-
随着社会经济的飞速发展和城市化进程的不断推进,湖泊富营养化问题尤为突出[1-2]。磷元素是导致湖泊富营养化的关键因子[3-4],去除湖泊中磷元素的主要形态-磷酸盐对于湖泊富营养化治理意义重大。目前常用的除磷技术有化学沉淀法[5]、生物处理法[6]和吸附法[7-8]。化学沉淀法是通过药剂与磷酸盐形成沉淀达到除磷的目的,除磷效果容易受到水体pH的影响且药剂费用较高;生物处理法容易受到水体中COD影响;吸附法是通过吸附、离子交换等过程将水体中的磷转移到固体材料中达到除磷功效,其工艺操作简单,处理效果较好。改性粘土[9-10]、改性生物炭[11-12]等在国内外除磷案例中应用较为广泛。天然膨润土是以蒙脱石为主要成分的粘土矿物,因其具有较大的比表面积和较强的吸附能力而被广泛用于废水处理[13]、土壤修复[14]等领域。
层状双金属氢氧化物(layered double hydroxides, LDHs)以其独特的结构和吸附性能被用于去除水中的含氧阴离子[15-17],其化学通式可表示为[M2+1-xM3+x(OH)2]x+(An-)x/n·mH2O,其中 M2+和M3+分别代表 LDHs 主体层板上的二价金属阳离子(Ca2+、Mg2+、Mn2+、Ni2+、Cu2+、Zn2+)和三价金属阳离子(Fe3+、Al3+、Mn3+、Ni3+、La3+),金属氢氧化物层板因部分二价金属离子被三价金属离子同晶置换而带正电荷,位于层间的阴离子An-则起到平衡电荷的作用。带正电荷的层板和层间阴离子的可交换性为LDHs应用于吸附磷酸根提供了有利条件。除此之外,可根据目标污染物的种类选择形成不同LDHs的金属阳离子组合[18],强化除磷效果。目前,国内外关于LDHs的研究逐渐由二元LDHs过渡到三元LDHs,三元LDHs具有比二元LDHs更优秀的物化性质和吸附性能。REZAK[19]用共沉淀法制备Zn-Al-Fe-LDHs,该吸附剂对P(Ⅴ)的最大吸附容量为140.85 mg·g−1,明显高于二元LDHs。但是,将粉末态的LDHs直接投加到湖泊中吸附水体污染物,水体会长时间浑浊,难以固液分离[20]。一些学者研究发现,Zn系LDHs的磷吸附能力最强[21-22],铝盐和镧盐对P(Ⅴ)有极强的亲和性使其经常被用于湖泊除磷[23-24]。笔者前期研究发现,镧很难用于制备二元LDHs,经尝试可以用于制备三元LDHs。因此,本研究制备了三元Zn-Al-La-LDHs,将其负载在吸附性能较好的膨润土表面用于水体锁磷,探究了Zn-Al-La-LDHs改性膨润土的除磷性能及其吸附机理,以期为湖泊富营养化的治理提供参考。
-
本研究使用的所有化学药品均为分析纯级试剂。氯化镧(LaCl3∙xH20)从上海麦克林生化科技有限公司购买,氯化锌(ZnCl2)、氯化铝(AlCl3∙6H20)、氢氧化钠(NaOH)和磷酸二氢钾(KH2PO4)均从国药化学试剂有限公司购买。实验材料选用人工钠基膨润土,从中国东莞市瑞恒矿产品有限公司购置。
-
根据前期研究得到的阶段性成果,采用恒定pH共沉淀法制备Zn-Al-La-LDHs改性膨润土。按Zn : (Al + La)摩尔比为3,Al : La摩尔比为10,称取一定量ZnCl2、AlCl3∙6H20和LaCl3∙xH20配制金属盐的混合溶液100 mL于烧杯中,采用2.5 mol∙L−1的NaOH溶液作为沉淀剂。加入10 g膨润土和一定量的底液(200 mL去离子水)到1 000 mL烧杯中,搅拌均匀,置于磁力搅拌水浴锅中,保持水浴温度40 ℃;分别采用蠕动泵将混合盐溶液和NaOH溶液缓慢的滴入到烧杯的底液中,边滴加边搅拌,并控制反应体系pH 在9 左右,反应2 h,然后100 ℃陈化18 h;陈化产物用去离子水洗涤至中性、过滤、烘干、研磨,即得Zn-Al-La-LDHs改性膨润土。
-
采用扫描电子显微镜(JSM-IT300,日本) 、能谱仪 (X-MaxN20,牛津)、 X射线衍射仪(D8 Advance,德国布鲁克AXS公司)表征样品的微观形貌、元素组成及晶体结构。
-
使用分析纯KH2PO4配置磷质量浓度为0、1、2、4、8、16、32、64 mg∙L−1的不同溶液100 mL,分别加入到一系列250 mL的锥形瓶中,称取0.1 g Zn-Al-La-LDHs改性膨润土投加到反应溶液中,然后将锥形瓶放置于温度25 ℃、转速160 r∙min−1恒温水浴振荡器振荡24 h,过滤后的溶液中磷浓度按照钼锑铵分光光度法测定。改性膨润土的磷吸附量采用式(1)进行计算。
式中:qe为平衡吸附容量,mg·g−1;C0为磷的初始质量浓度,mg·L−1;Ce为磷的平衡质量浓度,mg·L−1;V为溶液体积,L;m为吸附剂干质量,g。
采用Langmuir模型(式(2))、Freundlich 模型(式(3))对不同初始质量浓度下磷酸盐的平衡吸附实验结果进行拟合。
式中:qm为改性膨润土的最大吸附容量,mg·g−1;KL为Langmuir吸附平衡常数;n为非线性系数;KF是Freundlich吸附平衡常数。
-
本研究选取武汉市汤逊湖水样和底泥样为研究对象。在汤逊湖中心取带底泥和上覆水的实验柱5根,柱内水样初始水温20.1 ℃,pH为8.68,溶解氧为5 mg·L−1,柱子内径为8.4 cm,控制上覆水高度为130 cm。然后,测定水柱中底泥和上覆水中的磷含量。汤逊湖上覆水中磷素超标很大程度上来源于底泥中内源磷的释放[25]。因此,以上覆水中溶解性磷酸盐(SRP)以及表层0~5 cm深度底泥中活性磷(NaOH-P)[26-27]作为投加药剂待去除对象计算投药量。采用5根实验柱进行平行对比实验,不同实验柱中Zn-Al-La-LDHs改性膨润土的投加量所依据的除磷量分别为:1#为上覆水中SRP含量;2#为上覆水中SRP含量+33.3%的0~5 cm底泥NaOH-P含量;3#为上覆水中SRP含量+66.7%的0~5 cm底泥NaOH-P含量;4#为上覆水中SRP含量+100%的0~5 cm底泥NaOH-P含量;5#为上覆水中SRP含量+133.3%的0~5 cm底泥NaOH-P含量。
按照Zn-Al-La-LDHs改性膨润土的吸附容量计算5根实验柱各自药剂投加量。将Zn-Al-La-LDHs改性膨润土溶于蒸馏水中,以悬浊液方式投入实验柱,用玻璃棒搅拌表层水10 min使药剂均匀扩散至泥水界面,持续20 d观测水柱中TP、SRP的含量变化(在第1、2、4、6、8、12、16、20天取样)。
-
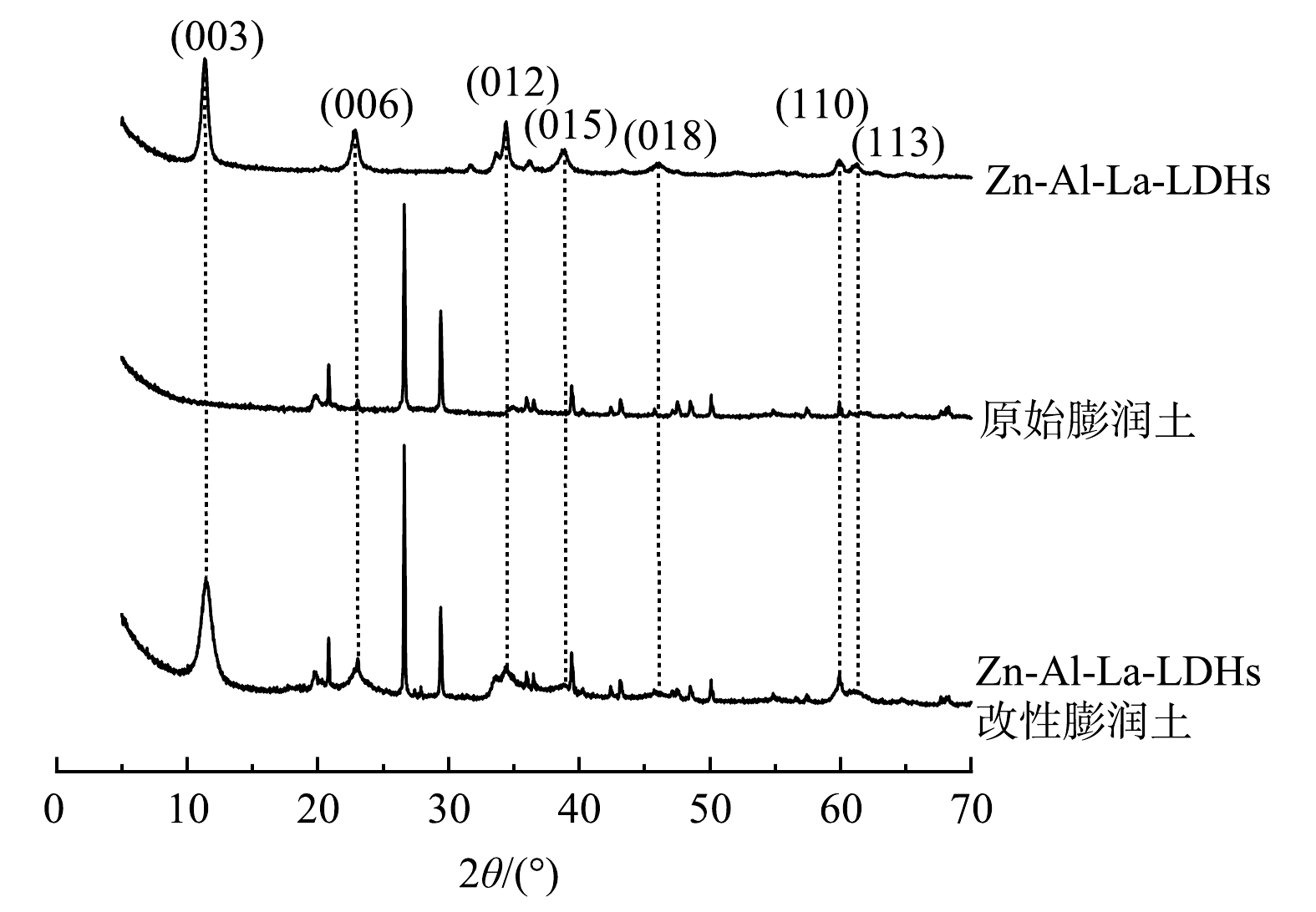
对Zn-Al-La-LDHs、Zn-Al-La-LDHs改性膨润土和原始膨润土进行了X射线衍射(XRD)表征,结果如图1所示。由图1可以看出,Zn-Al-La-LDHs改性膨润土的X射线衍射图谱基线平稳,峰形尖锐,结晶度较好。在11.4°、22.9°、34.4°、38.9°、 46.1°、 60.0° 和 61.3°出现了LDHs的典型特征衍射峰[28-29],Zn-Al-La-LDHs改性膨润土的图谱具有Zn-Al-La-LDHs和原始膨润土强度减弱的特征峰。上述结果表明,Zn-Al-La-LDHs已成功合成并负载于膨润土表面。
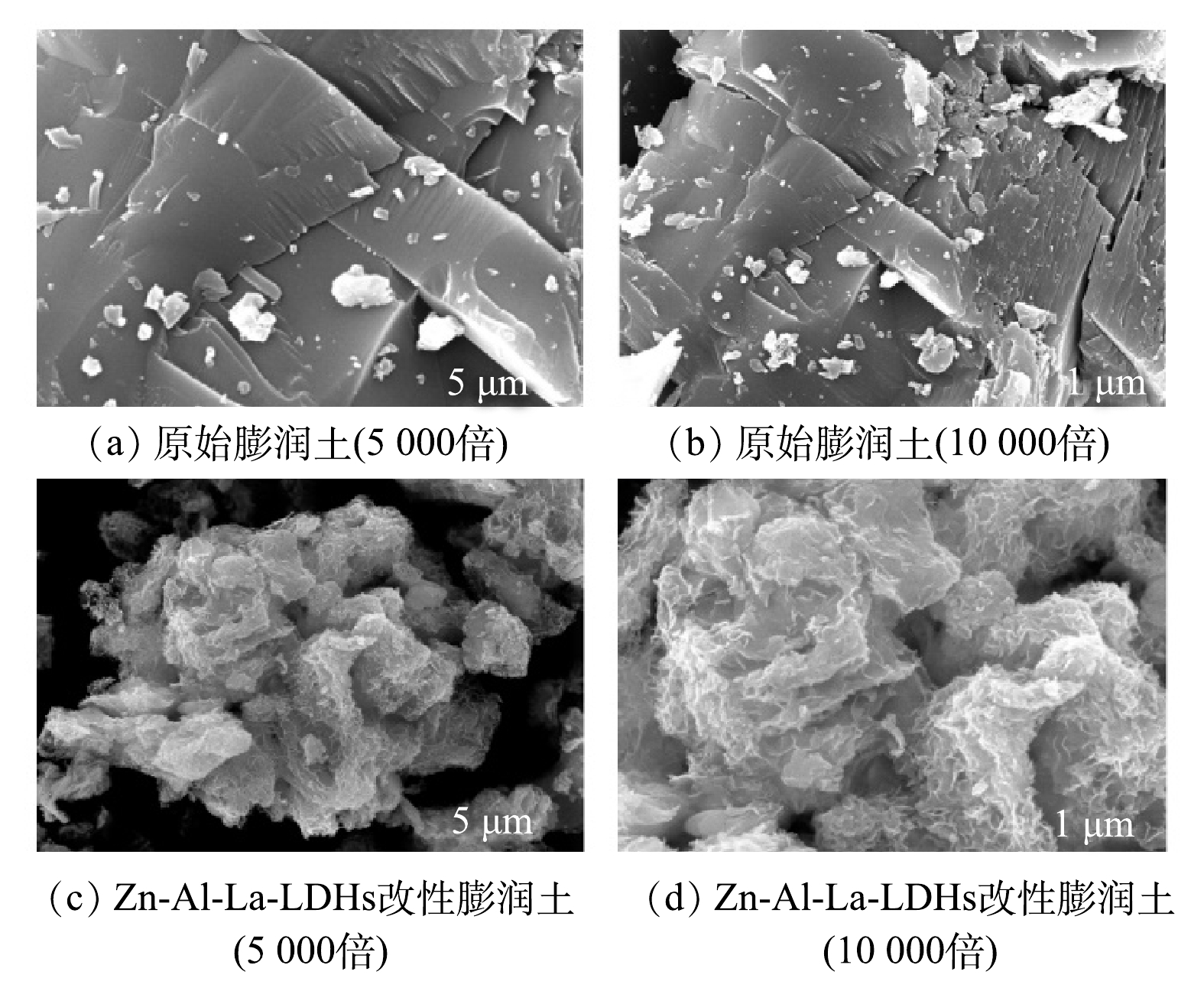
原始膨润土和Zn-Al-La-LDHs改性膨润土的扫描电镜图如图2所示。由图2可清晰看到,除了存在少许细小颗粒之外,原始膨润土表面较为平整;Zn-Al-La-LDHs改性膨润土表面均匀附着多层絮状结晶体,孔隙较多。LDHs改性使膨润土表面的形貌特征发生明显变化。
原始膨润土和Zn-Al-La-LDHs改性膨润土的能谱分析图如图3所示。膨润土主要成分是蒙脱石,主要组成元素为O、Si、Ca,并含有少量Na、Mg、Al、Fe等金属元素。在膨润土表面负载Zn-Al-La-LDHs后,Zn-Al-La-LDHs改性膨润土中检测出了La和Cl元素,而且Zn、Al含量明显增多。改性处理显著改变了原始膨润土的化学成分组成。采用X射线衍射、扫描电镜及能谱分析等技术手段对Zn-Al-La-LDHs改性膨润土进行表征。以上结果表明,Zn-Al-La-LDHs成功合成,且可负载于膨润土表面。
-
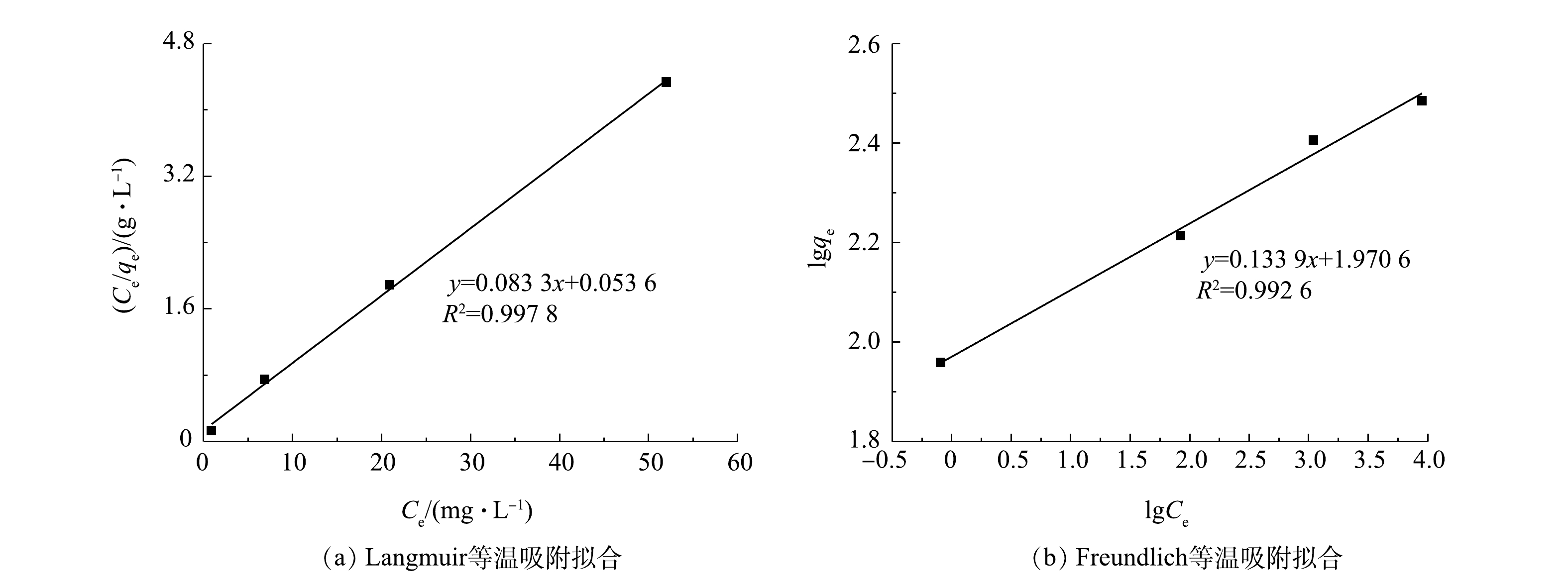
为了探究Zn-Al-La-LDHs改性膨润土的吸附机制和最大吸附容量,以便于进行后续汤逊湖实验水柱的药剂初始投加量计算,对等温吸附实验数据进行了Langmuir和Freundlich吸附模型线性拟合,结果如图4所示,拟合参数如表1所示。
由图4可见,Freundlich和Langmuir 2个吸附模型均能很好地拟合Zn-Al-La-LDHs改性膨润土对磷酸盐的吸附实验结果。这说明吸附过程既有单层吸附又有多层吸附[30]。此外,Zn-Al-La-LDHs改性膨润土吸附能力较强。在静电吸引作用下,带正电荷的LDHs层板会吸引溶液中的含氧阴离子;LDHs层板与层间阴离子存在范德华力、氢键等较弱的作用力[31],水中的阴离子很容易与层间阴离子交换,从而得到去除;LDHs前驱金属种类同样会影响LDHs对含氧阴离子的吸附能力,金属阳离子与6个OH−通过共价作用形成正八面体结构[18],而LDHs层板就是由若干个这样的正八面体叠加而成。由于La3+与磷酸根极强的亲和性[32],水中的磷酸根会与OH−竞争,代替OH−与位于八面体配位中心的La3+形成表面络合物[33]。在静电吸引、表面配合和离子交换作用下,Zn-Al-La-LDHs改性膨润土的最大理论吸附容量达到12 mg∙g−1。目前,国内外应用较为广泛的除磷药剂Phoslock®[34]是一种由膨润土(95%)和稀土镧(5%)的化合物组成的混合物。Phoslock®主要作用原理由化学活性成分镧化合物与水体的磷酸根反应,生成难溶解的磷酸镧沉淀,钝化水土界面的磷,抑制内源磷释放。已有研究表明,Phoslock®的磷吸附能力较强。任琪琪等[9]根据Langmuir 吸附模型计算得出Phoslock®的最大磷吸附量为10.4 mg∙g−1。陈小燕等[35]通过Langmuir吸附等温线对实验数据进行拟合,计算得出Phoslock®的吸附容量为9.88 mg∙g−1。国内西湖、滇池等已经进行了Phoslock®性能测试与应用,效果较为理想[36-37],相比于Phoslock®, Zn-Al-La-LDHs改性膨润土在吸附能力和吸附机理上是具备一定优势的。从分析结果可见,Zn-Al-La-LDHs改性膨润土含镧量(0.56%)小于Phoslock®的含镧量(5%左右)[9-10],吸附容量却略大于Phoslock®,因此,Zn-Al-La-LDHs改性膨润土是更具竞争力的除磷药剂。
-
1)投药量第1阶段实验结果与分析。对从汤逊湖所取的带底泥和上覆水的实验柱进行了上覆水水质和底泥(0~5 cm)泥质检测分析。结果表明,上覆水TP为0.32 mg∙L−1,SRP为0.23 mg∙L−1;底泥密度为1.049 g∙cm−3,相对含水率为75.06%,底泥中NaOH-P为0.566 mg∙g−1。上覆水和底泥含磷量分别采用式(4)和式(5)进行计算。
式中:m1为实验柱内上覆水SRP含量,mg;q1为上覆水SRP浓度,mg·L−1;s为采样柱截面积,cm2;h1为上覆水的计算深度,cm;m2为实验柱内底泥NaOH-P含量,mg;q2为底泥NaOH-P浓度,mg·g−1;h2为底泥的计算深度,cm;ρ为湿泥密度,g∙cm−3;ω为相对含水率。
经过计算,上覆水中SRP含量为1.66 mg。0~5 cm深度的底泥中NaOH-P为40.95 mg。按照Zn-Al-La-LDHs改性膨润土的最大理论吸附量(12 mg∙g−1)对药剂投加量进行计算:1#实验柱为0.14 g;2#实验柱为1.26 g;3#实验柱为2.42 g;4#实验柱为3.55 g;5#实验柱为4.68 g。经过20 d的采样检测,第1阶段不同实验柱上覆水中TP含量变化和SRP含量变化分别如图5和图6所示。
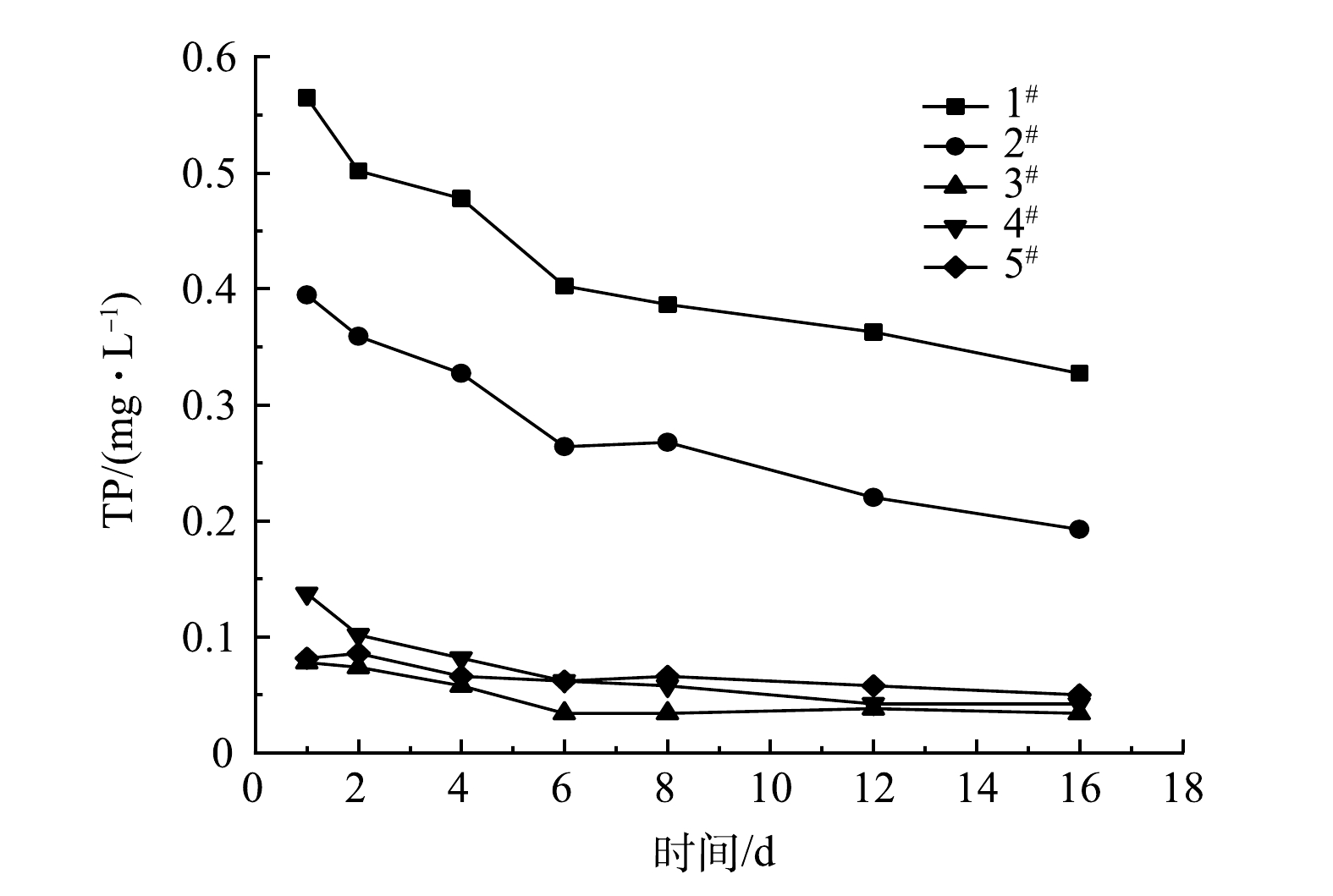
由图5可知,5根实验柱上覆水中TP含量在前2 d迅速降低,然后逐渐增加,第16天达到较为稳定的状态。这是因为Zn-Al-La-LDHs改性膨润土投入实验柱以后,上覆水中磷会迅速被完全吸附,底泥和上覆水中磷浓度平衡遭到破坏,底泥中累积的活性磷大量释放且超出了所投加吸附剂的吸附能力,导致上覆水中磷含量不断增加,直至达到新的磷浓度平衡。据此推断,吸附剂已处于吸附饱和的状态。图6中SRP含量变化趋势与图5中TP含量变化趋势基本一致,说明汤逊湖底泥释磷以溶解性磷酸盐释放为主。依据第20天上覆水含磷量和吸附剂的吸附量,可计算5根实验柱的底泥释磷量,结果见表2。
不同实验柱Zn-Al-La-LDHs改性膨润土初始投药量不同,底泥释磷量也不同。投加量较少的水柱会提前吸附饱和,达到平衡。结果表明,5个实验柱内的吸附剂已经全部达到饱和,投加量越多,稳定时上覆水中的含磷量越少。但5#的投加量较大,其上覆水也没有达到Ⅲ类水的TP水质标准(TP≤0.05 mg∙L−1),说明底泥还有一定程度的磷可释放,但未能完全释放。根据第1阶段初始投药量计算,对汤逊湖底泥释磷量的预估存在一定偏差。
2)投药量第2阶段实验结果与分析。为了使汤逊湖水体达到地表Ⅲ类水的水体功能,对5根实验柱已有的20 d实验结果进行拟合,以推算底泥可释磷量。前20 d内,上覆水中TP检测结果如表3所示。
以相应柱号实验柱实验末期TP浓度(表3中第20天数据)为X,以相应柱号实验柱20 d天内底泥释磷量(表2最后一列)为Y,进行线性拟合,结果如图7所示。
将地表水Ⅲ类水TP=0.05 mg∙L−1作为计算浓度代入线性拟合方程y=-45.40x+66.73中,可计算得到汤逊湖实验柱底泥释磷量为64.46 mg。取底泥活性磷(NaOH-P)含量为64.46 mg,上覆水溶解性磷酸盐(SRP)含量为1.66 mg,需去除磷量为66.12 mg。按照Zn-Al-La-LDHs改性膨润土最大磷吸附量(12 mg∙g−1)计算投药量,设置安全系数10%。若全部去除,计算得到应投加药剂量为6.06 g,5根实验柱的投加量梯度及二次追加投加量如表4所示。
在第1阶段实验结束第2天,以悬浊液方式向5根实验柱追加投药并搅拌表层水10 min,分别在第1、2、4、6、8、12、16天取样测试 TP和SRP含量。第2阶段不同实验柱上覆水中TP含量变化和SRP含量变化分别如图8和图9所示。
5根实验柱在追加相应质量Zn-Al-La-LDHs改性膨润土后,上覆水中TP、SRP含量同步降低,鲜有回升迹象。这说明吸附剂的吸附能力已强于底泥释磷能力,并持续到平衡阶段。3#、4#、5#的上覆水TP含量均达到地表水Ⅲ类水标准且较为稳定,3#上覆水甚至接近Ⅱ类水要求(TP≤0.025 mg·L−1),同时3#、4#、5#溶解性磷酸盐(SRP)基本去除,说明推算出的底泥释磷量比较准确。根据实验柱投药量计算所依据的底泥释磷量,进而可推算底泥有效释磷深度。不同深度底泥NaOH-P的含量为:0~5 cm为0.566 mg·g−1;5~10 cm为0.532 mg·g−1;10~15 cm为0.463 mg·g−1;15~20 cm为0.424 mg·g−1。
当底泥释磷量为64.46 mg时,据式(5)和底泥中NaOH-P含量的垂直分布情况可计算得到底泥有效释磷深度为8 cm。Zn-Al-La-LDHs改性膨润土的投药量根据式(6)进行计算。
式中:mt为Zn-Al-La-LDHs改性膨润土的投药量,g;m1为上覆水SRP含量,g;m2为0~8 cm表层底泥NaOH-P含量,g;θ为安全系数,取10%;qm为Zn-Al-La-LDHs改性膨润土的最大吸附容量,mg·g−1。
底泥有效释磷深度与沉积物本身活性磷的含量以及底泥活性磷在不同深度的垂直分布结构密切相关。底泥深度越大,活性磷的含量越小。首先,沉积物0~10 cm的磷是可以参与到湖泊新陈代谢的,甚至深度达20 cm的磷也可以释放到上覆水中,这取决于湖泊磷污染的高负荷历史[38]。其次,湖泊环境条件同样影响内源磷的释放[39-40]。水力扰动会引起底泥再悬浮,增加泥-水界面磷交换,磷酸盐从高浓度向低浓度扩散,加剧底泥中内源磷释放;温度升高会加速内源磷释放的物理化学反应速率,加快内源磷的释放,这也是许多湖泊夏季富营养化程度增强的原因;pH和溶解氧通过改变活性磷的形态促进内源磷的释放。本研究取夏季汤逊湖5根带底泥和上覆水的实验柱,将Zn-Al-La-LDHs改性膨润土作为新的除磷药剂进行投药量实验,推算得到汤逊湖底泥有效释磷深度约为8 cm,作为投药量公式的计算依据,相比于按照湖泊面积或者湖泊体积计算投药量的传统方式,本研究所提出的基于除磷成因的投药量计算公式可为解决湖泊富营养化关键因子磷的问题提供参考。
-
1)采用X射线衍射仪、扫描电镜、能谱仪对原始及改性膨润土进行表征,检测结果表明:Zn-Al-La-LDHs具有双金属氢氧化物(LDHs)的典型特征峰,将其负载在膨润土表面在技术上是可行的。
2) Langmuir和Freundlich吸附模型均能很好地拟合Zn-Al-La-LDHs改性膨润土吸附磷酸盐的实验结果,吸附过程同时存在单层吸附和多层吸附。在静电吸引、表面配合和离子交换作用下,Zn-Al-La-LDHs改性膨润土的最大吸附容量可达到12 mg∙g−1。
3)在汤逊湖中心取5根带底泥和上覆水的样柱进行投药量实验。经过两阶段药剂投加实验,推算得到底泥的有效释磷深度为8 cm。根据上覆水中SRP含量、表层8 cm底泥中NaOH-P含量、吸附剂最大吸附容量及投药量安全系数10%,可计算得到所需的改性膨润土投加量,投药后可将水柱上覆水中TP浓度锁定在0.05 mg∙L−1以下,达到或优于地表水Ⅲ类水体有关TP的水质要求在技术上是可行的。
Zn-Al-La-LDHs改性膨润土对富营养化湖泊中磷的锁定效果
Locking effect of phosphorus in eutrophic lakes by bentonite/Zn-Al-La-LDHs
-
摘要: 采用恒定pH共沉淀法制备了Zn-Al-La-LDHs改性膨润土,通过X射线衍射仪(XRD)、扫描电子显微镜(SEM)、能谱仪(EDS)对样品微观形貌、元素组成、晶体结构进行了表征。结果表明:Zn-Al-La-LDHs已成功合成并负载于膨润土表面;Langmuir和Freundlich吸附模型均能很好地拟合Zn-Al-La-LDHs改性膨润土对磷酸根的吸附过程,吸附过程中同时存在单层吸附和多层吸附,最大吸附容量可达12 mg∙g−1。在富营养化程度较高的汤逊湖中心取5根带底泥和上覆水的样柱,进行了除磷药剂投加量实验。结果表明,根据上覆水溶解性磷酸盐(SRP)含量、表层8 cm底泥中活性磷(NaOH-P)含量、投药量安全系数(取10%)、改性膨润土吸附容量,可计算得到投药量;据前述计算结果投加所需的除磷药剂后,汤逊湖实验柱上覆水总磷(TP)质量浓度锁定在0.05 mg∙L−1以下,达到或优于地表水Ⅲ类水体有关总磷的水质要求(TP≤0.05 mg∙L−1)。以上结果说明所研制的Zn-Al-La-LDHs改性膨润土可用于富营养化水体的控磷。
-
关键词:
- 湖泊 /
- Zn-Al-La-LDHs /
- 改性膨润土 /
- 吸附剂 /
- 除磷
Abstract: Bentonite/Zn-Al-La-LDHs was prepared by constant pH coprecipitation method. The microstructure, elemental composition and crystal structure of the bentonite/Zn-Al-La-LDHs samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). The results showed that Zn-Al-La-LDHs was successfully synthesized and loaded on the surface of bentonite. Langmuir and Freundlich models could well fit the isothermal adsorption process of bentonite/Zn-Al-La-LDHs toward phosphate, which contained the single-layer and multi-layer adsorption modes at the same time, and the maximum adsorption capacity was 12 mg∙g−1. Five sample columns with sediment and overlying water were taken from the center of Tangxun Lake with high eutrophication degree to test the dosage of phosphorus removal agent. The results showed that the dosage could be calculated according to the content of dissolved phosphate (SRP) in overlying water, the content of active phosphorus (NaOH-P) in 8 cm surface sediment, the dosage saftey coefficient of 10% and the adsorption capacity of modified bentonite. After the required phosphorus removal agent was added, the concentration of total phosphorus (TP) in the overlying water of Tangxun Lake test column was locked below 0.05 mg∙L−1, which met or exceeded the water quality requirements of TP content in class III water body of surface water (TP≤0.05 mg∙L−1). The bentonite/Zn-Al-La-LDHs can be used for phosphorus control in eutrophic water.-
Key words:
- lake /
- Zn-Al-La-LDHs /
- modified bentonite /
- adsorbent /
- phosphorus removal
-

-
表 1 等温吸附模型拟合参数
Table 1. Fitting parameters of adsorption isothermal model
吸附剂 Langmuir Freundlich qm/(mg·g−1) KL R2 1/n KF R2 Zn-Al-La-LDHs
改性膨润土12.00 1.55 0.997 8 0.13 7.17 0.992 6 表 2 20 d内5根汤逊湖实验柱的底泥释磷量
Table 2. Phosphorus release from sediment in five Tangxun Lake test columns within 20 days
实验
柱号TP/
(mg∙L−1)上覆水
TP/mg原上覆水
TP/mg吸附剂
吸附量/mg底泥释
磷量/mg1# 1.27 9.15 2.30 1.66 9.15 2# 0.94 6.78 2.30 15.17 19.65 3# 0.69 4.95 2.30 29.10 31.75 4# 0.66 4.75 2.30 42.61 45.06 5# 0.19 1.36 2.30 56.12 55.18 表 3 上覆水中TP浓度随时间变化的检测结果
Table 3. Test results of TP concentration in overlying water over time
mg∙L−1 实验
柱号不同时间下TP浓度 第1天 第2天 第4天 第6天 第8天 第12天 第16天 第20天 1# 0.29 0.26 0.56 0.86 1.16 1.29 1.31 1.27 2# 0.17 0.13 0.25 0.41 0.54 0.76 0.93 0.94 3# 0.14 0.13 0.37 0.46 0.49 0.58 0.68 0.69 4# 0.14 0.10 0.15 0.30 0.46 0.61 0.66 0.66 5# 0.14 0.10 0.26 0.24 0.25 0.21 0.22 0.19 表 4 二次投加量梯度设置及追加投药量
Table 4. Gradient setting of secondary dosage and additional dosage
实验柱号 投药量梯度/%m 总投药量/g 一次投药量/g 追加投药量/g 1# 50 3.03 0.14 2.89 2# 75 4.54 1.26 3.28 3# 150 9.09 2.42 6.67 4# 125 7.58 3.55 4.03 5# 100 6.06 4.68 1.38 -
[1] LIU Y M, CHEN W, LI D H, et al. Cyanobacteria-/cyanotoxin-contaminations and eutrophication status before Wuxi drinking water crisis in Lake Taihu, China[J]. Journal of Environmental Sciences, 2011, 23(4): 575-581. doi: 10.1016/S1001-0742(10)60450-0 [2] 秦伯强. 我国湖泊富营养化及其水环境安全[J]. 科学对社会的影响, 2007(3): 17-23. [3] PU J, WANG S R, NI Z K, et al. Implications of phosphorus partitioning at the suspended particle-water interface for lake eutrophication in China's largest freshwater lake, Poyang Lake[J]. Chemosphere, 2021, 263: 128334. doi: 10.1016/j.chemosphere.2020.128334 [4] AUVRAY F, VAN HULLEBUSCH E D, DELUCHAT V, et al. Laboratory investigation of the phosphorus removal (SRP and TP) from eutrophic lake water treated with aluminium[J]. Water Research, 2006, 40(14): 2713-2719. doi: 10.1016/j.watres.2006.04.042 [5] 孟顺龙, 裘丽萍, 陈家长, 等. 污水化学沉淀法除磷研究进展[J]. 中国农学通报, 2012, 28(35): 264-268. doi: 10.3969/j.issn.1000-6850.2012.35.049 [6] ZHANG C, GUISASOLA A, BAEZA J A. Achieving simultaneous biological COD and phosphorus removal in a continuous anaerobic/aerobic A-stage system[J]. Water Research, 2021, 190: 116703. doi: 10.1016/j.watres.2020.116703 [7] GAN F Q, ZHOU J M, WANG H Y, et al. Removal of phosphate from aqueous solution by thermally treated natural palygorskite[J]. Water Research, 2009, 43(11): 2907-2915. doi: 10.1016/j.watres.2009.03.051 [8] GOSCIANSKA J, PTASZKOWSKA-KONIARZ M, FRANKOWSKI M, et al. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash[J]. Journal of Colloid and Interface Science, 2018, 513: 72-81. doi: 10.1016/j.jcis.2017.11.003 [9] 任琪琪, 唐婉莹, 殷鹏, 等. 镧改性膨润土对底泥内源磷控制效果[J]. 中国环境科学, 2021, 41(1): 199-206. doi: 10.3969/j.issn.1000-6923.2021.01.023 [10] 林娟, 姚佳雯, 魏笑, 等. 镧改性膨润土对磷吸附特性、机理与影响因素[J]. 环境科学与技术, 2021, 44(1): 7-12. [11] WANG Z J, MIAO R R, NING P, et al. From wastes to functions: A paper mill sludge-based calcium-containing porous biochar adsorbent for phosphorus removal[J]. Journal of Colloid and Interface Science, 2021, 593: 434-446. doi: 10.1016/j.jcis.2021.02.118 [12] ZHU D C, CHEN Y Q, YANG H P, et al. Synthesis and characterization of magnesium oxide nanoparticle-containing biochar composites for efficient phosphorus removal from aqueous solution[J]. Chemosphere, 2020, 247: 125847. doi: 10.1016/j.chemosphere.2020.125847 [13] 王泽龙, 李顺义, 吴朕君. 膨润土改性和复配及在废水处理中的应用进展[J]. 工业水处理, 2022, 42(2): 11-18. [14] 赵军超, 王权, 任秀娜, 等. 钙基膨润土辅助对堆肥及土壤Cu、Zn形态转化和白菜吸收的影响[J]. 环境科学, 2018, 39(4): 1926-1933. [15] ZHANG X L, XUE Y, GAO J T, et al. Comparison of adsorption mechanisms for cadmium removal by modified zeolites and sands coated with Zn-layered double hydroxides[J]. Chemical Engineering Journal, 2020, 380: 122578. doi: 10.1016/j.cej.2019.122578 [16] TANG Y Q, LIAO X S, ZHANG X L, et al. Enhanced adsorption of hexavalent chromium and the microbial effect on quartz sand modified with Al-layered double hydroxides[J]. Science of the Total Environment, 2021, 762: 143094. doi: 10.1016/j.scitotenv.2020.143094 [17] 胡美艳, 张翔凌, 姬筠森, 等. 两种碳酸系Fe-LDHs负载改性沸石对Cd(Ⅱ)吸附特性对比研究[J]. 环境科学研究, 2021, 34(11): 2655-2664. [18] GOH K H, LIM T T, DONG Z. Application of layered double hydroxides for removal of oxyanions: a review[J]. Water Research, 2008, 42(6/7): 1343-1368. [19] REZAK N, BAHMANI A, BETTAHAR N. Adsorptive removal of P(V) and Cr(VI) by calcined Zn-Al-Fe ternary LDHs[J]. Water Science and Technology, 2021, 83(10): 2504-2517. doi: 10.2166/wst.2021.123 [20] 付瑜玲, 严晗璐, 姚天启, 等. 改性镁铝水滑石对黑臭水体中磷的去除效果研究[J]. 环境科学学报, 2021, 41(10): 4032-4038. [21] 张翔凌, 黄华玲, 郭露, 等. Zn系LDHs覆膜改性人工湿地沸石基质除磷机制[J]. 环境科学, 2016, 37(8): 3058-3066. [22] 张翔凌, 陈俊杰, 郭露, 等. 垂直流人工湿地LDHs覆膜改性沸石基质强化除磷效果及其机制[J]. 环境科学, 2014, 35(12): 4553-4559. [23] YIN H, REN C, LI W. Introducing hydrate aluminum into porous thermally-treated calcium-rich attapulgite to enhance its phosphorus sorption capacity for sediment internal loading management[J]. Chemical Engineering Journal, 2018, 348: 704-712. doi: 10.1016/j.cej.2018.05.065 [24] WANG Z, LU S, WU D, et al. Control of internal phosphorus loading in eutrophic lakes using lanthanum-modified zeolite[J]. Chemical Engineering Journal, 2017, 327: 505-513. doi: 10.1016/j.cej.2017.06.111 [25] 范中亚, 王文才, 蒋锦刚, 等. 华阳河湖群沉积物内源磷释放风险及控制策略[J]. 环境科学研究, 2020, 33(5): 1170-1178. [26] 刘辉, 胡林娜, 朱梦圆, 等. 沉积物有效态磷对湖库富营养化的指示及适用性[J]. 环境科学, 2019, 40(9): 4023-4032. [27] 金晓丹, 吴昊, 陈志明, 等. 长江河口水库沉积物磷形态、吸附和释放特性[J]. 环境科学, 2015, 36(2): 448-456. [28] RAHMAN S, NAVARATHNA C M, KRISHNA D N, et al. High capacity aqueous phosphate reclamation using Fe/Mg-layered double hydroxide (LDH) dispersed on biochar[J]. Journal of Colloid and Interface Science, 2021, 597: 182-195. doi: 10.1016/j.jcis.2021.03.114 [29] WANG G R, JIN Z L, ZHANG W X. Ostensibly phosphatized NiAl LDHs nanoflowers with remarkable charge storage property for asymmetric supercapacitors[J]. Journal of Colloid and Interface Science, 2020, 577: 115-126. doi: 10.1016/j.jcis.2020.05.032 [30] WANG P, DU M L, ZHU H, et al. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism[J]. Journal of Hazardous Materials, 2015, 286: 533-544. doi: 10.1016/j.jhazmat.2014.12.034 [31] PENG X M, LUO W D, WANG M, et al. Insights into the adsorption mechanism of carbon cellulose fiber loaded globular flowers bimetallic layered double hydroxide for efficiency pollutant removal[J]. Journal of Molecular Liquids, 2019, 290: 111201. doi: 10.1016/j.molliq.2019.111201 [32] CHEN H Y, LU C, YANG H M. Lanthanum compounds-modified rectorite composites for highly efficient phosphate removal from wastewater[J]. Applied Clay Science, 2020, 199: 105875. doi: 10.1016/j.clay.2020.105875 [33] ZHANG X L, GUO L, HUANG H L, et al. Removal of phosphorus by the core-shell bio-ceramic/Zn-layered double hydroxides (LDHs) composites for municipal wastewater treatment in constructed rapid infiltration system[J]. Water Research, 2016, 96: 280-291. doi: 10.1016/j.watres.2016.03.063 [34] ROBB M, GREENOP B, GOSS Z, et al. Application of PhoslockTM, an innovative phosphorus binding clay, to two Western Australian waterways: Preliminary findings[J]. Hydrobiologia, 2003, 494(1/2/3): 237-243. [35] 陈小燕, 刘键熙, 苏玉萍, 等. 两种锁磷剂锁磷效果对比研究[J]. 福建师范大学学报(自然科学版), 2016, 32(4): 56-60. [36] 朱广伟, 李静, 朱梦圆, 等. 锁磷剂对杭州西湖底泥磷释放的控制效果[J]. 环境科学, 2017, 38(4): 1451-1459. [37] 余先旭, 孙珮石, 朱宝平, 等. 锁磷剂(Phoslock)对滇池水体的除磷试验研究[J]. 贵州环保科技, 2006(1): 6-9. [38] SONDERGAARD M, JENSEN J P, JEPPESEN E. Role of sediment and internal loading of phosphorus in shallow lakes[J]. Hydrobiologia, 2003, 506(1/2/3): 135-145. [39] 蔡顺智, 李大鹏, 王忍, 等. 多重扰动对湖泊内源磷迁移转化的影响[J]. 环境科学, 2016, 37(11): 4203-4211. [40] 高湘, 李妍, 何怡. 湖泊底泥磷释放及磷形态变化[J]. 环境工程学报, 2015, 9(7): 3350-3354. doi: 10.12030/j.cjee.20150745 -



 下载:
下载: