-
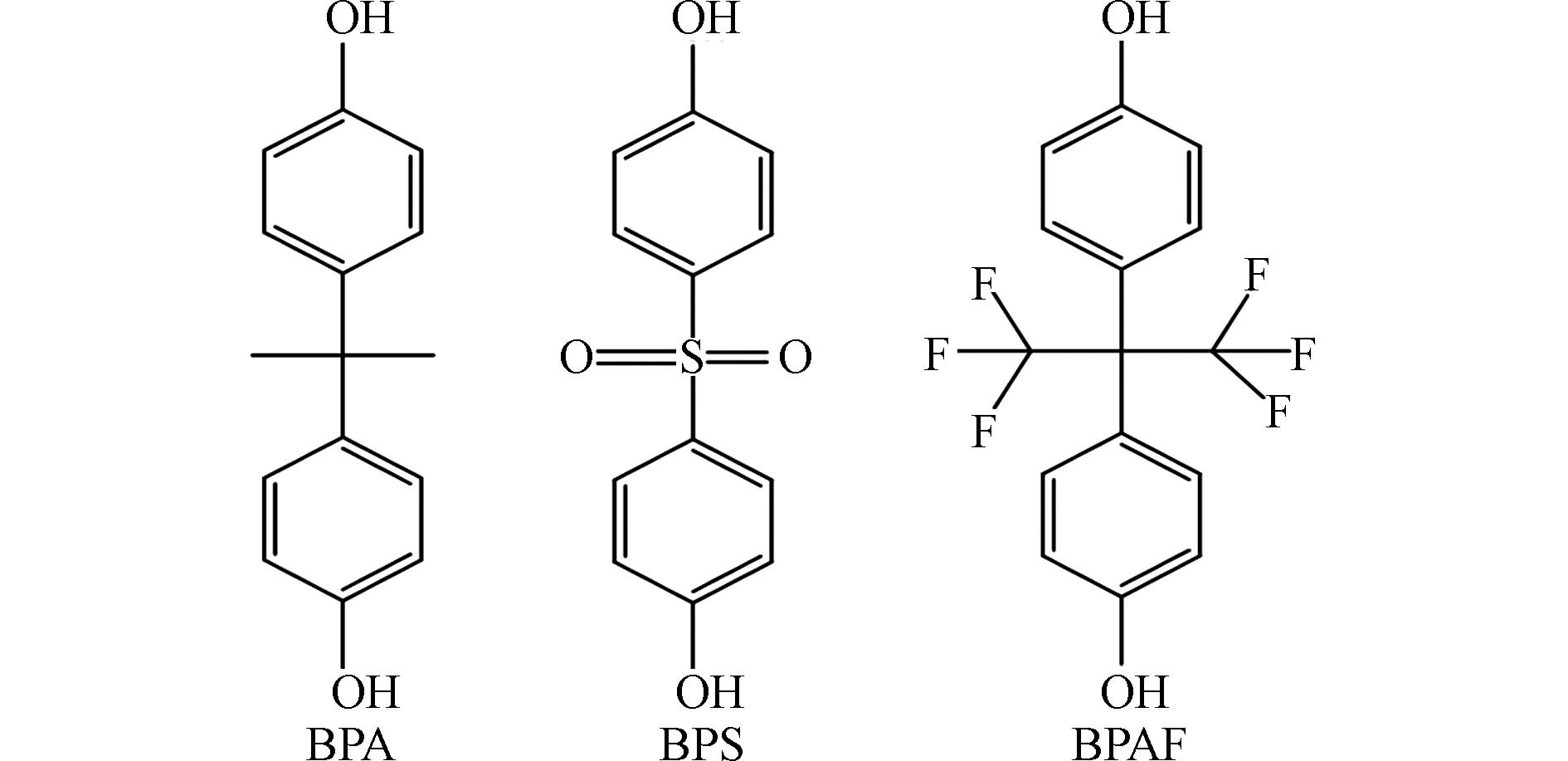
双酚类物质是常见的内分泌干扰物,作为添加剂被广泛用于塑料、树脂、涂料、胶水和防腐剂等工业产品的生产中[1 − 3],在环境介质、野生动物和人群中均有检出[4 − 7]. 双酚类物质主要经口、呼吸道和皮肤等途径进入体内[8 − 11],可通过干扰内分泌系统、生殖系统、神经系统的正常功能而影响健康 [12 − 15]. 双酚A(bisphenol A, BPA)是目前应用最广的双酚类物质[1]. 双酚S(bisphenol S, BPS)和双酚AF(bisphenol AF, BPAF)作为重要的BPA替代品(结构如图1所示),是目前人体和环境样品中检出率较高的双酚类物质[16 − 19]. 研究表明,BPA在孕妇血液和尿液中的浓度分别可以达到291 nmol·L−1 和567 nmol·L−1 [20 − 21],BPS在男性尿液样本中浓度可达48.3 nmol·L−1 [22],BPAF在人血浆中浓度可达49.3 nmol·L−1 [23]. 有研究表明,BPA在
1000 nmol·L−1浓度下暴露HepG2细胞,5 d后细胞活力显著降低,在100 nmol·L−1浓度下可诱导细胞发生胰岛素抵抗[24]. BPS和BPAF分别在80 μmol·L−1和45 μmol·L−1的浓度下暴露细胞,72 h后导致20%的细胞死亡[25]. 体内实验结果显示,BPA在5 mg·kg−1·d−1的暴露剂量下对小鼠生殖和发育无明显损害作用,但会导致小鼠脾脏中T helper cell 17 (Th17)细胞数量的增加[26-27]. 基于BPA对免疫的影响欧洲食品安全局设立了可耐受每日摄入量(TDI)为0.2 ng·kg−1·d−1 [28]. 目前的研究显示,BPA进入人体后主要被代谢成葡萄糖醛酸盐和硫酸盐缀合物,并最终通过尿液排出体外,其人体半衰期大约为1.5 h[26]. BPS主要在肝脏中代谢为葡糖苷酸,半衰期大约为6.8 h[29]. BPAF在体内主要代谢为相应的葡糖苷酸[30],但BPAF人体代谢周期目前未见报道。一项在大鼠体内的研究表明,BPAF在血浆中半衰期约为3.35 h[31]. 虽然双酚类物质在人体中代谢速度较快,但在长期稳定暴露下的情况下,双酚类物质仍然存在环境暴露风险.外泌体是由细胞分泌的直径为30—150 nm的双层膜囊泡,包含蛋白质、核酸、脂质等功能性分子,在细胞通讯中发挥重要作用[32 − 38]. 外泌体包含的分子是其信息传递的物质基础,而外泌体的数量决定了信号的强弱. 外泌体由细胞器多泡体向内出芽形成,通过多泡体与质膜融合释放[32],该过程受外界刺激干扰[39 − 41]. 已有研究证明,多环芳烃可促进肝细胞释放外泌体[41],表明环境污染物可通过干扰外泌体的分泌过程导致毒性,促进疾病的发生发展. 然而,目前双酚类物质对外泌体分泌的影响尚不明确. 因此,本文研究了BPA、BPS、BPAF在人体可检出浓度范围内对外泌体分泌的影响,从而更全面地评估双酚类物质的毒性效应,为相关政策的制定提供更全面的依据.
-
甲基纤维素(R097528-100g)、草酸(R096488-25g),购自上海易恩化学技术有限公司;戊二醛(P1126),购自北京索莱宝科技有限公司;细胞增殖/毒性检测试剂盒(BMU106-CN),购自亚科因(武汉)生物技术有限公司;CD81抗体(ab109201)、TSG101抗体(ab125011)、钙连蛋白(Calnexin)抗体(ab133615),购自艾博抗(上海)贸易有限公司;DMEM培养基(C11995500BT)、胎牛血清(FBS,
10099141 )、青霉素-链霉素溶液 (15140 ‐122),购自赛默飞世尔科技(中国)有限公司;活性炭吸附血清(CS‐FBS, 04‐201‐1B),购自Biological Industries;双酚A(BPA, CAS: 80-05-7,B108652,>99.0%),购自上海阿拉丁生化科技股份有限公司;双酚S(BPS, CAS: 80-09-1,B0495,>98.0%)、双酚AF(BPAF,CAS:1478 -61-1,B0945,>98.0%),购自梯希爱(上海)化成工业发展有限公司.实验所用无外泌体血清使用超滤管(UFC910024, 100K 默克密理博公司)在
3000 g,55 min,4 ℃条件下超滤获得[42]. -
BPA、BPS、BPAF溶于二甲基亚砜(DMSO)配制成100 mmol·L−1的原液,并在−20 ℃下保存. 使用时,在无菌条件下对各原液进行梯度稀释以得到不同浓度的工作液. 根据这3类物质的人体检测浓度,BPA暴露浓度为50、100、200、500 nmol·L−1,BPS和BPAF暴露浓度为50 nmol·L−1. 细胞暴露时DMSO的最终浓度不超过0.1%. 细胞培养和污染物的暴露实验所用细胞培养液配方见表1.
人肝癌细胞系HepG2使用完全培养液在37 ℃ 5% CO2条件下培养. 细胞汇合度达到90%时进行传代. 双酚类物质暴露前24 h将完全培养液换成暴露培养液以消耗激素成分,排除其对双酚类物质效应的干扰. 在检测双酚类物质对细胞活性的影响时,用分别含有0、10、100、
1000 、10000 、10000 nmol·L−1双酚类物质的暴露培养液处理细胞24 h;在检测双酚类物质对外泌体分泌的影响时,用含有双酚类物质的外泌体提取培养液处理细胞24 h,以0.1% DMSO处理的细胞为对照组. -
取对数生长期细胞,以每孔
8000 个的细胞密度铺于96孔板中,铺板时使用暴露培养液以消耗激素成分. 24 h后弃掉原培养基,用磷酸盐缓冲液(PBS)清洗细胞1次,分别使用含有0、10、100、1000 、10000 、10000 nmol·L−1双酚类物质的暴露培养液培养24 h. 培养结束后将培养基换成含有10% 细胞增殖/毒性检测试剂的培养基在细胞培养箱中孵育40 min. 使用酶标仪检测各个孔在450 nm波长处的OD值,计算各剂量组相对于对照组的相对细胞活性. -
(1)外泌体提取
取对数生长期细胞,以每皿3×105个的细胞密度在60 mm 皿中铺板,24 h后用PBS清洗细胞3次,并换用外泌体提取培养液培养细胞,24 h后收集细胞培养上清液并对细胞进行计数. 将收集到的细胞培养上清液在4 ℃条件下依次经1983 r·min−1,5 min;
3967 r·min−1,10 min;8870 r·min−1,30 min,28000 r·min−1,70 min离心,以获取外泌体. 向外泌体沉淀加入PBS以重悬外泌体,再次于4 ℃,28000 r·min−1,70 min以达到清洗外泌体的目的. 弃去上清液用100 μL PBS重悬外泌体用于后续表征或放于−80 ℃冰箱保存.(2)外泌体透射电镜表征
本实验使用Théry改良方法进行外泌体透射电镜样品制备[43]. 使用4% PFA和1%戊二醛对外泌体样本进行固定并装载至铜网,使用pH为7的草酸铀酰溶液和甲基纤维素-UA包埋样品并增强样品反差,将铜网转移到滤纸上干燥后使用透射电子显微镜进行观察.
(3)外泌体粒径与浓度表征
本实验使用纳米颗粒追踪分析仪(NanoSight NS300,英国马尔文公司)对外泌体的粒径范围和浓度进行测量,其有效检测浓度大约在107—109 个·mL−1. 测量不同样本时,使用超纯水对样品池及管路进行充分清洗. 单次采集影像1 min,每个样品采集5次,软件自动计算样品中外泌体的浓度.
(4)免疫印迹试验
采用免疫印迹技术对外泌体标志性蛋白进行检测,以表征外泌体. 使用RIPA裂解液(Radio Immunoprecipitation Assay Lysis buffer)裂解外泌体或细胞以获得蛋白,使用BCA(Bicinchoninic Acid Assay)蛋白定量试剂盒对其浓度定量. 使用SDS-PAGE凝胶电泳对蛋白进行分离,并转移至硝酸纤维素膜上. 使用脱脂牛奶粉封闭. 外泌体表征使用TSG101、CD81、Calnexin抗体孵育,其中Calnexin作为外泌体的阴性对照蛋白. 抗体孵育结束后使用化学发光剂进行显色,并使用成像系统(ChemiDocTM MP,美国伯乐)进行成像. 通过ImageJ软件对图像进行定量分析.
-
所有结果由 GraphPad Prism 9软件进行统计分析和作图. 实验数据以平均值±标准差表示. 多组间比较采用单因素方差分析(ANOVA)进行统计分析. P≤0.05(*)表示组间有统计学差异,P≤0.01(* *)表示组间有显著统计学差异,P≤0.001(* * *)表示组间有极显著统计学差异.
-
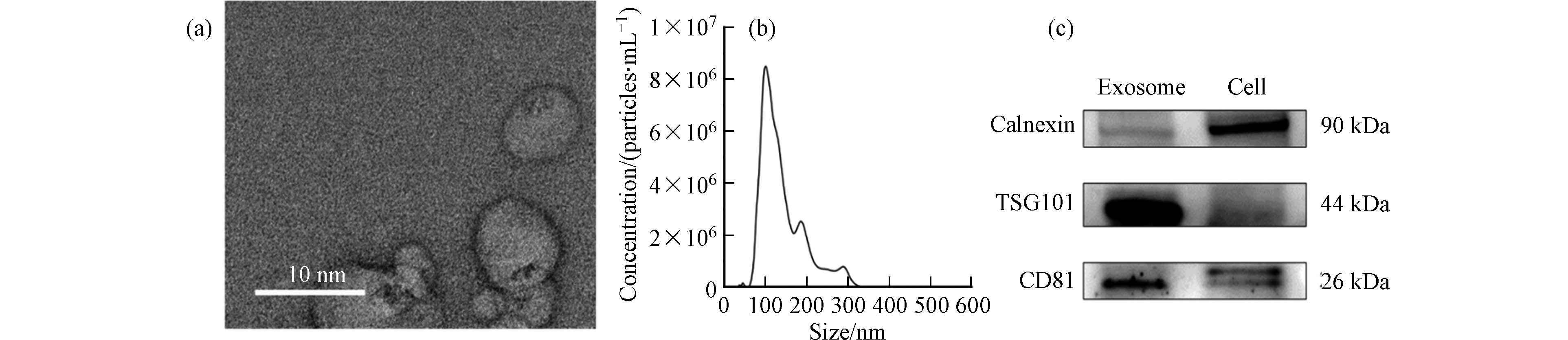
通过超速离心法获得的外泌体形态如图2(a),其整体呈圆形并有明显的凹陷. NTA的结果表明其粒径主要分布为(141.3±54.1)nm(图2(b)). 正常情况下外泌体的粒径在30—150 nm左右,在我们的结果中观察到的粒径略大,可能是由于在超高速离心过程中部分外泌体发生了团聚[44]. 进一步通过免疫印迹技术对所提取的外泌体进行表征,以外泌体来源的HepG2细胞作为参照,检测了外泌体的标志性蛋白TSG101和CD81,以及内质网标志性蛋白Calnexin. 如图2(c)所示,外泌体样本的TSG101和CD81含量高于细胞样本,而Calnexin含量低于细胞样本,这表明本实验提取的外泌体没有受到破碎细胞的污染.
-
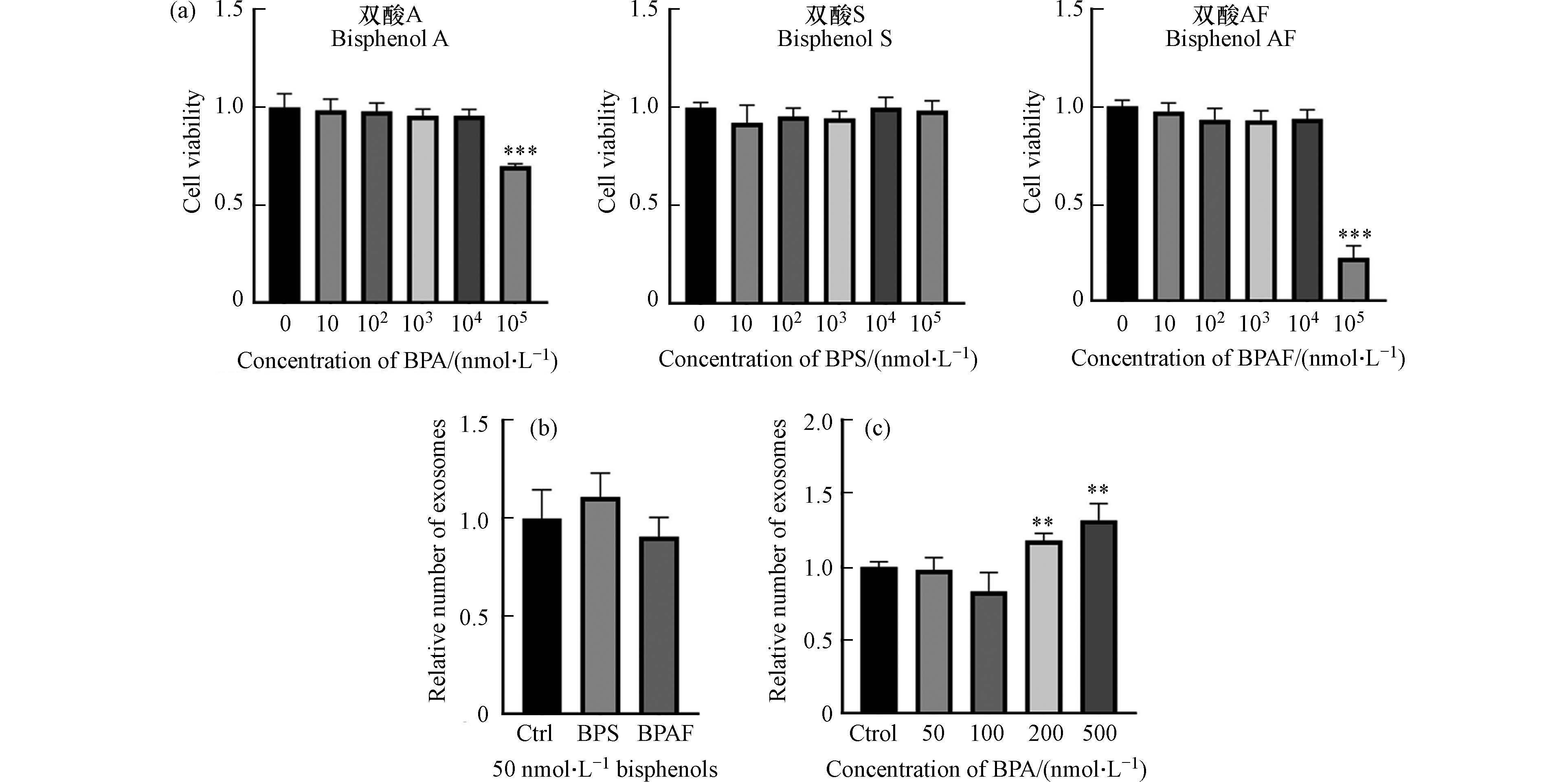
细胞毒性检测结果表明,BPA、BPS和BPAF在10 μmol·L−1及以下暴露剂量下对细胞活性无影响(图3a). 这表明在人体相关浓度下,BPA、BPS、BPAF在24 h内对HepG2细胞的活性不会产生显著影响.
在不影响细胞活性的情况下,进一步检测BPA、BPS、BPAF对细胞外泌体分泌的影响. 实验结果显示,在人体相关浓度(50 nmol·L−1)下,BPS和BPAF对细胞外泌体的分泌没有明显影响(图3b). 然而,当BPA的浓度达到200 nmol·L−1或500 nmol·L−1时,外泌体的分泌量显著增加(图3c). 以上结果表明在人体相关浓度范围内,BPS和BPAF对HepG2细胞外泌体的分泌不产生影响,而BPA可以促进外泌体的分泌.
外泌体释放过程具有复杂性. 首先,细胞质膜发生内陷并形成内体,内体经历物质筛选和内向出芽等步骤,进而演变为多泡体. 多泡体可与溶酶体或自噬体融合被降解,或者与细胞质膜融合,从而释放内腔囊泡,形成外泌体[32]. 该过程涉及多个蛋白质和分子的调控,例如磷脂和四次跨膜蛋白等[32]. 有研究表明,BPA可抑制磷脂合成酶的活性,降低细胞内磷脂的合成[45],进而可能对外泌体的释放产生影响. 外泌体作为细胞之间重要的信息传递媒介,可释放代谢调节和神经调节相关分子[34,36]. 已知双酚类物质可干扰激素作用并进一步导致代谢紊乱、神经毒性等不良效应[12,14]. 因此,双酚类物质可能通过影响细胞外泌体的分泌来干扰细胞代谢或神经调节过程,进而导致相关疾病的发生,其相关生物学过程和潜在分子机制还需要进一步探究.
目前针对内分泌干扰物对外泌体分泌的影响研究很少. 已知一项研究在体外利用鼠原代肝细胞研究了3种多环芳烃,包括苯并[a]芘(Benzo[a]pyrene, BP)、二苯并[a,h]蒽(Dibenzo[a,h]anthracene, DBA)、芘(Pyrene, PYR),在100 nmol·L−1浓度下对以外泌体为主的细胞外囊泡分泌的影响[41]. 该研究表明在100 nmol·L−1浓度下,暴露5 h后,3种物质均可促进以外泌体为主的细胞外囊泡的分泌,并呈现出时间依赖性. 结合本实验结果,这提示了特定污染物的暴露可影响外泌体的分泌.
需要注意的是,本实验仅检测了双酚类物质暴露24 h对外泌体分泌的影响,而污染物对机体的暴露往往是长期低剂量的,因此在未来的研究中需要延长暴露时间以进一步评估BPA、BPS、BPAF影响外泌体分泌的长期效应. 此外,本研究结果仅基于HepG2细胞模型,双酚类物质可能对其它细胞类型有不同的效应. 人群中存在个体差异且暴露途径多样,这些因素可能对双酚类物质的毒性作用产生影响. 进一步的研究应该更多考虑实际暴露场景,并扩大样本规模以提高结果的可靠性和结论的普适性.
综上所述,本研究结果显示,BPA在人体内暴露相关浓度下可以促进细胞分泌更多外泌体,虽然不直接影响细胞活性,但它的暴露可能通过影响细胞外泌体的正常分泌而对机体产生不良影响. 这对于深入了解双酚类物质的潜在健康风险具有重要意义.
-
本文研究了BPA、BPS、BPAF在人体相关浓度下对细胞外泌体分泌的影响. 结果表明,3种双酚类物质对外泌体的分泌干扰表现出差异. BPS、BPAF不会影响外泌体的分泌,而BPA可显著促进外泌体分泌. BPA干扰细胞外泌体正常分泌的分子机制有待进一步探究.
双酚类物质对外泌体分泌的影响
The effect of bisphenols on the secretion of exosomes
-
摘要: 双酚类物质是一种常见的内分泌干扰物,广泛应用于工业生产. 其中,双酚A(bisphenol A,BPA)及其替代物双酚S(bisphenol S,BPS)和双酚AF(bisphenol AF,BPAF)可通过饮食、呼吸、皮肤吸收等途径进入人体,并影响机体健康. 外泌体作为细胞分泌的囊泡,在细胞通讯中起重要作用,参与多种生理和病理过程. 研究表明,环境污染物可影响外泌体的分泌过程. 然而,双酚类物质对外泌体分泌的影响未见报道. 因此,本文以双酚类物质为研究对象,研究其对细胞外泌体分泌的影响. 通过超高速离心法提取高浓度外泌体,并结合透射电子显微镜、纳米颗粒追踪分析技术(Nanoparticle Tracking Analysis,NTA)和蛋白免疫印迹技术对外泌体进行表征. 结果表明,200 nmol·L−1 和500 nmol·L−1 BPA暴露HepG2细胞24 h后,会显著促进外泌体的分泌,而BPS和BPAF在人体相关浓度下(50 nmol·L−1)对外泌体的分泌无影响. 本研究结果将为双酚类物质的毒性评估和机制研究提供新的思路.Abstract: Bisphenols are common endocrine-disrupting contaminants that are widely used in industrial production. Among them, bisphenol A (BPA) and its substitutes bisphenol S (BPS) and bisphenol AF (BPAF) can enter the body through diet, respiration, and skin absorption, and affect the health of the body. Exosomes, as vesicles secreted by cells, play an important role in cellular communication and are involved in a variety of physiological and pathological processes. The secretion of exosomes can be influenced by environmental pollutant. However, the effects of bisphenols on exosome secretion have not been reported. This study focuses on bisphenols and investigates their effects on the secretion of exosome. High-concentration exosomes are extracted using ultracentrifugation method, and the exosomes are characterized through transmission electron microscopy, nanoparticle tracking analysis (NTA), and western blot techniques. Our results demonstrated that BPA significant promoted exosome secretion of HepG2 cells at the concentration of 200 nmol·L−1 and 500 nmol·L−1 after exposed for 24 h, while BPS and BPAF had no effect on exosome secretion at physiologically relevant concentrations (50 nmol·L−1). The study will provide new view for toxicity evaluation and mechanism research of bisphenols.
-
Key words:
- bisphenols /
- exosome /
- human-relevant concentration
-

-
表 1 细胞培养所用试剂配方
Table 1. Reagent formula for cell culture
名称
Name配方
Formula完全培养液 DMEM(高糖)培养基加入10%FBS和1%双抗 暴露培养液 无酚红DMEM培养基加入10% CS‐FBS和1%双抗 外泌体提取培养液 无酚红DMEM培养基加入10% 无外泌体CS‐FBS和1%双抗 -
[1] HAHLADAKIS J N, IACOVIDOU E, GERASSIMIDOU S. An overview of the occurrence, fate, and human risks of the bisphenol-a present in plastic materials, components, and products[J]. Integrated Environmental Assessment and Management, 2023, 19(1): 45-62. doi: 10.1002/ieam.4611 [2] MOLINA-MOLINA J M, AMAYA E, GRIMALDI M, et al. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-a congeners and derivatives via nuclear receptors[J]. Toxicology and Applied Pharmacology, 2013, 272(1): 127-136. doi: 10.1016/j.taap.2013.05.015 [3] CAO X L, CORRIVEAU J, POPOVIC S. Migration of bisphenol A from can coatings to liquid infant formula during storage at room temperature[J]. Journal of Food Protection, 2009, 72(12): 2571-2574. doi: 10.4315/0362-028X-72.12.2571 [4] ARIS A. Estimation of bisphenol A (BPA) concentrations in pregnant women, fetuses and nonpregnant women in Eastern Townships of Canada[J]. Reproductive Toxicology, 2014, 45: 8-13. doi: 10.1016/j.reprotox.2013.12.006 [5] NAHAR M S, LIAO C Y, KANNAN K, et al. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans[J]. Journal of Biochemical and Molecular Toxicology, 2013, 27(2): 116-123. doi: 10.1002/jbt.21459 [6] EDLOW A G, CHEN M, SMITH N A, et al. Fetal bisphenol A exposure: Concentration of conjugated and unconjugated bisphenol A in amniotic fluid in the second and third trimesters[J]. Reproductive Toxicology, 2012, 34(1): 1-7. doi: 10.1016/j.reprotox.2012.03.009 [7] LI J F, WU C S, ZHAO H Z, et al. Exposure assessment of bisphenols in Chinese women during pregnancy: A longitudinal study[J]. Environmental Science & Technology, 2019, 53(13): 7812-7820. [8] GEENS T, GOEYENS L, COVACI A. Are potential sources for human exposure to bisphenol-a overlooked?[J]. International Journal of Hygiene and Environmental Health, 2011, 214(5): 339-347. doi: 10.1016/j.ijheh.2011.04.005 [9] ZALKO D, JACQUES C, DUPLAN H, et al. Viable skin efficiently absorbs and metabolizes bisphenol A[J]. Chemosphere, 2011, 82(3): 424-430. doi: 10.1016/j.chemosphere.2010.09.058 [10] VASILJEVIC T, HARNER T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels[J]. Science of the Total Environment, 2021, 789: 148013. doi: 10.1016/j.scitotenv.2021.148013 [11] CHEN D, KANNAN K, TAN H L, et al. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-a review[J]. Environmental Science & Technology, 2016, 50(11): 5438-5453. [12] SANTORO A, CHIANESE R, TROISI J, et al. Neuro-toxic and reproductive effects of BPA[J]. Current Neuropharmacology, 2019, 17(12): 1109-1132. doi: 10.2174/1570159X17666190726112101 [13] BARBONETTI A, D'ANDREA S, BERNABÒ N, et al. Editorial: Bisphenols and male reproductive health[J]. Frontiers in Endocrinology, 2020, 11: 597609. doi: 10.3389/fendo.2020.597609 [14] McDONOUGH C M, XU H S, GUO T L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison[J]. Critical Reviews in Toxicology, 2021, 51(4): 283-300. doi: 10.1080/10408444.2021.1908224 [15] DALLIO M, MASARONE M, ERRICO S, et al. Role of bisphenol A as environmental factor in the promotion of non-alcoholic fatty liver disease: Invitro and clinical study[J]. Alimentary Pharmacology & Therapeutics, 2018, 47(6): 826-837. [16] SONG S M, DUAN Y S, ZHANG T, et al. Serum concentrations of bisphenol A and its alternatives in elderly population living around e-waste recycling facilities in China: Associations with fasting blood glucose[J]. Ecotoxicology and Environmental Safety, 2019, 169: 822-828. doi: 10.1016/j.ecoenv.2018.11.101 [17] ZHANG H F, ZHANG Y P, LI J B, et al. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China[J]. Science of the Total Environment, 2019, 655: 607-613. doi: 10.1016/j.scitotenv.2018.11.053 [18] YANG Y J, GUAN J, YIN J, et al. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in South China[J]. Chemosphere, 2014, 112: 481-486. doi: 10.1016/j.chemosphere.2014.05.004 [19] JIN H B, ZHU J, CHEN Z J, et al. Occurrence and partitioning of bisphenol analogues in adults’ blood from China[J]. Environmental Science & Technology, 2018, 52(2): 812-820. [20] GERONA R, VOM SAAL F S, HUNT P A. BPA: Have flawed analytical techniques compromised risk assessments?[J]. The Lancet Diabetes & Endocrinology, 2020, 8(1): 11-13. [21] LEE Y J, RYU H Y, KIM H K, et al. Maternal and fetal exposure to bisphenol A in Korea[J]. Reproductive Toxicology, 2008, 25(4): 413-419. doi: 10.1016/j.reprotox.2008.05.058 [22] LIAO C Y, LIU F, ALOMIRAH H, et al. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures[J]. Environmental Science & Technology, 2012, 46(12): 6860-6866. [23] LIANG X X, YIN N Y, LIANG S X, et al. Bisphenol A and several derivatives exert neural toxicity in human neuron-like cells by decreasing neurite length[J]. Food and Chemical Toxicology, 2020, 135: 111015. doi: 10.1016/j.fct.2019.111015 [24] GENG S S, WANG S J, ZHU W W, et al. Curcumin attenuates BPA-induced insulin resistance in HepG2 cells through suppression of JNK/p38 pathways[J]. Toxicology Letters, 2017, 272: 75-83. doi: 10.1016/j.toxlet.2017.03.011 [25] HERCOG K, MAISANABA S, FILIPIČ M, et al. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells[J]. Science of the Total Environment, 2019, 687: 267-276. doi: 10.1016/j.scitotenv.2019.05.486 [26] HENGSTLER J G, FOTH H, GEBEL T, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A[J]. Critical Reviews in Toxicology, 2011, 41(4): 263-291. doi: 10.3109/10408444.2011.558487 [27] LUO S M, LI Y, LI Y P, et al. Gestational and lactational exposure to low-dose bisphenol A increases Th17 cells in mice offspring[J]. Environmental Toxicology and Pharmacology, 2016, 47: 149-158. doi: 10.1016/j.etap.2016.09.017 [28] LAMBRÉ C, BARAT BAVIERA J M, et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs[J]. EFSA Journal, 2023, 21(4): e06857. [29] OH J, CHOI J W, AHN Y A, et al. Pharmacokinetics of bisphenol S in humans after single oral administration[J]. Environment International, 2018, 112: 127-133. doi: 10.1016/j.envint.2017.11.020 [30] GRAMEC SKLEDAR D, TRONTELJ J, TROBERG J, et al. Data on biosynthesis of BPAF glucuronide, enzyme kinetics of BPAF glucuronidation, and molecular modeling[J]. Data in Brief, 2019, 22: 977-986. doi: 10.1016/j.dib.2018.12.033 [31] WAIDYANATHA S, BLACK S R, AILLON K, et al. Toxicokinetics and bioavailability of bisphenol AF following oral administration in rodents: A dose, species, and sex comparison[J]. Toxicology and Applied Pharmacology, 2019, 373: 39-47. doi: 10.1016/j.taap.2019.04.015 [32] KALLURI R, LeBLEU V S. The biology , function , and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. [33] GUAY C, REGAZZI R. Exosomes as new players in metabolic organ cross-talk[J]. Diabetes, Obesity & Metabolism, 2017, 19(Suppl 1): 137-146. [34] CASTAÑO C, KALKO S, NOVIALS A, et al. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(48): 12158-12163. [35] KAPUSTIN ALEXANDER N, MICHAEL S, SCHURGERS LEON J, et al. Prothrombin loading of vascular smooth muscle cell-derived exosomes regulates coagulation and calcification[J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2017, 37(3): e22-e32. [36] WANG X Y, ZHOU Y X, GAO Q N, et al. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases[J]. Oxidative Medicine and Cellular Longevity, 2020, 2020: 3232869. [37] LI X, LI C Y, ZHANG L P, et al. The significance of exosomes in the development and treatment of hepatocellular carcinoma[J]. Molecular Cancer, 2020, 19(1): 1. doi: 10.1186/s12943-019-1085-0 [38] ZOU J L, PENG H Y, LIU Y Z. The roles of exosomes in immunoregulation and autoimmune thyroid diseases[J]. Frontiers in Immunology, 2021, 12: 757674. doi: 10.3389/fimmu.2021.757674 [39] NGALAME N N O, LUZ A L, MAKIA N, et al. Arsenic alters exosome quantity and cargo to mediate stem cell recruitment into a cancer stem cell-like phenotype[J]. Toxicological Sciences, 2018, 165(1): 40-49. doi: 10.1093/toxsci/kfy176 [40] DU X H, ZHANG Q L, JIANG Y X, et al. Characterization of plasma-derived exosomal miRNA changes following traffic-related air pollution exposure: A randomized, crossover trial based on small RNA sequencing[J]. Environment International, 2022, 167: 107430. doi: 10.1016/j.envint.2022.107430 [41] van METEREN N, LAGADIC-GOSSMANN D, CHEVANNE M, et al. Polycyclic aromatic hydrocarbons can trigger hepatocyte release of extracellular vesicles by various mechanisms of action depending on their affinity for the aryl hydrocarbon receptor[J]. Toxicological Sciences, 2019, 171(2): 443-462. doi: 10.1093/toxsci/kfz157 [42] KORNILOV R, PUHKA M, MANNERSTRÖM B, et al. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum[J]. Journal of Extracellular Vesicles, 2018, 7(1): 1422674. doi: 10.1080/20013078.2017.1422674 [43] THÉRY C, AMIGORENA S, RAPOSO G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids[J]. Current Protocols in Cell Biology, 2006, Chapter 3: Unit3.22. [44] JEPPESEN D K, HVAM M L, PRIMDAHL-BENGTSON B, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation[J]. Journal of Extracellular Vesicles, 2014, 3(1): 25011. doi: 10.3402/jev.v3.25011 [45] LI X Y, HE X X, LIN X N, et al. Effects of bisphenols on lipid metabolism and neuro-cardiovascular toxicity in marine medaka larvae[J]. Aquatic Toxicology, 2023, 259: 106551. doi: 10.1016/j.aquatox.2023.106551 -



 下载:
下载: