-
溶解性有机质(dissolved organic matter,DOM)是指能够通过0.45 μm滤膜的有机碳组分,是一类以苯环和脂肪烃为骨架并含有多种官能团的复杂多组分有机物[1-2]。DOM广泛存在于环境水体中,约占水体中总有机碳的90%,对全球碳循环具有重要的影响[1-2]。此外,DOM还能显著影响水环境的生物地球化学过程[3-4],包括为微生物循环供给碳源和能源、通过螯合吸附等作用影响重金属和有机污染物的迁移转化、吸收光从而减少光的穿透和引起光化学反应、控制水体里的溶解氧、pH以及营养元素的循环和生物有效性等。因受来源、水质及环境过程的影响,DOM在水环境中的浓度、组成结构及性质差异很大,不同来源和不同组分的聚集进一步加大了DOM组成结构的复杂度和异质性[1]。
DOM在生物地球化学循环中的重要作用推进了人们对其组成和结构的研究,并发展了光谱分析、核磁共振和超高分辨质谱等多种表征DOM的技术[4-7],极大促进了对DOM结构特征、反应活性、参与环境过程以及区域和全球碳循环的认识。尽管如此,人们对DOM的化学组成结构以及DOM在不同环境条件下的迁移转化仍缺乏全面理解[4]。由于DOM本身组成结构极其复杂,且通常以低浓度(1—20 mg·L−1)存在于盐含量高的复杂水体中(淡水盐度约为0.01‰—0.5‰,海水平均盐度为35‰)[4, 8],同时部分表征方法需要低盐、高纯度或高质量的DOM样品[4, 9-10],因此需要发展分离富集DOM的高效前处理方法。分离指将DOM组分与无机组分分开,富集指通过降低溶液总体积从而提高DOM的浓度。对环境水体DOM进行分离富集需要克服3个困难[11]:完全去除水体尤其是海水中的盐、去除水和保留DOM中大部分甚至全部有机物。目前用于分离富集水体中DOM的方法有固相萃取法(solid phase extraction,SPE)[10, 12-13]、超滤法[4, 14-15]、透析法[16]和反渗透/电解析联用法[11, 17-19],且已有文献从原理、步骤和应用等方面对DOM分离技术进行了总结[4, 20-22]。其中SPE能有效分离富集DOM,且简单快捷,重现性好,是目前应用最广的DOM分离富集方法[4]。SPE方法目前存在的主要问题是吸附剂对部分组分的保留性差,或部分组分的洗脱率低,导致SPE在分离富集部分水体DOM的效率仍不理想,近年来相关学者为提升SPE分离富集DOM的效率做了大量工作,然而目前尚缺乏针对SPE分离富集环境水体DOM的进展介绍。
本研究主要归纳和总结了SPE分离富集DOM的原理和步骤、使用的吸附剂、洗脱溶剂和步骤、影响因素,以及提高SPE效率的改进方法,并对SPE萃取DOM的效率和选择性进行了探讨。
-
SPE是目前应用最广泛的DOM分离富集方法,该方法首先将DOM组分选择性地保留在SPE的吸附材料上,然后用选择性洗脱的方式对DOM进行分离、富集和净化[4],SPE具有操作简便、分离物纯度高、萃取效率高等优点[10, 23]。
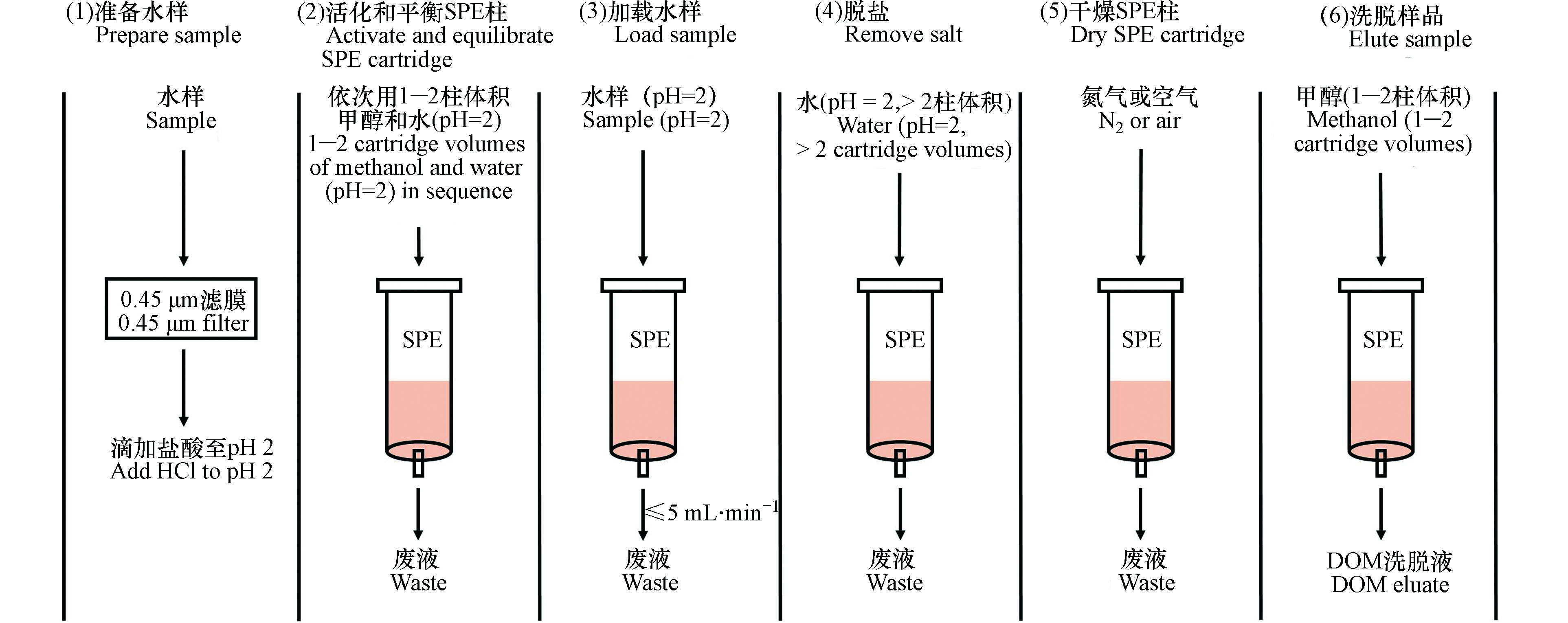
SPE分离富集水样中DOM的流程如图1所示,总共分为6步[10, 12, 24-25]。(1)水样过滤和酸化,水样首先通过滤膜去除颗粒物,常用的滤膜材料有醋酸纤维、聚碳酸酯、玻璃纤维等,滤膜孔径一般为0.45 μm,也有研究使用0.2 μm和0.7 μm的滤膜。过滤后的水样用甲酸或者盐酸酸化至pH 2,酸化的主要目的是让DOM中有机酸或者酚类物质等组分呈中性状态[26-27],从而提高SPE的萃取效率;(2)SPE柱活化和平衡,活化是用1—2柱体积甲醇淋洗SPE小柱,以使吸附剂保持湿润,平衡是用与水样一致的酸化超纯水(pH=2)淋洗SPE小柱,以除去甲醇并为上样创造适宜的溶剂环境;(3)上样,即利用抽真空或者重力作用使酸化水样流经吸附剂并保留DOM,该步骤需要控制流速和水样加载量;(4)除盐,即利用至少2柱体积的酸化超纯水(pH=2)过柱以除去保留在吸附剂上的无机盐;(5)干燥,即利用空气或者氮气吹干已加载完样品并去除盐的SPE小柱,该步骤不适用于硅胶基吸附剂;(6)洗脱,即利用至少1柱体积甲醇洗脱吸附剂所保留的DOM。洗脱溶剂通常用冻干或者氮吹方式干燥,保留的DOM组分然后溶于水测溶解性有机碳(dissolved organic carbon,DOC)含量,或定容至特定的溶液中进行光谱或者质谱表征。DOC回收率常用于评估SPE分离富集水体中DOM的效率,其计算公式如下所示[4]:
其中,DOC萃取是萃取液的DOC浓度,DOC空白是空白对照(加入与样品相同体积的超纯水)萃取液的DOC浓度,V萃取是萃取液的体积;DOC样品是原始水样的DOC浓度,V样品是过SPE柱的水样体积。
-
DOM的组成和结构极其复杂,选择合适的吸附剂对于SPE萃取DOM具有重要的影响。目前用于分离富集水体DOM的SPE吸附剂主要以硅胶基和高聚物为基质(表1)[10, 12, 28]。以硅胶基为基质的常见反相SPE柱有C8、C18、C18OH(不封端的十八烷基键合相)等,正相SPE柱有氨基、氰基、二醇基柱等[28]。高聚物吸附剂具有疏水性结构和高比表面积,并能引入不同修饰的官能团,因此能保留从极性到非极性的目标物[10, 12, 28]。苯乙烯-二乙烯基苯(styrene-divinylbenzene,SDVB)共聚物是商用SPE柱使用最广的骨架基质材料,常见的高聚物SPE柱有Bond Elut PPL、Oasis HLB等。相比于高聚物吸附剂,硅胶基吸附剂对极性物质的提取效率低、在极端pH值下不稳定、以及残存硅醇基[28],因此在DOM分离富集的应用不如高聚物吸附剂广泛。
为从淡水和海水中分离具有代表性的DOM样品,多种以硅胶基和高聚物为基质的SPE柱被用于DOM的提取。Dittmar等以经过修饰的SDVB共聚物(PPL和ENV柱)和键合不同长度碳链的硅胶基(C8、C18、C18OH和C18EWP柱)为吸附剂,提出了基于SPE柱提取海水DOM的简便方法(图1)[10]。结果表明,硅胶基吸附剂对DOM的提取效率低于高聚物吸附剂,C18是硅胶基吸附剂中回收率最高的吸附剂,PPL柱对DOM的萃取效率最高,以溶解性有机碳(dissolved organic carbon,DOC)计回收率能达到62%,比C18柱效率高30%;C18OH柱在低pH值下不稳定,引起了样品的严重污染[10]。ENV柱对DOM的回收率低于PPL柱,这与两种吸附剂的孔径有关(45 nm vs 15 nm),孔径大的ENV柱不利于保留占DOM 70%以上的小分子组分(<1 kDa)[10],这说明吸附剂孔径也能影响DOM的分离富集。对海水而言,因PPL柱提取组分的C/N比和水样接近,而C18柱提取组分则具有更高的C/N比,因此PPL更适合含氮量高的DOM的提取,对于含氮量低的DOM例如陆源DOM,C18和PPL柱都适合用于DOM的分离富集[10]。Perminova等比较了3种高聚物吸附剂(XAD-8、PPL、C18)和阴离子交换树脂二乙胺基乙基纤维素(diethylaminoethyl-cellulose,DEAEC)对北极地区河流DOM的分离效果,发现DEAEC树脂分离DOM的DOC回收率最高(82%),C18和PPL柱的回收率接近(67%—68%),XAD-8树脂的回收率最低仅有50%[23]。C18和PPL柱提取组分的C/N比接近[23],高于Dittmar等的研究结果,说明陆源DOM中的含氮量低于海洋DOM[10]。相比于XAD-8树脂,C18和PPL柱能更好地保留脂肪类物质,DEAEC树脂提取组分的氧含量更高,该结果进一步表明PPL和C18柱能有效分离DOM组分,且操作简便[23]。DEAEC树脂作为一种弱阴离子交换吸附剂,对DOM具有较高的DOC回收率,对羧基碳组分的保留性好,因此也常用于DOM的萃取[29-30]。Raeke等利用SPE柱PPL、HLB和C18对淡水DOM进行了萃取,发现DOC回收率在20%—65%之间,DOM中大分子化合物与PPL和HLB吸附剂的强作用力导致其无法被洗脱,而C18柱不利于保留小分子物质[31]。Yang等评估了五种吸附剂(ENVI-Carb、HLB、PPL以及XAD-8和ENVI-18)对高盐度湖泊DOM的萃取选择性,结果表明HLB和PPL柱对DOM的萃取效率最高,PPL和ENVI-Carb柱表现出了对含氮化合物和具有极性官能团的脂类化合物的高亲和力,HLB柱更易保留氧含量高的化合物和含硫化合物,XAD-8树脂和ENVI-18柱萃取的组分含有较高比例的脂肪族、烯烃和芳香族化合物[32]。Zou等利用HLB和PPL柱对大气气溶胶里的DOM进行了分离富集,发现SPE方法能有效提取具有芳香结构和对紫外可见光有强吸收的组分[33]。
在先前研究基础上,Li等进一步从DOC回收率、分子组成和浸出行为考察了24种市售SPE柱对淡水和海水DOM的分离富集[12]。该研究采用相同的分离步骤和条件,发现高聚物吸附剂对DOM的分离富集效率普遍高于硅胶基吸附剂,DOM回收率(以DOC计)随着SPE吸附剂的极性增大而减小[12]。应用该SPE萃取方法,部分SPE柱如2OH、C18OH、DPA-6S、HLB、SCX、Strata XC和WAX的吸附剂浸出到洗脱液中,导致了较高的表观DOC回收率,HLB柱浸出的组分主要是脂类物质,吸附剂的浸出在应用SPE提取DOM时需引起重视[12]。SPE柱对淡水DOM的回收率在20%—90%之间,远高于海水DOM的回收率(10%—50%),回收率的显著差异与海水和淡水DOM的组成差异有关,海水DOM含有不同极性的组分,淡水DOM含有更多的非极性化合物,因此淡水DOM更容易被非极性的SPE吸附剂例如C8、C18、HLB和PPL保留[10, 12, 23, 34]。海水DOM回收率低还与其高盐度有关,但早期通过向淡水加NaCl或MgSO4仅降低了DOM中大分子组分的回收率[35],因此DOM组成和结构是影响其萃取效率的主要因素[12]。对24种SPE柱分离富集的DOM进行组分分析发现[12],萃取组分的平均H/C和O/C比随SPE吸附剂的极性呈规律性变化,说明极性大的吸附剂更容易保留氧含量低的化合物,而非极性吸附剂则更容易富集氧含量高和不饱和度高的化合物;对比PPL、C18和HLB柱,C18柱萃取组分具有更高的饱和度,HLB柱萃取组分具有更高的O/C比。除了淡水和海水,SPE也常用于分离富集生活污水和工业废水中DOM。Wang等从DOC回收率、分子组成比较了18种商用SPE吸附剂在相同萃取和洗脱条件下对城市污水处理厂中出水DOM的分离富集,结果发现C18OH、PPL、HLB以及ENV柱能有效提取出水DOM(DOC回收率均>56%)[36]。Zhang等利用SPE方法萃取了化工园区污水处理厂中的DOM,发现PPL柱提取组分的DOC回收率均>54%[37]。
在众多商用SPE柱和吸附材料中,PPL和C18柱在分离富集多种环境水体DOM中表现出了更高的效率,是目前应用最广的DOM萃取柱,本研究也主要聚焦于这两种SPE柱在DOM分离富集中的应用。PPL是一种典型的官能化修饰的SDVB吸附剂,能够提取从极性到非极性的化合物,并表现出了可观的回收率,同时具有操作简单、提取DOM组分重复性好的优点,目前已广泛用作解析DOM来源和组成的前处理柱[10, 23, 25, 33]。C18柱是一种十八烷基键合硅胶的吸附剂,含碳密度高,能有效保留如烷烃/烯烃等饱和度高的组分和芳香化合物[15, 23],而对极性小分子物质、脂肪胺/酰胺和含氧量高的单宁类化合物的保留效果差[13, 15],因此C18柱更适合萃取陆源DOM。XAD树脂早期常用来提取DOM,并可根据DOM性质将DOM分为不同组分,然而由于XAD树脂提取DOM操作繁琐,处理样品时间周期长,且频繁使用强酸或强碱易破坏DOM的结构,因此后期逐渐被PPL、C18等吸附剂取代[4];其它吸附剂如硅胶基吸附剂和聚合物吸附剂也被用于DOM分离,然而因预处理费时费力、或只能萃取特定组分导致回收率低、或吸附剂浸出等缺点,未被广泛接受并应用于DOM的分离富集[4, 10, 12, 23, 26]。
-
在经典SPE分离富集DOM的方法中,通常用至少2柱体积以上的甲醇对保留在吸附剂上的DOM进行洗脱。但有研究表明,用SPE萃取DOM并用甲醇洗脱组分损失了大量的DOM组分[4, 12, 38-39],例如氧含量高的单宁类物质[13, 40]、极性大的含氮化合物[40]、脂肪酸类化合物[16]以及大分子物质[31]。除因吸附剂不能有效保留极性大的组分外,导致组分损失的另一个重要原因是部分DOM组分与SPE吸附剂结合力太强,导致其无法被有效洗脱[8, 31]。例如有研究表明,脂肪酸类和低氧类有机硫化合物不易被甲醇洗脱[13, 15-16, 40]。为此,为回收更多的DOM组分,相关人员尝试了用不同溶剂或不同流程洗脱SPE吸附组分。
为防止疏水性更强的组分不能被甲醇洗脱,Dittmar等在用甲醇洗脱后,进一步用丙酮洗脱保留在PPL柱的组分,发现丙酮洗脱的组分使DOM回收率提高了近3%[10]。尽管该组分对海水DOM的回收率提升不大,其具体组成仍值得研究。Zou等在提取大气气溶胶DOM组分的研究中,发现用2%氨水甲醇溶液(体积分数)洗脱HLB保留的组分,显著提升了DOC回收率,最高由43%±0.2%提升到92%±0.3%[33],该研究结果表明了溶剂对SPE洗脱的影响,需要注意的是该研究并没有考虑HLB吸附剂浸出的影响。Coppola等利用SDVB高聚物作为吸附剂(HP-20柱,孔径为20 nm),对淡水、沿海海水和海洋海水DOM进行分离富集,并依次用甲醇、丙酮、乙酸乙酯和二氯甲烷逐步洗脱组分,其对应的组分为极性到半极性、中性脂类和非极性组分[26]。该方法对淡水和海水DOM回收率分别在61%和43%左右,4种溶剂洗脱组分中,甲醇洗脱组分对整体DOC的贡献最大,分别占淡水和海水总DOC的99%和>89%,其余3种溶剂洗脱的低极性组分仅占沿海海水DOC的8%,二氯甲烷洗脱组分占海水DOC的9%[26]。Gao等利用PPL柱对国际腐殖酸协会的天然有机质进行分离富集,然后依次用10 mL 50%甲醇水溶液(体积分数)、纯甲醇和50%甲醇二氯甲烷混合溶剂(体积分数)洗脱[38]。与纯甲醇一次洗脱获得的DOC回收率(53.5%)相比,逐步洗脱的DOC回收率分别为26.4%,31.5%和5.7%,总DOC回收率为63.6%[38],说明多步洗脱有效提升了DOM组分的洗脱效率。
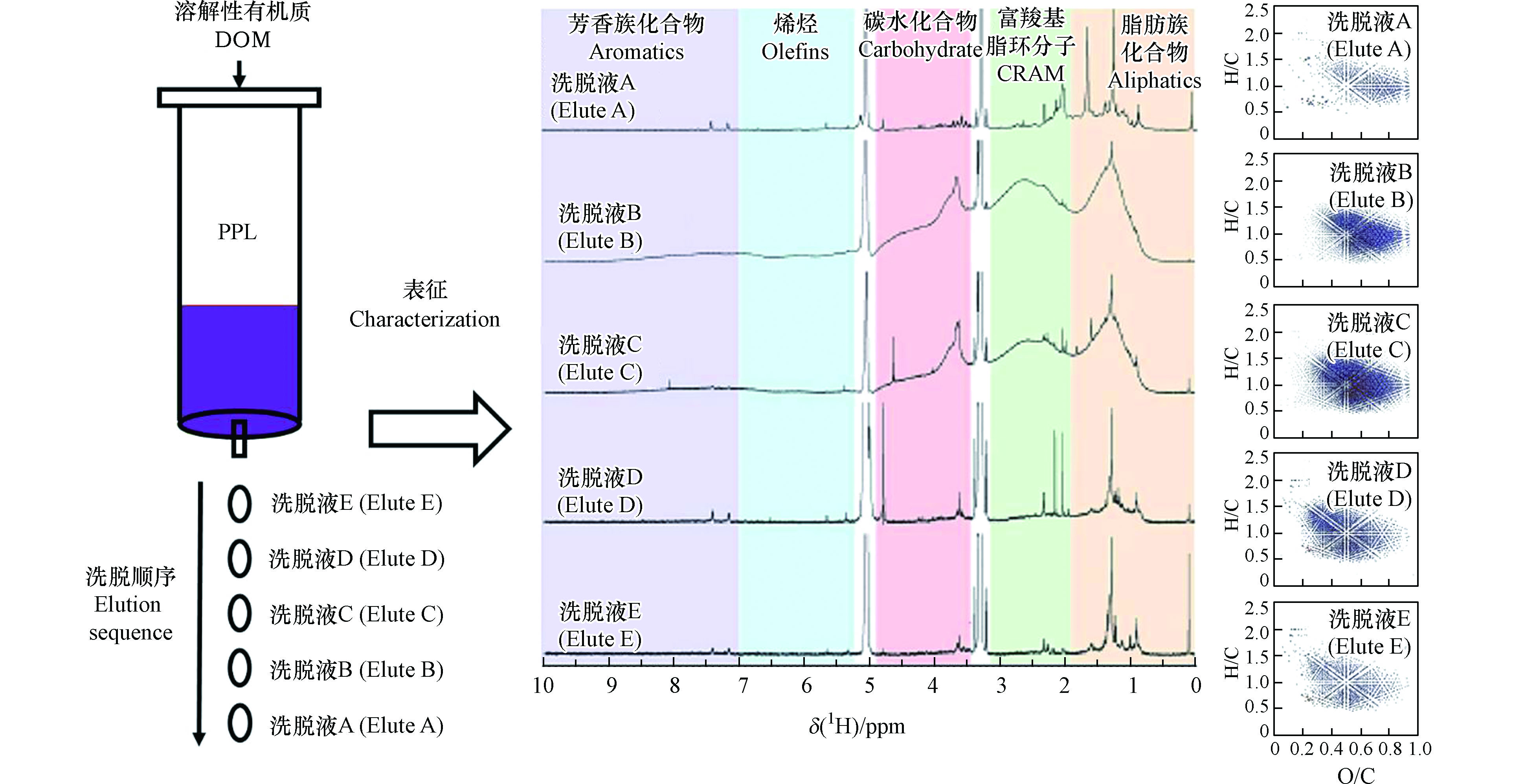
利用相同或不同溶剂多步洗脱SPE提取的DOM组分不仅能提升洗脱效率,对每步洗脱组分单独表征还可减少DOM分子组成的复杂性和相互作用,从而更有利于全面表征DOM的组成和结构[8, 39]。Li等利用PPL柱分离富集312.5 mL萨瓦尼河的有机质,然后用50 mL甲醇分5次洗脱获得5份相同体积的洗脱液,总DOC回收率达到91%[8]。对5种组分进行光谱和分子表征的结果显示[8],5种洗脱液的组分在组成和结构上表现出相似的层级聚类特点,中度疏水性组分是构成DOM的主要组成部分,其他重要组成物质包括脂类物质、富含羧基的脂环分子、碳水化合物和芳烃等;最先洗脱的少量亲水性组分(洗脱液A)由高脂肪类和多种脂环族羧酸类化合物组成,后两次洗脱组分(洗脱液D和E)的疏水性高,可能主要源自去官能团的脂质类化合物(图2)。Lv等根据DOM组成的极性和电离效率,提出了一种简单有效的SPE-逐步洗脱法,该方法分别用12 mL 20%甲醇水溶液(体积分数)、50%甲醇水溶液(体积分数)和纯甲醇洗脱PPL柱吸附的DOM,并将其分成3个组分[39]。傅里叶变换离子回旋共振质谱(Fourier transform ion cyclotron resonance mass spectrometry,FT-ICR MS)分析结果表明[39],与传统一步洗脱方法相比,该方法检出了更多稠环芳烃、黑碳氧化产物和脂类等含氧量低的化合物,另外具有高度亲水性的碳水化合物和单宁类化合物也被大量检出,其中仅含碳、氢和氧以及含氮化合物的识别率提高了50%以上[39]。为洗脱不易被甲醇保留的组分,Gao等用甲醇二氯甲烷混合溶剂洗脱出了弱极性的组分,该组分包含大量的脂类物质且分子量更大[38]。
-
除了SPE吸附剂和洗脱溶剂对DOM萃取有显著影响外,SPE萃取过程的影响参数也需要探究。Li等应用PPL柱富集萨瓦尼河水体的DOM,基于DOC分析、FT-ICR MS和核磁共振波谱法(nuclear magnetic resonance,NMR)等表征手段对SPE分离富集的影响因素进行了系统调查[25]。研究结果表明[25],水样浓度和加载体积对SPE萃取效率有很大影响。当加载溶液(DOC浓度为80 mg·L−1)体积超过25 mL时,SPE的DOC萃取效率随着体积增大而减小。FT-ICR MS和NMR分析结果表明,水样加载体积过大时,与PPL吸附剂作用力较弱的DOM组分会逐渐被与吸附剂作用力较强的组分取代,前者包括含氧量高的组分(O/C=0.5—0.97),例如含多羧基的芳香结构和碳水化合物,以及含有甲氧基官能团的脂类物质和小分子物质,后者主要是氧含量低的组分(O/C=0.4—0.6)和大分子组分,其脂化度和芳香度较高。当水样加载体积一定,SPE萃取DOM的回收率随着水样DOC的增加呈现先升高后降低的趋势,而且低含量和高含量DOC分离的组分也有显著差异,低含量DOC水样萃取组分含有更多的脂类物质,高含量DOM水样(≥10 mg·L−1 C)提取的组分含有更多含氮化合物。水样加载体积和浓度对SPE萃取效率的影响根本上是受DOM总有机碳(total organic carbon,TOC)含量与吸附剂质量比值(TOC/吸附剂质量比)的影响,该比值在1/100—1/200之间时,SPE提取组分的回收率和组分都相对稳定,该比值为1/800时回收率达到了最高的89%。先前也有研究建议将TOC/吸附剂质量比设定在1%—5%之间能获得更均匀的混合物[41]。Dittmar等在其研究中,也建议1 g吸附剂加载水样体积不要超过10 L或者DOC浓度不要超过2 mmol·L−1[10]。
在SPE萃取DOM的过程中,流速过大将导致目标物不能在吸附剂上充分保留,流速过小则会导致处理速度太慢。Li等继续考察了流速对SPE萃取效率的影响,发现流速(0.5—5 mL·min−1)对DOM的萃取效率几乎无影响[25]。SPE吸附剂的质量对DOM的萃取影响较小,不同质量吸附剂提取的相同组分占94%,其影响主要体现在当吸附剂质量≤500 mg时,提取组分含有更高的脂肪类物质;当吸附剂质量≥1 g时,提取组分含有更多的甲氧基官能团;这可能与高质量吸附剂下发生的自酯化反应有关[25]。由于DOM含有的官能团主要以酸性基团(羧基、酚羟基、酮基)为主[1],因此SPE方法提取DOM之前通常需将水样pH调至酸性(pH=2),从而使酸性官能团保持中性并提高SPE吸附剂对DOM的保留效率,因此近年来的研究很少就水样pH的调节进行专门探究。
-
尽管基于PPL和C18吸附剂的SPE方法被认为是分离富集水体DOM的有效方法,但DOM组分的复杂性和异质性使得SPE方法在分离富集不同水体DOM时表现出了很大的差异性。前期研究表明,SPE萃取DOM组成差异小的水样时有相似的DOC回收率,但萃取DOM组成差异大的水样时DOC回收率有显著差异,例如SPE萃取淡水DOM的DOC回收率明显高于海水,对陆源DOM的DOC回收率明显高于水源[4, 10, 12]。这与海水DOM或者内源性DOM含有高氧单宁类、亲水性含氮、脂肪胺/酰胺、多糖分子等极性组分有关,这些组分难以被吸附剂保留,易在加载样品和洗脱过程中大量损失[4, 13, 15-16]。SPE吸附剂对DOM组分的选择性保留说明单一吸附剂难以全面地提取DOM不同组分,因此串联多个SPE柱或多种吸附剂以更有效提取水体中DOM得到了重视和研究。
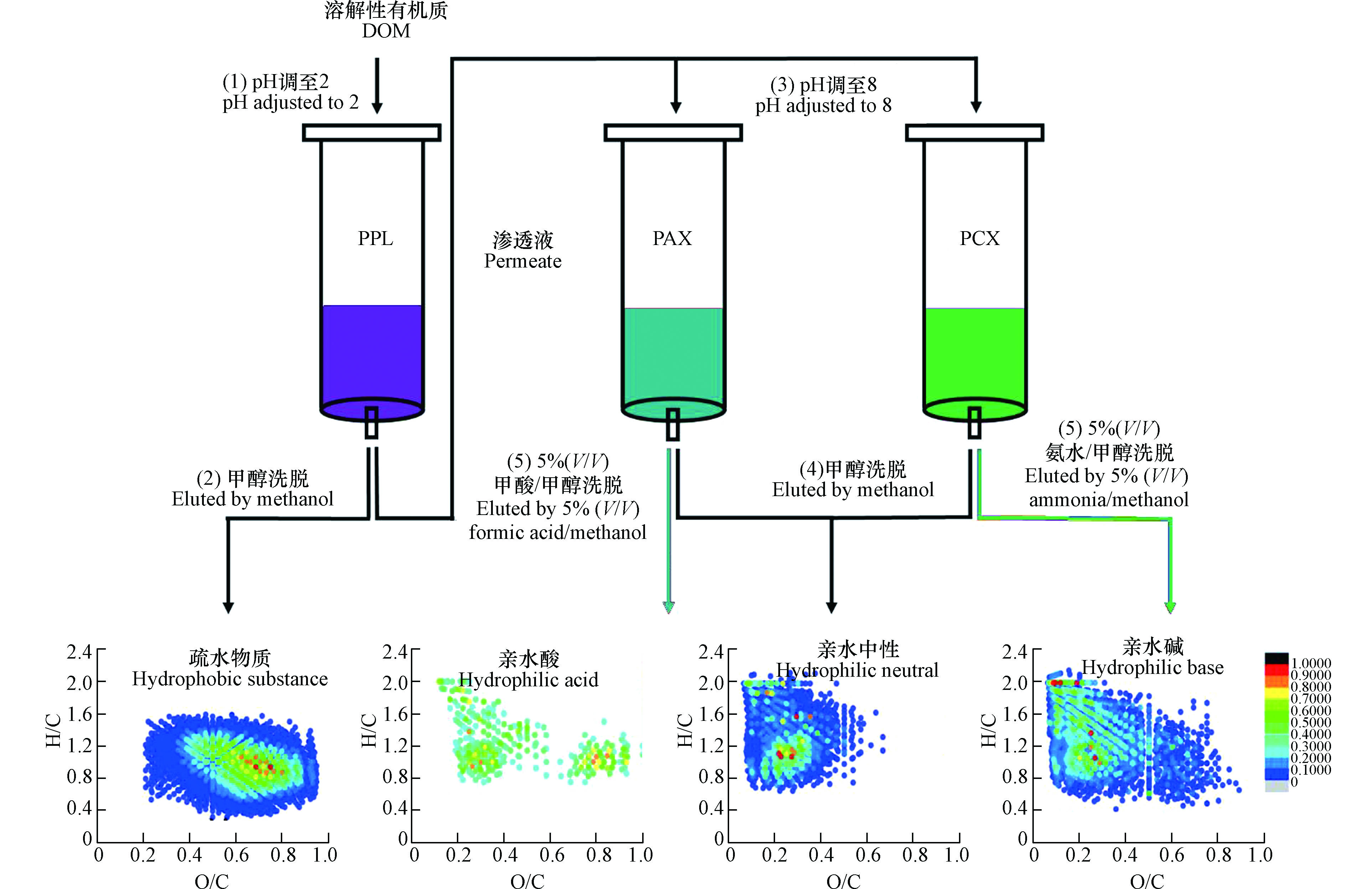
为防止样品过载进而导致穿透,Dittmar等用两支PPL柱对同一海水样品的DOM进行连续萃取,发现仅有不到1%的DOC被保留在第二支PPL柱上,说明PPL柱能高效保留选择性富集的组分[10]。结合兼具反相保留和离子交换吸附功能的SPE吸附剂提取DOM也得到了尝试。Fang等利用Oasis MCX和MAX两种固相萃取柱依次提取洗脱炼油废水DOM,并将其分为疏水酸、疏水中性和疏水碱三个组分,DOC回收率分别为22.0%、36.7%和13.9%,该方法显著提高了炼油废水DOM的回收率[42]。对3种组分进行分子表征发现,酸性化合物和含氮碱性化合物分别被富集到疏水酸和疏水碱组分中,含有多个氧原子的化合物是疏水酸的主要组分,疏水中性组分中化合物含氧量较少[42]。由于部分亲水性强的组分不易被PPL和C18柱等保留,Wang等在用PPL柱提取DOM后,进一步用兼具反相保留和离子交换吸附功能的PAX和PCX柱对DOM进行了萃取,并将DOM组分分为疏水物质、亲水酸、亲水中性以及亲水碱的4个组分(图3)[43]。DOC分析结果显示,4种组分的回收率分别为74.6%、2.1%、1.8%和1.6%;光谱和分子表征结果显示,亲水性组分和疏水性组分在分子组成上有显著差异,亲水性组分主要由类酪氨酸和类色氨酸组成,亲水酸主要由高O/C比的单宁酸类化合物组成,亲水碱主要由脂肪族胺/酰胺类化合物组成,亲水中性主要由低O/C比的木质素和脂类化合物组成[43]。因在前期研究中发现不同源腐殖质中的含羧基组分在Van Krevelen图上具有相似的分布[44-45],Zherebker等用PPL柱连续萃取了不同pH(7、5、3和2)值下的溶解性腐殖质,即先将溶液pH调到7,然后过PPL柱,过柱后的渗透液继续调pH值和过PPL柱,该方法有利于分离脂肪族、芳香族和含羧基化合物,分离的4个组分饱和度逐渐降低,在pH=5时分离组分主要以脂肪族羧酸为主,在pH=3时分离组分主要以芳香羧酸为主[46-47]。尽管多柱联用或者结合不同吸附剂提取DOM能提高DOC回收率和分离效率,但由于其效果不显著且增加了成本和操作流程,目前关注仍然有限。
-
SPE方法目前已被广泛用于萃取水体DOM,但由于DOM的复杂性和异质性以及吸附剂对部分结构的选择性,使得SPE萃取DOM的回收率仍不理想,尤其在海水中,DOC回收率通常低于50%[10, 12, 48]。另外以SPE萃取组分解析DOM的整体组成结构和环境行为,会遗漏其他未被保留的组分从而导致很大偏差[48]。为提高环境水体DOM的DOC回收率和获得更具代表性的DOM样品,研究人员开展了SPE结合其他分离富集方法萃取DOM的研究[15]。
大分子组分(>1 kDa)占总DOC浓度的20%—70%,但SPE方法不能有效回收DOM中的大分子组分[15, 31]。超滤法使用的截留膜能有效保留大分子组分,因此SPE结合超滤法能有效保留DOM中的大分子和小分子组分,从而提升DOM的回收率。Simjouw等将1 L河口水样依次通过超滤(1 kDa)和C18柱对DOM进行分离富集,两种萃取方式的DOC回收率分别为50%和20%,C18柱萃取组分与超滤法萃取大分子组分存在显著差异,前者是以芳香类物质为主要组成的小分子组分,后者组分主要包括多糖和氨基糖类物质[15]。Broek等首先用超滤法分离出大分子组分(≥2.5 kDa),然后用PPL柱萃取剩余组分并获得小分子组分[48],两种方法结合提高了对不同深度海水DOM的提取效率,平均DOC回收率为37%。对其组分进行分析,发现超滤分离的高分子组分主要由年代较短的半不稳定化合物组成,而PPL柱萃取的低分子组分主要由年代较长的难降解化合物组成。
前期研究表明,渗析法萃取的DOM组分与SPE法有显著差异,其中SPE不能有效萃取羧酸类、有机硫和碳水化合物等极性化合物,而渗析法萃取的组分含有大量的羧酸类化合物[16];反渗透/电渗析联用法在萃取海水DOM上表现出了高于其它方法的DOC回收率,最高能达到90%,同时还能萃取超滤法和SPE不能保留的物质[11, 18]。因此Green等结合PPL柱和反渗透/电渗析联用法对海水中DOM进行了分离富集,表层水和深层水DOC回收率分别达到了98%和101%,同时该组合方法提取组分的C/N比更接近原始水样[9]。
由于氧含量高和极性大的单宁类物质是海水DOM的重要组分,且不容易被SPE吸附剂保留,Li等利用该类物质易与铁(Ⅲ)(氧)氢氧化物共沉淀,发展了基于Fe(OH)3共沉淀与SPE相结合分离富集海水DOM的方法[49]。该方法向海水里加入1 mmol·L−1的Fe(Ⅲ),然后得到上清液和沉淀物,前者利用SPE方法进行富集得到组分F1,后者溶解在pH=2的溶液里再用SPE方法富集得到组分F2。与不加Fe(Ⅲ)仅用SPE前处理的方式对比,该方法将DOC回收率提高了25%,FT-ICR MS检出的分子式数量增加了51%,组分分析结果表明海水DOM具有更高的饱和度,F2含有更多的单宁类物质。该方法有望用于海水DOM稳定组分的前处理。
-
DOM由于其巨大的复杂性和异质性,以及通常以低浓度存在于基质复杂的水环境中,目前仍无法完整地从分子水平上对其进行定性定量表征。SPE因其操作简单快捷,是目前应用最广的DOM分离富集方法。为获得高回收率和更具代表性的DOM样品,多种吸附剂被应用于分离富集DOM,其中PPL和C18柱因具有更高的DOC回收率和重现性,是目前应用最广的SPE柱。应用多种表征手段对SPE萃取组分进行表征,揭示了SPE萃取DOM的效率和选择性。围绕着PPL和C18两种SPE柱,多种影响DOM回收率和代表性的因素得到了探索和优化,包括洗脱溶剂和洗脱条件、样品加载量和体积、流速以及吸附剂用量。由于SPE吸附剂对DOM组分的选择性,单一的吸附剂难以满足对水体尤其是海水中DOM的高效分离,因此发展了多种SPE柱串联以及SPE联用其他方法分离富集DOM,有效提高了DOM的萃取效率。综合考虑DOC回收率、萃取时间和成本,水体中基于SPE分离富集DOM仍推荐Dittmar等提出的操作方法,对于以内源性DOM为主的水体例如海水和富营养化水体,PPL柱是更优的选择,对于以陆源性DOM为主的水体,PPL柱和C18柱都是合适的选择。为保证DOM的SPE回收率,水样pH通常需调至2,洗脱溶剂首选甲醇,水样过SPE柱的流速≤5 mL·min−1,溶剂洗脱的流速≤2 mL·min−1,样品DOC总量与吸附剂质量比值建议保持在1/100—1/200之间。尽管SPE分离富集DOM提升了对DOM组成结构和生物地球化学循环的认识,但SPE对极性小分子、强极性物质和大分子物质的低保留率或低洗脱率仍是DOM完整表征亟需克服的难点,对海水和内源性DOM的高效SPE分离富集方法仍需不断发展和优化。由于经典SPE洗脱的DOM会导致在谱图上产生大量叠加和投影的信息,以及无法将原始数据与DOM分子特征显著关联,因此如何提高洗脱组分的分辨率和识别率也需要重点关注。
固相萃取法分离富集环境水体中溶解性有机质的研究进展
Isolation and concentration of dissolved organic matter by using solid phase extraction method in environmental waters
-
摘要: 溶解性有机质(dissolved organic matter,DOM)是具有芳香族和脂肪族碳氢化合物结构以及富含多种官能团和元素的复杂有机混合物,在陆生和水生环境中的物理、化学和生物过程中起着至关重要的作用。DOM巨大的复杂性和异质性,以及在环境水体中的浓度较低并与无机盐共存,给分离富集DOM并对其组成和结构进行全面深入分析带来了巨大的挑战。本文围绕DOM分离富集应用最广的固相萃取法(solid phase extraction,SPE),阐述了该方法的原理和步骤,萃取DOM的吸附剂、洗脱和影响因素、以及多SPE柱串联和SPE结合其他方法萃取DOM的进展,以期促进对基于SPE分离富集水体中DOM及其表征的认识和理解。
-
关键词:
- 固相萃取法(SPE) /
- 溶解性有机质(DOM) /
- 表征方法 /
- 分离富集
Abstract: Dissolved organic matter (DOM) is a complex mixture with aromatic and aliphatic hydrocarbon structures and rich in various functional groups and elements. DOM plays a vital role in physical, chemical and biological processes in terrestrial and aquatic environments. The huge complexity and heterogeneity of DOM, as well as its low concentration in environmental waters and coexistence with inorganic salts, bring great challenges to the isolation and concentration of DOM, as well as the further comprehensive analysis of its compositions and structures. This article focuses on the solid phase extraction (SPE), which is the most widely used method for DOM isolation and concentration, describes the principle and basic processes of this method, the adsorbents for DOM extraction, elution and influencing factors, and summarizes the research progress on the improvement of extraction efficiency by using multiple SPE adsorbents and combining the SPE with other methods for DOM extraction. This article will provide a scientific basis for enhancing the understanding of DOM extraction by SPE and DOM characterization. -

-
吸附剂类别
Category吸附剂
Adsorbent组成
Composition平均孔径/nm
Mean pore
size粒径和形状/μm
Particle size
and shape封端
Endca-
pped非极性 PPL 官能化修饰的SDVB/聚合物 15 125,半球形 是 C18OH 十八烷基/硅胶基 15 40和120,不规则 否 C18 官能化的十八烷基/硅胶基 6 40和120,不规则 是 C18EWP 官能化修饰的SDVB 50 40,不规则 是 HLB N-乙烯基吡咯烷酮-二乙烯基苯/聚合物 8 30 — ENVI-Carb 石墨化无孔碳黑 n.a. 80—100 — ENV 非官能化聚SDVB/聚合物 45 125,球形 — ENVI-18 十八烷基/硅胶基 6 45,不规则 是 中等非极性 C8 辛基/硅胶基 6 40和120,不规则 是 CH 环己基/硅胶基 6 40和120,不规则 是 PH 苯基/硅胶基 6 40—120,不规则 是 阴离子交换混合 WAX 哌嗪基,N-乙烯基吡咯烷酮-二乙烯基苯/聚合物 8 30 否 MAX 季胺基,N-乙烯基吡咯烷酮-二乙烯基苯/聚合物 8 30 否 PAX 官能化亲水SDVB 10 45,球形 — SAX 三甲基氨丙基/硅胶基 6 40和120,不规则 否 阳离子交换混合 PCX 官能化亲水SDVB 10 45,球形 — MCX 磺酸基,N-乙烯基吡咯烷酮-二乙烯基苯/聚合物 8 30 否 WCX 羧基,N-乙烯基吡咯烷酮-二乙烯基苯/聚合物 8 30 否 Strata XC 磺酸基/聚合物 n.a. 33 — SCX 苯磺酸/硅胶基 6 40和120,不规则 否 弱非极性和中极性 CN-E 氰丙基/硅胶基 6 40—120,不规则 是 CN-U 氰丙基/硅胶基 6 40—120,不规则 否 CBA 羧酸/硅胶基 6 40,不规则 是 极性和强极性 NH2 氨丙基/硅胶基 6 40,不规则 否 2OH 二醇基/硅胶基 6 40,不规则 否 SI 自然硅/硅胶基 6 40和120,不规则 否 DPA-6S 聚酰胺/聚合物 n.a. 50—160 — 注:n.a.(not available)表示未获得该数据,“—”表示吸附剂没有该性质。封端是指:加入单官能团化合物使吸附剂的端基功能团消失.
Note: n.a. indicates that the data is not available, “-” indicates that the sorbent does not have this property. Endcapped refers to the disappearance of the end-group of the adsorbent by the addition of monofunctional compounds. -
[1] LEENHEER J A, CROUE J P. Peer reviewed: Characterizing aquatic dissolved organic matter [J]. Environmental Science & Technology, 2003, 37(1): 18A-26A. [2] MOPPER K, STUBBINS A, RITCHIE J D, et al. Advanced instrumental approaches for characterization of marine dissolved organic matter: extraction techniques, mass spectrometry, and nuclear magnetic resonance spectroscopy [J]. Chemical Reviews, 2007, 107(2): 419-442. doi: 10.1021/cr050359b [3] HASSETT J P. Dissolved natural organic matter as a microreactor [J]. Science, 2006, 311(5768): 1723-1724. doi: 10.1126/science.1123389 [4] MINOR E C, SWENSON M M, MATTSON B M, et al. Structural characterization of dissolved organic matter: A review of current techniques for isolation and analysis [J]. Environmental Science. Processes & Impacts, 2014, 16(9): 2064-2079. [5] DERRIEN M, BROGI S R, GONçALVES-ARAUJO R. Characterization of aquatic organic matter: Assessment, perspectives and research priorities [J]. Water Research, 2019, 163(Octa15): 114908.1-114908.17. [6] NEBBIOSO A, PICCOLO A. Molecular characterization of dissolved organic matter (DOM): A critical review [J]. Analytical and Bioanalytical Chemistry, 2013, 405(1): 109-124. doi: 10.1007/s00216-012-6363-2 [7] DERENNE S, NGUYEN TU T T. Characterizing the molecular structure of organic matter from natural environments: An analytical challenge [J]. Comptes Rendus Geoscience, 2014, 346(3): 53-63. [8] LI Y, HARIR M, LUCIO M, et al. Comprehensive structure selective characterization of dissolved organic matter by reducing molecular complexity and increasing analytical dimensions [J]. Water Research, 2016, 106: 477-487. [9] GREEN N W, MICHAEL PERDUE E, AIKEN G R, et al. An intercomparison of three methods for the large-scale isolation of oceanic dissolved organic matter [J]. Marine Chemistry, 2014, 161: 14-19. doi: 10.1016/j.marchem.2014.01.012 [10] DITTMAR T, KOCH B, HERTKORN N, et al. A simple and efficient method for the solid phase extraction of dissolved organic matter (SPE-DOM) from seawater [J]. Limnology and Oceanography: Methods, 2008, 6(6): 230-235. doi: 10.4319/lom.2008.6.230 [11] GURTLER B K, VETTER T A, PERDUE E M, et al. Combining reverse osmosis and pulsed electrical current electrodialysis for improved recovery of dissolved organic matter from seawater [J]. Journal of Membrane Science, 2008, 323(2): 328-336. doi: 10.1016/j.memsci.2008.06.025 [12] LI Y, HARIR M, UHL J, et al. How representative are dissolved organic matter (DOM) extracts? A comprehensive study of sorbent selectivity for DOM isolation [J]. Water Research, 2017, 116: 316-323. doi: 10.1016/j.watres.2017.03.038 [13] SLEIGHTER R L, HATCHER P G. Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization fourier transform ion cyclotron resonance mass spectrometry [J]. Marine Chemistry, 2008, 110(3-4): 140-152. doi: 10.1016/j.marchem.2008.04.008 [14] BENNER R, PAKULSKI J D, MCCARTHY M, et al. Bulk chemical characteristics of dissolved organic matter in the ocean [J]. Science, 1992, 255(5051): 1561-1564. doi: 10.1126/science.255.5051.1561 [15] SIMJOUW J P, MINOR E C, MOPPER K. Isolation and characterization of estuarine dissolved organic matter: Comparison of ultrafiltration and C18 solid phase extraction techniques [J]. Marine Chemistry, 2005, 96(3): 219-235. [16] TFAILY M M, HODGKINS S, PODGORSKI D C, et al. Comparison of dialysis and solid-phase extraction for isolation and concentration of dissolved organic matter prior to Fourier transform ion cyclotron resonance mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2012, 404(2): 447-457. doi: 10.1007/s00216-012-6120-6 [17] VETTER T A, PERDUE E M, INGALL E, et al. Combining reverse osmosis and electrodialysis for more complete recovery of dissolved organic matter from seawater [J]. Separation and Purification Technology, 2007, 56(3): 383-387. doi: 10.1016/j.seppur.2007.04.012 [18] KOPRIVNJAK J F, PFROMM P H, INGALL E, et al. Chemical and spectroscopic characterization of marine dissolved organic matter isolated using coupled reverse osmosis–electrodialysis [J]. Geochimica et Cosmochimica Acta, 2009, 73(14): 4215-4231. doi: 10.1016/j.gca.2009.04.010 [19] KOPRIVNJAK J F, PERDUE E M, PFROMM P H. Coupling reverse osmosis with electrodialysis to isolate natural organic matter from fresh waters [J]. Water Research, 2006, 40(18): 3385-3392. doi: 10.1016/j.watres.2006.07.019 [20] 王立英, 吴丰昌, 张润宇. 应用XAD系列树脂分离和富集天然水体中溶解有机质的研究进展 [J]. 地球与环境, 2006, 34(1): 90-96. WANG L Y, WU F C, ZHANG R Y. A method of separate and concentrate dissolved organic matter by xad resin in natural aquatic systems [J]. Earth and Environment, 2006, 34(1): 90-96(in Chinese).
[21] 王立英, 吴丰昌, 黎文, 等. 水体溶解有机质富集分离方法的研究进展 [J]. 地球与环境, 2008, 36(2): 171-178. WANG L Y, WU F C, LI W, et al. Advances in research on the methods of concentrating and isolating dissolved organic matter in aquatic water [J]. Earth and Environment, 2008, 36(2): 171-178(in Chinese).
[22] 卫丹丹, 王映辉, 许云平. 海水溶解有机质分离富集方法的发展与比较 [J]. 海洋环境科学, 2019, 38(6): 977-984. doi: 10.12111/j.mes20190623 WEI D D, WANG Y H, XU Y P. Development and comparison of pretreatment methods for separation and enrichment of marine dissolved organic matter [J]. Marine Environmental Science, 2019, 38(6): 977-984(in Chinese). doi: 10.12111/j.mes20190623
[23] PERMINOVA I V, DUBINENKOV I V, KONONIKHIN A S, et al. Molecular mapping of sorbent selectivities with respect to isolation of arctic dissolved organic matter as measured by Fourier transform mass spectrometry [J]. Environmental Science & Technology, 2014, 48(13): 7461-7468. [24] MÉNDEZ-DÍAZ J D, SHIMABUKU K K, MA J, et al. Sunlight-driven photochemical halogenation of dissolved organic matter in seawater: A natural abiotic source of organobromine and organoiodine [J]. Environmental Science & Technology, 2014, 48(13): 7418-7427. [25] LI Y, HARIR M, LUCIO M, et al. Proposed guidelines for solid phase extraction of suwannee river dissolved organic matter [J]. Analytical Chemistry, 2016, 88(13): 6680-6688. doi: 10.1021/acs.analchem.5b04501 [26] COPPOLA A I, WALKER B D, DRUFFEL E R M. Solid phase extraction method for the study of black carbon cycling in dissolved organic carbon using radiocarbon [J]. Marine Chemistry, 2015, 177: 697-705. doi: 10.1016/j.marchem.2015.10.010 [27] LOUCHOUARN P, AMON R M W, DUAN S, et al. Analysis of lignin-derived phenols in standard reference materials and ocean dissolved organic matter by gas chromatography/tandem mass spectrometry [J]. Marine Chemistry, 2010, 118(1): 85-97. [28] FONTANALS N, MARCE R M, BORRULL F. New hydrophilic materials for solid phase extraction [J]. Trac-Trends in Analytical Chemistry, 2005, 24(5): 394-406. doi: 10.1016/j.trac.2005.01.012 [29] BREZONIK P L, BLOOM P R, SLEIGHTER R L, et al. Chemical differences of aquatic humic substances extracted by XAD-8 and DEAE-cellulose [J]. Journal of Environmental Chemical Engineering, 2015, 3(4, Part B): 2982-2990. doi: 10.1016/j.jece.2015.03.004 [30] KIDA M, SATO H, OKUMURA A, et al. Introduction of DEAE Sepharose for isolation of dissolved organic matter [J]. Limnology, 2019, 20(2): 153-162. doi: 10.1007/s10201-018-0561-3 [31] RAEKE J, LECHTENFELD O J, WAGNER M, et al. Selectivity of solid phase extraction of freshwater dissolved organic matter and its effect on ultrahigh resolution mass spectra [J]. Environmental Science. Processes & Impacts, 2016, 18(7): 918-927. [32] YANG K L, ZHANG Y L, DONG Y P, et al. Selectivity of solid phase extraction for dissolved organic matter in the hypersaline Da Qaidam Lake, China [J]. Environmental Science. Processes & Impacts, 2017, 19(11): 1374-1386. [33] ZOU C, LI M, CAO T, et al. Comparison of solid phase extraction methods for the measurement of humic-like substances (HULIS) in atmospheric particles [J]. Atmospheric Environment, 2020, 225: 117370. doi: 10.1016/j.atmosenv.2020.117370 [34] LAM B, BAER A, ALAEE M, et al. Major structural components in freshwater dissolved organic matter [J]. Environmental Science & Technology, 2007, 41(24): 8240-8247. [35] KRUGER B R, DALZELL B J, MINOR E C. Effect of organic matter source and salinity on dissolved organic matter isolation via ultrafiltration and solid phase extraction [J]. Aquatic Sciences, 2011, 73(3): 405-417. doi: 10.1007/s00027-011-0189-4 [36] WANG X, JI Y, SHI Q, et al. Characterization of wastewater effluent organic matter with different solid phase extraction sorbents [J]. Chemosphere, 2020, 257: 127235. doi: 10.1016/j.chemosphere.2020.127235 [37] ZHANG B, SHAN C, HAO Z, et al. Transformation of dissolved organic matter during full-scale treatment of integrated chemical wastewater: Molecular composition correlated with spectral indexes and acute toxicity [J]. Water Research, 2019, 157: 472-482. doi: 10.1016/j.watres.2019.04.002 [38] GAO Y, WANG W, HE C, et al. Fractionation and molecular characterization of natural organic matter (NOM) by solid-phase extraction followed by FT-ICR MS and ion mobility MS [J]. Analytical and Bioanalytical Chemistry, 2019, 411(24): 6343-6352. doi: 10.1007/s00216-019-01943-7 [39] LV J, ZHANG S Z, LUO L, et al. Solid-phase extraction-stepwise elution (SPE-SE) procedure for isolation of dissolved organic matter prior to ESI-FT-ICR-MS analysis [J]. Analytica Chimica Acta, 2016, 948: 55-61. doi: 10.1016/j.aca.2016.10.038 [40] REEMTSMA T, THESE A, LINSCHEID M, et al. Molecular and structural characterization of dissolved organic matter from the deep ocean by FTICR-MS, including hydrophilic nitrogenous organic molecules [J]. Environmental Science & Technology, 2008, 42(5): 1430-1437. [41] THURMAN E M, MARGARET S M. Solid phase extraction: Principles and practice[M]. New York: Wiley, 1998. [42] FANG Z, HE C, LI Y Y, et al. Fractionation and characterization of dissolved organic matter (DOM) in refinery wastewater by revised phase retention and ion-exchange adsorption solid phase extraction followed by ESI FT-ICR MS [J]. Talanta, 2017, 162: 466-473. doi: 10.1016/j.talanta.2016.10.064 [43] WANG W, HE C, GAO Y, et al. Isolation and characterization of hydrophilic dissolved organic matter in waters by ion exchange solid phase extraction followed by high resolution mass spectrometry [J]. Environmental Chemistry Letters, 2019, 17(4): 1857-1866. doi: 10.1007/s10311-019-00898-6 [44] ZHEREBKER A, KOSTYUKEVICH Y, KONONIKHIN A, et al. Enumeration of carboxyl groups carried on individual components of humic systems using deuteromethylation and Fourier transform mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2017, 409(9): 2477-2488. doi: 10.1007/s00216-017-0197-x [45] KOSTYUKEVICH Y, ACTER T, ZHEREBKER A, et al. Hydrogen/deuterium exchange in mass spectrometry [J]. Mass Spectrometry Reviews, 2018, 37(6): 811-853. doi: 10.1002/mas.21565 [46] ZHEREBKER A, SHIRSHIN E, KHARYBIN O, et al. Separation of benzoic and unconjugated acidic components of leonardite humic material using sequential solid-phase extraction at different pH values as revealed by Fourier transform ion cyclotron resonance mass spectrometry and correlation nuclear magnetic resonance spectroscopy [J]. Journal of Agricultural and Food Chemistry, 2018, 66(46): 12179-12187. doi: 10.1021/acs.jafc.8b04079 [47] ZHEREBKER A, SHIRSHIN E, RUBEKINA A, et al. Optical properties of soil dissolved organic matter are related to acidic functions of its components as revealed by fractionation, selective deuteromethylation, and ultrahigh resolution mass spectrometry [J]. Environmental Science & Technology, 2020, 54(5): 2667-2677. [48] BROEK T A B, WALKER B D, GUILDERSON T P, et al. Coupled ultrafiltration and solid phase extraction approach for the targeted study of semi-labile high molecular weight and refractory low molecular weight dissolved organic matter [J]. Marine Chemistry, 2017, 194: 146-157. doi: 10.1016/j.marchem.2017.06.007 [49] LI L J, FANG Z, HE C, et al. Separation and characterization of marine dissolved organic matter (DOM) by combination of Fe(OH)3 co-precipitation and solid phase extraction followed by ESI FT-ICR MS [J]. Analytical and Bioanalytical Chemistry, 2019, 411(10): 2201-2208. doi: 10.1007/s00216-019-01663-y -



 下载:
下载: