-
氧氟沙星(ofloxacin,OFX)因其良好的药物动力学特性、广谱抗菌性以及高效的杀菌效果被广泛应用于人畜疾病防治、畜牧及水产养殖等领域[1]。这类药物的半衰期长、化学性质稳定且在生物体内无法被完全地吸收和利用,将通过生物体代谢及废水排放等途径进入水环境中并且长期积累[2-3]。环境中的氧氟沙星将导致抗性微生物的出现,威胁生态平衡和人类健康[4]。因此,急需探索一种高效环保、经济简便的技术以解决此类水污染问题。
电芬顿技术凭借反应条件温和、操作简便、可控性强和环境友好等优点,在诸多难降解废水的处理技术中脱颖而出[5]。传统的电芬顿技术通过阴极原位产H2O2与Fe2+构成Fenton体系,产生·OH来降解污染物[6]。相比于传统的电芬顿技术,异相电芬顿技术反应效率高、不产生铁泥并且可重复利用,受到研究者的青睐。研究表明,在异相电芬顿系统中引入可见光能够有效提高其催化活性[7]。当异相催化剂的带隙能小于可见光的能量时,在可见光的照射下将产生大量光生电子和空穴,不仅能加速催化剂表面金属离子的氧化还原循环来提高催化效率,还可以直接活化H2O2产生高活性自由基,促进污染物的降解[8]。因此,构建异相光电Fenton体系在污染物的降解领域具有极大的发展潜力。
铜铁矿CuFeO2异相催化剂是近年来水处理领域研究的热点材料,其在地球上储量丰富、环境友好且化学性质稳定[9]。不仅如此,CuFeO2禁带宽度较为狭窄,常作为光催化剂用于可见光下制氢[10],还原水中重金属[11]和降解有机染料[12],是一种具有可见光响应的高效催化剂。然而,纯相CuFeO2本身较高的表面能导致其颗粒易团聚,光催化效率较低且对总有机碳(TOC)的去除效果也不理想[13-15]。为提高CuFeO2的光催化性能,研究者提出诸多不同的策略用于催化剂的改性,如控制催化剂的暴露晶面[16]、最小化自由基的迁移距离[9]和微波辅助[17]等。近年来,聚乙烯吡咯烷酮(polyvinylpyrrolidone,PVP)由于无毒、高稳定性、可生物降解性和空间位阻效应在催化剂改性方面被广泛应用。将PVP与CuFeO2复合,利用PVP可避免团聚、调节形貌和能带隙、促进载流子分离及产生丰富的氧空位等作用对CuFeO2进行改性有望显著提高其催化效率[18-20]。
本研究以PVP作为改性剂,通过简单的低温水热法成功合成了表面具有丰富氧空位的CuFeO2@PVP催化剂。通过多种表征测试了CuFeO2@PVP的形貌、微观结构、光学性能和光电性能等。以CuFeO2@PVP为催化剂、铂片为阳极、碳毡电极为阴极、可见光为光源以及以氧氟沙星(OFX)作为目标污染物,构建了异相光电-Fenton体系;研究了不同体系下OFX的降解率和反应速率;探讨了催化剂投加量、电流密度、pH、共存离子等限制性因素对氧氟沙星降解效果的影响;探究了CuFeO2@PVP催化剂在光电芬顿体系下降解OFX的循环稳定性;揭示了CuFeO2@PVP光电Fenton体系降解OFX的主要活性物质以及OFX的降解机理。
-
采用低温水热法合成了CuFeO2@PVP催化剂。称取4.0 g聚乙烯吡咯烷酮溶于30 mL去离子水中,60 ℃下磁力搅拌20 min至PVP溶解完全,冷却至室温备用。按照化学计量比(摩尔比为Cu:Fe=1:1)准确称取3.62 g Cu(NO3)2·3H2O和6.06 g Fe(NO3)3·9H2O置于烧杯中,加入30 mL去离子水,室温下磁力搅拌10 min至完全溶解,得到黄绿色盐溶液。加入0.5 g无水葡萄糖,继续磁力搅拌至完全溶解,记为A液。将A液倒入PVP溶液中,室温下磁力搅拌15 min得到B液。称取5 g NaOH溶于30 mL去离子水中,室温下磁力搅拌10 min至完全溶解,待其冷却至室温后缓慢加入到B液中,剧烈搅拌30 min得到溶解完全、混合均匀的C液。将C液缓慢倒入100 mL的内衬并装入不锈钢反应釜中,在180 ℃下保持24 h,冷却至室温后将产物倒入烧杯中。加入适量稀硝酸调节产物pH至中性,分别用去离子水和无水乙醇对黑色产物进行多次清洗和离心。清洗完全的产物在70 ℃下干燥1~2 h得到CuFeO2@PVP催化剂,并装袋备用。
-
1)催化剂的晶形结构、形貌、表面元素组成及化学状态的表征。通过40 kV和30 mA的X射线衍射光谱(XRD, D8 ADVANCE, German)对CuFeO2@PVP的晶形结构进行分析。采用美国Thermo Fisher Scientific公司的Nicolet IS10傅立叶变换红外 (Fourier Transform Infrared, FT-IR) 光谱仪对CuFeO2@PVP的官能团组成进行测定,扫描范围为4 000~500 cm−1。采用扫描电子显微镜(SEM, JEOL-7500F, Japan)和透射电子显微镜(TEM, Tecnai G2 f20 s-twin)对催化剂的形貌结构进行表征。采用X射线光电子能谱仪(XPS, ESCALAB 250, American)测定CuFeO2@PVP的表面元素组成及化学状态。采用电子自旋共振波谱仪(ESR,FA200)验证CuFeO2@PVP表面氧空位的形成。
2)催化剂的光学性能表征。采用紫外可见光谱仪(UV-Vis/DRS, Lambda 900, American)在300~800 nm内测定催化剂的紫外可见漫反射光谱,分析CuFeO2@PVP的光吸收性能和禁带宽度。以Mg Kα射线(1 253.6 eV)作为激发源,采用X射线光电子能谱仪(XPS, ESCALAB 250, American)测定CuFeO2@PVP的价带谱。通过荧光光谱仪(Hitachi F-4600和Edinburgh FL-920)测定催化剂的稳态和瞬时光致发光光谱,对催化剂的光生载流子分离和重组情况进行了分析。
3)催化剂的光电性能表征。利用上海辰华仪器有限公司的CHI660E型电化学工作站在三电极体系内对催化剂的光电性能进行测试。以Ag/AgCl为参比电极,铂片为对电极,涂有催化剂的导电ITO玻璃为工作电极,0.5 mol·L−1的Na2SO4溶液为电解液,300 W的氙灯为可见光源测定异相光电Fenton体系的电化学阻抗谱和瞬时光电流响应,分析CuFeO2@PVP的光电催化性能。
-
以300W的氙灯作为可见光光源,分别采用铂片为阳极、碳毡为阴极,在石英反应器中进行光电类芬顿体系降解OFX的实验。OFX初始浓度为10 mg·L−1,电解液为0.05 mol·L−1的Na2SO4溶液。称取0.04 g的CuFeO2@PVP加入100 mL OFX溶液中,在室温下磁力搅拌60 min,以达到CuFeO2@PVP催化剂和OFX之间的吸附-解吸平衡。而后接通电极电源和曝气装置电源、打开光源,在降解过程中持续磁力搅拌。在一定的时间间隔取样,并用0.45 µm的滤膜过滤,利用紫外可见分光光度计在293 nm下测定OFX的吸光度并计算其降解率。对反应后的溶液进行抽滤分离,使用酒精和去离子水多次清洗催化剂并烘干处理,得到反应后的CuFeO2@PVP。再次按照上述步骤反复 4 次,考察催化剂的重复性和稳定性。通过XRD谱图对反应前后催化剂的晶形结构进行表征。采用电子自旋共振波谱仪(ESR,FA200)检测不同降解体系中活性自由基的形成情况。
-
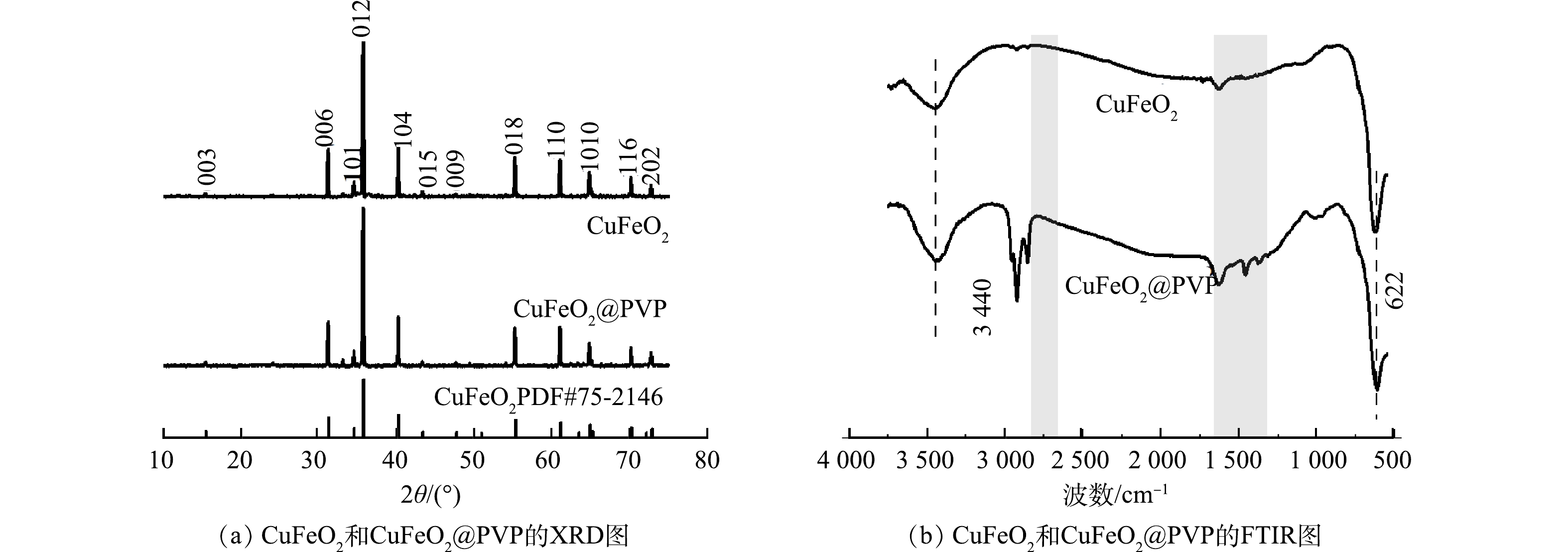
1)晶貌结构与形貌分析。采用XRD技术确定了纯相CuFeO2和CuFeO2@PVP复合催化剂的晶形结构。如图1(a)所示,2个样品均在2θ为15.5°、31.1°、35.6°、40.2°、43.3°、47.6°、50.8°、55.2°、61.0°、64.8°、65.1°、70.1°和72.7°处出现的特征峰,分别对应于(003)、(006)、(101)、(012)、(104)、(015)、(009)、(107)、(018)、(110)、(1010)、(116)和(202)晶面。与标准谱图PDF#75-2416标准谱图进行比对,发现所制催化剂在10~75°出现的特征峰与CuFeO2的峰型一一匹配,没有任何杂质峰的出现。这表明水热法成功合成了CuFeO2@PVP复合催化剂,且PVP的引入并未改变CuFeO2的晶形结构。由图1(b)可知,CuFeO2和CuFeO2@PVP在3 440 nm处的特征峰是—OH基团的拉伸振动引起的,622 nm处的特征峰是由于Cu—O的拉伸振动和O—Cu—O的不对称振动引起的。CuFeO2@PVP含有CuFeO2的所有特征峰,其在2 924 nm和1 632 nm的特征峰明显强于CuFeO2,分别归因于聚合物主链的C—H的拉伸振动和C=O官能团的拉伸振动[21],进一步证实了CuFeO2@PVP复合催化剂的形成。
通过SEM、TEM等手段获得了CuFeO2和CuFeO2@PVP的晶形结构和表面形貌。图2(a)和图2(c)反映了纯相CuFeO2呈不规则的斜方六面体结构。可见,CuFeO2粒径在1~3 µm,其表面光滑且不同粒度的颗粒之间产生比较严重的团聚作用,故以颗粒较大的团簇状形式存在。由图2(b)可见,CuFeO2@PVP复合催化剂粒径较小,表面粗糙、具有大量的孔隙结构,颗粒分布明显更为分散。由图2(d)可见,CuFeO2@PVP表面均匀的覆盖着非晶PVP涂层。证实PVP成功负载到CuFeO2上,并且起到抑制CuFeO2颗粒的生长、促进颗粒分散的作用,使催化剂具有更强的分散性、更大的比表面积和更多催化活性位点。
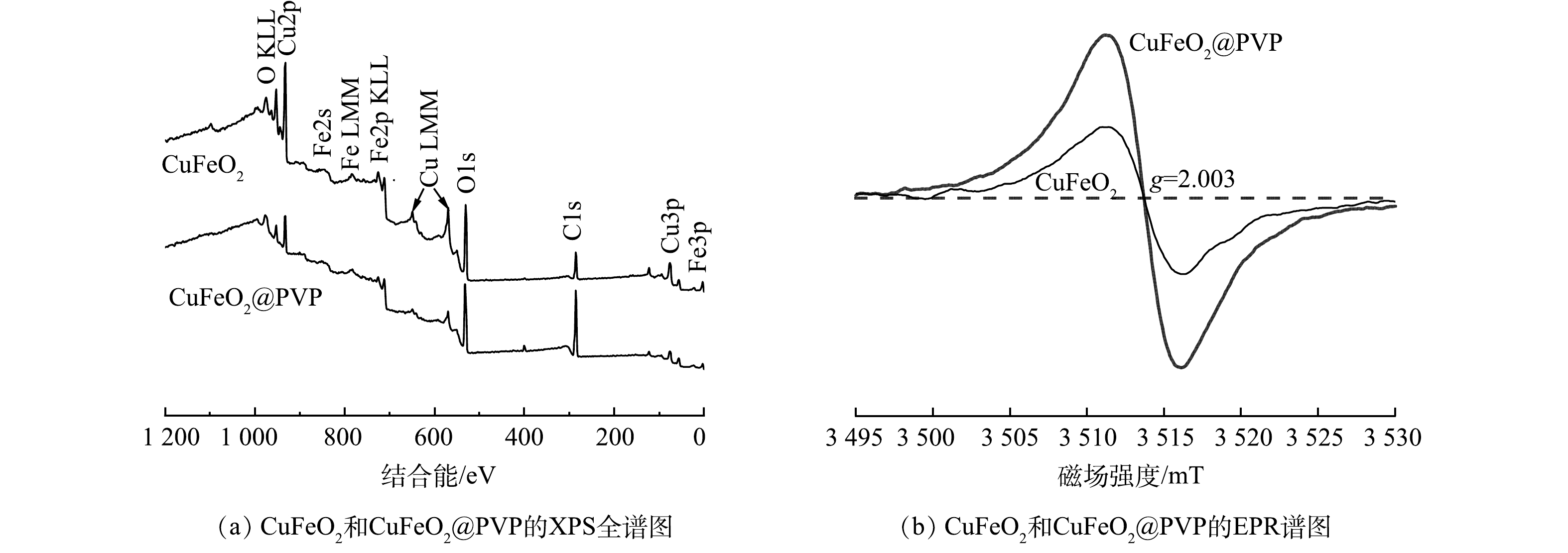
2)表面结构与性能分析。通过XPS技术分析了CuFeO2和CuFeO2@PVP的元素组成和表面价态。由图3(a)可见,CuFeO2和CuFeO2@PVP特征峰的位置和数量保持不变,均包含C、O、Cu、Fe 4种元素,但个别峰强度波动较大。C的特征峰明显有所增强,O、Cu、Fe对应的特征峰减弱,再次表明PVP的成功引入。根据电荷平衡规律,Cu和Fe的反应往往伴随着氧空位(OVs)的生成,于是进一步采用了EPR技术验证了CuFeO2和CuFeO2@PVP中氧空位的存在。如图3(b)所示,在g=2.003处检测到OVs的共振信号[22],而CuFeO2@PVP的信号强度明显大于CuFeO2。这表明PVP的引入使得催化剂表面成功生成了丰富的OVs。
利用高分辨XPS光谱研究了引入OVs前后CuFeO2表面的基本组成成分和化学状态的变化。由图4(a)可知,CuFeO2@PVP的Fe2p高分辨率光谱中结合能为709.8 eV和723.3 eV的峰属于Fe2+;结合能为711.8 eV和726 eV的峰属于Fe3+;718.9 eV和732.5 eV为卫星峰,均归属于Fe3+。由图4(b)可知,结合能为932.6 eV和952.6 eV的峰属于Cu+;结合能为936.1 eV和956.1 eV的峰属于Cu2+;940、943.5和963.1 eV为卫星峰,均属于Cu2+。值得注意的是,CuFeO2@PVP的Fe2p和Cu2p谱均向结合能更低的方向移动,Cu+的含量由83.6%增至88.7%,Fe2+含量由0增至15%。这归因于OVs在CuFeO2@PVP表面形成时留下电子,每生成1个氧空位有2个电子留下,电子将相邻金属离子还原至低氧化态以维持电荷平衡[23]。此外,由图4(c)可见,结合能为529.9、531.8和533.0 eV分别属于晶格氧(Olatt)、氧空位(OVs)和表面吸附氧(Oads)。相比于纯相CuFeO2,CuFeO2@PVP中OVs的相对含量由25.4%增至54.5%,进一步证实PVP有效的在CuFeO2表面引入大量的OVs。由图4(d)可知,结合能为288.6 eV和284.9 eV的峰分别属于C=O键和C—C键。而在CuFeO2@PVP的C1s谱中,除了C=O和C—C键的峰,在285.6 eV处出现1个新峰,可能归因于CuFeO2@PVP表面PVP涂层的C—N键。上述结果表明,PVP在催化剂表面形成均匀的涂层,从而促进CuFeO2表面大量OVs的生成,并将部分Fe3+和Cu2+位点还原为Fe2+和Cu+,进而有利于H2O2的活化。
3)光响应和能带结构分析。采用UV-Vis DRS吸收光谱研究了CuFeO2和CuFeO2@PVP对可见光的捕获能力。如图5(a)所示,2个样品在可见光区域均表现出一定的吸收能力。纯相CuFeO2的可见光吸收范围主要集中在420~465 nm,而在波长大于465 nm的范围内几乎不吸收。与纯相CuFeO2相比,CuFeO2@PVP的可见光吸收边缘发生了红移,在波长大于450 nm的可见光区吸收明显增强,表明CuFeO2@PVP对可见光的捕获能力显著增强。此外,基于UV-Vis DRS数据,根据Kubelka-Munk公式(式(1))估计了CuFeO2和CuFeO2@PVP的带隙值。由所得的(αhν)2~hν曲线可知,CuFeO2和CuFeO2@PVP的禁带宽度Eg分别为2.05 eV和1.81 eV。带隙变窄意味着激发电子从价带(VB)跃迁至导带(CB)所需的能量更少,从而增强光吸收。上述结果表明,PVP的引入可提高催化剂的光响应和缩小禁带宽度。另外,由XPS价带谱(图6(a))可知,CuFeO2和CuFeO2@PVP的价带位置分别是0.89 eV和1.41 eV。根据禁带宽度和价带位置进一步计算出CuFeO2和CuFeO2@PVP的导带位置分别是−1.16 eV和−0.67 eV,由此得到CuFeO2@PVP的能带结构图(图6(b))。
式中:α为吸光度;h为普朗克常量;ν为光的频率;Eg为带隙能量值;A为常数;n=1/2或2分别对应间接带隙型半导体和直接带隙型半导体。
4)荧光性能分析。利用PL光谱从电荷转移动力学角度评估了CuFeO2@PVP光生电子和空穴的分离效率。PL峰值强度与电子复合速率成正比,与电荷分离效率成反比,当PL光谱中吸收峰下降时表明催化材料显著抑制了电子空穴复合速率[24]。如图7(a)所示,纯相CuFeO2在320~500 nm有较强的吸收峰,表明在此范围内光生载流子复合严重。相比之下,CuFeO2@PVP的光致发光强度明显降低。这表明PVP的引入有效地抑制了光生电子和空穴的复合,增强了催化剂的光催化活性。进一步通过时间分辨荧光衰减曲线研究了CuFeO2和CuFeO2@PVP光生载流子的寿命。如图7(b)所示,CuFeO2@PVP表现出更高的平均载流子寿命。以上结果表明,PVP的引入可以促进光生载流子的分离和重组并提高载流子寿命,这有利于提高催化剂的光催化活性。
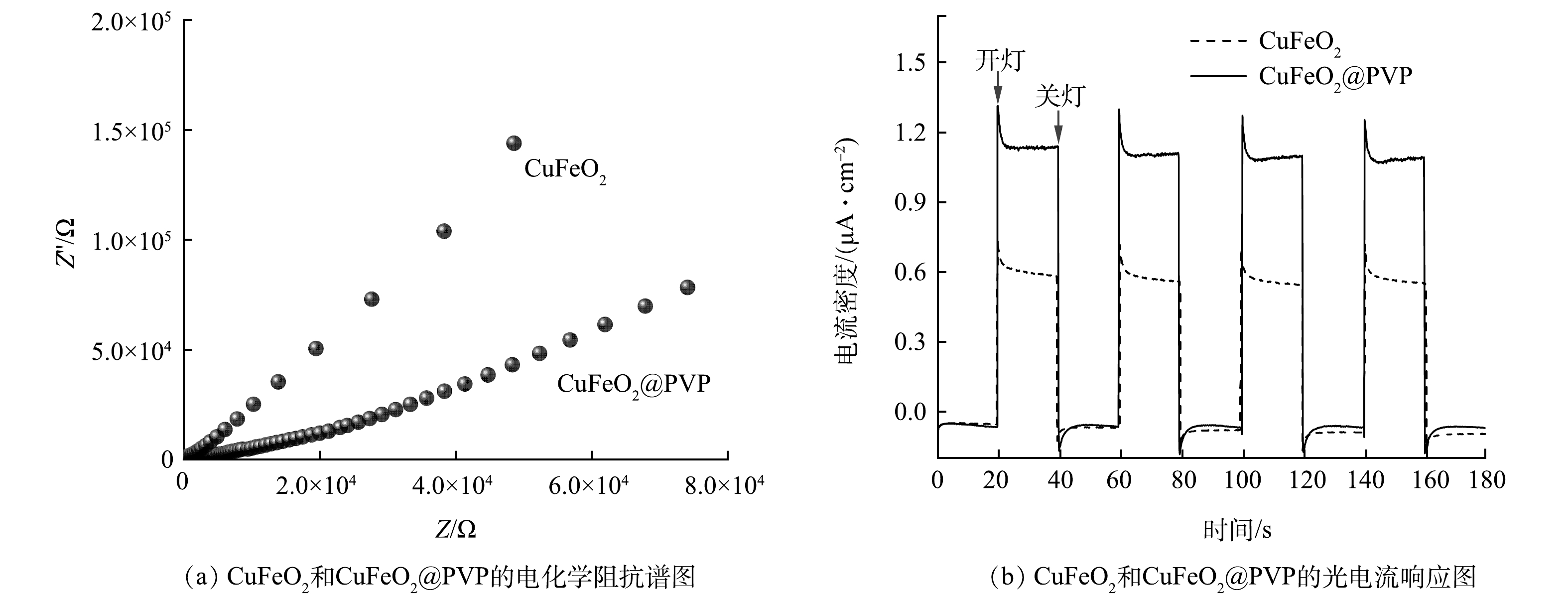
5)电化学阻抗和光电流分析。通过电化学阻抗测试和光电流响应测试,进一步研究CuFeO2@PVP复合材料的电荷转移和分离效率。在电化学阻抗谱中,半圆弧对应的半径越小代表电荷转移电阻越小[25]。如图8(a)所示,CuFeO2@PVP复合材料的EIS曲线半圆弧小于CuFeO2的EIS曲线半圆弧。这表明PVP的引入降低了CuFeO2的电子转移电阻,使其具有更快的电子迁移速率。图8(b)反映了在定时开关灯状态下,催化剂的瞬态光电流响应。在开灯状态下,CuFeO2@PVP的光电流响应明显高于CuFeO2,达到1.3 μA·cm−2。这是因为PVP的引入促进了光生载流子的分离和光电子的转移,从而产生更强的光电流响应。
-
为了探索CuFeO2@PVP的光电催化活性,以碳毡为阴极、铂片为阳极,在催化剂投加量为0.6 g·L−1、电流密度为4 mA·cm−2的条件下,考察了不同体系对OFX的降解效果并对相应的动力学进行了分析。图9(a)反映了OFX在不同反应体系中的降解效果。单独CuFeO2@PVP的吸附实验结果表明催化剂的吸附作用对污染物的去除效果甚微,反应120 min的去除率为6.4%。不添加碳毡电极,单独阳极氧化体系反应120 min的降解率为26.1%。当体系不添加可见光和催化剂时,反应120 min的电催化降解率为27.3%。这是因为没有催化剂活化H2O2的情况下,具有较低的氧化还原电位(E0=1.77 eV)的H2O2对有机污染物的降解作用有限[26]。体系不添加电的作用时,CuFeO2@PVP催化剂在可见光照射条件下,反应120 min的光催化降解率为27.0%。这表明在没有H2O2产生的情况下,单独CuFeO2@PVP在可见光下的催化能力较差。无论是CuFeO2@PVP还是H2O2单独存在的情况下,体系对于OFX的降解效率均较低。在异相电芬顿体系和异相光电芬顿体系中,反应120 min时降解率分别达到84.5%和94.3%。可见光的引入进一步提高了降解效率,归因于光电协同体系中产生了·OH、·O2 –、光生电子和光生空穴等更多的活性物种辅助催化降解OFX。相比于纯相CuFeO2,CuFeO2@PVP作为光电芬顿体系中的催化剂时降解率提高了9.4%。这表明OVs的引入有效提高了CuFeO2活化H2O2的催化活性。准一级动力学方程反映了不同体系的OFX降解速率。如图9(b)所示,CuFeO2@PVP异相光电Fenton体系的反应速率常数,分别是CuFeO2异相光电芬顿(0.016 18 min−1)、电芬顿(0.015 89 min−1)、电催化(0.002 71 min−1)、光催化(0.002 42 min−1)、阳极氧化(0.002 46 min−1)和吸附(0.000 56 min−1)的1.79、1.83、10.7、11.99、11.8和51.82倍。结果表明,可见光、电场和异相催化剂CuFeO2@PVP之间存在协同效应,以CuFeO2@PVP为催化剂的异相光电Fenton体系可高效降解OFX。
-
1)催化剂投加量对OFX降解的影响。由于催化剂用量与活性位点和光生载流子的数量密切相关,因此有必要通过单因素实验找出降解性能最佳的催化剂投加量。由图10(a)所示,当CuFeO2@PVP的投加量由0.05 g·L−1增加到0.4 g·L−1时,CuFeO2@PVP异相光电Fenton体系对OFX的反应速率随之提高。因为催化剂的投加量增多意味着提供了更多的活性位点,加速H2O2的生成与转化,从而加速OFX的分解。然而,当催化剂的投加量进一步增加至0.6 g·L−1时,OFX的降解率轻微降低。这是由于粉末状催化剂投加量过多时会降低溶液的透光性,影响催化剂对光的捕获性能,导致OFX的降解率有所下降。此外,由于受到H2O2浓度的限制,过量的催化活性位点并不能被利用,进而限制了OFX的降解效率。
2)电流密度对OFX降解的影响。在CuFeO2@PVP催化剂投加量为0.4 g·L−1时,考察了电流密度对异相光电芬顿体系降解OFX的影响。如图10(b)所示,当电流密度由1 mA·cm−2增加到4 mA·cm−2时,OFX的降解效率也随之增加。这表明在一定范围内增加电流密度能够促进异相光电Fenton体系产H2O2,提高活性自由基的产量,从而提高体系的催化活性。但当电流密度从4 mA·cm−2增加到8 mA·cm−2时,OFX的降解效率下降,继续增加电流密度至12 mA·cm−2时,降解效率基本不变。这是因为通过曝气的方式从外部供应到阴极表面的O2有限,过高的电流密度并不能被充分利用。另外,电流密度过高会导致H2O2发生如式(2)所示的副反应,限制H2O2的积累、消耗部分·OH,对OFX的降解产生抑制作用。
3)溶液pH对OFX降解的影响。在CuFeO2@PVP催化剂投加量为0.4 g·L−1、电流密度为4 mA·cm−2时,考察了溶液pH对异相光电 Fenton 体系降解OFX的影响。如图10(c)所示,当溶液的pH从3增加到9时,异相光电Fenton体系对OFX的降解效率呈现持续下降的趋势,在降解120 min后,降解率由94.3%下降到61.2%。这是因为pH较高时,溶液中高浓度的OH−导致H2O2发生脱质子反应,降低了H2O2在溶液中的浓度[27]。当溶液未调节pH(pH=3.6),该体系在0~60 min内的降解效率明显低于溶液pH=3时,这归因于pH=3的溶液中酸性条件更强,有足量的H+参与H2O2的生成,促使体系在短时间内产生更多的活性自由基用于降解OFX。然而当反应进行到120 min时,降解效率达到了92.2%,这与pH=3时的降解效率(94.3%)基本一致。这是因为随着反应的持续进行,H+的产生量少于消耗量,反应速率逐步下降。考虑到溶液调节pH将增加繁琐的步骤和耗材,在此种情形下,本研究其他实验均未调节溶液的初始pH。
4)共存阴离子对OFX降解的影响。无机阴离子广泛存在于天然水体中,其存在可能会对OFX的降解产生干扰。因此,本研究考察了四种常见无机阴离子(Cl−、NO3−、CO32−和PO43−)对异相光电Fenton 体系降解OFX的影响。如图10(d)所示,与不添加任何阴离子的对照组相比,Cl−的存在对OFX的降解率有微弱的促进作用,在反应进行120 min时降解效率达到99%,比对照组提高了4.7%。这是因为添加到溶液中的Cl−与·OH反应生成·ClOH−,但·ClOH−将以更大的速率常数( (4.3±0.4)×109)快速解离成·OH[28]。另外,Cl−相当于电解质,可以促进溶液中电子的转移,减小电子迁移对于电化学活性物质传质的影响,从而提高体系的催化活性。与Cl−相反,添加NO3−、PO43−和CO32−后,OFX的降解率相对于对照组分别降低了28.1%、82.9%和86.2%。其中,NO3−可通过与催化剂发生络合影响H2O2的生成,降低OFX的降解效率[29]。PO43−在水中发生水解反应,形成PO43−-HPO4·−-H2PO4·−-H3PO4平衡体系。其中,H2PO4·−和HPO4·−能够通过式(3)和式(4)反应淬灭·OH[30-31],此外,还可以通过占据CuFeO2@PVP表面的活性位点,从而抑制H2O2的活化[32]。CO32−在水中会发生水解,在电离作用下,溶液中形成一个CO32−-HCO3−-H2CO3平衡体系。而CO32−和HCO3−对活性自由基具有很强的清除作用,通过式(5)和式(6)的反应,CO32−和HCO3−实现对·OH的转化,并生成氧化活性较低的CO3·−,从而降低催化效率[30]。
-
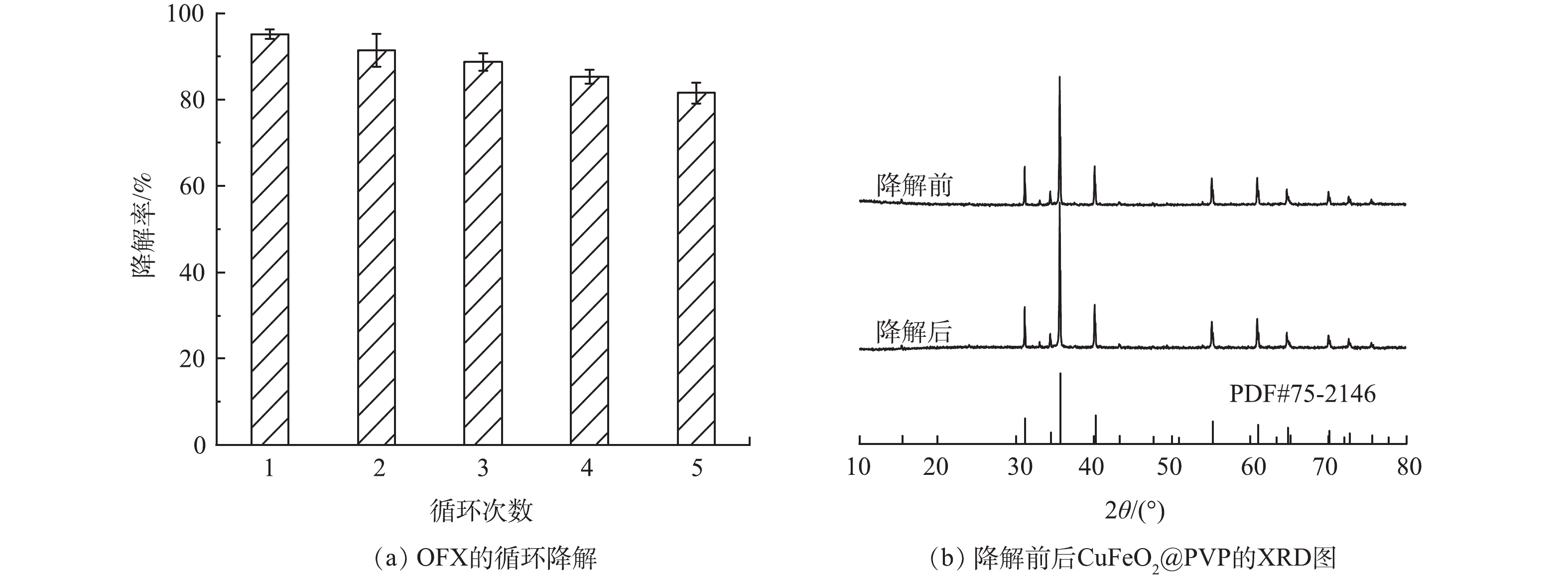
为了评价异相光电Fenton体系在长期运行下降解OFX的性能,在CuFeO2@PVP催化剂投加量为0.4 g·L−1、电流密度为4 mA·cm−2的条件下,进行了5次循环降解实验。每次降解实验结束后,通过抽滤的方法回收溶液中的催化剂,经过清洗和烘干后用于下一轮的降解实验。如图11(a)所示,CuFeO2@PVP在5次连续反应中对OFX的降解率分别为94.3%、91.4%、88.7%、85.3%和81.5%。表明CuFeO2@PVP具有良好的稳定性。此外,通过测定反应前后CuFeO2@PVP的XRD谱图进一步分析了催化剂的循环稳定性。如图11(b) 所示,降解前后CuFeO2@PVP的XRD谱图特征峰仍然一一对应,这表明反应后的CuFeO2@PVP仍然能够保持原有的晶型结构。上述结果表明,CuFeO2@PVP在异相光电Fenton体系降解OFX的过程中有较高的稳定性,不易造成二次污染,具有经济环保的优良特性。
-
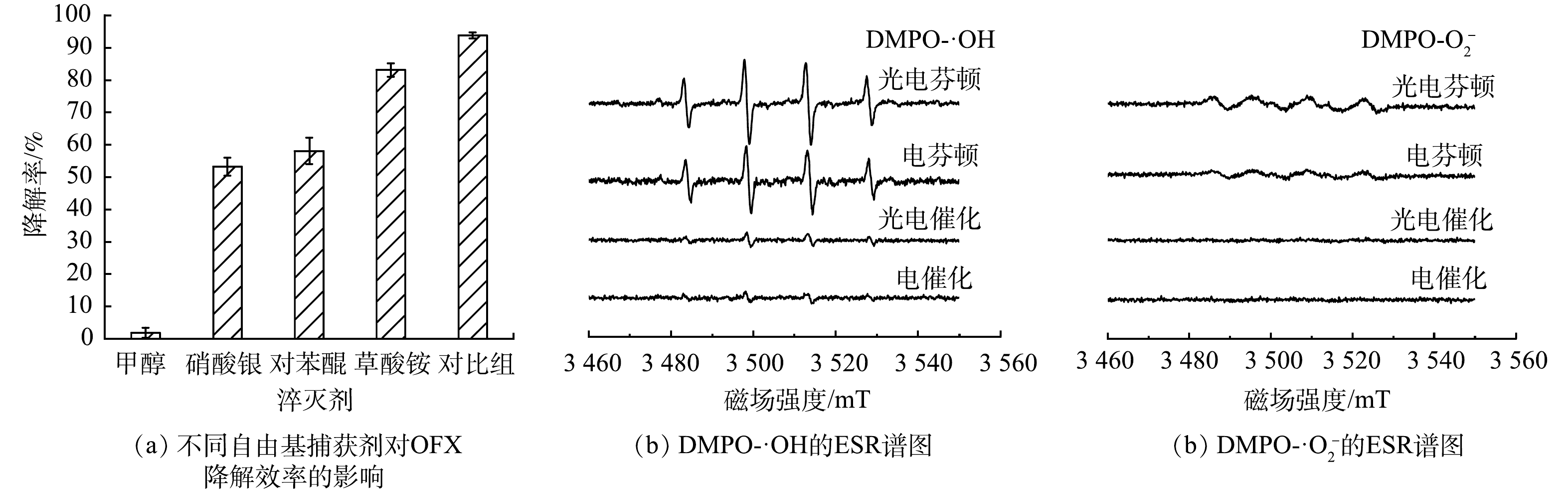
1)活性自由基的鉴定。为了确定CuFeO2@PVP异相光电Fenton体系中降解OFX的主要活性自由基,进行了自由基淬灭实验。采用浓度均为50 mmoL·L−1的甲醇、硝酸银、对苯醌和草酸铵分别作为·OH、光生电子、·O2−和光生空穴的淬灭剂。如图12(a)所示,在不添加任何淬灭剂的情况下,OFX的去除率达到了94.3%,当添加甲醇、硝酸银、对苯醌和草酸铵后,OFX的降解率分别降至1.9%、53.2%、58.1%和82.1%。这表明·OH、光生电子和·O2−在反应过程中起主要作用。为了进一步研究异相光电芬顿体系中的活性自由基,以二甲基吡啶N-氧化物(DMPO)为捕获剂,分别在光电芬顿、电芬顿、光电催化、电催化体系下进行了ESR实验。如图12(b)和图12(c)所示,光电催化和电催化体系中DMPO-·OH和DMPO-·O2−信号十分微弱。这表明2个体系中·OH和·O2−的产量极低,降解效率较低。在光电芬顿和电芬顿体系中均检测到强度比为1:2:2:1的DMPO-·OH加合物的特征峰和强度比为1:1:1:1的DMPO-·O2−加合物的特征峰,且光电芬顿体系的特征峰峰值大于电芬顿体系。这表明2个体系中均可以产生·OH和·O2−,而可见光的引入促进了2种自由基的产生,进一步提高降解效率,这与不同体系的降解实验结果相一致。另外,在相同反应时间内,光电芬顿和电芬顿体系中DMPO-·OH特征峰的强度比DMPO-·O2−特征峰强度大,表明·OH是异相芬顿和异相光芬顿体系中的主要活性自由基,这与自由基捕获实验的结果相符合。
2)反应机理推测。基于上述分析,提出了一种合理的异相光电Fenton体系降解OFX的机理,如图13所示。在电场的作用下,碳毡阴极通过二电子反应将O2转化成H2O2,并扩散到电解液中。体系中的CuFeO2@PVP表面先发生式(7)~式(8)所示反应形成表面活性位点≡Cu+/Cu2+-OH−和≡Fe3+/Fe2+-OH−。≡Cu+/Cu2+-OH−和≡Fe3+/Fe2+-OH−分别与H2O2发生如式(9)~式(12)所示反应生成·OH和O2·−活性自由基,并且实现他们两两之间的转化。产生的O2·−能够将≡Fe3+-OH还原成≡Fe2+-OH。除此之外,由于Cu2+/Cu+的氧化还原电位(0.17 V)低于Fe3+/Fe2+的氧化还原电位(0.77 V),≡Cu+-OH可通过式(14)将≡Fe3+-OH还原成≡Fe2+-OH。≡Cu+-OH−和≡Fe3+-OH−的协同作用有利于促进整体的氧化还原循环,提高CuFeO2@PVP活化H2O2的性能。然而,由于通过式(10)、式(13)、式(14)实现Cu+和Fe2+再生的速率较慢,体系的降解效率仍然受限。值得注意的是,CuFeO2@PVP表面大量的OVs的存在有效提高了催化效率。一方面OVs的引入能够增强相邻活性位点与H2O2之间的相互作用,生成氧空位所留下的电子将促进表面Cu+和Fe2+的生成,促进氧化还原循环。另一方面,有研究[21]表明,氧空位对H2O2具有较强的吸附作用,有助于H2O2中O-O键的伸长和裂解,从而实现对H2O2的活化(式(15))。另外,可见光和电场的存在也发挥了重要作用。首先,可见光激发CuFeO2@PVP产生光生电子和空穴(式(16)),光生电子和空穴能够活化H2O2生成高活性的·OH,直接促进污染物的降解(式(17)~(18))。另外,光生电子和电场极化产生的电子能够通过式(19)~(20)加速≡Fe3+-OH/Fe2+-OH和≡Cu2+-OH/Cu+-OH的氧化还原循环反应,进而提高催化效率。最后,OFX在·OH、O2·−、光生电子、光生空穴、OVs的共同作用下被降解为中间产物,CO2和H2O(式(21))。
-
1)以聚乙烯吡咯烷酮作为改性剂,通过水热法成功制备了CuFeO2@PVP复合催化剂。通过表征发现PVP能够提高催化剂的分散性和稳定性,在CuFeO2表面引入大量氧空位,缩小CuFeO2的禁带宽度,促进光生载流子的分离与重组。
2)在催化剂投加量为0.4 g·L−1、电流密度为4 mA·cm−2、初始pH为3.6的条件下,CuFeO2@PVP异相光电Fenton体系在120 min时对OFX的去除率达到94.3%。另外,Cl−的添加对OFX降解无明显影响,而NO3−、PO43−和CO32−的添加对OFX降解产生抑制作用。
3)经5次循环降解实验后,CuFeO2@PVP异相光电Fenton体系对OFX的降解率仅降低了12.3%;降解后CuFeO2@PVP仍能保持原有的晶型结构,表明CuFeO2@PVP在具有良好的稳定性和可重复性。
4) CuFeO2@PVP异相光电Fenton体系中存在·OH、·O2−、e−、h+ 4种自由基,·OH发挥主要作用。可能的反应机理为:OVs、可见光和电场的引入均能加速CuFeO2@PVP表面Fe3+/Fe2+和Cu+/Cu2+的氧化还原循环,快速催化碳毡电极通过二电子氧反应产生的H2O2,生成的大量活性自由基可促进OFX的降解。此外,除了通过辅助氧化还原循环的途径提高催化活性外,OVs、光生电子、光生空穴和电场极化产生的电子也可直接活化H2O2,从而产生更多的活性氧自由基实现OFX的高效降解。
CuFeO2@PVP的制备及其基于光电芬顿降解氧氟沙星
Preparation of CuFeO2@PVP for the photo-electro- Fenton degradation of ofloxacin
-
摘要: 以聚乙烯吡咯烷酮作为改性剂,利用水热法合成了表面具有丰富氧空位的CuFeO2@PVP复合催化剂。通过XRD、FT-IR、SEM、TEM和EPR等方法证实了催化剂的成功合成及确定了催化剂的形貌和微观结构。采用UV-vis DRS、PL、EIS和IT等方法证实了CuFeO2@PVP比CuFeO2具有更好的光学性能及光电性能。不同体系下的降解实验结果表明,CuFeO2@PVP的光电催化活性比纯相CuFeO2有明显的提升,反应速率是纯相CuFeO2的1.79倍,去除率相比于单独的吸附、阳极氧化、光催化、电催化和电芬顿体系分别提高了87.9%、68.2%、67.3%、67%和9.8%,说明可见光、电场和异相催化剂间存在协同效应。进一步探究了催化剂投加量、电流密度、溶液pH、共存离子种类对异相光电芬顿体系降解氧氟沙星(OFX)的影响。结果表明,在最佳催化剂投加量为0.4 g/L、最佳电流密度为4 mA/cm2的条件下,CuFeO2@PVP光电Fenton体系在120 min时对10 mg·L−1 OFX的降解率达到94.3%。pH在5-9之间时对OFX的降解呈现抑制作用,pH在3-3.6之间时降解效果基本持平。溶液中的Cl−对OFX的降解起到轻微的促进作用,而NO3−、PO43−和CO32−会抑制体系对OFX的降解。此外,5次循环降解实验后,CuFeO2@PVP的降解效率降低了13.8%,表明其具有良好的稳定性。自由基淬灭实验和电子顺磁共振结果表明·OH是最主要的活性自由基并基于上述结果推测出可能的降解机理。
-
关键词:
- CuFeO2@PVP /
- 氧空位 /
- 光电芬顿 /
- 氧氟沙星 /
- 降解机理
Abstract: The CuFeO2@PVP catalyst with rich oxygen vacancies was synthesized via a simple one-step hydrothermal method with polyvinylpyrrolidone as modifier. The successful synthesis of the catalyst and its morphology and microstructure were confirmed by XRD, FT-IR, SEM, TEM and EPR methods. UV-vis DRS, PL, EIS and IT were used to confirmed that CuFeO2@PVP has better photocatalytic activity and photoelectrochemical properties than CuFeO2. The results of degradation experiments with different systems showed that the degradation efficiency by CuFeO2@PVP in photo-electro-Fenton system increased significantly, which was 1.79 times as much as pure CuFeO2. The removal rate by photo-electro-Fenton system was 66.8%, 66.5% and 9.3% higher than photocatalysis, electrocatalysis and electro Fenton system, respectively, which indicated that there was a synergistic effect among the visible light, electro and heterogeneous catalyst. The effects of catalyst dosage, current density, solution pH values and coexistence ions on the degradation of OFX were further discussed. The removal rate for 10 mg·L-1 OFX was 94.3% after 120 min oxidation at catalyst dosage of 0.4 g·L−1 and current density of 4 mA/cm2. At pH 5-9, OFX degradation was inhibited, while at pH 3-3.6, the degradation was almost unchanged. Cl− had a slight acceleration effect on OFX degradation, while NO3-, PO43− and CO32− inhibited the reaction. In addition, the degradation efficiency of CuFeO2@PVP decreased by 13.8% after 5 cyclic degradation experiments, indicating that it has good stability. The results of radical quenching experiment and electron paramagnetic resonance showed that ·OH was the main active radical, and the possible degradation mechanism was inferred based on the above results.-
Key words:
- CuFeO2@PVP /
- Oxygen Vacancies /
- photo-electro Fenton /
- ofloxacin /
- degradation mechanism
-

-
-
[1] NGUYEN T D, ITAYAMA T, RAMARAI R, et al. Chronic ecotoxicology and statistical investigation of ciprofloxacin and ofloxacin to Daphnia magna under extendedly long-term exposure[J]. Environmental Pollution, 2021, 291: 118095. doi: 10.1016/j.envpol.2021.118095 [2] LIU Y, YUAN Y, WANG Z, et al. Removal of ofloxacin from water by natural ilmenite-biochar composite: A study on the synergistic adsorption mechanism of multiple effects[J]. Bioresource Technology, 2022, 363: 127938. doi: 10.1016/j.biortech.2022.127938 [3] RASOULZADEH H, AZARPIRA H, ALINEJAD N, et al. Efficient degradation of Ocufloxin a neutral photo oxidation/reduction system based on the enhanced heterogeneous-homogeneous sulfite-iodide cycle[J]. Optik, 2022, 257: 168878. doi: 10.1016/j.ijleo.2022.168878 [4] MATHUR P, SANYAL D, CALLAHAN D L, et al. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ- ment and human health[J]. Environmental Pollution, 2021, 291: 118233. doi: 10.1016/j.envpol.2021.118233 [5] LU M, XU Z, ZHAO H, et al. Heterogeneous electro-Fenton catalysis with novel bimetallic CoFeC electrode[J]. Separation and Purification Technology, 2022, 302: 122069. doi: 10.1016/j.seppur.2022.122069 [6] WEN Z, REN S, ZHANG Y, et al. Performance of anode materials in electro-Fenton oxidation of cefoperazone in chloride medium: New insight into simultaneous mineralization and toxic byproducts formation[J]. Journal of Cleaner Production, 2022, 377: 134225. doi: 10.1016/j.jclepro.2022.134225 [7] 龚月湘, 兰华春, 李久义, 等. 光电芬顿矿化草甘膦有机废水[J]. 环境工程学报, 2016, 10(8): 3999-4003. doi: 10.12030/j.cjee.201503080 [8] JIA X, BAI X, JI Z, et al. Insight into the Effective Removal of Ciprofloxacin Using a Two-Dimensional Layered NiO/g-C3N4 Composite in Fe-Free Photo-Electro-Fenton System[J]. Acta Physico Chimica Sinica, 2021, 37(8): 2010042. [9] SCHMACHTENBERG N, SILVESTRI S, SILVERIRA S J, et al. Preparation of delafossite–type CuFeO2 powders by conventional and microwave-assisted hydrothermal routes for use as photo–Fenton catalysts[J]. Journal of Environmental Chemical Engineering, 2019, 7(2): 102954. doi: 10.1016/j.jece.2019.102954 [10] YOUNSI M, AIDER A, BOUGUELIA A, et al. Visible light-induced hydrogen over CuFeO2 via S2O32− oxidation[J]. Solar Energy, 2005, 78(5): 574-580. doi: 10.1016/j.solener.2004.01.012 [11] XU Q, LI R, WANG C, et al. Visible-light photocatalytic reduction of Cr(Ⅵ) using nano-sized delafossite (CuFeO2) synthesized by hydrothermal method[J]. Journal of Alloys and Compounds, 2017, 723: 441-447. doi: 10.1016/j.jallcom.2017.06.243 [12] DARKHOSH F, LASHANIZAGEGAN M, MAHJOUB A R, et al. One pot synthesis of CuFeO2@expanding perlite as a novel efficient floating catalyst for rapid degradation of methylene blue under visible light illumination[J]. Solid State Sciences, 2019, 91: 61-72. doi: 10.1016/j.solidstatesciences.2019.03.009 [13] XIN S, MA B, LIU G, et al. Enhanced heterogeneous photo-Fenton-like degradation of tetracycline over CuFeO2/biochar catalyst through accelerating electron transfer under visible light[J]. Journal of Environmental Management, 2021, 285: 112093. doi: 10.1016/j.jenvman.2021.112093 [14] DENG Q, CHEN H, WANG G, et al. Structural, optical and photoelectrochemical properties of p type Ni doped CuFeO2 by hydrothermal method[J]. Ceramics International, 2020, 46(1): 598-603. doi: 10.1016/j.ceramint.2019.09.008 [15] TU L W, CHANG K S. Hydrothermal fabrication and photocatalytic study of delafossite (CuFeO2) thin films on fluorine-doped tin oxide substrates[J]. Materials Chemistry and Physics, 2021, 267: 124620. doi: 10.1016/j.matchemphys.2021.124620 [16] WANG R, AN H, ZHANG H, et al. High active radicals induced from peroxymonosulfate by mixed crystal types of CuFeO2 as catalysts in the water[J]. Applied Surface Science, 2019, 484: 1118-1127. doi: 10.1016/j.apsusc.2019.04.182 [17] DAI C, NIE Y, TIAN X, et al. Insight into enhanced Fenton-like degradation of antibiotics over CuFeO2 based nanocomposite: To improve the utilization efficiency of ·OH/O2·- via minimizing its migration distance[J]. Chemosphere, 2022, 294: 133743. doi: 10.1016/j.chemosphere.2022.133743 [18] ZHANG B, ZHANG M, ZHANG L, et al. PVP surfactant-modified flower-like BiOBr with tunable bandgap structure for efficient photocatalytic decontamination of pollutants[J]. Applied Surface Science, 2020, 530: 147233. doi: 10.1016/j.apsusc.2020.147233 [19] HU X, ZHANG Y, WANG B, et al. Novel g-C3N4/BiOClxI1-x nanosheets with rich oxygen vacancies for enhanced photocatalytic degradation of organic contaminants under visible and simulated solar light[J]. Applied Catalysis B:Environmental, 2019, 256: 117789. doi: 10.1016/j.apcatb.2019.117789 [20] SENASU T, CHANKHANITTHA T, HEMAVIBOOL K, et al. Solvothermal synthesis of BiOBr photocatalyst with an assistant of PVP for visible-light-driven photocatalytic degradation of fluoroquinolone antibiotics[J]. Catalysis Today, 2022, 384-386: 209-227. doi: 10.1016/j.cattod.2021.04.008 [21] WANG M, LIU C, SHI H, et al. Facile synthesis of chitosan-derived maillard reaction productions coated CuFeO2 with abundant oxygen vacancies for higher Fenton-like catalytic performance[J]. Chemosphere, 2021, 283: 131191. doi: 10.1016/j.chemosphere.2021.131191 [22] ZHUANG G, CHEN Y, ZHUANG Z, et al. Oxygen vacancies in metal oxides: recent progress towards advanced catalyst design[J]. Science China Materials, 2020, 63(11): 2089-2118. doi: 10.1007/s40843-020-1305-6 [23] BAIANO C, SCHIAVO E, GERBALDI C, et al. Role of surface defects in CO2 adsorption and activation on CuFeO2 delafossite oxide[J]. Molecular Catalysis, 2020, 496: 111181. doi: 10.1016/j.mcat.2020.111181 [24] NARKBUAKAEW T, SATTAYAPORN S, SAITO N, et al. Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV–Visible light irradiation[J]. Applied Surface Science, 2022, 573: 151617. doi: 10.1016/j.apsusc.2021.151617 [25] MOLOTO W, MAFA P J, MBULE P, et al. Photoinduced electrochemical effect of porous BiPOM on TiO2 photoanode performance for dye-sensitized solar cells application[J]. Materials Today Communications, 2022, 30: 103001. doi: 10.1016/j.mtcomm.2021.103001 [26] LIU Y, ZHANG J, SHENG C, et al. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process[J]. Chemical Engineering Journal, 2010, 162(3): 1006-1011. doi: 10.1016/j.cej.2010.07.009 [27] XIN S, HUO S, XIN Y, et al. Heterogeneous photo-electro-Fenton degradation of tetracycline through nitrogen/oxygen self-doped porous biochar supported CuFeO2 multifunctional cathode catalyst under visible light[J]. Applied Catalysis B:Environmental, 2022, 312: 121442. doi: 10.1016/j.apcatb.2022.121442 [28] LIU Y, HE X, FU Y, et al. Degradation kinetics and mechanism of oxytetracycline by hydroxyl radical-based advanced oxidation processes[J]. Chemical Engineering Journal, 2016, 284: 1317-1327. doi: 10.1016/j.cej.2015.09.034 [29] XU P, WANG P, LI X, et al. Efficient peroxymonosulfate activation by CuO-Fe2O3/MXene composite for atrazine degradation: Performance, coexisting matter influence and mechanism[J]. Chemical Engineering Journal, 2022, 440: 135863. doi: 10.1016/j.cej.2022.135863 [30] HU L, ZHANG G, LIU M, et al. Enhanced degradation of Bisphenol A (BPA) by peroxymonosulfate with Co3O4-Bi2O3 catalyst activation: Effects of pH, inorganic anions, and water matrix[J]. Chemical Engineering Journal, 2018, 338: 300-310. doi: 10.1016/j.cej.2018.01.016 [31] HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications[J]. Applied Catalysis B:Environmental, 2016, 181: 103-117. doi: 10.1016/j.apcatb.2015.07.024 [32] LAI L, JI H, ZHANG H, et al. Activation of peroxydisulfate by V-Fe concentrate ore for enhanced degradation of carbamazepine: Surface ≡V(III) and ≡V(IV) as electron donors promoted the regeneration of ≡Fe(II)[J]. Applied Catalysis B:Environmental, 2021, 282: 119559. doi: 10.1016/j.apcatb.2020.119559 -



 下载:
下载: