-
纳米氧化锌(nZnO)作为一种应用广泛的纳米材料,全球年产量达3.15—3.40万吨,预计8%—20%被排入水环境中[1]. nZnO是一种带电型可溶性纳米颗粒,其颗粒态及离子态均可对水生生物产生不利影响[2]. 由于实际水环境的复杂性,纳米材料的环境行为与生物效应会受其他带电共存物的影响. 带负电的污染物如纳米二氧化钛[3]、多溴二苯醚[4]、十二烷基硫酸钠[5]可通过改变带正电荷的nZnO的ζ-电位,干扰nZnO与其他物质之间的相互作用从而减弱其生物毒性. 而带负电荷的污染物如nCu[6]可通过促进带负电荷的nZnO的Zn2+的溶出及生物积累,增强nZnO的生物毒性. 目前大部分研究集中于相反电性共存物与nZnO之间的生物毒性[7]及其对nZnO溶出行为的影响[8],对于相同电性或不带电的共存物对nZnO的影响还有待研究.
表面活性剂因具有优良的清洁和增溶性能被广泛应用于浮选剂和洗涤剂等产品中. 作为纳米材料生产使用过程中的助剂之一,表面活性剂是最有可能在水环境中与纳米材料接触的带电荷污染物之一[9]. 前期研究发现,带正电荷的十六烷基三甲基氯化铵(CTAC)可吸附在带负电荷的nZnO表面,改变其与带负电荷的藻细胞间的静电力作用,抑制nZnO的溶出,使nZnO主要以颗粒态吸附在细胞表面[10]. 为此,本研究以水生生态系统中常见的初级生产者小球藻作为受试生物,探究了3种不同电荷表面活性剂,阳离子型的CTAC、阴离子型的十二烷基苯磺酸钠(SDBS)、非离子型的聚乙二醇辛基苯基醚(TX-100)存在下nZnO对小球藻毒性效应及细胞分布的影响机制,以期为评价纳米颗粒在不同电荷水环境中的生物效应提供理论依据.
-
普通小球藻(Chlorella vulgaris F1068)购自中国科学院武汉水生生物研究所;CTAC(CAS:112-02-7,纯度:97%)、SDBS(CAS:25155-30-0,纯度:95%)、TX-100(CAS:9002-93-1,纯度:98%)及nZnO(CAS:1314-13-2,纯度:99.9%)均购自于上海阿拉丁生化科技股份有限公司.
-
参照OECD标准方法进行培养[11],培养箱光照强度设定为2500 lux,光暗比为L:D=12 h:12 h,培养温度设置为(25±1)℃,每天人工摇瓶3次.
-
取对数生长期的小球藻接种至OECD培养基中,调节藻细胞密度为1.0×106 cells·mL–1. 有研究表明nZnO的环境浓度为0.1—8.6 mg·L–1[5]. 基于此,本研究将藻细胞暴露于浓度分别为0.00、0.81、1.62、3.24、6.48、9.72 mg·L–1 的nZnO中;加入3种表面活性剂使得3组复合体系中CTAC、SDBS和TX-100最终浓度分别为0.2、20、100 mg·L–1,每组实验设3个平行. 每隔24 h测定一次藻密度,计算各实验组的96 h生长抑制率并绘制生长曲线.
-
细胞表面疏水性(CSH)参照Rosenberg的方法[12]. 藻液培养6 h后用磷酸缓冲液(PBS,0.01 mol·L–1,pH:7.4)洗涤两遍,重悬浮于PBS并加入三氯甲烷(藻液:三氯甲烷体积比为6:1),室温下剧烈振荡3 min后静置20 min,分别于680 nm下测定加入三氯甲烷前后的吸光度A1、A2. 计算公式如下.
-
使用毒性单位法(TU)、相加指数法(AI)、相似性参数法(λ)和混合毒性指数法(MTI)评价nZnO/CTAC、nZnO/SDBS、nZnO/TX-100的3种复合体系对小球藻急性毒性的联合作用类型[13].
-
将少量nZnO颗粒悬浮于无水乙醇中,超声分散后将悬浮液滴在粘附有碳膜的铜网上,干燥后使用透射电子显微镜(JEM-2100,日本电子,日本)对nZnO的形态进行表征.
使用原子吸收光谱仪(AA6300C,苏州岛津,中国)测定复合体系下nZnO溶解Zn2+浓度. nZnO/表面活性剂混合溶液恒温振荡12 h后离心(1000 r·min–1,15 min,25 ℃),用0.22 μm聚醚砜水相滤膜过滤,滤液用0.2%硝酸酸化后测量溶液中Zn2+含量.
使用荧光分光光度计(F–7000,日立,日本)测定复合体系中nZnO的疏水性. 芘溶解于丙酮后取一定量于瓶中干燥,加入nZnO/表面活性剂混合溶液(混合溶液与芘的体积比为4:5),室温振荡6 h后测量. 设置激发和发射光栅分别为3.0 nm和1.5 nm,激发波长为338 nm,在350—500 nm采集发射光谱,扫描速率为240 nm·min–1. 测量芘荧光光谱的第一个峰与第三个峰(I1/I3)的强度比.
前期研究表明,在0.81 mg·L–1浓度条件下nZnO粒子间不会因压缩双电层作用而影响实测值,且nZnO能够较稳定地分散于介质中[13]. 使用马尔文激光粒度仪(ZEN3700,马尔文,英国)测定0.81 mg·L–1 nZnO/不同电荷表面活性剂复合体系中nZnO的水力直径和ζ-电位.
-
取培养第4天的藻液离心(4000 r·min–1,15 min,4 ℃),上清液按1:1体积比加入10%硝酸酸化,0.45 μm聚醚砜水相滤膜过滤后使用原子吸收光谱仪测滤液中Zn元素含量,视为溶液中Zn元素浓度. 藻泥用0.02 mmol·mL–1 EDTA溶液重悬浮后再次离心(10000 r·min–1,15 min,4 ℃),上清液按1:1体积比加入10%硝酸酸化,0.45 μm聚醚砜水相滤膜过滤后使用原子吸收光谱仪测滤液中Zn元素含量,视为小球藻细胞表面Zn元素浓度. 藻泥加入5%硝酸消解,待24 h消解完全后,用0.45 μm聚醚砜水相滤膜过滤并测量滤液中Zn元素含量,视为小球藻细胞内Zn元素浓度.
-
研究发现单一体系的nZnO、阳离子表面活性剂CTAC、阴离子表面活性剂SDBS及非离子表面活性剂TX-100均对小球藻的生长有抑制作用,其96 h-EC50值分别为3.1、0.2、21.4、141.4 mg·L–1. 基于此,设定nZnO/表面活性剂复合体系中CTAC、SDBS和TX-100的浓度分别为0.2、20、100 mg·L–1,测定nZnO/表面活性剂复合体系对小球藻生长的影响. 发现nZnO/CTAC、nZnO/SDBS、nZnO/TX-100复合体系对小球藻的96 h-EC50值分别为1.8、1.2、2.8 mg·L–1,表明CTAC与TX-100缓解了nZnO对小球藻生长的抑制,SDBS则使nZnO的抑制作用增强(图1).
进一步通过毒性单位法、相加指数法、相似性参数法和混合毒性指数法进行联合毒性评价证实nZnO/CTAC,nZnO/TX-100复合体系对小球藻的联合毒性表现为拮抗作用,而nZnO/SDBS复合体系表现为协同作用,结果见表1.
-
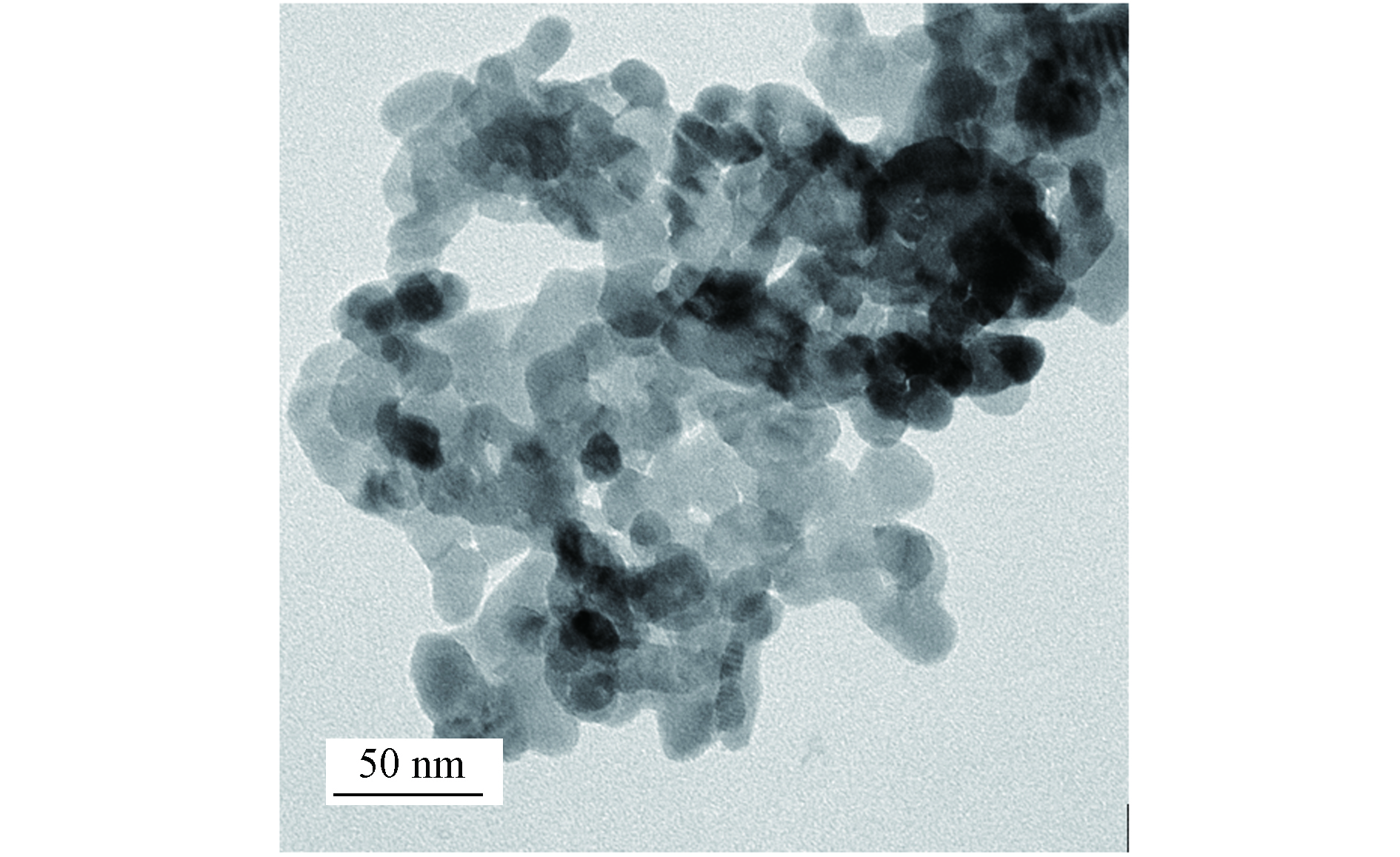
如图2所示,nZnO在无水乙醇中呈椭球状并发生团聚现象,颗粒平均粒径为20—30 nm.
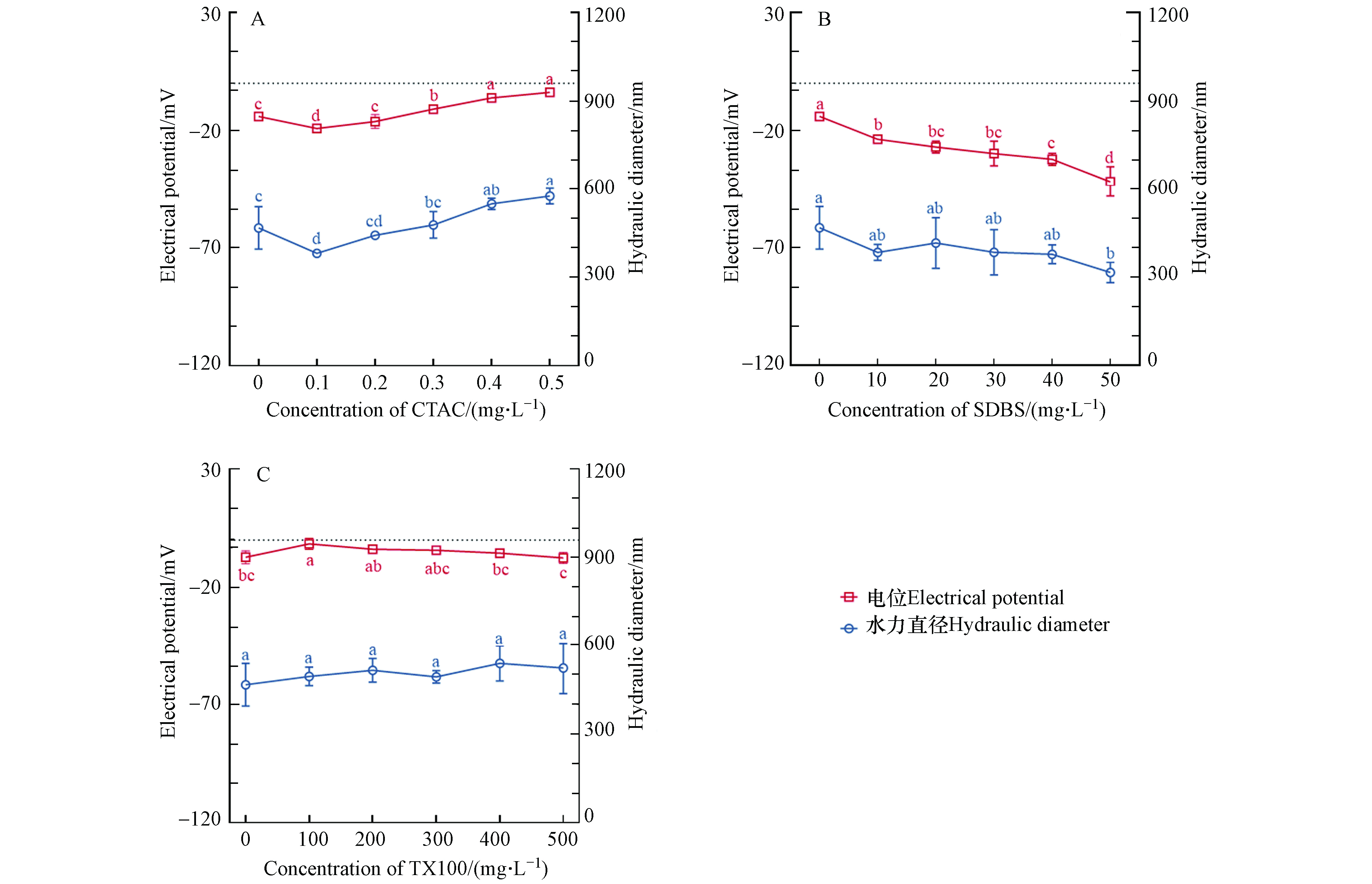
表面活性剂与nZnO相互作用可改变nZnO的理化性质,从而影响nZnO的环境行为及生物有效性[14]. 如图3A所示,随着CTAC浓度由0 mg·L–1增加至0.5 mg·L–1,nZnO的ζ-电位由–14.1 mV增大至–3.9 mV,粒径由466.6 nm增大至574.9 nm. 阳离子表面活性剂CTAC可通过静电力和表面能作用吸附在带负电荷的nZnO表面,减少nZnO的聚集并增强nZnO的稳定性[10]. 而阴离子表面活性剂SDBS使nZnO的ζ-电位由–14.1 mV降至–41.8 mV,粒径由466.6 nm减小至315.4 nm(图3B). EI-Lateef等[15]也发现了类似的现象并提出表面活性剂SDBS可吸附在带相同电荷的零价铁纳米粒子表面. 由于nZnO表面电荷分布不均匀,带正电的双十二烷基二甲基溴化铵(DDAB)可能吸附在带相同电荷的nZnO未带电区域表面,因而进一步增加了nZnO的表面电荷使其稳定性增加[16]. 本实验中非离子型表面活性剂TX-100对nZnO的电位及粒径没有显著影响(图3C).
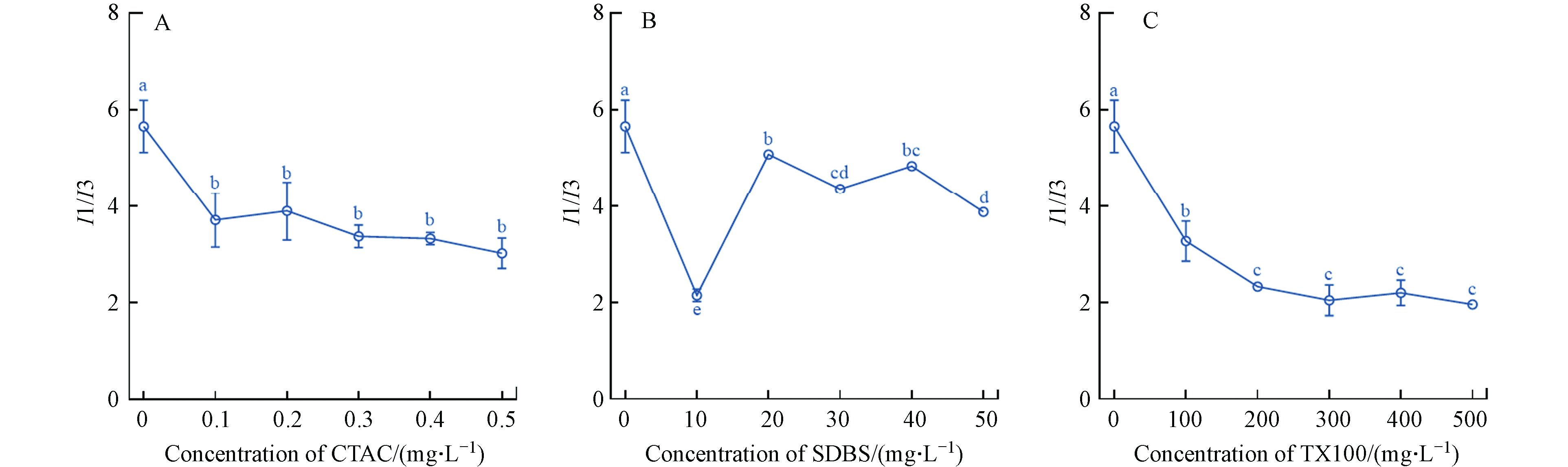
芘是最常用的极性荧光探针之一,可通过I1/I3的强度比来跟踪环境极性的变化,比值降低表明疏水性增强[17]. 如图4所示,3种复合体系下I1/I3均显著下降(P < 0.05),0.5 mg·L–1 CTAC、50 mg·L–1 SDBS、500 mg·L–1 TX-100时I1/I3由5.7分别降至3.1、3.9和1.9. 这表明3种表面活性剂均可增强nZnO的疏水性. CTAC含有烷基疏水基和带正电的亲水基,在液固界面易吸附于带负电荷的物质表面,可通过静电和疏水相互作用吸附在纳米颗粒表面,从而增强其疏水性[18–19]. I1/I3在10 mg·L–1 SDBS时最小,并随SDBS浓度增大而增大,这可能是由于低浓度时SDBS吸附在nZnO表面使其疏水性增大. 随着SDBS浓度增加,SDBS与nZnO间的静电排斥作用逐渐强于疏水相互作用,部分SDBS从nZnO表面脱离而重新游离于溶液中,从而导致nZnO表面疏水性减小. TX-100可通过氢键和疏水相互作用力吸附在纳米颗粒表面导致纳米颗粒疏水性增大[20].
Zn2+的溶出是nZnO产生毒性效应的主要原因[21]. 前期研究表明nZnO溶出在12 h之内达到平衡[10],因此测量了12 h时OECD介质中nZnO溶解的Zn2+随表面活性剂浓度的变化. 如图5所示,CTAC显著降低了nZnO的溶出,而SDBS增加了Zn2+的溶出(P < 0.05). 当CTAC浓度增加到0.5 mg·L–1时,0.81、1.62、9.72 mg·L–1 nZnO溶出Zn2+的浓度分别由0.4、0.8、1.4 mg·L–1降低至0.2、0.3、0.5 mg·L–1;25 mg·L–1 SDBS则使其增高至0.5、0.9、1.8 mg·L–1. CTAC与nZnO间的静电和疏水相互作用(图3和图4)使nZnO被“包裹”,从而抑制Zn2+的释放. SDBS存在下nZnO水力直径降低(图3),表明nZnO的比表面积增加. 根据Noyes-Whitney方程可知,nZnO的比表面积增加,Zn2+的溶出量增加[22]. 本实验中非离子表面活性剂TX-100对nZnO的溶出行为没有显著影响.
-
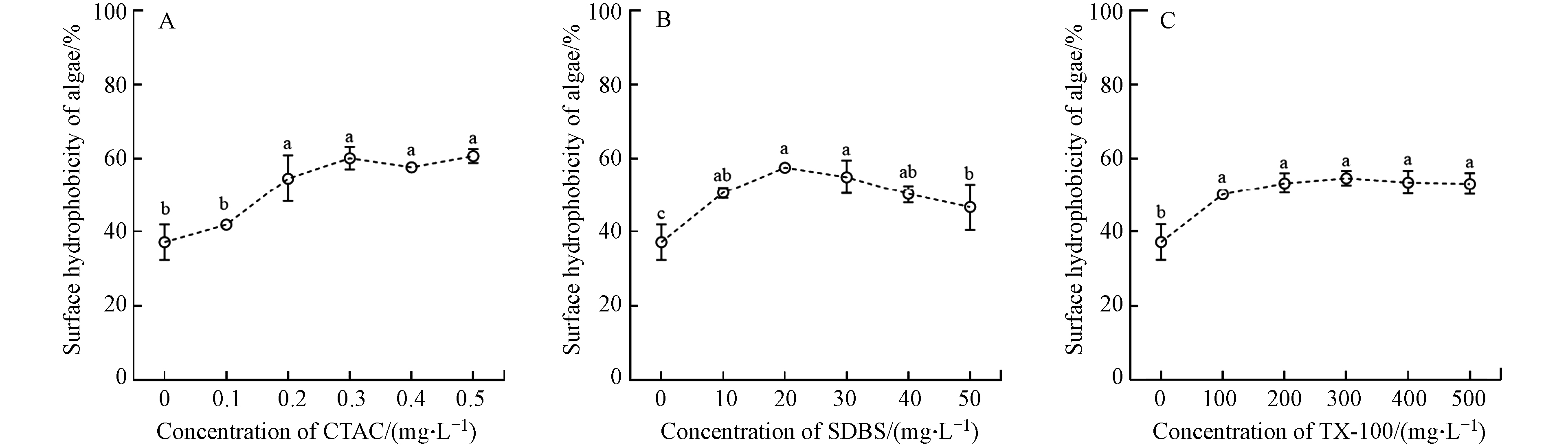
CSH是影响污染物与藻类细胞亲和力的关键因素,CSH越大则亲和力越大[23]. 如图6所示,3种表面活性剂均显著提高藻细胞的CSH(P < 0.05),0.5 mg·L–1 CTAC,50 mg·L–1 SDBS和500 mg·L–1 TX-100使CSH由37.1%分别提高至60.7%、46.6%和53.1%. SDBS胁迫下CSH先增大后减小. Liu等[24]提出SDBS可与细菌细胞膜相互作用,降低胞外蛋白和脂多糖的含量,导致CSH增加. 随着SDBS浓度增大,其与细菌的疏水作用力增强,SDBS的疏水端吸附于细胞表面而亲水端暴露于水相,因而细胞CSH降低. TX-100浓度高于200 mg·L–1后CSH变化趋于稳定,这可能是由于TX-100在细胞膜表面吸附达到饱和. Masakorala等[25]提出,阳离子型表面活性剂十六烷基三甲基溴化铵(HDTMA)与藻细胞的结合涉及离子交换和静电相互作用,因而较阴离子型和非离子型表面活性剂相比对细胞的疏水性更大.
nZnO可通过细胞壁和细胞膜进入细胞,从而抑制细胞生长[26]. 因此测定了nZnO单一体系和nZnO/表面活性剂复合体系下Zn元素在藻细胞的分布,进一步探究了不同电荷表面活性剂对nZnO颗粒态及离子态(相同Zn浓度的ZnCl2)毒性和细胞分布的影响. 如表2所示,生物量与Zn2+积累量呈显著负相关(–0.916). ZnCl2/表面活性剂复合体系下Zn2+浓度与生物量、积累量的相关性(–0.946、–0.979)均明显高于nZnO/表面活性剂复合体系下Zn2+浓度与生物量、积累量的相关性(–0.593、–0.669),表明在nZnO/表面活性剂复合体系中离子态nZnO并不是唯一的毒性来源,颗粒态nZnO也具有一定的影响. 而生物量与Zn元素积累量(包括nZnO的颗粒态和离子态)的相关性不高可能是由于nZnO颗粒态与离子态对小球藻毒性效应的影响程度不同. 上述结果证实nZnO离子态与颗粒态均会对毒性产生影响,且离子态的毒性效应较颗粒态更大.
nZnO/不同电荷表面活性剂和ZnCl2/不同电荷表面活性剂复合体系中Zn元素的细胞分布如图7所示. 0.81 mg·L–1 nZnO/CTAC复合体系中Zn元素在溶液、细胞表面和细胞内的分布比例分别为54.8%、31.3%和13.9%,与nZnO单一体系相比,Zn元素在细胞表面的含量增加12.7%,细胞内的含量降低3.3%,表明CTAC存在下,Zn元素更趋向于分布在细胞表面,这与Liu等[10]的研究结论相符;与ZnCl2/CTAC复合体系相比,细胞内含量降低14.9%. 一方面CTAC促进nZnO的团聚使颗粒态nZnO难以进入细胞,同时细胞与颗粒表面疏水及静电相互作用的增加使nZnO主要以颗粒态附着在小球藻表面;另一方面CTAC抑制了nZnO的溶出,减少细胞对Zn2+的内化,因而降低nZnO对细胞的毒性作用.
0.81 mg·L–1 nZnO/SDBS复合体系中Zn元素在溶液、细胞表面和细胞内的分布比例分别为45.7%、24.3%和30.1%,与nZnO单一体系相比,Zn元素在细胞表面和细胞内的含量分别增加5.6%和12.9%;与ZnCl2/SDBS复合体系相比,Zn元素在细胞表面的含量降低16.6%,细胞内的含量增加7.2%. 这一结果主要归因于SDBS可促进nZnO的分散和Zn2+溶出,增加了细胞对Zn2+的内化. 已有研究表明,Zn2+可以损伤细胞并在细胞膜上形成“孔洞”,进一步促进nZnO进入细胞[27–28]. 此外,SDBS增强nZnO与藻细胞间静电排斥的同时也增强了两者的疏水相互作用,从而促进了部分nZnO在细胞表面的吸附. 因此SDBS主要通过促进Zn2+的内化来增强nZnO对细胞的毒性作用.
0.81 mg·L–1 nZnO/TX-100复合体系中Zn元素在溶液、细胞表面和细胞内的分布比例分别为64.9%、18.1%和16.9%,与nZnO单一体系相比细胞表面和细胞内Zn元素含量无显著变化(P < 0.05). 而在1.62 mg·L–1、9.72 mg·L–1 nZnO/TX-100复合体系中Zn元素在溶液、细胞表面和细胞内的分布比例分别为48.5%、21.9%、27.9%和51.5%、21.5%、27.1%,与nZnO单一体系相比细胞表面和细胞内Zn元素含量分别降低1.3%、3.2%和6.7%、6.4%. 随着nZnO浓度增大,Zn元素分布趋势逐渐由细胞表面向细胞内转移,但细胞Zn元素含量仍低于nZnO单一体系. 通过对比ZnCl2/TX-100复合体系与ZnCl2单一体系中Zn元素的分布可知,TX-100存在对Zn2+的细胞分布无显著影响. 因而在nZnO浓度较低时,由于TX-100增大了nZnO与藻细胞间的疏水相互作用,Zn元素在细胞表面的含量增加. 随着nZnO浓度增大,吸附在nZnO表面的TX-100可能是通过增大空间位阻效应减少纳米颗粒与细胞间的有效接触[29],从而导致细胞内颗粒态nZnO减少,细胞毒性减弱.
-
(1)nZnO的颗粒态和离子态均能对小球藻产生毒性作用,且离子态较颗粒态的毒性影响更大. nZnO/CTAC、nZnO/SDBS和nZnO/TX-100复合体系对小球藻的联合毒性分别为拮抗作用、协同作用和拮抗作用.
(2)阳离子型表面活性剂CTAC促进nZnO的团聚并抑制Zn2+溶出,增强nZnO与藻细胞间的静电和疏水相互作用,使细胞表面吸附的颗粒态nZnO增加,细胞内Zn2+减少,nZnO毒性降低;阴离子型表面活性剂SDBS促进nZnO的分散及Zn2+溶出,增强nZnO颗粒与藻细胞间的静电排斥,细胞内Zn2+增加,nZnO毒性增强. 非离子表面活性剂TX-100对nZnO的理化性质和Zn2+的细胞分布无显著影响,可能是通过空间位阻作用减少nZnO与藻细胞的有效接触从而减少nZnO颗粒与细胞的接触,细胞内颗粒态nZnO减少导致nZnO毒性减弱.
不同电荷表面活性剂影响下纳米氧化锌对小球藻的毒性效应与细胞分布
The toxic effect and cell distribution of nano-zinc oxide on Chlorella vulgaris under the presence of different-charged surfactants
-
摘要: 水环境中的带电物质可改变可溶性纳米颗粒的理化性质和累积分布,从而影响纳米颗粒对水生生物的毒性效应. 本文探究了3种不同电荷表面活性剂,阳离子型的十六烷基三甲基氯化铵(CTAC)、阴离子型的十二烷基苯磺酸钠(SDBS)、非离子型的聚乙二醇辛基苯基醚(TX-100)存在下纳米氧化锌(nZnO)对小球藻(Chlorella vulgaris)的毒性效应,探明了3种表面活性剂对nZnO性质(电位粒径、Zn2+溶出等)及Zn元素细胞分布的影响. 结果表明,带有不同电荷的表面活性剂与nZnO复合具有不同的联合毒性效应及机制. 阳离子型CTAC促进nZnO的团聚并抑制Zn2+的溶出,增强nZnO与藻细胞间的静电相互作用,使细胞表面吸附的颗粒态nZnO增加,其联合毒性表现为拮抗作用;阴离子型SDBS促进nZnO的分散和Zn2+的溶出,增强nZnO与藻细胞间的静电排斥,使细胞内Zn2+含量增加,其联合毒性表现为协同作用;非离子型TX-100对Zn2+的细胞分布无显著影响,可能是通过空间位阻作用减少nZnO颗粒与细胞接触,使细胞内颗粒态nZnO降低,其联合毒性表现为拮抗作用. 研究结果为更准确、真实地评价纳米颗粒在含有不同电荷物质的水环境中的生态风险提供了理论依据.Abstract: The charged species in aqueous environments can alter the physicochemical properties, accumulation, and distribution of solubel nanoparticles, thereby affecting their toxicity on aquatic life. This study investigated the toxicity of zinc oxide nanoparticles (nZnO) on Chlorella vulgaris (C. vulgaris) under the stress of three different-charged surfactants, including the cationic surfactant cetyltrimethyl ammonium chloride (CTAC), anionic surfactant sodium dodecyl benzene sulfonate (SDBS), and nonionic surfactant octyl phenoxy polyethoxyethanol (TX-100). Meanwhile, we determined the effect of these surfactants on the properties of nZnO (potential, particle size, dissolution of Zn2+, etc.) and the intracellular distribution of Zn. Results showed that nZnO presented different combined toxicities and mechanisms after interacting with different-charged surfactants. Cationic CTAC promoted the agglomeration of nZnO but inhibited the dissolution of Zn2+. Moreover, CTAC enhanced the electrostatic attraction between nZnO and the algal cells, reducing the accumulation of nZnO in cells. This indicated an antagonistic effect of nZnO and CTAC on algal growth. In contract, a synergistic effect of anionic SDBS and nZnO was comfirmed because SDBS promoted the dispersion and dissolution of nZnO, and increased intracellular accumulation of Zn2+. Furthermore, nonionic TX-100 exhibited no significant effect on the cell distribution of Zn2+ and decreased intracellular accumulation of granular nZnO due to the steric hindrance. Thus the combined toxicity of nZnO and TX-100 on C. vulgaris exhibited an antagonistic effect. These results will provide more accurate and authentic evidence of the biological effects of nanoparticles in aqueous environments with different charged species.
-

-
表 1 nZnO/不同电荷表面活性剂复合体系对小球藻的联合毒性评价
Table 1. Joint toxicity evaluation of nZnO/surfactant with different charges on Chlorella vulgaris
实验组
The experimental group评价方法
Evaluation method评价参数
Evaluation parameters评价结果
Evaluation resultsnZnO+0.2 mg·L–1 CTAC
(阳离子型Cationic)毒性单位法 M>M0(3.22>1.41) 拮抗作用 相加指数法 AI<0(AI= –2.23) 相似性参数法 0<λ<1(λ= 0.28) 混合毒性指数法 MTI<0(MTI= –2.35) nZnO+20 mg·L–1 SDBS
(阴离子型Anionic)毒性单位法 M<1(M= 0.97) 协同作用 相加指数法 AI>0(AI= 0.03) 相似性参数法 λ>1(λ= 1.05) 混合毒性指数法 MTI>1(MTI= 1.05) nZnO+100 mg·L–1 TX-100(非离子型Nonionic) 毒性单位法 M>M0(5.56>1.14) 拮抗作用 相加指数法 AI<0(AI= –4.56) 相似性参数法 0<λ<1(λ= 0.71) 混合毒性指数法 MTI<0(MTI= –11.62) 表 2 不同试验组生物量、积累量、投加量和溶出量间的相关性分析
Table 2. Correlation analysis among different test groups' biomass, accumulation, dosage, and dissolution
生物量
BiomassZn元素积累量
Accumulation of ZnZn2+积累量
Accumulation of Zn2+Zn2+投加量
Dosage of Zn2+Zn2+溶出量
Dissolution of Zn2+生物量 1 –0.538 –0.916** –0.946** –0.593* Zn元素积累量 1 0.813** 0.669* Zn2+积累量 1 0.979** 0.852** Zn2+投加量 1 Zn2+溶出量 1 注:*P<0.05, **P<0.01. -
[1] JANKOVIĆ N Z, PLATA D L. Engineered nanomaterials in the context of global element cycles [J]. Environmental Science:Nano, 2019, 6(9): 2697-2711. doi: 10.1039/C9EN00322C [2] YE N, WANG Z, WANG S, et al. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions [J]. Nanotoxicology, 2018, 12(5): 423-438. doi: 10.1080/17435390.2018.1458342 [3] XIN X Y, HUANG G H, ZHANG B Y, et al. Trophic transfer potential of nTiO2, nZnO, and triclosan in an algae-algae eating fish food chain [J]. Aquatic Toxicology, 2021, 235: 105824. doi: 10.1016/j.aquatox.2021.105824 [4] KHAN R, INAM M A, KHAN S, et al. Interaction between persistent organic pollutants and ZnO NPs in synthetic and natural waters [J]. Nanomaterials (Basel, Switzerland), 2019, 9(3): 472. doi: 10.3390/nano9030472 [5] ZHU K Y, ZHANG L, MU L, et al. Antagonistic effect of zinc oxide nanoparticle and surfactant on anaerobic digestion: Focusing on the microbial community changes and interactive mechanism [J]. Bioresource Technology, 2020, 297: 122382. doi: 10.1016/j.biortech.2019.122382 [6] HERNÁNDEZ-MORENO D, VALDEHITA A, CONDE E, et al. Acute toxic effects caused by the co-exposure of nanoparticles of ZnO and Cu in rainbow trout [J]. Science of the Total Environment, 2019, 687: 24-33. doi: 10.1016/j.scitotenv.2019.06.084 [7] ZHAO L, JI Y, SUN P Z, et al. Effects of individual and complex ciprofloxacin, fullerene C60, and ZnO nanoparticles on sludge digestion: Methane production, metabolism, and microbial community [J]. Bioresource Technology, 2018, 267: 46-53. doi: 10.1016/j.biortech.2018.07.024 [8] KHAN A U H, LIU Y J, NAIDU R, et al. Interactions between zinc oxide nanoparticles and hexabromocyclododecane in simulated waters [J]. Environmental Technology & Innovation, 2021, 24: 102078. [9] LI M, PEI J C, TANG X M, et al. Effects of surfactants on the combined toxicity of TiO2 nanoparticles and cadmium to Escherichia coli [J]. Journal of Environmental Sciences, 2018, 74: 126-133. doi: 10.1016/j.jes.2018.02.016 [10] LIU N, WANG Y P, GE F, et al. Antagonistic effect of nano-ZnO and cetyltrimethyl ammonium chloride on the growth of Chlorella vulgaris: Dissolution and accumulation of nano-ZnO [J]. Chemosphere, 2018, 196: 566-574. doi: 10.1016/j.chemosphere.2017.12.184 [11] OECD. Current Approaches in the Statistical Analysis of Ecotoxicity Data[M]. OECD, 2006. [12] ROSENBERG M. Basic and applied aspects of microbial adhesion at the hydrocarbon: Water interface [J]. Critical Reviews in Microbiology, 1991, 18(2): 159-173. doi: 10.3109/10408419109113512 [13] 王懿鹏. 纳米ZnO/CTAC复合污染体系对小球藻生长的影响[D]. 湘潭: 湘潭大学, 2016.WANG Y P. The joint effect of binary mixtures of nano-ZnO and cetyltrimethyl ammonium chloride on the growth of Chlorella vulgaris[D]. Xiangtan: Xiangtan University, 2016(in Chinese). [14] LI X K, YONEDA M, SHIMADA Y, et al. Effect of surfactants on the aggregation and sedimentation of zinc oxide nanomaterial in natural water matrices [J]. Science of the Total Environment, 2017, 581/582: 649-656. doi: 10.1016/j.scitotenv.2016.12.175 [15] ABD EL-LATEEF H M, KHALAF ALI M M, SALEH M M. Adsorption and removal of cationic and anionic surfactants using zero-valent iron nanoparticles [J]. Journal of Molecular Liquids, 2018, 268: 497-505. doi: 10.1016/j.molliq.2018.07.093 [16] JIA H, WU H Y, WU T, et al. Investigation on the adsorption mechanism and model of didodecyldimethylammonium bromide on ZnO nanoparticles at the oil/water interface [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2020, 585: 124159. doi: 10.1016/j.colsurfa.2019.124159 [17] VERA-LÓPEZ S, MARTÍNEZ P, SAN ANDRÉS M P, et al. Study of graphene dispersions in sodium dodecylsulfate by steady-state fluorescence of Pyrene [J]. Journal of Colloid and Interface Science, 2018, 514: 415-424. doi: 10.1016/j.jcis.2017.12.052 [18] LI R M, WANG Z R, GU X F, et al. Study on the assembly structure variation of cetyltrimethylammonium bromide on the surface of gold nanoparticles [J]. ACS Omega, 2020, 5(10): 4943-4952. doi: 10.1021/acsomega.9b03823 [19] 许银, 葛飞, 陶能国, 等. 十六烷基三甲基氯化铵抑制小球藻生长的效应及作用机制 [J]. 环境科学, 2009, 30(6): 1767-1772. doi: 10.3321/j.issn:0250-3301.2009.06.036 XU Y, GE F, TAO N G, et al. Growth inhibition and mechanism of cetyltrimethyl ammonium chloride on Chlorella vulgaris [J]. Environmental Science, 2009, 30(6): 1767-1772(in Chinese). doi: 10.3321/j.issn:0250-3301.2009.06.036
[20] JIA H, HUANG W J, HAN Y G, et al. Systematic investigation on the interaction between SiO2 nanoparticles with different surface affinity and various surfactants [J]. Journal of Molecular Liquids, 2020, 304: 112777. doi: 10.1016/j.molliq.2020.112777 [21] AHAMED A, LIANG L L, LEE M Y, et al. Too small to matter?Physicochemical transformation and toxicity of engineered nTiO2, nSiO2, nZnO, carbon nanotubes, and nAg [J]. Journal of Hazardous Materials, 2021, 404: 124107. doi: 10.1016/j.jhazmat.2020.124107 [22] ADITYA A, CHATTOPADHYAY S, GUPTA N, et al. ZnO nanoparticles modified with an amphipathic peptide show improved photoprotection in skin [J]. ACS Applied Materials & Interfaces, 2019, 11(1): 56-72. [23] YIN J Y, DONG Z M, LIU Y Y, et al. Toxicity of reduced graphene oxide modified by metals in microalgae: Effect of the surface properties of algal cells and nanomaterials [J]. Carbon, 2020, 169: 182-192. doi: 10.1016/j.carbon.2020.07.057 [24] LIU J B, XU L M, ZHU F F, et al. Effects of surfactants on the remediation of petroleum contaminated soil and surface hydrophobicity of petroleum hydrocarbon degrading flora [J]. Environmental Engineering Research, 2021, 26(5): 200384. [25] MASAKORALA K, TURNER A, BROWN M T. Toxicity of synthetic surfactants to the marine macroalga, Ulva lactuca [J]. Water, Air, & Soil Pollution, 2011, 218(1/2/3/4): 283-291. [26] WAN J P, WANG R T, WANG R L, et al. Comparative physiological and transcriptomic analyses reveal the toxic effects of ZnO nanoparticles on plant growth [J]. Environmental Science & Technology, 2019, 53(8): 4235-4244. [27] GODOY-GALLARDO M, ECKHARD U, DELGADO L M, et al. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications [J]. Bioactive Materials, 2021, 6(12): 4470-4490. doi: 10.1016/j.bioactmat.2021.04.033 [28] ZHAO J, ZHANG B W, ZUO J E. Response of anammox granules to ZnO nanoparticles at ambient temperature [J]. Environmental Technology & Innovation, 2019, 13: 146-152. [29] WANG D L, LIN Z F, YAO Z F, et al. Surfactants present complex joint effects on the toxicities of metal oxide nanoparticles [J]. Chemosphere, 2014, 108: 70-75. doi: 10.1016/j.chemosphere.2014.03.010 -




 下载:
下载: