-
在水处理技术中,混凝是一种常见的工艺,其可以与许多其他处理方法(膜滤、氧化等)相结合,从而达到净水目的[1]。近年来,不同的混凝变量(混凝剂的类型和浓度)以及絮体的特性(再生强度和再生能力)受到了相当大的关注[2-4]。铝盐和铁盐均为常见的混凝剂,与铝盐混凝剂相比,铁盐混凝剂可以在更宽的pH和温度范围内使用,而且铁盐更容易生成较大的絮体,更利于沉降分离[5-6]。在混凝过程中,Fe(Ⅱ)/Fe(Ⅲ)盐会快速水解成铁氢氧化物纳米颗粒,而后聚集形成絮体沉淀,而且随着纳米颗粒的聚集和结晶,其结构也会发生变化[7-8]。大量研究表明,铁(氢)氧化物的结晶和转化,会显著影响污染物(磷、砷等)的迁移[9-10]。比如,老化结晶过程会导致絮体的比表面积变小,进而导致其去除磷酸盐的效率变低。结晶和转化过程一般可以用2种不同的生长模式来描述:一种是奥斯瓦尔德熟化,其描述了一种非均匀结构随时间所发生的变化,溶质中的小型晶体或溶胶颗粒溶解并再次沉积到大型的晶体或溶胶颗粒上;另一种是定向附着机制,体系中的微晶通过旋转合适的角度附着到沿相同晶向生长的较大晶体的晶面上,最终聚集体可以看作是原始晶体颗粒以不可逆和高度统一取向的方式构建而成的大单晶[11-14]。
Fe盐水解沉淀及其后续结晶转化过程会受到有机质、pH、无机盐和光照等环境条件的影响[15] 。其中,有机质对Fe沉淀的转化受到了广泛的关注,具有不同分子质量、不同官能团和含量的有机质对沉淀的形成和转化有不同的影响。许多研究[16-17]表明,在混凝过程中有机物所含的羧基官能团是其重要的反应位点。
然而,小分子有机物如何影响混凝过程,目前还没有明确或充分的解释。虽然小分子有机物通常不是混凝去除的目标污染物,但其对混凝性能的影响(颗粒聚集时间、絮体大小和结晶度等)不容忽视。因此,本研究选用有2个羧基和1个氨基的天冬氨酸,以亚铁盐(Fe(Ⅱ))作为混凝剂,探讨了不同浓度的天冬氨酸对絮体形成过程和对磷的去除效果的影响。
-
七水合硫酸亚铁(Ⅱ) (FeSO4·7H2O,CAS:7782-63-0)、碳酸氢钠(NaHCO3,CAS:144-55-8)、四水钼酸铵(4MoO3·3H2MoO4·4H2O·6H3N,CAS:12054-85-2)、抗坏血酸(C6H8O6,CAS:50-81-7)、浓硫酸(H2SO4,CAS:7664-93-9)和磷酸二氢钾(KH2PO4,CAS: 7758-11-4)均购自国药化学试剂有限公司(中国上海),天冬氨酸(Asp, Aspartic acid, CAS: 6899-03-2)购自百灵威。所有试剂均为分析级。
-
混凝实验中,在含0.42 g碳酸氢钠的纯水中分别加入不同浓度的天冬氨酸(Asp),超声至完全溶解并定容至1 L,最终制得浓度分别为0、0.4、1、1.5、2.5、5和10 mmol·L−1梯度的天冬氨酸溶液,然后使用稀盐酸(HCL, 0.2 mol·L−1)或氢氧化钠溶液(NaOH, 0.2 mol·L−1)将所得溶液调至pH为7,溶液中的溶解氧使用便携式溶解氧仪(F4, METTLERTOLEDO, Switzerland, with LE621 IP67 dissolved oxygen sensor)测定。使用硫酸亚铁(0.1 mmol·L−1)作为混凝剂,通过混凝装置(MY3000-2N,武汉梅宇,中国)控制混凝体系中絮体的生长和破碎。在此过程当中用光度分散分析仪Photometric Dispersion Analyzer (PDA2000, Rank Brothers Ltd,UK)对絮体的生长情况进行实时监测,该仪器通过连续循环检测水中颗粒的变化和波动;每秒收集均方根(rms)和平均透射光强(dc)的比值。rms与dc的比值称为絮凝指数(FI), FI可以反映絮凝体在絮凝实验过程中的实时生长情况。
投加0.1 mmol·L−1的FeSO4絮凝剂后,以200 r·min−1的速度快速搅拌1 min使溶液混合均匀,而后以50 r·min−1 的速度缓慢搅拌观察絮体生长情况;当絮体生长至平稳值持续500 s后以200 r·min−1的速度对絮体进行1 min的剪切破碎,然后恢复转速至50 r·min−1使絮体再次生长,直至所得絮体的大小不再发生明显变化终止实验。光度色散分析仪连接电脑的bubble4软件上实时记录检测所得数据,PDA的读数大小不再发生较大波动时,认为絮体生长完成。所有的混凝实验均在室温(25±1) ℃下进行。
磷的去除实验中,称取0.252 g二水合磷酸二氢钠 (NaH2PO4·2H2O)于100 mL容量瓶中加纯水定容至刻度线作为磷酸储备液(0.5 g·L−1)。在混凝实验中絮体生长稳定至峰值时,取1 mL磷酸储备液加入到1 L的混凝体系当中,此时磷酸盐的初始质量浓度为0.5 mg·L−1,以50 r·min−1的速度搅拌10 min后用0.45 μm的微孔滤膜(聚偏二氟乙烯(PVDF)、直径50 mm、津腾)进行固液分离,液相用来测定反应后磷酸盐的浓度,絮体用来进行表面官能团、成分和结晶度分析。
-
在混凝实验中,当絮凝指数(FI, flocculation index)达到稳定的峰值时,通过使用0.45 μm微滤膜进行抽滤将所得絮体沉在膜表面,然后将其冷冻后进行真空冷冻干燥,经过约48 h后将完全冷干的样品取出。对冷干之后的絮体样品进行如下表征:采用扫描电镜(Hitachi SU8020, Japan)观察絮体的生长和表面形貌变化;采用透射电镜(Tecnai G2 F30 S-TWIN)观察絮体的微观形貌,鉴定其物相和结晶度;采用X射线衍射仪 (XRD, X’Pert PRO MPD, PAN alytical)在40 kV、40 mA的条件下对其进行测定分析,以获得材料的结晶程度、成分及其所占比例和晶体结构信息,(衍射角为5~90°,扫描速度为6o·min−1)。使用x射线光电子能谱仪(XPS, PHI Quantera II, ULVAC, Japan)在150 W Al-K α辐射条件下测定样品的化学状态信息,不同元素的结合能由C1s校准 (284.8 eV)。傅里叶红外变换光谱(FTIR)被用于分析冷冻干燥后絮体的表面官能团性质及相对含量(扫描范围为400~4 000 cm−1)。
在磷的去除实验中,磷的测定采用钼酸铵分光光度法(GB 11893-89)测定,通过钼酸铵与正磷酸盐反应生成黄色磷酸杂多酸,而后被抗坏血酸还原成磷钼蓝,于700 nm处用分光光度计测其吸光度,带入标准曲线计算出溶液中的磷酸盐含量。
-
PDA结果如图1所示。可见,在DO为5 mg·L−1、pH为7的混凝体系中添加天冬氨酸后,絮体的颗粒尺寸显著增大,随着体系中有机物浓度的增加,絮体生长的滞后时间延长,由于排列在絮体外侧的铁离子率先水解,天冬氨酸与—OH竞争占据铁离子表面的活性位点,从而改变铁离子在水中的形态。加入天冬氨酸等同于在体系中加入了大量带负电荷的物质,提高了絮凝指数,由于天冬氨酸的桥接作用,絮体的尺寸相较于不添加天冬氨酸时均有明显的增加,当天冬氨酸的浓度为1.5 mmol·L−1时,絮体的尺寸在2 500 s左右达到峰值,絮体的FI指数由1.5%左右上升到3%,说明此时絮凝后絮体会更大。破碎后絮体可以再生长到更接近于破碎前的大小。这可能是由于氢氧根沉淀物内的化学键仅有少量断裂、未引起絮体表面性质改变[18],更有利于絮体的沉淀。随着天冬氨酸的浓度继续增加,可能由于天冬氨酸的静电斥力和空间位阻作用占主导地位,导致絮体的FI指数开始减少直至无明显的絮体生长。在混凝过程中,当搅拌速率从50 r·min−1增加到200 r·min−1时,絮体破碎后,纳米颗粒表面活性减小(或失去),致使絮体无法生长至破碎前的尺寸[18-19]。
-
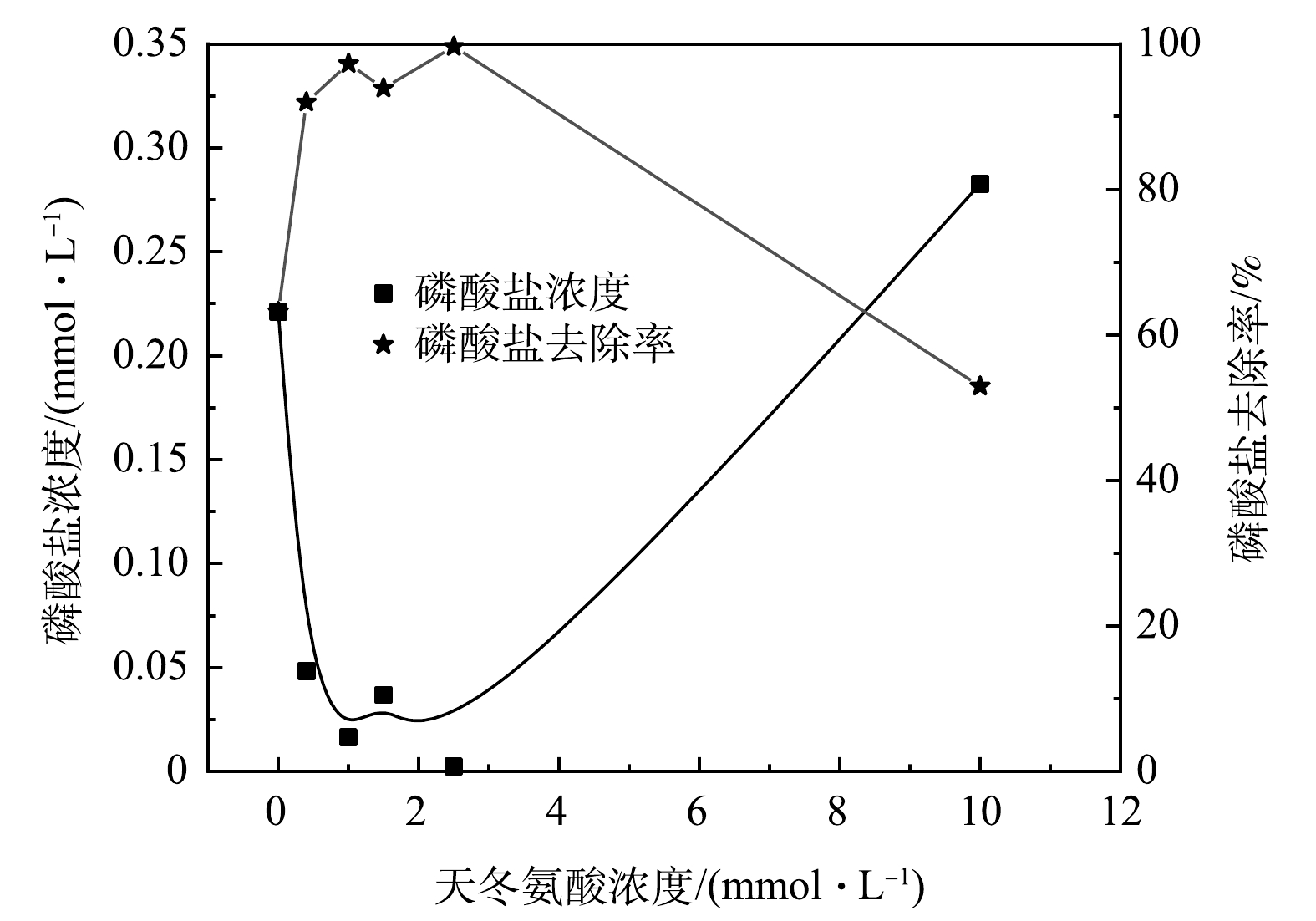
在DO为5 mg·L−1、pH为7的条件下,分别添加0、0.4、1、1.5、2.5、10 mmol·L−1天冬氨酸,当絮体生长且稳定到最大FI值时,加入0.5 g·L−1磷酸盐储备液1 mL于混凝杯中,此时体系中的磷酸盐初始值为0.5 mg·L−1,磷酸盐去除效果如图2所示。结果表明,在不添加天冬氨酸时,去除磷酸盐后其质量浓度保持在0.2 mg·L−1;添加天冬氨酸混凝后磷酸盐残余质量浓度降低至0.02 mg·L−1,有效提高了磷酸盐的去除效果。经过计算在不添加天冬氨酸时生成絮体对磷酸盐的去除率为63.16%。在添加天冬氨酸浓度为0.4、1、1.5、2.5 mmol·L−1时,生成的絮体对于磷酸盐的去除率分别为91.96%、97.26%、93.9%、99.6%,而当天冬氨酸的浓度为10 mmol·L−1时,磷酸盐的去除率仅有52.91%。这是由于过多的天冬氨酸不仅在絮体的形成过程中起到了桥接作用,而且在后续的磷酸盐去除阶段也占据了絮体表面的活性位点与其形成了竞争吸附的关系,从而导致了对于磷酸盐的去除效果不理想。
-
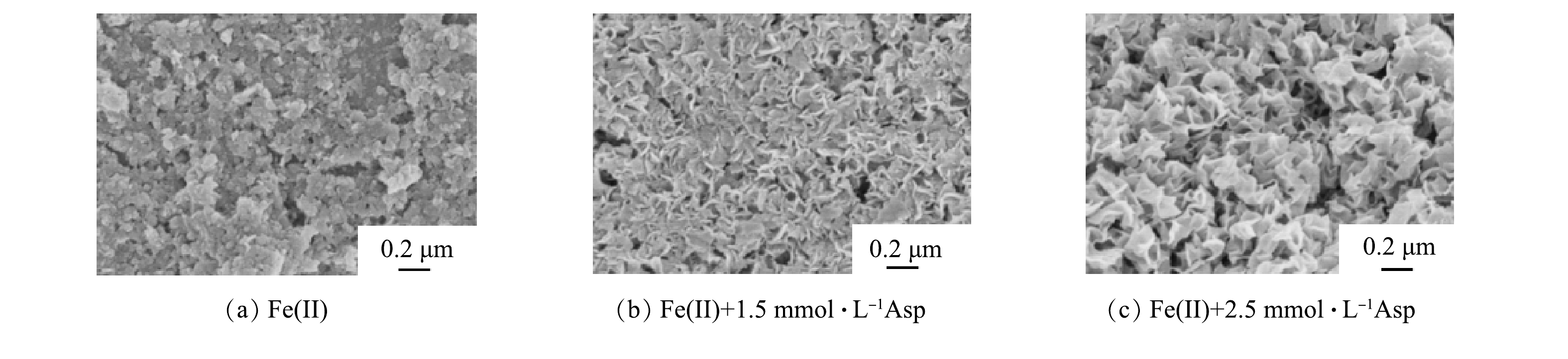
在DO为5、pH为7时絮体表面形貌的SEM表征结果如图3(a)所示。由图3(a) 可见,无添加天冬氨酸体系内,絮体呈现出50 nm左右的小球和100 nm大小的片状混合体;如图3(b)所示,和没有添加天冬氨酸相比,添加1.5 mmol·L−1天冬氨酸后絮体尺寸变大(约200 nm),这与PDA的结果一致。如图3(c)所示,当天冬氨酸浓度增加到2.5 mmol·L−1时,絮体的纳米颗粒相互连接聚拢,呈现出更加清晰的褶皱状结构。
-
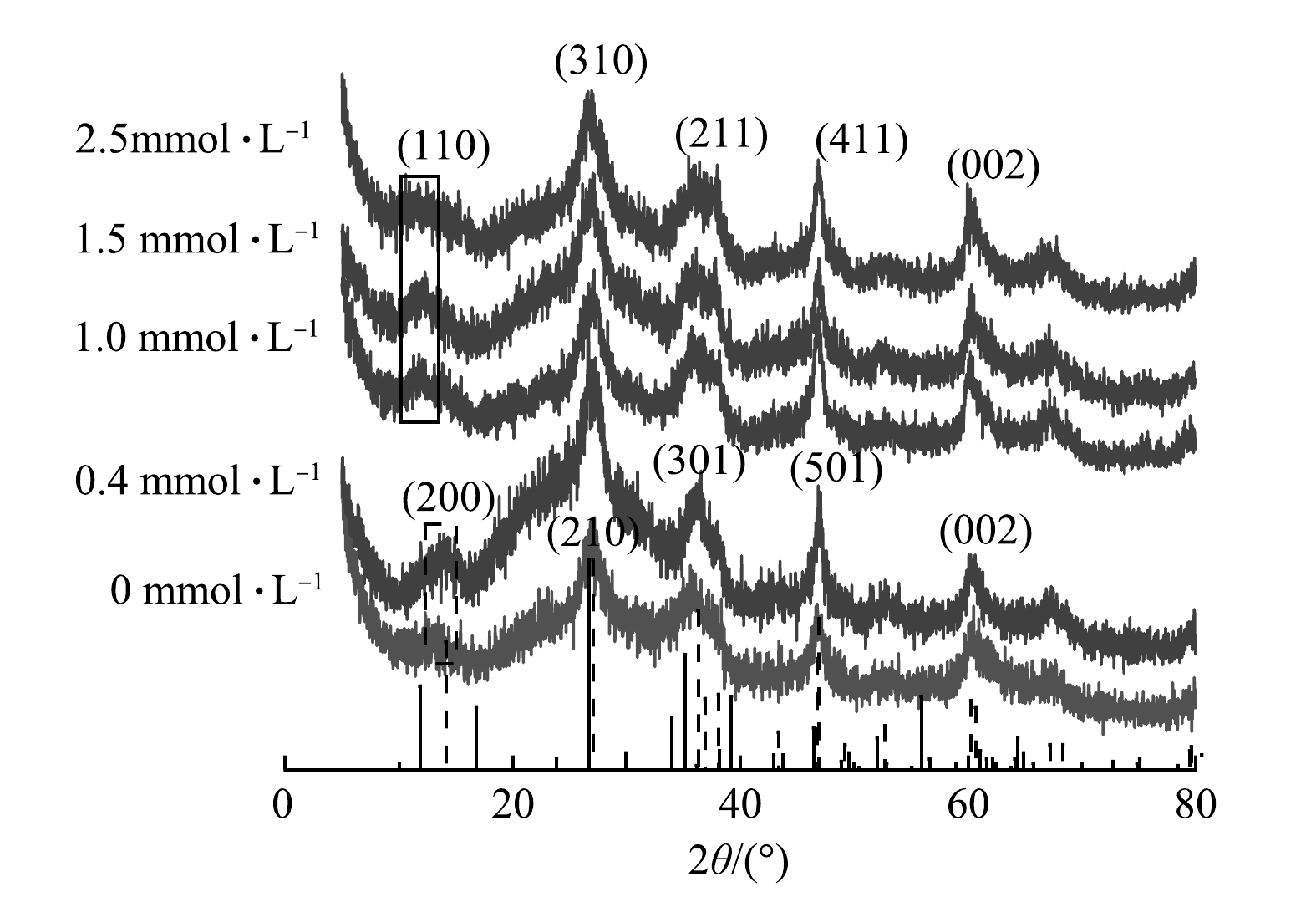
如图4所示,在不添加天冬氨酸和添加0.4 mmol·L−1天冬氨酸时,所对应的XRD图中2θ为14.113°、27.047°、39.296°、46.778°、52.714°、67.218°处,鉴定为纤铁矿(JCPDS card 44-1415),晶格常数a为12.52 Å、b为3.873 Å、c为3.071 Å。添加0.4 mmol·L−1 天冬氨酸与不添加天冬氨酸相比,结晶度明显增强。这可能是由于天冬氨酸诱导了其矿物的转化,使其具有更加良好的晶型。此外磷酸盐去除率显著提升,说明了少量的天冬氨酸的存在间接促进了磷酸盐的去除。这可能是由于天冬氨酸中的羧基促进絮体向纤铁矿(γ-FeOOH)的转化,为磷酸盐提供了更多去除位点[20]。当体系中天冬氨酸的浓度为1 mmol·L−1及以上时,絮体所对应的衍射峰位于2θ为11.842°、26.725°、35.161°、46.433°、61.097°处,这表明絮体的主要成分为四方纤铁矿(JCPDS card 34-1266),晶格常数a为10.535 Å、b为10.535 Å、c为3.03 Å。值得注意的是,当体系中天冬氨酸的浓度为小于0.4 mmol·L−1时,随着天冬氨酸浓度增加絮体结晶性增加,然而当天冬氨酸浓度增加到1 mmol·L−1,絮体的结晶度降低。这可能是由于在低C/Fe比时,有机物在与铁离子络合在一起,对亚铁离子转化为三价的羟基氧化铁有促进作用[21-22];然而在体系中有较高浓度的有机物时,纳米颗粒的表面活性位点更多的被封锁,限制了纳米颗粒之间的吸附,同时有机物的静电排斥和空间位阻的影响阻止纳米颗粒的聚集,因此,限制了羟基氧化铁的生长[23],导致絮体结晶性降低。
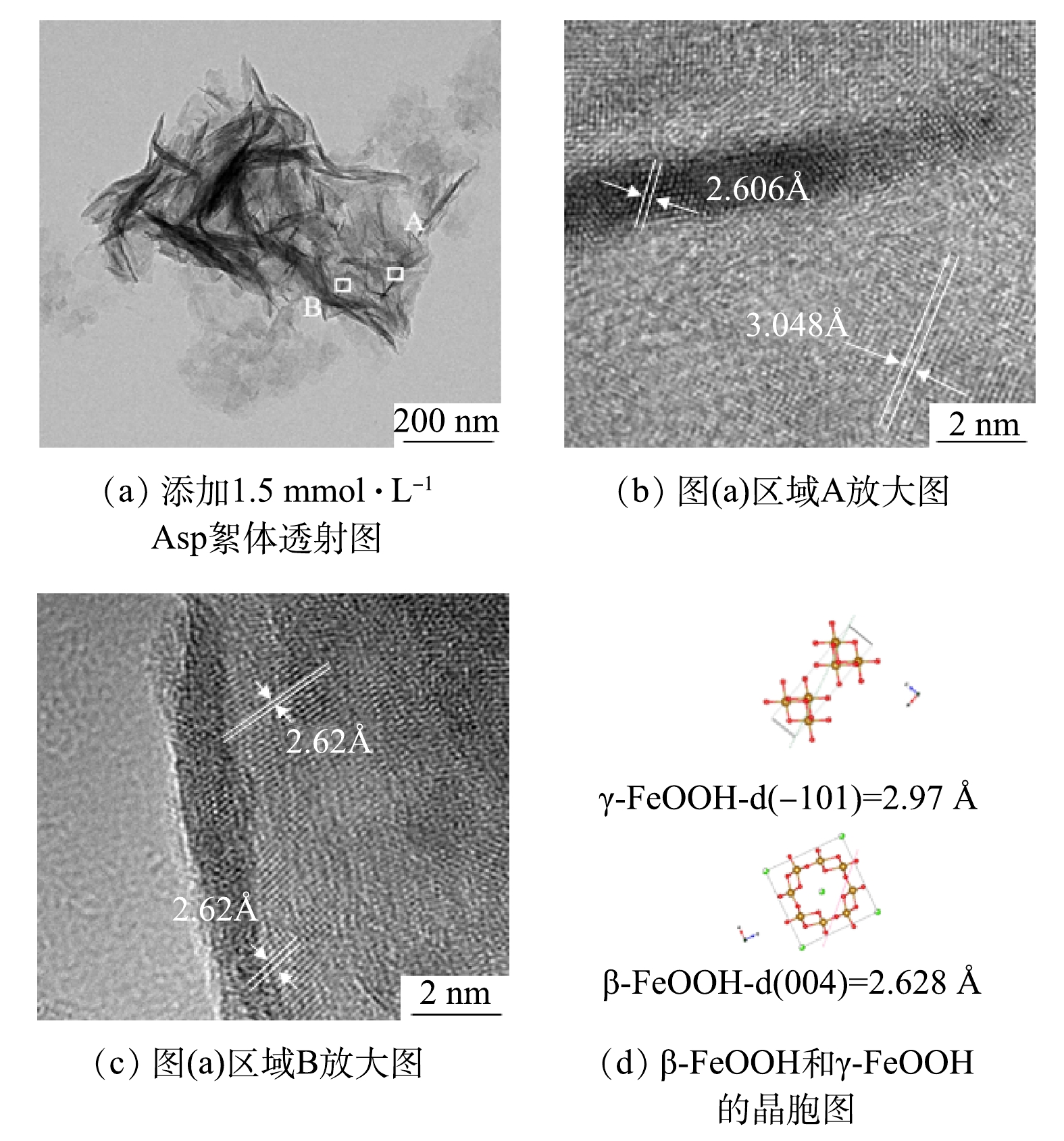
为了提供γ-FeOOH和β-FeOOH存在的直接证据,采用HR-TEM对pH为7、DO为 5.0 mg·L−1、含1.5 mmol·L−1 天冬氨酸混凝后的絮体进行了细致的研究。如图5(a)所示,絮体在纳米尺度下的形貌与SEM(图3(b))下的形貌皆呈现出较大的板片状,密集的堆叠在一起形成大的聚集体,图5(a)中区域A如图5(b)所示,分别对应β-FeOOH (004)和γ-FeOOH (-101)的晶格面,晶面间距为2.606 Å和3.048 Å;图5(a)中区域B如图5(c)所示,对应于β-FeOOH (004) 晶面,晶面间距为2.62 Å,与图5(d)中晶胞结构图分别对应。
-
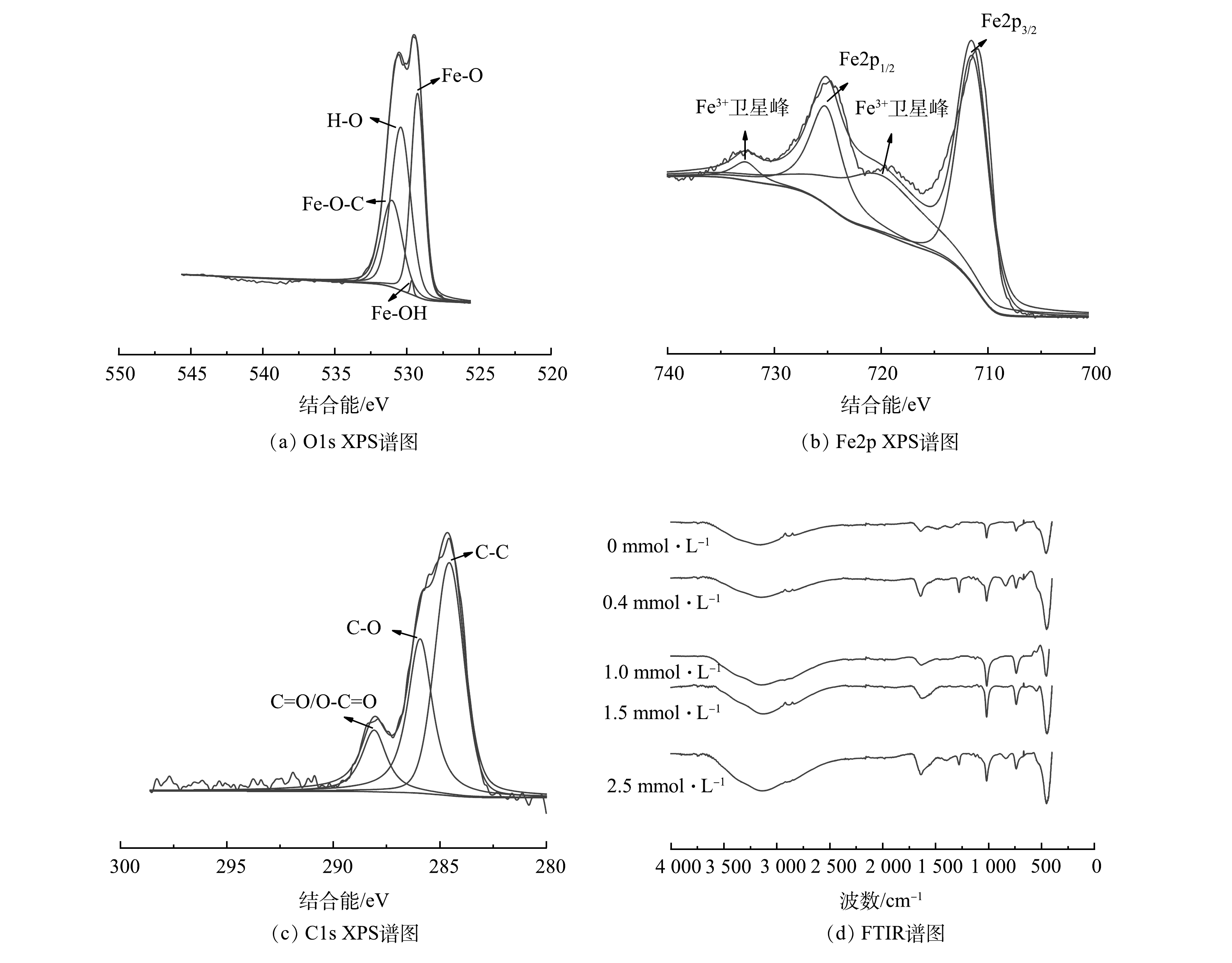
采用XPS和FTIR对pH为7、DO为5.0 mg·L−1、含1.5 mmol·L−1 天冬氨酸混凝后的絮体进行了表面官能团分析。如图6(a)所示,Fe2p的XPS光谱中含有2个主峰,分别为Fe2p3/2(711.4 eV),Fe2p1/2(725.4 eV),连同2个重要的卫星峰,其分别在720.1 eV和733.1 eV,此外,未发现有其他杂质峰的存在。结合O光谱图6(b),进一步证实了Fe—O—C键的存在。O1s可以分为Fe—O(531.2 eV)、Fe—O—C(532.4 eV)、Fe—OH(533.1 eV) 3个峰,说明了絮体的表面的确形成了羟基氧化铁。如图5(c)所示,C1s峰显示有3个峰,分别为C—C(284.1 eV)、C—O(286.6 eV)、C=O(288.7 eV),证实了天冬氨酸存在于絮体的表面,与絮体形成了络合物。进一步对絮体进行了FTIR分析,结果如图6(d)所示。3 177 cm−1处归属于羟基峰[24-25],在1 642 cm−1处的峰是由于晶体结构中的H2O的震动引起的,1 020 (面内弯曲振动)、746 (面外弯曲振动)、476 cm−1对应于Fe—O—H不同的弯曲振动[26]。结合XRD的表征结果,可进一步证明γ-FeOOH和β-FeOOH的存在。
-
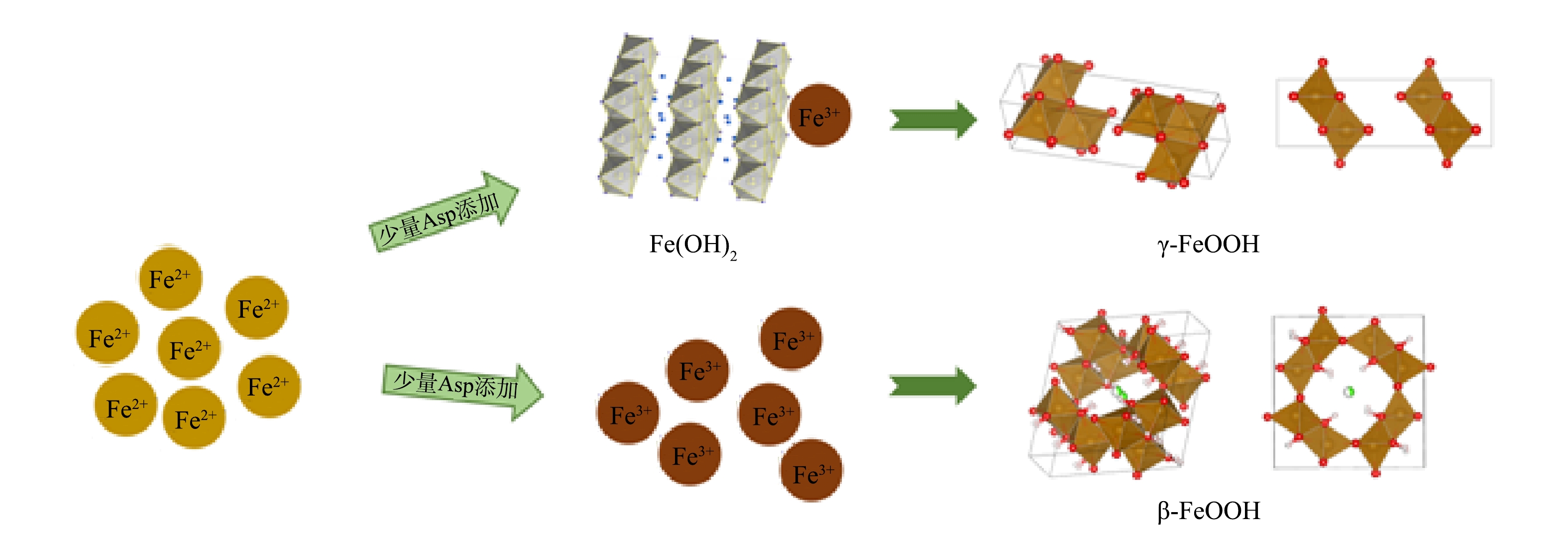
综上所述,Fe(Ⅱ)混凝中产生了形貌相似的片状絮凝体,但其主要成分存在差异。当体系中存在少量天冬氨酸时,由于Fe(Ⅱ)能在水溶液中快速水解,体系中部分Fe(Ⅱ)会被包裹,Fe(OH)2纳米颗粒由于铁化合物的部分饱和快速形成,随后作为晶核外延生长,Fe(OH)2纳米离子表面被氧化转化成三价铁,当溶液颜色完全变黄时,最终形成γ-FeOOH或者β-FeOOH(图7)。此外,少量的天冬氨酸添加明显增加了絮体γ-FeOOH的结晶性(图4)。然而当天冬氨酸的浓度增加到0.4 mmol·L−1以上时,絮体的主要成分不再是γ- FeOOH而是β-FeOOH。这可能是由于增多的有机物会将部分亚铁离子完全包裹,导致剩余的亚铁离子被快速氧化为三价铁,在天冬氨酸的作用下转化成了β-FeOOH。
-
1)在pH为7,DO为5 mg·L−1的条件下,添加天冬氨酸可使絮体的生长显著延后,不同浓度天冬氨酸的添加使絮体的形成过程存在差异从而影响絮体的主要成分。添加0.4 mmol·L−1以下天冬氨酸时,Fe(OH)2纳米颗粒由于铁化合物的部分饱和快速形成,随后作为晶体的核心外延生长,此时絮体的主要成分是γ- FeOOH;在添加大于0.4 mmol·L−1时,未被天冬氨酸完全包裹的Fe(Ⅱ)被迅速氧化,同时,在天冬氨酸的作用下絮体的主要成分是β-FeOOH。
2)添加1.5 mmol·L−1天冬氨酸时的磷酸盐去除率93.9%,此时絮体的FI指数最高,絮体更加容易沉降分离。
3)相较于不添加天冬氨酸生成的絮体,经过天冬氨酸诱导后形成的絮体对磷酸盐去除能力约为前者的1.57倍,磷酸盐的质量浓度降至0.02 mg·L−1,有效降低了富营养化的累积。
天冬氨酸对亚铁混凝剂絮体结构及磷酸盐去除效果的影响
Effect of aspartic acid on the floc composition of Fe(II) coagulant and its deep phosphorus removal
-
摘要: 铁混凝剂广泛应用于水处理领域,形成的絮体大小、形态和结晶度直接决定其混凝效果。然而小分子有机物对混凝的影响机制尚不清楚。本研究以FeSO4作为混凝剂,通过添加特定的小分子有机物(分子质量<1 000 Da)天冬氨酸,研究了天冬氨酸对絮体生长过程及其产物的影响。结果表明,天冬氨酸通过影响金属的水解和纳米颗粒的性质进而影响其混凝性能。在pH=7时,天冬氨酸的存在延缓了絮体的初始生长时间,但增大了絮凝体的最大粒径(从0.05 μm大小的碎片和小球状参杂的絮体变化至0.1 μm的褶皱状絮体)和磷酸盐的去除率。当添加0.4 mmol·L−1的天冬氨酸时,纤铁矿(γ-FeOOH)为絮体的主要成分,这可能少量的天冬氨酸存在时,体系中部分亚铁离子会被包裹, Fe(OH)2纳米颗粒由于铁化合物的饱和快速形成,随后作为晶核生长; Fe(OH)2纳米颗粒表面通过被氧化转化成三价铁,最终形成γ-FeOOH;当添加天冬氨酸大于0.4 mmol·L−1时,絮体的主要成分为四方纤铁矿(β-FeOOH),此时部分亚铁离子被完全包裹,剩余的亚铁离子被氧化成三价铁后形成β-FeOOH。经过天冬氨酸诱导后形成的絮体对磷酸盐的去除率均增加了1.57倍左右,处理后的磷酸盐浓度降低到了0.02 mg·L−1,且在添加1.5 mmol·L−1 天冬氨酸时絮体的尺寸最大,此时的絮体更加容易沉降分离。Abstract: Iron flocculants are widely used in water treatment applications where the size, morphology, and crystallinity of the flocs directly determine the adsorption activity and settling performance. However, the effect mechanism of small molecular organics on coagulation is still unclear. In this study, FeSO4 was used as coagulant, its effects of adding specific small molecule organic matter (molecular weight <1000 Da) of aspartic acid (containing two carboxyl groups and one amino group) on flocs growth process and products were investigated. The results show that aspartic acid affected metal hydrolysis, nanoparticles properties and the coagulation performance. At pH=7, the presence of aspartic acid delayed the initial growth time of flocs, but increased the maximum particle size of flocs, changing from 0.05 μm sized flocs with fragments and small spherical participations to 0.1 μm folded flocs, and the phosphate removal rate. When 0.4 mmol·L−1 aspartic acid was added, lepidocrocite (γ-FeOOH) was the main component of the floc. Due to the rapid hydrolysis of Fe(Ⅱ) ions in aqueous solution, when there was a small amount of aspartic acid in the system, part of the Fe(Ⅱ) ions in the system would be wrapped, and Fe(OH)2 nanoparticles would form rapidly due to the saturation of iron compounds. Afterwards, Fe(OH)2 nanoparticles continued to grow as the crystal, its surface was oxidized into Fe(Ⅲ), finally γ-FeOOH formation occurred. When the dosage of aspartic acid was higher than 0.4 mmol·L−1, the main component of flocs was akaganeite (β-FeOOH). At this time, part of Fe(Ⅱ) ions were completely wrapped, and the remaining Fe(Ⅱ) ions were oxidized into Fe(Ⅲ) irons and hydrolyzed to form β-FeOOH. The phosphorus removal rate by the flocs induced by aspartic acid increased by about 1.57 times, and the phosphate concentration decreased to 0.02 mg·L−1 after treatment. Moreover, the FI index of flocs was the highest when 1.5 mmol·L−1 aspartic acid was added, and the flocs were easier to settle and separate.
-
Key words:
- aspartic acid /
- floc /
- lepidocrocite and akageneite /
- phosphorus removal
-

-
-
[1] SILLANPAA M, NCIBI M C, MATILAINEN A, et al. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review[J]. Chemosphere, 2018, 190: 54-71. doi: 10.1016/j.chemosphere.2017.09.113 [2] XU W, GAO B, YUE Q, et al. Effect of shear force and solution pH on flocs breakage and re-growth formed by nano-Al(13) polymer[J]. Water Research, 2010, 44(6): 1893-1899. doi: 10.1016/j.watres.2009.11.029 [3] YU W, GREGORY J, CAMPOS L C. Breakage and re-growth of flocs: Effect of additional doses of coagulant species[J]. Water Research, 2011, 45(20): 6718-6724. doi: 10.1016/j.watres.2011.10.016 [4] YU W, GREGORY J, CAMPOS L C, et al. Dependence of floc properties on coagulant type, dosing mode and nature of particles[J]. Water Research, 2015, 68: 119-126. doi: 10.1016/j.watres.2014.09.045 [5] WU M, YUAN J, WU H, et al. Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability[J]. Journal of Membrane Science, 2019, 576: 131-141. doi: 10.1016/j.memsci.2019.01.040 [6] HSU P H. Comparison of iron(III) and aluminum in precipitation of phosphate from solution[J]. Water Research, 1976: 903-907. [7] SU Z, LIU T, YU W, et al. Coagulation of surface water: Observations on the significance of biopolymers[J]. Water Research, 2017, 126: 144-152. doi: 10.1016/j.watres.2017.09.022 [8] YU W, XU L, LEI K, et al. Effect of crystallization of settled aluminum hydroxide precipitate on "dissolved Al"[J]. Water Research, 2018, 143: 346-354. doi: 10.1016/j.watres.2018.06.063 [9] BOLAND D D, COLLINS R N, MILLER C J, et al. Effect of solution and solid-phase conditions on the Fe(II)- accelerated transformation of ferrihydrite to lepidocrocite and goethite[J]. Environmental Science & Technology, 2014, 48(10): 5477-5485. [10] SHU Z, LIU L, TAN W, et al. Solar irradiation induced transformation of ferrihydrite in the presence of aqueous Fe2[J]. Environmental Science & Technology, 2019, 53(15): 8854-8861. [11] MIRABELLO G, IANIRO A, BOMANS P H H, et al. Crystallization by particle attachment is a colloidal assembly process[J]. Nature Materials, 2020, 19(4): 391-396. doi: 10.1038/s41563-019-0511-4 [12] OSTWALD W. Studien über die bildung und umwandlung fester Körper: 1. Abhandlung: Übersättigung und Überkaltung[J]. Zeitschrift für Physikalische Chemie, 1897, 22U(1): 289-330. [13] ZHANG X, SHEN Z, LIU J, et al. Direction-specific interaction forces underlying zinc oxide crystal growth by oriented attachment[J]. Nature Communications, 2017, 8(1): 835. doi: 10.1038/s41467-017-00844-6 [14] ZHU C, LIANG S, SONG E, et al. In-situ liquid cell transmission electron microscopy investigation on oriented attachment of gold nanoparticles[J]. Nature Communications, 2018, 9(1): 421. doi: 10.1038/s41467-018-02925-6 [15] XING B, GRAHAM N, YU W. Transformation of siderite to goethite by humic acid in the natural environment[J]. Communications Chemistry, 2020, 3(1): 38. doi: 10.1038/s42004-020-0284-3 [16] GUAN X H, CHEN G H, SHANG C. Combining kinetic investigation with surface spectroscopic examination to study the role of aromatic carboxyl groups in NOM adsorption by aluminum hydroxide[J]. Journal of Colloid and Interface Science, 2006, 301(2): 419-427. doi: 10.1016/j.jcis.2006.05.031 [17] SHENG A, LIU F, XIE N, et al. Impact of proteins on aggregation kinetics and adsorption ability of hematite nanoparticles in aqueous dispersions[J]. Environmental Science & Technology, 2016, 50(5): 2228-2235. [18] YU W Z, GREGORY J, CAMPOS L, et al. The role of mixing conditions on floc growth, breakage and re- growth[J]. Chemical Engineering Journal, 2011, 171(2): 425-430. doi: 10.1016/j.cej.2011.03.098 [19] YU W Z, GREGORY J, GRAHAM N. Regrowth of broken hydroxide flocs: Effect of added fluoride[J]. Environmental Science & Technology, 2016, 50(4): 1828-1833. [20] XING B, OUYANG M, GRAHAM N, et al. Enhancement of phosphate adsorption during mineral transformation of natural siderite induced by humic acid: Mechanism and application[J]. Chemical Engineering Journal, 2020, 393: 124730. doi: 10.1016/j.cej.2020.124730 [21] THOMASARRIGO L K, BYRNE J M, KAPPLER A, et al. Impact of organic matter on iron(II)-catalyzed mineral transformations in ferrihydrite-organic matter coprecipitates[J]. Environmental Science & Technology, 2018, 52(21): 12316-12326. [22] CHEN C, KUKKADAPU R, SPARKS D L. Influence of coprecipitated organic matter on Fe2+(aq)-catalyzed transformation of ferrihydrite: Implications for carbon dynamics[J]. Environmental Science & Technology, 2015, 49(18): 10927-10936. [23] CHEN C, DYNES J J, WANG J, et al. Properties of Fe-organic matter associations via coprecipitation versus adsorption[J]. Environmental Science & Technology, 2014, 48(23): 13751-13759. [24] WECKLER H D L. Lattice vibration spectra. Part XCV. Infrared spectroscopic studies on the iron oxide hydroxides goethite (α), akaganéite (β), lepidocrocite (γ), and feroxyhite (δ)[J]. European Journal of Solid State and Inorganic Chemistry, 1998, 35(8-9): 531-544. doi: 10.1016/S0992-4361(99)80017-4 [25] LI X, GRAHAM N J D, DENG W, et al. The formation of planar crystalline flocs of γ-FeOOH in Fe(II) coagulation and the influence of humic acid[J]. Water Research, 2020, 185: 116250. doi: 10.1016/j.watres.2020.116250 [26] Bio-inspired fabrication of hierarchical FeOOH nanostructure array films at the air-water interface, their hydrophobicity and application for water treatment[J]. ACS nano, 2013, 72: 1368-1378. -




 下载:
下载: