-
随着世界各地的原子能技术以及原子能工业的快速发展,原子能在军工、医疗、农作物生产、环保等领域中得到了广泛的应用。由于其产生的大量有毒有害废弃物危害到人们的生活环境和人身健康安全,核废料的后期处理问题倍受关注。国内外应用比较广泛的高放射性废物地质处置包含了天然屏障和人工屏障体系[1]。天然屏障主要是指深层的岩石等地质体。这些地质中间介质在整个系统中起到保护污染源和生物圈的两重功能。而人工屏障指的是核废料的包装容器,缓冲、回填材料以及处置库工程构建物[1]。目前用于处理高放射性废弃物地质处理的方法主要是选择一个地质环境好,不易发生地壳运动、水分较少的、与城市较远且远离人类活动区域的地点。该工程通过挖掘出较大面积的水平坑道;在坑道中建造洞室或分支道,再将高放射性废弃物放置在特殊的容器中储存于此,最后利用回填缓冲材料进行永久性的封闭隔离[1]。
工程回填材料实际上是使用硅酸铝盐黏土等矿物质作为铀吸附剂。铀离子具有很强的迁移性。其在地下水的环境中会产生铀酰离子且极易与其他物质产生络合反应。铀酰离子UO22+及其所形成的络合物是高放射性废弃物中最为主要的污染物之一。因此研究铀酰离子在各种吸附剂上的吸附行为是十分必要的。研究铀酰离子在多种矿物质表面的吸附行为有助于对核污染点的放射性铀酰离子的扩散进行评估。从而使人们加深对铀酰离子吸附机理的理解,可以更好更安全地发展核技术,并将它应用于生产活动中,保护地球环境。
国内外许多学者对铀酰离子在各种吸附介质上的吸附行为做了大量的研究[2-7],大致可以分为以下两种,一种是通过采集样品进行实验[8-12],从宏观水平方面对铀酰离子吸附的原理进行阐述;另一种是通过软件进行模拟,使用分子模拟的方法模拟 [2-3, 13-17],研究铀酰离子在各种不同的基地物质上的吸附,继而从原子水平上解释铀酰离子吸附过程的机制。Catalano等[8]的研究表明,在较低的pH和离子强度下,吸附过程中的阳离子交换会比预计的表面络合模拟更加容易。这使得铀酰离子更容易吸附于介质。刘艳等[9]在研究膨润土吸附铀的过程中发现,铀的吸附量随着铀初始浓度的增加而增加,而吸附率则随着初始浓度的增加而减少。当初始浓度确定后,铀的吸附量会随着吸附剂的增加而减少,而吸附率则恰好相反。崔瑞萍等[11]的实验研究表明,在控制一些环境条件不变的情况下,高岭石吸附铀的过程会受到pH的影响。陈阳等[10]也研究了pH对吸附率的影响,当pH值小于7的时候,高岭石对铀的吸附率很低;当pH大于3时,高岭石对于铀的吸附量逐渐上升;直到当pH处于中性附近时,高岭石对于铀的吸附率可达90%[10]。张金流等[12]在研究凹凸棒黏土对于铀的吸附过程中发现,该黏土有很好的铀吸附性能,并且当对凹凸棒黏土进行后期的加热或者酸化处理后可以大幅度的提升其吸附铀的能力。他确定了5%盐酸和400 ℃的热活化温度是最合适的条件。从上述的报道可知酸性条件对高岭石吸附铀不利,而酸化处理有利黏土对于铀的吸附。
许多实验从宏观上测量吸附后溶液里铀含量减少来,从而研究不同环境条件对铀吸附的影响。但是这些研究没有对所吸附物的种类和结构方面给予解析。当表面是由多种吸附构型的物质混合吸附时,通过光谱实验得到的只能是各种吸附结构的平均结构参数。Kremleva等[2]通过密度泛函理论方法对中性高岭石四面体[SiO4]表面和高岭石的八面体[A1O2(OH)4]表面上铀酰离子的吸附进行研究,发现结构中的四面体[SiO4]表面的反应活性比八面体[A1O2(OH)4]表面的反应活性低。Glezakou等[16]计算得到铀与α-Al2O3表面直接配位形成的铀酰内层双齿配位结构是最稳定的吸附构型。中性的α-Al2O3表面去掉两个H质子的缺陷表面作为吸附位。该表面在吸附铀酰离子后重新从吸附的水合铀酰结构获得H原子,而使得铀酰的两个水分子配体转化为氢氧根配体[16]。铀酰倾向于吸附在去质子化的高岭土Al(O)表面,而在高岭土的Si(O)表面的吸附作用比较弱[2-3, 13, 15]。
三水铝石的化学式为Al(OH)3,它在晶体中属于单斜晶系、P21/n空间群,它也是最常见的氢氧化铝矿物之一,从热力学角度来说三水铝石是最稳定的形态[17]。由此可见Al(O)表面具有吸附铀酰离子的特性。三水铝石的结构是由OH—Al—OH配位八面体周期性排列而成的层状重叠结构。其中,Al3+只占据了2/3的八面体空隙。三水铝石有望成为优质的铀吸附剂。
本课题组前期[18]采用密度泛函理论研究铀酰离子在羟基化α-石英(1 0 1)表面的吸附行为。本论文研究将作为之前工作的延续,继续开展铀酰离子与Al(O)表面的作用的研究,进一步解释铀酰离子与矿石表面的吸附行为,从而探究铀酰吸附机制。
-
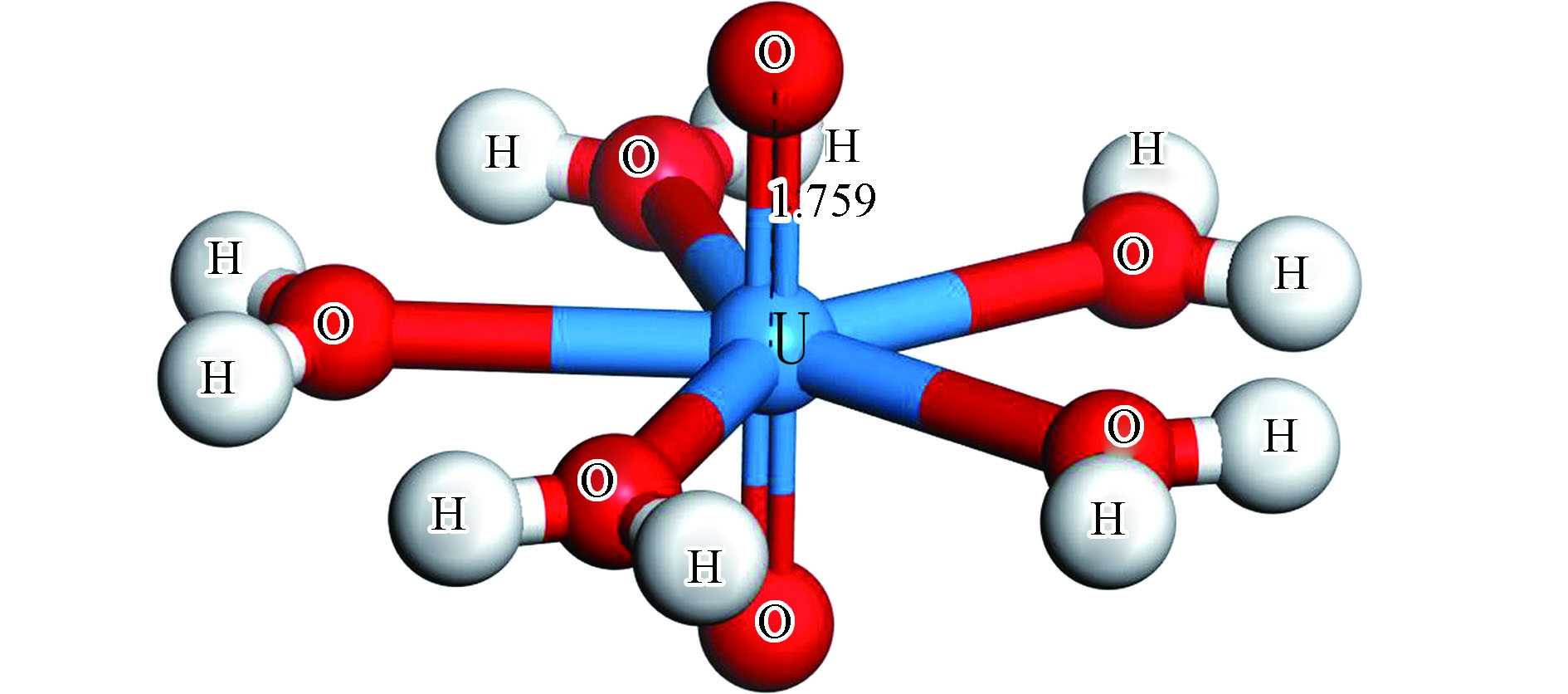
前期课题对铀酰离子的配位方式和配位数进行了系统的研究[18-21],得知铀酰离子极易与其他物质产生络合效应。在地下水中易形成5个水分子与铀酰离子(O=U=O)的铀配位的结构(图1)。前期研究也表明不同配体直接影响到铀酰配合物的稳定性。本课题所研究的三水铝石(0 0 1)面含大量的Al—OH吸附位,很可能与铀酰离子配位。同时,该表面的—OH基团易脱氢得到去质子化的Al—O吸附位。因此有必要考虑三水铝石(0 0 1)吸附位的去质子化程度对铀酰吸附的影响。计算过程中必须保证体系的电荷为零,否则会对超重金属离子的吸附产生较大的影响。所以铀酰离子吸附必须考虑电荷的规避问题。在计算过程采用保持吸附面结构的情况下,对吸附面底层氧进行部分去质子化的方式来保证整个吸附体系显电中性。
本论文研究所构建的完整三水铝石表面基底如图2。课题通过研究三水铝石表面吸附水合铀酰离子过程的能量变化,探究水合铀酰离子在三水铝石 (0 0 1)面上最佳吸附位置和吸附机理。应用Materials Studio软件的Dmol3模块中的广义梯度近似(GGA)中的PW91泛函的交换相关势[22-23],结合双数值基极化函数(DNP)展开的价电子波函数进行周期性密度泛函计算[24-25]。Al、O、H原子采用全电子DNP基组。本论文对铀原子采用内层60个电子冻芯处理,外层32电子为价电子的有效核赝势(ECP60MWB)。结构优化以位移、能量和力的收敛为依据,收敛值分别为5.0×10−4 nm、1.0×10−5 Ha和2.0×10−4 Ha·nm−1。
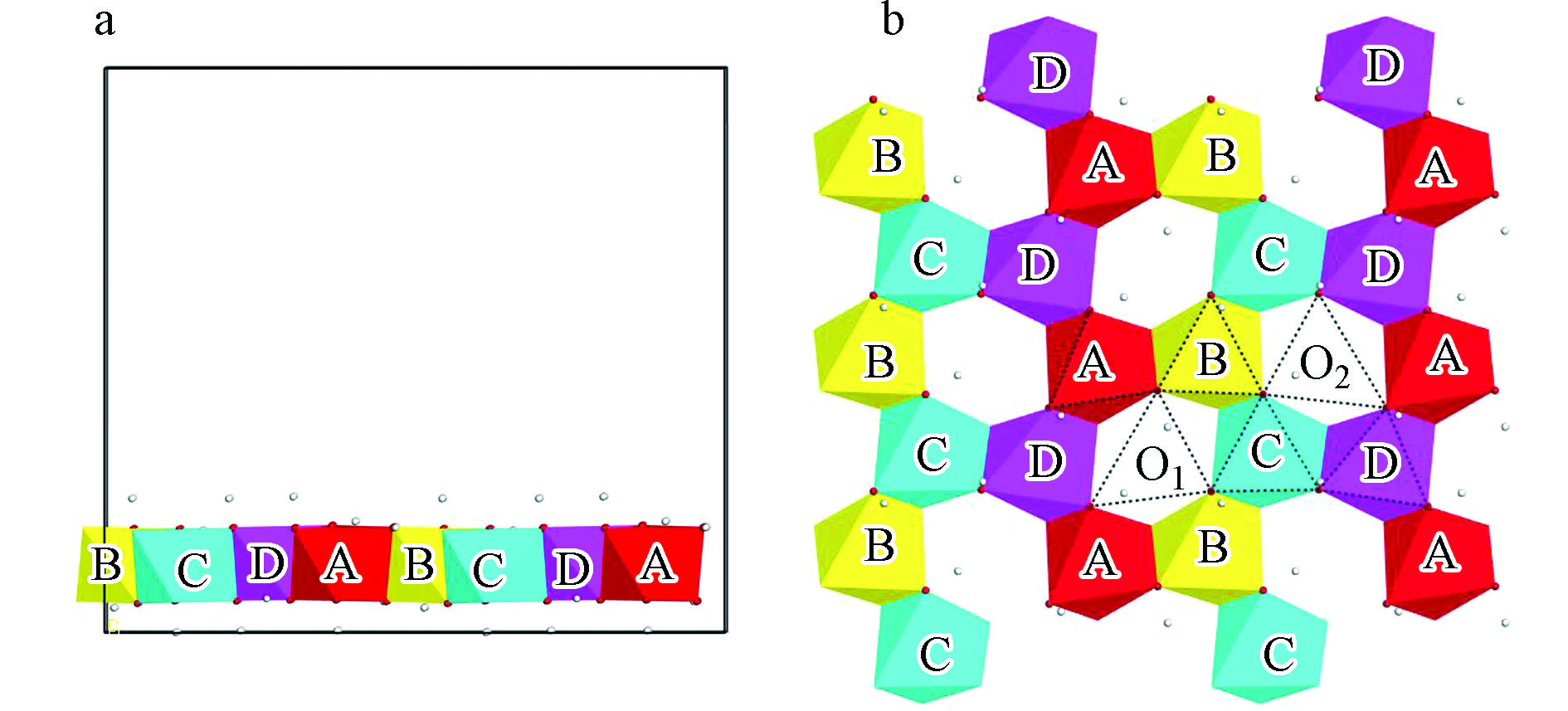
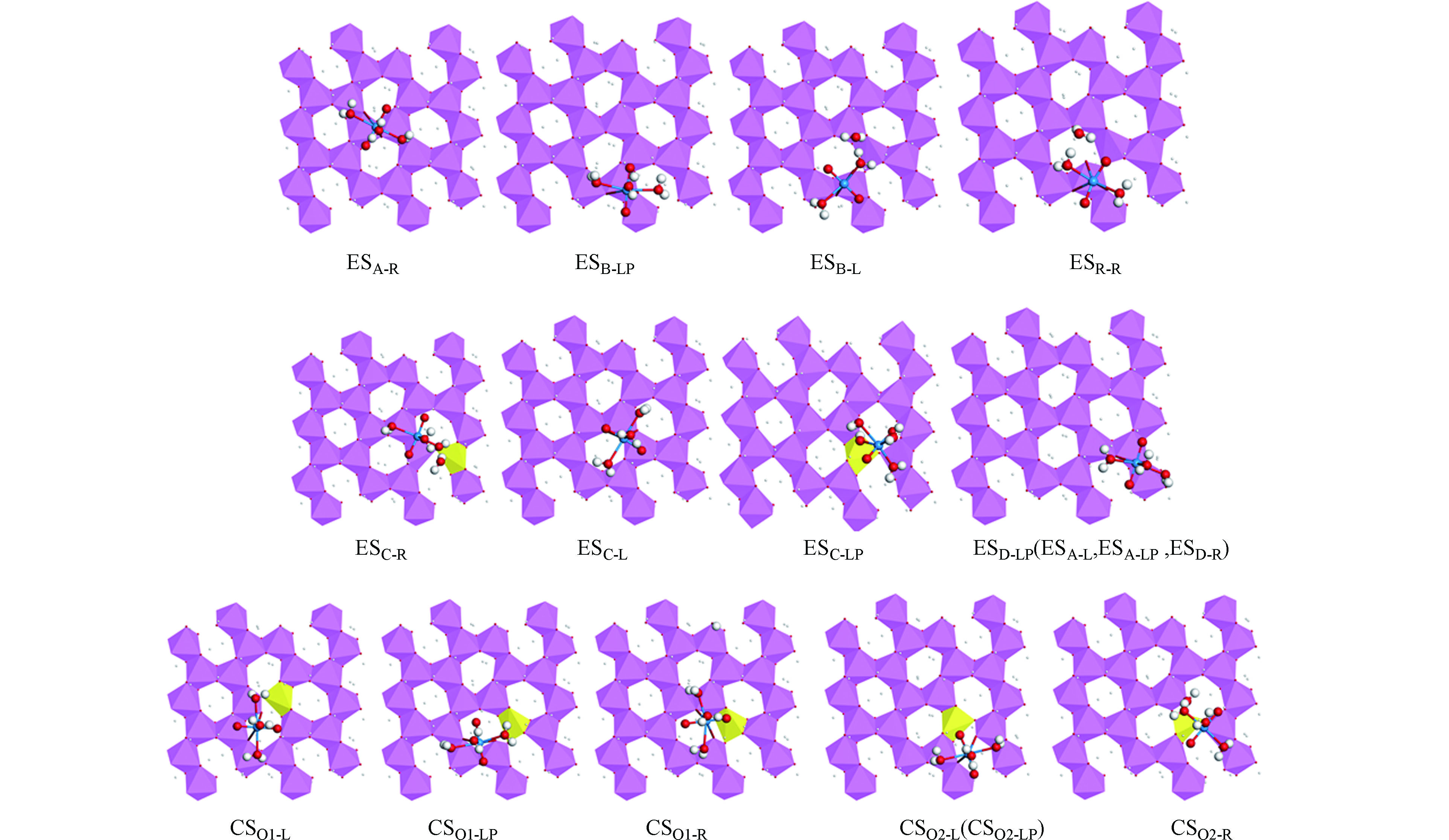
吸附前水合铀酰的结构如图1所示。经过优化后的水合铀酰离子,U=O键的键长为0.1759 nm,U—O键的键长为0.2557 nm,O—U—O的键角为72º。考虑到铀酰配合物的结构属于大分子结构,吸附面太小会造成吸附物之间排斥力的影响。计算过程使用较大的三水铝石(0 0 1)面,并采用2×3的超晶胞结构,同时设置了真空层厚度为15Å。通过对称性分析,得知三水铝石 (0 0 1)面以Al(OH)6八面体通过一定的结构顺序组成的网状结构(图2),根据AlO6八面体的边长不同(边长为八面体中O与O之间的距离)的情况,将边长不一的四个八面体用分别命名为A、B、C、D。吸附位置分为两大类:第一类为两个吸附点处于不同的Al(OH)6八面体上,称为Corner-sharing吸附(简称CS吸附类型);第二类为两个吸附点处于相同的Al(OH)6八面体上,称为Edge-sharing吸附(简称ES吸附类型)。根据3条边的位置分为左方的Left、右方的Right以及下方的Lower Part,可能的吸附位置有以单个Al(OH)6八面体为中心的ES吸附类型,吸附位置个数为3×4=12,分别为ESA-L、ESA-R、ESA-LP、ESB-L、ESB-R、ESB-LP、ESC-L、ESC-R、ESC-LP、ESD-L、ESD-R、ESD-LP和与相邻两个Al(OH)6八面体共享两个Al中心的的CS吸附类型,6个吸附位置分别为CSO1-L、CSO1-R、CSO1-LP、CSO2-L、CSO2-R、CSO2-LP,共18种不同的吸附位置。
-
完整三水铝石(0 0 1)表面基底即表面吸附位置不进行去质子化处理,简称为x=0吸附面。优化得到18个吸附位置的铀酰吸附构型如图3所示,详细的结构参数列于表1。吸附后x=0吸附面的结构发生了一定程度的变形,部分吸附位置上的水合铀酰离子在优化过程向邻近吸附位置转移。ESA-L、ESA-LP 和ESD-R吸附位置的水合铀酰离子转移到了ESD-LP位置,CSO2-LP吸附位置的水合铀酰离子向CSO2-L转移。
大部分吸附构型保持铀酰中心与3个水分子和x=0吸附面的吸附位置形成五配位结构。这与之前关于铀酰离子的配位方式及配位数研究一致。水合铀酰离子在ESB-R吸附位置上发生脱水反应,倾向形成铀氧化物(UO3的形式)沉积在x=0吸附面。其结构中铀与吸附面形成3个稳定的U—O键,吸附后O=U=O键的键角发生变化并出现明显的弯曲。角度由180°转变为158.4°。水合铀酰离子在ESB-L吸附位置上同样发生了脱去配体水的反应,有形成铀氧化物的倾向。上述两种结构特点可看成水合铀酰离子的配位中心铀直接与吸附表面作用。原有的水分子配体与吸附面的—OH存在氢键作用而吸附在表面。研究发现吸附过程也存水合铀酰离子的水分子配体与x=0吸附面发生反应的情况。在ESC-LP和ESC-R吸附位置上,水合铀酰离子的水分子配体通过H原子与x=0吸附面的—OH发生作用生成水,使吸附表面发生脱—OH造成明显结构变化,水合铀酰的水分子配体由于失去H原子转化为氢氧根配体。
经过数据的整理和对比后发现吸附作用后x=0吸附面上的Oa—Ob距离相比于吸附作用前要缩短了。其中ESB-L、CSO1-L、ESB-LP等构型吸附前后Oa—Ob距离缩短的变化较为明显。但也存在个别情况如ESC-R的吸附位置出现了Oa—Ob的距离增加的情况,这主要是由于ESC-R位置吸附后x=0吸附面上发生脱—OH造成明显结构变化。吸附前后吸附物质水合铀酰的结构也发生了较大的变化。吸附前水合铀酰的O=U=O键的键角为180°、铀与两个水配体形成配位键的Oa—U—Ob的键角为72°、U—O配位键长为0.2557 nm。而吸附后O=U=O键的键角出现了明显的弯曲,铀酰配位中心直接与吸附表面作用所形成的Oa—U—Ob键角小于72°。
针对电荷布局分析对于整个吸附过程的原理和理论研究是十分重要的,Mulliken电荷布局分析(MPA)计算得到的相关电荷分布数据见表2。吸附前铀酰中铀所带正电荷量为1.336,x=0吸附面的各个吸附位置的Oa和Ob的电荷量在−0.732— −0.776之间。吸附后配位中心的铀原子所带的正电荷量减少,x=0吸附面配位的Oa和Ob所带的带负电荷量增加。配位中心的铀原子由于得到了电子,所带的正电荷量减少。配位的Oa和Ob得到电子,所带的负电荷量增加。从电荷的变化可看出,水合铀酰整体得到电子。吸附后,x=0吸附面的Al所带正电荷量减少,而吸附面上的H正电荷量增加。当水合铀酰与x=0吸附面吸附时,吸附底物Al(OH)6八面体上H和O离子化程度增大,分别带更多的正电荷和负电荷。电荷的重新分布使表面的H—O和Al—O共价键强度减弱,键长增长。
-
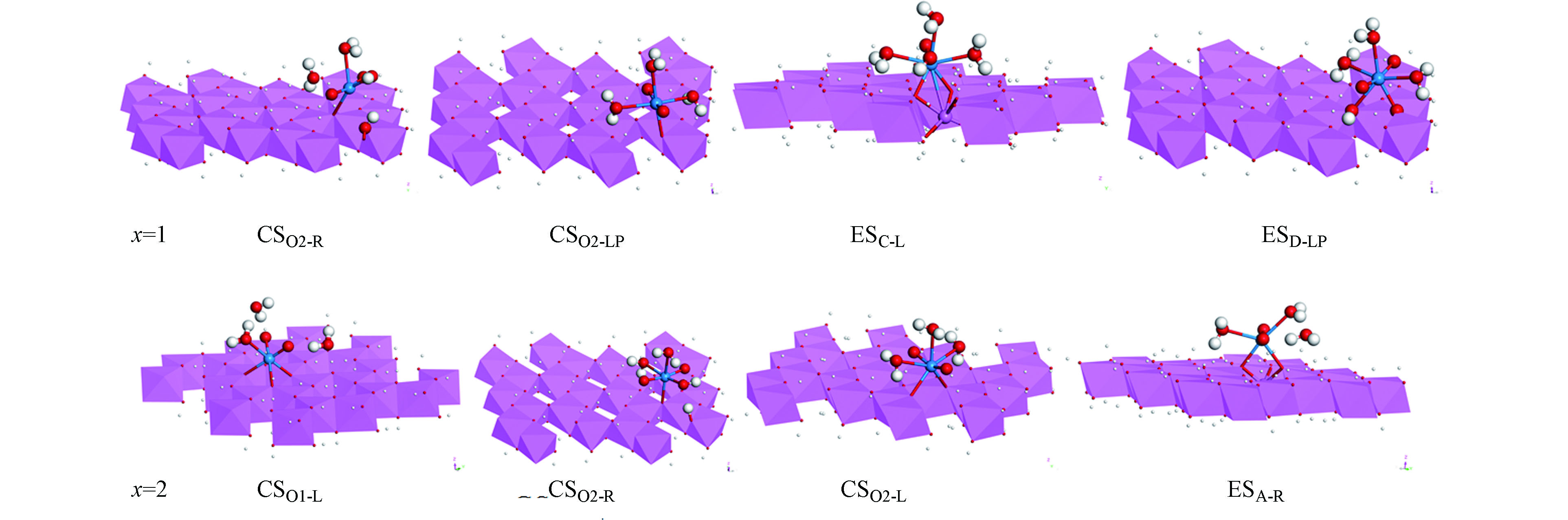
由于吸附过程受到溶液酸碱性的影响,吸附位置很可能发生去质子化现象。因此理论计算采用对三水铝石(0 0 1)表面的吸附位置进行部分去质子化模拟pH值对吸附面的作用。对三水铝石(0 0 1)表面的吸附位置进行不同程度的去质子化得到x=0、x=1和x=2吸附面(x表示三水铝石(0 0 1)表面吸附位置上去质子化的个数)。本论文为了保持整个体系电荷的平衡,在x=0吸附面上,铀酰直接与表面的两个—OH基团配位,并对吸附面底部两个—OH基团进行去质子化;在x=1吸附面上,将与铀酰直接配位的其中一个—OH基团进行去质子化处理,并对吸附面底部一个—OH基团进行去质子化;在x=2吸附面上,将与铀酰直接配位的两个—OH基团进行去质子化。部分去质子化的三水铝石(0 0 1)表面(x=1、2吸附面)的吸附构型如图4所示。在这两个吸附面上,CSO2-R位与水合铀酰离子进行单齿配位。同时铀酰离子的水分子配体失去H原子转化为氢氧根配体使得去质子化的吸附面重新获得H原子。x=1吸附面的大部分吸附构型如(图4)CSO2-LP吸附构型所示。铀酰离子与去质子化的表面O进行单齿配位。同时,铀酰离子的水分子配体与表面有氢键作用。x=2吸附面的大部分吸附构型如(图4)CSO2-L吸附构型所示。铀酰离子与表面的两个去质子化的O形成双齿配位。在x=1、2吸附面上,部分吸附位置的水合铀酰离子发生脱水反应。具有形成铀氧化物的趋势(如x=1 吸附面上的ESC-L吸附位和 x=2 吸附面上的CSO1-L吸附位)。
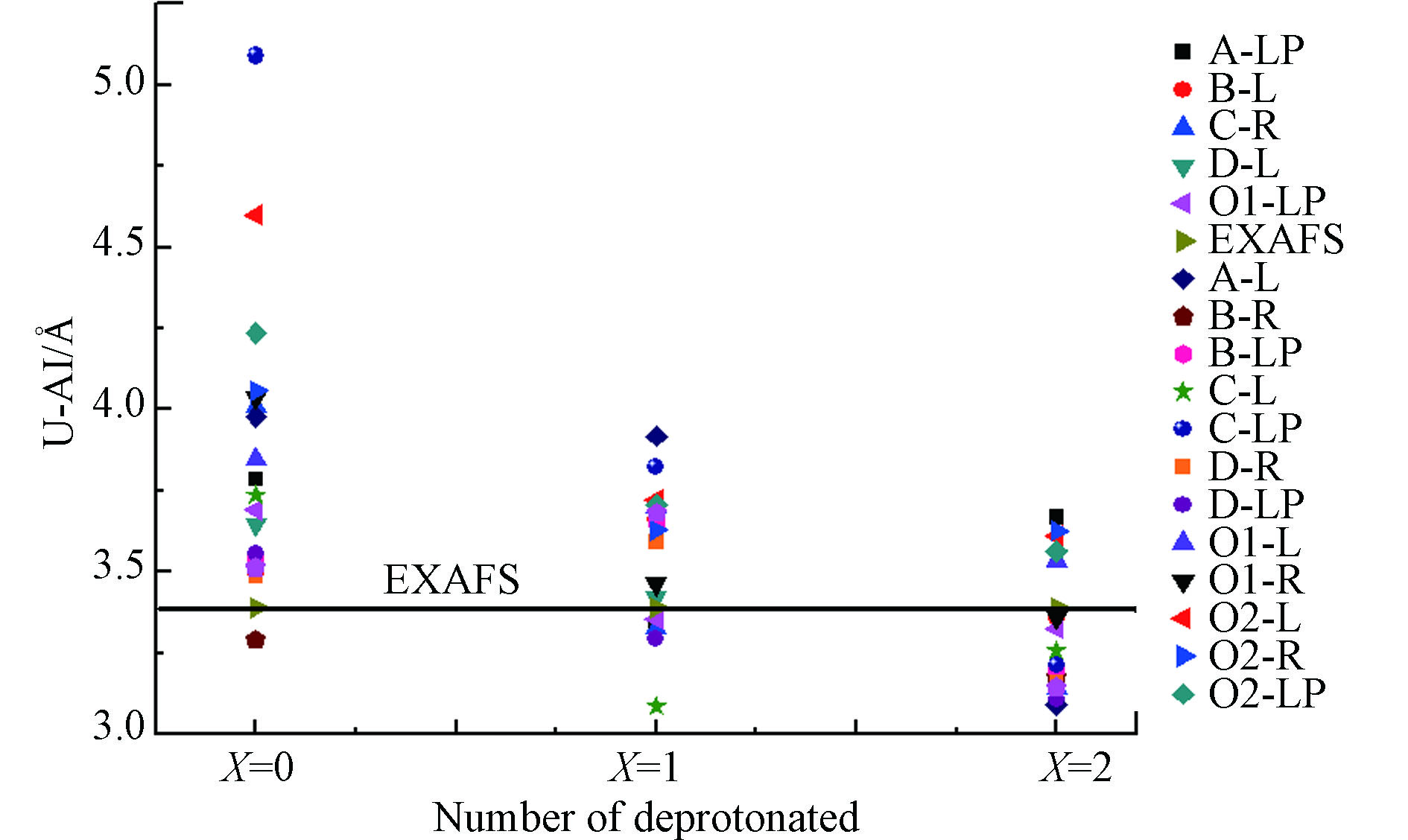
三水铝石(0 0 1)表面吸附位置去质子后与铀酰离子吸附结构的U—Al、U—Oa和U—Ob键键长均缩短。这说明该吸附结构更加稳定,吸附作用更强。在Edge-sharing的D-R吸附结构中,当x=0时U—O1键长为0.2564 nm、U—O2的键长为0.332 nm、U—Al的键长为0.3487 nm;当x=2时U—O1的键长为0.2172 nm、U—O2键长为0.2279 nm、U—Al的键长为0.3161 nm。根据化学键越短,键越稳定的理论,得出x=2吸附面的铀酰离子吸附结构越稳定。通过EXAFS实验测试分析[26]得出的RU—Al≈0.339 nm。表3中列出了铀酰在三水铝石(0 0 1)面吸附过程中不同质子化对U—Al距离的影响。图5为吸附构型的U—Al距离变化图。从图5可看出,质子化的3个面中,以x=0到 x=2,U—Al的距离整体呈下降的趋势,并且与实验结果更为接近。
水合铀酰在x=1和x=2吸附面吸附结构的Mulliken电荷布居分析(MPA)计算得到相关电荷分布数据见表4。吸附前铀酰中铀所带正电荷量为1.336,x=0吸附面的各个吸附位置的Oa和Ob的负电荷量在−0.732— −0.776之间。随着去质子化程度的增加,x=1和x=2吸附面上的吸附的水合铀酰整体得到电子,铀所带的正电荷量明显减少,配位的Oa和Ob所带的带负电荷量进一步增加,Al所带正电荷量也总体减少了。由此可见,当水合铀酰在x=1、2吸附面吸附时,吸附底物Al(OH)6八面体上氧原子离子化程度增大,带更多的负电荷。这使吸附面H—O和Al—O共价键减弱;O与水合铀酰的铀原子成键,最终形成稳定吸附结构。通过对比吸附面上吸附前后H—O和Al—O的键长,存在键长增大的趋势,这与电荷分析一致。
-
对吸附结构的进一步分析研究发现,三水铝石(0 0 1)表面不同吸附位置与铀酰离子的成键方式分为单齿配位吸附、双齿配位吸附和形成铀氧化物吸附。为了能比较直观地比较每个吸附位置稳定性,现将不同吸附位与铀酰离子的吸附特征和吸附能量的计算结果列于表5。从x=0吸附位的吸附能可以看出ESB-L、ESC-R和ESD-R吸附位能负值较大,所以这3个是比较稳定的吸附位置;反之吸附能负值较小的是CSO1-LP、ESC-LP和ESO1-L这3个吸附位;而其他的吸附位置(包括了:ESA-L、ESA-LP、ESB-R、ESB-LP、ESC-L、ESD-LP等)的吸附能负值则变化较为平稳。在x=0吸附面上,吸附位的吸附能最大差值在40KJ·mol−1左右,Edge-sharing的吸附总体比Corner-sharing吸附的更稳定,并且以双齿配位吸附为主;在x=1吸附面上,仍然显现出大部分的Edge-sharing的吸附比Corner-sharing吸附的更稳定,该面以单齿配位吸附为主;在x=2吸附面上,各个吸附位均表现出良好的吸附能力,该面以双齿配位吸附为主,但形成铀氧化物吸附的可能性增大。
-
在本论文采用周期性密度泛函理论对铀酰在三水铝石(0 0 1)表面的吸附进行了研究。不同吸附构型铀酰中的键长以及键角的变化,都说明了在铀酰吸附于三水铝石(0 0 1)表面后整体结构趋于稳定。电荷分析发现,三水铝石(0 0 1)表面发生铀酰离子吸附后的铀所带正电荷数相比于吸附前有所减少,而直接配位的氧原子所带负电荷数增加。三水铝石(0 0 1)表面与铀酰离子通过配位键的作用形成稳定吸附。发生吸附配位后吸附面的电荷分布发生变化,吸附底物Al(OH)6八面体上氧原子离子化程度增大,带更多的负电荷,并通过减弱吸附面H—O和Al—O共价键强度,增强表面O与水合铀酰的配位键强度,最终形成稳定吸附结构。考虑不同质子化对于铀酰吸附在三水铝石(0 0 1)表面的影响,优化计算了去除不同质子数x=1、2时的吸附构型。随着去质子化程度的增加,各个吸附位均表现出良好的吸附能力,吸附能变大。三水铝石(001)表面不同吸附位置与铀酰离子成键的主要方式分为单齿配位吸附、双齿配位吸附和形成铀氧化物吸附。
水合铀酰离子在三水铝石(0 0 1)表面的吸附
Adsorption of hydrated uranyl on gibbsite (0 0 1) surface
-
摘要: 本文采用周期性密度泛函理论研究三水铝石(0 0 1)面吸附水合铀酰离子过程中的能量和结构的变化以及质子化对吸附的影响。对三水铝石(0 0 1)表面的吸附位置进行不同程度的去质子化,得到x=0、x=1和x=2吸附面(x表示三水铝石(0 0 1)表面吸附位置上去质子化的个数)。x=0吸附面上各吸附位的吸附能最大差值在40 KJ·mol-1左右,Edge-sharing吸附总体比Corner-sharing吸附的更稳定,并且以双齿配位吸附为主。在x=1吸附面的吸附位上,也显现出大部分的Edge-sharing吸附比Corner-sharing吸附得更稳定。该面以单齿配位吸附为主。在x=2吸附面上,各个吸附位均表现出良好的吸附能力。该面以双齿配位吸附为主,但形成铀氧化物吸附的可能性增大。三水铝石(0 0 1)表面与铀酰离子通过配位键的作用形成稳定吸附。发生吸附配位后吸附面的电荷分布发生变化。吸附底物Al(OH)6八面体上氧原子离子化程度增大即带更多的负电荷。吸附通过减弱三水铝石表面H—O和Al—O共价键强度,增强其表面氧与水合铀酰的配位键强度,来形成稳定吸附结构。随着去质子化程度的增加,各个吸附位均表现出良好的吸附能力,吸附能变大。Abstract: In this paper, periodic density functional theory was used to study the changes of energy and structure during the adsorption of hydrated uranyl ions on gibbsite (0 0 1) surface and the effect of protonation on adsorption. The adsorption surfaces x=0, x=1 and x=2 were obtained by deprotonating the adsorption sites on the gibbsite (0 0 1) surface in different degrees (x represents the number of deprotonations on the adsorption sites of gibbsite (0 0 1) surface). For x=0 adsorption surface, the maximum difference of adsorption energy was about 40 KJ·mol-1, and the adsorption of Edge-sharing site was generally more stable than that of Corner-sharing site, moreover the dominant adsorption type was bi-dentate coordination. For x=1 adsorption surface, it still showed that most of the Edge-sharing adsorption was more stable than the Corner-sharing adsorption, but mono-dentate coordination adsorption was dominated on this surface. It could also find that x=2 adsorption surface showed good adsorption capacity. The surface was mainly bidentate coordination adsorption, and the possibility to form uranium oxide adsorption increases. The surface of gibbsite (0 0 1) formed stable adsorption with uranyl ion through coordination bond. After adsorption coordination, the charge distribution on the adsorption surface changed, and the ionization degree of oxygen atoms on the adsorption substrate Al(OH)6 octahedron increased, that was, it carried more negative charges. By weakening the covalent bonds strength between H—O and Al—O on the adsorption surface, the coordination bond strength between surface oxygen and hydrated uranyl was enhanced, and finally a stable adsorption structure was formed. With the increase of deprotonation degree, each adsorption site showed good adsorption capacity, and the adsorption energy become larger.
-
Key words:
- gibbsite /
- density functional theory /
- uranyl adsorption
-

-
表 1 吸附前后完整三水铝石(0 0 1)表面(x=0吸附面)和吸附物质铀酰结构的参数变化
Table 1. The parameters changes in gibbsite (0 0 1) surface(Adsorption surface x=0)and the hydrated uranyl after adsorptions
吸附位
Adsorption sites吸附前Before-adsorption 吸附后After-adsorption O—O/nm O—O/nm O=U=O/(°) Oa—U—Ob/(°) U—Oa/nm U—Ob/nm ESA-L 0.2747 0.2555 159.6 54.0 0.3260 0.2606 ESA-R 0.2716 0.2612 166.8 60.5 0.2536 0.2646 ESA-LP 0.2828 — 161.3 45.8 0.3000 0.3950 ESB-L 0.2824 0.2584 165.2 63.3 0.2530 0.2390 ESB-R 0.2899 0.2765 153.4 66.1 0.2530 0.2540 ESB-LP 0.2716 0.2579 167.4 60.1 0.2650 0.2500 ESC-L 0.2838 0.2607 154.9 62.0 0.2720 0.2550 ESC-R 0.2851 — 173.3 84.1 0.2460 0.2190 ESC-LP 0.2770 — 172.4 29.6 0.4360 0.2220 ESD-L 0.2749 0.2652 151.2 63.4 0.2490 0.2600 ESD-R 0.2754 0.2643 166.6 52.4 0.2560 0.3320 ESD-LP 0.2833 0.2639 167.8 65.2 0.2410 0.2490 CSO1-L 0.3458 0.279 168.7 67.5 0.2640 0.2350 CSO1-R 0.2956 0.2960 158.2 73.0 0.2580 0.2380 CSO1-LP 0.3139 0.2941 159.4 72.8 0.3870 0.2380 CSO2-L 0.2983 0.2794 160.3 67.4 0.2600 0.2450 CSO2-R 0.3430 — 173.0 50.3 0.2390 0.5010 CSO2-LP 0.3198 0.2825 161. 9 62.3 0.2590 0.3390 表 2 吸附前后完整三水铝石(0 0 1)晶面(x=0吸附面)和水合铀酰离子的Mulliken电荷布局分析
Table 2. Mulliken charges distributions on gibbsite (0 0 1) surface (Adsorption Surface x=0) and hydrated uranyl ion
Oa(前) Ob(前) Al(前) Ha(前) Hb(前) Oa(后) Ob(后) U(后) Al(后) Ha(后) Hb(后) 铀酰离子
Urayl ionU(前) −1.336 — — — — — — — — — ESA-L −0.750 −0.732 1.418 0.265 0.285 −0.768 −0.775 1.270 1.368 0.332 0.333 ESA-R
ESA-LP−0.750
−0.760−0.760
−0.7321.418
1.4180.293
0.2650.316
0.293−0.821
−0.833−0.821
−0.8041.260
1.2611.349
1.3970.380
0.3460.402
0.362ESB-L −0.776 −0.760 1.417 0.293 0.277 −0.784 −0.826 1.315 1.378 0.373 0.350 ESB-R −0.772 −0.776 1.417 0.316 0.277 −0.831 −0.799 1.255 1.364 0.400 0.343 ESB-LP −0.772 −0.760 1.417 0.293 0.316 −0.754 −0.761 1.283 1.234 0.371 0.372 ESC-L −0.772 −0.776 1.417 0.316 0.277 −0.773 −0.804 1.274 1.304 0.358 0.372 ESC-R
(表面脱-OH)−0.772 −0.746 1.417 — — −0.821 −0.728 1.272 1.297 — — ESC-L
(表面脱-OH)−0.776 −0.746 1.417 — — −0.777 −0.810 1.258 1.275 — — ESD-L −0.746 −0.732 1.417 0.265 0.264 −0.808 −0.774 1.273 — — — ESD-R −0.732 −0.750 1.417 0.265 0.285 −0.756 −0.781 1.286 1.317 0.338 0.348 ESD-LP −0.746 −0.750 1.417 0.264 0.285 −0.762 −0.814 1.274 1.326 0.308 0.367 CSO1-L −0.760 −0.750 1.417/
1.4180.293 0.285 −0.756 −0.816 1.225 1.372/
1.3790.379 0.392 CSO1-R −0.776 −0.760 1.417/
1.4180.293 0.277 −0.790 −0.794 1.293 1.282/
1.2590.387 0.371 CSO1-LP −0.750 −0.776 1.418/
1.4180.285 0.277 −0.809 −0.768 1.263 1.380/
1.3700.384 0.332 CSO2-L −0.772 −0.746 1.417/
1.4180.264 0.316 −0.805 −0.754 1.249 1.262/
1.3690.336 0.392 CSO2-R −0.746 −0.732 1.417/
1.4180.264 0.265 −0.822 −0.755 1.248 1.347/
1.3610.380 0.289 CSO2-LP −0.772 −0.732 1.417/
1.4170.316 0.265 −0.805 −0.745 1.243 1.360/
1.3820.392 0.300 表 3 质子化过程中U—Al距离变化
Table 3. U-Al distance on depronated sites of gibbsite surface after adsorptions
吸附位Adsorption site x=0 x=1 x=2 U—Al/nm U—Alb/nm U—Al/nm U—Alb/nm U—Al/nm U—Alb/nm ESA-L 0.3977 — 0.3918 — 0.3092 — ESA-RESA-LP 0.35130.3785 — 0.36820.3336 — 0.31450.3670 — ESB-L 0.3495 — 0.3662 — 0.3356 — ESB-R 0.3290 — 0.3698 — 0.3.175 — ESB-LP 0.3539 — 0.3652 — 0.3200 — ESC-L 0.3736 — 0.3086 — 0.3256 — ESC-R 0.4011 — 0. 3330 — 0.3142 — ESC-LP 0.5089 — 0.3824 — 0.3212 — ESD-L 0.3648 — 0.3420 — 0.3551 — ESD-R 0.3487 — 0.3592 — 0.3161 — ESD-LP 0.3555 — 0.3294 — 0.3109 — CSO1-L 0.3848 0.3957 0.3699 0.4584 0.3536 0.3585 CSO1-R 0.4037 0.4178 0.3462 0.4176 0.3361 0.3453 CSO1-LP 0.3691 0.3725 0.3354 0.4099 0.3274 0.3326 CSO2-L 0.4599 0.4689 0.3721 0.4002 0.3613 0.3618 CSO2-R 0.4057 0.5438 0.3629 0.4005 0.3625 0.4775 CSO2-LP 0.4236 0.4771 0.3709 0.4650 0.3564 0.3656 表 4 去质子化三水铝石(0 0 1)表面(x=1、x=2吸附面)和水合铀酰离子的部分原子电荷分析
Table 4. Mulliken charges distributions of atoms on deprotonated gibbsite (0 0 1) surface (x=1, 2 adsorption surface) and hydrated uranyl ion
吸附位
Adsorption sitex=1吸附面 x=2吸附面 Oa(后) Ob(后) U(后) Al(后) Oa(后) Ob(后) U(后) Al(后) ESA-L −0.806 −0.735 1.190 1.303 −0.832 −0.804 1.203 1.291 ESA-R −0.816 −0.800 1.192 1.286 −0.806 −0.805 1.218 1.319 ESA-LP −0.809 −0.775 1.191 1.280 −0.840 −0.755 1.171 1.301 ESB-L −0.835 −0.817 1.217 1.296 −0.853 −0.753 1.206 1.266 ESB-R −0.844 −0.797 1.210 1.287 −0.858 −0.875 1.148 1.308 ESB-LP −0.801 −0.811 1.199 1.317 −0.922 −0.804 1.189 1.302 ESC-L −0.798 −0.731 1.271 1.327 −0.928 −0.814 1.216 1.337 ESC-R −0.801 −0.768 1.183 1.267 −0.845 −0.810 1.177 1.321 ESC-LP −0.803 −0.787 1.204 1.301 −0.871 −0.805 1.242 1.333 ESD-L −0.800 −0.795 1.233 1.302 −0.819 −0.781 1.167 1.344 ESD-R −0.805 −0.729 1.180 1.268 −0.842 −0.797 1.232 1.353 ESD-LP −0.790 −0.768 1.199 1.257 −0.791 −0.723 1.309 1.328 CSO1-L −0.811 −0.815 1.211 1.303/1.284 −0.813 −0.786 1.320 1.315/1.296 CSO1-R −0.823 −0.796 1.185 1.290/1.290 −0.803 −0.808 1.231 1.312/1.308 CSO1-LP −0.794 −0.789 1.210 1.300/1.282 −0.817 −0.778 1.280 1.316/1.286 CSO2-L −0.798 −0.782 1.210 1.280/1.255 −0.864 −0.886 1.235 1.315/1.281 CSO2-R −1.746 −1.740 1.215 1.262/1.286 −0.838 −0.759 1.179 1.327/1.281 CSO2-LP −0.827 −0.823 1.212 1.307/1.269 −0.837 −0.810 1.250 1.302/1.275 表 5 不同吸附位的吸附特征与吸附能计算
Table 5. The adsorption characteristics and the calculated adsorption binding energy in different sites
吸附位
Adsorption sitex=0 Eb/(kJ·mol−1) x=1 Eb/(kJ·mol−1) x=2 Eb/(kJ·mol−1) ESA-R 双齿配位吸附 −950.1 单齿配位吸附 −1053.1 双齿配位吸附 −1144.4 ESA-L 双齿配位吸附 −931. 8 双齿配位吸附 −1070.6 双齿配位吸附 −1101.2 ESA-LP 双齿配位吸附 −927.8 单齿配位吸附 −1042.3 单齿配位吸附,表面得到H −1199.1 ESC-L 双齿配位吸附 −931.8 单齿配位吸附 −1031.4 双齿配位吸附 −1117.5 ESC-R 双齿配位吸附,表面脱羟基 −976.4 双齿配位吸附 −1073.9 双齿配位吸附 −1140.2 ESC-LP 双齿配位吸附,表面脱羟基 −896.2 单齿配位吸附 −1005.6 双齿配位吸附 −1118.8 ESD-LP 双齿配位吸附 −938.1 双齿配位吸附 −1072.6 铀氧化物吸附 −1159.2 ESD-R 双齿配位吸附 −967.0 单齿配位吸附 −1053.9 双齿配位吸附 −1161.4 ESD-L 双齿配位吸附 −847.8 单齿配位吸附 −1059.1 单齿配位吸附,表面得到H −1178.3 ESB-LP 双齿配位吸附 −930.2 单齿配位吸附 −1076.8 双齿配位吸附 −1119.7 ESB-R 铀氧化物吸附 −925.9 单齿配位吸附 −1039.3 双齿配位吸附 −1086.1 ESB-L 双齿配位吸附,失去一个水分子配体 −970.7 单齿配位吸附 −1072.4 单齿配位,表面得到H −1192.6 CSO1-L 双齿配位吸附 −908.6 单齿配位吸附 −992.4 铀氧化物吸附 −1160.9 CSO1-R 双齿配位吸附 −930.3 单齿配位吸附 −1088.1 铀氧化物吸附 −1160.0 CSO1-LP 双齿配位吸附 −867.7 单齿配位吸附 −1037.5 铀氧化物吸附 −1148.0 CSO2-L 双齿配位吸附 −942.4 单齿配位吸附,表面得到H −1038.5 双齿配位吸附 −1110.9 CSO2-R 双齿配位吸附 −923.0 单齿配位吸附 −1048.7 单齿配位吸附,表面得到H −1181.5 CSO2-LP 双齿配位吸附 −949.1 单齿配位吸附 −1073.4 双齿配位吸附 −1141.8 -
[1] 周焱. 国内外高放废物地质处置的介绍及国内进展 [J]. 中国机械, 2014, 7: 165-166. ZHOU Y. Introduction and development of geological disposal of high level radioactive waste in the domestic and foreign country [J]. Machine China, 2014, 7: 165-166(in Chinese).
[2] KREMLEVA A, KRüGER S, RöSCH N. Uranyl adsorption at (010) edge surfaces of kaolinite: A density functional study [J]. Geochimica Et Cosmochimica Acta, 2011, 75(3): 706-718. doi: 10.1016/j.gca.2010.10.019 [3] YANG W, ZAOUI A. Uranyl adsorption on (0 0 1) surfaces of kaolinite: A molecular dynamics study [J]. Appl Clay Sci, 2013, 80-81: 98-106. doi: 10.1016/j.clay.2013.04.007 [4] STEWART B D, MAYES M A, FENDORF S. Impact of uranyl-calcium-carbonato complexes on uranium(VI) adsorption to synthetic and natural sediments [J]. Environ Sci & Technol, 2010, 44(3): 928-934. [5] GALINDO C, NERO M D, BARILLON R, et al. Mechanisms of uranyl and phosphate (co)sorption: Complexation and precipitation at [alpha]-Al2O3 surfaces [J]. J Colloid Interf Sci, 2010, 347(2): 282-289. doi: 10.1016/j.jcis.2010.03.045 [6] COMARMOND M J, PAYNE T E, HARRISON J J, et al. Uranium sorption on various forms of titanium dioxide – Influence of surface area, surface charge, and impurities [J]. Environ Sci & Technol, 2011, 45(13): 5536-5542. [7] DRISKO G L, CHEE KIMLING M, SCALES N, et al. One-Pot preparation and uranyl adsorption properties of hierarchically porous zirconium titanium oxide beads using phase separation processes to vary macropore morphology [J]. Langmuir, 2010, 26(22): 17581-17588. doi: 10.1021/la103177h [8] CATALANO J G, BROWN G E. Uranyl adsorption onto montmorillonite: Evaluation of binding sites and carbonate complexation [J]. Geochimica Et Cosmochimica Acta, 2005, 69(12): 2995-3005. doi: 10.1016/j.gca.2005.01.025 [9] 刘艳, 易发成, 王哲. 膨润土对铀的吸附研究 [J]. 非金属矿, 2010, 33(1): 52-53,7. doi: 10.3969/j.issn.1000-8098.2010.01.018 LIU Y, YI F C, WANG Z. Study on sorption of bentonite to uranium [J]. Non-Metallic Mines, 2010, 33(1): 52-53,7(in Chinese). doi: 10.3969/j.issn.1000-8098.2010.01.018
[10] 陈阳, 程宏飞, 邓宇涛, 等. 黏土矿物对铀的吸附作用研究进展 [J]. 化工矿产地质, 2015(2): 93-98. doi: 10.3969/j.issn.1006-5296.2015.02.005 CHEN Y, CHENG H F, DENG Y T, et al. Progress in uranium adsorption by clay minerals [J]. Geology of Chemical Minerals, 2015(2): 93-98(in Chinese). doi: 10.3969/j.issn.1006-5296.2015.02.005
[11] 崔瑞萍, 李义连, 景晨. 伊利石对水溶液中低浓度铀的吸附 [J]. 环境化学, 2015, 34(2): 314-320. doi: 10.7524/j.issn.0254-6108.2015.02.2014060305 CUI R P, LI Y L, JING Chen. Adsorption of uranium from aqueous solution on illite [J]. Environmental Chemistry, 2015, 34(2): 314-320(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.02.2014060305
[12] 张金流. 凹凸棒黏土对铀吸附性能的研究 [J]. 净水技术, 2006, 25(5): 66-68. doi: 10.3969/j.issn.1009-0177.2006.05.020 ZHANG J L. Study on the adsorption of uranium on the attapulgite clay [J]. Water Purification Technology, 2006, 25(5): 66-68(in Chinese). doi: 10.3969/j.issn.1009-0177.2006.05.020
[13] MARTORELL B, KREMLEVA A, KRÜGER S, et al. Density functional model study of uranyl adsorption on the solvated (001) surface of kaolinite [J]. J Phys Chem C, 2010, 114(31): 13287-13294. doi: 10.1021/jp101300w [14] 师琳琳, 房晓红, 曾凡桂. 钠蒙脱石(0 0 1)面铀酰吸附的分子模拟 [J]. 煤炭技术, 2015, 34(10): 314-316. SHI L L, FANG X H, ZENG F G. Molecular simulation of uranyl adsorption on (001) surface of Na-montmorillonite [J]. Coal Technology, 2015, 34(10): 314-316(in Chinese).
[15] KREMLEVA A, KRüGER S, RöSCH N. Density functional model studies of uranyl adsorption on (0 0 1) surfaces of kaolinite [J]. Langmuir, 2008, 24(17): 9515-9524. doi: 10.1021/la801278j [16] GLEZAKOU V-A, DEJONG W A. Cluster-models for uranyl(Ⅵ) adsorption on α-alumina [J]. J Phys Chem A, 2011, 115(7): 1257-1263. doi: 10.1021/jp1092509 [17] 杨晓芳, 王东升, 孙中溪. 三水铝石(γ-Al(OH)3)和α-Al2O3表面酸碱性质与磷酸根吸附研究 [J]. 环境科学学报, 2007, 27(4): 637-642. doi: 10.3321/j.issn:0253-2468.2007.04.016 YANG X F, WANG D S, SUN Z X. Studies on the surface acid-base properties and phosphate adsorption behavior of gibbsite (γ-Al(OH)3) and α-Al2O3 [J]. Acta Scientiae Circumstantiae, 2007, 27(4): 637-642(in Chinese). doi: 10.3321/j.issn:0253-2468.2007.04.016
[18] 辜家芳, 陈文凯. 铀酰离子在羟基化α-石英(1 0 1)表面的吸附 [J]. 物理化学学报, 2014, 30(10): 1810-1820. doi: 10.3866/PKU.WHXB201408221 GU J F, CHEN W K. Adsorption of the uranyl ion on the hydroxylated α-quartz (101) surface [J]. Acta Physico-Chimica Sinica, 2014, 30(10): 1810-1820(in Chinese). doi: 10.3866/PKU.WHXB201408221
[19] 辜家芳, 陆春海, 陈文凯, 等. 水溶液中碳酸铀酰化合物的电子结构(英文) [J]. 物理化学学报, 2012, 28(4): 68-74. GU J F, LU C H, CHEN W K, et al. Electronic structures of uranyl(Ⅵ) carbonate complexes in the aqueous phase [J]. Acta Physico-Chimica Sinica, 2012, 28(4): 68-74(in Chinese).
[20] 辜家芳, 满梅玲, 陆春海, 等. 一系列水合和非水合铀酰卤族(F, Cl, Br)配合物在水溶液中的结构和紫外吸收光谱性质的密度泛函理论研究 [J]. 无机化学学报, 2012, 28(7): 31-39. GU J F, MAN M L, LU C H, et al. A DFT study on properties of geometries and UV-Vis spectra of hydrated and non-hydrated uranyl halides (F, Cl, Br) in aqueous phase [J]. Chinese Journal of Inorganic Chemistry, 2012, 28(7): 31-39(in Chinese).
[21] 辜家芳, 陆春海, 陈文凯, 等. 气相和水溶液中铀酰配合物UO2Ln2-n*a (L=F-, CO32-, NO3-;n=0-6, a=1, 2)的结构和振动光谱 [J]. 物理化学学报, 2009, 25(4): 655-660. doi: 10.3866/PKU.WHXB20090419 GU J F, LU C H, CHEN W K, et al. Structures and vibrational frequencies of gas phase and solvated uranyl complexesUO2Ln2-n*a (L=F-, CO32-, NO3-;n=0-6, a=1, 2) [J]. Acta Physico-Chimica Sinica, 2009, 25(4): 655-660(in Chinese). doi: 10.3866/PKU.WHXB20090419
[22] PERDEW J P, WANG Y. Accurate and simple analytic representation of the electron-gas correlation energy [J]. Phys Rev B, 1992, 45: 13244-13249. doi: 10.1103/PhysRevB.45.13244 [23] PERDEW J P, WANG Y. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation [J]. Phys Rev B, 1986, 33: 8800-8802. doi: 10.1103/PhysRevB.33.8800 [24] DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules [J]. J Chem Phys, 1990, 92: 508-517. doi: 10.1063/1.458452 [25] DELLEY B. From molecules to solid with the Dmol3 approach [J]. J Chem Phys, 2000, 113: 7756-7764. doi: 10.1063/1.1316015 [26] HATTORI T, SAITO T, ISHIDA K, et al. The structure of monomeric and dimeric uranyl adsorption complexes on gibbsite: A combined DFT and EXAFS study [J]. Geochimica Et Cosmochimica Acta, 2009, 73(20): 5975-5988. doi: 10.1016/j.gca.2009.07.004 -



 下载:
下载: