-
微量有机污染物(micro organic pollutants, MOPs),如药物与个人护理品、内分泌干扰物等,具有生物累积性、环境持久性和难降解性,在微量浓度下即会威胁环境和人群健康。臭氧(O3)已被广泛应用于水深度处理中[1-2],可高效降解大多数MOPs[3-4],而O3与溶液的接触方式是其应用的关键。传统的鼓泡反应器通过底部的O3曝气使O3与水充分混合,具有操作便捷、安装方便等优点,但曝气头表面的微孔易堵塞,需经常清洗,同时还会出现泡沫、液泛等问题。此外,混合塔也被广泛采用,其将水从高处喷下形成雾状,O3气体从塔下方通入,与水流逆行并充分接触,这种方式有利于O3传质,但该设备成本高,能量损失大。O3膜接触器是一种新型O3/溶液接触装置。其基于带微孔的中空纤维膜管,通过“外液内气”方式,使得O3在浓度差作用下以无泡方式从气相扩散至液相[5]。相较于传统O3接触方式,O3膜接触器具有传质效率高、O3分布均匀、占地面积小、能耗低等优点,可有效避免泡沫、液泛等问题,应用前景广阔[6-7]。同时,其亦存在膜易被污染、设备结构复杂等问题,应用中需予以关注。
O3工艺存在的重要问题是无法降解耐O3 MOPs(O3 resistant MOPs, OR-MOPs),且出水中残余O3会影响后续工艺。O3的紫外(UV)摩尔吸光系数高(约3 000 L·(mol·cm)–1)[8],在UV辐照下可被快速光解并生成羟基自由基(HO·),过程见式(1)~式(7)[9−10]。HO·具有强氧化性,可降解绝大多数MOPs(包括OR-MOPs)。因此,在O3氧化后增加UV辐照,可将残余O3转化为HO·,强化OR-MOPs降解,提高O3利用率。
目前,有关膜接触O3-UV联用的研究鲜有报道,而且用于开展相关研究的实验装置也并不多见。细管流光反应系统是新型的UV装置。它具有剂量测定准确、样品量少、剂量率均一等优点[11]。该装置过流式的运行模式方便与O3膜接触器进行连接,以开展膜接触O3-UV联用的相关研究。
本研究搭建了O3膜接触器与细管流光反应系统相结合的膜接触O3-UV实验装置,以探究O3膜接触器中各因素对液相O3浓度的影响,并准确测定细管流光反应系统输出的辐照剂量;之后,以一种耐O3氧化且广泛应用于临床治疗的非甾体抗炎药—布洛芬为代表性OR-MOP [12-13],用以考察膜接触O3-UV联用对水中OR-MOPs的强化降解效果。通过分析O3利用率、pH对联用工艺降解MOPs的影响及各降解途径的贡献比,以期为工艺应用与优化提供参考。
-
布洛芬、尿苷、靛蓝三磺酸钾、磷酸二氢钠、磷酸氢二钠和亚硫酸氢钠均为分析纯。所用仪器包括:Milli-Q超纯水设备(Advantage A10, Millipore, 美国)、UV-Vis分光光度计(UV-2600, Shimadzu公司, 日本)、高效液相色谱(HPLC, Agilent1200, 美国)、O3发生器(COM-AD-01, Anseros, 德国)、O3分析仪(M-OEM, Anseros, 德国)、O3尾气破坏器(F800, 同林臭氧, 中国)。
-
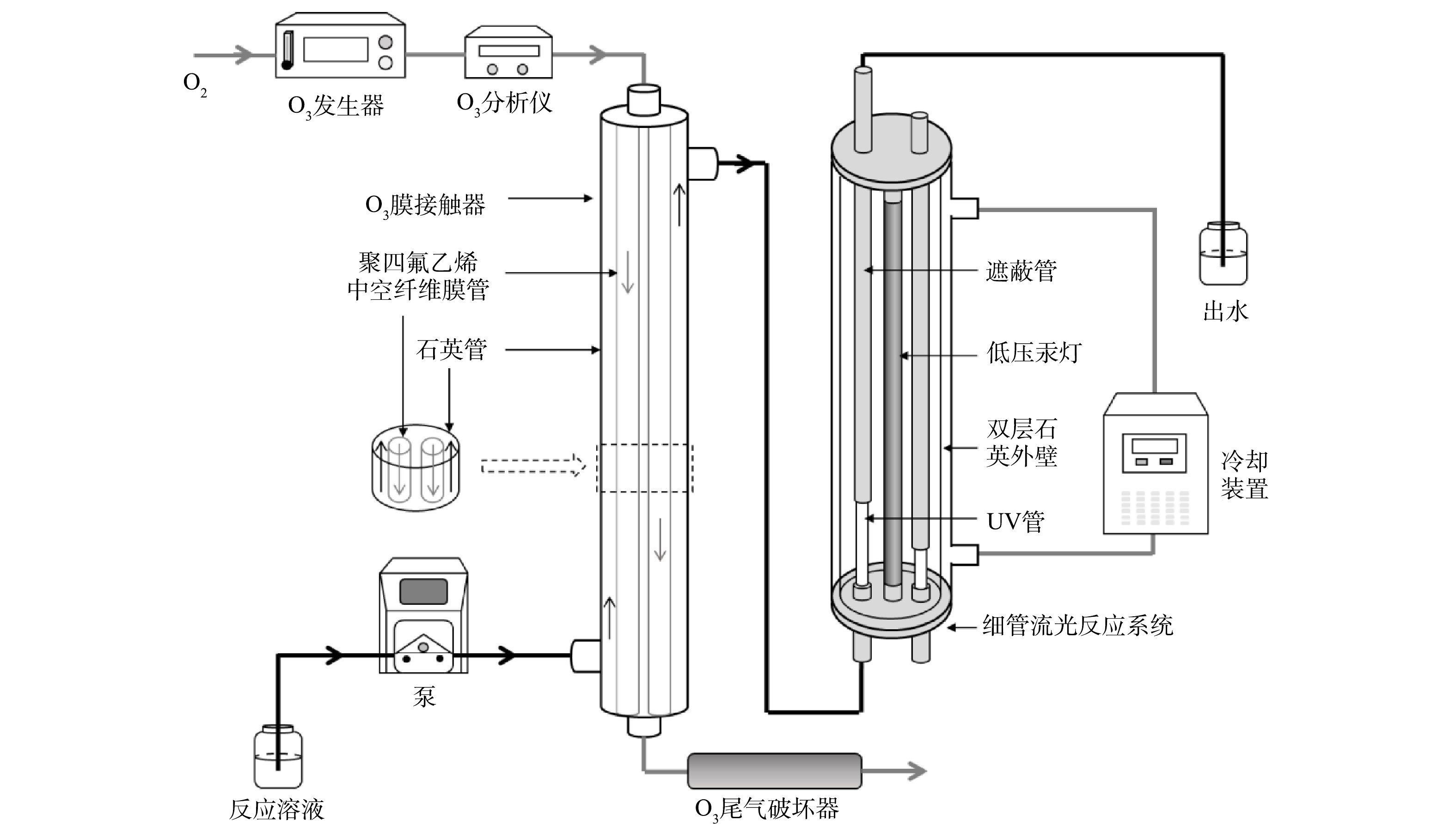
膜接触O3-UV实验装置主要由O3发生器、O3分析仪、O3膜接触器、O3尾气破坏器、细管流光反应系统和冷却装置组成,如图1所示。O3膜接触器由内部的聚四氟乙烯中空纤维膜管及外部的石英管组成。聚四氟乙烯膜是耐O3的永久疏水性材料,pH耐受范围广泛,稳定性强且易于加工[14]。O3发生器产生的O3,经O3分析仪在线监测气相质量浓度后,进入O3膜接触器的膜管内腔,与反应溶液进行膜接触传质后排入O3尾气破坏器。反应溶液从O3膜接触器的膜管与石英管中间流过,膜管内O3分子跨过膜微孔进入膜管外溶液边界层,最终进入溶液主体,期间不产生肉眼可见的O3气泡[15-16]。通过改变O3发生器功率调节气相O3质量浓度。反应溶液从O3膜接触器流出后通过连接管进入后续细管流光反应系统,在连接管不同位置取样可获得不同的O3接触时间。
细管流光反应系统主体由双层石英管壁、中心处的低压汞灯、以及平行于低压汞灯安装的UV管(含钛石英材料)组成[17]。反应溶液流经UV管接受UV辐照,UV管外套有不锈钢遮蔽管,控制遮蔽管位置可调节UV剂量。冷却水(25 ℃)从双层石英管壁间自下而上流过,保持系统恒温,从而保证UV辐照的稳定性。
-
采用磷酸缓冲溶液,通过在O3膜接触器出口溶液中测定液相O3质量浓度,探究气相O3质量浓度(20、60、100 g·m−3)、溶液流量(3~60 mL·min−1)、pH(5.5~9.0)、磷酸根等因素对O3膜接触器中O3溶解的影响。之后,利用化学剂量法测定UV剂量。在搭建的膜接触O3-UV实验装置上,通过在不同取样口取样获得不同的O3接触时间(0~65 s),通过控制细管流光反应系统遮光管位置获得不同的UV剂量(0~3.3 × 10–3 Einstein·m–2),并通过控制气相O3质量浓度(20、60 g·m−3)来获得不同的液相O3质量浓度(1.0、2.0 mg·L−1),用于探讨布洛芬在膜接触O3-UV联用工艺中的降解效果。
采用靛蓝三磺酸钾法测定液相O3质量浓度 [18];采用化学剂量法,以尿苷(0.12 mmol·L−1)作为感光剂 [11,17],通过循环流运行模式测定细管流光反应系统的UV剂量率(Ep,UV, Einstein·m−2·s−1);利用HPLC测定布洛芬质量浓度,检测器为UV二极管阵列检测器,色谱柱为SB-C18柱(2.1 mm × 50 mm, 1.8 μm, Waters公司,美国),流动相为超纯水(30%)与乙腈(70%),流速0.8 mL·min−1,进样量为100 μL,柱温30 ℃。
-
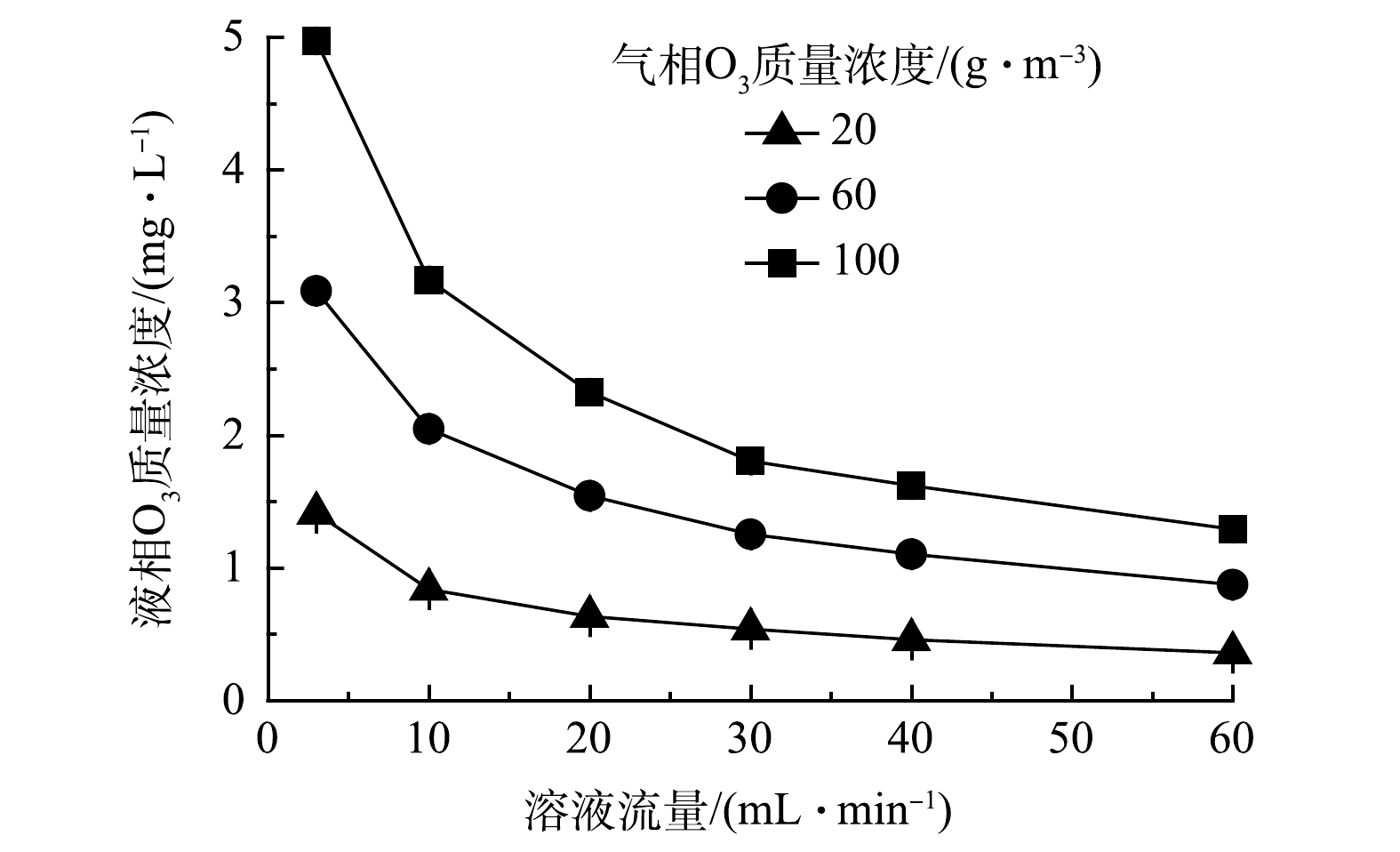
为揭示O3膜接触器的性能,探讨了溶液流量、气相O3质量浓度、溶液pH以及磷酸缓冲溶液对出水液相O3质量浓度的影响。图2为不同气相O3质量浓度下,10 mmol·L–1磷酸缓冲溶液(pH = 7.5)通过O3膜接触器后,出水液相O3质量浓度与溶液流量的关系。结果表明,在相同气相O3质量浓度下,出水液相O3质量浓度随溶液流量的增加而降低。这主要受2方面影响[7]:首先,溶液在O3膜接触器中的接触时间随着溶液流量增加而缩短,造成O3溶解量减少;其次,气液相界面处的O3迁移阻力随着溶液流量的增加而减小,促进O3扩散溶解。因此说明,在本研究的实验条件范围内,前者对O3溶解的影响更显著。
在相同的溶液流量下,气相O3质量浓度提高会增加O3溶解。这是因为,气、液两相间O3质量浓度差增大,加大了传质的推动力,从而促进O3扩散进入反应溶液[19−20]。值得注意的是,气相O3利用率会随着气相O3质量浓度增加而降低,尽管工程反应器较实验装置膜管长度要更长,但更高的气相O3质量浓度容易造成更多的O3进入O3尾气破坏系统,导致O3不能充分利用。
溶液pH对O3溶解的影响如图3所示,当气相O3质量浓度为60 g·m−3、溶液流量为10 mL·min−1时,O3膜接触器的出水液相O3质量浓度随着pH的升高而降低。这是因为,OH−的增多会促进溶液中O3的分解,从而降低出水液相O3质量浓度,如式(5)~式(7)所示[21-23]。
污染物降解实验通常采用磷酸缓冲溶液来控制反应过程的pH。因此,本实验对比了磷酸缓冲溶液和超纯水中O3溶解的差异。由于超纯水偏酸性(pH在5.3~5.5),故采用pH为5.5、浓度为10 mmol·L−1的磷酸缓冲溶液。实验结果如图4所示,在溶液流量为3~60 mL·min−1时,磷酸缓冲溶液对出水液相O3质量浓度几乎无影响。这说明,磷酸根在10 mmol·L−1浓度时,对O3的溶解影响可忽略。
-
Ep,UV(Einstein·m−2·s−1)计算公式见式(8)。
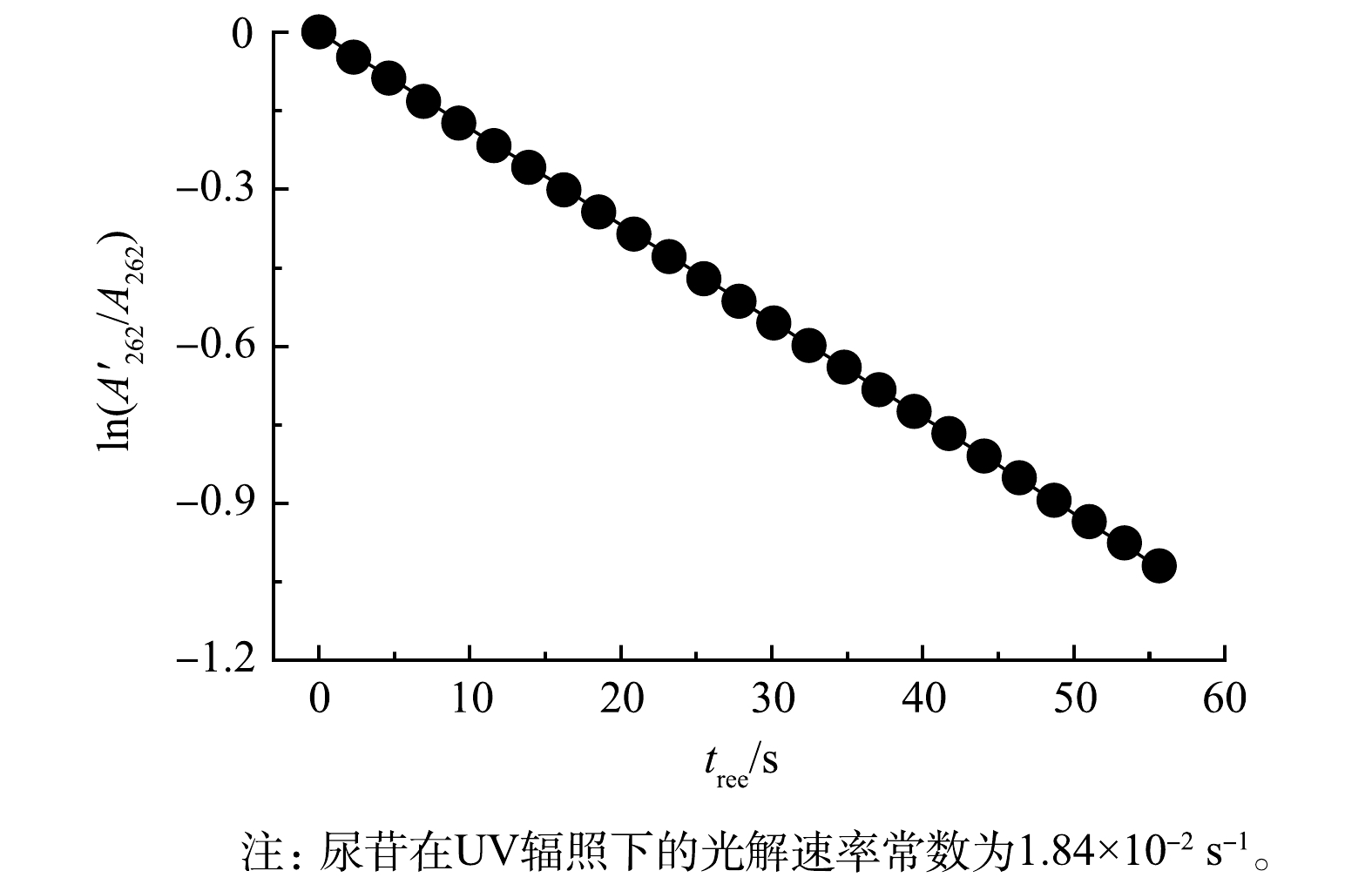
式中:ε254为尿苷在254 nm的摩尔吸光系数,8 740 L·(mol·cm)−1;Φu为尿苷光解反应的量子产率,0.02 mol·Einstein−1;
$A'_{262} $ 和A262分别是UV辐照前后尿苷在262 nm处的吸光度,cm−1;tree是等效辐照时间,s,可由总反应时间(ttot, s)乘以接受辐照的UV管容积(πr2h, mL, r = 0.18 cm, h = 38 cm)与反应溶液体积(V,50 mL)之比获得。图5为细管流光反应系统(循环流运行模式)中尿苷的直接UV光解结果,通过拟合可得准一级光解速率常数为1.84 × 10−2 s−1。代入式(9)计算得到Ep,UV = 4.56×10−4 Einstein·m−2·s−1,剂量可通过公式(9)计算。
式中:t为辐照时间,s,可通过UV管中反应溶液接受辐照的体积(
${\text{π}{r}^{2}h'} $ , mL, r = 0.18 cm)与溶液流量(Q = 10 mL·min−1)的比值得到,其中$h' $ 为UV管感光部分长度(未被遮光管遮挡部分),如式(10)所示。 -
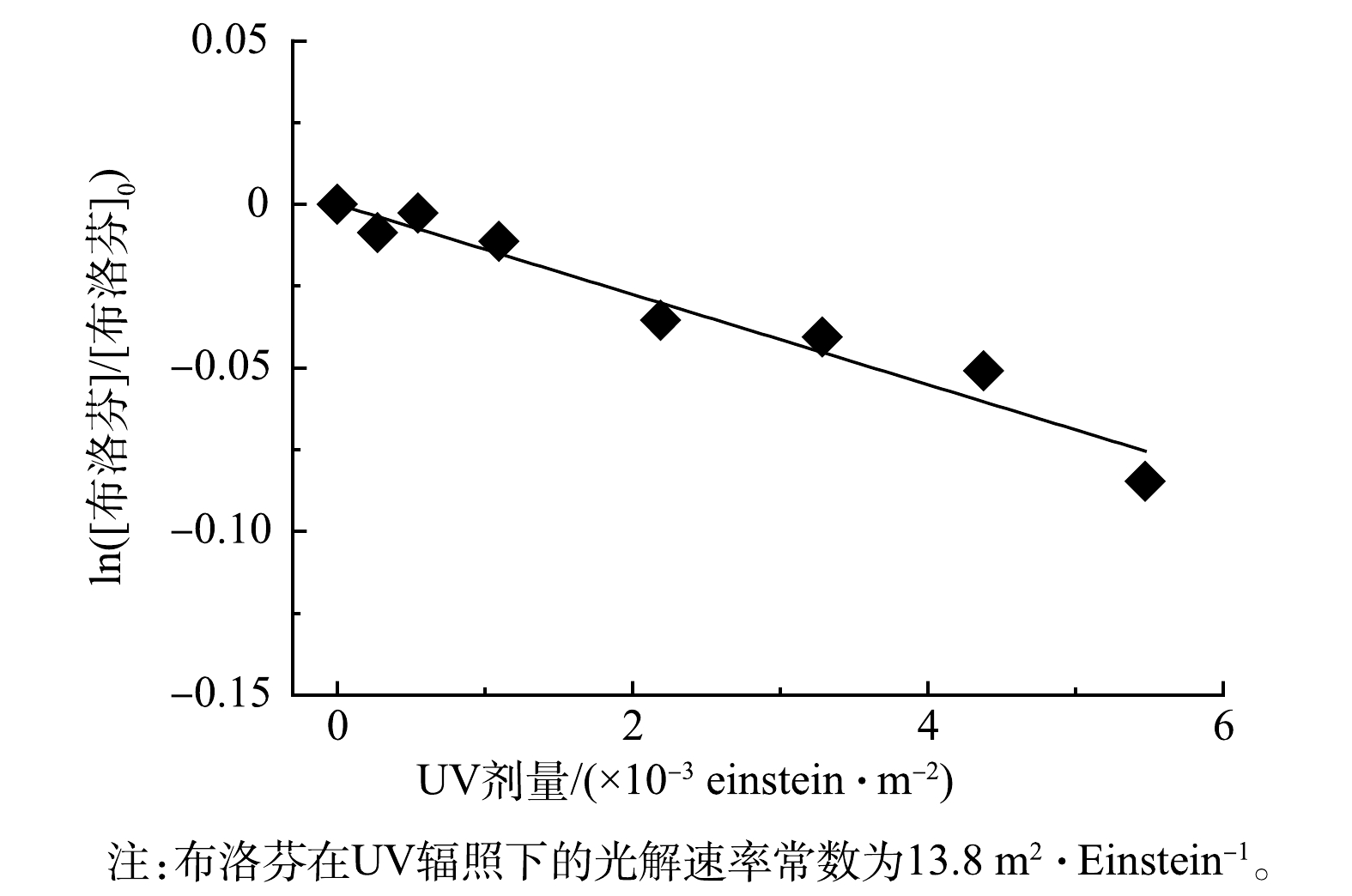
在细管流光反应系统中,通过调节遮光管位置,测定布洛芬在磷酸缓冲溶液中(pH=7.5)的直接UV光解动力学。图6结果表明,布洛芬光解过程符合准一级动力学,光解速率常数为13.8 m2·Einstein–1。这表明,直接UV光解对布洛芬降解的贡献十分有限。
-
膜接触O3-UV联用降解磷酸缓冲溶液中(pH=7.5)布洛芬(2.0 mg·L–1)及液相O3质量浓度变化的结果分别见图7。布洛芬降解过程分为2个阶段,UV辐照(细管流光反应系统)前的O3氧化阶段和残余O3在UV辐照下生成HO·,进而形成的O3/UV高级氧化阶段。在O3氧化阶段,液相O3质量浓度越大,接触时间越长,布洛芬的去除率越高。然而,O3氧化阶段对布洛芬的去除率与O3利用率均不高,液相O3质量浓度为1.0和2.0 mg·L–1时,布洛芬接触O3 65 s后去除率仅达到25.1%和38.4%,残余O3质量浓度分别为0.9和1.7 mg·L–1。这是由于布洛芬与O3的二级反应速率较低(kO3,IBP=9.6 L·(mol·s)–1 [24]),难以被O3氧化降解。在O3/UV高级氧化阶段,液相O3质量浓度为1.0和2.0 mg·L–1时,增加UV剂量,使布洛芬和O3的质量浓度同步降低。当UV剂量达到3.3 × 10–3 Einstein·m–2时,O3被完全消耗。此时,布洛芬的去除率分别为63.2%和82.9%,相对于O3氧化阶段分别增加了38.1%和44.5%。这是因为,O3在UV辐照下转化为HO·,布洛芬与HO·的二级反应的速率常数高(kHO·,IBP=7.4×109 L·(mol·s)–1 [24]),使得布洛芬的去除率增加(图7),同时提高O3的利用率,防止O3进入环境或后续工艺。实验表明,膜接触O3-UV联用可强化OR-MOPs的降解,可实现残余O3的高效利用。
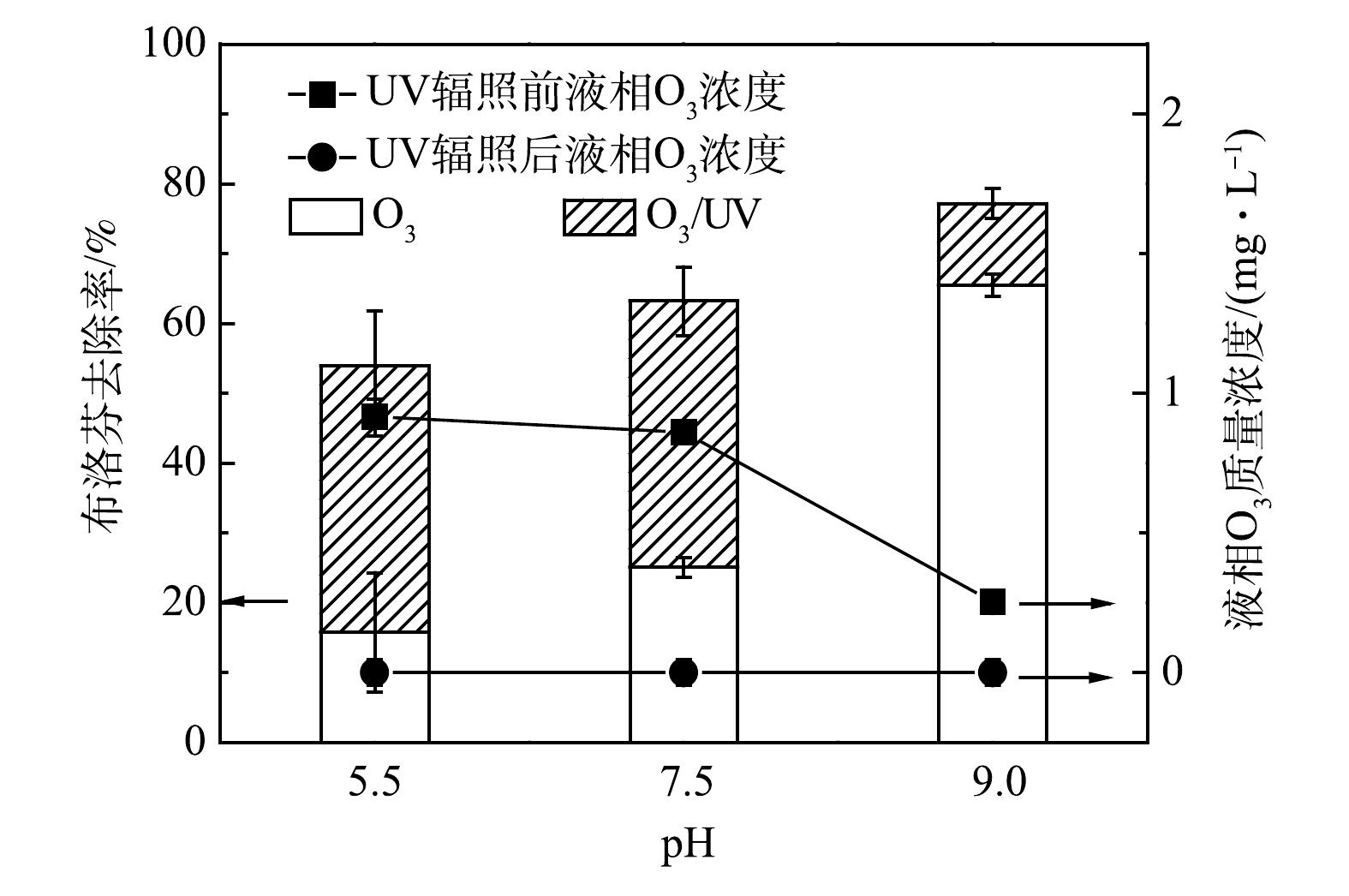
在O3/UV高级氧化阶段,布洛芬的去除率在pH为5.5和7.5时基本相同(约38.1%),而pH = 9.0时去除率仅为11.7%,残余O3在所有pH下均被完全利用。本阶段主要降解途径为UV光解O3产生的HO·氧化,故pH对布洛芬降解的影响主要取决于进入细管流光反应系统的残余O3质量浓度。在pH = 9.0时,残余O3质量浓度最低,布洛芬去除率亦最低。对于膜接触O3-UV整体过程,在pH = 5.5、7.5、9.0时,布洛芬的去除率分别为48.8%、63.2%、77.2%。这说明OR-MOPs在产生HO·更多的碱性条件下更易被降解。
-
膜接触O3-UV联用降解布洛芬的过程中,O3氧化阶段含有O3分子氧化和HO·氧化2种降解途径,而O3/UV高级氧化阶段含有HO·氧化和直接UV光解2种降解途径。
O3氧化阶段,布洛芬的降解可由式(11)表示。
式中:[IBP]0和[IBP]分别是布洛芬在初始和反应时间t时的浓度,mol·L−1;kO3,IBP和kHO·,IBP分别为布洛芬与O3及HO·的二级反应速率常数(kO3,IBP = 9.6 L·(mol·s)–1,kHO·,IBP = 7.4×109 L·(mol·s)–1);∫[O3]dt和∫[HO·]dt分别为O3和HO·的浓度随时间的积分。
Rct为∫[HO·]dt与∫[O3]dt的比值,在给定水中一定反应时间内是定值,其数量级为10–8 [25–26],见式(12)。
将式(12)代入式(11)可以得到组合形式,见式(13)。
前文测定了膜接触O3氧化布洛芬过程中液相O3质量浓度的变化(图8),将该过程液相O3质量浓度进行积分,结合布洛芬的降解结果代入式(13),即可得到液相O3质量浓度为1.0和2.0 mg·L−1时,Rct值分别为3.0×10–8和3.2×10–8。O3氧化阶段布洛芬被O3分子氧化占比(fO3)和HO·氧化占比(fHO·)可分别用式(14)和式(15)计算。
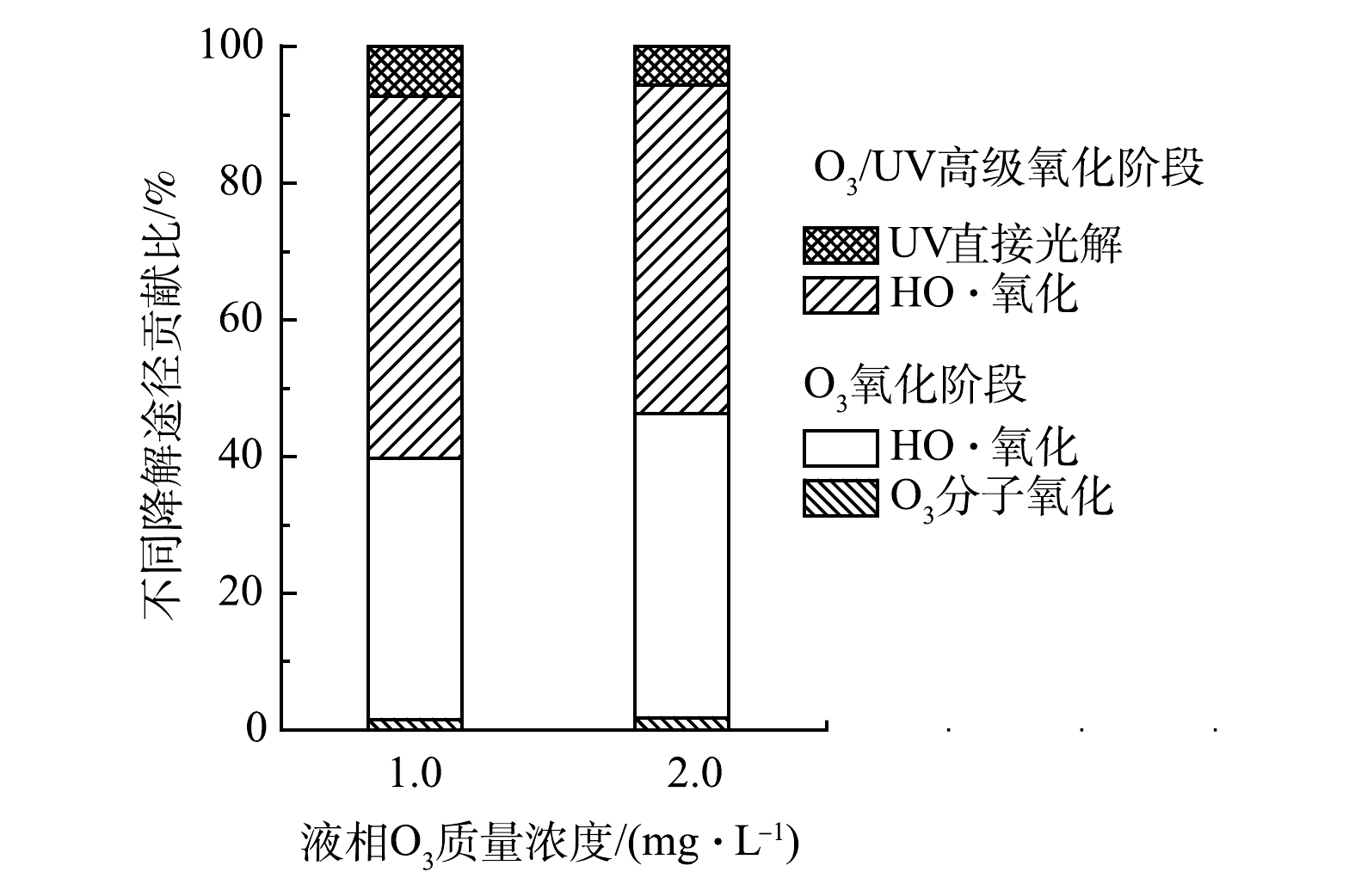
结果表明,O3氧化阶段,液相O3质量浓度为1.0和2.0 mg·L−1、缓冲溶液pH = 7.5时,fO3分别为95.9%和96.1%,相对应的fHO·分别为4.1%和3.9%。结合前文O3/UV高级氧化阶段和直接UV光解的结果,可计算膜接触O3-UV联用中布洛芬主要降解途径(O3氧化阶段的O3分子氧化、HO·氧化以及高级氧化阶段的HO·氧化和直接UV光解)的贡献占比。图9结果表明,布洛芬的降解途径以HO·氧化为主,贡献比可以达到90%以上。这说明,随着液相O3质量浓度增加,HO·氧化对布洛芬的降解贡献比略有增加。
-
1)通过对O3膜接触器进行评估发现,随着气相O3质量浓度增加、溶液流量和pH的降低,液相O3质量浓度升高,而磷酸盐对O3溶解没有影响。通过化学感光剂标定细管流光反应系统的Ep,UV=4.56×10−4 Einstein·m−2·s−1。
2)膜接触O3-UV联用降解布洛芬可分为O3氧化和O3/UV高级氧化2个阶段,O3/UV高级氧化由于HO·的生成,布洛芬去除效果和O3利用率较O3氧化阶段明显提升。此外,膜接触O3-UV联用对布洛芬的降解效果随pH升高而提升。这表明,OR-MOPs在产生HO·更多的碱性条件下更易被降解。
3)膜接触O3-UV联用的主要降解途径包括O3氧化阶段的O3分子氧化、HO·氧化以及O3/UV高级氧化阶段的HO·氧化和直接UV光解,HO·氧化的贡献比可达90%以上。
膜接触臭氧-紫外联用强化降解耐臭氧微量有机污染物
Combination of membrane contact ozone and ultraviolet processes for enhanced degradation of ozone-resistant micro organic pollutants
-
摘要: 臭氧(O3)已被广泛应用于水深度处理中,但其无法有效降解耐O3微量有机污染物(OR-MOPs)。针对OR-MOPs的强化降解,搭建了由O3膜接触器和细管流光反应系统组成的膜接触O3-紫外(UV)实验装置,并对O3膜接触器的处理效果进行评估。结果表明,随着气相O3质量浓度增加、溶液流量和pH的降低,液相O3质量浓度升高;磷酸盐对O3溶解没有影响;测得细管流光反应系统的UV剂量率为4.56 × 10−4 Einstein·m−2·s−1。当液相O3质量浓度为1.0和2.0 mg·L−1时,膜接触O3-UV联用对代表性OR-MOP—布洛芬的去除率分别为63.2%和82.9%,比单独O3氧化增加了38.1%和44.5%,且O3被完全利用。布洛芬的降解效果随pH升高而提升,当液相O3质量浓度为1.0 mg·L−1、pH = 9.0时,布洛芬的整体去除率最高(77.2%);在布洛芬各主要降解途径中,羟基自由基(HO·)氧化的贡献比达90%以上。膜接触O3-UV联用可强化OR-MOPs的降解并提高O3利用率,可为O3在水深度处理中的应用提供新方法。
-
关键词:
- 膜接触臭氧 /
- 紫外 /
- 臭氧氧化 /
- 高级氧化 /
- 耐臭氧微量有机污染物
Abstract: Ozone (O3) has been widely used in advanced water treatment but can hardly degrade O3 resistant micro organic pollutants (OR-MOPs). For enhanced degradation of OR-MOPs, a membrane contact O3-UV experimental system was developed by combining an O3 membrane contactor (OMC) and a mini-fluidic photoreaction system (MFPS). The performance of OMC was evaluated, showing that the dissolved O3 increases with the rise of gaseous O3 mass concentration, or decreases with the solution flow rate and pH; phosphate has no effect on the O3 dissolution; photon fluence rate of MFPS was determined as 4.56×10−4 Einstein·m−2·s−1. By using the combination of membrane contact O3 and UV processes, the removal efficiencies of ibuprofen, a representative OR-MOP, were 63.2% and 82.9% at aqueous O3 mass concentrations of 1.0 and 2.0 mg·L−1, which were 38.1% and 44.5% higher than those by sole ozonation, respectively, with O3 fully utilized. The degradation of ibuprofen was enhanced as the pH increased, and the highest removal efficiency (77.2%) of ibuprofen was achieved at aqueous O3 mass concentration of 1.0 mg·L−1 and pH = 9.0. Hydroxyl radical (HO•) oxidation contributed more than 90% of the main degradation pathways of ibuprofen. This study demonstrated that the combination of membrane contact O3 and UV processes could enhance the degradation of OR-MOPs and improve the O3 utilization efficiency, which provided a new method for application of O3 in water advanced treatment.-
Key words:
- membrane contact O3 /
- UV /
- ozonation /
- advanced oxidation /
- O3 resistant micro organic pollutants
-

-
-
[1] VOLKER J, STAPF M, MIEHE U, et al. Systematic review of toxicity removal by advanced wastewater treatment technologies via ozonation and activated carbon[J]. Environmental Science & Technology, 2019, 53(13): 7215-7233. [2] MESTANKOVA H, PARKER A M, BRAMAZ N, et al. Transformation of contaminant candidate list (CCL3) compounds during ozonation and advanced oxidation processes in drinking water: Assessment of biological effects[J]. Water Research, 2016, 93(15): 110-120. [3] LIU Z, DEMEESTERE K, HULLE S V. Enhanced ozonation of trace organic contaminants in municipal wastewater plant effluent by adding a preceding filtration step: Comparison and prediction of removal efficiency[J]. ACS Sustainable Chemistry and Engineering, 2019, 7(17): 14661-14668. doi: 10.1021/acssuschemeng.9b02571 [4] TAKASHINA T A, LEIFELD V, ZELINSKI D W, et al. Application of response surface methodology for coffee effluent treatment by ozone and combined ozone/UV[J]. Ozone Science & Engineering, 2018, 40(4): 293-304. [5] WENTEN I G, JULIAN H, PANJAITAN N T. Ozonation through ceramic membrane contactor for iodide oxidation during iodine recovery from brine water[J]. Desalination, 2012, 306(18): 29-34. [6] SCHMITT A, MENDRET J, ROUSTAN M, et al. Ozonation using hollow fiber contactor technology and its perspectives for micropollutants removal in water: A review[J]. Science of the Total Environment, 2020, 729: 138664. doi: 10.1016/j.scitotenv.2020.138664 [7] 张勇, 王军, 侯得印, 等. 膜接触反应器臭氧传质及其对模拟印染废水的降解[J]. 环境工程学报, 2017, 11(8): 4453-4458. doi: 10.12030/j.cjee.201607215 [8] VON SONNTAG C, VON GUNTEN U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications[M]. London: IWA Publishing, 2012. [9] PEYTON G R, GLAZE W H. Destruction of pollutants in water with ozone on combination with ultraviolet-radiation. 3. Photolysis of aqueous ozone[J]. Environmental Science & Technology, 1988, 22(7): 761-767. [10] BELTRAN F J, ENCINAR J M, GONZALEZ J F. Industrial wastewater advanced oxidation. Part 2. Ozone combined with hydrogen peroxide or UV radiation[J]. Water Research, 1997, 31(10): 2415-2428. doi: 10.1016/S0043-1354(97)00078-X [11] LI M K, QIANG Z M, HOU P, et al. VUV/UV/chlorine as an enhanced advanced oxidation process for organic pollutant removal from water: Assessment with a novel mini-fluidic VUV/UV photoreaction system (MVPS)[J]. Environmental Science & Technology, 2016, 50(11): 5849-5856. [12] LOOS R, LOCORO G, CONTINI S. Occurrence of polar organic contaminants in the dissolved water phase of the Danube River and its major tributaries using SPE-LC-MS2 analysis[J]. Water Research, 2010, 44(7): 2325-2335. doi: 10.1016/j.watres.2009.12.035 [13] ANDINI S, BOLOGNESE A, FORMISANO D, et al. Mechanochemistry of ibuprofen pharmaceutical[J]. Chemosphere, 2012, 88(5): 548-553. doi: 10.1016/j.chemosphere.2012.03.025 [14] 田岳林. 无机膜与有机膜分离技术应用特性比较研究[J]. 过滤与分离, 2011, 21(1): 45-48. doi: 10.3969/j.issn.1005-8265.2011.01.015 [15] ATCHARYAWUT S, PHATTARANAWIK J, LEIKNES T, et al. Application of ozonation membrane contacting system for dye wastewater treatment[J]. Separation & Purification Technology, 2009, 66(1): 153-158. [16] 税奕瑜. 聚四氟乙烯中空纤维膜臭氧曝气的传质过程研究[D]. 成都: 西南石油大学, 2017. [17] LI M K, LI W T, WEN D, et al. Micropollutant degradation by the UV/H2O2 process: Kinetic comparison among various radiation sources[J]. Environmental Science & Technology, 2019, 53(9): 5241-5248. [18] BADER H. Determination of ozone in water by the indigo method: A submitted standard method[J]. Ozone Science & Engineering, 1982, 4(4): 169-176. [19] ZHANG Y, LI K L, WANG J, et al. Ozone mass transfer behaviors on physical and chemical absorption for hollow fiber membrane contactors[J]. Water Science & Technology, 2017, 76(6): 2017254. [20] STYLIANOU S K, KOSTOGLOU M, ZOUBOULIS A I. Ozone mass transfer studies in a hydrophobized ceramic membrane contactor: Experiments and analysis[J]. Industrial & Engineering Chemistry Research, 2016, 55(28): 7587-7597. [21] EGOROVA G V, VOBLIKOVA V A, SABITOVA L V, et al. Ozone solubility in water[J]. Moscow University Chemistry Bulletin, 2015, 70(5): 207-210. doi: 10.3103/S0027131415050053 [22] RAO Y F, CHU W. A new approach to quantify the degradation kinetics of linuron with UV, ozonation and UV/O3 processes[J]. Chemosphere, 2009, 74(11): 1444-1449. doi: 10.1016/j.chemosphere.2008.12.012 [23] ELOVITZ M S, VON GUNTEN U, KAISER H P. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties[J]. Ozone Science & Engineering, 2000, 22(2): 123-150. [24] HUBER M M, CANONICA S, PARK G Y, et al. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes[J]. Environmental Science & Technology, 2003, 37(5): 1016-1024. [25] ELOVITZ M S, VON GUNTEN U. Hydroxyl radical/ozone ratios during ozonation processes. I. The Rct concept[J]. Ozone:Science & Engineering, 1999, 21(3): 239-260. [26] ELOVITZ M S, VON GUNTEN U, KAISER H P. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties[J]. Ozone:Science & Engineering, 2000, 22(2): 123-150. -



 下载:
下载: