-
近年来,重金属-有机物复合污染物广泛地存在水环境中。例如,由于规模化、集约化养殖需求,大量的抗生素类药剂和含重金属(Cu等)饲料被长期用于动物的治疗、预防和促进生长[1-2]。然而,绝大部分的药剂和含重金属饲料未能被充分利用,而是通过畜禽粪便、尿液直接排入周边环境,再经过土壤渗透和径流进入地表水或地下水[3-5]。在此过程中,富含羧基、羟基、氨基等基团或电子供体原子的抗生素分子极易与重金属离子发生络合反应,形成结构稳定和毒性更强的复合污染物[6]。而且,与单一污染物相比,这些复合污染物的吸附降解行为和生物利用度更复杂,导致复合污染物的去除难度更大,给生态环境安全和人类健康带来更加严峻的威胁[7]。因此,亟待研发去除水体重金属-抗生素复合污染的技术。
目前,吸附法是最受关注的复合污染水体处理技术之一[1]。其中,吸附材料的性能优劣是高效去除复合污染物的关键[8-9]。在众多的吸附材料中,广泛存在、生物量巨大的植物纤维因具有多孔、比表面积大和无毒性的优点,从而成为极具潜力的重金属吸附材料。然而,纤维结构中大量的活性羟基被纤维分子内和分子间的氢键束缚,导致其吸附能力不尽如人意,并在实际应用中受到很大的限制[10]。为此,CHEN等[11]使用环氧氯丙烷醚化处理玉米秸秆纤维,使其对Cr(VI) 的吸附量达到200.00 mg·g−1;SUN等[12]利用硬脂酸和丙烯酰胺接枝改性蔗渣纤维,将其吸附Hg(Ⅱ) 的容量提升到178 mg·g−1;胡东英[13]采用十二叔胺接枝修饰亚麻纤维,实现环丙沙星的最大吸附量为247.41 mg·g−1。由此可见,醚化、接枝等改性技术不但可以破坏纤维的氢键结构,而且能够赋予更多的功能性基团,从而有效地改善材料吸附重金属和抗生素的性能[14-16]。但是,上述纤维材料仅具备吸附去除单一污染物的性能,缺乏吸附重金属复合污染物的功能。究其原因,是因为重金属离子主要通过静电、配位络合和离子交换等作用被吸附到材料的亲水位点[17-18]。而抗生素分子不仅与亲水位点之间产生氢键和静电作用,而且还会与疏水位点发生π-π相互作用等[19-20]。而且,重金属离子和抗生素在水环境中还可以发生氧化还原反应和络合反应[21],极易与吸附材料产生架桥作用,导致材料难以发挥吸附效果。由此可见,复合污染的复杂性对纤维醚化和接枝化改性方法提出了更高的要求。此外,改性纤维材料吸附重金属复合污染物的行为机制尚不清晰,尤其机理的定量阐述更是研究甚少。为此,非常有必要利用理论化学计算进一步量化其吸附行为机制,以期为纤维吸附材料设计提供参考。
基于以上分析,本研究首先充分利用来源广泛的玉米秸秆纤维(RCS),通过环氧氯丙烷的醚化和次氮基三乙酸接枝方法,成功地制备出多基团修饰秸秆纤维(NCS)。然后,表征分析NCS材料的微观样貌、比表面积和元素基团,并考察了在不同pH值下NCS对SMZ、Cu(Ⅱ)和SMZ-Cu络合物的吸附性能,再进一步拟合分析其吸附等温线和吸附动力学模型,定性分析吸附行为过程。最后,采用DFT理论计算定量分析NCS的吸附行为机制。
-
材料选取广州市郊区的玉米秸秆 (RCS) 。经过择除残叶、洗涤附着的泥土砂石,以及在50 ℃条件下烘干后,秸秆被粉碎成粉末,再筛分选取0.45 mm粒径的秸秆样品。实验采用的化学药品如硝酸(HNO3)、盐酸(HCl)、氢氧化钠(NaOH)、碳酸氢钠(NaHCO3),购买于广州化学试剂厂。N,N-二甲基甲酰胺(C3H7NO,DMF)、环氧氯丙烷(C3H5ClO,EPI)购买于天津大茂化学试剂厂。次氮基三乙酸(C6H9NO6,NTA)、三水合硝酸铜(Cu(NO3)2·3H2O)、吡啶(C5H5N,PY) 购买于安耐吉化学试剂厂。磺胺甲恶唑(C10H11N3O3S,SMZ)购买于德国Dr.Ehrenstorfer公司。实验中的溶液均使用超纯水配制,化学试剂纯度均为分析纯,使用前没有经过进一步提纯。在实验室条件下,称取0.3802 g Cu(NO3)2·3H2O晶体,加入去离子水溶解后定容至100 mL容量瓶,配制1 000 mg·L−1的Cu(Ⅱ)储备液。另外,称取0.2 g SMZ固体,加入800 mL超纯水,在水浴锅90 ℃下加热搅拌30 min。当溶液澄清和自然冷却到室温后,移液至1 000 mL容量瓶定容得到SMZ储备液。其他浓度的SMZ溶液和Cu(Ⅱ)溶液均通过稀释上述的储备液制得。
-
1)在NaOH溶液中完全浸泡秸秆样品,碱化处理24 h,使其充分去除大部分的木质素、半纤维素,并破坏纤维素的结晶区。再用去离子水洗涤至中性、过滤,在60 ℃下烘干恒重。
2)称取4 g碱化样品,加入60 mL DMF和40mL EPI。在磁力水浴锅中85 ℃下,搅拌反应1 h。再加入20 mL PY,保持同样温度继续反应1 h。
3)称取7.6 g NaOH固体,用40 mL去离子水在烧杯配制浓NaOH溶液。然后,加入17 g NTA,待完全溶解后,将混合溶液加入步骤2)的体系中,在85 ℃下继续搅拌反应1 h。
4)反应结束后,抽滤处理。再依次用去离子水、DMF、饱和NaHCO3溶液冲洗。最后,在60 ℃下烘干,所得产物为NCS。
-
利用扫描电子显微镜-能量色散X射线光谱(SEM-EDS,Car Zeiss EVO LS10, INCA ENERGY 350,英国)观察材料表观形貌和测定元素含量。使用傅里叶变换红外光谱(FTIR,Nicolet 6700,美国),在扫描范围为400~4 000 cm−1、分辨率为2 cm−1的条件下获得材料功能性基团变化情况。运用全自动比表面(积)分析测试仪(BET,Micromeritics TriStar II,美国)获取材料的表面积、平均孔径和总孔径数据。在X射线光电子能谱仪(XPS,Thermo SCIENTIFIC ESCALAB 250Xi,美国)中,采用功率230 W的单色Al K alpha 光源测试材料表面元素的变化情况,并定性分析材料的吸附行为。
-
1)溶液pH对吸附性能的影响实验。在单一吸附体系中,称取0.01 g NCS于锥形瓶中,加入10 mL 50 mg·L−1的SMZ溶液(pH分别调至2、3、5、6、8、11);同样,称取0.01g NCS加入10 mL 30 mg·L−1的Cu(Ⅱ)溶液(pH分别调至2、3、5、6)。在二元吸附体系中,称取0.01 g NCS加入10 mL SMZ(浓度为50 mg·L−1)和Cu(Ⅱ)(浓度为30 mg·L−1)的混合溶液(pH分别调至2、3、5、6)。每个pH设置3组平行样,在298 K、160 r·min−1转速的遮光摇床中反应24h。反应结束后,立即测定溶液的pH值、SMZ和Cu(II)的浓度。其中,使用高效液相色谱仪(HPLC,LC-10AT,日本)测定SMZ的浓度,色谱条件为:Zorbax Eclipse Plus C18 型色谱柱(4.6 µm×150 mm, 5 μm);流动相为甲酸溶液(0.1% v:v): 乙腈=70:30;进样量为20 μL;流速为 1 mL·min−1;柱温保持为30 ℃;检测波长为 269 nm。测试前,先对流动相进行脱气处理。然后,将SMZ溶液经过 0.22 μm 聚醚砜膜过滤后再放入进样口。测试时,设置 SMZ 保留时间为 4.473 min。同时,使用原子吸收光谱分析仪(AAS,ICE-3500,美国)测定Cu(Ⅱ)的浓度。测试前,将吸附后的Cu(Ⅱ)溶液经过0.22 μm 聚醚砜膜过滤,并使用2%稀硝酸稀释20倍后再测定。最后,采用式(1)计算吸附剂的吸附容量[22]。
式中:
$ {q}_{\mathrm{e}} $ 是平衡吸附容量,mg·g−1;C0和Ce分别是初始和平衡时的浓度,mg·L−1;V是溶液体积,L;m是所用吸附剂的质量,g。2)吸附等温实验:在单一吸附体系中,分别配制浓度为10、30、50、70、90 mg·L−1的SMZ溶液,以及浓度为20、25、30、35、40 mg·L−1的Cu(Ⅱ)溶液。在二元吸附体系中,先设定SMZ浓度为50 mg·L−1,再设置Cu(Ⅱ)浓度分别为20、25、30、35、40 mg·L−1的混合溶液;随后设定Cu(Ⅱ)浓度为30 mg·L−1,再设定SMZ浓度分别为10、30、50、70、90 mg·L−1的混合溶液。接着,分别取上述溶液10 mL与0.01 g NCS在锥形瓶中进行摇瓶实验,每个浓度设置3个平行样,均在298 K、160 r·min−1转速的遮光摇床中反应24 h。反应结束后,取上清液分别测定SMZ和Cu(Ⅱ)浓度。最后,采用Langmuir(式(2))和Freundlich(式(3))模型拟合实验数据[22]。
式中:Qe为平衡时吸附容量,mg·g−1;Ce为平衡时吸附浓度,mg·L−1,
$ {{Q}}_{\text{max}} $ 为最大理论吸附量,mg·g−1;$ {{K}}_{\text{L}} $ 为Langmuir吸附常数,L·mg−1,一般与吸附键结合能有关;$ {{K}}_{\text{F}} $ 为Freundlich吸附常数,mg(1−n)·Ln·g−1;$ \dfrac{\text{1}}{{n}} $ 为与温度相关的常数;KF和$ \dfrac{\text{1}}{{n}} $ 都反映吸附非线性程度和吸附机理存在的差异。其中,$ \dfrac{\text{1}}{{n}} $ 与吸附难易程度有关。3)吸附动力学实验:在单一吸附体系中,配制浓度为50 mg·L−1、200 mL的SMZ溶液,以及配制浓度为30 mg·L−1、200 mL的Cu(Ⅱ)溶液。在二元吸附体系中,配制200 mL含 50 mg·L−1 SMZ和30 mg·L−1 Cu(Ⅱ) 的混合溶液。在上述体系中分别加入0.2 g NCS,设置反应时间为0 、0.1、0.25、0.5、1、3、6、12、24 h,再测定SMZ和Cu(Ⅱ)的浓度。最后,采用准一级动力学方程(式(4))和准二级动力学方程(式(5))对所得实验数据进行拟合[22]。
式中:
$ {{q}}_{\text{t}} $ 为在t时刻的吸附容量,mg·g−1;t为取样时间,h;k1为准一级吸附动力学方程吸附速率常数,min−1;k2为准二级吸附动力学方程吸附速率常数,g·(mg·min)−1。 -
采用Materials Studio 2017 R2 的 Dmol3 模块开展密度泛函理论(density functional theory, DFT)计算定量吸附机理。基于第一性原理和 DFT 考察了平衡构型和结合能,利用基于广义梯度近似 (GGA) 的 Perdew-Burke-Ernzerhof (PBE) 函数。其中,电子自洽场收敛值(SCF tolerance)为 2.6255×10−3 kJ·mol−1,收敛容差值为 2.6255×10−2 kJ·mol−1,0.05 nm(最大位移)和 52.51 kJ·(mol·nm)−1(最大力)。考虑到溶剂化效应,选择介电常数为 78.54(水)的类导体屏蔽模型(COSMO)来模拟水层包裹的结构。电子基础集选定为双数值极化(DNP 3.5),DFT-D校正选择过渡状态(TS)方案。正式旋转用作初始状态,并且旋转无限制模式用于执行计算。采用全电子核心处理和优良质量。通过式(6)计算不同吸附质在吸附剂上的结合能[23]。
式中:Eab是吸附后的复合物的总能量,kJ·mol−1;Ea和Eb分别是吸附质和吸附剂的总能量,kJ·mol−1。
-
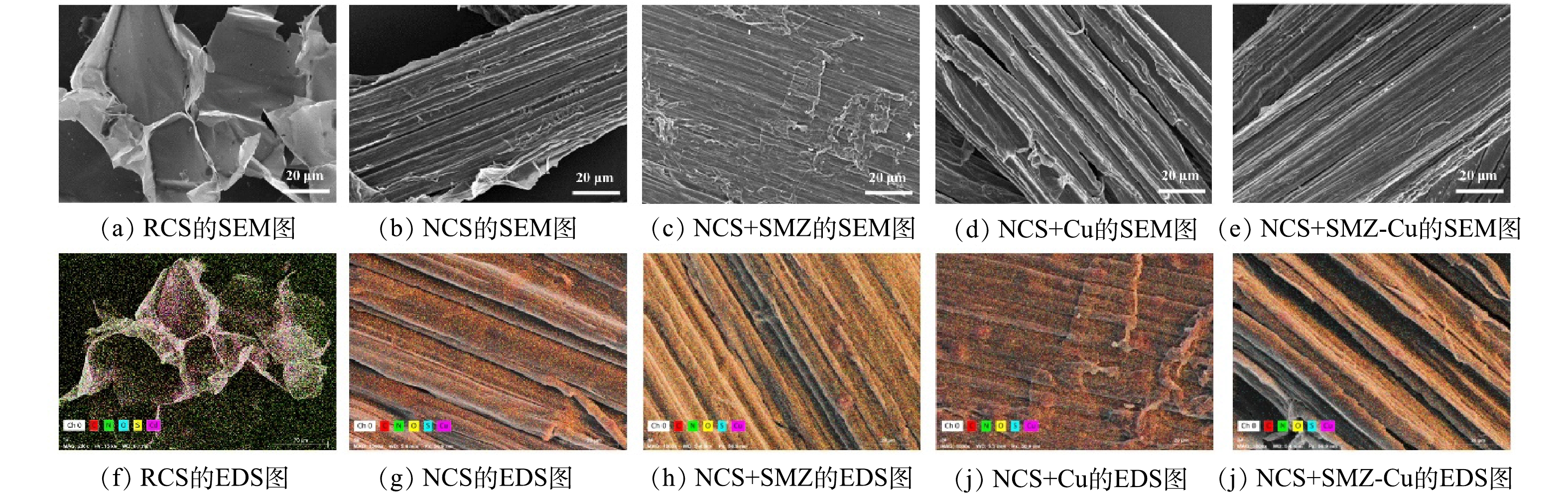
NCS和RCS的微观样貌如图1所示。与RCS相比,NCS的表面沟壑更加明显和粗糙,具有不均匀且凸起的珠粒样貌,表明材料具有较多的表面吸附位点(图1(b)和图1(g))。吸附后,NCS表面产生一些晶体物(图1(c)~(e)和图1(h)~(j))。这可能是由于Cu和SMZ与NCS表面的基团形成配位或氢键而出现结晶现象[24]。EDS能谱分析结果表明:在一元吸附体系中,NCS的Cu和S含量分别由0.02%和0.84%增加到4.41%和1.40%;而在二元吸附体系中,Cu和S含量则增加到2.0%和1.96%(表1)。其中,S来源于SMZ中的磺酰胺基团(-SO2NH-),这表明NCS吸附Cu(Ⅱ)和SMZ的效果显著。RCS、NCS吸附前后的比表面积、总孔隙体积和平均孔径结果表明:NCS的比表面积和总孔隙体积均有明显的下降,这说明NCS吸附过程不同于多孔吸附材料,物理吸附不是其主要吸附行为。此外,与单一吸附Cu相比,二元吸附体系的比表面积和总孔隙体积都略微增加,则说明体系中SMZ结构可能会增加材料对Cu的吸附位点,从而促进NCS对Cu的吸附。
-
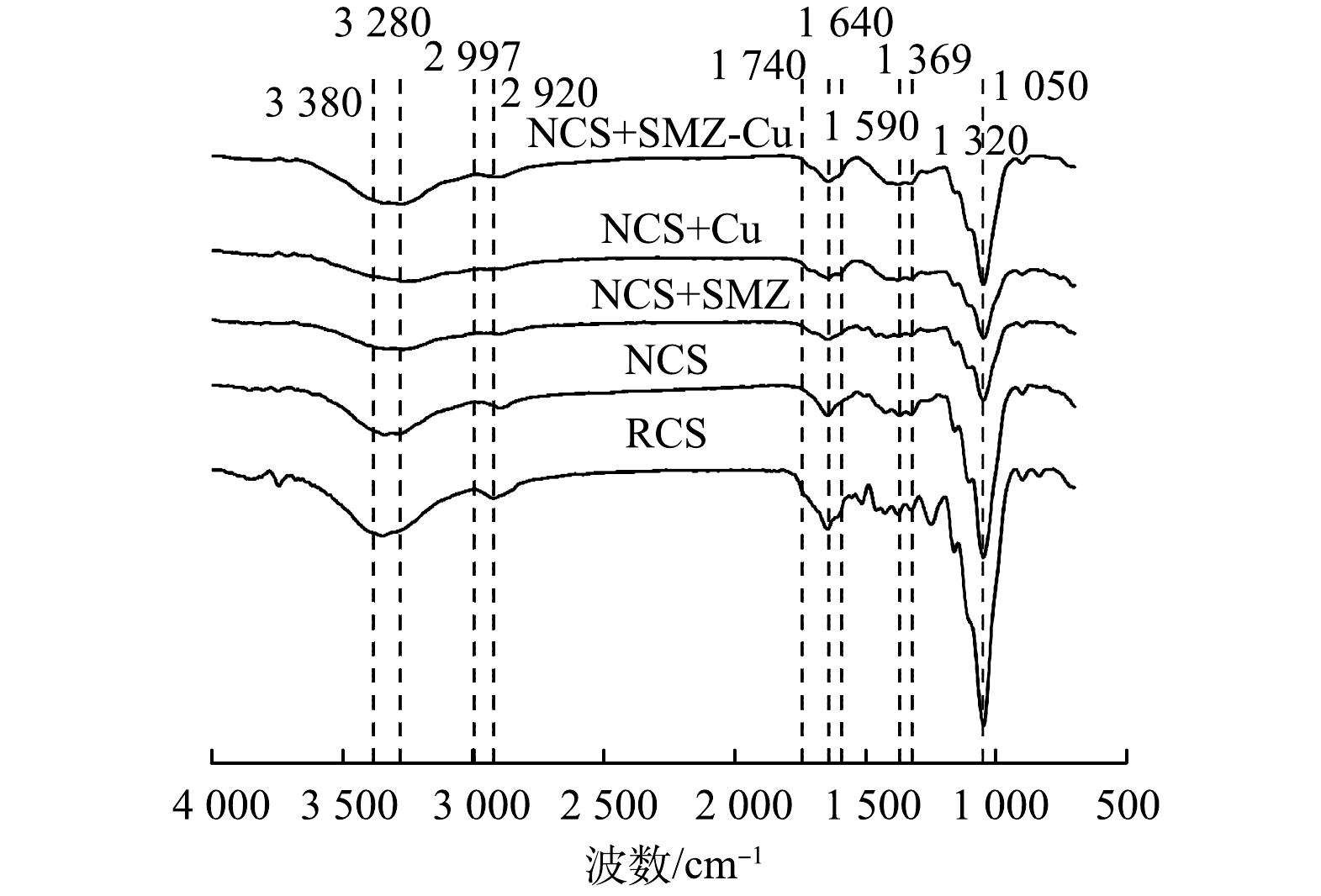
材料表面的功能性基团变化如图2的红外图谱所示。在2 920 cm−1和1 050 cm−1附近属于RCS纤维素的—CH和C—O—C基团,而在2 997 cm−1附近的谱带是纤维素和半纤维素的—OH基团[25-26] 。吸附前的NCS在1 740 cm−1和1 369 cm−1附近形成C=O的伸缩振动小峰[27],而在1 320 cm−1处是C—N的伸缩峰,以及在3 280~3 380 cm−1和1 590~1 640 cm−1均出现季铵盐(—N+)的特征吸收峰[28-29]。由此可见,NCS结构具有—COOH和含N基团。其中,含N基团可能分为2种:一种是由于NTA接枝程度的差异性而形成的—N+基团,另一种是C—N结构。吸附后这些NCS的特征峰都发生明显的变化,说明含N基团和—COOH基团均参与NCS对Cu(Ⅱ)和SMZ的吸附过程。
-
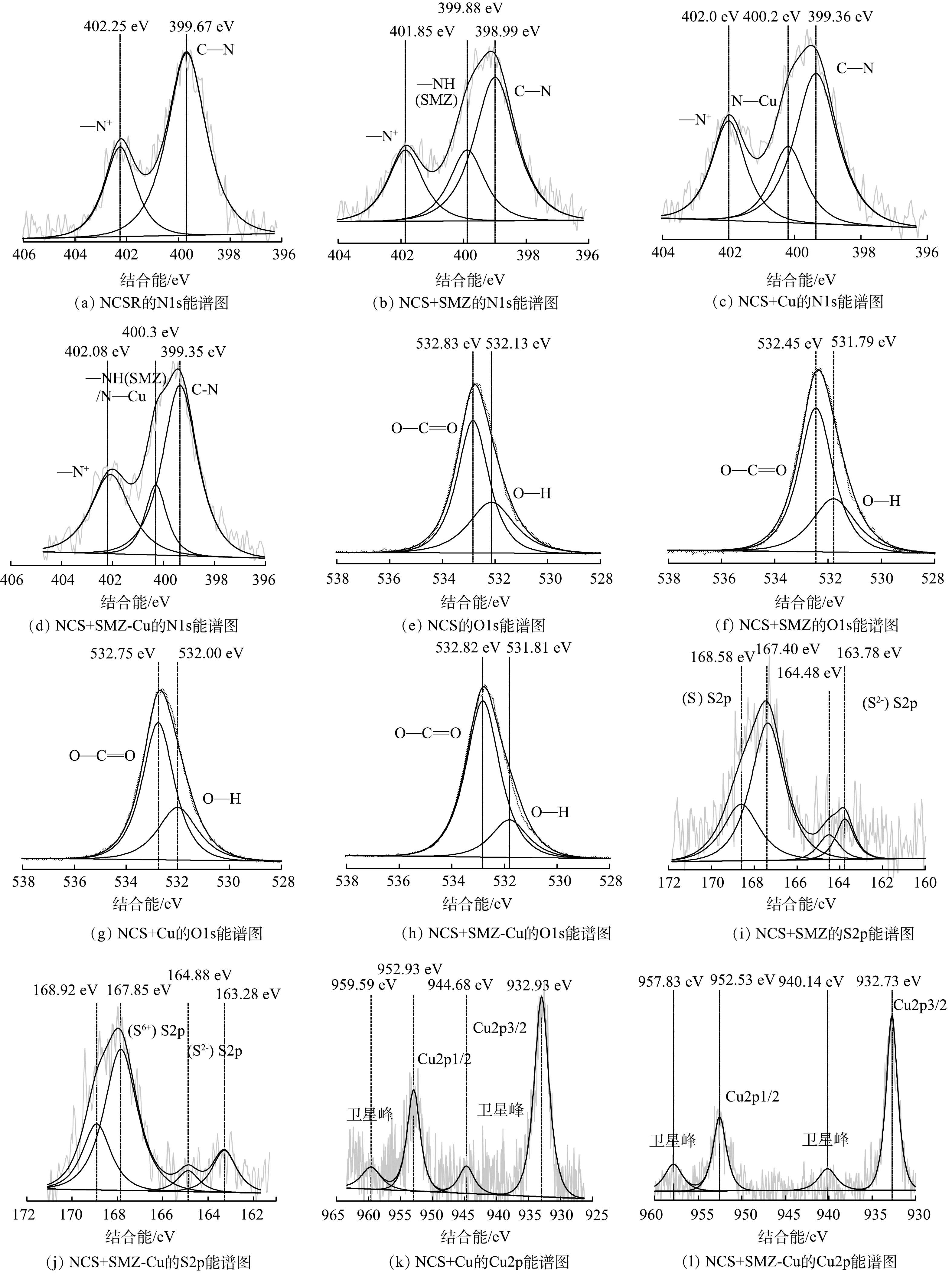
XPS表征结果如图3所示。对于N1s,在402.25 eV属于NCS的-N+特征峰,而399.67 eV处则为中性胺C—N的特征峰[30-31] ,即NCS存在N+结构和C-N结构,这进一步印证了FTIR的表征结果。在单一吸附体系中,在吸附SMZ后,NCS的—N+峰和C—N峰分别移至401.85 eV和398.99 eV,而在399.88 eV处为SMZ的特征峰—NH。当Cu(Ⅱ)被吸附后,NCS的C—N峰移动到399.36 eV,-N+峰则偏移至402.00 eV,这表明Cu(Ⅱ)通过配位反应与C—N和—N+相互作用[18],而SMZ可分别通过氢键和静电作用与—N+以及C—N发生结合[32]。在二元吸附体系中,各个峰的位置都发生了显著偏移,这是多重相互作用叠加在NCS吸附过程的效果[18]。总的来说,无论是一元还是二元吸附体系,NCS的—N+特征峰发生偏移,这意味着在吸附过程中—N+基团附近的结构发生变化(图3(a)~(d))。对于O1s,NCS在单一吸附体系中分别吸附SMZ和Cu(II) 后,其—COO—的特征峰(532.83 eV)[33]分别偏移到532.45 eV和532.75 eV(图3(e)~(g))。但是,该特征峰在二元吸附体系中几乎发生没有偏移(532.82 eV)(图3(h))。N1s和O1s的分峰结果表明:在单一吸附体系中,相比于Cu(II),NCS对SMZ的吸附性能更好。在二元吸附体系中,NCS主要为吸附Cu与SMZ的络合物,而不倾向吸附单一污染物。此外,在2个体系中均出现Cu的新峰,即952 eV附近的Cu2p1/2和932 eV附近Cu2p3/2(图3(i)和图3(j)),以及SMZ中S的新峰,即168 eV附近的 (S6+) S2p和163 eV附近的 (S2−) S2p[4,34]。这充分说明NCS对SMZ和Cu(Ⅱ) 具有良好的吸附效果(图3(k)和图3(l))。
-
单一的SMZ在不同pH下具有不同的形态结构。如图4(a)所示,在强酸性条件下,SMZ的碱性胺基(-NH2)获得一个H+,会发生质子化变成SMZ+。在pH大约为4时,SMZ+的碱性胺基(-NH3+)失去一个H+,会发生第1步去质子化变为SMZ0。当SMZ0中N1位上的酸性亚氨基(-NH-)失去一个质子时,则会发生第2步的去质子化变为SMZ−。如果SMZ与Cu(II)同时存在时,SMZ结构中N1上的-NH-可发生去质子化,极易通过磺酰胺氮与Cu(II)在N1上进行络合;或者通过磺胺氧(S=O),即N1上的N和磺胺基上O位点与Cu(Ⅱ)发生络合作用。当pH=4.5时,已有SMZ-Cu络合物存在。随pH增大,SMZ-Cu+所占比例逐渐增大。当pH=7.0时,SMZ-Cu+约占SMZ总形态分布的10% [35]。同时,Zeta电位分析显示NCS的表面基本带正电荷(图4(b)),这可能会导致重金属离子与有机物分子的竞争吸附[36]。由此可见,水体pH会对NCS对SMZ和Cu(Ⅱ)的吸附性能产生重要的影响。
在一元吸附体系中,NCS在强酸性(pH=2~3)条件下仅有少部分的SMZ吸附量。这是由于质子化作用使其表面官能团的氢键和π-π相互作用被抑制,NCS只能依靠较弱的疏水作用和静电作用来吸附SMZ [37]。随着pH增大,NCS吸附量也迅速增大,并在pH为5时达到最大吸附量39.55 mg·g−1。此时,溶液中SMZ0和SMZ−的比例相应增大,静电作用、氢键包括阴离子辅助氢键作用的协同效果最强。随后pH升到11时,逐渐增多的氢氧根离子使NCS表面的官能团逐渐去质子化,而SMZ完全以SMZ−的形式存在。因此,静电斥力的增大和氢键作用的减弱导致吸附量降低[31]。在二元吸附体系中,Cu(Ⅱ)的存在会抑制NCS吸附SMZ,尤其在强酸溶液中的抑制程度更高。这主要归结于Cu(Ⅱ)和SMZ0对亲水性吸附位点的竞争关系。在较高pH时,氢键作用极大地促进对SMZ的吸附。然而,Cu(Ⅱ)对这些位点的亲和力更强,即配位络合作用强于氢键作用[18],这会减弱NCS对SMZ的吸附能力(图4(c))。
随着pH从2升至5,NCS在一元吸附体系中对Cu(Ⅱ)的吸附量显著提升。当pH由5增至6时,其吸附量增长逐渐变缓,并在pH为6时达到最大值4.27 mg·g−1。这是因为强酸性环境下大量的 H+会与 Cu(II)竞争 NCS表面基团的吸附位点。与此同时,其表面基团的质子化作用也会抑制NCS与 Cu(II)的配位络合作用。在二元吸附体系中,SMZ对Cu(Ⅱ)的吸附性能具有明显的促进作用,这是SMZ−静电屏蔽效应引起的结果[18,36] (图4(d)) 。
总的来看,NCS更倾向于吸附SMZ,进而在二元吸附体系中可促进对Cu(II)的吸附性能。相反,NCS吸附Cu(II)的能力较弱,故在二元吸附体系中Cu(II)会较小程度上抑制NCS对SMZ的吸附。这与XPS表征结果一致。在图4(c)和图4(d)中,对比空白样品,在初始pH小于5时,NCS吸附后的溶液平衡pH没有发生明显变化。而当初始pH为5时,平衡pH均有所升高,而单一吸附体系的平衡pH提高最大。这是因为在单一吸附体系中,NCS表面吸附位点可同步提供给Cu(II)和H+,他们之间的竞争吸附关系较弱;而SMZ−的存在则会吸附一部分的H+。在二元吸附体系中,平衡pH的变化相对较小,这可能与SMZ−络合作用有关。
-
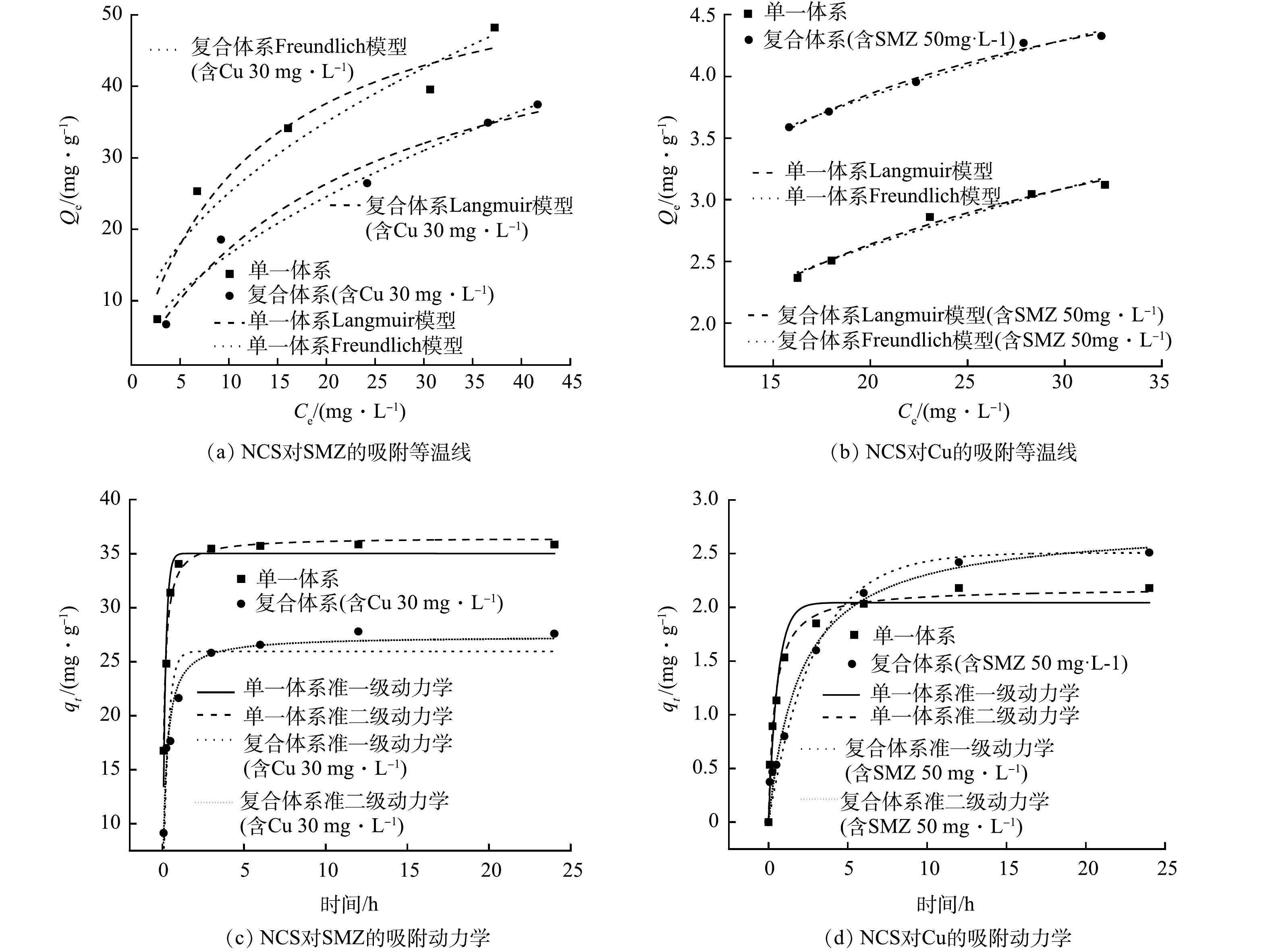
整体来看,NCS对SMZ和Cu(Ⅱ)的吸附是一个快速吸附过程。如图5所示,在前1 h内,NCS对SMZ和Cu(Ⅱ)的吸附量达到总吸附量的90%以上,随后缓慢增长,直至6 h后达到吸附平衡。在这个过程中,NCS对SMZ和Cu(Ⅱ)的吸附等温线拟合结果及相关参数如图5(a)~(b)和表2所示。相比于Freundlich方程,Langmuir方程可更好地拟合NCS对Cu(Ⅱ)和SMZ的吸附数据。结果表明,NCS在单一吸附体系中对SMZ和Cu(Ⅱ)的最大理论吸附量分别为59.76 mg·g−1和4.71 mg·g−1;在二元吸附体系中的最大理论吸附量分别可以达到56.21 mg·g−1和5.54 mg·g−1。可见,NCS能够适应重金属-抗生素复合污染物的复杂环境行为且保持与一元吸附体系相同的吸附性能。吸附动力学模型及相关参数如图5(c)~(d)和表3所示。拟合结果表明:准二级动力学方程的相关性(R2)均大于采用准一级动力学方程的,而且Qe值也与实验值更接近,这表明NCS对于单一污染物和复合污染物的吸附速率是与污染物浓度的二次方成正比关系,其吸附过程主要是化学吸附[22],这与NCS中功能性基团的作用密切相关。
-
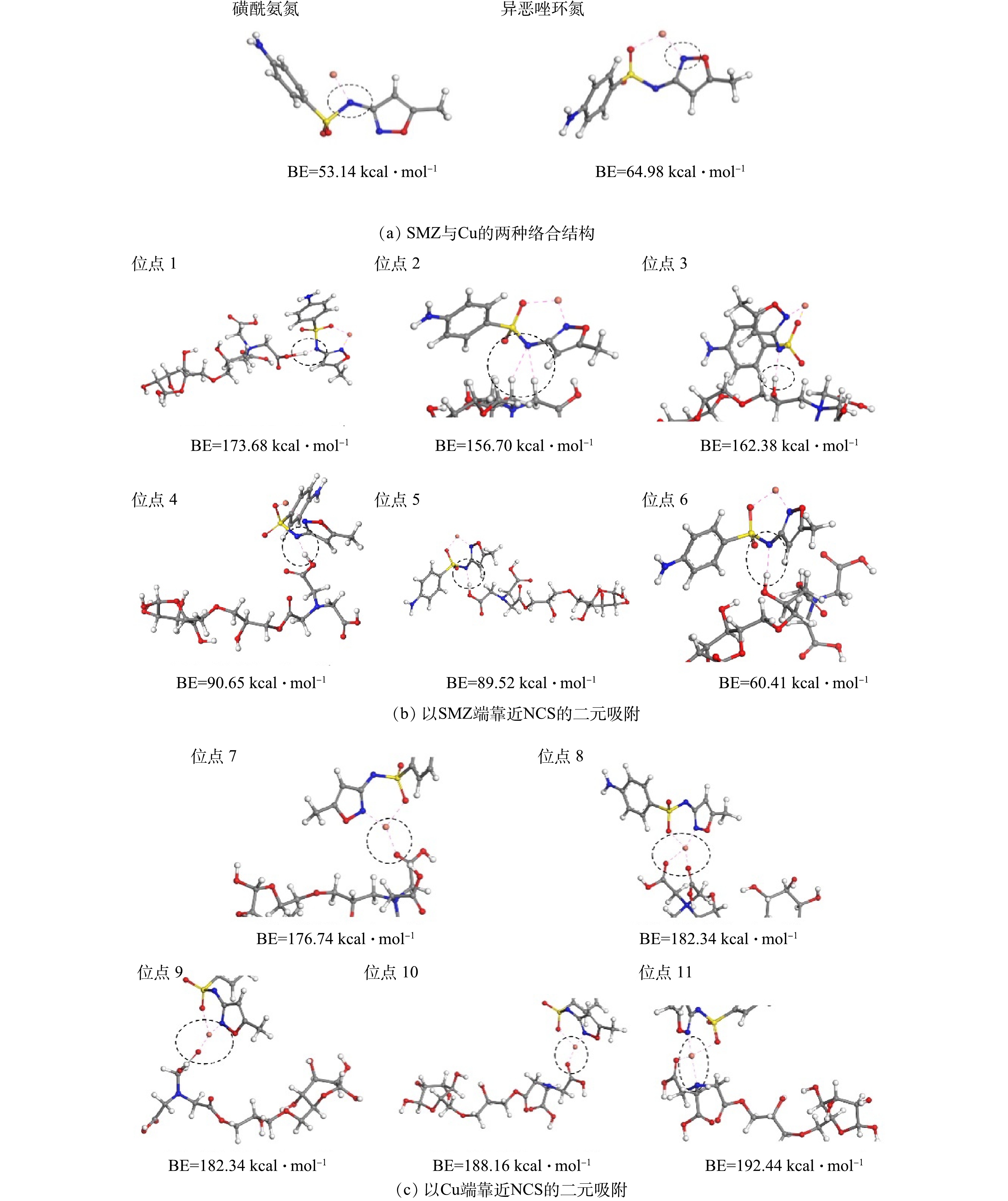
基于XPS的定性分析结果,NCS表面的基团可作为Cu(II)和SMZ的吸附位点。为此,将上述单一和二元吸附体系的吸附过程拟合到这些吸附位点,再通过结合能(BE)的计算分析,能够更好地定量阐明每个位点对吸附的贡献。一般来说,BE的绝对值越高,结合能就越高,表明吸附剂与吸附质的结合力越强[23]。如图6(a)~(d)所示,NCS 结构中的6个代表性结合位点(1~6号)为吸附SMZ的位点,5个代表性结合位点(7~11号)归为吸附Cu(II) 的位点。在单一吸附体系中,SMZ中的磺酰胺氮和异恶唑环氮分别与NCS中-N+和C-N 中的—COOH相互作用,即1~6号结合位点均含有上述2种可作用于SMZ的结构。其中,1号位点(—N+结构中—COOH)与SMZ中磺酰氨氮的BE值最大(41.39 kcal·mol−1),而其他不同位置的吸附位点BE值较小。同样,Cu与NCS所有吸附位点的BE值相差不大,最大的BE值(187.96 kcal·mol−1)是11号位点,即C-N与Cu的相互作用。尽管NCS吸附Cu的结构比吸附SMZ−的更稳定,但是吸附位点的不足导致NCS对Cu(II)的吸附容量较低[38-39],而且NCS表面的正电荷与Cu(II)的排斥作用也阻碍吸附发生[40]。
如图7(a)~(c)所示的二元吸附体系中,相比于SMZ中磺酰胺氮Cu(II)形成络合 (BE=53.14 kcal·mol−1),异恶唑环氮与Cu(II)(BE=64.98 kcal·mol−1)的络合会使整体结构更稳定。因此,SMZ-Cu络合物结构可设定为异恶唑环氮与Cu的结合方式。而且,无论是以Cu端接近NCS的方式还是SMZ端接近NCS的方式,NCS吸附SMZ-Cu络合物的结构都比单一吸附Cu或SMZ的BE值高。这是由于SMZ−和Cu(II)只能利用NCS表面一小部分的吸附位点,而SMZ-Cu络合物可利用绝大部分的吸附位点。因此,NCS在二元吸附体系中以吸附SMZ-Cu络合物为主,这也与XPS定性分析结果一致。进一步来看,SMZ-Cu络合物以Cu接近NCS结合方式的BE值(均>175 kcal·mol−1)显然比以SMZ−接近NCS结合方式的BE值(<175 kcal·mol−1)更高。这意味着NCS主要是以Cu接近NCS的吸附结构存在[41]。同时,NCS在二元吸附体系中对Cu的吸附量有所增加,是因为SMZ−先与Cu(II)形成络合物以减少整体的正电性,有利于NCS的静电吸附和络合。然后,NCS与SMZ-Cu络合物的结合与络合物中Cu的络合为主,而与SMZ−的结合能较低。而且,大量的Cu(II)还会破坏络合物的形态,从而造成SMZ−重新释放到溶液中(即解络合效应)。接着,释放出来的SMZ−继续与Cu(II)发生络合效应形成SMZ-Cu络合物,并再次被NCS通过络合作用所吸附。以此不断推进吸附进程,直至NCS吸附Cu(II)达到最大容量。在此过程中,SMZ−不断的被释放和被吸附会引起其吸附量稍微降低,因此,导致NCS在二元吸附体系中对SMZ的吸附量(56.21 mg·g−1)略低于单一吸附体系(59.76 mg·g−1)。这种吸附行为机制与前期研究的络合-解络合-再络合模式相符[37]。综上所述,SMZ-Cu络合物以Cu接近NCS的吸附结构更为稳定,而以SMZ−接近NCS的吸附结构更易发生变化,导致络合-解络合-再络合的发生,不但促进NCS对Cu(II)的吸附,而且使得其吸附SMZ-Cu络合物的结构主要是以Cu接近NCS方式存在。
-
1)与RCS相比,NCS因具有含N基团(—N+或C—N)和—COOH基团,从而表现出良好的吸附性能。其等温吸附过程符合Langmuir模型,即在单一吸附体系中,并在pH=5、固液比1g·L−1的条件下,NCS对SMZ和Cu(Ⅱ)的最大理论吸附量分别为59.76 mg·g−1和4.71 mg·g−1。而在相同条件的二元吸附体系中,NCS对络合物中SMZ和Cu(II)的最大理论吸附量分别为56.21 mg·g−1和5.54 mg·g−1。而且,NCS的吸附过程符合准二级动力学方程,属于化学吸附,这与其结构中的功能性基团密切相关。
2)在单一吸附体系中,Cu(Ⅱ)与NCS中的含N基团(—N+基或C—N)和—COOH发生配位作用,而SMZ可通过氢键和静电相互作用与以上基团结合。相比而言,NCS对SMZ的吸附亲和性要强于Cu(II)。而在二元吸附体系中,多重相互作用叠加效果导致NCS以吸附络合物SMZ-Cu为主,不倾向对单一污染物的吸附。
3)在单一吸附系统中,NCS的—N+结构中—COOH与SMZ中磺酰氨氮结合最稳定;而C—N直接与Cu的相互作用最大。而在二元吸附体系中,SMZ-Cu络合物以Cu位置接近NCS方式是最主要的吸附结构,由此引起的络合-解络合-再络合模式导致NCS对Cu的吸附量增加,但稍微降低了对SMZ的吸附效果。
多基团秸秆纤维对水体中铜/磺胺甲恶唑复合污染物的定量吸附性能
Quantitative adsorption behavior of copper/sulfonamides compound pollutants in aqueous solution by multi-groups straw fibers
-
摘要: 以秸秆纤维为研究对象,通过醚化和接枝化修饰方法赋予其纤维结构的季胺基(-N+)、羧基(-COOH)等,从而制备出多基团纤维材料(NCS)。在一元和二元吸附体系中,考察了NCS对不同pH水体中铜/磺胺甲恶唑(SMZ-Cu)复合污染物的去除效果。Langmuir吸附等温线拟合结果表明,NCS对SMZ和Cu(Ⅱ)的最大理论吸附量分别为59.76 mg·g−1和4.71 mg·g−1,以及对络合物中SMZ和Cu(Ⅱ)的最大理论吸附量分别为56.21 mg·g−1和5.54 mg·g−1。吸附过程也符合准二级动力学方程,属于化学吸附。相比而言,NCS在一元吸附体系中更倾向单一吸附SMZ而不是Cu(II)。而且,多重相互作用使NCS在二元吸附体系中主要吸附SMZ-Cu为主,而不是单一污染物。密度泛函理论(DFT)计算不但验证了上述结果,还定量地解释在单一吸附系统中,NCS的-N+结构中-COOH与SMZ中磺酰氨氮结合最稳定,而C-N则与Cu结合能最大;在二元吸附体系中,最主要的吸附结构是SMZ-Cu以Cu接近NCS方式,而络合-解络合-再络合模式是该体系中Cu被吸附增加的原因。
-
关键词:
- 秸秆纤维 /
- 铜/磺胺类抗生素复合物 /
- 基团 /
- 吸附行为 /
- 定量计算
Abstract: Straw fiber was selected as the research object, and then multigroup fiber materials (NCS) were prepared by endowing quaternary amine groups (-N+) and carboxyl groups (-COOH) onto fiber structures through etherification and grafting modification methods. In the single and binary adsorption systems, the removal effects of NCS on copper/sulfamethoxazole (SMZ-Cu) combined pollutants in water at different pHs were investigated. The fitting results of Langmuir adsorption isotherms showed that the maximum theoretical adsorption capacities of NCS to SMZ and Cu (Ⅱ) were 59.76 mg·g−1 and 4.71 mg·g−1, respectively, and the maximum theoretical adsorption capacities of SMZ and Cu (Ⅱ) in the complex were 56.21 mg·g−1 and 5.54 mg·g−1, respectively. The adsorption process also conformed to the pseudo-second-order kinetic equation, and belonged to chemical adsorption. In contrast, NCS was more likely to adsorb SMZ than Cu(Ⅱ) in the single system. Moreover, the multiple interactions caused NCS to mainly adsorb SMZ-Cu in the binary system rather than a single pollutant. Density functional theory (DFT) calculations not only verified the above analysis, but also quantitatively explained the following results in the single adsorption system: In the -N+ structure of NCS, -COOH had the most stable binding energy with sulfonamide nitrogen in SMZ, while C-N had the largest binding energy with Cu. In the binary adsorption system, the most dominant adsorption mechanism was that SMZ-Cu approached NCS in the form of Cu, and the complexation–decomplexation–complexation mode was the reason for increasing Cu adsorption.-
Key words:
- straw fiber /
- copper/sulfa antibiotic complex /
- groups /
- adsorption behavior /
- quantitative calculation
-

-
表 1 材料的SEM-EDS和BET数据
Table 1. SEM-EDS and BET data of materials
材料类型 C含量/% N含量/% O含量/% S含量/% Cu含量/% 比表面积/(m2·g−1) 总孔隙体积/(cm3·g−1) 平均孔径/nm RCS 51.43 4.67 42.72 0.52 0.66 2.670 2.3×10-3 2.670 NCS 63.03 4.24 31.89 0.84 0.02 0.924 1.53×10-4 6.028 NCS+SMZ 62.15 2.69 33.73 1.40 0.03 2.063 6.78×10-4 29.310 NCS+Cu 66.67 0.42 28.28 0.22 4.41 1.564 5.08×10-4 5.444 NCS+SMZ-Cu 60.81 2.80 32.43 1.96 2.00 1.880 5.2×10-5 4.257 表 2 Langmuir和Freundlich模型拟合参数
Table 2. Langmuir and Freundlich model fitting constants
吸附体系 Langmuir模型 Freundlich模型 Qmax/(mg·g−1) KL/(L·mg−1) R2 Kf/(mg(1−n)·Ln·g−1) 1/n R2 Cu(Ⅱ) 4.71 0.064 0.986 0.778 0.405 0.972 Cu(Ⅱ)(SMZ 50 mg·L−1) 5.54 0.115 0.991 1.662 0.279 0.986 SMZ 59.76 0.048 0.952 8.255 0.482 0.923 SMZ (Cu(Ⅱ) 30 mg·L−1) 56.21 0.044 0.978 4.359 0.577 0.976 表 3 准一级、准二级动力学模型拟合参数
Table 3. Pseudo-first-order and Pseudo-second-order model fittings constants
吸附体系 准一级动力学模型 准二级动力学模型 qe/(mg·g−1) k1/min−1 R2 qe/(mg·g−1) k2/(g·(mg·min)−1) R2 Cu(Ⅱ) 2.043 1.772 0.960 2.179 1.167 0.991 Cu(Ⅱ)(SMZ 50 mg·L−1) 2.508 0.344 0.975 2.774 0.177 0.984 SMZ 35.027 5.827 0.939 36.492 0.274 0.989 SMZ (Cu(Ⅱ) 30 mg·L−1) 25.962 3.303 0.861 27.360 0.189 0.967 -
[1] 徐西蒙, 陈远翔. 水体重金属-有机物复合污染的协同处理技术研究进展[J]. 化工环保, 2020, 40(5): 467-473. doi: 10.3969/j.issn.1006-1878.2020.05.002 [2] 徐舟影, 孟发科, 吕意超, 等. 抗生素与重金属复合污染废水处理的研究进展[J]. 环境科学研究, 2021, 34(11): 2686-2695. [3] JIA S Y, YANG Z, YANG W B, et al. Removal of Cu(II) and tetracycline using an aromatic rings-functionalized chitosan-based flocculant: enhanced interaction between the flocculant and the antibiotic[J]. Chemical Engineering Journal, 2016, 283: 495-503. doi: 10.1016/j.cej.2015.08.003 [4] YANG Z, JIA S Y, ZHANG T T, et al. How heavy metals impact on flocculation of combined pollution of heavy metals-antibiotics: a comparative study[J]. Separation and Purification Technology, 2015, 149: 398-406. doi: 10.1016/j.seppur.2015.06.018 [5] QIAN M, WU H, WANG J, et al. Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China[J]. Science of the Total Environment, 2016, 559: 174-181. doi: 10.1016/j.scitotenv.2016.03.123 [6] 马芳. 小麦秸秆及其纤维素的改性与吸附水体中Pb(Ⅱ)和As(Ⅴ)的机理研究[D]. 咸阳: 西北农林科技大学, 2017. [7] YUAN L, YAN M, HUANG Z, et al. Influences of pH and metal ions on the interactions of oxytetracycline onto nano-hydroxyapatite and their co-adsorption behavior in aqueous solution[J]. Journal of Colloid and Interface Science, 2019, 541: 101-113. doi: 10.1016/j.jcis.2019.01.078 [8] LU H, ZHU Z, ZHANG H, et al. Simultaneous removal of arsenate and antimonate in simulated and practical water samples by adsorption onto Zn/Fe layered double hydroxide[J]. Chemical Engineering Journal, 2015, 276: 365-375. doi: 10.1016/j.cej.2015.04.095 [9] YU J, XIONG J, CHENG B, et al. Hydrothermal preparation and visible-light photocatalytic activity of Bi2WO6 powders[J]. Journal of Solid State Chemistry, 2005, 178(6): 1968-1972. doi: 10.1016/j.jssc.2005.04.003 [10] 桑丰, 刚淑艳, 李佳, 等. 改性玉米秸秆对海水中Cr(VI)的吸附应用[J]. 河北渔业, 2022(04): 1-5+13. doi: 10.3969/j.issn.1004-6755.2022.04.001 [11] CHEN S H, YUE Q Y, GAO B Y, et al. Removal of Cr(VI) from aqueous solution using modified corn stalks: Characteristic, equilibrium, kinetic and thermodynamic study[J]. Chemical Engineering Journal, 2011, 168(2): 909-917. doi: 10.1016/j.cej.2011.01.063 [12] SUN N, WEN X, YAN C J. Adsorption of mercury ions from wastewater aqueous solution by amide functionalized cellulose from sugarcane bagasse[J]. International Journal of Biological Macromolecules, 2018, 108: 1199-1206. doi: 10.1016/j.ijbiomac.2017.11.027 [13] 胡冬英. 两种季铵化纤维素的制备、表征及应用研究[D]. 哈尔滨: 东北林业大学, 2017. [14] YANG Y, ZHENG L, ZHANG T, et al. Adsorption behavior and mechanism of sulfonamides on phosphonic chelating cellulose under different pH effects[J]. Bioresource Technology, 2019, 288: 121510. doi: 10.1016/j.biortech.2019.121510 [15] HOKKANEN S, BHATNAGAR A, SILLANPÄÄ M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity[J]. Water Research, 2016, 91: 156-173. doi: 10.1016/j.watres.2016.01.008 [16] LIU M, JIA L, ZHAO Z, et al. Fast and robust lead (II) removal from water by bioinspired amyloid lysozyme fibrils conjugated with polyethyleneimine (PEI)[J]. Chemical Engineering Journal, 2020, 390: 124667. doi: 10.1016/j.cej.2020.124667 [17] ZHAO F, REPO E, YIN D, et al. EDTA-cross-linked beta-cyclodextrin: an environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes[J]. Environmental Science & Technology, 2015, 49(17): 10570-10580. [18] QIU H, LING C, YUAN R, et al. Bridging effects behind the coadsorption of copper and sulfamethoxazole by a polyamine-modified resin[J]. Chemical Engineering Journal, 2019, 362: 422-429. doi: 10.1016/j.cej.2019.01.043 [19] CHEN T, LIU F, LING C, et al. Insight into highly efficient coremoval of copper and p-nitrophenol by a newly synthesized polyamine chelating resin from aqueous media: competition and enhancement effect upon site recognition[J]. Environmental Science & Technology, 2013, 47(23): 13652-13660. [20] AHMED M, ZHOU J, NGO H, et al. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges[J]. Science of the Total Environment, 2015, 532: 112-126. doi: 10.1016/j.scitotenv.2015.05.130 [21] ZHOU Y, LIU X, TANG L, et al. Insight into highly efficient co-removal of p-nitrophenol and lead by nitrogen-functionalized magnetic ordered mesoporous carbon: Performance and modelling[J]. Journal of Hazardous Materials, 2017, 333: 80-87. doi: 10.1016/j.jhazmat.2017.03.031 [22] REN J, ZHENG L, SU Y, et al. Competitive adsorption of Cd(II), Pb(II) and Cu(II) ions from acid mine drainage with zero-valent iron/phosphoric titanium dioxide: XPS qualitative analyses and DFT quantitative calculations[J]. Chemical Engineering Journal, 2022, 445: 136778. doi: 10.1016/j.cej.2022.136778 [23] YU H, ZHENG L, ZHANG T, REN J, et al. Adsorption behavior of Cd (II) on TEMPO-oxidized cellulose in inorganic/ organic complex systems[J]. Environmental Research, 2021, 195: 110848. doi: 10.1016/j.envres.2021.110848 [24] BEDIAKO J K, WEI W, KIM S, et al. Removal of heavy metals from aqueous phases using chemically modified waste Lyocell fiber[J]. Journal of Hazardous Materials, 2015, 299: 550-561. doi: 10.1016/j.jhazmat.2015.07.033 [25] ZHENG L, ZHANG S, CHENG W, et al. Theoretical calculations, molecular dynamics simulations and experimental investigation of the adsorption of cadmium(ii) on amidoxime-chelating cellulose[J]. Journal of Materials Chemistry A, 2019, 7(22): 13714-13726. doi: 10.1039/C9TA03622A [26] ZHENG L, YANG Y, MENG P, et al. Absorption of cadmium (II) via sulfur-chelating based cellulose: Characterization, isotherm models and their error analysis[J]. Carbohydrate Polymers, 2019, 209: 38-50. doi: 10.1016/j.carbpol.2019.01.012 [27] ZHENG L, PENG D, MENG P. Promotion effects of nitrogenous and oxygenic functional groups on cadmium (II) removal by carboxylated corn stalk[J]. Journal of Cleaner Production, 2018, 201: 609-623. doi: 10.1016/j.jclepro.2018.08.070 [28] KUMAR M, SINGH S, SHAHI V K. Cross-linked poly(vinyl alcohol)-poly(acrylonitrile-co-2-dimethylamino ethylmethacrylate) based anion-exchange membranes in aqueous media[J]. Journal of Physical Chemistry B, 2010, 114(1): 198-206. doi: 10.1021/jp9082079 [29] 姚之侃. 叔胺两亲共聚物共混膜的制备及其过滤吸附功能的研究[D]. 杭州: 浙江大学, 2015. [30] HUANG B, LIU Y, LIU B, et al. Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite[J]. Carbohydrate Polymer, 2017, 157: 576-585. doi: 10.1016/j.carbpol.2016.10.025 [31] LI M, LIU Y, LIU S, et al. Cu(II)-influenced adsorption of ciprofloxacin from aqueous solutions by magnetic graphene oxide/nitrilotriacetic acid nanocomposite: competition and enhancement mechanisms[J]. Chemical Engineering Journal, 2017, 319: 219-228. doi: 10.1016/j.cej.2017.03.016 [32] LING C, LI X, ZHANG Z, et al. High adsorption of sulfamethoxazole by an amine-modified polystyrene-divinylbenzene resin and its mechanistic insight[J]. Environmental Science and Technology, 2016, 50(18): 10015-10023. doi: 10.1021/acs.est.6b02846 [33] WANG R Z, HUANG D L, LIU Y G, et al. Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar[J]. Journal of Hazardous Materials, 2020, 384: 121470. doi: 10.1016/j.jhazmat.2019.121470 [34] LI Z, LI M, WANG Z, LIU X. Coadsorption of Cu(II) and tylosin/sulfamethoxazole on biochar stabilized by nano-hydroxyapatite in aqueous environment[J]. Chemical Engineering Journal, 2020, 381: 122785. doi: 10.1016/j.cej.2019.122785 [35] 汪晨. 水中典型药物与重金属的络合行为[D]. 南京: 东南大学, 2016. [36] PUNAMIYA P, SARKAR D, RAKSHIT S, et al. Effect of solution properties, competing ligands, and complexing metal on sorption of tetracyclines on Al-based drinking water treatment residuals[J]. Environmental Science and Pollution Research, 2015, 22(10): 7508-7518. doi: 10.1007/s11356-015-4145-z [37] ZHANG T, ZHENG L, YU H, et al. Solution pH affects single, sequential and binary systems of sulfamethoxazole and cadmium adsorption by self-assembled cellulose: Promotion or inhibition?[J]. Journal of Hazardous Materials, 2021, 402: 124084. doi: 10.1016/j.jhazmat.2020.124084 [38] WEI J, SUN W, PAN W, et al. Comparing the effects of different oxygen-containing functional groups on sulfonamides adsorption by carbon nanotubes: Experiments and theoretical calculation[J]. Chemical Engineering Journal, 2017, 312: 167-179. doi: 10.1016/j.cej.2016.11.133 [39] SONG W, YANG T, WANG X, et al. Experimental and theoretical evidence for competitive interactions of tetracycline and sulfamethazine with reduced graphene oxides[J]. Environmental Science:Nano, 2016, 3(6): 1318-1326. doi: 10.1039/C6EN00306K [40] HOSSAIN M R, HASAN M M, NOOR-E-ASHRAFI, et al. Adsorption behaviour of metronidazole drug molecule on the surface of hydrogenated graphene, boron nitride and boron carbide nanosheets in gaseous and aqueous medium: A comparative DFT and QTAIM insight[J]. Physica E, 2021: 126. [41] LI S, WANG F, PAN W, et al. Molecular insights into the effects of Cu(II) on sulfamethoxazole and 17 β-estradiol adsorption by carbon nanotubes/CoFe2O4 composites[J]. Chemical Engineering Journal, 2019, 373: 995-1002. doi: 10.1016/j.cej.2019.05.111 -



 下载:
下载: