-
地表水是我国东部河网地区饮用水的主要来源,包括河流、湖泊、水库等。虽然水量丰富,但上述水源容易受到周边工农业生产过程排放的有机污染物的威胁。其中,农药、全氟化合物等微量有机污染物(TrOCs,trace organic contaminants)浓度虽低,但由于具有较高的生物毒性、环境持久性和生物累积性,成为当前水处理行业的关注热点。对我国东部地区饮用水源地52个采样点的112种微量有机污染物进行了调查,发现水中共检出104种化学物质,检出浓度范围为0~156.5 ng·L−1[1]。饮用水带来的TrOCs暴露可能会对人体发育代谢、免疫功能、内分泌功能等产生不利影响。另一方面,饮用水厂普遍采用的氯消毒工艺可能诱导水中有机物反应生成消毒副产物(DBPs,disinfection by-products),增加人体罹患膀胱癌和先天性畸形等重大疾病的风险[2]。目前我国饮用水厂净水工艺仍以混凝-沉淀-过滤-消毒的常规工艺为主,对水源水中出现的消毒副产物前体物、农药、工业化学品等控制效果不佳。因此,在常规处理的基础上增加深度处理单元,是保障和提升河网地区饮用水水质的重要途径。近年来,基于紫外的高级氧化技术成为水深度处理领域的研究热点,包括紫外/过氧化氢、紫外/过硫酸盐、紫外/氯等[3]。次氯酸钠作为饮用水厂常用的消毒剂,其与紫外线组成的紫外/氯高级氧化工艺具有氧化效率高、氧化剂余量可直接利用等优点,成为饮用水深度处理工艺的重要选择[4-6]。然而,有关紫外/氯工艺在饮用水厂现场中试及以上规模的研究还比较少,实际水质条件下不同工艺参数对有机物降解效率的影响及机理还有待探究。尤其是水中消毒副产物(生成势)的变化及工艺对全氟化合物等新污染物的作用效果值得关注。本研究采用紫外/氯中试系统对浙江某饮用水厂滤后水进行深度处理,考察了该工艺对水中TrOCs、DBPs和消毒副产物生成势(DBPsFP,formation potential of DBPs)等化学风险物质的控制效果,为其在饮用水厂的推广应用提供技术支持。
-
本中试研究在浙江东北部某饮用水厂中进行,水厂原水取自附近一个集灌溉、供水、养鱼等功能为一体的综合性水库。水厂日均供水量约为1.7×104 t,净水工艺单元包括水力混合和折板反应池、平流沉淀池、V型炭砂滤池和清水池等,其中涉及的加药过程包括原水加矾/碱/氯、炭砂滤后水加氯等。以加氯前的炭砂滤池出水(简称滤后水)为紫外/氯中试系统进水,采样分析时间为2022年10月。期间,滤后水中无明显杂质颗粒, pH为7.0±0.1、CODMn为(0.83±0.08) mg·L−1、DOC为(1.27±0.10) mg·L−1、UV254为(0.016±0.002) cm−1。
-
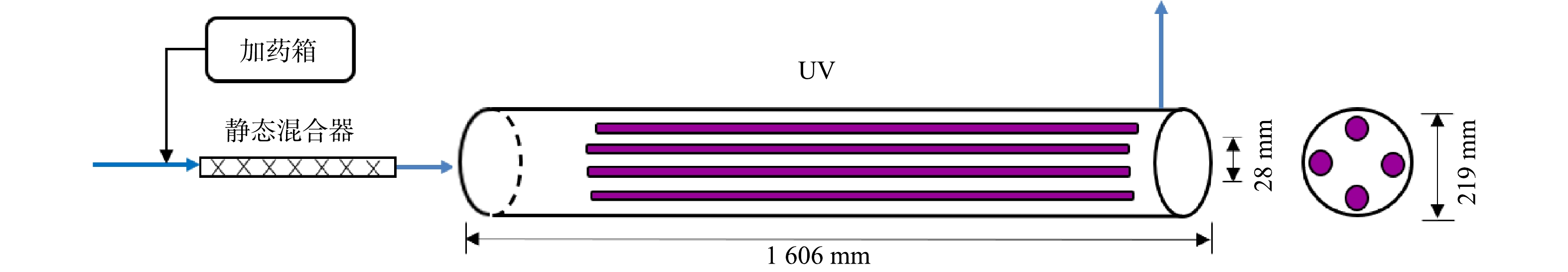
图1为现场紫外/氯中试系统的工艺流程示意图。以水厂消毒用的次氯酸钠为氧化剂,其有效质量分数约为5%,即有效氯浓度约50 g·L−1。采用计量泵将次氯酸钠原液投加到紫外反应器(北京安力斯,EX-260M)的进水管道中,经静态混合器与滤后水充分混合后进入反应器腔体。紫外反应器长度为1 606 mm,直径为219 mm;内置4支低压高强紫外灯(美国莱劭思,GPHHA1554T6L),单灯功率为320 W,UVC效率约为33%,套管外径为28 mm;反应器有效容积约为0.07 m3。定义现场紫外/氯中试系统的基础工况为:灯管输出功率100%(即P0为11.5 mW·cm−2),加氯量为5 mg·L−1,进水流量为3 m3·h−1。此时,水流在反应器腔体内的水力停留时间约为84 s。为考察不同加氯量的效果,研究期间有效氯投加量分别为0、5、10、15 mg·L−1。采用可调镇流器对紫外灯输出功率进行调节(P1、P2和P3),得到对应的紫外强度分别为11.2、10.8和9.1 mW·cm−2。此外,改变进水流量(1、2、3、4 m3·h−1)以评估系统在不同处理规模下的运行效果。如表1所示实验工况总共10个,分为3个对照组。每个工况下首先运行20 min待系统稳定后,在反应器出口取样并间隔10 min取平行样,分析水样的常规有机物指标(如UV254、溶解性有机碳DOC、三维荧光光谱)以及TrOCs、DBPs和DBPsFP等。
-
余氯(特指自由余氯)采用哈希USEPA DPD法测定,方法编号为8021。UV254采用紫外分光光度计(美国哈希,DR6000)。DOC利用总有机碳分析仪(日本岛津,TOC-VCPH)测定。水中的荧光性有机物通过荧光分光光度计(美国瓦里安,Cary Eclipse)获得的三维谱图进行表征分析。消毒副产物生成势测定方法如下:取50 mL水样置于无顶空棕色玻璃瓶中,投加5 mg·L−1次氯酸钠(以有效氯计)溶液,置于室温(25 ℃)下避光环境下反应24 h,后用抗坏血酸淬灭余氯,立即使用液液萃取方法测定DBPs浓度。液液萃取过程以甲基叔丁基醚(MTBE)为萃取剂,取20 mL水样,投加6 g无水硫酸钠,加入2 mL MTBE(含内标),振荡10 min后取有机相1 mL至进样瓶中待测。DBPs浓度测定采用配备HP 5毛细管色谱柱(30 m×0.25 mm,0.25 μm)的气相色谱仪和电子捕获检测器(美国安捷伦,Agilent 7890A),进样体积为10 μL,升温程序为:初始温度35 ℃,保持15 min;25 ℃·min−1 升温至145 ℃,保持3 min;再以35 ℃·min−1的速度上升至240 ℃。进样口温度为200 ℃,检测器温度为300 ℃,后运行2 min[7]。实验检测的共有13种DBPs,其中三氯甲烷(TCM, trichloromethane)、二氯一溴甲烷(BDCM,bromodichloromethane)、二溴一氯甲烷(DBCM,dibromochloromethane)、二氯乙腈(DCAN,dichloroacetonitrile)、1,1,1-三氯丙酮(1,1,1-TCP,1,1,1-trichlorpropanone)和三氯硝基甲烷(TCNM,trichloronitromethane)的检测限分别为0.50、0.07、0.09、0.03、0.03和0.04 μg·L−1,回收率分别为78.9%、89.2%、92.7%、90.2%、80.2%和75.4%。TrOCs分析首先需经过SPE富集:取1 L水样过玻璃纤维滤膜,调pH至2.5,加2.5 mL EDTA-2Na(0.2 mol·L−1)溶液;采用固相萃取柱(Oasis HLB, 500 mg·6 mL)富集,进样流速为5 mL·min−1,富集结束后进行淋洗、抽干,用10 mL甲醇洗脱小柱,洗脱液氮吹吹干后用400 μL甲醇和600 μL超纯水复溶,上机测样。TrOCs的定量分析采用超高液相色谱串联四极杆质谱仪(美国安捷伦,Agilent 6420),进样体积为5 μL。样品前处理过程中每10个样品设置1个方法空白。测定时,每10个样品设置1个溶剂空白,检测限为0.1 ng·L−1[8]。
-
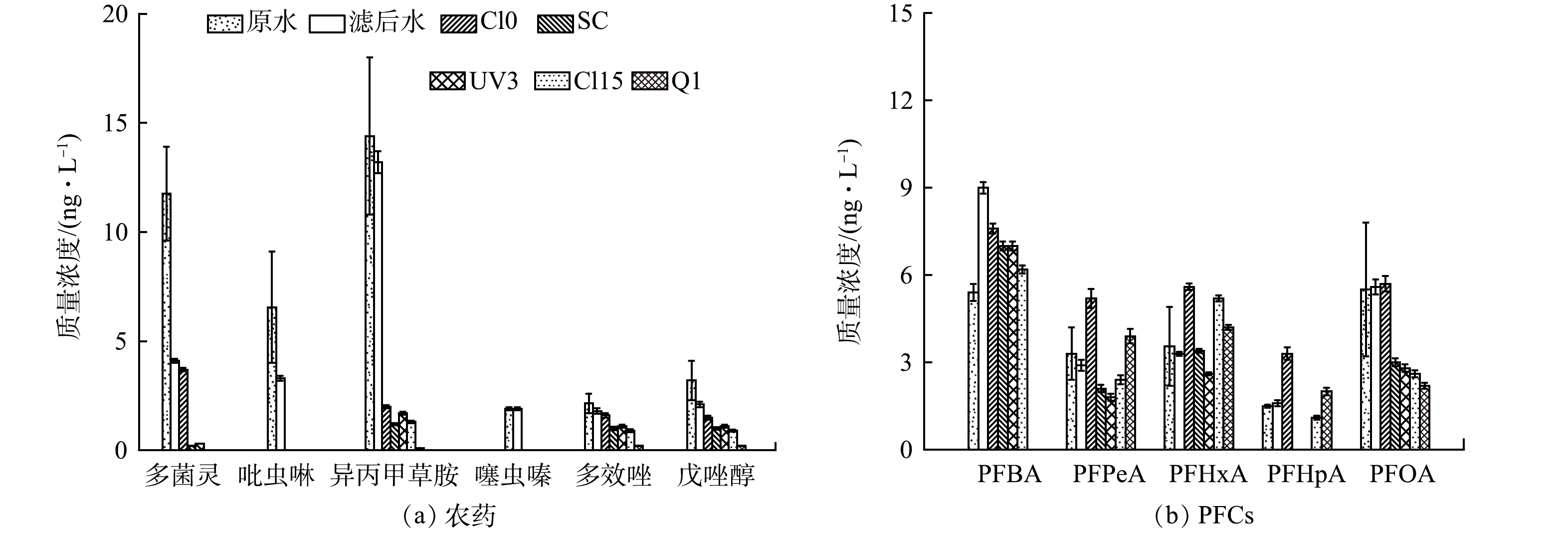
参考文献报道的环境水体TrOCs的检出情况,对水厂原水及滤后水中TrOCs的种类浓度进行筛查,进而评估典型工况下紫外/氯工艺对TrOCs的降解去除效果,结果如图2所示。筛查清单包括29种农药、29种抗生素以及14种全氟化合物(PFCs,perfluorinated compounds)[1,9]。水厂原水及滤后水中共检测出16种农药(多菌灵、吡虫啉、异丙甲草胺、噻虫嗪、多效唑、戊唑醇、异丙威、啶虫脒、甲霜灵、三唑醇、噻嗪酮、己唑醇、丙环唑、烯酰吗啉、嘧菌酯、苯醚甲环唑,其中前6种浓度高于1.0 ng·L−1)、5种PFCs(PFBA,perfluorobutyric acid、PFPeA,perfluorovaleric acid、PFHxA,perfluorocaproic acid、PFHpA,perfluoroheptanoic acid和PFOA,perfluorooctanoic acid;浓度均大于1.0 ng·L−1)和2种抗生素类有机物(磺胺甲恶唑、洛美沙星,浓度均小于1.0 ng·L−1)。这表明,作为水厂水源的水库水主要受到农药以及工业化学品污染,而生活源排放的抗生素等药物对其影响较小。水厂常规处理工艺对水中的农药类TrOCs有一定的去除作用,其中多菌灵、吡虫啉和戊唑醇的去除率在34.4%~65.1%,推测主要机理为混凝吸附以及活性炭吸附。滤后水中检出的农药以异丙甲草胺、多菌灵和吡虫啉等为主,浓度分别为13.2、4.1和3.3 ng·L−1,占比分别为42.3%、13.1%和10.6%。PFBA、PFOA、PFHxA的浓度分别为9、5.6和3.3 ng·L−1,占比分别为40.2%、25.0%和14.7%。滤后水中仅检测出超痕量的洛美沙星和磺胺甲恶唑(浓度均<1 ng·L−1),经紫外/氯工艺处理后未有检出,因此,后续不再对抗生素类TrOCs进行讨论分析。
紫外/氯工艺可以高效去除水厂滤后水中的农药类TrOCs,基础工况下16种农药的总体去除率为87.1%(图2(a))。其中,异丙甲草胺以及吡虫啉、噻虫嗪在单独紫外辐照下即发生显著的降解去除(>84.8%),而多菌灵在不同紫外/氯工况下均可实现高效去除(>92.7%)。多效唑和戊唑醇的降解效率与紫外剂量、加氯量高度相关,且提升紫外剂量相比加氯量能更好地提升降解效果,在此工况下2种污染物的最高去除率分别为88.9%和90.5%。上述降解去除规律的差异主要与TrOCs不同的分子结构及其在紫外直接光降解和自由基氧化下的反应活性有关。异丙甲草胺和吡虫啉、噻虫嗪分属酰胺类和烟碱类农药,前者对紫外光吸收一般但光降解量子产率很高(ε254=503 mol−1·cm−1,Φ254=0.302 mol·Einstein−1)[10];后两者的ε254分别为11 433 mol−1·cm−1和17 867 mol−1·cm−1,而Φ254分别为0.048 mol·Einstein−1和0.073 mol·Einstein−1 [11]。基于相关参数及低浓度污染物紫外光降解原理[12],可以确定去除90%上述3种农药所需的紫外剂量分别为3 104、859和362 mJ·cm−2。本中试系统紫外反应器在基础工况下提供的有效剂量超过966 mJ·cm−2(壁面光强乘以水力停留时间),因此,吡虫啉和噻虫嗪的去除率接近100%。紫外辐射对多菌灵的直接光降解能力较弱(ε254 = 3 043 mol−1·cm−1,Φ254 = 0.0016 mol·Einstein−1),而氯自由基(如Cl•、ClO•、Cl2•-)[13]的产生可显著强化对多菌灵的去除,这与多菌灵在UV/H2O2和UV/S2O82-处理过程中的去除规律类似[14]。饮用水中多效唑和戊唑醇的氧化去除在前期研究中关注较少。基于本研究结果,可以推断多效唑和戊唑醇在基础工况的紫外辐射及氯自由基氧化条件下的降解速率均偏低。降低进水流量至1 m3·h−1可显著提升水流接收到的紫外剂量(相对基础工况提升2倍),此时出水中包括多效唑等在内的农药总浓度降至0.6 ng·L−1,对应的总体去除率为98.1%。
相对而言,水中PFCs类TrOCs的降解去除更难且过程机理更为复杂。从图2(b)中可以看到,紫外/氯工艺对滤后水中PFCs的去除率并不理想,尤其是对于分子质量较低的短链PFCs(如PFBA),部分工况下甚至出现PFCs浓度升高的情况。具体地,PFOA在紫外/氯条件下可实现超过46.4%的降解,PFBA的降解率约为30%,而PFHxA等在部分工况下出现了浓度升高的情况。出现这一结果的原因在于PFCs分子结构中主要存在的C—F键键能过高,其降解过程主要围绕末端的-COOH进行,包括脱羧-羟基化-消除反应-水解系列反应,产物为减碳后的PFCs[15]。由于对254 nm波长的光近似无吸收,PFOA在单独紫外辐照下基本不发生降解[16],而其在紫外/氯条件下的降解主要与工艺过程产生的Cl•和Cl2•-有关[17]。相似的结果在CAO等[18]研究中出现,紫外光照射对PFOA的降解率仅为9%,但降解中间产物PFHxA和PFHpA的去除率随着照射时间的延长明显增加,进一步反应后PFHxA开始减少。此外,水中存在的未知PFCs前体物可能会在紫外/氯工艺的作用下转化为PFHxA和PFHpA等,导致该类PFCs的浓度升高甚至超标[19]。虽然本工艺对PFCs有一定的降解效果,但总体去除率不高。考虑到饮用水中允许的PFCs限值通常很低(如美国纽约州饮用水水质标准要求PFOA浓度低于10 ng·L−1),对于PFCs检出浓度较高的水源,需要考虑采用其他工艺以降低饮用水的化学风险。
-
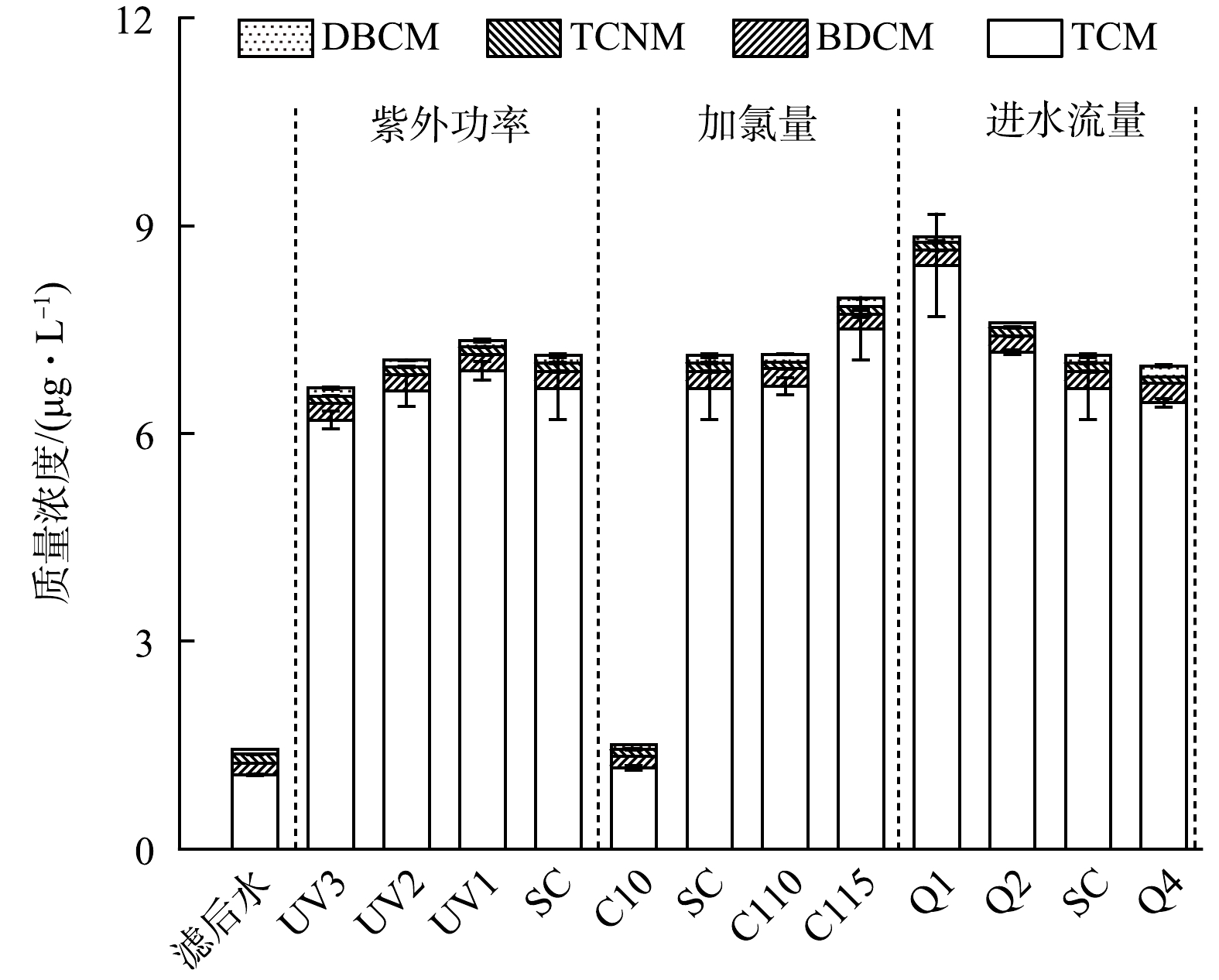
如前所述,采用紫外/氯工艺可以有效降解去除水中大部分的TrOCs。然而,由于该工艺过程主要依赖强氧化性的活性氯物种,期间水中DBPs的生成量可能会有所增加,需要特别关注。图3给出了紫外/氯工艺深度处理水厂滤后水过程中典型DBPs的生成情况。其中,以三卤甲烷(THMs)作为含碳消毒副产物的代表,以卤乙腈(HANs)作为含氮消毒副产物的代表。在未加氯的情况下(滤后水及单独紫外辐射),水中DBPs的浓度很低,可能来源为水厂原水的预氯化过程。滤后水经紫外/氯工艺处理后,水中DBPs浓度有较为显著的上升,且在不同工况下三氯甲烷(TCM)的占比均超过90%。含溴及含氮DBPs的浓度很低甚至低于检出限,这可能在于水中Br−浓度较低,而上述高级氧化过程以活性氯物种的反应为主。提高灯管输出功率、增大加氯量以及减少进水流量,水中DBPs浓度均呈现增加趋势,在进水流量为1 m3·h−1时,DBPs最高浓度为8.8 μg·L−1,相对基础工况时增加了约24%。紫外/氯工艺处理过程中产生的活性氯物种会通过氧化和亲电卤化作用攻击水中的有机物从而形成氯代消毒副产物或者前驱物。然而,随着加氯量的提高,THMs并未出现预期中的大量增加的情况。究其原因,可能在于该水厂滤后水DOC浓度(1.27 mg·L−1)及SUVA值(1.28 L·(mg·m)−1)较小,即水中有机物含量少且芳香性低,因此,对应的DBPs生成量少[20]。总体而言,现场紫外/氯工艺深度处理滤后水时虽然会增加水中DBPs的含量,但其浓度仍显著低于《生活饮用水卫生标准》(GB 5749-2022)的限值。
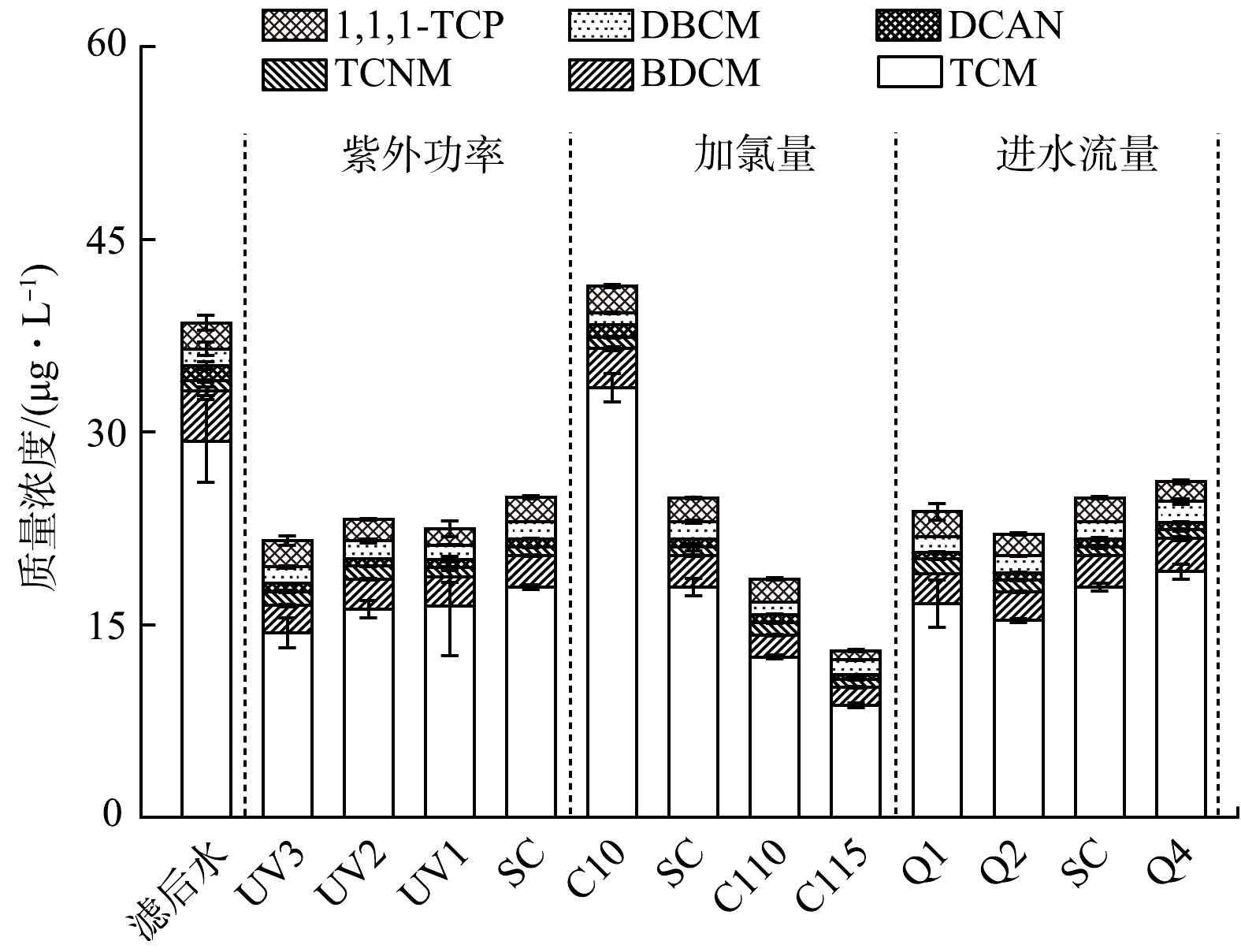
为分析紫外/氯工艺对饮用水DBPs的综合控制效果,对工艺处理前后水样的消毒副产物生成势(DBPsFP)进行评估。为保证不同水样的初始有效氯浓度一致,首先通过投加硫代硫酸钠猝灭相关水样中的余氯,再投加适宜的次氯酸钠以保证最终的有效氯浓度为5 mg·L−1。置于避光环境下反应24 h,检测相关DBPs生成浓度。图4给出了不同水样中DBPsFP的分布,检测到的DBPs种类包括TCM、BDCM、TCNM、DCAN、DBCM、1,1,1-TCP和BCAN等。与直接检测到的DBPs分布结果类似,紫外/氯处理前后水样DBPsFP同样以TCM为主(占比超过65%)。滤后水的DBPsFP为38.5 μg·L−1,经单独紫外处理后略有上升(41.3 μg·L−1)。而高级氧化过程明显降低了水样后续生成消毒副产物的能力,且加氯量越高最终的DBPsFP越低,最小值仅为12.9 μg·L−1,相对滤后水的降幅达到66.3%。紫外/氯工艺将减少天然有机物的疏水性和芳香性并增加DBPs前体物浓度,但生成的DBPs及前体物在紫外辐射和自由基氧化的作用下可进一步降解去除[21]。中试及以上规模的处理条件下,紫外/氯工艺过程中DBPs及DBPsFP的形成与实际水质及现场操作条件息息相关。基于本研究结果,当水厂滤后水中有机物含量及芳香性较低时,采用紫外/氯工艺可明显削减DBPs的综合风险,进而提高饮用水化学安全性。值得一提的是,紫外/氯工艺处理后适宜浓度的余氯可直接进入后续管网输配系统。本研究现场中试系统出水余氯浓度介于0~5.6 mg·L−1,其中基础工况下余氯浓度为1.07 mg·L−1,且改变加氯量或紫外剂量可以调节出水余氯浓度,从而满足管网微生物控制需求。
-
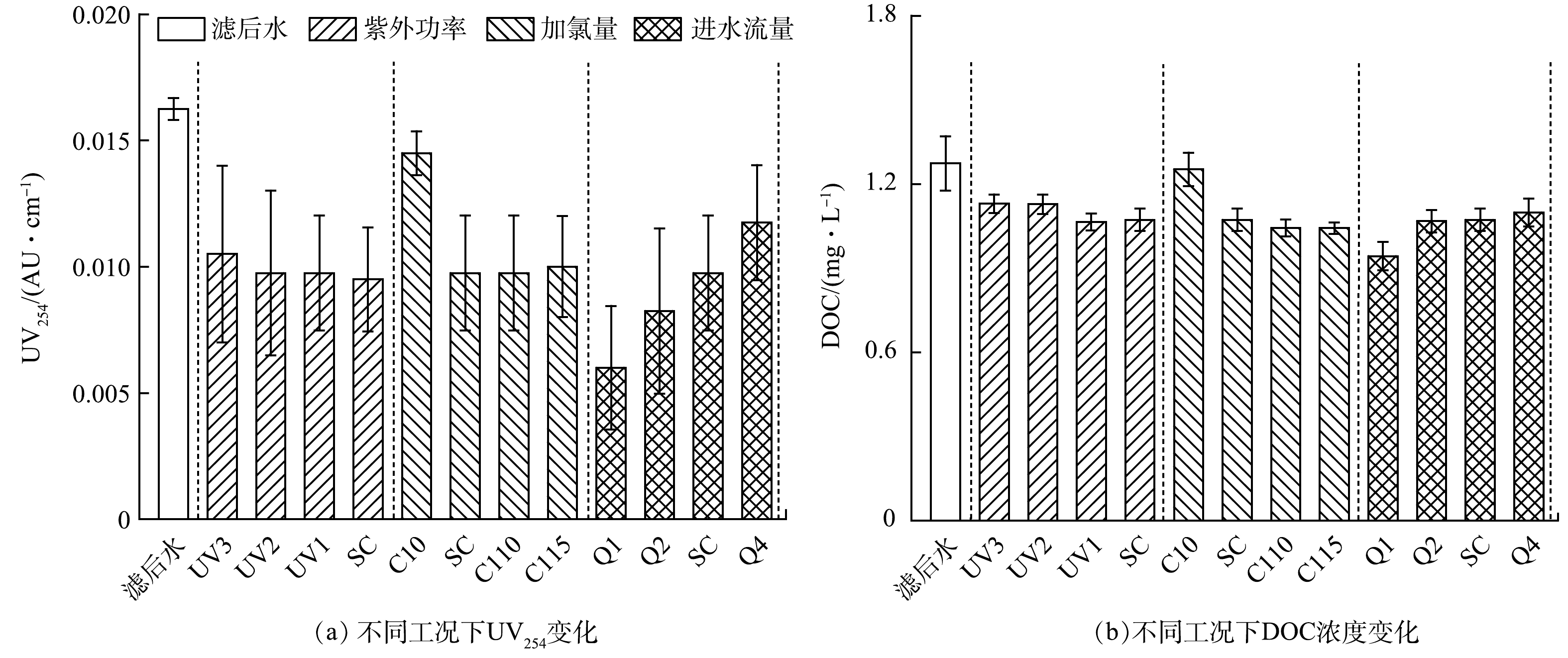
为进一步分析紫外/氯中试系统中微量有机污染物及消毒副产物的变化规律和机理,对中试系统进出水的常规有机物指标进行了同步监测。图5为不同工况紫外/氯工艺处理前后水中UV254和DOC的变化。由图5可知,当紫外灯输出功率增加或处理流量降低时,水中UV254的去除率均有不同程度的提升,在流量为1 m3·h−1时去除率达到最大,约为63%。紫外/氯工艺过程中增加加氯量对UV254的削减作用影响甚微。这表明,紫外剂量而非有效氯浓度是影响水中有机物芳香性去除的关键。这与前述农药类TrOCs的去除规律类似,即污染物(除吡虫啉和噻虫嗪)的最大去除率出现在流量最小时(剂量最大时)。相对而言,不同工况下水中DOC的变化较小,其去除率基本在11.3%~18.1%,最大去除率同样在进水流量最低时得到,为26.0%。采用比紫外吸光度(SUVA)进一步量化上述变化。现场滤后水的SUVA值约为1.28 L·(mg·m)−1,经紫外/氯深度处理后SUVA值降至1.0 L·(mg·m)−1以下,最小值仅为0.64 L·(mg·m)−1,表明水中有机物芳香性进一步降低。有研究[20-21]表明,水体DBPsFP与有机物SUVA值具有良好的正相关性。这解释了上述紫外/氯工艺处理后DBPsFP减少的原因。
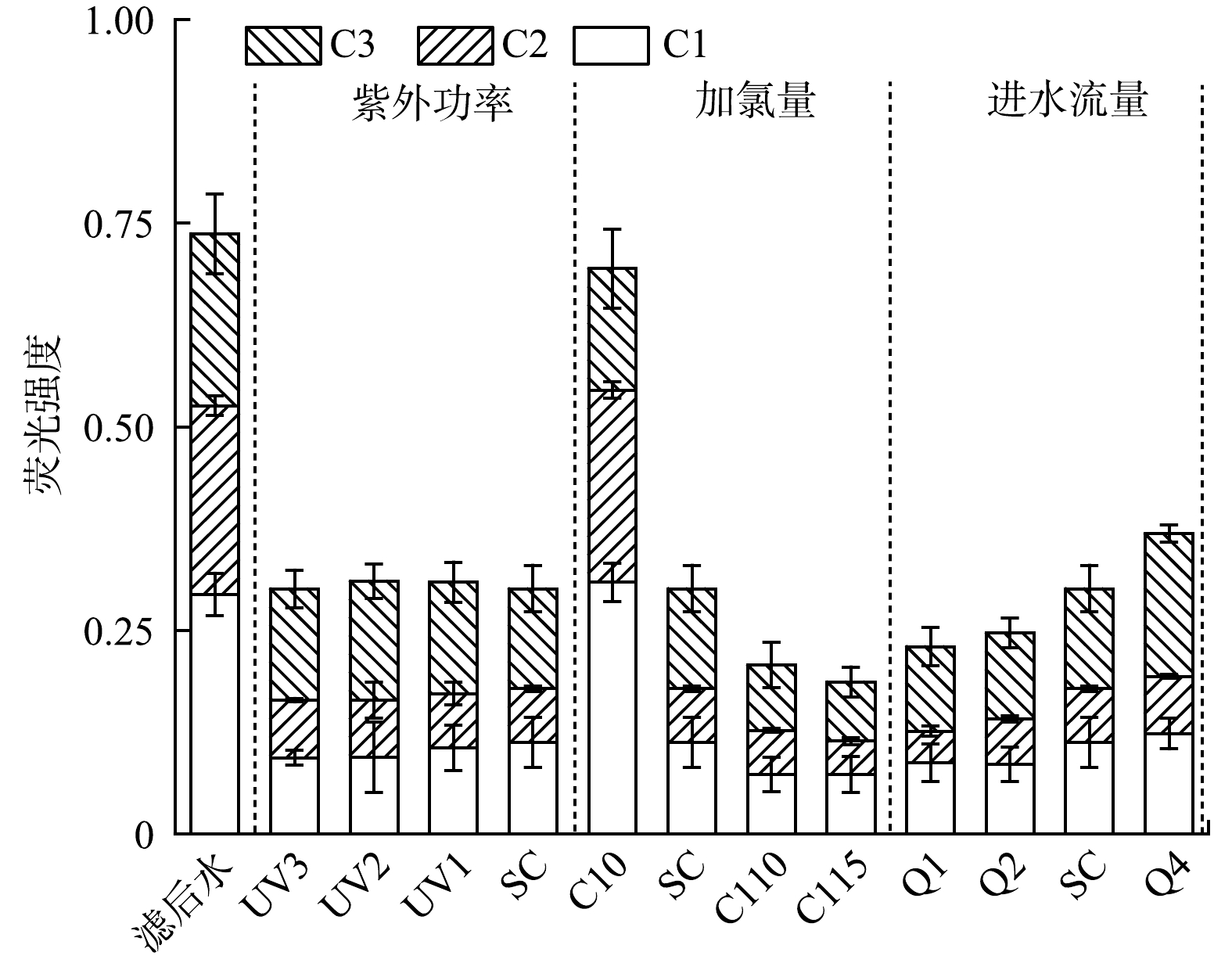
图6为不同工况紫外/氯工艺处理前后水样荧光光度的变化。由图6(a)可知,水厂滤后水中主要含有富里酸类物质(区域III)、腐殖酸类物质(区域V)和蛋白质类物质(区域I和II)。单独紫外辐射对水中荧光性有机物的去除效果有限,而同时投加次氯酸钠形成紫外/氯高级氧化工艺后各类荧光性物质均得到显著去除(图6(b)和图6 (c))。降低灯管输出功率、增大加氯量或延长水力停留时间均可进一步降低有机物的荧光强度(图3(d)~(f))。对不同工况下水样的荧光光谱数据进行PARAFAC建模分析[22],确定了3种荧光组分,相应荧光强度如图7所示。其中,C1为腐殖酸类物质,Ex/Em最大值为245 nm/440 nm;C2为富里酸类有机物,Ex/Em最大值为250 nm/420 nm;C3为芳香族蛋白质有机物,Ex/Em最大值为220 nm/330 nm。紫外/氯工艺对水中各类荧光组分均具有良好的去除效果,基础工况下C1、C2、C3组分的去除率分别为61.7%、71.6%和41.8%。腐殖酸类、富里酸类和芳香族蛋白质类有机物均是DBPs的重要前体物[23],这些有机物的去除有助于减少后续加氯消毒过程中相关DBPs的生成。
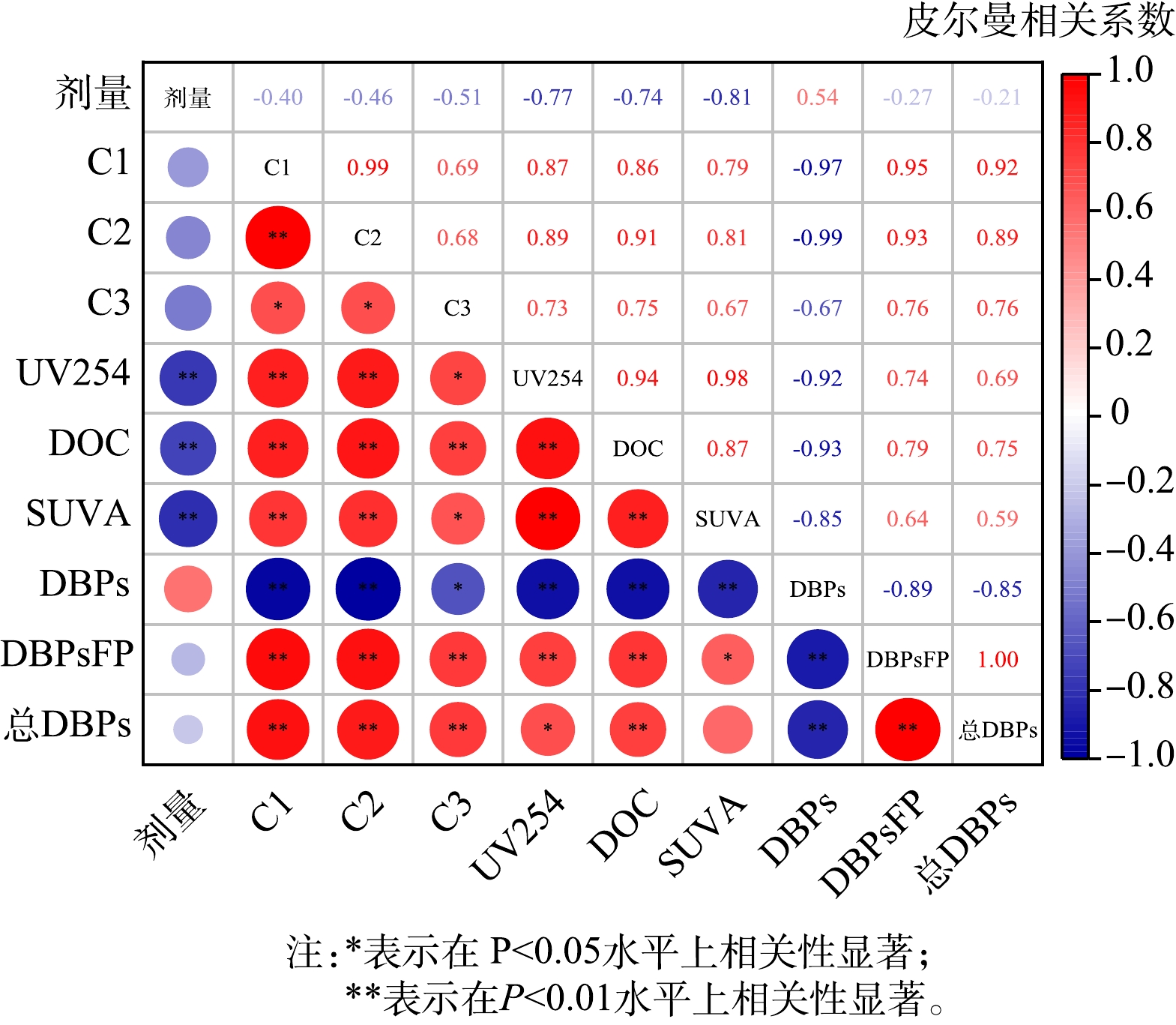
为量化评估紫外/氯工艺中DBPs生成与常规有机物指标之间的相关性,采用皮尔曼相关系数进行分析,结果如图8所示。相关系数越接近+1(-1),表明正(负)相关性越强,相关系数越接近0表明相关性越弱。由图8可见,在紫外/氯工艺中紫外剂量与UV254、DOC、SUVA之间呈显著负相关。紫外剂量越大,UV254、DOC、SUVA的去除效果越好,这与紫外/氯工艺反应原理相一致。C1、C2、C3组分与UV254、DOC、SUVA之间呈显著正相关,说明荧光组分是水中有机物尤其是芳香性有机物的重要组成部分。紫外/氯工艺过程产生的DBPs及后续加氯的DBPsFP分别与水中常规有机物指标呈显著负相关和强正相关,表明随着有机物反应的进行,紫外/氯生成的DBPs会增多,但后续DBPsFP因为水中荧光性/芳香性组分的减少而降低。综合水中TrOCs及总DBPs的削减效果,紫外/氯工艺在饮用水深度处理中具有良好的应用前景。
-
1)紫外/氯中试系统对滤后水中检出的农药类TrOCs具有良好的去除效果,基础工况下水中异丙甲草胺、多菌灵、吡虫啉等的整体去除率为87.1%,提高紫外剂量可以进一步提高其去除率;紫外/氯工艺对检测到的5种PFCs的去除效果较为一般,其中PFOA去除率相对最高(46.4%),而部分工况下PFHxA等出现浓度增加的情况,说明在紫外/氯高级氧化过程中可能存在长链PFCs向短链PFCs转化的降解途径。
2)滤后水紫外/氯高级氧化过程会生成一定量的以TCM为主的DBPs(≤ 8.8 μg·L−1),但处理后水样的DBPsFP浓度降低,且在此过程中加氯量越高DBPsFP浓度越低,最低仅为12.9 μg·L−1,相较于处理前降幅达到66.3%。
3)紫外/氯工艺可以强化去除滤后水中的芳香性和荧光性有机物,工艺出水的SUVA值降至1.0 L·(mg·m)−1以下,3类荧光组分的去除率在41.8%~71.6%。通过皮尔曼相关系数分析,确认了紫外/氯处理过程DBPs生成及后续DBPsFP降低与水中荧光性/芳香性有机物组分含量变化的强相关性。
中试紫外/氯工艺对饮用水中微量有机污染物及消毒副产物的削减
Elimination of trace organic contaminants and disinfection by-products in drinking water by a pilot-scale UV/chlorine process
-
摘要: 针对水源微污染等问题,在某饮用水厂开展紫外/氯高级氧化工艺深度处理滤后水的中试研究,探究不同工艺条件下水中微量有机污染物(TrOCs,trace organic contaminants)的去除及消毒副产物(DBPs,disinfection by-products)的控制机制。结果表明,紫外/氯工艺可高效去除滤后水中的异丙甲草胺、多菌灵、吡虫啉等农药,上述基础工况下其总体去除率为87.1%。提高紫外剂量对各类农药去除率的提升效果优于增大加氯量。水中全氟化合物(PFCs,perfluorinated compounds)的去除效率一般,其中全氟辛酸(PFOA,Perfluorooctanoic acid)去除率(46.4%)相对最高,而部分工况下全氟己酸(PFHxA,perfluorocaproic acid)等出现浓度升高的情况,说明在紫外/氯高级氧化过程中可能存在长链PFCs向短链PFCs转化的降解途径。紫外/氯高级氧化过程伴随着一定量的三氯甲烷(TCM,trichloromethane)等DBPs的生成(≤ 8.8 μg·L−1),但工艺出水的消毒副产物生成势(DBPsFP,formation potential of DBPs)下降明显,且加氯量越高消毒副产物的生成势越低,其最低值(12.9 μg·L−1)相较于处理前降幅达到66.3%。对滤后水中常规有机物指标进行分析,发现紫外/氯工艺可以强化去除滤后水中的芳香性和荧光性有机物,工艺出水的比紫外吸光度(SUVA)降至1.0 L·(mg·m)−1以下,3类荧光组分的去除率在41.8%~71.6%。相关性分析结果表明,紫外/氯处理过程中DBPs的生成及后续DBPsFP的大小主要取决于水中荧光性/芳香性有机物组分的含量。Abstract: Against the background of water sources being contaminated by micropollutants, a pilot-scale UV/chlorine system was set up in a drinking water treatment plant to explore the efficacy and mechanism of trace organic contaminants removal as well as the elimination of disinfection by-products and the formation potentials under different process conditions. Results show that the UV/chlorine process was efficient in removing metolachlor, carbendazim, imidacloprid and other pesticides found in the sand-filtered water. A gross removal efficiency of 87.1% was achieved under the benchmark operation condition, while an elevated UV fluence was more beneficial than an increased chlorine dose. The removal of perfluorinated compounds (PFCs) by UV/chlorine was relatively low with the largest removal efficiency found with perfluorooctanoic acid (PFOA), which was 46.4%. The content of perfluorohexanoic acid (PFHxA) was found to increase under some operation conditions. This indicates that the transformation of long-chain PFCs to short-chain PFCs could exist during the process. Meanwhile, disinfection by-products (DBPs) consisting mainly of trichloromethane (TCM) were generated with a maximum concentration of 8.8 μg·L−1, yet the disinfection by-products formation potential (DBPsFP) of the effluent decreased significantly. The minimum DBPsFP occurred with the largest chlorine dose, which was 12.9 μg·L−1, 66.3% lower than that before UV/chlorine treatment. Moreover, the changes in dissolved organic matter in the sand-filtered water were characterized with parameters such as the UV absorbance at 254 nm (UV254) and fluorescence excitation emission matrix. The aromatic and fluorescent constitutions of the organics were reduced significantly by UV/chlorine treatment as evidenced by the lowered SUVA (< 1.0 L·mg−1·m−1) and the prominent removal efficiencies of the three identified fluorescent compositions (41.8%~71.6%). A correlation analysis was conducted which revealed the strong correlations between the formed DBPs or the subsequent DBPsFP and the contents of aromatic and fluorescent constitutions in the water.
-

-
表 1 实验工况参数
Table 1. Parameters of experimental conditions
工况 紫外功率
/(mW·cm−2)加氯量/(mg·L−1) 进水流量/(m3·h−1) SC 11.5 5 3 UV1 11.2 5 3 UV2 10.8 5 3 UV3 9.1 5 3 Cl0 11.5 0 3 Cl10 11.5 10 3 Cl15 11.5 15 3 Q1 11.5 5 1 Q2 11.5 5 2 Q4 11.5 5 4 注:SC表示标准工况;UV表示紫外功率;Cl表示加氯量;Q表示进水流量。 -
[1] MA S J, DONG H Y, LI D, et al. Priority screening of contaminants of emerging concern in drinking water sources of eastern China: Assessing risks based on exposure-activity ratios (EARs)[J]. Science of the Total Environment, 2023, 896: 164881. [2] WU S N, DONG H Y, ZHANG L P, et al. Formation characteristics and risk assessment of disinfection by-products in drinking water in two of China’s largest basins: Yangtze River Basin Versus Yellow River Basin[J]. ACS ES& T Water, 2024, 4(1): 79-90. [3] 李慧琴. 基于紫外的高级氧化技术在水处理中的应用进展[J]. 化工设计通讯, 2020, 46(11): 28-30. doi: 10.3969/j.issn.1003-6490.2020.11.015 [4] 张淼, 李文涛, 连军锋, 等. 紫外/氯工艺去除煤化工高盐废水中有机物的研究[J]. 中国给水排水, 2021, 37(21): 82-88. [5] ZHOU Y, CHENG F, HE D, et al. Effect of UV/chlorine treatment on photophysical and photochemical properties of dissolved organic matter[J]. Water Research, 2021, 192(19): 116857. [6] YIN R, SHANG C. Removal of micropollutants in drinking water using UV-LED/chlorine advanced oxidation process followed by activated carbon adsorptiont[J]. Water Research, 2020, 185: 116297. doi: 10.1016/j.watres.2020.116297 [7] WANG Y, DONG H, WU Z, et al. UV activated monochloramine promotes metribuzin degradation and disinfection by-products formation[J]. Chemical Engineering Journal, 2019, 385: 123846. [8] WU S N, YUAN T T, FU W, et al. Perfluorinated compound correlation between human serum and drinking water: Is drinking water a significant contributor? [J]. Science of the Total Environment, 873: 162471. [9] 顾允轩, 仇付国, 王大伟, 等. 典型农业小流域中29种农药类微污染物检出、时空变化与生态风险评估[J]. 生态毒理学报, 2022, 17(3): 326-338. doi: 10.7524/AJE.1673-5897.20210815001 [10] WU C L, SHEMER H, Linden K G. Photodegradation of Metolachlor Applying UV and UV/H2O2[J]. Journal of Agricultural and Food Chemistry, 2007(10): 55. [11] ACERO J L, REAL J F, BENITEZ J F, et al. Degradation of neonicotinoids by UV irradiation: Kinetics and effect of real water constituents[J]. Separation and Purification Technology, 2019, 211: 218-226. doi: 10.1016/j.seppur.2018.09.076 [12] BOLTON J R, STEFAN M I. Fundamental photochemical approach to the concepts of fluence (UV dose) and electrical energy efficiency in photochemical degradation reactions[J]. Research on Chemical Intermediates, 2002, 28(7-9): 857-870. doi: 10.1163/15685670260469474 [13] FANG J, FU Y, SHANG C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system[J]. Environmental Science & Technology, 2014, 48(3): 1859-1868. [14] STARLING V C V. M, SOUZA P P, PERSON L A, et al. Intensification of UV-C treatment to remove emerging contaminants by UV-C/H2O2 and UV-C/S2O82-: Susceptibility to photolysis and investigation of acute toxicity[J]. Chemical Engineering Journal, 2019, 376: 120856. doi: 10.1016/j.cej.2019.01.135 [15] TAKASHI Y, YUKIO N, SHIN-ICHI S, et al. Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol[J]. Environmental Science & Technology, 2007, 41(16): 5660-5. [16] 刘明, 黄妍妍, 史金玉, 等. 真空紫外/紫外/过硫酸盐工艺降解水中全氟辛酸[J]. 环境工程学报, 2023, 17(5): 1409-1417. doi: 10.12030/j.cjee.202301039 [17] METZ J, ZUO P X, WANG B, et al. Perfluorooctanoic acid Degradation by UV/ChlorineJordin[J]. Environmental Science & Technology Letters, 2022, 9(8): 673-679. [18] CAO M H, WANG B B, YU H S, et al. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 1143-1146. [19] VENKATESAN A K, LEE C S, CHRISTOPHER G, et al. Hydroxyl-radical based advanced oxidation processes can increase perfluoroalkyl substances beyond drinking water standards: Results from a pilot study.[J]. Science of the Total Environment, 2022, 847: 157577. doi: 10.1016/j.scitotenv.2022.157577 [20] KRISTIANA I, HERVE G, JOLL C, et al. The formation of halogen-specific TOX from chlorination and chloramination of natural organic matter isolates[J]. Water Research, 2009, 43(17): 4177-4186. doi: 10.1016/j.watres.2009.06.044 [21] SUN J, KONG D, AGHDAM E, et al. The influence of the UV/chlorine advanced oxidation of natural organic matter for micropollutant degradation on the formation of DBPs and toxicity during post-chlorination[J]. Chemical Engineering Journal, 2019, 373: 870-879. doi: 10.1016/j.cej.2019.05.096 [22] ISHII S K L, BOYER T H. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: A critical review[J]. Environmental Science & Technology, 2012, 46(4): 2006-17. [23] XU X T, KANG J, SHEN J M, et al. EEM–PARAFAC characterization of dissolved organic matter and its relationship with disinfection by-products formation potential in drinking water sources of northeastern China[J]. Science of the Total Environment, 2021, 774(1): 145297. -



 下载:
下载: