-
全氟辛烷磺酸(perfluorooctane sulphonate,PFOS)是一种新污染物,广泛存在于空气、水、土壤和底泥中[1-5],特别是氟化工生产企业附近的环境介质中[6-7]。PFOS的持久稳定性、潜在生物毒性和生物累积性等[8-10],会对危害人群健康[11-12],已引起相关学者的关注[13-14]。2000年,美国3M公司宣布禁止生产和应用PFOS[15]。欧盟化学品限制指令中规定PFOS作为产品成分的质量浓度应低于10 mg·kg−1[16]。一些国家和地区环保部门出台的规定中PFOS浓度限值标准为痕量级(1~1 000 μg·L−1),甚至远低于痕量级。如美国USEPA规定饮用水中PFOS和全氟辛酸(perfluoro caprylic acid,PFOA)的总浓度不超过0.07 μg·L−1[17];欧盟规定内陆地表水中PFOS的年平均质量浓度限值为0.000 65 μg·L−1,其中淡水可接受的最大值为36 μg·L−1,海洋年平均质量浓度限值为0.000 13 μg·L−1[18]。另外,在PFOS处理技术的研究中,如吸附[19-20]、高级氧化、零价铁还原和电化学处理等[21-26],PFOS的处理目标浓度亦为痕量级或低于痕量级。然而,测定痕量甚至远低于痕量级的浓度,对仪器分析及分析预处理过程的要求也更高。
测定痕量级的PFOS时,其浓度越低,相对标准偏差会越大,影响因素可能有以下4个方面。1)在实验和测定过程中,PFOS与各种化学试剂接触并混合,可能对PFOS浓度测定结果产生干扰。2)检测仪器自身问题,如基质效应产生离子抑制、流动相洗脱强度不同、出峰情况等,均可能对PFOS浓度测定产生影响[27]。3)在采样或实验时使用的容器或器材通常由特定材料制成,如玻璃、塑料等,可能影响测定结果。在涉及全氟化合物(PFASs,PFOS为PFASs中的一种)的实验测定过程中不能使用玻璃仪器及含聚四氟乙烯(polytetrafluoroethylene,PTFE)材料,原因是玻璃材质会吸附PFASs造成浓度损失;而PTFE材质的器皿因含有PFASs残留造成含量增多,均可导致测定数据误差增大[28-30]。USEPA及ISO标准方法中也规定PFASs的样品采集和检测分析中,样品不得与任何玻璃容器或注射器等转移仪器的材料接触,并建议使用聚丙烯(polypropylene,PP)容器用于样品溶液的制备和储存[31-32]。与之相反,有报道称,PP材质及含有PTFE过滤器对PFOA的吸附损失反而比玻璃大[33],这与ISO建议的标准方法相矛盾。4)针对PFOS样品中存在明显干扰杂质,以及环境样品中PFOS浓度低于痕量级等问题,一般需要进行萃取预处理,主要为固相萃取、液液萃取。固相萃取主要用于PFOS浓度低于痕量级的环境样品,使富集浓缩、净化后的PFOS甲醇溶液浓度达到仪器分析的检出限,并使相对标准偏差和回收率在合理范围内[6,29,34];液液萃取可有效去除样品中的细胞、组织或杂质,并使组分或杂质中吸收的PFOS得以释放[35-36]。上述2种预处理方式步骤多,易产生累积误差。针对无干扰杂质或组分且浓度为痕量级及以上的PFOS溶液,无需复杂的预处理过程,可采用水-甲醇混合液测定,即以PFOS溶液与甲醇按一定比例混合过滤后直接上机测定,如PARK等[37]将PFOS水溶液与甲醇以体积比为1∶1的比例混合过滤后上机测定,溶液中含有过硫酸盐;BRUTON等[38]将PFOS溶液先用水稀释5~10倍后,再用甲醇稀释5~25倍上机测定,且溶液中也含有过硫酸盐;另外,以避免出现操作过程中的误差,还可采用水溶液直接上机测定的方法[39]。
本研究针对PFOS痕量浓度测定中的误差问题,以PFOS标准溶液开展了探索性实验,对PFOS痕量级浓度分析误差的影响进行评估,明确测定过程中存在的问题,确定主要影响因素,以及解决影响因素的依据与方法,为PFOS及相关全氟化合物的测定提供参考。
全文HTML
-
1)主要试剂。四丁基硫酸氢铵(TBAHS,C16H37NO4S),过硫酸钠(Na2S2O8),碳酸氢钠(NaHCO3),九水合硫化钠(Na2S·9H2O)无水硫酸钠(Na2SO4)均为分析纯;甲醇(CH3OH),甲基叔丁基醚(MTBE,C5H12O)均为色谱纯;PFOS(C8F17SO3)为标准液;地下水(采集苏州市某地区的地下水样品,pH 为7.30)。
2)主要仪器。液相色谱-三重串联四级杆液质联用仪(LC-MS/MS,LC:Agilent 1290 Infinity Ⅱ,MS:AB SCIEX 4 500),配合LC-MS/MS使用的色谱柱(ZORBAX RRHD Eclipse Plus 95Å C18,Φ2.1 mm × 50 mm,1.8 µm,120 MPa,美国Agilent公司),氮吹浓缩仪(HGC-12A,天津恒奥科技发展有限公司),超纯水仪(A10,美国Milli-Q公司)。
3)主要材料。主要实验器材有针式过滤器、离心管、注射器、移液枪枪头。针式过滤器中滤膜的相关材质见表1,滤膜材质主要有聚四氟乙烯(PTFE)、尼龙(Nylon)2种材质,购自不同商家,颜色各不相同;离心管(聚丙烯PP,2 mL,南京泰普瑞仪器设备有限公司);注射器(PP,2 mL,常州市回春医疗器材有限公司;玻璃,2 mL,金坛市五星医疗器械有限公司),针式过滤器与注射器为配套使用;移液枪枪头(PP,200、1 000 μL,德国Eppendorf公司);样品瓶(棕色玻璃,2 mL,美国Agilent公司);微量注射器(玻璃,10、100、500 μL,上海高鸽工贸有限公司)。
-
1)样品制备方法。PFOS标准溶液初始浓度为1 000 μg·L−1,本研究主要以PFOS标准溶液开展。测定前采用水-甲醇混合液,即取 0.1 mL的PFOS溶液与0.9 mL甲醇混合,溶剂中水与甲醇体积比为1∶9的混合液(简称“1∶9混合液”),混合溶液中PFOS的理论浓度为100 μg·L−1。其他标准溶液的配制与此类似。
2) PFOS测定方法。本研究源于笔者在对PFOS氧化降解实验体系的研究中发现测定数据存在误差较大,故针对误差产生的影响因素展开分析。该氧化降解实验体系的采样和测定的流程为:先使用注射器抽取PFOS溶液进行采样,采集的PFOS溶液样品注入离心管中临时存储;然后使用移液枪向离心管中吸取 0.1 mL的PFOS溶液至样品瓶中,加入0.9 mL的甲醇充分震荡混合后,再用注射器配套针式过滤器过滤后的滤液进行上机测定。在上述流程中,PFOS溶液会一次或多次接触针式过滤器、注射器、移液枪枪头、离心管等。对测定中不同影响因素的分析通过如下方法开展。
① LC-MS/MS仪器测定。主要分析仪器的误差,相对标准误差和置信区间,明确仪器误差的可接受范围。PFOS标准溶液的质量浓度为1 000 μg·L−1,使用微量注射器配制0、10、20、40、60、80、100 μg·L−1的PFOS溶液样品(1∶9混合液)。此外,使用移液枪重复配制6个100 μg·L−1的PFOS溶液样品(1∶9混合液)及3个100 μg·L−1的PFOS甲醇溶液样品,每个样品均连续测定3次。
② PFOS溶液(1∶9混合液)质量浓度的测定。配制0.1 mLPFOS溶液与0.9 mL甲醇混合液(1∶9混合液),用注射器取1 mL的1∶9混合液,并使用针式过滤器过滤,过滤后的滤液上机测定,分析针式过滤器的影响;再使用注射器取样后不进行过滤即直接上机测定,分析注射器的影响。PFOS样品的理论质量浓度为100 μg·L−1。
③不同水与甲醇体积比下PFOS溶液质量浓度的测定。与上述2)步骤相似,设定水与甲醇的体积比例分别为0.025∶0.975、0.05∶0.95、0.075∶0.925。对应的PFOS样品理论浓度为25、50、75 μg·L−1。使用有机系针式过滤器过滤,过滤后的滤液上机测定,分析注射器和针式过滤器的影响。
④以甲醇为溶剂的PFOS溶液质量浓度的测定。用质量浓度为1 000 μg·L−1的PFOS甲醇标准溶液配制100 μg·L−1的PFOS甲醇溶液,使用有机系针式过滤器过滤,过滤后的滤液上机测定,分析注射器和针式过滤器的影响。
⑤以水为溶剂的PFOS溶液质量浓度的测定。用质量浓度为1 000 μg·L−1的PFOS标准溶液配制100 μg·L−1的PFOS水溶液,使用水系针式过滤器过滤,过滤后的滤液上机测定,分析注射器和针式过滤器的影响。另外,采样过程不仅使用注射器,还要使用离心管,故增加了对离心管对PFOS水溶液的影响。
⑥地下水及其过硫酸钠溶液中PFOS质量浓度的测定。用质量浓度为1 000 μg·L−1的PFOS标准溶液配制浓度为100 mmol·L−1的过硫酸钠(Na2S2O8)溶液,且不参与激活反应,同时以质量浓度为1 000 μg·L−1的PFOS标准溶液配制浓度为100 mmol·L−1的硫酸钠(Na2SO4)溶液。另外,以地下水配制PFOS溶液,分析PFOS标准溶液中含有Na2S2O8对PFOS测定的影响,同时以液液萃取的方式分析其对PFOS溶液质量浓度测定的影响。
3)液液萃取。取0.5 mL的PFOS溶液至10 mL样品瓶内,加入1 mL浓度为0.5 mol·L−1 TBAHS溶液和2.5 mL浓度为0.25 mol·L−1 NaHCO3溶液,振荡3 min后,加入3 mL 的MTBE,再振荡5 min,静置5 min后分层,取上清液,共萃取3次。将萃取后的MTBE萃取液进行氮吹,吹干后,加入5 mL甲醇溶解震荡混匀,取2 mL待测液用有机相针式过滤器过滤,弃去前1 mL,保留后1 mL过滤后上机测定[35-36]。
4)样品测定方法。采用LC-MS/MS在负电喷雾电离(ESI)模式下进行分离目标分析物,可获得目标分析物的浓度梯度曲线。设置仪器自动注入5 μL样品,流动相为2 mmol·L−1乙酸铵(A)和乙腈(B)。设置初始条件为80%的A和20%的B,流动相流速为0.3 mL·min−1,保持1.5 min,然后A相在3.5~4.0 min时降至10%,并在4.5 min回到初始条件为80%的A和20%的B,并保持6 min,柱温始终保持在40 ℃不变。色谱图采用多反应监测模式(MRM)记录。仪器的气体参数:源气体温度(350 ℃)、源气体流速(9 L·min−1)、雾化器压力(0.276 MPa)、毛细管(3 500 V负极)、Delta EMV(-)(200 V)。
5)数据分析方法。本研究均采用1 000 μg·L−1的PFOS标准溶液作为母液进行分析,分析得到的PFOS浓度可直接反映回收率,无需再计算回收率。但需要提前测定PFOS溶液的标准曲线并进行相关性分析,采用溶液浓度的偏差、标准偏差,t检验法(P=0.95,α=0.05)显著性差异分析,4
$\bar d$ 法判断可疑值、平均值的置信区间等[40],进行样品数据的处理和说明。若无特殊要求,每组样品连续测定3个平行样。
1.1. 实验材料
1.2. 实验方法
-
使用玻璃材质的微量注射器配制0、10、20、40、60、80、100 μg·L−1的PFOS溶液(1∶9混合液),并进行上机测定。以PFOS溶液(1∶9混合液)浓度为横坐标,仪器测定结果的对应峰面积为纵坐标,得到PFOS浓度与峰面积的标准曲线为y = 88 577x + 134 708,相关系数r = 0.998 8。根据检验相关系数临界值表,当置信度为99.9%,自由度f = 5(7个样品)时,r临界值= 0.951 < 0.998 8,表明该标准曲线具有很好的线性关系。此外,根据计算得出决定系数R2 = 0.997 7,表明一元线性回归拟合度高。因此,PFOS溶液(1∶9混合液)配制的标准溶液可作为仪器分析的标准工作曲线使用。玻璃材质的微量注射器对PFOS测定是否存在等比例的影响尚未知,但可确定无其他明显影响。
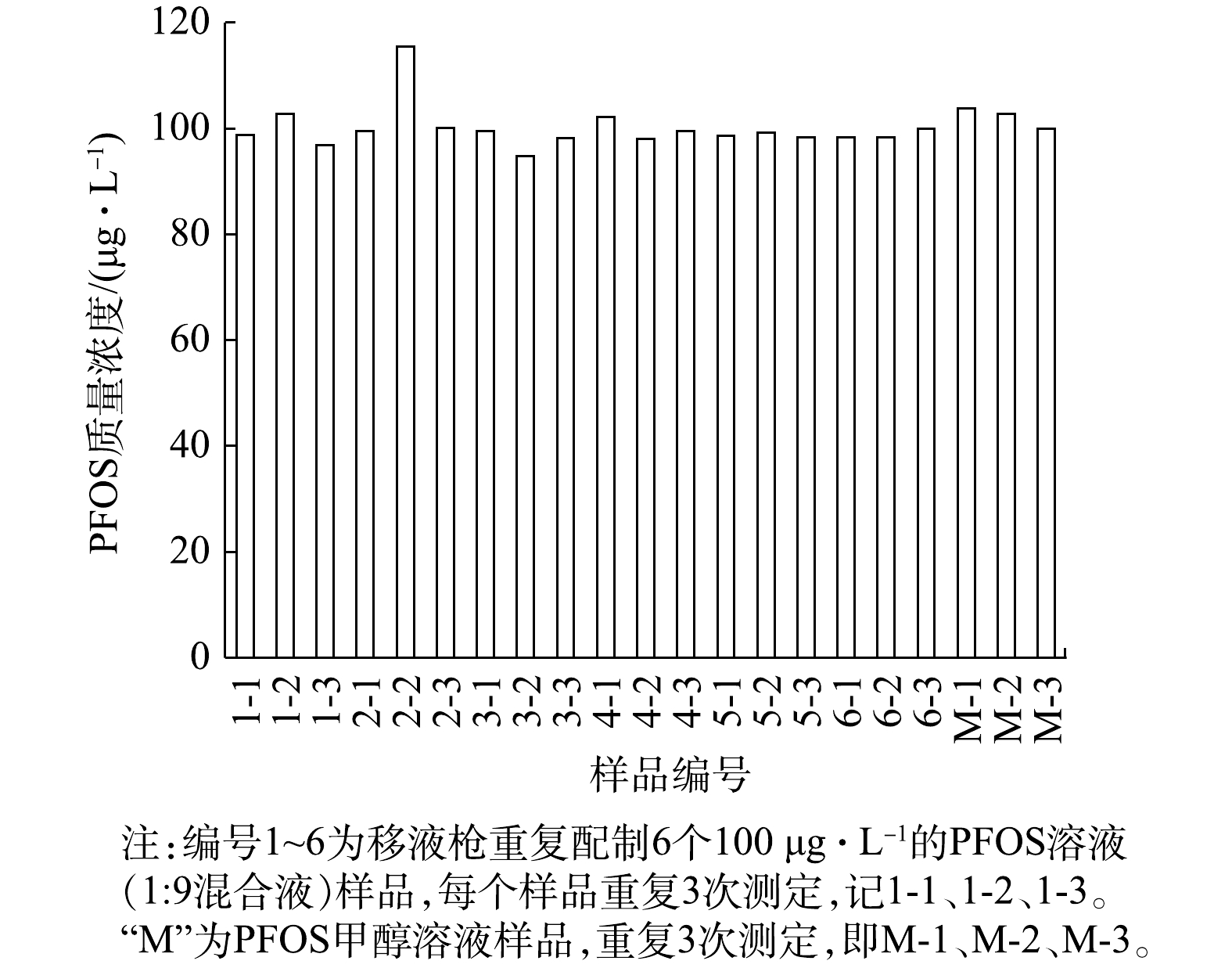
此外,使用移液枪重复配制6个100 μg·L−1的PFOS溶液(1∶9混合液),每个样品连续测定3次,以及3个100 μg·L−1的PFOS甲醇溶液,结果见图1。
由图1可知,样品的20个数据均接近100 μg·L−1,仅2-2数据偏高。21个数据的平均值
$\bar x$ 为100.19 μg·L−1,表明分析数据准确度高;s为标准偏差4.08,RSD为相对标准偏差4.07%,表明测定测的数据精密度高。由于测定样品次数为21次,测定次数越多,所获得的平均值精密度越高,影响每个数据的判断,因此,需计算t分布以进一步明确是否存在显著性差异和系统误差。根据t检验法,当自由度f = 20(21个样品),$\bar x$ = 100.19,理论值μ = 100,s= 4.07时,计算得t =0.214。以分析化学中95%的置信度作为检验标准,显著性水准为5%,即当P = 0.95、α = 0.05、f = 20时,根据tα,f值表可知t0.05,20 = 2.09 > 0.214,表明测定值的$\bar x$ 与理论值不存在显著性差异,没有引起明显的系统误差和操作误差。图1中2-2的数值比理论值高15.49。根据4$\bar d$ 法判断可疑值,除2-2样品外,其他20个样品的$\bar x$ = 99.43,平均偏差$\bar d$ = 1.52,则4$\bar d$ = 6.08。因此,可接受的测定值范围为99.24±6.08,即93.16~105.51,说明仪器测定质量浓度为100 μg·L−1的PFOS溶液可接受误差约为±6%。而根据正态分布规律,测定值115.49超出4$\bar d$ 计算范围检验值,且理论检出概率低于0.3%[40],此数值可以正常弃舍。另外,本文实验均以3个平行样品测定,则测定的21个样品中每组3个实验平行样品测定数据,当P = 0.95,α=0.05,根据tα,f表(双边)计算求得总体平均值置信区间最大范围为99.44±5.61,即93.83~105.05。此外,由移液枪枪头配制的PFOS溶液测定数据与玻璃材质的微量注射器得出的标准曲线数据比较,两者数据有很好的兼容性,可推断玻璃与PP的移液枪枪头两者对PFOS溶液的测定无显著影响。 -
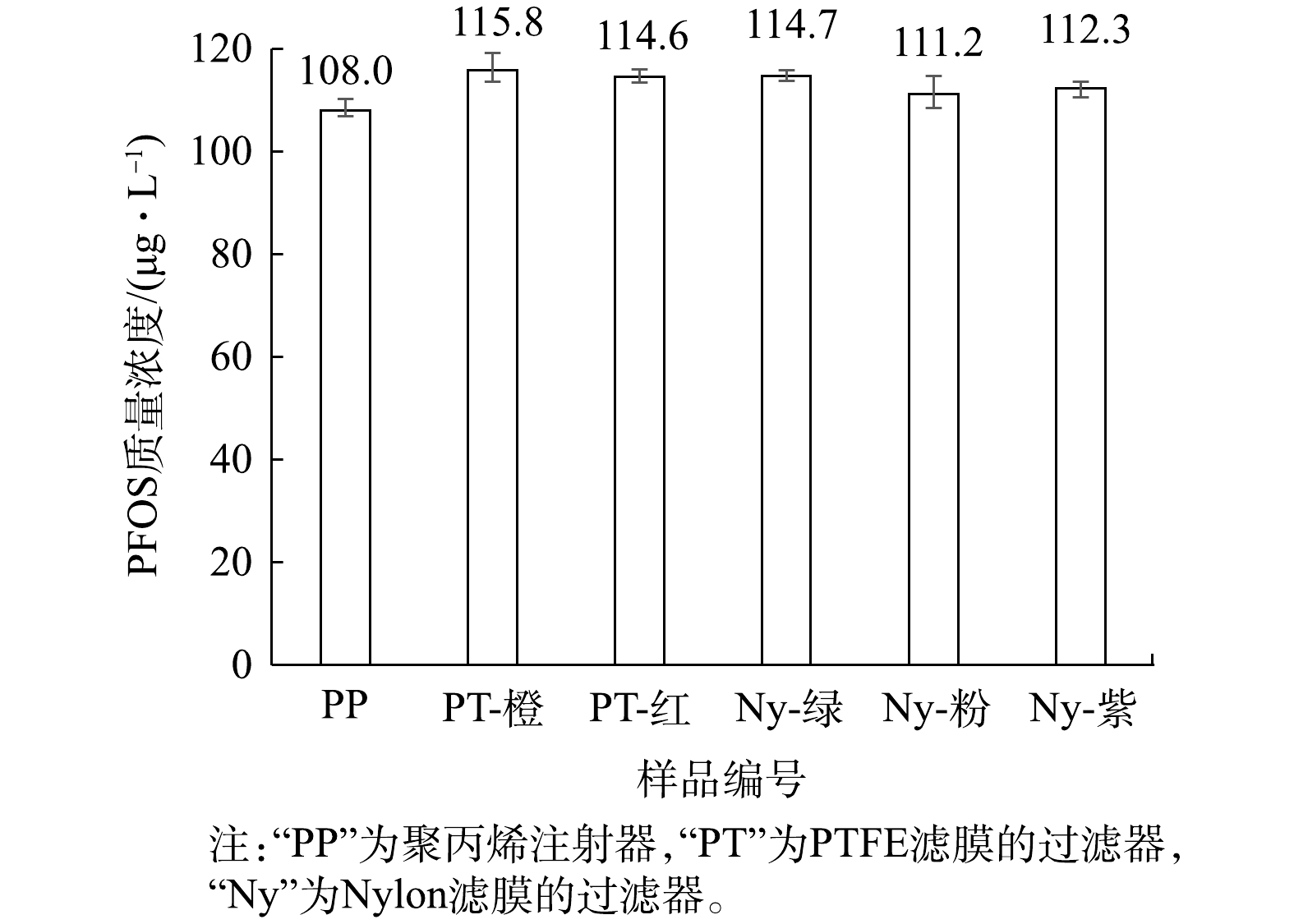
用聚丙烯(PP)注射器取1 mL的100 μg·L−1的PFOS溶液(1∶9混合液),直接上机测定;再用注射器取1 mL的100 μg·L−1的PFOS溶液(1∶9混合液),使用针式过滤器过滤,过滤后上机测定,分析注射器与分析针式过滤器的影响,结果见图2。由于1∶9混合液中同时含有水相和有机相,故同时采用水系和有机系的针式过滤器使用。
由图2可知,PP注射器、PTFE和Nylon滤膜的过滤器均使100 μg·L−1的PFOS(1∶9混合液)浓度升高,表明针式过滤器同样会影响PFOS的浓度。另外,文献[30]报道PTFE或相关材料中,存在少量PFOS残留使得溶液的测定浓度偏高,本研究亦进行了相关分析。
-
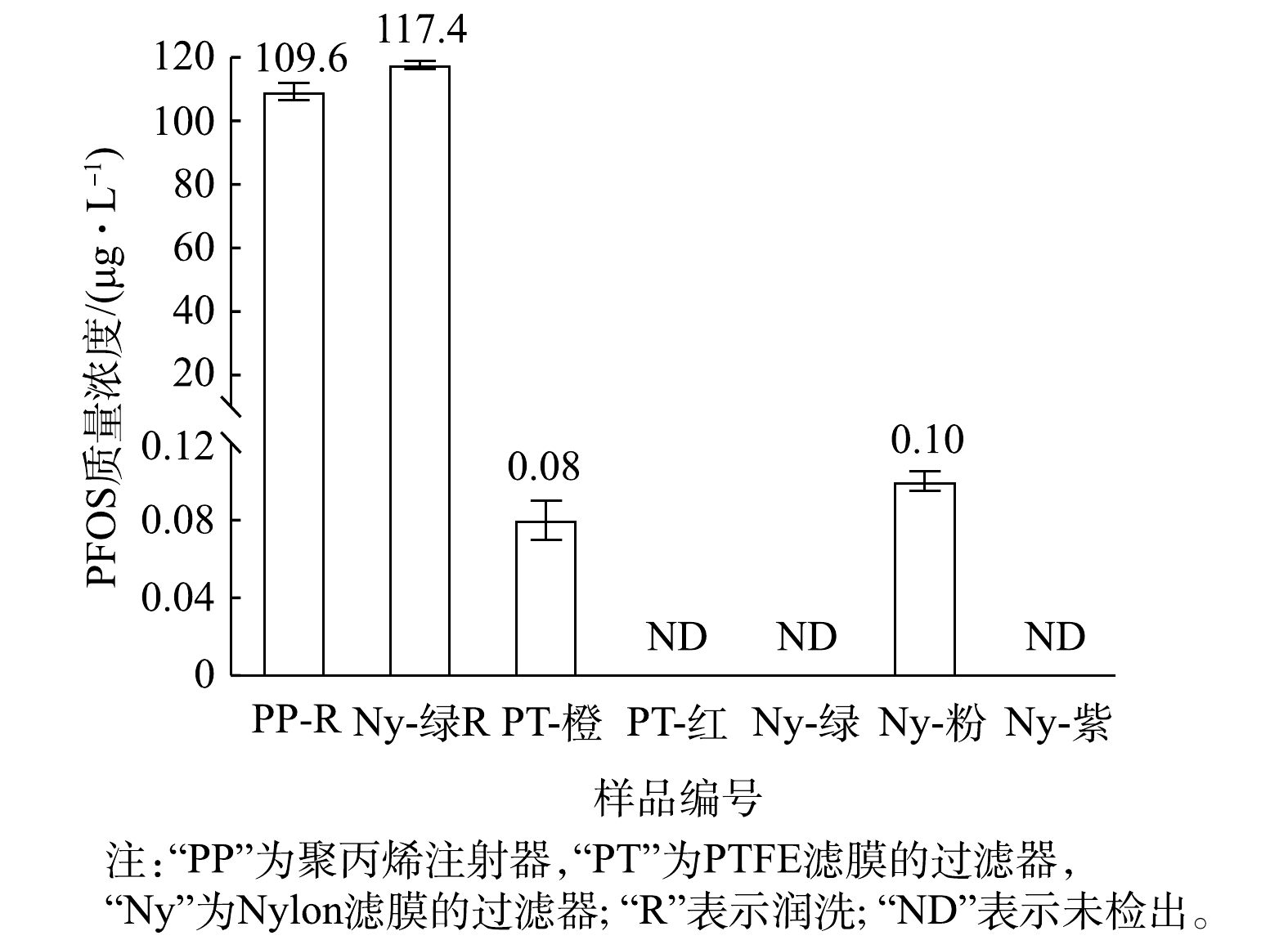
进一步分析PP注射器与不同针式过滤器对PFOS痕量浓度测定的影响。首先用PP注射器取1 mL不含PFOS的1∶9混合液,并使用针式过滤器过滤,过滤后的滤液上机测定,确认PP注射器与针式过滤器自身是否存在PFOS残留会导致溶液中PFOS浓度升高;其次,取1 mL质量浓度为100 μg·L−1的PFOS溶液(1∶9混合液)润洗PP注射器后,再取1 mL该溶液直接上机测定;然后用注射器取2 mL该溶液,并用Nylon-绿色的针式过滤器过滤,前1 mL用于润洗注射器与过滤器,后1 mL过滤后的滤液上机测定,结果见图3。
由图3可知,5种针式过滤器中,PTFE-橙色与Nylon-粉色的过滤器中PFOS(1∶9混合液)的测定浓度分别为0.08和0.10 μg·L−1,影响仅约0.1%(远低于6%的允许误差),其他3种过滤器低于检出限,故对材料中残留的PFOS干扰可忽略不计。此外,润洗注射器与针式过滤器后,PFOS(1∶9混合液)测定浓度分别为109.6和117.4 μg·L−1,与未润洗实验的PFOS(1∶9混合液)测定浓度接近,所以润洗并未使PFOS(1∶9混合液)的测定回归正常。
以上结果表明,使用注射器及针式过滤器会导致PFOS溶液(1∶9混合液)测定浓度增大,并非材料中含有PFOS残留造成,即PFOS作为溶质并未明显增多。而是否为注射器和针式过滤器干扰了1∶9混合液中水与甲醇导致,目前尚未证实。因此,下一步研究当配制PFOS溶液的质量浓度等比例变化时,注射器及针式过滤器的干扰是否会形成等比例变化或一个稳定常数。
-
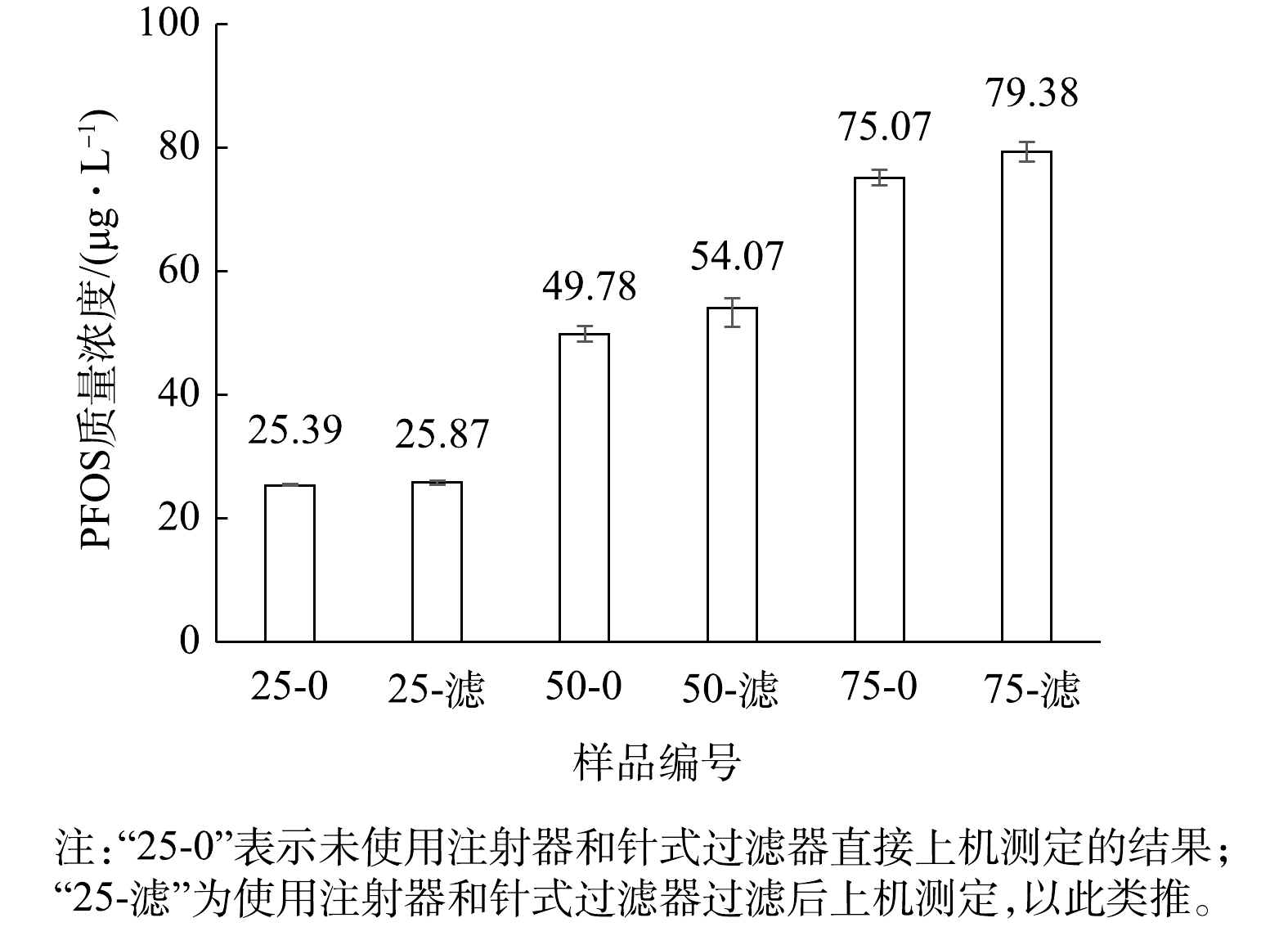
用1 000 μg·L−1的PFOS标准溶液与甲醇混合配制不同比例的PFOS溶液,水与甲醇混合液体积分数比例分别为0.025∶0.975、0.05∶0.95、0.075∶0.925。即对应的PFOS溶液的理论质量浓度为25、50、75 μg·L−1。选用有机系Nylon-绿色为代表的针式过滤器过滤,过滤后的滤液上机测定,分析PP注射器和针式过滤器的影响,结果见图4。图4中“25-0”表示未使用注射器和针式过滤器直接上机测定,“25-滤”为使用注射器和针式过滤器过滤后上机测定,以此类推。
由图4可知,将水与甲醇按不同体积比混合后制备质量浓度分别为25、50、75 μg·L−1的PFOS标准溶液。在未使用与使用注射器及针式过滤器测定这2种条件下,测定3种不同质量浓度标准溶液的痕量浓度。测定质量浓度为25 μg·L−1的PFOS标准溶液时,使用注射器及针式过滤器未明显提高测定结果。对于质量浓度为50和75 μg·L−1的PFOS标准溶液,使用注射器及针式过滤器后,升高幅度约为6%~7%。因此,质量浓度为25、50、75、100 μg·L−1的PFOS标准溶液浓度呈梯度变化时,注射器及针式过滤器的干扰并没有形成等比例变化或一个稳定的变化常数,故不排除仪器分析和实验操作存在的误差。
然而,由图4可知,质量浓度为25 μg·L−1的PFOS标准溶液的痕量测定浓度无明显升高,表明注射器及针式过滤器在其中未产生明显干扰。配制PFOS溶液时,除了PFOS浓度出现变化,溶剂中水与甲醇的浓度也有变化。该实验用到的标准溶液中水与甲醇混合液体积分数比例分别为0.025∶0.975、0.05∶0.95、0.075∶0.925、0.1∶0.9,即质量浓度为25 μg·L−1的PFOS标准溶液中甲醇的体积分数为97.5%。由此假设,在PFOS标准溶液中甲醇含量越大,注射器和针式过滤器的干扰就越小;水含量越大,注射器及针式过滤器的干扰越大。针对该假设,进行下一步实验研究。
-
用质量浓度为1 000 μg·L−1的PFOS甲醇标准溶液配制质量浓度为100 μg·L−1的PFOS甲醇溶液,用PP注射器取1 mL该溶液直接上机测定;用注射器取1 mL溶液使用有机系Nylon-绿色针式过滤器过滤,过滤后的滤液直接上机测定;用PP注射器取1 mL溶液润洗注射器后,再取1 mL直接上机测定;用注射器取2 mL溶液于Nylon-绿色针式过滤器中过滤,前1 mL用于润洗注射器与过滤器,后面1 mL过滤后的滤液直接上机测定。分析注射器及针式过滤器的影响。测定结果见图5。
由图5可知,以甲醇为溶剂配制的PFOS标准溶液,使PP注射器和针式过滤器的材质干扰明显降低;PFOS甲醇溶液润洗后,PFOS溶液的痕量浓度接近仪器测定平均值,并且误差在6%范围内,而玻璃仪器对PFOS的测定无显著影响。
-
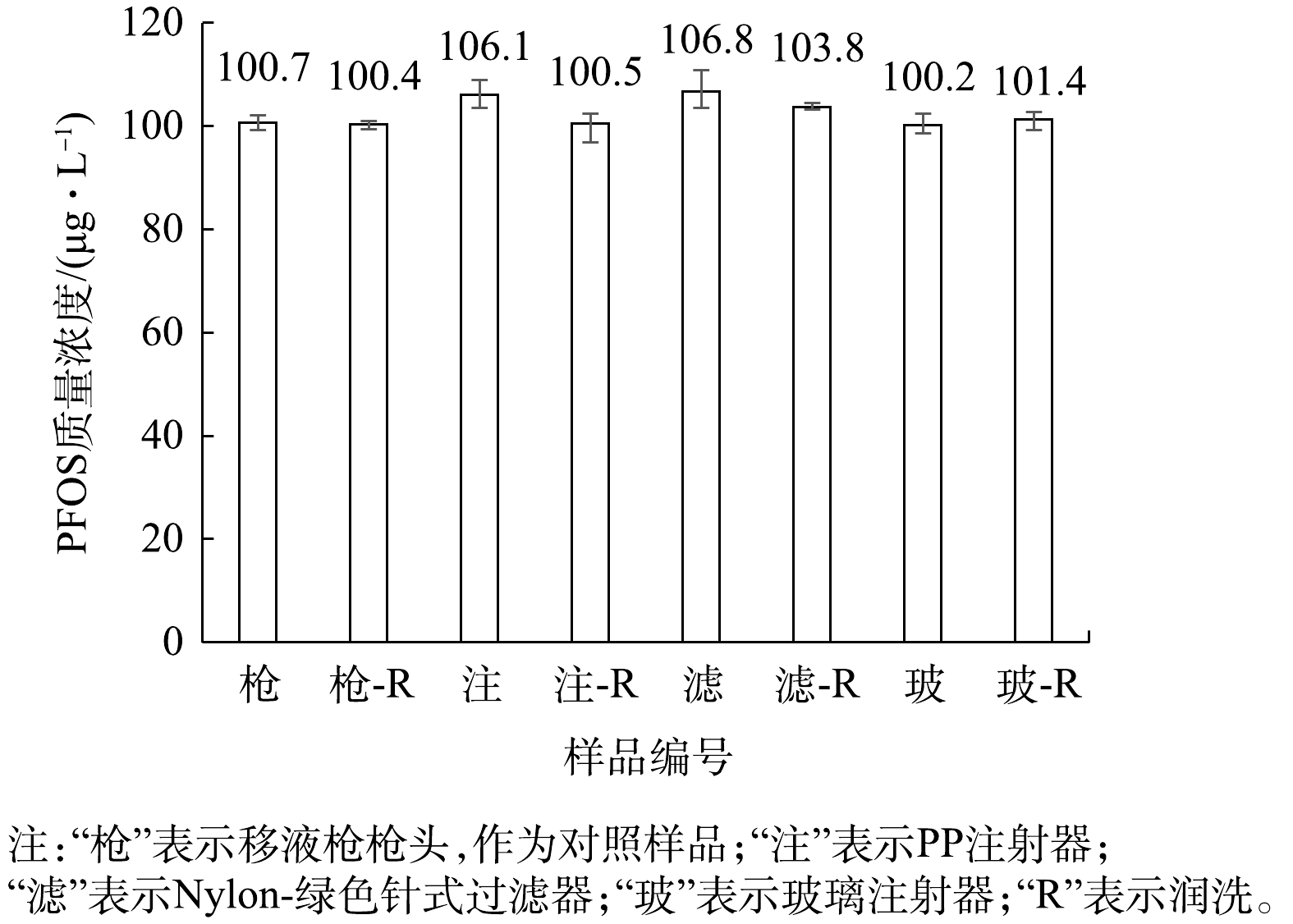
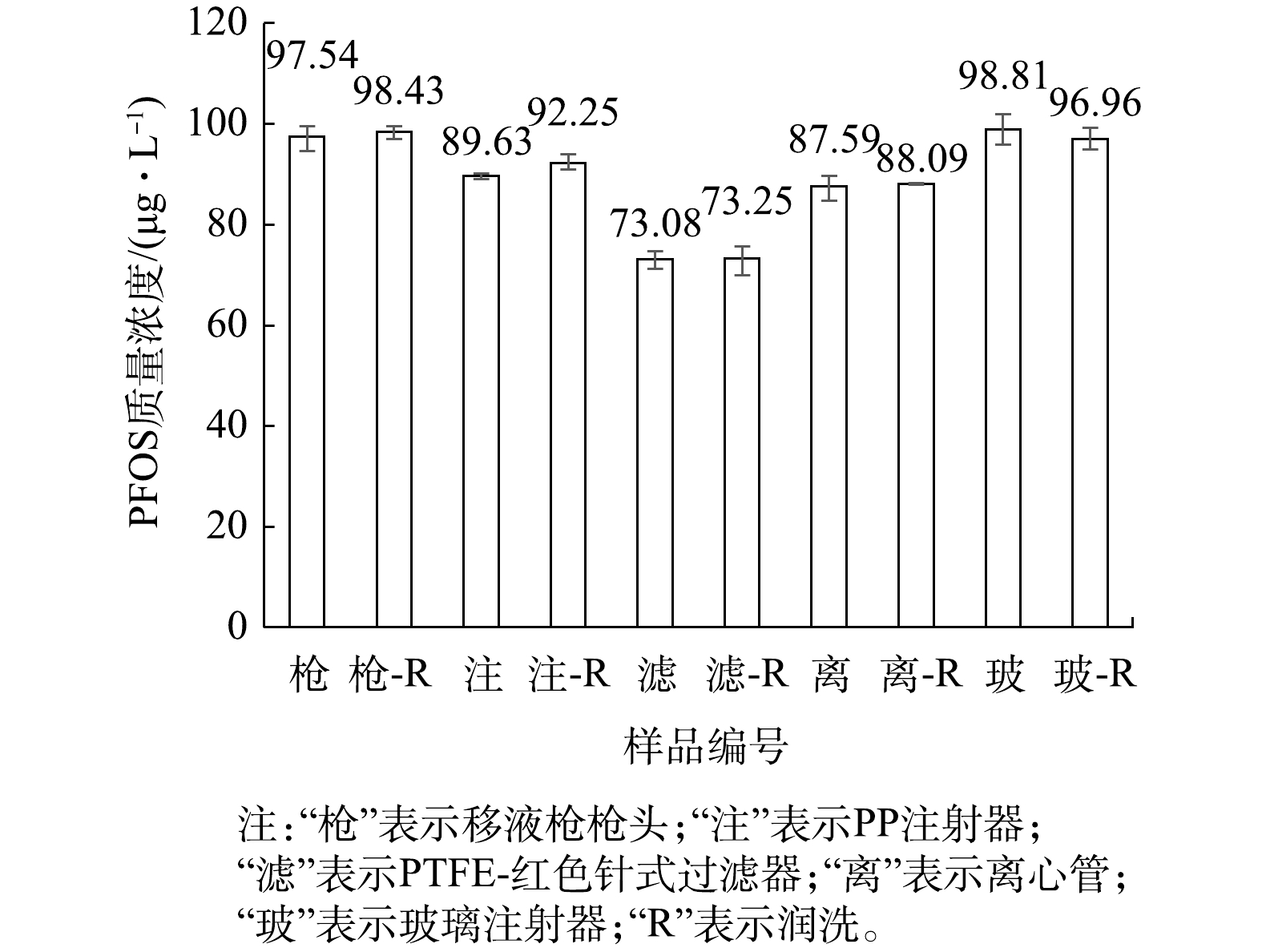
用质量浓度为1 000 μg·L−1的PFOS标准水溶液配制质量浓度为100 μg·L−1的PFOS水溶液。先用PP注射器取1 mL该溶液直接上机测定;注射器取1 mL溶液使用水系PTFE-红色针式过滤器过滤,过滤后滤液上机测定;再用PP注射器取1 mL溶液润洗注射器,再取1 mL溶液直接上机测定;用注射器取2 mL溶液至PTFE-红色针式过滤器中过滤,前1 mL用于润洗注射器与过滤器,后1 mL经过滤后直接上机测定。分析注射器及分析针式过滤器对测定结果的影响,结果见图6。
在以过硫酸钠为主体的PFOS氧化降解体系中,采样环节不仅使用注射器、移液枪,还会用到离心管,样品为水溶液。实验过程为:用移液枪取1 mL质量浓度为100 μg·L−1的PFOS水溶液放入离心管,振荡15~30 s后直接上机测定;另将离心管用待测液润洗后,用移液枪取1 mL质量浓度为100 μg·L−1的PFOS水溶液至离心管,重复上述步骤。
由图6可知,测定质量浓度为100 μg·L−1的PFOS水溶液时,使用移液枪枪头及润洗,与使用玻璃注射器及润洗会略微降低溶液中的PFOS浓度测定结果;使用PP离心管、PP注射器及PTFE-红色针式过滤器对PFOS水溶液中的测定结果有影响,尤其是针式过滤器的影响较大。由此推测,PP和PTFE可能对水溶液中的PFOS存在吸附作用[29],从而导致测得的水溶液中PFOS浓度偏低。
与LATH等[33]研究PP离心管及PTFE针式过滤器吸附PFOA的结论相似,水溶液体系中PP材质的离心管与PTFE材质的针式过滤器对PFOS同样有吸附影响。此外,该实验操作是在数秒钟的短时间内完成的,若有吸附作用,还会存在吸附动力学与吸附饱和度的联系[39],因此本研究中PP材质的离心管与PTFE材质的针式过滤器对PFOS可能存在还未吸附完全的状态。文献[31-32]建议使用PP材质的容器采集含有痕量PFOS的天然水体样品并存储数天,不建议使用玻璃容器。而本实验结果表明PP材质的容器会显著降低PFOS含量,不建议用PP材质的容器存储PFOS溶液,建议可使用玻璃容器存储。
-
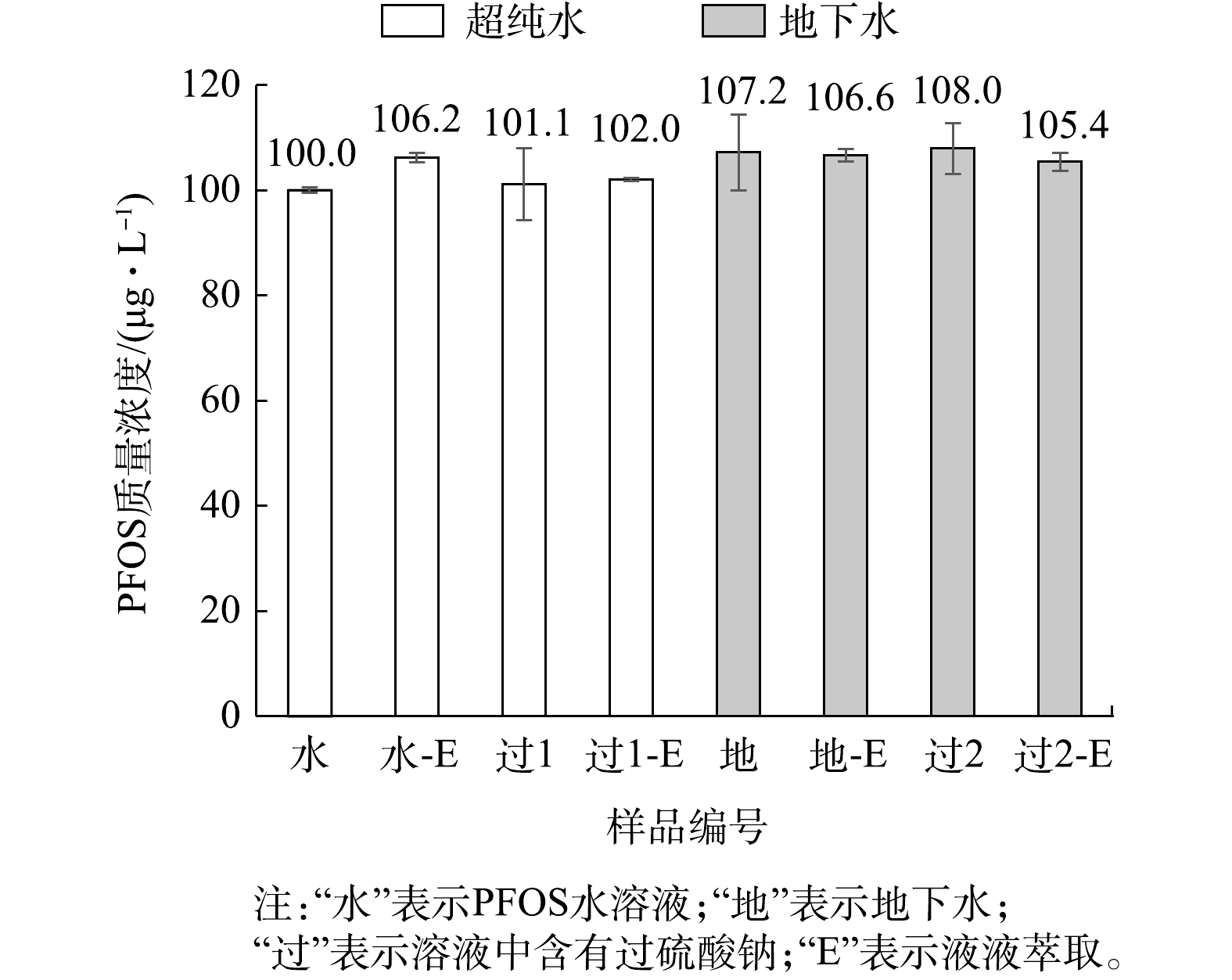
用质量浓度为1 000 μg·L−1的PFOS标准溶液配制100 mmol·L−1的过硫酸钠(Na2S2O8)与溶液,取1 mL与甲醇配制1∶9混合液(PFOS质量浓度的理论值为100 μg·L−1),用Nylon-绿色针式过滤器过滤后上机测定;取0.5 mL浓度为100 mmol·L−1Na2S2O8溶液的PFOS标准溶液(质量浓度为1 000 μg·L−1)进行液液萃取,萃取后得到5 mL甲醇溶液(PFOS质量浓度的理论值为100 μg·L−1),以Nylon-绿色针式过滤器过滤后直接上机测定。另外,将质量浓度为100 μg·L−1的PFOS标准溶液直接上机测定,以及液液萃取后直接上机测定作为空白对照。配制质量浓度为1 000 μg·L−1的PFOS地下水溶液及浓度为100 mmol·L−1的Na2S2O8溶液,按上述操作方式再进行一套实验。上述操作过程的每个环节中,均用待测液润洗各个器材,测定结果见图7。
由图7可知,液液萃取后的PFOS测定浓度偏高。由于液液萃取涉及更多操作步骤,且接触的化学药剂增多,导致操作累积误差增大,因此液液萃取后PFOS的测定浓度总平均值较大,允许误差至少大于6%。
含有Na2S2O8溶液的PFOS标准溶液(质量浓度为100 μg·L−1)过滤、及液液萃取后过滤的PFOS测定浓度较为接近。然而,从误差棒来看,未进行液液萃取的PFOS溶液的RSD为9.57%,说明过硫酸钠对PFOS痕量浓度的测定有显著影响。一方面,1∶9混合液中注射器及针式过滤器仍存在干扰作用;另一方面,过滤后的待测溶液中含有Na2S2O8会随着PFOS一起进入分析仪器,而Na2S2O8具有强氧化性,故存在干扰作用[41];此外,Na2S2O8在水溶液中会自身分解或化学反应生成的 Na2SO4,经实验(质量浓度为100 mmol·L−1的Na2SO4)证明,在1∶9混合液中,Na2SO4会形成大量沉淀,因此可能存在物理吸附、化学反应或对色谱柱污染等多因素共同干扰作用,导致结果存在较大误差。
另外,地下水中含有颗粒物、无机物、有机物、微生物等各种杂质,过滤只能滤掉部分物质,未被过滤的物质同样可能存在物理吸附[39]、化学反应,甚至微生物作用(如流动相中醋酸铵可作为微生物的培养基成分)等,在多因素的共同作用下,导致较大误差的产生。质量浓度为100 μg·L−1的PFOS溶液经过滤后测定的浓度为101.1 μg·L−1,虽然在允许误差范围,但RSD达到9.52%,测定精确度降低,说明地下水对PFOS浓度的测定有显著影响。同样,以地下水配制的过硫酸钠溶液对PFOS的痕量测定有着相似的影响。
液液萃取本质上是将水溶液经过振荡、萃取后,转变为PFOS甲醇溶液的过程。PFOS甲醇溶液的优点在2.4节中已有详细描述。此外,液液萃取中添加TBAHS和NaHCO3是碱解析和脱附的过程,如PFOS被微生物吸收,碱液可将微生物细胞破裂释放PFOS;同时可将PFOS从吸附的物质中脱附出来。液液萃取中MTBE为不溶于水的有机萃取剂,可将水中PFOS转移至有机相。而很多溶于水的物质,如Na2S2O8、Na2SO4等盐类均无法溶入MTBE中,由此可去除无机盐和其他不溶物。
尽管液液萃取操作步骤较多,使得累积误差增大,但由图7可知,液液萃取后PFOS总体平均值误差仅增大约1%(总体平均值置信区间最大范围为93.83~105.05),但RSD较低(0.33%~2.29%),说明测定精确度高。因此,液液萃取在PFOS的痕量测定中具有较好的可行性。
2.1. LC-MS/MS仪器的误差分析
2.2. 1∶9混合液中实验器材对PFOS浓度的影响
2.2.1. 注射器与针式过滤器的影响
2.2.2. PFOS测定浓度(1∶9混合液)偏高原因分析
2.3. 采用不同体积比混合液时器材对测定的影响
2.4. 甲醇溶剂配置标准液时器材对测定的影响
2.5. 水溶剂配置标准溶液时器材对测定的影响
2.6. 地下水及其水溶液中过硫酸钠对测定的影响
-
1)仪器分析表明,质量浓度为100 μg·L−1的PFOS溶液可接受的仪器分析误差即允许误差在±6%左右。无论溶剂采用1∶9混合液或是甲醇溶液,无论移液工具采用玻璃材质的微量注射器或是PP材质移液枪枪头,均未引起明显的系统误差和操作误差。
2)质量浓度为100 μg·L−1的PFOS溶液(溶剂为水与甲醇体积比为1∶9的混合液)实验表明,使用PP注射器、PTFE和Nylon滤膜的过滤器均使PFOS的测定浓度升高,而且并非上述材料中含有PFOS残留造成,同时润洗上述材料也并未将PFOS的测定浓度回归正常范围。PFOS溶液中溶剂甲醇含量增大,会减小注射器及针式过滤器的干扰;溶剂中水的含量增大,会增大注射器及针式过滤器的干扰。
3)仅甲醇配制的PFOS溶液浓度测定实验表明,以甲醇为溶液的PFOS浓度误差在允许范围内,用PFOS待测液润洗相关材料后测定的PFOS浓度更接近平均值,精确度更高。PFOS上机测定使用溶剂建议仅使用甲醇溶液,并用待测液润洗PP材质的注射器与针式过滤器,对PFOS溶液的测定无明显影响。
4)仅水配制的PFOS溶液实验表明,移液枪枪头和玻璃注射器使水溶液中PFOS的浓度略低,但未产生显著影响。PP离心管、PP注射器和PTFE-红色针式过滤器对PFOS的浓度有显著影响,尤其是针式过滤器的影响较大。针对收集、存储或制备含有痕量级或低于痕量级的PFOS水溶液的样品,相关文献标准中建议使用PP材质,不建议使用玻璃容器采集。但此实验结果表明PP材质容器会显著降低PFOS的含量,不建议用PP材质的容器,建议使用玻璃容器。
5)当水溶液中含有Na2S2O8及地下水含有各种杂质的多因素作用下,PFOS浓度测定会存在较大误差,测定的精确度降低。液液萃取可去除无机盐和其他不溶物,将被吸附的PFOS脱附出来。将水溶剂替换甲醇为溶剂,虽然液液萃取由于操作步骤增多,但测定精确度高。因此,液液萃取可行性较好。



 下载:
下载: