-
氮氧化物(NOx)是造成酸雨、雾霾等的主要大气污染物,对人类的生活和生产造成危害[1]。目前,国内外普遍采用选择性催化还原(SCR)技术对NOx进行控制[2]。SCR技术具有污染物去除率高、技术成熟的优点,但同时也存在氨逃逸、N资源无法回收利用等不足。NOx主要以一氧化氮(NO)的形式存在,因此要实现低成本烟气脱硝,最简便的方法是利用烟气中剩余的O2,在催化剂的作用下将NO氧化为可溶性的NO2(催化氧化SCO技术),再用碱液吸收湿法脱除,其中氧化效率达到50%左右时可实现最大的吸收率[3]。SCO技术因其催化性能和产物稳定,成本较低且不会造成二次污染,最具工业应用前景,因而近年来吸引了众多研究者的关注。
在诸多SCO催化剂中,过渡金属氧化物因其活性高、原料来源广和价格低等优点,成为近年来NO催化氧化研究的热点。研究较多的有锰(Mn)系、钴(Co)系和铬(Cr)系金属氧化物,其均具有较高的NO催化氧化率。Meng等[4]研究了不同煅烧温度对MnOx-CeO2催化剂催化氧化NO效率的影响。结果表明,煅烧温度为650 ℃下煅烧得到的产物具有最高的活性,反应温度为300 ℃时,NO氧化效率可达78%左右,然而过高的煅烧温度会导致无定形MnOx的烧结,并使得无定形态MnOx转变为块状Mn2O3,而无定形态MnOx的高含量是高效催化氧化NO的关键因素。Shang等[5]以柠檬酸为络合剂,采用一锅法制备了Co/Zr0.2Ce0.8O2催化剂,研究不同Co负载量对催化氧化NO性能的影响。结果表明,当负载量达到10% wt.时,催化剂具有最高的活性,反应温度为300 ℃时NO氧化效率可达76%。然而,烟气中二氧化硫(SO2)的存在会占据催化剂表面的活性位,降低氧化效率。在过渡金属氧化物中,Cr系催化剂因其具有较高的抗硫性能而备受关注[6]。Cai等[7]研究了不同水热反应溶剂对Cr-Ce复合氧化物催化氧化NO性能的影响,结果表明,当乙醇作为反应溶剂时催化剂具有最高的活性,反应温度为300 ℃下的NO氧化效率可达64%。同时该催化剂具有较高的抗硫性能,当(4×10−4)%vol. SO2通入反应气氛中时,催化剂在300 ℃下仍可保持在高于50%的NO氧化活性。同时,该研究表明较高的催化剂表面粗糙度利于表面活性位点的充分暴露,是提升其NO氧化活性的关键因素。因此,通过改变制备条件来促使更多的活性位点暴露出来,便更有利于提升NO的氧化活性。有研究表明,纳米结构催化材料的合成能够进一步提升催化剂的催化性能,尤其是低温下的催化活性[8-10]。针对水热反应,众所周知,水热温度是水热反应的核心参数,是控制催化剂纳米结构形态的关键因素。到目前为止,水热温度对Cr系催化剂的纳米结构形态及其NO氧化活性影响的研究鲜有报道。
本文采用一锅水热法制备了一种纳米结构的Cr2O3/CeO2复合氧化物,并考察了不同水热温度对低温催化氧化NO效率的影响,并通过多种表征研究了水热温度、催化剂纳米结构与催化性能之间的构效关系。
-
主要试剂:硝酸铈(Ce(NO3)3·6H2O)、硝酸铬(Cr(NO3)3·9H2O)、脲(CO(NH2)2)、乙醇(C2H5OH),以上试剂均为分析纯。
主要气体:氮气N2(纯度99.99%),氧气O2(纯度99.99%),一氧化氮NO(纯度1.01%,剩余平衡气为N2)。
-
取适量的硝酸铈、硝酸铬和脲,将其加入到60 mL乙醇溶液中,搅拌1 h至溶液澄清,接着将混合溶液转移至100 mL高压釜中进行水热反应,水热温度分别为100、120、140、160、180、200 ℃,水热时间均为24 h。待反应釜自然冷却至室温后,将混合液取出,倒掉上层溶液,收集下层产物。产物经去离子水、乙醇分别清洗5次后,60 ℃下真空烘干过夜,而后将产物置于马弗炉中500 ℃下空气煅烧4 h。最后产物经研磨后收集。控制硝酸铬与硝酸铈的物质的量比为1∶3.03(相当于10 %wt.的Cr负载在CeO2上),脲与总金属(包括Cr与Ce)的物质的量比为3∶1。将产物分别命名为CC100、CC120、CC140、CC160、CC180、CC200,分别对应水热温度为100、120、140、160、180、200 ℃。
-
X射线衍射仪(XRD),XD-3型,北京普析通用仪器有限公司;透射电子显微镜(TEM),JEOL-2100型,日本电子株式会社;X射线光电子能谱仪(XPS),ESCALAB 250型,美国赛默飞世尔科技公司;紫外可见漫反射光谱仪(UV-vis DRS),UV-2550型,日本岛津公司;程序升温脱附仪(TPD),ChemBET Pulsar型,美国康塔仪器公司;常压固定床反应器,HP-WF51型,南京皓而普分析设备有限公司;烟气分析仪,NOVA Plus型,德国MRU公司。
-
催化剂的脱硝活性评价在常压固定床反应器中进行,反应管内径为20 mm、高为550 mm的竖式石英管,由管式电炉加热。活性评价装置由模拟烟气、固定床反应器和分析检测3部分组成。催化剂用量为0.3 g,模拟烟气由NO、O2、N2、SO2(需要时)、H2O(g)(需要时)混合而成,由质量流量计控制各个反应气体流量:总流量为100 mL·min−1,其中N2流量为88 mL·min−1,O2流量为8 mL·min−1,NO流量为4 mL·min−1,在以上参数下,NO浓度(体积分数)为4×10−4% vol,O2浓度为8% vol。反应温度为150— 400 ℃,在第一个温度点150 ℃时,反应体系先稳定2 h,以排除催化剂的吸附性能对氧化效率的影响,而后通过集气袋集气,用烟气分析仪检测反应器进、出口的NO浓度。然后逐步程序升温,每隔50 ℃作为1个反应温度点,升温速率为2 ℃·min−1。在各个测试温度下,先稳定0.5 h后通过集气袋进行集气,采用烟气分析仪检测进、出口的NO浓度。
催化剂的脱硝活性以NO的氧化率(η)衡量,计算公式如下:
式中,(NO)in、(NO)out分别代表NO的进口、出口浓度。
-
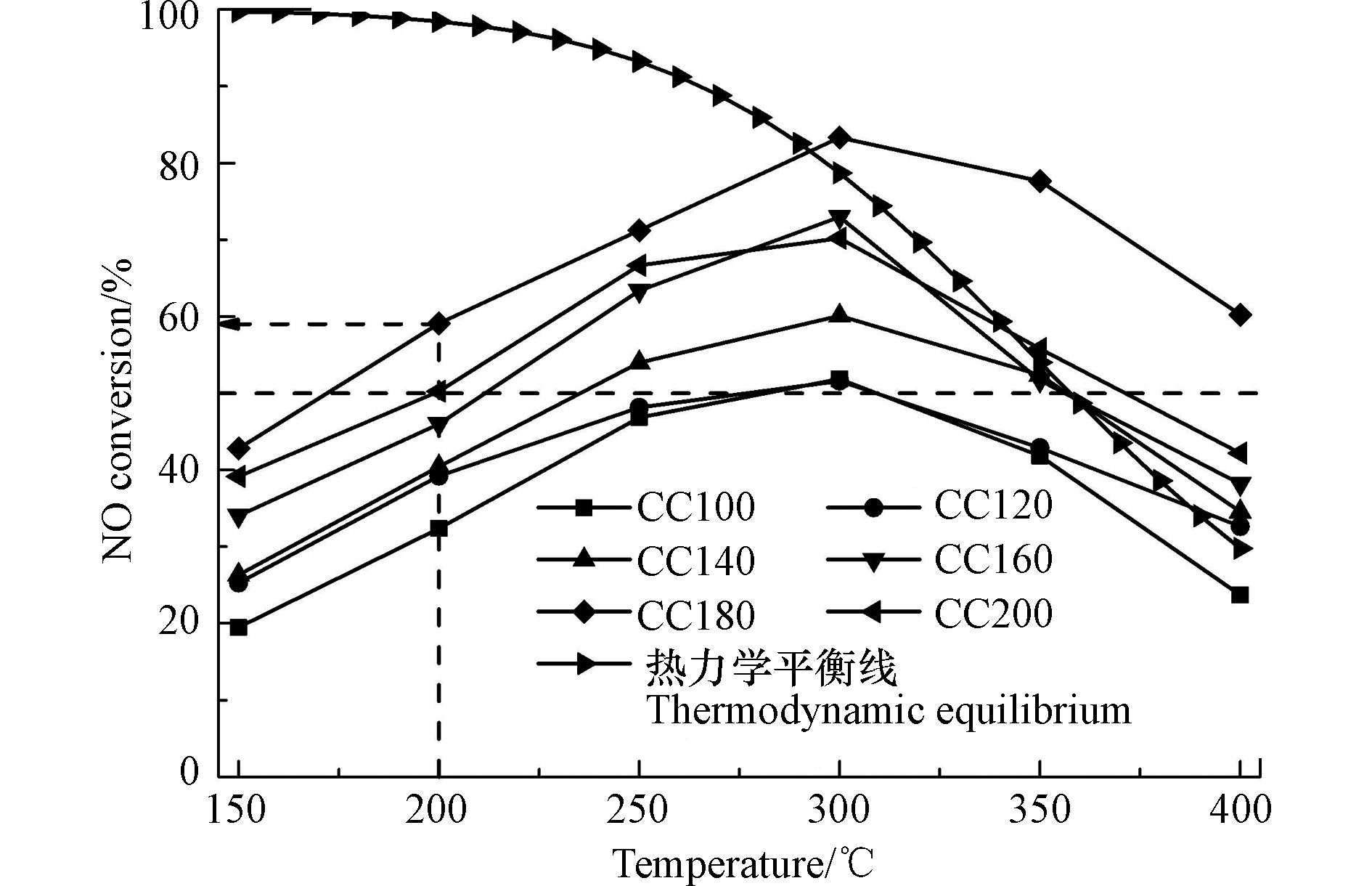
不同水热温度对NO氧化率的影响如图1所示。
由图1可知,所有催化剂的氧化率在150 ℃到400 ℃的温度区间内均呈现先上升后下降的趋势,高温下氧化率的下降受热力学平衡所控制[11]。所有催化剂均在300 ℃下呈现最高活性。随着水热温度的升高,催化剂的整体NO氧化效率也逐渐提升。当水热温度为180 ℃时,CC180催化剂在全反应温度区间呈现最高的催化活性,温度为200 ℃时氧化率可达60%左右,温度为300 ℃时,最高氧化率高达83%。而当进一步提高水热温度至200 ℃时,CC200催化剂的NO氧化活性开始下降。与已报道的Mn基和Co基催化剂催化氧化NO的性能对比见表1。由表1可知,所有催化剂在对应的反应温度下普遍具有较高的氧化效率,针对各自催化剂的最高活性下所对应的反应温度时,可看出本文制备的Cr基催化剂与文献报道的Co基催化剂的氧化活性接近,但二者的最佳反应温度均高于Mn基催化剂所对应的温度,表明Mn基催化剂的低温催化活性高于Cr基与Co基催化剂。
众所周知,在水热过程中,水热温度是影响催化剂纳米结构形态的关键因素[12],通过改变纳米结构形态,来促使活性位点充分暴露,从而提升NO氧化活性。此外,从图1中还可以看出,除CC100和CC120催化剂外,其余催化剂在250—350 ℃的反应区间内均呈现高于50%的NO氧化效率,表明在该制备条件下,大部分催化剂具有较高的催化活性。
-
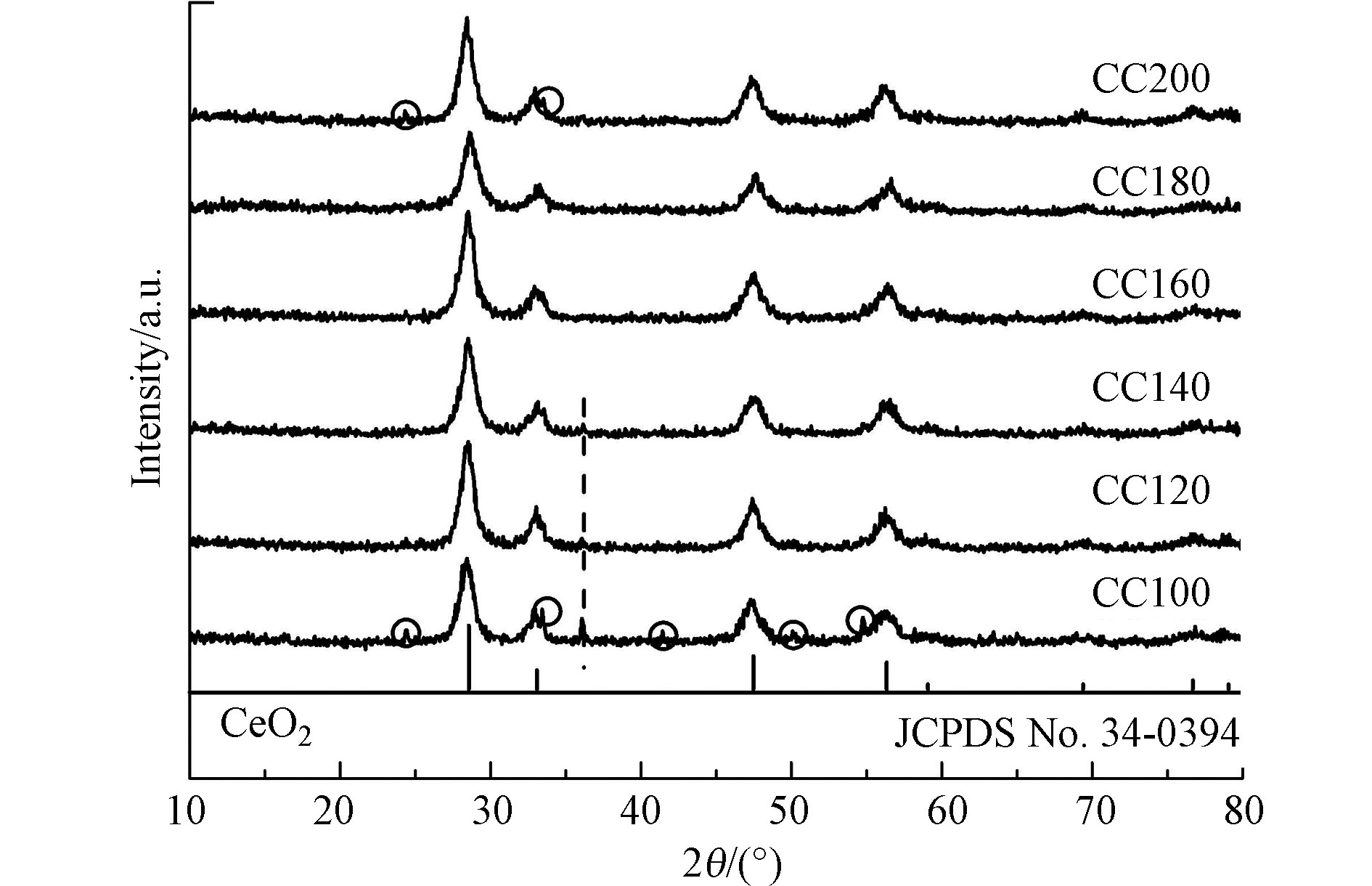
采用XRD谱图对不同水热温度制备条件下的催化剂的晶型进行表征,结果如图2所示。由图2可知,所有催化剂均出现了明显的4个衍射峰,其衍射角2θ角分别位于28.55°、33.08°、47.48°和56.33°附近,分别对应着立方相CeO2的(1 1 1)、(2 0 0)、(2 2 0)和(3 1 1)晶面(标准卡片号:34-0394)。该结果表明,即使在水热温度为100 ℃下,立方相的CeO2仍可形成。此外,Cr物种添加后,CeO2的XRD衍射峰并未发生明显的偏移,结合布拉格公式2dsinθ=nλ(式中,d为晶胞参数,θ为衍射角,n取正整数,λ为Cu靶的激发波长)可知,对于固定的仪器,n和λ为常数值,因此若θ未发生改变,则d也不变。上述结果表明Cr物种以表面分散的形式为主。然而值得注意的是,当水热温度小于140 ℃时,CC140、CC120和CC100催化剂在2θ=36°处均出现了1个峰,该峰为Cr2O3的(1 1 0)晶面的峰(标准卡片号:38-1479),而当水热温度仅为100 ℃时,CC100催化剂还在2θ=24°、33°、42°、50°和55°处出现了属于Cr2O3的峰,分别对应Cr2O3的(0 1 2)、(1 0 4)、(1 1 3)、(0 2 4)和(1 1 6)晶面。此外,水热温度为200 ℃时,CC200催化剂在2θ=24°和33°也出现了较为明显的属于Cr2O3的峰。上述现象表明,水热温度较低(≤140 ℃)或较高(≥200 ℃)时均不利于Cr物种在CeO2的表面分散不均匀,而适中的水热温度则有助于提升Cr物种的表面分散程度,从而增加活性位点的数量,从而提升催化剂的活性。
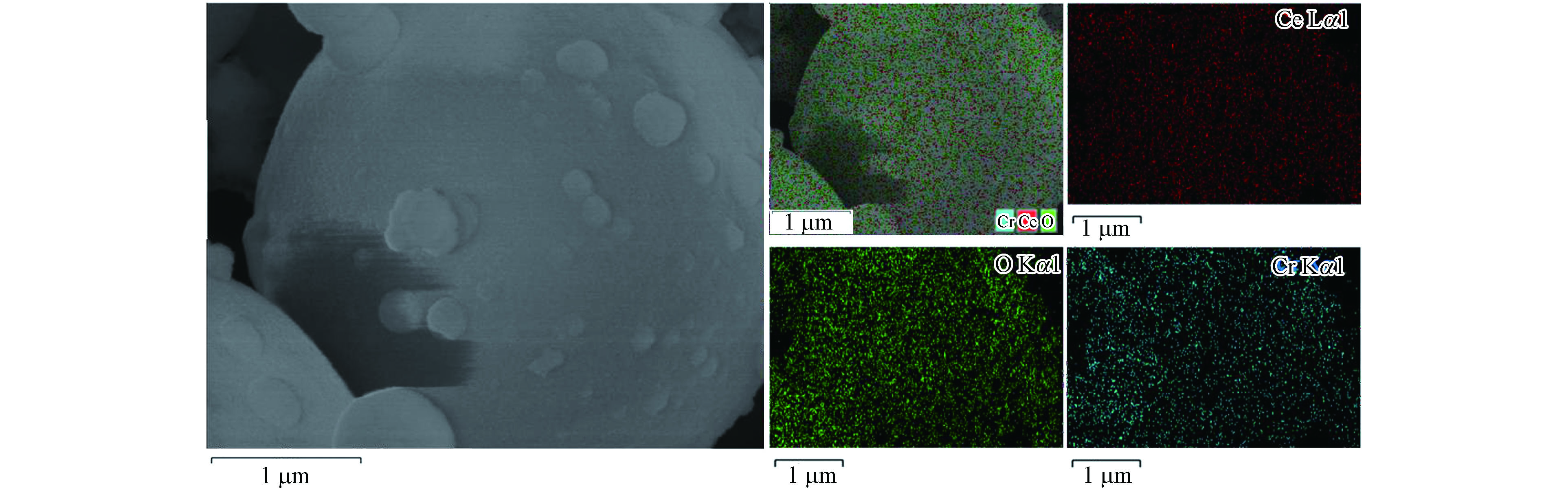
为了进一步证明Cr物种在CeO2表面的分散程度,对CC180催化剂进行了mapping测试,如图3所示。由图3可知,CC180催化剂中的Cr物种在CeO2表面分散程度高,与XRD结论相一致。
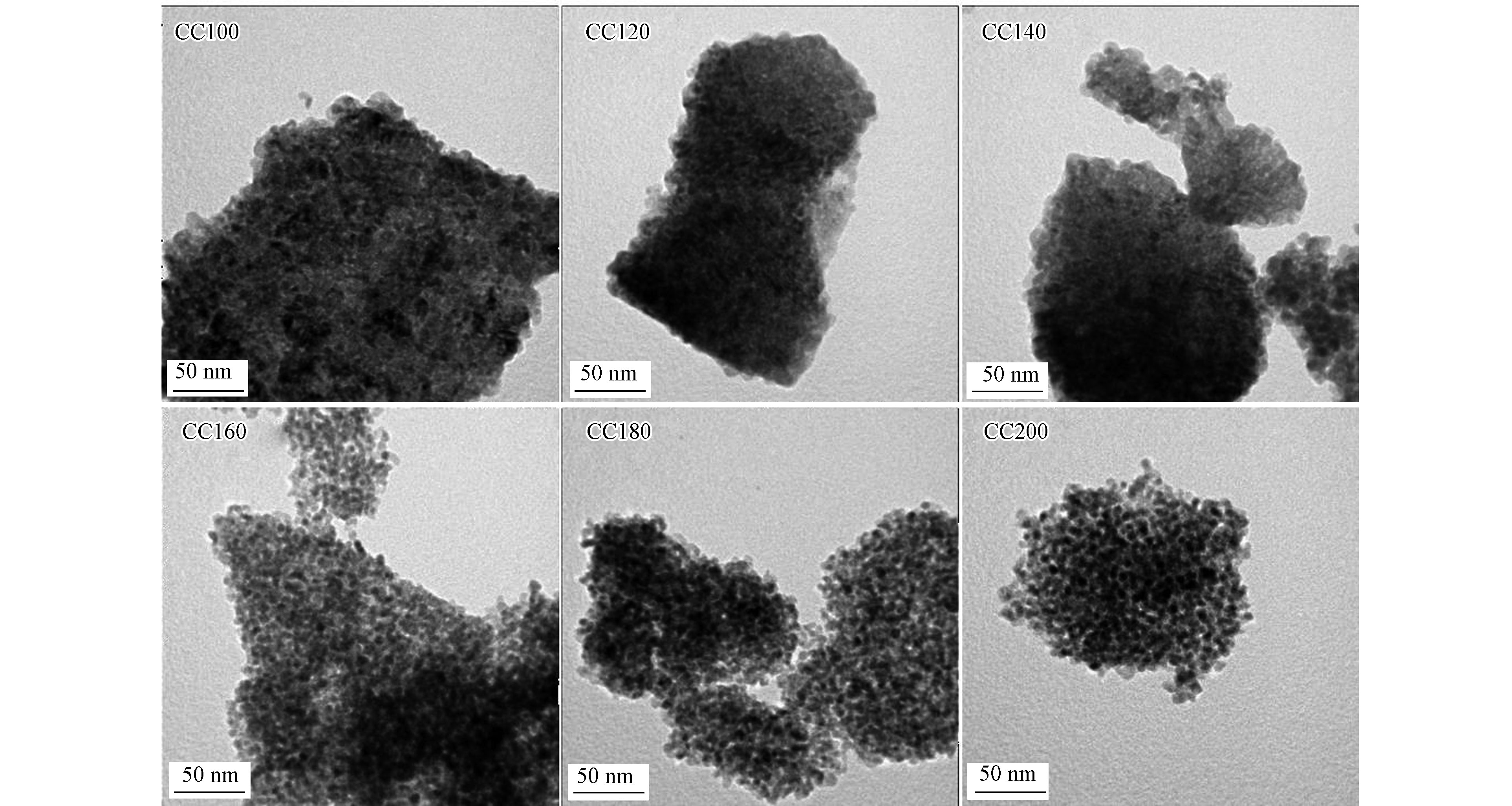
采用TEM对不同水热温度制备条件下的催化剂的形貌进行表征,结果如图4所示。由图4可知,所有催化剂均呈现小颗粒的团聚形状(小颗粒尺寸约为10 nm),但随着水热温度的升高,小颗粒的边界愈加明显,表明较高的水热温度利于Cr或Ce物种晶核的生长,同时颗粒边界的明显化也有助于活性位点的充分暴露,从而提升催化活性。采用高倍透射电镜(HRTEM)和选区电子衍射(SAED)对CC180催化剂的高倍形貌作进一步分析,如图5所示。由HRTEM可知,小颗粒的催化剂主要以CeO2为主,呈现出CeO2的(1 1 1)晶面,晶面间距为0.326 nm。由XRD可知,(1 1 1)晶面为立方相CeO2的最强晶面。此外,在HRTEM中也出现了属于Cr2O3的(1 0 4)晶面,晶面间距0.280 nm。图中属于Cr2O3的小颗粒的占比较少,证明了Cr2O3的高分散性。由SAED图可知,衍射环的出现表明Cr2O3/CeO2催化剂以多晶态形式存在,四个衍射环分别对应立方相CeO2的(1 1 1)、(2 0 0)、(2 2 0)和(3 1 1)晶面,晶面间距分别为0.325、0.283、0.209和0.185 nm,该数值与于纯CeO2接近,同样证明Cr物种以表面分散的形式为主,与XRD结论一致。
-
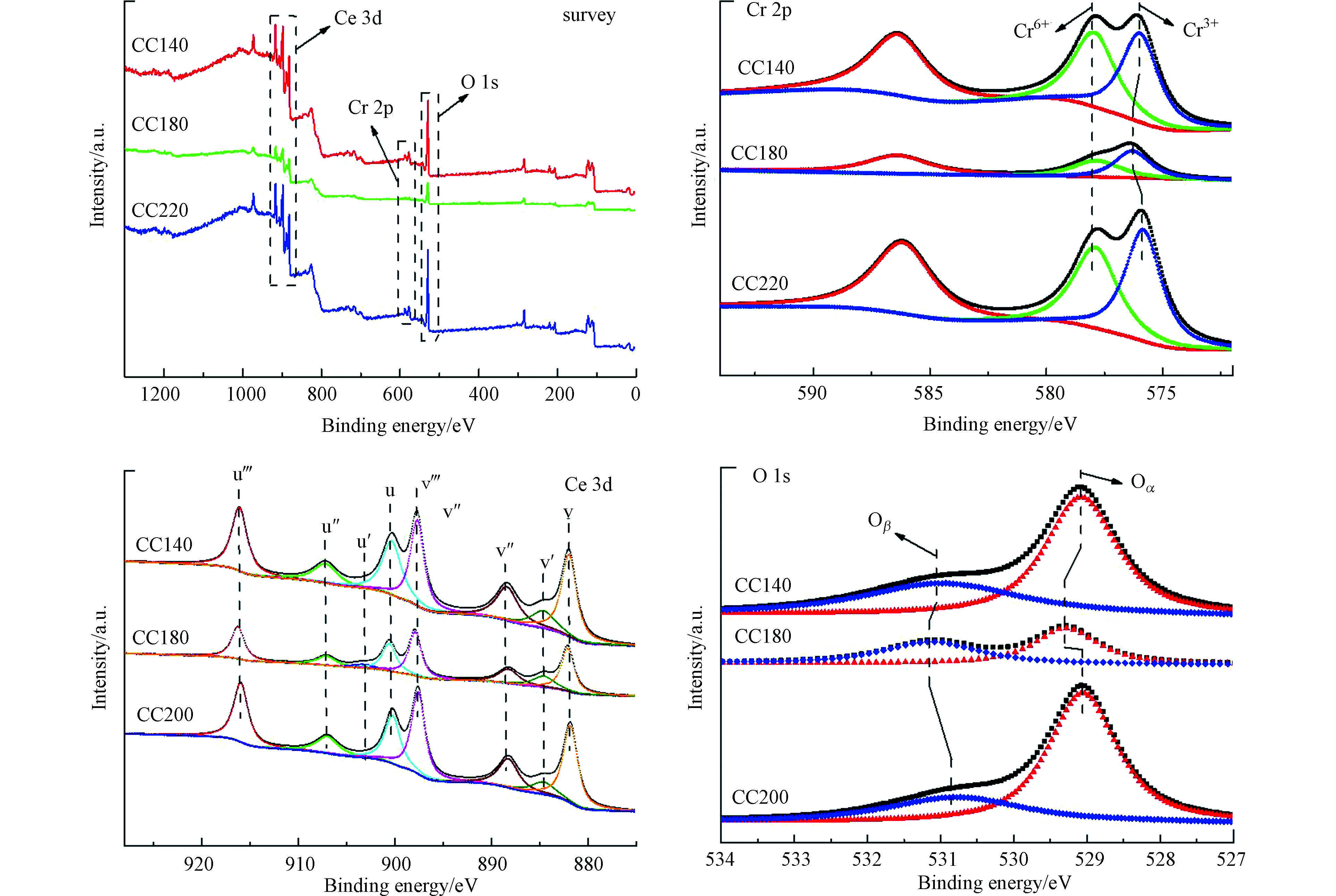
采用XPS对催化剂CC140、CC180和CC200的表面电子形态进行分析,结果如图6所示。
由图6可知,3个催化剂表面均出现了从属于Ce 3d、Cr 2p和O 1s的峰,证实3个催化剂表面均存在Ce、Cr和O元素。由Cr 2p谱图可知,催化剂表面上存在着两种价态的铬,Cr6+和Cr3+,分别处于579.08 eV和576.80 eV[7]。对含Ce的催化剂进行Ce 3d高斯拟合,最终得到3d3/2和3d5/2两类谱峰,分别以v和u作为标记,各4个峰,其中(v, u)、(v’’, u’’)和(v’’’, u’’’)归属于Ce4+的特征峰,(v’, u’)归属于Ce3+的特征峰[16]。从O 1s谱图可知,O 1s可拟合为两种峰,其中低结合能处的峰归属于金属离子键合的晶格氧的特征峰(Oα)[17],高结合能处的峰归属于表面化学吸附氧(Oβ),如O2−,O−等[18]。研究表明[7],催化剂表面的Cr6+和Oβ含量是决定NO催化氧化活性的关键因素,其中催化剂表面的Cr位点为吸附NO的主要活性位,而催化剂表面的Oβ则可以有效地将吸附态NO进行氧化,从而催化剂表面高含量的Cr6+和Oβ有助于NO的催化氧化过程。诸多研究结果证实,表面氧物种(例如Oβ)是由于气态氧吸附在催化剂表面缺陷位的氧空位所形成的[19-21]。而在Cr2O3/CeO2催化剂中,表面氧空位不仅来源于Ce3+与Ce4+之间的转换,也源于催化剂内部晶格尺寸变化所生成的表面缺陷位,但主要来源于前者。在CeO2基材料中,Ce4+大量存在,如果改性后促进了Ce3+的生成,则会增加表明氧空位的含量,从而利于增加表面的Oβ含量。鉴于上述结论,基于峰面积计算可得催化剂表面的Cr6+、Ce3+和Oβ含量,如表2所列。结合表2中的Cr6+、Ce3+和Oβ含量与NO氧化活性结果可知,3个催化剂表面的Cr6+、Ce3+和Oβ含量的变化趋势与其氧化活性的变化趋势相一致,因而表明NO的氧化过程与催化剂表面的Cr6+、Ce3+和Oβ含量密切相关。3个催化剂中CC180具有最高的Cr6+、Ce3+和Oβ含量,因此CC180催化剂呈现出高的NO催化活性。
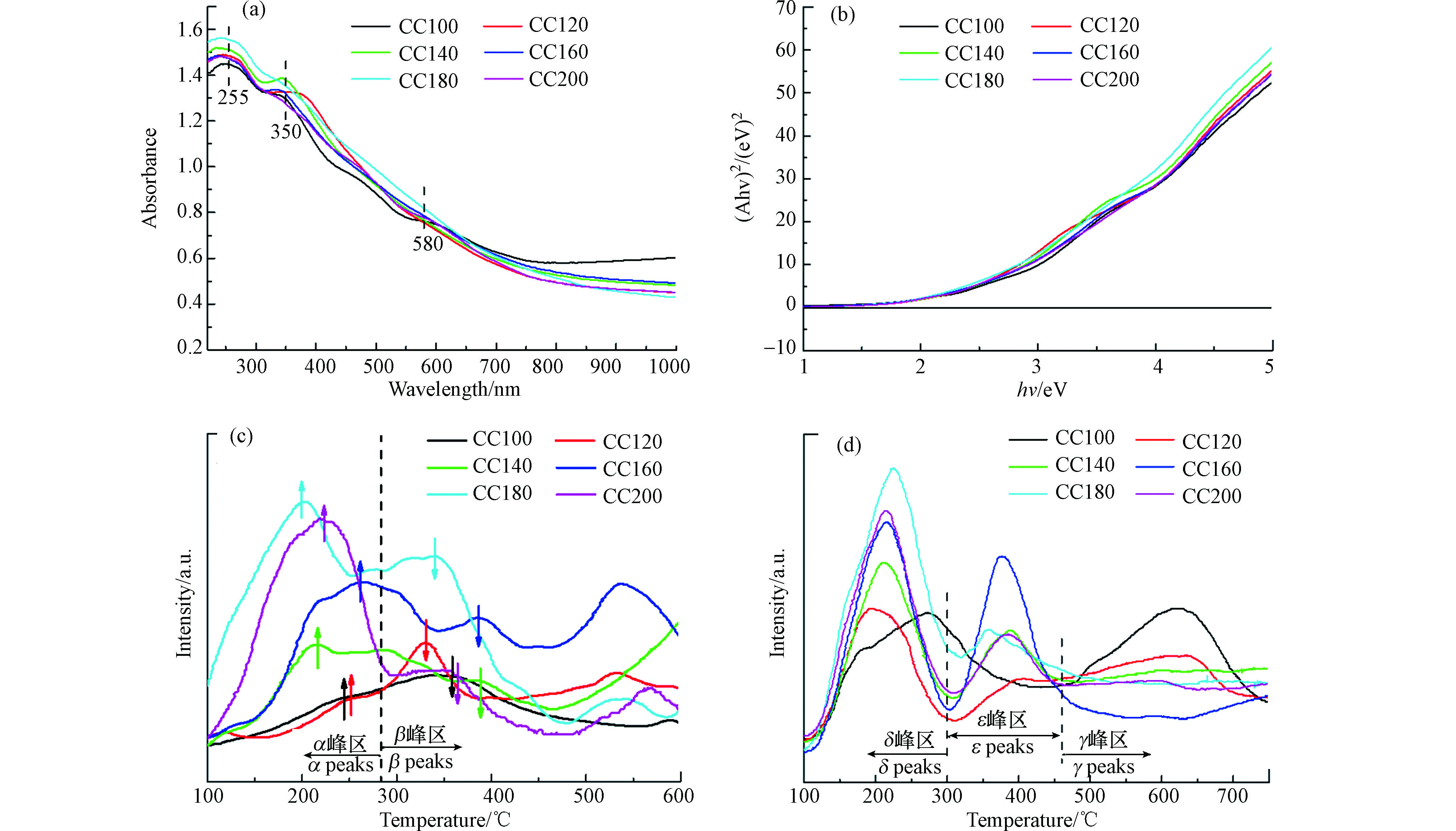
采用UV-vis DRS表征对催化剂表面的电子结构进一步分析,如图7所示。由图7(a)可知,所有催化剂在255 nm和350 nm附近处均出现了吸收峰,其中前者属于O2→Ce3+的电荷跃迁峰[22],后者属于O2→Ce4+的电荷跃迁峰[23]。该现象进一步证明催化剂表面存在Ce3+物种,与XPS结论相一致。此外,所有催化剂在580 nm处额外出现了一个弱峰,该峰属于Cr2O3中的Cr3+的带隙跃迁峰[24]。该峰的出现表明Cr物种以表面分散的形式为主,而峰强度较弱则表明Cr物种的表面分散程度较高,与XRD、TEM结论一致。将图7(a)中的曲线经过Kubelka-Munk公式转换后可得催化剂的禁带宽度值,如图7(b)所示。由图可知,所有催化剂的禁带宽度值之间的差异较小,结合NO氧化活性数据可以推断,禁带宽度值的大小并不是影响NO氧化活性高低的决定因素。
-
采用TPD对不同水热温度制备条件下的催化剂的NO和O2吸附性能进行分析,结果如图7所示。由图7(c)可知,在100—600 ℃范围内,所有催化剂均出现两个NO脱附峰,其中位于200—250 ℃范围内的脱附峰归属于催化剂表面的单齿硝酸盐和桥接硝酸盐的分解峰,而位于300—400 ℃范围内的脱附峰归属于表面桥接硝酸盐和双齿硝酸盐的分解峰[25]。在所有催化剂中,较高水热温度条件下制备的催化剂呈现出较高的脱附峰面积,其中CC180催化剂的峰面积最大,且其脱附峰温较低,因而该催化剂具有最高的低温NO氧化活性。由图7(d)可知,所有催化剂大体呈现出3个O2脱附峰。一般而言,Ce基催化剂表面存在3种活性氧类型,低于300 ℃的峰归属于催化剂表面化学吸附氧,高于500 ℃的峰归属于催化剂内部晶格氧,而介于两者温度之间的峰则归属于催化剂表面的缺陷氧[20]。在所有催化剂中,水热温度高于140 ℃时制备出的催化剂呈现出较高的表面化学吸附氧峰及缺陷氧峰,而这两种类型的氧含量与催化氧化活性呈正相关,因而CC160、CC180和CC200催化剂表现出较高的NO氧化效率。在3个催化剂中,CC180催化剂的化学吸附氧峰强度最高,因此该催化剂具有最高的低温NO氧化活性。
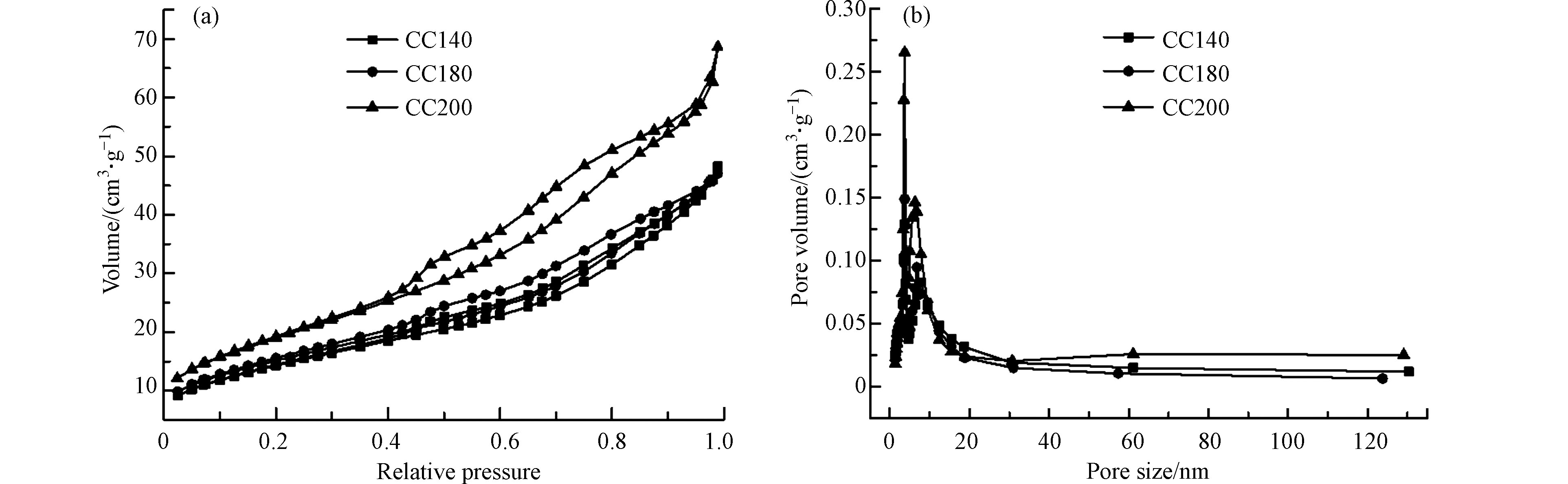
采用BET表征对催化剂CC140、CC180和CC200的比表面积、孔容积和孔径进行分析,测试结果如图8所示和表3所列。由测试结果可知,在3个催化剂中,CC200具有最高的比表面积和孔容积,结合NO氧化性能测试可知,催化剂的比表面积和孔容积与其NO氧化活性并不成正比,由此可以推断,在Cr2O3/CeO2催化剂体系中,较高的比表面积和孔容积并不是促进NO氧化活性提升的关键因素,再结合TPD和XPS结果可知,提升NO和O2的吸附量的主要因素为催化剂表面的Cr6+和Oβ的高含量。
-
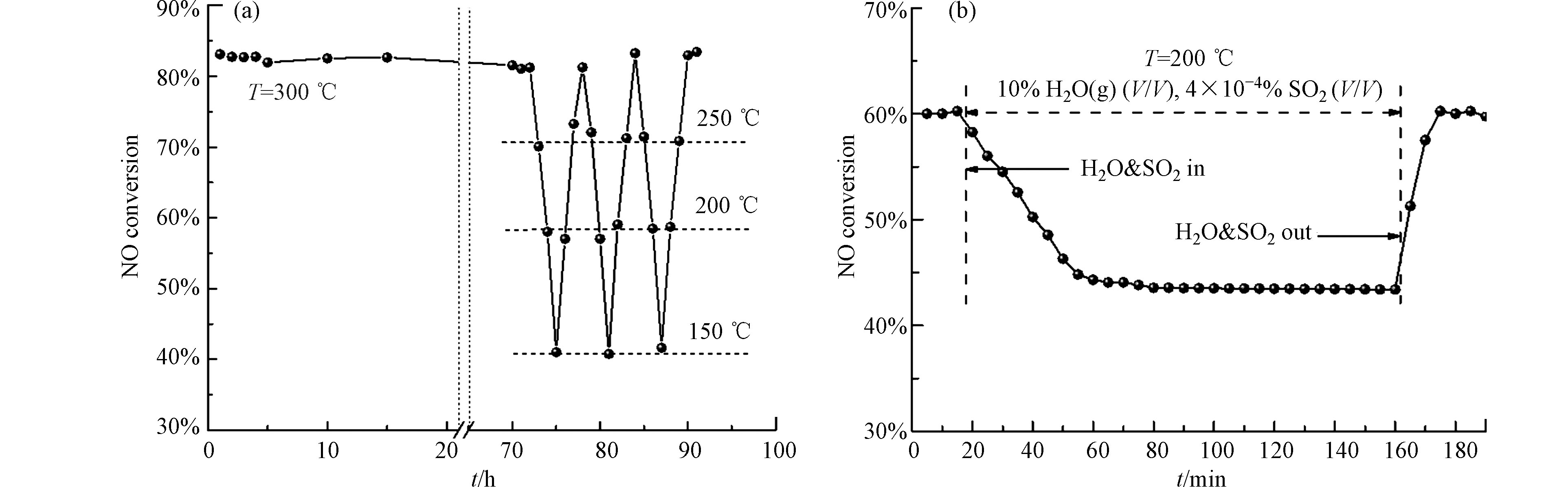
为了推进SCO技术的工业应用进程,选取催化活性最高的CC180催化剂,对其进行稳定性及抗硫抗水性能测试,结果如图9所示。由图9(a)所示,反应温度为300 ℃时,在20 h的反应时间内,CC180催化剂的NO氧化效率下降不明显,且在经过3次“降温-升温”程序后,在每个温度点,催化剂均能保持基本不变的催化活性,这表明CC180催化剂具有较高的稳定性能。催化剂CC180的抗硫抗水测试如图9(b)所示。由图可知,当通入4×10−4 %vol. SO2和10 %vol. H2O(g)的混合气后,催化剂的活性开始下降。通入30 min后,活性不再下降,NO氧化效率稳定的保持在42%左右。当SO2和水蒸气混合气撤出反应体系后,催化剂能够在不到20 min内恢复至初始活性水平。该测试结果表明该催化剂具有较强的自恢复特性,但在SO2和H2O(g)存在时,其NO氧化活性尽管仍能保持在较高的水平,但距离实际应用仍存在一段距离,待进一步提高。
-
(1)采用步骤简单、成本低廉的一锅法制备出纳米颗粒状Cr2O3/CeO2复合氧化物,将其用于催化氧化烟气脱硝研究,并考察了不同水热温度对催化活性的影响。结果表明,水热温度为180 ℃时,催化剂CC180具有最高的氧化效率,反应温度为300 ℃时的氧化效率高达83%,同时该催化剂具有最高的低温催化活性,反应温度200 ℃时氧化效率高达59%。
(2)采用XRD、TEM、XPS、UV-vis、TPD、BET等表征手段对催化剂的结构进行分析。XRD、TEM和UV-vis结果表明,Cr物种主要以表面分散的形式分散在催化剂表面,且适中的水热温度有利于Cr物种的高分散性,增加活性位点数量,促进NO氧化效率,此外,禁带宽度值并不是影响NO氧化活性的关键因素;XPS结果表明,催化剂表面的Cr主要以Cr6+形式存在,且表面存在较高的化学吸附氧Oβ含量,Ce元素主要以Ce4+存在,但存在一定含量的Ce3+;TPD结果表明,较高的水热温度下制备出的催化剂具有较高的低温NO和O2吸附容量,利于NO在催化剂表面的氧化过程;稳定性和抗硫抗水性能测试结果表明,该条件下制备出的催化剂具有较高的稳定性能以及较强的抗硫抗水测试后自恢复特性。
(3)结合NO氧化活性测试与上述等表征结果,可以得出结论:CC180催化剂所呈现的高NO氧化活性与Cr物种的高分散、Cr6+和Oβ的高含量以及较高的反应组分吸附容量有关,其中Cr物种的高分散、Cr6+和Oβ的高含量提升了NO和O2在催化剂表面的吸附容量,是提高催化活性的直接因素。
水热温度对纳米Cr2O3/CeO2复合氧化物低温催化氧化NO性能的影响
Effect of different hydrothermal temperatures on catalytic performance for NO oxidation at low temperature over nano-structured Cr2O3/CeO2
-
摘要: 采用一锅水热法合成了纳米颗粒状铬铈复合氧化物(Cr2O3/CeO2),并将其应用于催化氧化烟气脱硝(SCO),考察了不同水热温度对催化剂低温催化活性的影响。结果表明,当水热温度为180 ℃时制得的催化剂(CC180)具有较高的低温SCO活性,其在200 ℃下NO氧化率高达59%。采用X-射线衍射、透射电镜、X-光电子能谱、程序升温脱附等表征手段探讨了催化剂的结构形貌与催化活性之间的构效关系。结果表明,Cr物种主要以表面分散的形式存在于催化剂表面,同时适中的水热温度利于活性位点Cr物种的表面高分散,提升活性位点数量,而催化剂表面较高的六价铬Cr6+和化学吸附氧Oβ含量则提升了低温下NO和O2的吸附容量,促进了低温下的NO氧化效率。此外,催化剂较高的稳定性及较强的抗硫抗水测试后自恢复性推进了SCO技术的工业化进程。
-
关键词:
- 脱硝 /
- 催化氧化 /
- Cr2O3/CeO2催化剂 /
- 抗硫抗水
Abstract: The Cr2O3/CeO2 composites were synthesized via a one-pot hydrothermal method, and were evaluated for denitration on catalytic oxidation of NO (SCO). The influence of different hydrothermal temperatures on catalytic performance at low temperature was investigated. The results showed that the sample with hydrothermal temperature of 180 ℃ (CC180) displayed the highest activity among all samples, which exhibited 59% oxidation efficiency at 200 ℃. Kinds of characterizations such as X-ray diffraction, transmission electron microscopy, X-ray photoelectron spectroscopy, temperature-programmed desorption, et al. were applied to explore the relationship between the structure and the activity over the catalysts. The results displayed that Cr species mainly existed on the surface of catalysts in the form of surface dispersion, and the proper temperature of hydrothermal process benefits for the well dispersion of Cr species. The high ratios of Cr6+ and chemisorbed oxygen (Oβ) on the surface promoted the adsorption capacity of NO and O2 at low temperature, thus enhancing the NO oxidation efficiency at low temperature. Moreover, the high stability and the strong recovery capacity after the remove of SO2 and H2O of this series catalysts promoted the industrial application progress of SCO technology.-
Key words:
- denitration /
- catalytic oxidation /
- Cr2O3/CeO2 catalyst /
- SO2 & H2O(g) resistance
-

-
表 1 本文制备的Cr2O3/CeO2催化剂与已报道的Mn基和Co基催化剂催化氧化NO的性能对比
Table 1. The comparison of NO oxidation efficiency among the reported Mn-based and Co-based catalysts, and the synthesized Cr2O3/CeO2 catalyst in this work
催化剂
CatalystsNO氧化率/%
NO conversion温度1/℃
Temperature参考文献
ReferenceCr/CeO2 83 300 本文 MnCe 82 220 [13] CoCe 80 300 [14] LaMnO3 78 314 [15] 注:1.该温度为测试温度区间内的最高NO氧化率所对应的反应温度。
Note: 1. The temperature here refers to the reaction temperature corresponding to the highest NO oxidation efficiency in the range of testing temperature.表 2 催化剂CC140、CC180和CC200表面的Cr6+、Ce3+和Oβ含量
Table 2. The Cr6+, Ce3+and Oβ ratios on the surface of CC140, CC180 and CC200 catalysts
催化剂
CatalystsCr6+含量
Cr6+ ratiosCe3+含量
Ce3+ ratiosOβ含量
Oβ ratiosCC140 48.97% 7.40% 38.40% CC180 57.14% 13.72% 54.24% CC200 47.01% 5.83% 29.70% 表 3 催化剂CC140、CC180和CC200的BET比表面积、孔容积和孔径
Table 3. The BET surface area, pore volume and pore size of CC140, CC180 and CC200 catalysts
催化剂
CatalystsBET比表面积/(m2·g−1)
BET surface area孔容积/(cm3·g−1)
Pore volume孔径/nm
Pore sizeCC140 51.9 0.076 3.716 CC180 54.5 0.074 3.714 CC200 69.5 0.109 3.719 -
[1] 周佳丽, 王宝冬, 马静, 等. 锰基低温SCR脱硝催化剂抗硫抗水性能研究进展 [J]. 环境化学, 2018, 37(4): 782-791. doi: 10.7524/j.issn.0254-6108.2017091904 ZHOU J L, WANG B D, MA J, et al. SO2 and H2O poisoning resistance of manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx [J]. Environmental Chemistry, 2018, 37(4): 782-791(in Chinese). doi: 10.7524/j.issn.0254-6108.2017091904
[2] 张先龙, 马康, 蔡程, 等. MnOx/PG低温SCR催化剂二氧化硫中毒及再生特性 [J]. 环境化学, 2019, 38(6): 1403-1412. doi: 10.7524/j.issn.0254-6108.2018090503 ZHANG X L, MA K, CAI C, et al. Sulfur dioxide poisoning and regeneration characteristics of MnOx/PG low temperature SCR catalysts [J]. Environmental Chemistry, 2019, 38(6): 1403-1412(in Chinese). doi: 10.7524/j.issn.0254-6108.2018090503
[3] LI K, TANG X, YI H, et al. Low-temperature catalytic oxidation of NO over Mn-Co-Ce-Ox catalyst [J]. Chemical Engineering Journal, 2012, 192: 99-104. doi: 10.1016/j.cej.2012.03.087 [4] MENG L, WANG J, SUN Z, et al. Active manganese oxide on MnOx-CeO2 catalysts for low-temperature NO oxidation: Characterization and kinetics study [J]. Journal of Rare Earths, 2018, 36(2): 142-147. doi: 10.1016/j.jre.2017.05.017 [5] SHANG D, ZHONG Q. High performance of NO oxidation over Co/Zr0.2Ce0.8O2 catalysts prepared by one-pot method[C]. IOP Conference Series: Earth and Environmental Science. IOP Publishing, 2018, 186(2): 012003. [6] ZHONG L, YU Y, CAI W, et al. Structure-activity relationship of Cr/Ti-PILC catalysts using a pre-modification method for NO oxidation and their surface species study [J]. Physical Chemistry Chemical Physics, 2015, 17(22): 15036-15045. doi: 10.1039/C5CP00896D [7] CAI W, ZHAO Y, CHEN M, et al. The formation of 3D spherical Cr-Ce mixed oxides with roughness surface and their enhanced low-temperature NO oxidation [J]. Chemical Engineering Journal, 2018, 333: 414-422. doi: 10.1016/j.cej.2017.10.002 [8] MA M, HUANG H, CHEN C, et al. Highly active SBA-15-confined Pd catalyst with short rod-like micro-mesoporous hybrid nanostructure for n-butylamine low-temperature destruction [J]. Molecular Catalysis, 2018, 455: 192-203. doi: 10.1016/j.mcat.2018.06.016 [9] ANDREOLI S, DEORSOLA F A, GALLETTI C, et al. Nanostructured MnOx catalysts for low-temperature NOx SCR [J]. Chemical Engineering Journal, 2015, 278: 174-182. doi: 10.1016/j.cej.2014.11.023 [10] VENKATASWAMY P, RAO K N, JAMPAIAH D, et al. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures [J]. Applied Catalysis B:Environmental, 2015, 162: 122-132. doi: 10.1016/j.apcatb.2014.06.038 [11] ATRIBAK I, GUILLEN-HURTADO N, BUENO-LOPEZ A, et al. Influence of the physic-chemical properties of CeO2-ZrO2 mixed oxides on the catalytic oxidation of NO to NO2 [J]. Applied Surface Science, 2010, 256: 7706-7712. doi: 10.1016/j.apsusc.2010.06.042 [12] LEKPHET W, KE T C, SU C, et al. Morphology control studies of TiO2 microstructures via surfactant-assisted hydrothermal process for dye-sensitized solar cell applications [J]. Applied Surface Science, 2016, 382: 15-26. doi: 10.1016/j.apsusc.2016.04.115 [13] SHEN Q, ZHANG L, SUN N, et al. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation [J]. Chemical Engineering Journal, 2017, 322: 46-55. doi: 10.1016/j.cej.2017.02.148 [14] MEGARAJAN S K, RAYALU S, TERAOKA Y, et al. High NO oxidation catalytic activity on non-noble metal based cobalt-ceria catalyst for diesel soot oxidation [J]. Journal of Molecular Catalysis A:Chemical, 2014, 385: 112-118. doi: 10.1016/j.molcata.2014.01.026 [15] WU L, JIANG Q, WANG L, et al. Formation mechanism of yolk-shell LaMnO3 microspheres prepared by P123-template and oxidation of NO [J]. Frontiers of Materials Science, 2019, 13(1): 77-86. doi: 10.1007/s11706-019-0451-6 [16] WANG N, CHU W, ZHANG T, et al. Manganese promoting effects on the Co-Ce-Zr-Ox nano catalysts for methane dry reforming with carbon dioxide to hydrogen and carbon monoxide [J]. Chemical Engineering Journal, 2011, 170(2): 457-463. [17] AVGOUROPOULOS G, IOANNIDES T. Effect of synthesis parameters on catalytic properties of CuO-CeO2 [J]. Applied Catalysis B:Environmental, 2006, 67(1): 1-11. [18] REDDY B M, RAO K N, BHARALI P. Copper promoted cobalt and nickel catalysts supported on ceria-alumina mixed oxide: Structural characterization and CO oxidation activity [J]. Industrial & Engineering Chemistry Research, 2009, 48(18): 8478-8486. [19] TAN R, ZHU Y. Poisoning mechanism of perovskite LaCoO3 catalyst by organophosphorous gas [J]. Applied Catalysis B:Environmental, 2005, 58(1): 61-68. [20] ZHAO Z, YANG X, WU Y. Comparative study of nickel-based perovskite-like mixed oxide catalysts for direct decomposition of NO [J]. Applied Catalysis B:Environmental, 1996, 8(3): 281-297. doi: 10.1016/0926-3373(95)00067-4 [21] WU Y, YU T, DOU B S, et al. A comparative study on perovskite-type mixed oxide catalysts A′xA1-xBO3-λ (A′= Ca, Sr, A= La, B= Mn, Fe, Co) for NH3 oxidation [J]. Journal of Catalysis, 1989, 120(1): 88-107. doi: 10.1016/0021-9517(89)90253-4 [22] SI R, ZHANG Y W, LI S J, et al. Urea-based hydrothermally derived homogeneous nanostructured Ce1-xZrxO2 (x=0-0.8) solid solutions: A strong correlation between oxygen storage capacity and lattice strain [J]. The Journal of Physical Chemistry B, 2004, 108(33): 12481-12488. doi: 10.1021/jp048084b [23] REDDY B M, BHARALI P, SAIKIA P, et al. Structural characterization and catalytic activity of nanosized CexM1-xO2 (M= Zr and Hf) mixed oxides [J]. The Journal of Physical Chemistry C, 2008, 112(31): 11729-11737. doi: 10.1021/jp802674m [24] POOLE JR C P, ITZEL JR J F. Optical reflection spectra of chromia-alumina [J]. The Journal of Chemical Physics, 1963, 39(12): 3445-3455. doi: 10.1063/1.1734213 [25] LIU L J, LIU B, DONG L H, et al. In situ FT-infrared investigation of CO or/and NO interaction with CuO/Ce0.67Zr0.33O2 catalysts [J]. Applied Catalysis B:Environmental, 2009, 90: 578-586. doi: 10.1016/j.apcatb.2009.04.019 -



 下载:
下载: