-
挥发性有机化合物(volatile organic compounds,VOCs)主要来源于工业生产、溶剂使用、机动车尾气和生物质燃烧等领域[1-2],其无序排放会造成雾霾、光化学烟雾、臭氧损耗、全球变暖,同时也会危害人群健康[3]。生态环境部在制《重点行业挥发性有机物综合治理方案》中指出:石油化工、工业喷涂和印刷包装等是VOCs治理的重点行业,要求综合治理印刷包装在内的重点行业排放的VOCs,推行强化源头控制和提升末端治理水平[2,4]。现行的工业VOCs末端治理技术分为回收技术和销毁技术2大类。回收技术包括膜分离、吸收、吸附和冷凝技术等;销毁技术包括蓄热式热力氧化、催化氧化、低温等离子体催化和生物技术等[5]。
催化氧化法因对中小气量和中高浓度VOCs的处理具有无选择性和降解效率高等特点[6-9],已成为国家推荐和企业青睐的技术[10]。传统的VOCs催化氧化技术利用电加热器预热将VOCs加热至起燃点并送入催化剂床层,在催化剂表面发生氧化反应,整个过程能耗较高[11]。而微波加热只对吸波性物质起作用,且催化剂介电常数越大,微波加热作用越强,因此会在催化剂床层形成“局部热点”,从而有利于VOCs降解;另外,微波的“偶极极化”作用可促进极性VOCs分子振荡,降低其反应自由能而有助于氧化反应的进行。因此,微波加热具有选择性加热、即时加热、加热均匀和能耗低等优点[12-13],可代替传统的电加热应用于催化氧化技术中。本课题组贺利娜和杨力等[14-15]利用小型微波催化燃烧装置和Cu-Mn-Ce催化剂处理单组分和多组分VOCs废气均取得了良好的去除效果,单组分甲苯在床层温度270 ℃下的去除率为90%,双组份的甲苯和氯苯在层温度273 ℃下的去除率分别为95%和90%。ZHU等[16]发现,在实验室用浸渍法制备的Pt/CuMnCe整体式催化剂对甲苯、乙酸乙酯和正己烷具有良好的催化活性,其T90(T90指VOCs去除率为90%时的反应温度)分别为216、200和260 ℃。
为解决工程应用中可能出现的尾气带走过多热量、燃烧装置保温效果较差、能量损耗严重,以及微波源端的高温废气严重影响磁控管正常工作等突出问题,以整体式蜂窝状堇青石和纳米陶瓷为载体,用等量浸渍法制备了Pt/CuMnCeOx/堇青石整体式蜂窝状(Pt/CMC/CH)催化剂和Pt/CuMnCeOx/纳米陶瓷整体式蜂窝状(Pt/CMC/NH)催化剂。进一步地,将制备的催化剂应用于自主研发的大型微波催化燃烧装置,在印刷包装企业现场开展了含甲苯、二甲苯、乙酸乙酯、甲缩醛、二氯丙烷、醇、树脂和少量丁酮等[17-19]VOCs的废气治理研究,考察了进气质量浓度、进气量和床层温度等因素的变化对VOCs去除率的影响,通过表征催化剂观察其表面形貌特征及活性组分成分,以期为微波催化燃烧技术的应用推广提供参考。
-
采用等量浸渍法制备催化剂:将硝酸铜(Cu(NO3)2·3H2O)、硝酸锰(Mn(NO3)2)、硝酸铈(Ce(NO3)3·6H2O)和氯铂酸(H2PtCl6·6H2O)等按照一定的质量比加入到容器中,混入定量水后搅拌均匀;然后将纳米陶瓷(145 mm×145 mm×50 mm,7目,NH)和堇青石(150 mm×150 mm×150 mm,7目,CH)载体浸泡在其中,上下翻转以使浸渍液被完全吸收;置于120 ℃烘箱中过夜烘干,500 ℃马弗炉(RT4-200-12型,武汉市汉口电炉有限责任公司)中焙烧5 h后即制得成品催化剂。
催化剂的比表面积、平均孔径及孔体积等采用比表面积与孔径测定仪(BET,V-sorb 2800P型,北京金埃谱公司)测定;表面形貌、活性组分颗粒大小及形状通过扫描电镜(SEM,JSM-6510LV型,日本电子)进行观察;活性组分的物相组成、晶粒粒径和分散度采用X射线衍射仪(XRD,D/MAX-2400型,日本理学)进行测试,所用射线为铜Kα射线,加速电压和应用电流分别为40 kV和40 mA。
-
自主研制的微波催化燃烧VOCs装置主要由燃烧装置和自动化控制系统组成。燃烧腔体由2层中间填充耐高温硅酸铝保温棉的不锈钢构成,腔体顶部和底部采用四面体收口,中间为正方形柱体。在顶部的每个斜面上安装4个功率均为850 W的微波发射器,其磁控管处采用水冷套管冷却,以保证微波发射的正常运行。所激发产生的微波通过波导管和云母片(一种透波材料,将燃烧腔体与微波发生器隔开,防止高温废气腐蚀微波发生装置)传输到腔体内部,作用于整体式催化剂固定床;波导管与腔体连接处也采用水冷套管予以冷却,以阻止热量向微波发生装置传递。结合催化剂体积和腔体尺寸,Pt/CMC/CH催化剂摆2层,共98块;Pt/CMC/NH催化剂摆6层,共294块;催化剂床层体积为1.05 m×1.05 m×0.5 m。腔体保温层壁厚0.17 m,且处于完全封闭状态,以保证热量尽量不损失;同时,腔体外部安装有不锈钢外壳,可有效防止漏波现象的发生。腔体周围的空隙用催化剂填满,VOCs废气量和微波功率可通过自动控制系统调控,催化剂床层温度、VOCs进气温度和出气温度均通过自动控制屏显示。
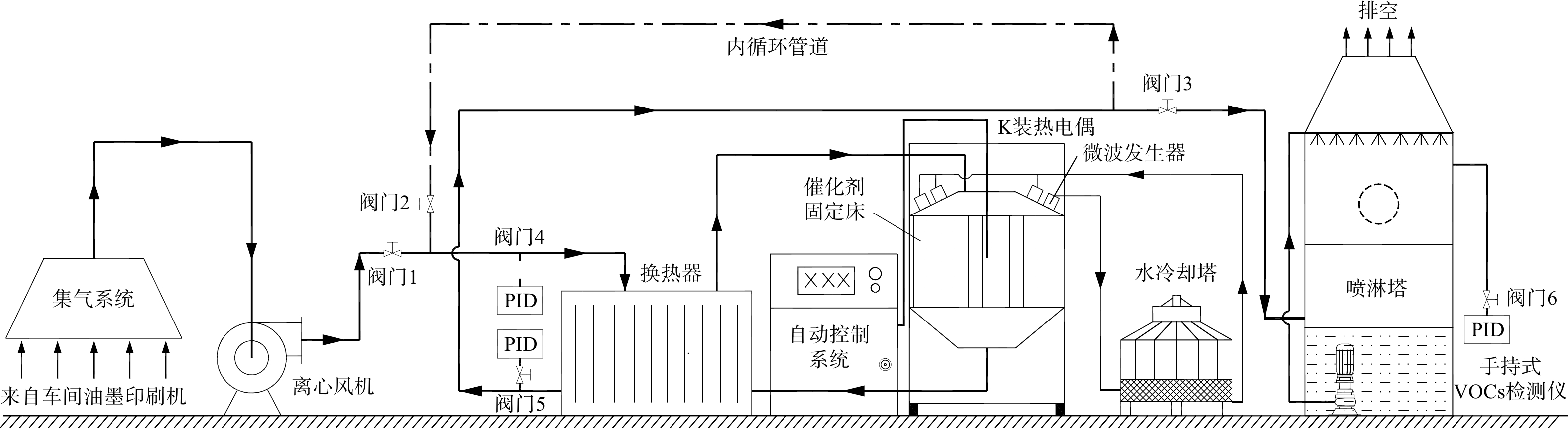
现场测试工艺如图1所示。实验开始前,先开启冷却塔运行水冷系统,以冷却磁控管和波导管;接着开启离心风机(9-19型,上海耐腾电器科技有限公司),此时阀门1、阀门3打开,阀门2关闭,车间的低温、中高浓度VOCs废气经过换热器(YLW-COBH200,亚利威环保科技(苏州)有限公司)预热后从燃烧装置顶端进入固定床反应器;然后开启微波源,对催化剂床层进行升温,VOCs在催化剂表面进行催化燃烧,燃烧后的高温尾气从装置底部流经换热器进行换热,然后进入喷淋塔,经水吸收后排空。测试结束后,先关闭微波源,接着关闭离心风机,此时阀门1、阀门3关闭,阀门2打开,热气体在腔体和管道内循环,达到催化剂床层保温的目的。冷却塔一般不关闭,始终使微波发生装置保持低温。测试过程中进气端、出气端及催化剂床层温度均由K装热电偶探针测试,通过自动控制屏显示,微波源通过自动控制系统可即时开启或关停。
-
现场微波燃烧装置进出口的VOCs质量浓度均由手持式VOCs检测仪测定(PGM-7320型,上海翼航智能科技有限公司),检测器为光离子化检测器(PID)。每隔15 min对进出口(阀门4、阀门5)处各测1次样,并记录各管段和催化剂床层温度。VOCs的流速用风速仪(6006型,沈阳加野科技有限公司)进行检测,再根据管径计算出处理气量,计算方法如式(1)所示。VOCs的净化效果和催化剂活性均以VOCs去除效率为指标,计算方法如式(2)所示。
式中:Q为处理气量,m3·h−1;v为流速,m·s−1;d为管径,m;η 为VOCs去除率,%;C0、C分别为进出口处VOCs废气总质量浓度,mg·m−3。
-
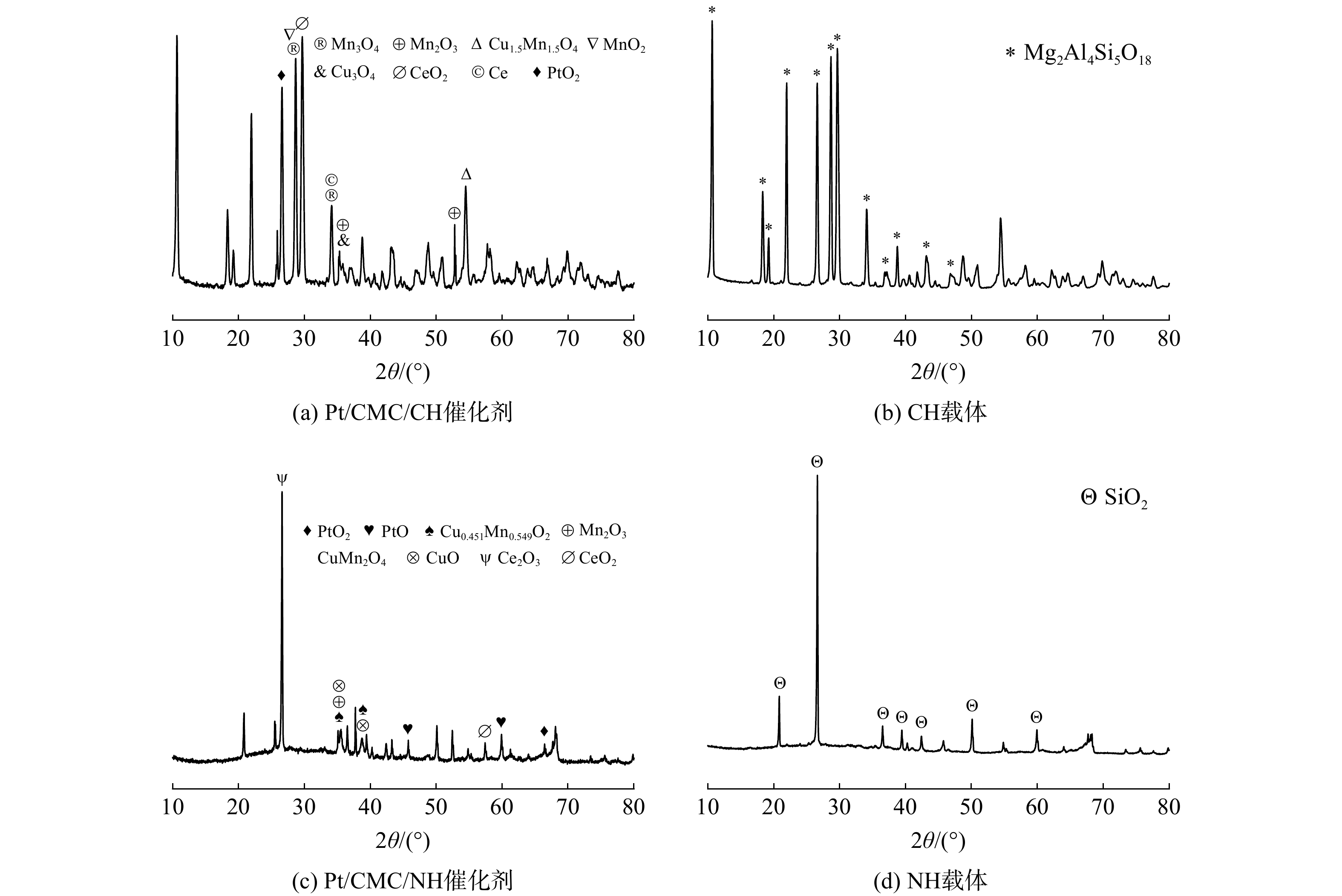
1)SEM分析结果。本研究对Pt/CMC/CH和Pt/CMC/NH 2种催化剂的表面形貌和活性组分颗粒的大小进行了观察,结果如图2所示。2种蜂窝载体表面凹凸不平、层状结构明显且存在缝隙(6 μm)和孔隙(4 μm)。这些凹凸不平和孔缝结构有利于活性组分的附着和阻隔有机废气的通过,进而延长污染物在载体表面的停留时间,并增加其与活性组分的接触机会。负载活性组分并经高温焙烧后的催化剂表面缝隙(10 μm)和孔径(7 μm)更大更明显。这是因为催化剂焙烧过程中载体内挥发性和氧化性杂质被清除掉了,从而扩大了孔径,增加了催化剂的比表面积和孔隙[20]。上述结果与表1中比表面积结果一致。不同大小和几何形状的金属氧化物颗粒不均匀地分布在载体表面,结合后续XRD谱图的测试结果可知,这些颗粒物是铂、铜、锰、铈的金属氧化物及其多元混合氧化物。根据文献[21-24]的报道,PtO2、CuO、Mn3O4、CeO2等氧化物可提高催化剂对芳香族和氯化物的催化活性及其低温活性。
2)BET分析结果。表1为2种载体及其催化剂的比表面积、孔体积以及平均孔径等数据。负载活性组分的催化剂比表面积相对于其载体明显增大,这与SEM照片(图2)结果一致,且分别增加了约27倍和2倍,极大地增强了催化剂对VOCs的吸附能力。然而,Pt/CMC/NH催化剂的微孔面积和微孔体积相较于其载体都有所减小,这可能是Pt/CMC/NH催化剂在高温煅烧的过程中发生了烧结现象,可挥发分和氧化性物质在逃逸的过程中撑大了微孔[20],导致微孔数目减少,而使微孔面积和微孔体积减小。
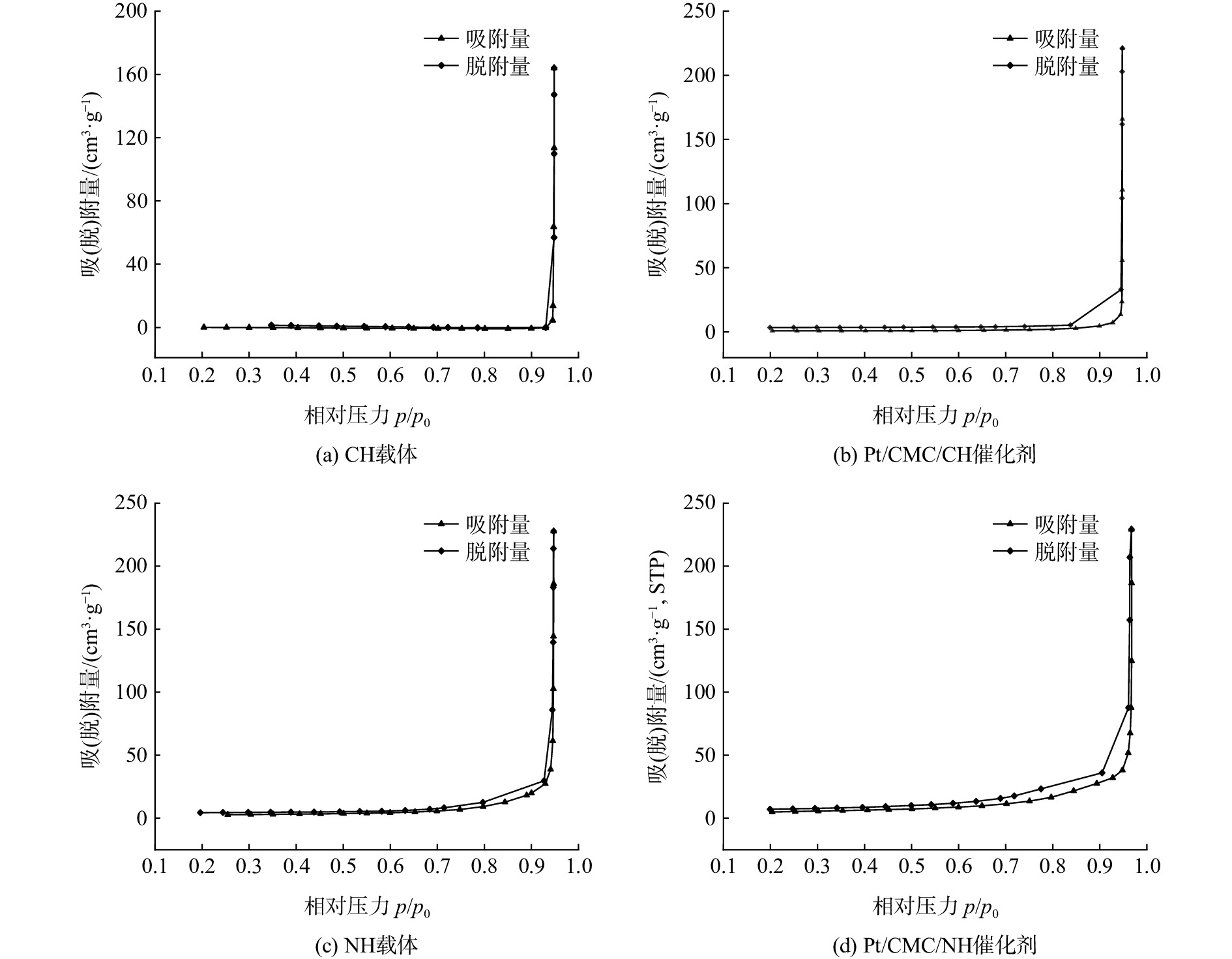
图3为2种载体及其催化剂的吸附-脱附等温线。相关反应均属于第Ш类反Langmuir 型曲线,证明其均具有多分子层吸附现象[25]。在p/p0=0.8~1.0时,有一个C类回滞环,说明催化剂是由粒状微粒烧结成的材料,且存在介孔结构(2~50 nm)[26]。从表1中的平均孔径数值来看,2种催化剂均以介孔为主,从而有利于污染物在介孔内部的扩散,可减小内扩散阻力的影响[27]。
3) XRD分析结果。对2种载体和催化剂表面活性组分的晶体结构及组成进行了表征,结果如图4所示。这2种催化剂的XRD谱图均存在其载体的特征峰,说明负载活性组分后并未破坏载体的主体晶形。2种催化剂的谱图中出现了MnO2(2θ=28.851°,JCPDS39-0375)、Mn3O4(2θ=28.870°、34.061°,JCPDS13-0162)、Mn2O3(2θ=35.597°、53.210°、35.597°,JCPDS33-0900)、Cu0.451Mn0.549O2(2θ=35.393°、38.696°,JCPDS41-0184)、CeO2(2θ=29.981°、57.322°,JCPDS44-1001)、Ce2O3(2θ=26.426°,JCPDS44-1086)、PtO2(2θ=27.722°、66.493°,JCPDS37-1087)和PtO(2θ=45.079°、59.597°,JCPDS42-0866)等活性物质的衍射峰,但并未出现单质Pt的衍射峰,说明Pt金属高度分散在载体表面[28]。有研究者指出,Mn2O3和Mn3O4氧化物可提高催化反应的低温活性[23,29],2种催化剂中锰的氧化态有所不同,推测与载体中不同金属氧化物的存在有关;铜锰复合氧化物中Cu和Mn间存在着电子传递,增强了催化剂降解VOCs的活性,并且Cu、Mn氧化物和PtO的混合结构分散在铈基固溶体的表面能激活有机分子和运输活性氧。另外,铈氧化物具有良好的储氧能力,当结构中存在Ce3+和Ce4+时,氧空位丰富,从而有利于氧吸附和晶格氧传递到催化剂表面[30]。因此,这些活性物质对提高催化剂活性起着重要作用,同时也说明该催化剂可有效降解VOCs。
-
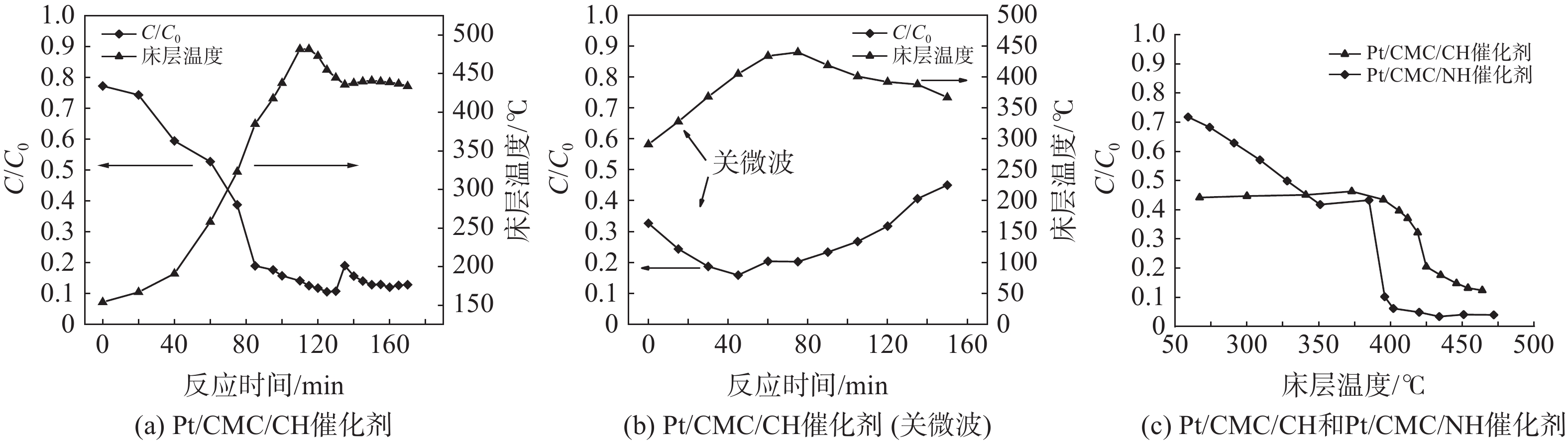
在催化剂床层体积330 L、微波功率13.6 kW、进气质量浓度1 520 mg·m−3和处理气量440 m3·h−1条件下,现场测试了催化剂床层温度对VOCs去除率的影响,结果如图5所示。床层温度与去除率之间有着密切联系,在0~115 min时,随着床层温度的升高,VOCs去除率也随之增加;当床层温度在420~450 ℃时,VOCs去除率保持在90%左右。当床层温度达到350 ℃时,微波源依据设置而关闭,随后床层温度与VOCs去除率的变化如图5(b)所示。关闭微波后,床层温度和去除率仍保持上升趋势。这是因为VOCs燃烧释放的热量是腔体热量来源的重要部分,即使关闭了微波,也可在短时间内(1 h)依靠VOCs燃烧释放的热量来维持床层温度;随着床层温度的升高,废气带走的热量与装置散热量增加,当损失热量大于燃烧放热时,床层温度开始下降,此时VOCs的去除率减小。另外,微波“偶极极化”与“热点效应”的消失也是VOCs去除率减小的重要原因。VOCs的去除率与床层温度之间并非线性关系。当VOCs去除率约为55%时,其去除率快速增加,且床层温度对2种催化剂的影响效果不同:低温时Pt/CMC/CH催化剂的活性较高;高温时Pt/CMC/NH催化剂活性较高。其原因是:2种催化剂中锰的氧化态有所不同,Pt/CMC/CH催化剂中Mn3O4、Mn2O3和MnO2(图4(a)),从而降低了T50(T50指VOCs去除率为50%时的反应温度),提高了催化剂的低温活性[23];然而Pt/CMC/NH催化剂的比表面积远大于Pt/CMC/CH催化剂且微孔数目较多,VOCs在空隙内的停留时间较长,而有利于其降解,因此,当Pt/CMC/NH催化剂不受低温限制时其去除率较高[14]。催化剂床层温度既会影响VOCs去除率,也会受到VOCs燃烧释放的热量影响;床层温度在未达到起燃温度(T50)之前,主要靠微波加热维持;当床层温度达到起燃温度后,VOCs快速燃烧释放出大量热量,从而有助于床层温度和VOCs去除率的升高;更高的去除率又会释放出更多热量,如此反复,形成良性循环。
-
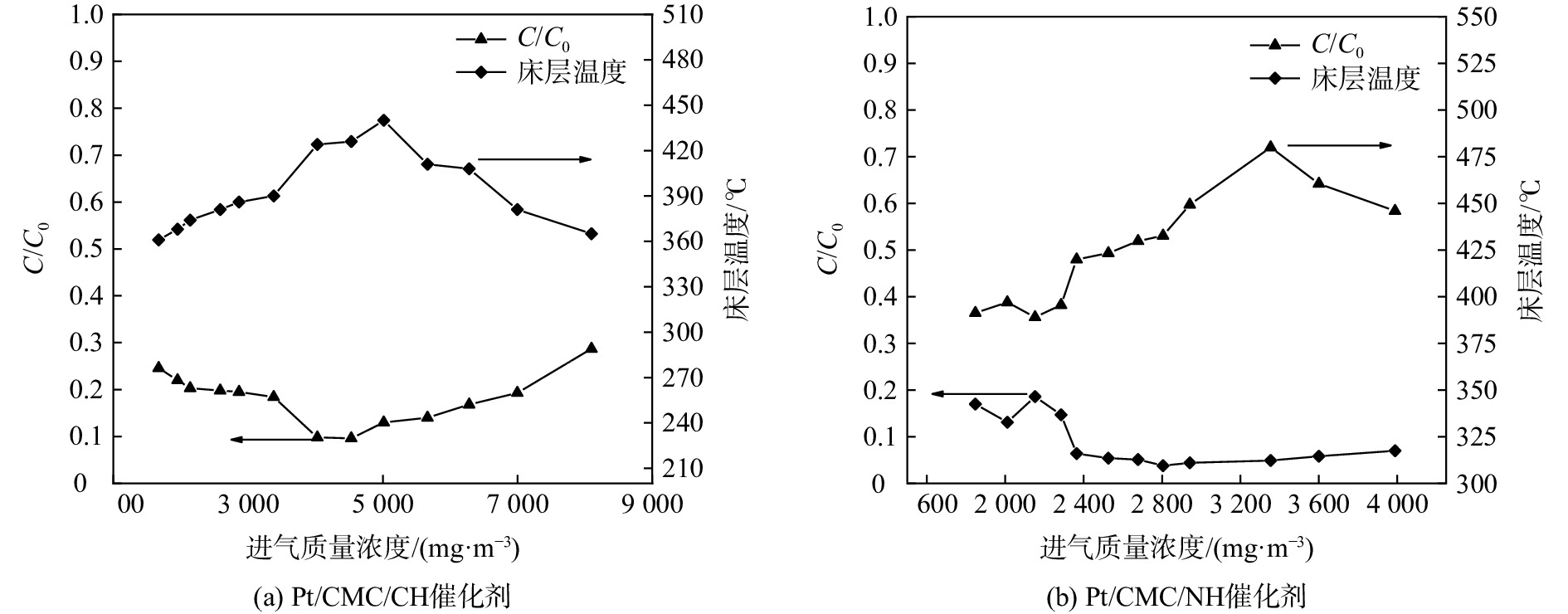
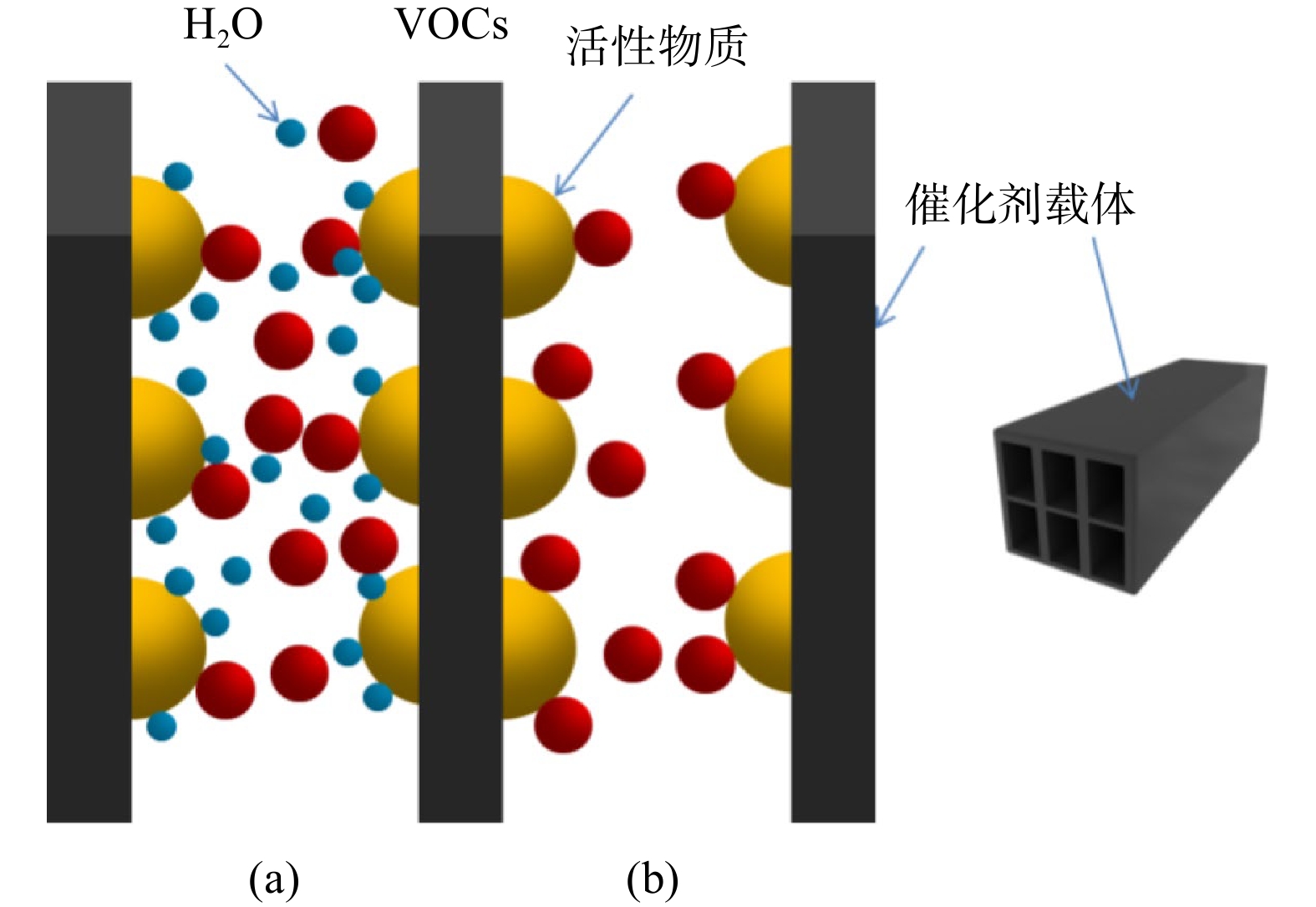
通过现场实验考察了Pt/CMC/CH和Pt/CMC/NH这两种催化剂对不同进气质量浓度VOCs的降解情况,结果如图6所示。VOCs的去除率随着进气质量浓度的增大,先呈现一定程度的上升,随后又逐渐降低。当进气质量浓度分别约为4 500 mg·m−3和2 800 mg·m−3时,VOCs去除率达到最高,分别为90%和96%,此时的床层温度分别为426 ℃和432 ℃。其原因是,VOCs质量浓度越高,即腔体内燃料越多,燃烧释放的热量则越多,此时催化剂床层温度升高的幅度越大,从而有利于VOCs去除率的升高,即可通过调节进气质量浓度影响床层温度来间接影响去除率。然而,在给定床层体积下,催化剂的活性位点是有限的[31],当VOCs的进气质量浓度达到4 500 mg·m−3时,其去除率达到最大值(90%)。这说明此温度(T90)下,催化剂所有活性位点被最大程度地利用。此后,继续增大VOCs的进气质量浓度,由于活性位点不足,加之过剩的VOCs分子会引起孔隙堵塞,导致催化剂活性降低,进而引起VOCs的去除率下降。去除率下降必然会导致VOCs燃烧释放热量减少,一段时间后,床层温度开始下降。这也说明,VOCs去除率与床层温度之间的关系是双向而复杂的。另外,随着VOCs质量浓度的增加,作为降解产物之一的水蒸气量也在增多,水蒸气与VOCs分子竞争吸附在活性位点上[32],并且水蒸气对活性位点的覆盖率更高,于是VOCs的可用活性位点数量减少,导致VOCs去除率降低,两者的竞争机理如图7所示。当VOCs进气质量浓度大于最佳浓度后,VOCs去除率下降的幅度不同(图6)。这是由于不同催化剂对水蒸气的吸附能力不同[33],相较于Pt/CMC/CH催化剂,水蒸气对Pt/CMC/NH催化剂的影响较小,推测这与极性水分子在非极性NH载体(SiO2)表面吸附力较弱有关。
-
在催化剂床层体积330 L,微波功率13.6 kW, VOCs质量浓度为2 000 mg·m−3的条件下,通过现场测试考察了不同进气量对VOCs去除率的影响,结果如图8所示。VOCs去除率与进气量有着一定联系,当进气量为565 m3·h−1时,去除率可稳定在80%左右;当进气量分别达到600 m3·h−1和712 m3·h−1时,去除率分别为60%~80%和40%~90%。由此可见,进气量越大,VOCs去除率波动幅度越大,即去除率越不稳定。在测试过程中,保持VOCs的进气质量浓度不变,若突然增大进气量,则VOCs去除率会降低,并且进气量增加得越多,去除率下降的越快;同样,若减小进气量,VOCs去除率则会升高。随着进气量的增大,VOCs去除率在降低,这证明气量不仅影响去除率的稳定性,还影响去除率的高低。分析其原因后可认为:进气量越大,VOCs的催化燃烧越容易受到高温干扰和传质阻力的影响[34],其去除率及对CO2的选择性会呈现出不规律的增长或降低;其次,进气量越大,出气带走的热量也越多,床层温度低且不稳定,从而导致去除率低且不稳定;最后,进气量越大,VOCs在催化剂表面停留的时间越短,气体还没有充分燃烧就已流出腔体了。经对比发现,较低气量下会出现二次燃烧,可增大VOCs去除率和对CO2的选择性;而在高气量条件下,VOCs去除率降低并伴随更多VOCs副产物的生成。
-
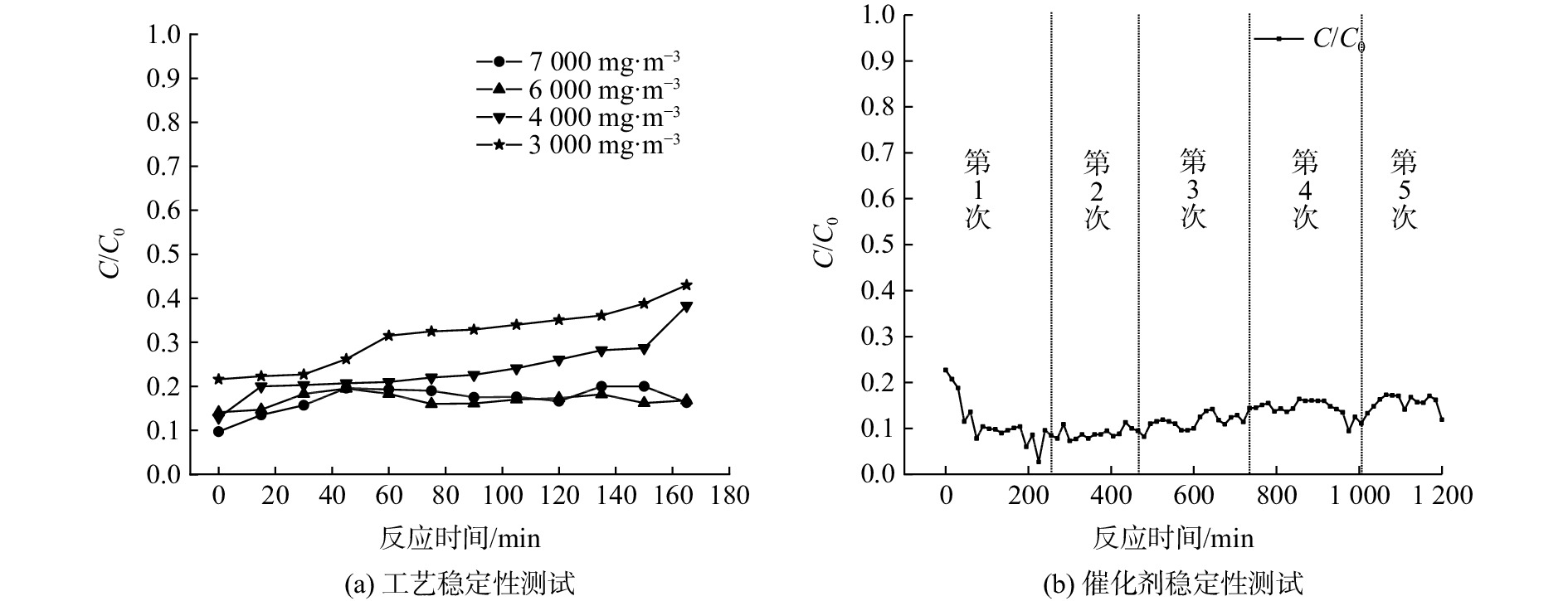
在现场实验基础上进一步提高VOCs处理气量,并增大催化燃烧装置的尺寸,在Pt/CMC/CH催化剂床层体积1 094 L、微波功率13.6 kW、进气量800 m3·h−1的条件下,测试该工艺是否能稳定处理更高浓度和更大气量的VOCs,结果如图9(a)所示。当进气质量浓度为6 000 mg·m−3和7 000 mg·m−3时,VOCs去除率较高且稳定在85%左右,而当进气质量浓度为3 000 mg·m−3和4 000 mg·m−3时,VOCs去除率随时间逐渐降低。这是由于高浓度VOCs在燃烧时释放热量多,可补偿出口废气带走的热量,从而维持床层温度稳定;而低浓度VOCs燃烧时释放的热量不足以维持床层温度稳定而导致VOCs去除率降低,若要提高去除率则需要开启微波来补充额外热量。当VOCs进气质量浓度越高,其去除率则相对越高,且高质量浓度条件下VOCs的去除率保持相对稳定。
在催化剂体积1 094 L、微波功率13.6 kW、进气量800 m3·h−1、进气质量浓度5 000 mg·m−3的条件下,对Pt/CMC/CH催化剂进行了5次重复性实验,结果如图9(b)所示。催化剂经5次连续性实验后,VOCs去除率基本无变化,仍保持在90%左右,说明催化剂活性没有减弱,可稳定高效地处理实际印刷包装VOCs。虽然催化燃烧的高温环境会影响催化剂结构,容易使活性组分发生团聚现象,但由于微波加热均匀性好,不会因为局部过热而导致催化剂烧结,因此催化剂表面形貌和结构无明显变化[14,20,35],从而保证了催化剂活性的稳定,延长了催化剂的使用寿命。
-
1)现场实验证实,微波催化燃烧VOCs技术对于印刷包装行业VOCs治理是可行的。当固定床温度低于450 ℃时,VOCs去除率随着床层温度的升高而增大;在较高的去除率下,关闭微波源,VOCs燃烧释放的热量可在一定时间内维持床层温度和去除率的稳定,床层温度与去除率之间是相互影响的关系。
2)在催化剂床层体积330 L、微波功率13.6 kW、进气量440 m3·h−1的条件下,Pt/CMC/CH和Pt/CMC/NH 2种催化剂的最佳进气质量浓度分别为4 500 mg·m−3和2 800 mg·m−3;在催化剂活性位点充足的情况下,VOCs去除率受进气质量浓度的影响较大,当VOCs进气质量浓度超过某一范围时,由于床层活性位点不足,VOCs去除率下降。
3) VOCs进气量是影响VOCs去除率的重要因素之一。进气量越大,去除率越易受到高温的干扰和传质阻力的影响,从而越不稳定;在固定床层体积下,进气量增大会导致VOCs在催化剂表面的停留时间变短,其氧化不彻底和燃烧放热量减少,从而导致VOCs去除率降低。
4)在企业现场工况条件下,当满足进气质量浓度和催化剂活性位点要求时,床层温度达到起燃温度后可关闭微波源,进而依靠VOCs燃烧放热以维持床层温度和稳定VOCs去除率,从而达到降低成本和节约能源的目的。
微波催化燃烧技术处理印刷包装行业VOCs
Microwave catalytic combustion of VOCs exhaust from printing and packaging industry
-
摘要: 微波催化燃烧技术将微波辐照与吸波型催化剂相结合,可用于对挥发性有机化合物(VOCs)进行催化燃烧处置。研制了Pt/CuMnCeOx/堇青石和Pt/CuMnCeOx/纳米陶瓷整体式蜂窝状催化剂,并开发了微波催化燃烧VOCs的装置,将其应用于印刷包装行业的VOCs治理。通过操作条件的优化,考察了微波催化燃烧技术对VOCs的实际处理效果。同时,对催化剂表面形貌、比表面积和晶体结构等进行了测试分析。结果表明:Mn3O4/Mn2O3、CeO2/Ce2O3、CuMn2O4和PtO等尖晶石的存在降低了反应温度、提高了储氧释氧能力和催化剂活性;催化剂的介孔结构和较大的比表面积有利于VOCs在孔隙内部的扩散,并可延长VOCs在催化剂上的停留时间。在催化剂床层体积330 L、微波功率13.6 kW、进气质量浓度1 520 mg·m-3和进气量440 m3·h−1的条件下,床层温度可达到420 ℃,此时催化剂床层温度及VOCs去除率保持稳定。当进气质量浓度分别为约4 500 mg·m−3和2 800 mg·m−3时,VOCs的去除率分别为90%和96%。考察燃烧热量发现,大气量的VOCs在催化剂表面的停留时间短且带走热量多,从而导致VOCs去除率下降;高浓度VOCs在燃烧时会因释放出更多热量,从而提高床层温度和VOCs去除率。在确保催化剂表面活性位点充足的条件下,微波催化燃烧工艺适合处理中高浓度的印刷包装行业VOCs。同时,利用VOCs燃烧释放的热量来保持床层高温,还可达到节能降耗的目的。本研究可为印刷包装行业的VOCs治理提供参考。Abstract: Microwave catalytic combustion is a new catalytic combustion technology that combining microwave irradiation with microwave-absorbing catalyst closely. Based on the development of microwave catalytic combustion device and the exploitation of monolithic honeycomb catalysts of both Pt/CuMnCeOx/cordierite and Pt/CuMnCeOx/nano ceramic, this new technology was applied to treat volatile organic compounds (VOCs) exhausting from a printing and packaging factory on the spot. The real removal rate of VOCs by microwave catalytic combustion was investigated through an optimization of different operating conditions. Subsequently, surface morphology, specific surface area and crystal structure of the catalysts were characterized in this study. It indicated that the existence of Mn3O4/Mn2O3, CeO2/Ce2O3, CuMn2O4 and PtO spinels reduced reaction temperature and improved oxygen reserving-releasing ability and catalytic activity of the catalysts greatly. The mesoporous structure and abundant specific surface area of the catalysts are beneficial to the diffusion of pollutants in the inner pores and can prolong the retention time of targets on the surface of the catalysts simultaneously. The research work showed that bed temperature reached 420 ℃ and VOCs removal rate kept at steady under the conditions(330 L of bed volume, 13.6 kW of microwave power, 1 520 mg·m-3 of inlet concentration and 440 m3·h−1 of airflow). The removal rates of VOCs exhaust were 90% and 96% when the inlet concentration was about 4 500 mg·m−3 and 2 800 mg·m−3, respectively. The retention time of VOCs on the surface of the catalyst was shortened and much more heat was taken away with the increase of airflow, which resulted in a decrease of VOCs removal rate. VOCs exhaust with high concentration released more heat while burning, which increased bed temperature apparently and improved VOCs removal rate obviously. The new technology was confirmed to be suitable for the treatment of VOCs with medium or high concentration on condition that sufficient active sites are provided onto the surface of the catalyst. Simultaneously, heat releasing from VOCs combustion can maintain high temperature of catalyst bed so that additional energy do not need to be provided, which save energy and reduce cost in the operation. This study carves out a new way for the treatment of VOCs from printing and packaging industry.
-

-
表 1 催化剂的比表面积和孔结构参数
Table 1. The specific surface area and pore parameters of the carriers and catalysts
样品 比表面积
/(m2·g−1)微孔面积
/(m2·g−1)孔体积
/(cm3·g−1)微孔体积
/(cm3·g−1)平均孔径
/nmCH载体 0.110 0.000 0.254 0.000 000 36.789 Pt/CMC/CH催化剂 2.945 1.115 0.342 0.000 473 36.853 NH载体 9.355 1.794 0.352 0.000 721 34.486 Pt/CMC/NH催化剂 17.585 1.441 0.355 0.000 482 36.472 -
[1] SCIRE S, LIOTTA L F. Supported gold catalysts for the total oxidation of volatile organic compounds[J]. Applied Catalysis B:Environmental, 2012, 125: 222-246. doi: 10.1016/j.apcatb.2012.05.047 [2] LIU Y X, DENG J G, XIE S H, et al. Catalytic removal of volatile organic compounds using ordered porous transition metal oxide and supported noble metal catalysts[J]. Chinese Journal of Catalysis, 2016, 37(8): 1193-1205. doi: 10.1016/S1872-2067(16)62457-9 [3] ZHANG S H, YOU J P, KENNES C, et al. Current advances of VOCs degradation by bioelectrochemical systems: A review[J]. Chemical Engineering Journal, 2018, 334: 2625-2637. doi: 10.1016/j.cej.2017.11.014 [4] “十三五”挥发性有机物污染防治工作方案[J]. 石油石化绿色低碳, 2017, 2(5): 68. [5] LI X Q, ZHANG L, YANG Z Q, et al. Adsorption materials for volatile organic compounds (VOCs) and the Key factors for VOCs adsorption process: A review[J]. Separation and Purification Technology, 2020, 235: 116213. doi: 10.1016/j.seppur.2019.116213 [6] CHEN G Y, WANG Z, LIN F W, et al. Comparative investigation on catalytic ozonation of VOCs in different types over supported MnOx catalysts[J]. Journal of Hazardous Materials, 2020, 391: 122218. doi: 10.1016/j.jhazmat.2020.122218 [7] HE C, CHENG J, ZHANG X, et al. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources[J]. Chemical Reviews, 2019, 119(7): 4471-568. doi: 10.1021/acs.chemrev.8b00408 [8] LIU X L, CHEN L, ZHU T U, et al. Catalytic oxidation of chlorobenzene over noble metals (Pd, Pt, Ru, Rh) and the distributions of polychlorinated by-products[J]. Journal of Hazardous Materials, 2019, 363: 90-98. doi: 10.1016/j.jhazmat.2018.09.074 [9] FANG R M, HUANG W J, HUANG H B, et al. Efficient MnOx/SiO2@AC catalyst for ozone-catalytic oxidation of gaseous benzene at ambient temperature[J]. Applied Surface Science, 2019, 470: 439-447. doi: 10.1016/j.apsusc.2018.11.146 [10] SHU Y J, XU Y, HUANG H B, et al. Catalytic oxidation of VOCs over Mn/TiO2/activated carbon under 185nm VUV irradiation [J]. 2018, 208: 550-558. [11] 卜龙利, 刘海楠, 王晓辉, 等. 不同加热方式下催化氧化甲苯的性能研究[J]. 环境化学, 2013, 32(8): 1524-1531. doi: 10.7524/j.issn.0254-6108.2013.08.017 [12] 周德良, 刘洁. 微波加热及其量子特性[J]. 黑龙江八一农垦大学学报, 2019, 31(1): 74-77. doi: 10.3969/j.issn.1002-2090.2019.01.013 [13] 普婧, 张之筠, 刘钱钱, 等. 钛渣在微波加热过程中的升温特性和吸收行为[J]. 钢铁钒钛, 2019, 40(1): 18-22. doi: 10.7513/j.issn.1004-7638.2019.01.004 [14] 贺利娜, 卜龙利, 都琳, 等. 微波催化燃烧气态甲苯特性及床层温度分布[J]. 中国环境科学, 2019, 39(8): 3242-3248. doi: 10.3969/j.issn.1000-6923.2019.08.014 [15] 杨力, 卜龙利, 孙剑宇, 等. 双组分甲苯、氯苯的微波辅助催化氧化及机理[J]. 环境工程学报, 2014, 8(11): 4871-4879. [16] ZHU A M, ZHOU Y, WANG Y, et al. Catalytic combustion of VOCs on Pt/CuMnCe and Pt/CeY honeycomb monolithic catalysts[J]. Journal of Rare Earths, 2018, 36(12): 1272-1277. doi: 10.1016/j.jre.2018.03.032 [17] 严雪峰. 某包装厂印刷有机废气处理工程设计 [D]. 武汉: 武汉工程大学, 2015. [18] 王俊. 苏州市印刷包装行业挥发性有机污染物处理对策研究 [D]. 苏州: 苏州科技大学, 2016. [19] 王家德, 吕建璋, 李文娟, 等. 浙江省包装印刷行业挥发性有机物排放特征及排放系数[J]. 环境科学, 2018, 39(8): 3552-3556. [20] 卜龙利, 张钰彩, 王晓晖, 等. 微波辅助催化氧化苯高性能催化剂实验研究[J]. 燃料化学学报, 2012, 40(7): 878-885. doi: 10.3969/j.issn.0253-2409.2012.07.018 [21] SANZ O, DELGADO J J, NAVARRO P, et al. VOCs combustion catalysed by platinum supported on manganese octahedral molecular sieves [J]. Applied Catalysis B: Environmental, 2011, 110; 231-237. [22] DAI Q G, WANG X Y, LU G Z. Low-temperature catalytic destruction of chlorinated VOCs over cerium oxide[J]. Catalysis Communications, 2007, 8(11): 1645-1649. doi: 10.1016/j.catcom.2007.01.024 [23] KIM S C, SHIM W G. Catalytic combustion of VOCs over a series of manganese oxide catalysts[J]. Applied Catalysis B:Environmental, 2010, 98(3-4): 180-185. doi: 10.1016/j.apcatb.2010.05.027 [24] ZENG Y Q, WANG Y N, SONG F J, et al. The effect of CuO loading on different method prepared CeO2 catalyst for toluene oxidation[J]. Science of the Total Environment, 2020, 712: 135635. doi: 10.1016/j.scitotenv.2019.135635 [25] 张晖, 吴春笃. 环境工程原理 [M]. 武汉: 华中科技大学出版社, 2011. [26] 金彦任, 黄振兴. 吸附与孔径分布 [M]. 北京: 国防工业出版社, 2015. [27] 徐铭遥. 单元式CuMn2CenOx/Cord催化剂的研制及其对甲苯催化燃烧性能 [D]. 广州: 华南理工大学, 2011. [28] GE Y L, FU K X, ZHAO Q, et al. Performance study of modified Pt catalysts for the complete oxidation of acetone[J]. Chemical Engineering Science, 2019, 206: 499-506. doi: 10.1016/j.ces.2019.05.051 [29] 刘秀珍. MnOx-CeO2/蜂窝沸石对甲苯的吸附-低温催化氧化一体化研究 [D]. 广州: 华南理工大学, 2012. [30] 刘鹏. 多孔矿物负载型/过渡金属复合型锰氧化物热催化氧化挥发性有机物的研究 [D]. 北京: 中国科学院大学, 2019. [31] ZHANG X, WU D F. Ceramic monolith supported Mn-Ce-M ternary mixed-oxide (M=Cu, Ni or Co) catalyst for VOCs catalytic oxidation[J]. Ceramics International, 2016, 42(15): 16563-16570. doi: 10.1016/j.ceramint.2016.07.076 [32] PAN H Y, XU M Y, LI Z, et al. Catalytic combustion of styrene over copper based catalyst: Inhibitory effect of water vapor[J]. Chemosphere, 2009, 76(5): 721-726. doi: 10.1016/j.chemosphere.2009.04.019 [33] LI X, WANG L J, XIA Q B, et al. Catalytic oxidation of toluene over copper and manganese based catalysts: Effect of water vapor[J]. Catalysis Communications, 2011, 14(1): 15-19. doi: 10.1016/j.catcom.2011.07.003 [34] ELKHALIFA E A, FRIEDRICH H B. Oxidative dehydrogenation of n-octane over a vanadium-magnesium oxide catalyst: Influence of the gas hourly space velocity[J]. Arabian Journal of Chemistry, 2019, 12(8): 2464-2469. doi: 10.1016/j.arabjc.2015.03.017 [35] 刘海楠, 卜龙利, 王晓晖, 等. 二氧化钛复合型催化剂制备及其微波辅助催化氧化甲苯性能[J]. 环境科学学报, 2013, 33(6): 1720-1727. -



 下载:
下载: