-
近年来,为解决化石资源不可再生及全球生态环境的问题,人们提出了许多减缓碳排放的方法[1 − 4]. 其中,利用可持续太阳能将CO2转化为高效的能源物质(如甲烷、甲酸、甲醇等)的技术方案受到众多研究人员的关注[5 − 8]. 因此,如何提高光催化还原CO2的反应性能和选择性成为研究热点[9 − 10].
一般来说,光催化剂的反应位点对光催化的反应性能起着重要的作用. 但是大多数多光催化催化还原CO2的催化剂存在着颗粒尺寸大、不易分散等问题,这些问题容易导致反应位点减少,从而降低光催化性能[11 − 12]. 近年来,在载体上高度分散金属原子在催化领域引起了越来越多的关注[13 − 15]. 原子层沉积(ALD)可以实现单原子催化剂的精准可控合成,然而其存在着合成设备成本高、产率低的问题[16 − 18]. 因此,从实际应用的角度出发,开发出具有操作简单、可批量生产等优点的湿化学制备技术尤为重要. 例如调控配位不饱和位点以增强载体与金属原子间的相互作用[19 − 20],在载体表面构建合适的缺陷[21 − 22],将金属原子限域在框架材料的分子笼中[23 − 26]以及设计金属前驱体的锚定位点[27 − 28]等.
二维层状结构的类石墨烯氮化碳(g-C3N4)中三嗪环上的N原子存在孤对电子,可以锚定金属原子[29 − 35]. Huang等[36]利用光沉积法在g-C3N4上制备了单个金属位点,其表现出优异的光催化还原CO2的反应性能. Lu等[37]以2-巯基-5-丙基嘧啶为配体,通过煅烧Ni2+配位聚合物,成功合成了具有丰富硫空位的复合光催化剂NiS@g-C3N4. 其中,Ni2+和含巯基的N杂环分子在配位聚合物中紧密连接,有利于NiS颗粒的均匀分布. 在煅烧过程中,邻近的N含巯基的杂环分子限制了NiS纳米颗粒的生长,从而成功合成了小尺寸的NiS纳米颗粒. Gao等[38]用沉积法将钯和铂单原子负载在g-C3N4上进行光催化还原CO2,发现了其具有良好的光催化还原CO2性能.
稀土氧化铈物(CeO2)具有丰富的氧空位和碱性位点,优异的Ce3+/Ce4+之间相互转化的氧化还原能力[39 − 40],这可以促进光催化CO2的吸附和活化[41 − 43]. 本课题组前期对CeO2光催化还原CO2进行了一些研究[11,43 − 44],发现氧空位可以作为Lewis酸性,表面官能团可以作为碱性位点,这可以加快CO2的吸附和活化. 最近有文献也提出Ce物种的存在状态在光催化反应中起着重要作用. Xie等[45]制备了Ce掺杂g-C3N4催化剂,提出Ce物种和表面吸附的羟基分别是草酸络合和臭氧活化的重要催化位点. He等[46]从理论上证实了负载在g-C3N4上的Ce单原子是一种潜在的ORR催化剂. 然而,g-C3N4上高分散Ce物种应用于光催化还原CO2生成CH4的反应机理研究较少.
因此,本工作通过利用尿素和铈盐前驱体的配位作用,制备了在g-C3N4上高度分散的Ce物种催化剂,再将其负载到g-C3N4,记为CeCN-urea-N2,通过浸渍法制备了CeCN-N2样品进行对比. 采用TEM、STEM-HAADF、XPS、FT-IR、同步辐射表征和DFT计算研究了Ce的存在状态. 结果发现,CeCN-urea-N2的三嗪环与Ce之间的电子相互作用有利于内建电场的形成,加快了光生电荷转移和·CO2−自由基的生成. 此外,O 1s XPS和水接触角的结果表明,具有更多Lewis碱位的CeCN-urea-N2能够吸附更多的H2O分子,其作为质子源攻击·CO2−自由基,从而提高CH4的选择性. 结合CO2吸附原位红外揭示反应机理. 本研究内容为开发和制备高效的铈基光催化剂促进二氧化碳资源化利用提供一种可行性的方案.
-
尿素、六水合硝酸铈(Ce(NO3)3·6H2O)、无水乙醇均为分析级.
-
在马弗炉中,将尿素在550 ℃下煅烧4 h(升温速率为2.5 ℃·min−1)得到g-C3N4. 取0.95 g的g-C3N4,加入适量无水乙醇中,充分搅拌. 分别将0.126 g的Ce(NO3)3·6H2O和0.2 g的尿素加入到适量无水乙醇中,完全溶解后,将Ce(NO3)3·6H2O的乙醇溶液缓慢滴入尿素的乙醇溶液,搅拌1 h. 将上述溶液缓慢滴入g-C3N4悬浮液中,搅拌1 h,油浴加热蒸干. 最后将固体粉末均匀铺至瓷舟内,将瓷舟置于管式炉中,在氮气气氛下升温至500 ℃煅烧4 h,得到CeCN-urea-N2催化剂.
-
在马弗炉中,将Ce(NO3)3·6H2O在550 ℃下煅烧4 h得到CeO2颗粒. 将CeO2 (60.05 g)和g-C3N4 (0.95 g)分别放入一定量的去离子水中,搅拌均匀后,将CeO2缓缓滴加到g-C3N4悬浊液中,搅拌加热蒸干. 最后将固体粉末均匀铺至瓷舟内,将瓷舟置于管式炉中,在氮气气氛下升温至500 ℃煅烧4 h,得到CeCN-N2催化剂.
-
称取20 mg催化剂样品,置于石英玻璃砂反应器上,滴入1 mL去离子水. 将空气排净后,注入4 bar高纯CO2进入反应装置,在300 W氙灯下光照8 h,用气相色谱检测光催化还原CO2反应的产物.
-
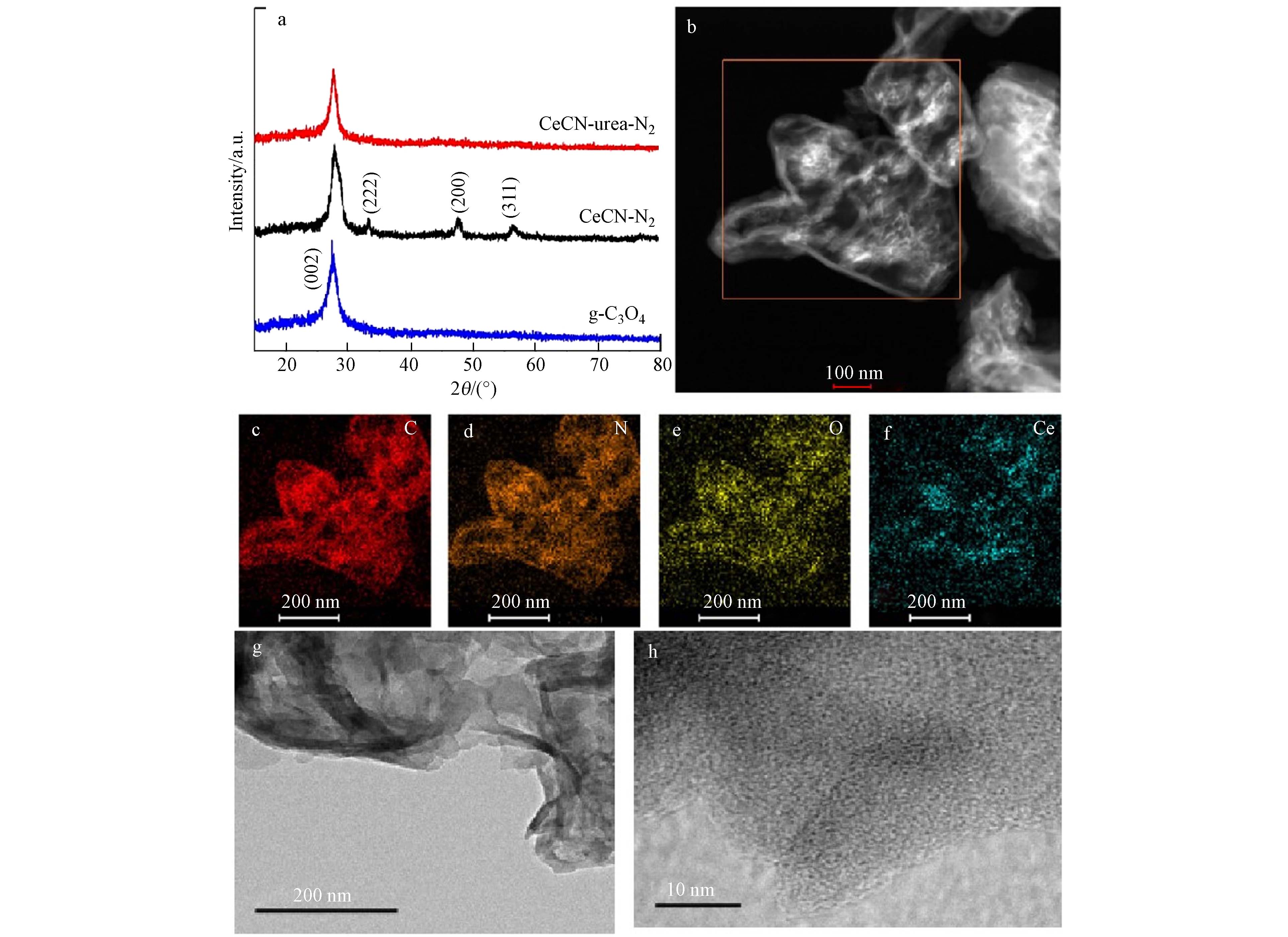
在CeCN-urea-N2样品的STEM-HAADF和TEM图像结果中未观察到CeO2颗粒(图1b、g、h),EDX图中的元素分布表明除了C、N元素外,CeCN-urea-N2样品中的Ce物种均匀地分散在g-C3N4表面. 由此推断,与CeCN-N2相比,尿素配位保护下有利于Ce物种在g-C3N4表面高度分散,因此在CeCN-urea-N2上没有明显团聚的CeO2纳米颗粒.
此外,CeCN-N2和CeCN-urea-N2的XRD衍射图证实了上述结果. 图1a中27.3°处的峰对应于g-C3N4的(002)面,28.8°、33.5°、47.8°和56.8°处的峰分别对应于CeO2 (JCPDS No.04-0802)的(111)、(222)、(200)和(311)晶面[47 − 48]. 可以看出,在CeCN-N2样品上出现了CeO2的衍射峰,而在CeCN-urea-N2样品上除了g-C3N4的衍射峰外,没有CeO2的衍射峰. 结合TEM和HRTEM的结果,进一步证实了CeCN-urea-N2样品中Ce物种的高度分散.
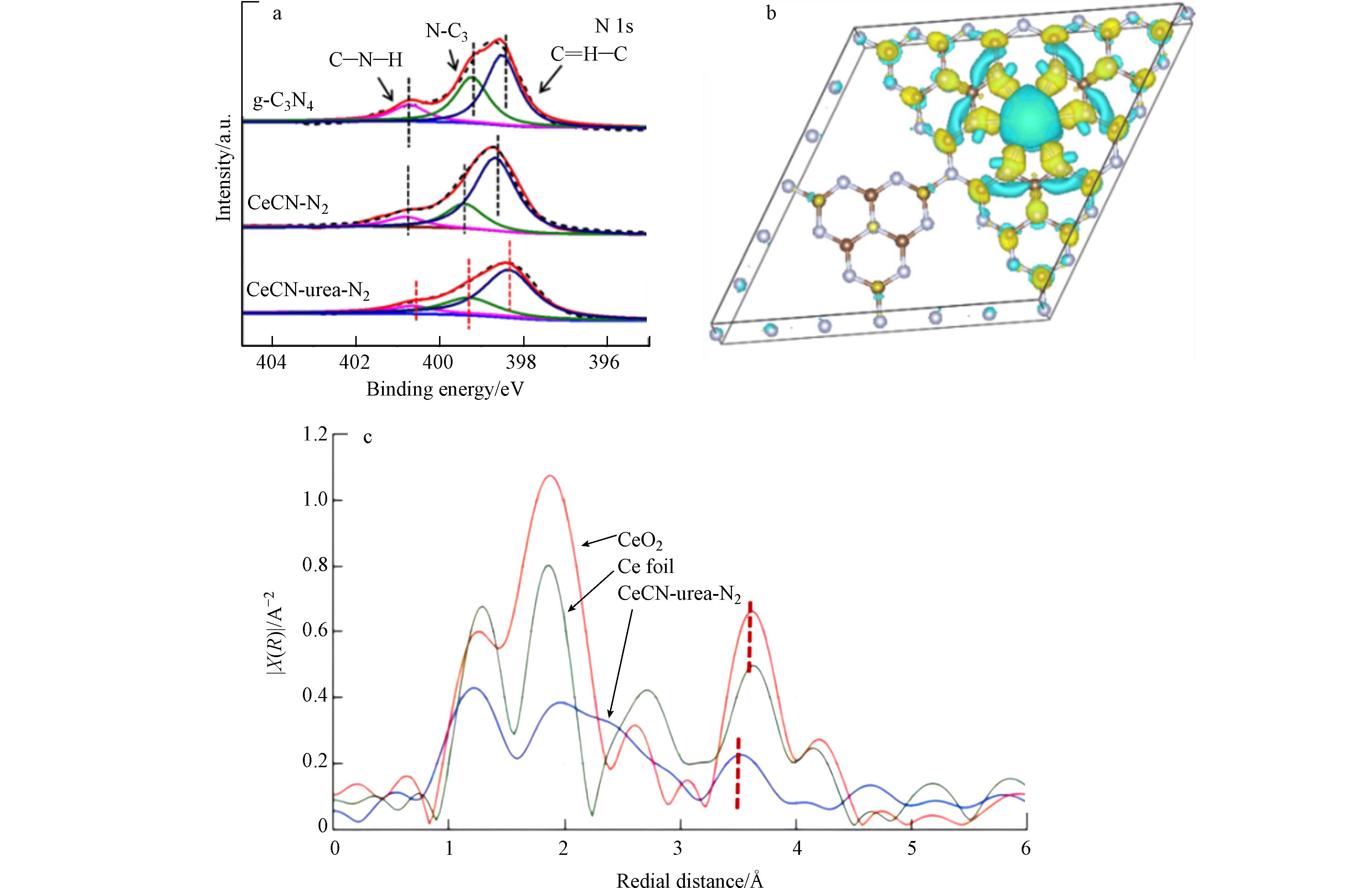
通过XPS、X射线吸收近边结构(XANES)和DFT理论计算确定了CeCN-urea-N2中Ce物种的存在状态. 将N 1s的XPS能谱信号拟合为3个峰(图2a),位于398.6、399.4、400.8 eV的峰分别归属于N原子(C=N—C)、三级氮原子(H—N—C2或N—C3)和氨基官能团. 与g-C3N4相比,CeCN-urea-N2样品中N 1s的峰向低结合能偏移. 此外,如图2c所示,XANES测定了CeCN-urea-N2中Ce L3的吸收边,相对于Ce和CeO2,CeCN-urea-N2中峰形发生了变化,并左移,这是由于CeCN-urea-N2中Ce—N键的形成,Ce的配位数降低,结构无序程度增加,振幅减小[49]. 因此,可以认为Ce进入g-C3N4骨架形成Ce—N键,Ce的4f电子转移到界面上的N原子上,CeCN-urea-N2样品界面相互作用增强. 为了确定CeCN-urea-N2界面处的内建电场的电子转移方向,通过DFT理论计算了Ce与g-C3N4界面间的差分电荷. 如图2b所示,青色和蓝色分别代表电荷密度的减小和增大. 界面处g-C3N4中N原子上电荷聚集,而Ce上电荷减少,说明界面处电子从Ce转移到g-C3N4的N原子上,形成了界面内建电场.
-
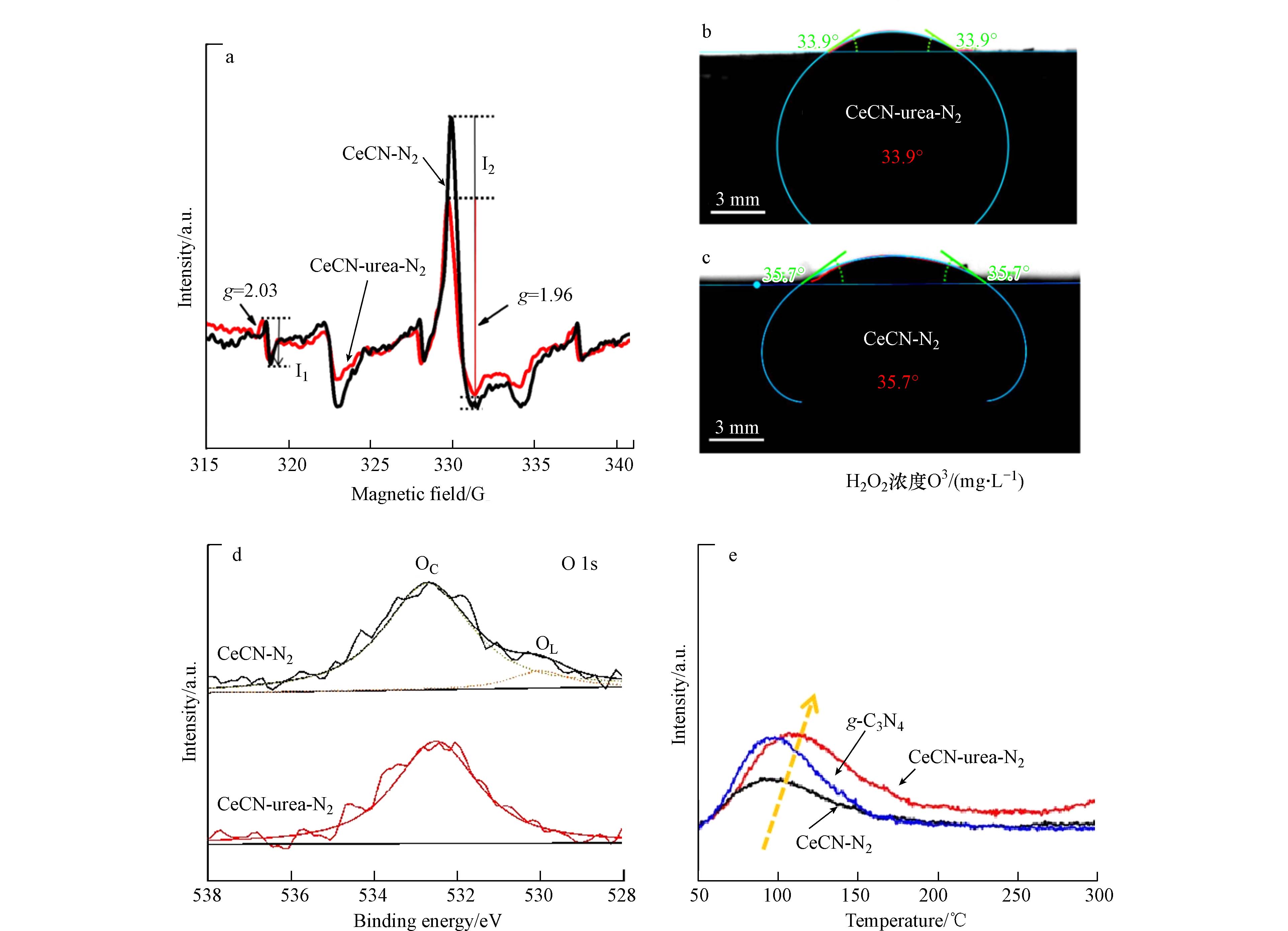
众所周知,在光催化还原CO2反应过程中,催化剂表面吸附的水分子可以提供质子,有利于提高CH4的选择性. 通过低温ESR、水接触角、O 1s 的XPS、O2-TPD研究了CeCN-urea-N2和CeCN-N2催化剂表面吸附的水分子. 水接触角结果(图3b、c)显示,CeCN-urea-N2的水接触角较小,说明CeCN-urea-N2表面亲水性较好,含氧物质较多. O 1s 的XPS谱图(图3d)给出了表面吸附氧信息. 将位于530.0 eV和532.6 eV左右处的两个峰分别属于晶格氧(OL)和吸附氧(OC). 通常情况下,吸附氧包括羟基和吸附水分子. 吸附氧物种和晶格氧的峰面积如表1所示,CeCN-N2中还有小部分晶格氧物种,而CeCN-urea-N2中都是吸附的水分子和羟基物种. O2-TPD结果如图3e所示,在100—200 ℃之间的宽峰归属于表面化学吸附氧的脱附[50 − 53]. 相比于纯g-C3N4的O2-TPD结果,CeCN-urea-N2和CeCN-N2样品中的吸附氧物种的脱附温度更高,更难脱附,说明铈物种和g-C3N4间具有更强的相互作用,上述现象在CeCN-urea-N2样品中更明显,间接说明高度分散的铈物种增强了CeCN-urea-N2样品界面相互作用. 此外,表面化学吸附氧的脱附峰面积也说明CeCN-urea-N2催化剂表面吸附氧物种更多,具有更多的羟基物种.
为什么CeCN-urea-N2具有更多的表面羟基物种呢?在图3a中,将g=2.03和g=1.96处的峰分别归属于总的物种浓度,包括表界面和体相的超氧自由基和Ce3+[54 − 57]. 对比两个峰强,可以发现,在CeCN-urea-N2的峰强较高,说明Ce3+含量高于CeCN-N2,而Ce3+可以作为Lewis碱位吸附羟基和水分子. 遗憾的是,尽管实验测试了Ce 3d的XPS谱图,但是可能是由于Ce的载量较低,Ce 3d能谱信号的信噪比较大,无法对其进行分峰拟合处理. 因此,CeCN-urea-N2中表面吸附的羟基和水分子丰富,有利于光催化还原CO2为CH4.
-
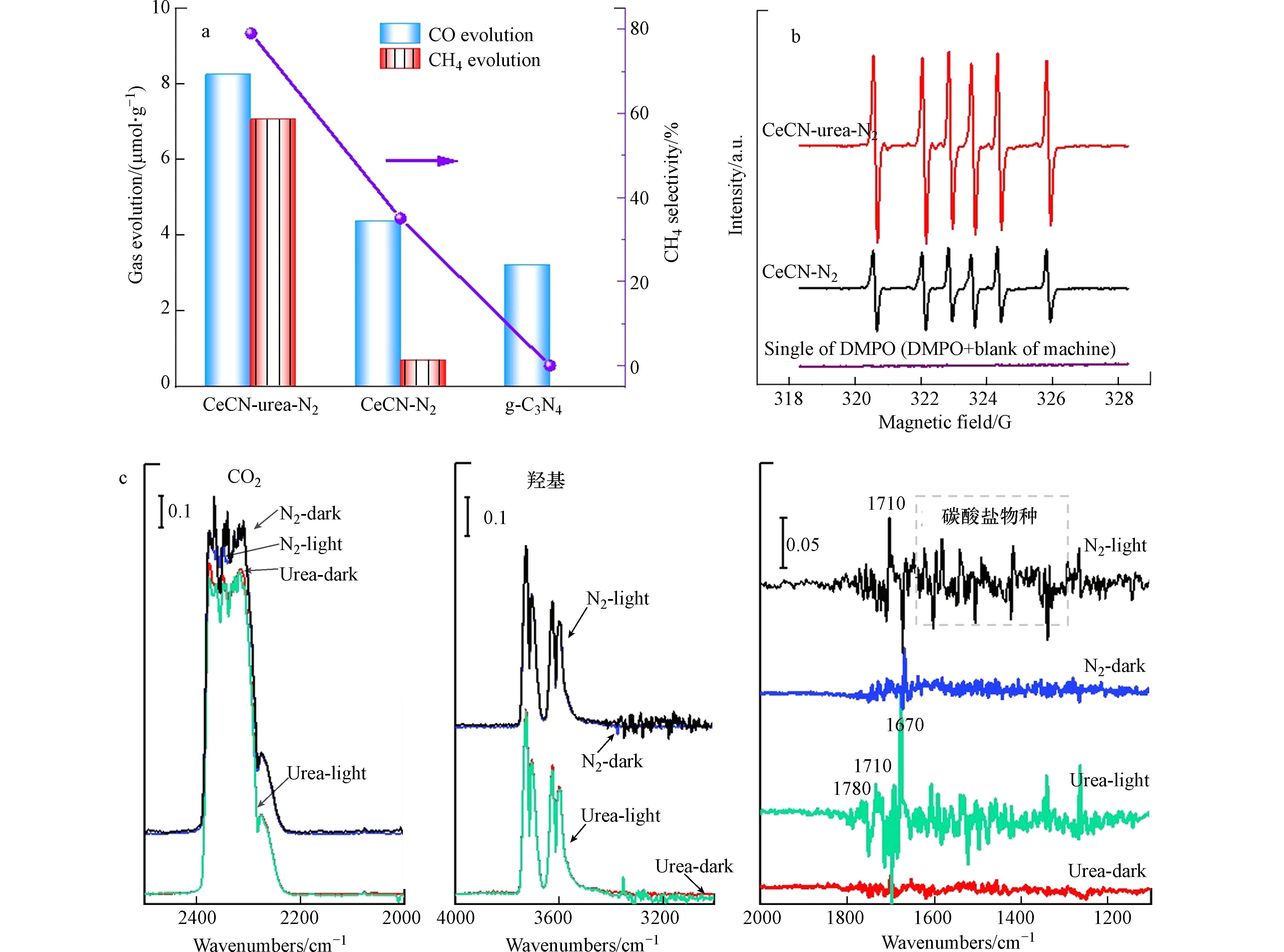
CeCN-urea-N2、CeCN-N2和g-C3N4催化剂的活性结果如图4所示. 光照8 h后,g-C3N4催化剂上只能检测到CO,而CeCN-urea-N2和CeCN-N2催化剂上能同时检测出CO和CH4,且CO产率显著高于g-C3N4. 而对比CeCN-urea-N2和CeCN-N2催化剂,CeCN-urea-N2样品的CO产率是CeCN-N2样品的2倍左右,而CH4产率是CeCN-N2样品的10倍以上,这表明高分散性的CeCN-N2样品的CH4选择性得到了显著提高. 同时相比于文献中不加牺牲剂和光敏剂的工作,CeCN-urea-N2光催化剂具有较好的CO2还原性能(表2)[58 − 59]. 原位ESR检测CeCN-urea-N2和CeCN-N2样品中的二氧化碳自由基. 在图4b中,5,5-二甲基-1-吡咯烷氧氮化物(DMPO)捕获的信号归因于生成的·CO2 -自由基. CeCN-urea-N2 上的·CO2 -自由基的信号强度比CeCN-N2上的信号强度更高,表明·CO2 -自由基更容易在 CeCN-urea-N2上生成. 结合上述结果,可以得出CeCN-urea-N2上Ce与g-C3N4间形成的强烈的相互作用促进了光生电荷转移和·CO2−自由基的产生,同时更多的表面吸附羟基和水分子促使质子攻击·CO2 -自由基,提高了CH4的选择性.
-
为了探究光催化还原CO2反应机理,进行了黑暗和光照下CeCN-urea-N2和CeCN-N2样品的原位CO2吸附DRIFTS. 如图4c所示,分别比较了光照前后,两个样品表面CO2的信号,可以看到CeCN-urea-N2表面CO2的峰值强度略微减弱,说明光照促使CO2在CeCN-N2上发生光催化还原过程,主要生成HCO3−(
1670 cm−1)和*CHO(1780 cm−1)关键中间物种,这对于CO和CH4的产生非常重要. 而CeCN-N2样品,光照后主要形成了碳酸盐(1600 —1300 cm−1)和羧基(1710 cm−1)中间物种[60 − 61],但是HCO3−(1670 cm−1)和*CHO(1780 cm−1)关键中间物种较少,所以CO和CH4的产量低.对于CeCN-urea-N2,光照后3800 —3200 cm−1处的吸附羟基峰强稍微减弱,说明吸附的CO2更容易与吸附的羟基发生反应,促进HCO3−和*CHO关键中间物种生成. 因此,CeCN-urea-N2更有利于CO2反应物的吸附和活化,同时表面丰富的含氧官能团(OH/H2O)充当质子源,攻击活性中间物种 HCO3−和*CHO产生大量CH4. -
通过配位法成功地制备了g-C3N4上高度分散Ce物种的光催化剂,与浸渍法合成的CeCN样品相比,由于尿素的配位作用,在CeCN-urea-N2的样品表面没有观测到CeO2团聚颗粒. 同时CeCN-urea-N2界面处强烈的电子作用,促进了光生电子的迁移,以及表面丰富的吸附氧物种有利于CH4生成.
高分散铈物种催化剂的可控制备及其光催化还原CO2为CH4的促进机制
Highly dispersed Ce species on g-C3N4 for the enhanced selectivity of photocatalytic CO2 reduction to CH4
-
摘要: 调控金属氧化物与载体间的界面作用可以有效提升光催化还原CO2的反应性能. 在本工作中,使用配位法将铈(Ce)物种嵌入g-C3N4的三嗪环(CeCN-urea-N2)中,相比于浸渍法制备的CeCN-N2样品,CeO2的颗粒尺寸变小,界面之间的相互作用增强. 结果表明,CeCN-urea-N2样品中存在高度分散的Ce物种,同时理论计算表明,Ce原子的f电子转移到七氮环上,促进光生电子流入Ce位点,并与吸附在其上的CO2分子发生反应. 因此,CeCN-urea-N2样品可以形成更多·CO2−自由基,进而与表面H2O/OH产生质子化过程,提高了光催化还原CO2为CH4的选择性.Abstract: Enhanced interfacial interaction between metal oxide and support is a promising alternative to photocatalysis. In the work, the coordination method was used to synthesize the photocatalyst of heptazine rings chelating Ce atoms on g-C3N4 (CeCN-urea-N2) for CO2 photoreduction. On CeCN-urea-N2, Ce species were highly dispersed, while for the impregnated sample of CeCN-N2, agglomerated CeO2 particles were found. The highly dispersed Ce species on g-C3N4 was beneficial for the generation of in-built electronic field, i.e., f electrons of Ce atoms transferred to heptazine rings. Therefore, the photogenerated electrons flowed into Ce and reacted with the adsorbed CO2 molecules, leading to more ·CO2− radicals on CeCN-urea-N2, which were attacked by the surface enriched protons of H2O/OH to enhance CH4 selectivity, confirmed by the in situ DRIFTS result. The work provides a simple way to design efficient photocatalysts.
-
Key words:
- highly dispersed Ce /
- CO2 photoreduction /
- in-built electronic field /
- CH4 selectivity.
-

-
图 1 (a) CeCN-urea-N2、CeCN-N2和g-C3N4样品的XRD结果, (b) CeCN-urea-N2的STEM-HAADF图,CeCN-urea-N2的EDX图:(c) C,(d) N,(e) O,(f) Ce元素,和(g) 、(h)TEM图像
Figure 1. (a) XRD result of CeCN-urea-N2, CeCN-N2 and g-C3N4 samples, (b) STEM-HAADF image of CeCN-urea-N2, EDX mapping images of (c) C, (d) N, (e) O and (f) Ce on CeCN-urea-N2, (g)、(h) TEM images of CeCN-urea-N2
图 3 (a) CeCN-urea-N2和CeCN-N2样品在77 K时的ESR结果;水接触角结果:(b) CeCN-urea-N2和(c) CeCN-N2样品;(d) O 1s的XPS图,(e) CeCN-urea-N2、CeCN-N2和g-C3N4的O2-TPD结果
Figure 3. (a) ESR result at 77 K of CeCN-urea-N2 and CeCN-N2 samples; The contact angle results of (b) CeCN-urea-N2 and (c) CeCN-N2. High-resolution XPS image of (d) O 1s; (e) O2-TPD result of CeCN-urea-N2, CeCN-N2 and g-C3N4
图 4 (a) CeCN样品和g-C3N4的光催化还原CO2的反应性能, (b)·CO2−自由基的ESR信号,(c) CeCN-urea-N2和CeCN-N2的黑暗和光照下的CO2吸附原位红外
Figure 4. (a) Activities of photocatalytic CO2 reduction on CeCN samples and g-C3N4, (b) ESR signals of the radicals in the reaction and (c) in situ DRIFTS of CO2-adsorption in dark or light of CeCN-urea-N2 and CeCN-N2
表 1 CeCN-urea-N2和CeCN-N2样品O 1s的XPS的峰面积信息
Table 1. Peak information of O 1s XPS spectra of CeCN-urea-N2 and CeCN-N2
吸附氧(OC) 晶格氧(OL) 峰位置/eV
Peak position峰面积/a.u
Peak area峰位置/eV
Peak position峰面积/a.u
Peak areaCeCN-urea-N2 532.5 1360 - - CeCN-N2 532.7 1450 530.0 150 表 2 本工作光催化还原CO2的反应性能与文献的性能对比结果
Table 2. Comparison of CeCN-urea-N2 production with relevant literature
-
[1] SUN D R, FU Y H, LIU W J, et al. Studies on photocatalytic CO2 reduction over NH2-Uio-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal-organic frameworks[J]. Chemistry, 2013, 19(42): 14279-14285. doi: 10.1002/chem.201301728 [2] LI D D, KASSYMOVA M, CAI X C, et al. Photocatalytic CO2 reduction over metal-organic framework-based materials[J]. Coordination Chemistry Reviews, 2020, 412: 213262. doi: 10.1016/j.ccr.2020.213262 [3] YOUNAS M, REZAKAZEMI M, DAUD M, et al. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs)[J]. Progress in Energy and Combustion Science, 2020, 80: 100849. doi: 10.1016/j.pecs.2020.100849 [4] KURIKI R, YAMAMOTO M, HIGUCHI K, et al. Robust binding between carbon nitride nanosheets and a binuclear ruthenium(II) complex enabling durable, selective CO2 reduction under visible light in aqueous solution[J]. Angewandte Chemie (International Ed. in English), 2017, 56(17): 4867-4871. doi: 10.1002/anie.201701627 [5] LI A, CAO Q, ZHOU G Y, et al. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface[J]. Angewandte Chemie (International Ed. in English), 2019, 58(41): 14549-14555. doi: 10.1002/anie.201908058 [6] ZHANG F, LI Y H, QI M Y, et al. Boosting the activity and stability of Ag-Cu2O/ZnO nanorods for photocatalytic CO2 reduction[J]. Applied Catalysis B:Environmental, 2020, 268: 118380. doi: 10.1016/j.apcatb.2019.118380 [7] HAN Z, TANG C Z, WANG J J, et al. Atomically dispersed Ptn+ species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation[J]. Journal of Catalysis, 2021, 394: 236-244. doi: 10.1016/j.jcat.2020.06.018 [8] de GREGORIO G L, BURDYNY T, LOIUDICE A, et al. Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities[J]. ACS Catalysis, 2020, 10(9): 4854-4862. doi: 10.1021/acscatal.0c00297 [9] HE F, ZHU B C, CHENG B, et al. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity[J]. Applied Catalysis B:Environmental, 2020, 272: 119006. doi: 10.1016/j.apcatb.2020.119006 [10] WANG X W, LI Q C, GAN L, et al. 3D macropore carbon-vacancy g-C3N4 constructed using polymethylmethacrylate spheres for enhanced photocatalytic H2 evolution and CO2 reduction[J]. Journal of Energy Chemistry, 2021, 53: 139-146. doi: 10.1016/j.jechem.2020.05.001 [11] PU Y, LUO Y D, WEI X Q, et al. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect[J]. Applied Catalysis B:Environmental, 2019, 254: 580-586. doi: 10.1016/j.apcatb.2019.04.093 [12] ZOU W X, DENG B, HU X X, et al. Crystal-plane-dependent metal oxide-support interaction in CeO2/g-C3N4 for photocatalytic hydrogen evolution[J]. Applied Catalysis B:Environmental, 2018, 238: 111-118. doi: 10.1016/j.apcatb.2018.07.022 [13] YANG X F, WANG A Q, QIAO B T, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research, 2013, 46(8): 1740-1748. doi: 10.1021/ar300361m [14] KAISER S K, CHEN Z P, FAUST AKL D, et al. Single-atom catalysts across the periodic table[J]. Chemical Reviews, 2020, 120(21): 11703-11809. doi: 10.1021/acs.chemrev.0c00576 [15] LI X G, BI W T, ZHANG L, et al. Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution[J]. Advanced Materials, 2016, 28(12): 2427-2431. doi: 10.1002/adma.201505281 [16] GAO C, LOW J, LONG R, et al. Heterogeneous single-atom photocatalysts: Fundamentals and applications[J]. Chemical Reviews, 2020, 120(21): 12175-12216. doi: 10.1021/acs.chemrev.9b00840 [17] ZHANG N Q, YE C L, YAN H, et al. Single-atom site catalysts for environmental catalysis[J]. Nano Research, 2020, 13(12): 3165-3182. doi: 10.1007/s12274-020-2994-3 [18] LU B Z LIU Q M CHEN S W. Electrocatalysis of single-atom sites: Impacts of atomic coordination[J]. ACS Catalysis, 2020, 10(14): 7584-7618. doi: 10.1021/acscatal.0c01950 [19] ZHANG J, WU X, CHEONG W C, et al. Cation vacancy stabilization of single-atomic-site Pt1/Ni(OH) x catalyst for diboration of alkynes and alkenes[J]. Nature Communications, 2018, 9: 1002. doi: 10.1038/s41467-018-03380-z [20] WAN J W, CHEN W X, JIA C Y, et al. Defect effects on TiO2 nanosheets: Stabilizing single atomic site Au and promoting catalytic properties[J]. Advanced Materials, 2018, 30(11): 1705369. doi: 10.1002/adma.201705369 [21] TANG N F, CONG Y, SHANG Q H, et al. Coordinatively unsaturated Al3+ sites anchored subnanometric ruthenium catalyst for hydrogenation of aromatics[J]. ACS Catalysis, 2017, 7(9): 5987-5991. doi: 10.1021/acscatal.7b01816 [22] PETERSON E J, DeLARIVA A T, LIN S, et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina[J]. Nature Communications, 2014, 5: 4885. doi: 10.1038/ncomms5885 [23] CHEN Y J, JI S F, WANG Y G, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie , 2017, 56(24): 6937-6941. doi: 10.1002/anie.201702473 [24] ZHANG W J, JIANG P P, WANG Y, et al. Bottom-up approach to engineer a molybdenum-doped covalent-organic framework catalyst for selective oxidation reaction[J]. RSC Advances, 2014, 4(93): 51544-51547. doi: 10.1039/C4RA09304F [25] LIU L C, DÍAZ U, ARENAL R, et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D[J]. Nature Materials, 2017, 16(1): 132-138. doi: 10.1038/nmat4757 [26] LU J, AYDIN C, BROWNING N D, et al. Imaging isolated gold atom catalytic sites in zeolite NaY[J]. Angewandte Chemie (International Ed. in English), 2012, 51(24): 5842-5846. doi: 10.1002/anie.201107391 [27] ZHANG B, ASAKURA H, ZHANG J, et al. Stabilizing a Platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity[J]. Angewandte Chemie (International Ed. in English), 2016, 55(29): 8319-8323. doi: 10.1002/anie.201602801 [28] HAN Y H, WANG Y G, CHEN W X, et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: Superior electrocatalysts for oxygen reduction[J]. Journal of the American Chemical Society, 2017, 139(48): 17269-17272. doi: 10.1021/jacs.7b10194 [29] YUAN X J, DUAN S L, WU G Y, et al. Enhanced catalytic ozonation performance of highly stabilized mesoporous ZnO doped g-C3N4 composite for efficient water decontamination[J]. Applied Catalysis A:General, 2018, 551: 129-138. doi: 10.1016/j.apcata.2017.12.011 [30] JIN R R, HU S Z, GUI J Z, et al. A convenient method to prepare novel rare earth metal Ce-doped carbon nitride with enhanced photocatalytic activity under visible light[J]. Bulletin of the Korean Chemical Society, 2015, 36(1): 17-23. doi: 10.1002/bkcs.10001 [31] YUE B, LI Q Y, IWAI H, et al. Hydrogen production using zinc-doped carbon nitride catalyst irradiated with visible light[J]. Science and Technology of Advanced Materials, 2011, 12(3): 034401. doi: 10.1088/1468-6996/12/3/034401 [32] LI X Z, ZHU W, LU X W, et al. Integrated nanostructures of CeO2/attapulgite/g-C3N4 as efficient catalyst for photocatalytic desulfurization: Mechanism, kinetics and influencing factors[J]. Chemical Engineering Journal, 2017, 326: 87-98. doi: 10.1016/j.cej.2017.05.131 [33] WANG S Z, XU L J, WANG J L. Enhanced activation of peroxymonosulfate through exfoliated oxygen-doping graphitic carbon nitride for degradation of organic pollutants[J]. Chemical Engineering Journal, 2022, 428: 131066. doi: 10.1016/j.cej.2021.131066 [34] LI Y, LI X, ZHANG H W, et al. Porous graphitic carbon nitride for solar photocatalytic applications[J]. Nanoscale Horizons, 2020, 5(5): 765-786. doi: 10.1039/D0NH00046A [35] TANG Q J, SUN Z X, DENG S, et al. Decorating g-C3N4 with alkalinized Ti3C2 MXene for promoted photocatalytic CO2 reduction performance[J]. Journal of Colloid and Interface Science, 2020, 564: 406-417. doi: 10.1016/j.jcis.2019.12.091 [36] HUANG P P, HUANG J H, PANTOVICH S A, et al. Selective CO2 reduction catalyzed by single cobalt sites on carbon nitride under visible-light irradiation[J]. Journal of the American Chemical Society, 2018, 140(47): 16042-16047. doi: 10.1021/jacs.8b10380 [37] LU L L, XU X X, AN K L, et al. Coordination polymer derived NiS@g-C3N4 composite photocatalyst for sulfur vacancy and photothermal effect synergistic enhanced H2 production[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 11869-11876. [38] GAO G P, JIAO Y, WACLAWIK E R, et al. Single atom (Pd/Pt) supported on graphitic carbon nitride as an efficient photocatalyst for visible-light reduction of carbon dioxide[J]. Journal of the American Chemical Society, 2016, 138(19): 6292-6297. doi: 10.1021/jacs.6b02692 [39] WANG M, SHEN M, JIN X X, et al. Exploring the enhancement effects of hetero-metal doping in CeO2 on CO2 photocatalytic reduction performance[J]. Chemical Engineering Journal, 2022, 427: 130987. doi: 10.1016/j.cej.2021.130987 [40] 邹伟欣, 于平平, 董林. 稀土铈基纳米材料在光催化消除环境污染物中的研究进展[J]. 环境化学, 2022, 41(8): 2505-2515. doi: 10.7524/j.issn.0254-6108.2021041903 ZOU W X, YU P P, DONG L. Research progress on ceria-based nanomaterials for photocatalytic elimination of environmental pollutants[J]. Environmental Chemistry, 2022, 41(8): 2505-2515 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021041903
[41] LI M L, ZHANG L X, WU M Y, et al. Mesostructured CeO2/g-C3N4 nanocomposites: Remarkably enhanced photocatalytic activity for CO2 reduction by mutual component activations[J]. Nano Energy, 2016, 19: 145-155. doi: 10.1016/j.nanoen.2015.11.010 [42] REN J, LIU X, GAO R H, et al. Morphology and crystal-plane effects of Zr-doped CeO2 nanocrystals on the epoxidation of styrene with tert-butylhydroperoxide as the oxidant[J]. Journal of Energy Chemistry, 2017, 26(4): 681-687. doi: 10.1016/j.jechem.2017.01.007 [43] LI W Q, JIN L, GAO F, et al. Advantageous roles of phosphate decorated octahedral CeO2{111}/g-C3N4 in boosting photocatalytic CO2 reduction: Charge transfer bridge and Lewis basic site[J]. Applied Catalysis B:Environmental, 2021, 294: 120257. doi: 10.1016/j.apcatb.2021.120257 [44] PU Y, LI W Q, CAI Y D, et al. Effects of different treatment atmospheres on CeO2/g-C3N4 photocatalytic CO2 reduction: Good or bad?[J]. Catalysis Science & Technology, 2021, 11(8): 2827-2833. [45] XIE Y, PENG S H, FENG Y, et al. Enhanced mineralization of oxalate by highly active and Stable Ce(Ⅲ)-Doped g-C3N4 catalyzed ozonation[J]. Chemosphere, 2020, 239: 124612. doi: 10.1016/j.chemosphere.2019.124612 [46] HE F, LI H Q, DING Y C, et al. The oxygen reduction reaction on graphitic carbon nitride supported single Ce atom and Ce xPt6- x cluster catalysts from first-principles[J]. Carbon, 2018, 130: 636-644. doi: 10.1016/j.carbon.2018.01.071 [47] YAO X J, KONG T T, CHEN L, et al. Enhanced low-temperature NH3-SCR performance of MnO x/CeO2 catalysts by optimal solvent effect[J]. Applied Surface Science, 2017, 420: 407-415. doi: 10.1016/j.apsusc.2017.05.156 [48] LI L L, TAN W, WEI X Q, et al. Mo doping as an effective strategy to boost low temperature NH3-SCR performance of CeO2/TiO2 catalysts[J]. Catalysis Communications, 2018, 114: 10-14. doi: 10.1016/j.catcom.2018.05.015 [49] KOETTGEN J, MARTIN M. Coordination numbers in Sm-doped ceria using X-ray absorption spectroscopy[J]. The Journal of Physical Chemistry C, 2019, 123(11): 6333-6339. doi: 10.1021/acs.jpcc.8b10494 [50] ZHANG Y X, YOU R, LIU D S, et al. Carbonates-based noble metal-free lean NO x trap catalysts MO x-K2CO3/K2Ti8O17 (M = Ce, Fe, Cu, Co) with superior catalytic performance[J]. Applied Surface Science, 2015, 357: 2260-2276. doi: 10.1016/j.apsusc.2015.09.224 [51] QU X Y, HU S Z, LI P, et al. The effect of embedding N vacancies into g-C3N4 on the photocatalytic H2O2 production ability via H2 plasma treatment[J]. Diamond and Related Materials, 2018, 86: 159-166. doi: 10.1016/j.diamond.2018.04.027 [52] CHEN X, CHEN X, YU E Q, et al. in situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion[J]. Chemical Engineering Journal, 2018, 344: 469-479. doi: 10.1016/j.cej.2018.03.091 [53] WANG B W, CHI C M, XU M, et al. Plasma-catalytic removal of toluene over CeO2-MnO x catalysts in an atmosphere dielectric barrier discharge[J]. Chemical Engineering Journal, 2017, 322: 679-692. doi: 10.1016/j.cej.2017.03.153 [54] LIU B, LIU J, XIN L, et al. Unraveling reactivity descriptors and structure sensitivity in low-temperature NH3-SCR reaction over CeTiO x catalysts: a combined computational and experimental study[J]. ACS Catalysis, 2021, 11: 7613-7636. doi: 10.1021/acscatal.1c00311 [55] ZHAO K, QI J, YIN H J, et al. Efficient water oxidation under visible light by tuning surface defects on ceria nanorods[J]. Journal of Materials Chemistry A, 2015, 3(41): 20465-20470. doi: 10.1039/C5TA05817A [56] PENG M M, GANESH M, VINODH R, et al. Solvent free oxidation of ethylbenzene over Ce-BTC MOF[J]. Arabian Journal of Chemistry, 2019, 12(7): 1358-1364. doi: 10.1016/j.arabjc.2014.11.024 [57] CHEN Y L, CAO X X, LIN B Z, et al. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies[J]. Applied Surface Science, 2013, 264: 845-852. doi: 10.1016/j.apsusc.2012.10.160 [58] WANG Y G, BAI X, WANG F, et al. Nanocasting synthesis of chromium doped mesoporous CeO2 with enhanced visible-light photocatalytic CO2 reduction performance[J]. Journal of Hazardous Materials, 2019, 372: 69-76. doi: 10.1016/j.jhazmat.2017.10.007 [59] WANG K, FU J L, ZHENG Y. Insights into photocatalytic CO2 reduction on C3N4: Strategy of simultaneous B, K co-doping and enhancement by N vacancies[J]. Applied Catalysis B:Environmental, 2019, 254: 270-282. doi: 10.1016/j.apcatb.2019.05.002 [60] DICCIANNI J B, HU C T, DIAO T N. Insertion of CO2 mediated by a (xantphos)NiI-alkyl species[J]. Angewandte Chemie (International Ed. in English), 2019, 58(39): 13865-13868. doi: 10.1002/anie.201906005 [61] ZHU C Z, WEI X Q, LI W Q, et al. Crystal-plane effects of CeO2{110}and CeO2{100}on photocatalytic CO2 reduction: Synergistic interactions of oxygen defects and hydroxyl groups[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(38): 14397-14406. -



 下载:
下载: