-
2019年,我国城市生活垃圾的清运量已达2.4亿吨[1];在垃圾分类收运、处理处置与资源化过程中,均会产生特征性的二次污染物——渗滤液。渗滤液是指固体废物在填埋、堆存或受挤压条件下,伴随生物化学等作用下产生的一种液相污染物[2]。我国的渗滤液产率通常为每吨垃圾产生0.2—0.33 t渗滤液[3]。渗滤液含有大量的可生物降解及难生物降解有机物质(例如,腐殖质、微量有机污染物)、氨氮、重金属及无机盐。
渗滤液中的溶解性有机物(dissolved organic matters, DOM)是其主要的污染物组分。DOM成分复杂,可能包含了数万种有机物,且存在着因相互作用形成的“超分子”结构。因其复杂性,DOM显著地影响着各类渗滤液处理工艺的运行与处理效果。据此,本文总结了渗滤液DOM表征方法的技术特点,及其在渗滤液处理工艺中的应用,以期为DOM性质解构、污染与环境安全控制发展提供新思路。
-
DOM解析是仪器分析领域的永恒话题,且不断发展中。渗滤液DOM性质表征的主要思路是:基于其物理化学性质的分离预处理后,采用总有机碳量[4]、光谱[5]、质谱[6]等表征方法获取其分子结构信息;定量定性分析以DOM形式存在的特定化合物,可主要分为靶向分析(target analysis,有标准品)[7-10]、怀疑分析(suspect analysis,无标准品)[9-13]和非靶向分析(non-target analysis,未知污染物分析)[14-18]。
-
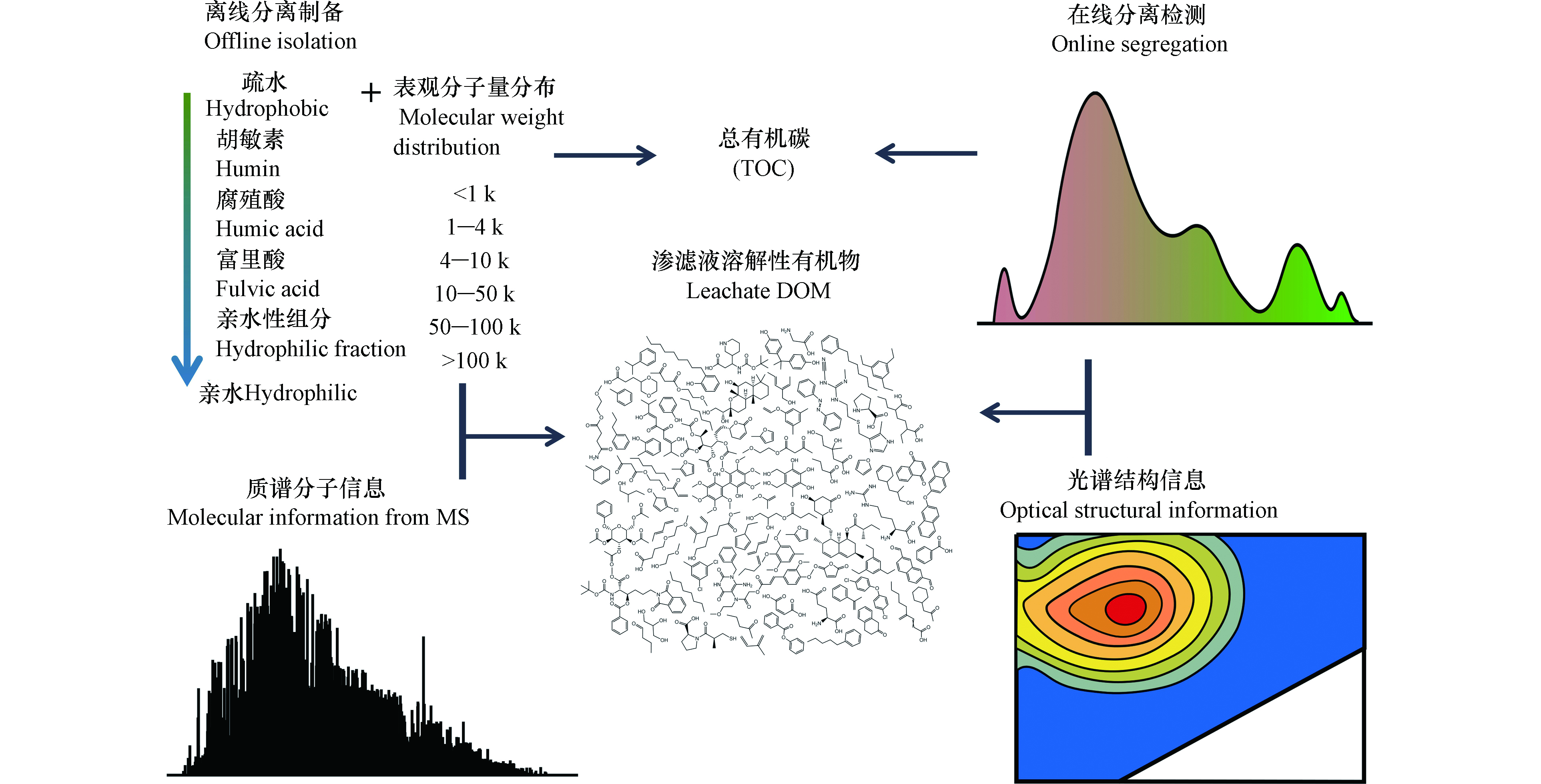
借鉴地球科学中对土壤腐殖质分离的定义[19],参见图1。渗滤液DOM可以分为:酸性条件(pH<2)下可沉淀的腐殖酸(humic acid, HA);可由XAD-8树脂提取的富里酸(fulvic acid,FA);以其他剩余的亲水性组分(hydrophilic acid,HyI)。基于此分离方法,识别出混凝沉淀工艺的主要去除对象是渗滤液中的疏水性腐殖酸[20]。采用超滤膜分离或凝胶色谱的方法,可获悉渗滤液DOM中1‒100 k Da的表观分子量分布[4-5, 21]。此方法揭示了渗滤液中难生物降解DOM主要是大分子的腐殖酸,并且可通过电解的方式将部分大分子HA转化为可生物降解的小分子有机物[4]。上述分离方法还可以与紫外(ultra violet,UV)或有机碳(organic carbon detector,OCD)等检测器在线连接[22],获取DOM的表观分子量或极性组分分布。特别地,还可以采用有机氮检测器(organic nitrogen detector,OND)获取渗滤液DOM中的含氮有机物分布。此外,这些分离方法也可进行离线操作,与后续的其他光谱(例如,三维荧光光谱)[5]与质谱分析仪器耦合分析[23]。
许多特征性的DOM具有在紫外至可见光波长范围内的特征性吸收光谱、发射光谱和荧光光谱。例如,芳香族化合物含量与单位浓度有机质在254 nm下的紫外光吸收度斜率(specific ultraviolet absorbance at 254 nm,SUVA254)具有很好的指示相关性[24]。针对DOM具有的光学特性,同时测定其激发与发射光谱,可获得其三维荧光光谱(excitation-emission matrix, EEM)[25]。不同发射光谱和激发光谱的区域可分为:酪蛋白区、可生物降解DOM区、类腐殖酸区、类富里酸区和类微生物代谢产物区[26];从而依据不同区域荧光强度来评判DOM的分子结构特征。例如,凝胶色谱离线组合EEM的方法揭示了不同龄期渗滤液DOM的差异性,并识别出了其中的难降解组分[5]。Wu等进一步结合荧光淬灭现象,解析了渗滤液中重金属与腐殖质交互关系,揭示了重金属在渗滤液DOM中的分布规律[27]。光谱方法具有非破坏性与快速检测的优点,同时,还可以一定程度地反映DOM的结构信息,例如,光学吸光度斜率与DOM的分子量分布具有相关性[28]。但是,并非所有DOM都具有光学性质[29],即光谱学分析方法有时会忽略此类DOM组分。
(超)高分辨率质谱仪被认为是21世纪最先进的高通量DOM分析方法[30],其数据结果被称为“分子信息”。(超)高分辨率质谱,如傅里叶变换离子回旋共振质谱仪(Electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry, ESI FT-ICR MS)的分辨率高达500000—3000000[31],从而可区分诸如[CHO]N2O2、[CHO]C5和[CHO]C2H4S间的微小分子量差异[32]。亦或是,轨道阱质谱(electrostatic axially harmonic orbital trapping mass spectrometry, Orbitrap MS)可与液相色谱在线连接[33],依据检测到的有机物分子精确分子量,推算其分子式[32, 34-35]。该方法识别出渗滤液DOM的化合物种类可高达数万种[6]。相比于凝胶色谱分离法获取的分子量分布谱,(超)高分辨率质谱获取的渗滤液DOM分子分布是100—1000 Da。这其中存在矛盾之处,即是渗滤液DOM中的腐殖质分子是否是实际存在的大分子(>10 kDa)化合物。例如,根据“超分子”假说[36],腐殖质是由小分子化合物经氢键和范德华力形成的,该结构不能被凝胶色谱的分离过程破坏;而质谱分析的离子化过程可消除其中的分子间作用力,从而获取其本质的分子量。分子动力学模拟计算结果也印证了该观点[37]。但是,(超)高分辨率质谱是半定量的分析方法[32];软电离过程受仪器操作条件影响[38];存在实验室间和实验室内不同时间分析结果重现性差的问题[39],尚待进一步解决。
-
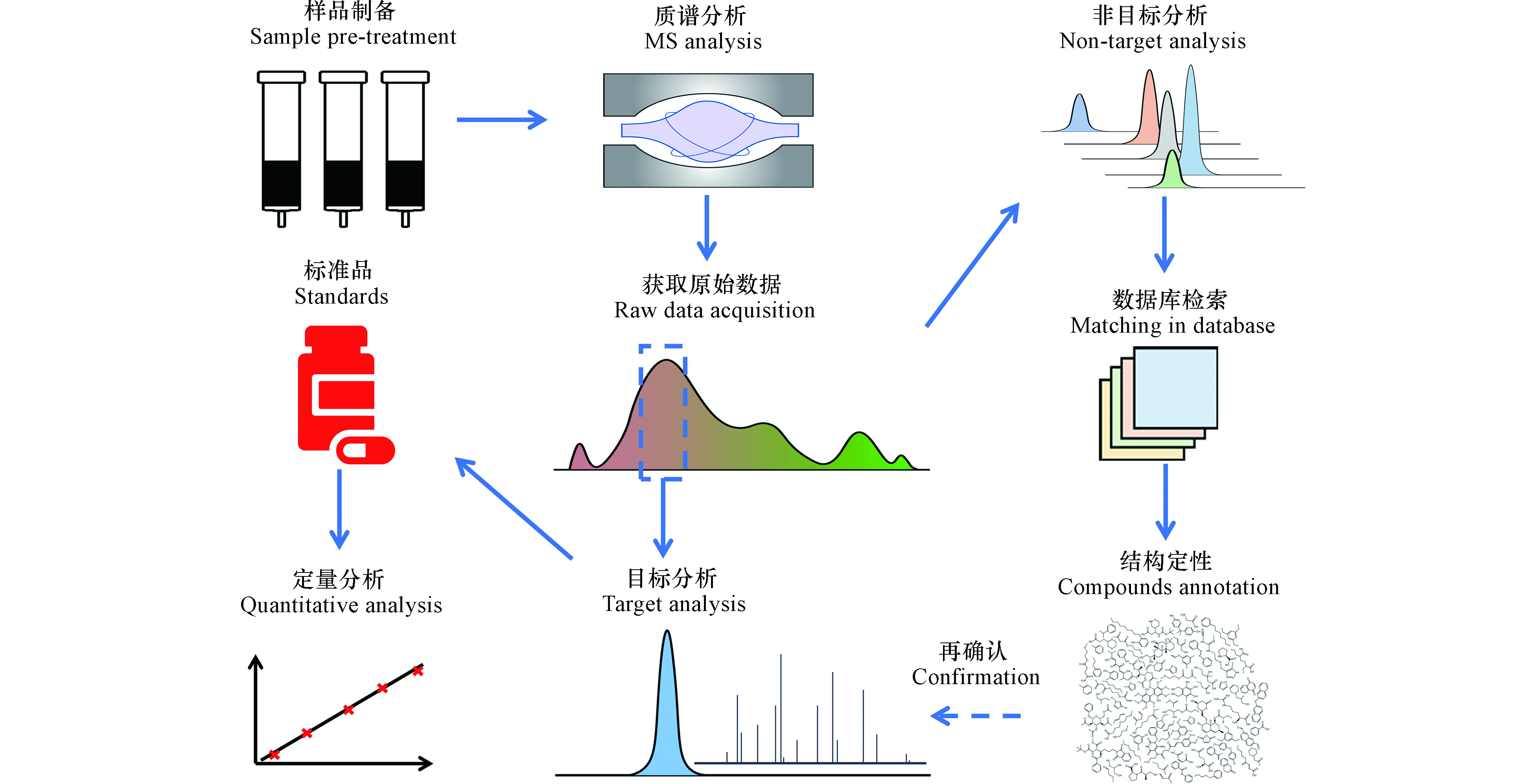
塑料制品、药物、个人护理品、电子产品等工业化产品的大量使用、淘汰与更新,极大地提高了生活垃圾组成的复杂程度及其中微量污染物的污染风险[7, 40-41]。这些微量有机污染物会以DOM的形式进入渗滤液,并在后续的处理系统内产生新的衍生物,可能成为固体废物污染控制过程中未知的环境健康风险。对于DOM中特定化合物(例如,微量有机污染物)的定性分析流程可参见图2。
靶向分析是面向渗滤液中已知的微量有机污染物,对照标准物质进行定性定量分析。基于该思路,识别了渗滤液中存在主要源于塑化剂的高浓度内分泌干扰素,例如,邻苯二甲酸酯类化合物和双酚A[7, 21];以及石油化工过程产生的苯系物,如甲苯、氯酚、氯苯等[42];还存在颗粒态和微纳米级的微塑料[41],以及生物大分子类的可移动基因元件和抗性基因[40, 43]。但是,面向渗滤液中存在的数万种化合物,怀疑分析或非靶向分析是识别其中以DOM形态存在的微量有机污染物的关键方法。
靶向分析通常要求尽可能纯化分离样品中的目标物质[7, 44]。但是,怀疑分析或非靶向分析则需要全量提取DOM;主要地,有固相萃取[45]和反渗透-电渗析[46]方法。其中,固相萃取吸附剂历经第一代XAD树脂和改进的DAX树脂吸附剂[47]、第二代硅基吸附剂[48]和第三代聚合物类吸附剂[45]。其中,聚合物类吸附剂,如改性的苯乙烯二乙烯基苯聚合物(PPL),因其对DOM回收率可达50%—80%,应用最为广泛[49]。相比国际腐殖质协会标准化(International Humic Substances Society, IHSS)的反渗透-电渗析方法,固相萃取操作简便、便于现场操作[50]。但是,提取到的溶解性有机物可能受吸附剂的选择性[51]和操作条件干扰[49],同样存在重现性问题[39, 52]。
耦合高通量分析手段,利用大数据分析方法,可定性识别未知的微量有机污染物。事实上,怀疑分析和非靶向分析之间的边界较模糊[39]。怀疑分析通常可根据某种标准(如,生物毒性、辛醇-水分配系数等)拟定一系列优先可疑组分,进行针对性分析,削减数据分析工作量[12]。非靶向分析则面向未知化合物,其定性主要通过气相色谱-质谱和液相色谱-质谱进行后验式的全球质谱图谱数据库检索确定。根据其分子离子碎裂机制不同,两者的解谱过程各不相同。对于液相色谱-高分辨串联质谱(LC-HRMS/MS)分析,主要分为数据依赖型(Data-dependent acquisition, DDA)[53]和数据独立型(Data-independent acquisition, DIA)[14-15, 54]两种数据获取模式。其中的依赖型数据获取模式应用广泛。但是,受限于质谱扫描速度,并非所有母离子均可检测到二级碎片[14-15];而独立型数据获取模式的困难在于二级质谱图污染,解卷积困难[14]。相较而言,气相色谱-高分辨串联质谱(GC-HRMS)[55]或全二维气相色谱-高分辨质谱(GC×GC-HRMS)[16, 56]分析,通过电子电离(EI)产生的谱图可与美国国家标准和技术研究院(National Institute of Standards and Technology, NIST)标准谱库进行对比检索,定性效果好[9, 55];但是,对于难气化组分则需采用LC-HRMS/MS进行分析。基于该思路,研究人员在调研了中国东部8个填埋场后,在跨度20年的填埋过程中,基于二级质谱图的碎片筛查方法,识别出N-丁基苯磺酰胺在渗滤液中的释放与填埋龄具有显著单调相关性[6]。上述结果表明,基于高分辨质谱的碎片分析是识别渗滤液中未知微量有机污染物的重要方法。
-
上述DOM的表征手段是辅助探究渗滤液DOM在各类处理工艺过程中转化机制的关键。但是,渗滤液水质复杂、处理难,故其处理流程通常是夹带多股回流的多级串联处理流程。其处理工艺流程的复杂性,使得探究渗滤液DOM在其中的转化规律极具挑战性。
-
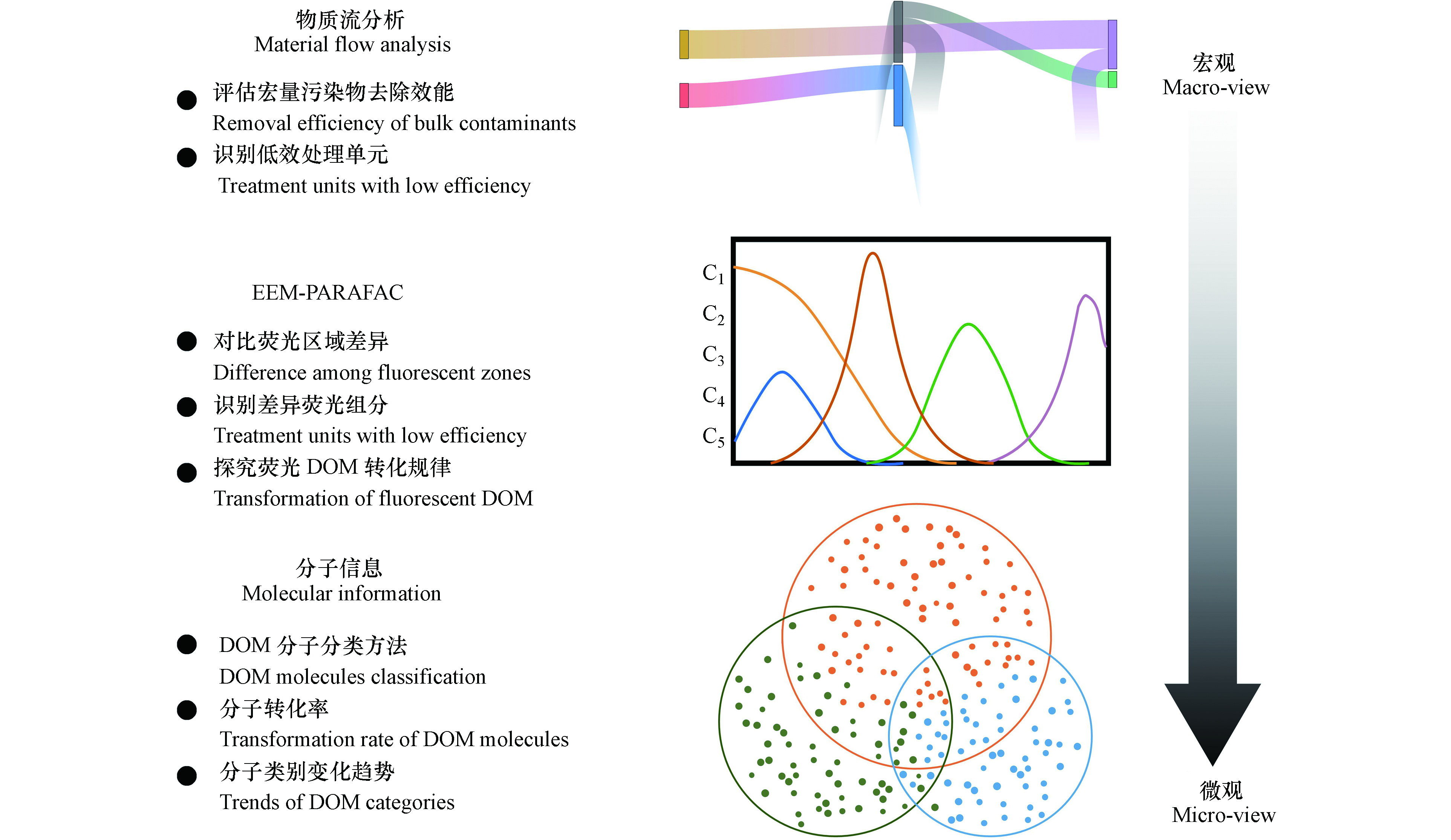
厘清渗滤液中主要污染物在处理工艺流程中的通量与去除贡献率是梳理渗滤液DOM去除机制的先决条件。不同于生活污水的处理流程,渗滤液处理流程中的污染物浓度(例如,化学需氧量(chemical oxygen demand, COD)和总氮(total nitrogen, TN))的降低并不意味着污染物的去除。这其中可能是由于硝化液、超滤污泥、膜处理浓液或部分清液回流产生的“稀释”作用[57-58]。因此,特定断面的质量流量是体现渗滤液某一工艺单元处理能力的本质参数(参见图3)。Shao等采用物质流分析方法评估了1座中后期渗滤液处理工艺,发现两级硝化-反硝化单元可去除75%的TN;COD在生物处理过程无显著性变化,而超滤分离单元可贡献92%的COD去除效能[57]。显然,该方法对于评估好氧生物处理过程中,中后期渗滤液或经厌氧处理后的新鲜渗滤液的去除效率,表现得不敏感[57-58]。
由此,基于DOM光谱特性的分析方法可应用于上述渗滤液DOM发生部分转化的情形。半定量的EEM图谱可直接对比不同激发与发射波长区域内的荧光强度(代表不同类别的DOM组分),验证反应前后某一类别DOM组分是否被去除。该研究思路探明了芬顿(Fenton)法与紫外双氧水氧化法优先去除渗滤液中蛋白类与酪氨酸类DOM[59]。但是,不同激发与发射波长区的DOM可能存在峰的叠加。平行因子分析方法(Parallel factor, PARAFAC)可识别一系列DOM样品中的荧光差异组分,即具有解卷积的数据分析功效[60]。尽管COD指标已经“钝化”,但基于此方法,在中后期渗滤液处理工艺流程中,揭示了生物处理单元可使得蛋白类DOM发生部分转化;从而,使得生物处理出水中主要是芳香结构含量高的微生物衍生DOM[57]。同样,基于解卷积的荧光差异组分,Yu等初步提出了,在渗滤液处理过程中,DOM“超分子”结构解体过程[61]。
但是,并非所有的DOM均存在光学敏感性[29]。基于高分辨质谱的分子信息方法则可规避该问题,且可从分子层面解析渗滤液DOM的转化规律。通过对比处理前后渗滤液DOM分子的存在性,可以区分原生与衍生的DOM。He等采用该分类方法,评估了Fenton处理、电解技术、膜处理、活性炭吸附等多种深度处理技术对生物稳定渗滤液DOM的去除效果。结果表明,Fenton对各类DOM均有80%以上的去除效果,探明了不同处理单元对生物稳定渗滤液DOM去除的选择性[62]。相比于单一处理单元,带回流的两级处理单元更为复杂。例如,新鲜渗滤液在产甲烷同时反硝化工艺中,DOM可进一步被分为7类。进一步结合分子信息学高通量数据集与物质流分析方法,提出了微生物衍生DOM的产生与去除率关系满足双曲对偶方程,在理论层面确定了好氧衍生DOM通过厌氧单元去除的可行性与热力学极限[58]。对于更为复杂的实际工程规模的“膜生物反应器+纳滤”中后期渗滤液处理设施,宜分单元(或模块)进行分析比对各处理单元的贡献和作用[63]。尽管COD指标数值在生物处理段无显著变化,但事实上83.2%—92.2%的DOM分子发生了部分转化。其中,木质素类、脂质类和蛋白类化合物是中后期渗滤液DOM的主要组分。微生物易于转化饱和性相对较高的木质素类与多羰基类化合物,致使处理出水中主要是亲水性的高芳香性DOM。上述研究结果表明,尽管渗滤液处理工艺流程复杂,分子信息方法可适用于解析不同规模及工艺流程的渗滤液处理过程中的DOM转化规律。
-
在渗滤液处理过程中,以DOM形式存在的微量有机污染物会发生在相际迁移[7, 64]、去除转化[65-67]。研究微量有机污染物在该过程中的赋存规律与转化机理是其污染控制过程的关键(见图4)。例如,渗滤液中的邻苯二甲酸酯类化合物,因其脂溶性特点,主要以HA形式存在[7],并多赋存于3—14 kDa“超分子”形态的渗滤液DOM中[21]。因而,邻苯二甲酸酯类化合物可与疏水性的HA同步被混凝沉淀工艺去除;但是,该过程仅发生了邻苯二甲酸酯类化合物在固液两相的分馏分配过程[67]。相较而言,非选择性的高级氧化技术(如Fenton处理)可去除渗滤液中的邻苯二甲酸酯类化合物和双酚A。但因渗滤液中其他DOM的掩蔽效应,邻苯二甲酸酯类化合物和双酚A的去除率受其初始浓度限制[66]。但是,邻苯二甲酸酯类化合物和双酚A是完全去除还是部分转化,其去除行为的机理与产物仍不明晰。
特别地,生物处理是渗滤液的主流处理工艺[2]。近期的研究结果表明,新鲜渗滤液经生物处理后,卤代微量有机污染物是主要的高丰度原生不可降解DOM[58]。同时,采用高分辨质谱碎片结合全球数据库检索的方法,确定了生物处理出水中的部分高丰度化合物为含磷阻燃剂、涂料及粘合试剂,其浓度分布为5—200 μg·L−1。值得注意的是,这些微量有机污染物可能在生物处理过程中发生转化。例如,在生活污水厂的生物处理过程中,含Cl的糖皮质激素类药物可能发生部分氧化、脱水、羟基化等过程,部分官能团转化后形成新的Cl代微量有机污染物,转化产物的环境健康风险不明[68]。在自然环境中,海洋微生物可能产生天然的卤代化合物[69],非卤代微量有机污染物可能在生物处理过程氯化,卤代微量有机污染物也可能在这一过程中发生部分还原[70]。这些经过处理后残余的原生难降解和生物处理衍生的卤代微量有机污染物,甚至可能成为潜在的持久性有机污染物的“源”。这些经处理后的微量有机污染物衍生产物,因其未知,解析与研究方法具有挑战性,而基于高分辨质谱的非靶向分析技术具有解决该问题的潜力。
-
DOM是渗滤液的关键污染特征与影响其处理过程的主要因素。近二十年来,因渗滤液DOM的复杂性,其解析方法经由基于亲疏水性分离、分子量分级的主体DOM量化指标,发展为紫外-可见光谱、三维荧光光谱的光学解析手段,并在近年来深入到了分子级别。这些一系列的渗滤液DOM解析手段,可适用于揭示各类复杂渗滤液处理工艺流程中的DOM转化规律。但是,仍然存在一些需要克服的关键科学和技术问题。
在未来的研究中以下方面值得重点考虑。关于渗滤液DOM解析方法,基于光谱与高分辨质谱的渗滤液DOM的解析结果是半定量的,故而实验室间结果难以比较,同一实验室内不同时间的分析结果也可能缺乏重现性。因此,需要建立标准化的渗滤液DOM分析流程以及仪器信号调谐依据。进一步地,尽管高分辨质谱可以获取渗滤液DOM的分子式谱,但是,分子间的作用力及DOM团聚状态尚无实验手段可以评估。关于微量有机污染物衍生产物,渗滤液中各类以DOM形式存在的微量有机污染物均可能在其处理过程中发生迁移转化。目前,大多研究可计算物流通量,从而探明其迁移赋存规律。部分研究也可揭示特定微量有机污染物在简单基质(如模拟废水)中的衍生产物与转化途径。但是,渗滤液中共存着多种微量有机污染物,它们发生转化时是否存在交互作用仍未可知,有待进一步探索。
渗滤液溶解性有机物解析及其转化研究前瞻
Property and transformation of DOM in solid waste leachate
-
摘要:
渗滤液中的溶解性有机物(dissolved organic matters, DOM)是其污染控制的关键对象,并影响着渗滤液处理工艺的效能。渗滤液DOM的解析方法经历了20多年的发展历程,并用于认识渗滤液各类复杂处理工艺流程中的DOM转化规律。本综述分别从主体DOM解析与微量有机污染物识别两方面,总结了渗滤液DOM解构方法学的发展历程;进一步地,评述了研究渗滤液各类处理流程中DOM转化规律的研究进展。据此,本文展望了渗滤液DOM解析方法的未来发展方向,并提出了现有渗滤液处理过程中微量有机污染物转化研究存在的问题。
Abstract:Dissolved organic matters (DOM) are the key fraction of waste leachate to be treated, and affect various treatment technologies inversely. The methodologies of leachate DOM characterization have made a great progress to disclose the DOM transformation during leachate treatment processes in the two decades. This review summarizes development of DOM characterization from the perspectives of bulk DOM and micropollutants. Additionally, the application of these characterization methods is critically reviewed. It mentions that future of leachate DOM characterization and micropollutants transformation during treatment processes.
-
Key words:
- solid waste /
- characterization /
- material flow analysis /
- high resolution mass spectrum
-

-
-
[1] 国家统计局. 中国统计年鉴[M]. 北京: 中国统计出版社, 2020. National Bureau of Statistics of China. China statistical yearbook[M]. China Statistics Press, 2020(in Chinese).
[2] RENOU S, GIVAUDAN J G, POULAIN S, et al. Landfill leachate treatment: Review and opportunity [J]. Journal of Hazardous Materials, 2008, 150(3): 468-493. doi: 10.1016/j.jhazmat.2007.09.077 [3] YANG N, DAMGAARD A, KJELDSEN P, et al. Quantification of regional leachate variance from municipal solid waste landfills in China [J]. Waste Management, 2015, 46: 362-372. doi: 10.1016/j.wasman.2015.09.016 [4] HE P J, XUE J F, SHAO L M, et al. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill [J]. Water Research, 2006, 40(7): 1465-1473. doi: 10.1016/j.watres.2006.01.048 [5] LU F, CHANG C H, LEE D J, et al. Dissolved organic matter with multi-peak fluorophores in landfill leachate [J]. Chemosphere, 2009, 74(4): 575-582. doi: 10.1016/j.chemosphere.2008.09.060 [6] QIU J J, LÜ F, ZHANG H, et al. UPLC Orbitrap MS/MS-based fingerprints of dissolved organic matter in waste leachate driven by waste age [J]. Journal of Hazardous Materials, 2020, 383: 121205. doi: 10.1016/j.jhazmat.2019.121205 [7] ZHENG Z, HE P J, SHAO L M, et al. Phthalic acid esters in dissolved fractions of landfill leachates [J]. Water Research, 2007, 41(20): 4696-4702. doi: 10.1016/j.watres.2007.06.040 [8] LANG J R, ALLRED B M, FIELD J A, et al. National estimate of per- and polyfluoroalkyl substance (PFAS) Release to U. S. municipal landfill leachate [J]. Environmental Science & Technology, 2017, 51(4): 2197-205. [9] MIYAKE Y, TOKUMURA M, WANG Q, et al. Identification of novel phosphorus-based flame retardants in curtains purchased in Japan using orbitrap mass spectrometry [J]. Environmental Science & Technology Letters, 2018, 5(7): 448-455. [10] LIU A F, SHI J B, QU G B, et al. Identification of emerging brominated chemicals as the transformation products of tetrabromobisphenol A (TBBPA) derivatives in soil [J]. Environmental Science & Technology, 2017, 51(10): 5434-5444. [11] ALYGIZAKIS N, SAMANIPOUR S, HOLLENDER J, et al. Exploring the potential of a global emerging contaminant early warning network through the use of retrospective suspect screening with high-resolution mass spectrometry [J]. Environmental Science & Technology, 2018, 52(9): 5135-5144. [12] GAGO-FERRERO P, KRETTEK A, FISCHER S, et al. Suspect screening and regulatory databases: A powerful combination to identify emerging micropollutants [J]. Environmental Science & Technology, 2018, 52(12): 6881-6894. [13] RAPP-WRIGHT H, MCENEFF G, MURPHY B, et al. Suspect screening and quantification of trace organic explosives in wastewater using solid phase extraction and liquid chromatography-high resolution accurate mass spectrometry [J]. Journal of Hazardous Materials, 2017, 329: 11-21. doi: 10.1016/j.jhazmat.2017.01.008 [14] TSUGAWA H, CAJKA T, KIND T, et al. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis [J]. Nature Methods, 2015, 12(6): 523-526. doi: 10.1038/nmeth.3393 [15] SAMANIPOUR S, REID M J, BÆK K, et al. Combining a deconvolution and a universal library search algorithm for the nontarget analysis of data-independent acquisition mode liquid Chromatography−High-resolution mass spectrometry results [J]. Environmental Science & Technology, 2018, 52(8): 4694-4701. [16] VEENAAS C, BIGNERT A, LILJELIND P, et al. Nontarget screening and time-trend analysis of sewage sludge contaminants via two-dimensional gas chromatography-high resolution mass spectrometry [J]. Environmental Science & Technology, 2018, 52(14): 7813-7822. [17] PETER K T, TIAN Z Y, WU C, et al. Using high-resolution mass spectrometry to identify organic contaminants linked to urban stormwater mortality syndrome in Coho Salmon [J]. Environmental Science & Technology, 2018, 52(18): 10317-10327. [18] RATHGEB A, CAUSON T, KRACHLER R, et al. From the peat bog to the estuarine mixing zone: Common features and variances in riverine dissolved organic matter determined by non-targeted analysis [J]. Marine Chemistry, 2017, 194: 158-167. doi: 10.1016/j.marchem.2017.06.012 [19] van ZOMEREN A, COMANS R N J. Measurement of humic and fulvic acid concentrations and dissolution properties by a rapid batch procedure [J]. Environmental Science & Technology, 2007, 41(19): 6755-6761. [20] LABANOWSKI J, PALLIER V, FEUILLADE-CATHALIFAUD G. Study of organic matter during coagulation and electrocoagulation processes: Application to a stabilized landfill leachate [J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 166-172. [21] ZHANG H, CHANG C H, LÜ F, et al. Estrogenic activity of fractionate landfill leachate [J]. Science of the Total Environment, 2009, 407(2): 879-886. doi: 10.1016/j.scitotenv.2008.09.055 [22] XIAO K K, ABBT-BRAUN G, HORN H. Changes in the characteristics of dissolved organic matter during sludge treatment: A critical review [J]. Water Research, 2020, 187: 116441. doi: 10.1016/j.watres.2020.116441 [23] SPRANGER T, PINXTEREN D V, REEMTSMA T, et al. 2D liquid chromatographic fractionation with ultra-high resolution MS analysis resolves a vast molecular diversity of tropospheric particle organics [J]. Environmental Science & Technology, 2019, 53(19): 11353-11363. [24] WEISHAAR J L, AIKEN G R, BERGAMASCHI B A, et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon [J]. Environmental Science & Technology, 2003, 37(20): 4702-4708. [25] MATTHEWS B J H, JONES A C, THEODOROU N K, et al. Excitation-emission-matrix fluorescence spectroscopy applied to humic acid bands in coral reefs [J]. Marine Chemistry, 1996, 55(3-4): 317-32. doi: 10.1016/S0304-4203(96)00039-4 [26] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence Excitation−Emission matrix regional integration to quantify spectra for dissolved organic matter [J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [27] WU J, ZHANG H, HE P J, et al. Insight into the heavy metal binding potential of dissolved organic matter in MSW leachate using EEM quenching combined with PARAFAC analysis [J]. Water Research, 2011, 45(4): 1711-1719. doi: 10.1016/j.watres.2010.11.022 [28] HELMS J R, STUBBINS A, RITCHIE J D, et al. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter [J]. Limnology and Oceanography, 2008, 53(3): 955-969. doi: 10.4319/lo.2008.53.3.0955 [29] KELLERMAN A M, KOTHAWALA D N, DITTMAR T, et al. Persistence of dissolved organic matter in lakes related to its molecular characteristics [J]. Nature Geoscience, 2015, 8(6): 454-457. doi: 10.1038/ngeo2440 [30] SCHMIDT M W, TORN M S, ABIVEN S, et al. Persistence of soil organic matter as an ecosystem property [J]. Nature, 2011, 478(7367): 49-56. doi: 10.1038/nature10386 [31] SMITH D F, PODGORSKI D C, RODGERS R P, et al. 21 Tesla FT-ICR mass spectrometer for ultrahigh-resolution analysis of complex organic mixtures [J]. Analytical Chemistry, 2018, 90(3): 2041-2047. doi: 10.1021/acs.analchem.7b04159 [32] HAWKES J A, DITTMAR T, PATRIARCA C, et al. Evaluation of the Orbitrap mass spectrometer for the molecular fingerprinting analysis of natural dissolved organic matter [J]. Analytical Chemistry, 2016, 88(15): 7698-7704. doi: 10.1021/acs.analchem.6b01624 [33] PATRIARCA C, BERGQUIST J, SJÖBERG P J R, et al. Online HPLC-ESI-HRMS method for the analysis and comparison of different dissolved organic matter samples [J]. Environmental Science & Technology, 2018, 52(4): 2091-2099. [34] STENSON A C, MARSHALL A G, COOPER W T. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra [J]. Analytical Chemistry, 2003, 75(6): 1275-1284. doi: 10.1021/ac026106p [35] KOCH B P, DITTMAR T, WITT M, et al. Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter [J]. Analytical Chemistry, 2007, 79(4): 1758-1763. doi: 10.1021/ac061949s [36] SUTTON R, SPOSITO G. Molecular structure in soil humic substances: the new view [J]. Environmental Science & Technology, 2005, 39(23): 9009-9015. [37] DEVARAJAN D, LIANG L Y, GU B H, et al. Molecular dynamics simulation of the structures, dynamics, and aggregation of dissolved organic matter [J]. Environmental Science & Technology, 2020, 54(21): 13527-13537. [38] KRUVE A. Strategies for drawing quantitative conclusions from nontargeted liquid chromatography-high-resolution mass spectrometry analysis [J]. Analytical Chemistry, 2020, 92(7): 4691-4699. doi: 10.1021/acs.analchem.9b03481 [39] HITES R A, JOBST K J. Is nontargeted screening reproducible? [J]. Environmental Science & Technology, 2018, 52(21): 11975-11976. [40] YU Z F, HE P J, SHAO L M, et al. Co-occurrence of mobile genetic elements and antibiotic resistance genes in municipal solid waste landfill leachates: A preliminary insight into the role of landfill age [J]. Water Research, 2016, 106: 583-592. doi: 10.1016/j.watres.2016.10.042 [41] HE P J, CHEN L Y, SHAO L M, et al. Municipal solid waste (MSW) landfill: A source of microplastics?-Evidence of microplastics in landfill leachate [J]. Water Research, 2019, 159: 38-45. doi: 10.1016/j.watres.2019.04.060 [42] OMAN C B, JUNESTEDT C. Chemical characterization of landfill leachates--400 parameters and compounds [J]. Waste Management, 2008, 28(10): 1876-1891. doi: 10.1016/j.wasman.2007.06.018 [43] HE P, HUANG J, YU Z, et al. Antibiotic resistance contamination in four Italian municipal solid waste landfills sites spanning 34 years [J]. Chemosphere, 2021, 266: 129182. doi: 10.1016/j.chemosphere.2020.129182 [44] 郑仲, 何品晶, 章骅, 等. 城市生活垃圾中邻苯二甲酸酯的源分布特征 [J]. 同济大学学报(自然科学版), 2007, 35(12): 1646-1650. doi: 10.3321/j.issn:0253-374X.2007.12.013 ZHENG Z, HE P J, ZHANG H, et al. Distribution of phthalic acid esters in municipal solid waste [J]. Journal of Tongji University (Natural Science), 2007, 35(12): 1646-1650(in Chinese). doi: 10.3321/j.issn:0253-374X.2007.12.013
[45] DITTMAR T, KOCH B, HERTKORN N, et al. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater [J]. Limnology and Oceanography-Methods, 2008, 6(6): 230-235. doi: 10.4319/lom.2008.6.230 [46] GURTLER B K, VETTER T A, PERDUE E M, et al. Combining reverse osmosis and pulsed electrical current electrodialysis for improved recovery of dissolved organic matter from seawater [J]. Journal of Membrane Science, 2008, 323(2): 328-336. doi: 10.1016/j.memsci.2008.06.025 [47] THURMAN E M, MALCOLM R L. Preparative isolation of aquatic humic substances [J]. Environmental Science & Technology, 1981, 15(4): 463-466. [48] KRUGER B R, DALZELL B J, MINOR E C. Effect of organic matter source and salinity on dissolved organic matter isolation via ultrafiltration and solid phase extraction [J]. Aquatic Sciences, 2011, 73(3): 405-417. doi: 10.1007/s00027-011-0189-4 [49] LI Y, HARIR M, LUCIO M, et al. Proposed guidelines for solid phase extraction of Suwannee River dissolved organic matter [J]. Analytical Chemistry, 2016, 88(13): 6680-6688. doi: 10.1021/acs.analchem.5b04501 [50] GREEN N W, MICHAEL PERDUE E, AIKEN G R, et al. An intercomparison of three methods for the large-scale isolation of oceanic dissolved organic matter [J]. Marine Chemistry, 2014, 161: 14-19. doi: 10.1016/j.marchem.2014.01.012 [51] LI Y, HARIR M, UHL J, et al. How representative are dissolved organic matter (DOM) extracts? A comprehensive study of sorbent selectivity for DOM isolation [J]. Water Research, 2017, 116: 316-323. doi: 10.1016/j.watres.2017.03.038 [52] HOLLENDER J, SCHYMANSKI E L, SINGER H P, et al. Nontarget screening with high resolution mass spectrometry in the environment: Ready to go? [J]. Environmental Science & Technology, 2017, 51(20): 11505-11512. [53] SCHYMANSKI E L, SINGER H P, SLOBODNIK J, et al. Non-target screening with high-resolution mass spectrometry: Critical review using a collaborative trial on water analysis [J]. Analytical and Bioanalytical Chemistry, 2015, 407(21): 6237-6255. doi: 10.1007/s00216-015-8681-7 [54] LI H, CAI Y P, GUO Y, et al. MetDIA: targeted metabolite extraction of multiplexed MS/MS spectra generated by data-independent acquisition [J]. Analytical Chemistry, 2016, 88(17): 8757-8764. doi: 10.1021/acs.analchem.6b02122 [55] SILVA L K, HILE G A, CAPELLA K M, et al. Quantification of 19 aldehydes in human serum by headspace SPME/GC/high-resolution mass spectrometry [J]. Environmental Science & Technology, 2018, 52(18): 10571-10579. [56] DROLLETTE B, BRENNEIS R J, PLATA D L. Oligomer-specific, short chain linear alcohol ethoxylate quantification via comprehensive two-dimensional gas chromatography [J]. Environmental Science & Technology Letters, 2018, 5(9): 539-545. [57] 邵立明, 邓樱桃, 仇俊杰, 等. 工程规模长填龄渗滤液膜生物-纳滤组合设施各单元污染物去除效能 [J]. 环境科学, 2021, 42(3): 1469-1476. SHAO L M, DENG Y T, QIU J J, et al. Pollutant removal efficiency of different units along a mature landfill leachate treatment process in a membrane biological reactor-nanofiltration combined facility [J]. Environmental Science, 2021, 42(3): 1469-1476(in Chinese).
[58] QIU J J, LÜ F, ZHANG H, et al. Persistence of native and bio-derived molecules of dissolved organic matters during simultaneous denitrification and methanogenesis for fresh waste leachate [J]. Water Research, 2020, 175: 15705. [59] 何品晶, 徐延春, 吕凡, 等. 芬顿和双氧水紫外处理稳定渗滤液的光谱特征 [J]. 同济大学学报(自然科学版), 2016, 44(2): 249-254. doi: 10.11908/j.issn.0253-374x.2016.02.013 HE P J, XU Y C, LV F, et al. Spectroscopic characteristics of mature leachate during Fenton and H2O2-UV treatment process [J]. Journal of Tongji University(Natural Science), 2016, 44(2): 249-254(in Chinese). doi: 10.11908/j.issn.0253-374x.2016.02.013
[60] STEDMON C A, BRO R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial [J]. Limnology and Oceanography: Methods, 2008, 6(11): 572-579. doi: 10.4319/lom.2008.6.572 [61] YU M D, XI B D, ZHU Z Q, et al. Fate and removal of aromatic organic matter upon a combined leachate treatment process [J]. Chemical Engineering Journal, 2020, 401: 126157. doi: 10.1016/j.cej.2020.126157 [62] HE P J, LIU W Y, QIU J J, et al. Improvement criteria for different advanced technologies towards bio-stabilized leachate based on molecular subcategories of DOM [J]. Journal of Hazardous Materials, 2021, 414: 125463. doi: 10.1016/j.jhazmat.2021.125463 [63] SHAO L M, DENG Y T, QIU J J, et al. DOM chemodiversity pierced performance of each tandem unit along a full-scale “MBR+NF” process for mature landfill leachate treatment [J]. Water Research, 2021, 195: 117000. doi: 10.1016/j.watres.2021.117000 [64] KLEIN S, WORCH E, KNEPPER T P. Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-main area in Germany [J]. Environmental Science & Technology, 2015, 49(10): 6070-6076. [65] OTURAN N, van HULLEBUSCH E D, ZHANG H, et al. Occurrence and removal of organic micropollutants in landfill leachates treated by electrochemical advanced oxidation processes [J]. Environmental Science & Technology, 2015, 49(20): 12187-12196. [66] HE P J, ZHENG Z, ZHANG H, et al. PAEs and BPA removal in landfill leachate with Fenton process and its relationship with leachate DOM composition [J]. Science of the Total Environment, 2009, 407(17): 4928-4933. doi: 10.1016/j.scitotenv.2009.05.036 [67] ZHENG Z, ZHANG H, HE P J, et al. Co-removal of phthalic acid esters with dissolved organic matter from landfill leachate by coagulation and flocculation process [J]. Chemosphere, 2009, 75(2): 180-186. doi: 10.1016/j.chemosphere.2008.12.011 [68] WEIZEL A, SCHLUSENER M P, DIERKES G, et al. Analysis of the aerobic biodegradation of glucocorticoids: Elucidation of the kinetics and transformation reactions [J]. Water Research, 2020, 174: 115561. doi: 10.1016/j.watres.2020.115561 [69] GRIBBLE G W. Naturally occurring organohalogen compounds [J]. Accounts of Chemical Research, 1998, 31(3): 141-152. doi: 10.1021/ar9701777 [70] SCHOLLÉE J E, SCHYMANSKI E L, AVAK S E, et al. Prioritizing unknown transformation products from biologically-treated wastewater using high-resolution mass spectrometry, multivariate statistics, and metabolic logic [J]. Analytical Chemistry, 2015, 87(24): 12121-12129. doi: 10.1021/acs.analchem.5b02905 -



 下载:
下载: