-
氯酚类化合物是广泛应用的工业原料与中间体,其化学稳定性与高生物毒性已引起研究者们的关注[1]。自然界中的氯酚类化合物一方面来自于富含氯酚类工业废水的排放废水,另一方面源自地球化学作用的自然合成[2-3]。目前污水处理厂难以完全去除工业废水中的氯酚类化合物,导致该类化合物随尾水进入环境,危及人类及生态环境的健康。
高级氧化技术是去除氯酚类污染物的主要方法,包括臭氧氧化、芬顿氧化、光催化氧化与过硫酸盐氧化等[4]。SUNG等利用臭氧氧化土壤中的氯酚[5]。KUAN等将FeOx负载到TiO2和CuFe2O4上,再与紫外联用可产生羟基自由基,从而实现对4-CP的去除[6]。BARAKAT 等使用Co(Ⅲ)掺杂TiCl4与紫外联用实现2-氯酚的去除[7]。但以上方法存在制备工艺复杂、能耗高和有毒金属离子浸出等问题。Mn作为代表性的过渡金属元素之一,其生物毒性低、在自然界中分布广泛,是一种天然和高活性的氧化剂[8]。最常见的锰氧化物为二氧化锰晶体。有研究表明,影响二氧化锰催化降解性能的可能因素有锰的平均氧化态(Mn AOS)、三价锰元素的丰度等[8-9]。然而截至目前,并没有直接证据证明他们与MnO2反应活性之间的关系。因此,探究MnO2反应活性的影响因素十分必要。但二氧化锰晶体易团聚导致催化活性位点减少,在一定程度上降低了其催化活性。BC掺杂过渡金属可有效抑制金属离子浸出,并且BC原材料丰富、制备工艺简单、不存在二次污染[10-12]。
本研究利用水热法将γ-MnO2负载到BC表面,合成了γ-MnO2@BC,且考察了其活化PMS对4-CP的氧化降解性能,此外,分析了BC对不同晶相二氧化锰结晶的影响,探讨了γ-MnO2@BC活化PMS的机理和不同晶相二氧化锰催化性能差异的主要原因。
-
KMnO4、MnSO4、MnSO4.H2O、(NH4)2S2O8、KHSO5(PMS)、ClC6H4OH(4-CP)、NaNO3、NaHCO3、KH2PO4、NaCl、腐殖酸 (HA)、CH3OH、CH2Cl2、NaSO4、CF3COOH、5,5-二甲基-1-吡咯啉-N-氧化物 (DMPO)、2,2,6,6-四甲基哌啶氧化物 (TEMP)均为分析级试剂,购买自上海阿拉丁生物科技有限公司。
-
α-MnO2制备:将1.94 g KMnO4与和0.845 g MnSO4在100 mL 去离子水中充分溶解后,转移至反应釜中,在160 ℃下反应12 h。MnCO3@BC制备:待KMnO4与MnSO4充分溶解后加入2.46 g BC并搅拌均匀,制备方法与α-MnO2相同。
软锰矿MnO2制备:1.325 g MnSO4 .H2O和1.826 g (NH4)2S2O8充分溶于40 mL去离子水后,转移至反应釜中,140 ℃反应12 h。nMnOx-1@BC制备:待MnSO4 .H2O和(NH4)2S2O8充分溶解后加入3.28 g BC并搅拌均匀,制备方法与软锰矿MnO2相同。nMnOx中n与x为变量,1指代未定型锰氧化物1。
γ-MnO2制备:3.375 g MnSO4.H2O,4.575 g (NH4)2S2O8在80 mL去离子水中搅拌30 min,随后转移至反应釜中,在90 ℃下反应24 h。γ-MnO2@BC制备:待MnSO4.H2O和(NH4)2S2O8充分溶解后,将5 g BC加入到混合溶液中搅拌均匀,制备方法与γ-MnO2相同。
δ-MnO2制备:0.948 g KMnO4溶解在去离子水中,然后将0.169 g (NH4)2S2O8加入该溶液,混合搅拌30 min,转移至反应釜中在160 ℃下反应12 h。nMnOx-2@BC制备:将KMnO4和(NH4)2S2O8充分溶解后加入2.87 g BC搅拌均匀,制备方法与δ-MnO2相同(2指代未定型锰氧化物2)。
以上材料均在烘箱中60 ℃下干燥12 h,生物炭的制备是以稻壳为原料,稻壳在惰性气体保护下500 ℃反应4 h,升温速率为5 ℃·min−1。得到的黑色固体用研钵研磨至粉末状,过100目筛。过筛后的粉末分别用去离子水与乙醇洗涤5次,在烘箱中60 ℃干燥12 h,并将收集到的粉末命名为生物炭(BC)。BC初始掺杂量以制备各晶相二氧化锰的原料中锰元素的含量为依据,以1:5为质量比设置。
在探究BC掺杂量对γ-MnO2@BC催化性能的影响时,仅改变BC掺杂量,制备方法与γ-MnO2相同。
-
将10 mg 4-CP在烧杯中充分溶解,转移至1 L容量瓶中定容后,加入0.1537 g PMS,超声溶解后准确量取40 mL溶液,倒入装有24 mg催化剂的锥形瓶中(设置3个平行样),迅速转移至恒温水浴锅中振荡,水浴锅转速为160 r·min−1。相应时间取出对应锥形瓶,用注射器吸取1 mL样品,使用0.22 μm滤膜过滤到装有1 mL 1 mol·L−1 NaN3的离心管中,冷藏保存。使用高效液相色谱(HPLC, Shimadzu, LC-20AT)检测反应前后4-CP的浓度(流动相中甲醇∶三氟乙酸=70%∶30%,检测波长279 nm,流速0.5 mL·min−1,三氟乙酸为2 mL·L−1)。在不同实验中4-CP浓度均为10 mg·L−1,催化剂投加量和PMS浓度见表1。
-
使用转靶X-射线衍射仪(XRD,日本理学D/MAX2500VL/PC)鉴定MnO2晶相;使用X射线荧光光谱仪(XRF,日本岛津)分析各样品中主要元素的含量;使用冷场发射扫描电子显微镜(SEM,日本Hitachi公司)观察各晶型二氧化锰形态和生物炭表面二氧化锰的负载情况;使用高分辨场发射扫描电子显微镜(EDS,日本Hitachi公司)映射锰、氧、炭的元素分布;使用X射线光电子能谱(XPS,Thermo ESCALAB 250Xi)分析样品中Mn(Ⅲ)与Mn(Ⅳ)的含量;使用电子自旋共振(ESR/EPR,JEOL JES-FA200 ESR)鉴定活性氧物种。
-
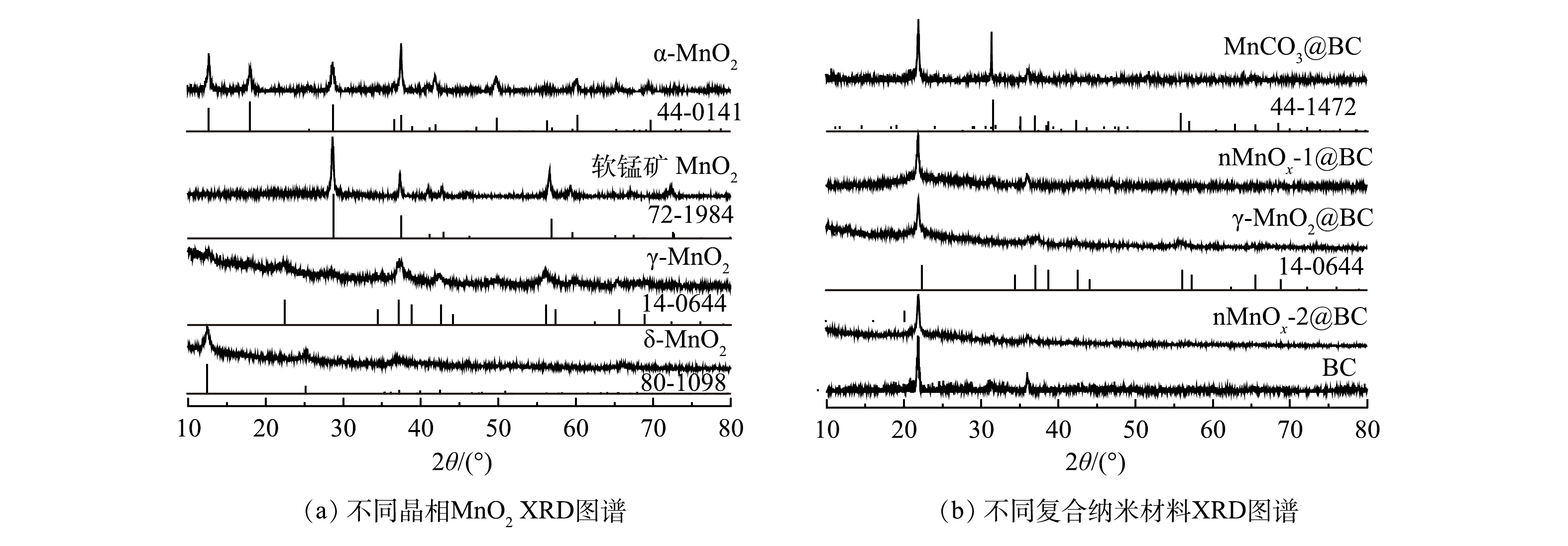
1) XRD分析。如图1所示,α-MnO2,γ-MnO2、δ-MnO2和软锰矿MnO2XRD衍射峰的位置与α-MnO2,γ-MnO2和δ-MnO2 JCPDS卡片中衍射峰的位置匹配。α-MnO2、γ-MnO2、δ-MnO2和软锰矿MnO2对应的JCPDS卡片号分别为44-0141,14-0644,80-1098和72-1984 [13]。上述结果表明,利用水热法能够合成这4种晶相二氧化锰。图1(b)为4种二氧化锰掺杂BC后合成的催化剂。如图1(b)所示,掺杂BC后分别合成了MnCO3@BC、nMnOx-1@BC、nMnOx-2@BC与对应晶相二氧化锰的衍射峰发生改变,但合成的γ-MnO2@BC与γ-MnO2具有相同的衍射峰。这说明本实验已成功合成γ-MnO2@BC。
2) SEM表征。利用SEM观察各晶相结构二氧化锰和复合纳米材料的微观形貌。由图2(a)与图2(b)可以看出,α-MnO2和软锰矿MnO2呈棒状或纤维状。γ-MnO2为海胆状(图2(c)), δ-MnO2为层状(图2(d))。MnCO3和nMnOx-2为絮状物附着在BC表面(图2(e),图2(h))。由图2(f)可见,nMnOx-1表面较为光滑,几乎没有可见的锰氧化物附着在BC的表面和破损部位。XRF分析结果也表明该材料中Mn含量仅为0.57%。而由图2(g)可见,BC孔洞处形成了海胆状的γ-MnO2。这说明γ-MnO2能够附着在BC表面。EDS实验结果表明,Mn、O、C元素均匀分布在γ-MnO2@BC表面。
-
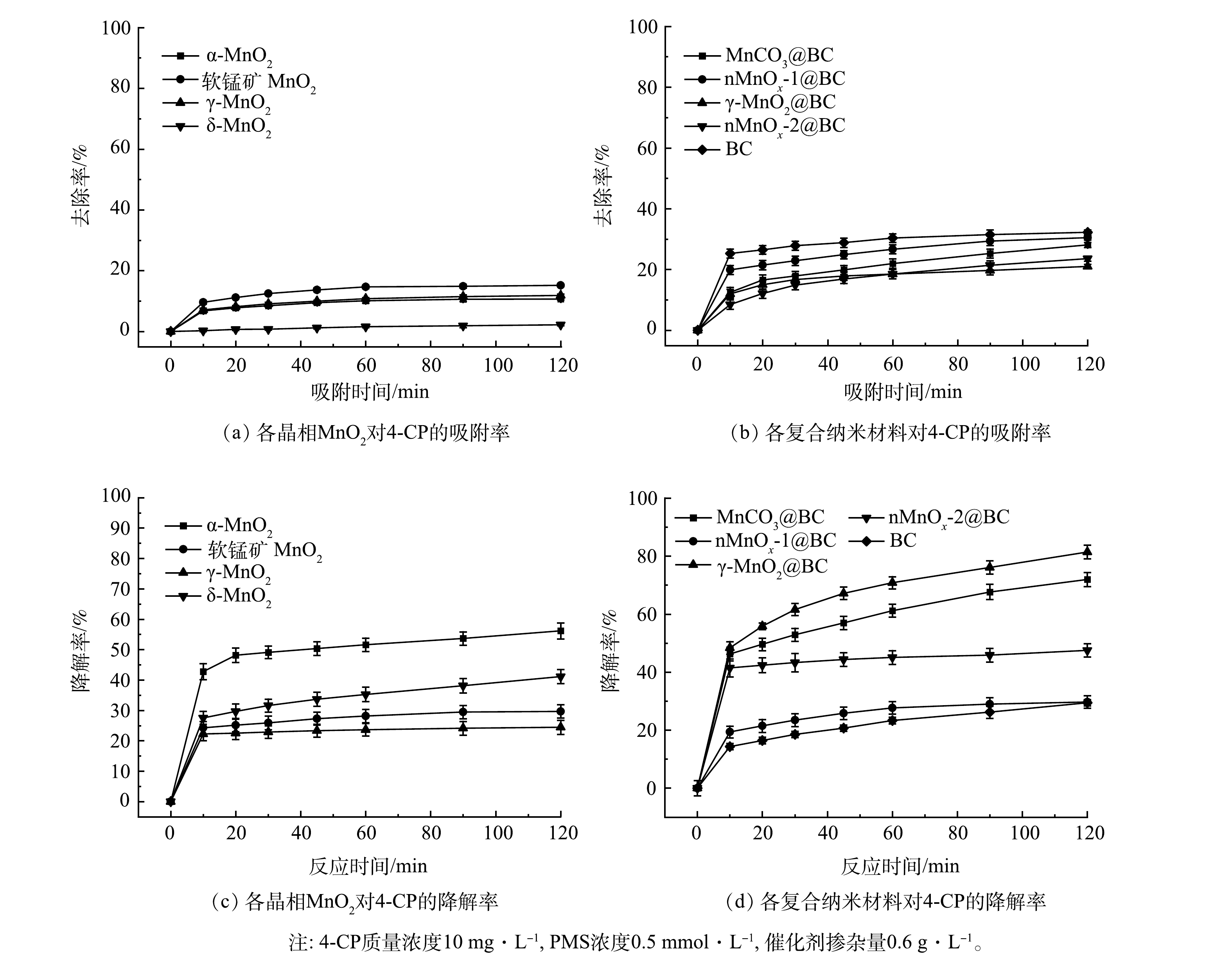
1)材料筛选实验。不同材料对4-CP的去除率如图3(a)和图3(b)所示,掺杂BC后各复合纳米材料对4-CP的去除率均得到提升,其中BC对4-CP的去除率最高(32.28%)。不同晶相二氧化锰和4种复合纳米材料活化PMS 对4-CP的降解效果如图3(c)和图3(d)所示。由图3(c)可见,α-MnO2降解率最高(58.86%)。这可能得益于α-MnO2大孔径隧道,为PMS的活化提供了较多的活性位点[13]。由图3(d)可见,γ-MnO2@BC活化PMS对4-CP的降解率最高,达到81.48%。MnCO3@BC、 nMnOx-1@BC、nMnOx-2@BC活化PMS对4-CP的降解率分别为71.92%、29.4%、29.68%,BC在相同条件下对4-CP的降解率仅为32.72%。通过对上述8种材料吸附率与降解率的对比可以发现,这些材料均能够在不同程度上活化PMS从而降解4-CP。而γ-MnO2@BC活化PMS对4-CP的降解率最高,并且仅有γ-MnO2能够负载在BC表面,因此,选定γ-MnO2@BC进行后续的实验研究。
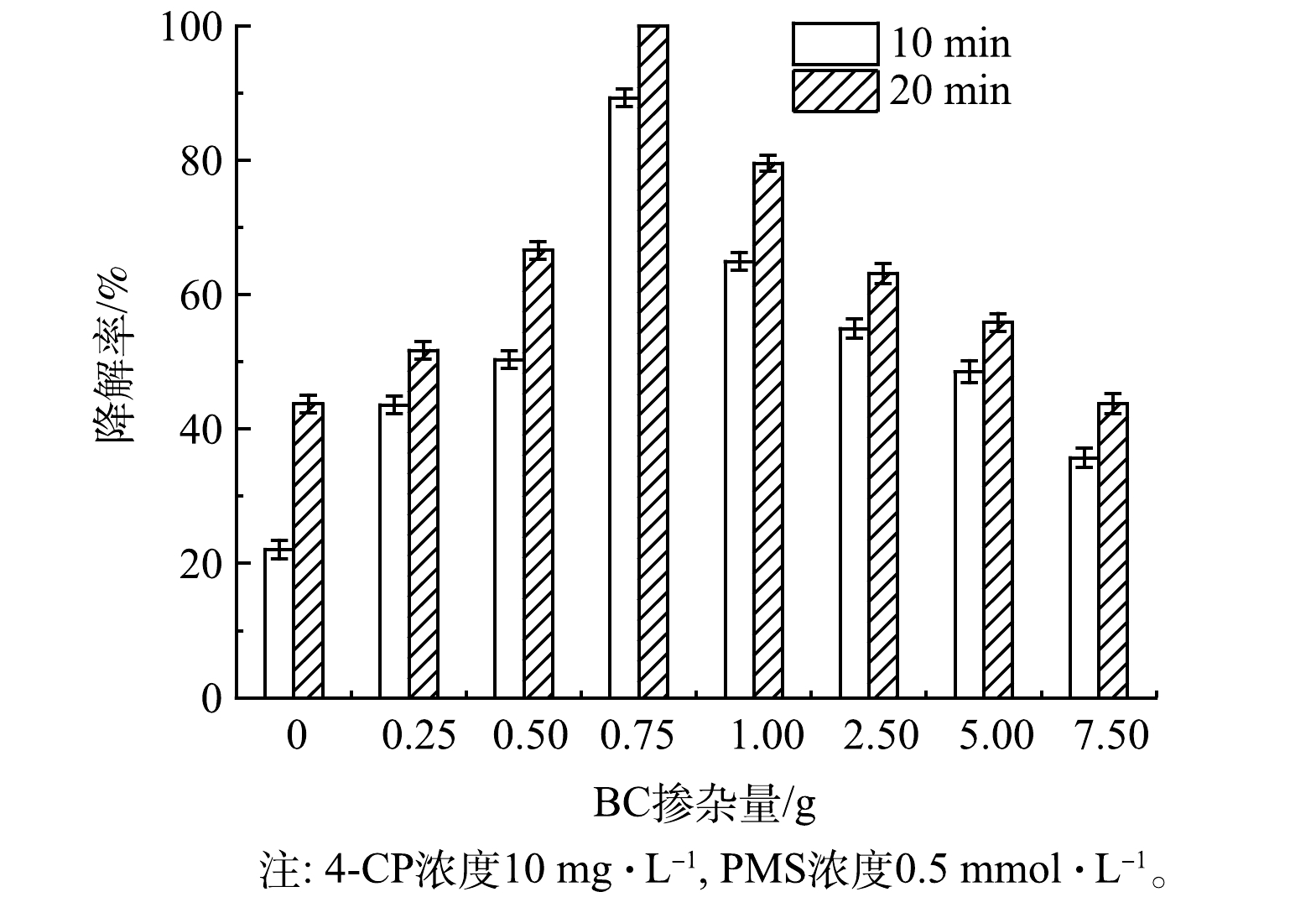
2) BC掺杂量对γ-MnO2@BC降解4-CP效率的影响。如图4所示,在0.75~7.5 g内,随着BC掺杂量的减少,4-CP的降解率逐渐提高。当BC的掺杂量为0.75 g时,4-CP降解率最高;但BC的掺杂量小于0.75 g时,4-CP的降解率逐渐下降。因此,确定0.75 g BC为最佳掺杂量。在γ-MnO2@0.75BC的用量为0.6 g·L−1、PMS浓度为0.5 mmol·L−1时,10 mg·L−1 4-CP可在20 min内完全降解。以下实验中使用的催化剂均为γ-MnO2@0.75BC,故将其简称为γ-MnO2@BC。
如表2所示,在各晶相的MnO2中掺杂BC后,其中的锰元素含量均有所下降。以γ-MnO2为例,未掺杂BC时Mn元素含量高达86.43%,但4-CP降解率在120 min时仅为24.48%。经过调整BC掺杂量后γ-MnO2@BC中Mn元素含量虽然仅为42.59%,但在相同条件下能够在20 min时完全去除4-CP。因此,BC与γ-MnO2两者之间存在良好的协同反应。
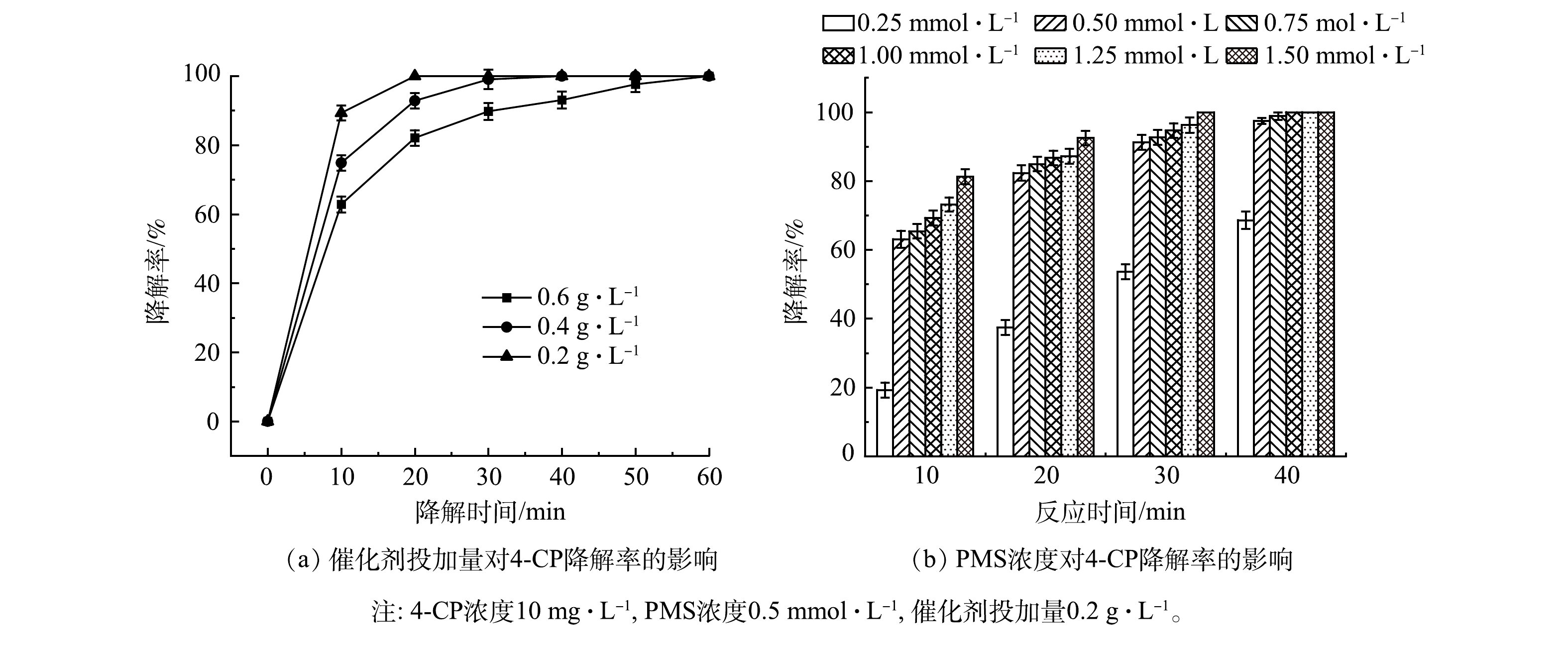
3)单因素实验。如图5(a)所示,虽然γ-MnO2@BC掺杂量逐渐减少,但γ-MnO2@BC依然表现出良好的降解效果。当催化剂掺杂量为0.2 g·L−1时,60 min时4-CP的降解率依然达到100%。因此,在后续的实验中选择催化剂质量浓度为0.2 g·L−1。如图5(b)所示,随着PMS浓度的增加,反应速率也逐渐增加。这是因为PMS浓度的提高增加了活性反应物种的产生量,加快了污染物的降解。从缩短反应时间的角度考虑,最佳PMS浓度为1 mmol·L−1。
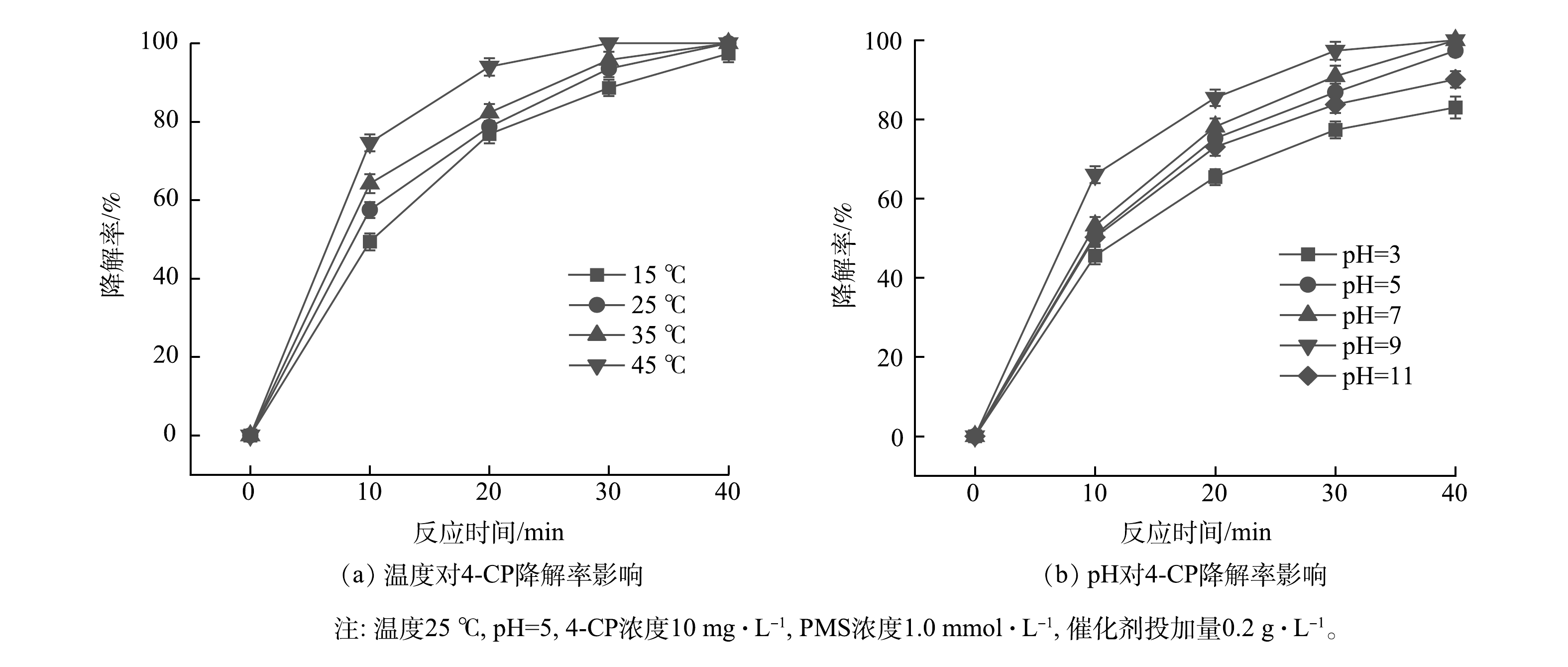
如图6(a)所示,当反应温度为45 ℃,4-CP的降解率在30 min时可接近100%;当温度降低到15 ℃时,降解率下降并不明显,40 min时降解率为97.2%。可见,污染物的去除速率会随着温度升高而增加,但温度过高将会影响PMS的稳定性,同时还会增加能耗。因此,25 ℃为合适的反应温度。
有研究[13-15]表明,当pH在5~9时,催化剂活化PMS降解污染物的效率随pH的增加而增加。在酸性条件下,大量的氢离子与O—O键形成氢键,从而抑制生物碳基催化剂对PMS 的活化效率[16]。但当pH大于9时,产生大量SO52−,从而影响污染物的降解效率[14];当pH在8.5左右时,有利于HSO5−向SO52−的转化,促进产生更多的单线态氧[17]。本研究考察了pH为3~11时反应体系对4-CP的降解率(图6(b))。当pH为9时4-CP的降解率最高,其次是pH为7时。因此,γ-MnO2@BC/PMS体系的最佳pH为7~9。但γ-MnO2@BC/PMS体系在pH=3或pH=11时依然表现出较高的降解率,这说明该体系应用时pH范围较宽。
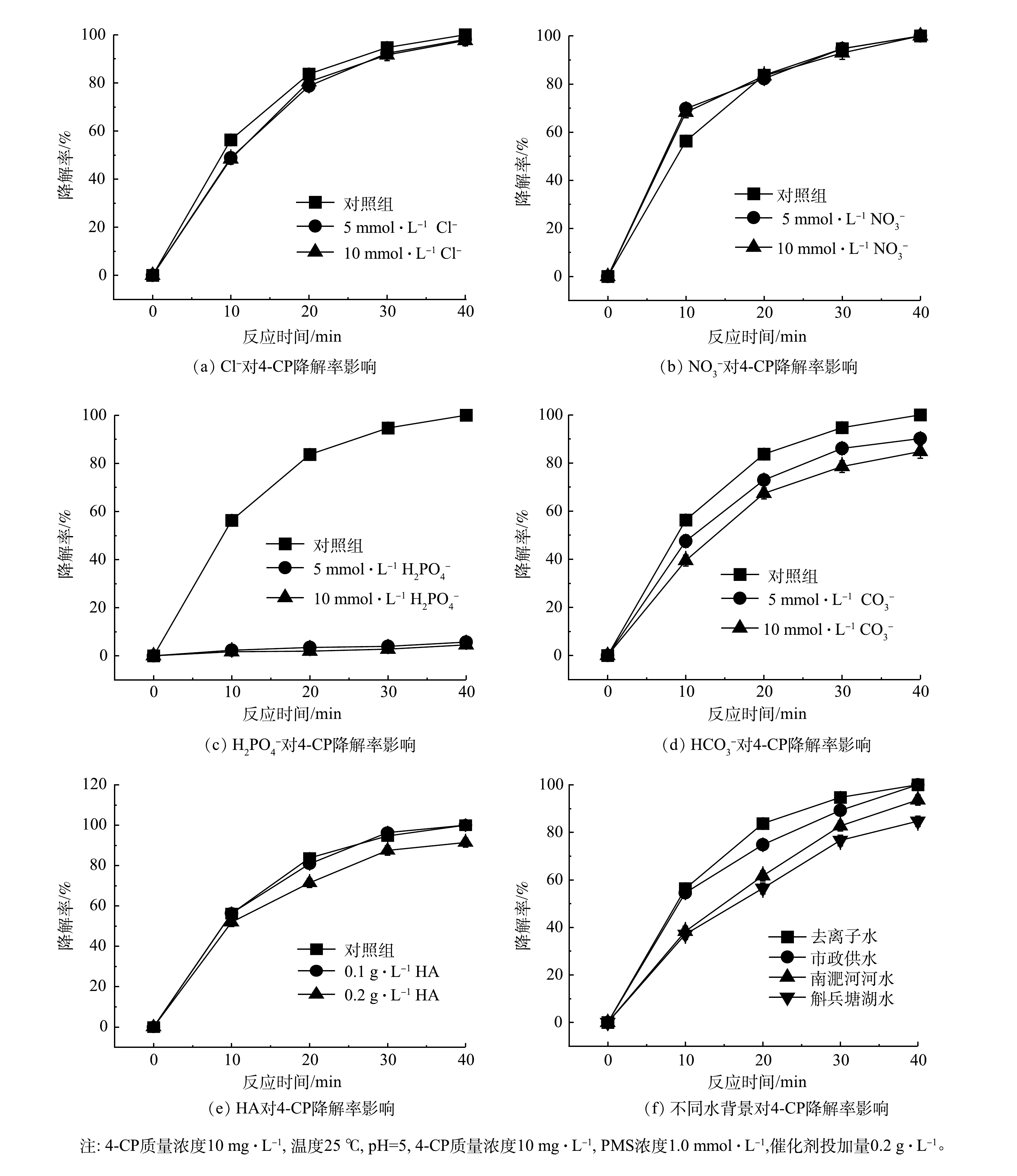
如图7所示,在γ-MnO2@BC/PMS体系中加入NaCl、NaNO3、NaHCO3、KH2PO4和腐殖酸(HA)。除H2PO4−几乎淬灭了反应外,其他阴离子和HA对体系影响较小,NO3−甚至在一定程度上促进了反应。氯离子是硫酸根自由基和羟基自由基的清除剂,可消耗硫酸根自由基和羟基自由基并产生.Cl和HOCl.−,但当活性氧物种以非自由基为主时,氯离子对反应体系的影响可以忽略[9,15,18]。如图7(a)~图7(b)所示,当反应体系中含有Cl−时,前20 min降低了反应速率,而当反应体系中含有NO3−时前20 min则提升了反应速率。这在一定程度上表明该体系并不是羟基自由基主导的反应体系。HCO3−和H2PO4−能淬灭硫酸自由基和羟基自由基,分别生成碳酸氢盐自由基和磷酸二氢自由基,但其氧化电位低于硫酸自由基和羟基自由基,从而抑制反应的进行[3,19]。反应体系中HCO3−的加入量会影响体系的pH。但大多数情况下HCO3−和H2PO4−仍对反应体系起到抑制作用[16]。如图7(c)~图7(d),H2PO4−对反应体系影响较大,几乎淬灭了降解反应,随着HCO3−浓度的提升降解速率也随之降低,当HCO3−为10 mmol·L−1时4-CP的降解率依然可达到84.75%。如图7(e)所示,HA的投加量分别为10 mmol·L−1和0.2 g·L−1时,γ-MnO2@BC/PMS体系对4-CP的降解率分别为91%和91.4%。
采用去离子水、南淝河水、城市供水和斛兵塘水研究不同水质对催化剂降解4-CP的影响,以模拟实际应用场景。斛兵塘水与南淝河河水水质指标见表3,水背景对反应体系降解4-CP效率的影响见图7(f)。斛兵塘水对系统的影响较大,这可能是因为底泥中释放的微量的磷溶解,可抑制γ-MnO2@BC/PMS体系对4-CP的降解,使4-CP降解率下降到84.66%。河水对降解有轻微的抑制作用,可能是生物炭吸附了溶解在河水中的大分子物质,覆盖了催化剂表面的活性反应位点,从而使4-CP的降解率下降至93.64%,也可能受微量的磷元素的影响导致4-CP的降解效率降低。
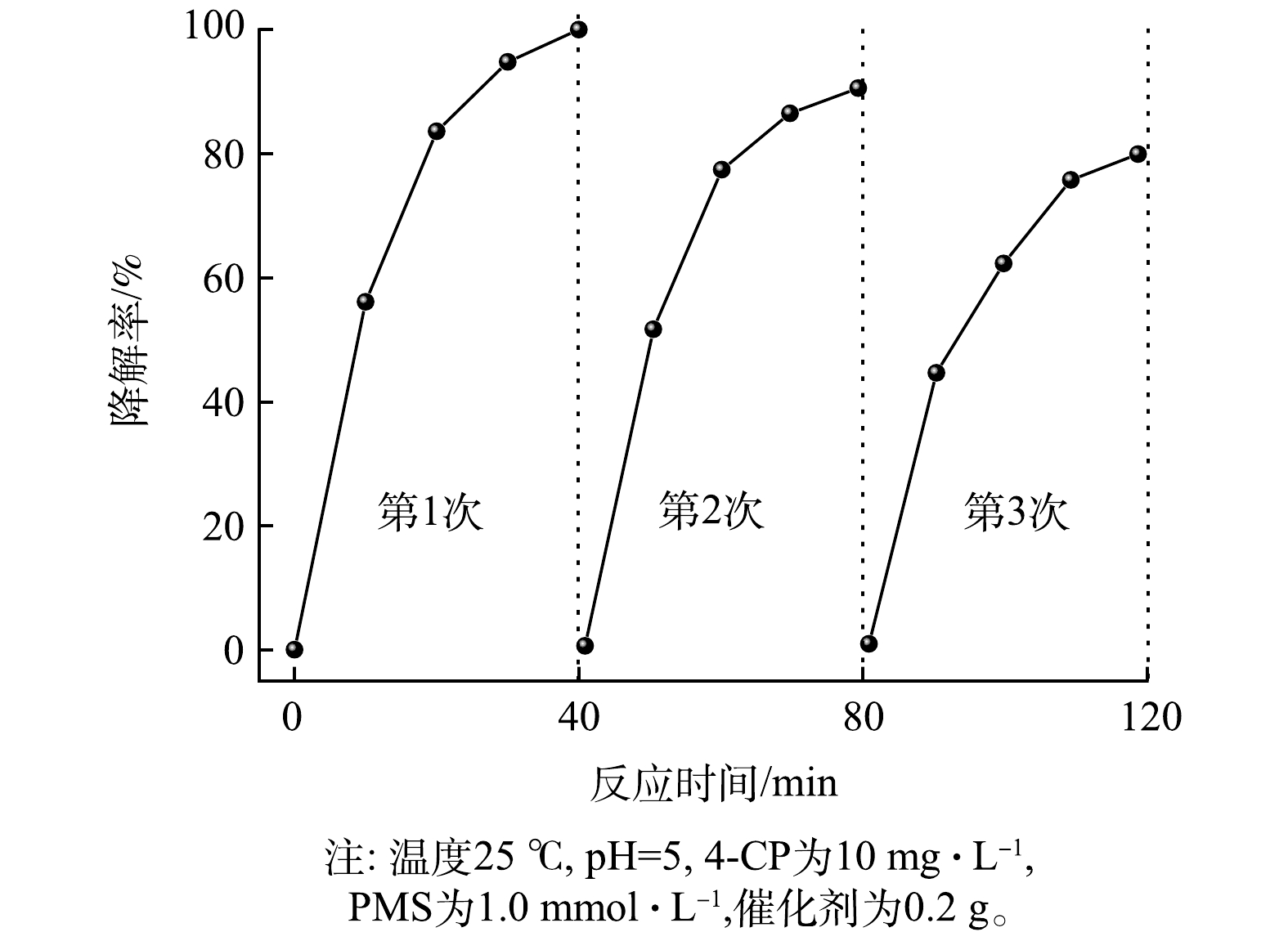
通过3次重复实验对γ-MnO2@BC/PMS降解4-CP的稳定性进行了研究。如图8,4-CP降解率分别为100%, 86.1%, 75.1%。降解效率随着反应次数的增加而逐渐降低,这可能是由于中间产物覆盖了反应的活性位点导致降解效率降低,但经3次重复利用后依然表现出了较高的降解效率。另一方面,MnO2活化PMS降解4-CP的能力与Mn(Ⅲ)/Mn(Ⅳ)的比例有关,反应后Mn(Ⅲ)/Mn(Ⅳ)的比值增大也是导致γ-MnO2@BC催化降解效率的降低主要原因之一。
-
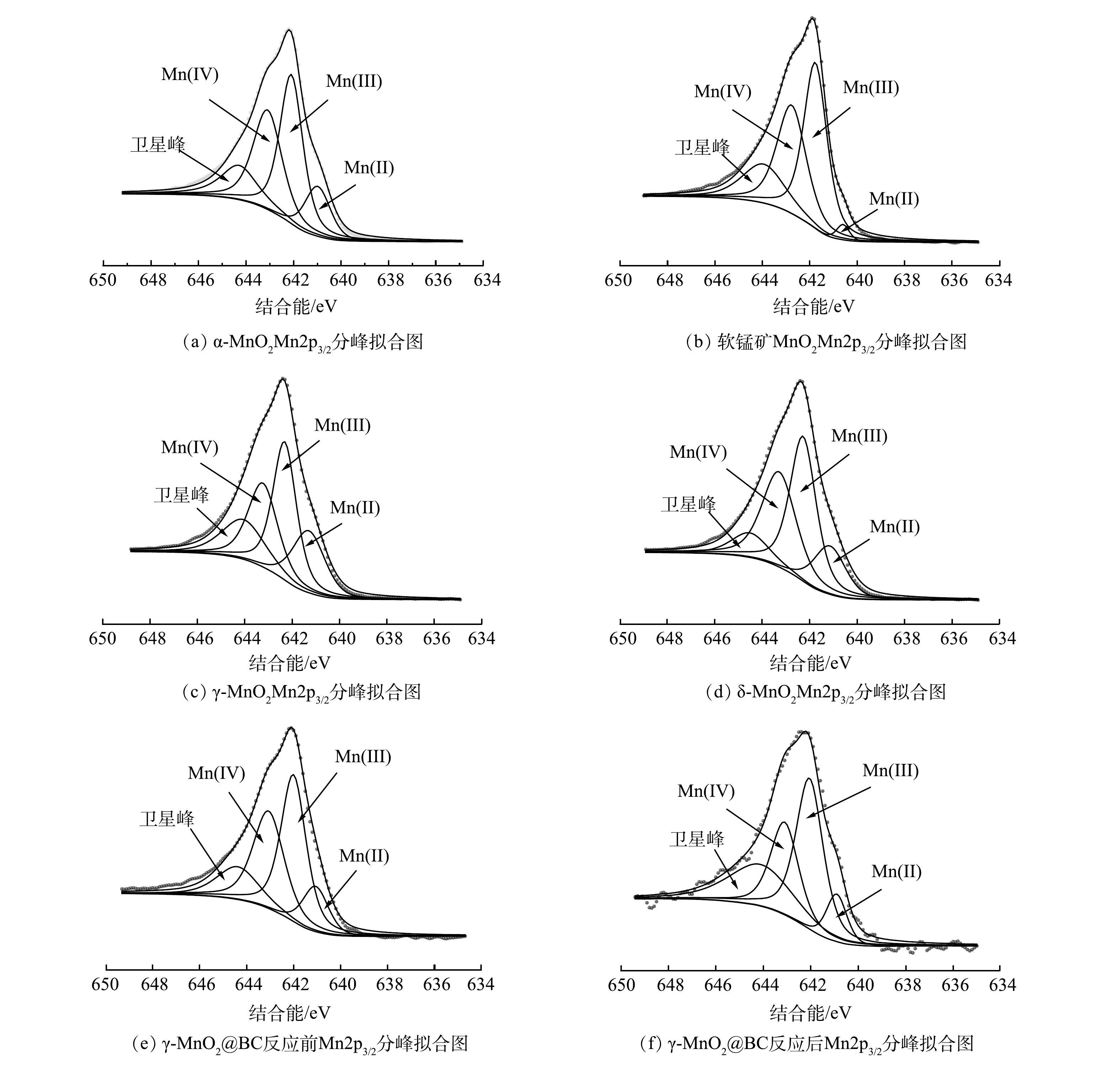
1) XPS分析。为探究γ-MnO2@BC反应前后不同价态Mn含量的变化,利用XPS对不同晶相中Mn2p轨道进行了分析。因为Mn2p1/2曲线受卫星区域的混合价效应影响较大[20],所以仅对Mn2p3/2曲线进行分峰拟合。如图9所示,各峰对应的结合能可匹配出相应锰元素的价态,其中Mn(II)、Mn(Ⅲ)、Mn(Ⅳ)对应键能分别为640.7~641.7、641.7~642.4和642.4~643.4 eV,各峰对应的面积可计算该价态锰的含量[20-21]。经计算,4种晶相二氧化锰中Mn(Ⅲ)与Mn(Ⅳ)的比值分别为α-MnO2(1.2)<δ-MnO2(1.21)<软锰矿MnO2(1.22)<γ-MnO2 (1.31)。但由图3(a)可见,在120 min时4种晶相二氧化锰对4-CP降解率为α-MnO2>δ-MnO2>软锰矿MnO2>γ-MnO2。这与Mn(Ⅲ)与Mn(Ⅳ)比值变化趋势正好相反。而γ-MnO2@BC中Mn(Ⅲ)与Mn(Ⅳ)比值为1.21比γ-MnO2 (1.31)低,但对4-CP去除效率相较于γ-MnO2显著提高。并且γ-MnO2@BC反应后Mn(Ⅲ)与Mn(Ⅳ)比值增加到了1.61,在重复使用中催化能呈现了下降趋势。因此,MnO2催化降解效率与Mn(Ⅲ)与Mn(Ⅳ)比值为反比。
如图9(e)和图9(f)所示,反应前Mn(Ⅲ)和Mn(Ⅳ)含量分别为40.78%和33.72%;反应后含量分别为43.87%和27.29%,所以推测锰在反应中获得电子,起到了氧化剂的作用。BC提升了催化剂的电子转移能力,较好的提高了γ-MnO2@BC的催化性能。
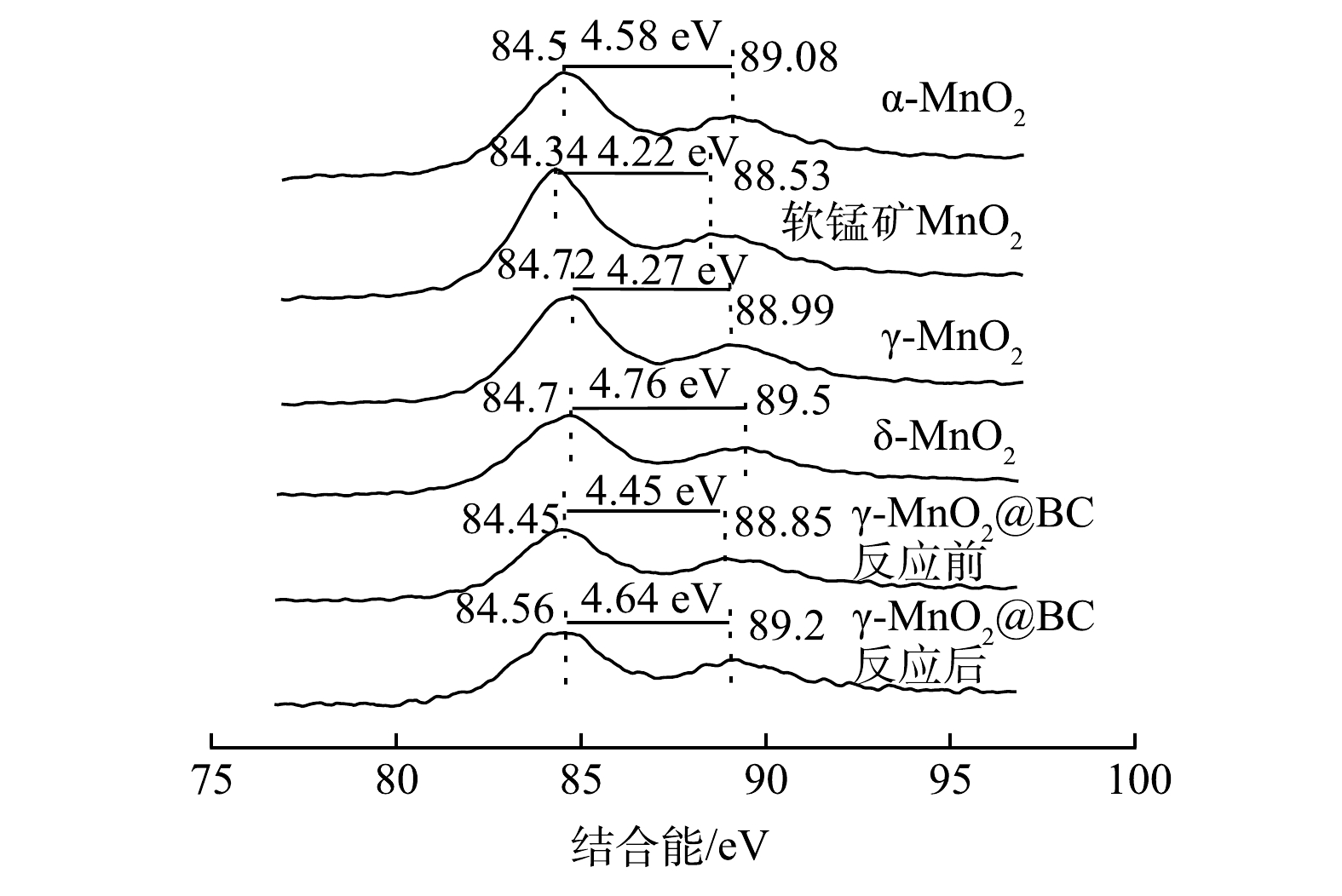
为了探究不同晶相MnO2中Mn AOS与二氧化锰催化活性直接的关系,还利用XPS检测了各材料中Mn3s轨道,因为Mn3s轨道对Mn AOS非常敏感。Mn AOS可依据
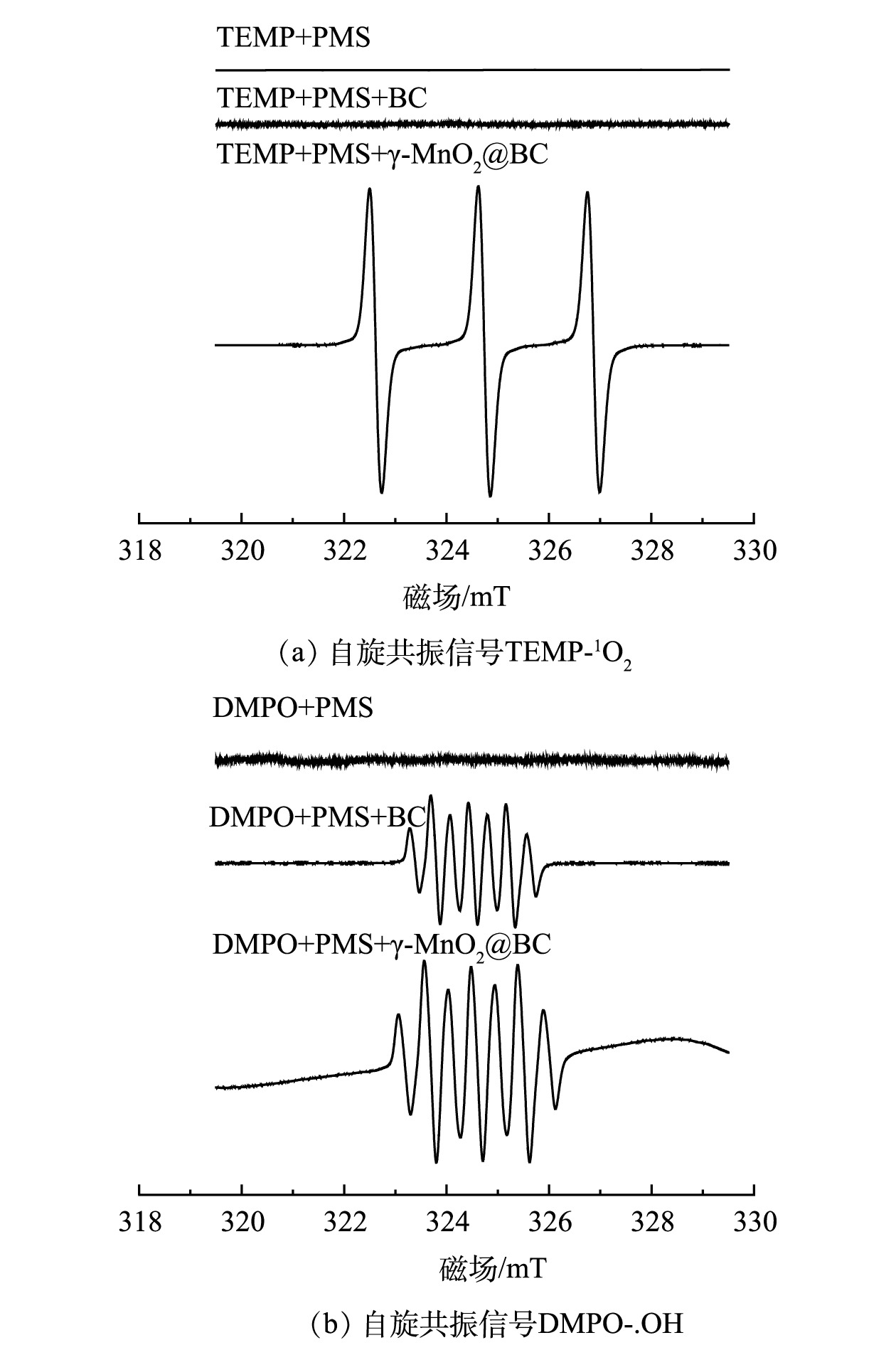
$ \mathrm{A}\mathrm{O}\mathrm{S}=8.95-1.13\Delta E\mathrm{s} $ [25]计算($ \Delta E\mathrm{s} $ 为Mn3s轨道主峰与卫星峰的键能差)。如图10所示,计算得出:α-MnO2(3.77 eV)、β-MnO2 (4.18 eV)、γ-MnO2 (4.12 eV)、δ-MnO2 (3.57 eV)、γ-MnO2@BC反应前(3.92 eV)、γ-MnO2@BC反应后(3.71 eV)。如图3(a)所示,α-MnO2 (3.77 eV)>δ-MnO2 (3.57 eV)>β-MnO2 (4.18 eV)>γ-MnO2(4.12 eV)。不难发现Mn AOS偏低,MnO2的催化性能越好,但γ-MnO2@BC反应前Mn AOS(3.92 eV)大于γ-MnO2@BC反应后Mn AOS (3.71 eV),与之前结论似乎矛盾,所以Mn AOS与催化降解速率之间并无线性关系。但催化剂反应后Mn AOS的降低验证了该催化剂中γ-MnO2起到了吸收电子的作用。2)活性氧物种的确定及分析。为了确定本研究中γ-MnO2@BC/PMS体系对4-CP的降解机制,使用TEMP和DMPO作为自由基捕获剂,采用自旋捕获电子顺磁共振光谱(ESR)鉴定降解体系中的活性物种。如图11(a)所示,当使用TEMP作为电子自旋捕获剂时,产生了较强的TEMPO(1∶1∶1)信号,而在未添加γ-MnO2@BC的情况下均未产生信号。如图11(b)所示,以DMPO为自旋捕获剂时,DMPO+γ-MnO2@BC检测出7个峰,其峰强比为1∶2∶1∶2∶1∶2∶1。这归因于氧化的DMPO(DMPO-X)。在DMPO+BC体系中也检测到相同的峰,但BC活化PMS对4-CP的降解率仅为32.72%,比BC吸附4-CP的去除率仅提升了0.44%。而在相同条件下γ-MnO2@BC在20 min时对4-CP降解率接近100%。有研究表明,氯离子是SO4·-和.OH的清除剂[17-18],而硝酸根离子会促进.OH的产生[22]。如图7(b)所示,在氯离子共存时,前20 min 4-CP降解率低于对照组;如图7(d)所示,在硝酸根离子共存时,前20 min 4-CP降解率高于对照组,而两者在40 min 时对4-CP的降解率均达到100%。
综上所述,使用TEMP作为单线态氧捕获剂时,γ-MnO2@BC+PMS体系产生了较强的信号;而使用DMPO捕获羟基自由基时,γ-MnO2@BC+PMS信号强度与BC+PMS类似。因此,该体系是以单线态氧为主导的非自由基氧化体系。
3)可能的降解机理。生物炭可作为电子传递的桥梁,还可以吸附污染物缩短污染物与催化剂之间的反应距离。据报道,当生物炭在反应时贡献电子,可产生SO·-和.OH(式(1)~式(2))。当生物炭在反应时吸收电子时会产生1O2(式(3)~式(6))[17]。
如图11(b)所示,以DMPO为电子自旋捕获剂捕获SO4.-和.OH时γ-MnO2@BC+PMS与BC+PMS产生.OH信号的强度差异并不显著,而以TEMP为电子自旋捕获剂捕获γ-MnO2@BC/PMS体系产生的单线态氧时,出现了较为明显的三重峰,如图11(a)所示。结合图3(b)中BC活化PMS对4-CP的降解率(120 min时为32.72%)来看,γ-MnO2@BC/PMS是以单线态氧为主导的反应体系。基于γ-MnO2@BC/PMS体系反应前后Mn2P 3/2和活性组分的分析结果,得出以下2点结论:1)在该体系中,生物炭不仅可作为电子受体,而且可通过吸附污染物缩短催化剂与污染物之间的距离,生物炭的破损位点为γ-MnO2提供了负载位点,同时抑制了金属锰离子的浸出;2)负载在BC上的γ-MnO2 中Mn(Ⅲ)与Mn(Ⅳ)的比值由反应前的1.21升高至反应后的1.61,γ-MnO2@BC作为氧化剂可催化氧化4-CP。综上所述,推测γ-MnO2@BC活化PMS产生单线态氧降解4-CP的可能机理如式(8)~式(11)所示。
当γ-MnO2@BC得到电子,PMS失去电子时,产生单线态氧与4-CP反应,实现4-CP的去除,该反应过程占主导地位。γ-MnO2@BC也会失去电子,当PMS得到电子时会产生羟基自由基。
-
1) γ-MnO2@BC活化PMS体系对4-CP的降解效率最高,当BC掺杂量为0.75 g时,γ-MnO2@BC的降解率有较大提升。当pH为5~9、催化剂掺杂量为0.2 g·L−1、PMS为1 mmol·L−1、温度为25 ℃时,在反应40 min后4-CP降解率接近100%。
2) γ-MnO2@BC/PMS体系是以单线态氧为主导的非自由基体系,但也会产生少量羟基自由基。当体系中存在阴离子和HA以及在不同水样背景条件下,除H2PO4−有较大影响外,其他条件下对4-CP均能保持较高的降解率。
3)随着Mn(Ⅲ)与Mn(Ⅳ)的比值的减少,MnO2氧化反应活性逐渐提高。并且γ-MnO2@BC在反应中BC与γ-MnO2在反应中均吸收电子,形成了良好的协同反应。
生物炭负载γ-MnO2纳米复合材料活化过一硫酸盐降解对氯苯酚的性能及机理
Performance and mechanism of biochar doped γ-MnO2 nanocomposite activated peroxymonsulfate on 4-Chlorophenol degradation
-
摘要: 采用水热法合成了4种不同晶相结构MnO2及生物炭负载γ-MnO2复合纳米材料,并对其活化过硫酸盐(PMS)降解4-CP的性能进行了研究。采用XRD、SEM、EDS以及XRF等手段对不同复合纳米材料进行了表征分析,发现仅有γ-MnO2成功负载到生物炭材料表面形成γ-MnO2@BC复合纳米材料。在优化条件下,γ-MnO2@BC活化PMS体系能在20 min内将10 mg·L−1对氯苯酚完全降解。γ-MnO2@BC对H2PO4−之外的阴离子均表现出较强的抗干扰性。采用自由基捕获及电子自旋共振波谱(ESR)、X射线光电子能谱(XPS)等手段研究了该复合纳米材料活化PMS降解污染物的机理。结果表明,γ-MnO2@BC活化PMS产生的活性氧物种为单线态氧,并发现Mn(III)与Mn(IV)的比值是影响不同晶相二氧化锰催化性能的主要因素。Abstract: Four kinds of MnO2 with different crystal phase structure and biochar supported γ-MnO2 nanocomposites were synthesized by hydrothermal method, and their performance on activating peroxymonsulfate(PMS) and degrading 4-CP was studied. XRD, SEM, EDS and XRF were used to characterize different nano-composite materials. It was found that only γ-MnO2 was successfully loaded on the surface of biochar to form γ-MnO2@BC. Under the optimum conditions, 10 mg·L−1 4-chlorophenol could be completely degraded by γ-MnO2@BC activated PMS in 20 min. γ-MnO2@BC showed the strong anti-interference against anions except for H2PO4−. Finally, the mechanism of reactive oxygen species (ROS) generated by γ-MnO2@BC activated PMS was studied by means of free radical capture, electron spin resonance spectroscopy (ESR) and X-ray energy spectroscopy (XPS). The results showed that the ROS produced by γ-MnO2@BC activated PMS was singlet oxygen. It is found that the ratio of Mn(III) to Mn(IV) was the main factor affecting the catalytic performance of MnO2 with different crystalline phases.
-
Key words:
- MnO2 /
- crystal phase /
- biochar /
- peroxymonsulfate /
- degradation of 4-chlorophenol
-

-
表 1 不同实验中4-CP,催化剂和PMS掺杂量
Table 1. Doping amount of 4-CP, catalyst and PMS in different experiments
实验 催化剂/(g·L−1) PMS/(mmol·L−1) 筛选实验
吸附实验0.6
0.60.5
0.5γ-MnO2BC配比实验 0.6 0.5 催化剂掺杂量实验 0.2~0.6 0.5 PMS掺杂量实验 0.2 0.5~1.5 单因素和阴离子干扰实验 0.2 1.0 表 2 不同材料的主要元素含量
Table 2. Contents of main elements in different materials
材料 Mn C O K Si 其他 α-MnO2 77.14 0.86 10.08 9.41 0.49 2.02 软锰矿 MnO2 81.01 0.47 17.16 0 0.32 1.04 γ-MnO2 86.43 0.43 11.38 0 0.13 1.63 δ-MnO2 70.54 0.53 15.35 11.74 0.77 1.07 MnCO3@BC 49.16 7.72 11.78 3.33 26.48 1.53 nMnOx-1@BC 0.57 31.22 17.13 1.38 46.46 3.42 γ-MnO2@BC 42.59 11.16 10.6 0.85 32.5 2.3 nMnOx-2@BC 27.1 17.99 12.44 3.02 37.52 1.93 表 3 水质指标
Table 3. Water quality parameters
水样 TP/(mg·L−1) COD/(mg·L−1) pH 氨氮/(mg·L−1) 斛兵塘湖水 0.110 78.3 7.55 0.066 南淝河河水 0.038 91.3 7.3 0.262 -
[1] 钟少芬, 莫健文, 李阳苹, 等. 粉末活性炭对水中氯酚的吸附[J]. 环境工程学报, 2016, 10(6): 2927-2932. doi: 10.12030/j.cjee.201501023 [2] GAO X, CHEN J, CHE H, et al. Rationally constructing of a novel composite photocatalyst with multi charge transfer channels for highly efficient sulfamethoxazole elimination: Mechanism, degradation pathway and DTF calculation[J]. Chemical Engineering Journal 2021, 273: 128506 [3] XIAO S, CHENG M, ZHONG H, et al. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review[J]. Chemical Engineering Journal, 2020, 384: 123265. doi: 10.1016/j.cej.2019.123265 [4] 王晓东, 张光辉, 顾平, 等. 水体中氯酚类污染物的生物降解性研究进展[J]. 中国给水排水, 2008, 24(16): 4. [5] SUNG M, HUANG C. In situ removal of 2-chlorophenol from unsaturated soils by ozonation[J]. Environmental Science & Technology, 2002, 36(13): 2911-2918. [6] KUAN C, CHANG S, SCHROEDER S. Fenton-Like oxidation of 4-chlorophenol: Homogeneous or heterogeneous[J]. Industrial & Engineering Chemistry Research, 2015, 54(33): 8122-8129. [7] BARAKAT M, SCHAEFFER H, HAYES G, et al. Photocatalytic degradation of 2-chlorophenol by co-doped TiO2 nanoparticles[J]. Applied Catalysis B Environmental, 2005, 57(1): 23-30. doi: 10.1016/j.apcatb.2004.10.001 [8] HUANG J, ZHONG S, DAI Y, et al. Effect of MnO2 phase structure on the oxidative reactivity toward bisphenol A degradation[J]. Environmental Science & Technology, 2018, 52(19): 11309-11318. [9] MCKENDRY I, KONDAVEETI S, SHUMLAS S, et al. Decoration of the layered manganese oxide birnessite with Mn(II/III) gives a new water oxidation catalyst with fifty-fold turnover number enhancement[J]. Dalton Transactions, 2015, 44(29): 12981-12994. doi: 10.1039/C5DT01436K [10] LEE H, KIM H I, WEON S, et al. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds[J]. Environmental Science & Technology, 2016, 50(18): 10134-10142. [11] DUAN X, SU C, ZHOU L, et al. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds[J]. Applied Catalysis B:Environmental, 2016, 194: 7-15. doi: 10.1016/j.apcatb.2016.04.043 [12] FANG G, LIU C, GAO J, et al. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation[J]. Environmental Science & Technology, 2015, 49(9): 5645-5653. [13] MA J, ZHANG S, DUAN X, et al. Catalytic oxidation of sulfachloropyridazine by MnO2: Effects of crystalline phase and peroxide oxidants[J]. Chemosphere, 2021, 267: 129287. doi: 10.1016/j.chemosphere.2020.129287 [14] WANG Z, YUAN R, GUO Y, et al. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone reagent: Kinetic analysis[J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 1083-1087. [15] MA J, YANG Y, JIANG X, et al. Impacts of inorganic anions and natural organic matter on thermally activated persulfate oxidation of BTEX in water[J]. Chemosphere, 2018, 190: 296-306. doi: 10.1016/j.chemosphere.2017.09.148 [16] ZHAO C, SHAO B, YAN M, et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review[J]. Chemical Engineering Journal, 2021, 416: 128829. doi: 10.1016/j.cej.2021.128829 [17] 王静, 邢梦林, 邰超, 等. 不同光谱区间日光照射下水体成分的光致羟基自由基生成研究[J]. 环境化学, 2015, 34(12): 2162-2169. doi: 10.7524/j.issn.0254-6108.2015.11.2015060402 [18] YUAN R, RAMJAUN SN, WANG Z, et al. Effects of chloride ion on degradation of acid orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds[J]. Journal of Hazardous Materials. 2011, 196: 173-179. [19] FAHEEM, DU J, KIM S, et al. Application of biochar in advanced oxidation processes: supportive, adsorptive, and catalytic role[J]. Environmental Science And Pollution Research International 2020, 27(30): 37286-37312. [20] ILTON E, POST J, HEANEY P, et al. XPS determination of Mn oxidation states in Mn (hydr)oxides[J]. Applied Surface Science, 2016, 366: 475-485. doi: 10.1016/j.apsusc.2015.12.159 [21] Al-NU'AIRAT J, OLUWOYE I, ZEINALI N, et al. Review of chemical reactivity of singlet oxygen with organic fuels and contaminants[J]. Chemical Record, 2021, 21(2): 315-342. doi: 10.1002/tcr.202000143 [22] SUN C, SHI Z. Transition-metal-free coupling reactions[J]. Chemical Reviews, 2014, 114(18): 9219-9280. doi: 10.1021/cr400274j -



 下载:
下载: