-
砷(As, arsenic)在自然界中广泛存在,是一种对环境和生物有毒的元素。环境中的砷主要来源于自然过程和人为活动,如岩石风化、化石燃料燃烧、含砷农药的使用等。长期暴露在砷污染环境中,可能会诱发癌症、皮肤病、神经问题等疾病。环境中的砷主要为无机砷和有机砷,价态分为三价和五价,而天然水体中的砷通常以As(Ⅲ)的无机砷形态存在。由于As(Ⅲ)比 As(Ⅴ)更具有毒性、可溶性和流动性[1],As(Ⅲ)的去除受到国内外的广泛关注。目前,常用的除砷方法有吸附法、混凝沉淀法、离子交换法、膜分离法[2]。其中,吸附法因其操作简单、去除效果好而被广泛应用于环境中砷的去除。常用的吸附剂有铁氧化物、氢氧化物、锰氧化物及粘土矿物等[3]。铁和锰的化学性质密切相关,在环境中往往以铁锰二元氧化物的形式存在,常用于去除水体中的砷[4,5]。这是由于铁氧化物具有表面电荷高、比表面积大、吸附能力强等优点[6],而锰氧化物则可以将毒性高且不易被去除的As(Ⅲ)转化为低毒性的As(Ⅴ),从而提高对砷的去除效率。LAKSHMIPATHIRAJ等[4]制备了一种铁锰化合物MnFeOOH,可以将水中的As(Ⅲ)氧化为As(Ⅴ),然后As(Ⅴ)通过铁锰化合物吸附去除。CHAKRAVARTY等[7]采用天然存在的廉价铁锰矿石来处理含砷废水,可通过吸附作用将地下水中砷质量浓度降至0.02 μg·L−1以下。然而,天然铁锰矿对砷的吸附性能存在一定的局限性,采用物理或化学方法对天然锰矿进行改性,可增强其表面活性,提高其吸附能力。水合肼作为一种良好的还原剂,在酸性介质中可以对天然铁锰矿进行还原溶解作用,将天然铁锰矿表面的杂质物质溶出。而加入的高锰酸钾溶液可将Mn2+氧化成高价态锰,使得天然铁锰矿具有高活性表面,以提高天然铁锰矿的界面吸附能力。马子川等[8]采用水合肼对天然锰矿进行改性,极大提高了天然锰矿对重金属离子的吸附能力。但针对不同铁含量的天然锰矿水合肼的改性效果及其除砷机制的研究尚少见报道。为此,本研究以含有不同铁锰比例的天然锰矿为材料,探讨了水合肼改性铁锰含量不同的天然锰矿对砷的吸附性能及影响因素,并结合X射线衍射(XRD, X-ray diffraction)、傅立叶变换红外光谱(FTIR, Fourier-transform infrared spectroscopy)、X射线光电子能谱(XPS, X-ray photoelectron spectroscopy)等表征手段,分析吸附砷前后以及反应过程中可能的吸附氧化机制,以期为天然铁锰矿的应用与砷污染的治理提供参考。
-
实验中使用的水合肼(N2H4·H2O)、硫酸(H2SO4)、高锰酸钾(KMnO4)、硝酸钠(NaNO3)、硝酸(HNO3)、氢氧化钠(NaOH)、三氧化二砷(As2O3)均为分析纯试剂,购自于国药化学试剂有限公司。As(Ⅲ)储备液采用As2O3制备而成。实验中所有水溶液均使用18.25 MΩ·cm的超纯水配制。
-
天然铁锰矿石采自广西某地,经水洗、自然风干后,研磨至100目,代号为NFM。本实验选用2种铁锰占比差异较大的天然铁锰矿为材料,分别为NFM-I和NFM-II。NFM-I的铁、锰质量占比分别为6.64%和25.5% (Fe/Mn=1:3.8),而NFM-II的铁、锰质量占比为0.862%和44.5% (Fe/Mn=1:51.6)。分别简称为高铁低锰矿(NFM-I)和低铁高锰矿(NFM-II)。
为减少含铁锰矿表面杂质成分,提高矿物表面活性铁锰成分含量,从而提升其砷去除能力,本研究采用水合肼法进行铁锰矿石的改性。称取50 g NFM与0.5 mol·L−1水合肼(N2H4-H2O)和3 mol·L−1硫酸(H2SO4)溶液混合,在100 ℃条件下加热搅拌,反应60 min后,向冷却的反应液中加入1 mol·L−1高锰酸钾(KMnO4),直至上清液颜色不再褪去。沉淀物经水洗、烘干后,研磨至100目,即为改性的天然锰矿(MNFM)。本研究中2种不同类型的天然含铁锰矿NFM-I和NFM-II,经改性后分别为MNFM-I和MNFM-II。
-
首先,将0.100 0 g MNFM-I (或MNFM-II) 加入含有100 mL、200 µg∙L−1 As(Ⅲ)溶液的150 mL锥形瓶中。密封后,置于恒温振荡器(25 ℃、200 r∙min−1)中反应180 min。然后,在预定的时间间隔采样,经0.45 µm的滤膜过滤,测定滤液中的总砷、As(Ⅲ)、As(Ⅴ)浓度。实验中,采用稀盐酸和氢氧化钠调节溶液pH,背景离子强度为0.01 mol·L−1 NaNO3。总砷浓度和As(Ⅲ)浓度采用原子荧光光谱仪(AFS, Beijing Titan Instrument Co., Ltd., China)测定,As(Ⅴ)的浓度根据其差值计算,砷吸附量和砷去除率分别按式(1)和式(2)进行计算,分别使用一级动力学模型方程(式(3))和二级动力学模型方程(式(4))进行数据拟合。
式中:qe为吸附剂的平衡吸附量,mg·g−1;C0和Ce分别为As(Ⅲ)溶液的初始质量浓度和平衡质量浓度,mg∙L−1;V为As(Ⅲ)溶液的体积,L;R为去除率,%;m为吸附剂质量,g。
式中:qt为时间t时,As在吸附剂上的吸附量,mg∙g−1;qe为平衡时的吸附容量,mg∙g−1;K1为一级动力学模型速率常数,min−1;K2为二级动力学模型速率常数,g∙(mg∙min)−1。
-
将0.100 0 g NFM (或MNFM)分别加入含有100 mL,As(Ⅲ)溶液质量浓度为5、10、20、40、60、80、100、120 mg∙L−1的锥形瓶中。密封置于恒温振荡器(25 ℃、200 r∙min−1)中反应24 h后,经0.45 µm的滤膜过滤,测定滤液中平衡砷浓度。实验中,采用稀盐酸和氢氧化钠调节溶液pH为7.0±0.1,背景离子强度为0.01 mol·L−1 NaNO3。采用Langmuir模型(式(5))和Freundlich模型(式(6))进行数据拟合。
式中:Ce为平衡溶液中砷的质量浓度,mg·L−1;qm为吸附剂最大平衡吸附容量,mg∙g−1;b为吸附平衡常数,L∙mg−1;Kf 为Freundlich常数,mg∙g−1;n为非均质系数。
为了比较不同材料对砷的吸附能力,将0.100 0 g NFM (或MNFM) 分别加入含有100 mL、200 µg∙L−1 As(Ⅲ)溶液的锥形瓶中。密封置于恒温振荡器(25 ℃、200 r∙min−1)中反应24 h后,经0.45 µm的滤膜过滤,测定滤液中平衡As(Ⅲ)浓度。实验中,采用稀盐酸和氢氧化钠调节溶液pH为7.0±0.1,背景离子强度为0.01 mol·L−1 NaNO3。As(Ⅲ)浓度采用原子荧光光谱仪(AFS)测定。
-
为了探究Cl−、NO3−、SO42−、CO32−、PO43−、SiO32−共存离子对As(Ⅲ)去除的影响,将0.100 0 g MNFM-I (或MNFM-II) 加入含有100 mL、200 µg∙L−1 As(Ⅲ)溶液的锥形瓶中,再分别添加Cl−、NO3−、SO42−、CO32−、PO43−、SiO32−六种共存离子,每种共存离子分别设置0、0.1、1 mmol·L−1三种浓度,密封置于恒温振荡器(25 ℃、200 r∙min−1)中反应24 h后,经0.45 µm的滤膜过滤,测定滤液中的As(Ⅲ)浓度。实验中,采用稀盐酸和氢氧化钠调节溶液pH为7.0±0.1,背景离子强度为0.01 mol·L−1 NaNO3。
为了探究pH对As(Ⅲ)去除的影响,将0.100 0 g NFM (或MNFM) 加入含有100 mL、200 µg∙L−1 As(Ⅲ)溶液的锥形瓶中,溶液初始pH分别设置为1、3、5、7、9、11、13,以0.01 mol·L−1 NaNO3作为背景离子。密封置于恒温振荡器(25 ℃、200 r∙min−1)中反应24 h后,经0.45 µm的滤膜过滤,测定滤液中的As(Ⅲ)浓度。
-
采用X射线荧光光谱仪(XRF, Bruker)对改性前后天然铁锰矿的组分与含量进行分析测定。检测条件为:电压30~60 kV,电流50~100 mA,温度25 ℃,湿度55% RH。根据陆泗进等[9]的方法测试材料的电荷零点。称取0.500 0g NFM和MNFM于离心管中,加入适量的去离子水,调节溶液pH至1~6,保持最终体积为10 mL;在25 ℃条件下,将溶液平衡3~4 d,每天振荡1 h,测其pH,记为pH;向溶液中加入0.5 mL、2 mol·L−1 KCl溶液,并振荡4 h,测其pH,记为pH1;计算样品pHPZC:ΔpH (ΔpH =pH1-pH)。以pH为横坐标,ΔpH为纵坐标作图,ΔpH=0时对应的pH即为该样品的pHPZC。
改性前后材料利用D8Advance(Holland) X射线衍射仪进行XRD分析鉴定。测试条件为:Cu Kα辐射,管压40 kV,管电流20 mA,扫描速度为10°·min−1,步长0.02°。材料的FTIR光谱通过Bruker Vertex70型傅里叶变换红外光谱仪进行测试。取适量干燥样品以质量比为1:100与干燥的溴化钾粉末混合均匀,压片后测试,扫描波数范围为4 000~400 cm−1,分辨率为4 cm−1,扫描次数为32。数据处理利用OPUS软件。
材料的光电子能谱(XPS)采用XSAM800型(Kratos,Britain)光电子能谱仪检测。测试条件为:Al靶,真空度为2×10−7 Pa。采用C1s(284.8eV)作为荷电校正的标准,分别采集试样XPS全谱和各元素高精度窄区谱,图谱利用Avantage软件分析,采用Shirley背景扣除,进行重叠峰分离、峰形拟合。
-
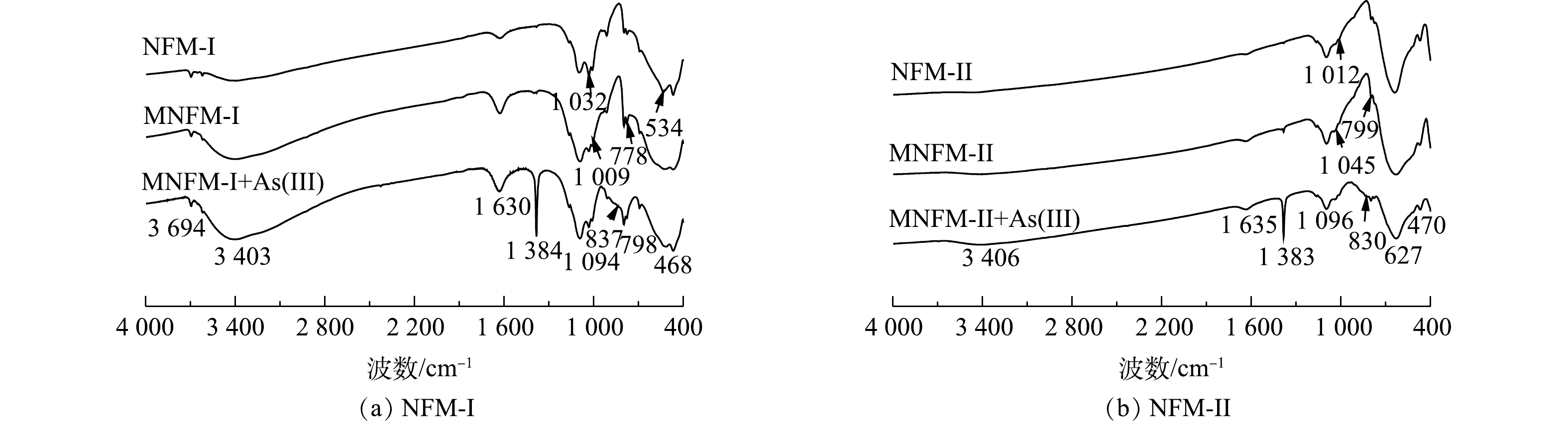
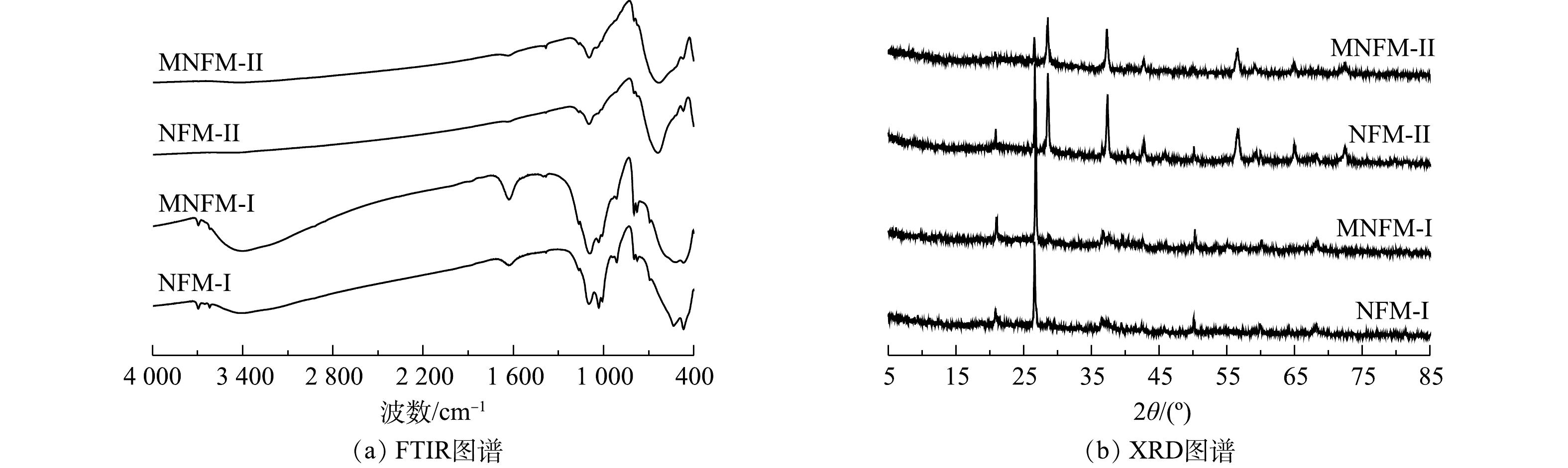
2种天然铁锰矿改性前后及吸附砷前后的元素含量变化如表1所示。从表1可见,材料改性后,MNFM-I和MNFM-II中的Fe含量均有轻微降低,而Mn含量则有所增加。这可能是由于水合肼的还原溶解作用溶解了结构中的部分Fe,并被释放到溶液中,导致Fe含量有所减少。而在高锰酸钾的再氧化过程中,材料表面可能生成了新的MnO2。NFM-I、NFM-II、MNFM-I、MNFM-II的FT-IR光谱如图1(a)所示。可见,NFM-I在3 403 cm−1、1 630 cm−1处的特征峰与NFM-II在3 406 cm−1、1 635 cm−1处的特征峰可归属为H—O—H的伸缩振动和弯曲振动[10]。然而,改性后的MNFM-I和MNFM-II的H—O—H特征峰明显变宽且变强,表明含有不同铁锰比例的材料在改性过程中表面的羟基含量均有所增加。NFM-I在1 009、1 032、1 094 cm−1的特征峰与NFM-II在1 012、1 045、1 096 cm−1的特征峰均被认为是铁(氢)氧化物的羟基官能团的弯曲振动[11]。NFM-I的468 cm−1与NFM-II 的470 cm−1特征峰为Fe—O伸缩振动引起的,而NFM-I在534 cm−1和NFM-II在627 cm−1处的特征吸收峰可归因于Mn—O键的振动[12-13]。对比NFM与MNFM光谱曲线发现,两者的主要特征吸收峰峰位一致,但是改性后的MNFM的H—O—H和Mn—O特征峰更宽且强,谱线变得平滑,其中,NFM-I改性前后特征峰变化效果更加明显,表明改性过程未改变材料的组成结构,可能部分杂质被溶解去除,材料表面暴露出更多的活性位点。
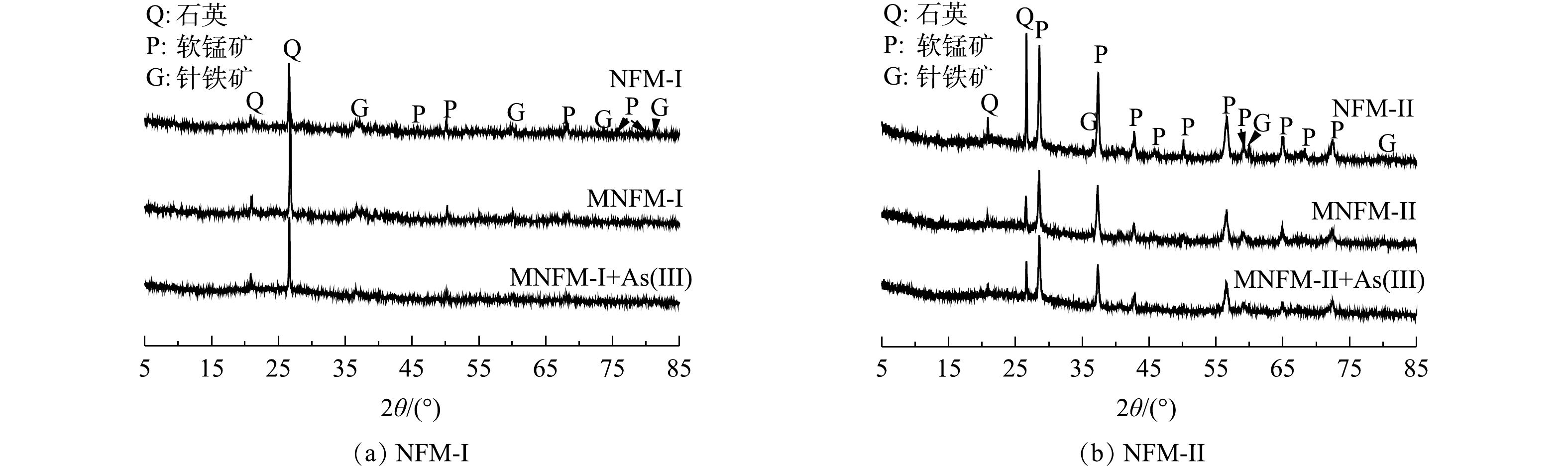
4种材料(NFM-I、NFM-II、MNFM-I、MNFM-II)的XRD图谱如图1(b)所示。可见,NFM-I和NFM-II主要由SiO2 (quartz, PDF #70-3755)、MnO2 (pyrolusite, PDF #01-0799)、Fe2O3·H20 (goethite, PDF #17-0536)组成,但锰含量较高的NFM-II的主要峰型为MnO2峰。
-
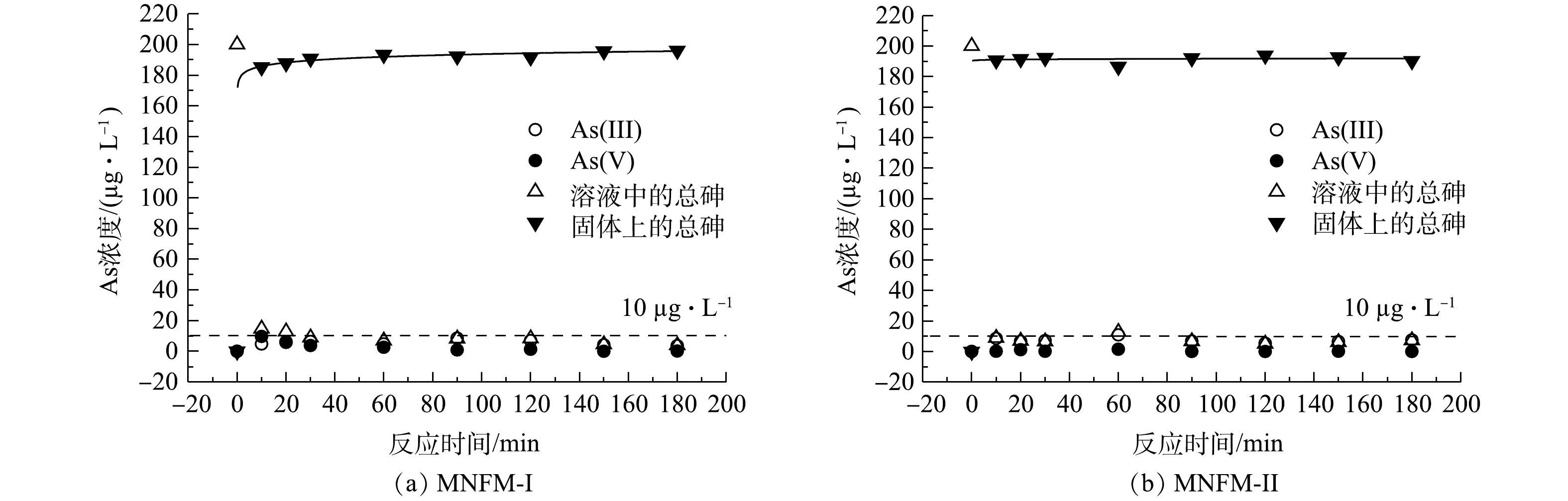
测定了2种铁锰比例不同的天然铁锰矿改性材料对去除水中砷的动力学特征(图2)。结果表明,在30 min内,溶液中总砷质量浓度迅速由200 µg·L−1降至10 µg·L−1以下。MNFM-I在1 h内达到吸附平衡,而相比MNFM-I,MNFM-II能在20 min内达到吸附平衡。然而,反应180 min后,MNFM-I吸附后溶液中总砷的最终平衡质量浓度为4 µg·L−1,而MNFM-II为9.1 µg·L−1,显然MNFM-I能更好的使水体总As质量浓度达到10 µg·L−1的国际饮用水标准[14]。
二级动力学模型假定吸附容量与吸附剂活性位点的数量成正比,广泛用于描述吸附剂对重金属的吸附行为[15-16]。使用一级动力学模型和二级动力学模型对本研究所得数据进行拟合发现,二级动力学模型能更好的拟合2种材料对As(Ⅲ)的吸附过程(R2>0.99)。
As(Ⅴ)的浓度变化可反映As(Ⅲ)的氧化过程。在本研究中,反应10 min时,MNFM-I将200 µg·L−1的总砷质量浓度迅速降至186 µg·L−1,此时As(Ⅲ)质量浓度为4.9 µg·L−1,As(Ⅴ)为9.1 µg·L−1,说明反应初期的氧化速率大于吸附速率。随着反应的进行,As(Ⅴ)的浓度持续减小,180 min时,As(Ⅴ)的质量浓度为0.25 µg·L−1,As(Ⅲ)为3.7 µg·L−1,表明溶液中的As(Ⅴ)优先吸附到MNFM-I表面。有研究[17-19]发现铁(氢)氧化物对As(Ⅴ)的亲和力远大于As(Ⅲ),两者共存时,As(Ⅴ)能被优先去除。相较MNFM-I,在反应过程中,MNFM-II处理组中的As(Ⅴ)浓度呈现先增加后减少的趋势,180 min时,溶液中未检测到As(Ⅴ),As(Ⅲ)质量浓度为9.1 µg·L−1。这可能是由于锰氧化物对溶液中游离的As(Ⅲ)亲和力较弱,以至于溶液中As(Ⅲ)的残留含量更高[20]。有研究[21]表明,As(Ⅲ)的毒性比As(Ⅴ)高出60多倍。因此,MNFM-I更适合用于去除水中的砷。
-
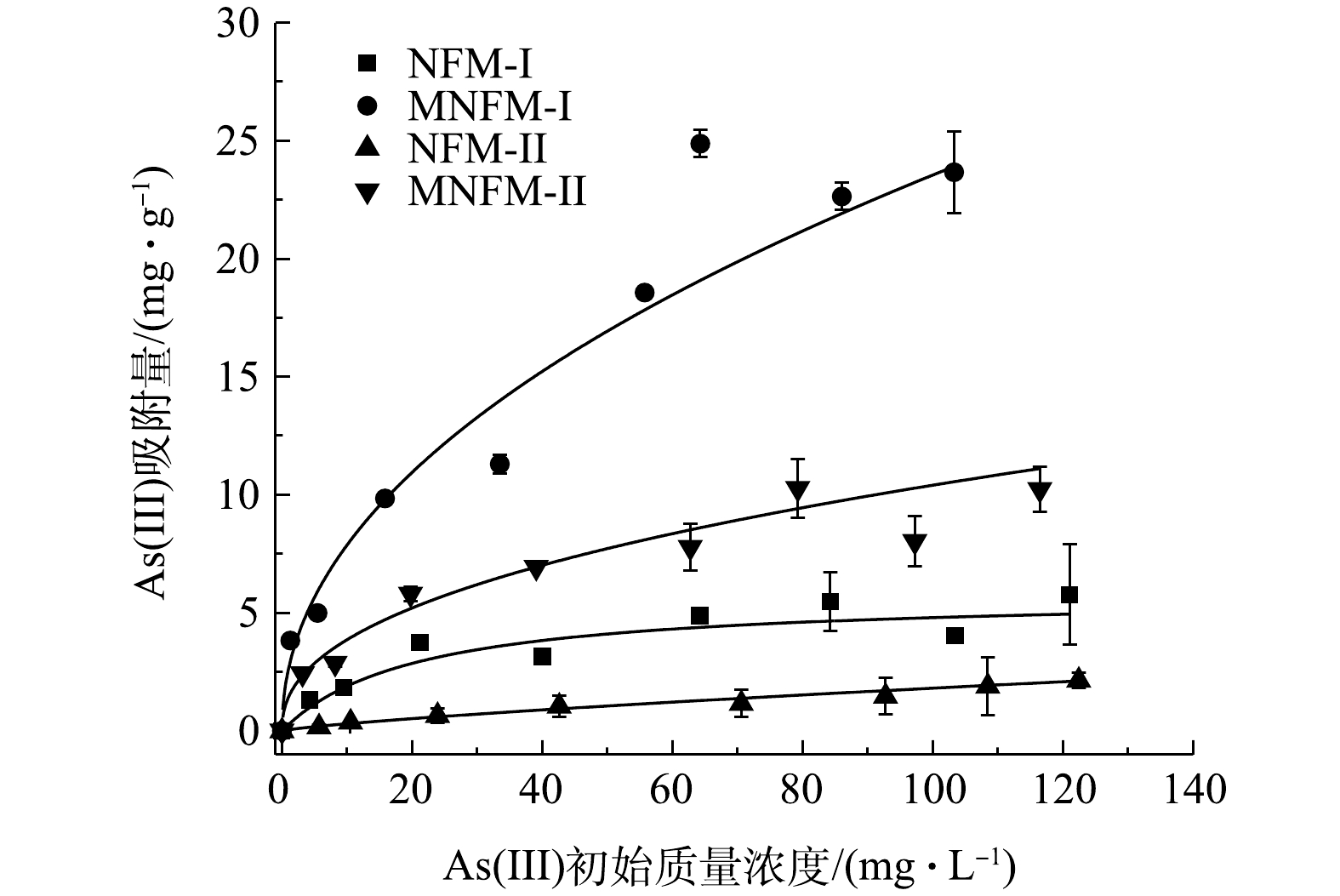
NFM-I、MNFM-I、NFM-II、MNFM-II对As (Ⅲ) 的吸附量与平衡质量浓度之间关系如图3所示。由图3可知,MNFM-I和MNFM-II的平衡吸附容量均比NFM-I、NMF-II高出4.7倍左右,说明改性后材料的吸附容量远高于未改性的材料,通过改性明显可提高材料的除砷性能。
吸附等温线的模拟结果表明,除NFM-II材料Langmuir模型R2较低外,2种模型Langmuir和Freundlich模型均能较好地拟合等温吸附曲线(表2)。可见,MNFM-I (30.9 mg·g−1)的Langmuir吸附容量比MNFM-II (12 mg·g−1)高近2.6倍。比较4种材料的Freundlich模型Kf值发现,改性后材料的Kf值明显增大,NFM-I由1.23上升到2.62,NFM-II由0.05上升到1.37,其中MNFM-I最大,NFM-II最小,表明MNFM-I对As(Ⅲ)有较强的亲和力。
表3为本研究与其他材料的除砷效果对比。由表3可见,YU等[5]采用磁性铁锰二元氧化物纳米片去除水溶液中的As(Ⅲ),其最大吸附量可达56.1 mg·g−1。CHAO等[22]合成的磁性铁锰氧化物对水溶液中的As(Ⅲ)也有较好的去除效果,吸附容量可达47.8 mg·g−1。由此可见,人工合成的磁性铁锰纳米材料具有较强的除As(Ⅲ)能力。但是,人工合成的材料成本高、产量低,其在水体修复中的应用前景有限。本研究采用天然含铁锰矿作为除砷原材料去除水溶液中的As(Ⅲ),其吸附容量最高可达30.9 mg·g−1。相比于人工合成磁性铁锰二元氧化物,MNFM-I成本低、储量大,更适合作为水体修复的理想材料。不过,含铁量较少的MNFM-II的砷吸附容量仅为12 mg·g−1,与相似除砷材料相比,其去除能力明显较弱。
-
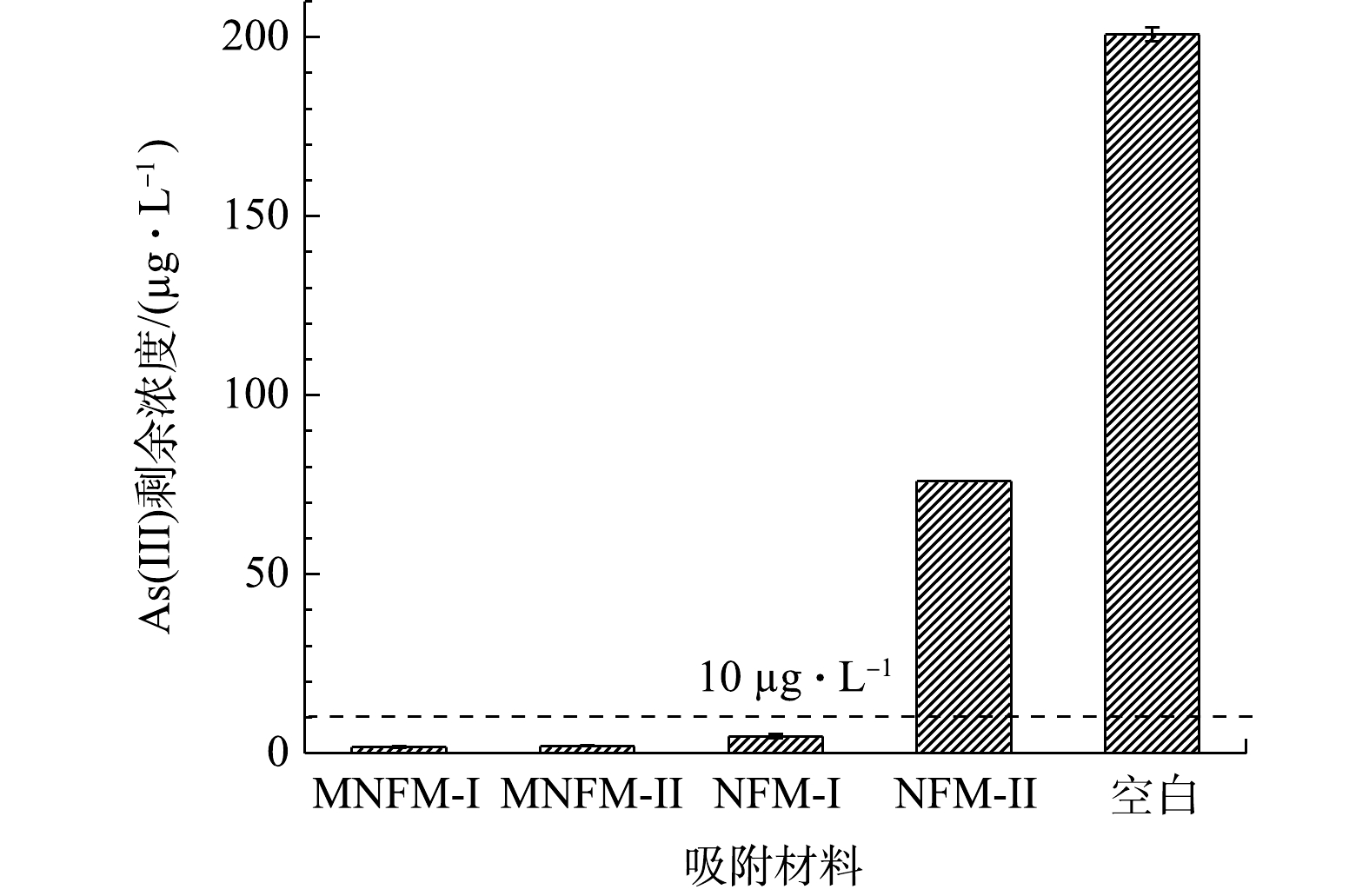
为了进一步考察4种材料对砷的去除能力,依据过去研究报道的地下水砷浓度[28],设置初始As(Ⅲ)质量浓度为200 µg·L−1。1 g·L−1的材料(NFM-I、MNFM-I、NFM-II、MNFM-II)去除As(Ⅲ)后的剩余质量浓度如图4所示。可见,在未添加吸附剂的情况下,As(Ⅲ)溶液在相同的实验条件下浓度未发生变化。由图4可见,在NFM-I、MNFM-I去除As(Ⅲ)后,溶液的剩余总As质量浓度均小于污染物浓度最大限值(10 µg·L−1),但NFM-I的剩余总As质量浓度(4.74 µg·L−1)是MNFM-I (1.8 µg·L−1)的2.6倍,MNFM-I的去除能力明显优于NFM-I。NFM-II去除As(Ⅲ)后,其剩余总As质量浓度远大于10 µg·L−1。然而,改性后的MNFM-II (2.04 µg·L−1)能满足10 µg·L−1的质量浓度限值。比较NFM-I和NFM-II发现,NFM-II (76 µg·L−1)的剩余总As质量浓度是NFM-I (4.74 µg·L−1)的16倍,说明NFM-I可能存在更多的可用活性吸附位点用于去除水溶液中的As(Ⅲ),从而有较强的去除As(Ⅲ)的能力。NFM-II表面严重钝化,然而改性后MNFM-II的除As(Ⅲ)能力增强,说明改性过程可能更新了钝化表面,暴露出NFM-II的活性吸附位点。对比4种材料的除As(Ⅲ)效果发现,MNFM-I去除效果最好,MNFM-II次之,这说明MNFM-I更具去除砷的潜力。
-
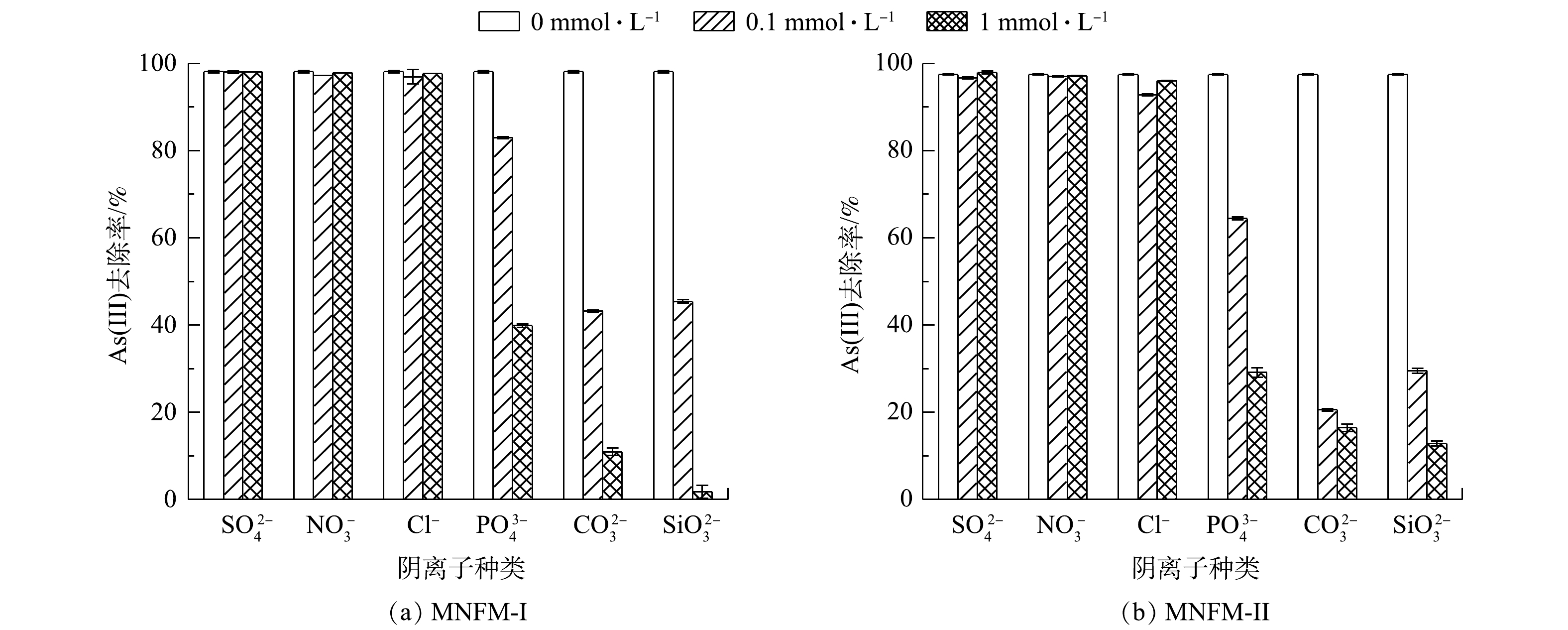
采用批处理实验研究了6种共存离子(SO42−、NO3−、Cl−、PO43−、CO32−、SiO32−)对MNFM-I和MNFM-II去除As(Ⅲ)的影响(图5)。结果表明,SO42−、NO3−对材料去除As(Ⅲ)没有明显影响,但Cl−存在下As(Ⅲ)的去除率有轻微下降。然而,PO43−、CO32−、SiO32存在时As(Ⅲ)的去除率显著降低;当共存离子浓度为1 mmol·L−1时,As去除率被降低到1.5%~39%。这与ZHANG等[29]报道的研究结果类似;4种共存离子(SO42−、PO43−、CO32−、SiO32−)中,PO43−、CO32−、SiO32−对MnFe2O4 As(Ⅲ)的吸附影响最大。
有报道表明,SO42−、NO3−、Cl−可在材料表面形成外球型络合物,相反,砷氧阴离子可与材料形成结合牢固的内球型络合物[30]。因此,在本实验条件下,即使NO3−、Cl−、SO42−的浓度远大于As(Ⅲ),也对As(Ⅲ)的去除影响不大。有研究[31]表明,PO43−和SiO32−可与材料形成内球型络合物,从而减少对As(Ⅲ)的吸附,因为PO43−的结构与砷氧阴离子类似,均为四面体结构,可与砷氧阴离子竞争材料表面活性位点[32]。有研究[33]表明,CO32−可与砷氧阴离子形成砷-碳酸盐络合物,如AsCO3+、As(CO3)2−、As(CO3)(OH)2−,从而阻碍砷氧阴离子在材料表面形成内球型络合物。另外,水溶液中存在大量CO32−时,CO32−水解产生的OH−离子使溶液pH上升,增加了H2AsO4−与表面负电荷改变的材料之间的静电排斥力。
-
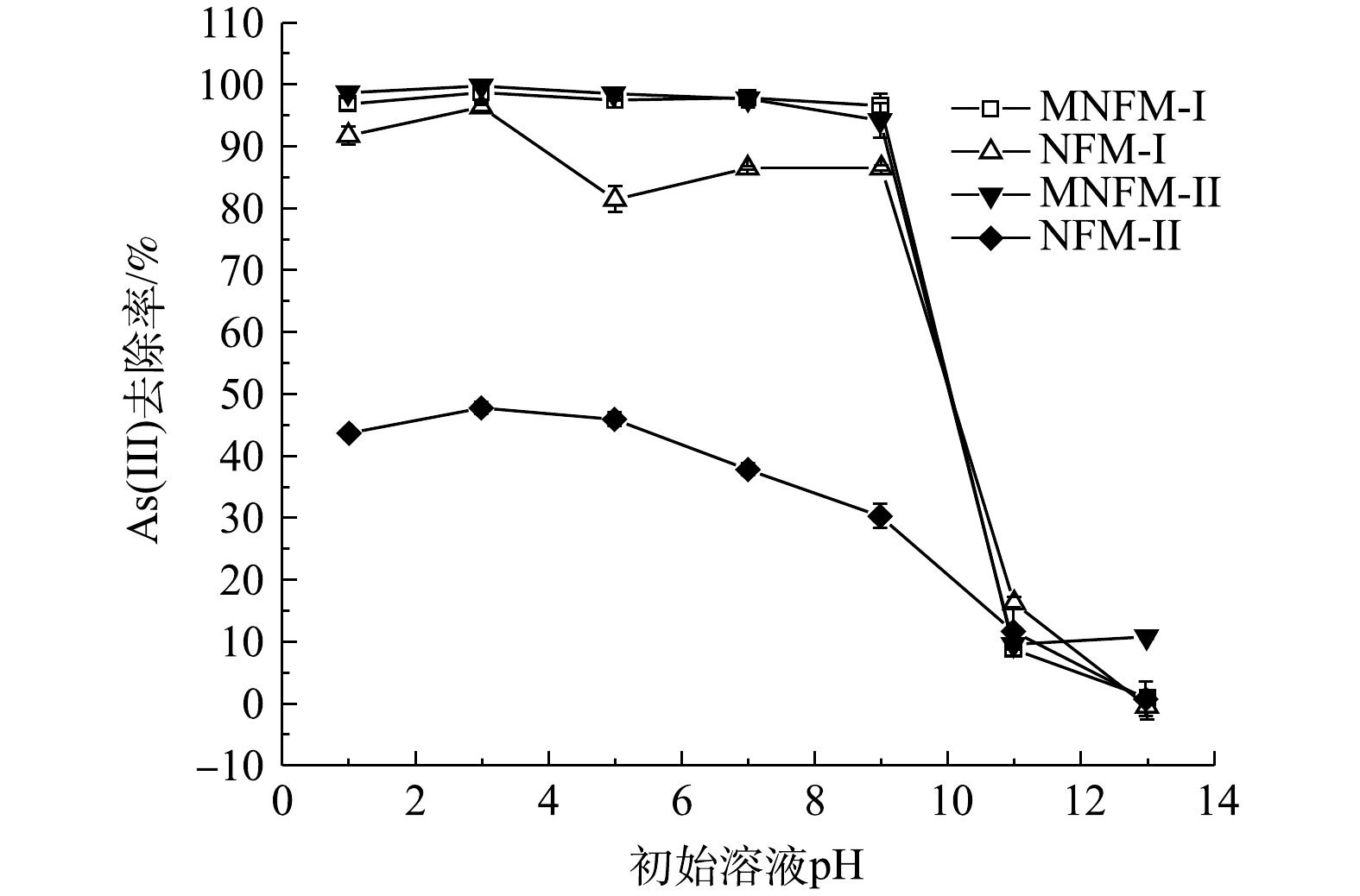
在本研究中下,初始溶液的pH显著影响材料对砷的去除效率(图6)。当pH在1~9内,MNFM-I的As去除率在96%以上,MNFM-II在94%以上,此时溶液中剩余总As质量浓度均小于10 µg·L−1的国家地下水限定标准[14]。但是,4种材料对As(Ⅲ)的去除率均在酸性条件下最高,而在碱性条件下最低。MNFM-I和MNFM-II的As吸附量,在pH=3时均达到最大值,而在pH>9时As的去除率迅速降低。CHAO等[25]用磁性纳米铁锰二元氧化物去除As(Ⅲ)的结果也表明,在pH=4~9内As去除率在95%以上,而pH>9时迅速降低至30%以下。砷的存在形态受溶液pH影响,在pH<9.2时,中性的H3AsO30是As(Ⅲ)的主要存在形式,而在高pH下(pH>9),带负电荷的H2AsO3−是As(Ⅲ)的主要存在形式。由于4种材料的pHPZC均小于6(表1),在高pH条件下,吸附剂去质子化,表面发生负电荷改变,从而增加了材料表面与砷物种之间的静电排斥力,导致As(Ⅲ)吸附量的减少。
-
2种材料及改性后吸附As(Ⅲ)前后的XRD图谱表明(图7),MNFM-I在27°、50°的吸收峰有所减弱,而MNFM-II在吸附As(Ⅲ)前后没有新的吸收峰出现,表明改性天然铁锰矿表面没有产生新物质,只是发生了表面吸附。
溶液中砷的吸附机理包括静电相互作用[34]、配位[35]和氢键[36]。为了表征砷在改性天然铁锰矿分子上的相互作用,采用FTIR光谱探索了其吸附机理(图8)。结果表明,625 cm−1处的带是H—O—H的弯曲振动,表明氧化物上存在物理吸附的水;1 384 cm−1处的峰值是由于硝酸钠调节溶液离子强度而引起的NO3−的振动所致。与吸附As(Ⅲ)前的改性天然铁锰矿相比较,吸附As(Ⅲ)后红外光谱图在468 cm−1、470 cm−1的特征峰分别为Mn—O的振动,MNFM-Ⅰ与MNFM-ⅠI在吸附前后该峰没有明显变化。MNFM-Ⅰ+As(Ⅲ)(图8(a))在837 cm−1处的特征峰与MNFM-Ⅱ+As(Ⅲ)(图8(b))在830 cm−1处的特征峰均被认为是As—O的伸缩振动谱[37],说明吸附As(Ⅲ)后的矿物中存在As—O键结构,表明As(Ⅲ)与改性天然铁锰矿结合为表面络合物。1 132 cm−1、1 045 cm−1处的峰对应于羟基(Fe—OH)的弯曲振动。与As(Ⅲ)反应后,1 032 cm−1和1 045 cm−1处的峰值明显减弱。以上结果表明,As (Ⅲ)离子在吸附过程中可能与铁中的羟基发生了配体交换。
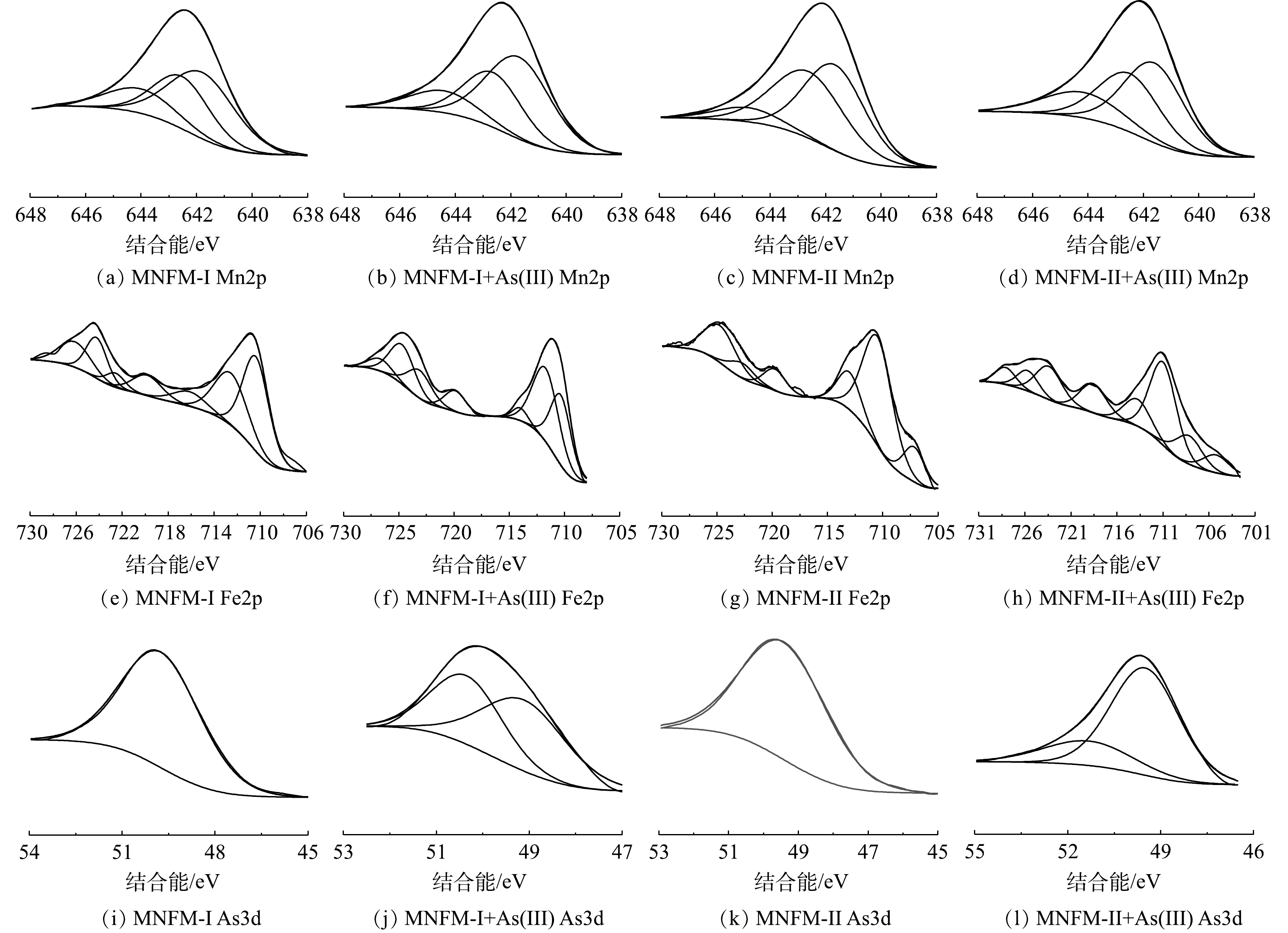
采用XPS进一步研究了As在改性天然铁锰矿表面的行为特征,以确定天然铁锰矿表面的元素含量和氧化情况(图9)。一般认为,位于52.9、533.9、646.2和717.2 eV的峰,分别对应于As3d、O1s、Mn2p2/3和Fe2p的特征峰[38-39]。图9分别为MNFM-I、MNFM-II在吸附As(Ⅲ)前后Fe2p、Mn2p、As3d窄区光谱图及分峰拟合图。通过拟合结果可得出吸附As(Ⅲ)前后各元素的含量及价态变化。对图9的结果进行分析可见,吸附As(Ⅲ)前后的Mn2p3/2峰可以分解为3个峰,结合能在641.79、642.53和644 eV处分别为Mn2+、Mn3+和Mn4+的特征峰。MNFM-I吸附As(Ⅲ)前后Mn2+所占百分含量由46.30%增加到51.55%,Mn4+由17.13%减少到13.92%;MNFM-II吸附As(Ⅲ)后高价态锰含量减少,低价态锰含量增加,由此初步推断改性天然铁锰矿在吸附As(Ⅲ)过程中锰元素可能发生了氧化反应。
MNFM-I吸附As(Ⅲ)前后,Fe2+所占百分比分别为54.68%和52.15%,而Fe3+分别为45.32%和47.85%,表明吸附As(Ⅲ)前后Fe2+、Fe3+所占百分含量变化不大,说明Fe主要起到吸附作用。MNFM-II吸附As(Ⅲ)前后的情况有所不同。MNFM-II吸附As(Ⅲ)前后,Fe2+所占百分比分别为34.47%和58.77%,Fe3+分别为65.53%和41.23%。表明Fe2+所占百分含量增加,初步判断Fe可能发生氧化反应。
值得注意的是,改性天然铁锰矿在吸附砷后,在50.35 eV和49.11 eV处的2个拟合峰均为As(Ⅴ)的特征峰[40],这说明吸附在改性铁锰矿表面的As(Ⅲ)被氧化为As(Ⅴ)。这与本研究中FT-IR的结果一致。
-
1) 2种天然铁锰矿对水体中砷的去除结果表明,水合肼法改性显著提高了材料的除砷性能,且MNFM-I对砷的吸附容量显著高于MNFM-II。
2)改性后的材料对As(Ⅲ)的吸附过程符合二级动力学模型,而等温吸附曲线符合Freundlich模型。酸性条件更有利材料对水体砷的去除,而共存离子PO43−、SiO32−、CO32−抑制材料对As(Ⅲ)的去除。
3)改性能去除材料表面的部分杂质,暴露出更多的活性位点。2种改性材料在吸附As(Ⅲ)过程中,Mn将As(Ⅲ)氧化为As(Ⅴ),从而提高了砷的去除效率,Fe主要参与As的吸附反应过程中。
不同铁锰比例天然锰矿的改性及对As(III)吸附和氧化的行为与机制
Adsorption/oxidation behavior and mechanism of As(III) by modified natural manganese ores with different Fe/Mn ratios
-
摘要: 砷污染地下水和废水严重影响人体健康和生态环境,开发新型砷污染水体的修复材料具有重要意义。含铁锰矿来源广,具有较高的砷吸附容量和氧化特性,常用于吸附去除水体砷污染。但是,天然铁锰矿成分复杂,杂质含量多,对砷的吸附容量小,通常需要改性以提高其除砷性能。以不同铁锰含量比例的天然锰矿为材料,研究了水合肼改性法提高天然铁锰矿砷吸附容量的效果,采用批处理法研究了影响改性铁锰矿吸附砷的过程和影响因子,并结合XPS和FTIR等光谱学手段探究改性天然铁锰矿对As(III)的去除机制。结果表明,两种不同铁锰比例的改性天然铁锰矿对砷的吸附容量分别为30.9 mg·g−1和12 mg·g−1,远高于未改性的材料。改性后的材料对As(III)的吸附过程符合二级动力学模型,等温吸附曲线符合Freundlich模型。影响因子结果表明,共存离子PO43−、SiO32−、CO32−对不同铁锰比改性材料去除As(III)均有抑制作用。4种材料的pHpzc均小于6。pH为1~9时,2种改性含铁锰矿As去除率在94%以上,而pH>9时,材料对砷的去除率显著降低。FT-IR分析发现,改性后材料的H—O—H和Mn—O特征峰增宽增强,谱线变得平滑,表明改性后材料表面部分杂质被溶解去除,暴露出更多的活性位点。XPS分析发现,改性后材料吸附As(III)后低价态锰含量增加,而Fe2+、Fe3+所占百分含量变化不大,说明不同铁锰比改性材料在吸附As(III)过程中Mn将As(III)氧化为As(V),Fe主要参与吸附反应。以上研究结果可为水体砷污染修复技术的开发提供参考。Abstract: Arsenic-contaminated groundwater and wastewater seriously affect human health and ecological environment, and it is important to develop new remediation materials for arsenic-contaminated water bodies. Ferromanganese ore has a wide source, high arsenic adsorption capacity and oxidation characteristics, and is commonly used to adsorb and remove arsenic pollution from water bodies. However, natural ferromanganese ores have complex compositions, high impurity content and low adsorption capacity for arsenic, and usually need to be modified to improve their arsenic removal performance. In this study, natural manganese ores with different iron and manganese content ratios were used as materials to investigate the effect of hydrazine hydrate modification on arsenic adsorption capacity of natural ferromanganese ores. The process and influencing factors affecting arsenic adsorption of modified ferromanganese ores were studied by batch processing method, and the related removal mechanism of As (III) was explored by XPS, FTIR and other spectroscopic means. The results showed that the arsenic adsorption capacities of two modified natural ferromanganese ores with different Fe/Mn ratios were 30.9 mg·g−1 and 12 mg·g−1, respectively, which were much higher than those of the unmodified materials. The adsorption process of As (III) by the modified material conformed to the second-order kinetic model, and the isothermal adsorption curves were in accordance with the Freundlich model. The experimental results of influence factors showed that the coexisting ions of PO43−, SiO32− and CO32− all inhibited As (III) removal by the modified materials with different iron manganese ratios. The pHpzc of all the four materials were lower than 6, and the As removal rates of the two modified materials were above 94% at pH 1~9, while decreased significantly at pH>9. FT-IR analysis showed that the H—O—H and Mn—O characteristic peaks of the modified materials were broadened and enhanced, and the spectral lines became smooth, indicating that some impurities on the surface of the modified materials were dissolved and removed, and more active sites were exposed. XPS analysis revealed that the content of low-valent manganese increased after the adsorption of As(III) by the modified materials, and a slight change occurred on the percentage content of Fe2+ and Fe3+, indicating that Mn oxidized As(III) to As(V) during the adsorption of As(III) by different Fe/Mn ratio materials, and Fe mainly participated in the adsorption reaction. In this study, new arsenic pollution remediation materials were developed and their mechanisms were clarified to provide scientific basis for the development of arsenic pollution remediation technology in water bodies.
-
Key words:
- modification /
- natural iron manganese ore /
- arsenic /
- adsorption /
- oxidation
-

-
表 1 供试材料的组成成分及电荷零点pHPZC
Table 1. Composition and pHPZC of the tested materials
材料 部分组成成分/% pHPZC Fe Mn K Ca Zn Cu As NFM-I 6.640 25.500 0.634 0.679 0.068 0.003 0.000 5.58 MNFM-I 6.490 27.400 0.214 0.092 0.012 0.000 0.000 2.18 NFM-II 0.862 44.500 0.000 0.132 0.000 0.000 0.000 3.72 MNFM-II 0.452 45.800 0.126 0.129 0.000 0.000 0.000 2.76 表 2 材料吸附As(Ⅲ)的Langmuir和Freundlich模型参数
Table 2. Langmuir and Freundlich model parameters of As(Ⅲ) adsorption by four materials
材料 Langmuir模型 Freundlich 模型 qm b R2 Kf n R2 NFM-I 4.89 0.080 0 0.854 1.23 0.385 0.798 MNFM-Ⅰ 30.9 0.034 4 0.898 2.62 0.487 0.959 NFM-II 3.81 0.007 06 0.455 0.051 1 0.743 0.957 MNFM-Ⅱ 12.0 0.054 5 0.963 1.37 0.451 0.936 表 3 不同材料对As(III)的吸附能力比较
Table 3. Comparison of As(III) adsorption capacities of different materials
-
[1] WEERASUNDARA L, OK Y S, BUNDSCHUH J. Selective removal of arsenic in water: A critical review[J]. Environmental Pollution, 2021, 268: 115668. doi: 10.1016/j.envpol.2020.115668 [2] RATHI B S, KUMAR P S. A review on sources, identification and treatment strategies for the removal of toxic arsenic from water system[J]. Journal of Hazardous Materials, 2021, 418(1): 126299. [3] LI M, KUANG S, KANG Y, et al. Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment[J]. Science of the Total Environment, 2022, 819: 153157. doi: 10.1016/j.scitotenv.2022.153157 [4] LAKSHMIPATHIRAJ P, NARASIMHAN B, PRABHAKAR S, et al. Adsorption studies of arsenic on Mn-substituted iron oxyhydroxide[J]. Journal of Colloid & Interface Science, 2006, 304(2): 317-322. [5] YU L, LIU H, LIU C, et al. Magnetically–confined Fe–Mn bimetallic oxide encapsulation as an efficient and recoverable adsorbent for arsenic (III) Removal[J]. Particle & Particle Systems Characterization, 2016, 33(6): 323-331. [6] GUPTA A D, RENE E R, GIRI B S, et al. Adsorptive and photocatalytic properties of metal oxides towards arsenic remediation from water: A review[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106376. doi: 10.1016/j.jece.2021.106376 [7] CHAKRAVARTY S, DUREJA V, BHATTACHARYYA G, et al. Removal of arsenic from groundwater using low cost ferruginous manganese ore[J]. Water Research, 2002, 36(3): 625-32. doi: 10.1016/S0043-1354(01)00234-2 [8] 马子川, 王颖莉, 贾密英, 等. 提高天然锰矿吸附水中重金属离子能力的方法[J]. 金属矿山, 2006, 41(9): 78-80. doi: 10.3321/j.issn:1001-1250.2006.09.022 [9] 陆泗进, 谭文峰, 刘凡, 冯雄汉. 一种改进的盐滴定法测定氧化锰矿物的电荷零点[J]. 土壤学报, 2006, 43(5): 756-763. doi: 10.3321/j.issn:0564-3929.2006.05.008 [10] WEN Z, ZHANG Y, DAI C, et al. Synthesis of ordered mesoporous iron manganese bimetal oxides for arsenic removal from aqueous solutions[J]. Microporous and Mesoporous Materials, 2014, 200: 235-244. doi: 10.1016/j.micromeso.2014.08.049 [11] ZHANG Y, YANG M, DOU X, et al. Arsenate adsorption on an Fe-Ce bimetal oxide adsorbent: role of surface properties.[J]. Environmental Science & Technology, 2005, 39(18): 7246-7253. [12] HOU J, LUO J, SONG S, et al. The remarkable effect of the coexisting arsenite and arsenate species ratios on arsenic removal by manganese oxide[J]. Chemical Engineering Journal, 2017, 315: 159-166. doi: 10.1016/j.cej.2016.12.115 [13] YU M, WANG Y, KONG S, et al. Adsorption kinetic properties of As(III) on synthetic nano Fe-Mn binary oxides[J]. Journal of Earth Science, 2016, 27(4): 699-706. doi: 10.1007/s12583-016-0714-4 [14] GALAL-GORCHEV H, OZOLINS G. WHO guidelines for drinking-water quality[J]. Water Supply, 1993, 11(3): 1-16. [15] WANG J, GUO X. Adsorption kinetic models: Physical meanings, applications, and solving methods[J]. Journal of Hazardous materials, 2020, 390: 122156. doi: 10.1016/j.jhazmat.2020.122156 [16] HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465. doi: 10.1016/S0032-9592(98)00112-5 [17] GUO H, STÜBEN D, BERNER Z. Removal of arsenic from aqueous solution by natural siderite and hematite[J]. Applied Geochemistry, 2007, 22(5): 1039-1051. doi: 10.1016/j.apgeochem.2007.01.004 [18] SHI Y J, HUANG R L, HUANG Y H. Adsorptive removal of arsenic using a novel akhtenskite coated waste goethite[J]. Journal of Cleaner Production, 2015, 87: 897-905. doi: 10.1016/j.jclepro.2014.10.065 [19] LIU Z, CHEN J, WU Y, et al. Synthesis of magnetic orderly mesoporous α-Fe2O3 nanocluster derived from MIL-100 (Fe) for rapid and efficient arsenic (III, V) removal[J]. Journal of Hazardous Materials, 2018, 343: 304-314. doi: 10.1016/j.jhazmat.2017.09.047 [20] MA L, CAI D, TU S. Arsenite simultaneous sorption and oxidation by natural ferruginous manganese ores with various ratios of Mn/Fe[J]. Chemical Engineering Journal, 2020, 382: 123040. doi: 10.1016/j.cej.2019.123040 [21] JAIN C K, ALI I. Arsenic: occurrence, toxicity and speciation techniques[J]. Water Research, 2000, 34(17): 4304-4312. doi: 10.1016/S0043-1354(00)00182-2 [22] CHAO S, TONG M. Efficient removal of trace arsenite through oxidation and adsorption by magnetic nanoparticles modified with Fe-Mn binary oxide[J]. Water Research, 2013, 47(10): 3411-3421. doi: 10.1016/j.watres.2013.03.035 [23] QI J, ZHANG G, LI H. Efficient removal of arsenic from water using a granular adsorbent: Fe-Mn binary oxide impregnated chitosan bead[J]. Bioresource Technology, 2015, 193: 243-249. doi: 10.1016/j.biortech.2015.06.102 [24] LEI M, QIN P, PENG L, et al. Using Fe–Mn binary oxide three-dimensional nanostructure to remove arsenic from aqueous systems[J]. Water Science and Technology:Water Supply, 2016, 16(2): 516-524. doi: 10.2166/ws.2015.163 [25] RYU S R, JEON E K, YANG J S, et al. Adsorption of As(III) and As(V) in groundwater by Fe–Mn binary oxide-impregnated granular activated carbon (IMIGAC)[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 72: 62-69. doi: 10.1016/j.jtice.2017.01.004 [26] ZHI B, DING H, WANG D, et al. Ordered mesoporous MnO2 as a synergetic adsorbent for effective arsenic(III) removal[J]. Journal of Materials Chemistry A, 2014, 2(7): 2374-2382. doi: 10.1039/c3ta13790b [27] LIN L, QIU W, WANG D, et al. Arsenic removal in aqueous solution by a novel Fe-Mn modified biochar composite: characterization and mechanism[J]. Ecotoxicology and Environmental Safety, 2017, 144: 514-521. doi: 10.1016/j.ecoenv.2017.06.063 [28] RAJU N J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies[J]. Environmental Research, 2022, 203: 111782. doi: 10.1016/j.envres.2021.111782 [29] ZHANG S, NIU H, CAI Y, et al. Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4[J]. Chemical Engineering Journal, 2010, 158(3): 599-607. doi: 10.1016/j.cej.2010.02.013 [30] ZHANG G, LIU H, LIU R, et al. Adsorption behavior and mechanism of arsenate at Fe–Mn binary oxide/water interface[J]. Journal of Hazardous Materials, 2009, 168(2/3): 820-825. [31] STRAUSS R, BRÜMMER G W, BARROW N J. Effects of crystallinity of goethite: II. Rates of sorption and desorption of phosphate[J]. European Journal of Soil Science, 1997, 48(1): 101-114. doi: 10.1111/j.1365-2389.1997.tb00189.x [32] ZHENG Q, TU S, HOU J, et al. Insights into the underlying mechanisms of stability working for As (III) removal by Fe-Mn binary oxide as a highly efficient adsorbent[J]. Water Research, 2021, 203: 117558. doi: 10.1016/j.watres.2021.117558 [33] APPELO C A J, VAN DER WEIDEN M J J, TOURNASSAT C, et al. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic[J]. Environmental Science & Technology, 2002, 36(14): 3096. [34] CHEN W R, HUANG C H. Surface adsorption of organoarsenic roxarsone and arsanilic acid on iron and aluminum oxides[J]. Journal of Hazardous Materials, 2012, 227-228: 378-385. doi: 10.1016/j.jhazmat.2012.05.078 [35] JUN J W, TONG M, JUNG B K, et al. Effect of central metal ions of analogous metal–organic frameworks on adsorption of organoarsenic compounds from water: plausible mechanism of adsorption and water purification[J]. Chemistry-A European Journal, 2015, 21(1): 347-354. doi: 10.1002/chem.201404658 [36] LIU B, YANG F, ZOU Y, et al. Adsorption of phenol and p-nitrophenol from aqueous solutions on metal-organic frameworks: effect of hydrogen bonding[J]. Journal of Chemical & Engineering Data, 2014, 59(5): 1476-1482. [37] ZHENG Q, HOU J, HARTLEY W, et al. As (III) adsorption on Fe-Mn binary oxides: are Fe and Mn oxides synergistic or antagonistic for arsenic removal?[J]. Chemical Engineering Journal, 2020, 389: 124470. doi: 10.1016/j.cej.2020.124470 [38] JOSHI T P, ZHANG G, JEFFERSON W A, et al. Adsorption of aromatic organoarsenic compounds by ferric and manganese binary oxide and description of the associated mechanism[J]. Chemical Engineering Journal, 2017, 309: 577-587. doi: 10.1016/j.cej.2016.10.084 [39] YU X, WEI Y, LIU C, et al. Ultrafast and deep removal of arsenic in high-concentration wastewater: A superior bulk adsorbent of porous Fe2O3 nanocubes-impregnated graphene aerogel[J]. Chemosphere, 2019, 222: 258-266. doi: 10.1016/j.chemosphere.2019.01.130 [40] WANG H, WANG Y N, SUN Y, et al. A microscopic and spectroscopic study of rapid antimonite sequestration by a poorly crystalline phyllomanganate: differences from passivated arsenite oxidation[J]. RSC Advances. 2017, 7(61): 38377-38386. -



 下载:
下载: