-
2022年,我国的皮革出口总额达
1075.3 亿美元,生产和贸易量均居世界首位[1]. 据统计,我国制革行业每年约排放出14亿t的含铬(Cr)废水[2],并产生约3.5万t的含铬污泥[3]. 目前对制革污泥的处理方法有填埋和焚烧等,其中填埋是最主要的处置方式[4]. 在美国和日本,约40%—60%的制革污泥是采用填埋的方式进行处理[5]. 然而,制革污泥中的Cr具有含量高、易迁移的特点,填埋场的防渗能力随着时间衰减可能发生含Cr渗滤液向堆场周边扩散,造成生态环境污染[6 − 7].制革污泥填埋场周边土壤和地下水的Cr污染严重,会发生深层迁移. 例如Guo和Ye等利用Hydrus-1D模型预测发现表层土壤中Cr会不断向下迁移,对土壤和地下水造成严重污染[8 − 9];土柱实验[10]也表明Cr会沿土壤剖面向深层地下环境运移,进而对周边环境造成潜在风险. 然而,目前针对制革污泥填埋场周边的环境影响和生态风险仍存在认识不深入,研究不系统等问题. 在河北省辛集市某皮革制造厂的污泥堆场中,污泥中的总Cr含量高达
20700 —21400 mg·kg−1 [11]. 环境中Cr并非处于静止状态,而是会随降水、土壤溶液和地下水等对流和扩散发生迁移. Cr的赋存形态随环境条件的变化而转变,如土壤中的无机矿物(如MnO2)可以将Cr(Ⅲ)氧化为Cr(Ⅵ),极大地提高Cr的迁移风险[12]. 此外,制革污泥中高有机质含量会促进污泥胶体颗粒对Cr的吸附,Cr还可与低分子可溶性有机酸形成配合物存在于土壤中,其溶解性和迁移能力显著提高[13]. 污泥胶体颗粒可以改变重金属形态、毒性和运移过程,有机/无机胶体可以通过吸附和共沉淀作用携带Cr在环境介质之间运移[14],扩大Cr污染范围,导致污泥堆场周边土壤、地表水及地下水环境的污染,威胁周边人居环境的生态安全.污泥胶体吸附Cr在环境介质中迁移的过程,可能与土壤颗粒、胶体、带电离子等物质发生交互作用,因此识别主要环境因子是研判土-水环境中Cr污染负荷及生态风险的关键. 一般情况下,微生物硝化作用使制革污泥在不受外界扰动的情况下呈弱酸性[11]. 制革污泥中除了含有大量的Cr外,还有Fe、Al、Ca等氧化物及氢氧化物形成的无机矿物胶体[15]. 赤铁矿(Hem)胶体在制革污泥中广泛存在,含量范围在8.3%—55%[11, 16]. Hem胶体在自然环境中通常携带正电荷,而Cr主要以带负电的CrO42−和Cr2O72−形式存在,因此两者间具有很强的亲和力[17],可以作为Cr有效载体[17]. 污泥中有机质(如腐殖酸(HA)等)的存在也会增加Cr(Ⅵ)的迁移风险[18,19]. 此外,腐殖酸表面带有可变电荷,具有比表面积大、物质活性高等特点,可显著影响Cr在胶体上的吸附行为[20]. 当污泥胶体协同Cr从有氧环境扩散至缺氧环境中,氧化还原条件也会影响胶体颗粒的形成、稳定性以及流动性[21]. 目前对污泥胶体协同污染运移过程研究多为单一要素对Cr迁移特性的影响,而对于多要素下Cr的环境风险研究相对缺乏[22].
本研究结合制革污泥酸性、Hem胶体和有机质含量高的特性,通过吸附批实验和柱迁移实验,探究Cr(Ⅵ)在Hem胶体上的吸附机制及在多孔介质中的迁移行为. 基于DLVO理论,探究Cr(Ⅵ)、HA、Hem在二元和三元体系中协同运移机制. 此次工作将为深入理解制革污泥场中Hem胶体和Cr(Ⅵ)协同运移过程奠定基础,也为制革污泥中污染物的防治提供科学依据.
-
Cr(Ⅵ)储备液的配制:称取
0.0707 g重铬酸钾(K2Cr2O7,天津市大茂化学试剂厂),使用超纯水(DW,18 MΩ,Milli-Q)制备浓度为100 mg·L−1的Cr(Ⅵ)储备液. 将Cr(Ⅵ)储备液稀释至0.25、0.5、1、3、5、10 mg·L−1.Hem胶体的制备:根据Schwertmann和Cornell[23]的标准化方法合成Hem胶体,将0.2 mol·L−1的Fe(NO3)3·9H2O(上海阿拉丁生化科技股份有限公司)溶液加热至90 ℃,随后依次加入预热至90 ℃的1 mol·L−1的KOH溶液和1 mol·L−1的NaHCO3(国药集团化学试剂有限公司)溶液,将混合后的溶液密封置于90 ℃的烘箱中恒温老化48 h,老化后的溶液离心后透析至电导率小于2 μs·cm−1,最后将溶液冷冻干燥成固体颗粒研磨成粉末备用.
HA储备液的配制:使用蒸馏水将0.02 g的HA(上海北诺生物科技有限公司)搅拌后超声处理,加入1 mol·L−1的NaOH溶液使HA固体颗粒充分溶解,得到200 mg·L−1的HA溶液,过0.22 μm PTFE滤膜以去除未溶解的颗粒,得到HA储备液,将HA储备液转移至棕色试剂瓶中密封并于−4 ℃条件下避光保存. 实验中使用的所有化学试剂均为分析纯.
-
在15 mL的离心管中加入背景溶液为1 mmol·L−1的NaCl溶液和浓度为20 mg·L−1的超声分散均匀的Hem胶体. 基于河北省辛集市某污泥堆场作的前期调查,制革污泥的pH为4—5[11]. 因此,使用0.01 mol·L−1的HCl将pH调至5. 随后加入不同浓度的Cr(Ⅵ)(0.25、0.5、1、3、5、10 mg·L−1),将振荡2 h后的上述悬浮液过0.22 μm PTFE 滤膜去除Hem胶体颗粒,测定上清液中的Cr(Ⅵ)的浓度. 实验设置3个重复. 为探究HA存在下,Cr(Ⅵ)在Hem胶体上的吸附性能,上述试验在加入Cr(Ⅵ)溶液之前,先加入0.25 mg·L−1的HA,其它试验条件不变.
-
使用0.01 mol·L−1的HCl溶液将一定浓度的Hem胶体悬浮液pH值调至5,水浴超声15 min使其充分分散;然后依次加入一定体积的Cr(Ⅵ)储备液和1 mmol·L−1的NaCl溶液,快速取一定量混合悬浮液置于纳米粒度仪中测量. 30 min内每30 s测定1次,每个实验条件设置2个重复. 悬浮液中Hem胶体的浓度为20 mg·L−1,Cr(Ⅵ)浓度分别为0.5、1、3、5、10、15、20 mg·L−1. 为探究HA存在时Cr(Ⅵ)对Hem胶体稳定性的影响,上述试验在加入Cr(Ⅵ)溶液之前,先加入0.25 mg·L−1的HA反应10 min,其它试验条件不变.
-
使用2 mol·L−1的HCl溶液去除石英砂(粒径:425—600 μm)表面的有机物、碳酸盐以及金属氧化物等杂质,清洗至石英砂电导率保持在2 μS·cm−1以下. 柱迁移实验所用填充柱为不锈钢柱(内径:2.6 cm,高度:12.6 cm),砂柱容重为1.46—1.52 g·cm−3. 试验中液体的流动方向为下进上出. 设定蠕动泵流速为1 mL·min−1,每隔5 min收集一管出流液. 使用紫外可见分光光度计在测定出流液中的胶体浓度.
其中,含Cr(Ⅵ)的Hem胶体流入液的配制如下:调节Hem胶体悬浮液的pH为5,依次加入一定体积的Cr(Ⅵ)储备液(表1)和1 mmol·L−1的NaCl溶液,实验设置2个重复. 为探究HA存在时Cr(Ⅵ)对Hem胶体稳定性的影响,在加入Cr(Ⅵ)溶液之前,先加入0.25 mg·L−1的HA反应10 min,其它试验条件不变(表1). 收集完毕出流液以后,将不锈钢柱中的石英砂均等剖分成12份;测定石英砂中滞留的Hem胶体浓度.
-
胶体在不同电解质溶液中的水合粒径、Zeta(ζ)电势使用纳米粒度仪(Zetasizer Nano ZS90,马尔文,英国)测试,测试结果用于DLVO势能计算. DLVO理论中总相互作用能(ФDLVO)包括范德华引力势能(ФvdW)与双电层力势能(Фdl)(公式1)[24]. 当DLVO总势能为正时,说明胶体间斥力大于引力,当总势能为负时,说明胶体间引力大于斥力.
采用球-球模型计算赤铁矿胶体颗粒之间的势能(公式(2)和(3)),采用球-板模型计算赤铁矿胶体与石英砂之间的势能(公式(4)和(5)).
式中:A为Hamaker常数(J);R表示胶体半径(m);h表示胶体颗粒和固相介质之间的距离(m);H表示胶体颗粒相互之间的距离(m),λ0表示德拜特征长度(m);ε表示液相的介电常数(C·V−1m−1),k表示玻尔兹曼常数(J·K−1);T表示液相的绝对温度(K);v是离子价态;e表示电子电荷(C);κ表示德拜长度的倒数(m−1);γ1和γ2分别表示赤铁矿胶体和石英砂的表面电势(V)[25].
颗粒粒径和ζ电位提供了胶体颗粒稳定性信息. 胶体稳定性可以通过两个相互作用粒子之间的静电排斥力和范德华吸引力的总体平衡来评估. 胶体颗粒的稳定性取决于其能量势垒[26].
-
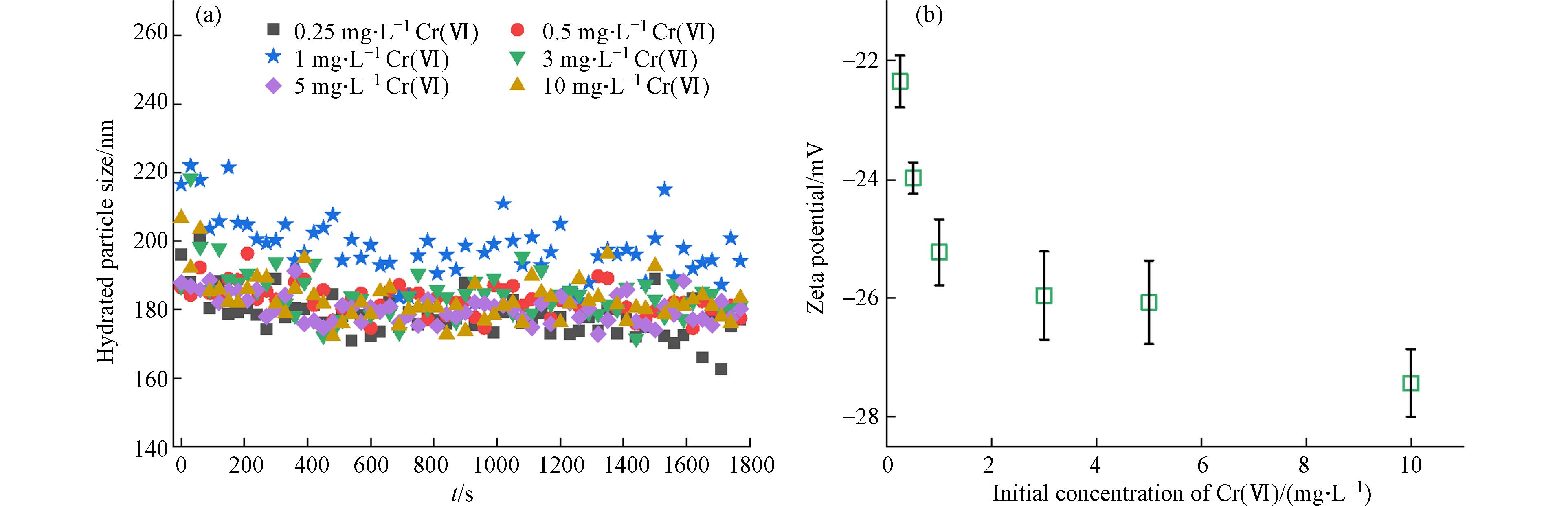
酸性条件下,随着Cr(Ⅵ)初始浓度从0.25 mg·L−1增加到10 mg·L−1,Hem胶体对Cr(Ⅵ)的吸附量呈上升趋势(图1),最大吸附量为2.72 mg·g−1. Hem胶体对Cr(Ⅵ)的吸附机制主要依托于二者的赋存形式. 在pH=5条件下存在大量的H+,使得Hem胶体表面高度质子化;而此时Cr(Ⅵ)主要以HCrO4−和少量Cr2O72−形式存在[27],带正电的Hem胶体与HCrO4−产生强烈的静电吸引作用[28],从而使Hem胶体表现出对Cr(Ⅵ)较强的吸附能力.
当吸附体系中添加HA后,Hem胶体对不同浓度Cr(Ⅵ)的吸附量随着Cr(Ⅵ)初始浓度的增大而增大,与未预吸附HA的Hem胶体相比,在相同Cr(Ⅵ)初始浓度下,预吸附HA的Hem胶体对Cr(Ⅵ)的吸附量均降低(图1). HA在其柔性有机骨架上具有高容量的酸性官能团,通常带负电荷[29],HA对Cr(Ⅵ)离子具有很强的络合能力[30],并且对矿物表面也具有很高的亲和力[31]. 因此,HA可以吸附在Hem胶体表面,改变Hem胶体的表面特性:一方面占据Hem表面的吸附位点,从而与Cr(Ⅵ)形成竞争吸附机制;另一方面,HA通过与Hem胶体反应位点结合改变了Hem胶体的静电特性,导致反应位点与CrO42−之间的静电排斥增加. 在以往的研究中也发现了类似规律:HA对黄铁矿吸附Cr(Ⅵ)具有抑制作用[32];HA和水铁矿的络合物对Cr(Ⅵ)的吸附量随C/Fe比率的增加而降低[33];柠檬酸-施氏矿物络合物对Cr(Ⅵ)的吸附量和吸附速率明显低于施氏矿物本身,并且复合材料对Cr(Ⅵ)的吸附量和吸附速率与复合材料的载碳量呈负线性相关[34]. 基于以上分析,HA的存在降低了Hem胶体对Cr(Ⅵ)的吸附,使游离的Cr(Ⅵ)离子浓度增加.
-
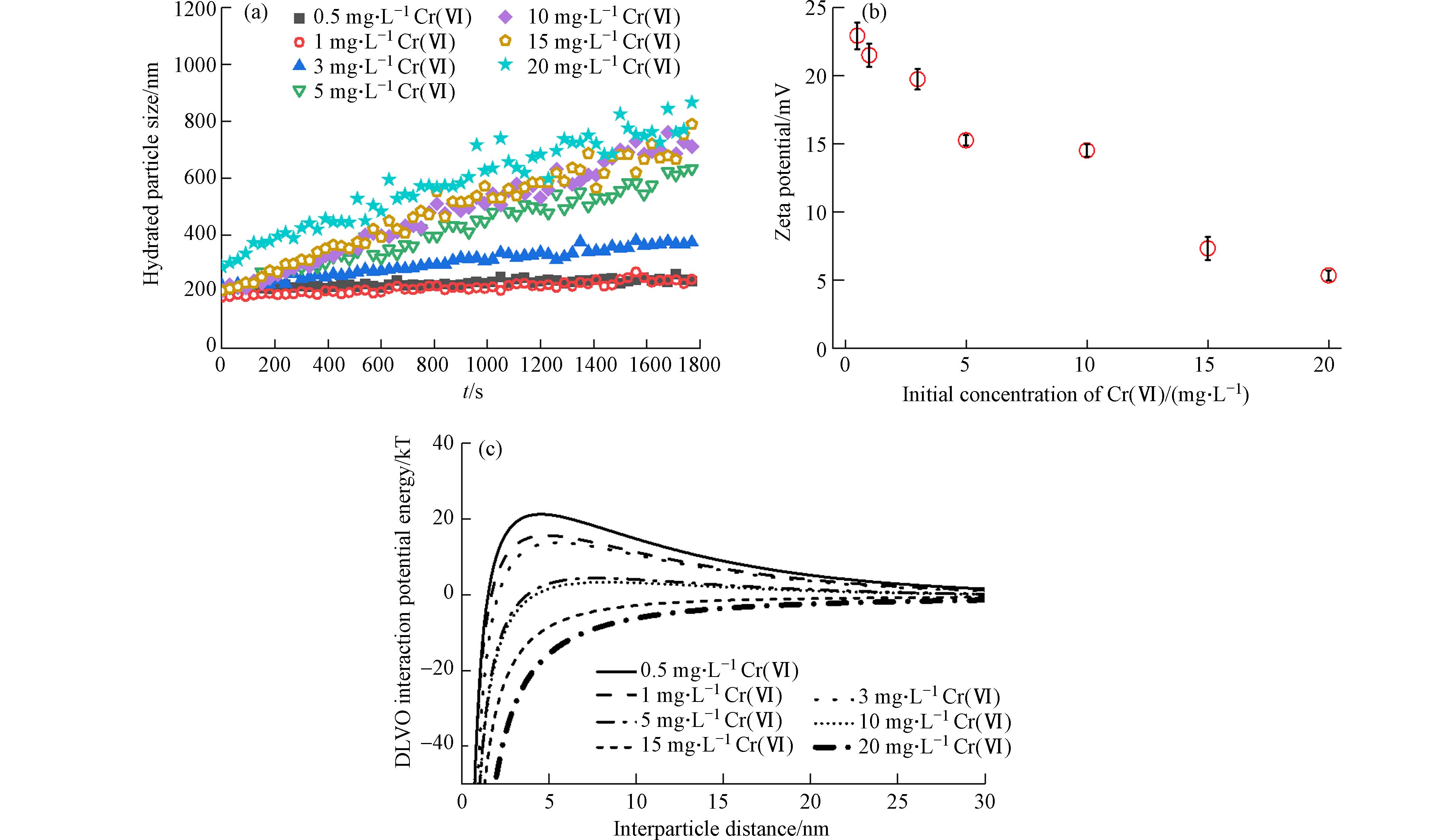
在1 mmol·L−1 NaCl溶液中,Hem胶体在不同浓度的Cr(Ⅵ)溶液中水合粒径随时间的变化如图2a所示.
当Cr(Ⅵ)的浓度≤1 mg·L−1时,Hem胶体的水合粒径随时间的变化不大,表明该浓度下Hem胶体处于相对稳定的状态. 随着Cr(Ⅵ)浓度继续增大,Hem胶体的水合粒径增加的速率逐渐增大,稳定性越来越弱. 这与Hem胶体电势随Cr(Ⅵ)浓度变化趋势一致(图2b),即:随着Cr(Ⅵ)吸附量的逐渐增大,更多的Cr2O72−和HCrO4−吸附到Hem胶体表面,使得胶体的表面正电荷被中和;未吸附到Hem胶体上的含氧酸根离子游离在溶液中也会压缩Hem胶体的双电层. 因此zeta电势逐渐下降,Hem胶体表面电势绝对值变小,并随着Cr(Ⅵ)浓度的增大而趋于“0”,胶体颗粒间的最大排斥势垒逐渐降低(图2c),胶体间斥力减弱,胶体悬浮液稳定性降低[35]. 上述结果表明,在酸性条件下,Cr(Ⅵ)浓度的增加促进了Hem胶体的聚集.
与Cr(Ⅵ)-Hem悬浮液体系相比,HA-Cr(Ⅵ)-Hem悬浮液体系中的Hem胶体更加稳定. 在不同Cr(Ⅵ)浓度下,Hem胶体的水合粒径没有随时间发生明显变化(图3a). 预吸附HA后,随着Cr(Ⅵ)浓度从0.25 mg·L−1增加到10 mg·L−1,Hem胶体的电势从−22.35 mV降低到−27.43 mV(图3b),这可能是由于Cr2O72−和HCrO4−吸附到了Hem胶体表面. 虽然Hem胶体的电势随Cr(Ⅵ)浓度增加逐渐下降,但是图3a中显示其水合粒径并未随Cr(Ⅵ)浓度增加而减小,这可能是由于HA对Hem胶体具有较强的分散作用,Hem胶体粒径已经达到最小,微小的电位变化幅度不足以使Hem胶体水合粒径进一步减小.
-
以通入溶液的孔隙体积(PV)为横坐标,出流液中Hem胶体浓度C与入流液Hem胶体浓度C0的比值C/C0为纵坐标,绘制穿透曲线(图4a). 结果表明,3种Cr(Ⅵ)浓度下,在出流液中,均未检测到Hem胶体. 以无量纲化距离(石英砂的位置距离入水口的相对位置)为纵坐标,Hem胶体的相对滞留量(N/Ni)为横坐标绘制滞留曲线(图4c). 大部分Hem胶体沉积在石英砂柱流入液的入口附近,并随着深度的增加而下降. Hem胶体和Cr(Ⅵ)络合后使得Hem胶体的粒径增加,zeta电位降低且带正电荷,与带负电荷的石英砂发生静电吸引,这将使得更多的Hem胶体被吸附滞留在石英砂柱中[35]. Hem胶体与石英砂之间的最大排斥势垒消失(图4e),而稳定性的降低也进一步促使Hem胶体在石英砂中附着滞留,运移能力减弱. 实验结果发现,砂柱中胶体的回收率较高(82.9%—87.4%)也证实了这一结果.
预先吸附0.25 mg·L−1的HA后,随着Cr(Ⅵ)浓度从0.25 mg·L−1增加至10 mg·L−1,Hem胶体的穿透率从53.12%增加至61.70%(图4b),可能是由于Cr(Ⅵ)浓度越高,Hem胶体表面负电荷越多,其与石英砂之间的斥力逐渐越大,因而有更多的Hem胶体迁出. Hem胶体的zeta电势变化(表2)证实了上述观点,即:随着Cr(Ⅵ)浓度增加,Hem胶体的zeta电势的绝对值增加. 但Cr(Ⅵ)浓度为10 mg·L−1的穿透率比5 mg·L−1时的穿透率仅增加了2.38%,推测胶体已经达到了该pH条件下的能够迁出的最大值,表明酸性条件下Cr(Ⅵ)能够促进预先吸附了HA的Hem胶体的运移. 当Cr(Ⅵ)浓度从0.25 mg·L−1增加至10 mg·L−1时,Hem胶体与石英砂的最大排斥势垒从98.32 kT增大到125.78 kT(表2,图4f),较大的排斥势垒引起了Hem胶体更多的迁出(图4b).
与Cr(Ⅵ)-Hem体系(图4a、4c、4e)相比,在相同浓度的Cr(Ⅵ)溶液中,HA-Cr(Ⅵ)-Hem悬浮液(图4b、4d、4f)中Hem胶体的运移能力都发生了不同程度的增强. 这表明HA促进了环境中Hem胶体的运移能力. 除了电荷之间的静电力相互作用外,Cr(Ⅵ)-Hem配合物可以减小Hem胶体在石英砂表面的吸附,进一步促进Hem胶体在多孔介质中的运移,这可能是Cr(Ⅵ)促进Hem胶体迁移的主要机制之一. 研究表明,HA可以促进U(Ⅵ)在花岗岩颗粒柱中的运移[36]. U(Ⅵ)在多孔介质中的迁移实验表明,当流入液中为U(Ⅵ)-高岭石胶体时,38.8%的U(Ⅵ)可以穿过石英砂柱;当流入液中为U(Ⅵ) -HA-高岭石胶体时,73.7%的U(Ⅵ)可以穿过石英砂柱,表明在高岭石胶体和HA共同作用下显著提高了U(Ⅵ)的移动性[37]. 由此推断,Cr(Ⅵ)-HA-Hem悬浮液体系中,HA可以促进体系中Hem胶体携带大量的Cr(Ⅵ)从石英砂柱中的穿出. 在实际场地中,由于制革过程中有机酸、染料和辅助鞣剂的大量使用[6],这会导致污泥池中含有大量的可溶性有机质,从而增加了Hem胶体协同Cr(Ⅵ)迁移风险.
-
本研究通过研究酸性条件下,HA对Hem胶体吸附Cr(Ⅵ)和垂向迁移的影响,主要结论如下:
1)Hem胶体对Cr(Ⅵ)的吸附量随Cr(Ⅵ)浓度的增加而增加,相同Cr(Ⅵ)初始浓度下,HA在一定程度上降低了Hem胶体对Cr(Ⅵ)的吸附.
2)Cr(Ⅵ)浓度的增加促进了Hem胶体的聚集,而HA的存在能有效抑制Hem胶体的聚集,增加Hem胶体颗粒的稳定性.
3)在酸性条件下,Hem胶体带正电,所有胶体在石英砂柱中均未迁出,而HA会促进Hem胶体在石英砂柱中的运移能力.
因此,在酸性Cr(Ⅵ)污染土壤环境中,如果土壤中几乎没有可溶性有机质时,制革污泥中Hem胶体协同Cr(Ⅵ)迁移风险较小;而当可溶性有机质大量共存时,Hem胶体协同Cr(Ⅵ)迁移风险较高,对堆场周边的生态环境构成潜在风险.
酸性条件下饱和多孔介质中赤铁矿胶体和Cr(Ⅵ)的协同运移及影响机制
Co-transport and influence mechanism of hematite colloids and Cr(Ⅵ) in saturated porous media under acidic conditions
-
摘要: 含铬污泥的长时间填埋堆存可能对周边环境带来污染风险. 制革污泥中富含胶体物质和腐殖酸,有研究表明腐殖酸能通过吸附和静电作用影响胶体和Cr(Ⅵ)在多孔介质中的协同运移,使Cr(Ⅵ)向深层环境介质迁移. 然而,制革污泥中赤铁矿胶体等共存物质对Cr(Ⅵ)运移的影响尚不清楚. 本研究选取制革污泥中特征组分(赤铁矿胶体和腐殖酸),通过吸附实验和柱迁移实验等方法模拟酸性条件下,腐殖酸和Cr(Ⅵ)浓度对赤铁矿胶体稳定性和运移行为,探究赤铁矿胶体协同Cr(Ⅵ)运移的环境风险. 结果表明,赤铁矿胶体对Cr(Ⅵ)的最大吸附量为2.72 mg·g−1;Cr(Ⅵ)浓度越高,赤铁矿胶体聚集越明显,腐殖酸能够有效抑制Cr(Ⅵ)-赤铁矿胶体聚集,使Cr(Ⅵ)-赤铁矿胶体在石英砂柱中的穿透量增加,增强了Cr(Ⅵ)-赤铁矿胶体悬浮液的稳定性,提高了赤铁矿胶体携带Cr(Ⅵ)的运移能力. 基于DLVO理论,综合柱流出物的zeta电位和颗粒尺寸的演变规律,揭示了Cr(Ⅵ)、赤铁矿胶体和腐殖酸在二元和三元体系中协同运移的作用机理. 研究结果将为评估制革污泥中的赤铁矿胶体在实际环境中携带Cr(Ⅵ)运移的风险提供理论支持.Abstract: Long-term landfill storage of chrome-containing sludge may cause pollution risks to the surrounding environment. Tanning sludge contains abundant colloidal substances and humic acid. Studies have shown that humic acid can affect the co-transport of colloid and Cr(Ⅵ) in porous media through adsorption and electrostatic interaction, causing Cr(Ⅵ) transport to deep underground environment. However, the effect of coexisting substances such as hematite (Hem) colloids in tannery sludge on the transport of Cr(Ⅵ) remains unclear. In this study, the characteristic components (Hem colloids and humic acid (HA)) in tannery sludge were selected to simulate the effects of HA and Cr(Ⅵ) concentration on the stability and transport behavior of Hem colloids under acidic conditions through adsorption and column transport experiments, exploring the environmental risk of Cr(Ⅵ)-Hem colloids co-transporting. The results showed that the maximum adsorption of Cr(Ⅵ) by Hem colloids was 2.72 mg·g−1; the higher the concentration of Cr(Ⅵ), the more obvious the aggregation of Hem colloids. However, HA effectively inhibited the aggregation of Cr(Ⅵ)-Hem colloid, therefore increased their penetration in quartz sand columns and enhanced the transport capacity of Hem colloids carrying Cr(Ⅵ). The co-transport mechanisms of Cr(Ⅵ), Hem colloids and HA in binary and ternary systems were comprehensively revealed by evaluating the evolution of zeta potential and particle size of the column effluent as well as DLVO theoretical calculations. The results will provide theoretical support to evaluate the risk of Cr(Ⅵ) transport being carried by Hem colloids in tannery sludge under the real-life situation.
-
Key words:
- Hem colloids /
- Cr(Ⅵ) /
- pH /
- saturated porous media /
- transport
-

-
表 1 柱迁移实验条件设置
Table 1. Column experiment conditions
HA浓度/ (mg·L−1)
HA concentrationHem浓度/ (mg·L−1)
Hem concentrationCr(Ⅵ)浓度/ (mg·L−1)
Cr(Ⅵ) concentrationpH NaCl浓度/ (mmol·L−1)
NaCl concentration孔隙度
Porosity— 20 0 5 1 0.47 — 20 0.25 5 1 0.47 — 20 5 5 1 0.49 — 20 10 5 1 0.47 0.25 20 0.25 5 1 0.46 0.25 20 5 5 1 0.47 0.25 20 10 5 1 0.48 表 2 不同浓度Cr(Ⅵ)溶液中Hem胶体与石英砂之间的DLVO势能计算及运移实验中Hem胶体的回收率(pH=5)
Table 2. DLVO interaction energy between Hem colloid and quartz sand at different concentrations of Cr(Ⅵ) and recovery rates of hematite colloid in column experiments
HA/
(mg·L−1)Cr(Ⅵ)/
(mg·L−1)D/nm ζ/mV ζs/mV Ф/kT M/% Фmax Фmin2 Meff Mret Mtot 0 0 199 ± 10 25.3 ± 0.8 −33.2 ± 0.9 — — 1.5 87.4 88.8 0 0.25 213 ± 17 23.6 ± 0.8 −33.2 ± 0.9 — — 1.7 86.0 87.7 0 5 207 ± 20 15.3 ± 0.4 −33.2 ± 0.9 — — 1.3 82.9 84.2 0 10 202 ± 27 14.5 ± 0.5 −33.2 ± 0.9 — — 1.4 85.2 86.6 0.25 0 203 ± 10 −25.3 ± 0.4 −33.2 ± 0.9 109.0 −0.08 56.4 33.2 89.7 0.25 0.25 196 ± 22 −22.4 ± 0.5 −33.2 ± 0.9 98.3 −0.08 53.1 29.7 82.9 0.25 5 188 ± 9 −26.1 ± 0.7 −33.2 ± 0.9 118.5 −0.08 59.3 26.7 86.0 0.25 10 207 ± 31 −27.4 ± 0.6 −33.2 ± 0.9 125.8 −0.08 61.7 23.0 84.7 D为Hem胶体的水合粒径;ζ为Hem胶体的zeta电势;ζs为石英砂的zeta电势;Ф为Hem胶体与石英砂之间的总相互作用势能,其中Фmax为最大排斥势垒,Фmin2为次级势阱;M为Hem胶体的质量回收率,其中Meff为出流液中胶体的回收率,Mret为砂柱中胶体的回收率,Mtot为总回收率.
D is the hydrated particle size of Hem colloid. ζ is the zeta potential of the Hem colloid. ζs is the zeta potential of quartz sand. Ф is the total interaction potential energy between Hem colloid and quartz sand, of which Фmax is the maximum repulsive barrier, Фmin2 is the secondary potential well; M is the mass recovery rate of Hem colloid, where Meff is the recovery rate of colloid in the outflow liquid, Mret is the recovery rate of colloid in the sand column, and Mtot is the total recovery rate. -
[1] 杨本晓, 梁思哲, 张建华. 制革企业节能诊断实例研究[J]. 皮革科学与工程, 2023, 33(3): 94-98. YANG B X, LIANG S Z, ZHANG J H. Case study of energy-saving diagnosis in leather enterprise[J]. Leather Science and Engineering, 2023, 33(3): 94-98 (in Chinese).
[2] 杨朝, 崔俊云. 制革污水智能处理方法及清洁化生产技术研究[J]. 中国皮革, 2023, 52(4): 124-128,132. YANG Z, CUI J Y. Treatment method and clean production of tannery wastewater[J]. China Leather, 2023, 52(4): 124-128,132 (in Chinese).
[3] 黑静. 制革工业重金属污染治理技术概述[J]. 西部皮革, 2022, 44(21): 18-20. HEI J. Overview of heavy metal pollution control technology in leather industry[J]. West Leather, 2022, 44(21): 18-20 (in Chinese).
[4] 姚庆达, 黄鑫婷, 周华龙, 等. 制革含铬污泥处置及资源化技术进展[J]. 中国皮革, 2023, 52(5): 7-14. YAO Q D, HUANG X T, ZHOU H L, et al. Disposal and recycling technology of chromium-containing sludge from tannery: A review[J]. China Leather, 2023, 52(5): 7-14 (in Chinese).
[5] ZHANG F Z, PENG Y Z, WANG Z, et al. An innovative process for mature landfill leachate and waste activated sludge simultaneous treatment based on partial nitrification, in situ fermentation, and anammox (PNFA)[J]. Environmental Science & Technology, 2022, 56(2): 1310-1320. [6] GUO S S, TIAN Y Q, WU H, et al. Spatial distribution and morphological transformation of chromium with coexisting substances in tannery landfill[J]. Chemosphere, 2021, 285: 131503. doi: 10.1016/j.chemosphere.2021.131503 [7] 王兴润, 李磊, 颜湘华, 等. 铬污染场地修复技术进展[J]. 环境工程, 2020, 38(6): 1-8,23. WANG X R, LI L, YAN X H, et al. Progress in remediation of chromium-contaminated sites[J]. Environmental Engineering, 2020, 38(6): 1-8,23 (in Chinese).
[8] GUO S S, XU Y H, YANG J Y. Simulating the migration and species distribution of Cr and inorganic ions from tanneries in the vadose zone[J]. Journal of Environmental Management, 2021, 288: 112441. doi: 10.1016/j.jenvman.2021.112441 [9] YE T T, LI H B, WANG Z X, et al. Transport and fate of hexavalent chromium in slag-soil system[J]. Environmental Earth Sciences, 2019, 78(7): 239. doi: 10.1007/s12665-019-8245-9 [10] 潘俊, 张玉祥, 王昭怡. 滞洪型平原水库底泥中铬的迁移转化[J]. 环境科学与技术, 2019, 42(增刊2): 93-97. PAN J, ZHANG Y X, WANG Z Y. Migration and transformation of chromium in sediments of flood detention plain reservoirs[J]. Environmental Science & Technology, 2019, 42(Sup 2): 93-97 (in Chinese).
[11] 徐铁兵, 师碧玲, 吕媛, 等. 制革污泥堆场中含Cr污泥胶体在不同pH和离子强度条件下稳定性及迁移行为研究[J]. 环境科学学报, 2023, 43(3): 244-254. XU T B, SHI B L, LÜ Y, et al. Stability and migration behavior of Cr-containing sludge colloids in tannery sludge landfill under different pH and ionic strength conditions[J]. Acta Scientiae Circumstantiae, 2023, 43(3): 244-254 (in Chinese).
[12] 夏平平. δ-MnO2对Cr(Ⅲ)氧化动力学的研究[D]. 武汉: 华中农业大学, 2012. XIA P P. Kinetics study of Cr(Ⅲ) oxidation by δ-MnO2[D]. Wuhan: Huazhong Agricultural University, 2012 (in Chinese).
[13] YANG F, GUO J, DAI R N, et al. Oxidation of Cr(III)-citrate/tartrate complexes by δ-MnO2: Production of Cr(Ⅵ) and its impact factors[J]. Geoderma, 2014, 213: 10-14. doi: 10.1016/j.geoderma.2013.07.022 [14] SHI M Q, MIN X B, KE Y, et al. Recent progress in understanding the mechanism of heavy metals retention by iron (oxyhydr)oxides[J]. Science of the Total Environment, 2021, 752: 141930. doi: 10.1016/j.scitotenv.2020.141930 [15] 孔祥科. 制革污泥中Cr(Ⅲ)和氨氮在包气带的迁移转化及微生物响应机制[D]. 北京: 中国地质科学院, 2019. KONG X K. Migration and transformation of Cr(Ⅲ) and ammonia-nitrogen from tannery sludge and the corresponding microbial response mechanisms in the vadose zone[D]. Beijing: Chinese Academy of Geological Sciences, 2019 (in Chinese).
[16] ZHOU Y C, CHEN Z Z, GONG H J, et al. Chromium speciation in tannery sludge residues after different thermal decomposition processes[J]. Journal of Cleaner Production, 2021, 314: 128071. doi: 10.1016/j.jclepro.2021.128071 [17] 朱龙辉, 李航, 田锐, 等. 正电荷胶体(赤铁矿)凝聚动力学机制的激光散射研究[J]. 西南大学学报(自然科学版), 2016, 38(7): 70-75. ZHU L H, LI H, TIAN R, et al. Kinetics mechanism of aggregation of the positively charged colloid(hematite)by laser scattering technology[J]. Journal of Southwest University (Natural Science Edition), 2016, 38(7): 70-75 (in Chinese).
[18] AGUELMOUS A, LAHSAINI S, FELS L, et al. Biodegradation assessment of biological oil sludge from a petroleum refinery[J]. Journal of Materials and Environmental Science, 2016, 7(9): 3421-3430. [19] ZOU D A, CHI Y, DONG J, et al. Supercritical water oxidation of tannery sludge: Stabilization of chromium and destruction of organics[J]. Chemosphere, 2013, 93(7): 1413-1418. doi: 10.1016/j.chemosphere.2013.07.009 [20] YANG Y, SAIERS J E, BARNETT M O. Impact of interactions between natural organic matter and metal oxides on the desorption kinetics of uranium from heterogeneous colloidal suspensions[J]. Environmental Science & Technology, 2013, 47(6): 2661-2669. [21] LIAO P, PAN C, DING W Y, et al. Formation and transport of Cr(Ⅲ)-NOM-Fe colloids upon reaction of Cr(Ⅵ) with NOM-Fe(II) colloids at anoxic-oxic interfaces[J]. Environmental Science & Technology, 2020, 54(7): 4256-4266. [22] GUO S S, YU C Y, ZHAO X Y, et al. The chromium migration risk from tannery sludge into shallow soil and groundwater: Influence factors, modeling, and microbial response[J]. Journal of Cleaner Production, 2022, 374: 133776. doi: 10.1016/j.jclepro.2022.133776 [23] CORNELL R M, SCHWERTMANN U. The iron oxides: structure, properties, reactions, occurrences, and uses[M]. 2nd, completely rev. and extended ed. Weinheim: Wiley-VCH, 2003. [24] ADAMCZYK Z, WEROŃSKI P. Application of the DLVO theory for particle deposition problems[J]. Advances in Colloid and Interface Science, 1999, 83(1/2/3): 137-226. [25] FRITZ G, SCHÄDLER V, WILLENBACHER N, et al. Electrosteric stabilization of colloidal dispersions[J]. Langmuir, 2002, 18(16): 6381-6390. doi: 10.1021/la015734j [26] FRENCH R A, JACOBSON A R, KIM B, et al. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles[J]. Environmental Science & Technology, 2009, 43(5): 1354-1359. [27] SHAHID M, SHAMSHAD S, RAFIQ M, et al. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review[J]. Chemosphere, 2017, 178: 513-533. doi: 10.1016/j.chemosphere.2017.03.074 [28] AQUINO C L E, BONGAR M J C, SILVESTRE A B, et al. Synthesis of hematite (α-Fe2O3) nanostructures by thermal oxidation of iron sheet for Cr (VI) adsorption[J]. Key Engineering Materials, 2018, 775: 395-401. doi: 10.4028/www.scientific.net/KEM.775.395 [29] 谢青青, 马晓燕, 艾迪娅·阿不来提, 等. 腐殖酸对二甲基胂酸在磁铁矿上吸附过程的影响[J]. 环境化学, 2023, 42(2): 658-670. doi: 10.7524/j.issn.0254-6108.2022072201 XIE Q Q, MA X Y, HADIYA A, et al. Effect of humic acid on the adsorption of dimethylarsinic acid by magnetite[J]. Environmental Chemistry, 2023, 42(2): 658-670 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022072201
[30] LI Y, YUE Q Y, GAO B Y, et al. Adsorption thermodynamic and kinetic studies of dissolved chromium onto humic acids[J]. Colloids and Surfaces B:Biointerfaces, 2008, 65(1): 25-29. doi: 10.1016/j.colsurfb.2008.02.014 [31] SALMAN M, EL-ESWED B, KHALILI F. Adsorption of humic acid on bentonite[J]. Applied Clay Science, 2007, 38(1/2): 51-56. [32] KANTAR C, BULBUL M S, KESKIN S. Role of humic substances on Cr(Ⅵ) removal from groundwater with pyrite[J]. Water, Air, & Soil Pollution, 2017, 228(1): 48. [33] WANG X R, LI L, YAN X H, et al. Processes of chromium (Ⅵ) migration and transformation in chromate production site: A case study from the middle of China[J]. Chemosphere, 2020, 257: 127282. doi: 10.1016/j.chemosphere.2020.127282 [34] LI X Y, ZHOU T, LI Z, et al. Legacy of contamination with metal(loid)s and their potential mobilization in soils at a carbonate-hosted lead-zinc mine area[J]. Chemosphere, 2022, 308(Pt 3): 136589. [35] 崔申申, 杜晓丽, 刘殿威, 等. 降雨入渗对下渗设施土壤胶体-重金属共释放迁移的影响[J]. 环境化学, 2022, 41(9): 2842-2849. doi: 10.7524/j.issn.0254-6108.2021100806 CUI S S, DU X L, LIU D W, et al. Influence of rainfall infiltration on soil colloids-heavy metals co-release and co-migration in infiltration column[J]. Environmental Chemistry, 2022, 41(9): 2842-2849 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021100806
[36] YANG J W, ZHANG Z, CHEN Z Y, et al. Co-transport of U(Ⅵ) and gibbsite colloid in saturated granite particle column: Role of pH, U(Ⅵ) concentration and humic acid[J]. Science of the Total Environment, 2019, 688: 450-461. doi: 10.1016/j.scitotenv.2019.05.395 [37] CHEN C, ZHAO K, SHANG J Y, et al. Uranium (Ⅵ) transport in saturated heterogeneous media: Influence of kaolinite and humic acid[J]. Environmental Pollution, 2018, 240: 219-226. doi: 10.1016/j.envpol.2018.04.095 -




 下载:
下载: