-
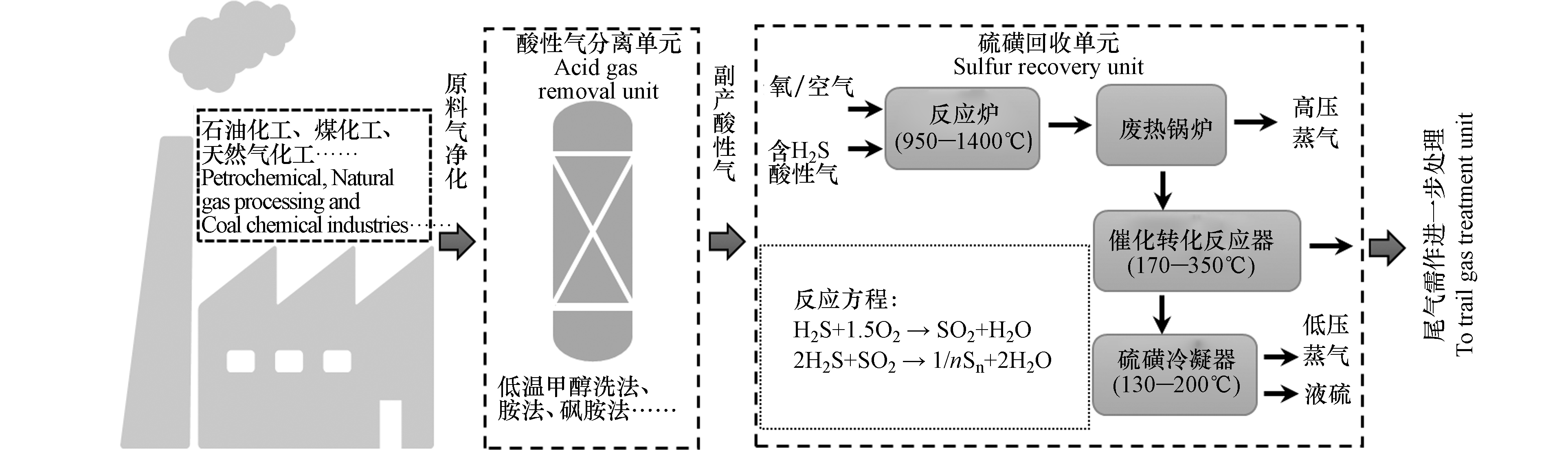
世界范围内能源需求的增加以及优质化石能源的日渐枯竭迫使人们将目光投向储量丰富的高含硫酸性原料的开发利用。中东地区拥有世界已探明石油储量的64%,而这些原油大多为含硫量高的“酸性油”(硫含量超过2 % 质量分数)[1]。目前已探明的天然气世界储量中H2S含量1%—15%,CO2含量0—15%的酸性天然气占30%以上,H2S及CO2含量高于15%的酸性气体占4%以上[2]。我国含硫气田(含硫2%—4%)气产量更是占全国气产量的60%以上,普光、元坝气田为典型的高含硫气田,H2S含量最高可达17%[3]。这些酸性原料在加工利用过程中会产生大量酸性气体(H2S和CO2),对环境及人体健康危害极大。目前工业上主要采用溶剂法分离出H2S和CO2,再利用克劳斯硫回收法处理H2S,将H2S转化为低值的硫磺和水,典型处理流程图如图1所示。酸性气体中伴生杂质(CO2、烃类)的存在常导致克劳斯硫回收过程的技术问题[4]。此外,上述酸性气体的治理过程忽略了CO2的治理,导致CO2的大量排放。当前,全球气候变化的威胁不断升级,极端气候灾害更趋严峻。为应对气候危机,我国力争于2030年前实现CO2排放达到峰值,2060年前实现碳中和,将用全球历史上最短的时间实现碳达峰到碳中和,任务艰巨而紧迫。碳中和目标下,如何同时实现硫化氢污染控制与二氧化碳减排是化工行业酸性气体治理过程中面临的一项非常紧迫和充满挑战的任务。
因此,有必要开发更具经济与环境效益的酸性气体治理与资源利用的替代手段,尤其是针对不同行业、工况下酸性气体的特点开发多资源协同回收利用技术,实现污染减排及资源利用的最大化。目前,针对含H2S酸性气体的多组分转化与资源综合利用,研究者们开展了酸性气体(H2S、CO2、CH4等混合气)直接转化为高价值产品——氢气以及高附加值的硫衍生物等的相关研究,主要包括以下几个方面。
全文HTML
-
CO2是含硫酸性气体中一种常见的伴生杂质,且大多数酸性气体CO2含量很高,如我国普光天然气田中CO2和H2S含量分别高达8.6%和14.1%[5],煤化工过程产生的酸性气体中CO2含量甚至高达30%以上,给克劳斯工艺的运行带来严重的技术问题,甚至不适于用克劳斯工艺进行处理,通常需采用耗能较高的胺吸收法将CO2进行选择性去除[2]。由于其热力学稳定性和低的化学价值,CO2的工业用途较少,通常被直接排放到大气中。据估算,炼油厂、天然气加工行业每年约释放1200 Mt CO2[6]。此外,在一些石油化工过程中CO2也作为主流副产品产生,如甲烷蒸汽重整制氢。CO2是温室气体,会引起气候变暖等诸多生态环境问题,因此化工行业酸性气体中CO2减排问题也亟待解决。
考虑到酸性气体中同时存在H2S与CO2,且H2S和CO2可分别作为还原剂与氧化剂,研究者们提出通过酸性气体(H2S和CO2)热解或氧化还原反应将其转化为单质硫和有工业价值的合成气(H2 + CO),总反应方程式如反应(1),并对此过程进行了理论模拟和实验研究。
Groisil等[7-8]对1000—2000 ℃条件下酸性气体(H2S和CO2)的热解进行了数值模拟研究,结果表明H2/CO比值随温度的升高而降低,只有在高温(1550 ℃以上)及特定酸性气体组成(H2S含量<60%)条件下,才能产生适合于氨合成或燃料发动机的理想合成气(H2/CO比值在0.33—1.26)。此外,1600 ℃以上热解过程中CO2分解释放出的氧会氧化S2形成COS、SO2等含硫化合物。该研究结果说明只有将反应温度控制在1550—1600 ℃狭窄的温度区间内才可能达到较理想的状态,反应条件苛刻且难以控制。El-Melih等[9]进一步对该过程进行了实验研究,研究表明热解温度及酸性气体组成对合成气的产量及H2/CO比值影响很大,酸性气体热解法很适合于处理Claus工艺难以处理的贫酸性气体。但其未考察反应停留时间的影响以及反应过程中其他硫化合物的生成情况。
Ibrahim等[10]基于动力学模拟分析了酸性气体组成及工艺条件对酸性气体热分解制合成气和硫磺的影响,尤其是合成气及COS、SO2等含硫化合物随停留时间(0—2 s)及温度(1373—1800 K)的演变情况。研究结果表明H2S与CO2的热解存在协同效应,即H2S可促进CO2被还原为CO,CO2可促进H2S的转化。升高温度和延长停留时间有助于促进合成气的生成,但同时也促进了含硫化合物的生成,而温度高于1600 K,COS的生成会减少。
国内研究者们尝试了采用催化的方法来实现酸性气体(H2S和CO2)的协同转化。Su等[11]研究了400—800 ℃条件下系列氧化物(如CaO,NiO/MgO,NiO/γ-Al2O3等)催化剂上H2S与CO2催化转化性能,研究结果表明催化剂可有效地促进H2S和CO2的转化,提高CO和硫的选择性,但温度高于600 ℃才会有少量H2的生成。此外,催化剂的化学组成对催化性能影响不大,因此其推测H2S和CO2反应的机理是催化剂引发的自由基机理,但该过程并未得到验证。Ma等[5]建立了太阳能驱动的电化学体系,在中性条件下利用石墨烯包覆的氧化锌催化剂还原CO2及石墨烯催化剂氧化H2S,使CO2和H2S同时转化为高活性的CO及高稳定性的硫,但该过程中H2S中的“H”并未得到回收利用。Zhao等[12]利用Mo2O3/Al2O3催化剂结合低温等离子体辅助,在低温常压条件下实现了H2S和CO2高选择性地转化为合成气,CO2转化率高达60%以上,并实现H2S接近完全转化(98%—100%),且反应过程不会有COS及SOx等硫化物的生成。其研究结果显示可通过改变比能量输入(SEI)、进料气体组成等反应条件对合成气H2/CO的比值进行调控以满足不同应用需求。该过程为酸性气体的资源化利用提供了新策略,对H2S污染控制及CO2减排具有重要价值,但其能量成本较高。
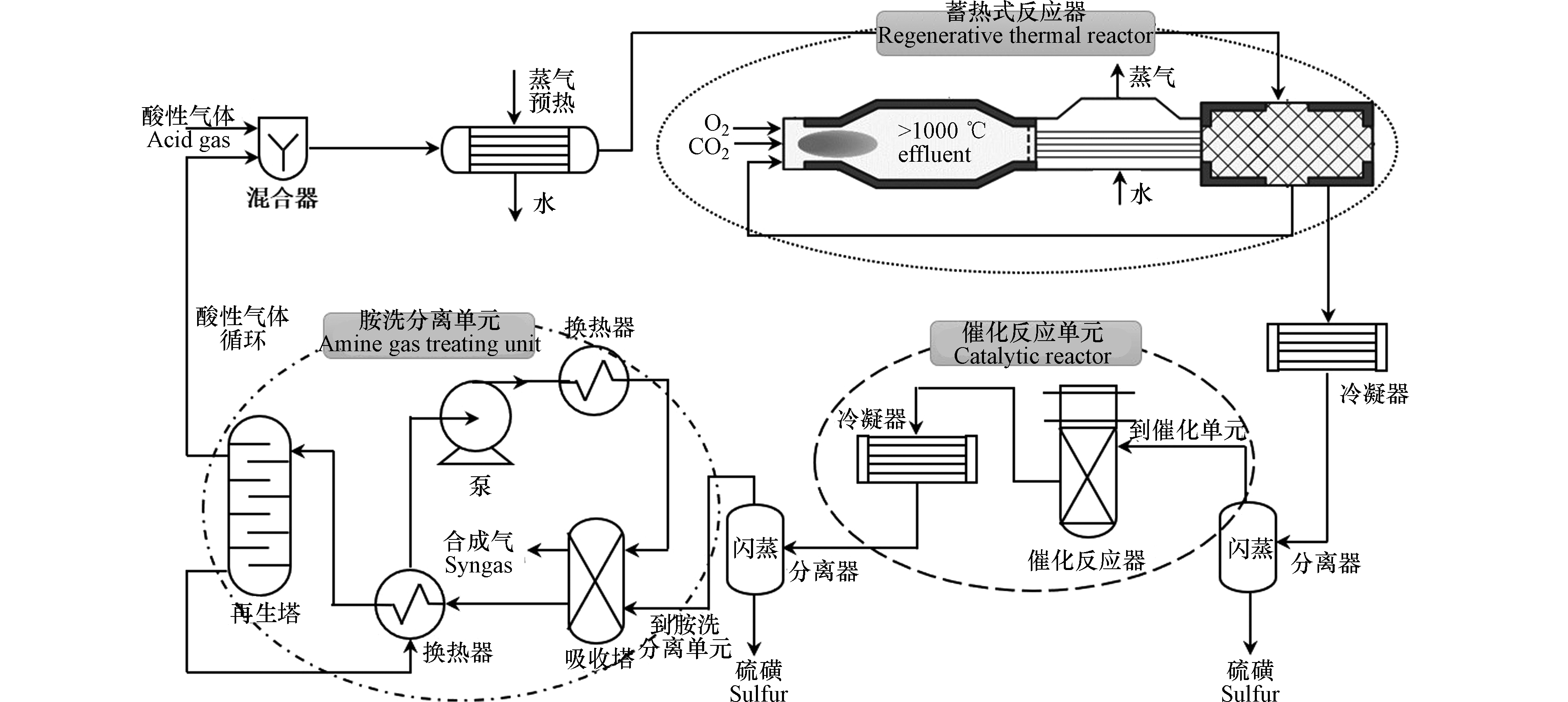
米兰理工大学Manenti团队[13-15]基于H2S与CO2的氧化还原反应过程,创新性地开发了酸性气体制合成气工艺技术(AG2STM),如图2所示,该工艺包含蓄热式反应器,催化单元和胺洗分离单元[16]. 该工艺的核心是蓄热式反应器(RTR),其与传统的Claus反应炉配置不同,该反应器更有利于H2的生成,关键点在于提供合适比例的酸性气体(H2S和CO2)组成,并对酸性气体进行预热。其模拟结果表明,合成气H2/CO比值随停留时间变化较大,反应物的转化率则与RTR的工作温度有关。
该团队通过对煤气化工艺进行改造,直接用AG2STM工艺替代传统的胺洗分离+克劳斯脱硫工艺,模拟结果显示整个煤气化工艺的CO2排放量下降了11.8%,H2S减少了99.3%,同时还富余了3.16%的合成气[17-18]。Manenti等进一步将AG2STM工艺应用于天然气重整制氢过程中,使得燃料用量减少了0.63%,二氧化碳总排放量减少了0.84%,天然气原料用量减少1.06%,并且实现了出口H2S和SO2零排放,同时还产生额外的合成气[19]。该工艺在更绿色地使用煤炭及减轻二氧化碳的影响方面开辟了新前景,尤其是针对一些含硫量高的原料的利用,含硫量高意味着二氧化碳排放量减少,但该技术目前尚未得到实际应用。
-
硫化氢(H2S)的结构与水(H2O)相似,也能与甲烷(CH4)发生类似于甲烷水蒸气重整(SMR)的反应,产生氢气,反应方程式如反应(2)和(3):
但与传统甲烷水蒸气重整不同的是,硫化氢甲烷重整(H2SMR)不会产生CO2,而是获得易于液化存贮的二硫化碳(CS2)。CS2是一种比硫磺更具商业价值的硫衍生物,价格约为硫磺的4倍。其是一种重要的化工原料,在人造纤维、玻璃纸、橡胶、化工、农药、医药、冶金及炼油等行业广泛应用。同时也是一种目前且长时间内无法替代的良好的溶剂,近年来被广泛用于原油的提采(EOR)。此外,CS2与H2还能进一步反应制得汽油或甲硫醇等[20-21]。
H2S-CH4重整反应最早被人们关注也是在以H2S和CH4为原料生产CS2的研究中,其研究结果表明只有在反应温度高于1000 ℃以上才可达到较高的CS2收率。H2S-CH4重整反应为强吸热反应,且受热力学平衡限制,当温度高于930 ℃时反应才能自发进行。同时,该反应过程还伴随着H2S热分解(反应(4))和CH4热裂解(反应(5))副反应的发生。为此,研究者们对H2S-CH4重整反应过程进行了大量的热力学和模拟分析[22-24]。研究结果表明温度是影响CH4和H2S转化率的主要因素,且任何温度下,H2S的转化率均低于CH4的转化率;CH4在577 ℃以上即能达到一定的转化率,800 ℃以上转化率可达100%;温度低于1000 ℃,H2S的转化率不超过20%。此外,研究者们普遍认为H2S-CH4重整反应包含两个步骤,即通过H2S热分解形成S2(反应(4)),然后S2再与CH4反应生成CS2和H2(反应(6))。H2S-CH4重整受限于H2S的热分解。Karan等[25]的实验研究结果也表明CH4-S2反应生成CS2很快,H2S的分解是CH4-H2S反应生成CS2的速控步骤。针对上述反应过程,目前尚缺乏更深入的微观反应过程及反应动力学的研究。
El-Melih等[26]探究了不同因素对H2S-CH4重整反应过程的影响,结果表明温度是影响CH4和H2S转化率的主要因素,温度大于1100 ℃,CH4完全转化,H2产量急剧增加,H2S的转化率也显著提高;同时反应器内壁有炭沉积。此外,随着H2S/CH4物质的量比的增加,H2S的转化率降低,H2和CS2产生量减少;反应最佳比例为化学计量比,可实现H2收率高达95%,CS2收率60%。国内Li等[27]基于反应模拟分析研究了停留时间359—367 ms 条件下H2S/CH4摩尔比对产氢性能的影响,结果表明900—1150 ℃内,H2S/CH4 = 3是产氢最佳的比例,反应温度为1250 ℃时,反应最佳比例为H2S/CH4 = 2,但其未考虑停留时间对该过程的影响。El-Melih等[28]对停留时间影响的研究结果表明反应温度越高,CH4、H2S转化达到渐近稳态值所需停留时间越短,例如1100 ℃时CH4和H2S分别需要0.5 s和0.7 s。
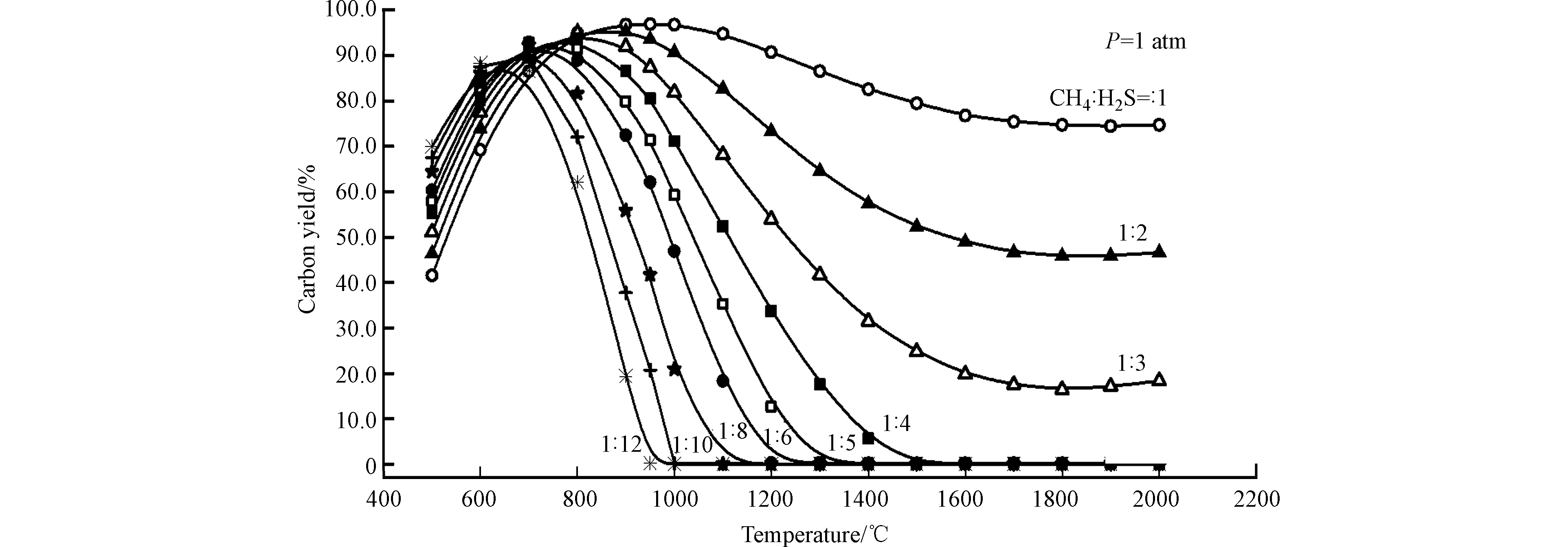
为解决反应过程中积炭失活的问题,Huang等[29]对H2S-CH4反应体系进行了热力学和化学平衡分析,通过窄点分析,确定了零积炭的反应条件(如图3所示),研究结果表明高的H2S/CH4摩尔比有利于防止积炭的生成,例如当CH4/H2S = 1/4时,反应温度高于1500 ℃则不会有炭生成;当CH4/H2S = 1/10时,温度高于1000 ℃则不会有炭生成。
针对反应机理方面,国内Li等[30]建立了包括515个反应和85种反应物的H2S-CH4反应机理模型,模拟研究结果表明SH自由基是H2S和CH4转化最重要的中间产物,其来源于H2S的分解和CH4与S自由基的相互作用,并进一步参与H2、S2和CS2的生成。但对于该模拟结果尚无实验数据证实。
此外,为加速H2S-CH4重整反应的进行,研究者们引入了催化剂。研究涉及的催化剂包括:(1)负载型的金属及金属氧化物催化剂,Pt/Al2O3[31],Mo,Cr/ZrO2-La2O3[32],Mo,Cr/ZrO2-SBA-15[33],Fe2O3/γ-Al2O3,MoO3-NiO/CeO2[34],Fe2O3-NiO/MgO;(2)金属硫化物催化剂,MoS2[35],Cr2S3和Ce2S3[20];(3)金属复合氧化物催化剂,La2NiFeO6[36]等。Erekson等[20]研究了一系列硫化物催化剂的H2S-CH4重整反应性能,结果显示Cr2S3、Ce2S3催化剂抑制CH4裂解及催化剂积炭后用H2S再生产生CS2的效果最好,其催化性能也最好。在1100 ℃、H2S/CH4 = 4条件下可实现CS2收率为100%,H2收率为60%。Martínez-Salazar等[33]研究了800—950 ℃、H2S/CH4=12条件下Mo,Cr/ZrO2-La2O3,Mo,Cr/ZrO2-SBA-15催化剂的催化性能,研究结果也表明Cr的添加可提高催化剂的CS2选择性,Mo则有利于产H2。但反应过程中催化剂载体的晶体结构坍塌(SBA-15 → ZrSiO4),活性组分也发生明显的相变,形成相应的硫化物和碳化物的复杂混合物,如:MoS2、ZrS2和MoC、Mo3C2等,难以推断反应活性物种的本质。Galindo- Hernández等[37]报道的Fe2O3/γ-Al2O3催化剂在950 ℃、H2S/CH4 = 12条件下运行了14 h,其CH4转化率为100%,H2S转化率为60%;研究结果表明,催化剂对CS2的选择性与八面体配位的Fe3+有关,而四面体配位的Fe3+则与产H2有关;反应后催化剂中Fe以Fe1-xS形式存在,且没有金属相Fe0的生成。目前文献报道的这些H2S-CH4重整催化剂大多都是基于单纯制氢目的设计的,且对反应过程催化剂积炭情况几乎没有研究,相关反应机理的探索也比较缺乏,今后催化剂的设计方面应更注重催化剂的抗积碳性能,提高催化剂的选择性,更深入地研究反应机理及反应动力学,实现多资源协同回收利用。
-
利用硫化氢为原料生产高附加值有机硫化工产品不仅市场前景广阔,而且具有很高的经济效益和环境效益[38]。作为精细化工生产的重要原料以及工业合成有机中间体,甲硫醇以用途最广、经济效益最高、发展前景最好而受到广泛关注,可用于合成农药、医药、食品、合成材料和饲料添加剂等[39]。其最主要的用途在于生产蛋氨酸,且每吨蛋氨酸需消耗甲硫醇0.4 t。近年来,蛋氨酸合成工业快速发展,对甲硫醇的需求量很大,甲硫醇的生产也引起了人们的重视。目前国内外甲硫醇的制备主要采用甲醇-硫化氢法,通常采用碱金属修饰的过渡金属硫化物作为催化剂,但其反应流程多,成本高[40-41]。考虑到甲醇是由合成气制得,直接以合成气和硫化氢为原料一步法合成甲硫醇可使资源得到最大化利用,因而成为当前化工及环境领域的研究热点[42]。此外,研究者们对以CO2为碳源制备甲硫醇(CO2,H2S和H2反应)[43-44]以及以碳硫化合物(COS/CS2)[21, 45]为原料制甲硫醇等方法也进行了一系列的研究。这对酸性气体的转化与综合利用(酸性气体制合成气、硫化氢甲烷制二硫化碳和氢气),进而进行深加工(制甲硫醇),向高附加值有机硫化工产品方向发展提供了新的思路(如图4所示)。
合成气法制甲硫醇最早由Olin等[46]在1962年提出,其在专利中报道了以Fe、Co、Ni、Mo等金属硫化物为催化剂促成CO(或CO2)与H2和H2S反应制甲硫醇,反应方程式如反应(7):
此后国内外研究者们对合成气和H2S反应合成甲硫醇的催化剂组成及助剂、反应条件及反应过程机理方面进行了大量的研究,取得了一系列的研究成果。
在催化剂组成及助剂方面,目前研究最为广泛的为负载型的钼基、钨基与过渡金属硫化物催化剂,并以碱金属和过渡金属等分别为第一和第二助剂,表1列出了不同催化剂的性能比较。Liu等[47]比较了碱金属对Mo/SBA-15催化剂性能的影响,发现甲硫醇选择性顺序为Cs>K>Na,Cs修饰的催化剂上甲硫醇选择性最高(可达66%)。Yang等[48]研究了K2MoS4/SiO2催化剂上合成气和H2S反应制甲硫醇,XRD和XPS结果表明Mo-S-K物种为合成甲硫醇的活性相。该课题组进一步研究了过渡金属(Fe、Co、Ni、Mn)及稀土元素(La和Ce)作为助剂对K2MoS4/SiO2催化剂性能的影响,研究结果表明过渡金属和稀土元素助剂的加入有利于Mo-S-K活性相的形成,从而提高甲硫醇的收率[49-50]。Cordava等[51]研究了一系列γ-Al2O3负载的Mo-、K-与Mo-K-基催化剂,通过XPS分析发现,K+倾向于插入到MoS2层间形成1T-MoS2(KxMoS2)物种,并且该物种数量越多,甲硫醇产率越高,1T-MoS2物种为活性相。其制备的负载型K2WO4和K2WS4催化剂也发现类似的K+插层的1T-WS2活性相,与合成甲硫醇的催化性能有相关性[52]。
然而,Yu等[53]基于XPS及EXAFS分析发现反应条件下K2Mo催化剂中的1T-MoS2相并不稳定,尽管反应后的催化剂中1T-MoS2相消失,但K2Mo催化剂的高活性仍能在长时间内保持稳定,由此说明1T-MoS2相与甲硫醇收率没有相关性,其认为K的作用可能在于位于MoS2边缘的K是选择性生成CH3SH的关键。碱金属助剂在合成甲硫醇的反应中起着重要作用,但其作用本质尚需进一步深入研究。
此外,载体的性质对催化剂性能也有很大影响。Zhang等[55]研究了α-Al2O3,γ-Al2O3,Cr2O3,HZSM-5,SiO2等载体的活性,发现未修饰的α-Al2O3具有最佳的催化性能,甲硫醇选择性高达98%,并首次发现噻吩副产物的生成。同时其发现载体中的酸位会促进甲硫醇加氢生成烃类,因此为了达到甲硫醇选择性最大,载体表面要相对非功能化,具有最小的酸度。黄锶等[60]比较了SBA-15和SiO2两种载体负载K-Mo-Co基催化剂的性能,发现与无定型SiO2相比,SBA-15由于具有更大的比表面积和均一的孔径分布,有利于活性组分分散及反应物和产物的扩散,从而表现出更佳的催化活性和甲硫醇选择性。
在反应条件及反应过程机理方面。甲硫醇的合成通常需要适宜的温度、一定的压力及过量H2和H2S,CO和CO2均可作为原料,H2S也可用硫磺替代,反应方程式如反应(8)、(9)和(10):
Buchholz等[61]1983年专利中报道的硫化的CsOH-NiO/Al2O3催化剂上,以CO2为碳源,在290 ℃,压力约4.76 MPa,CO2/H2S/H2 = 1/8/4条件下,CO2转化率达50%,甲硫醇收率高达92%,同时还提出以气态硫代替H2S作为原料。Mul等[56]研究了负载型钒基催化剂上CO和H2S反应合成甲硫醇的影响,结果表明催化剂活性及甲硫醇选择性最佳的温度为342 ℃,温度低于342 ℃,COS和H2为主要产物,高于342 ℃则易生成CH4。在反应过程机理方面目前有3种说法:COS中间产物机理、改性Fischer-Tropsch机理和合成醇机理。
(1)COS中间产物机理
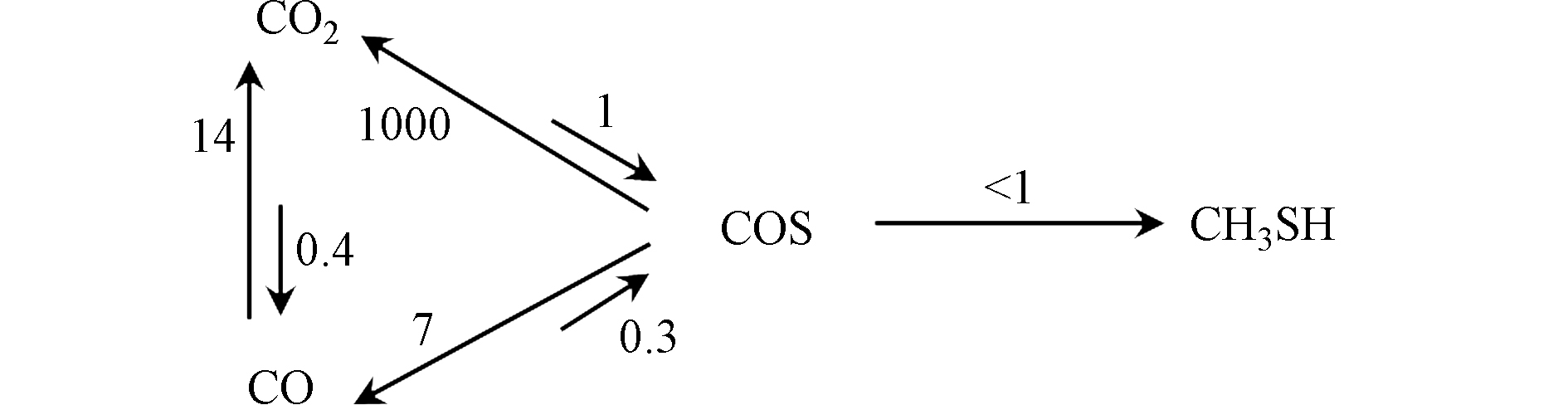
Barrault等[43]研究了K-W/Al2O3催化剂上CO(CO2),H2和H2S反应合成甲硫醇的反应过程,提出COS是合成甲硫醇的中间产物,CO(CO2)首先和H2S反应生成COS,COS再与H2加氢生成甲硫醇(反应路径如图5),同时基于分步反应的速率常数证明COS加氢为合成甲硫醇的速控步骤;CO为碳源制甲硫醇的主要副产物是CO2,COS水解反应速率比COS加氢制甲硫醇大两个数量级;CO2为碳源时,反应生成的H2O无法去除,抑制了COS的生成,进而导致甲硫醇产量降低。其后,Mul等[56]及Chen等[62]也得到了与Barrault一致的结论,并进一步指出了CS2,CH4,CH3SCH3等副产物的来源,即COS的歧化反应和CH3SH的加氢反应。但目前尚无对该机理更深入的研究。
(2)改性Fischer-Tropsh机理
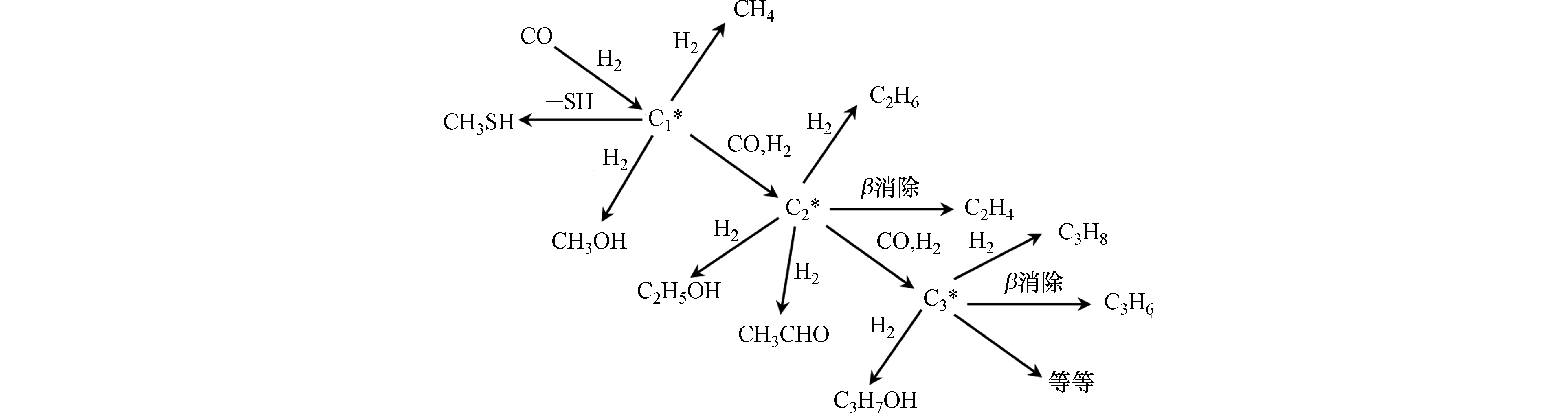
Zhang等[55]探究了α-Al2O3催化剂上合成气与H2S反应制甲硫醇,其基于合成气反应的Fisher-Tropsch过程(即表面C1中间物种反应形成C2中间物种的表面聚合过程),提出了改性的Fisher-Tropsch机理(如图6所示)。当合成气中引入H2S时,α-Al2O3表面会形成比—OH基团更具亲核性的—SH基团,该—SH基团会抢先与CO加氢形成的表面C1中间物种形成CH3S−ads中间物种或吸附态的CH3SH。该研究表明-SH基团中高亲核性的S可阻止链增长,从而只形成C1产物,即CH3SH,与Schulz-Flory聚合动力学一致。
(3)合成醇机理
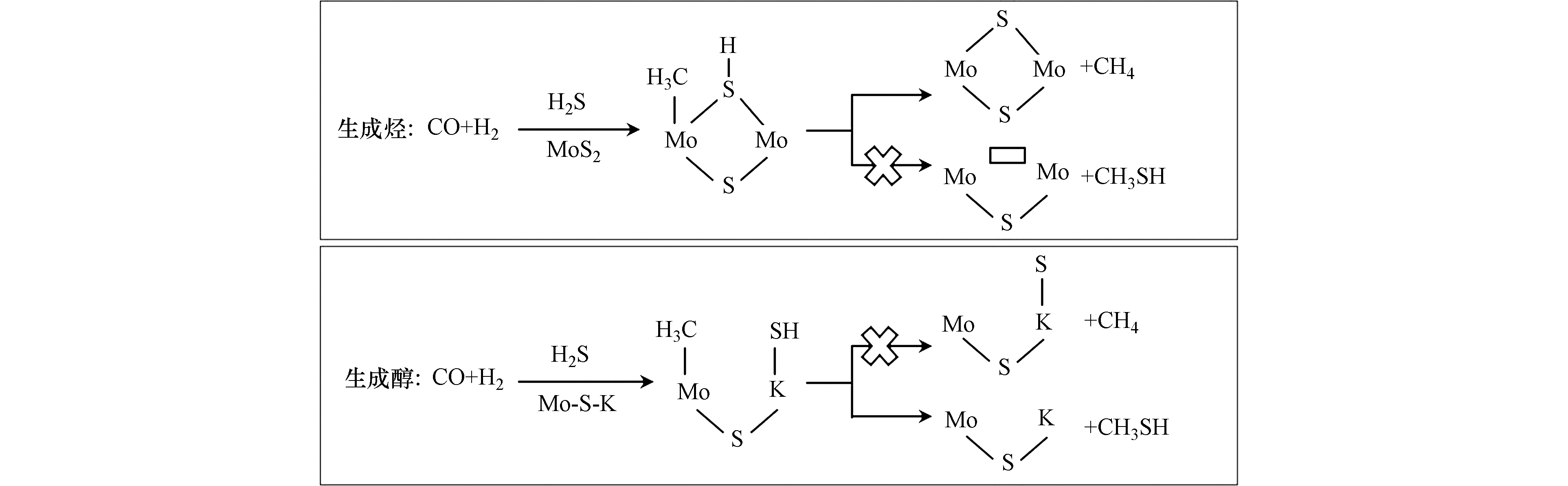
Yang等[50]基于K-Mo催化剂上合成气和H2S制甲硫醇的研究认为甲硫醇的生成与Mo-S-K密切相关,而烃的生成与MoS2相关。当CO加氢生成表面-CH3物种时,在MoS2活性位点上,断裂S-H键使H向-CH3转移形成CH4要比断裂邻位的Mo-SH键使-SH基团与-CH3基团结合形成CH3SH要容易,因而优势产物为烃类;而在Mo-S-K活性位点上,断裂邻位上的K-SH键实现-SH基团向-CH3基团转移形成CH3SH则容易很多,因而主要产物为甲硫醇,反应机理图如图7所示。该机理合理地解释了碱金属修饰前后产物的差别,但并未对其他副产物如CO2作出表述。
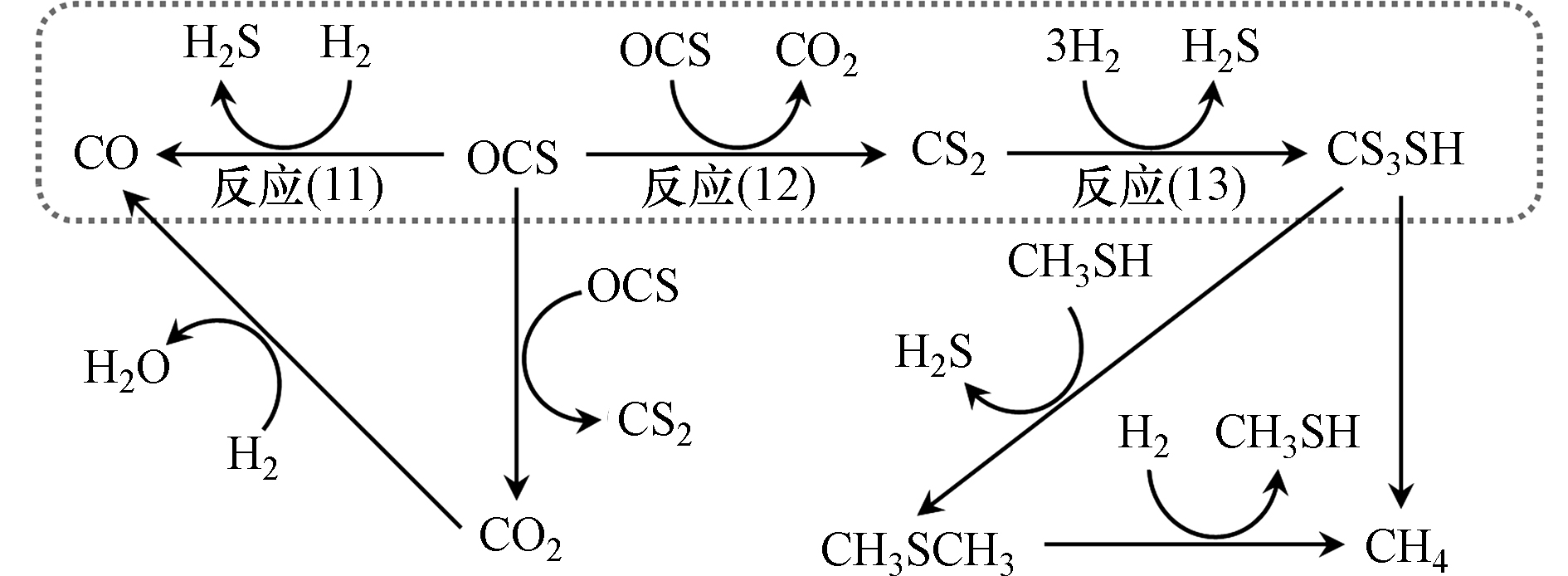
基于甲硫醇合成的COS中间产物机理,慕尼黑理工大学Lercher课题组[45, 63-65]探究了K-Mo基催化剂上COS和CS2为原料和H2S、H2反应合成甲硫醇的过程,催化剂性能如表2所示。然而该团队的研究结果打破了前人的观点,其研究发现以COS为原料合成甲硫醇时,COS并不是直接与H2反应一步生成甲硫醇的,而是一个复杂的反应过程(反应路径如图8所示),主要包括两个并行反应羰基硫氢解反应(反应(11)和歧化反应(反应(12))以及二硫化碳加氢反应(反应(13)),并通过巧妙的实验设计详细论证了该反应路径。
该机理的提出对甲硫醇合成机理作出了更加合理的解释。针对活性相及反应机理的探索,如今已有更加先进的原位反应、原位表征技术及理论计算等,因此今后的研究人员应结合前人的研究基础与先进的表征分析技术,从更深入的原子分子水平上去解析催化剂的活性相与反应过程机理。
-
结合当前酸性气体污染控制面临的问题和挑战,针对不同行业、工况下酸性气体的特点开发多资源协同回收利用技术,实现酸性气体污染减排及资源利用最大化具有重要意义。本文综述了利用酸性气体(H2S、CO2、CH4等混合气体)多组分直接转化为高价值产品——氢气以及高附加值硫衍生物的研究进展,现对各技术路径的现状及未来方向进行总结与展望:
针对H2S和CO2制合成气:(1)利用高含硫含碳酸性气体中H2S和CO2组分不经分离直接转化获得更有价值的工业产品,如氢气、一氧化碳和硫磺,可大大减少复杂的分离工序产生的能耗和费用,具有非常大的经济价值和环境效益。(2)但该反应过程受热力学限制比较严重,需要非常高的反应温度,同时副产物的生成使分离更加困难。因此若想实现工业化,还需要解决很多问题。(3)今后可借助辅助手段如光电催化、等离子体或微波辅助的方法等。
针对高含硫天然气硫化氢甲烷重整:(1)对于高含硫天然气可考虑硫化氢甲烷重整以回收其中的硫碳资源(CS2)和氢资源,目前的研究集中于对其可行性的热力学或实验探究以及相关催化剂上的动力学研究,结果证明该反应制氢气是可行的。(2)然而对于硫化氢甲烷重整反应催化剂的筛选、催化剂性能改善、反应机理及反应条件优化等目前尚无有系统的研究报道,尤其是国内对该反应的研究尚浅,今后国内同行可增加些科研投入,以期未来可实现该工艺的工业应用。
针对有机硫化工产品——甲硫醇的制备:(1)有机硫化工产品的价值是硫磺的数倍乃至数十倍,且需求日益增大,前景十分广阔。甲硫醇是生产精细化工生产的重要原料以及工业合成有机中间体,具有多种合成途径(合成气法、CO2为碳源、COS/CS2反应路径)。(2)目前针对甲硫醇的合成相关的催化剂体系、催化剂性能调控、反应过程机理已有较多研究,但仍存在一些争议。(3)未来研究者们应结合当前先进的表征技术及理论计算等,从更深入的原子分子水平上去解析催化剂的活性相与反应过程机理.
酸性气体的转化与综合利用(酸性气体制合成气、硫化氢甲烷制二硫化碳和氢气)应该和进行深加工(如制甲硫醇等)相结合,以便向高附加值方向发展。总之,酸性气体的利用一定要从长远发展着想,既要符合社会经济效益,也要符合环境效益。



 下载:
下载: