-
目前,氨气选择性催化还原(NH3-SCR)技术被广泛应用于燃煤电厂、生物质电厂和垃圾焚烧等固定源的烟气脱硝,为降低我国的氮氧化物排放总量做出了巨大贡献。NH3-SCR技术的核心是催化剂,VW(Mo)Ti催化剂[V2O5-WO3(MoO3)TiO2]是最广泛使用的商业催化剂之一[1],该催化剂在300~450 ℃可以显示出较高的活性。因此,目前NH3-SCR脱硝装置通常布置在锅炉之后、静电除尘器之前,从而可以利用烟气热量以保持运行所需温度[2]。然而,这会导致催化剂暴露于高浓度粉尘和SO2等中。随着运行时间的增加,催化剂活性逐渐降低,导致烟气中NOx浓度无法达到排放要求。脱硝催化剂的使用寿命一般为2~3 年[3],失活脱硝催化剂由于含有有毒金属元素钒和多种吸附的杂质元素(砷和铅等)被归类为国家危险废物(废物代码772-007-50)。目前的主要处理方法是填埋,这不仅浪费钒、钨和钼等重要的金属资源,还会占用土地资源并对环境和人类健康造成潜在危害[4-5]。随着烟气脱硝需求的不断增加,文献[6]估算,2020年后中国每年将产生大约250 000~300 000 m3废SCR催化剂。脱硝催化剂中V2O5含量为1%~3%,WO3含量为5%~10%,TiO2含量为80%~90%。Ti、V和W是重要的金属资源,广泛应用于现代高科技设备、合金等[7]。失活脱硝催化剂的再生和资源化利用可以有效减少废催化剂量、降低处置成本和减轻对环境的影响,具有重要的环境和经济效益。
-
脱硝催化剂的失活是指在催化剂的使用过程中,由于受到复杂烟气条件的影响,催化剂的活性逐渐降低的现象[8]。造成催化剂失活的原因主要可以分为物理失活和化学失活。
物理失活主要是指脱硝催化剂磨损、通道堵塞、“覆盖层”中毒以及高温烧结[9]。催化剂磨损是指在运行过程中由于烟气烟尘的冲刷而导致催化剂表面整体损坏。烟气中的飞灰随着烟气流经脱硝装置时速度变小,灰粒会首先聚集在脱硝装置上游,积累到一定程度后掉落到催化剂通道中,造成催化剂通道堵塞;通道堵塞会使脱硝装置的压降增加,对脱硝装置甚至锅炉的安全运行产生极为不利的影响。“覆盖层”中毒主要是指飞灰中的SiO2、Al2O3、CaO和硫酸盐等沉积在催化剂表面,堵塞催化剂内部微孔,覆盖住催化剂表面的活性位,进而阻碍NOx与NH3在催化剂表面发生反应。脱硝催化剂需长时间在高温下运行,当温度高于450 ℃时,会造成催化剂载体TiO2烧结和晶型转变,引起催化剂比表面积急剧下降、孔隙率变低,导致活性降低。另外高温还会引起催化剂中活性组分V2O5的聚合和挥发损失,从而引起催化活性的急剧下降[10]。此外,聚合后的V2O5还会促进SO2氧化为SO3[11],从而对下游的管道产生不利影响。
化学失活主要是指烟气中含有的某些有害杂质元素使脱硝催化剂的活性、选择性出现明显下降或丧失的现象[12]。固定源烟气中含有大量的杂质元素,如碱和碱土金属元素K、Na、Ca和Mg,重金属元素Pb、Hg、Fe和Zn,以及其他As、S、Cl和P等元素,这些元素大部分在脱硝催化剂活性位点有很强的化学吸附作用,可以与活性位发生反应,改变活性位的结构或通过电子相互作用改变NOx、NH3吸附行为,从而导致催化剂失活[13]。脱硝催化剂失活的不同类型及其失活机理,见表1。
-
对失活脱硝催化剂进行再生可以延长催化剂的使用寿命,降低更换催化剂产生的成本,是处理失活脱硝催化剂的首要选择。由于脱硝催化剂失活的原因复杂,对催化剂进行再生时,需要针对催化剂的失活原因选择相应的再生方法,才能高效地恢复催化剂活性。
-
燃煤或者生物质烟气中的碱(土)金属元素可以造成脱硝催化剂失活。研究表明,钒基催化剂上的酸性位特别是Brønsted酸性位在NH3-SCR反应中起着关键作用:喷入的NH3首先吸附在催化剂表面的酸性位上,继而活化与烟气中NOx和O2反应生成N2和H2O。飞灰中碱(土)金属元素主要为K、Na、Ca和Mg等,这些元素容易与催化剂表面Brønsted酸性位发生反应,改变酸性位结构,阻碍NH3在催化剂表面的吸附,使NH3-SCR反应无法发生,从而导致催化剂活性明显降低[14-15]。目前,针对脱硝催化剂碱和碱土金属元素失活的再生方法主要是去除碱(土)金属元素,恢复催化剂上的Brønsted酸性位,从而提高失活催化剂的活性。常用方法有:水洗再生、SO2硫酸化再生和酸洗再生等。
YUE et al[16]制备了K和Na中毒的商业钒钨钛催化剂,并用热水洗涤对其进行再生。催化剂表面的碱金属可以在热水中得到去除,失活催化剂的活性得到有效恢复。KHODAYARI[17]研究了SO2硫酸化对失活催化剂的再生,发现SO2硫酸化可以在催化剂表面形成硫酸盐从而增加表面酸性位的数量和强度,提高了催化剂的活性。ZHENG et al[18]比较了SO2硫酸化稀硫酸和氯化铵洗涤对K失活的商业脱硝催化剂的再生效果;SO2硫酸化可以增加催化剂的酸性位,但NH3存在时容易与SO2发生反应生成NH4HSO4堵塞催化剂微孔,并且SO2硫酸化无法有效地去除K;稀硫酸和氯化铵洗涤则可以有效地恢复K失活催化剂的活性,0.5 mol/L的H2SO4再生可以有效去除催化剂表面的K并且产生新的Brønsted酸位点,再生后催化剂在温度高于300 ℃时显示出比新鲜催化剂更高的活性。GAO et al[19]也制备了K和Na失活的脱硝催化剂,采用去离子水和超声辅助H2SO4洗涤再生,发现超声波辅助洗涤可以增加SO42−的分散性从而提高再生效果。
稀硫酸可以有效去除催化剂中的碱金属,是目前常用的再生液。但H2SO4在再生过程中容易引起设备腐蚀、催化剂活性成分流失和机械强度降低[20]。YUE et al[21]用稀硫酸对K失活的商业催化剂进行再生,当稀硫酸浓度高于0.2 mol/L时,再生后催化剂中K含量与新鲜催化剂几乎相同,但催化剂中的钒含量则开始下降。除硫酸外,研究者还对中等强酸和酸性较弱的杂多酸进行了研究。WANG et al[22]制备了2% K2O失活的催化剂,比较了不同类型与强度的酸(强酸:H2SO4,中等强酸:H3PO4和C2H2O4,杂多酸:H6O39SiW12、H3O40PW12•xH2O和H5Mo12O41P)的再生效果;结果表明,0.1% H2SO4、0.5% C2H2O4和1% H6O39SiW12的再生效果相近,再生后催化剂NOx转化率在300~350 ℃时超过90%。商雪松等[23]的研究表明(NH4)2SO4溶液可以有效去除催化剂上的K从而恢复催化剂的活性;催化剂在再生过程中没有出现明显的活性物质流失现象,并且机械强度的降低较小。YUE et al[24]还采用电泳法对K、Na失活的催化剂进行再生,发现碱金属离子通过电泳处理几乎全部溶解在水中,使活性得到较好恢复;并且处理过程中不会产生有害的溶液或气体。
实际运行条件下,碱土金属在SO2的作用下会在催化剂上形成硫酸盐。这些硫酸盐难溶或者不溶于水,因此无法用水洗或者酸洗的方式对其进行再生。LI et al[25]采用制备了CaSO4失活的催化剂,用1wt%羟基亚乙基二膦酸(HEDP)溶液进行再生;HEDP液体在弱酸性环境下可以定向络合Ca2+从而提高CaSO4的溶解性,具有较高的Ca去除率。LI et al[26]采用1wt%的OP-10(烷基酚聚氧乙烯醚)溶液再生Ca中毒失活的催化剂,发现聚醚中的桥接氧原子可以与Ca2+相互作用,从而选择性地去除Ca2+;与H2SO4相比,OP-10具有更高的Ca2+去除率和更低的V2O5损失,再生后脱硝催化剂的活性显著提高。
-
燃煤电厂或者垃圾焚烧电厂烟气中含有的重金属元素如Pb、As、Hg和Zn等。目前的研究发现Pb可以导致脱硝催化剂失活。Pb在燃烧过程中转化为PbO、Pb3O4或PbCl2,通过在酸性位的竞争性化学吸附毒化活性位,阻碍催化剂表面NH3的吸附,导致催化剂失活[27]。与碱金属氧化物相比,Pb对脱硝催化剂的毒性介于Na2O和K2O之间[28]。在煤燃烧过程中,煤炭中的As会形成气态氧化物As2O3扩散到催化剂微孔内,吸附在催化剂表面的活性位上,改变活性位结构从而引起催化剂的失活[29]。另外,吸附的As2O3还会被氧化成As2O5覆盖住催化剂活性位,抑制NH3的吸附活化并促进N2O的生成,从而降低脱硝催化剂的活性[30]。目前,关于重金属失活脱硝催化剂的再生方法主要有:水洗再生、酸洗再生、碱洗再生、H2O2清洗再生、热还原法再生和电化学法再生等。
YAN et al[31]采用水、乙酸和硝酸溶液对Pb失活的催化剂进行了再生,结果表明硝酸溶液可增加催化剂表面积,并产生新的酸性位,恢复效果较好。QI et al[32]采用去离子水、乙酸和三亚乙基四胺混合液洗涤对Pb失活的催化剂进行了再生,混合液有效地去除了催化剂微孔中的PbO,再生后催化剂350 ℃的NOx转化率从59.49%增加到95.70%;乙酸可以增加催化剂的酸度,三亚乙基四胺可以络合固定PbO,从而高效地恢复失活催化剂活性。LI et al[33]通过比较乙酸、柠檬酸、Na2CO3、H2SO4、NH3•H2O、NaOH、H2O2和Ca(NO3)2溶液对As失活催化剂的再生效果,结果表明H2O2溶液可以有效地去除As2O5,但容易造成催化剂活性成分V2O5的流失;碱性溶液可以更有效地去除As2O5,1wt% NaOH溶液具有最高的As去除效率,但也会造成较高的V2O5流失,并且Na元素还会沉积在催化剂上造成二次中毒,后续需要采用酸洗将其去除以恢复催化剂的活性;Ca(NO3)2溶液在碱性条件下能有效去除As2O3,且活性成分损失较低,但后续也需要采用酸洗以去除沉积的Ca元素。LI et al[13]也研究了H2O2溶液对As失活催化剂的再生,发现当H2O2浓度高于4 mol/L时,催化剂中As含量保持不变,钒含量开始下降。TiAN et al[34]采用H2SO4、H2O2、H2C2O4、CH3COOH、HCl和NaOH溶液再生As失活的催化剂,发现稀H2SO4有效地去除了覆盖在催化剂表面的As2O3,从而恢复催化剂酸性位点。LU et al[35]采用氨水洗涤-H2还原-空气煅烧法对燃煤电厂As失活催化剂进行再生,结果表明,该方法能有效地去除催化剂表面的As2O3,增强Lewis酸性位和提高表面吸附氧含量,可使再生后催化剂的NOx去除率恢复至96%。XUE et al[36]采用电化学法在碱性条件下对As失活的催化剂进行再生,通过电化学高级氧化过程实现了As的有效去除,并且催化剂载体的晶体结构和原始组成没有发生明显变化,用活性成分浸渍后得到的再生催化剂的活性与新鲜催化剂几乎相同。
-
固定源的烟气中除含有碱、碱土元素和重金属等金属元素外,也含有S、Cl和P等无机非金属元素,在烟气中主要以SO2、HCl、P2O5和H3PO4等气体形式存在,也会引起催化剂活性的下降。
烟气中SO2在低于300 ℃的温度下容易与NH3和H2O反应形成(亚)硫酸铵盐,覆盖催化剂活性位,导致催化剂活性降低[37]。HCl可以与还原剂NH3反应生成NH4Cl,抑制NH3在催化剂表面的吸附活化并可以堵塞微孔;HCl还可与催化剂活性位点发生反应生成金属氯化物,这些都会造成催化剂失活。P在燃烧过程中形成的气态P2O5和H3PO4可以堵塞微孔,并与活性位反应生成碱性磷酸盐沉积在催化剂表面,导致催化剂失活[38]。目前,针对此类失活催化剂的再生研究较少,主要有水洗再生和高温热处理再生等。
XU et al[39]制备了SO2中毒的催化剂,在400 ℃N2气氛下加热30 min有效地分解了催化剂表面的NH4HSO4,使催化活性得到恢复。但金属硫酸盐的分解温度较高,一般热处理难以使金属硫酸盐沉积的催化剂再生[40]。ZHAO et al[41]使用SO2连续吹扫催化剂3 h,得到硫中毒的催化剂样品;分别采用H2/Ar和NH3/Ar还原气体在350 ℃处理3 h对其进行热还原再生,2种方法都能显著恢复催化剂活性,采用NH3还原的催化剂活性更好。不同原因导致脱硝催化剂失活的再生方法及其特点,见表2。
以稀酸和络合剂溶液为代表的溶液清洗可以去除催化剂表面的杂质金属,恢复催化剂表面的酸性位,从而恢复催化剂的活性,是目前脱硝催化剂再生中常用的再生方式。但溶液清洗会引起催化剂活性组分流失,溶液清洗后一般还需进行活性物质植入以提高再生后催化剂中活性物质的含量。另外溶液清洗会产生大量的废水,需要对废水进行进一步处理。碱溶液去除As等杂质元素的效果较好,但碱溶液会对催化剂产生二次失活,碱溶液再生后需要采用酸溶液来去除残留的碱金属离子。电泳法对杂质元素的去除效率高,活性恢复效果好,并且产生废水量较少,应该是一种有前景的催化剂再生技术。
-
目前,脱硝催化剂再生在我国已经有多项工程应用,再生工艺主要包括5个步骤,见图1。
A公司采用的钒钛基脱硝催化剂在运行约36 000 h后对其进行了再生处理,工艺可分为5步:负压吸尘;去离子水清洗;超声波化学清洗;超声波活性载体激活、负载;干燥,与失活催化剂相比,再生后催化剂的脱硝效率提高了40%~50%,NOx的排放浓度可以达到电厂烟气排放标准[42]。B公司对某电厂的废失活催化剂进行了再生,工艺分为7步:酸洗、鼓泡;碱洗;酸洗中和;去铁;漂洗;活性浸渍;干燥、煅烧,再生催化剂活性恢复到初始值的90%以上,并且SO2/SO3转化率低于新鲜催化剂,可以满足电厂的使用要求[43]。C公司对As失活的脱硝催化剂进行了再生,工艺共5个步骤:预处理去除催化剂表面飞灰;物理化学处理去除主要导致催化剂失活的化学物质;中间热处理巩固催化剂微孔结构;负载活性物质;最终热处理,再生后的催化剂脱硝效率达到96%以上,运行8 000 h后催化剂活性未出现明显下降[44]。某300 MW燃煤机组采用蜂窝式钒钨钛脱硝催化剂,在运行约30 000 h后对催化剂进行了再生:除灰;酸洗;活性物质浸渍;干燥,再生后催化剂活性恢复至新催化剂活性的99%,其他各项指标均满足脱硝工程的要求[45]。可以看出,在实际工程应用中,需要针对不同类型失活的催化剂采取针对性的再生方法,从而提高催化剂再生的效果。
-
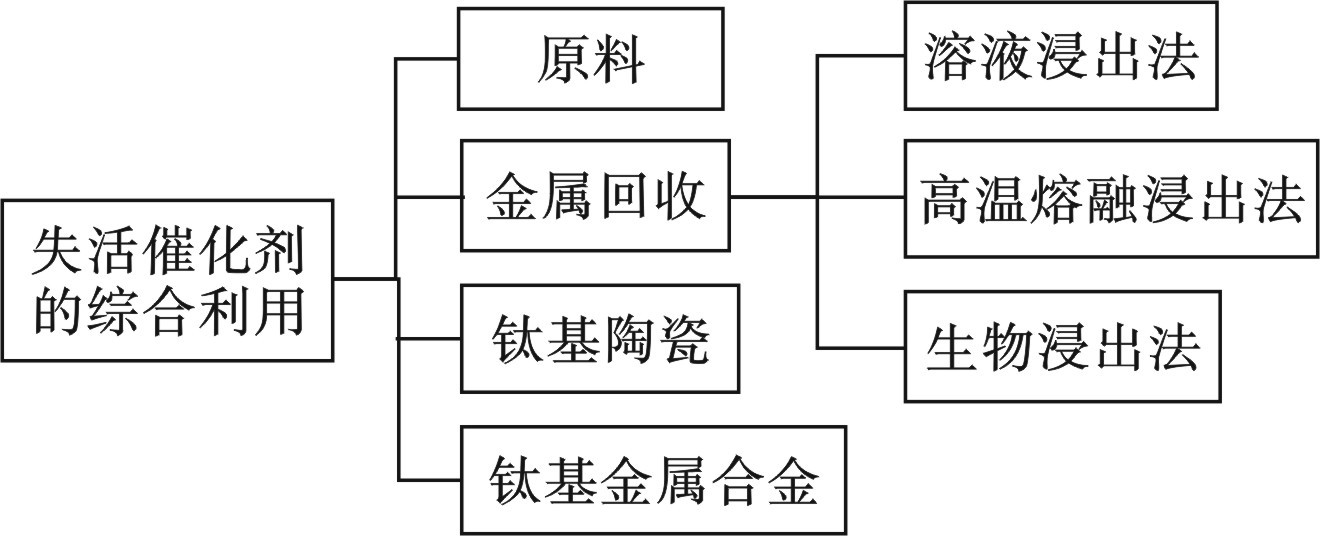
虽然再生可以使失活催化剂的活性得到明显提升,但再生过程有可能引起催化剂结构损坏、材料流失,再生后催化剂的寿命一般低于新鲜催化剂的寿命。经过3~4个再生循环使用后,脱硝催化剂会永久失去催化功能,成为废催化剂[46]。脱硝催化剂组分中主要包含1%~3%的V2O5,5%~10%的WO3或者MoO3,80%~90%的TiO2,以及微量的Si、Ca和Al等元素[47]。使用过后的脱硝催化剂中还会含有K、Na、Pb、As和S等多种元素,因此废脱硝催化剂被列为危险固体废弃物,其综合利用受到很大的限制。目前综合利用的方式主要有作为脱硝催化剂的生产原料、回收有价金属资源、作为钛基陶瓷和钛基金属合金的原料等。失活催化剂的再生方法和综合利用方式,见图2。
-
废脱硝催化剂经过适当处理去除催化剂中的K、Na和Pb等杂质元素后可以按照一定比例与其他原料混合后用于生产脱硝催化剂。何川等[48]将废脱硝催化剂破碎、研磨、过筛后,按不同比例与催化剂生产原料NH4VO3、(NH4)6W7O24•6H2O和TiO2混合后制备脱硝催化剂,研究了废催化剂使用量对新鲜催化剂性能的影响,结果表明,当废催化剂的含量<5wt%时,制备的催化剂脱硝活性没有明显降低。QI et al[49]将运行约35 000 h的失活催化剂研磨并筛分后,采用1.5 mol/L的H2C2O4溶液浸渍除去沉积的碱金属和其他杂质,然后将浸提后的残渣与NH4VO3的H2C2O4溶液共浸渍制备新催化剂,制备的催化剂的脱硝活性几乎达到新鲜催化剂水平,在300 ℃时NOx转化率为91%,且表现出良好的抗SO2/H2O性能。CAO et al[50]用HNO3洗涤粉碎后的废脱硝催化剂,再用NH4VO3和H2C2O4混合溶液浸渍制备新催化剂,负载1.60wt%的V2O5催化剂其脱硝效率达到85%以上。林晓等[51]申请了一种废脱硝催化剂的回收方法,将废脱硝催化剂去除杂质和钒钨钼等组分后,浸渍得到富钛浸出渣和含钨钼钒的浸出液,对浸出液中有价组分同步纯化后,对各个物质含量的比例进行调节,得到催化组分的混合物,用于进一步制备新催化剂。
将废催化剂作为脱硝催化剂的生产原料,处理成本较低,并且可以降低催化剂的生产成本,是可行的利用方式。但其对废催化剂的物理化学性能尤其是比表面积有较高的要求,而运行后的催化剂比表面积一般会出现不同程度的降低,比表面积降低严重的催化剂无法用作生产脱硝催化剂的原料。
-
废脱硝催化剂中的V、W或Mo是重要的金属资源,可以将V、W、Mo与载体TiO2分离后,分别进行回收。回收得到的金属可以直接利用,TiO2可以用于涂料、油漆、油墨、塑料、橡胶和造纸等行业。目前的脱硝催化剂金属资源回收的方法主要有溶液浸出法、高温熔融法和生物浸出法等。
溶液浸出法根据浸出液的酸碱性分为酸浸法和碱浸法。酸浸法一般使用盐酸、硝酸、硫酸和草酸等无机酸和有机酸。草酸相对无机强酸的酸性较弱,是环境友好的浸出剂。TiO2和WO3不能溶解在稀酸溶液中,酸浸法主要用于回收废脱硝催化剂中的钒。韩奇等[52]用草酸将催化剂中的钒浸出后回收,然后使用残留的钛钨粉制备了可以高效降解罗丹明B的Bi2WO6/钛钨粉复合光催化剂。ZHANG et al[53]使用“H2SO4+Na2SO3”酸还原系统选择性浸出废弃商用催化剂中的钒,在最佳浸出条件:95 ℃下浸出2 h后,催化剂中的钒完全溶解到浸出液中;然后使用残渣作为原料,制备出具有较高光催化效率的WO3-TiO2光催化剂。酸浸法虽然可以有效地浸出废脱硝催化剂中的钒,但不能将钨和钛分离回收,并且处理过程中会产生大量的酸性废液。
碱浸法常用于处理较难分解的矿物和固体废弃物。NaOH是废脱硝催化剂碱浸处理过程中广泛使用的试剂,主要用于浸出催化剂中的V和W。戚春萍等[54]发现NaOH溶液可以高效浸出废催化剂中的V和W以及杂质Al、Si等,载体TiO2则几乎完全留存在浸出渣中;经稀酸洗涤后得到纯度较高、孔道较好的锐钛矿型TiO2;同时研究了高温和高压等辅助技术对V和W在NaOH溶液中浸出率的影响;加压可以降低所需NaOH溶液的浓度和缩短浸出时间,提高浸出温度则可以明显缩短浸出时间并提高浸出率[55]。JIAO et al[56]将废脱硝催化剂先后用NaOH和H2SO4浸泡,除去WO3、V2O5和Al2O3等大部分杂质而制得TiO2;通过碳热还原法将TiO2转化为高纯度的Ti2CO,并使用油酸捕获进一步纯化;最后,Ti2CO在NaCl-KCl熔融盐中被电解精炼,金属钛沉积在阴极上,纯度可达99.5wt%。碱浸法可以从废脱硝催化剂中有效回收钒和钨,需要进一步对V和W分离提纯,并且产生的强碱性废水处理难度较大。
高温熔融法通过高温下熔盐的氧化作用强化难分解矿物和碱性浸出剂的化学反应,从而提取目标金属。废脱硝催化剂通常采用Na2CO3和NaOH作为碱源;在焙烧过程中,V、W、Si、Al和其他两性金属可以与熔融碱发生反应转化为水溶性金属钠盐,而Ti则会与熔融碱反应形成不溶的钛酸钠,这些钠盐可以通过溶解分离从而分别回收。ZHANG et al[57]用NaOH焙烧法从废脱硝催化剂中提取TiO2,在最佳焙烧条件下(550 ℃、10 min),Ti的提取率为97.8%,最终得到的TiO2纯度为99.15%。MA et al[46]采用钠盐焙烧法、酸浸法和液相沉淀法相结合的混合工艺回收废脱硝催化剂中的纳米TiO2:首先在650 ℃焙烧使催化剂的组分与碳酸钠反应:Ti转化为不溶于水的Na2TiO3,其他组分转化为可溶于水的盐;不溶部分在80 ℃下用稀硫酸重复浸出;然后加入Na2CO3将富钛酸溶液调节至中性,以获得悬浮在溶液中的偏钛酸颗粒;将沉淀物进行处理得到含有锐钛矿型及少量金红石型TiO2。碱性焙烧可以有效地回收废催化剂的金属资源,然而其需要耐高温、耐碱腐蚀等的特殊设备并且能耗较高,从而导致回收成本较高。
此外,生物浸出法是一种环境友好和成本低的技术,由嗜酸微生物利用铁硫氧化代谢促进固体基质中的金属溶解[58]。WANG et al[59]将废脱硝催化剂粉碎后作为微生物培养基的一部分原料,研究了从废脱硝催化剂中提取V和W的5种不同生物浸出途径,包括硫介导途径、硫铁混合介导途径、碳介导途径、富碳介导途径和铁介导途径;由生长在不同电子供体上的不同功能微生物群落产生不同的浸提液,各个浸出途径在10 d内产生的最大生物浸出效率分别约为90%、35%、33%、20%和7%。但生物浸出法所需时间过长,并且浸出过程中存在较多干扰因素,需要进一步的研究。总结了从废脱硝催化剂中回收有价金属的各种方法,见表3。目前由于操作复杂、回收成本高、产生废液多,从废弃脱硝催化剂中回收有价金属和TiO2仍处于研究阶段,还没有得到实际应用。亟需流程简单、能耗低和环境友好的回收方式。
-
钛基陶瓷具有高熔点、高硬度、优良的耐磨性和化学稳定性等特点,可用于制作切削刀具、磨料、装甲和热保护涂层等。废脱硝催化剂中的TiO2含量很高,有可能作为制备钛基陶瓷的原料,但需要首先解决催化剂中V等有毒元素的固化。
沈岳松等[60]申请了一种以废脱硝催化剂为原料制备钛基陶瓷的方法,将质量百分比30%~80%的废催化剂与其他原料经粉碎、配料、混炼、成型、干燥和煅烧等工艺制备得到性能优异的钛基陶瓷制品;通过添加外加剂,经过高温处理使废脱硝催化剂中的有毒元素V得到固化,得到了符合标准的钛基陶瓷,可作为纺织瓷、建卫瓷及艺术瓷等。刘海沛等[61]将废脱硝催化剂进行吹灰处理、球磨破碎后,与其他原料混合制备钛基陶瓷:分别使用CuO、B2O3和 Nb2O5作为烧结助剂,考察不同外加剂对陶瓷性能的影响;结果表明,添加2% Nb2O5的产品致密化程度最高,V和其他Zn、Hg、As等有毒元素浸出率均达到国家标准;并且体积密度、吸水率、抗压强度和抗弯强度等性能均达到商用钛基陶瓷的标准。刘海沛等[62]还将废催化剂吹灰、破碎处理后,与高岭土、石英和长石等混合球磨,经过成型、焙烧等工艺制备瓷质砖;研究了原料配比、烧制温度和保温时长等对产品性能的影响;结果表明,当废催化剂含量为20%,烧制温度为1 120 ℃,保温时长2 h时,样品的吸水率和抗弯强度最佳,满足行业标准;瓷砖砖中V的浸出率为0.32 mg/L,优于国家标准(1 mg/L)。张陆文[63]将废催化剂预处理后与陶瓷废料制成混料,经过成型、干燥后烧制多孔陶瓷,催化剂中的碱金属、碱土金属氧化物,在一定程度上可起到烧结助剂的作用从而降低烧结温度,并且可以促进陶瓷样品的致密性;废催化剂掺入量在10wt%~30wt%时,采用发泡法选用SiC作为发泡剂制备的多孔陶瓷可以形成闭孔,从而强化废催化剂中重金属的固化。王彩兰[64]将废催化剂依次用15wt% NH3•H2O、5wt% HNO3溶液和H2O清洗,以CuO、B2O3或Nb2O5作为V元素的固化剂,经过破碎、过筛后与其他原料组分混合均匀,进行湿法球磨得到混合粉体,压制成型后进行高温烧结得到钛基陶瓷,产品抗压强度达到653 MPa以上,抗弯强度达到184 MPa以上,并且V浸出率远优于国家标准。
利用废脱硝催化剂制备陶瓷加以利用,适用范围广,产品性能优异,并且该资源化方法对催化剂的物理化学性能要求较低,具有一定的应用前景。但需要高温操作条件,工艺复杂、成本较高,目前处于探索研究阶段。
-
钛基金属合金密度较低,具有良好的耐蚀性、优异的高温氧化性和耐燃性,在航空、航天、航海和交通运输等高温结构材料领域具有广泛的应用[65]。陈广玉等[66]将废脱硝催化剂破碎成粉末,与CaO、CaF2、Na2CO3和Al粉按配比混合作为炉料,将纯铁在1 600 ℃高温电阻炉熔化后投入炉料,Al粉作为还原剂将废催化剂中的TiO2还原为Ti;冶金过程中,还原的产物Ti通过传质进入铁液中形成铁钛合金,最终大量TiO2转化为Ti,钢渣中的Al粉被氧化为Al2O3;对冶金过程进行热力学计算的结果表明,Al还原废催化剂中TiO2的反应在923 ℃左右发生,属于液-固反应;合金中Ti的含量随着炉料中Al量的增加而增加,当炉料中Al粉含量为40wt%时,合金中的Ti含量达到最大值约为4.25%[67]。朴荣勋等[68]将废催化剂粉碎后与CaO、Al和Cr混合,采用铝热还原-重熔法制备Ti-Al基合金,并研究了Cr添加量对合金性能的影响;将混合物1 580 ℃熔炼后得到粗合金和残渣,CaO的添加有利于合金和渣的分离;将粗合金在1 600 ℃高纯氩气下进行重熔除去氧化物和碳化物等杂质,得到钛铝基合金,合金中Ti的含量为44%;添加Cr元素可以减少高含铝型钛铝化合物产生,增加Ti3Al的生成,同时减少钙铝氧化物含量。朴荣勋等[69]为了对钛铝合金进一步除杂,采用真空电磁悬浮精炼法对粗合金进行除氧除杂,将粗合金在高纯氩气气氛下于1 500 ℃真空电磁悬浮熔化炉中进行精炼,精炼3次后,合金中Ti的含量为52.6%,杂质总体去除率明显增加。W和V的固溶可以强化合金的总体力学性能。然而,随着精炼次数的增加,TiAl相会向没有工业应用价值的富铝化合物相TiAl2转变。彭军等[70]对废弃脱硝催化剂进行吹灰处理、粉碎和过筛后,与MgO、Na2CO3、CaF2和含钙合金混合作为钛渣,用于冶炼铁钛钒合金;将低碳钢加热到1 600 ℃后分批加入钛渣,含钙合金作为还原剂将废弃脱硝催化剂中的TiO2和V2O5还原为Ti和V,经过传质进入钢液形成合金。
废脱硝催化剂作为钛基金属合金的原料进行资源化,适用范围广,对催化剂的物理化学性能要求也较低。然而该方法同样需要高温条件且成本较高。此外,制备钛铝合金需要控制钛铝基金属间化合物相TiAl2和TiAl3等没有工业应用价值的富铝化合物相的形成,合金的高温力学性能还需进一步研究。
-
近年来,失活脱硝催化剂的产量逐年递增,如何实现失活脱硝催化剂的有效再生和废催化剂的资源化综合利用得到了国内外科研工作者的广泛关注。目前,失活脱硝催化剂的再生方法主要是使用溶液清洗再生。针对不同的失活原因选择相应的溶液进行清洗,从而可以有效提高催化剂再生的效果。目前溶液清洗再生已有工业应用的案例。电泳法对杂质元素的去除效率高并且产生废水量较少,是一种有一定前景的催化剂再生技术。在废脱硝催化剂资源化综合利用方面,将废催化剂用作生产脱硝催化剂的原料工艺简单、处理成本低,可以实现废脱硝催化剂的循环利用,是一种可行的利用方式。从废脱硝催化剂中提取有价金属和TiO2也可以实现催化剂的循环利用。催化剂中有价金属的回收方法主要有酸浸法、碱浸法、高温熔融法和生物浸出法等,但目前缺乏工业应用案例,仍需进一步寻找回收效率高、成本低、对环境影响小的回收方式。对于物理化学性质特别是比表面积下降严重的废脱硝催化剂,可将其制成钛基陶瓷和钛基金属合金等材料,该利用方式具有较高的应用前景。
失活脱硝催化剂再生和综合利用研究进展
Research progress of regeneration and comprehensive utilization of deactivated denitrification catalysts
-
摘要: 随着钒基脱硝催化剂被大量应用于烟气脱硝中,作为危险固体废弃物的失活脱硝催化剂处置引起了人们的广泛关注。首先概述了脱硝催化剂失活的机理,然后总结了针对不同失活类型催化剂的再生方法,并介绍了部分工业再生案例,最后比较了废脱硝催化剂各种综合利用方式的优缺点。根据催化剂的失活程度和原因,合理选择再生方式和综合利用方式,以达到尽可能避免填埋处理的目的。Abstract: With the application of V-based catalysts in the flue gas denitrification, deactivated catalysts as the hazardous solid-waste have drawn much attention of human beings. In this paper, the mechanism of deactivation for denitrification catalysts was analyzed at first. The effective regeneration methods for different types of deactivated catalysts were summarized based on the reasons of catalyst deactivation and some industrial regeneration cases were introduced. The advantages and disadvantages of different comprehensive utilizations for waste catalysts were also studied. The regeneration and comprehensive utilization should be based on the level of the deactivated catalysts, thus avoiding the landfill treatment as far as possible.
-
Key words:
- denitrification catalyst /
- deactivation /
- regeneration /
- comprehensive utilization
-

-
表 1 不同类型的失活原因及失活机理
失活类型 失活原因 失活机理 物理失活 磨损 催化剂表面整体损坏 孔内堵塞 飞灰聚集、掉落到催
化剂表面,造成堵塞“覆盖层”中毒 各类杂质沉积在催化剂
表面上,覆盖活性位点高温烧结 载体烧结和晶型转变引
起催化剂比表面积急剧
下降,V2O5聚合和挥发化学失活 碱金属、碱土金属、
重金属和其他元素改变活性位结构或通过
电子相互作用改变NOx、
NH3的吸附行为表 2 不同原因导致脱硝催化剂失活的再生方法及其特点
中毒原因 再生方法 特点 碱与碱土金属 去离子水洗涤[15] 简单有效 SO2硫酸化[16] 可以增加酸性位点,但存在NH3、O2或H2O
时,与SO2反应生成NH4HSO4堵塞催化剂稀硫酸洗涤[17] 有效去除表面碱金属,提供酸性位点,同时造成
设备腐蚀,催化剂活性成分和机械强度的损失电泳再生[23] 能耗较高 重金属 水、乙酸和硝酸溶液[28] 酸洗可以更有效地去除重金属,并提供酸性位点 去离子水、乙酸和三亚
乙基四胺混合溶液[29]有效地去除微孔中的重金属,并络合固定PbO 砷 Ca(NO3)2[32] 碱性条件下有效去除As2O3,活性成分损失较低 NaOH溶液[32] 不能有效清除催化剂微孔中的As,
可能导致催化剂二次中毒稀硫酸洗涤[33] 有效去除As2O3 高温H2还原再生[34] 有效恢复深度As失活催化剂的活性,但会对其物化性能产生影响 SO2 热处理 有效去除硫酸铵 表 3 回收废弃V2O5-WO3/TiO2催化剂中有价金属的方法
回收方法 溶液浸出法 高温熔融法 生物浸出法 酸浸法 碱浸法 主要试剂 HCl、HNO3、H2SO4、H2C2O4 NaOH NaOH Na2CO3 嗜酸微生物 优点 有效浸出V金属 有效浸出V和W 有效回收VW和Ti 环境友好成本低 不足 V和Ti不能分离回收,大量酸性废液 V和W难以分离提纯,
大量强碱性废液能耗高、且设备需耐
高温、耐碱腐蚀可行性尚未得到充分研究 -
[1] 郝吉明, 马广大, 王书肖, 等. 大气污染控制工程[M]. 北京: 高等教育出版社, 2010: 378. [2] CHEN Z H, YANG Q, LI H, et al. Cr-MnOx mixed-oxide catalysts for selective catalytic reduction of NOx with NH3 at low temperature[J]. Journal of Catalysis, 2012, 276(1): 56 − 65. [3] YU Y K, HE C, CHEN J S, et al. Deactivation mechanism of de-NOx catalyst (V2O5-WO3/TiO2) used in coal fired power plant[J]. Journal of Fuel Chemistry and Technology, 2012, 40(11): 1359 − 1365. doi: 10.1016/S1872-5813(13)60003-1 [4] WU W F, WANG C Y, WANG X R, et al. Removal of V and Fe from spent denitrification catalyst by using oxalic acid: Study of dissolution kinetics and toxicity[J]. Green Energy & Environment, 2020, 3(11): 1 − 3. [5] FERELLA F. A Review on management and recycling of spent selective catalytic reduction catalysts[J]. Journal of Cleaner Production, 2019, 246: 118990. [6] ZHANG Q J, WU Y F, YUAN H R. Recycling strategies of spent V2O5-WO3/TiO2 catalyst: A review[J]. Resources Conservation and Recycling, 2020, 161: 104983. doi: 10.1016/j.resconrec.2020.104983 [7] KIM J W, LEE W G, HWANG I S, et al. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching[J]. Journal of Industrial and Engineering Chemistry, 2015, 28: 73 − 77. doi: 10.1016/j.jiec.2015.02.001 [8] 于艳科. 商用SCR脱硝催化剂的失活和再生机理研究[D]. 北京: 中国科学院大学, 2016. [9] 李想, 李俊华, 何煦, 等. 烟气脱硝催化剂中毒机制与再生技术[J]. 化工进展, 2015, 34(12): 4129 − 4138. [10] HAN L P, CAI S X, GAO M, et al. Selective catalytic reduction of NOx with NH3 by using novel catalysts: state of the art and future prospects[J]. Chemical Reviews, 2019, 119(19): 10916 − 10976. doi: 10.1021/acs.chemrev.9b00202 [11] NOVA I, DALLACQUA L, LIETTI L, et al. Study of thermal deactivation of a de-NOx commercial catalyst[J]. Applied Catalysis B:Environmental, 2002, 35(1): 31 − 42. [12] 竹涛, 张星, 高放, 等. 废弃SCR催化剂再生研究进展[J]. 环境工程, 2018, 36(10): 97 − 101+188. [13] LI J H, PENG Y, CHANG H Z, et al. Chemical poison and regeneration of SCR catalysts for NOx removal from stationary sources[J]. Frontiers of Environmental Science & Engineering, 2016, 10(3): 413 − 427. [14] LISI L, LASORELLA G, MALLOGGI S, et al. Single and combined deactivating effect of alkali metals and HCl on commercial SCR catalysts[J]. Applied Catalysis B:Environmental, 2004, 50(4): 251 − 258. doi: 10.1016/j.apcatb.2004.01.007 [15] LISI L, CIMINO S. Poisoning of SCR catalysts by alkali and alkaline earth metals[J]. Catalysts, 2020, 10(12): 1475. doi: 10.3390/catal10121475 [16] YUE P, LI J H, CHEN L, et al. Alkali metal poisoning of a CeO2-WO3 catalyst used in the selective catalytic reduction of NOx with NH3: an experimental and theoretical study[J]. Environmental Science & Technology, 2012, 46(5): 2864 − 2869. [17] KHODAYARI R. Regeneration of commercial SCR catalysts by washing and sulphation: effect of sulphate groups on the activity[J]. Applied Catalysis B:Environmental, 2001, 33(4): 277 − 291. doi: 10.1016/S0926-3373(01)00193-X [18] ZHENG Y J, JENSEN A D, JOHNSSON J E, et al. Laboratory investigation of selective catalytic reduction catalysts: deactivation by potassium compounds and catalyst regeneration[J]. Industrial & Engineering Chemistry Research, 2004, 43(4): 941 − 947. [19] GAO F Y, TANG X L, Yi H H, et al. The poisoning and regeneration effect of alkali metals deposed over commercial V2O5-WO3/TiO2 catalysts on SCR of NO by NH3[J]. Chinese Science Bulletin, 2014, 59(31): 3966 − 3972. doi: 10.1007/s11434-014-0496-y [20] ZHANG Y J, GAN T, HU H Y, et al. Effective treatment and utilization of hazardous waste sulfuric acid generated from alkylation by lignocellulose ester-catalyzed oxidative degradation of organic pollutants[J]. Journal of Hazardous Materials, 2019, 380(15): 120892. [21] YUE P, LI J H, SI W Z, et al. Deactivation and regeneration of a commercial SCR catalyst: Comparison with alkali metals and arsenic[J]. Applied Catalysis B:Environmental, 2015, 168: 195 − 202. [22] WANG X X, MA H Y, SHI Y, et al. Regeneration of alkali poisoned TiO2-based catalyst by various acids in NO selective catalytic reduction with NH3[J]. Fuel, 2021, 285(1): 119069. [23] 商雪松, 陈进生, 胡恭任, 等. 商用SCR脱硝催化剂K2O中毒后再生: (NH4)2SO4溶液[J]. 燃料化学学报, 2012, 40(6): 750 − 756. doi: 10.3969/j.issn.0253-2409.2012.06.018 [24] YUE P, LI J H, SHI W B, et al. Design strategies for development of SCR catalyst: improvement of alkali poisoning resistance and novel regeneration method[J]. Environmental Science & Technology, 2012, 46(22): 12623 − 12629. [25] LI X, LI X S, CHEN J J, et al. An efficient novel regeneration method for Ca-poisoning V2O5-WO3/TiO2 catalyst[J]. Catalysis Communications, 2016, 87: 45 − 48. doi: 10.1016/j.catcom.2016.06.017 [26] LI X S, LIU C D, LI X, et al. A neutral and coordination regeneration method of Ca-poisoned V2O5-WO3/TiO2 SCR catalyst[J]. Catalysis Communications, 2017, 100: 112 − 116. doi: 10.1016/j.catcom.2017.06.034 [27] GUO R T, LU C Z, PAN W G, et al. A comparative study of the poisoning effect of Zn and Pb on Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3[J]. Catalysis Communications, 2015, 59: 136 − 139. doi: 10.1016/j.catcom.2014.10.006 [28] 邓双, 张凡, 刘宇, 等. 燃煤电厂铅的迁移转化研究[J]. 中国环境科学, 2013, 33(7): 1199 − 1206. [29] SENIOR C L, LIGNELL D O, SAROFIM A F, et al. Modeling arsenic partitioning in coal-fired power plants[J]. Combustion & Flame, 2006, 147(3): 209 − 221. [30] KONG M, LIU Q C, WANG X Q, et al. Performance impact and poisoning mechanism of arsenic over commercial V2O5-WO3/TiO2 SCR catalyst[J]. Catalysis Communications, 2015, 72: 121 − 126. doi: 10.1016/j.catcom.2015.09.029 [31] YAN D J, GUO T, YA Y U, et al. Lead poisoning and regeneration of Mn-Ce/TiO2 catalysts for NH3-SCR of NO at low temperature[J]. Journal of Fuel Chemistry and Technology, 2021, 49(1): 113 − 120. doi: 10.1016/S1872-5813(21)60003-8 [32] QI L Q, LI J T, YAO Y, et al. Heavy metal poisoned and regeneration of selective catalytic reduction catalysts[J]. Journal of Hazardous Materials, 2019, 366(15): 492 − 500. [33] LI X, LI J H, PENG Y, et al. Regeneration of commercial SCR catalysts: probing the existing forms of arsenic oxide[J]. Environmental Science & Technology, 2015, 49(16): 9971 − 9978. [34] TIAN Y M, YANG J, LIU L, et al. Insight into regeneration mechanism with sulfuric acid for arsenic poisoned commercial SCR catalyst[J]. Journal-Energy Institute, 2020, 93(1): 387 − 394. doi: 10.1016/j.joei.2019.02.002 [35] LU Q, ALI Z, TANG H, et al. Regeneration of commercial SCR catalyst deactivated by arsenic poisoning in coal-fired power plants[J]. Korean Journal of Chemical Engineering, 2019, 36(3): 377 − 384. doi: 10.1007/s11814-018-0227-9 [36] XUE Y D, ZHANG Y, ZHANG Y, et al. Electrochemical detoxification and recovery of spent SCR catalyst by in-situ generated reactive oxygen species in alkaline media[J]. Chemical Engineering Journal, 2017, 325: 544 − 553. doi: 10.1016/j.cej.2017.05.113 [37] ZHANG L, LI L L, CAO Y, et al. Getting insight into the influence of SO2 on TiO2/CeO2 for the selective catalytic reduction of NO by NH3[J]. Applied Catalysis B:Environmental, 2015, 165: 589 − 598. doi: 10.1016/j.apcatb.2014.10.029 [38] BECK J. Thebehaviour of phosphorus in the flue gas during the combustion of high-phosphate fuels[J]. Fuel, 2006, 85(10): 1541 − 1549. [39] XU L W, WANG C Z, CHANG H Z, et al. New insight into SO2 poisoning and regeneration of CeO2-WO3/TiO2 and V2O5-WO3/TiO2 catalysts for low-temperature NH3-SCR[J]. Environmental Science & Technology, 2018, 52(12): 7064 − 7071. [40] WANG Y Z, YI W, YU J, et al. A novel method for assessing SO2 poisoning effect and thermal regeneration possibility of MOx-WO3/TiO2 (M= Fe, Mn, Cu, V) catalysts for NH3-SCR[J]. Environmental Science & Technology, 2020, 54: 12612 − 12620. [41] ZHAO N, LI L, PAN S W, et al. Sulfur poisoning and regeneration of SCR catalyst based on V2O5/TiO2[J]. Advanced Materials Research, 2014, 1010-1012: 880 − 884. doi: 10.4028/www.scientific.net/AMR.1010-1012.880 [42] 李树田. 燃煤锅炉烟气SCR脱硝催化剂再生工程实践[J]. 发电设备, 2013, 27(2): 133 − 135. [43] 任启柏. 废旧脱硝催化剂再生工艺及工程案例[J]. 山东化工, 2019, 11(48): 195 − 200. [44] 宋文雷, 李扬, 王伟. 燃煤电站锅炉脱硝催化剂再生技术的研究应用[J]. 电站系统工程, 2018, 34(1): 5 − 8. [45] 熊丽仙, 栗莉. 燃煤电厂SCR脱硝催化剂失活分析及再生应用[J]. 能源环境保护, 2017, 31(6): 23 − 26. [46] MA B T, QIU Z F, YANG J, et al. Recovery of nano-TiO2 from spent SCR catalyst by sulfuric acid dissolution and direct precipitation[J]. Waste & Biomass Valorization, 2019, 10: 3037 − 3044. [47] WU W F, WANG C Y, BAO W J, et al. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2 catalyst[J]. Hydrometallurgy, 2018, 179: 52 − 59. doi: 10.1016/j.hydromet.2018.05.021 [48] 何川, 王乐乐, 杨晓宁, 等. 废弃选择性催化还原催化剂混掺对新催化剂脱硝性能的影响[J]. 化工进展, 2018, 37(2): 581 − 586. [49] QI C P, BAO W J, WANG L G, et al. Study of the V2O5-WO3/TiO2 catalyst synthesized from waste catalyst on selective catalytic reduction of NOx by NH3[J]. Catalysts, 2017, 7(12): 110. doi: 10.3390/catal7040110 [50] CAO Y B, HAN F, WANG M X, et al. Regeneration of the waste selective catalytic reduction denitrification catalyst by nitric acid washing[J]. ACS Omega, 2019, 4: 16629 − 16637. doi: 10.1021/acsomega.9b02288 [51] 林晓, 刘晨明, 李志强, 等. 一种SCR废烟气脱硝催化剂的回收方法, CN 104805298A[P]. 2015-07-29. [52] 韩奇, 冯丽娟, 翟文艳, 等. 草酸浸取废SCR催化剂制备光催化剂[J]. 化工环保, 2020, 40(4): 406 − 410. [53] ZHANG Q J, WU Y F, LI L L, et al. Sustainable approach for spent V2O5-WO3/TiO2 catalysts management: selective recovery of heavy metal vanadium and production of value-added WO3-TiO2 photocatalysts[J]. ACS Sustainable Chemistry & Engineering, 2018, 6: 12502 − 12510. [54] 戚春萍, 武文粉, 王晨晔, 等. 燃煤电厂废旧SCR脱硝催化剂中TiO2载体的回收与再利用[J]. 化工学报, 2017, 68(11): 10. [55] LIU N N, XU X Y, LIU Y. Recovery of vanadium and tungsten from spent selective catalytic reduction catalyst by alkaline pressure leaching[J]. Physicochemical Problems of Mineral Processing, 2020, 56(3): 405 − 418. doi: 10.37190/ppmp/118475 [56] JIAO L, SONG W L, CHEN H, et al. Sustainable recycling of titanium scraps and purity titanium production via molten salt electrolysis[J]. Journal of Cleaner Production, 2021, 58: 557 − 563. [57] ZHANG Q J, WU Y F, ZUO T Y. Titanium extraction from spent selective catalytic reduction catalysts in a NaOH molten-salt system: thermodynamic, experimental, and kinetic studies[J]. Metallurgical and Materials Transactions B, 2019, 50: 471 − 479. doi: 10.1007/s11663-018-1475-5 [58] FONTI V, DELLANNO A, BEOLCHINI F, et al. Does bioleaching represent a biotechnological strategy for remediation of contaminated sediments[J]. Science of the Total Environment, 2016, 563-564: 302 − 319. doi: 10.1016/j.scitotenv.2016.04.094 [59] WANG S H, XIE Y L, YAN W F, et al. Leaching of vanadium from waste V2O5-WO3/TiO2 catalyst catalyzed by functional microorganisms[J]. Science of the Total Environment, 2018, 639: 497 − 503. doi: 10.1016/j.scitotenv.2018.05.168 [60] 沈岳松, 刘海沛, 沈树宝, 等. 以废旧钒钛脱硝催化剂为原料的钛基陶瓷及制备方法, CN105347785B[P]. 2016-02-24. [61] 刘海沛, 李众, 沈岳松, 等. 不同外加剂对废弃脱硝催化剂制备钛基陶瓷性能的影响[J]. 热力发电, 2017, 46(7): 66 − 71. [62] 刘海沛, 严巍, 李众等. 废弃脱硝催化剂制备瓷质砖的研究[J]. 环境工程2017(增刊2), 2017: 242-246. [63] 张陆文. 废弃SCR脱硝催化产品的无害化处理与再利用[D]. 南京: 南京工业大学, 2013. [64] 王彩兰. 一种钛基陶瓷及其绿色制备方法, CN108341657A[P]. 2018-07-31. [65] KOTHARI K, RADHAKRISHNAN R, WERELEY N M. Advances in gamma titanium aluminides and their manufacturing techniques[J]. Progress in Aerospace Sciences, 2012, 55: 1 − 16. doi: 10.1016/j.paerosci.2012.04.001 [66] 陈广玉, 康嘉龙, 刘俊杰, 等. 废弃脱硝催化剂直接合金化研究[J]. 钢铁钒钛, 2018, 39(6): 99 − 102. doi: 10.7513/j.issn.1004-7638.2018.06.016 [67] 陈广玉, 康嘉龙, 吴世杰, 等. 废弃脱硝催化剂直接合金化的热力学计算和动力学研究[J]. 钢铁钒钛, 2019, 40(2): 87 − 91. doi: 10.7513/j.issn.1004-7638.2019.02.014 [68] 朴荣勋, 马兰, 杨绍利, 等. 废SCR钛基脱硝催化剂铝热还原重熔制备含铬钛铝基合金的试验研究[J]. 钢铁钒钛, 2019, 40(2): 79 − 86. [69] 朴荣勋, 马兰, 杨绍利, 等. 废弃脱硝催化剂铝热还原—真空磁悬浮精炼制备钛铝基合金试验研究[J]. 钢铁钒钛, 2019, 40(3): 39 − 45. [70] 彭军, 陈广玉, 刘丽霞, 等. 一种含废弃脱硝催化剂的冶金钛渣和铁钛钒合金的制备方法, CN108677016B[P]. 2018-10-19. -




 下载:
下载: