-
近年来,随着工业化进程不断向前,有机染料被广泛应用,我国是染料应用大国[1],其年产量约占全球7成以上[2]. 然而,高色度染料废水的大量排放会干扰自然水体的透光率,降低水生生物的光合作用强度,降低水体的复氧能力[3]. 其中,亚甲基蓝(methylene blue,MB)是使用普遍的染料之一,在水环境中高度稳定,能与多数无机盐生成复盐,具有抗生物降解特性[4 − 5]. 在生态系统中积累,会对人类及其他生物体产生有害影响. 因此,为避免生态环境健康遭到威胁,低能耗、高去除率的染料废水处理技术的研究具有重要意义.
在各种高级氧化技术(advanced oxidation process,AOPs)中电化学氧化由于环境友好、氧化效率高、易于控制等优点,已成为处理含生物难降解有机污染物废水的最有前途的技术[6]. 其中,阳极材料是影响电化学氧化效率的关键因素,与Pt、Ir等贵金属相比,B掺杂金刚石(boron-doped diamond,BDD)阳极由于具有极宽的电位窗口、腐蚀稳定性、强氧化能力等特性在众多材料中脱颖而出[7 − 9],已被广泛用于处理各种有机废水处理. 此外,基于Fenton反应的电化学氧化可利用碳毡(carbon felt ,CF)、生物炭[10]等碳质材料作为阴极,原位生成H2O2和Fe2+反应产生的·OH,以提高体系的氧化能力. 同时,避免了传统Fenton反应中必须外加H2O2带来的高昂成本以及高浓度爆炸性H2O2的处理、运输和储存相关的安全风险[11]. 因此,以BDD为阳极和CF为阴极的电Fenton体系(electro-Fenton,EF)已经成功地应用于有机污染物的降解[12 − 13]. 然而,Fe2+的再生是促进·OH生成的重要因素,但常规的铁基催化材料中Fe3+/Fe2+循环缓慢,成为电Fenton体系反应速率的限速条件. 目前,金属硫化物(FeS2、MoS2、WS2、Cr2S3、CoS2或PbS)已被证明是提高有机污染物降解效率和显著降低H2O2和Fe2+所需量的优良催化剂[14 − 15]. 其中,Cr2S3、CoS2、PbS都存在毒性,环境风险大的问题,相比之下,FeS2毒性较低,对环境危害小,且已有研究表明S2−作为电子供体,可促进Fe3+的连续还原和Fe2+的再生[16],便于在催化过程中反复利用,可作为一种含铁材料替代品,但单一金属硫化物由于活性中心和电子转移能力有限,S物种对Fe3+/Fe2+的循环不足以支持反应的进行. 而纳米花状MoS2具有比表面积高,反应位点丰富[17],电荷阻力较低,有利于反应过程中电子的转移[18]的优点,可作为助催化剂,Xing等[19]的研究表明,MoS2表面的不饱和硫原子可俘获溶液中的质子从而暴露出Mo4+,从而促进Fe3+/Fe2+循环,并将H2O2分解效率显著提高47.2%,降低AOPs中H2O2(0.4 mmol·L−1)和Fe2+(0.07 mmol·L−1)的消耗.
本研究将FeS2负载到MoS2上,成功合成双金属硫化物MoS2@FeS2,并将其引入BDD-CF电化学系统,对催化剂剂量、pH值、支持电解质等反应参数进行系统地探究,以确定最佳实验条件. 此外,对反应过程中Fe2+与·OH含量进行测定,以探究其在降解过程中的作用机理.
-
亚甲基蓝((methylene blue,MB)、无水硫酸钠、氯化钠、硝酸钠、碳酸氢钠、磷酸氢二钠、氢氧化钠、浓硫酸、七水合硫酸亚铁、硫粉、硫代硫酸钠、四水合钼酸铵、硫脲、乙二醇、还原性铁粉、盐酸羟胺、一水1,10-菲啰啉、无水乙酸钠均购于国药集团化学试剂有限公司;N,N二甲基-p-亚硝基苯胺购于上海麦克林生化科技有限公司,所用试剂均为分析纯.
BDD电极(20 mm×20 mm×1.5 mm)购于德国CONDIAS GmbH公司,不锈钢电极(20 mm×20 mm×1.5 mm)购于上海越磁电子科技有限公司,碳毡电极购自天津碳素厂.
电子天平(FA1004)购自上海上平仪器,磁力搅拌器(JB-1A)购自雷磁-上海仪电科学仪器有限公司,直流稳压稳流电源(DH1765-1)购自北京大华无线电仪器厂,数控超声波清洗器(KQ-100DE)购自昆山市超声仪器有限公司,紫外可见分光光度计(V-750)购自日本JASCO公司,pH计(PHS-2F)购自上海雷磁仪器有限公司,X射线衍射仪(XD-DI)产自日本岛津公司,扫描电子显微镜(Zeiss Sigma 300)产自德国卡尔蔡司.
-
将等物质的量0.01 mol的FeSO4·7H2O(2.780 g)和 Na2S2O3·5H2O(2.480 g)以及S粉(0.320 g)混合分散在20 mL乙二醇溶液中并连续搅拌30 min. 将等Fe/Mo质量比的 (NH4)6Mo7O24·4H2O(1.023 g)和CH4N2S(1.886 g)溶解于20 mL去离子水中,并连续搅拌30 min. 将二者混合并连续搅拌15 min,超声处理15 min,然后将混合溶液转移到100 mL聚四氟乙烯衬里的不锈钢高压釜中,于200 ℃加热24 h. 反应完成后,将高压釜自然冷却至室温,并用去离子水、无水乙醇离心洗涤3次以去除杂质. 最后将所得的材料MoS2@FeS2转移到真空干燥箱中干燥10 h. 在相同情况下合成纯FeS2和MoS2. 最后将所制得的材料密封保存以备后续使用.
-
采用X射线衍射仪(X-ray diffraction,XRD)对材料的晶体结构、物相组成和所含元素进行分析,使用扫描电子显微镜(scanning electronic microscopy,SEM)观察材料的表面结构.
-
以BDD电极为阳极,CF电极为阴极,两块电极板大小一致,平行放置,相距2 cm,有效电解面积均为4 cm2. 并在电流密度为5 mA·cm−2、MoS2@FeS2的投加量为0—1.0 g·L−1、磁力搅拌条件下将300 mL电解液(50 mg·L−1 MB和0.05 mol·L−1 Na2SO4)降解140 min,以设定的时间间隔取样,并用0.45 μm的过滤头过滤,并稀释5倍,用分光光度计于665 nm处测定MB残余浓度.
MB浓度变化使用一级动力学方程拟合:
式中,C0和Ct 分别代表初始时间和电解时间为t时的MB浓度,kobs为反应速率常数,min−1.
-
采用邻菲啰啉分光光度法对降解过程溶液中的铁离子浓度进行测定.
-
在电Fenton体系中,主要依靠产生的强氧化性·OH对有机污染物进行降解,本研究采用R-NO(N,N-二甲基-p-亚硝基苯胺)法对降解过程中的·OH生成量进行测定,溶液中R-NO的减少量与·OH生成量呈对应的量化关系:
-
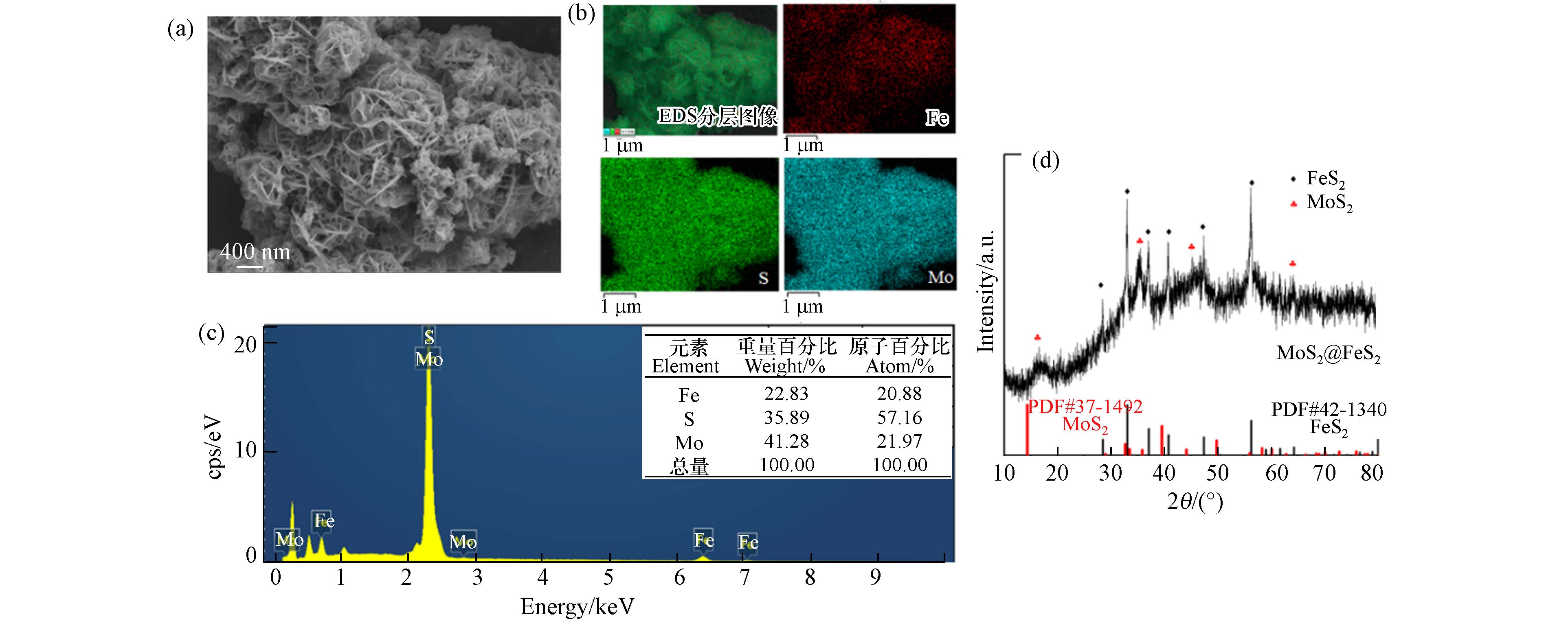
MoS2@FeS2的SEM、XRD表征结果图1所示,由图1(a)可知, MoS2@FeS2呈纳米花球状网络结构,可使比表面积增加,暴露更多的活性边缘和反应位点,从而加快反应速率. 此外,经EDS元素分布图证实Mo、Fe、S元素均匀分散,FeS2均匀负载在MoS2上,可有效避免在反应过程中FeS2团聚现象. 从图1(d)可知,33.08°、37.10°、40.78°、56.28°和64.28°处有明显的衍射峰与FeS2(PDF#42-
1340 )的特征峰对应,且峰型尖锐,表明结晶度较好. 其次,16.38°、33.50°、44.20°、58.32°为MoS2(PDF#37-1492 )的特征峰,表明MoS2@FeS2成功合成. -
采用BDD-CF体系进行实验,首先测试CF阴极对MB吸附的影响,结果显示在3 h后MB几乎未降解,说明CF阴极对于MB的吸附效果很弱,可以忽略不计. 从图2可知,在电流密度为5 mA·cm−2条件下,以BDD为阳极,CF为阴极的体系中,对MB的降解效果远高于以BDD为阳极,SS为阴极的体系. 当反应进行到140 min时,BDD-CF系统对MB的降解率达64%,而BDD-SS仅为10%,kobs分别为0.008 min−1和0.001 min−1,这是由于CF阴极产生的H2O2能够在酸性环境中得到电子生成·OH,从而去除MB. 而BDD-SS系统仅依靠BDD阳极产生的·OH进行反应. 实验继续测试了还原性铁粉、MoS2、FeS2、MoS2@FeS2作为催化剂时BDD-CF体系降解MB的效果,可以看出,加入催化剂后,MB的降解速率有了很明显的提升,表明材料会向溶液中浸出Fe2+、Mo4+,在酸性溶液中与H2O2反应产生·OH,促进了·OH的生成速率,进而加速MB的氧化降解. 且在相同作用条件下,MoS2@FeS2作为催化剂时的降解效果(94.7%)明显优于其余3种催化剂,kobs值为0.019 min−1,这可能是因为与Fe0相比金属硫化物MoS2@FeS2释放的Fe2+速率缓慢可控[20],与单一的FeS2和MoS2相比,复合材料中由于双金属协同作用,Mo4+可还原Fe3+产生Fe2+,从而可确保体系中有足够多的Fe2+用于有效活化,这说明本实验的复合催化剂相比于单一催化剂效果更优.
-
在构建的电Fenton降解MB体系中,助催化剂MoS2@FeS2的作用是双重的. 其一是提供Fe2+,其二是促进Fe3+/Fe2+循环. Fe2+将与阴极产生的H2O2反应得到·OH,从而降解MB. 其中,Fe2+的浓度对降解速率有着决定作用. 由此本实验对催化剂MoS2@FeS2的剂量进行分析讨论,以确定最适剂量. 如图3所示,在MoS2@FeS2投加量为0.1—1.0 g·L−1时,MB的去除率随着MoS2@FeS2投加量的增加而增加,这是由于催化剂剂量增加后可暴露出更多的活性位点,加快了·OH的生成. 此外,催化剂投加量为0.3 g·L−1和0.5 g·L−1时体系降解效果差别不大,且80 min后0.5 g·L−1的效果相较于0.3 g·L−1开始变缓. 而催化剂剂量为0.3 g·L−1和0.7 g·L−1、1.0 g·L−1在反应80 min时MB的去除率分别为83.9%、90.7%和98.0%,可见当催化剂剂量扩大2—3倍时,MB的去除率并没有大幅度提高,其原因可能是溶液中析出的过量Fe2+可能会消耗一部分自由基(式3)[21 − 22]. 基于以上讨论,综合环境效益及经济效益角度,实验将0.3 g·L−1的MoS2@FeS2作为最优剂量进行后续实验.
-
在电Fenton体系中,pH是一个重要的影响因素,较窄的pH范围是限制Fenton实际应用的关键因素之一,因此,开发对宽pH范围具有高耐受性的催化剂具有重要意义. 为此,本实验探究溶液初始pH值为3、5、6.9(原始pH值)、9时对MB降解效果的影响. 如图4所示,除pH为5时(83.3%),MB去除率均高于91.4%,对应的kobs分别为0.016、0.012、0.019、0.015 min−1,表明MoS2@FeS2复合材料具有较宽的pH值适用范围. 此外,当溶液pH接近中性(6.9)时,反应效果明显好于酸性和碱性,这与Qu等[23] FeOCl/MoS2类Fenton体系去除MB的研究结果一致. 其原因可能是由于MB在水溶液中表现为带正电的阳离子形态,在酸性环境中,H+会与CF表面的羧基等含氧基团结合,对带正电的MB产生一定的静电排斥作用,从而影响对MB的降解;然而,MB在不同pH下的降解不仅受H+的影响,而且受催化剂的控制,在pH=5时,浸出的Fe2+可能会发生水解(方程4),形成铁絮体,导致H2O2分解受到抑制,·OH的生成量减少,阻碍MB降解. 因此,在H+影响和H2O2分解的双重作用下,pH=3比pH=5的条件下降解效果更好. 在碱性环境下,除催化剂MoS2@FeS2水解外,溶液中OH−的存在会与CF阴极产生的H2O2反应生成O2,从而使·OH的生成受到影响[24],反应速率减慢.
-
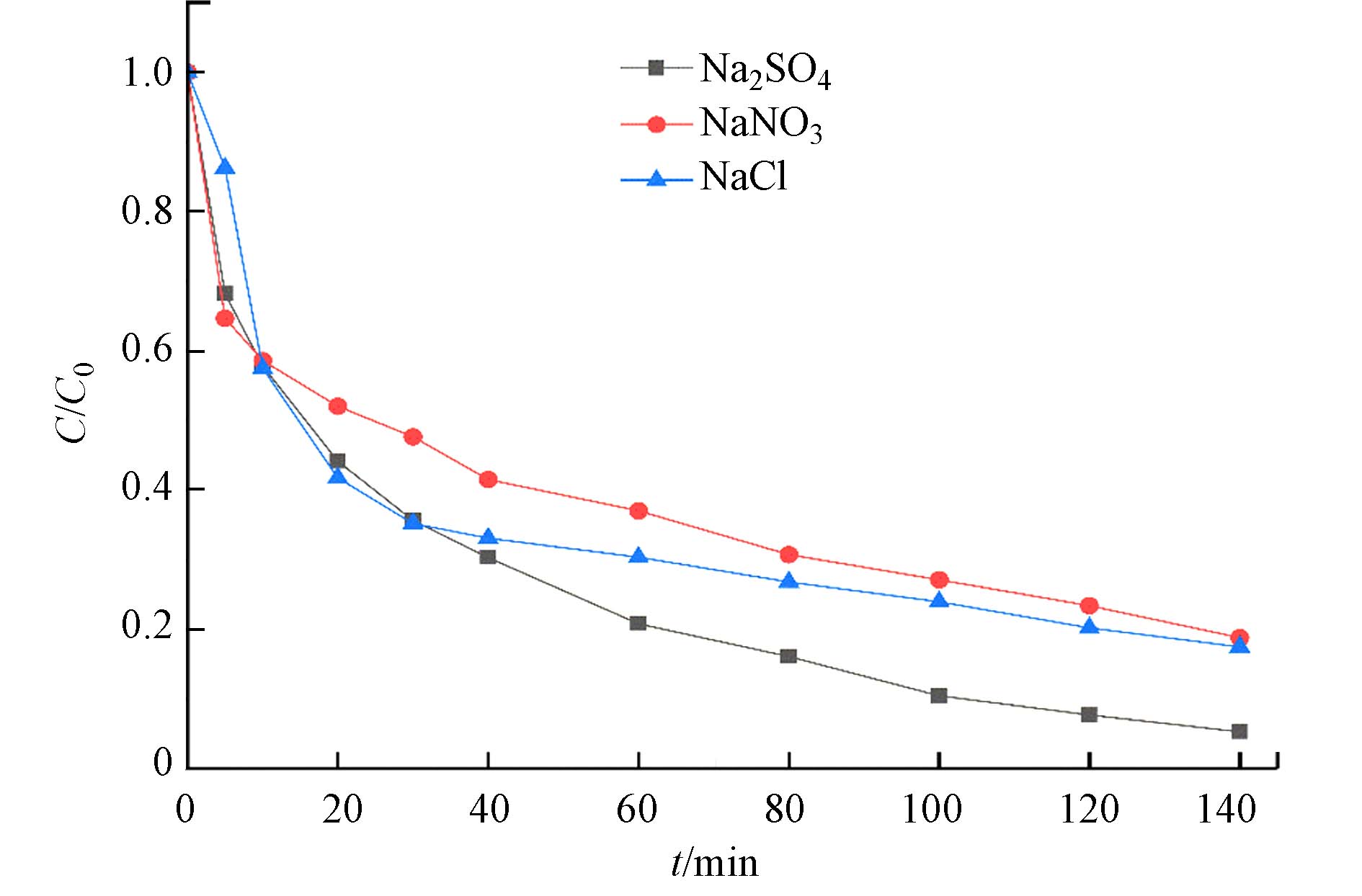
不同电解质对于电Fenton系统降解MB会有不同的效果. 本研究探究了Na2SO4、NaCl、NaNO3 3种电解质对于MB去除的影响. 如图5所示,Na2SO4支持体系中MB去除率为94.7%,NaCl、NaNO3支持体系中MB去除率分别为81.2%、82.5%,这表明硫酸盐可能被电极激活,产生额外的SO4·−,降解更多的MB分子. 其次,Cl−和NO3−会与·OH反应生成其他氧化能力较弱的活性物质(式5, 6)[25 − 26]. 此外,Liu 等[27]研究表明, Cl−的存在还可能造成铁-阴离子络合物的生成,从而使MB的去除速率减缓. 但是其降解效率仍高于无催化剂的BDD-CF/Na2SO4体系(64%),表明尽管Cl−和NO3−对·OH有清除作用,MoS2@FeS2仍能表现出良好的催化性能.
-
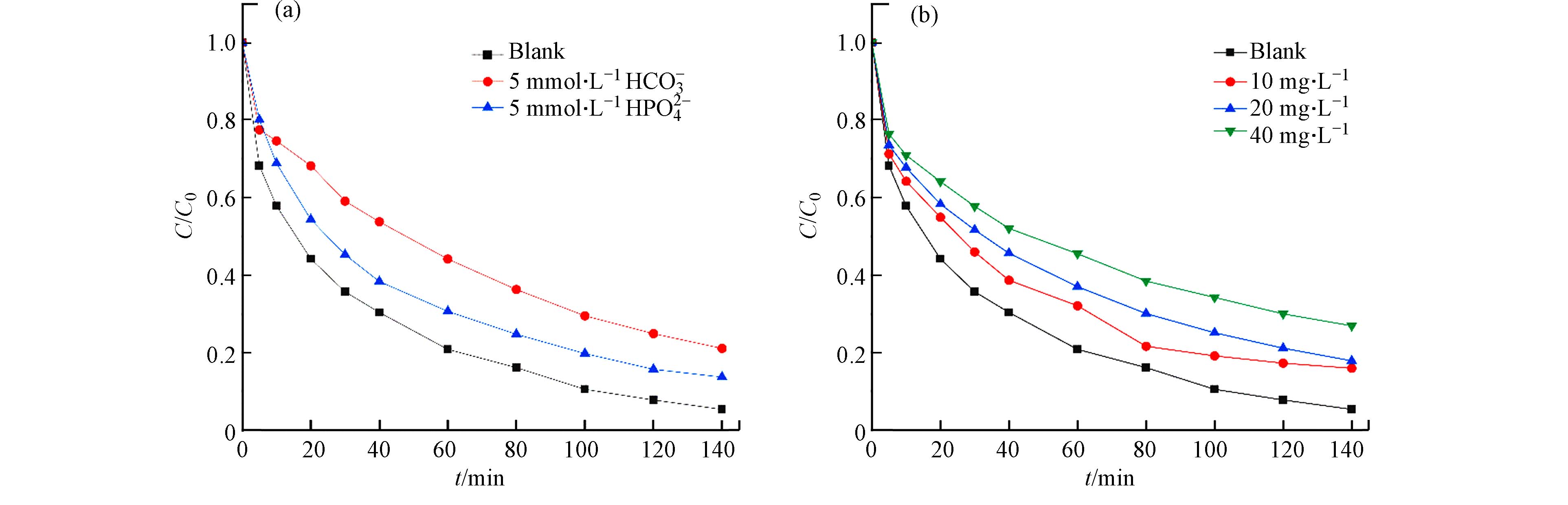
染料废水中通常还含有各种无机盐离子及天然有机物质,一般情况下这些共存物质会对污染物的去除产生干扰,因此本实验分别探究了HCO3−、HPO42−和富里酸(Humic acid,HA)共存体系下MB去除的影响. 如图6所示,HPO42−和HCO3−的存在会对MB的降解产生不同程度的抑制,且溶液中HA浓度由10 mg·L−1增加到40 mg·L−1时,抑制作用随之增强,当体系中加入40 mg·L−1的HA后,MB的去除率下降至20%. 这主要是因为它们对·OH的淬灭作用(式7—9)以及对活性Fe2+位点的破坏. 此外,HPO42−和HCO3−的水解会增加pH值,从而使反应速率减慢.
-
在电Fenton体系中,Fe2+含量及·OH生成量是决定反应速率的关键因素,因此本实验对MoS2@FeS2/BDD-CF体系中的Fe2+含量与·OH生成量进行测定,探究其作用机理.
-
对体系中铁离子浓度测定的结果如图7所示,MoS2@FeS2复合材料能够在溶液中缓慢释放铁离子,主要以Fe2+形式存在. 在整个反应体系中Fe2+的浓度基本上维持在一个稳定的范围,说明材料表面的不饱和硫原子俘获溶液中的质子暴露出的Mo4+可促进Fe3+还原为Fe2+,从而可确保体系中有足够多的活性Fe2+用于H2O2的分解. 在80 min后,Fe2+和总Fe的含量出现一个下降的趋势,可能是由于水溶液的PH接近于7,在此条件下Fe2+和Fe3+会发生部分水解,从而导致了浓度的降低. 此外,实验测试了反应结束后CF阴极对于铁离子的吸附作用. 将CF阴极取下在超声条件下用去离子水清洗,过滤洗涤液并测试水洗液中的铁离子浓度. 如图7(b)所示,CF阴极对于铁离子的吸附作用很小,表明MoS2@FeS2的不易被CF吸附,方便后续回收重复利用.
-
在相同条件下,通过R-NO法对MoS2@FeS2/BDD-CF、BDD-SS两个体系·OH的生成量进行测定. 由图8可知,·OH的生成验证了对MB的降解主要依赖于该种强氧化性自由基. 此外,20 min内,BDD-CF体系·OH(1.43×10−5 mol·L−1)生成量远高于BDD-SS(0.41×10−5 mol·L−1)体系. 这是因为在以BDD为阳极,CF为阴极的电化学系统中加入MoS2@FeS2后,·OH的来源主要有两个方面,一方面是在BDD阳极氧化,另一方面是MoS2@FeS2浸出的Fe2+作用于CF阴极的H2O2产生·OH,而在SS电极上并不能生成H2O2,仅依靠BDD阳极氧化生成·OH. 这也表现出MoS2@FeS2/BDD-CF体系的优越性.
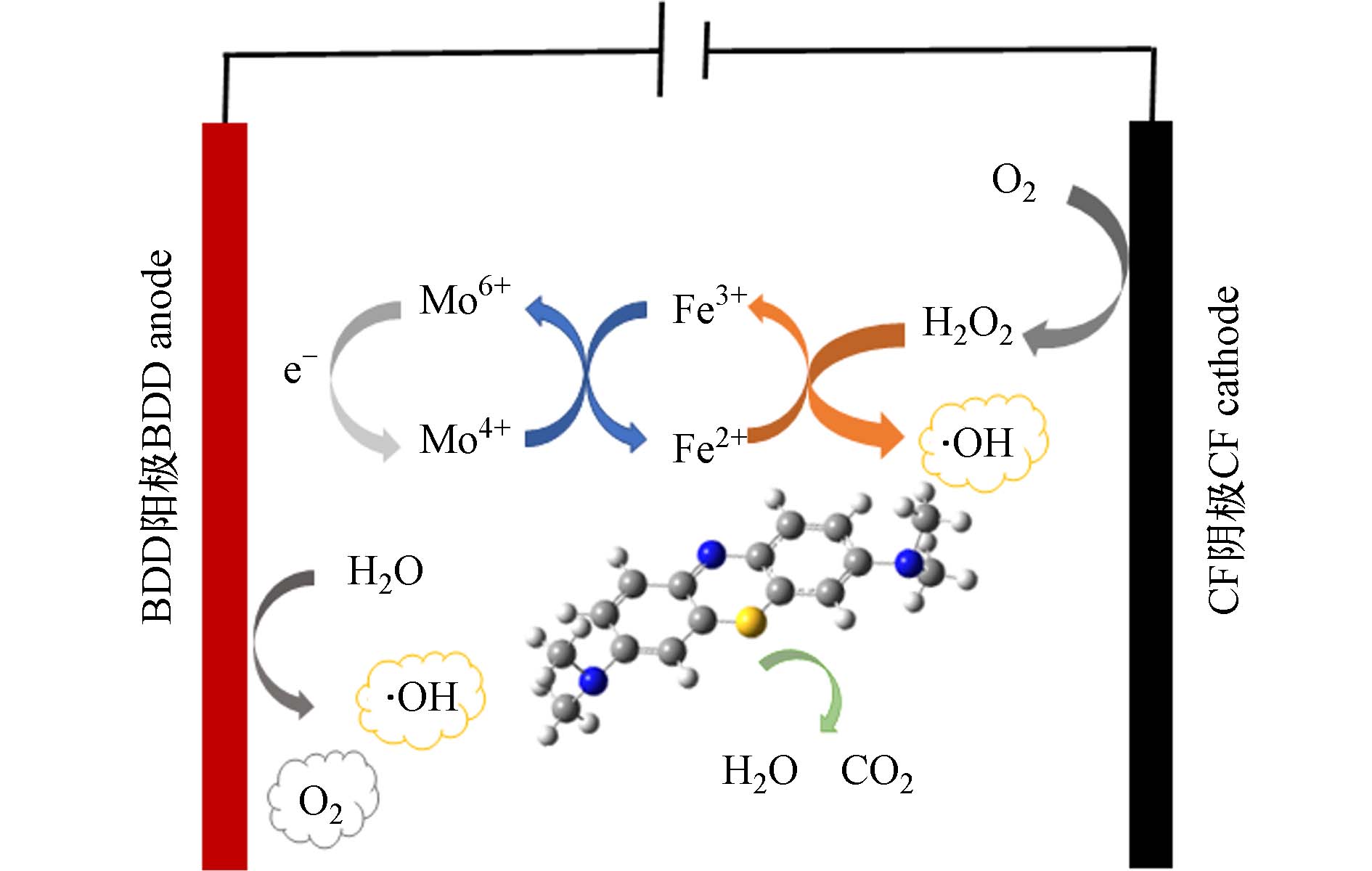
基于上述实验结果,对实验体系降解MB的机理进行推断. 如图9所示,在MB的降解中,BDD阳极直接电解水产生·OH和O2,·OH可直接氧化MB. CF阴极通过捕获阳极产生的O2和溶液中的溶解氧还原产生H2O2,并在Fe2+的催化下产生·OH来氧化MB. 此外,MoS2@FeS2中暴露的Mo4+与Fe3+发生反应生成Fe2+,从而使反应持续进行.
-
1)通过简单的溶剂热法成功制备出双金属硫化物MoS2@FeS2催化剂,SEM表征结果表明该材料形貌呈纳米花球状网络结构,暴露更多的活性边缘和反应位点,促进反应的进行.
2)通过系统的对比BDD-SS、BDD-CF、Fe0/BDD-CF、FeS2/BDD-CF、MoS2/BDD-CF和MoS2@FeS2/BDD-CF 6种体系对MB的降解效果,证实了MoS2@FeS2具有良好的催化作用,其kobs值为0.019 min−1,是未投加催化剂BDD-CF(0.008 min−1)和BDD-SS(0.001 min−1)体系的19倍与2.38倍,表明是一种优良的催化剂,而且在降解染料废水方面具有较好的应用前景.
3)机理分析表明MoS2@FeS2复合材料结构可有效避免在反应过程中FeS2团聚现象,并利用MoS2表面的不饱和硫原子俘获溶液中的质子暴露出的Mo4+的原理,促进Fe2+再生,以确保体系中有足够Fe2+用于活化H2O2,使体系中的·OH不断增强,20 min时·OH的浓度达到1.43×10−5 mol·L−1.
MoS2@FeS2催化电Fenton降解染料废水
Dye wastewater degradation in electro-Fenton system catalyzed by MoS2@FeS2
-
摘要: 本研究以掺硼金刚石(boron-doped diamond,BDD)电极为阳极,碳毡(carbon felt,CF)为阴极,并采用简单的一步溶剂热法合成Fe、Mo双金属复合材料,构建电Fenton系统,处理亚甲基蓝(methylene blue,MB)污染物. 扫描电子显微(scanning electron microscopy,SEM)和X射线衍射(X-ray diffraction,XRD)表征结果表明MoS2@FeS2双金属硫化物成功合成. 实验结果表明MoS2@FeS2表现出优异的催化性能,在电流密度为5 mA·cm−2,投加量为0.3 g·L−1时, MB降解效率达94.7%,反应速率常数为0.019 min−1,是未投加催化剂的BDD-CF体系(0.008 min−1)的2.38倍. 此外,该催化剂在pH为3—9的范围内对MB均具有较高的去除率,在实际应用中不需要调节pH. 其机理分析表明在整个反应体系中Fe2+的浓度基本上维持在一个稳定的范围,表明该催化剂可通过Mo4+氧化为Mo6+促进Fe3+与Fe2+之间的氧化还原循环,从而可确保体系中有足够多的Fe2+用于有效活化,最大化H2O2的分解效率以促进·OH的产生,20 min时·OH的浓度达到1.43×10−5 mol·L−1.
-
关键词:
- 电Fenton /
- BDD /
- CF /
- 亚甲基蓝 /
- MoS2@FeS2.
Abstract: In the present study, Fe and Mo bimetallic composite was prepared by one-step solvothermal method and applied in electro-Fenton system with boron-doped diamond (BDD) anode and carbon felt (CF) cathode for methylene blue (MB) degradation. The characterization results by scanning electron microscopy (SEM) and X-ray diffraction (XRD) indicated that bimetallic sulfide MoS2@FeS2 had been synthesized successfully. MoS2@FeS2 exhibited excellent catalytic performance, with 94.7% MB removed when the current density was 5 mA·cm−2 and the dosage is 0.3 g·L−1. Degradation rate constant of kobs (0.019 min−1) was about 2.38 times compared with BDD-CF system without catalyst (0.008 min−1). Moreover, MB removal rate kept high in the pH range of 3—9, which demonstrated that MoS2@FeS2 could be used in practical without the requirement of pH adjustment. The concentration of Fe2+ in the whole reaction system was maintained in a stable range. This result was mainly due to the redox cycle between Fe3+ and Fe2+ through the oxidation of Mo4+ to Mo6+, which ensured enough Fe2+ existing for effective Fenton reaction in the system. Concentration of ·OH reached 1.43×10−5 mol·L−1 at 20 min in the system coming form the efficient decomposition of H2O2 produced at cathode.-
Key words:
- electro-Fenton /
- BDD /
- CF /
- MB /
- MoS2@FeS2.
-

-
-
[1] HOU Y P, ZHANG R D, YU Z B, et al. Accelerated azo dye degradation and concurrent hydrogen production in the single-chamber photocatalytic microbial electrolysis cell[J]. Bioresource Technology, 2017, 224: 63-68. doi: 10.1016/j.biortech.2016.10.069 [2] 袁思杰, 张芮铭. 染料废水处理技术研究进展[J]. 染料与染色, 2022, 59(4): 55-62. YUAN S J, ZHANG R M. Research progress of dye wastewater treatment technology[J]. Dyestuffs and Coloration, 2022, 59(4): 55-62 (in Chinese).
[3] MEILI L, LINS P V S, COSTA M T, et al. Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling[J]. Progress in Biophysics and Molecular Biology, 2019, 141: 60-71. doi: 10.1016/j.pbiomolbio.2018.07.011 [4] KULKARNI P, WATWE V, DOLTADE T, et al. Fractal kinetics for sorption of Methylene blue dye at the interface of Alginate Fullers earth composite beads[J]. Journal of Molecular Liquids, 2021, 336: 116225. doi: 10.1016/j.molliq.2021.116225 [5] KHAN I, SAEED K, ZEKKER I, et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation[J]. Water, 2022, 14(2): 242. doi: 10.3390/w14020242 [6] WEI J C, SHI L, WU X. Electrochemical advanced oxidation process with simultaneous persulfate and hydrogen peroxide on-site generations for high salinity wastewater[J]. Separation and Purification Technology, 2023, 310: 123147. doi: 10.1016/j.seppur.2023.123147 [7] OTURAN M A. Outstanding performances of the BDD film anode in electro-Fenton process: Applications and comparative performance[J]. Current Opinion in Solid State and Materials Science, 2021, 25(3): 100925. doi: 10.1016/j.cossms.2021.100925 [8] TITCHOU F E, ZAZOU H, AFANGA H, et al. Electro-Fenton process for the removal of Direct Red 23 using BDD anode in chloride and sulfate media[J]. Journal of Electroanalytical Chemistry, 2021, 897: 115560. doi: 10.1016/j.jelechem.2021.115560 [9] ZHU Y S, QIU S, DENG F X, et al. Degradation of sulfathiazole by electro-Fenton using a nitrogen-doped cathode and a BDD anode: Insight into the H2O2 generation and radical oxidation[J]. Science of the Total Environment, 2020, 722: 137853. doi: 10.1016/j.scitotenv.2020.137853 [10] KUANG C Z, ZENG G S, ZHOU Y J, et al. Integrating anodic sulfate activation with cathodic H2O2 production/activation to generate the sulfate and hydroxyl radicals for the degradation of emerging organic contaminants[J]. Water Research, 2023, 229: 119464. doi: 10.1016/j.watres.2022.119464 [11] ZHANG Q Z, ZHOU M H, REN G B, et al. Highly efficient electrosynthesis of hydrogen peroxide on a superhydrophobic three-phase interface by natural air diffusion[J]. Nature Communications, 2020, 11: 1731. doi: 10.1038/s41467-020-15597-y [12] MIAO D T, LI Z S, CHEN Y H, et al. Preparation of macro-porous 3D boron-doped diamond electrode with surface micro structure regulation to enhance electrochemical degradation performance[J]. Chemical Engineering Journal, 2022, 429: 132366. doi: 10.1016/j.cej.2021.132366 [13] 徐进, 李方舟, 陈梓慧, 等. 铁碳复合材料催化电Fenton处理抗生素废水的效果和机理研究[J]. 功能材料, 2022, 53(7): 7175-7181. XU J, LI F Z, CHEN Z H, et al. Antibiotic wastewater treatment in electro-Fenton system catalyzed by iron-carbon composite[J]. Journal of Functional Materials, 2022, 53(7): 7175-7181 (in Chinese).
[14] LUO H P, ZHOU X, GUO X J, et al. WS2 as highly active co-catalyst for the regeneration of Fe(II) in the advanced oxidation processes[J]. Chemosphere, 2021, 262: 128067. doi: 10.1016/j.chemosphere.2020.128067 [15] 刘怀浩. 二硫化钨/二氧化钛复合材料光催化降解水中硝酸盐氮的研究[D]. 泰安: 山东农业大学, 2022. LIU H H. Photocatalytic degradation of nitrate nitrogen in water by WS2/TiO2 composites[D]. Taian: Shandong Agricultural University, 2022 (in Chinese).
[16] XU H D, SHENG Y Q. New insights into the degradation of chloramphenicol and fluoroquinolone antibiotics by peroxymonosulfate activated with FeS: Performance and mechanism[J]. Chemical Engineering Journal, 2021, 414: 128823. doi: 10.1016/j.cej.2021.128823 [17] 徐祥福, 陈佳, 赖国霞, 等. 单层MoS2在合金化及应力调控下光催化裂解水产氢的理论研究[J]. 燃料化学学报, 2020, 48(3): 321-327. doi: 10.1016/S1872-5813(20)30015-3 XU X F, CHEN J, LAI G X, et al. Theoretical study on enhancing the monolayer MoS2 photocatalytic water splitting with alloying and stress[J]. Journal of Fuel Chemistry and Technology, 2020, 48(3): 321-327 (in Chinese). doi: 10.1016/S1872-5813(20)30015-3
[18] WANG Z Y, MI B X. Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets[J]. Environmental Science & Technology, 2017, 51(15): 8229-8244. [19] XING M Y, XU W J, DONG C C, et al. Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes[J]. Chem, 2018, 4(6): 1359-1372. doi: 10.1016/j.chempr.2018.03.002 [20] DU M M, KUANG H N, ZHANG Y Q, et al. Enhancement of ball-milling on pyrite/zero-valent iron for persulfate activation on imidacloprid removal in aqueous solution: A mechanistic study[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105647. doi: 10.1016/j.jece.2021.105647 [21] DIRANY A, SIRÉS I, OTURAN N, et al. Electrochemical abatement of the antibiotic sulfamethoxazole from water[J]. Chemosphere, 2010, 81(5): 594-602. doi: 10.1016/j.chemosphere.2010.08.032 [22] SIRÉS I, GARRIDO J A, RODRÍGUEZ R M, et al. Catalytic behavior of the Fe3+/Fe2+ system in the electro-Fenton degradation of the antimicrobial chlorophene[J]. Applied Catalysis B:Environmental, 2007, 72(3/4): 382-394. [23] QU S Y, WANG W H, PAN X Y, et al. Improving the Fenton catalytic performance of FeOCl using an electron mediator[J]. Journal of Hazardous Materials, 2020, 384: 121494. doi: 10.1016/j.jhazmat.2019.121494 [24] FENG J Y, HU X J, YUE P L. Effect of initial solution pH on the degradation of Orange II using clay-based Fe nanocomposites as heterogeneous photo-Fenton catalyst[J]. Water Research, 2006, 40(4): 641-646. doi: 10.1016/j.watres.2005.12.021 [25] QU J H, XU Y, ZHANG X B, et al. Ball milling-assisted preparation of N-doped biochar loaded with ferrous sulfide as persulfate activator for phenol degradation: Multiple active sites-triggered radical/non-radical mechanism[J]. Applied Catalysis B:Environmental, 2022, 316: 121639. doi: 10.1016/j.apcatb.2022.121639 [26] WANG Z X, HAN Y F, FAN W L, et al. Shell-core MnO2/Carbon@Carbon nanotubes synthesized by a facile one-pot method for peroxymonosulfate oxidation of tetracycline[J]. Separation and Purification Technology, 2021, 278: 119558. doi: 10.1016/j.seppur.2021.119558 [27] LIU Y D, ZHOU A G, GAN Y Q, et al. Effects of inorganic anions on carbon isotope fractionation during Fenton-like degradation of trichloroethene[J]. Journal of Hazardous Materials, 2016, 308: 187-191. doi: 10.1016/j.jhazmat.2016.01.044 -



 下载:
下载: