-
近年来,在污水和自然水体中频繁发现各种与人类生产生活息息相关、质量浓度在ng·L−1~μg·L−1的微量有机污染物被检出,这些微量有机物逐渐受到广泛关注[1-2]。微量有机物主要包括医药药物、个人护理品、农药、工业添加剂、内分泌干扰物以及饮用水消毒副产物等[3]。虽然污水和自然水体中微量有机物含量远低于氮磷等常规污染物,但由于其具有较大的毒性和更高的环境风险以及对人类可能存在的健康隐患而备受关注。因此,在水处理过程中对微量有机物的去除尤为重要。
作为水处理的常用工艺,微滤虽然可以有效去除浊度、悬浮颗粒物等污染物,但对于微量有机物的去除则非常有限。外加电场与膜分离工艺相结合,可以提高对微量有机物的去除效果。一方面,电场力作用可以使得水中的带电荷有机物发生定向迁移,减少或增加膜表面的微量有机物附着[4];另一方面,尤其是在沿海地区,因水中含有的较高浓度的氯离子,可在电极表面氧化生成活性氯组分,因而可氧化去除微量有机物[5]。
虽然很多研究者采用电化学结合膜过滤系统在处理微量有机物方面获得了优异的去除效果,但大多数报道主要将氧化过程作为关注点[6-7],而对于电场所具有的物理特性诸如电场力、电极吸附等作用以及分离膜本身对微量有机物的去除特点的研究较少。电场力对带电荷微量有机物的定向迁移作用可以减少出水中的微量有机物含量,提高整个系统的去除效果[8-9]。虽然微滤对于微量有机物去除效果有限,并且膜吸附去除作用集中于过滤前期,但研究这一过程对于考察膜本身对不同微量有机物的去除机理仍有意义。此外,很多研究者采用的微量有机物质量浓度(mg·L−1)远高于实际水中的质量浓度(ng·L−1~μg·L−1级),这样很容易忽视微滤吸附作用对整体去除效果的贡献。针对单一的目标有机物的去除研究虽然有利于解析其在电化学过程中的降解途径,但实验设定并不符合实际过程中多种低浓度微量有机物共存的复杂情况。目前也少有研究将微滤和电化学过程相结合用来同时去除低浓度的多种微量有机物。
因此,本研究选取实际污水处理厂二级出水中经常检出的5种微量有机物:舒必利、咖啡因、美托洛尔、利古隆以及卡马西平作为目标有机物[10-11],并对该污水处理厂二级出水取样3次,测得上述有机物的质量浓度分别为(387.1±8.7)、(35.8±1.7)、(442.2±47.4)、(14.9±3.0)和(151.0±27.2) ng·L−1。基于上述浓度水平,本研究采用500 ng·L−1作为进水浓度,用来考察微滤结合电场对微量有机物的处理效果。首先,探究了不同膜材料、酸碱度以及不同离子添加条件下微滤短期过滤对微量有机物的去除效果,解析了不同性质微量有机物的膜吸附机理;其次,通过改变电压和添加氯捕获剂来进一步了解单独电场、电化学氧化以及电极吸附作用对微量有机物的去除效果;最后,将微滤和电场整合在单一装置中,对比研究了电极和膜的空间位置关系对微量有机物去除效果的影响以及对膜表面可能存在的电化学侵蚀。
-
微量有机物:舒必利、美托洛尔、利古隆以及卡马西平购自德国Dr. Ehrenstorfer公司,1 g·L−1咖啡因标准品(溶解在1 mL甲醇溶液)购自上海安谱公司。微量有机物的物理化学性质见表1。固相萃取柱为Oasis HLB(500 mg)萃取柱,购自美国waters公司。实验所用电极均为2 mm厚的石墨毡,购自北京晶龙特碳石墨厂。实验采用的氯化钠、硫酸钠、氯化钙以及硫代硫酸钠等药剂均为分析纯。实验进水中5种微量有机物的含量均为500 ng·L−1,进水pH由盐酸或者氢氧化钠调节。
-
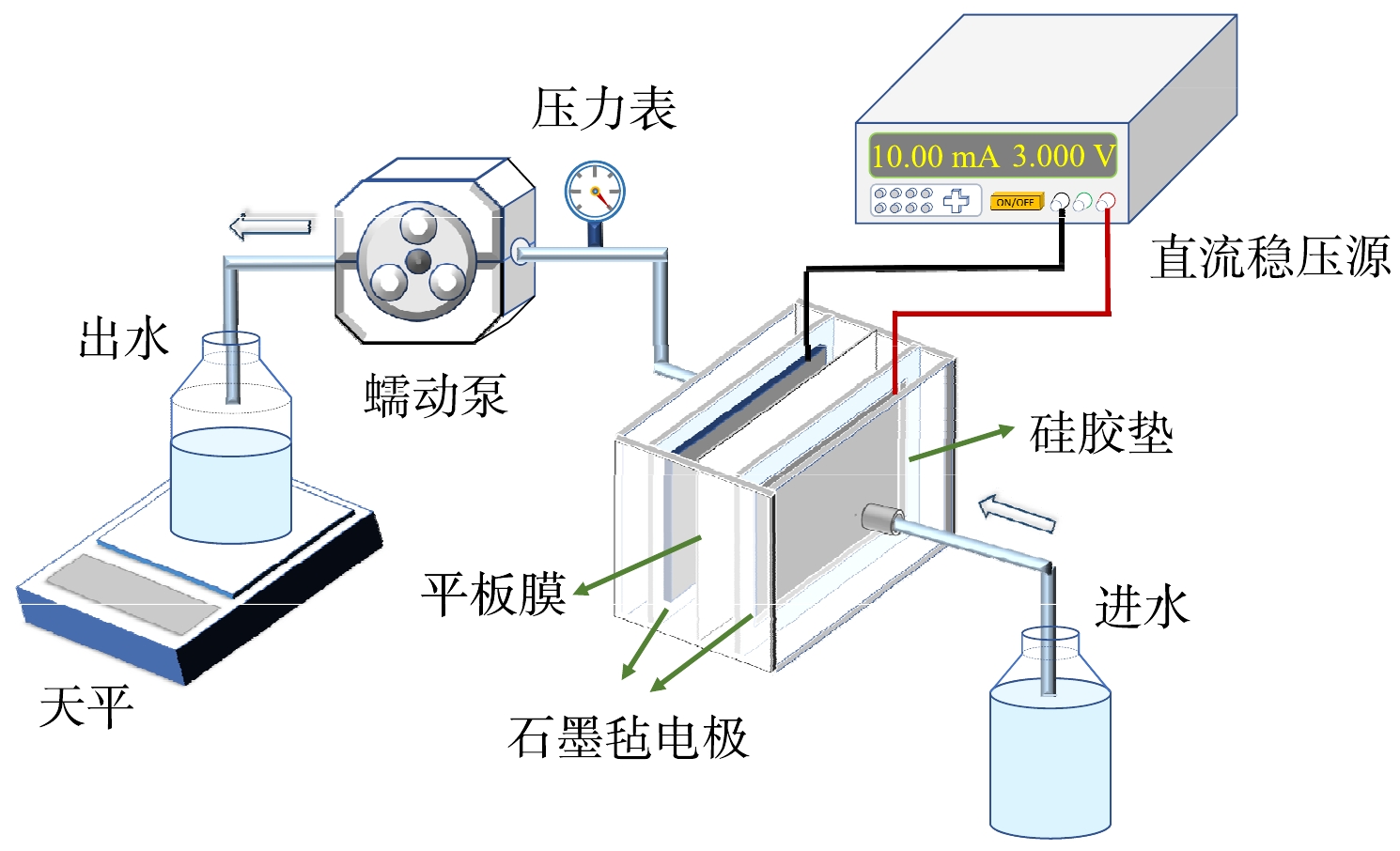
实验所采用的聚偏氟乙烯(polyvinylidene fluoride,PVDF)和聚醚砜(polyethersulfone,PES)材质的平板膜,孔径均为0.1 μm,分别购自日本东丽和美国科氏公司。微滤采用死端过滤,膜片位于进水和出水廊道之间,廊道尺寸为长100 mm,宽50 mm,廊道深度可通过插入不同厚度硅胶垫调节,组件的有效膜过滤面积为50 cm2 (图1)。电极材料为厚度2 mm石墨毡,阴阳电极距离保持在4 mm。实验在(25±1) °C的条件下进行,每组实验至少重复3次。实验之前,平板膜在超纯水中浸泡24 h,然后过滤超纯水直至通量稳定,用来去除膜表面附着的防腐剂等物质。膜分离装置通过蠕动泵负压恒压运行,跨膜压力为30 kPa,每次出水200 mL,通过天平以质量计,每次运行时间约为20 min,具体时长受不同膜片性能的微小差异而有所波动。单独电场实验采用与膜过滤相同的实验装置,添加阴阳电极,去掉微滤膜片,连续运行,运行时间与膜分离实验保持一致。
-
微量有机物检测:取200 mL水样,加入浓度500 mg·L−1的乙二胺四乙酸二钠混匀,放置1 h。然后用浓度为1 mol·L−1盐酸将水样的pH调至3左右,混匀后进行固相萃取。依次用5 mL乙腈、10 mL甲醇和5 mL超纯水(pH调至3左右)淋洗活化萃取柱,之后将水样以5 mL·min−1的流量通过萃取柱。结束后,用10 mL超纯水(pH调至3左右)淋洗萃取柱,再真空干燥萃取柱30 min。之后依次用3 mL和4 mL的0.5%甲酸乙腈溶液淋洗萃取柱,收集洗脱液并经过氮气流吹扫浓缩至干燥。将吹干的样品采用超高效液相色谱初始流动相配比(95%的0.3%甲酸水溶液+5%的乙腈)复溶并定容至1 mL,将定容后的样品混合均匀后,再经0.22 µm滤膜过滤后放置于−18 ℃冰箱中保存,采用超高效液相色谱-三重四级杆质谱UPLC-MS/MS(AQUITY TQD,Waters,美国)在正离子模式下检测,以体积分数为0.3%甲酸溶液为水相流动相,以乙腈为有机流动相,柱温设定为40 ℃。液相色谱柱型号为ACQUITY UPLC BEH C18色谱柱(1.7 μm,2.1 mm×100 mm)。
膜表面的Zeta电位通过SurPASS电动分析仪(Anton Paar GmbH,Graz,奥地利)检测。膜表面接触角由自动接触角测定仪(SL200KS,Kino,美国)检测。出水中的总氯浓度通过哈希试剂包显色经由哈希DR900便携式光度计比色得出。
-
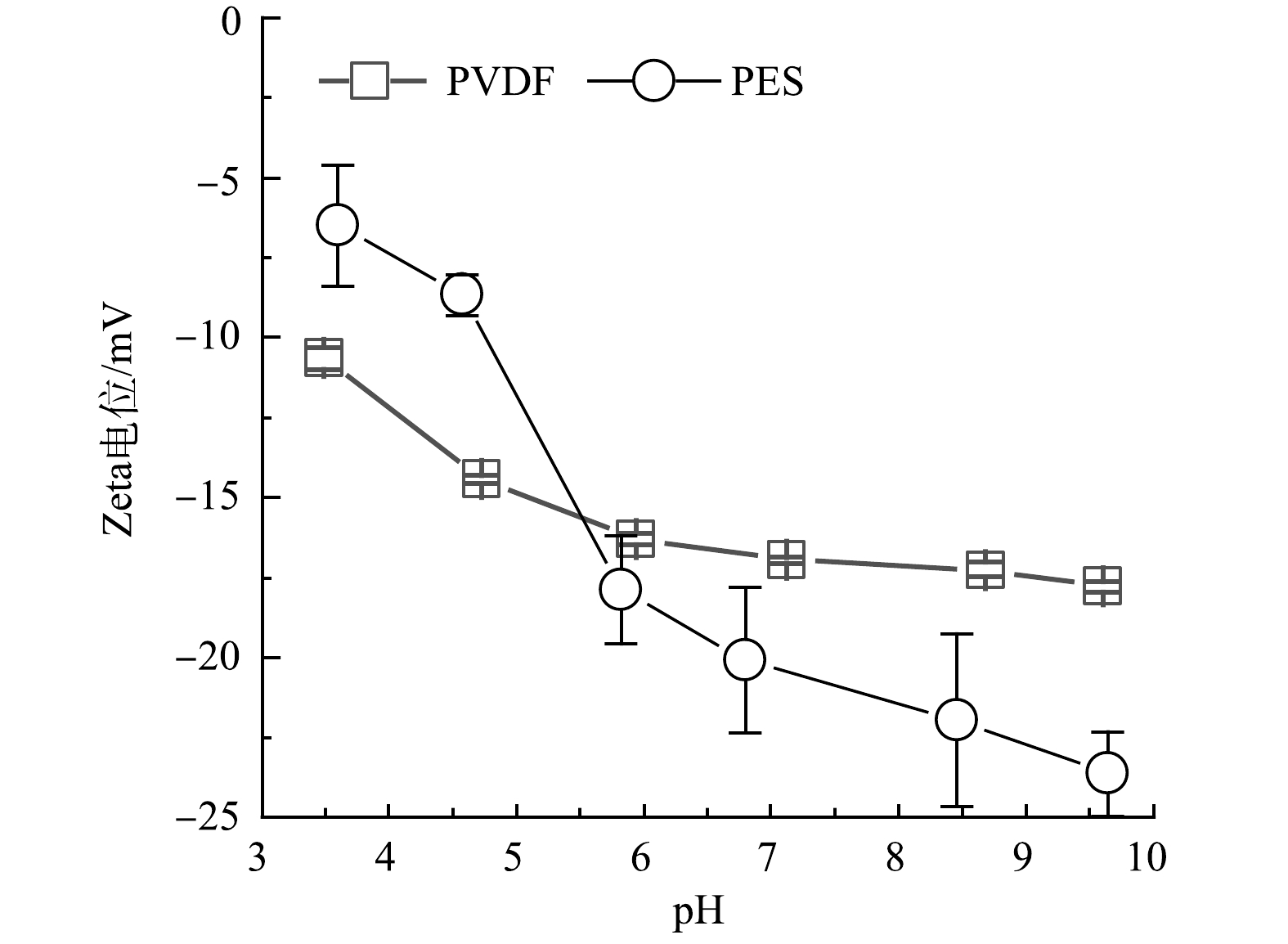
如图2所示,在PVDF和PES膜表面Zeta电位在pH为3~10,均为负值。这说明2种膜在使用过程中表面均带有负电荷。为了避免因膜表面电位不同而干扰微量有机物去除机理分析,本实验在pH为5.5的条件下进行,在此条件下2种膜表面的Zeta电位均接近−15 mV。虽然微滤膜孔径远大于微量有机物的分子质量,难以通过空间位阻效应拦截微量有机物,但依然有9.9%~62.3%的微量有机物被微滤去除(图3)。这说明在微滤膜分离过程中存在其他作用去除部分微量有机物。在反渗透或者纳滤中,微量有机物的去除机理主要包括空间位阻、电性相互作用以及疏水作用[12-13]。所以,本实验中电性作用和疏水性作用可能是微量有机物去除的主要机理。
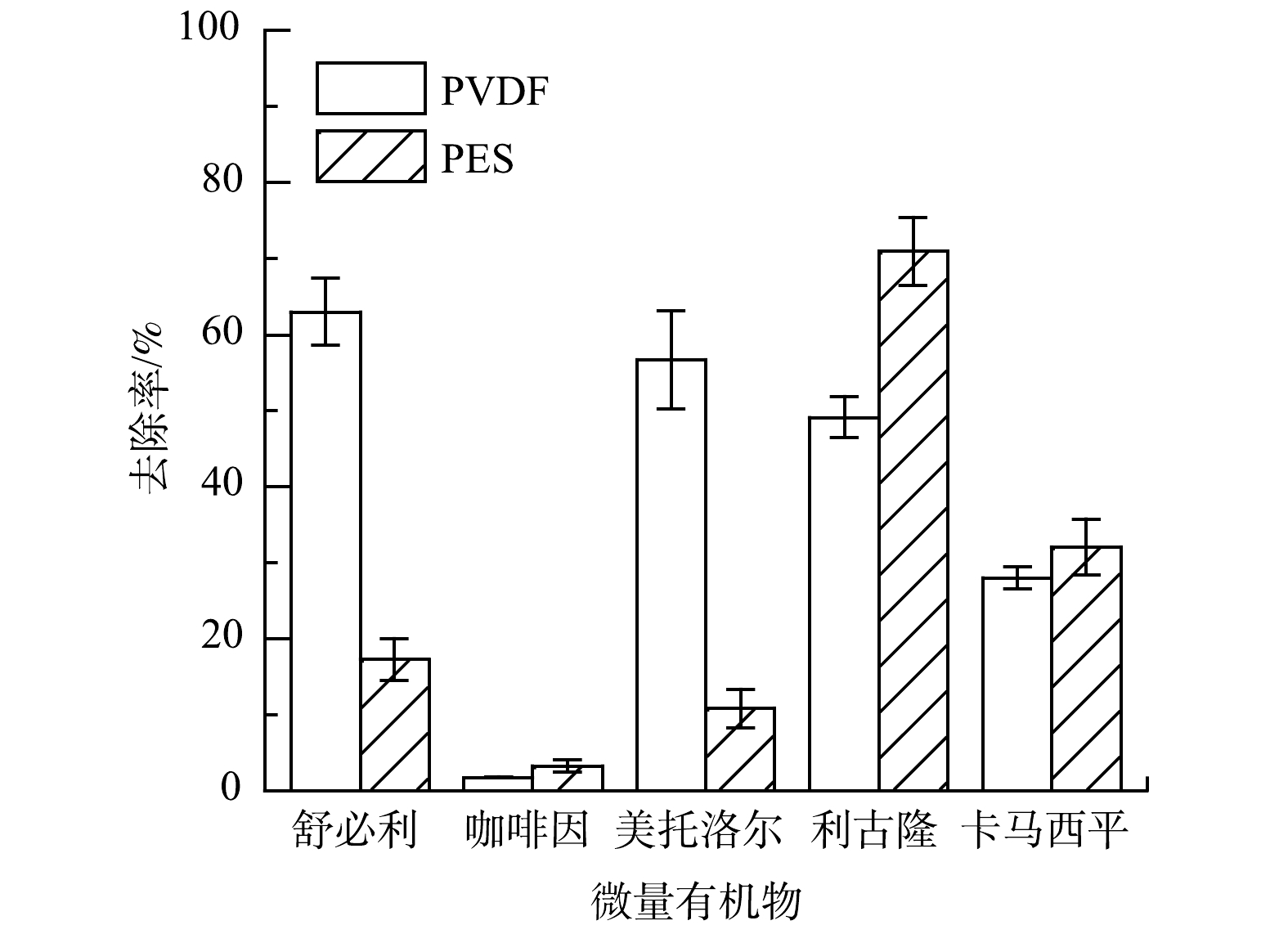
如图3所示,2种材料膜对不同微量有机物的去除效果存在明显差异。考虑到在本实验条件下2种材料膜片的Zeta电位相当,所以,可认为二者与微量有机物之间的电性作用类似。因此,2种膜材料的不同亲疏水性可能是造成微量有机物去除效果差异的主要原因。本实验检测了2种膜材料的接触角,用来分辨二者的亲疏水性。PVDF膜和PES膜的表面接触角分别为69.5°±2.4°和75.8°±3.9°,PVDF膜表面接触角明显小于PES膜表面的接触角。这说明PES材料的疏水性更强,也意味着PES膜表面与微量有机物的疏水作用更强。
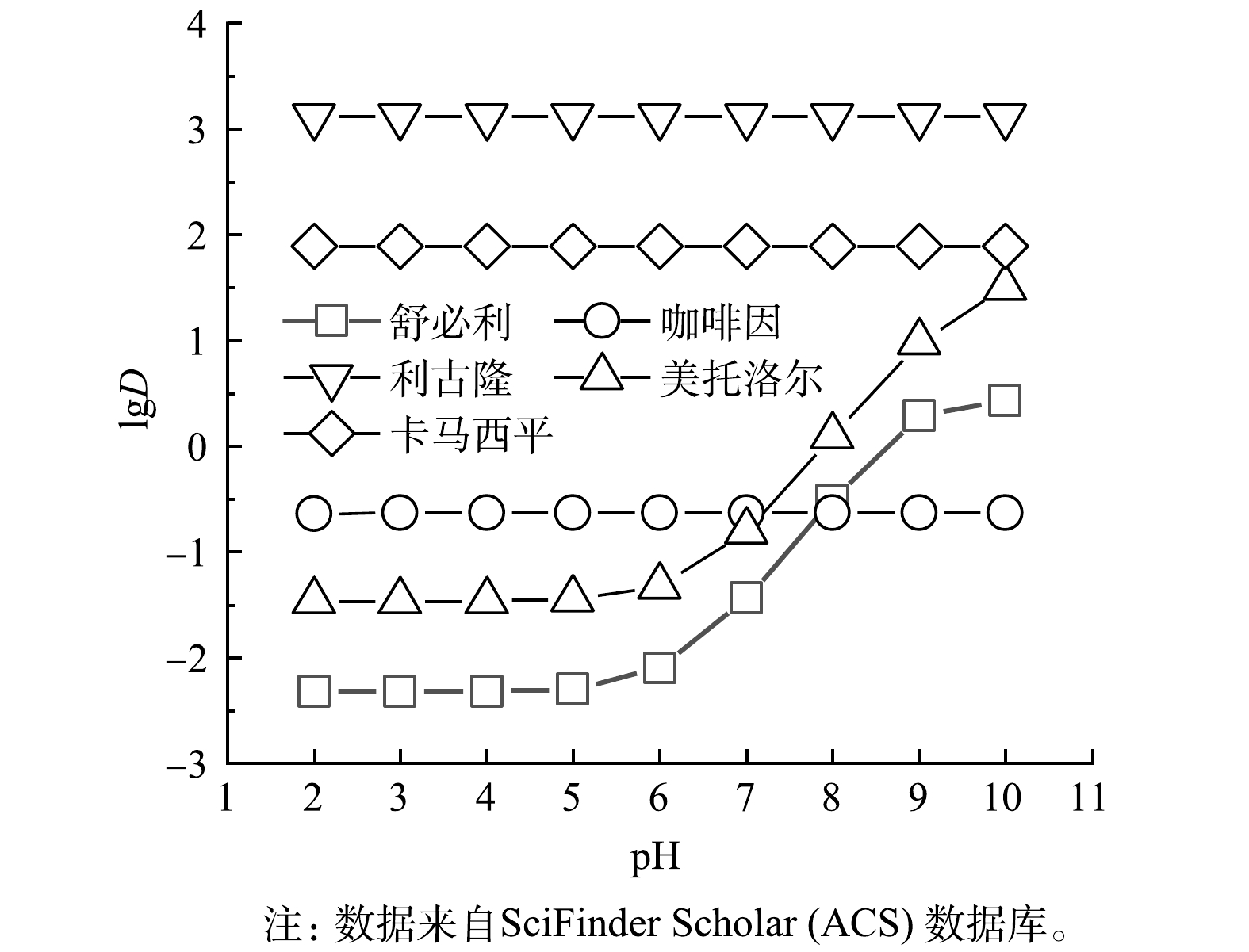
由图4可知,利古隆和卡马西平的正辛醇/水分配系数lgD在5种目标微量有机物中最高。这说明,此2种化合物的疏水性在5种物质中最强,而几乎不带有电荷。所以,相比于电性相互作用,可能疏水作用为这2种物质去除的主要机理。由图3可知,PES膜片对于这2种微量有机物的去除效果要明显优于PVDF膜片,而PES膜的疏水性又强于PVDF膜。这说明,疏水作用使得利古隆和卡马西平更易吸附在膜表面,因此,去除率也更高。而舒必利和美托洛尔的亲水性在这5种物质中最强,所以,疏水作用可能对这2种物质的去除贡献较少。但由表1可知,这2种物质在实验条件下明显带有正电荷,可以通过电性吸引作用吸附在带有负电荷的膜表面来实现去除。
由图3可知,即使PVDF和PES表面Zeta电位相当,舒必利和美托洛尔在PVDF膜过滤中明显去除效果更好。这说明,除了电性吸引作用和疏水作用,可能还存在其他去除机理。有研究[14-15]表明,微量有机物还可以与膜表面发生其他作用,例如形成氢键。DOLAR等[16]通过傅里叶红外光谱检测证实了亲水性微量有机物与膜表面会生成氢键。虽然PVDF中的F原子以及PES材料中的O原子均可以作为氢键受体,但F原子夺取电子的能力更强,因此,PVDF膜可能比PES膜更容易与微量有机物之间形成氢键。由表1可知,舒必利和美托洛尔分子中分别有3个和2个氢键供体,具备与膜表面形成氢键的条件,可使PVDF膜对微量有机物更好的去除效果。在5种微量有机物中,咖啡因的去除率最低。这是因为:一方面,咖啡因是亲水性分子,与膜表面的疏水作用弱,而且不带有电荷,很难通过电性相互作用去除;另一方面,咖啡因分子上没有氢键供体,故不能与膜表面形成氢键。
-
根据以上实验结果,选取对微量有机物整体去除率更好的PVDF微滤膜作为接下来实验的主要膜材料。由于在以上实验中未能体现电性相互作用在微量有机物去除过程中的贡献,而水中常见无机离子可以吸附在膜表面从而影响其Zeta电位[17]。为了更进一步分析电性相互作用对于不同微量有机物微滤去除的影响,本实验选取常见的氯化钠(NaCl)、硫酸钠(Na2SO4)以及氯化钙(CaCl2)作为背景电解质,采用0、2 000、5 000 mg·L−1浓度梯度,在pH为5.5±0.2时进行实验,探究了不同无机盐对微量有机物去除的影响,以揭示电性相互作用对微量有机物去除的影响。
无机盐的种类和浓度对微量有机物去除效果的影响有明显的差异(图5)。有研究[18]表明,随着无机盐浓度的提高,电性屏蔽作用会导致膜表面Zeta电位下降。对比图5(a)和图5(b)可以看出,氯化钠和硫酸钠对于微量有机物去除率的影响类似。这说明阴离子对微量有机物去除的影响可能有限,而阳离子才是影响微量有机物去除的主要因素。阳离子的电性屏蔽作用可能导致膜表面对微量有机物的电性吸附能力降低。由于疏水性相互作用可能是利古隆和卡马西平去除的主要机理,膜表面的电位变化对于这2种物质的去除影响不明显,因此,在硫酸钠和氯化钠浓度变化时,这2种物质的去除率基本不变,这也与GUR-REZNIK等[19]的研究结果相符。而咖啡因的去除率本来就很低,所以无机盐浓度及种类对其去除率的影响并不明显。舒必利和美托洛尔则正好相反,随着盐浓度的提高,舒必利和美托洛尔的去除率则明显下降。
对比图5(a)和图5(c) 可以看出,钙离子对微量有机物去除的影响明显高于钠离子。由于钙离子带有2个电荷,同时其离子半径小于钠离子[20],因此,钙离子相比钠离子更容易吸附在膜表面,降低膜表面的Zeta电位,从而降低膜表面与微量有机物的静电引力。在钙离子存在的情况下,利古隆和卡马西平的去除率也呈现出了下降的趋势,这可能是由于大量钙离子吸附在膜表面,干扰了利古隆以及卡马西平与膜表面的疏水性相互作用。对于舒必利和美托洛尔,钙离子的影响则更为明显。随着钙离子浓度的升高,这2种微量有机物的去除率由60%左右降到10%以下。这说明,钙离子在膜表面的吸附会明显降低膜表面负电荷量,从而抑制膜表面对这2种有机物的吸附作用。由此可见,电性吸引作用是带正电荷微量有机物去除的重要机理。
-
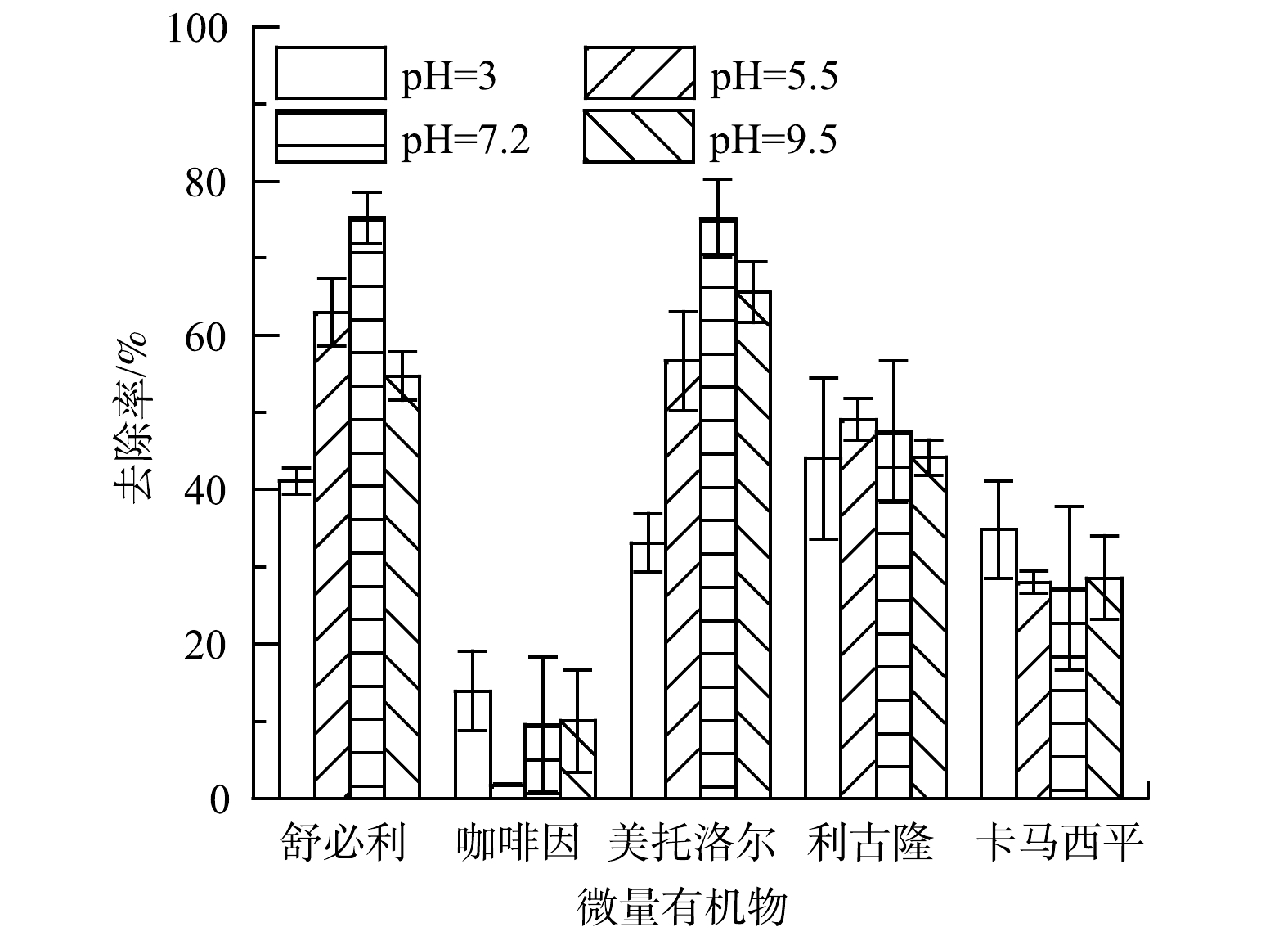
除了膜材料以及电解质种类和浓度,进水pH同样也会影响微量有机物的去除效果。进水pH的变化不仅会引起膜表面Zeta电位的变化,还会影响到微量有机物的亲疏水性和所带电荷,从而可能导致膜表面与微量有机物的相互作用发生改变。所以,调节进水pH分别为3、5.5、7.2以及9.5,采用PVDF膜进行了膜过滤实验。
由图6可以看出,pH的变化对舒必利和美托洛尔去除率影响明显,且表现出一定的趋势,而对利古隆和卡马西平去除率的影响并不明显。由图3可以看出,利古隆和卡马西平的lgD不随pH变化而变化。这说明,这2种物质具有恒定的亲疏水性,因此,这2种物质与膜表面的疏水性相互作用可能不会受到pH变化的影响。需要指出的是,卡马西平在酸性条件下可能会带有正电荷,所以,电性吸引作用也会对卡马西平的去除作出贡献。由表1可看出,在pH 3~7.2时,舒必利和美托洛尔均带有正电荷。而膜表面带有的负电荷量随pH的升高而升高,可知这2种微量有机物与膜表面的电性吸引作用逐渐增强,所以其去除率也逐渐提高。LIU等[21]也提出,带正电荷的微量有机物的去除主要依赖于电性吸引作用。而当pH为9.5时,舒必利和美托洛尔带有负电荷,与膜表面的电性吸引作用变为电性排斥作用。由于微量有机物分子量远小于微滤膜孔径,所以,电性排斥作用很难拦截微量有机物。但是,从图4中可看出,碱性条件下这2种微量有机物疏水性显著提高,与膜表面的疏水性相互作用也会增强,也能实现对微量有机物的吸附去除。因此,在电性吸引作用减弱,疏水性相互作用增强的情况下,微量有机物的去除率没有明显下降,甚至其去除率高于酸性条件下的去除率。
-
为了研究单一电场对于微量有机物的去除效果,采用与膜过滤相同的实验装置,添加阴阳电极,去掉微滤膜片,并且保持进水在装置中的停留时间一致,在pH为7.0±0.2条件下进行实验。由图7(a)可以看出,随着外加电压的升高,微量有机物的去除率也随之逐渐升高,可以将微量有机物的去除率由4.1%~21.7%提高到3 V电压下的44.2%~82.8%。当电压由0升高到1 V时,除卡马西平外,其他微量有机物去除率均有提升。这可能是由于:电场力的作用使得带电荷微量有机物倾向于留在电场中,同时,电极表面带有的电荷也可以与微量有机物产生电性吸引作用,所以,相应微量有机物的去除率得到了提高。对于不带电荷的微量有机物,电场的极化作用会使得电荷在有机物分子上不均匀分布,使得原本呈现电中性的有机物呈现出带电荷有机物的性质[22],从而强化电场对微量有机物的去除效果。当电压继续升高到2 V,在电场中开始发生电化学反应后,微量有机物的去除率明显升高。
由图7(b)可以看出,在电压为2 V和3 V情况下,在装置的出水中检测到有总氯生成。氯作为氧化剂可以提高微量有机物的去除效果[23],尤其是对咖啡因、美托洛尔以及卡马西平而言。总氯的生成说明进水中含有的氯离子在阳极表面被氧化,主要反应如式(1)~式(3)所示。
为了探究总氯以及电场力在微量有机物去除过程中的作用,在进水中加入氯捕获剂硫代硫酸钠来抑制氯对微量有机物的氧化,在2 V和3 V电压下进行与之前的实验结果进行了对比。由图7(a)可以看出,外加硫代硫酸钠之后,所有微量有机物的去除率出现不同程度的下降。此外,由图7(b)可以看出,添加硫代硫酸钠后出水中未检出总氯。这说明,添加捕获剂后装置中已不存在总氯,从而也证实了总氯对微量有机物的去除有一定贡献。在添加氯捕获剂后,虽然微量有机物的除率有所下降,但在没有氧化剂存在的条件下,微量有机物的去除率仍然随着电压的升高而升高,说明电场对微量有机物的去除也起到重要作用。此外,由于0 V电压下的微量有机物主要通过电极吸附去除,3 V电压下微量有机物主要通过电极吸附、电场以及电化学氧化的共同作用去除,而3 V电压下添加氯捕获剂后微量有机物主要通过电极吸附以及电场共同作用去除,故据此对3 V电压下的微量有机物的去除贡献进行了分析(图7(c))。
为了进一步探究单独电场作用对微量有机物去除的影响,在进水中加入过量氯捕获剂进行连续进出水实验,运行20 min后切断电源,连续监测出水中微量有机物的含量。由图7(d)可知,外加3 V电压时,出水中微量有机物相比进水明显下降。其原因为:一方面,电极表面带有电荷可以吸引带有相反电荷的微量有机物;另一方面,阴阳极之间的距离只有4 mm,形成的电场较强,使得微量有机物可能会发生极化,从而强化电极对微量有机物的吸引作用。以上2种去除作用均依赖于电场的存在。当切断电源后,这2种作用消失,微量有机物可能又会被释放回水中,造成出水浓度高于进水的现象。但图7(d)表明,只有美托洛尔出水浓度高于了进水浓度,其余物质均不存在出水浓度高于进水的情况。这说明,外加电场除了通过电性吸引作用提高微量有机物的去除率,同时还有可能通过改变电极性质增强电极本身的吸附效果[24]。同时,在电场力的作用下,微量有机物的定向迁移使得电极附近微量有机物浓度升高,降低了传质阻力,从而也有利于提高电极的吸附效果。
-
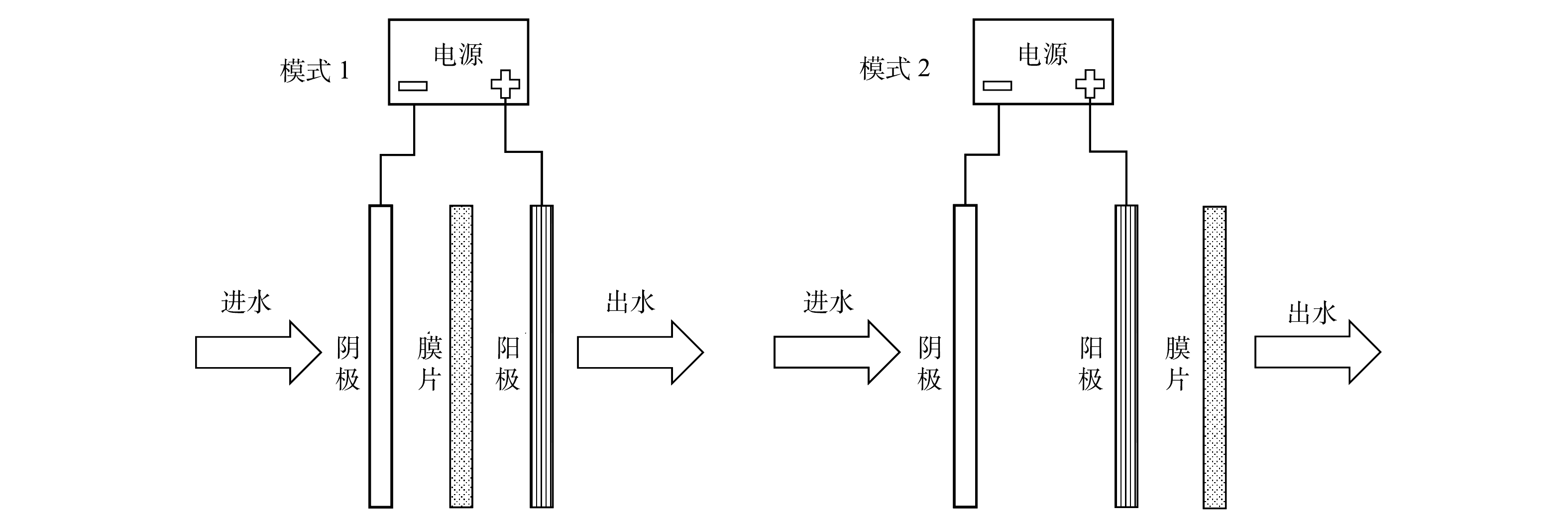
将微滤和电场相结合,以2 000 mg·L−1的氯化钠为背景电解质,在pH为7.0±0.2的条件下进行了实验。实验分为2种模式,根据膜与电极之间的空间位置关系,分为模式1和模式2(图8)。模式1是将膜片置于阴阳极之间,进水依次通过阴极、膜片以及阳极;而模式2是不改变模式1中阴阳极位置,将膜片置于阳极之后,进水依次通过阴极、阳极以及膜片。
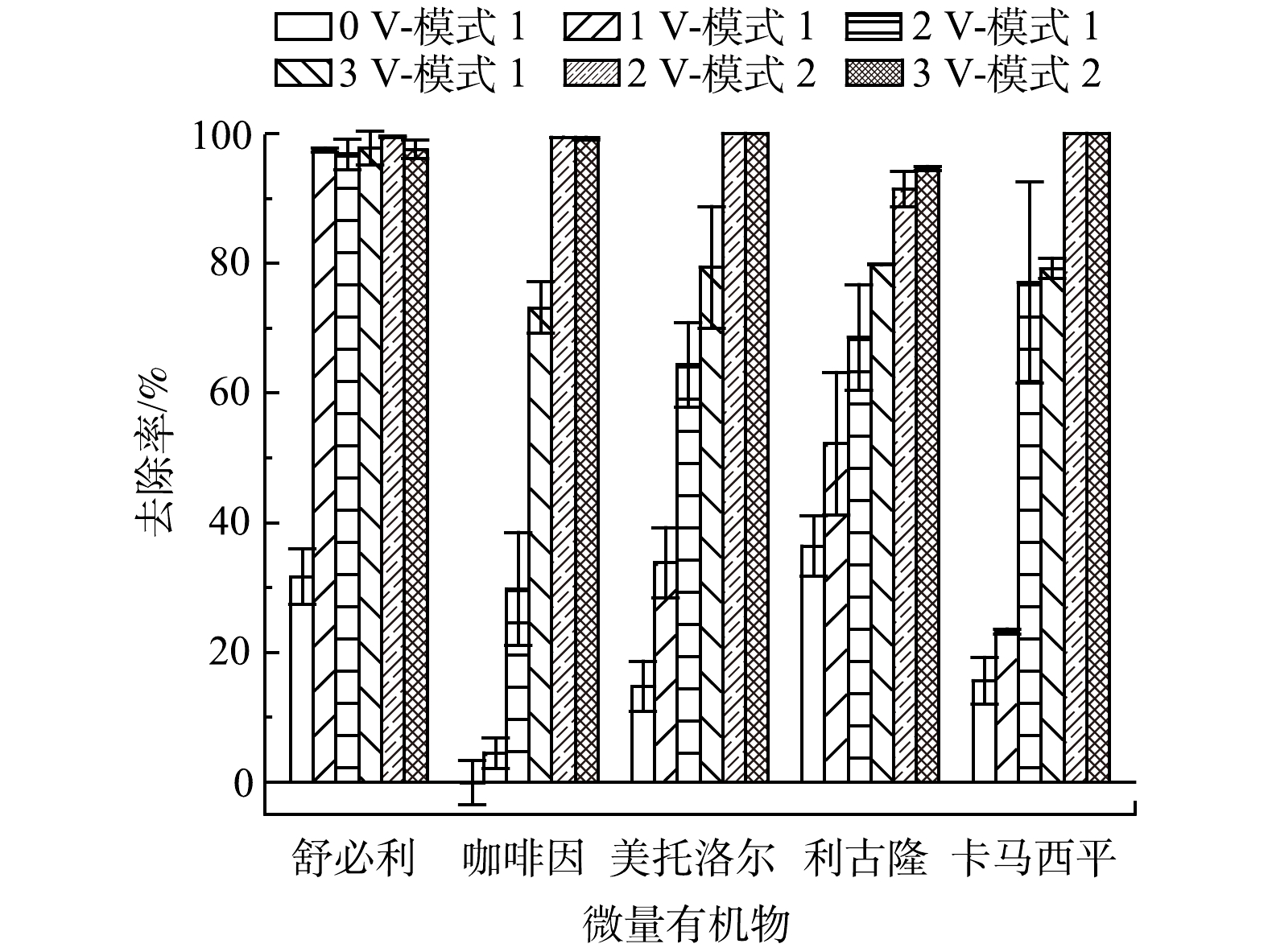
由图9可看出,模式1未加电压时微量有机物去除率均低于40%;而在外加电压后,微量有机物的去除效果优于单一微滤和电场的去除效果,尤其是舒必利,外加1 V电压下去除率就接近100%。这说明微滤和电场结合可以有效提高微量有机物的去除率。由于电场使得带电以及电场中发生极化的非带电微量有机物在场中实现定向迁移,导致微量有机物被保留在电场中,可以达到更久的停留时间,使得微量有机物与膜的接触时间变长,有利于微量有机物和膜表面之间的电性吸引作用和疏水性相互作用,故可增强微量有机物的去除效果。
随着电压的提高,阳极表面电解氧化氯离子会产生总氯。一方面,总氯可以直接氧化目标有机物;另一方面,由于电场对微量有机物的保留作用增加了氧化剂和微量有机物的接触时间,提高了微量有机物的氧化去除效果。由于外加1 V时微量有机物的去除效果明显低于2 V和3 V条件下的去除率,所以模式2只在2 V和3 V情况下运行。由图9可以看出,模式2下微量有机物的去除效果明显优于模式1,去除率均高于90%。模式1下,膜片放置于阴阳极之间,由于膜本身是绝缘体,所以可能会抑制电场对微量有机物的去除作用[25]。绝缘膜片位于电场中,一方面会降低电场力对微量有机物的迁移作用,另一方面则增大了阴阳极之间的传质阻力,可能会降低氯离子的氧化速率和氧化剂的生成。而在模式2条件下,膜片位于电场外侧,电场对微量有机物的保留能力此时很难促进膜对微量有机物的去除,但阳极生成的总氯可以实现与膜的有效接触,从而氧化去除膜表面吸附的微量有机物。然而,这种空间位置关系也增加了膜表面被氧化剂侵蚀的隐患和风险。在模式2模式下,水流先通过阳极之后再经过膜,这样就会增加阳极生成的总氯与膜表面的接触时间,造成膜片的侵蚀,减少膜的使用寿命[26]。对于模式1来说,虽然微量有机物的去除效果不如模式2,但可以具有相对更长的使用寿命。
-
1)单独微滤过程中,利古隆和卡马西平这2种疏水性有机物主要通过与膜表面的疏水相互作用去除,pH和溶液中的无机盐对其去除几乎没有影响。带正电荷的微量有机物舒必利和美托洛尔与膜表面存在电性吸引作用,pH和溶液中的无机盐可以通过改变膜表面Zeta电位以及微量有机物亲疏水性来影响其去除。微量有机物还可能与膜表面形成氢键,从而提高其去除率。
2)电场去除微量有机物的作用包含2个方面:一方面是带电荷微量有机物受电场力作用的定向迁移,可促进电极对微量有机物的吸附;另一方面是电极氧化氯离子生成的总氯导致微量有机物氧化去除。在3 V电压下,微量有机物的去除率可以达到44.2%~82.8%。
3)电场结合微滤可以明显提高微量有机物的去除效果。在外加电压为3 V时,在进水依次通过阴极、阳极以及膜片的模式下,可以实现对微量有机物90%以上的去除,但因生成的总氯对膜表面造成侵蚀,因而可能会减少膜的使用寿命。
电场强化微滤工艺去除水中微量有机物的效果
Removal of trace organic compounds in water by an electric field enhanced microfiltration
-
摘要: 微滤工艺虽然可以有效去除浊度、悬浮颗粒物等污染物,但对于微量有机物的去除则非常有限。外加电场与膜分离相结合,可以提高对微量有机物的去除效果。为此,研究了单独微滤、单独电场以及电场结合微滤3种工艺对水中5种典型低浓度微量有机物(舒必利、咖啡因、美托洛尔、利古隆、卡马西平)的去除效果;并考察了不同膜材料、进水中离子种类、进水离子浓度以及pH条件对微量有机物去除效果的影响。结果表明,微滤主要通过吸附作用去除水中微量有机物,吸附机理主要包括疏水性相互作用和静电引力作用,分别对应去除疏水性微量有机物(利古隆和卡马西平)和带正电荷微量有机物(舒必利和美托洛尔),而且微量有机物还可能通过与微滤膜表面形成的氢键,被吸附在膜表面。在单独电场中,电场力可以促进微量有机物在电极上的吸附,同时阳极上生成的总氯氧化剂也能强化微量有机物的去除,在3 V电压下微量有机物的去除率可以达到44.2%~82.8%。电场结合微滤可以明显提高微量有机物的去除效果。在外加电压为3 V时,在进水依次通过阴极、阳极以及膜片的模式下,可以实现对微量有机物90%以上的去除率,但阳极生成的总氯对膜表面造成的侵蚀可能会减少膜的使用寿命。Abstract: Although microfiltration can effectively remove turbidity and suspended particulate matters, its trace organic compounds removal was very limited. The combination of applied electric field and microfiltration could improve the removal effect of trace organic compounds. The removal of five typical low concentration trace organic compounds (sulpiride, caffeine, metoprolol, linuron, and carbamazepine) in water by three processes of sole microfiltration, sole electric field, and electric field combined microfiltration were investigated. The effects of membrane material, ion species, ion concentration, and pH in the feed water on the removal of trace organic compounds were studied. The results showed that membrane adsorption was the main approach for trace organic compounds removal. The adsorption mechanism included hydrophobic interaction and electrostatic attraction, which corresponded to the removal of hydrophobic trace organic compounds (linuron and carbamazepine) and positively charged trace organic compounds (sulpiride and metoprolol), respectively. Moreover, the formation of hydrogen bonds between trace organic compounds and the membrane surface could enhance the adsorption of trace organic compounds on the membrane. In the sole electric field, the electric field force promoted the adsorption of trace organic compounds on the electrodes, and the chlorine generated on the anode could also strengthen the removal of trace organic compounds. At 3 V, the removal rate of trace organic compounds was 44.2%~82.8%. Electric field combined with microfiltration could significantly improve the removal of trace organic compounds. In the mode of the feed water successively passed through the cathode, anode, and membrane, and over 90% removal rate of trace organic compounds occurred when 3 V voltage was applied. However, the corrosion on the membrane surface by chlorine produced on anode might reduce the service life of the membrane.
-
Key words:
- microfiltration /
- electric field /
- trace organic compounds /
- water treatment
-

-
表 1 目标微量有机物的基本信息及物理化学性质
Table 1. Basic information and physicochemical properties of the target TrOCs
微量有机物 分子式 分子质量/Da pKa(25 °C)1) 氢键
供体1)酸性 碱性 舒必利 C15H23N3O4S 341.43 9.98±0.0 8.97±0.50 3 咖啡因 C8H10N4O2 194.19 — 0.52±0.70 0 美托洛尔 C15H25NO3 267.36 13.89±0.20 9.43±0.10 2 利古隆 C9H10Cl2N2O2 249.09 12.13±0.70 −1.04±0.50 1 卡马西平 C15H12N2O 236.27 13.94±0.20 −0.49±0.20 2 注:1)表示pKa以及氢键供体数据来自SciFinder Scholar (ACS)数据库。 -
[1] DU Y J, XU X, LIU Q Z, et al. Identification of organic pollutants with potential ecological and health risks in aquatic environments: Progress and challenges[J]. Science of the Total Environment, 2022, 806: 150691. doi: 10.1016/j.scitotenv.2021.150691 [2] SAIDULU D, GUPTA B, GUPTA A K, et al. A review on occurrences, eco-toxic effects, and remediation of emerging contaminants from wastewater: Special emphasis on biological treatment-based hybrid systems[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105282. doi: 10.1016/j.jece.2021.105282 [3] ROUT P R, ZHANG T C, BHUNIA P, et al. Treatment technologies for emerging contaminants in wastewater treatment plants: A review[J]. Science of the Total Environment, 2021, 753: 141990. doi: 10.1016/j.scitotenv.2020.141990 [4] LI C, ZHANG M H, SONG C W, et al. Enhanced treatment ability of membrane technology by integrating an electric field for dye wastewater treatment: A review[J]. Journal of AOAC International, 2018, 101(5): 1341-1352. doi: 10.5740/jaoacint.18-0050 [5] GANIYU S O, VAN HULLEBUSCH E D, CRETIN M, et al. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review[J]. Separation and Purification Technology, 2015, 156: 891-914. doi: 10.1016/j.seppur.2015.09.059 [6] 孙继成, 吴志超, 王志伟, 等. 电化学耦合膜工艺去除饮用水中卡马西平[J]. 中国环境科学, 2018, 38(1): 193-201. doi: 10.3969/j.issn.1000-6923.2018.01.023 [7] ZHENG J J, MA J J, WANG Z W, et al. Contaminant removal from source waters using cathodic electrochemical membrane filtration: Mechanisms and implications[J]. Environmental Science & Technology, 2017, 51(5): 2757-2765. [8] ENSANO B M B, BOREA L, NADDEO V, et al. Control of emerging contaminants by the combination of electrochemical processes and membrane bioreactors[J]. Environmental Science and Pollution Research, 2019, 26: 1103-1112. doi: 10.1007/s11356-017-9097-z [9] CHEN X, DENG H P. Effects of electric fields on the removal of ultraviolet filters by ultrafiltration membranes[J]. Journal of Colloid and Interface Science, 2013, 393: 429-437. doi: 10.1016/j.jcis.2012.10.055 [10] TRAN N H, REINHARD M, GIN K Y. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions: A review[J]. Water Research, 2018, 133: 182-207. doi: 10.1016/j.watres.2017.12.029 [11] BEXFIELD L M, BELITZ K, LINDSEY B D, et al. Pesticides and pesticide degradates in groundwater used for public supply across the United States: Occurrence and human-health context[J]. Environmental Science & Technology, 2021, 55(1): 362-372. [12] NGHIEM L D, SCHÄFER A I, ELIMELECH M. Pharmaceutical retention mechanisms by nanofiltration membranes[J]. Environmental Science & Technology, 2005, 39(19): 7698-7705. [13] SHAHID M K, KASHIF A, FUWAD A, et al. Current advances in treatment technologies for removal of emerging contaminants from water: A critical review[J]. Coordination Chemistry Reviews, 2021, 442: 213993. doi: 10.1016/j.ccr.2021.213993 [14] ZHAO Y Y, KONG F X, WANG Z, et al. Role of membrane and compound properties in affecting the rejection of pharmaceuticals by different RO/NF membranes[J]. Frontiers of Environmental Science & Engineering, 2017, 11(6): 20. [15] FUJIOKA T, KODAMATANI H, WANG Y J, et al. Assessing the passage of small pesticides through reverse osmosis membranes[J]. Journal of Membrane Science, 2020, 595: 117577. doi: 10.1016/j.memsci.2019.117577 [16] DOLAR D, DRAŠINAC N, KOŠUTIČ K, et al. Adsorption of hydrophilic and hydrophobic pharmaceuticals on RO/NF membranes: Identification of interactions using FTIR[J]. Journal of Applied Polymer Science, 2017, 134(5): 44426. [17] PINO-SOTO L, SCHWARZ A, VARGAS C, et al. Influence of multivalent-electrolyte metal solutions on the superficial properties and performance of a polyamide nanofiltration membrane[J]. Separation and Purification Technology, 2021, 272: 118846. doi: 10.1016/j.seppur.2021.118846 [18] HE Z W, KASEMSET S, KIRSCHNER A Y, et al. The effects of salt concentration and foulant surface charge on hydrocarbon fouling of a poly(vinylidene fluoride) microfiltration membrane[J]. Water Research, 2017, 117: 230-241. doi: 10.1016/j.watres.2017.03.051 [19] GUR-REZNIK S, KOREN-MENASHE I, HELLER-GROSSMAN L, et al. Influence of seasonal and operating conditions on the rejection of pharmaceutical active compounds by RO and NF membranes[J]. Desalination, 2011, 277(1/2/3): 250-256. doi: 10.1016/j.desal.2011.04.029 [20] SUGIURA Y, SAITO Y, ENDO T, et al. Effect of the ionic radius of alkali metal ions on octacalcium phosphate formation via different substitution modes[J]. Crystal Growth & Design, 2019, 19(7): 4162-4171. [21] LIU Y L, WANG X M, YANG H W, et al. Quantifying the influence of solute-membrane interactions on adsorption and rejection of pharmaceuticals by NF/RO membranes[J]. Journal of Membrane Science, 2018, 551: 37-46. doi: 10.1016/j.memsci.2018.01.035 [22] SUN J Q, HU C Z, TONG T Z, et al. Performance and mechanisms of ultrafiltration membrane fouling mitigation by coupling coagulation and applied electric field in a novel electrocoagulation membrane reactor[J]. Environmental Science & Technology, 2017, 51(15): 8544-8551. [23] RYAN D R, MAHER E K, HEFFRON J, et al. Electrocoagulation-electrooxidation for mitigating trace organic compounds in model drinking water sources[J]. Chemosphere, 2021, 273: 129377. doi: 10.1016/j.chemosphere.2020.129377 [24] SEO H, SON M, SHIN I, et al. Faster dye-adsorption of dye-sensitized solar cells by applying an electric field[J]. Electrochimica Acta, 2010, 55(13): 4120-4123. doi: 10.1016/j.electacta.2010.02.076 [25] DUDCHENKO A V, ROLF J, RUSSELL K, et al. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes[J]. Journal of Membrane Science, 2014, 468: 1-10. doi: 10.1016/j.memsci.2014.05.041 [26] RABILLER-BAUDRY M, LOULERGUE P, GIRARD J, et al. Consequences of membrane aging on real or misleading evaluation of membrane cleaning by flux measurements[J]. Separation and Purification Technology, 2021, 259: 118044. doi: 10.1016/j.seppur.2020.118044 -



 下载:
下载: