-
随着全球能源危机和环境污染威胁的日益加剧,寻找一种高效且无公害的环境治理技术是目前环境领域研究的热点[1-2]。自从1972年Fujishima首次发现在TiO2电极上水的光电化学反应将水分解为氢气和氧气以来,半导体光催化技术就以其绿色、可持续发展的优点引起了人们的广泛关注[3]。
在半导体光催化剂中,SrTiO3因为具有高化学稳定性和丰富的组成元素,被认为是一种很有前景的光催化剂[4]。此外,SrTiO3的导带和价带边缘具有较高的光化学稳定性和良好的生物相容性[5]。但由于SrTiO3具有较宽的禁带宽度(约为3.2 eV),导致其光吸收范围仅限于紫外光波段,严重降低了SrTiO3对太阳光的利用效率。此外,光生电子-空穴对复合速率快,量子效率低也是限制SrTiO3光催化性能的重要因素[6]。为了提高SrTiO3的光催化性能,科学研究人员开发了各种改性方法[7-9]。Qazi等[10]发现掺杂Cu后的SrTiO3,Cu+取代了Sr+,SrTiO3的带隙宽度从3.2 eV降低到2.96 eV,掺杂后的SrTiO3光催化活性大大提高。Atkinson等[11]通过溶胶-凝胶法合成了氮(N)掺杂的SrTiO3粉末,研究表明,由于结构、组织和形貌的协同作用,N掺杂使SrTiO3的带隙减小了1 eV左右。此外,利用S、Cr、Er和C3N4等多种材料,也提高了SrTiO3的光催化活性和化学稳定性[12-15]。目前还没有关于钨(W)和银(Ag)共掺杂SrTiO3的报道。
本文采用溶胶-凝胶法制备了W-Ag共掺杂SrTiO3纳米复合材料,以亚甲基蓝为目标污染物探究其光催化活性。并采用多种表征手段,探究W-Ag共掺杂SrTiO3样品光催化效率提升的机制。
-
酒石酸(C4H6O6),碳酸锶(SrCO3),钛酸丁酯(C16H36O4Ti),钨酸铵((NH4)10W12O41-xH2O),硝酸银(AgNO3),亚甲基蓝(MB)。所有试剂在本实验中均为分析级纯,无需进一步纯化。实验用水为蒸馏水。
-
溶胶凝胶法制备纯SrTiO3和W-Ag共掺杂SrTiO3粉末:取1.47 g碳酸锶与适量的酒石酸混合,加入20 mL蒸馏水,在水浴温度70 ℃的情况下均匀搅拌30 min,形成清澈透明的均匀溶液A。在磁力搅拌器持续搅拌的情况下,向溶液A中缓慢滴加3.4 mL钛酸丁酯,继续搅拌,直至形成透明的均质溶胶。将溶胶置于坩埚中,在烘箱中80 ℃的情况下烘干10 h,制得干凝胶。再将干凝胶置于马弗炉中,在700 ℃下煅烧2 h,冷却至室温,研磨后就制得了纯SrTiO3粉末。制备掺杂的SrTiO3粉末与上述步骤基本类似,只需在加入钛酸丁酯之前加入所需的钨酸铵和硝酸银试剂,其它步骤不变,就制备出了W-Ag共掺杂SrTiO3粉末。实验结果表明,SrTiO3中W和Ag的最佳摩尔比分别为2%和0.5%,此时样品光催化效果最佳。
-
使用D8 ADVANCE型X射线衍射仪表征SrTiO3材料的结晶性质。用扫描电子显微镜(SEM,ZEISS,Supra55)在200 kV下观察样品的结构形态。通过HITACHI的F-7000获得室温光致发光光谱(PL)。在Shimadzu UV-2550分光光度计上记录UV-Vis漫反射光谱(DRS)。利用ASAP 2020装置测量了样品的氮吸附-解吸等温线,以研究其(BET)表面积。使用ESCALAB 250型X-射线光电子能谱仪进行X射线光电子能谱(XPS)分析。
-
本研究以MB作为目标污染物,评价了W-Ag共掺杂SrTiO3样品的光催化活性。具体操作为:将0.1 g固体催化剂分散于100 mL MB溶液中。在光照前,将混合物在黑暗中搅拌30 min,进行避光吸附,以达到吸附-解吸平衡。将250 W高压汞灯作为模拟太阳光光源。每隔2 h,取5 mL反应溶液进行离心,取上层清液并测量其吸光度。通过计算MB水溶液在664 nm处的吸光度,考察纯SrTiO3和W-Ag共掺杂SrTiO3的光催化活性。
-
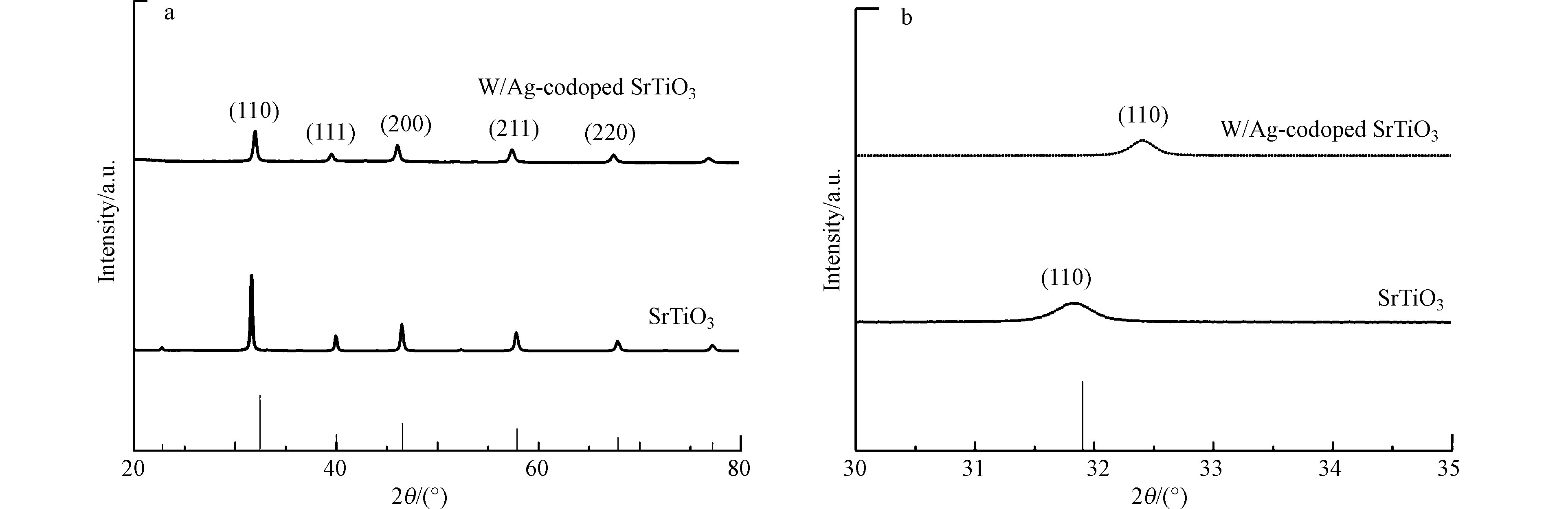
利用X射线衍射(XRD)分析了合成的纯SrTiO3和W-Ag共掺杂SrTiO3样品的晶体结构。如(图1)所示,衍射峰在2θ= 32.1°、39.6°、46.1°、57.2°和67.2°处分别对应SrTiO3标准卡(JCPDS No.35-0734)中(110)、(111)、(200)、(211)和(220)晶面,且没有观察到任何其他杂质峰。说明SrTiO3晶体是具有立方对称性的钙钛矿结构,且纯度和结晶度较高[16]。然而,图1中并没有观察到W和Ag峰,可能是W和Ag掺杂量过小,且均匀分散在SrTiO3晶格内。从图1(b)可以发现,与纯SrTiO3相比,W-Ag共掺杂SrTiO3的衍射峰强度降低且移动到了较高的衍射角区,根据Bragg公式,晶面间距变小,晶格收缩,晶体体积变小。这可能是由于W和Ag的离子半径小于Sr离子的半径,掺杂后使SrTiO3晶体尺寸减小[17]。一般来说,减小光催化剂的粒径可以缩短电荷载体的扩散路径,从而降低电子空穴对的复合几率,有助于提高光催化效率[18]。
-
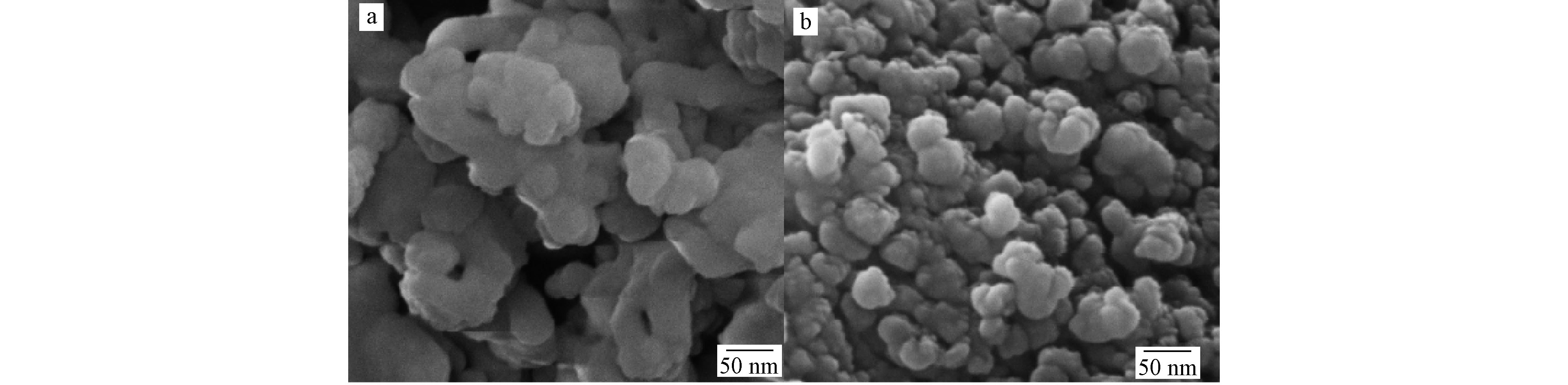
用SEM对制备的样品进行结构和形貌表征。如图2a所示,纯SrTiO3样品呈现为类球状,且有明显的团聚现象,粒径约为50 nm。从图2b中可以看出,共掺杂样品显示出与纯SrTiO3样品相似的微观形貌特征,区别在于其粒径更小,约为40 nm左右(这与XRD显示的结果高度一致),同时改善了团聚现象。比表面积增加有利于晶体暴露出更多的活性位点,增加了电子-空穴的捕获阱,抑制了他们的复合,与污染物结合的更加充分,从而提高光催化剂的光催化活性。
-
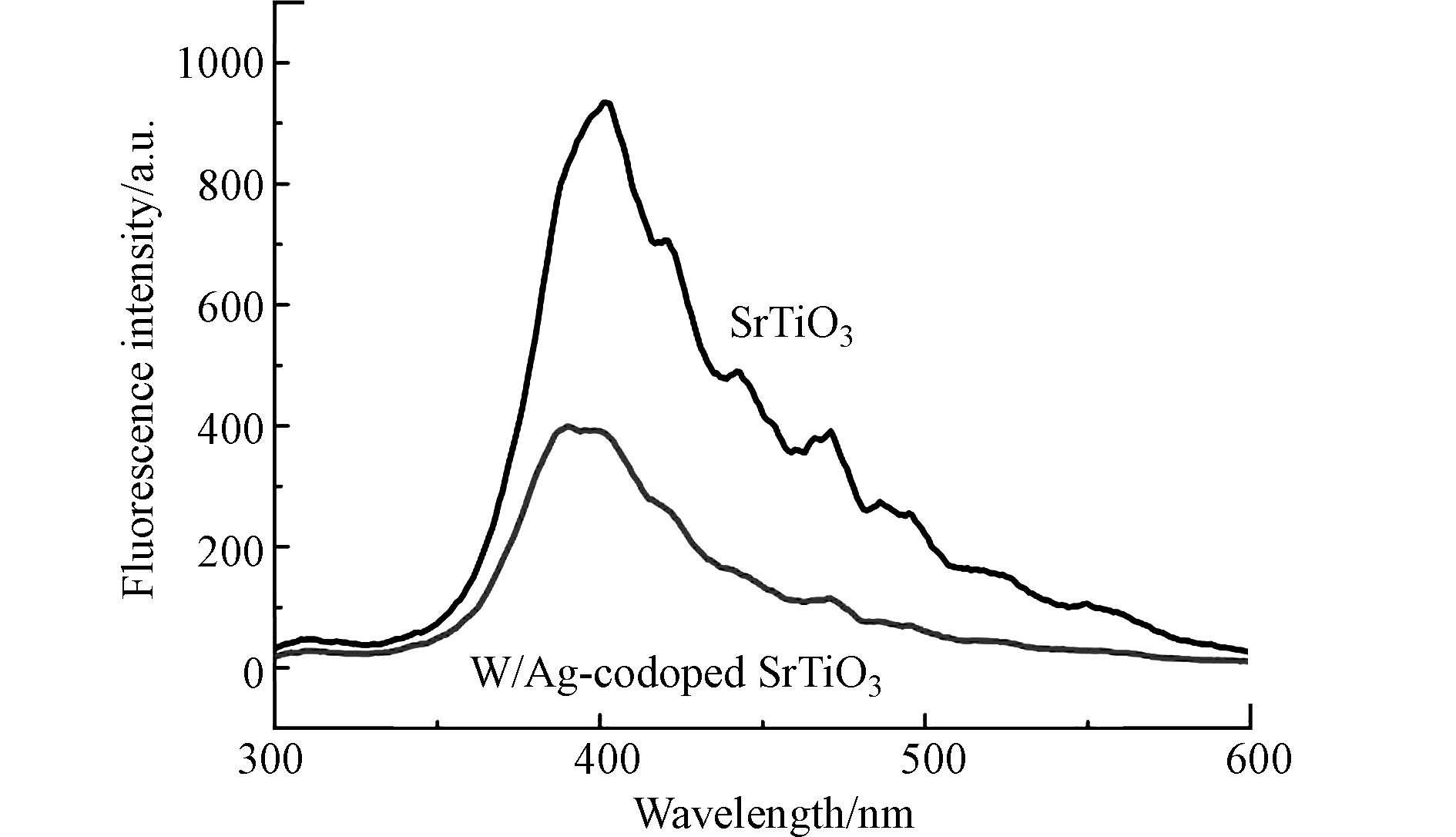
研究样品的电子-空穴对产生、分离和重组性质等光学性质,在室温下进行PL测量。其激发波长为365 nm。纯SrTiO3和W-Ag共掺杂SrTiO3样品的PL光谱如图3所示。与纯SrTiO3样品相比,W、Ag离子的掺杂使SrTiO3样品PL发生峰强度明显降低,这是由于W-Ag共掺杂SrTiO3抑制了光生电子-空穴对的复合,提高了光生载流子的分离效率[19]。降低电子-空穴对的复合速率更有利于电子空穴参与光催化反应,生成强氧化物质,从而提高光催化效率[20]。
-
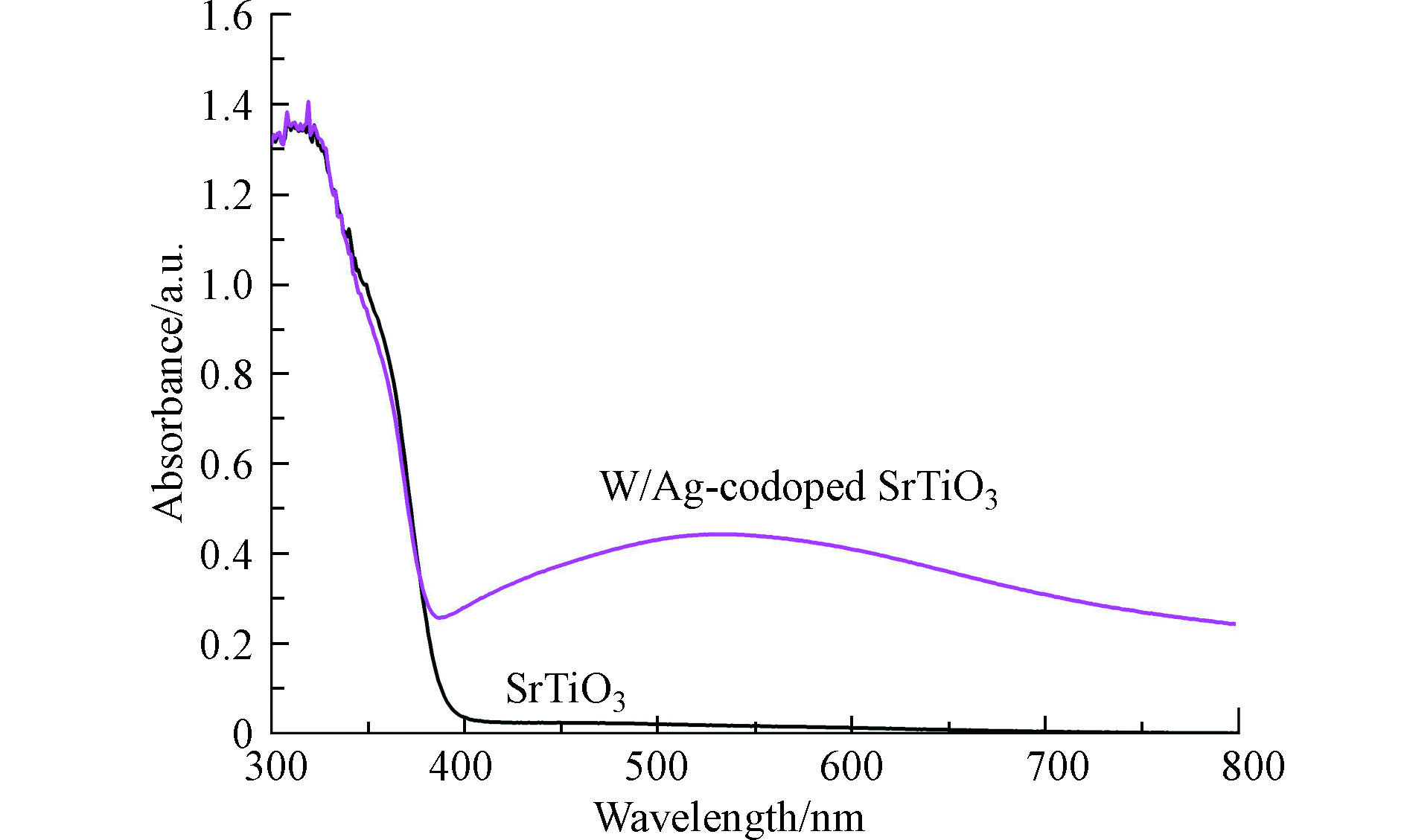
采用UV-vis DRS对纯SrTiO3和W-Ag共掺杂SrTiO3样品进行光学性能测试,如图4所示。纯SrTiO3样品在紫外区表现出明显的光吸收,这表明了纯SrTiO3仅由紫外光驱动的光催化活性,这可能与SrTiO3的带隙吸收有关[21]。对比掺杂后的SrTiO3可以发现,在紫外光波段显示出与纯SrTiO3几乎相同的光学吸收性质,但区别在于W-Ag共掺杂SrTiO3样品在可见光区具有明显的光吸收。可见光吸收增强的原因在于共掺杂在SrTiO3价带上方产生了缺陷能级,这表明了共掺杂可以有效提高SrTiO3的可见光响应[22]。
-
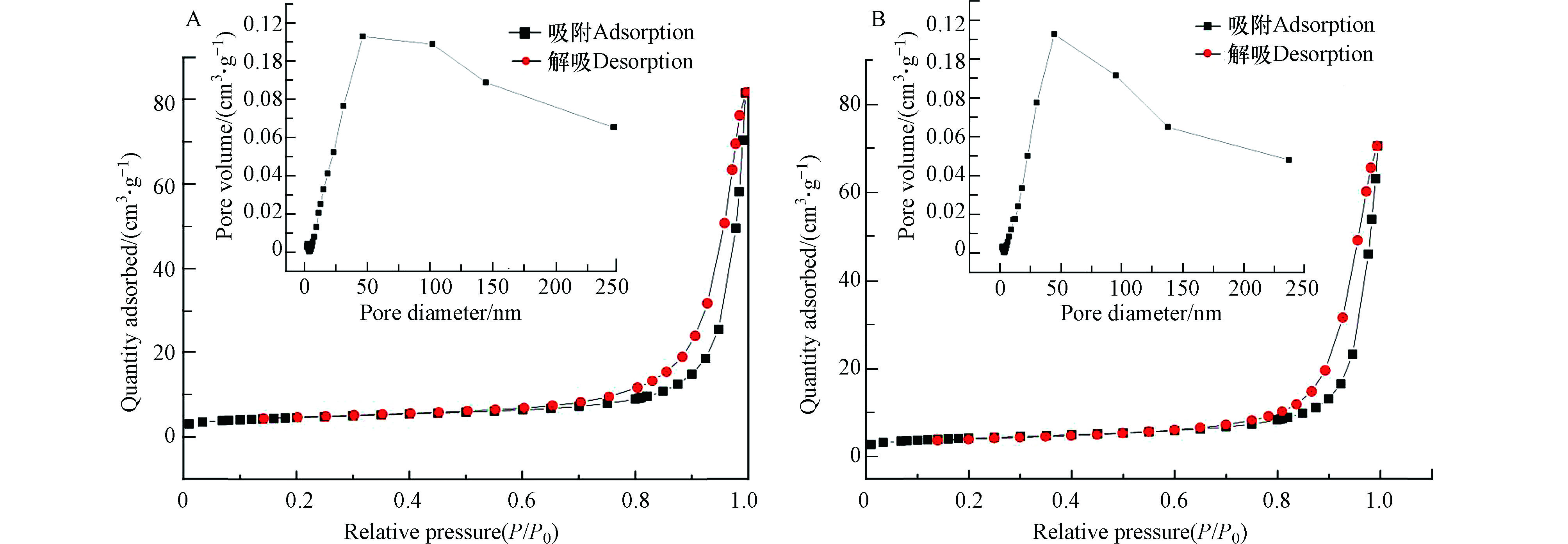
采用氮气吸附-解吸等温线分析纯SrTiO3和W-Ag共掺杂SrTiO3样品的BET表面积和孔径分布,如图5和表1所示。根据IUPAC分类,纯SrTiO3和W-Ag共掺杂SrTiO3样品具有IV型等温线,这是具有非常薄的H3滞回线的介孔材料的特征,其中解吸支向低压延伸,表明形成了狭缝形状的孔隙[23]。根据Barrett - Joyner - Halenda (BJH)模型分析,由等温线吸附支计算得到的多孔微球孔径分布较宽,约为10—200 nm,验证了样品为大孔、中孔结构。纯SrTiO3样品的BET表面积为15.200 m2·g−1,平均孔隙体积为0.086 cm3· g−1。对于W-Ag共掺杂SrTiO3样品,BET表面积为16.523 m2·g−1,平均孔隙体积为0.071 m3·g−1,这与SEM测定的结果高度一致。这些结果表明,W-Ag共掺杂SrTiO3样品比表面积增大,表面的活性中心随之增加,同时缩短了光生电子和空穴转移到样品表面的距离,从某种程度上也抑制了两种载流子的复合,氧化还原反应的效果增强,光催化活性得到提高。与此同时,提供了更多的吸附能力和表面活性。在W-Ag共掺杂后,由于离子的掺杂抑制了SrTiO3颗粒的团聚,因此SrTiO3的孔径相对减小。
-
为了确定W-Ag共掺杂SrTiO3样品的表面组成和价态,进行了XPS分析,如图6(a)所示。图6(a)中的XPS测量光谱表明存在Sr、Ti、W、Ag和O元素。C元素的存在可能是由于样品中残留的C或者是XPS设备中的污染造成的[24]。由图6(b)中观察到O 1s特征峰是不对称的,这表明样品中的表面氧存在两种以上的化学态,在533.24、531.68、529.23 eV处存在3个峰,分别为表面吸附氧、氧空位和晶格氧。相对于纯SrTiO3样品,W-Ag共掺杂SrTiO3样品中氧空位含量增加,且出现了表面吸附氧。其中空位氧和表面吸附氧的存在说明样品表面存在一定的表面缺陷以及羟基团,不同的氧缺陷浓度以及缺陷类型会影响光催化活性,而表面羟基的存在有利于捕获光生电子和空穴,抑制电子-空穴对的复合,有助于光催化活性的提高[25-26]。在图6(c)Sr 3d高分辨光谱中,出现132.41 eV和134.15 eV两个峰值,分别与Sr 3d5/2和Sr 3d3/2的结合能相关,意味着Sr在样品中仅以二价氧化态的形式存在[27]。图6(d)中的高分辨率光谱Ti 2p由Ti 2p1/2和Ti 2p3/2两个峰值组成,集中在463.63 eV和458.01 eV的结合能上,这是SrTiO3样品中Ti4+状态的特征,说明Ti+以+4价的形式存在。图中可以看出掺杂前后样品内部的Ti2p电子结合能没有明显变化,这证明少量金属W和Ag的掺杂不会改变Ti原子周围的宏观化学环境。由图6(e)可以发现,W 4f的峰值为35.55 eV和36.58eV,属于W6+氧化态,因此W以+6价的形式存在。由图6(f)可以观察到Ag 3d的峰值,373.07 eV和367.21 eV两处的能峰与Ag+相对应。以上结果说明W和Ag成功掺杂到了SrTiO3的晶格中,这也与XRD测定的结果一致。
-
纯SrTiO3和W-Ag共掺的SrTiO3样品降解MB的光催化活性和动力学如图7所示。为了排除MB溶液的自然降解,还在相同实验条件下,进行了单独光照实验。如图7(a)所示,在没有催化剂的情况下,MB的降解效率可以忽略不计,这表明MB溶液的降解效果均是由于光催化反应所提供。并且在相同的反应条件下,W-Ag共掺杂SrTiO3样品的光降解活性明显高于纯SrTiO3样品。W-Ag共掺杂SrTiO3样品在6 h内降解效率可达90.5%,而纯SrTiO3样品在6 h内降解效率仅为54.8%。如图7(b)所示,MB的降解遵循一级动力学方程[28-29]。降解速率常数k及其回归相关系数R2数据见表2,R2越接近1,降解过程,越高度符合准一级动力学模型。研究发现,W-Ag共掺杂SrTiO3样品的降解速率常数k值,几乎是纯SrTiO3的5倍。结果表明,在可见光条件下,W-Ag共掺杂可以大大提高SrTiO3的降解速率和光催化效率。
-
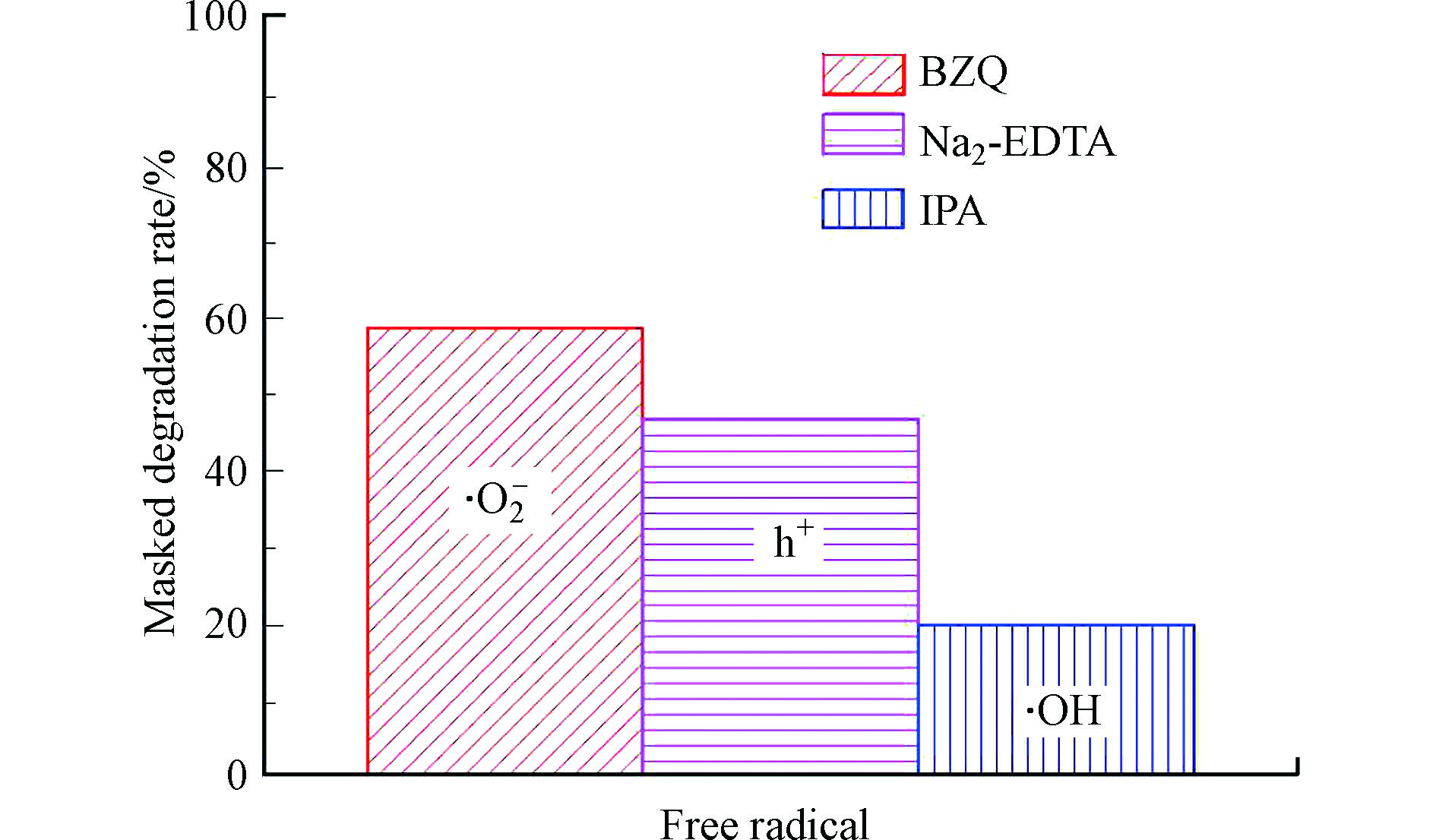
为了探索光催化机理,对W-Ag共掺杂SrTiO3复合催化剂进行自由基掩蔽实验。结果如图8所示。使用乙二胺四乙酸二钠(Na2-EDTA)、异丙醇(IPA)和对苯醌(BZQ),分别作为h+、·OH和·O2−的捕获剂。由图8可知,BZQ、Na2-EDTA、IPA和对降解实验的掩蔽率分别为58.77%、46.78%和19.62%,由此可知,光催化过程中起主要作用的是·O2−,其次为h+,而·OH对光催化效果影响较小。
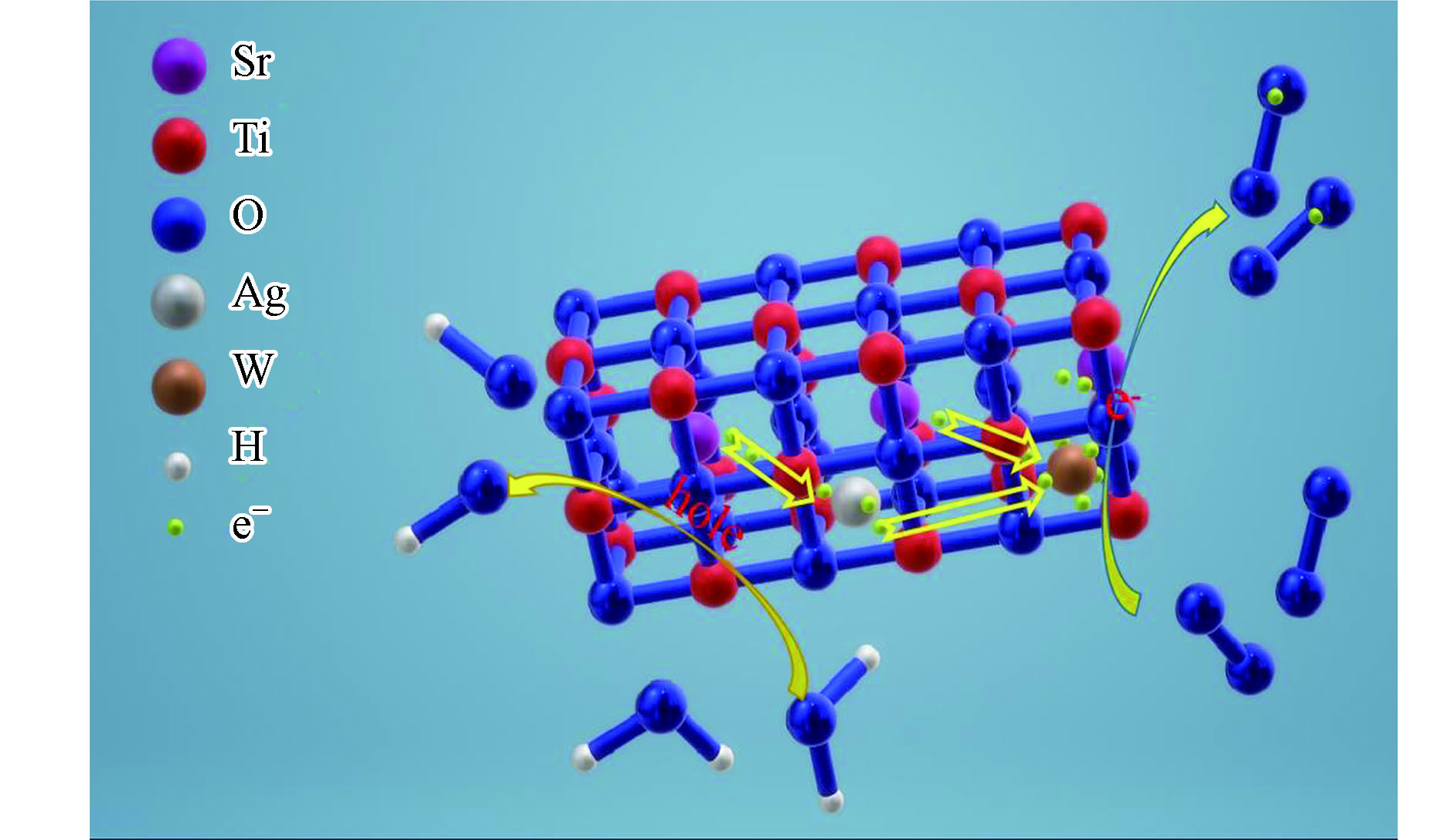
可以由Butler和Ginley模型[30]可知,SrTiO3的CB和VB分别为−1.2 eV和+2.1 eV。SrTiO3材料的价带电位(+2.1 eV)低于OH/·OH(+2.38 eV)和H2O/·OH(+ 2.72 eV)的还原电位,因此,SrTiO3材料的价带中的h+与OH−和H2O反应产生·OH的过程较为微弱。由·OH诱导的MB的降解也较为微弱。由于SrTiO3材料的导带电位(-1.2 eV)超过O2/·O2−(-0.33 eV)的氧化电位[31],SrTiO3的CB中的电子与周围的O2发生的反应强烈,从而进行强烈的光催化降解。W-Ag共掺杂SrTiO3复合材料对亚甲基蓝溶液可能的降解机制如图9所示。经光源照射后,在捕获中心中跃迁的电子与O2结合生成大量的·O2−,·O2−参与光催化降解反应。而电子跃迁后,留下的空穴与水分子反应生成·OH,·O2−和·OH共同作用于MB,直至分解。
-
(1) W和Ag掺杂使SrTiO3晶格发生畸变,降低其粒径尺寸,增大其比表面积,暴露更多的活性位点,提高其对污染物的吸附能力。
(2) W和Ag掺杂,在SrTiO3价带上方产生了缺陷能级,有效提高SrTiO3的可见光响应。
(3) W和Ag掺杂,增加SrTiO3氧空位和表面吸附氧的含量。有效抑制电子-空穴对的复合,提高其光催化活性。
(4)在模拟太阳光条件下,W-Ag共掺杂SrTiO3样品对MB的降解速率常数约为纯SrTiO3的5倍。
W-Ag共掺杂SrTiO3的制备及其光催化性能
Preparation and photocatalytic properties of W-Ag co-doped SrTiO3
-
摘要: 本研究采用溶胶-凝胶法制备W-Ag共掺杂SrTiO3,以亚甲基蓝(MB)为目标污染物,研究W-Ag共掺杂SrTiO3的光催化活性及其效率提升的机制。通过X射线衍射(XRD)、扫描电镜(SEM)、光致发光光谱分析(PL)、紫外-可见漫反射光谱(UV- vis DRS)、比表面积测试(BET)和X射线光电子能谱(XPS)对样品结构、组成和光学性能进行表征。结果表明,W和Ag成功掺入SrTiO3晶体结构中,并引起SrTiO3产生晶格畸变,粒径尺寸减小。相比纯SrTiO3比表面积15.200 m2·g−1,W-Ag共掺杂SrTiO3比表面积增大到16.523 m2·g−1,有利于活性位点的暴露。W和Ag的掺杂增加了SrTiO3晶格内氧空位和表面吸附氧含量,有效抑制电子-空穴对的复合。此外,在SrTiO3价带上方产生了缺陷能级,提高SrTiO3的可见光响应。因此,与纯SrTiO3相比,W-Ag共掺杂SrTiO3显示出了更强的光催化活性。在模拟太阳光条件下,相比纯SrTiO3在6 h内54.8%的降解效率,W-Ag共掺杂SrTiO3的降解效率可达到90.5%,而降解速率常数由0.0861 h−1增加到0.3895 h−1,几乎是纯SrTiO3的5倍。Abstract: In this paper, the novel W-Ag co-doped SrTiO3 samples were prepared by sol-gel method, the photocatalytic activity has been investigated for methylene blue degradation. Structure, composition and morphology of the as-prepared catalysts were characterized using several techniques including X-ray diffraction (XRD)、Scanning electron microscope (SEM)、Photoluminescence spectroscopy (PL)、Uv-visible diffuse reflectance (UV-vis DRS)、Specific surface area (BET) and X-ray photoelectron spectroscopy (XPS). The results show that the successful doping of W and Ag into SrTiO3 crystal lattice was achieved, and the dopants, and the distortion of SrTiO3 crystal lattice caused by doping resulted in the decrease of crystallite size of SrTiO3. W-Ag co-doped SrTiO3 sample has a surface area of about 16.523 m2·g−1, which is larger than that of pure SrTiO3 (15.200 m2·g−1). The relatively large specific surface area of W-Ag co-doped SrTiO3 sample provides a greater number of reactive sites for the photocatalytic process. Doping of W and Ag increases the oxygen vacancy concentration and surface absorbed oxygen, which suppress recombination rate of the photogenerated electron-hole pairs. Besides, the defect energy level is generated above valence band of SrTiO3, which improves its visible light response. So, W-Ag co-doped SrTiO3 show higher photocatalytic activity compared with pure SrTiO3. The photocatalytic degradation efficiency of W-Ag co-doped SrTiO3 to MB reaches 90.5% after 6 h under the simulated sunlight illumination, much higher than that of pure SrTiO3 (54.8%), and the degradation rate constant of MB for W-Ag co-doped SrTiO3 is 0.3895 h−1, which was almost 5 times as much as pure SrTiO3.
-
Key words:
- strontium titanate /
- simulated sunlight /
- doping /
- sol-gel process
-

-
图 7 (a)纯SrTiO3和W-Ag共掺杂SrTiO3样品降解MB的光催化活性比较,(b)纯SrTiO3和W-Ag共掺杂SrTiO3样品的相应一级动力学图
Figure 7. (a) Comparison of photocatalytic activity of pure and W-Ag-codoped SrTiO3 samples for the degradation of MB in aqueous solution and (b) the corresponding first-order kinetics plot of pure and W-Ag-codoped SrTiO3 samples
表 1 SrTiO3、W-Ag共掺杂SrTiO3的孔性质
Table 1. Pore properties of SrTiO3 and W-Ag co-doped SrTiO3
样品
Sample比表面/(m2 ·g−1)
Specific surface area孔容/(cm3 ·g−1)
Pore volume平均孔径/nm
Average apertureSrTiO3 15.200 0.086 21.129 W-Ag共掺杂SrTiO3 16.523 0.071 19.920 表 2 掺杂对SrTiO3催化降解动力学参数的影响
Table 2. Effect of doping on kinetic parameters of catalytic degradation of SrTiO3
k/h−1 R2 纯 SrTiO3 0.0861 0.9481 W-Ag共掺杂SrTiO3 0.3895 0.9415 -
[1] NORVIU K W, YU J K, CHI Y, et al. Effect of adding Au nanoparticles to TiO2 films on crystallization, phase transformation, and hotocatalysis [J]. Journal of Materials Research, 2018, 33(4): 467-481. doi: 10.1557/jmr.2018.16 [2] 齐中, 王熙, 李来胜, 等. 基于水热法制备的TiO2/MOS2复合光催化剂及其光催化制氢活性 [J]. 环境化学, 2016, 35(5): 1027-1034. doi: 10.7524/j.issn.0254-6108.2016.05.2015112403 QI Z, WANG X, LI L S. Development of TiO2 /MoS2 by hydrothermal method for photocatalytic hydrogen generation under solar light [J]. Environmental Chemistry, 2016, 35(5): 1027-1034(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.05.2015112403
[3] 赵真艺, 沈鑫怡, 陈定宁, 等. NH2-nFe3O4@ZnO@Ce磁性复合材料的制备及其对三氯酚污染物的光催化降解 [J]. 环境化学, 2020, 39(3): 643-652. doi: 10.7524/j.issn.0254-6108.2019080501 ZHAO Z Y, SHEN X Y, CHEN D N, et al. Preparation of NH2-nFe3O4@ZnO@Ce magnetic composite and its photocatalytic degradation of trichlorophenol [J]. Environmental Chemistry, 2020, 39(3): 643-652(in Chinese). doi: 10.7524/j.issn.0254-6108.2019080501
[4] GU L, WEI H H, PENG Z J, et al. Defects enhanced photocatalytic performances in SrTiO3 using laser-melting treatment [J]. Journal of Materials Research, 2016, 32(4): 748-756. [5] TAN H Q, ZHAO Z, ZHU W B, et al. Oxygen vacancyenhanced photocatalytic activity of pervoskite SrTiO3 [J]. Acs Appl Mater Interfaces, 2014, 21: 19184-19190. [6] FU X, LI H, LV R, et al. Synthesis of Mn2+ doped ZnS quantum dots/ZIF-8 composite and its applications as a fluorescent probe for sensing Co2+ and dichromate [J]. Journal of Solid State Chemistry, 2018, 264: 26-35. [7] JIANG J Z, JIA Y S, WANG Y B, et al. Insight into efficient photocatalytic elimination of tetracycline over SrTiO3(La, Cr) under visible-light irradiation: The relationship of doping and performance [J]. Applied Surface Science, 2019, 486: 93-101. doi: 10.1016/j.apsusc.2019.04.261 [8] JIN S W, SHU Y, QI W Z, et al. Influences of the factors on photocatalysis of fluorine-doped SrTiO3 made by mechanochemical [J]. Solid State Ionics, 2004, 172: 191-195. doi: 10.1016/j.ssi.2004.05.016 [9] SHAH Z H, GE Y, YE W, et al. Visible light activation of SrTiO3 by loading Ag/AgX (X = Cl, Br) for highly efficient plasmon-enhanced photocatalysis [J]. Materials Chemistry and Physics, 2017, 198: 36-73. [10] RAHMAN I Q, AHMAD M, MISRA S K, et al. Efficient degradation of methylene blue dye over highly reactive Cu doped strontium titanate (SrTiO3) nanoparticles photocatalyst under visible light [J]. Journal of Nanoence & Nanotechnology, 2012, 12: 7181-7186. [11] ATKINSON I, PARVULESCU V, PANDELE CUSU J, et al. Influence of preparation method and nitrogen (N) doping on properties and photo-catalytic activity of mesoporous SrTiO3 [J]. Journal of Photochemistry & Photobiology A Chemistry, 2018, 368: 12-41. [12] ZHANG C, JIA Y, JING Y, et al. DFT study on electronic structure and optical properties of N-doped, S-doped, and N/S co-doped SrTiO3 [J]. Physica B:Condensed Matter, 2012, 407: 4649-4654. doi: 10.1016/j.physb.2012.08.038 [13] YANG D, ZHAO X, ZOU X, et al. Removing Cr (Ⅵ) in water via visible-light photocatalytic reduction over Cr-doped SrTiO3 nanoplates [J]. Chemosphere, 2019, 215: 586-594. doi: 10.1016/j.chemosphere.2018.10.068 [14] ZhOU D D, ZHAI P P, HU G T, et al. Upconversion luminescence and enhanced photocatalytic hydrogen production for Er3+ doped SrTiO3 nanopaeticles [J]. Chemical Physics Letters, 2018, 711: 77-85. doi: 10.1016/j.cplett.2018.09.024 [15] LUO Y, DEG B, PU Y, et al. Interfacial coupling effects in g-C3N4/SrTiO3 nanocomposites with enhanced H2 evolution under visible light irradiation [J]. Applied Catalysis B:Environmental, 2019, 247: 1-25. doi: 10.1016/j.apcatb.2019.01.089 [16] SHAH Z H, GE Y, YE W, et al. Visible light activation of SrTiO3 by loading Ag/AgX (X = Cl, Br) for highly efficient plasmon-enhanced photocatalysis [J]. Materials Chemistry and Physics, 2017, 198: 73-78. doi: 10.1016/j.matchemphys.2017.05.002 [17] ANITHA B G, DEVI L G. Study of reaction dynamics of photocatalytic degradation of 4-Chlorophenol using SrTiO3, sulfur doped SrTiO3, silver metallized SrTiO3 and silver metallized sulfur doped SrTiO3 catalysts: Detailed analysis of kinetic results [J]. Surfaces and Interfaces, 2019, 16: 50-58. doi: 10.1016/j.surfin.2019.04.009 [18] KAVITHA V, MAHALINGAM P, JEYANTHINATH M, et al. Optical and structural properties of ungsten-doped barium strontium titanate [J]. Materials Today:Proceedings, 2019, 11: 1-4. doi: 10.1016/j.matpr.2018.12.097 [19] KAVIYARASAN K, VINOTHV, et al. Photocatalytic and photoelectrocatalytic performance of sonochemically synthesized Cu2O@TiO2 Heterojunction Nanocomposites [J]. Ultrasonics Sonochemistry, 2019, 51: 223-246. doi: 10.1016/j.ultsonch.2018.10.022 [20] WU G L, XIAO L S, GUO L S, et al. Fabrication and excellent visible-light-driven photodegradation activity for antibiotics of SrTiO3 nanocube coated CdS microsphere heterojunctions [J]. RSC Advances, 2016, 24: 19878-19902. [21] DUAN C L, SONG J L, WANGB Y, et al. Lactic acid assisted solvothermal synthesis of BiOClxI1–x solid solutions as excellent visible light photocatalysts [J]. Chemical Research in Chinese Universities, 2019, 236: 326-334. [22] HWANG D W, KIM H G, LEE J S, et al. Photocatalytic hydrogen production from water over M-Doped La2Ti2O7 (M = Cr, Fe) under visible light irradiation [J]. Journal of Physical Chemistry B, 2015, 109: 2093-2102. [23] GUAN X J, GUO L J, et al. Cocatalytic effect of SrTiO3 on Ag3PO4 toward enhanced photocatalytic water oxidation [J]. ACS Catalysis, 2014, 4: 3020-3027. doi: 10.1021/cs5005079 [24] LIU R R, JI Z J, WANG J, et al. Solvothermal synthesized Ag-decorated TiO2/sepiolite composite with enhanced UV–vis and visible light photocatalytic activity [J]. Microporous and Mesoporous Materials, 2018, 266: 268-275. doi: 10.1016/j.micromeso.2018.03.009 [25] DING J F, LONG G Y, LUO Y, et al. Photocatalytic reductive dechlorination of 2-chlorodibenzo-p-dioxin by Pd modified g-C3N4 photocatalysts under UV–vis irradiation: Efficacy, kinetics and mechanism [J]. Journal of Hazardous Materials, 2018, 355: 74-106. doi: 10.1016/j.jhazmat.2018.05.014 [26] WANG P, JIA C C J, LI J, et al. Ti3+-doped TiO2(B)/anatase spheres prepared using thioglycolic acid towards super photocatalysis performance [J]. Journal of Alloys and Compounds, 2018, 780: 660-690. [27] NG J W, XU S P, ZHANG X W, et al. Hybridized Nanowires and Cubes: A Novel Architecture of a Heterojunctioned TiO2/SrTiO3 Thin Film for Efficient Water Splitting [J]. Advanced Functional Materials, 2010, 20: 4287-4295. doi: 10.1002/adfm.201000931 [28] SHEN H F, WEI H Y, PAN Z D, et al. Preparation and characterization of SrTiO3-Ag/AgCl hybrid composite with promoted plasmonic visible light excited photocatalysis [J]. Applied Surface Science, 2017, 423: 403-446. doi: 10.1016/j.apsusc.2017.06.023 [29] HU C C, HUANG H X, LIN Y F, et al. Decoration of SrTiO3 nanofibers by BiOI for photocatalytic methyl orange degradation under visible light irradiation [J]. Journal of the Taiwan Institute of Chemical Engineers, 2019, 96: 264-273. doi: 10.1016/j.jtice.2018.11.020 [30] AKHUNDI A, AZIZ H A. Ternary g-C3N4/ZnO/AgCl nanocomposites: Synergistic collaboration on visible-light-driven activity in photodegradation of an organic pollutant [J]. Applied Surface Science, 2015, 358: 261-269. doi: 10.1016/j.apsusc.2015.08.149 [31] WAN S, OU M, ZHONG Q, et al. Z-scheme CaIn2S4/Ag3PO4 nanocomposite with superior photocatalytic NO removal performance: Fabrication, characterization and mechanistic study [J]. New Journal of Chemistry, 2018, 42(1): 318-326. doi: 10.1039/C7NJ03588H -



 下载:
下载: