-
随着人类社会快速发展,环境与资源问题日益凸显。目前普遍认为,人为因素造成的磷污染是水体富营养化的主要成因之一。水体富营养化将引起藻类快速繁殖,降低水体溶解氧,最终导致水生动植物死亡、生态系统失衡[1]。《2021年中国生态环境状况公报》显示,我国110个重要湖泊中,富营养状态湖泊占比高达27.3%[2]。湖泊富营养化已然成为我国亟待解决的重大环境问题之一。治理水体磷污染迫在眉睫。
近年来,国内外学者就水体磷污染控制开展了诸多研究,所采取的方法包括吸附法、化学沉淀法以及生物法等。其中,吸附法因其操作简单、处理迅速和效果稳定等优势受到关注。生物炭是近些年新兴的吸附材料,其由生物质热解碳化而得,具有较大的比表面积、发达的孔隙结构、丰富的官能团、强阳离子交换能力和低廉的价格等优势[3]。用农林废弃物等废弃生物质制备生物炭并用于去除废水中的磷,是当前的研究热点,具有固废资源化、固碳减排及磷资源回收等显著环境效益的优点。然而,大部分生物炭表面带负电,对磷酸盐的吸附效果不佳[4]。因此,需对生物炭实施改性,通过改变表面荷电性、增加吸附位点等方式提升其吸附磷的能力。常见的改性方法是在生物炭表面负载Ca[5]、Mg[6]、Ce[7]、Al[8]和La[9]等金属。在这些金属中,Mg具有在地壳中丰度高、对磷酸盐亲和力强的特点,非常适合用于生物炭改性[10]。在这方面,国内外已有较多相关研究。例如,研究者利用花生壳[11]、烟草秸秆[12]、槐木屑[13]、芦苇[14]和桉木茎片[15]等废弃生物质制备生物炭,然后用高浓度的Mg化学试剂对生物炭施以改性,并将改性生物炭用于废水除磷。这些研究表明,Mg改性生物炭对废水中磷的去除效果较好,具有一定实际工程应用的潜力。
虽然Mg改性生物炭除磷的研究取得了一定进展,但现有研究仍存在以下不足:以高浓度Mg化学试剂为改性剂增加了Mg改性生物炭的制备成本;Mg改性生物炭多呈粉末状,大量投加时易扬尘,且存在可操作性差、固液分离难的问题;多数研究集中于静态吸附实验,Mg改性生物炭的动态吸附研究相对较少[16-17]。可见,缺乏兼具经济性和实用性的Mg改性生物炭依然是该领域的瓶颈问题。开发低成本、具备一定尺寸且易于实际应用的Mg改性生物炭,并开展其在吸附柱中的动态吸附研究势在必行,是应对上述问题的有效策略之一。在前期研究中,ZHANG等[18]利用天然海水代替高浓度Mg化学试剂对生物炭粉末进行改性,发现不但改性效果好(改性后生物炭吸附容量提升了127倍),且改性过程几乎不增加生物炭的制造成本。这为制备兼具经济性和实用性的Mg改性生物炭提供了重要参考。据悉,目前尚无以海水为Mg源制备兼具经济性和实用性的Mg改性生物炭的研究报道。因此,本研究以天然海水为廉价Mg源、以生物炭颗粒(biochar granule,BC-g)为基材,制备低成本海水改性生物炭颗粒(seawater-modified biochar granule,SBC-g);利用表征技术考察SBC-g的物理化学性质及其吸附磷酸盐机理;在吸附柱中拟开展动态吸附实验,考察初始质量浓度、柱高、流量等因素对除磷效果的影响;并进行SBC-g制备成本的经济分析。本研究结果有望突破Mg改性生物炭除磷领域中存在的瓶颈问题,能够为兼具经济性和实用性Mg改性生物炭的制备及其在水体磷污染治理领域的实际应用提供参考。
-
收集福建省福州市周边的废弃杉木屑,清洗、干燥后用作制备生物炭的原料。海水采集自三沙湾海域,养殖尾水取自福州市某鳗鱼养殖场,二者的水质参数见表1。使用前,海水及尾水水样均经过0.45 μm滤膜过滤。分析纯磷酸二氢钾(KH2PO4)、氢氧化钠(NaOH)等化学试剂,购得即用,无进一步纯化。所有溶液均用去离子水配制。
-
1)生物炭颗粒(BC-g)的制备。将杉木屑放入坩埚,加盖后用锡箔纸包裹,放置于马弗炉(SXL-1008T,上海精宏实验设备有限公司)中,在限氧条件下以10 ℃·min−1的速率升温至600 ℃后,保持2 h。热解过程不使用额外惰性气体而以自身产生的热解气为热解保护气氛。冷却至室温后,取出用去离子水洗涤3次,在干燥箱中于80 ℃烘干过夜,取出研磨,筛选出大于50目的颗粒,即为BC-g。
2)海水改性生物炭颗粒(SBC-g)的制备。SBC-g的制备方法参考前期研究[18]的方法,具体步骤如下:首先将BC-g与海水以2 g·L−1的比例于烧杯中混合,在磁力搅拌器(LC-MAG-HS18,上海力辰邦西仪器科技有限公司)上搅拌1 h后,逐滴滴入1 mol·L−1NaOH,调节pH至10.50;再搅拌1 h后,将烧杯放入恒温水浴锅(HW2-26,上海一恒科学仪器有限公司),40 ℃下陈化过夜;然后取出抽滤,将固体置于真空干燥箱(DZF-6020MBE,上海博迅医疗生物仪器股份有限公司)中,于80 ℃真空干燥。待冷却至室温后,筛选出10~40目的颗粒,即为SBC-g。
-
用配备有能谱仪的扫描电镜(SEM,JMS-7500F,日本电子株式会社)观察SBC-g的形貌及表面元素分布;通过X射线衍射光谱(XRD,D8 Advance,德国Bruker公司)分析SBC-g的晶体成分;用X射线光电子能谱(XPS,250xi,美国Thermo公司)分析生物炭的元素组成;生物炭的比表面积通过N2吸附脱附系统(AutosobiQA3200-4,美国康塔仪器公司)测试并用Brunauer–Emmett–Teller(BET)方法计算;ζ电位用ζ电位仪(Nanoplus-AT,麦克默瑞提克仪器有限公司)测定。溶液中的磷酸盐浓度采用钼酸铵分光光度法(GB11893-89)测定。金属离子采用电感耦合等离子体-原子发射光谱(ICP-OES,Avio 200,珀金埃尔默仪器有限公司)。
-
1)柱实验方法。吸附柱为直径1 cm,高度10 cm的PMMA圆柱体,为均匀分配流量和防止SBC-g流失,在两端添加脱脂棉球。通过填充1.3、2.0、2.7 g的SBC-g以获得3.5、5.0、6.5 cm的吸附柱高度,在磷酸盐初始质量浓度为2.00 mg·L−1、流量为1.5 mL·min−1的条件下考察柱高对磷吸附情况的影响。然后在初始质量浓度为2.00 mg·L−1、柱高为6.5 cm时,分别设定流量为1.5、3.0和6.0 mL·min−1,得到不同流量下磷酸盐的穿透曲线。设定柱高为6.5 cm、流量为1.5 mL·min−1,在磷酸盐初始质量浓度为2.00、5.00和10.00 mg·L−1条件下,研究吸附柱对磷酸盐的吸附情况。最后,在最佳的柱高和流量条件下,使养殖尾水通过SBC-g吸附柱。在实验过程中,每隔一定的时间收集出水溶液并测定磷酸盐浓度。
2)柱实验参数计算。穿透曲线的穿透点一般按照相关标准限值或按照初始质量浓度的5%或10%选取。本研究以《污水综合排放标准》(GB 8979-1996)的一级标准限值为穿透点,即当出水的磷酸盐质量浓度大于0.5 mg·L−1时视为吸附柱穿透,所用时间可由线性插值法求得,此时吸附柱对应的吸附量可由穿透曲线与初始质量浓度的直线所围成的积分面积(式(1))进行计算;相应的平均吸附量可由式(2)计算;流经吸附柱的磷酸盐总量可由式(3)计算;吸附柱对磷酸盐的去除率可由式(4)计算;空床停留时间可由式(5)计算。
式中:qa为穿透时对应的吸附量,mg;Q为流量,mL·min−1;A为穿透曲线所围成的面积,mg·L·min−1;ta为穿透时间,min;C0、Ct分别为磷酸盐的初始质量浓度和t时刻的质量浓度,mg·L−1;qae为穿透时对应的平均吸附量,mg·kg−1;m为吸附柱中SBC-g的质量,g;ωa为穿透时流经吸附柱的磷酸盐总量,mg;r为吸附柱对磷酸盐的去除率,%;tb为空床停留时间,min;Vb为吸附柱床层体积,cm3。
3)穿透曲线的模型拟合。Thomas模型是以Langmuir方程为基础,用于估算吸附质的平衡吸附量和吸附速率常数的吸附动力学模型。通过对已有数据的拟合,可对吸附柱内的动态吸附行为和规律进行模拟和预测。研究采用Thomas模型(式(6))拟合不同柱高、初始质量浓度和流量的实验数据并对结果进行分析。
式中:KTH为吸附速率常数,L·(min·mg)−1;q为饱和吸附量,mg·kg−1;t为吸附时间,min。
-
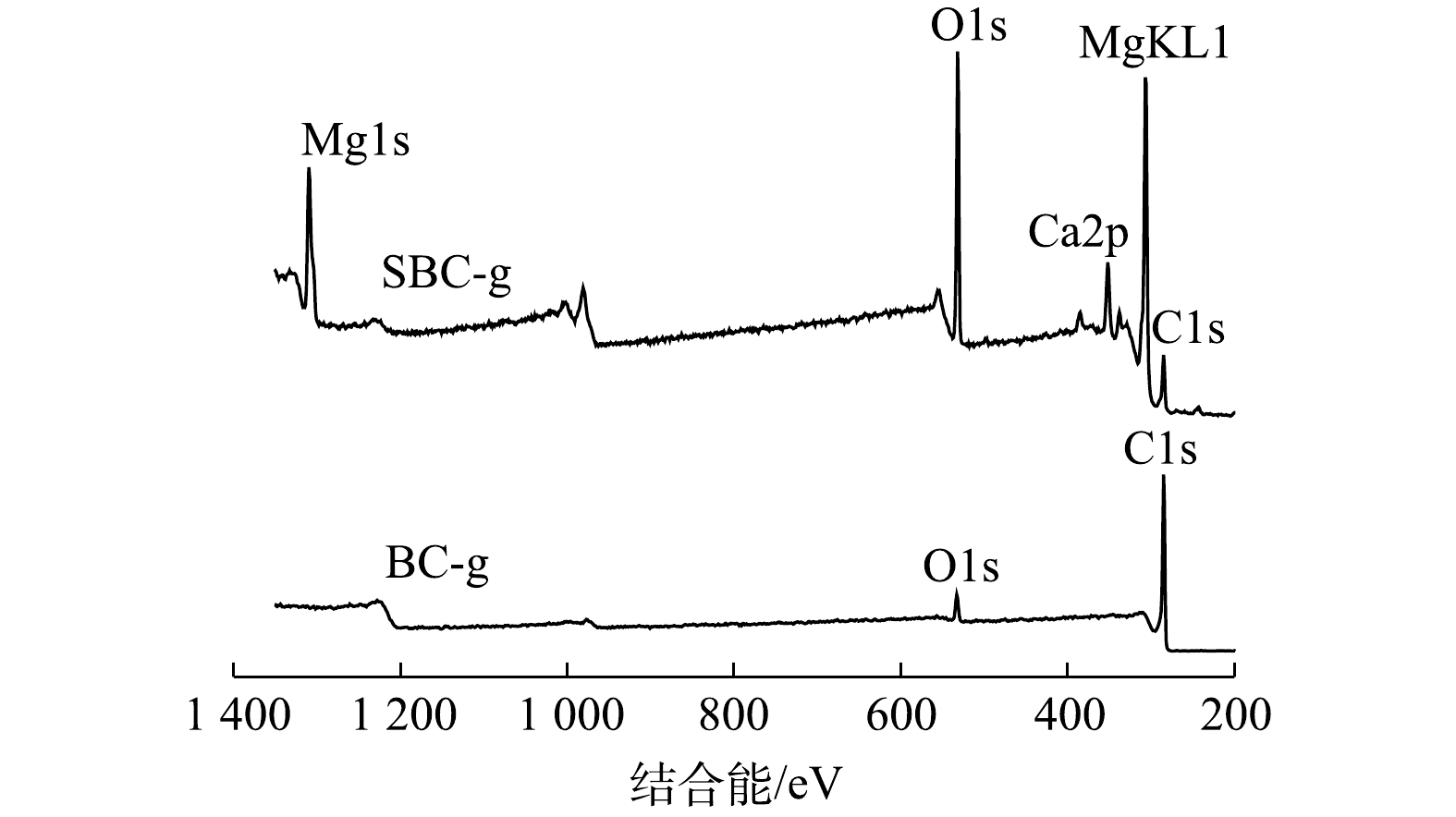
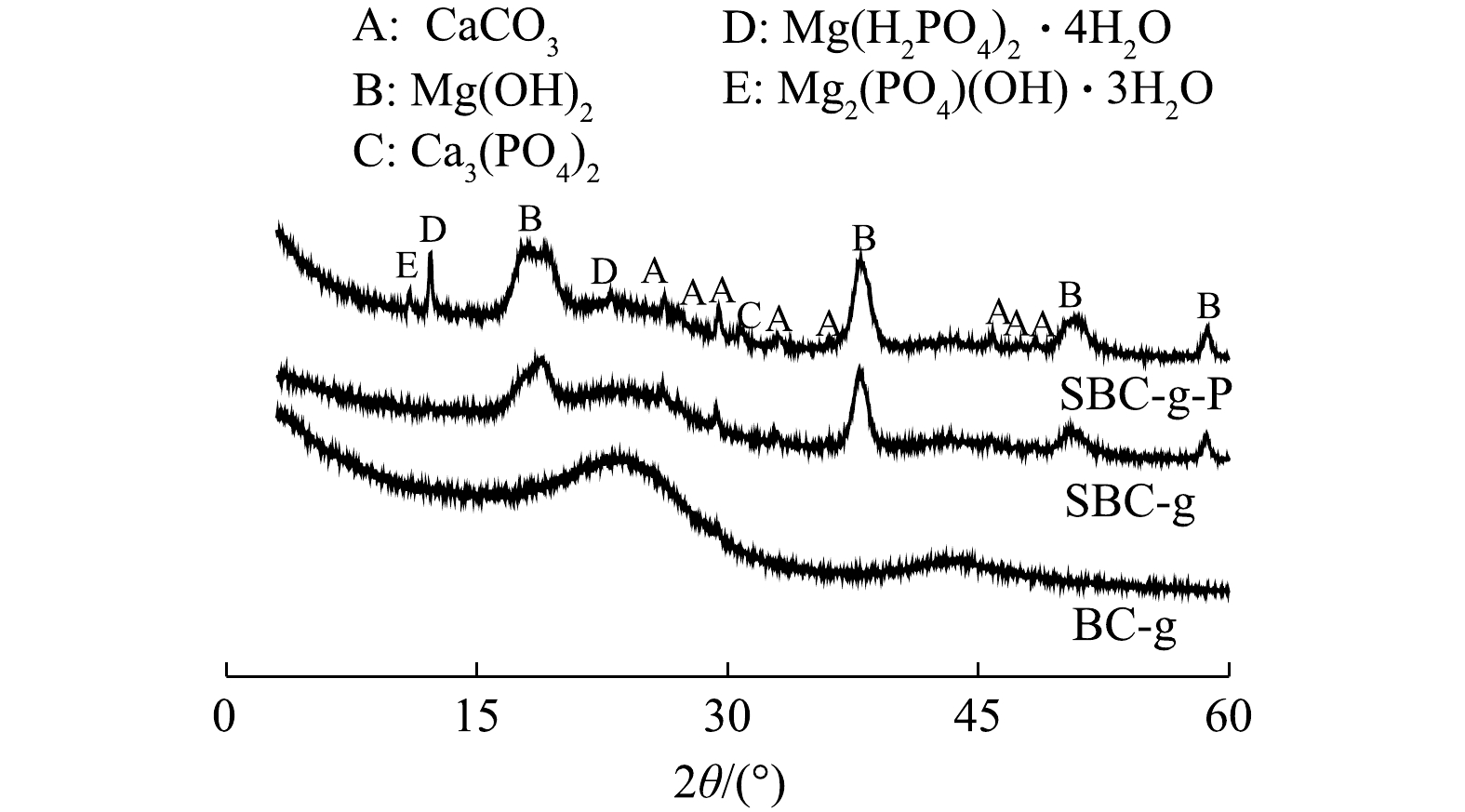
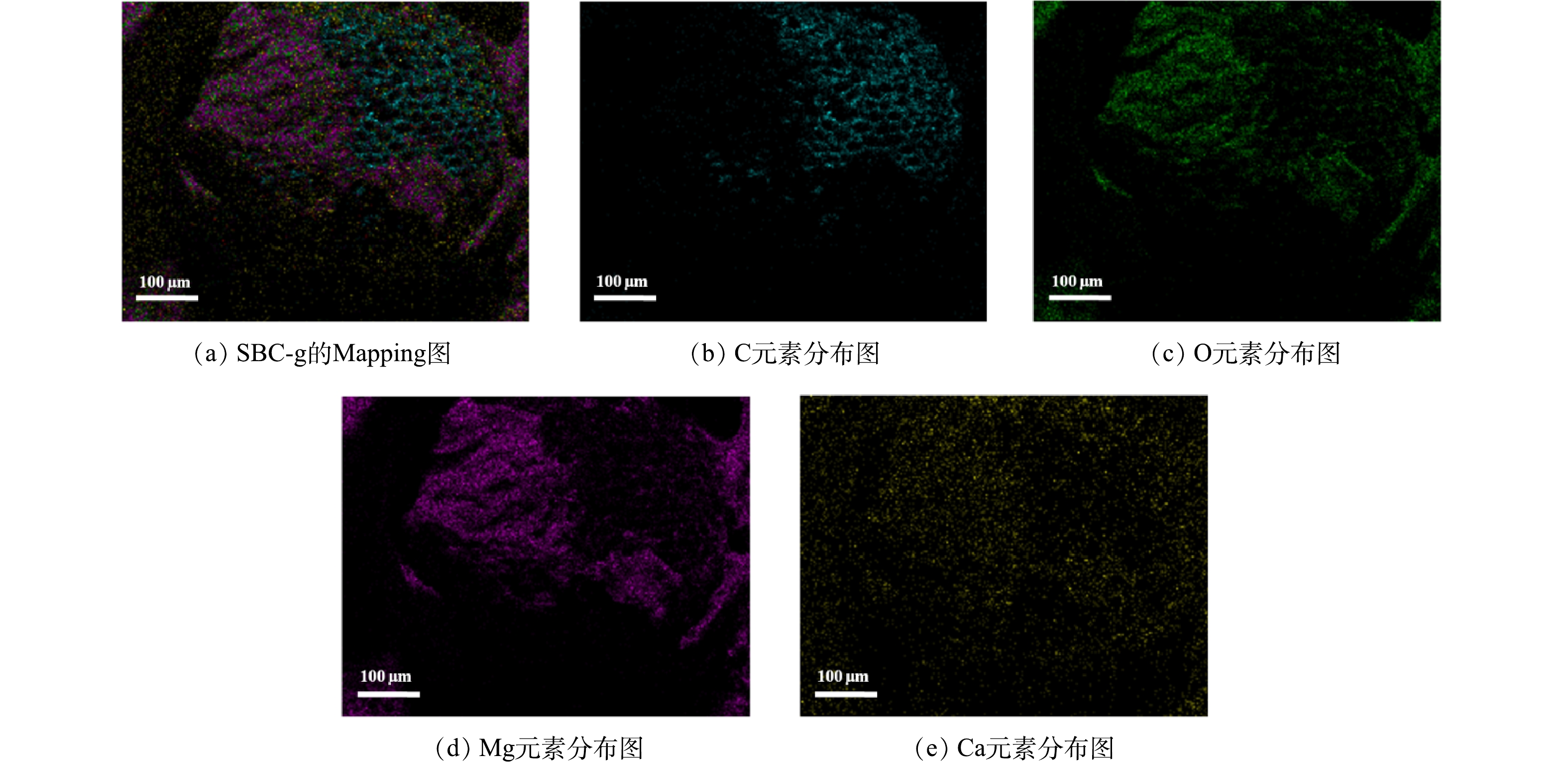
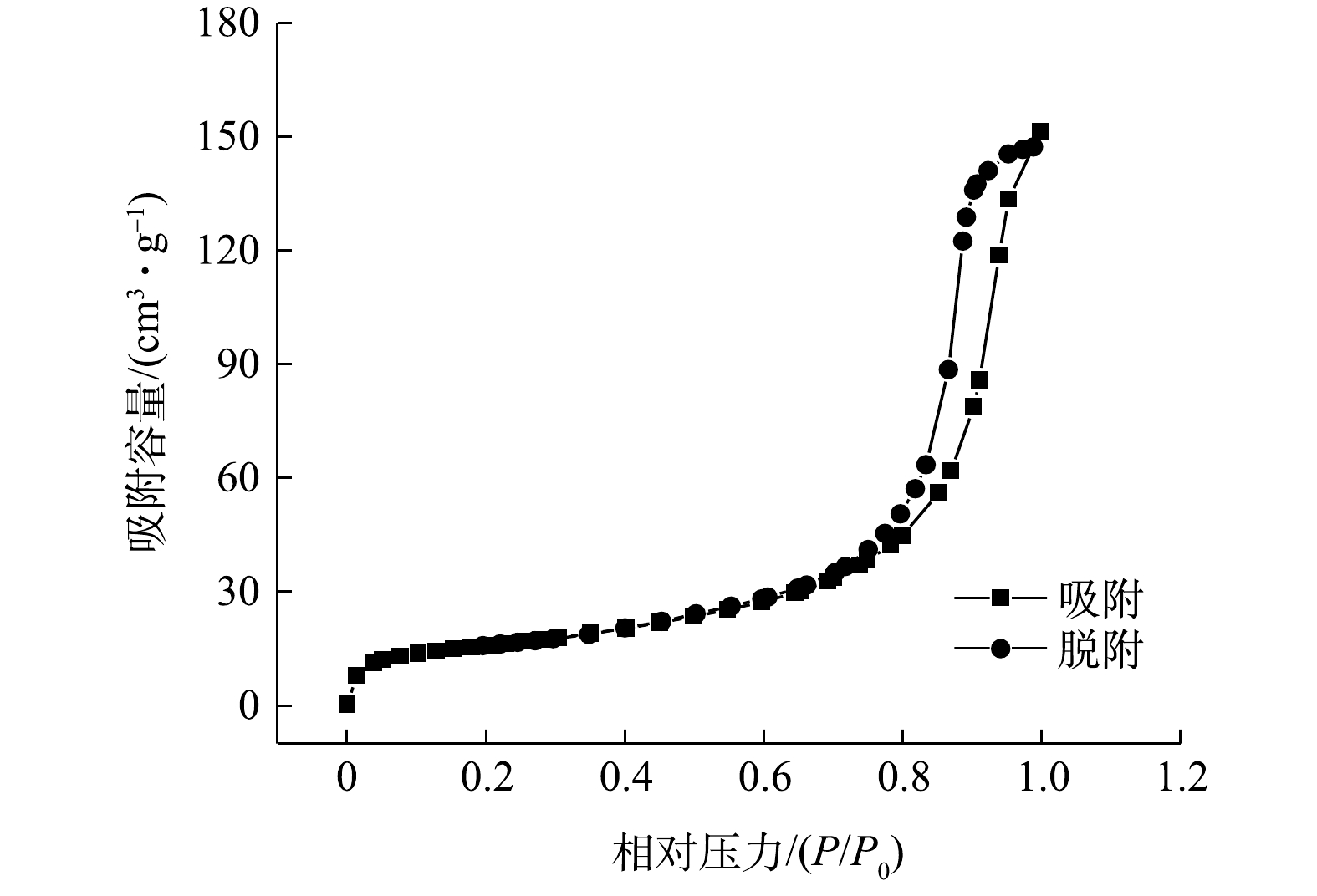
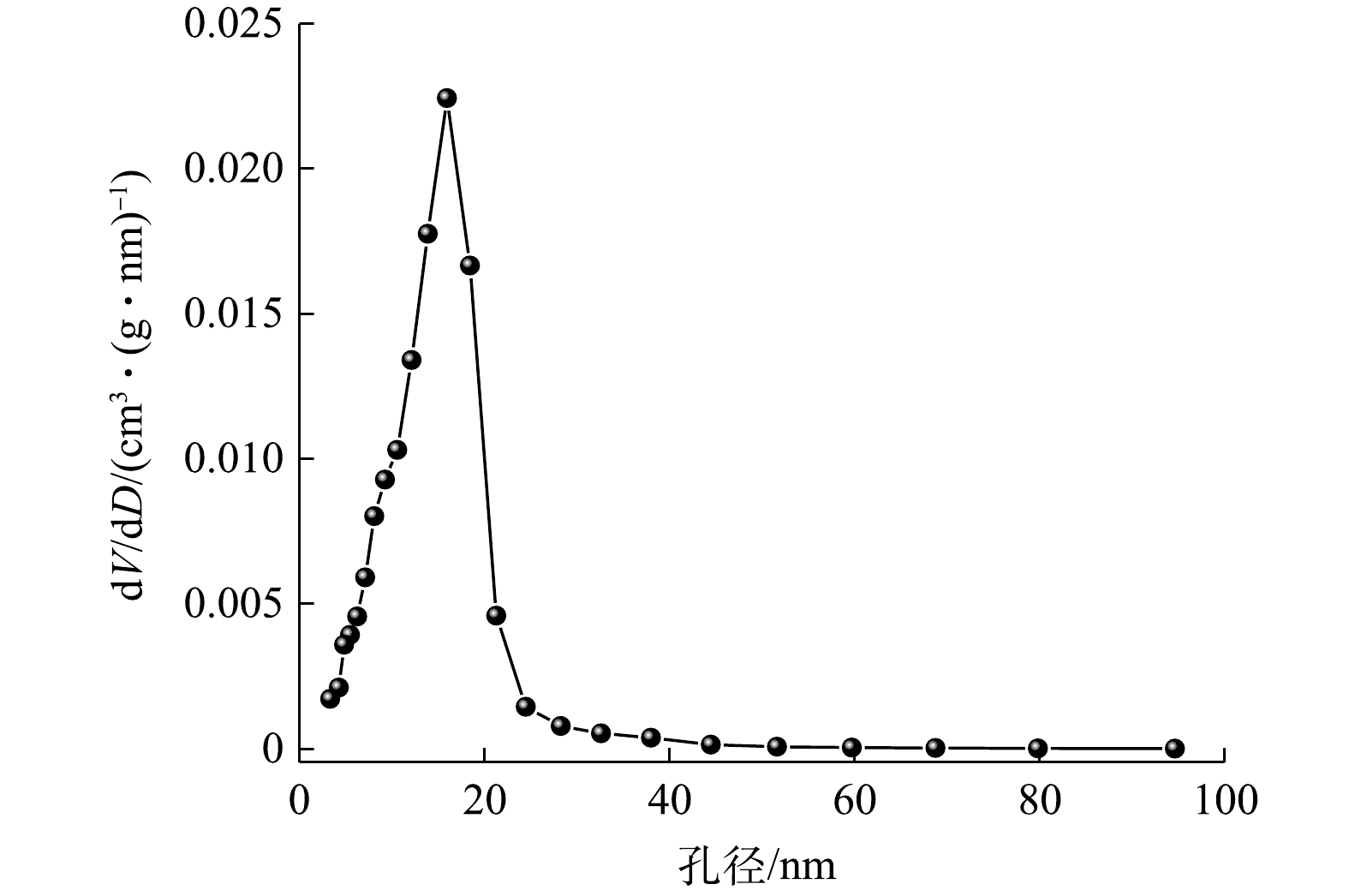
本研究以天然海水为改性剂,利用共沉淀法对BC-g实施改性。首先,BC-g和SBC-g的XPS表征结果如图1所示。由图1可以看出,和BC-g相比,在SBC-g表面O元素含量显著提高,并且谱图中出现Mg和Ca元素(前者含量高于后者)。由XRD图谱(图2)可以看出,相较于BC-g,SBC-g的峰形发生显著变化,且出现Mg(OH)2(PDF#44-1482)和CaCO3(PDF#41-1475)的特征峰。这表明改性后海水中Mg和Ca分别以Mg(OH)2和CaCO3形式负载到BC-g表面。此外,通过SEM照片(图3)可以看出,改性后新生成的物质将生物炭颗粒包裹,并堵塞了部分孔结构;由Mapping图(图4)可以看出,将生物炭颗粒包裹的物质主要元素为Mg和O。结合XRD的结果可推断,该物质可能为Mg(OH)2。此外,在SBC-g的孔隙外表面上还可观察到互相堆叠的纳米片(图3)。根据前期研究[18]结果表明,该纳米片为Mg(OH)2。正是由于Mg(OH)2的包裹及堵塞,使得SBC-g的比表面积(55.24 m2·g−1)小于BC-g的比表面积(350.15 m2·g−1)。从图5可以看出,SBC-g的氮气吸脱附曲线呈Ⅳ型;在相对压力达到1.0时,吸附尚未饱和,在相对压力接近0.8时,出现H3型回滞环。这说明材料含有大孔和狭缝形介孔[19]。由图3的表征结果推测,改性后的生物炭保留了原始生物炭丰富的大孔结构,而纳米片状的Mg(OH)2在其表面可堆叠形成狭缝形介孔,使得介孔数量增多。有研究[20]表明,磷酸盐吸附大多取决于材料的微孔和介孔,大孔中几乎不发生吸附。由孔径分布图(图6)可以看出,SBC-g的介孔孔径主要分布于3.32~24.47 nm。从表2得知,原始生物炭介孔的平均孔径仅为3.28 nm,经改性后增大到16.57 nm;相应地,介孔孔容从0.03 cm3·g−1增加到0.23 cm3·g−1。另外,BC-g和SBC-g的ζ电位值分别为−9.81 mV和4.32 mV,说明Mg(OH)2及CaCO3的负载改变了生物炭表面的带电状态,使生物炭从带负电变为带正电,这有利于吸附水中的磷酸盐。
-
由图2可以看出,与SBC-g相比,在SBC-g-P的XRD谱图中除了Mg(OH)2和CaCO3的峰之外,还出现了Mg(H2PO4)2·4H2O (PDF#87-0536)、Mg2(PO4)(OH)·3H2O(PDF#45-1380)和Ca3(PO4)2(PDF#70-0364)的特征衍射峰。其中,Mg(H2PO4)2·4H2O和Mg2PO4(OH)·3H2O的峰明显强于Ca3(PO4)2,这表明SBC-g上的Mg是吸附水中磷酸盐的主要活性位点。经对吸附磷酸盐后SBC-g的ζ电位(pH = 6)进行测定,发现吸附磷酸盐后SBC-g的ζ电位从4.32 mV降至−17.20 mV,这说明静电吸引在SBC-g吸附磷酸盐过程中起到一定作用。SBC-g对水中磷酸盐的吸附过程可能为:SBC-g首先通过静电作用吸附磷酸盐,磷酸盐再与SBC-g上的Mg(OH)2或CaCO3发生配体交换[21]、取代OH−或CO32−,最后与Mg或Ca通过内层络合作用结合生成磷酸镁或磷酸钙[22],其中Mg是主要活性位点。和原始生物炭相比,在相同条件下,海水改性可使生物炭吸附容量由0.91 mg·g−1升至25.21 mg·g−1。终上所述,改性后生物炭的吸附容量提升的原因可归结为:表面附着的Mg(OH)2可增加活性位点;介孔孔径及孔容的增大有助于磷酸盐分子的传输与吸附;表面荷电性的改变也有利于磷酸盐的吸附。
-
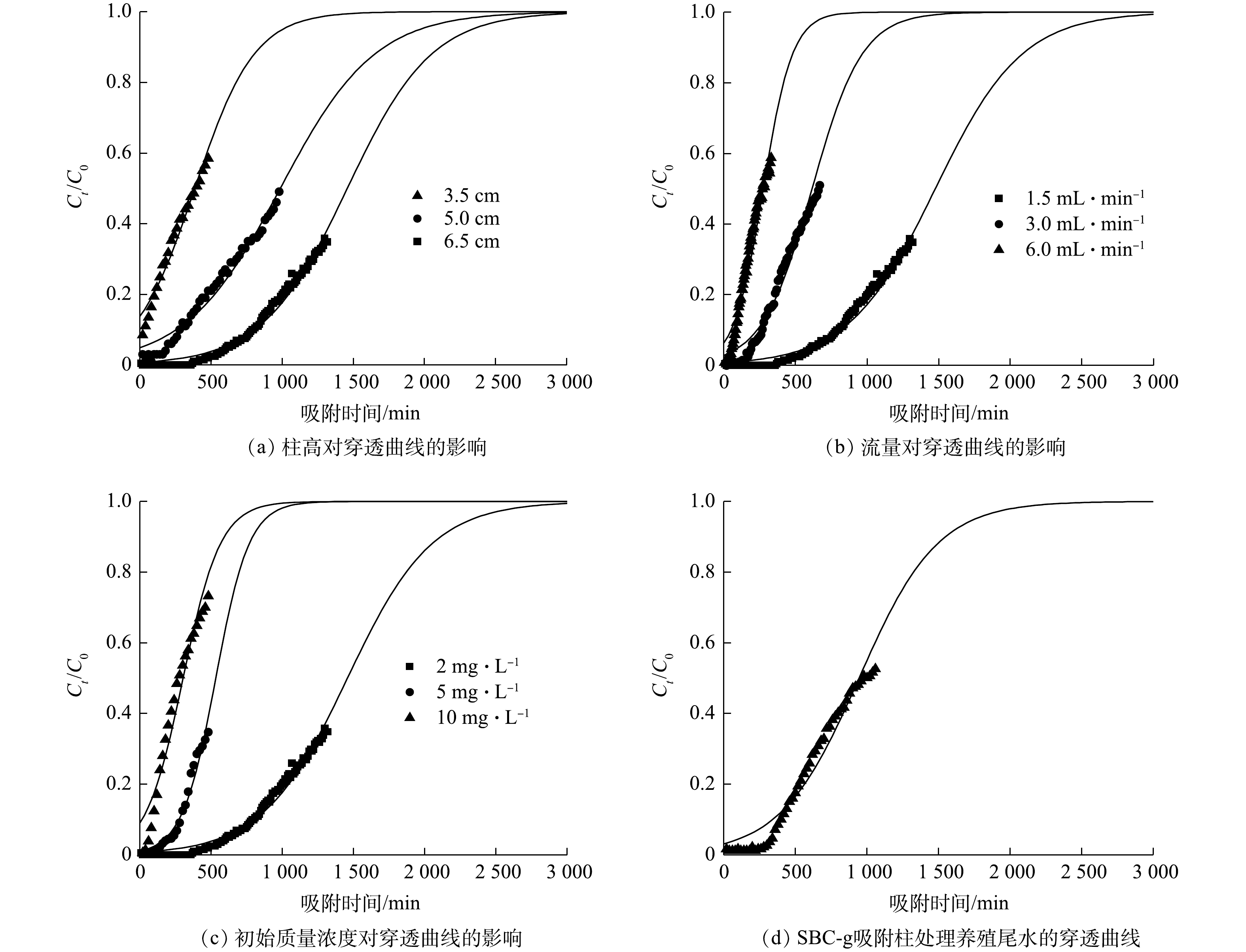
合适尺寸的颗粒状吸附剂可应用于吸附柱工艺中,因此与粉末状吸附剂相比具有操作简单和管理方便的优点。首先,将SBC-g填充于吸附柱中,研究不同的柱高、流量和初始质量浓度条件对磷酸盐吸附的影响,结果如图7所示。然后,探究SBC-g吸附柱对于含磷养殖废水的处理效果。在实验浓度范围内,吸附柱对磷酸盐具有良好的吸附效果,在吸附柱操作初期,出水磷酸盐浓度几乎为0。如表3所示,在不同操作条件下,吸附柱的穿透时间在141~1 112 min内,对磷酸盐的去除率均达到75%以上。使用Thomas模型对穿透曲线实验数据进行进一步拟合分析,其结果列于表3。由表3可看出,在柱高为6.5 cm、流量为1.5 mL·min−1和磷酸盐初始质量浓度为2.00 mg·L−1的条件下,由该模型拟合得到的吸附柱的饱和吸附量为1 619 mg·kg−1。处理实际养殖尾水时,吸附柱的穿透时间为589 min,饱和吸附量为1 051 mg·kg−1。Thomas模型对各条件下的穿透曲线均拟合良好。说明该模型能够较为准确地描述SBC-g吸附柱的动态吸附过程,可以用来预测动态吸附量和穿透时间。
1)柱高对吸附过程的影响。图7(a)为不同柱高条件下,吸附柱吸附磷的穿透曲线。由图7(a)可知,当柱高分别为3.0、5.0和6.5 cm时,穿透时间分别为141、570和1 112 min,相应的动态吸附量分别为244、641和927 mg·kg−1。由此可见,随着柱高的提升,相应的吸附活性位点增多,停留时间延长,使得磷酸盐与活性位点接触更充分。由Thomas模型拟合结果也可以看出,q随着柱高增加而增大,说明柱高的增大可提升吸附柱的吸附容量。然而,需要指出的是,吸附柱的高度并非越高越好,需要综合考虑运行成本、实际场地情况等因素,确定最终的柱高。
2)流量对吸附过程的影响。流量的变化对SBC-g吸附磷酸盐也有显著影响,如图7(b)所示。在流量为6.0 mL·min−1时,吸附柱在较短的时间内(144 min)即穿透。这是由于在较大的流量下,tb缩短,SBC-g吸附柱与进水中的磷酸盐接触时间有限。随着流量降至1.5 mL·min−1,SBC-g与进水中的磷酸盐的接触时间相应地增长,穿透时间延长至1 112 min,相应的动态吸附量也增加至927 mg·kg−1。由表3可以看出,随着流量降低,Thomas模型拟合得到的KTH减小,q增大。由此可见,适当地降低流量有助于磷酸盐与吸附柱内的SBC-g的活性位点充分接触,从而达到更好的吸附效果。
3)初始质量浓度对吸附过程的影响。在柱高为6.5 cm、流量为1.5 mL·min−1的条件下,将初始质量浓度分别为2.00、5.00和10.00 mg·L−1的磷酸盐溶液通过吸附柱,得到的穿透曲线如图7(c)所示。可以看出,当柱高和流量条件不变时,随着磷酸盐初始质量浓度降低,穿透点逐渐右移,穿透时间大幅增加,由145 min增长至1 112 min。由Thomas拟合结果可知,KTH随着初始质量浓度的升高而降低,q随着初始质量浓度的升高而增加。这是因为吸附柱中SBC-g的活性位点是有限的,当磷酸盐初始质量浓度增大时,传质驱动力增大[23],SBC-g在单位时间内吸附的磷酸盐增加,导致吸附柱在较短的时间内达到饱和。
4)养殖尾水的处理。在柱高为6.5 cm、流量为1.5 mL·min−1的条件下,将养殖尾水通过SBC-g吸附柱,得到的穿透曲线如图7(d)所示。由表1可知,养殖尾水的磷酸盐质量浓度为2.02 mg·L−1。从图7(d)可以看出,养殖尾水通过吸附柱的穿透时间为589 min,Thomas模型拟合得到的q为1 051 mg·kg−1。而在柱高和流量条件相同且初始质量浓度接近的情况下,磷酸盐溶液通过SBC-g吸附柱的穿透时间和q分别为1 112 min和1 619 mg·kg−1。这是由于实际养殖尾水中可能存在CO32−等共存离子和溶解性有机物,当有机物在吸附剂表面和水之间形成屏障时,易发生生物污染,从而不利于磷酸盐的吸附[24]。
-
生物炭是一种相对价廉易得的吸附材料。2021年,国内生物炭的生产价格约为2.37 元·kg−1[25]。用海水代替Mg对生物炭施以改性,可省去从海水到Mg化学试剂生产环节中所投入的人力和物力等,从而大幅降低生物炭的Mg改性成本。当生物炭产地为沿海地区时,海水的成本可忽略不计。按照本研究的方案,改性过程中NaOH(市场价为3.00 元·kg−1)的消耗量约为0.51 kg·kg−1,SBC-g的产率高达214.84%。由此计算得出,SBC-g的生产成本约为2.65 元·kg−1。可见,改性过程几乎不增加生物炭的制备成本。经比较,SBC-g的生产成本低于海泡石改性生物炭(4.60 元·kg−1)[26]、氢氧化钾改性蟹壳(3.80 元·kg−1)[24]和层状双氢氧化物改性高岭土(8.90~9.10 元·kg−1)[27]等除磷吸附剂以及市面上的商用除磷吸附剂(5.40~33.80元·kg−1)[24]。
-
1)以海水为Mg源对生物炭颗粒实施改性可在其表面负载Mg(OH)2纳米片增加活性位点,也可增大生物炭颗粒的介孔孔径和孔容、改变其表面荷电性,从而显著提升其对磷酸盐的吸附容量;吸附机理主要包括静电吸引、配体交换和内层络合作用。
2)柱高的增加、流量和初始质量浓度的降低均可延长穿透时间,有利于磷酸盐的去除;Thomas模型对穿透曲线拟合良好(R2 > 0.919),可用于预测穿透时间及吸附量。
3) SBC-g可有效去除养殖尾水中的磷酸盐,在最佳条件下吸附柱的穿透时间为589 min,饱和吸附量为1 051 mg·kg−1。
4) SBC-g是一种低成本吸附剂,生产成本约为2.65 元·kg−1,改性过程几乎不增加其制备成本;与其他除磷吸附剂相比,其价格优势突出。
低成本海水改性生物炭颗粒的制备及其对水中磷的动态吸附性能
Fabrication of low-cost seawater-modified biochar granule and its dynamic adsorption performance for phosphate in aqueous solution
-
摘要: 针对生物炭除磷领域中缺乏兼具经济性和实用性的Mg改性生物炭的问题,以海水为廉价Mg源,制备了海水改性生物炭颗粒(SBC-g),探究了其物理化学特性和吸附磷酸盐机理,考察了柱高、流量和初始质量浓度对SBC-g动态吸附磷酸盐的影响及对含磷养殖尾水的处理效果,并对SBC-g进行了经济性分析。结果表明,改性后SBC-g表面负载的Mg(OH)2纳米片可增加吸附的活性位点,增大了介孔的孔径和孔容,改变了表面电荷性质,从而提高了其对磷酸盐的吸附容量。在一定范围内,柱高的增加或流量和初始质量浓度的降低均可延长穿透时间。Thomas模型对穿透曲线拟合良好(R2 > 0.919),可以较为准确地反映动态吸附过程。SBC-g吸附柱对养殖尾水具有良好的除磷效果,在最佳条件下吸附柱的穿透时间为589 min,磷饱和吸附量为1 051 mg·kg−1。SBC-g的生产成本约为2.65 元·kg−1,和其他除磷吸附剂相比具有较大的价格优势,兼具经济性和实用性。该研究结果可为Mg改性生物炭的制备及其在水体磷污染治理领域的实际应用提供参考。Abstract: There is a lack of low-cost and easy-operation Mg-modified biochar in the field of phosphorus removal by biochar, and thus seawater-modified biochar granule (SBC-g) was prepared using seawater as a cheap Mg source. The physicochemical properties of SBC-g and mechanism of phosphate adsorption were investigated. The effects of column height, flow rate and initial concentration on the dynamic adsorption of phosphate by SBC-g, as well as the removal performance of phosphorus from aquaculture tail water, were studied. Finally, the economic analysis of SBC-g was carried out. The results showed that the Mg(OH)2 nanosheets loading on the surface of SBC-g after modification increased the active sites, enlarged the mesopore size and volume, and changed the surface charge properties, thereby increasing the adsorption capacity for phosphate. Within a certain range, the increase of column height or the decrease of flow rate and initial concentration could prolong the breakthrough time. The Thomas model fitted well with the breakthrough curve (R2 > 0.919), which could accurately reflect the dynamic adsorption process. The SBC-g-loaded adsorption column exhibited a good performance on phosphorus removal from the aquaculture tail water. Under the optimal conditions, the breakthrough time was 589 min, and the saturated adsorption capacity of phosphorus reached 1 051 mg·kg−1. The production cost of SBC-g was about 2.65 ¥·kg−1, which had a great price advantage against other phosphorus removal adsorbents, and thus SBC-g could be regarded as a low-cost and easy-operation adsorbent. The results of this study can provide a reference for the preparation of low-cost and easy-operation Mg-modified biochar and its practical application in the field of phosphorus pollution control for waterbodies.
-
Key words:
- biochar /
- seawater /
- Mg-modified /
- phosphate adsorption /
- adsorption column
-
随着人类社会快速发展,环境与资源问题日益凸显。目前普遍认为,人为因素造成的磷污染是水体富营养化的主要成因之一。水体富营养化将引起藻类快速繁殖,降低水体溶解氧,最终导致水生动植物死亡、生态系统失衡[1]。《2021年中国生态环境状况公报》显示,我国110个重要湖泊中,富营养状态湖泊占比高达27.3%[2]。湖泊富营养化已然成为我国亟待解决的重大环境问题之一。治理水体磷污染迫在眉睫。
近年来,国内外学者就水体磷污染控制开展了诸多研究,所采取的方法包括吸附法、化学沉淀法以及生物法等。其中,吸附法因其操作简单、处理迅速和效果稳定等优势受到关注。生物炭是近些年新兴的吸附材料,其由生物质热解碳化而得,具有较大的比表面积、发达的孔隙结构、丰富的官能团、强阳离子交换能力和低廉的价格等优势[3]。用农林废弃物等废弃生物质制备生物炭并用于去除废水中的磷,是当前的研究热点,具有固废资源化、固碳减排及磷资源回收等显著环境效益的优点。然而,大部分生物炭表面带负电,对磷酸盐的吸附效果不佳[4]。因此,需对生物炭实施改性,通过改变表面荷电性、增加吸附位点等方式提升其吸附磷的能力。常见的改性方法是在生物炭表面负载Ca[5]、Mg[6]、Ce[7]、Al[8]和La[9]等金属。在这些金属中,Mg具有在地壳中丰度高、对磷酸盐亲和力强的特点,非常适合用于生物炭改性[10]。在这方面,国内外已有较多相关研究。例如,研究者利用花生壳[11]、烟草秸秆[12]、槐木屑[13]、芦苇[14]和桉木茎片[15]等废弃生物质制备生物炭,然后用高浓度的Mg化学试剂对生物炭施以改性,并将改性生物炭用于废水除磷。这些研究表明,Mg改性生物炭对废水中磷的去除效果较好,具有一定实际工程应用的潜力。
虽然Mg改性生物炭除磷的研究取得了一定进展,但现有研究仍存在以下不足:以高浓度Mg化学试剂为改性剂增加了Mg改性生物炭的制备成本;Mg改性生物炭多呈粉末状,大量投加时易扬尘,且存在可操作性差、固液分离难的问题;多数研究集中于静态吸附实验,Mg改性生物炭的动态吸附研究相对较少[16-17]。可见,缺乏兼具经济性和实用性的Mg改性生物炭依然是该领域的瓶颈问题。开发低成本、具备一定尺寸且易于实际应用的Mg改性生物炭,并开展其在吸附柱中的动态吸附研究势在必行,是应对上述问题的有效策略之一。在前期研究中,ZHANG等[18]利用天然海水代替高浓度Mg化学试剂对生物炭粉末进行改性,发现不但改性效果好(改性后生物炭吸附容量提升了127倍),且改性过程几乎不增加生物炭的制造成本。这为制备兼具经济性和实用性的Mg改性生物炭提供了重要参考。据悉,目前尚无以海水为Mg源制备兼具经济性和实用性的Mg改性生物炭的研究报道。因此,本研究以天然海水为廉价Mg源、以生物炭颗粒(biochar granule,BC-g)为基材,制备低成本海水改性生物炭颗粒(seawater-modified biochar granule,SBC-g);利用表征技术考察SBC-g的物理化学性质及其吸附磷酸盐机理;在吸附柱中拟开展动态吸附实验,考察初始质量浓度、柱高、流量等因素对除磷效果的影响;并进行SBC-g制备成本的经济分析。本研究结果有望突破Mg改性生物炭除磷领域中存在的瓶颈问题,能够为兼具经济性和实用性Mg改性生物炭的制备及其在水体磷污染治理领域的实际应用提供参考。
1. 材料与方法
1.1 实验材料
收集福建省福州市周边的废弃杉木屑,清洗、干燥后用作制备生物炭的原料。海水采集自三沙湾海域,养殖尾水取自福州市某鳗鱼养殖场,二者的水质参数见表1。使用前,海水及尾水水样均经过0.45 μm滤膜过滤。分析纯磷酸二氢钾(KH2PO4)、氢氧化钠(NaOH)等化学试剂,购得即用,无进一步纯化。所有溶液均用去离子水配制。
表 1 海水和养殖尾水水质参数Table 1. Water quality parameters of seawater and aquaculture tail water海水 pH PO43−/(mg·L−1) K+/(mg·L−1) Na+/(mg·L−1) Ca2+/(mg·L−1) Mg2+/(mg·L−1) 8.4 0.05 349.2 8 561.00 353.7 1 027.83 养殖尾水 pH 硝氮/(mg·L−1) 总氮/(mg·L−1) 活性磷/(mg·L−1) 总磷/(mg·L−1) 总有机碳/(mg·L−1) 7.05 5.35 6.72 2.02 2.15 6.47 1.2 材料制备
1)生物炭颗粒(BC-g)的制备。将杉木屑放入坩埚,加盖后用锡箔纸包裹,放置于马弗炉(SXL-1008T,上海精宏实验设备有限公司)中,在限氧条件下以10 ℃·min−1的速率升温至600 ℃后,保持2 h。热解过程不使用额外惰性气体而以自身产生的热解气为热解保护气氛。冷却至室温后,取出用去离子水洗涤3次,在干燥箱中于80 ℃烘干过夜,取出研磨,筛选出大于50目的颗粒,即为BC-g。
2)海水改性生物炭颗粒(SBC-g)的制备。SBC-g的制备方法参考前期研究[18]的方法,具体步骤如下:首先将BC-g与海水以2 g·L−1的比例于烧杯中混合,在磁力搅拌器(LC-MAG-HS18,上海力辰邦西仪器科技有限公司)上搅拌1 h后,逐滴滴入1 mol·L−1NaOH,调节pH至10.50;再搅拌1 h后,将烧杯放入恒温水浴锅(HW2-26,上海一恒科学仪器有限公司),40 ℃下陈化过夜;然后取出抽滤,将固体置于真空干燥箱(DZF-6020MBE,上海博迅医疗生物仪器股份有限公司)中,于80 ℃真空干燥。待冷却至室温后,筛选出10~40目的颗粒,即为SBC-g。
1.3 材料表征
用配备有能谱仪的扫描电镜(SEM,JMS-7500F,日本电子株式会社)观察SBC-g的形貌及表面元素分布;通过X射线衍射光谱(XRD,D8 Advance,德国Bruker公司)分析SBC-g的晶体成分;用X射线光电子能谱(XPS,250xi,美国Thermo公司)分析生物炭的元素组成;生物炭的比表面积通过N2吸附脱附系统(AutosobiQA3200-4,美国康塔仪器公司)测试并用Brunauer–Emmett–Teller(BET)方法计算;ζ电位用ζ电位仪(Nanoplus-AT,麦克默瑞提克仪器有限公司)测定。溶液中的磷酸盐浓度采用钼酸铵分光光度法(GB11893-89)测定。金属离子采用电感耦合等离子体-原子发射光谱(ICP-OES,Avio 200,珀金埃尔默仪器有限公司)。
1.4 柱实验
1)柱实验方法。吸附柱为直径1 cm,高度10 cm的PMMA圆柱体,为均匀分配流量和防止SBC-g流失,在两端添加脱脂棉球。通过填充1.3、2.0、2.7 g的SBC-g以获得3.5、5.0、6.5 cm的吸附柱高度,在磷酸盐初始质量浓度为2.00 mg·L−1、流量为1.5 mL·min−1的条件下考察柱高对磷吸附情况的影响。然后在初始质量浓度为2.00 mg·L−1、柱高为6.5 cm时,分别设定流量为1.5、3.0和6.0 mL·min−1,得到不同流量下磷酸盐的穿透曲线。设定柱高为6.5 cm、流量为1.5 mL·min−1,在磷酸盐初始质量浓度为2.00、5.00和10.00 mg·L−1条件下,研究吸附柱对磷酸盐的吸附情况。最后,在最佳的柱高和流量条件下,使养殖尾水通过SBC-g吸附柱。在实验过程中,每隔一定的时间收集出水溶液并测定磷酸盐浓度。
2)柱实验参数计算。穿透曲线的穿透点一般按照相关标准限值或按照初始质量浓度的5%或10%选取。本研究以《污水综合排放标准》(GB 8979-1996)的一级标准限值为穿透点,即当出水的磷酸盐质量浓度大于0.5 mg·L−1时视为吸附柱穿透,所用时间可由线性插值法求得,此时吸附柱对应的吸附量可由穿透曲线与初始质量浓度的直线所围成的积分面积(式(1))进行计算;相应的平均吸附量可由式(2)计算;流经吸附柱的磷酸盐总量可由式(3)计算;吸附柱对磷酸盐的去除率可由式(4)计算;空床停留时间可由式(5)计算。
qa=QA1000=Q1000∫t=tat=0(C0−Ct)dt (1) qae=1000qam (2) ωa=C0Qta1000 (3) r=qaωa×100% (4) tb=VbQ (5) 式中:qa为穿透时对应的吸附量,mg;Q为流量,mL·min−1;A为穿透曲线所围成的面积,mg·L·min−1;ta为穿透时间,min;C0、Ct分别为磷酸盐的初始质量浓度和t时刻的质量浓度,mg·L−1;qae为穿透时对应的平均吸附量,mg·kg−1;m为吸附柱中SBC-g的质量,g;ωa为穿透时流经吸附柱的磷酸盐总量,mg;r为吸附柱对磷酸盐的去除率,%;tb为空床停留时间,min;Vb为吸附柱床层体积,cm3。
3)穿透曲线的模型拟合。Thomas模型是以Langmuir方程为基础,用于估算吸附质的平衡吸附量和吸附速率常数的吸附动力学模型。通过对已有数据的拟合,可对吸附柱内的动态吸附行为和规律进行模拟和预测。研究采用Thomas模型(式(6))拟合不同柱高、初始质量浓度和流量的实验数据并对结果进行分析。
CtC0=11+exp(KTHqm/Q−KTHC0t) (6) 式中:KTH为吸附速率常数,L·(min·mg)−1;q为饱和吸附量,mg·kg−1;t为吸附时间,min。
2. 结果与讨论
2.1 海水改性生物炭颗粒(SBC-g)的表征
本研究以天然海水为改性剂,利用共沉淀法对BC-g实施改性。首先,BC-g和SBC-g的XPS表征结果如图1所示。由图1可以看出,和BC-g相比,在SBC-g表面O元素含量显著提高,并且谱图中出现Mg和Ca元素(前者含量高于后者)。由XRD图谱(图2)可以看出,相较于BC-g,SBC-g的峰形发生显著变化,且出现Mg(OH)2(PDF#44-1482)和CaCO3(PDF#41-1475)的特征峰。这表明改性后海水中Mg和Ca分别以Mg(OH)2和CaCO3形式负载到BC-g表面。此外,通过SEM照片(图3)可以看出,改性后新生成的物质将生物炭颗粒包裹,并堵塞了部分孔结构;由Mapping图(图4)可以看出,将生物炭颗粒包裹的物质主要元素为Mg和O。结合XRD的结果可推断,该物质可能为Mg(OH)2。此外,在SBC-g的孔隙外表面上还可观察到互相堆叠的纳米片(图3)。根据前期研究[18]结果表明,该纳米片为Mg(OH)2。正是由于Mg(OH)2的包裹及堵塞,使得SBC-g的比表面积(55.24 m2·g−1)小于BC-g的比表面积(350.15 m2·g−1)。从图5可以看出,SBC-g的氮气吸脱附曲线呈Ⅳ型;在相对压力达到1.0时,吸附尚未饱和,在相对压力接近0.8时,出现H3型回滞环。这说明材料含有大孔和狭缝形介孔[19]。由图3的表征结果推测,改性后的生物炭保留了原始生物炭丰富的大孔结构,而纳米片状的Mg(OH)2在其表面可堆叠形成狭缝形介孔,使得介孔数量增多。有研究[20]表明,磷酸盐吸附大多取决于材料的微孔和介孔,大孔中几乎不发生吸附。由孔径分布图(图6)可以看出,SBC-g的介孔孔径主要分布于3.32~24.47 nm。从表2得知,原始生物炭介孔的平均孔径仅为3.28 nm,经改性后增大到16.57 nm;相应地,介孔孔容从0.03 cm3·g−1增加到0.23 cm3·g−1。另外,BC-g和SBC-g的ζ电位值分别为−9.81 mV和4.32 mV,说明Mg(OH)2及CaCO3的负载改变了生物炭表面的带电状态,使生物炭从带负电变为带正电,这有利于吸附水中的磷酸盐。
表 2 改性前后生物炭比表面积、介孔孔径及孔容Table 2. Specific surface area, mesopore size and volume of biochar before and after modification生物炭 比表面积/(m2·g−1) 平均孔径/nm 总孔容/(cm3·g−1) BC-g 350.15 3.28 0.03 SBC-g 55.24 16.57 0.23 2.2 海水改性生物炭颗粒(SBC-g)对水中磷酸盐的吸附机理
由图2可以看出,与SBC-g相比,在SBC-g-P的XRD谱图中除了Mg(OH)2和CaCO3的峰之外,还出现了Mg(H2PO4)2·4H2O (PDF#87-0536)、Mg2(PO4)(OH)·3H2O(PDF#45-1380)和Ca3(PO4)2(PDF#70-0364)的特征衍射峰。其中,Mg(H2PO4)2·4H2O和Mg2PO4(OH)·3H2O的峰明显强于Ca3(PO4)2,这表明SBC-g上的Mg是吸附水中磷酸盐的主要活性位点。经对吸附磷酸盐后SBC-g的ζ电位(pH = 6)进行测定,发现吸附磷酸盐后SBC-g的ζ电位从4.32 mV降至−17.20 mV,这说明静电吸引在SBC-g吸附磷酸盐过程中起到一定作用。SBC-g对水中磷酸盐的吸附过程可能为:SBC-g首先通过静电作用吸附磷酸盐,磷酸盐再与SBC-g上的Mg(OH)2或CaCO3发生配体交换[21]、取代OH−或CO32−,最后与Mg或Ca通过内层络合作用结合生成磷酸镁或磷酸钙[22],其中Mg是主要活性位点。和原始生物炭相比,在相同条件下,海水改性可使生物炭吸附容量由0.91 mg·g−1升至25.21 mg·g−1。终上所述,改性后生物炭的吸附容量提升的原因可归结为:表面附着的Mg(OH)2可增加活性位点;介孔孔径及孔容的增大有助于磷酸盐分子的传输与吸附;表面荷电性的改变也有利于磷酸盐的吸附。
2.3 SBC-g对磷的动态吸附过程分析
合适尺寸的颗粒状吸附剂可应用于吸附柱工艺中,因此与粉末状吸附剂相比具有操作简单和管理方便的优点。首先,将SBC-g填充于吸附柱中,研究不同的柱高、流量和初始质量浓度条件对磷酸盐吸附的影响,结果如图7所示。然后,探究SBC-g吸附柱对于含磷养殖废水的处理效果。在实验浓度范围内,吸附柱对磷酸盐具有良好的吸附效果,在吸附柱操作初期,出水磷酸盐浓度几乎为0。如表3所示,在不同操作条件下,吸附柱的穿透时间在141~1 112 min内,对磷酸盐的去除率均达到75%以上。使用Thomas模型对穿透曲线实验数据进行进一步拟合分析,其结果列于表3。由表3可看出,在柱高为6.5 cm、流量为1.5 mL·min−1和磷酸盐初始质量浓度为2.00 mg·L−1的条件下,由该模型拟合得到的吸附柱的饱和吸附量为1 619 mg·kg−1。处理实际养殖尾水时,吸附柱的穿透时间为589 min,饱和吸附量为1 051 mg·kg−1。Thomas模型对各条件下的穿透曲线均拟合良好。说明该模型能够较为准确地描述SBC-g吸附柱的动态吸附过程,可以用来预测动态吸附量和穿透时间。
表 3 不同条件下吸附柱参数及Thomas模型的拟合参数Table 3. Adsorption column parameters and fitting parameters of Thomas model at different conditionsC0/(mg·L−1) Q/(mL·min−1) H/cm m/g ta/min tb/min qae/(mg·kg−1) r/% KTH/(L·(min·mg)−1) q/(mg·kg−1) R2 2.00 1.5 3.5 1.3 141 1.83 244 75.00 3.60×10−3 531 0.922 2.00 1.5 5.0 2.0 570 2.62 641 75.00 1.86×10−3 676 0.919 2.00 1.5 6.5 2.7 1 112 3.40 927 75.00 1.68×10−3 1 619 0.981 2.00 3.0 6.5 2.7 393 1.70 654 75.00 2.81×10−3 1 396 0.963 2.00 6.0 6.5 2.7 144 0.85 478 75.00 4.97×10−3 1 201 0.949 5.00 1.5 6.5 2.7 377 3.40 943 90.00 1.70×10−3 1 479 0.967 10.00 1.5 6.5 2.7 145 3.40 765 95.00 0.76×10−3 1 683 0.940 2.02(养殖尾水) 1.5 6.5 2.7 589 3.40 497 75.25 1.82×10−3 1 051 0.955 1)柱高对吸附过程的影响。图7(a)为不同柱高条件下,吸附柱吸附磷的穿透曲线。由图7(a)可知,当柱高分别为3.0、5.0和6.5 cm时,穿透时间分别为141、570和1 112 min,相应的动态吸附量分别为244、641和927 mg·kg−1。由此可见,随着柱高的提升,相应的吸附活性位点增多,停留时间延长,使得磷酸盐与活性位点接触更充分。由Thomas模型拟合结果也可以看出,q随着柱高增加而增大,说明柱高的增大可提升吸附柱的吸附容量。然而,需要指出的是,吸附柱的高度并非越高越好,需要综合考虑运行成本、实际场地情况等因素,确定最终的柱高。
2)流量对吸附过程的影响。流量的变化对SBC-g吸附磷酸盐也有显著影响,如图7(b)所示。在流量为6.0 mL·min−1时,吸附柱在较短的时间内(144 min)即穿透。这是由于在较大的流量下,tb缩短,SBC-g吸附柱与进水中的磷酸盐接触时间有限。随着流量降至1.5 mL·min−1,SBC-g与进水中的磷酸盐的接触时间相应地增长,穿透时间延长至1 112 min,相应的动态吸附量也增加至927 mg·kg−1。由表3可以看出,随着流量降低,Thomas模型拟合得到的KTH减小,q增大。由此可见,适当地降低流量有助于磷酸盐与吸附柱内的SBC-g的活性位点充分接触,从而达到更好的吸附效果。
3)初始质量浓度对吸附过程的影响。在柱高为6.5 cm、流量为1.5 mL·min−1的条件下,将初始质量浓度分别为2.00、5.00和10.00 mg·L−1的磷酸盐溶液通过吸附柱,得到的穿透曲线如图7(c)所示。可以看出,当柱高和流量条件不变时,随着磷酸盐初始质量浓度降低,穿透点逐渐右移,穿透时间大幅增加,由145 min增长至1 112 min。由Thomas拟合结果可知,KTH随着初始质量浓度的升高而降低,q随着初始质量浓度的升高而增加。这是因为吸附柱中SBC-g的活性位点是有限的,当磷酸盐初始质量浓度增大时,传质驱动力增大[23],SBC-g在单位时间内吸附的磷酸盐增加,导致吸附柱在较短的时间内达到饱和。
4)养殖尾水的处理。在柱高为6.5 cm、流量为1.5 mL·min−1的条件下,将养殖尾水通过SBC-g吸附柱,得到的穿透曲线如图7(d)所示。由表1可知,养殖尾水的磷酸盐质量浓度为2.02 mg·L−1。从图7(d)可以看出,养殖尾水通过吸附柱的穿透时间为589 min,Thomas模型拟合得到的q为1 051 mg·kg−1。而在柱高和流量条件相同且初始质量浓度接近的情况下,磷酸盐溶液通过SBC-g吸附柱的穿透时间和q分别为1 112 min和1 619 mg·kg−1。这是由于实际养殖尾水中可能存在CO32−等共存离子和溶解性有机物,当有机物在吸附剂表面和水之间形成屏障时,易发生生物污染,从而不利于磷酸盐的吸附[24]。
2.4 海水改性生物炭颗粒(SBC-g)的经济分析
生物炭是一种相对价廉易得的吸附材料。2021年,国内生物炭的生产价格约为2.37 元·kg−1[25]。用海水代替Mg对生物炭施以改性,可省去从海水到Mg化学试剂生产环节中所投入的人力和物力等,从而大幅降低生物炭的Mg改性成本。当生物炭产地为沿海地区时,海水的成本可忽略不计。按照本研究的方案,改性过程中NaOH(市场价为3.00 元·kg−1)的消耗量约为0.51 kg·kg−1,SBC-g的产率高达214.84%。由此计算得出,SBC-g的生产成本约为2.65 元·kg−1。可见,改性过程几乎不增加生物炭的制备成本。经比较,SBC-g的生产成本低于海泡石改性生物炭(4.60 元·kg−1)[26]、氢氧化钾改性蟹壳(3.80 元·kg−1)[24]和层状双氢氧化物改性高岭土(8.90~9.10 元·kg−1)[27]等除磷吸附剂以及市面上的商用除磷吸附剂(5.40~33.80元·kg−1)[24]。
3. 结论
1)以海水为Mg源对生物炭颗粒实施改性可在其表面负载Mg(OH)2纳米片增加活性位点,也可增大生物炭颗粒的介孔孔径和孔容、改变其表面荷电性,从而显著提升其对磷酸盐的吸附容量;吸附机理主要包括静电吸引、配体交换和内层络合作用。
2)柱高的增加、流量和初始质量浓度的降低均可延长穿透时间,有利于磷酸盐的去除;Thomas模型对穿透曲线拟合良好(R2 > 0.919),可用于预测穿透时间及吸附量。
3) SBC-g可有效去除养殖尾水中的磷酸盐,在最佳条件下吸附柱的穿透时间为589 min,饱和吸附量为1 051 mg·kg−1。
4) SBC-g是一种低成本吸附剂,生产成本约为2.65 元·kg−1,改性过程几乎不增加其制备成本;与其他除磷吸附剂相比,其价格优势突出。
-
表 1 海水和养殖尾水水质参数
Table 1. Water quality parameters of seawater and aquaculture tail water
海水 pH PO43−/(mg·L−1) K+/(mg·L−1) Na+/(mg·L−1) Ca2+/(mg·L−1) Mg2+/(mg·L−1) 8.4 0.05 349.2 8 561.00 353.7 1 027.83 养殖尾水 pH 硝氮/(mg·L−1) 总氮/(mg·L−1) 活性磷/(mg·L−1) 总磷/(mg·L−1) 总有机碳/(mg·L−1) 7.05 5.35 6.72 2.02 2.15 6.47 表 2 改性前后生物炭比表面积、介孔孔径及孔容
Table 2. Specific surface area, mesopore size and volume of biochar before and after modification
生物炭 比表面积/(m2·g−1) 平均孔径/nm 总孔容/(cm3·g−1) BC-g 350.15 3.28 0.03 SBC-g 55.24 16.57 0.23 表 3 不同条件下吸附柱参数及Thomas模型的拟合参数
Table 3. Adsorption column parameters and fitting parameters of Thomas model at different conditions
C0/(mg·L−1) Q/(mL·min−1) H/cm m/g ta/min tb/min qae/(mg·kg−1) r/% KTH/(L·(min·mg)−1) q/(mg·kg−1) R2 2.00 1.5 3.5 1.3 141 1.83 244 75.00 3.60×10−3 531 0.922 2.00 1.5 5.0 2.0 570 2.62 641 75.00 1.86×10−3 676 0.919 2.00 1.5 6.5 2.7 1 112 3.40 927 75.00 1.68×10−3 1 619 0.981 2.00 3.0 6.5 2.7 393 1.70 654 75.00 2.81×10−3 1 396 0.963 2.00 6.0 6.5 2.7 144 0.85 478 75.00 4.97×10−3 1 201 0.949 5.00 1.5 6.5 2.7 377 3.40 943 90.00 1.70×10−3 1 479 0.967 10.00 1.5 6.5 2.7 145 3.40 765 95.00 0.76×10−3 1 683 0.940 2.02(养殖尾水) 1.5 6.5 2.7 589 3.40 497 75.25 1.82×10−3 1 051 0.955 -
[1] YIN Q Q, REN H P, WANG R K, et al. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content[J]. Science of the Total Environment, 2018, 631-632: 895-903. [2] 2021年中国生态环境状况公报(摘录)[J]. 环境保护, 2022, 50(12): 61-74. [3] SUN D Q, HALE L, KAR G, et al. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment[J]. Chemosphere, 2018, 194: 682-691. [4] TAN Z X, YUAN S N, HONG M F, et al. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd[J]. Journal of Hazardous Materials, 2020, 384: 121370. [5] FENG Y Y, LUO Y, HE Q P, et al. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water[J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105267. [6] QI X, YIN H, ZHU M H, et al. MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil[J]. Chemosphere, 2022, 294: 133733. [7] 王光泽, 曾薇, 李帅帅. 铈改性水葫芦生物炭对磷酸盐的吸附特性[J]. 环境科学, 2021, 42(10): 4815-4825. [8] NOVAIS S V, ZENERO M D O, BARRETO M S C, et al. Phosphorus removal from eutrophic water using modified biochar[J]. Science of the Total Environment, 2018, 633: 825-835. [9] JIA Z Y, ZENG W, XU H H, et al. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar[J]. Process Safety and Environmental Protection, 2020, 140: 221-232. [10] WU B L, WAN J, ZHANG Y Y, et al. Selective phosphate removal from water and wastewater using sorption: Process fundamentals and removal mechanisms[J]. Environmental Science & Technology, 2020, 54(1): 50-66. [11] LIU X Q, ZHOU W, FENG L, et al. Characteristics and mechanisms of phosphorous adsorption by peanut shell-derived biochar modified with magnesium chloride by ultrasonic-assisted impregnation[J]. ACS Omega, 2022, 7(47): 43102-43110. [12] HE Q P, ZHANG K Q, LUO Y L, et al. Magnetic biochar particles prepared by ion cross-linking to remove phosphate from water[J]. Materials Research Express, 2021, 8(7): 76102. [13] XU K N, LIN F Y, DOU X M, et al. Recovery of ammonium and phosphate from urine as value-added fertilizer using wood waste biochar loaded with magnesium oxides[J]. Journal of Cleaner Production, 2018, 187: 205-214. [14] 杭嘉祥, 李法云, 梁晶, 等. 镁改性芦苇生物炭对水环境中磷酸盐的吸附特性[J]. 生态环境学报, 2020, 29(6): 1235-1244. [15] ARBELAEZ B L, MAHDI Z, PRATT C, et al. Modification of hardwood derived biochar to improve phosphorus adsorption[J]. Environments, 2021, 8(5): 41. [16] CUI X Q, DAI X, KHAN K Y, et al. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata[J]. Bioresource Technology, 2016, 218: 1123-1132. [17] JENA J, DAS T, SARKAR U. Explicating proficiency of waste biomass-derived biochar for reclaiming phosphate from source-separated urine and its application as a phosphate biofertilizer[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104648. [18] ZHANG M D, HE M Z, CHEN Q P, et al. Feasible synthesis of a novel and low-cost seawater-modified biochar and its potential application in phosphate removal/recovery from wastewater[J]. Science of the Total Environment, 2022, 824: 153833. [19] BAG S, TRIKALITIS P N, CHUPAS P J, et al. Porous semiconducting gels and aerogels from chalcogenide clusters[J]. Science, 2007, 317: 490-493. [20] SURESH K P, KORVING L, KEESMAN K J, et al. Effect of pore size distribution and particle size of porous metal oxides on phosphate adsorption capacity and kinetics[J]. Chemical Engineering Journal, 2019, 358: 160-169. [21] DAI Y J, WANG W S, LU L, et al. Utilization of biochar for the removal of nitrogen and phosphorus[J]. Journal of Cleaner Production, 2020, 257: 120573. [22] ZHENG Q, YANG L F, SONG D L, et al. High adsorption capacity of Mg-Al-modified biochar for phosphate and its potential for phosphate interception in soil[J]. Chemosphere, 2020, 259: 127469. [23] 胡奇, 李玉立, 潘红玉, 等. 改性木屑对水中苯胺的动态吸附[J]. 环境工程学报, 2016, 10(9): 4663-4667. [24] PAP S, KIRK C, BREMNER B, et al. Low-cost chitosan-calcite adsorbent development for potential phosphate removal and recovery from wastewater effluent[J]. Water Research, 2020, 173: 115573. [25] LI J, CAO L, LI B, et al. Utilization of activated sludge and shell wastes for the preparation of Ca-loaded biochar for phosphate removal and recovery[J]. Journal of Cleaner Production, 2023, 382: 135395. [26] DENG W D, ZHANG D Q, ZHENG X X, et al. Adsorption recovery of phosphate from waste streams by Ca/Mg-biochar synthesis from marble waste, calcium-rich sepiolite and bagasse[J]. Journal of Cleaner Production, 2021, 288: 125638. [27] 干方群, 徐子昊, 杨一帆, 等. 高岭土对畜禽废水中磷的净化效果及其费效分析[J]. 生态与农村环境学报, 2019, 35(6): 795-800. 期刊类型引用(2)
1. 杨玉祥,宋可心,杨光,马晓娜,解思琦,刘子烁,冯志华. 高岭土-牡蛎壳粉改性材料的制备及其对海水、淡水中氨氮与磷酸盐的去除效果. 大连海洋大学学报. 2024(04): 634-647 .  百度学术
百度学术
2. 李小林,王毅力,刘晨阳,任立威. 镧负载纤维素水凝胶多级串联吸附柱回收二沉池出水中磷的性能及机制研究. 环境科学学报. 2023(12): 317-326 .  百度学术
百度学术
其他类型引用(4)
-






 DownLoad:
DownLoad: