-
随着时代的发展,我国许多城市以湖泊、水库等作为饮用水水源,然而大规模的藻类水华现象不仅严重破坏生态平衡,还对水质安全带来了严重的威胁[1]。铜绿微囊藻是水华现象的优势藻种,藻细胞及生长过程中分泌的有机物是主要的消毒副产物前驱体[2],并且可能产生大量的藻毒素,危害人体的身体健康,得到了社会广泛的关注。藻类有机质(AOM)可能是水华现象和浮游植物数量增加的结果,是构成藻类系统的重要组成部分。文献[3]介绍了AOM通过代谢排泄在细胞外形成胞外有机物(EOM)以及细胞自溶在细胞内形成的胞内有机物(IOM)的研究内容。目前,常见的除藻工艺有混凝-沉淀、混凝-气浮和膜过滤等[4]。氯气、高锰酸钾等预氧化方法进一步改善混凝除藻效果[5],但是研究表明氧化剂投加过量会导致藻细胞破裂,IOM大量释放,进一步加大对水质安全的威胁。EOM作为藻细胞的代谢产物,主要由蛋白质、多糖和类腐殖酸物质组成,对藻细胞脱稳和去除具有重要影响[1]。
芬顿反应是目前公认的最有效的去除有机物方法之一[6],该反应是在酸性条件下投加七水合硫酸亚铁(FeSO4·7H2O)和过氧化氢(H2O2),Fe2+氧化为Fe3+,生成具有较强氧化能力的羟基自由基(·OH),攻击氧化有机物,从而使有机物得到降解[7]。由于反应产生的Fe3+通常作为混凝剂,因此芬顿反应在处理过程中具有氧化与混凝的双重功能。此外,铁是一种非常丰富和无毒的元素,过氧化氢易于处理且对环境危害较小[8]。
文献[9]报道,EOM与混凝剂结合有助于网捕卷扫,起到较好的助凝效果。因此,深刻认识混凝过程对芬顿反应去除EOM的影响,对于进一步优化除藻工艺以及降低藻类及其代谢产物对水质安全带来的风险有重要意义。
本文以铜绿微囊藻为对象,研究Fe2+/H2O2摩尔比和Fe2+投加量对芬顿反应去除EOM的影响以及对比研究芬顿混凝反应与铁盐混凝反应对EOM的去除效果。采用总有机碳测定仪(TOC)、紫外-分光光度计、激光粒径分析仪、扫描电镜(SEM)、高效体积排阻色谱(HPSEC)和三维荧光(EEM)等仪器对芬顿-混凝反应去除藻类EOM的机理做了进一步研究分析。
-
铜绿微囊藻培养:铜绿微囊藻藻种购自中国科学院武汉水生生物研究所,采用标准BG-11培养基进行灭菌处理,并用灭菌的移液管取50 mL藻种至培养基中,并混合均匀,之后置于人工气候箱内培养。设置培养箱温度为25 ℃,并在昼夜比为12 h∶12 h条件下培养。取稳定期藻细胞进行实验。
藻细胞EOM分离:取将含有藻细胞的培养溶液置于高速离心机中,在转速为5 000 r/min条件下,离心15 min,用0.45 μm聚醚砜膜过滤进一步去除藻细胞得到上清液并冷藏保存[10]。
实验所用七水合硫酸亚铁、30%过氧化氢、盐酸、氢氧化钠和无水硫酸铁,试剂均为分析纯。
-
实验模拟配水的初始铜绿微囊藻悬浊液的OD680测定为0.2左右。藻浓度用680 nm处的吸光度(OD680)(紫外-分光光度计,HITACHI,日本)表示,见表1。
-
(1)芬顿反应:在500 mL烧杯中取300 mL EOM加入0.1 mol/L HCl调节pH至3,在六联搅拌器(MY3000-6,梅宇)下进行芬顿实验,步骤如下:①250 r/min快搅30 s;②200 r/min快搅2 min30 s;③40 r/min慢搅22 min;④静置30 min。反应进行至第②阶段投加一定量FeSO4·7H2O,之后加入一定浓度的H2O2,反应25 min。在第③阶段加入0.1 mol/L NaOH调节pH至7.5终止芬顿氧化反应。经0.45 μm滤膜过滤进行后续TOC,UV254等指标测定。
(2)芬顿混凝实验:在芬顿反应步骤①②③基础上,设置步骤④为40 r/min慢搅20 min;⑤静置30 min。经0.45 μm滤膜过滤进行后续指标测定。底部絮体经冷冻干燥后进扫描电镜(SEM,S-3000N,日立,日本)分析。
(3)铁盐混凝实验:将无水硫酸铁(Fe2(SO4)3)粉末溶于酸性水溶液中,配置0.1 mol/L储备液。在300 mL的EOM中投加5 mmol/L储备液,并置于六联搅拌器下,反应步骤同芬顿混凝实验一致。在pH=3,室温条件下进行混凝实验。
(4)絮体粒径动态变化检测:上述芬顿混凝实验中,利用蠕动泵(BT00-300M,兰格)将液面2 cm以下的水样连续输送至Mastersizer 2000激光粒度分析仪(Malvern,UK)样品室进行颗粒粒径分析,之后返回到烧杯中。选用体积平均粒径(d50)作为粒径参数。
-
pH测定采用720型pH计(Thermo Orion,USA);OD680以及UV254测定采用紫外-可见分光光度计(U-3900H,HITACHI,日本);TOC测定采用岛津TOC-LCPN型总有机碳分析仪测定;分子量分布采用高效体积排阻色谱(HPSEC,HITACHI,日本)测定:絮体形貌采用场发射扫描电镜(S-3000N,HITACHI,日本)表征。
EOM采用三维荧光光谱仪(F4500,HITACHI,日本)测定,激发波长与发射波长范围分别为200~400 nm、220~550 nm。并采用荧光区域积分法识别,将三维荧光(3D-EEM)区域分成5个部分[11],分别在相应波长范围内,对3D-EEM选i区域进行积分,见公式(1)。
式中,Φi为i区域的积分值;λex为激发波长,nm;λem为发射波长,nm;I(λexλem)是相对应的荧光强度。
-
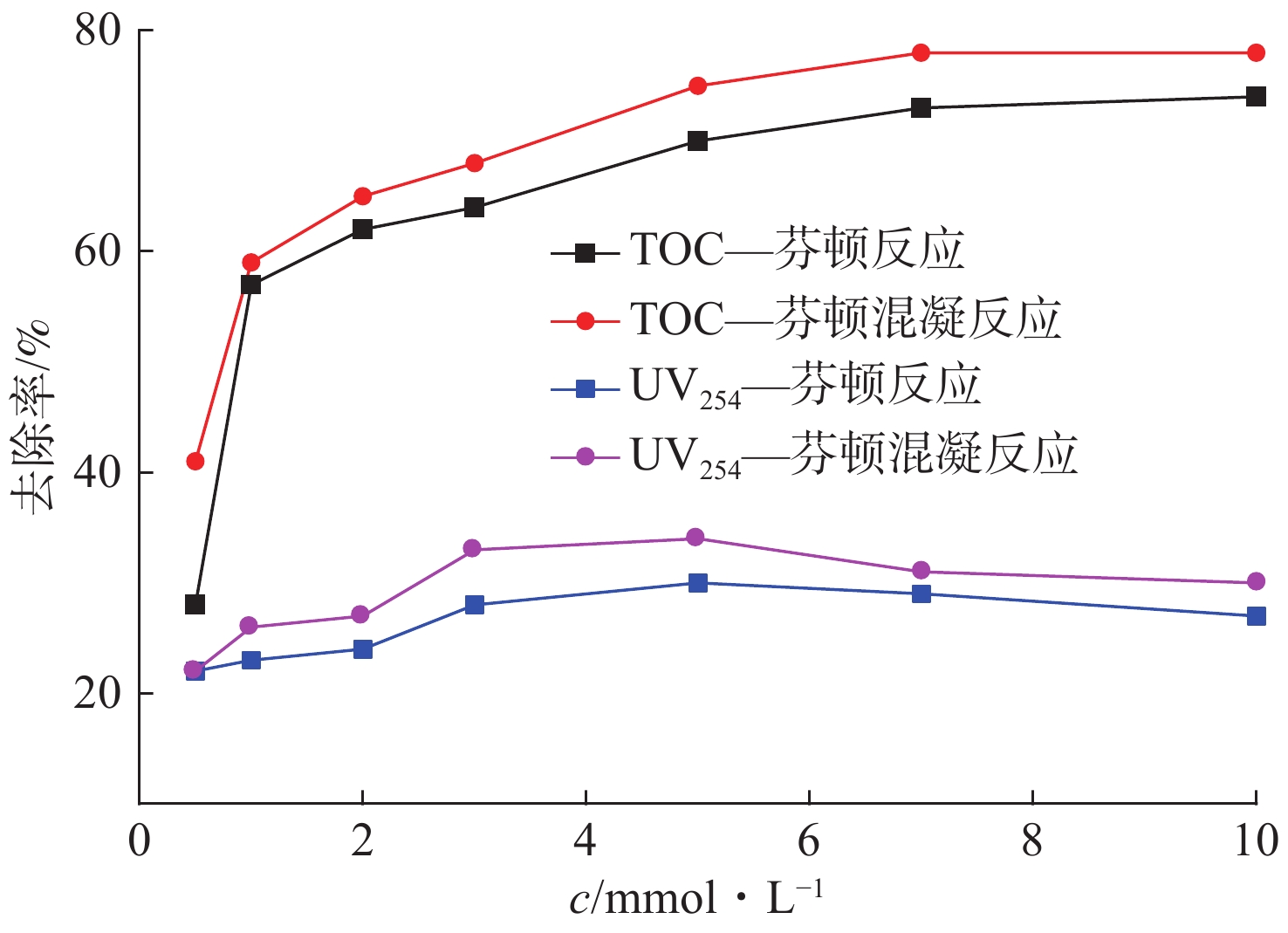
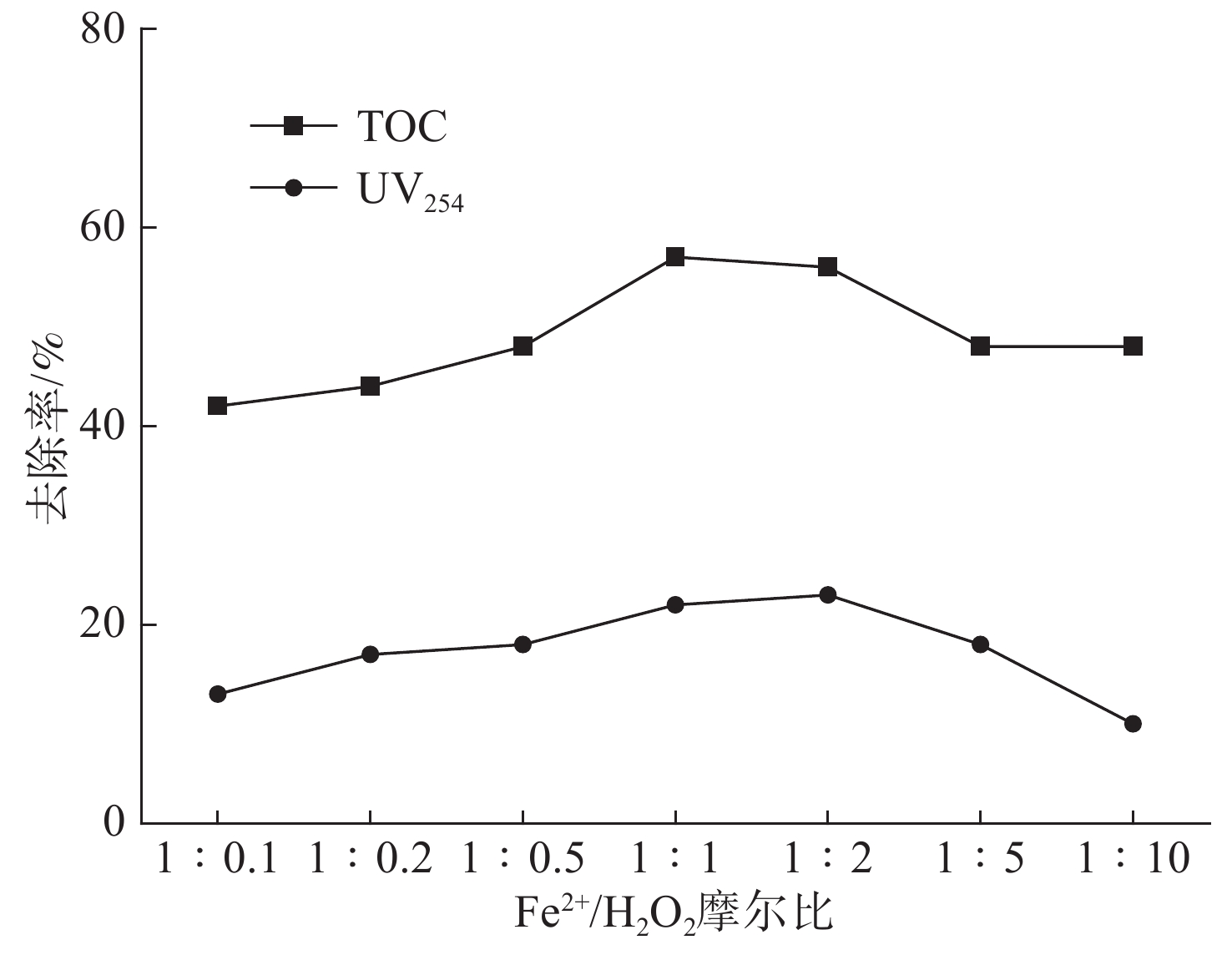
Fe2+/H2O2摩尔比对芬顿反应去除EOM的影响,见图1。
图1可见,在较高Fe2+/H2O2摩尔比(1∶0.1)条件下,TOC与UV254去除效果分别只有22%、13%。研究发现,浓度过高的Fe2+不仅会过量还原H2O2产生较多的Fe3+,并且会加速清除反应的进行,消耗药剂的同时增加铁系污泥产出量,由反应式(2)表明。
随着摩尔比增大,在1∶1摩尔比条件下,TOC与UV254去除率分别达到37%、22%,而在1∶2、1∶5和1∶10摩尔比条件下H2O2浓度不断增大,TOC与UV254去除率分别降低至23%、10%,这可能是过量的H2O2不仅不能通过反应式(3)分解产生更多的·OH,而且在反应开始阶段就把Fe2+迅速氧化为Fe3+,导致反应实际是在Fe3+的催化下,消耗H2O2的同时又抑制·OH的产生[12],并且过量的H2O2与Fe2+存在竞争关系,消耗·OH抑制反应(4)的进行,从而降低了TOC和UV254去除率[13]。
-
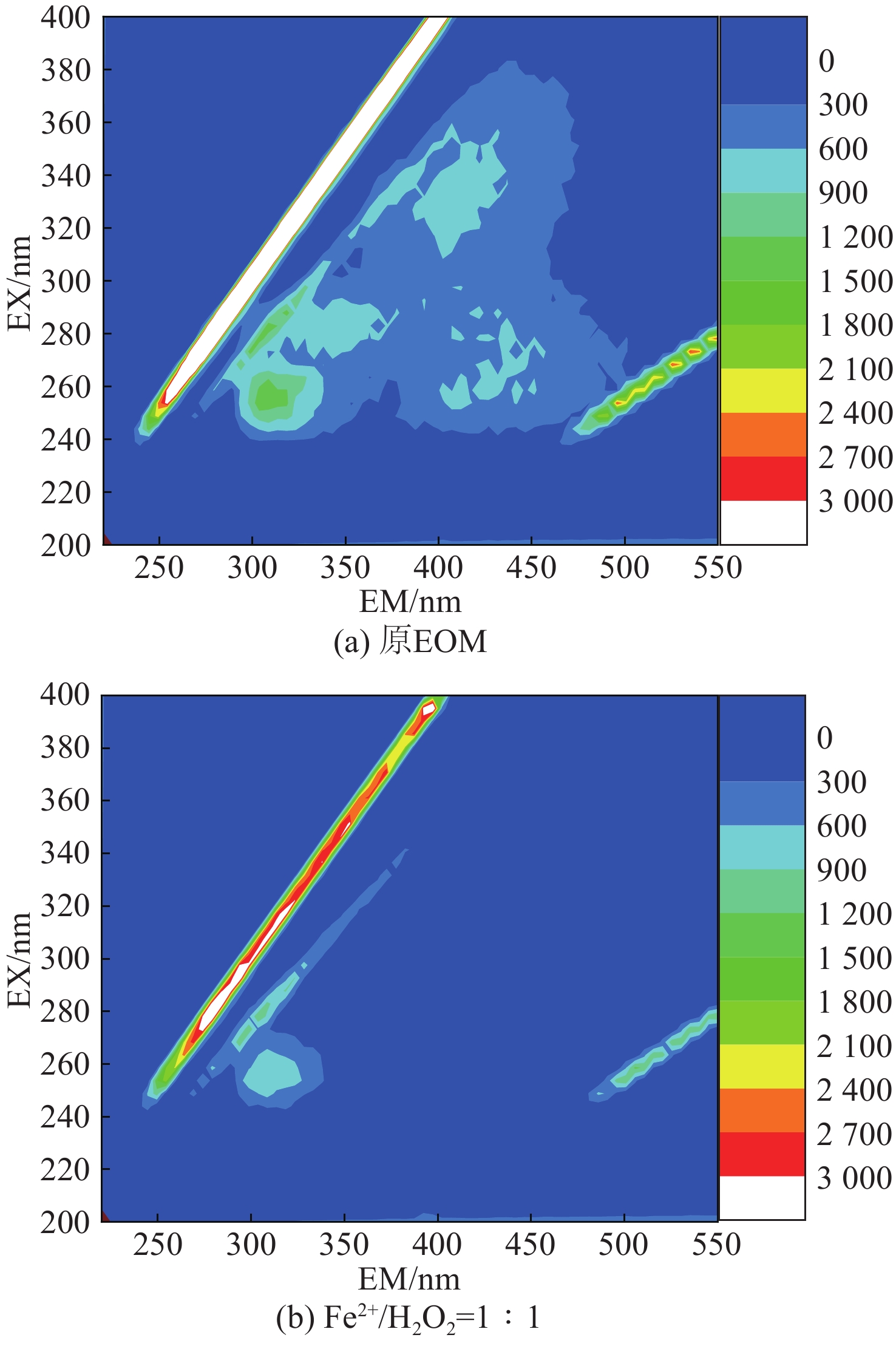
分析三维荧光是一种高灵敏度和选择性的分析工具,在生物源物质的表征方面具有独特的优势[14],处理前后三维荧光光谱图及不同反应条件三维荧光强度分布情况见图2和表2。
图2和表2可知,芬顿反应在Fe2+/H2O2=1∶1时,有机物Ⅲ和Ⅴ区的荧光强度分别减弱72%,80%,说明芬顿反应对EOM中类腐殖酸与类富里酸物质具有较强的去除能力。Ⅰ区类蛋白质与Ⅳ区微生物代谢产物区荧光强度分别降低40%,58%,说明芬顿反应对去除有机物类蛋白质与微生物代谢产物效果一般。
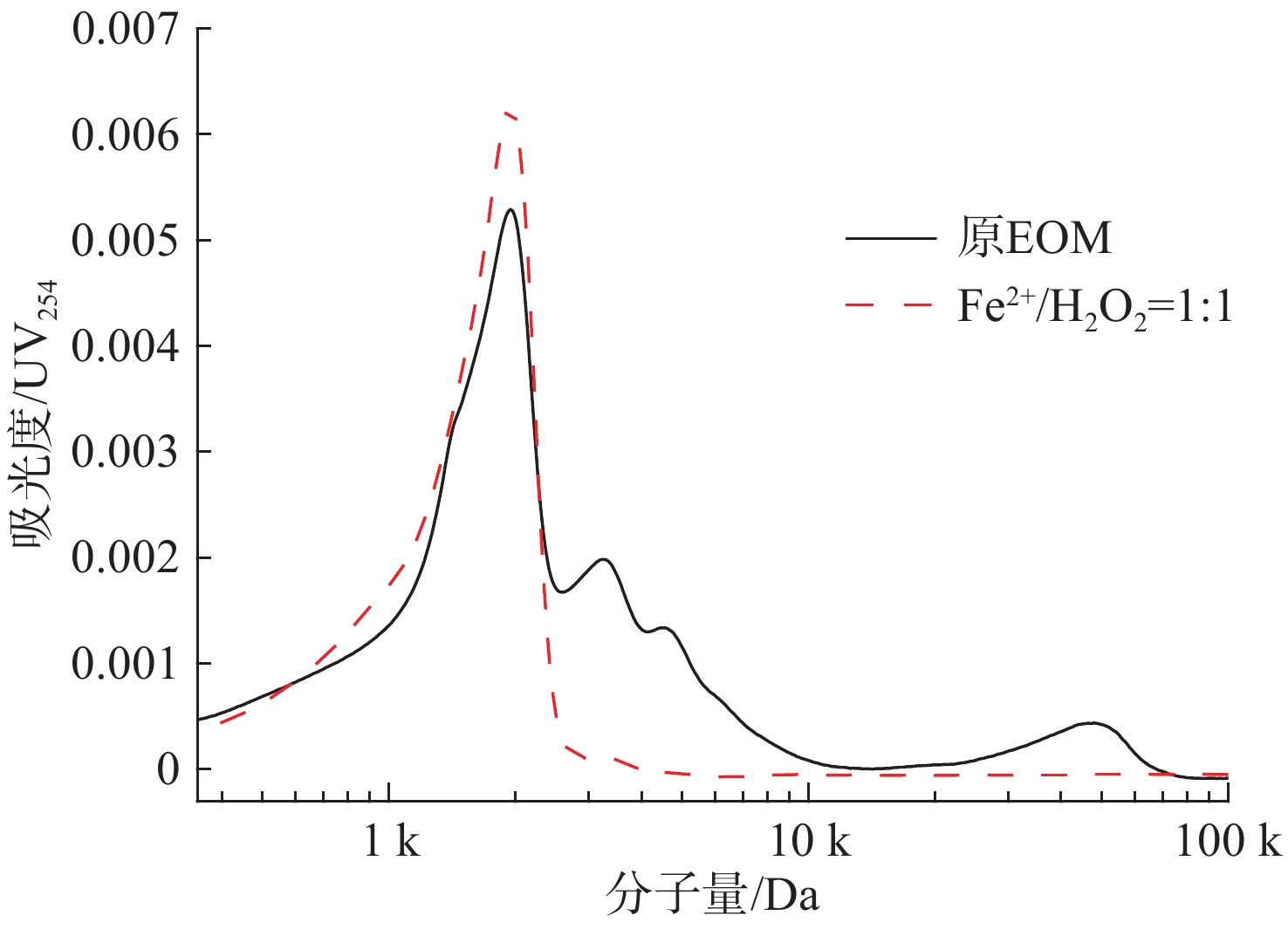
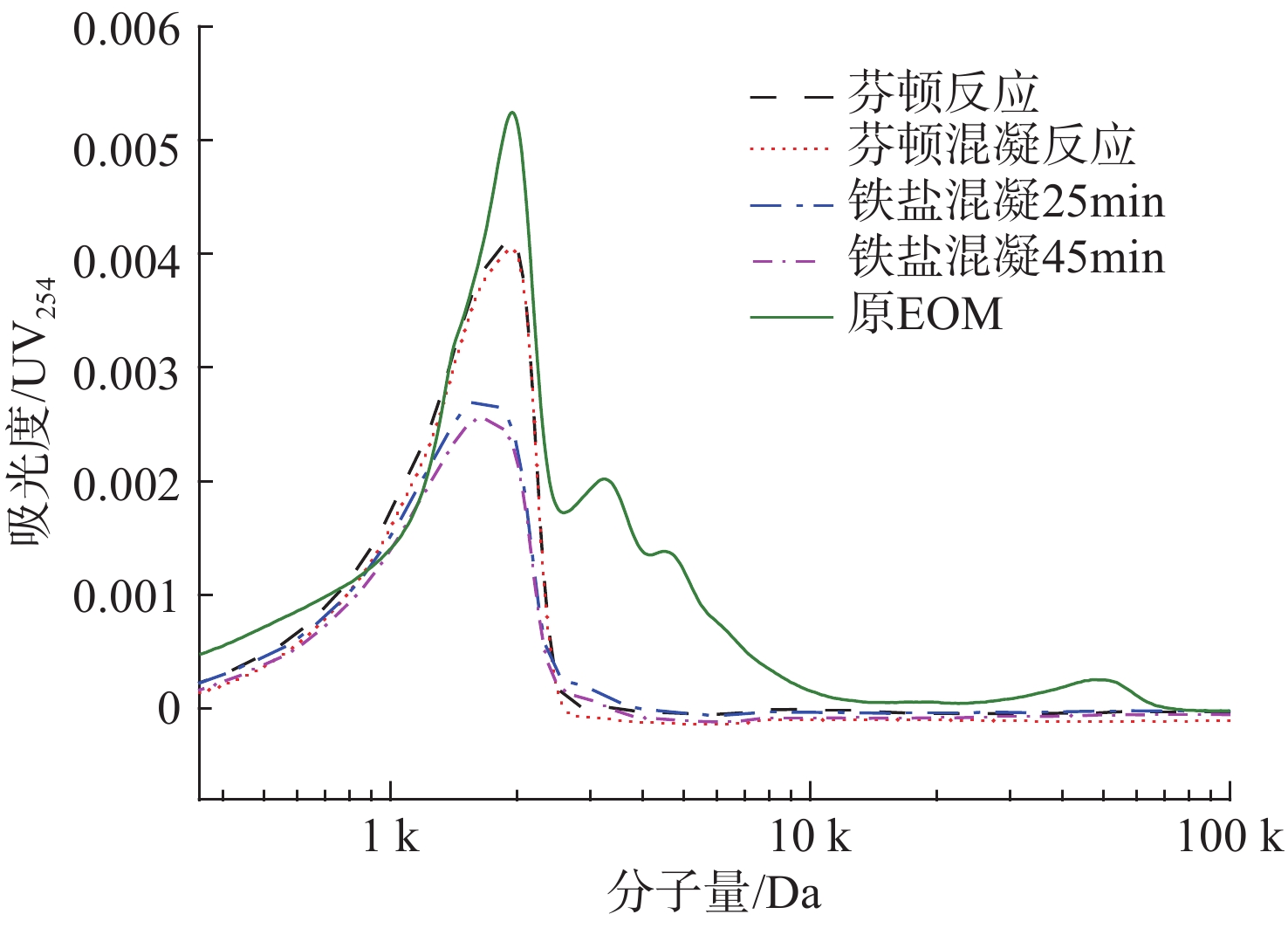
根据LYKO et al[15]的研究,有机物中大分子组分分布在>4 kDa范围内主要为蛋白质和多糖,中分子量组分在1 k~4 kDa范围主要为腐殖酸和低分子量组分,此外在<1 kDa范围内主要为分子骨架物质。处理前后三维荧光光谱分子量分布情况见图3。
图3可见,EOM中主要存在对紫外吸光度较低的分布在4 k~5 kDa、20 k~60 kDa范围内的大分子量有机物组分以及对紫外吸光度较高的分布在1~4 k内的中分子量物质。在Fe2+/H2O2=1∶1条件下,大分子量47 kDa吸光度由0.000 44降至0,4 kDa处吸光度由0.001 34降至0.000 03,结合荧光图谱说明芬顿反应对原始EOM中蛋白质类大分子物质具有一定的去除能力。在2 kDa处吸光度由0.005 29升高至0.006 75,可能是EOM中一部分蛋白质类大分子量物质降解不彻底从而产生中分子量物质,进而导致吸光度升高。
-
在Fe2+/ H2O2=1∶1基础上分别投加0.5、1、2、3、5、7和10 mmol/L的FeSO4·7H2O与H2O2,其去除效果见图4。
图4可见,随着Fe2+浓度的升高,TOC去除率由57%大幅提高至70%。投加量为0.5 mmol/L时,一方面可能是Fe2+作为催化剂,在较低投加量下对H2O2催化氧化能力较差,导致有机物去除效果一般,另一方面是由于溶液中产生的Fe3+含量较少,中和后生成较少的铁氢氧化物,导致混凝效果变差,从而影响TOC的去除。投加量为7、10 mmol/L时,去除率一定程度上提高,但是从经济可行性方面考虑以及Fe2+投加量过高会产生大量的铁系污泥[16],因此,确定最佳Fe2+投加量为5 mmol/L。UV254去除率的变化趋势也说明了上述的结论,不同的是在7、10 mmol/L投加量下,UV254的去除率达到30%后降低。文献[17]研究表明,使用高浓度的Fe2+可能会抑制芬顿氧化反应,阻碍芬顿过程中氧化与混凝协同作用,从而降低有机物去除效率。
考虑到Fe2+投加量对芬顿反应去除EOM有较大影响,Fe2+投加量越高,中和后Fe3+浓度越高,相应的混凝效果越好,去除有机物的能力越强。铁盐混凝对EOM去除效果见图5。
图5可见,TOC和UV254去除率分别为40%、35%,芬顿过程对应去除率为70%、30%。而铁盐混凝去除率为39%、37%,说明铁盐混凝对腐殖质类大分子物质以及含C=C双键和C=O双键的芳香族化合物具有较强的去除能力[18],而芬顿反应矿化有机物能力较强。由图4和图5表明,反应进行至45 min,芬顿混凝反应与铁盐混凝对TOC与UV254去除效果进一步提高,这可能是产生的铁氢氧化物经过20 min混凝反应,表面吸附了较多的EOM。
-
不同反应条件去除EOM的三维荧光EEM谱图见图6。
图6(a)以及表2分析表明,在芬顿反应投加量为5 mmol/L时,有机物Ⅲ和Ⅴ区的荧光强度分别减弱78%,92%,说明芬顿反应对EOM中类腐殖酸与类富里酸物质具有较强的去除能力。Ⅰ区类蛋白质荧光强度降低49%,Ⅳ区微生物代谢产物强度减弱67%,表明增大Fe2+投加量,可以进一步提高芬顿反应对蛋白质类与微生物代谢产物中大分子量物质的去除效果。由图6(c)分析可知,经过铁盐混凝后,Ⅰ区类蛋白质与Ⅳ区微生物代谢产物荧光强度分别降低32%、46%,说明对比芬顿反应,铁盐混凝对大分子量有机物具有较强的去除能力,一方面可能是Fe3+形成的铁氢氧化物易于吸附和网捕卷扫大分子量物质,另一方面可能是Fe3+易与蛋白质类物质发生络合反应形成铁蛋白复合物,消耗大分子量物质[19]。对比分析图6(a)~(d)以及表2,观察到Ⅰ、Ⅲ、Ⅳ和Ⅴ区特征峰强度进一步减弱,说明混凝反应进一步提高去除EOM的效果。
不同反应条件去除EOM的分子量分布见图7。
图7可见,Fe2+投加量为5 mmol/L时,4 kDa和47 kDa处蛋白质类大分子量以及3 kDa处腐殖质类中分子量物质基本去除,2 kDa处吸光度由0.005 29降至0.004 23,可能是由于提高Fe2+投加量促进EOM中大分子量物质降解彻底。铁盐混凝对蛋白质类大分子量物质具有一定的去除能力,在2 kDa处吸光度由0.005 29降至0.002 71,相比于芬顿反应吸光度变化较大,可能是Fe3+与中分子量物质中部分蛋白质类物质存在较强的络合作用。由芬顿混凝反应与铁盐混凝反应的分子量分布表明,在2 kDa、4 kDa和47 kDa处吸光度均进一步减小,与上述EEM结论一致。
-
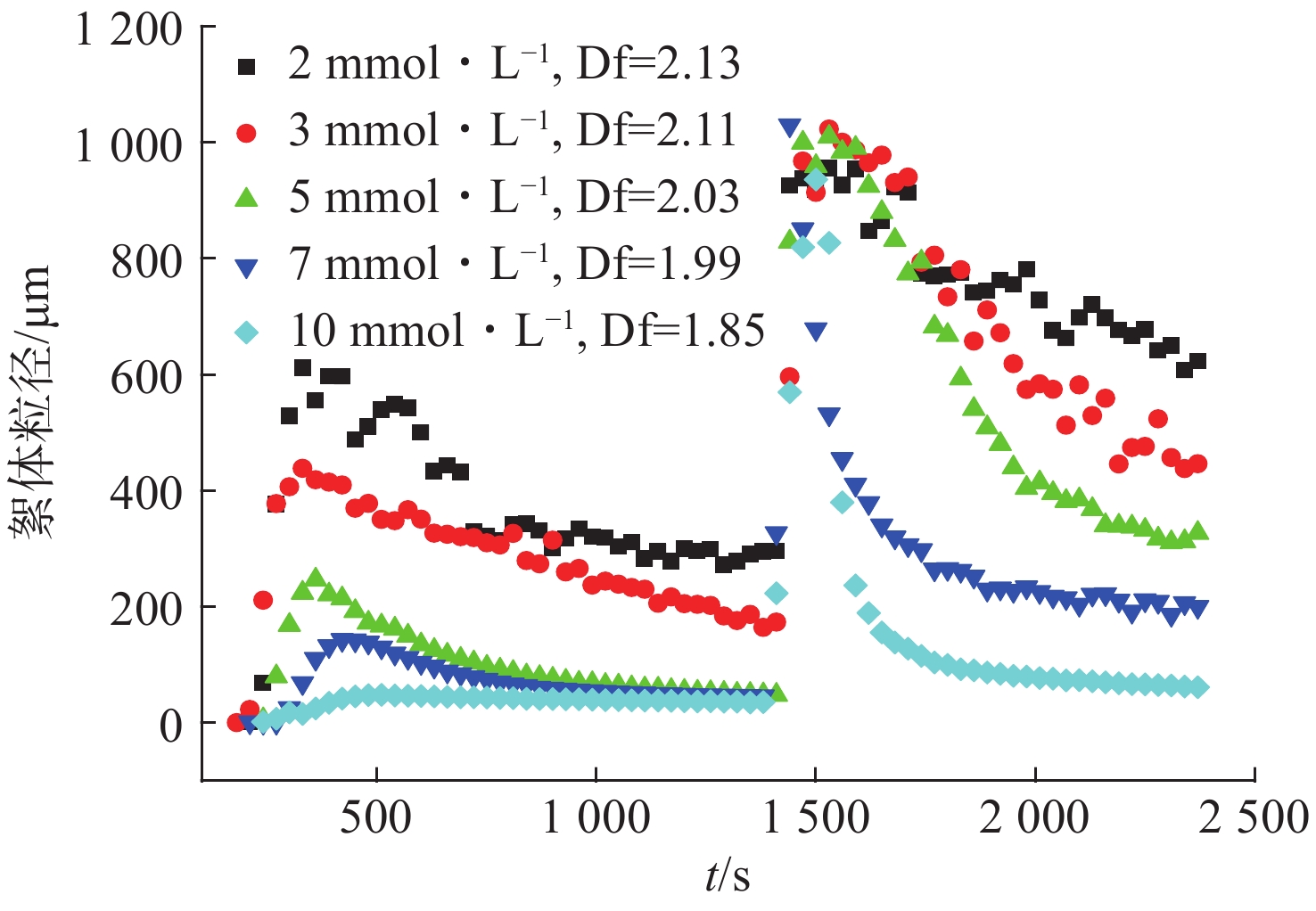
根据ZHANG et al[20]的研究,在去除生物质过程中其所形成的絮体与去除效果具有一定关系,因此研究芬顿反应过程中藻絮体结构的变化是有必要的。芬顿反应处理过程中藻絮体粒径和分型维数变化效果图见图8。
图8可见,进行2 min后,溶液中产生了粒径在1~6 μm的絮体,随着反应进行,絮体粒径进一步增大,在6 min左右絮体粒径达到第一个峰值,随后絮体粒径缓慢减小。反应进行25 min,由于发生中和反应,絮体粒径达到第2个峰值,即产生粒径较大的氢氧化物沉淀物,粒径分布在900~1 000 μm。反应进行至27 min,絮体粒径在达到最大值后逐渐减小,表明吸附有机物的铁氢氧化物在一定的剪切力下易破碎分散,且这种破碎不可逆。这种不可逆的破碎可能由于混凝体系中絮体的形成不仅因为简单的电中和而形成的物理键,其中还掺杂着部分化学键的形成[21]。图8分析了不同Fe2+投加量下生成絮体的分形维数,随着Fe2+浓度提高,分形维数减小。分形维数表征的是絮体的密实程度,分形维数越低,絮体结构越开放。说明在10 mmol/L投加量下,生成的絮体结构较松散开放。
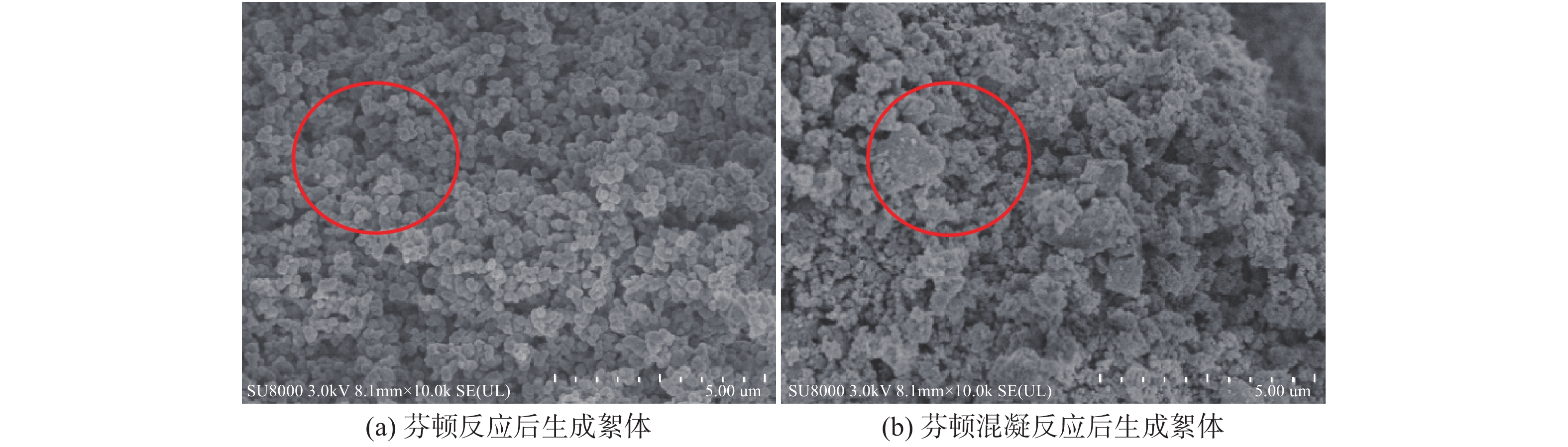
比较Fe2+投加量为5 mmol/L时,芬顿反应与芬顿混凝反应生成絮体的扫描电镜图见图9。
图9(a)可见,芬顿反应中和沉淀产生的絮体呈现圆球形结构较规则,絮体分布较密实。由图9(b)可见芬顿混凝反应后生成的絮体电镜图,可知经芬顿混凝反应产生的絮体结构开放不规则,絮体分布较松散,说明了絮体表面吸附了较多的EOM,表明了芬顿混凝反应中网捕、卷扫等机制发挥了主要作用。上述SEM图结果表明,芬顿混凝反应通过絮体对EOM的吸附进一步提高对EOM的去除效果。
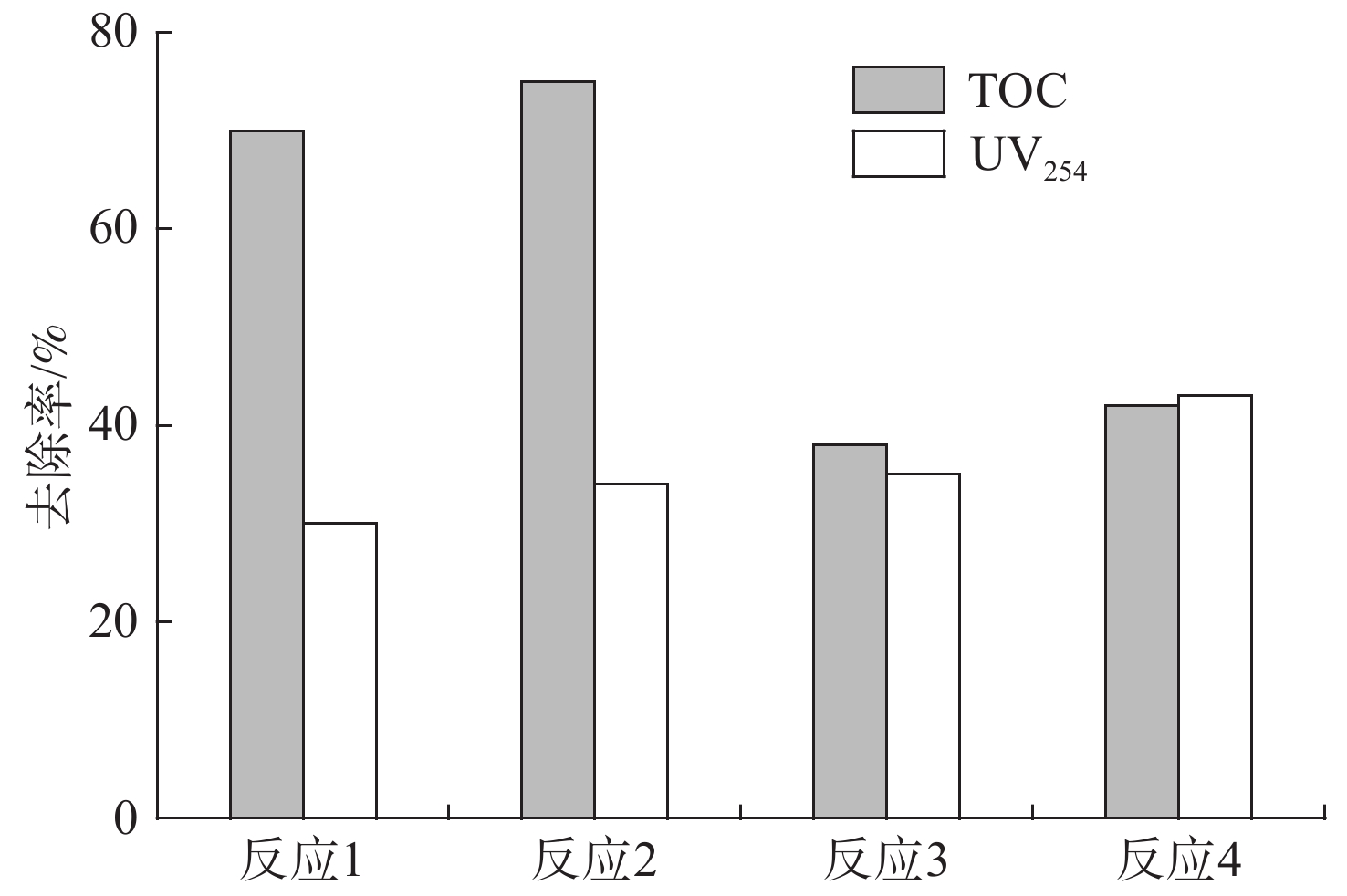
为了进一步说明生成的絮体粒径尺寸对胞外有机物去除影响,在反应进行6、25 min以及反应终止的时间点测定相应TOC值,结果见图10。
图10可知,反应进行6、25、和45 min时,Fe2+浓度为2 mmol/L产生的絮体粒径尺寸分别为610、900和655 μm,对应TOC去除率为42%、62%和65%,随着投加量增大,产生的絮体粒径减小,TOC去除率随之提高,在10 mmol/L投加量下,6、25和45 min产生的絮体粒径仅为70、885、和110 μm,对应TOC去除率为47%、74% 和80%,说明芬顿处理EOM过程中形成的絮体粒径越小,TOC去除率越高。徐磊等[22]研究发现,絮体的成长速率取决于溶液中颗粒的碰撞频率与碰撞效率,同时混凝过程中产生的絮体可能由于某种断裂力导致其在一定剪切力背景下,因为碰撞频率的变化而破碎[23]。上述现象可能是溶液中存在过量的Fe3+离子,中和反应后产生大量的絮体,一定程度上影响了颗粒物之间的碰撞频率与效率,加快了絮体破碎程度,进而提高对TOC的去除。通过分析芬顿反应中生成的絮体粒径分布,说明了芬顿反应中絮体结构对去除EOM有较大影响。
-
1)芬顿反应在室温,pH=3条件下,对EOM中TOC、UV254的去除率分别37%、22%,通过三维荧光与分子量分布分析,确定最优Fe2+/H2O2摩尔比为1∶1。藻类EOM中亲水性大分子多糖和蛋白质所占比例较大,而疏水性类腐殖酸物质所占比例较小。Fe2+在5 mmol/L投加量下TOC、UV254去除率分别达到70%、30%。
2)比较芬顿混凝反应与铁盐混凝反应对EOM的去除效果,表明混凝过程促进了芬顿反应对EOM的去除,由三维荧光和分子量分布表明,Fe3+与蛋白质类大分子量物质存在较强的络合作用。经过铁盐混凝反应UV254去除率达到35%,而芬顿反应对UV254去除仅为30%,推测EOM中亲水性大分子量物质的去除率很大程度是由Fe3+通过混凝过程贡献。
3)由絮体粒径(d50)动态分析,不同时间点产生的絮体粒径受Fe2+投加量影响较大,提高Fe2+投加量,絮体对有机物卷扫和吸附效果越好,对应TOC去除率越高,在芬顿处理藻液过程中形成的絮体粒径越小,TOC去除率越高。
芬顿-混凝反应对藻类胞外有机物去除机制研究
Study on Mechanism of Removal of Extracellular Organic Matter from Algae by Fenton-coagulation Reaction
-
摘要: 文章探究不同反应条件下芬顿-混凝反应与铁盐(Fe2(SO4)3)混凝反应对铜绿微囊藻胞外有机物(EOM)的去除效果。结果表明,在室温,pH=3,Fe2+/H2O2摩尔比为1∶1时,EOM的去除率达到最佳。Fe2+投加量为5 mmol/L时,TOC、UV254去除率分别达到70%、34%。通过三维荧光(EEM)与分子量分布(HPSEC)分析表明,藻类EOM中主要成分是亲水性大分子多糖和蛋白质,其次是疏水性类腐殖酸物质。单独的铁盐混凝反应表明混凝过程促进了芬顿反应对EOM的去除,EOM中亲水性大分子量物质的去除率很大程度是由Fe2+氧化为Fe3+通过混凝过程贡献。此外,在芬顿反应处理EOM过程中不同时间点絮体粒径受Fe2+投加量影响较大,形成的絮体粒径越小,TOC去除率越高。Abstract: This paper investigated the effect of Fenton-coagulation reaction and the iron salt (Fe2(SO4)3) coagulation reaction on the removal of extracellular organic matter (EOM) from Microcystis aeruginosa under different reaction conditions. The results showed that the removal rate of EOM was the best with room temperature and pH=3 as well as a 1:1 molar ratio of Fe2+/H2O2. The TOC and UV254 removal rates were 70% and 34% respectively with the Fe2+ dosage of 5mmol/L. Three-dimensional fluorescence (EEM) and high-performance size-exclusion chromatography (HPSEC) analysis indicated that the main components of algae EOM were hydrophilic macromolecular polysaccharides and proteins, followed by hydrophobic humic acid-like substances. The individual iron salt coagulation indicated that the coagulation process enhanced the Fenton reaction to remove EOM. The removal rate of hydrophilic large molecular weight substances in EOM was greatly contributed by the oxidation of Fe2+ to Fe3+ through the coagulation process. In addition, the particle size of the floc was greatly affected by Fe2+ dosage at different time in the EOM treatment process by Fenton reaction, the smaller particle size of the floc formed, the higher the removal rate of TOC.
-
Key words:
- EOM /
- Fenton Reaction /
- Coagulation /
- Particle Size of Floc /
- TOC
-

-
表 1 原水水质指标参数
pH UV254/cm−1 TOC/mg·L−1 SUVA OD680 7.86 0.0529 5.664 0.94 0.2 表 2 不同反应条件三维荧光强度区域积分
反应条件 区域分布 Ⅰ Ⅱ Ⅲ Ⅳ Ⅴ 原EOM 16 919.6 10 312.2 18 534.8 94 998.9 285 793.1 Fe2+/H2O2=1∶1 9 246.5 4 095.3 4 860.2 39 854.3 56 160.2 Fe2+投加量5 mmol·L−1 9 071.2 5 600.0 4 089.1 31 564.4 21 234.7 芬顿混凝反应 8 888.3 5 255.5 3 888.5 29 134.5 17 702.9 铁盐混凝25 min 11 649.5 8 402.4 12 940.6 50 924.1 174 451.1 铁盐混凝45 min 10 113.3 8 005.4 12 370.1 45 322.8 168 213.8 -
[1] QI J, LAN H, LIU R, et al. Prechlorination of algae-laden water: The effects of transportation time on cell integrity, algal organic matter release, and chlorinated disinfection byproduct formation[J]. Water Research, 2016, 102: 221 − 228. doi: 10.1016/j.watres.2016.06.039 [2] HENDERSON R, PARSONS S A, JEFFERON B. The impact of algal properties and pre-oxidation on solid-liquid separation of algae[J]. Water Research, 2008, 42(8/9): 1827 − 1845. [3] HENDERON R K, BAKER A, PARSONS S A, et al. Characterisation of algogenic organic matter extracted from cyanobacteria, green algae and diatoms[J]. Water Research, 2008, 42(13): 3435 − 3445. doi: 10.1016/j.watres.2007.10.032 [4] 张普, 乔俊莲, 王国强, 等. 聚二甲基二烯丙基氯化铵对铜绿微囊藻的去除效果研究[J]. 水处理技术, 2010, 36(11): 15 − 18. [5] 马敏, 刘锐平, 刘会娟, 等. 预氯化对铝盐混凝铜绿微囊藻过程中溶解性有机物和残余铝的影响[J]. 环境科学学报, 2014, 34(1): 73 − 78. [6] SANAYE S V, PISE N M, PAWAR A P, et al. Evaluation of antioxidant activities in captive-bred cultured yellow seahorse, Hippocampus kuda (Bleeker, 1852)[J]. Aquaculture, 2014, 434: 100 − 107. doi: 10.1016/j.aquaculture.2014.08.007 [7] PIGNATELLO J J, OLIVEROS E, MACKAY A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry[J]. Critical Reviews in Environmental Science and Technology, 2006, 36(1): 1 − 84. doi: 10.1080/10643380500326564 [8] BADAWY M I, ALI M E M. Fenton's peroxidation and coagulation processes for the treatment of combined industrial and domestic wastewater[J]. Journal of Hazardous Materials, 2006, 136(3): 961 − 966. doi: 10.1016/j.jhazmat.2006.01.042 [9] HENDERSON R K, PARSONS S A, JEFFERON B. The impact of differing cell and algogenic organic matter (AOM) characteristics on the coagulation and flotation of algae[J]. Water Research, 2010, 44(12): 3617 − 3624. doi: 10.1016/j.watres.2010.04.016 [10] QU F, LIANG H, WANG Z, et al. Ultrafiltration membrane fouling by extracellular organic matters (EOM) of Microcystis aeruginosa in stationary phase: influences of interfacial characteristics of foulants and fouling mechanisms[J]. Water Research, 2012, 46(5): 1490 − 1500. doi: 10.1016/j.watres.2011.11.051 [11] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter[J]. Environmental Science & Technology, 2003, 37(24): 5701 − 5710. [12] 吴彦瑜, 覃芳慧, 赖杨兰, 等. Fenton试剂对垃圾渗滤液中腐殖酸的去除特性[J]. 环境科学研究, 2010, 23(1): 94 − 99. [13] DENG Y. Physical and oxidative removal of organics during Fenton treatment of mature municipal landfill leachate[J]. Journal of Hazardous Materials, 2007, 146(1/2): 334 − 340. [14] HENDERSON R K, BAKER A, MURPHY K R, et al. Fluorescence as a potential monitoring tool for recycled water systems: a review[J]. Water research, 2009, 43(4): 863 − 881. doi: 10.1016/j.watres.2008.11.027 [15] LYKO S, WINTGENS T, Al-HALBOUNI D, et al. Long-term monitoring of a full-scale municipal membrane bioreactor-Characterisation of foulants and operational performance[J]. Journal of Membrane Science, 2008, 317(1/2): 78 − 87. [16] SUN J H, SUN S P, FAN M H, et al. A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process[J]. Journal of Hazardous Materials, 2007, 148(1/2): 172 − 177. [17] PARK S, YOON T. The effects of iron species and mineral particles on advanced oxidation processes for the removal of humic acids[J]. Desalination, 2007, 208(1−3): 181 − 191. doi: 10.1016/j.desal.2006.02.080 [18] 周玲玲, 张永吉, 孙丽华, 等. 铁盐和铝盐混凝对水中天然有机物的去除特性研究[J]. 环境科学, 2008, 29(5): 1187 − 1191. doi: 10.3321/j.issn:0250-3301.2008.05.006 [19] 丰桂珍, 董秉直. 水中藻类溶解性有机物特性研究[J]. 环境科学与技术, 2016, 39(11): 144 − 149. [20] ZHANG W, YANG P, YANG X, et al. Insights into the respective role of acidification and oxidation for enhancing anaerobic digested sludge dewatering performance with Fenton process[J]. Bioresource Technology, 2015, 181: 247 − 253. doi: 10.1016/j.biortech.2015.01.003 [21] JARVIS P, JEFFERSON B, PARSONS S A. Breakage, regrowth, and fractal nature of natural organic matter flocs[J]. Envionenmal Science & Technology, 2005, 39(7): 2307 − 2314. [22] 徐磊, 俞文正, 梁亮, 等. 天然有机物对混凝效果影响机制及絮体特性分析[J]. 环境科学, 2013, 34(11): 4290 − 4294. [23] MATSUO T, UNNO H. Closure to“Forces Acting on Floc and Strength of Floc” by Tomonori Matsuo and Hideaki Unno (June, 1981)[J]. Journal of Envionenmal Engineering, 1983, 109(1): 257 − 263. doi: 10.1061/(ASCE)0733-9372(1983)109:1(257) -



 下载:
下载: