-
大量氟化物的直接排放会对土壤、地下水及工农业生产等产生危害,尤其是能够影响植物的新陈代谢,抑制农作物的呼吸和光合作用。另外,人类长期吸收过量的氟化物会引起氟斑牙,甚至会造成骨膜增生、骨节硬化及骨质疏松等问题[1-2]。据调查,我国北方某地矿产业较为发达,煤矿开采中产生了大量的含氟矿井水,由于对矿井水的不当处置造成该地地表水氟化物超标,其检出质量浓度约为5.0 mg·L−1。除氟污染外,该地区地表水受人类活动影响较大,地表水中还含有一定量的有机污染物,有机物质量浓度可达4.0 mg·L−1,这增加了矿井水的处理以及综合利用难度[3]。
矿井水具有水量大,氟化物含量低的特点[4]。常用的处理方法主要有吸附法、混凝法、离子交换法、电渗析法、反渗透法和电凝聚法等[5-8]。吸附法虽然具有工艺简单、吸附速率快的特点,但主要用于处理低浓度的含氟废水,且由于吸附容量低、吸附剂再生及再生液处理复杂等问题导致处理成本较高,此外,有机物的存在会加速吸附剂的失活,因此,其实用性受到限制[9]。离子交换法通常可以获得较高的氟去除率,但无法有效地去除有机物,且离子交换树脂再生困难以及树脂再生产生二次污染的问题难以解决[1],并不适用于处理大量含有有机物的氟污染矿井水。电渗析法通过电解和交换除去水体中的杂质,但该方法存在废水处理产能低、能耗大以及工业化推广应用困难的问题。反渗透法通过渗透膜可以将水体中的细微杂质粒子去除,但却存在使用条件苛刻,需要前置预处理以及膜更换频繁、二次浓水难以妥善处理等。混凝工艺操作简单,可以有效地实现氟化物的去除。此外,混凝对天然有机物的去除也展现出优势,因此,选用混凝法对含有有机物的矿井废水进行处理更具有实际意义。目前广泛应用于除氟的混凝剂主要为铝基混凝剂,主要包括单体铝和聚合态铝。单体铝混凝剂有AlCl3、Al2SO3等,聚合态铝混凝剂主要包括聚合氯化铝(PAC)、Al13O14(OH)24(H2O)127+(Al13)等。单体铝混凝剂的使用往往伴随着出水余铝浓度过高的问题,高浓度的铝对人体有害,甚至会损伤人体的中枢神经系统。Al13已被多次证实可以高效去除氟化物,且出水能维持较低的余铝浓度。此外,陈孟等[10]证实,聚合态铝对三卤甲烷前体物具有良好的去除效果。
由于天然有机物是极其复杂的混合物,腐殖酸(HA)通常作为研究模型来代替天然有机物进行研究[11]。在本研究中,以腐殖酸为代表,对影响混凝过程的主要因素,包括混凝剂的投加量、水体pH和水样性质等,进行了逐一探究,系统地考察了腐殖酸的存在对铝系混凝剂去除氟化物机制的影响,以期为含氟矿井水的处理提供参考。
-
腐殖酸(HA),氯化铝(AlCl3),碳酸钠(Na2CO3),氟化钠(NaF),氢氧化钠(NaOH),盐酸(HCl),氯化钠(NaCl),碳酸氢钠(NaHCO3)均购于国药试剂有限公司,上述药品均为分析纯。
-
1)腐殖酸储备液的配置。用天平(FB224,上海舜宇恒平科学仪器有限公司)称取2.0 g腐殖酸粉末,随后加入0.5 mol·L−1的NaOH溶液不断搅拌至完全溶解,制备成1.0 L的腐殖酸储备液,调节pH至12.0。随后将腐殖酸储备液至于磁力搅拌器(90-4,上海振荣科学仪器有限公司)上连续搅拌5.0 h后,用0.45 μm膜过滤后避光置于冰箱(4 ℃)保存备用。
2)混凝剂的制备。在转速不低于300 r·min−1的磁力搅拌下,用蠕动泵以0.1~0.15 mL·min−1的速度向500 mL AlCl3(初始Al3+浓度为0.2 mol·L−1)溶液中滴加440 mL的Na2CO3(0.25 mol·L−1)溶液。利用恒温水浴器控制反应温度为60 ℃,待Na2CO3溶液全部滴加后,停止搅拌并将溶液置于常温下熟化2 h。随后加入一定量的硫酸钠固体,在常温下搅拌5.0 h,离心后获得白色沉淀(即硫酸铝十三),用超纯水清洗后冷冻干燥。称取一定量的硫酸铝十三并加入一定量的0.1 mol·L−1的BaCl2溶液,常温搅拌3 h后,过0.45 μm膜,即得纯化后的Al13溶液,测定总铝浓度。
3)实验水样的配置。实验水样用去离子水配制,加入适量的NaNO3(0.1 mol·L−1)和NaHCO3 (0.1 mol·L−1)储备液提供体系的离子强度和碱度,其中水样碱度控制为10 mg·L−1。随后加入一定量的腐殖酸储备液和氟化钠储备液,并用0.1 mol·L−1的NaOH溶液和0.1 mol·L−1的HCl溶液调节水样pH。
4)混凝过程。混凝于六联搅拌仪上进行,步骤参照XIANG等[12-13]的研究方法设定。具体如下:以250 r·min−1快搅30 s混匀水样,加入混凝剂后以200 r·min−1的速度快搅90 s,再以40 r·min−1慢搅10 min,结束后静置30 min。
-
pH用pH计(FE28,梅特勒-托利多仪器有限公司)测定;溶解性有机碳浓度(DOC)用总有机碳分析仪(TOC-L,日本岛津公司)测定;光谱分析用紫外分光光度计(UV-8000,上海精密仪器仪表有限公司)进行;氟离子浓度用氟离子选择电极(PXSJ-216F,上海仪电科学仪器股份有限公司)测定;Zeta电位用马尔文Zeta电位仪测定;铝形态分析用电喷雾飞行时间质谱(ESI-TOF-MS,ACQUITY UPLC/Xevo G2 Q TOF,美国沃特斯公司)进行;絮体形貌用扫描电镜(SU-8020,日本日立公司)测定;采用傅立叶变换红外光谱(FT-IR, Nicolet 8700,美国赛默飞公司)和X射线光电子能谱(XPS, ESCALAB250Xi,美国赛默飞公司)分析絮体的化学组成。
-
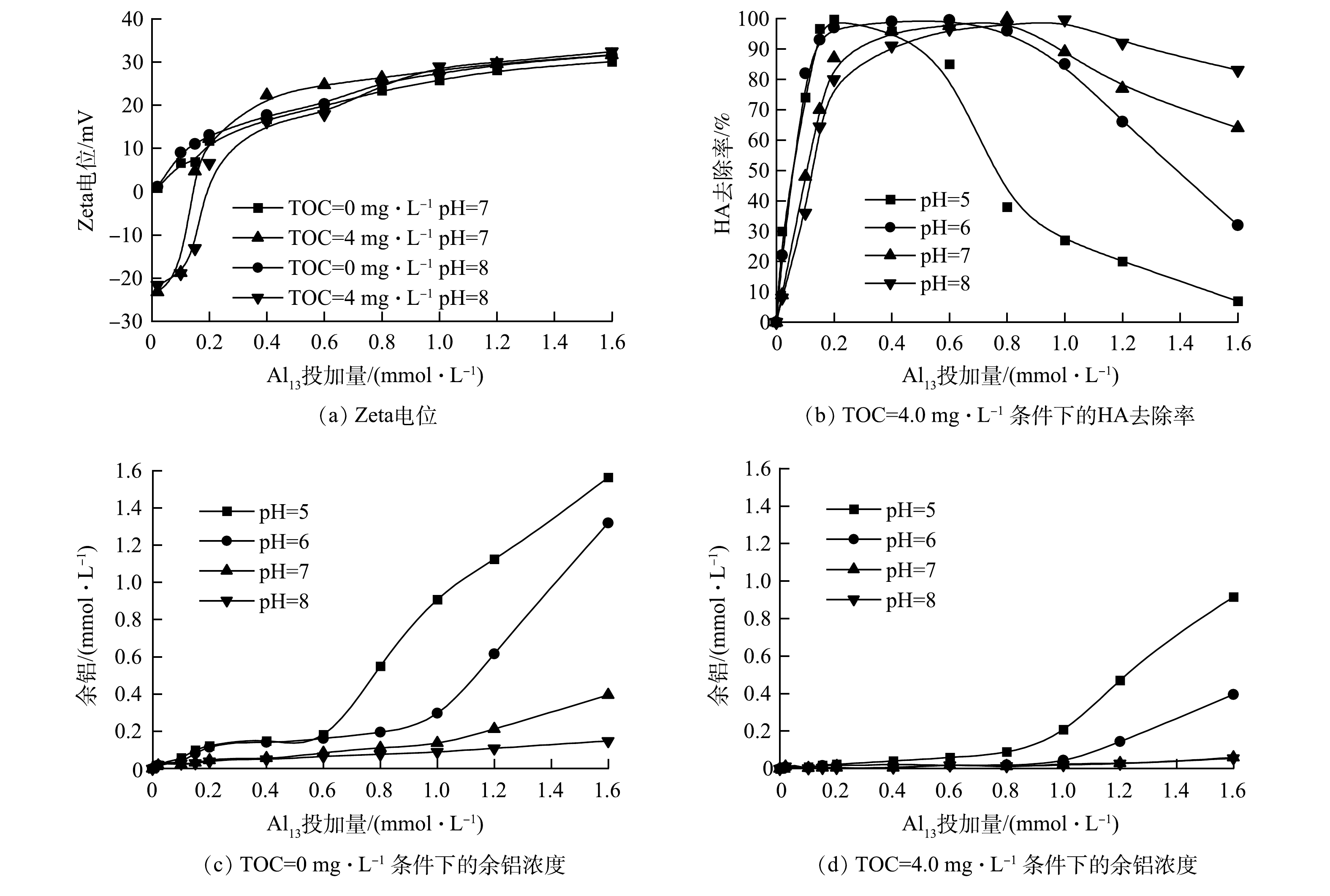
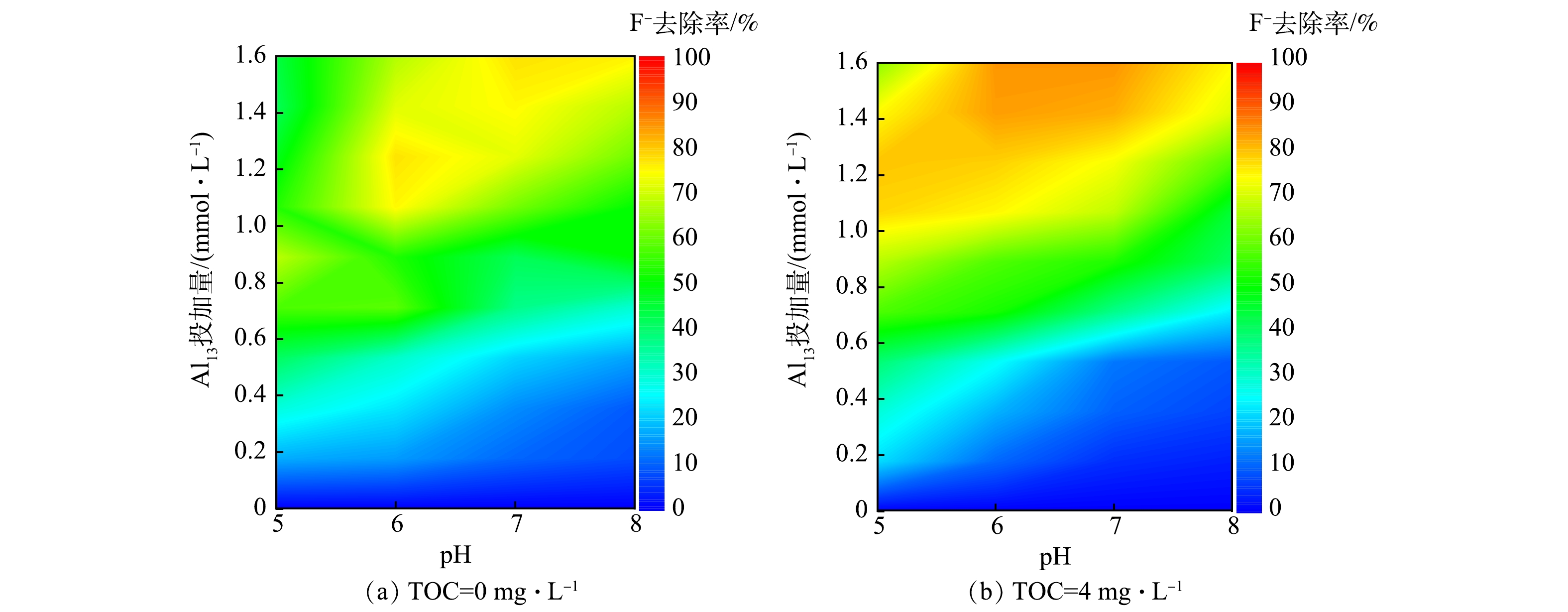
在TOC质量浓度分别为0 mg·L−1(T0体系)和4.0 mg·L−1(T4体系)的条件下,Al13在pH为5.0~8.0内的混凝除氟效果见图1。当Al13投加量小于0.40 mmol·L−1时,在2个体系中氟去除率均维持在较低水平(低于30%),此时有机物及pH的变化对氟的去除影响较小;当Al13投加量大于0.40 mmol·L−1时,氟去除率明显上升。如图1(b)所示,当pH为5.0时,HA的共存使氟化物的去除在Al13投加量在1.0~1.4 mmol·L−1出现了一个高效作用区域(氟化物去除率大于70%),最高去除率在Al13为1.0 mmol·L−1处达到80.40%,高于无腐殖酸体系在0.80 mmol·L−1 Al13取得的最高去除率68.12%(图1(a));继续增大pH,无腐殖酸体系在Al13高投加量处也出现了高效作用区域(氟化物去除率大于70%),在pH为7.0、Al13投加量为1.6 mmol·L−1时,无HA体系中氟化物最大去除率为77.68%。而有腐殖酸体系的高效作用区域的面积(氟化物去除率大于70%)和去除率均大于无腐殖酸体系,尤其在中性和碱性条件下,共存体系的氟化物最高去除率均在85%以上。由此可见,在混凝剂的高投加量下,无论是在酸性还是碱性环境中,HA的存在均可提高Al13对氟的去除率。
-
体系的Zeta电位、出水的余铝浓度及HA去除率变化情况如图2所示。由于在酸性环境无HA条件下混凝过程中几乎没有形成悬浮颗粒,Zeta电位测定值的标准偏差过大且无规律性[14],因此,仅测定了pH为7.0和8.0时体系的Zeta电位。在Al13低投加量下(低于0.2 mmol·L−1),T4体系的电负性远高于T0体系,且随着Al13投加量的增加,2个体系的Zeta电位均不断升高。在Zeta电位趋近于零时并没有取得最高除氟率和HA去除率(T4体系,图2(b)),在除氟的高效作用区域,2个体系的Zeta电位保持在20 mV以上。这表明氟化物以及HA的去除不完全是电中和作用的结果。在酸性条件下,在Al13低投加量下(0.2 mmol·L−1)就可获得HA的最大去除率;在碱性条件下,若要达到最高除氟率则需加大Al13投加量至0.8 mmol·L−1。如图2(c)所示,T0体系的残余铝浓度在达到最大除氟率时开始上升,尤其在酸性条件下,当Al13投加量超过0.8 mmol·L−1时,出水的残余铝浓度显著上升,而此时除氟效果也随之降低。在HA存在时,混凝出水的残余铝浓度均低于无HA体系(图2(d))。在酸性条件下,HA的去除率在投加量0.4 mmol·L−1(pH=5.0)和0.8 mmol·L−1(pH=6.0)开始下降,而此时出水残余铝浓度并未增加,而氟去除率开始上升。这进一步说明了在酸性条件下,HA与F−存在Al13的竞争结合。在投加量较低时,由于HA中的羧基等基团对Al13的作用更强,使其先被混凝去除。在中性和碱性条件下,在投加量达到1.6 mmol·L−1(HA的去除率下降到70%以下),其混凝出水的残余铝浓度仍维持较低水平,此时氟去除率也提升到80%以上。
-
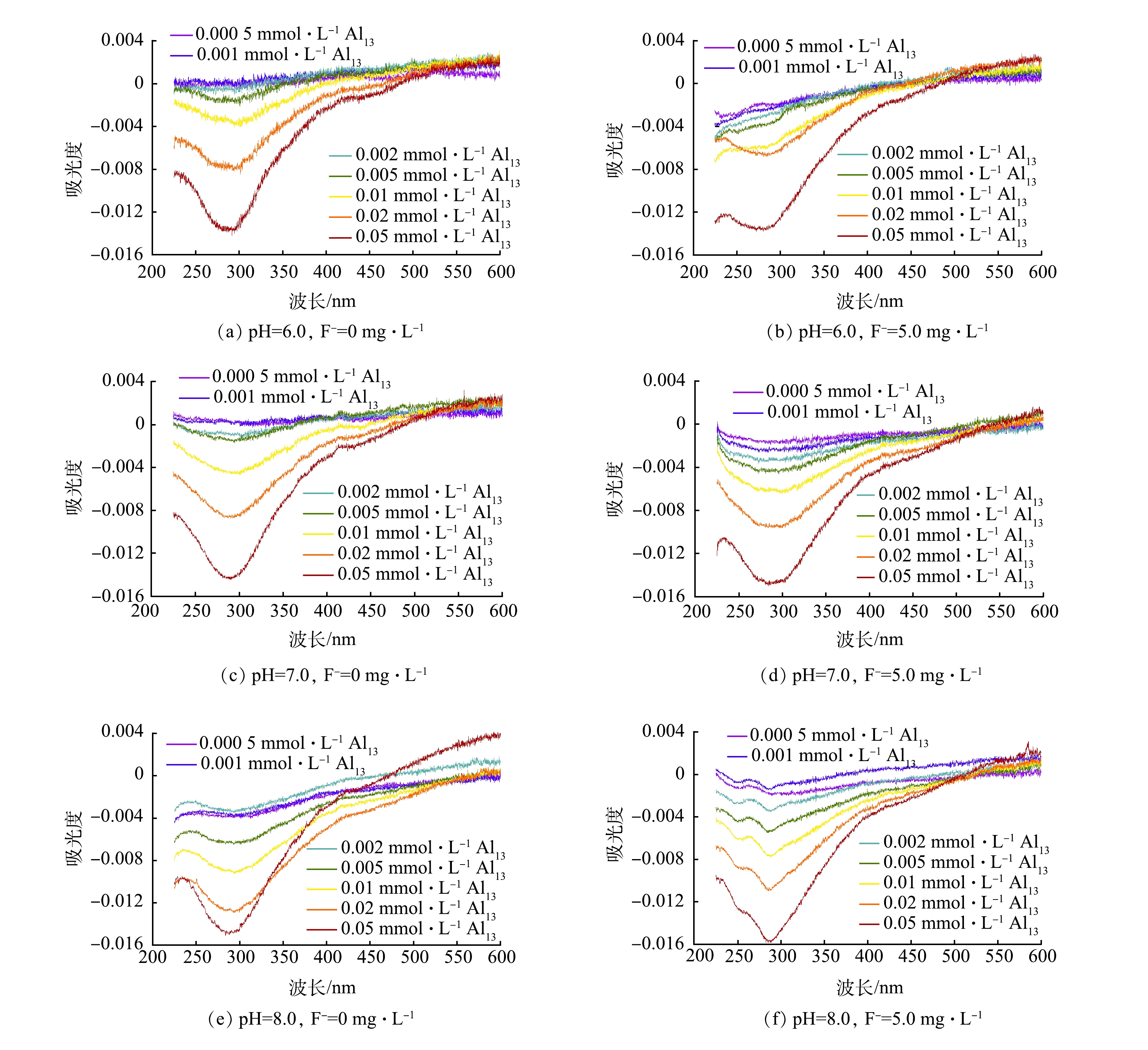
为进一步探究HA、F−与Al13的相互作用,测定了不同条件下的200~600 nm内的紫外吸收光谱并进行了差分光谱的分析[15],结果如图3所示。有研究表明,280 nm附近的谱带与有机物中的羧基的去质子化有关[16]。本研究中,随着Al13投加量的增加,吸光度降低,差分光谱在280 nm附近出现较强的响应。这表明Al13与HA中羧基的作用最为显著。而当体系中有氟离子存在时(图3(b)、图3(d)、图3(f)),虽然光谱中的波形有所改变,差分光谱在280 nm附近处的强响应几乎不受影响。这表明氟离子的存在并不影响HA分子中羧基与Al13的相互作用。KAZPARD等[17]的研究表明,当pH大于8.0时或Al13投加量过量时,酚羟基也会与Al13发生络合反应。本研究中,在差分光谱245 nm附近是与酚羟基有关的谱带,可以看到在pH为8.0时(图3(f)),含氟化物的样品中的差分光谱中在245 nm附近出现明显的响应峰,而未添加氟化物的差分光谱中则没有此现象。这说明溶液中F−对Al13与HA分子中的酚羟基的络合起到了一定的促进作用。
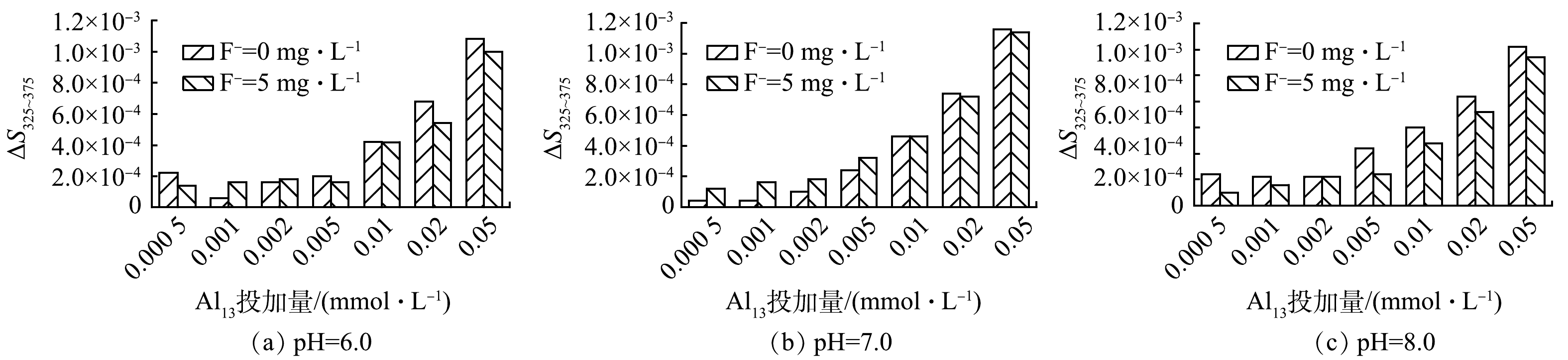
金属离子与有机物相互作用产生的光谱经对数变换后的光谱斜率变化较为显著,这种变化不受有机物浓度的影响,而是取决于有机物的化学性质及其与金属结合的波长[18-19]。在本研究中,参照YAN等[20]的方法,计算出325~375 nm处的吸光度的对数变换光谱线性斜率以及线性斜率的差值,记为ΔS325~375,ΔS325~375值的大小反映HA与Al3+的络合量,结果如图4所示。pH和F−浓度对ΔS325~375值均有一定的影响,随着Al13投加量的增加。随着Al13投加量的增加,ΔS325~375值呈现出持续增长的趋势。而当HA溶液中有F−存在时,在相同pH下ΔS325~375值略微有所下降,此时混凝剂Al13与HA络合的金属量降低。这表明有一部分Al13与F−结合为Al13-F物质。
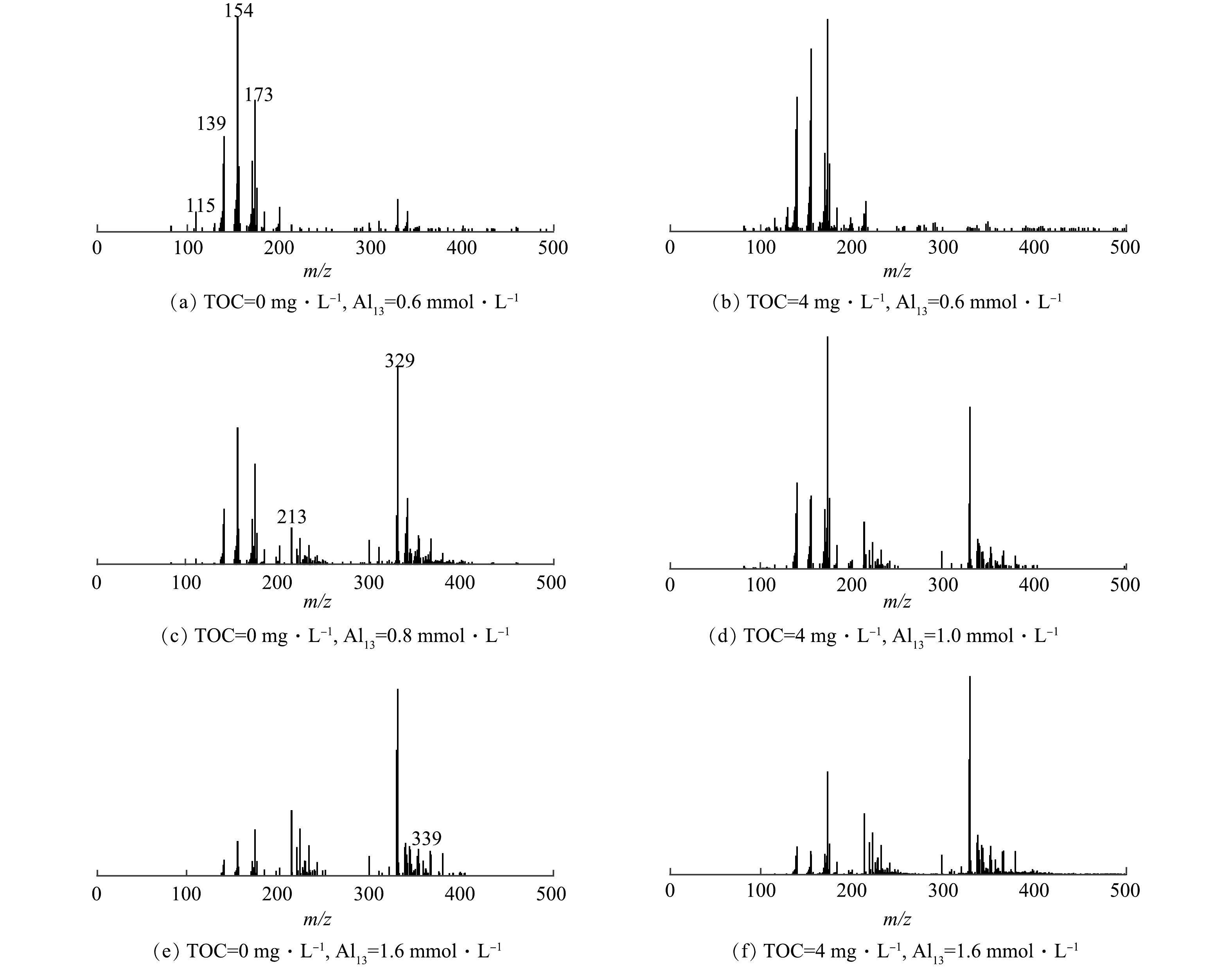
Al13由于配位作用和水解作用等会产生带不同电荷量和不同浓度的离子团,在质谱上能够产生位置和高度均不同的特征峰,故可利用ESI-TOF-MS进行解析并反推出溶液中的铝形态[21]。如2.1节所述,在酸性条件下(pH为5.0),有机物的加入明显改变了氟化物去除的作用区域,因此,选取了3种Al13投加量,分别为0.6 mmol·L−1 的低投加量、无HA存在体系的0.8 mmol·L−1和有HA共存体系的1.0 mmol·L−1的最佳投加量以及1.6 mmol·L−1的最大投加量,进行了ESI-TOF-MS的测定,结果如图5所示。表1对图5中出现的铝核离子形态进行了汇总,主要有Al1、Al2、Al3、Al5和Al13等[22-23]。同时,在光谱中也可以观察到其他强度相对较低的多电荷聚合物(Al4、Al6、Al7、Al9)。其中Al1主要以Al(OH)2(H2O)3+(m/z=115)为主;Al2中Al2O2(OH)(H2O)2+(m/z=139)和Al2(OH)F3(H2O)102+(m/z=154)含量最高;Al3中Al3O(OH)4F(H2O)92+(m/z=173)含量最丰富,Al13以AlO4Al12O14(OH)2+(m/z=328)和AlO4Al12O14F2+(m/z=329)为主要存在形式,出水中残余的各铝团簇种类中均存在Al-F结合形态。这说明,HA的存在并不会从根本上改变铝与氟主要的反应形式。
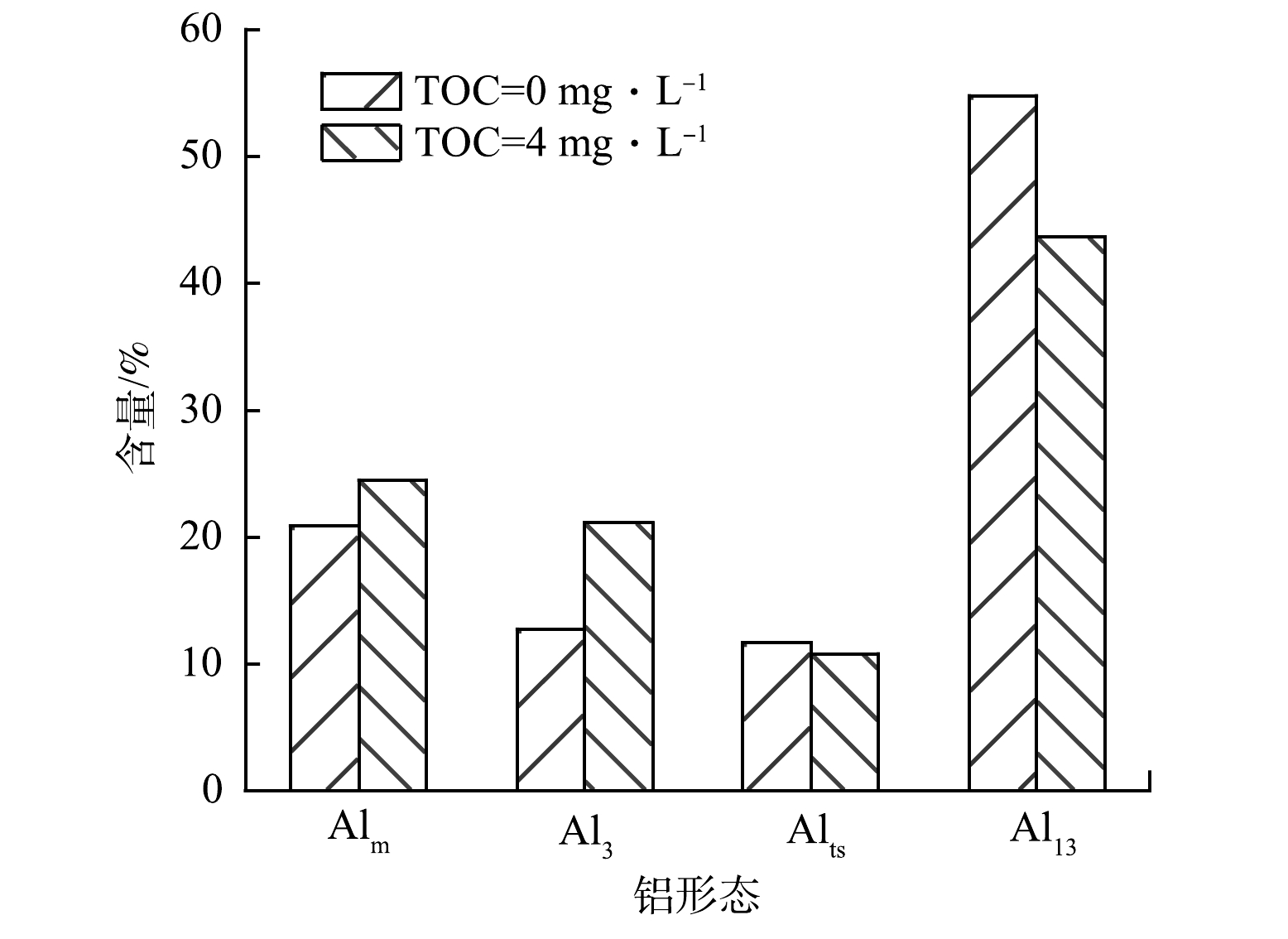
如图5(a)和图5(b)所示,在Al13低投加量(0.6 mmol·L−1)下,无HA存在的混凝出水中含量最高的是Al2(Al2(OH)F3(H2O)102+),同时其Al13离子以AlO4Al12O14F2+为主;而HA共存于体系中,混凝出水中Al3(Al3O(OH)4F(H2O)92+)含量最高,且Al13形态以AlO4Al12O143+为主。如图5(c)和图5(d)所示,当Al13投加量增加到最佳投加量时,AlO4Al12O13(OH)3-nFn2+(n=0~3)、AlO4Al12O14F2+等Al13离子含量大幅增加,相应的Al2、Al3等离子含量减少,同时Al1基本消失。为了更直观的了解出水中铝形态变化,对铝形态的分布变化进行了汇总,结果如图6所示。其中Alm为Al1和Al2的总称;Alts为新增的铝形态离子种类(Al5、Al9、Al12等),本质是在凝固过程中形成的中间簇。HA共存体系的混凝出水的Al13离子和Alts含量降低,而Alm和Al3含量增加,其中Al13含量降低了约11.2%,而Al3含量增加了8.9%。HA共存体系的混凝出水中Al13离子含量降低的结果表明,HA能够促进Al13的分解,产生了Al3、Alm等低聚体。当Al13投加量并达到1.6 mmol·L−1时,Al13含量急剧上升。在图5(c)与图5(f)中所含铝形态没有明显差别,主要以AlO4Al12O14(OH)2+和AlO4Al12O14F2+等为主。此时Al13过量,在溶液中占主导地位,同时HA和F−被大量去除,受其他因素的影响很小,所以在2种体系中出水铝的形态几乎没有差别。
-
收集在pH为7.0、Al13最佳投加量 下T0和T4体系中产生的絮体,将絮体冷冻干燥用于随后的分析。由于T0体系(无HA)产生的絮体为白色凝胶状物质,为避免破坏其形态结构,待其沉淀30 min后取其底部含絮体直接冷冻干燥;而T4体系(有HA)产生的絮体结构稳定,直接低速离心后,取其沉淀物冷冻干燥。
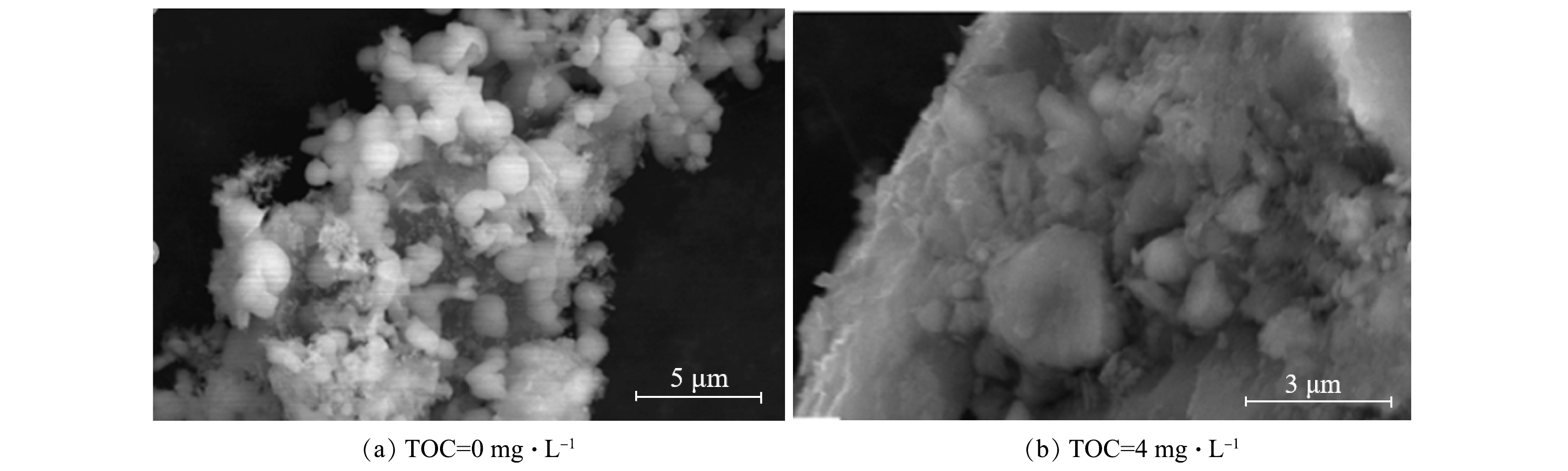
1)絮体形貌分析。如图7所示,当体系中无HA时,Al-F沉淀物的絮体孔隙较多,结构疏松;而当有HA共存于体系时,所形成的HA-Al-F沉淀的结构更为紧密。絮体孔隙度的减少表明在有HA的条件下,网捕卷扫和吸附架桥作用更强,絮体易于沉淀,有利于氟化物的去除。
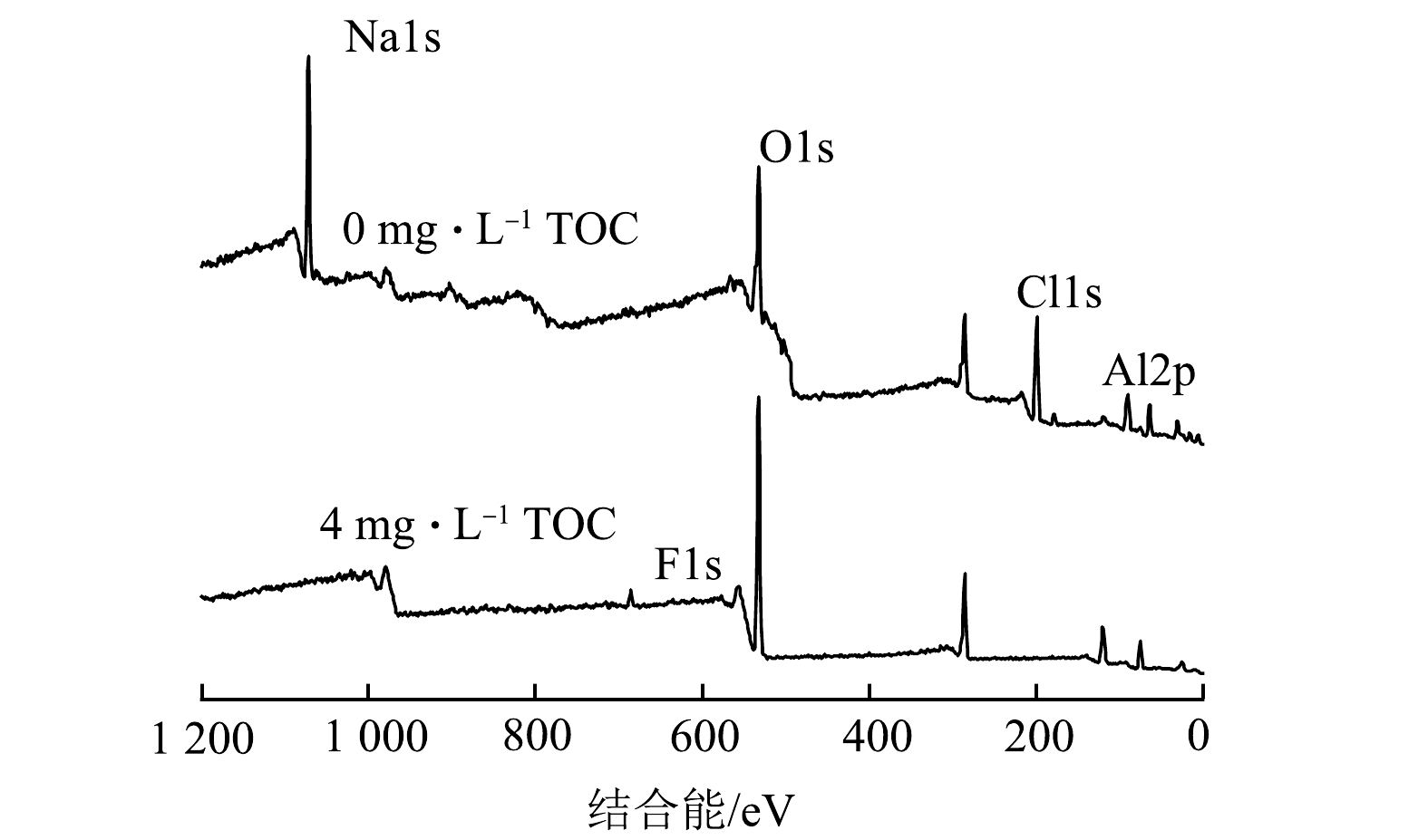
2)絮体成分分析。图8为pH为7.0条件下混凝后2中体系中絮体的XPS宽谱扫描图。由图8可以看出,絮体的主要元素组成有F、Al、O、C。表2为去除钠和氯元素后的剩余几种元素的含量对比。结果表明,2种体系中絮体元素含量占比差别较大,HA共存体系产生的絮体中氟和铝元素含量均有很大的提升。尽管氧元素含量占比下降较多,但根据图9中峰面积对比计算,有HA的絮体中O的原子数是无HA时的1.49倍。
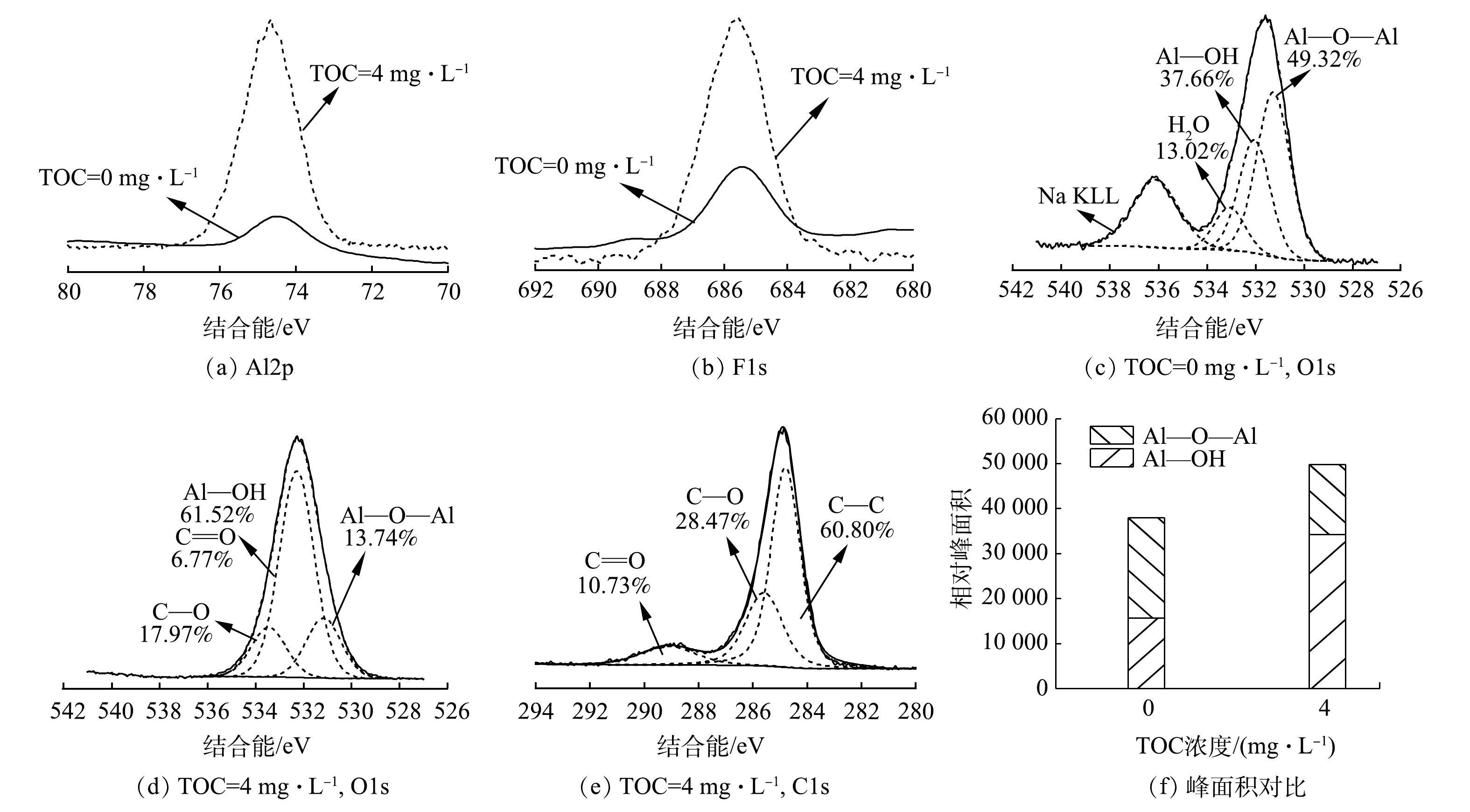
图9为絮体中单元素的XPS扫描谱图的分析结果。相比于无HA共存的体系产生的絮体,HA共存体系生成的絮体的Al2p的峰向更高结合能的位置移动了0.25 eV,这说明Al与HA中官能团的作用更胜于Al与F原子间的相互作用。2种絮体F1s的结合能均处于AlF3·H2O(686.30 eV)与Na3AlF6(685.50 eV)之间,说明F-与Al13之间发生了络合[24-25]。由图9(c)可见,无HA的絮体中O1s的形态主要是Al—O—Al(531.42 eV)和Al—OH(532.33 eV)[26]。而图9(d)显示,HA共存体系产生的絮体中O1s具有更多的形态。Al—O—Al键和C—O键的峰面积占比分别为1.74%和13.97%,然而Al—OH与C=O 2种形态的结合能相差低于0.3 eV,难以区分,故对C1s进行了分峰拟合(图9(e)),寻求存在的数学关系,发现其主要有3种形态:C—C(284.80 eV)、C=O(289.11 eV)、C—O(285.59 eV)[27]。如图9(e)所示,C=O键与C—O键的峰面积占比分别为10.73%和28.47%,比值为0.38。将该比值与图9(d)中C—O键的峰面积占比(17.97%)相乘得出C=O键的峰面积占比为6.77%,进而得出Al—OH键的峰面积占比为61.52%。 这一结果表明, 加入HA的絮体中O 1s主要有C—O(533.41 eV)、Al—O—Al(531.28 eV)、Al—OH和C=O(532.27 eV)这4种氧形态[26-28]。对絮体中Al—O—Al键和Al—OH键含量(图9(f))进行对比发现,HA共存体系生成的絮体中Al—OH键占比大幅提升,同时Al—O—Al键减少。这说明含AlO4基团的Al核物质大量水解。有研究[29]证实,絮体中Al—OH会与F-作用产生氢键,从而牢固的形成稳定的絮状物沉淀;更多的Al—OH会增大对F-的固定作用,从而提升氟的去除率。
-
1)在本研究中,中性和碱性条件更有利于氟化物的去除。在pH为7.0、Al13投加量为 1.6 mmol·L−1的条件下,在不含HA体系中,氟化物最大去除率可达80.18%;在该条件下,HA共存体系氟化物的最大去除率为85.19%;当Al13投加量大于1.0 mmol·L−1时,有HA共存体系的高效作用区域的面积(氟化物去除率大于70%)和最大氟去除率均大于无HA体系,表明HA可显著促进氟化物的去除。
2)在酸性条件下,当HA和F−在Al13投加量小于0.4 mmol·L−1(pH为5.0)和0.8 mmol·L−1(pH为6.0)时存在显著的竞争作用,HA在竞争中取得优势,可被大量去除。这是由于HA中存在的羧基、羟基等基团对Al13的作用较强,主要形成Al13与HA的络合体。
3) HA增强了网捕卷扫作用和吸附架桥作用,形成的絮体更为致密;HA共存体系生成的絮体中的氧元素形态更为复杂多样,Al-OH键占比大幅提升,增强了氟化物的固定。
水中腐殖酸对Al13混凝除氟的影响
Effects of humic acid on fluoride removal by Al13 coagulation
-
摘要: 针对含氟矿井水中有机物含量较高的问题,使用混凝区域图研究了在腐殖酸(HA)存在的条件下Al13混凝除氟的效果及相关的影响因素;使用电喷雾飞行时间质谱(ESI-TOF-MS)、差分吸收光谱法(DOAS)和X射线光电子能谱分析(XPS)考察了HA与F在混凝过程中的竞争关系,且表征了Al13与F的络合产物;分析了HA影响Al13除氟的可能机理。结果表明:在最佳投加量下,Al13对氟的去除率在HA共存时提高了10%以上,余铝浓度下降;同时,混凝过程中所形成的絮体结合更加紧密且易沉降。DOAS分析结果表明,在混凝初始阶段,HA对Al13的竞争具有一定的优势,此时主要形成Al13与有机物的络合体;随着反应的继续进行,Al13水解产生低聚态铝,此时氟主要通过与Al-OH的离子交换被去除,同时通过有机物絮体的网捕与吸附架桥作用进一步吸附游离态氟。Abstract: In order to improve the fluoride removal efficiency for fluorine-containing mine water with high organics content by using Al13 coagulation, the coagulation area diagram was used to study the fluoride removal effect by Al13 coagulation in the presence of humic acid (HA) and the related influencing factors. In order to investigate the mechanism of the influence of organic matter on the defluorination of Al13, electrospray time-of-flight mass spectrometry (ESI-TOF-MS), differential absorption spectroscopy (DOAS), and X-ray photoelectron spectroscopy (XPS) were used to analyze the competitive relationship between HA and fluoride in the coagulation process, and the complex product between Al13 and F was also characterized. The results indicated that at the optimal Al13 dosage, the removal rate of fluorine increased by over 10% in the presence of HA, the residual aluminum content decreased. The flocs formed in the coagulation process were compacted and easily settled. According to DOAS analysis, at the beginning of the coagulation, HA had an advantage in the competition with Al13, and Al13 -organic matter complex mainly occurred. As the reaction continued, Al13 was hydrolyzed to produce oligomeric Al nuclear material, and fluoride was mainly removed by ion exchange with Al-OH. At the same time, the flocs formed in the coagulation process containing organic matter further adsorbed free fluorine by netting and adsorption bridging effects.
-
Key words:
- humic acid /
- fluoride /
- coagulation /
- flocs /
- residual aluminum
-

-
表 1 在ESI-TOF-MS质谱中鉴定出的物种
Table 1. Identified species in ESI-TOF-MS spectra
质荷比 铝种类 分子式 质荷比 铝种类 分子式 81 Al1 Al(OH)F(H2O)+ 277 Al4 Al4O5(OH)(H20)4+ 83 Al1 AlF2(H2O)+ 170 Al5 Al5O5(OH)2F(H2O)42+ 115 Al1 Al(OH)2(H2O)3+ 196 Al5 Al5(OH)13H2O2+ 117 Al1 Al(OH)F(H2O)3+ 184 Al7 Al7O8(OH)32+ 139 Al2 Al2O2(OH)(H2O)2+ 298 Al9 Al9O4(OH)172+ 152 Al2 Al2(OH)3F(H2O)102+ 352 Al12 Al12O11(OH)122+ 153 Al2 Al2(OH)2F2(H2O)102+ 213 Al13 AlO4Al12O143+ 154 Al2 Al2(OH)F3(H2O)102+ 228 Al13 AlO4Al12O12F43+ 155 Al2 Al2F4(H2O)102+ 232 Al13 AlO4Al12O11(OH)4F23+ 163 Al2 Al2OF3(H2O)2+ 328 Al13 AlO4Al12O14(OH)2+ 175 Al2 Al2O2(OH)(H2O)4+ 329 Al13 AlO4Al12O14F2+ 173 Al3 Al3O(OH)4F(H2O)92+ 337 Al13 AlO4Al12O13(OH)32+ 183 Al3 Al3O3(OH)F(H2O)+ 339 Al13 AlO4Al12O13(OH)F22+ 219 Al3 Al3O3(OH)F(H2O)3+ 349 Al13 AlO4Al12O12(OH)2F32+ 223 Al3 Al3O2(OH)F3(H2O)2+ 357 Al13 AlO4Al12O11(OH)5F22+ 237 Al3 Al3O3(OH)F(H2O)4+ 365 Al13 AlO4Al12O10(OH)8F2+ 239 Al3 Al3O3F2(H2O)4+ 366 Al13 AlO4Al12O10(OH)7F22+ 241 Al3 Al3O2(OH)F3(H2O)3+ 379 Al13 AlO4Al12O11(OH)F(H2O)22+ 表 2 混凝絮体主要元素含量对比
Table 2. Comparison of main element content of coagulation floc
TOC/(mg·L−1) C/% Al/% O/% F/% 0.0 — 5.81 93.87 0.32 4.0 36.18 20.09 41.35 2.38 -
[1] 刘锐平. 饮用水氟污染控制原理与技术[J]. 应用生态学报, 2019, 30(1): 30-36. [2] BHATNAGAR A, KUMAR E, SILLANPÄÄ M. Fluoride removal from water by adsorption: A review[J]. Chemical Engineering Journal, 2011, 171(3): 811-840. doi: 10.1016/j.cej.2011.05.028 [3] 杨彪. 高氟病区水氟与土壤、作物氟积累的相关关系研究[D].太原: 山西大学, 2012. [4] 袁航, 石辉. 矿井水资源利用的研究进展与展望[J]. 水资源与水工程学报, 2008,19(5): 50-57. [5] VISWANATHAN N, MEENAKSHI S. Selective fluoride adsorption by a hydrotalcite/chitosan composite[J]. Applied Clay Science, 2010, 48(4): 607-611. doi: 10.1016/j.clay.2010.03.012 [6] 张威, 杨胜科, 费晓华. 反渗透技术去除地下水中氟的方法[J]. 长安大学学报(自然科学版), 2002,22(6): 116-118. [7] ISLAM M, PATEL R K. Evaluation of removal efficiency of fluoride from aqueous solution using quick lime[J]. Journal of Hazardous Materials, 2007, 143(1): 303-310. [8] LOGANATHAN P, VIGNESWARAN S, KANDASAMY J, et al. Defluoridation of drinking water using adsorption processes[J]. Journal of Hazardous Materials, 2013, 248-249: 1-19. doi: 10.1016/j.jhazmat.2012.12.043 [9] MEDELLIN-CASTILLO N A, LEYVA-RAMOS R, PADILLA-ORTEGA E, et al. Adsorption capacity of bone char for removing fluoride from water solution: Role of hydroxyapatite content, adsorption mechanism and competing anions[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(6): 4014-4021. doi: 10.1016/j.jiec.2013.12.105 [10] 陈孟, 张克峰, 张英芹, 等. 铝盐、铁盐和钛盐混凝对三卤甲烷前体物的去除研究[J]. 现代化工, 2021, 41(9): 178-184. [11] 王东升, 安广宇, 刘丽冰, 等. Al13 的分子学及其在环境工程中的应用[J]. 环境工程学报, 2018, 12(6): 1565-1584. doi: 10.12030/j.cjee.201803169 [12] 象豫, 徐慧, 李昆, 等. 铜绿微囊藻对混凝除氟的促进作用及机理分析[J]. 中国环境科学, 2021, 41(4): 1900-1908. doi: 10.3969/j.issn.1000-6923.2021.04.045 [13] XIANG Y, XU H, LI C F, et al. Effects and behaviors of Microcystis aeruginosa in defluorination by two Al-based coagulants, AlCl3 and Al13[J]. Chemosphere, 2022, 286: 131865. doi: 10.1016/j.chemosphere.2021.131865 [14] YU W Z, GREGORY J, GRAHAM N. Regrowth of broken hydroxide flocs: Effect of added fluoride[J]. Environmental Science & Technology, 2016, 50(4): 1828-1833. [15] GAO Y, YAN M, KORSHIN G. Effects of calcium on the chromophores of dissolved organic matter and their interactions with copper[J]. Water Research, 2015, 81: 47-53. doi: 10.1016/j.watres.2015.05.038 [16] SHI B, WEI Q, WANG D, et al. Coagulation of humic acid: The performance of preformed and non-preformed Al species[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2007, 296(1/2/3): 141-148. [17] KAZPARD V, LARTIGES B S, FROCHOT C, et al. Fate of coagulant species and conformational effects during the aggregation of a model of a humic substance with Al13 polycations[J]. Water Research, 2006, 40(10): 1965-1974. doi: 10.1016/j.watres.2006.03.014 [18] YAN M, KORSHIN G V. Comparative examination of effects of binding of different metals on chromophores of dissolved organic matter[J]. Environmental Science & Technology, 2014, 48(6): 3177-3185. [19] YAN M, BENEDETTI M F, KORSHIN G V. Study of iron and aluminum binding to Suwannee River fulvic acid using absorbance and fluorescence spectroscopy: Comparison of data interpretation based on NICA-Donnan and Stockholm humic models[J]. Water Research, 2013, 47(14): 5439-5446. doi: 10.1016/j.watres.2013.06.022 [20] YAN M, WANG D, KORSHIN G V, et al. Quantifying metal ions binding onto dissolved organic matter using log-transformed absorbance spectra[J]. Water Research, 2013, 47(7): 2603-2611. doi: 10.1016/j.watres.2013.02.044 [21] FENG C, BI Z, TANG H. Electrospray ionization time-of-flight mass spectrum analysis method of polyaluminum chloride flocculants[J]. Environmental Science & Technology, 2015, 49(1): 474-480. [22] HE Z, LAN H, GONG W, et al. Coagulation behaviors of aluminum salts towards fluoride: Significance of aluminum speciation and transformation[J]. Separation and Purification Technology, 2016, 165: 137-144. doi: 10.1016/j.seppur.2016.01.017 [23] WANG X, ZHANG G, FU X, et al. Aggregation and dissociation of aqueous Al13 induced by gluoride dubstitution[J]. Environmental Science & Technology, 2017, 51(11): 6279-6287. [24] HU C Y, LO S L, KUAN W H. Effects of the molar ratio of hydroxide and fluoride to Al(III) on fluoride removal by coagulation and electrocoagulation[J]. Journal of Colloid and Interface Science, 2005, 283(2): 472-476. doi: 10.1016/j.jcis.2004.09.045 [25] GONG W, QU J, LIU R, et al. Effect of aluminum fluoride complexation on fluoride removal by coagulation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 395: 88-93. [26] ZHANG X, WU Y, LIU G, et al. Investigation on sol–gel boehmite-AlOOH films on Kapton and their erosion resistance to atomic oxygen[J]. Thin Solid Films, 2008, 516(15): 5020-5026. doi: 10.1016/j.tsf.2008.01.023 [27] SAHOO N G, CHENG H K F, LI L, et al. Specific functionalization of carbon nanotubes for advanced polymer nanocomposites[J]. Advanced Functional Materials, 2009, 19(24): 3962-3971. doi: 10.1002/adfm.200901486 [28] WANG X, ZHANG G, LAN H, et al. Preparation of hollow Fe-Al binary metal oxyhydroxide for efficient aqueous fluoride removal[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 520: 580-589. [29] WANG X, XU H, WANG D. Mechanism of fluoride removal by AlCl3 and Al13: The role of aluminum speciation[J]. Journal of Hazardous Materials, 2020, 398: 122987. doi: 10.1016/j.jhazmat.2020.122987 -



 下载:
下载: