-
抗生素被大量应用于医疗和养殖等领域,导致环境中抗生素残留无处不在,甚至在饮用水和牛奶中,都普遍检测出抗生素残留[1-3]。纳米银是一种良好的抗菌剂,同样被广泛用于灭菌和消毒等过程,并在食品、医疗、纺织和建材等行业被大量使用[4-5],因此环境中不可避免地存在纳米银残留[6]。同时,由于抗生素和纳米银在医疗领域均被大量应用,导致二者在医疗环境中广泛共存[4]。并且,来自于医疗废水、生活污水和工业废水中的抗生素以及纳米银会汇集于污水处理厂,并经由污水处理厂的出水和污泥处置等方式进入环境水体或土壤中,造成二者在自然环境中的普遍共存[7]。鉴于此,抗生素和纳米银联合暴露对生态环境尤其是微生物群体的影响需要引起格外重视。以往的研究表明,抗生素和纳米银联合作用时会产生比各自单独作用时更强的抑菌活性。例如,Huang等[8]发现,纳米银和链霉素对大肠杆菌和金黄色葡萄球菌均表现出显著的协同抑制作用;王孟珍等[9]发现,纳米银复合材料和卡那霉素对大肠杆菌能够产生协同抑制作用。除直接的抑菌效应外,抗生素和纳米银还能够促进细菌耐药性的产生和传播[10-12],加剧环境中耐药细菌和耐药基因的污染问题,直接危害环境和人体健康。然而,纳米银和抗生素的联合暴露对细菌耐药性的影响尚未引起足够的重视。
突变是细菌产生抗生素耐药性的主要原因之一[13]。突变是指基因在复制或修复过程中发生碱基组成或排列的变化,包括碱基的替换、缺失、重复或插入等,其中导致细菌产生抗生素耐药表型的突变即为抗性突变。大量研究表明,细菌在抗生素以及各种非抗生素药物的作用下,抗性突变会被显著促进。例如,Manna等[14]发现,甲氧苄氨嘧啶能够通过诱导大肠杆菌folA基因的突变,促进其对抗生素的耐药性;Lu等[15]发现,三氯生通过促进fabI、frdD、marR、acrR和soxR等基因的突变而显著提高大肠杆菌对多种抗生素的耐药性。此外,最近的研究表明,纳米材料如纳米Al2O3和ZnO等能够通过诱导细菌胞内活性氧物种(ROS)的产生,促进细菌对抗生素的抗性突变[16]。然而,纳米银作为一种常用的纳米抗菌剂,其对细菌抗性突变的影响尚未见相关的报道。更重要的是,目前研究大都关注的是单一污染物对细菌抗性突变的影响,抗生素和纳米材料联合暴露对细菌抗性突变的影响仍有待进一步研究。
本文选取四环素(Tetracycline, TET)、氯霉素(Chloramphenicol, CHL)和纳米银(AgNPs)作为研究对象,以大肠杆菌(E. coli)作为模式细菌,测定了抗生素和纳米银分别单一和联合作用时对细菌的生长抑制效应以及抗性突变诱导效应,借助DNA测序和分子对接结合的方式验证了细菌的抗性突变位点和抗性机制,同时采用qPCR技术测定了细菌胞内与突变相关的基因表达(rpoS、recA、lexA、dinB、mutS和uvrD),对抗生素和纳米银的联合抗性突变诱导效应进行了初步的机制阐释。
-
实验所用的模式细菌为大肠杆菌MG1655,购买自Biovector公司。氯霉素(≥ 98%)、四环素(≥ 98%)、利福平(≥ 98%)和纳米银(10—15 nm)等化学试剂均购自阿拉丁试剂有限公司。
-
将大肠杆菌接种到5 mL的 MH培养基(酸水解酪蛋白17.5 g∙L−1、牛肉浸膏粉3 g∙L−1、淀粉1.5 g∙L−1、琼脂15 g∙L−1,pH=7.0—7.2)中,37 ℃条件下培养至OD590(590 nm处的光密度)达到0.5。随后,将菌液稀释105倍后加入含有不同浓度待测物质的96孔板中。每个浓度设置3个平行,同时设置空白对照组。将孔板置于37 ℃培养24 h,使用酶标仪测定每孔OD值。之后,计算不同浓度化合物对细菌的生长抑制率(式1),绘制浓度−效应曲线(concentration−response curves, CRCs)并获得半抑制浓度(50% inhibiting concentration, IC50)。
根据单一毒性测试得出的IC50值,将抗生素和纳米银按照等毒性比设计混合浓度,测定二元混合暴露体系对细菌的生长抑制作用,获得混合物的IC50值。此外,采用浓度加和(concentration addition, CA)模型,根据抗生素和纳米银单一毒性结果,预测联合作用的CRCs。CA模型适用于具有相似作用机制的化合物联合毒性的预测,并且被广泛应用于判别混合物的相互作用类型,具体的判别依据为:实验组曲线及其95%置信区间与CA模型曲线重叠时,二元混合毒性视为加和效应,位于CA模型曲线的左侧和右侧时,则分别视为协同和拮抗效应[17]。CA模型的计算如公式(2)所示[18]。
式中:ECy,m为达到y(%)抑制率时混合体系下的总浓度,ECy,1和ECy,2为达到y(%)抑制率时组分1和2的浓度,P1 和P2表示混合物中组分1和2所占的浓度比例。
-
大肠杆菌在96孔板中暴露于待测物质24 h后,将每孔的菌悬液(200 μL)收集到1.5 mL离心管中,离心并洗涤3次后重新悬浮菌液(20 μL)。将重悬后的菌液用移液枪转移至含有80 mg∙L−1利福平的MH固体培养基上,在37 ℃条件下培养48 h后统计菌落数Nm,Nm即为每孔菌液中发生突变的细菌个数。同时通过梯度稀释法,利用不含利福平的MH固体培养基,测定菌悬液中的细菌总数Nt。通过公式(3)计算突变频率η。
-
随机挑选抗性板上筛选出的耐药菌落,接种至含有利福平的MH液体培养基中,并于37 ℃恒温过夜培养后保存于离心管中。使用DNA提取试剂盒(生工生物,上海)提取细菌总DNA,利用引物5'−AAGCTCATCGATATCCGTAACG−3'和5'−GCACGTCGCCACGTTCAACC−3'对rpoB基因片段进行PCR扩增[19]。PCR扩增过程的升温程序为:在96 ℃下进行1个循环,保持时间为1 min;在96 ℃下进行26个循环,保持时间为10 s;在50 ℃保持5 s,最后在60 ℃延伸4 min。扩增后的产物使用琼脂糖凝胶电泳进行检测,并将产物纯化后进行基因测序(ABI 3730)。测序结果通过DNASTAR软件中MegAlign模块的多序列比对程序,将样本序列与标准rpoB序列(NCBI编号为NC_000913.3)进行比对,样本序列与标准序列中的碱基差异即为突变发生的位点。
-
从蛋白质结构数据库( https://www.rcsb.org)中获取大肠杆菌中利福平的靶标蛋白,即RNA聚合酶的三级结构(编号为5UAC)。根据细菌突变位点及编码氨基酸变化,使用PremPS(https://lilab.jysw.suda.edu.cn/research/PremPS/)在线服务构建以5UAC为模板的突变蛋白的三级结构。然后使用Discovery Studio软件中的CDOCKER模块,将野生型与突变后蛋白分别与利福平分子进行对接,获得野生型和突变后蛋白与利福平分子对接的相互作用能大小。
-
本研究选择了6个与突变相关的基因:rpoS、lexA、recA、dinB、mutS和uvrD,测定了抗生素和纳米银分别单独和联合作用时对这些基因表达的影响,所用的引物序列参考之前的文献[20]。具体实验步骤如下:离心收集不同处理组的细菌,使用 trizol 试剂盒提取总RNA,去除基因组中DNA杂质后逆转录,再通过PCR扩增获得每个样品循环数值(Ct),并用相对定量(relative quantitative, RQ)法对数据进行归一化。将对照组的RQ值设为1,计算基因表达差异,详细方程计算如式(4—6):
式中,∆Ct,暴露组和∆Ct,对照组分别表示目标基因与对照基因的循环阈值差值,∆∆Ct表示暴露组和对照组中每个样品循环阈值的差值,RQ是基因表达的倍数变化与对照相比。
-
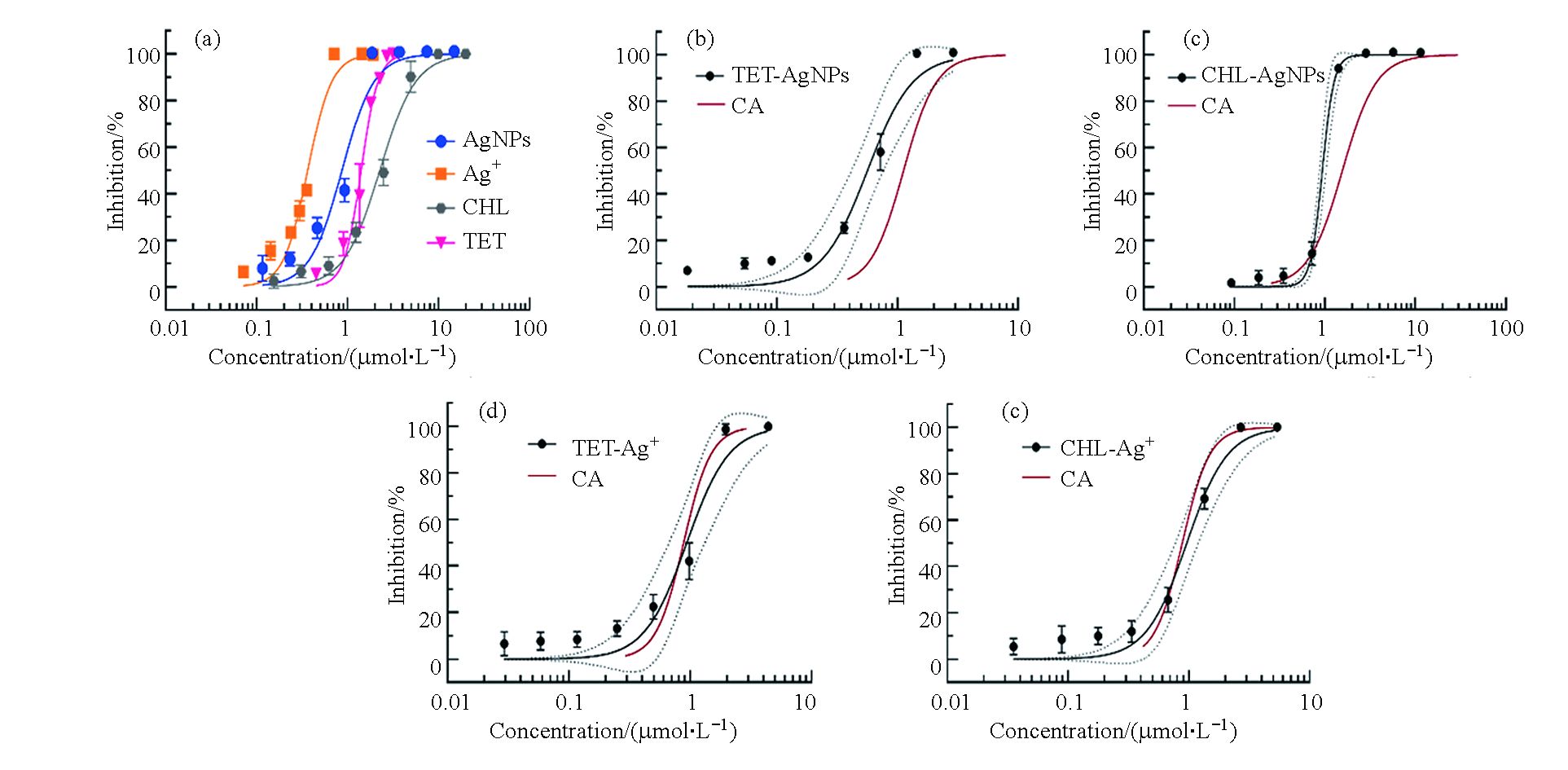
首先测定了抗生素和纳米银分别单独作用时对细菌生长的抑制作用。结果如图1(a)所示,抗生素和纳米银对细菌的生长抑制作用均呈现典型的S型浓度效应关系。纳米银对大肠杆菌的毒性显著大于四环素和氯霉素,其中纳米银的IC50值为0.61—1.18 μmol∙L−1,而四环素和氯霉素的IC50值分别为1.29—1.52 μmol∙L−1和1.92—2.64 μmol∙L−1 (表1)。此外,本研究还测定了银离子(AgNO3形式)对大肠杆菌的毒性,其IC50值为0.32—0.43 μmol∙L−1,显著大于纳米银和抗生素的抑菌效应。随后,测定了纳米银、银离子分别与氯霉素、四环素的联合抑菌效应。联合作用的浓度效应关系与单一化合物相似,均表现为典型的S型浓度效应曲线。二元混合物的IC50值在0.6—1.0 μmol∙L−1之间(表1),其中四环素和纳米银以及氯霉素和纳米银的二元联合对大肠杆菌的毒性效应相对较大,IC50值分别为0.41—0.74 μmol∙L−1和0.72—1.02 μmol∙L−1,而四环素和银离子及氯霉素和银离子的二元联合对大肠杆菌的毒性效应相对较弱,其IC50值分别为0.65—1.27 μmol∙L−1和0.77—1.18 μmol∙L−1。
随后比较了CA模型与实验获得的混合物剂量效应曲线的关系。如图1b和c所示,抗生素和纳米银联合作用的浓度效应曲线均在CA模型的左侧,二者的联合毒性呈现协同效应;与之不同的是,抗生素和银离子的CA模型曲线均在暴露组的95%置信区间内,表明二者联合毒性的相互作用类型为相加效应(图1d和e)。
-
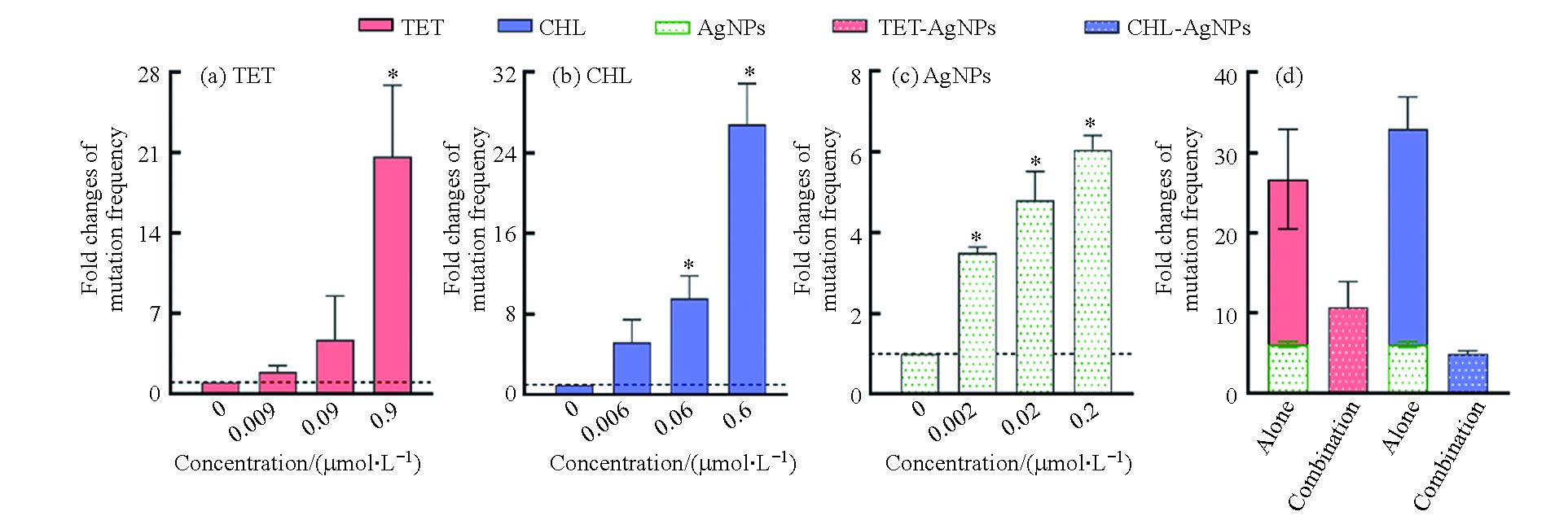
根据细菌毒性测试结果,选择亚抑制浓度的抗生素和纳米银,首先测定了抗生素和纳米银单独作用时,大肠杆菌对利福平的抗性突变频率。由图2(a—c)可知,抗生素和纳米银单独作用时对细菌的抗性突变频率随浓度的升高而增加。其中,四环素和氯霉素的浓度分别达到0.9 μmol∙L−1和0.6 μmol∙L−1时,细菌的抗性突变频率约是空白的20倍和27倍。纳米银对细菌的抗性突变诱导效应与抗生素相比较弱,其浓度达到0.2 μmol∙L−1时,细菌的抗性突变诱导频率约是空白组的6倍。
测定了四环素(0.9 μmol∙L−1)和氯霉素(0.6 μmol∙L−1)分别与纳米银(0.2 μmol∙L−1)联合作用时对细菌抗性突变频率的影响。结果如图2(d)所示,四环素和纳米银联合作用的抗性突变频率约是空白组自发突变频率的10倍;而氯霉素和纳米银联合作用下的突变频率约是空白组自发突变频率的5倍。与单独作用时相比,抗生素和纳米银的联合作用显著降低了细菌的抗性突变频率。
-
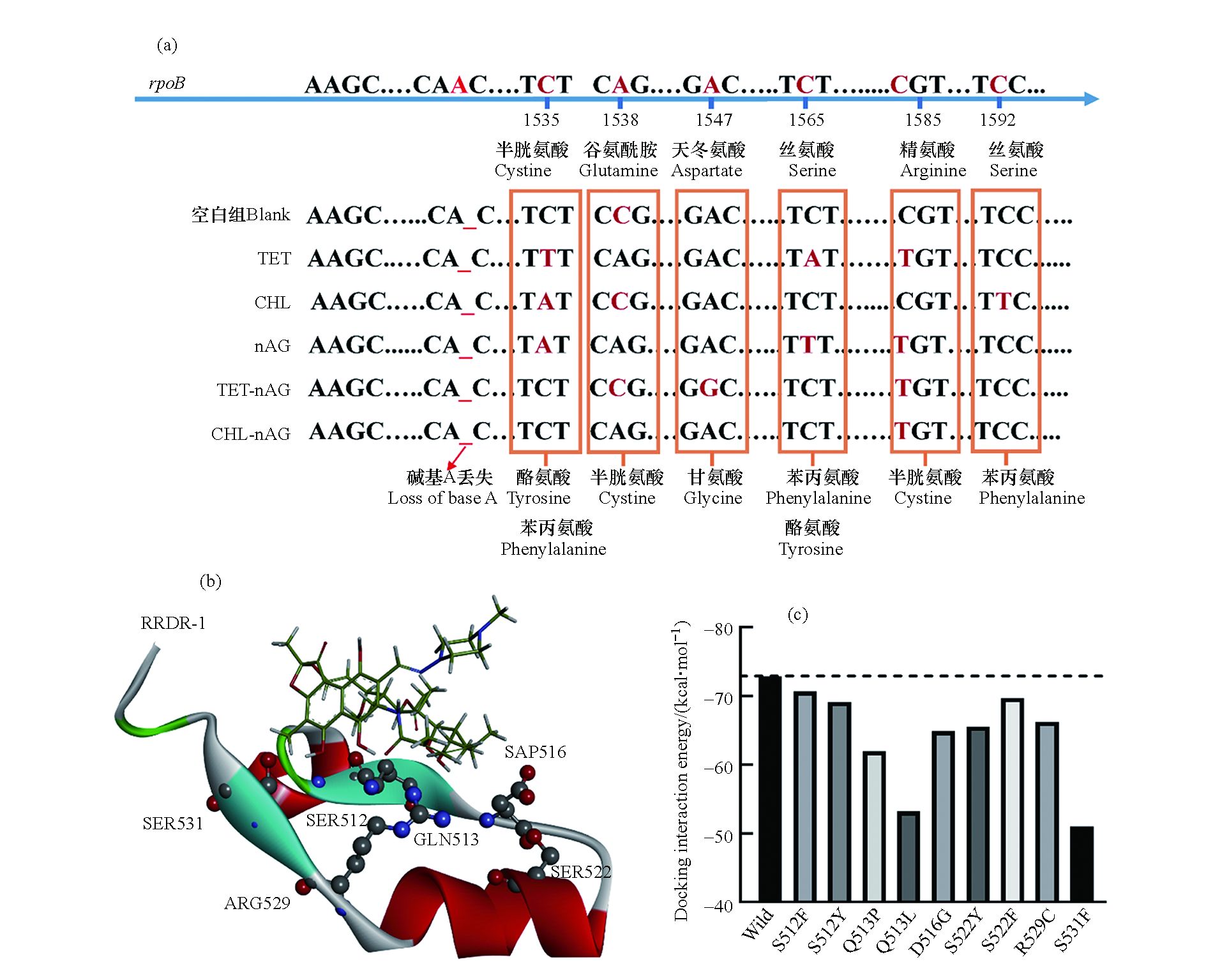
利福平是细菌抗性突变研究中最常用的抗性筛选药物之一,细菌对利福平的抗性大都由利福平靶蛋白RpoB(即RNA聚合酶β亚基)的突变所致[21]。本研究通过对抗性菌rpoB基因的测序,确定了rpoB基因上的突变位点。如图3(a)所示,在不同的暴露组中,细菌均发生了rpoB基因上的点突变,且突变位点均位于1535—1592区间内,其中1585和1538是突变发生的热点区域。这两个位点的突变分别导致RpoB蛋白上第529位的精氨酸突变为半胱氨酸以及513位的谷氨酰胺突变为脯氨酸。由图3(b)可知,本研究中检测到的突变氨基酸均位于RpoB蛋白的“利福平抗性决定区域”(Rifampicin resistance determining regions, RRDRs),即在空间上靠近利福平药物的结合位点。这表明这些氨基酸的变化很可能会影响RpoB蛋白的三级结构,改变利福平结合位点的构象,从而影响利福平与靶标蛋白结合,并最终导致细菌对利福平的耐药性。
根据大肠杆菌野生型RpoB蛋白构象(PDB ID:5UAC),结合实验确定的RpoB蛋白中突变的氨基酸位置,构建了突变型RpoB蛋白,并与利福平进行分子对接获得分子对接相互作用能,结果如图3(c)所示。未突变的RpoB蛋白(即5UAC)与利福平的相互作用能约为−73 kcal ∙mol−1,而突变后的RpoB蛋白与利福平结合的相互作用能在数值上均低于未突变的RpoB蛋白。这证实了RpoB蛋白突变后与利福平的亲和力降低,导致利福平的抑菌活性降低,即细菌对利福平的耐药性增加。
-
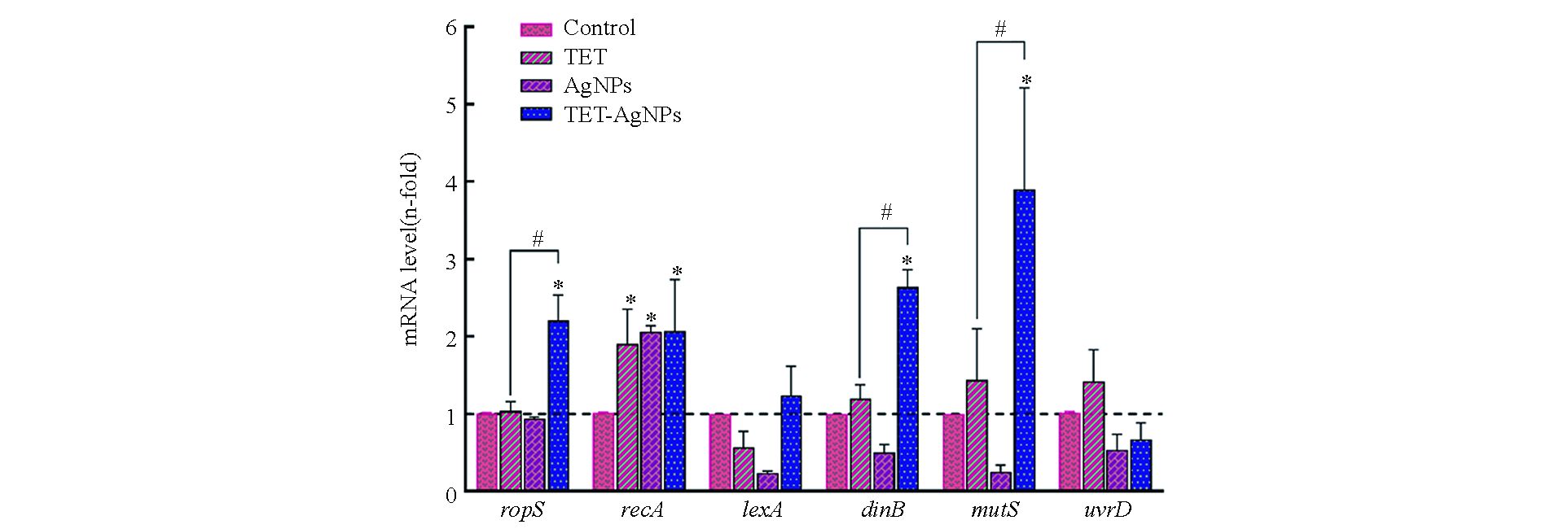
本研究测定了四环素和纳米银分别单独和联合作用下,细菌胞内与抗性突变相关的6种基因的表达,即dinB、lexA、mutS、recA、rpoS和uvrD。其中,rpoS是一种应激响应基因,同时调控细菌胞内多种基因的表达;lexA和recA是DNA损伤应激体系SOS中的主要调控基因;dinB调控参与DNA复制过程的 DNA聚合酶Ⅳ;mutS和uvrD是细菌DNA错配修复基因[22]。测试结果如图4所示,在四环素和纳米银的单独暴露组中,recA的基因表达显著增强,但是rpoS、dinB、mutS、lexA和uvrD的表达与空白组相比没有显著变化;而在四环素和纳米银的联合暴露组中,rpoS、recA、dinB、mutS的基因表达均显著增强,但是lexA和uvrD的表达相比较空白组没有显著变化。
-
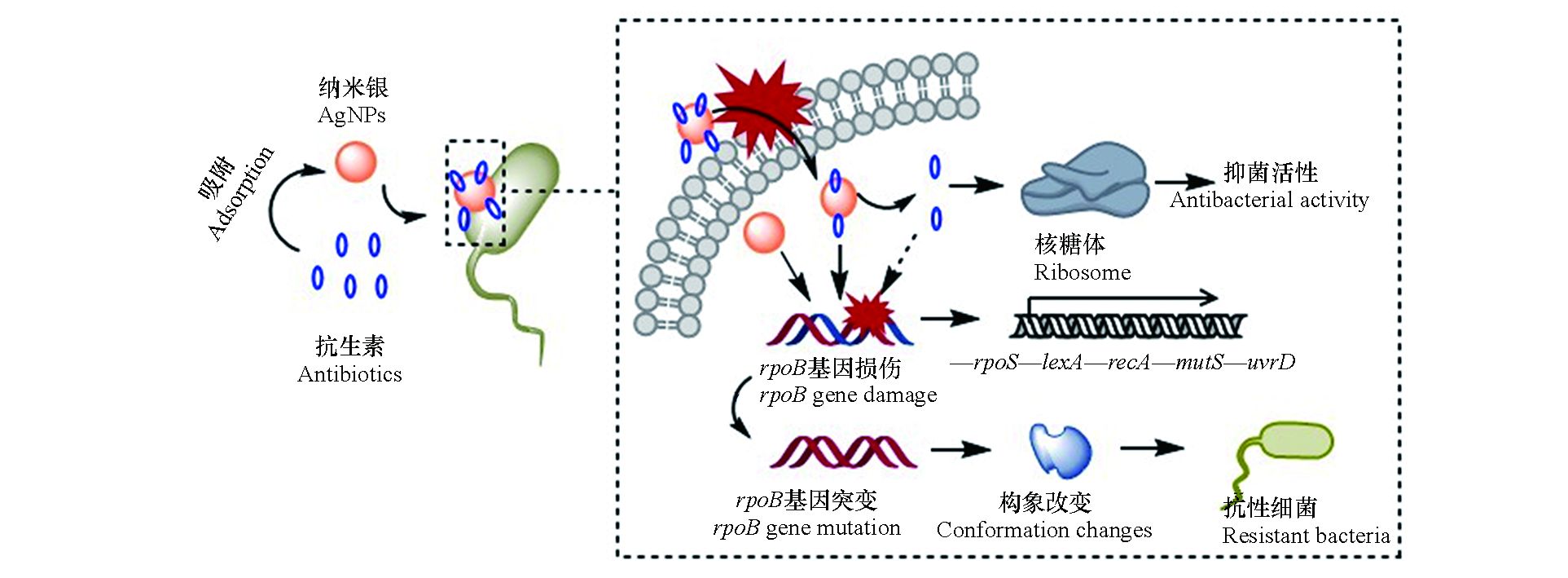
纳米银对细菌的毒性效应主要缘于其对细胞膜的物理损伤和在水介质中释放的银离子[4]。一方面,纳米银因其小尺寸效应而极易附着于细菌表面,引起细胞膜凹陷或形成穿孔,随后进入胞内对蛋白质和DNA等生物大分子造成损伤;另一方面,纳米银在水介质中能够释放出银离子,诱导产生活性氧物种(ROS),并与胞内含磷(P)、硫(S)和巯基(SH)的生物大分子结合,干扰细胞功能。本研究中,银离子的IC50值略低于纳米银,表现出更高的细菌毒性。这与之前的研究结果类似,例如Choi等[23]发现银离子对大肠杆菌的生长抑制作用显著大于纳米银。与纳米银和银离子不同,四环素和氯霉素能够特异性地作用于细菌的核糖体,分别与核糖体的30S和50S亚基结合,通过抑制蛋白质合成达到抑菌效果。本研究中,四环素和氯霉素的IC50值均大于纳米银和银离子,其细菌毒性相对较弱。在联合毒性测试中,纳米银与抗生素的联合作用类型表现出明显的协同效应,这可能源自于纳米银对抗生素分子的载体效应。如图5所示,在纳米银和抗生素的混合体系中,纳米银因具有较大的比表面积而容易吸附抗生素分子,随后携带抗生素分子附着于细菌表面,增加细菌与抗生素分子相互作用的机率;同时,纳米银对细胞膜的损伤增加了细胞膜的通透性,使抗生素分子更容易进入胞内,与靶蛋白结合,因此二者具有协同的抑菌效应。
-
本研究选用利福平用于测定抗生素和纳米银暴露对细菌抗性突变的影响,这是因为细菌对利福平的耐药性多由发生在rpoB基因上的点突变所致[21]。研究结果表明,在亚抑制浓度的抗生素和纳米银作用下,大肠杆菌对利福平的抗性突变频率显著增加。基因测序和分子对接结果证实了大肠杆菌对利福平的耐药性是由rpoB基因上的点突变引起的。抗生素和纳米银对细菌抗性突变诱导效应的机制如图5所示,首先,抗生素和纳米银进入细菌胞内后,通过诱导ROS或直接作用于DNA造成后者的损伤,导致细菌SOS系统中的recA基因表达显著上调。RecA蛋白是细菌DNA损伤的感应器,DNA损伤发生后,它能够和单链DNA(ssDNA)结合,促进LexA二聚体的裂解,从而解除LexA二聚体对DNA损伤修复系统的遏制作用,促进下游dinB、mutS和uvrD等基因的表达[24-25]。抗生素和纳米银造成的DNA损伤导致其在复制和修复过程中突变率增加,引起利福平靶蛋白RpoB结合位点的构象改变,最终导致细菌对利福平的耐药性增加。需要注意的是,在抗生素和纳米银联合作用于大肠杆菌时,其抗性突变频率相比于抗生素单独作用时有明显的下降趋势。结合基因表达的测试结果可以发现,在抗生素单独作用时,SOS系统的主要调控基因recA表达增强,而其他基因无明显变化;纳米银加入后,除recA基因依然呈现高表达外,rpoS、dinB和mutS的表达与抗生素单独作用时相比也显著增强。由此可以推断,在抗生素和纳米银的联合作用下,细菌胞内rpoS、dinB和mutS的表达增加,促进了细菌DNA损伤的修复进程,最终使细菌抗性突变维持在相对较低的水平。
-
细菌的抗生素耐药性已成为威胁人类健康的重大环境问题之一。细菌主要通过基因突变或从外源获取抗性基因(如质粒接合转移等)而产生抗生素耐药性。环境污染物能够通过促进细菌的抗性突变和抗性基因的水平传播而加剧环境中的耐药细菌和耐药基因污染。其中,抗生素作为特异性作用于细菌的一类污染物,已被大量研究证明在环境相关浓度下能够促进细菌的抗性突变。例如,环丙沙星、丝裂霉素、呋喃咀啶、氨苄西林、链霉素、甲氧苄氨嘧啶和新生霉素等抗生素能够显著增加大肠杆菌对利福平的抗性突变,其中环丙沙星和呋喃咀啶分别使抗性突变率增加了7倍和10倍[26]。本研究所关注的四环素和氯霉素是两种广谱性抗生素,常用于临床医药等领域,在环境介质中被频繁检出。根据实验结果,四环素和氯霉素均表现出显著的抗性突变诱导效应,在亚抑制浓度下对大肠杆菌耐利福平突变的促进率分别高达20倍和27倍。尤其值得关注的是,本实验中采用的抗生素浓度接近其环境检出浓度,表明环境中的抗生素残留具有促进细菌抗性突变的风险。纳米银作为一种高效的纳米抗菌剂,其抑菌活性显著高于四环素和抗生素,并且在亚抑制浓度下同样表现出显著的抗性突变诱导效应,对大肠杆菌的抗性突变产生高达5倍左右的促进率。这些研究结果表明,环境中广泛存在的抗生素和纳米银残留虽然多以亚抑制浓度形式存在,不足以直接对细菌造成毒性作用,但是能够通过促进细菌的抗性突变而增加细菌对抗生素的耐药性,最终对生态环境和人体健康构成更大的威胁。更重要的是,除抗生素和纳米银外,其他环境污染物,如杀生剂[27]、重金属[28]、纳米Al2O3和ZnO[16]、消毒副产物[29]以及纳米塑料[22]等均表现出对细菌抗性突变的促进作用。环境污染物对细菌的抗性突变诱导效应是否与日趋严峻的耐药细菌和耐药基因污染问题相关,是关乎人类健康的重大问题,需要引起足够的重视。此外,本研究还揭示了抗生素和纳米银的协同抑菌效应以及纳米银对抗生素诱导细菌抗性突变的遏制作用,研究结果能够为全面认识二者联合暴露的生态和健康风险提供参考。
-
本研究测定了两种抗生素(四环素和氯霉素)与纳米银对大肠杆菌的联合抑菌效应和抗性突变诱导效应,验证了抗生素和纳米银胁迫下大肠杆菌的抗性突变位点和抗性机制,得到以下主要结论:抗生素和纳米银的联合抑菌效应呈现协同作用,可能源自于纳米银对抗生素的载体效应;抗生素和纳米银在亚抑制浓度下能够引起大肠杆菌rpoB基因的利福平抗性决定区域(RRDR)发生点突变,导致利福平与靶标蛋白RpoB的结合能降低,从而促进大肠杆菌对利福平的耐药性;抗生素和纳米银联合作用对细菌的抗性突变诱导效应显著低于抗生素的单独作用,可能是由于联合作用下细菌胞内rpoS、dinB和mutS基因表达显著上调所致。需要注意的是,尽管本研究结合突变频率和基因表达的测试结果,对抗生素和纳米银联合抗性突变诱导效应的机制进行了初步地探讨,但是相关的分子调控机制仍需进一步研究。
抗生素和纳米银对大肠杆菌的联合毒性和抗性突变诱导效应
Joint toxicity and resistance mutation-inducing effect of antibiotics and AgNPs on Escherichia coli
-
摘要: 抗生素和纳米银因在各自领域的大量应用而不可避免地进入环境中,对生态环境尤其是微生物群体构成联合暴露的风险,但是二者对细菌生长和耐药性的联合作用,目前受到的关注较少。本研究测定了两种抗生素(四环素和氯霉素)以及纳米银(10—15 nm)对大肠杆菌的联合毒性及对细菌抗性突变的诱导作用,并对相关机制进行了初步探讨。结果表明,抗生素和纳米银对细菌的联合毒性呈现协同效应,但是二者联合作用下细菌对利福平的抗性突变频率显著降低。DNA测序和分子对接结果表明,编码利福平靶标蛋白的rpoB基因发生点突变,导致突变后的靶蛋白与利福平的结合能降低。同时,qPCR结果表明抗生素和纳米银联合作用时,细菌胞内rpoS、dinB和mutS等基因表达显著上调,可能是抗性突变频率在联合作用下降低的主要原因。本研究揭示了抗生素和纳米银联合作用对细菌抗性突变的影响,有利于全面认识二者联合暴露的环境和健康风险。Abstract: Antibiotics and nanosilver (AgNPs) are extensively applied in their respective fields and have high probability to co-exist in the environment, posing joint exposure risks on environmental organisms, especially on bacterial communities. However, the combined effects of antibiotics and AgNPs on bacterial growth and bacterial resistance mutations remain largely underexplored. In this study, the single and joint effects of two antibiotics (tetracycline and chloramphenicol) and AgNPs (10−15 nm) on bacterial growth and resistance mutations were investigated using Escherichia coli as a model bacterium, and the underlying mechanisms for the joint effects were explored. The results suggested that the antibiotics and AgNPs had synergistic effects on bacterial growth whilst antagonistic effects on the mutation frequency of E. coli against rifampicin, in comparison with their single actions. Furthermore, DNA sequencing and molecular docking results showed that point mutations occurred on rpoB gene that encodes the rifampicin target protein, leading to a decrease in the binding energy of the mutated protein with rifampicin. In addition, qPCR results showed that the gene expressions of rpoS, dinB and mutS in E. coli were significantly promoted upon exposure to antibiotics and AgNPs mixtures, which was likely a major cause for the significant decrease of the resistance mutations. This study is conducive to a comprehensive understanding of the environmental and health risks of combined exposure to antibiotics and AgNPs and provides a reference for assessment of their joint exposure risks.
-
Key words:
- nanosilver /
- antibiotics /
- Escherichia coli /
- combined toxicity /
- resistance mutation
-

-
图 3 (a)空白组和暴露组下大肠杆菌突变位点碱基变化及氨基酸变化;(b)大肠杆菌RpoB利福平抗性决定区域(RRDR—1)突变氨基酸位置与利福平结构图;(c)RpoB蛋白与利福平对接相互作用能
Figure 3. (a)Mutation sites and base changes of E. coli in the blank and exposed groups; (b)Amino acid location and rifampicin structure diagram of RpoB rifampicin resistance determination region(RRDR—I)mutant in E. coli; (c)Docking interaction energy between RpoB protein and rifampicin molecule.
表 1 化合物单独和联合作用对大肠杆菌的半数抑制浓度(IC50)
Table 1. The IC50 value of target substances against E. coli when acting alone or in combination
暴露组
Exposure group组分
ComponentsIC50(95% CI)/(μmol∙L−1) 单一暴露
Individual exposure四环素(TET) 1.41(1.29—1.52) 氯霉素(CHL) 2.26(1.92—2.64) 纳米银(AgNPs) 0.87 (0.61—1.18) 银离子(Ag+) 0.36 (0.32—0.43) 联合暴露
Combined exposureTET−AgNPs 0.56 (0.41—0.74) TET−Ag+ 0.94 (0.65—1.27) CHL−AgNPs 0.94 (0.72—1.02) CHL−Ag+ 0.96 (0.77—1.18) 注: IC50表示半抑制浓度,CI表示置信区间.
Note: IC50 is concentration for 50% of maximal inhibition, CI stands for confidence interval. -
[1] WANG H X, WANG N, WANG B, et al. Antibiotics in drinking water in Shanghai and their contribution to antibiotic exposure of school children [J]. Environmental Science & Technology, 2016, 50(5): 2692-2699. [2] DU B Y, WEN F, GUO X D, et al. Evaluation of an ELISA-based visualization microarray chip technique for the detection of veterinary antibiotics in milk [J]. Food Control, 2019, 106: 106713. doi: 10.1016/j.foodcont.2019.106713 [3] LYU J, YANG L S, ZHANG L, et al. Antibiotics in soil and water in China-a systematic review and source analysis [J]. Environmental Pollution, 2020, 266: 115147. doi: 10.1016/j.envpol.2020.115147 [4] RIZZELLO L, POMPA P P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines [J]. Chemical Society Reviews, 2014, 43(5): 1501-1518. doi: 10.1039/C3CS60218D [5] 吴宗山, 胡海洋, 任艺, 等. 纳米银的抗菌机理研究进展 [J]. 化工进展, 2015, 34(5): 1349-1356,1370. doi: 10.16085/j.issn.1000-6613.2015.05.028 WU Z S, HU H Y, REN Y, et al. Progress of antibacterial mechanisms of silver nanoparticles [J]. Chemical Industry and Engineering Progress, 2015, 34(5): 1349-1356,1370(in Chinese). doi: 10.16085/j.issn.1000-6613.2015.05.028
[6] 唐诗璟, 郑雄, 陈银广. 水体环境中纳米银的来源、迁移转化及毒性效应的研究进展 [J]. 化工进展, 2013, 32(11): 2727-2733. TANG S J, ZHENG X, CHEN Y G. Research progresses in aquatic environmental silver nanoparticles: Sources, transformation and toxicity [J]. Chemical Industry and Engineering Progress, 2013, 32(11): 2727-2733(in Chinese).
[7] MCGILLICUDDY E, MURRAY I, KAVANAGH S, et al. Silver nanoparticles in the environment: Sources, detection and ecotoxicology [J]. Science of the Total Environment, 2017, 575: 231-246. doi: 10.1016/j.scitotenv.2016.10.041 [8] HUANG W D, WANG J, WANG Z X, et al. Synergistic antimicrobial activity of silver nanoparticles combined with streptomycin sulfate against gram-negative and gram-positive bacteria [J]. Molecular Crystals and Liquid Crystals, 2021, 714(1): 80-88. doi: 10.1080/15421406.2020.1856610 [9] 王孟珍, 孙昊宇, 龙茜, 等. 纳米银复合材料与抗生素的联合抗菌性能及相关机制研究 [J]. 生态毒理学报, 2020, 15(2): 39-49. doi: 10.7524/AJE.1673-5897.20191204001 WANG M Z, SUN H Y, LONG X, et al. Combined antibacterial property and mechanism of nanosilver composites and antibiotics against bacteria [J]. Asian Journal of Ecotoxicology, 2020, 15(2): 39-49(in Chinese). doi: 10.7524/AJE.1673-5897.20191204001
[10] 卢文强, 孙昊宇, 王雅娟, 等. 抗生素的胁迫与抗生素抗性基因产生与传播关系的研究 [J]. 生态毒理学报, 2020, 15(4): 129-138. LU W Q, SUN H Y, WANG Y J, et al. The relationship of antibiotic stress with emergence and dissemination of antibiotic resistance genes [J]. Asian Journal of Ecotoxicology, 2020, 15(4): 129-138(in Chinese).
[11] 赖晓琳, 吴平霄, 阮博. 四环素对大肠杆菌抗生素抗性基因进化的影响 [J]. 环境科学学报, 2019, 39(8): 2475-2482. LAI X L, WU P X, RUAN B. Evolution of antibiotic resistance genes in E. coli by tetracycline [J]. Acta Scientiae Circumstantiae, 2019, 39(8): 2475-2482(in Chinese).
[12] 李旭飞, 巫晓丹, 孙昊宇, 等. 抗生素和纳米银对大肠杆菌耐药性的联合效应 [J]. 中国环境科学, 2020, 40(11): 5045-5054. doi: 10.3969/j.issn.1000-6923.2020.11.047 LI X F, WU X D, SUN H Y, et al. Joint effects of antibiotics and silver nanoparticles on resistance of Escherichia coli [J]. China Environmental Science, 2020, 40(11): 5045-5054(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.11.047
[13] MCGRATH M, van PITTIUS N C G, van HELDEN P D, et al. Mutation rate and the emergence of drug resistance in Mycobacterium tuberculosis [J]. Journal of Antimicrobial Chemotherapy, 2013, 69(2): 292-302. [14] MANNA M S, TAMER Y T, GASZEK I, et al. A trimethoprim derivative impedes antibiotic resistance evolution [J]. Nature Communications, 2021, 12: 2949. doi: 10.1038/s41467-021-23191-z [15] LU J, WANG Y, LI J, et al. Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera [J]. Environment International, 2018, 121: 1217-1226. doi: 10.1016/j.envint.2018.10.040 [16] ZHANG Y, GU A Z, XIE S S, et al. Nano-metal oxides induce antimicrobial resistance via radical-mediated mutagenesis [J]. Environment International, 2018, 121: 1162-1171. doi: 10.1016/j.envint.2018.10.030 [17] 刘树深, 刘玲, 陈浮. 浓度加和模型在化学混合物毒性评估中的应用 [J]. 化学学报, 2013, 71(10): 1335-1340. doi: 10.6023/A13040355 LIU S S, LIU L, CHEN F. Application of the concentration addition model in the assessment of chemical mixture toxicity [J]. Acta Chimica Sinica, 2013, 71(10): 1335-1340(in Chinese). doi: 10.6023/A13040355
[18] ESCHER B, BRAUN G, ZARFL C. Exploring the concepts of concentration addition and independent action using a linear low-effect mixture model [J]. Environmental Toxicology and Chemistry, 2020, 39(12): 2552-2559. doi: 10.1002/etc.4868 [19] KOTHARY V, SCHERL E J, BOSWORTH B, et al. Rifaximin resistance in Escherichia coli associated with inflammatory bowel disease correlates with prior rifaximin use, mutations in rpoB, and activity of Phe-Arg-β-naphthylamide-inhibitable efflux pumps [J]. Antimicrobial Agents and Chemotherapy, 2013, 57(2): 811-817. doi: 10.1128/AAC.02163-12 [20] NING Q, WANG D L, YOU J. Joint effects of antibiotics and quorum sensing inhibitors on resistance development in bacteria [J]. Environmental Science. Processes & Impacts, 2021, 23(7): 995-1005. [21] NING Q, WANG D L, CHENG F, et al. Predicting rifampicin resistance mutations in bacterial RNA polymerase subunit beta based on majority consensus [J]. BMC Bioinformatics, 2021, 22(1): 210. doi: 10.1186/s12859-021-04137-0 [22] NING Q, WANG D L, AN J H, et al. Combined effects of nanosized polystyrene and erythromycin on bacterial growth and resistance mutations in Escherichia coli [J]. Journal of Hazardous Materials, 2022, 422: 126858. doi: 10.1016/j.jhazmat.2021.126858 [23] CHOI O, DENG K K, KIM N J, et al. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth [J]. Water Research, 2008, 42(12): 3066-3074. doi: 10.1016/j.watres.2008.02.021 [24] 张岩, 盛多红. 细菌DNA损伤诱导反应(SOS反应)在控制细菌耐药性中的作用 [J]. 微生物前沿, 2013(2): 83-89. ZHANG Y, SHENG D H. Role of DNA damage response (SOS response) on the control of bacterial resistance [J]. Advance in Microbiol, 2013(2): 83-89(in Chinese).
[25] GALHARDO R S, HASTINGS P J, ROSENBERG S M. Mutation as a stress response and the regulation of evolvability [J]. Critical Reviews in Biochemistry and Molecular Biology, 2007, 42(5): 399-435. doi: 10.1080/10409230701648502 [26] MO C Y, MANNING S A, ROGGIANI M, et al. Systematically altering bacterial SOS activity under stress reveals therapeutic strategies for potentiating antibiotics [J]. mSphere, 2016, 1(4): e00163-e00116. [27] LU J, JIN M, NGUYEN S H, et al. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation [J]. Environment International, 2018, 118: 257-265. doi: 10.1016/j.envint.2018.06.004 [28] LI X Y, GU A Z, ZHANG Y, et al. Sub-lethal concentrations of heavy metals induce antibiotic resistance via mutagenesis [J]. Journal of Hazardous Materials, 2019, 369: 9-16. doi: 10.1016/j.jhazmat.2019.02.006 [29] LI D, GU A Z. Antimicrobial resistance: A new threat from disinfection byproducts and disinfection of drinking water? [J]. Current Opinion in Environmental Science & Health, 2019, 7: 83-91. -



 下载:
下载: