-
我国机动车保有量达3.4×108辆[1]。燃油挥发是机动车污染物的重要来源。目前,我国每辆汽油车年均燃油挥发量为8.8 kg,而美国的这一数据仅为0.5 kg[2]。虽然国内汽车都已装备碳罐,但受制于碳罐用活性炭吸附能力有限,燃油挥发造成的损失仍然巨大。为加强机动车排放污染物控制,响应国家打赢蓝天保卫战的号召[3],生态环境部颁发了轻型车排放法规,即国六排放标准[4],于2020年7月1日起在全国范围内实施。受此影响,汽车燃油蒸发有效控制及回收成为研究热点。汽车碳罐作为燃油蒸发排放污染物控制系统的关键零部件之一,正面临升级换代,迫切需要研发高性能的汽车碳罐用活性炭。
汽车碳罐用活性炭特指装填于汽车碳罐的活性炭,用于吸附化油器、缸等处挥发出的汽油蒸气,以防止燃油挥发并污染大气环境。活性炭性能对碳罐工作能力有决定性影响。然而,此类活性炭的相关研究却报道较少,主要原因是该产品门槛较高、研发难度大,且难于打破大企业的垄断壁垒。目前,国内使用的此类活性炭主要依赖进口。20世纪90年代,美国美德维实伟克公司报道了采用磷酸活化经浸渍、低温处理、成型、活化制备颗粒活性炭的方法[5-9]。该方法所制备产品的丁烷工作容量(butane work capacity,BWCv。v表示本文中涉及的BWC均为体积工作容量)较高,但其预处理时间较长,需要20~70 h,因而不利于产品的连续生产。国内学者对该方法进行了改进,但制备周期仍大于10 h,且高温活化时间过长,能耗较大[10]。朱光真等[11]通过在制备过程中加入6%的浓硫酸催化木屑解聚,使活性炭的BWCv从119 g·L−1提高至144 g·L−1,但硫酸的使用造成了设备腐蚀和环境污染,后续治理成本较高。贺德留[12]研究了活化气氛对活性炭丁烷吸附性能的影响,发现活化气氛为二氧化碳时制备的活性炭丁烷工作容量高、丁烷持附性低。活性炭BWCv受孔径影响较大,丁烷吸附的理想孔径为微孔上限和中孔下限[13-14]。刘晓敏等[15]发现,孔径分布为1.2~6.0 nm的孔容积高的活性炭BWCv高。
本课题组从提高活性炭有效吸附孔径和表观密度的角度出发,通过烘焙提质、粒度调控对原料进行预处理,并采用真空捏合、模孔设计等工艺过程,改进了传统磷酸活化法制备活性炭的方式,以期制备高BWCv、高强度的活性炭产品,满足国六排放标准下碳罐用活性炭TGZ1500指标[16]要求,实现对进口同类活性炭的替代。
全文HTML
-
杉木屑(江西天美生物科技有限公司提供):固定碳18.06%、挥发分81.20%、水分3.27%,灰分0.74%。磷酸(质量分数85%)、碘、亚甲基蓝均为分析纯,正丁烷(纯度大于95%)。一种磷酸活化法生产的商用活性炭(江西天美生物科技有限公司提供,碘吸附值860 mg·g−1,亚甲基蓝吸附值232.5 mg·g−1,BWCv为121 g·L−1)。
-
将杉木屑分别在100 ℃干燥和200、250、300 ℃下烘焙处理180 min,并标记为Dry-100 ℃、Trf-200 ℃、Trf-250 ℃、Trf-300 ℃;将处理后的杉木屑破碎至需要的尺寸并按照一定浸渍比与磷酸均匀混合,充分搅拌后,使用真空捏合机在140 ℃下真空捏合一定时间;捏合后将样品置于自制油压成型设备(压力16 t)中制成柱状颗粒;使柱状颗粒在140 ℃下硬化3 h,再转移至高温炉中进行活化;活化一定时间后冷却至室温,用去离子水洗涤至pH为5~7;再于150 ℃下烘干后,即得成品活性炭,待测。
-
碘吸附值(后文简写为Iv)、亚甲基蓝吸附值(后文简写为MBv)、强度分别根据国标GB/T 12496.8-2015、GB/T 12496.10-1999、GB/T 12496.6-1999的标准方法来测定,用以表征活性炭微孔和中孔的发达程度[17-18]及使用寿命。Iv和MBv的测定通过单位质量活性炭所能吸附碘液/亚甲基蓝溶液中碘/亚甲基蓝的最大质量计算;强度测定则是将活性炭置于含有钢球的钢筒中,通过转动摩擦,筛去受到破坏的活性炭粉末,求出保留颗粒质量分数后得到。丁烷工作容量(BWCv)和丁烷持附性(butane retentivity,BRv)根据GB/T 20449-2006中的标准方法来测定,用以表征活性炭对丁烷的吸附和脱附能力[4, 16, 19];采用Van Soest方法测定原料中木质素、半纤维素、纤维素的含量;使用S3400N-I型扫描电子显微镜,观察原料烘焙前后表面形貌,样品经喷金处理后在电压15 kV下放大1 000倍进行观察;采用D8 Focus X-ray衍射仪测定活性炭的衍射图,分析条件为辐射波长为0.154 nm,扫描范围为10°~80°,扫描速度为0.1(°)·s−1;使用ASAP2460型全自动比表面积分析仪测定活性炭的比表面积、孔径分布、孔容等,比表面积由BET法计算得出,孔容积、孔径分布采用密度函数理论(density functional theory,DFT)进行分析。
1.1. 实验材料
1.2. 活性炭的制备
1.3. 活性炭性能测试与表征
-
烘焙是在一定温度下脱除生物质中水分及惰性含氧物质的过程,常用于生物质存储和提高热值[20]。通过烘焙处理制备活性炭的研究目前较少。在原料粒度小于0.2 mm、浸渍比1.5∶1、真空捏合时间60 min、活化温度500 ℃、活化时间120 min的条件下,分别研究了不同烘焙温度对活性炭吸附性能的影响,结果见表1。由表1可知,原料经烘焙处理后制得活性炭的Iv、MBv、BWCv均高于仅进行烘干处理制备的活性炭,且随着烘焙温度的升高,活性炭Iv、MBv、BWCv呈现先提高后下降的趋势,其中,Trf-250 ℃活性炭产品的吸附性能最好。活性炭的强度随烘焙温度的提高呈小幅下降趋势,得率则相反,这可能与烘焙过程中水分、酯类、半纤维素的分解有关。考虑到烘焙对活性炭强度、得率影响较小,确定Trf-250 ℃条件来制备活性炭,以保证其产品吸附吸能。
-
为探究烘焙工艺提高活性炭性能的原因,研究了烘焙对原料半纤维素、木质素、纤维素质量分数的影响,结果见表2。由表2可知,原料中半纤维素、纤维素质量分数随烘焙温度的提高不断降低,半纤维素在250 ℃时基本分解完全,而纤维素在300 ℃时才能大部分分解。由于半纤维素和纤维素的不断分解,木质素的质量分数随烘焙温度的提高而不断提高。对比不同烘焙温度处理对原料中半纤维素、木质素、纤维素质量分数与活性炭性能的影响,发现两者存在密切关联。已有研究[21]表明,木质素质量分数的提高有利于增加活性炭的中大孔。Trf-250 ℃木质素质量分数提高至51.49%时,所制备活性炭的MBv、BWCv明显提高。说明活性炭中孔结构增加了,这可能与半纤维素被去除后木质素含量增加有关。同时,半纤维素的去除,破坏了纤维素和木质素的连接,进而导致细胞壁被破坏[22],为浸渍过程中活化剂的渗透提供了通道,也是此温度下制备活性炭性能较高的原因之一。
-
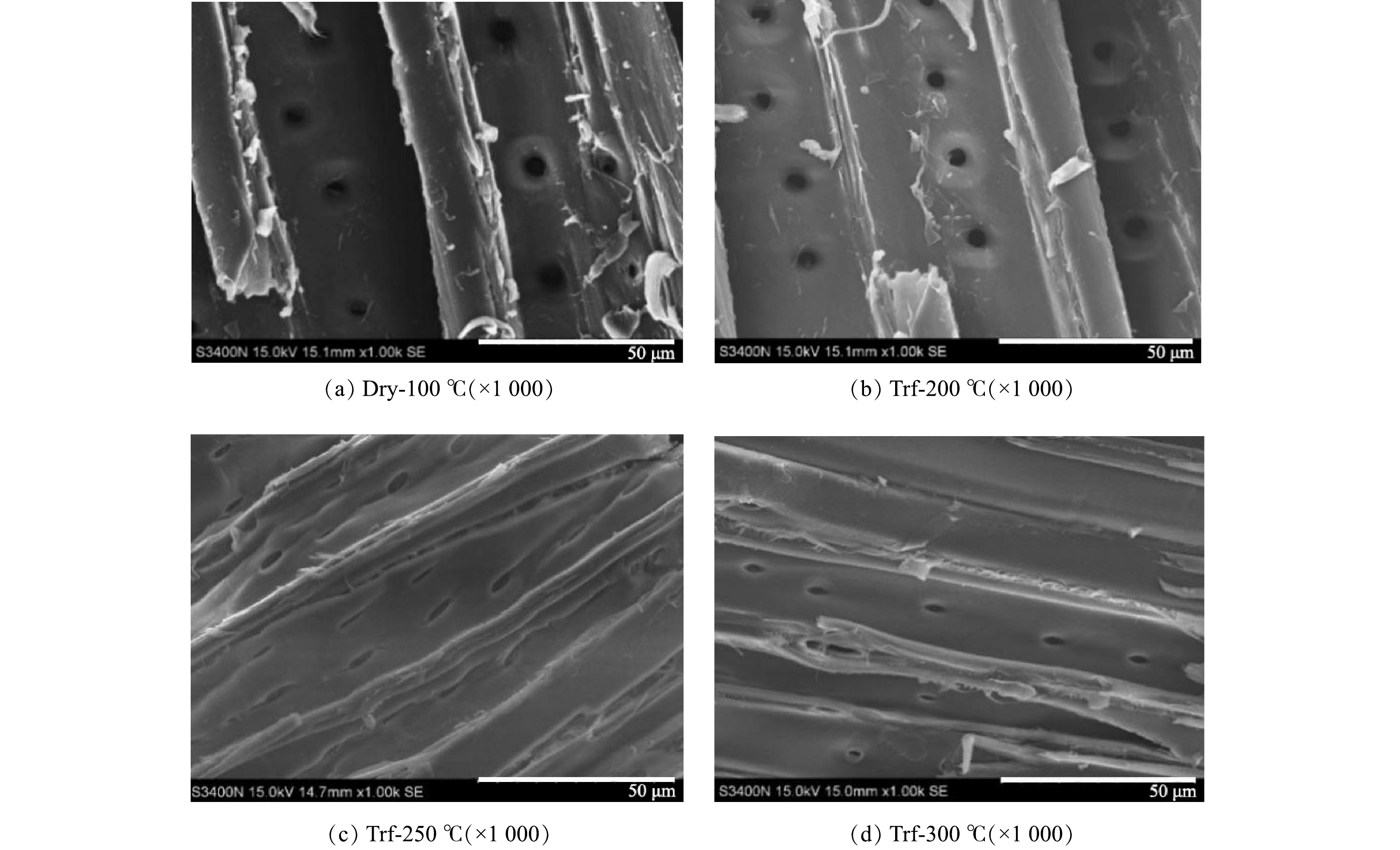
在烘焙过程中,伴随着半纤维素、木质素、纤维素不同程度的分解,使原料表面孔隙结构有一定发展[23]。研究了烘焙对活性炭表面形貌的影响,结果见图1。对比表面形貌可知,Dry-100 ℃表面粗糙、细小颗粒较多,部分孔为半闭合状态;Trf-200 ℃孔完全打开,表面细小颗粒减少,光滑度提高。当烘焙温度从200 ℃提高至250 ℃时,原料原生孔(交叉场纹孔)由圆形明显变为椭圆形,每排孔间距变小,且两排孔之间产生裂缝。这说明烘焙过程中温度的升高造成了原料收缩,原生孔受到挤压后变形;而新孔和裂缝的产生有利于磷酸渗透入原料内部,增加了原料和磷酸的接触面积,使制得的活性炭吸附性能提高。当烘焙温度提高至300 ℃时,原料进一步收缩,Trf-300 ℃产品的孔和裂隙不同程度地减少,导致制得的活性炭吸附性能有所下降。
-
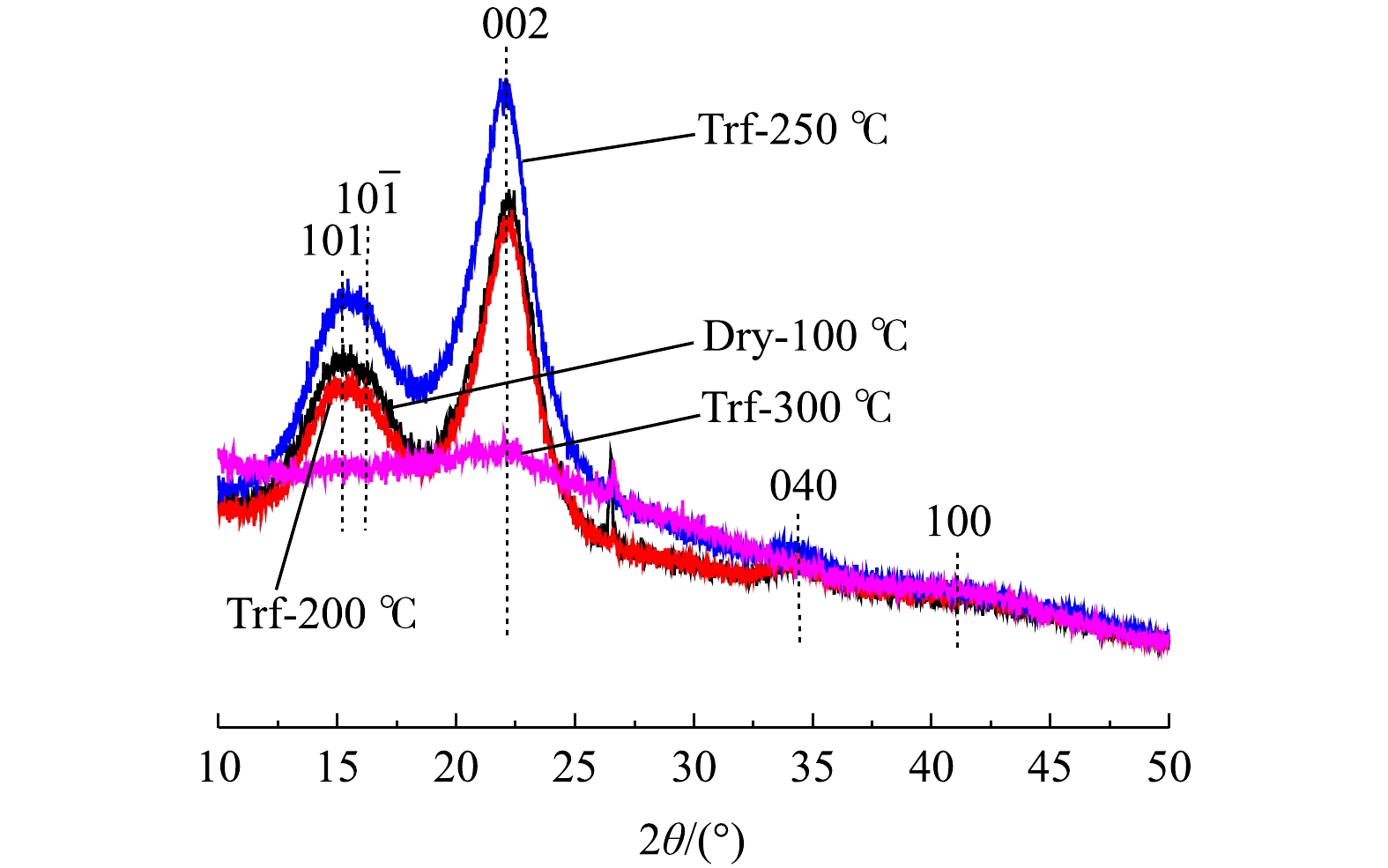
烘焙在导致原料部分降解的同时,也会对纤维素的结晶度产生影响。通过XRD研究了不同烘焙温度引起原料微晶结构的变化,结果如图2所示。由图2可知,烘焙温度低于250 ℃时,样品16°、22°、34°都有代表纤维素晶体的(101、10
$ \bar{1} $ )、(002)和(040)晶面,Trf-250 ℃峰值明显高于Dry-100 ℃和Trf-200 ℃,说明Trf-250 ℃纤维素的结晶度提高[24-25]。这可能是由于半纤维素在烘焙过程中最先降解,导致纤维素相对含量提高,进而引起结晶度提高[26]。Trf-300 ℃在24°、42°出现代表石墨化微晶碳的(002)衍射峰和(100)衍射峰,说明该烘焙温度下纤维素结晶区已被破坏,转化为无定形态,原料已炭化。原料炭化导致其与活化剂磷酸反应减弱,这可能也是导致Trf-300 ℃制备活性炭吸附性能下降的原因之一。 -
烘焙处理使原料细胞壁强度降低,可磨性提高[27],为原料粒度调控创造了条件。在经250 ℃烘焙后,对原料进行破碎筛分,研究了原料粒度对制得活性炭性能的影响,结果见表3。由表3可知,随着原料粒度的减小,成型活性炭的强度、表观密度则不断提高,Iv、MBv和BWCv也呈整体增加趋势。随着粒度的减小,磷酸通过扩散进入原料内部的距离缩短,原料吸酸率增大[28],磷酸与原料充分接触反应加速了原料物质形态结构的解离和热解,有利于孔结构的发展,使得活性炭的Iv、MBv和BWCv有所增加。另外,粉碎处理还会破坏原料中纤维素的结晶度,增加原料孔隙率[29],也起到增加活化剂渗透、提高活性炭性能的作用;另一方面,原料经粉碎筛分后,颗粒间距减小,颗粒间作用力增大,挤压后强度更高和表观密度更大。粒度小于0.2 mm的原料制得活性炭表观密度为0.413 g·mL−1,大于标准规定的0.350 g·mL−1[19]。继续减小粒度,会增加研磨成本,因而不利于工业化推广。
-
由于新标准中碳罐用柱状活性炭粒度指标以2~2.5 mm为主[19],考虑到挤压后成型料在干燥固化和高温活化过程中会收缩,故成型模具孔径的选择十分重要。分别研究了孔径为2~4 mm模具制得活性炭的粒度,结果见表4。由表4可知,随着模具孔径的增加,制备的活性炭粒度和表观密度随之增加,孔径2.5 mm模具制备的活性炭粒度集中分布于2.0~2.4 mm,表观密度0.413 g·mL−1亦可满足标准要求,因此选择孔径为2.5 mm的模具进行活性炭制备。
-
浸渍比是影响活性炭性能的重要因素。浸渍比在一定范围内的增加,会使磷酸占据更多潜在活化位点,有利于活化阶段造孔作用。然而,磷酸使用过量又会与原料间形成隔离层,阻碍活化作用的深入进行[30]。原料经烘焙调控(250 ℃)和粒度调控(<0.2 mm)后,在真空捏合时间60 min、磷酸活化温度500 ℃、磷酸活化时间120 min条件下,研究了不同浸渍比对所制活性炭性能的影响,结果见表5。由表5可知,活性炭的Iv、MBv、BWCv随浸渍比的提高而增加,强度和得率随浸渍比的提高而降低。当浸渍比从1.0∶1变为1.5∶1时,活性炭的Iv、MBv、BWCv都明显提高,MBv相比于Iv提高的更多,说明较大浸渍比有利于中孔生成[31]。当浸渍比为2.0∶1时,活性炭Iv、MBv、BWCv变化不大,而强度下降明显。这是由于多余的磷酸在漂洗过程中被洗去留下较大空腔,造成颗粒间接触降低,导致活性炭强度下降[32]。由于磷酸能与生物高分子发生交联反应,产生具有阻燃作用的磷酸酯键[33],降低了烧蚀反应,因而浸渍比提高,活性炭的得率有所提高。对比吸附性能,浸渍比对活性炭强度和得率的影响较大,故浸渍比优选为1.5:1。
-
在浸渍比为1.5∶1、磷酸活化温度500 ℃、磷酸活化时间120 min的条件下,研究了不同真空捏合时间对制备活性炭性能的影响,结果见表5。由表5可知,真空捏合30 min时,活性炭的Iv、MBv、BWCv及强度显著提高,并在真空捏合60 min时达到最大。这是由于真空捏合使物料间空气被排出,有利于磷酸渗透并与原料充分接触反应;同时,物料间的空气被排出后,物料结合得更加密实,成型效果更好,活性炭强度随之提高。但随着捏合时间延长(90 min),捏合过程产生的焦油量增加。虽然焦油量的增加有利于活性炭强度的提高,但较多的焦油会堵塞活性炭的部分孔隙,造成活性炭Iv、MBv、BWCv下降。真空捏合时间对活性炭得率影响较小。这是由于捏合温度相比活化温度要低很多,原料组分热分解较少。真空捏合60 min时,活性炭Iv为1 028 mg·g−1、MBv为270 mg·g−1、BWCv为152 g·L−1,为最优实验条件。
-
在浸渍比为1.5∶1、真空捏合时间60 min、磷酸活化时间120 min的条件下,研究了不同磷酸活化温度对制备活性炭性能的影响,结果见表5。由表5可知,活性炭的Iv、MBv、BWCv随活化温度的提高先增加后降低。这是由于活化温度较低时,活化反应不充分,活性炭孔结构还不发达;而活化温度过高,造成碳孔结构坍塌或磷酸盐结构降解,活性炭的孔结构遭到破坏,导致活性炭Iv、MBv、BWCv降低[34-35]。活化温度升高有利于活性炭强度提高。400~450 ℃时,活性炭强度提高较大,由86.0%提高至90.3%;500~550 ℃活性炭的强度提高较小,这可能与高温下磷酸催化木质纤维生成小分子缩聚成的交联网状结构减少有关。在活化过程中,随着温度不断升高,构成活性炭的骨架结构随之发生断裂、炭物质被过度烧蚀,导致得率降低。当温度为500 ℃时,制得活性炭的Iv、MBv、BWCv较好,强度提高较大,得率下降较小,故最优活化温度为500 ℃。
-
在浸渍比为1.5∶1、真空捏合时间60 min、磷酸活化温度500 ℃的条件下,研究了不同磷酸活化时间对制备活性炭性能的影响,结果见表5。由表5可知,活性炭的Iv、MBv、BWCv随磷酸活化时间的延长而增加,强度、得率随磷酸活化时间的延长而降低。这是由于在活化过程中,较长的活化时间更有利于磷酸溶液与原料的充分反应,促进孔隙结构的生成;但随着活化过程的进一步延长,磷酸侵蚀作用亦随之增强,炭烧失增加,强度和得率逐渐降低。有报道显示,活化时间延长还会使部分微孔转化为中孔[36],这一点可通过活性炭Iv下降得以证明。在磷酸活化时间为120 min时,制得的活性炭Iv、MBv、BWCv较好,强度和得率下降较少,故磷酸活化时间优选为120 min。
-
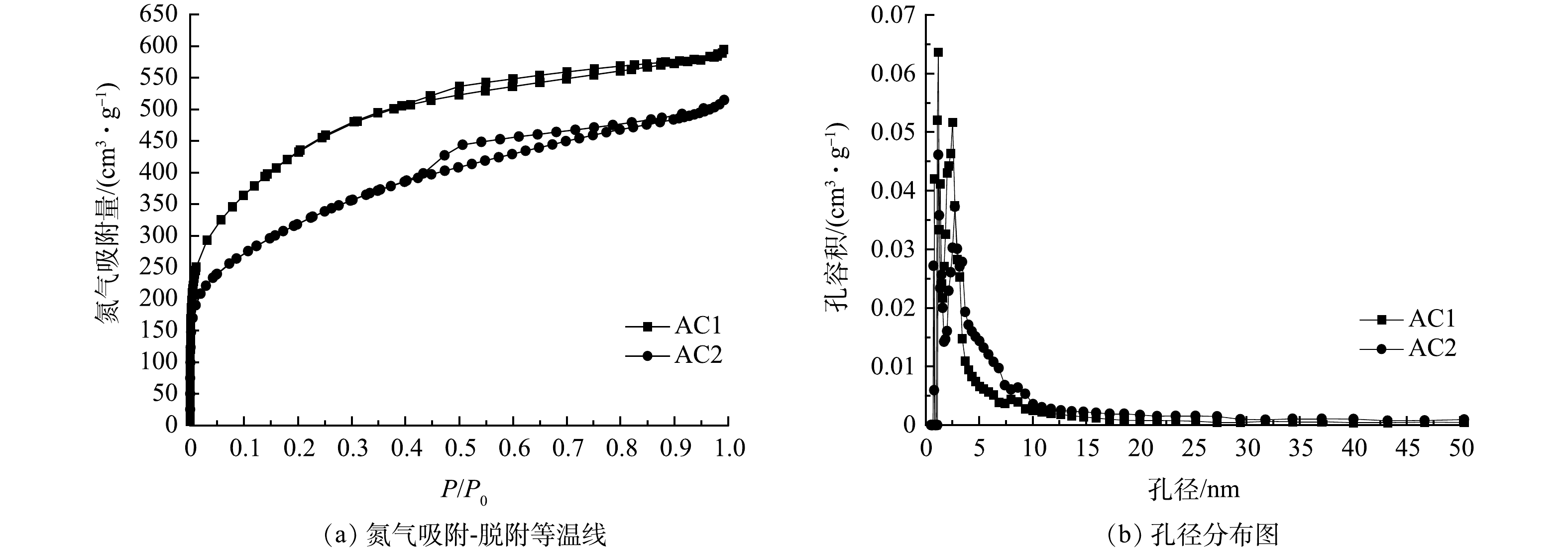
根据上文第2.1~2.6节的分析结果,对最优实验条件下(烘焙温度250 ℃、粒度<0.2 mm、浸渍比1.5∶1、真空捏合时间60 min、活化温度500 ℃、活化时间120 min)制得的活性炭(标记为AC1)和商用活性炭(标记为AC2)进行了孔结构表征,氮气吸附-脱附等温线和孔径分布图见图3。
由图3(a)可知,AC1和AC2的吸附等温线均为IV型[37]。在P/P0>0.4时出现的滞后回环表明AC1和AC2均具有一定数量的中孔。在同一相对压力下,AC1的N2吸附容量明显高于AC2,说明AC1具有更大的BET比表面积。由图3(b)可知,AC1和AC2的孔径集中分布于10 nm内,1.2~1.6 nm处的微孔分布密集,与活性炭具有较高Iv和MBv相符;2~4 nm处的中孔大量存在,与等温线出现明显滞后回环相符。对于<3 nm的孔径,AC1的孔容积高于AC2,而孔径>3 nm后结果正好相反。这说明烘焙提质和粒度调控可使>3 nm的中孔转变为更小的孔,而活性炭对丁烷的吸附能力变强可能与小于3 nm的孔增多有关。经计算,AC1和AC2的孔结构参数见表6。AC2的比表面积和总孔容积对比AC1小许多,这可能与其中孔容积较大(0.391 cm3·g−1)且小于3 nm孔占比较小有关。同时,根据文献[15]提出的影响活性炭丁烷工作吸附能力的有效孔径范围,列出了AC1、AC2的孔径为1.2~6 nm的孔占比,发现AC1有效孔径比例比AC2高14.32%,表明AC1的孔径分布更有利于丁烷吸附。
-
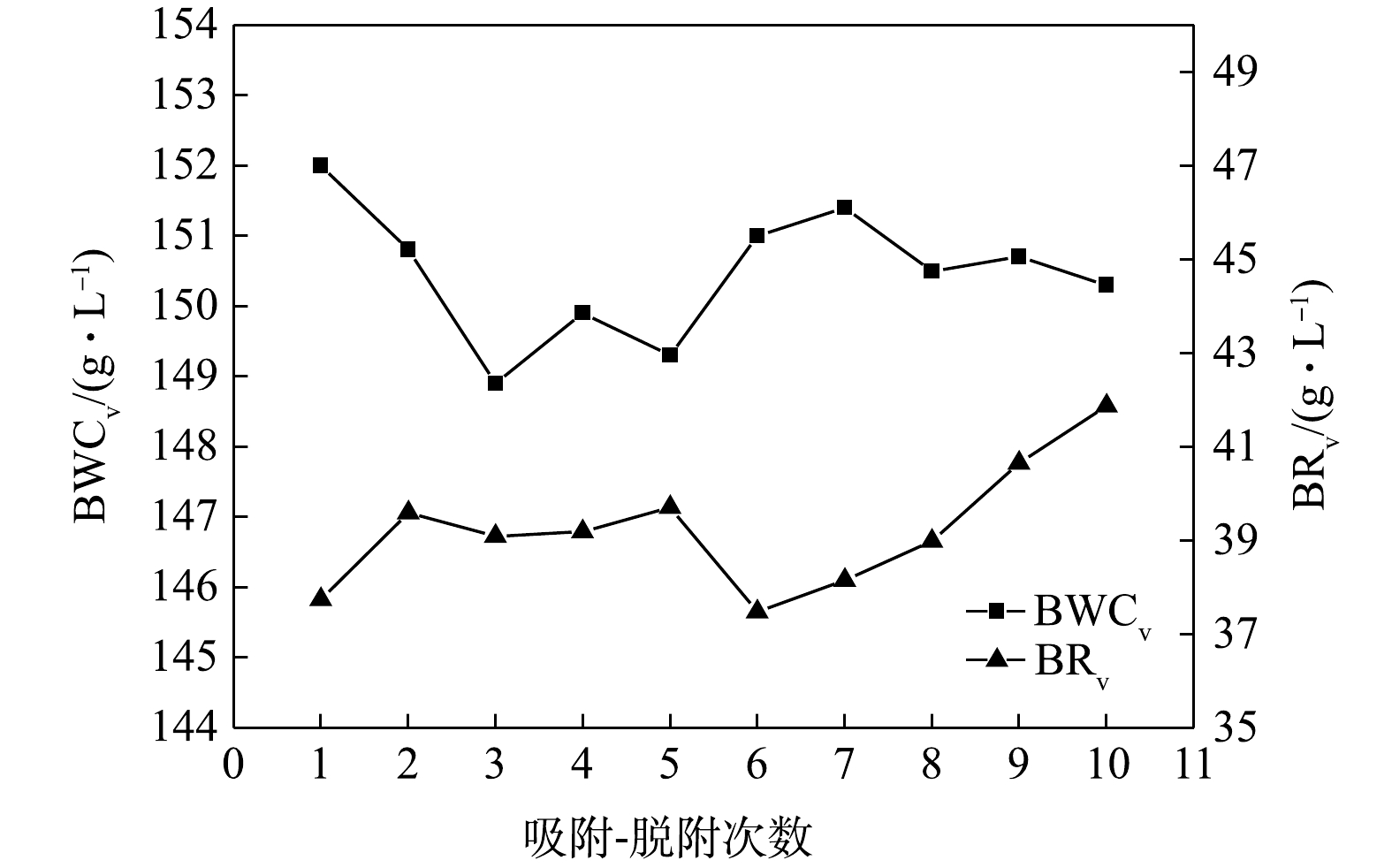
由于汽车碳罐用活性炭在使用过程中需要反复吸气和脱气,故要求制得的活性炭具有较高的循环稳定性。循环实验中,按照国标(GB/T 20449-2006)在活性炭吸附丁烷气体饱和后通过氮气吹扫再生,重复测量10次,通过吸附-脱附次数对活性炭BWCv和BRv的影响表征活性炭的循环稳定性,结果见图4。由图4可知,活性炭的BWCv随吸附-脱附次数的增加呈整体下降趋势,但下降过程没有规律性;吸附-脱附10次后,活性炭BWCv从150.3 g·L−1,降幅为1.12%。活性炭BRv随吸附-脱附次数的增加有所增加。吸附-脱附10次后,活性炭BRv从3.77%增至4.19%,变化较小,且远低于标准中BRv(<18%)的指标。吸附-脱附次数的增加会导致活性炭孔隙内的丁烷残留增多,这是导致活性炭BWCv下降和BRv上升的原因。本研究中制备的活性炭AC1在吸附-脱附10次后,仍能保持良好的循环稳定性,这与活性炭具有合理的孔径分布密切相关。
2.1. 烘焙预处理的影响
2.1.1. 烘焙对活性炭性能的影响
2.1.2. 烘焙对原料纤维素、半纤维素和木质素含量的影响
2.1.3. 烘焙对原料表面形貌的影响
2.1.4. 烘焙对原料微晶结构的影响
2.2. 对材料粒度的调控
2.2.1. 对原料粒度的调控
2.2.2. 对活性炭粒度的调控
2.3. 浸渍比的影响
2.4. 真空捏合时间的影响
2.5. 活化温度的影响
2.6. 活化时间的影响
2.7. 活性炭孔结构的表征
2.8. 活性炭的循环稳定性
-
1)活性炭原料经250 ℃烘焙去除了半纤维素,增加了木质素的比例。烘焙提质可使原料表面孔数量增加,纤维素的结晶区受到破坏,使活化剂能通过孔道渗透进原料内部并进行反应,从而有效提高活性炭的Iv、MBv和BWCv。
2)烘焙提质可降低活性炭原料细胞壁的强度,有利于原料破碎。优选粒度小于0.2 mm的原料来制备活性炭,目数的增加有利于提高活性炭的表观密度,进而提高活性炭的BWCv。较大的目数使原料颗粒间距减小,颗粒间作用力增大,成型后的活性炭有更高的强度。为使制备的活性炭粒径在标准规定范围内,成型时选择孔径为2.5 mm的模具。
3)活性炭原料经烘焙提质和粒度调控后,采用磷酸活化制备的成型活性炭的吸附性能、强度和表观密度都显著提高。在优选实验条件下制得的活性炭,可达到碳罐用活性炭TGZ1500指标要求,可实现对进口同类活性炭的替代。
4)活性炭的丁烷吸附-脱附性能与活性炭的孔结构分布密切相关。所制活性炭AC1孔径小于3 nm的孔占总孔容的74.30%,孔径为1.2~6 nm的孔占比高于常规方法制备的活性炭。孔径分布的改变与烘焙后原料有较高的木质素含量和采用较高的活化温度有关。
5)合理的孔径分布是活性炭有足够吸附能力的保证,又使丁烷能从孔道内脱附出来。通过10次吸附-脱附实验发现,AC1的BWCv和BRv变化较小,循环稳定性较好。



 下载:
下载: