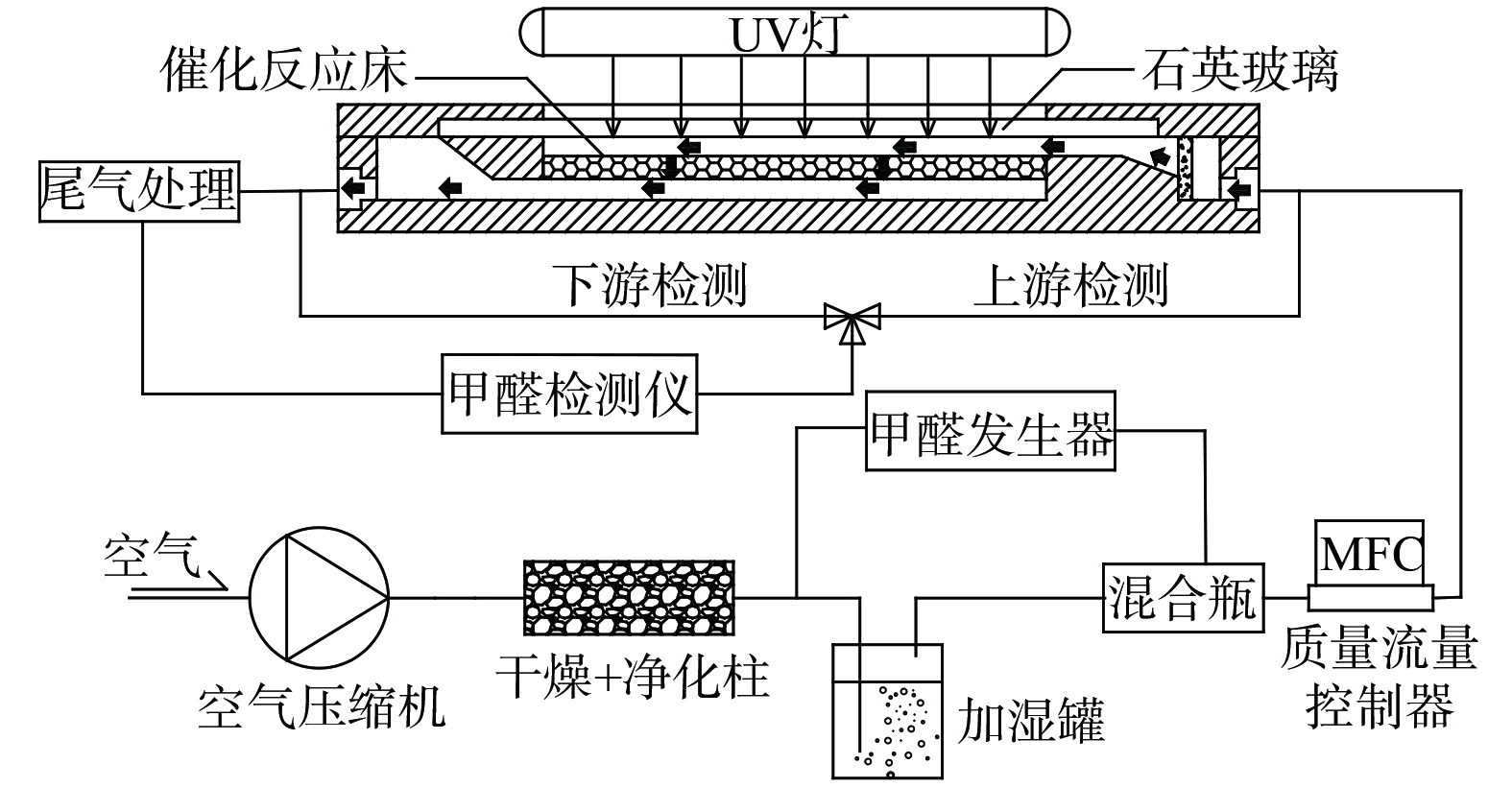
甲醛净化性能测试在连续流单通式反应系统 (图1) 中进行。催化反应器主要由透过式的填充块组成,150 mg催化剂均匀负载在5 cm×10 cm的玻璃纤维滤纸表面,气流自上而下穿过催化剂。催化剂上方设有石英玻璃透光窗,主波长为254 nm的汞灯作为光源,功率为9 W,紫外光强为3 mW·cm−2。自制甲醛发生器利用固体三聚甲醛粉末高温解聚的发生原理。甲醛检测采用美国Interscan公司生产的甲醛在线检测仪 (Interscan RM16) ,检测仪示数根据酚试剂分光光度法校准。
压缩空气先经过干燥净化柱除去背景污染物并降湿,通过控制加湿罐流量调节湿度,控制甲醛发生器流量调节甲醛浓度,质量流量控制器调节流量。在实验过程中,甲醛检测仪先接通上游,待上游甲醛浓度稳定1 h后,打开紫外灯,检测仪接通下游,连续监测。 每隔1 min记录1次数据,待下游甲醛浓度稳定1 h,取1 h平均值作为下游甲醛浓度。甲醛的单通净化效率根据式 (1) 计算。
式中:η为甲醛单通净化效率,%;Ci为反应器上游甲醛质量浓度,mg·m−3;Co为反应器下游甲醛质量浓度,mg·m−3。
-
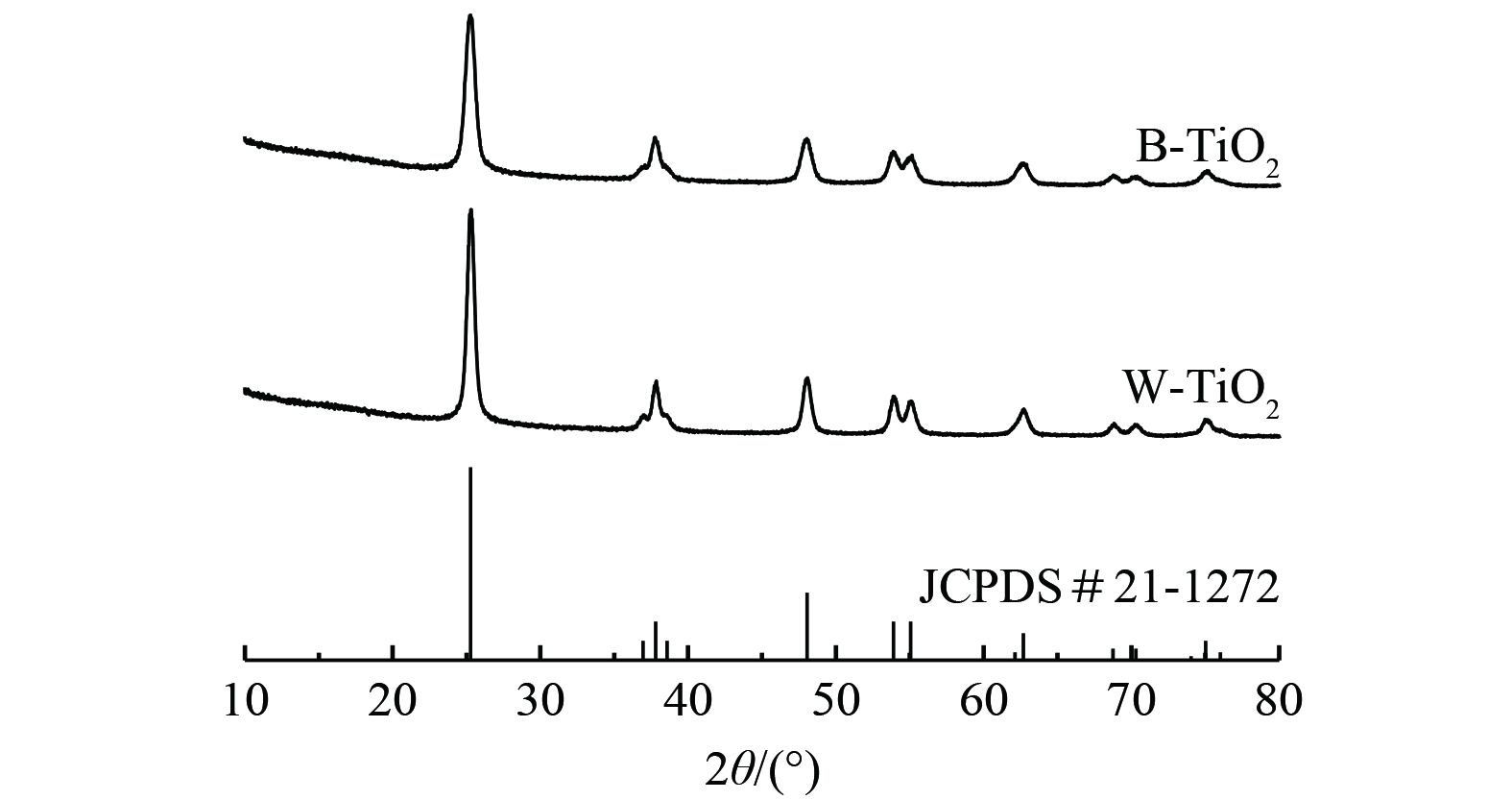
光催化剂的XRD图如图2所示。B-TiO2与W-TiO2位于2θ=25.3°、37.8°、48.1°、53.8°的衍射峰揭示了锐钛矿的主要特征,与JCPDS卡#21-1272一致。图2中W-TiO2的衍射峰强度比B-TiO2强,这表明W-TiO2的结晶度更好。而B-TiO2的半峰宽度更宽,则表明B-TiO2晶粒尺寸更小。根据Scherrer公式 (式(2)) ,计算出W-TiO2的晶粒尺寸为13.9 nm,B-TiO2的晶粒尺寸为10.8 nm。
式中:D为晶粒尺寸,nm;k为Scherrer常数;λ为X射线波长,nm;B为衍射峰半高宽度,nm;θ为布拉格角,(°)。
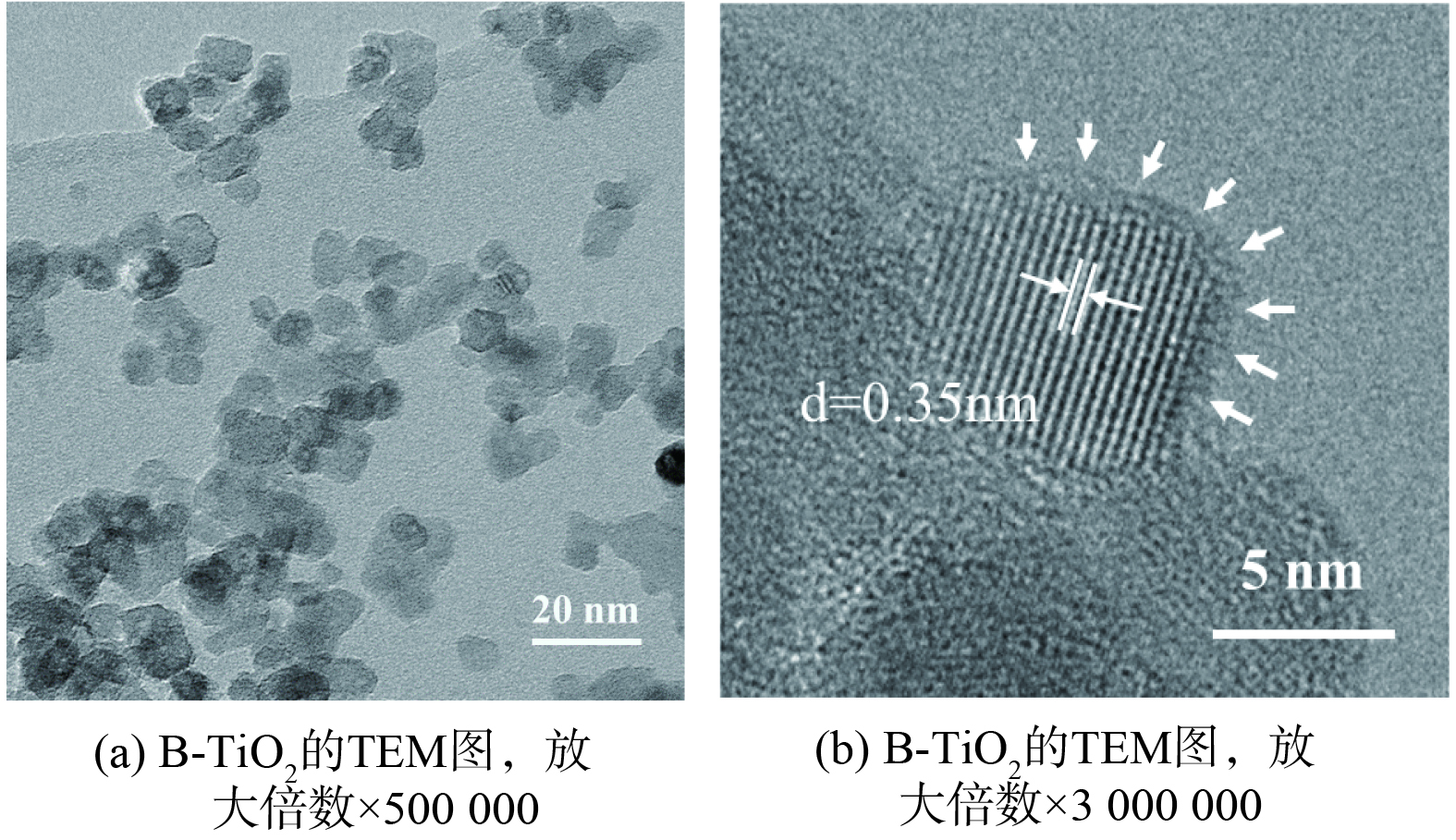
B-TiO2的TEM图如图3所示。黑色TiO2纳米粒子尺寸分布均匀,晶粒内部晶格条纹清晰均匀,这表明其内部有较好的结晶度。通过计算得到晶格间隙为0.35 nm。晶粒边缘晶格条纹弯曲,形成黑色TiO2典型的结晶核/无序壳结构。
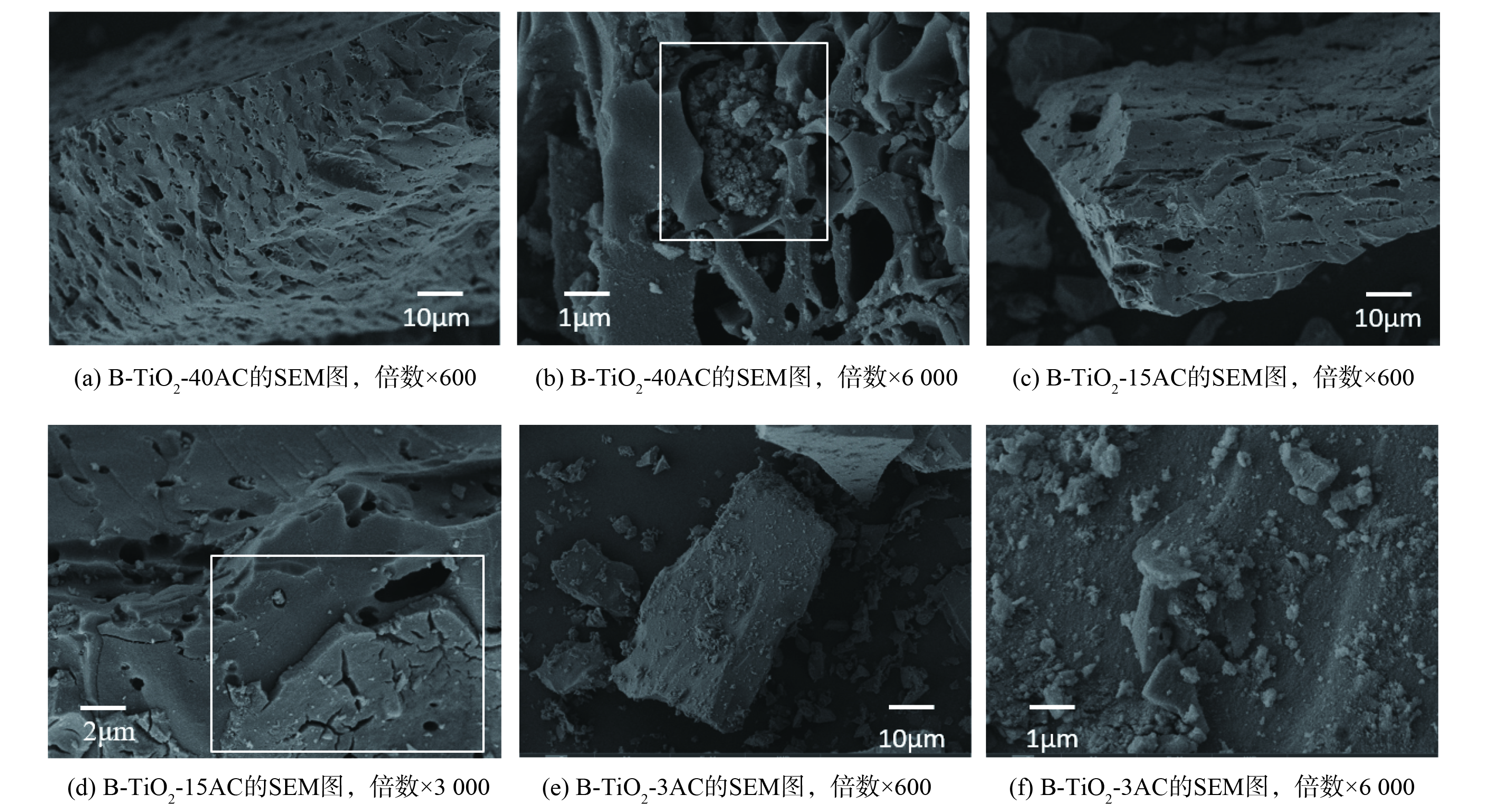
为观察B-TiO2与活性炭的复合结构,通过扫描电子显微镜获得B-TiO2-40AC、B-TiO2-15AC、B-TiO2-3AC的微观形貌 (图4) 。a、b为B-TiO2-40AC,c、d为B-TiO2-15AC,e、f为B-TiO2-3AC。活性炭与B-TiO2的相对含量不同,导致活性炭表面的形貌有较大差别。图4 (a) 表明B-TiO2-40AC表面具有清晰可见的孔隙结构;图4 (b) 表明部分纳米TiO2填充在活性炭的孔隙中。随着活性炭含量的减少,纳米TiO2相对含量增加。图4 (c) 表明活性炭孔隙明显变小,推测是纳米TiO2对孔隙的填充导致的。从放大图4 (d) 中发现,纳米TiO2以薄膜的形式负载在活性炭表面。随着活性炭含量继续减少,B-TiO2-3AC中纳米TiO2几乎完全覆盖了活性炭表面,观察不到活性炭表面的孔隙结构 (图4 (e) ) ,放大图中可观察到活性炭表面出现大量纳米TiO2团聚形成的微球 (图4 (f) ) 。
因此,黑色纳米TiO2在活性炭表面的复合主要包括孔隙填充与表面覆盖2种形式,当活性炭含量减少,黑色TiO2含量相对增加,可观察到活性炭孔隙逐渐减小,活性炭表面逐渐被黑色二氧化钛覆盖。
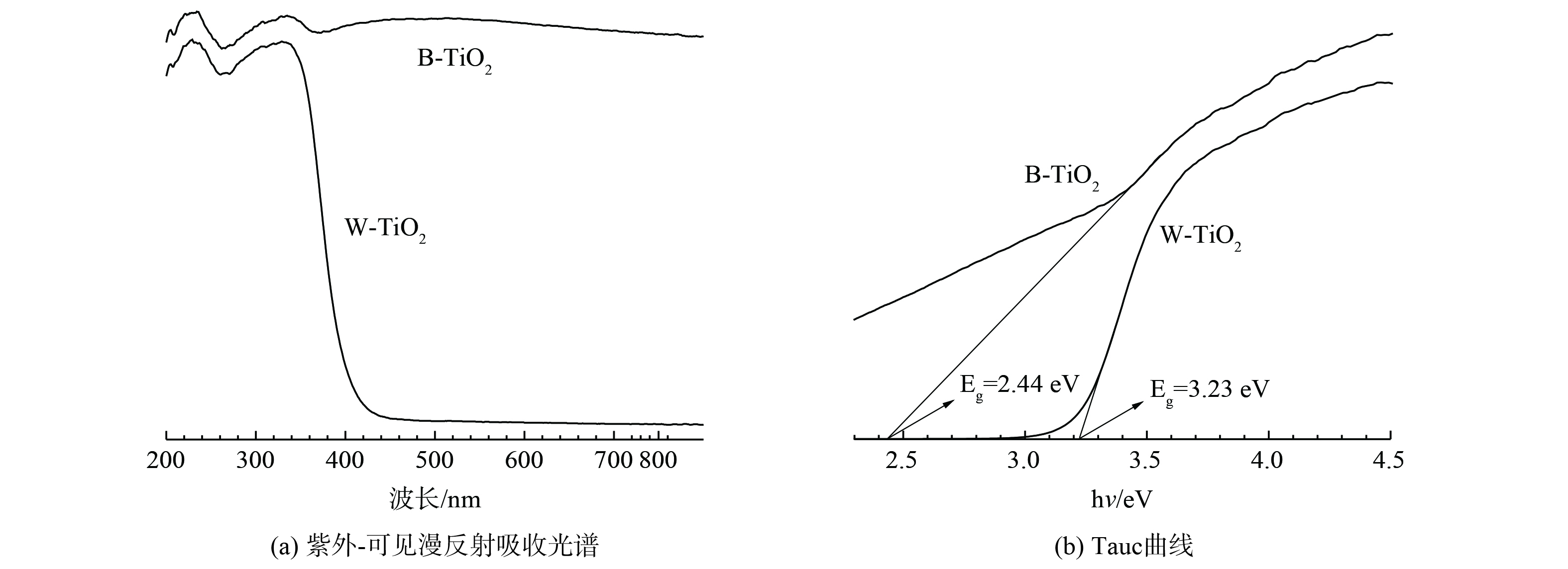
B-TiO2、W-TiO2的紫外-可见漫反射吸收光谱图如图5 (a) 所示。B-TiO2无论在紫外或是可见光部分都有更强的光吸收能力。结合TEM,B-TiO2可见光吸收能力的增强可能得益于表面黑色的无序壳结构。根据Tauc曲线 (图5 (b) ) 估算光催化剂的带隙, B-TiO2的带隙为2.44 eV,相比于W-TiO2的3.23 eV明显减小,这表明其对可见光有一定的响应能力。
为证明B-TiO2中氧空位的存在,利用EPR技术对比W-TiO2与B-TiO2的未成对电子运动状态,结果如图6所示。在g=2.003处可明显观察到B-TiO2的信号强度明显高于W-TiO2,这主要归因于B-TiO2表面氧空位的存在[14]。
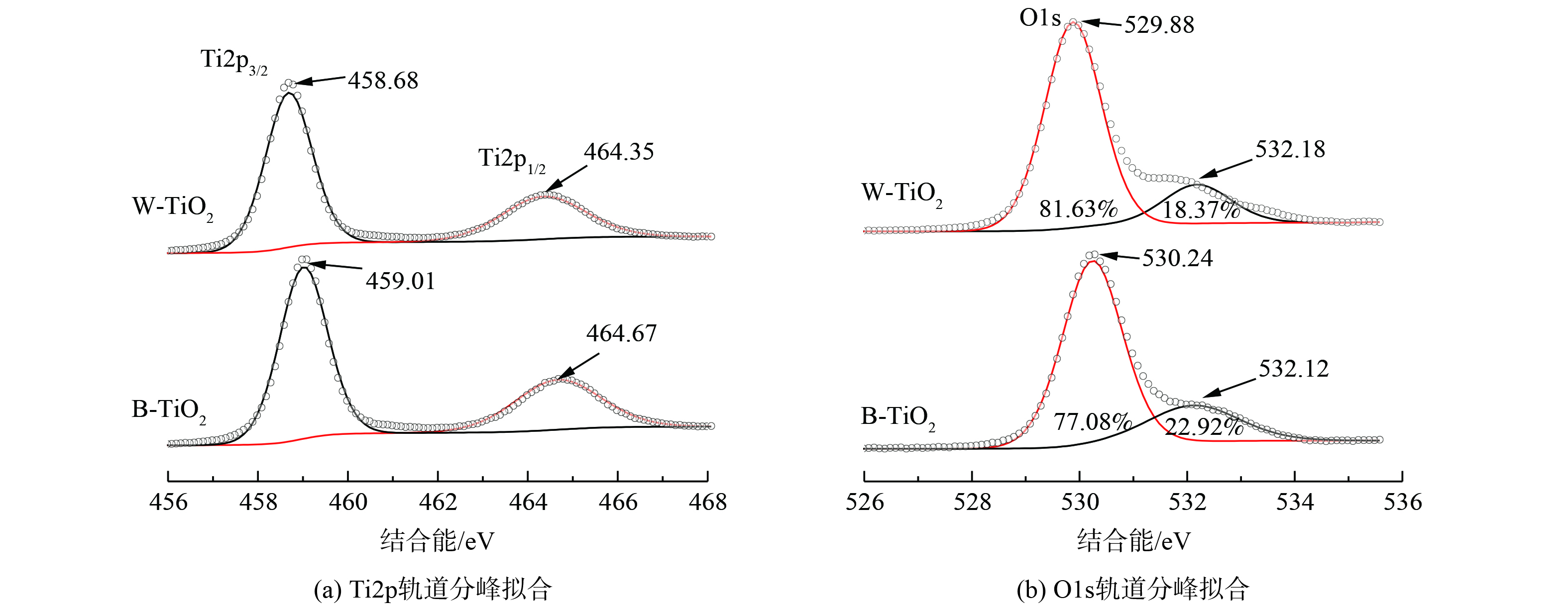
为确定W-TiO2、B-TiO2中Ti和O元素的化学状态,对Ti和O元素的XPS精细谱进行了分峰拟合,结果如图7所示。在Ti2p轨道 (图7 (a) ) 上,W-TiO2分别在458.68 eV (Ti 2p3/2) 和464.35 eV (Ti 2p1/2) 有一个单强峰和弱肩峰,这是TiO2中Ti—O—Ti键的结构特征,对应于Ti4+。氩气下煅烧得到的B-TiO2中Ti2p轨道的电子结合能分别增加到459.01 eV (Ti 2p3/2) 和464.67 eV (Ti 2p1/2) ,电子结合能的增大归因于缺氧环境下氧空位和Ti3+的形成[15]。
图7 (b) 为O1s轨道,2种催化剂在530 eV和532 eV处分别出现了晶格氧和吸附态氧的特征峰。W-TiO2晶格氧特征峰出现在529.88 eV。由于氧空位的存在,B-TiO2的晶格氧特征峰发生偏移,增加到530.24 eV。在532.1 eV附近拟合出的弱肩峰为吸附态氧的特征峰[16],根据元素定量,B-TiO2中吸附态氧的比例大于W-TiO2。这是由于氧空位的存在能促进空气中的氧转化为TiO2表面的吸附态氧[15-17],也进一步证明了氧空位的存在。
-
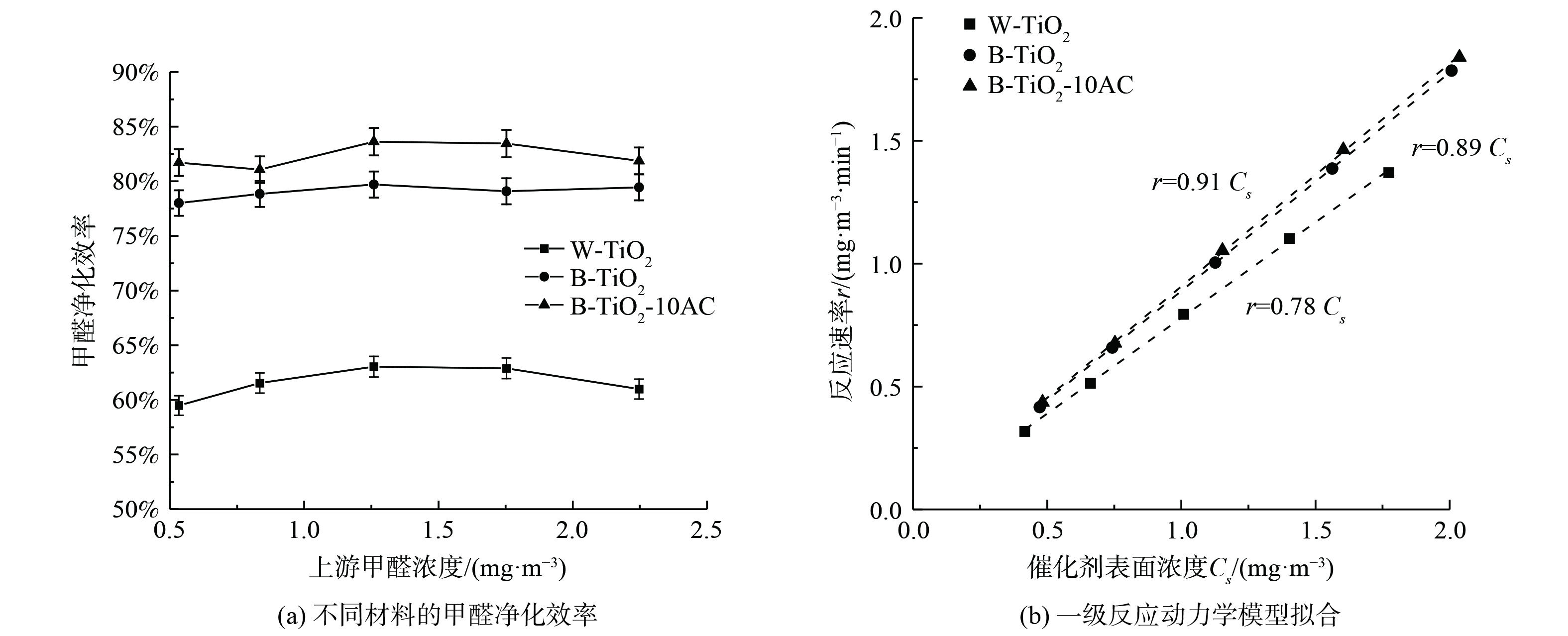
B-TiO2、W-TiO2、B-TiO2-10AC在不同浓度下的甲醛单通净化效率对比如图8 (a) 所示。考虑到室内实际甲醛污染水平,发生浓度选择国标规定的室内甲醛浓度限值的10倍左右数量级 (0.5~2.5 mg·m−3) ,湿度控制在 (50±2) %。在低浓度范围内,甲醛净化效率随浓度变化不明显。B-TiO2的甲醛净化效率在78%左右,相比于W-TiO2,甲醛净化效率提高了约16%。掺杂10%活性炭后,B-TiO2的净化效率提高了约5%。
通过不同浓度下获得的实验数据,可用于研究催化反应动力学,即可深入认识催化反应的反应机理,又可为实际催化反应装置的设计提供参考。Langmuir- Hinshelwood (L-H) 反应动力学模型 (式 (3) ) 被广泛用于解释气固相催化反应动力学机理[18-19]。在污染物浓度较低时,L-H模型可近似为准一级反应模型 (式 (4) ) ,即反应速率r与催化剂表面浓度Cs成正比。根据不同浓度下的降解效率拟合出的一级反应动力学平衡常数k´,如图8 (b) 所示,B-TiO2的k´值为0.89,明显高于W-TiO2,拟合度R2均大于0.99。
式中:r为甲醛降解速率,mg·m−3·min−1;k为L-H反应平衡常数,mg·m−3·min−1;k´为一级反应动力学平衡常数,min−1;K为吸附平衡常数mg−1·m3,Cs为催化剂表面甲醛质量浓度,mg·m−3。
B-TiO2相比于W-TiO2表现出更高的光催化活性,结合表征与相关研究,其增强作用主要归因于以下几点。首先,B-TiO2对于紫外光的吸收率增加;其次,氧空位处于富电子的化学还原态,能降低氧分子的化学吸附能,促进空气中的氧分子转化为吸附态氧,XPS结果 (图7 (b) ) 也表明B-TiO2表面吸附态氧含量增加,氧空位将电子转移到吸附态氧,并进一步转化为活性氧[20],从而加快甲醛的光催化降解速率。
-
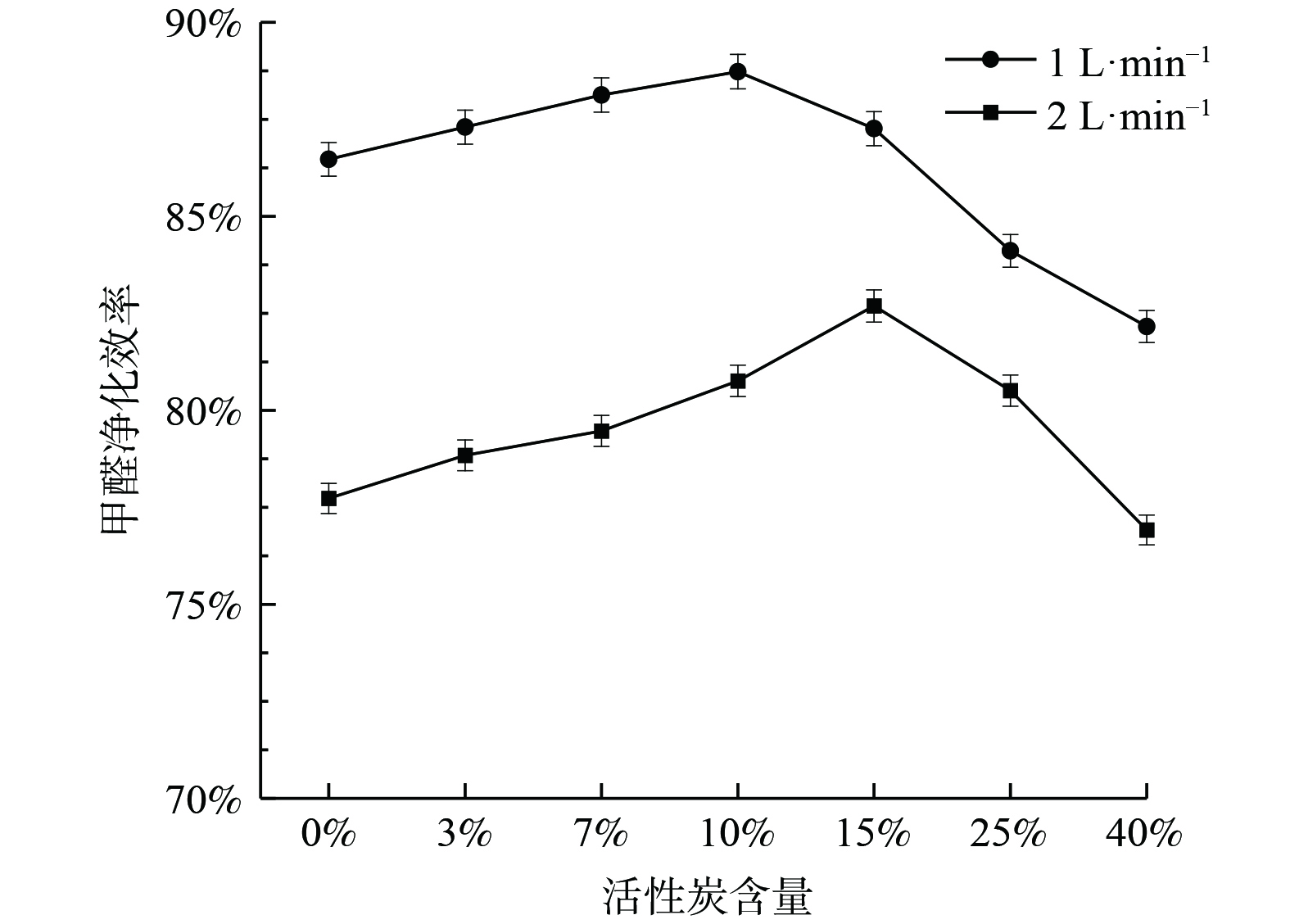
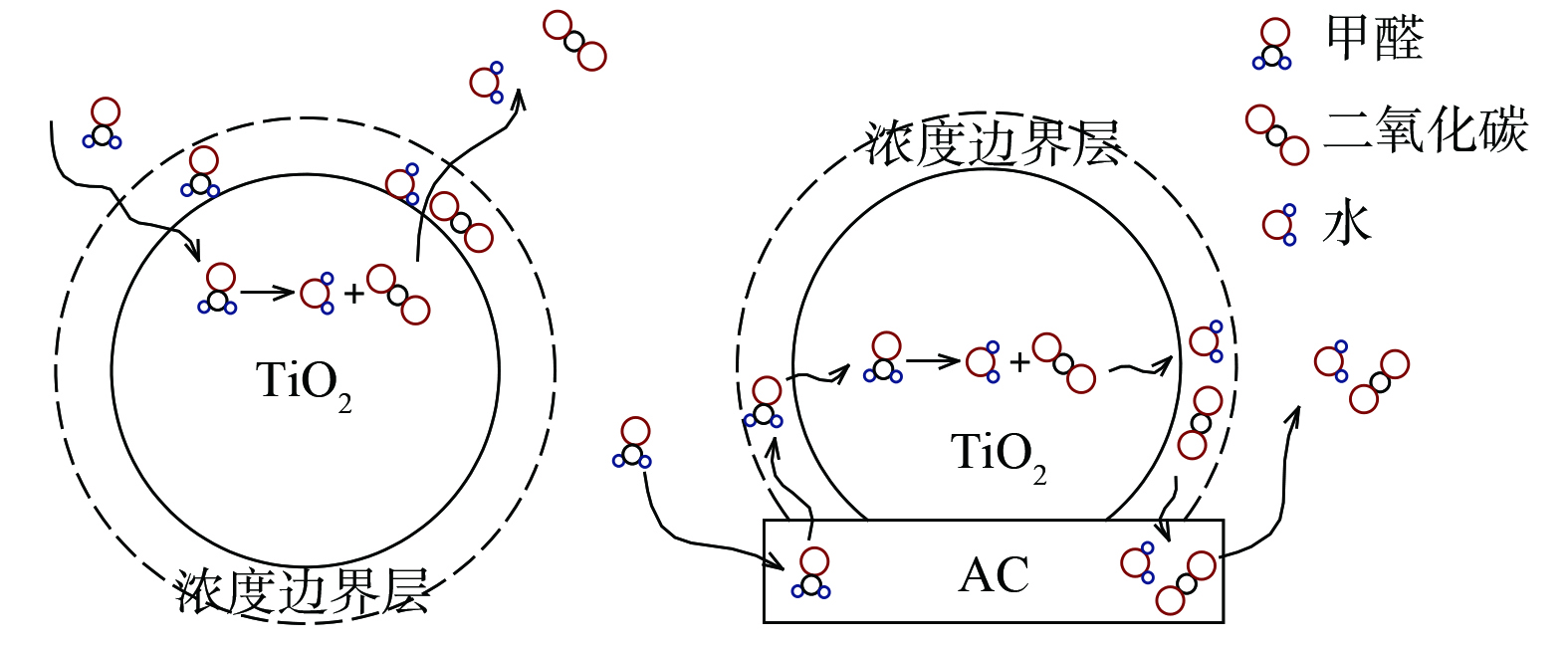
在紫外线照射下,不同活性炭比例的B-TiO2光催化降解甲醛效率如图9所示。当活性炭负载量小于10%时,甲醛净化效率随活性炭负载量增加而增大。这是由于活性炭的掺杂,一方面提高了催化剂的分散性和稳定性[21],防止催化剂团聚;另一方面,由于浓度边界层的存在 (图10) ,污染物从气流主体扩散到TiO2表面存在传质阻力,而在活性炭与TiO2的界面处,原本吸附在活性炭表面的污染物分子会由于浓度梯度和吸附力而转移到TiO2表面。这种活性炭吸附-界面转移-光催化反应的降解速率要高于普通的吸附催化速率,从而整体上提高光催化反应速率[22]。随着活性炭负载比例继续增加,催化活性位点相对减少,甲醛净化效率明显降低。因此,活性炭掺杂存在一个最佳比例。气流速度为1 L·min−1时的最佳负载量为10%,气流速度为2 L·min−1时的最佳负载量为15%。最佳负载量随着流速的增加而增加。这是由于最佳活性炭比例实质上是传质和催化2个反应步骤影响因素耦合的结果,活性炭因具有更大的比表面积和发达的孔隙结构而具有更好的吸附“浓缩”效果。当流速从1 L·min−1增加到2 L·min−1,污染物的停留时间缩短,需要更多的活性炭来有效捕集污染物,故最佳活性炭负载量增加。
综上所述,适量活性炭与黑色TiO2复合能提高甲醛净化效率,且这种催化-吸附协同净化能在一定程度上解决甲醛在催化剂表面停留时间短的问题。
-
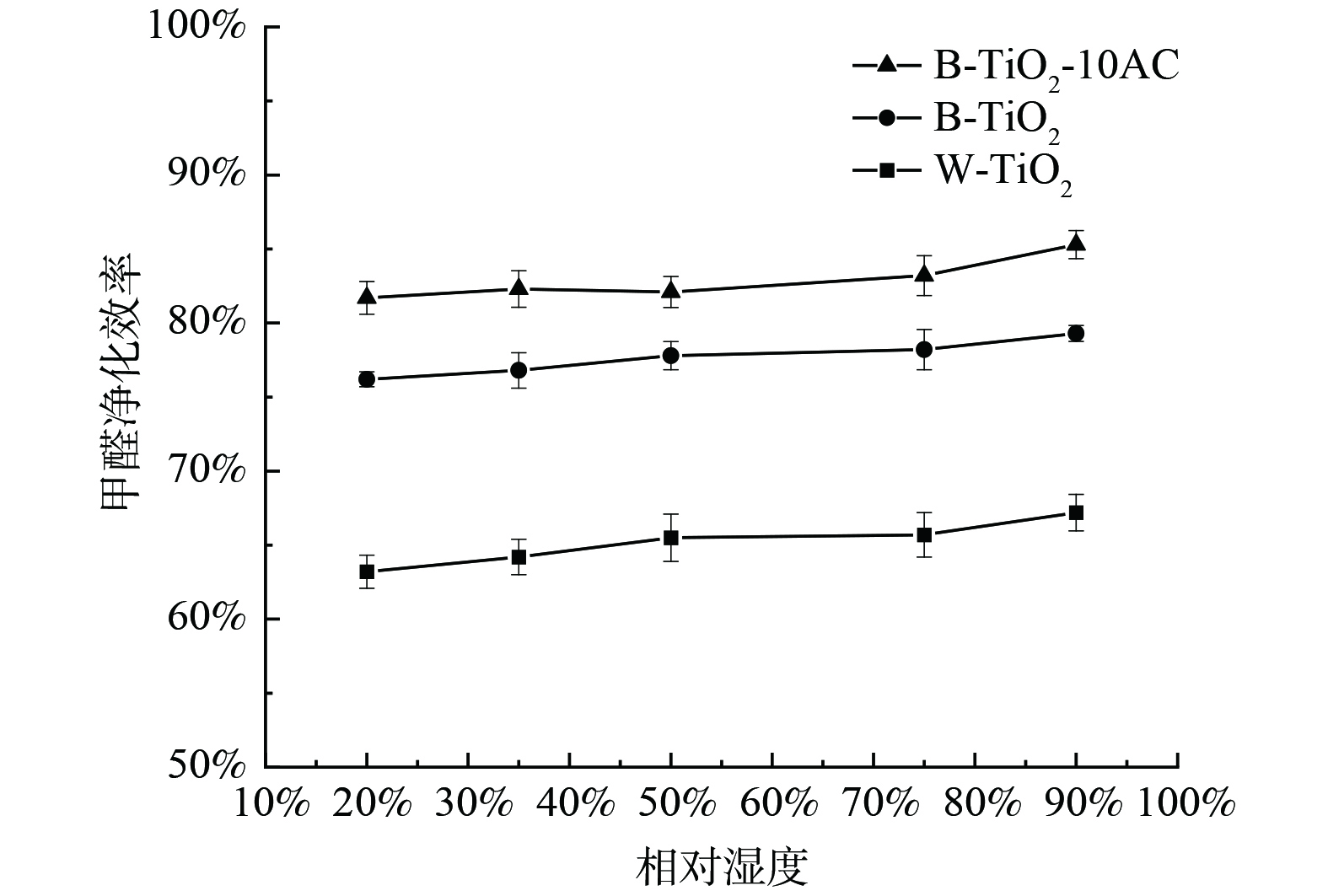
水分子对于光催化的影响较为复杂,与催化剂表面的结构特点及被降解的化合物结构都有关系,因此,针对不同的催化剂及测试系统,验证湿度对催化效率的影响是十分必要的。本实验在5种相对湿度条件下,测试W-TiO2、B-TiO2和B-TiO2-10AC的甲醛净化效率,实验结果如图11所示。随着相对湿度从20%升至90%,W-TiO2、B-TiO2和B-TiO2-10AC的甲醛的净化效率随湿度变化不明显,但总体来看,甲醛净化效率表现出上升的趋势。湿度从20%提高到90%,W-TiO2、B-TiO2和B-TiO2-10AC的甲醛净化效率分别提高了4%、3.1%和3.6%。
湿度通常被认为是影响光催化氧化速率的关键因素。一方面,羟基自由基作为光催化过程中的主要活性氧物种之一,是通过水分子与带正电的空穴反应得到的,故水分子的存在有利于合成更多的羟基自由基,从而加快催化反应速率;另一方面,水分子可能与污染物分子存在竞争吸附,从而抑制光催化反应速率[23],故湿度对于光催化反应速率的影响是双重的。在本研究中,甲醛分子质量浓度约为1.2 mg·m−3,水分子浓度为4 000~16 000 mg·m−3,两者的浓度相差3个数量级以上,因此两者竞争吸附的影响几乎可以忽略。在整体上,湿度的增加表现为对甲醛光催化降解速率的促进。该结果与HAN等[24]在低浓度污染物下,湿度对光催化速率影响的结论一致。
-
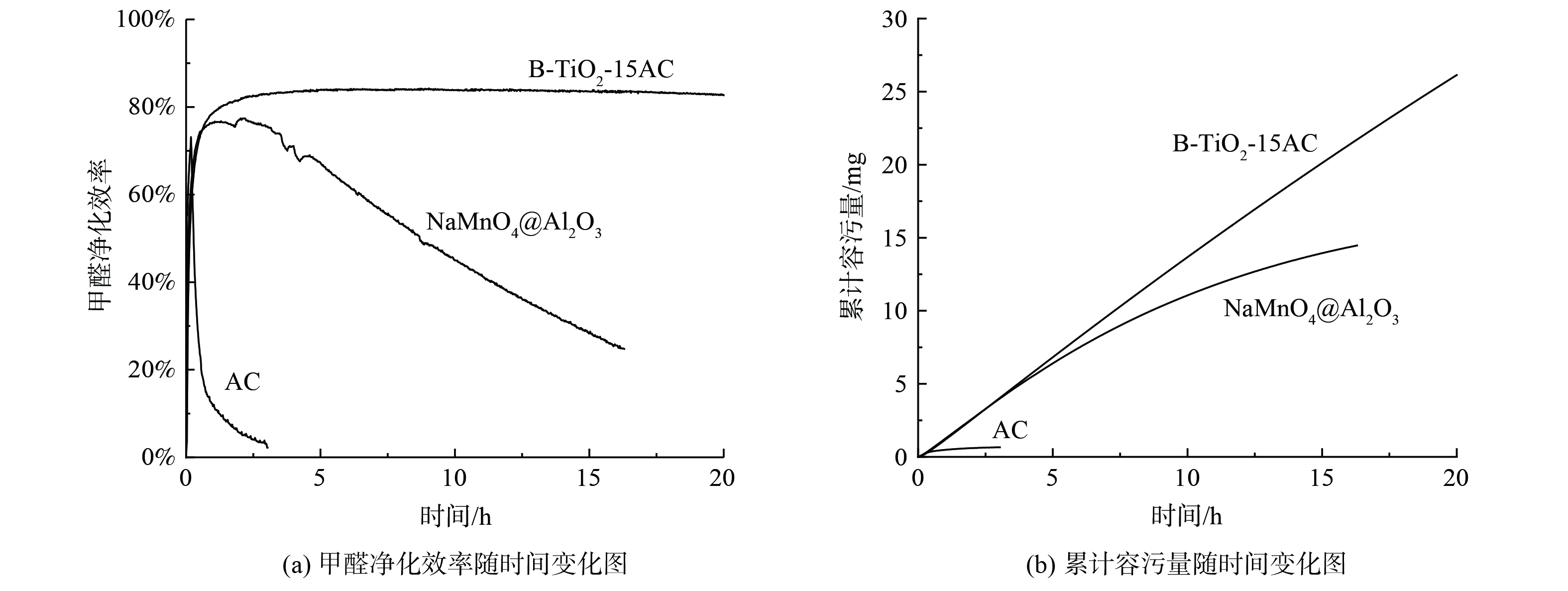
吸附和催化净化材料在实际应用中都存在失活的问题。为对比两类净化材料对于甲醛的长期净化性能,本研究采用一般吸附材料常用的容污量指标[25],将所研发的光催化材料与针对甲醛净化的吸附型材料在相同工况下进行对比。容污量是指净化材料效率衰减到一定程度时的累计净化量,该值受污染物浓度和空速等因素影响[6]。将B-TiO2-15AC与椰壳活性炭、高锰酸钠浸渍活性氧化铝3种材料在相同工况下进行长期性能测试。测试条件尽量接近实际室内环境工况:甲醛浓度1.2 mg·m−3,质量空速MHSV=800 L·g−1·h−1,紫外光强 (3 000±50) μW·cm−2,温度 (25±0.5) ℃,湿度 (50±2) %。
3种净化材料的效率与容污量随时间的变化趋势如图12所示。普通椰壳活性炭在2 h内效率从70%衰减到10%以下,单位质量容污量为4.3 mg·g−1。经过16 h,NaMnO4@Al2O3的净化效率从76%衰减至25%,单位质量容污量为96 mg·g−1。B-TiO2-15AC的效率一直保持在83%,没有出现明显衰减,16 h的单位质量容污量为147 mg·g−1。结果表明,B-TiO2-15AC催化剂在长期净化性能表现显著优于高锰酸钠浸渍活性氧化铝,普通活性炭吸附对甲醛净化效果较差。
-
1) 在常压、氩气气氛下制备了锐钛矿相黑色TiO2。黑色TiO2比白色TiO2的甲醛单通净化效率提升了16%,一级反应动力学常数提高了0.11。根据材料表征与相关研究推断,净化效率的提升归因于黑色TiO2对紫外线具有更强的吸收能力,同时氧空位的存在促进了氧分子的吸附与活化。因此,氩气气氛下煅烧得到的黑色TiO2对于室内甲醛气体有良好的净化效果。
2) 在掺杂活性炭后,B-TiO2-AC表现出催化吸附协同净化效果。活性炭的存在帮助TiO2催化剂的分散及污染物分子向催化剂表面的转移,但催化活性位点数减少,存在最佳活性炭负载量。气流速度为1 L·min−1时的最佳负载量为10%,气流速度为2 L·min−1时的最佳负载量为15%。因此,活性炭的掺入可解决B-TiO2因停留时间短而净化效率衰减的问题。
3) 在相同工况下对比了B-TiO2-15AC和吸附类净化材料对于甲醛的长期净化性能。在16 h内,B-TiO2-15AC效率维持在83%,而高锰酸钠浸渍活性氧化铝效率从76%衰减到25%,B-TiO2-15AC的容污量是高锰酸钠浸渍活性氧化铝的1.5倍。因此,对于室内甲醛净化,光催化相比于吸附技术在材料性能方面有很大优势。但光催化产品的结构形式、与传统HVAC系统如何匹配等问题仍有待优化,并需要经过综合效益的评估,才能更好地应用于空气净化产品。
黑色TiO2/活性炭复合材料的制备及其光催化净化甲醛性能
Preparation of black TiO2 / activated carbon and its photocatalytic purification performance for formaldehyde
-
摘要: 甲醛是最受关注的室内气态污染物之一。传统甲醛吸附类净化材料存在寿命短、二次污染、废弃物难处理等问题。以钛酸四丁酯为钛源,通过溶胶-凝胶法在氩气气氛下煅烧制备了黑色二氧化钛,采用TEM、XRD、DRS、XPS等技术手段表征了材料的物理、化学、光学特性,并利用连续流表面式光催化反应系统测试黑色TiO2的甲醛净化性能。结果表明,黑色TiO2存在表面无序层和氧空位等特征,并在紫外和可见光区域表现出更高的吸光度和更窄的带隙,紫外光下的甲醛单通净化效率可达78%。通过将活性炭与黑色TiO2复合,可进一步提升材料的甲醛净化效率,并得到在不同流速下活性炭的最佳负载量。黑色TiO2/活性炭与负载高锰酸钠的活性氧化铝经过16 h的长期性能测试对比,前者累计净化量是后者的1.5倍,且并未出现效率的衰减。本研究可为光催化技术在室内甲醛净化领域的应用提供参考。Abstract: Formaldehyde is one of the most widely concerned indoor gaseous pollutants. Traditional adsorption-based purification materials have the problems of short life-time, potential of secondary pollution and difficulty of waste disposal. In this study, black titanium dioxide was prepared by sol-gel calcination with tetrabutyl titanate as titanium source under argon atmosphere. The physical, chemical and optical properties of the prepared materials were characterized by TEM, XRD, DRS and XPS. Its formaldehyde purification performance was tested by continuous flow surface photocatalytic reaction system. The results show that black TiO2 has the characteristics of disordered surface layer and oxygen vacancy, and exhibits higher visible light absorbance and narrower band gap. Its single-pass purification efficiency of formaldehyde under ultraviolet light can reach 78%. By combining black TiO2 with activated carbon, the formaldehyde purification efficiency was further improved, and the optimal load of activated carbon at different flow rates were obtained. The long-term performance of black TiO2/ activated carbon and activated alumina loaded with potassium permanganate was compared for 16 hours, and the results show that the cumulative purification capacity of the former is 1.5 times that of the latter, and there is no efficiency decay. This study provides reference and comparison on material level for the application of photocatalytic technology in indoor formaldehyde purification.
-
Key words:
- black TiO2 /
- formaldehyde /
- photocatalysis
-

-
-
[1] International Agency for Research on Cancer. FORMALDEHYDE [EB/OL]. [2022-07-01]. https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-29.pdf,2018. [2] TANG X, BAI Y, DUONG A, et al. Formaldehyde in China: Production, consumption, exposure levels, and health effects[J]. Environment international, 2009, 36(8): 1210-1224. [3] SALTHAMMER T, MENTESE S, MARUTZKY R. Formaldehyde in the indoor environment[J]. Chemical Reviews, 2010, 110(4): 2536-2572. doi: 10.1021/cr800399g [4] LIU X, YAN M, DUAN H. Characteristics of indoor volatile organic compounds in urban residential microenvironments in China[J]. Research of Environmental Sciences, 2012, 25(10): 1077-1084. [5] ZHUANG X, HU X, LU X, et al. Analysis on the pollution characteristics of formaldehyde and alkyl benzenes in indoor air[J]. China Safety Science Journal, 2008, 18(5). [6] ZHANG X, GAO B, CREAMER A, et al. Adsorption of VOCs onto engineered carbon materials: A review[J]. Journal of Hazardous Materials, 2017, 338: 102-123. doi: 10.1016/j.jhazmat.2017.05.013 [7] CAO L X, GAO Z, SUIB S L, et al. Photocatalytic oxidation of toluene on nanoscale TiO2 catalysts: Studies of deactivation and regeneration[J]. Journal of Catalysis, 2000, 196: 253-261. doi: 10.1006/jcat.2000.3050 [8] HODGSON A T, DESTAILLATS H, SULLIVAN D P, et al. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications[J]. Indoor Air, 2007, 17(4): 305-316. doi: 10.1111/j.1600-0668.2007.00479.x [9] LIN T, YANG C, WANG Z, et al. Effective nonmetal incorporation in black titania with enhanced solar energy utilization[J]. Energy & Environmental Science, 2014, 7(3): 967. [10] CHOI W, TERMIN A, HOFFMANN M R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics[J]. Journal of Physical Chemistry, 1994, 98(51): 13669-13679. doi: 10.1021/j100102a038 [11] CHEN X, LIU L, YU P Y, et al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals[J]. Science, 2011, 331(6018): 746-750. doi: 10.1126/science.1200448 [12] ANDRONIC L, ENESCA A. Black TiO2 synthesis by chemical reduction methods for photocatalysis applications[J]. Frontiers in Chemistry, 2020, 8: 565489. doi: 10.3389/fchem.2020.565489 [13] KATAL R, SALEHI M, FARAHANI M, et al. Preparation of a new type of black TiO2 under a vacuum atmosphere for sunlight photocatalysis[J]. ACS Applied Materials & Interfaces, 2018, 10(41). [14] DONG J, HAN J, LIU Y, et al. Defective black TiO2 synthesized via anodization for visible-light photocatalysis[J]. Acs Applied Materials & Interfaces, 2014, 6(3): 1385-1388. [15] WEI Z, WANG W, LI W, et al. Steering electron–hole migration pathways using oxygen vacancies in tungsten oxides to enhance their photocatalytic oxygen evolution performance[J]. Angewandte Chemie-International Edition, 2021, 60: 8236-8242. doi: 10.1002/anie.202016170 [16] ABDULLA-AL-MAMUN M, KUSUMOTO Y, SHARIFUL ISLAM M, et al. Enhanced photocatalytic cytotoxic activity of Ag @ Fe-doped TiO2 nanocomposites against human epithelial carcinoma cells[J]. Journal of Materials Chemistry, 2012, 22: 5460-5469. doi: 10.1039/c2jm15636a [17] HE M, JI J, LIU B, et al. Reduced TiO2 with tunable oxygen vacancies for catalytic oxidation of formaldehyde at room temperature[J]. Applied Surface Science, 2019, 473: 934-942. doi: 10.1016/j.apsusc.2018.12.212 [18] KIM S B, HONG S C. Kinetic study for photocatalytic degradation of volatile organic compounds in air using thin film TiO2 photocatalyst[J]. Applied Catalysis B:Environmental, 2002, 35(4): 305-315. doi: 10.1016/S0926-3373(01)00274-0 [19] PEI J, XU H, YI L. Performance and kinetics of catalytic oxidation of formaldehyde over copper manganese oxide catalyst[J]. Building and Environment, 2015, 84: 134-141. doi: 10.1016/j.buildenv.2014.11.002 [20] ZENG L, SONG W L, LI M H, et al. Catalytic oxidation of formaldehyde on surface of H-TiO2/ H-C-TiO2 without light illumination at room temperature[J]. Applied Catalysis B:Environmental, 2014, 147: 490-498. doi: 10.1016/j.apcatb.2013.09.013 [21] LI J, ZHANG P, WANG J, et al. Birnessite-type manganese oxide on granular activated carbon for formaldehyde removal at room temperature[J]. The Journal of Physical Chemistry C, 2016, 120: 24121-24129. doi: 10.1021/acs.jpcc.6b07217 [22] MATOS J, LAINE J, HERRMANN J M. Association of activated carbons of different origins with titania in the photocatalytic purification of water[J]. Carbon, 1999, 37(11): 1870-1872. doi: 10.1016/S0008-6223(99)00198-0 [23] OBEE T N, BROWN R T. TiO2 photocatalysis for indoor air applications: effects of humidity and trace contaminant levels on the oxidation rates of formaldehyde, toluene, and 1, 3-butadiene[J]. Environmental Science & Technology, 1995, 29(5): 1223-1231. [24] HAN Z N, CHANG V W, WANG X P, et al. Experimental study on visible-light induced photocatalytic oxidation of gaseous formaldehyde by polyester fiber supported photocatalysts[J]. Chemical Engineering Journal, 2013, 218: 9-18. doi: 10.1016/j.cej.2012.12.025 [25] Test method for assessing the performance of gas-phase air cleaning media and devices for general ventilation—Part 1: Gas-phase air cleaning media: ISO10121-1-2014[S/OL]. The International Organization for Standardization, 2014. https://www.iso.org/standard/77498.html. -



 下载:
下载: