-
我国城市污水以及工业园区废水普遍存在碳氮比低的问题,常需投加补充碳源提高脱氮效果,增加了污水厂的运行成本。常用商品碳源有甲醇、乙酸钠、葡萄糖等[1]。这类碳源需要长期持续投加,投加量不足时出水中有残留硝态氮;投加过量时出水中残留碳源会造成二次污染[2]。近年来投加固态缓释碳源成为新的发展方向[3]。我国工业园区有机固废资源化利用率普遍较低,根据国家统计局发布的《2 022年中国统计年鉴》,全国一般工业固废综合利用率为57%。利用有机固废制备缓释碳源,在实现有机固废减量化和资源化的同时提高园区污水厂脱氮效果,降低污水处理成本,具有较好的应用价值和科学意义。
复合缓释碳源主要为骨架材料、有机底物、海藻酸钠(sodium alginate,SA)和氯化钙通过化学交联形成。常用的骨架材料有聚乙烯醇(polyvinyl alcohol, PVA)、聚乳酸(polylactic acid, PLA)、聚己内酯(polycaprolactone, PCL)等[4-5]。其中PCL多用于医学用途,价格相对较高;PLA强度较低,且相容性较差;PVA价格相对较低,机械强度高,因此,采用PVA为骨架材料的研究相对较多[6]。缓释碳源常用的有机底物有花生壳、淀粉、玉米芯等[7-9]。此外,在固体缓释碳源制备过程中加入乳化剂可以调节孔隙度、控制释碳速率、提高缓释效果[10]。用乳化剂发泡法制备的PVA具有均匀、高度贯通的孔隙结构[11]。常见的乳化剂有吐温80(tween80)和司盘80(span80)。
已有研究表明,复合缓释碳源具有较好的反硝化效果。XIONG等[7]利用花生壳做底物,PCL和PVA做骨架材料,与SA进行交联,花生壳:PCL:PVA:SA质量浓度比例为8:8:8:1制备的缓释碳源,反硝化速率达到0.178 mg·(L·h)−1,硝酸盐去除率为99.9%,出水溶解性有机碳(dissolved organic carbon, DOC)为38 mg·L−1。唐丹琦等[12]以淀粉为底物,PLA为骨架材料制备缓释碳源。按照淀粉:PLA=5 g·L−1:5 g·L−1的比例制备,反硝化速率为1.03 mg·(L·h)−1,硝酸盐去除率为99.0%,残余化学需氧量(chemical oxygen demand, COD)为 80 mg·L−1。王润众等[10]在醋酸酯淀粉/PVA体系中添加醋酸酯淀粉与PVA总质量5%的span80制备缓释碳源,反硝化速率为0.182 mg·(L·h)−1,硝酸盐去除率为85%,残余COD为16 mg·L−1。
酿酒产业是某些综合型工业园区中重要的产业类型。酒糟是经过第一道压榨工艺压榨,晾干后得到的固体物质,主要由酿酒原料以及在糖化、发酵过程中产生的代谢产物和残留的酵母细胞组分[13],主要成分为淀粉、蛋白质和氨基酸、纤维素、酒精等[14],具有良好的微生物可利用性。目前酒糟主要用途有作为饲料填充料、厌氧消化等[15-16],但缺乏利用酒糟制备缓释碳源的研究,因此本研究以酒糟做有机底物,以PVA-SA为骨架材料制备缓释,并考察其释碳和反硝化性能。研究结果可以为工业园区固废-污水协同治理模式提供参考。
-
有机底物酒糟取自江西省某工业园区某黄酒厂;PVA,分子质量67 000,上海麦克林生化科技股份有限公司;SA,上海麦克林生化科技股份有限公司;无水氯化钙(CaCl2),上海麦克林生化科技股份有限公司;硼酸(H3BO3),国药集团化学试剂有限公司;硝酸钾(KNO3),国药集团化学试剂有限公司;碳酸氢钠(NaHCO3),上海麦克林生化科技股份有限公司;tween80,上海麦克林生化科技股份有限公司;span80,上海麦克林生化科技股份有限公司;所有药品为分析纯级别。
-
酒糟在烘箱为50 ℃条件下,烘干2 h后备用。分别进行骨架材料配比和乳化剂优选两组实验。其中实验组1#~6#为骨架材料配比实验,缓释碳源溶液总体积为100 mL,加入1 g酒糟,PVA分别投加量为6、7、8 g,SA投加量分别为1、2 g,即PVA:SA质量比分别为6:1、6:2、7:1、7:2、8:1、8:2时制备缓释碳源,考察碳源释碳性能,优选最佳组。以最佳释碳骨架材料配比组为不加乳化剂的对照组7#,分别向7#加入缓释碳源制备溶液总体积0.25%、0.5%和1.0%的tween80(8#~10#)或span80(11#~13#)制备缓释碳源,考察碳源释碳、氮、磷性能。
-
根据设计的骨架材料配比,将适量的PVA和SA加入到容量为200 mL烧杯中,用超纯水定容至100 mL,将烧杯放入六联电动搅拌恒温水浴锅中。在搅拌速度为300 r·min−1,95 ℃恒温搅拌1 h,再加入相应质量的酒糟,继续搅拌1 h后,将凝胶注入10 mm×10 mm×10 mm硅胶模具中,将硅胶模具放入−20 ℃冰箱冷冻12 h成型。取出碳源材料置于4%氯化钙的饱和硼酸溶液中,4 ℃化学交联24 h。取出碳源材料用超纯水冲洗3次,去除碳源表面残留的交联剂。将制备好的缓释碳源放入烘箱中60 ℃干燥6 h至恒重,室温下自然冷却,密封存放于4 ℃冰箱备用。在乳化剂优选实验中,PVA和SA的投加量固定为80 g·L−1和10 g·L−1,制备碳源过程中在加入酒糟后投加相应量的乳化剂,其余步骤与骨架材料配比优化实验相同。
-
在环境温度为(20±2) ℃进行释碳性能评估。取1 g左右碳源,准确称量并记录碳源质量,分别放到锥形瓶里,用超纯水固定体积200 mL,密封锥形瓶放于摇床中,释放7 d,每24 h换水1次。释碳过程中释碳溶液pH为6.23±0.62。分别在0、2、4、6、8、12、24、48、72、96、120、144、168 h采集水样,分析pH、总溶解固体(TDS)、溶解性COD(记作COD)、溶解性有机碳、UV254、氨氮(NH4+-N)、硝态氮(NO3−-N)、亚硝氮(NO2−-N)、总氮(TN)以及正磷酸盐(PO43−-P),考察每日释碳量和累积释碳量变化,释碳量以每克缓释碳源释放的COD计。每组释碳实验做2组平行实验,取平均值进行分析。
-
采用二级动力学方程(式(1))对释碳动力学进行拟合分析(半衰期计算公式如式(2))[17]。
式中:Ct 为单位质量材料在t时间累计释放的碳量(以COD计),mg·(g·L)−1;C∞为饱和释碳量,mg·(g·L)−1;K为传质系数(表示释碳阻力),mg·(h·g·L)−1,K越大表明释碳过程受到的阻力越小,越容易释放有机碳;t1/2为半衰期,h,t1/2越小代表达到碳释放平衡的时间越短。
采用Ritger-Peppas方程(式(3))对缓释碳源的释碳机制进行分析[18]。
式中:Mt 为在t时间内累计释放的碳量,mg·g−1;M∞为饱和释碳量,mg·g−1;k为碳释放常数;N为碳释放指数。
-
在江西某市政污水处理厂氧化沟缺氧段收集活性污泥,用超纯水离心洗涤3次后,作为反硝化接种污泥。以人工配水作为反硝化实验进水。人工配水组分为KNO3、NaHCO3和微量元素[19]。取适量接种污泥加入到1 000 mL烧杯中,加入1 mL微量元素[20]溶液,将1粒缓释碳源(0.18~0.19 g·L−1)加入烧杯中,以人工配水定容到1 000 mL,以NaHCO3调节pH至8.0左右,污泥质量浓度(mixture liquid suspended solid, MLSS)为(3 613±181) mg·L−1,起始NO3−-N为40 mg·L−1。以不加碳源的实验组为空白组。杯口用保鲜膜密封,以200 r·min−1在室温下进行反硝化实验。每组反硝化实验做2组平行实验,取平均值进行分析。
-
水样pH和温度采用便携式水质分析仪(WTW,Germany)测定,COD通过哈希预制管消解及DR2 800分光光度计(HACH,USA)测定,DOC由TOC-VCPH分析仪(Shimadzu,Japan)测定,NH4+-N、NO2−-N、NO3−-N、TN、PO43−-P、TDS、MLSS等依据《水和废水监测分析方法》测定[21]。采用Lowry法测定蛋白质[22],Dubois法测定多糖[22]。通过气相色谱仪(Agilent,GC8 890,USA)测定可挥发性脂肪酸(volatile fatty acids, VFA)[23]。
缓释碳源释放溶解性有机质(dissolved organic matter, DOM)的紫外-可见光谱学特征采用紫外-可见吸收光谱分光光度计(Spectrum Lab 752sp,Lengquang Tech,China)测定,并计算下列参数:SUVA254以样品在254 nm处的吸光度乘100与DOC质量浓度比值表示DOM的芳香性等特征;A250/365为样品在250 nm和365 nm处吸光系数的比值,用来表征DOM分子质量大小;A300/400为样品在300 nm和400 nm处吸光系数的比值,用来表征DOM的腐殖化程度;A253/203为样品在253 nm和203 nm处吸光度比值,用来表征芳香环上取代基的种类和取代程度高低。
DOM的三维荧光光谱学特征采用三维荧光光谱仪(HITACHI,Japan)进行分析。测量激发波长(200~450 nm)和发射波长(220~550 nm)的荧光强度,生成三维激发发射荧光矩阵,激发和发射狭缝宽度设置为5 nm,样品稀释倍数为5倍。根据激发波长(excitation wavelength, Ex)和发射波长(emission wavelength, Em),可将荧光区域划分为5个区域[24]:区域I(200 nm<Ex<250 nm,280 nm<Em<330 nm)为酪氨酸类蛋白;区域II(200 nm<Ex<250 nm,330 nm<Em<380 nm)为色氨酸类蛋白;区域III(200 nm<Ex<250 nm,380 nm<Em<480 nm)为富里酸类;区域IV(250 nm<Ex<280 nm,280 nm<Em<380 nm)为溶解性微生物代谢物类;区域V(280 nm<Ex<420 nm,380 nm<Em<520 nm)为腐殖酸类。
采用阴离子色谱(Ion Chromatography,USA)对SO42−、Cl−、F−、Br−进行测定[22],通过电感耦合等离子体发射光谱仪(Inductively Coupled Plasma Optical Emission Spectrometer,ICP-OES,USA)检测水样中的Na、K、Ca、Mg[22]。
-
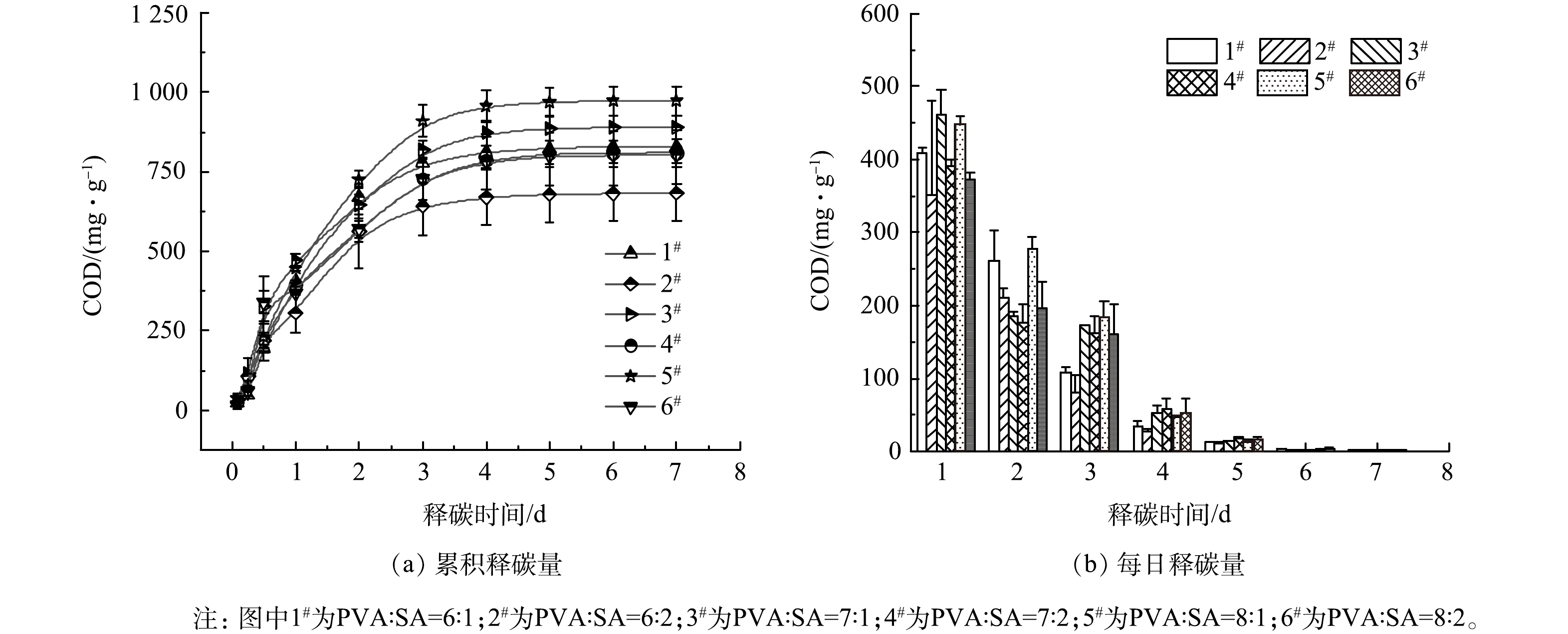
在不同PVA:SA骨架材料配比条件下,累积释碳量在前3 d持续增加,为快速释放期,此后趋于平缓(图1(a))。当SA为10 g·L−1时,随着PVA投加比例的增加,累积释碳量由828.72 mg·g−1增加到972.85 mg·g−1,增加了17.39%;当SA为20 g·L−1时,随着PVA投加比例增加,累积释碳量由681.88 mg·g−1增至802.44 mg·g−1,增加了17.68%。在PVA投加比例相同时,SA为20 g·L−1时累积释碳量均低于SA为10 g·L−1时的释放量:当PVA为60、70、80 g·L−1时,SA为20 g·L−1的累积释碳量分别降低了17.72%、9.08%、17.52%。以上结果表明,在SA投加量相同时,累积释碳量随着PVA投加量的增加而增加;在PVA投加量相同时,累积释碳量随着SA投加量的增加反而降低。当PVA:SA=8:1(5#)时的累积释碳量最高,达到972.85 mg·g−1。各组的每日释碳量均在24 h达到峰值(图1(b))。在1、2、3 d时,各组每日释碳量分别占累积释碳量的45.4%~59.7%、19.5%~29.1%、8.6%~22.1%。
-
1)缓释碳源释碳特征。根据骨架材料配比优化结果,以PVA:SA=8:1配比组为对照组(7#),考察tween80和span80这2种常用乳化剂对释碳效果的影响。与不加乳化剂的对照组(7#)相比,加入乳化剂后累积释碳量增加了1.3%~26.8%(图2(a))。当乳化剂为tween80时,随着加入量由0.25%增加到1%时,累积释碳量比对照组分别增加了1.3%、9.9%、15.7%,即累积释碳量随乳化剂用量的增加而增加。在乳化剂投加量相同时,加入span80比加入tween80的累积释碳量分别高出8.3%、8.2%、10.2%。与骨架配比实验结果相似,各组每日释碳量仍在24 h达到峰值(图2(b)),前3 d释碳量占累积释碳量的95.1%。对照组(7#)第1、2、3天的每日释碳量分别占累积释碳量的55.5%、25.8%、13.8%。加入1.0%的tween80、span80时,前3 d释碳量占累积释碳量的96.9%和96.3%;第1、2、3天的每日释碳量分别占累积释碳量的49.8%和53.1%、35.2%和32.7%、11.9%和10.5% (tween80和span80),即第1天和第3天的释碳量低于对照组,而第2天高于对照组。
LI等[9]研究采用玉米芯、PVA-SA、PCL、以及玉米芯+PVA-SA+PCL三者混合制备而成的复合缓释碳源,快速释放期最长为2 d,累积释放11.82 mg·(g·L)−1。XIONG等[25]对比PVA-SA、PCL、聚丁二酸丁二醇酯(polybutylene succinate, PBS)、玉米芯、花生壳、废弃大米,PVA-SA组缓释效果最好,快速释放期为2 d,累积释放DOC为17 mg·g−1 。凌宇等[17]考察水稻秸秆、小麦秸秆、玉米秸秆、玉米芯、大豆秸秆和大豆壳释碳特征,缓释效果最佳的玉米秸秆快速释放期仅为1 d,累积释碳量为344.83 mg·(g·L)−1。以上研究缓释碳源的快速释放期在1~2 d,而本研究采用酒糟结合PVA-SA制备的缓释碳源,快速释放期为3 d,且累积释碳量高达850~1 000 mg·g−1,释碳性能更好。
2)缓释碳源释氮、磷特征。缓释碳源作为补充碳源时,引入到反硝化系统中的氮和磷越低越好酒糟含有丰富的氮、磷、钾等营养物质[26],因此,研究考察了缓释碳源的氮磷释放特征。在乳化剂实验组释碳过程中,释放的TN随时间逐渐增加,在5 d后逐渐趋于平缓,7 d累积释放量达到5.24~8.50 mg·L−1(图3(d))。各组释放的TN均以NH4+-N和NO3−-N为主,7 d累积释放量分别为2.96~5.48 mg·L−1和1.15~4.25 mg·L−1,NO2−-N则均在0.2 mg·L−1以下。结合释碳结果,第7天各组释碳溶液的COD/TN在555~820,说明缓释碳源溶液具有较高的C/N比,在作为反硝化补充碳源时,引入反硝化系统的氮负荷较少,而碳源充足。此外,各实验组PO43−-P累积质量浓度均低于0.27 mg·L−1(数据未在图中展示),即缓释碳源释放的C/N比和C/P比较高,作为补充碳源时引入反硝化系统的氮磷负荷较低。
-
为进一步了解不同制备条件的缓释碳源释放动力学特征和释碳机制,采用拟二级动力学方程和Ritger-Peppas方程对缓释碳源释碳过程进行拟合分析。
1)释碳动力学研究。各实验组释碳过程经二级动力学方程拟合,R2均在0.96以上(表1),表明缓释碳源释碳过程符合二级动力学方程。骨架材料配比实验组(1#~6#),PVA:SA=8:1(5#)实验组饱和释碳量Cm值最大(972.85 mg·g−1),半衰期t1/2也最大(33.74 h),即达到释碳平衡的时间最长,缓释效果最好。在乳化剂优化实验中,加乳化剂后Cm均比对照组7#增加。0.5% tween80和1.0% tween80(9#和10#)实验组t1/2从23.07增加到24.87和28.53,缓释效果有所提高,并且这两组的K值(37.95和34.63)近似或低于对照组K值(37.22),说明释碳过程中受到的阻力相对较大,与缓释效果好的结果一致。0.5% span80和1.0% span80(12#和13#)实验组K值从37.22分别增加到48.73和43.61,表明释碳的阻力变小,更容易释放有机碳。1.0% span80、0.5% tween80和1.0% tween80(13#、9#和10#)组的t1/2值高于对照组,有更好的缓释效果,即加入适量乳化剂可以提高缓释效果和累积释碳量。LI等[9]采用玉米芯+PVA-SA+PCL制备的复合缓释碳源,传质系数K(以COD计)约为0.35 mg·(h·g·L)−1,t1/2值为8.11 h,Cm(以COD计)约为49 mg·g−1,均低于本研究的复合缓释碳源的相应指标,表明用酒糟制备复合缓释碳源具有更佳的释碳效果。
2)释碳机制研究。根据Ritger-Peppas方程释碳机制,当释放指数N<0.45时,释放过程属于Fick扩散;当0.45<N<0.89时,释放过程属于non-Fick扩散(即扩散和骨架溶蚀协同作用);当N>0.89时,释放过程为骨架溶蚀机制[27],即碳源的释放主要来自PVA-SA骨架材料的溶蚀作用[28]。Ritger-Peppas方程拟合结果见表2。在0~3 d的快速释碳阶段,骨架材料优化实验组N>0.89,均为骨架溶蚀释碳机制。乳化剂优化实验组,只有0.25% span80(11#)和0.50% span80(12#)组,0.45<N<0.89,为non-Fick扩散机制,其他组均为骨架溶蚀机制;在平缓释放阶段的3~7 d,各实验组均为Fick扩散释碳机制。这与已有研究结果相似,即复合缓释碳源在快速释放阶段为骨架溶蚀或non-Fick扩散,慢速释放期为Fick扩散[28-29]。在骨架溶蚀和non-Fick扩散阶段,碳源先在聚合物内部扩散,导致聚合物膨胀,产生聚合物弛豫现象;随着聚合物内部渗透压增加以及聚合物在水中分解,碳源快速释放;在平缓释放期的Fick扩散模式,碳源释放受到扩散和聚合物溶胀控制[30-31]。在同一复合碳源缓释体系中,可能同时发生一种或多种释放机制作用[32]。
-
为进一步了解缓释碳源释放的有机物组分特征,对乳化剂优化实验组单日释碳量峰值的24 h释碳溶液进行考察。
1)蛋白质、多糖、VFA的释放特征。所有碳源的24 h释碳溶液均未检测出VFA(未在表3中显示)。已有研究[7]也发现,采用6.25 g·L−1花生壳和PVA-SA与PCL三者混合制备而成的复合型固体缓释碳源仅能释放少量的VFA,乙酸仅在1.1 mg·L−1以下。与对照组(7#)相比,8#~13#实验组蛋白质和多糖含量均呈现上升趋势(表3),分别增加了10.7%~31.3%、3.5%~33.8%,但蛋白质和多糖的COD当量在缓释碳源释放的COD中占比小于7%,即缓释碳源释放的COD以未知组分为主。
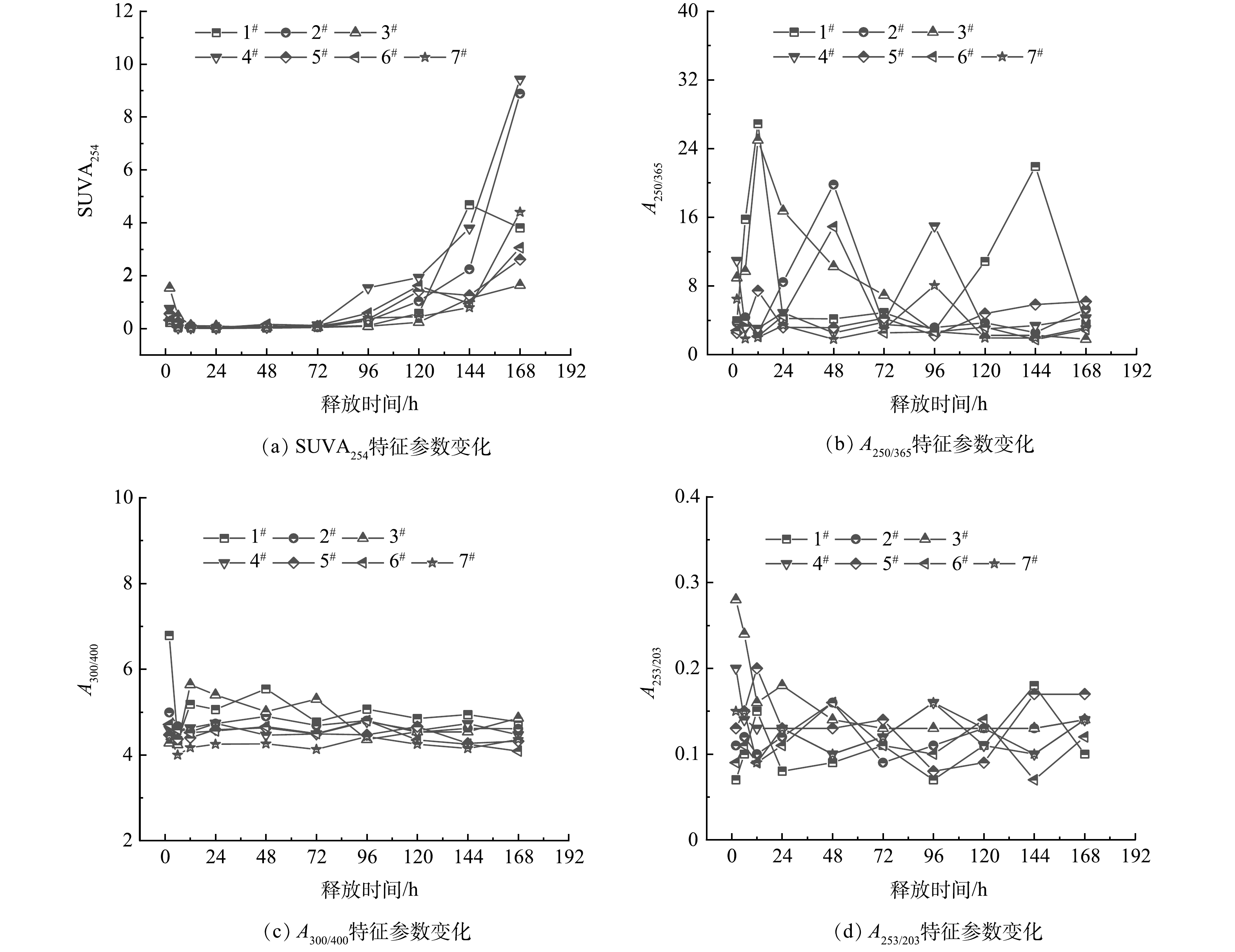
2)释放有机物UV-vis。乳化剂优化组释碳溶液的紫外-可见吸收光谱各参数指标随时间变化规律如图4所示。通常认为DOM的SUVA254越大,芳香度越高,SUVA254>4 L·(mg·m)−1为高芳香度、高分子质量和高疏水性的DOM;SUVA254<2 L·(mg·m)−1为低芳香度、低分子质量和亲水性的DOM[33]。由图4(a)可知,前4 d释碳溶液的SUVA254<2 L·(mg·m)−1,表明此期间缓释碳源释放的DOM的芳香性和分子质量相对较低,亲水性好,而5d后SUVA254>4 L·(mg·m)−1,表明此时释放的DOM芳香性和分子质量都相对较大,亲水性变差,可生化性也变弱。
A250/365可表征DOM分子质量大小和芳香性高低,该值越大表明分子质量越小[34-35]。A300/400可表征DOM的腐殖化程度,当A300/400>3.5时腐殖化较低,A300/400<3.5时腐殖化较高,腐殖化程度越高,可生物利用性越差[35-36]。A253/203是反映芳香环上取代基的种类和取代程度高低,该值越大表明取代度越高,DOM的可生物降解性越好[37]。由图4(b)可知,乳化剂对照组(7#)A250/365平均值最大,为9.90,说明释放的DOM分子质量最小。1.0% span80(13#)组A250/365平均值最小,为3.35,表明该组碳源释放的DOM分子质量最大。0.25% span80(11#)组A250/365值误差范围(1.64)显著小于其他实验组(2.07~8.27),表明该组碳源释放的DOM分子质量最稳定,其次为1.00% span80(13#)组。由图4(c)可知,对照组(7#)A300/400平均值最大,而1.0% span80(13#)组A300/400平均值最小,说明前者碳源释放的DOM腐殖化程度最低,后者腐殖化程度最高。由4(d)可知,加入适量乳化剂后A253/203平均值增加,芳香环取代度增加,即适量乳化剂可提高碳源释放DOM的可生物利用性。总体而言,加入适量乳化剂后,缓释效果有所增强,累积释碳量、DOM可生物利用性提高,但释放的DOM分子质量、腐殖化程度也有所增加。
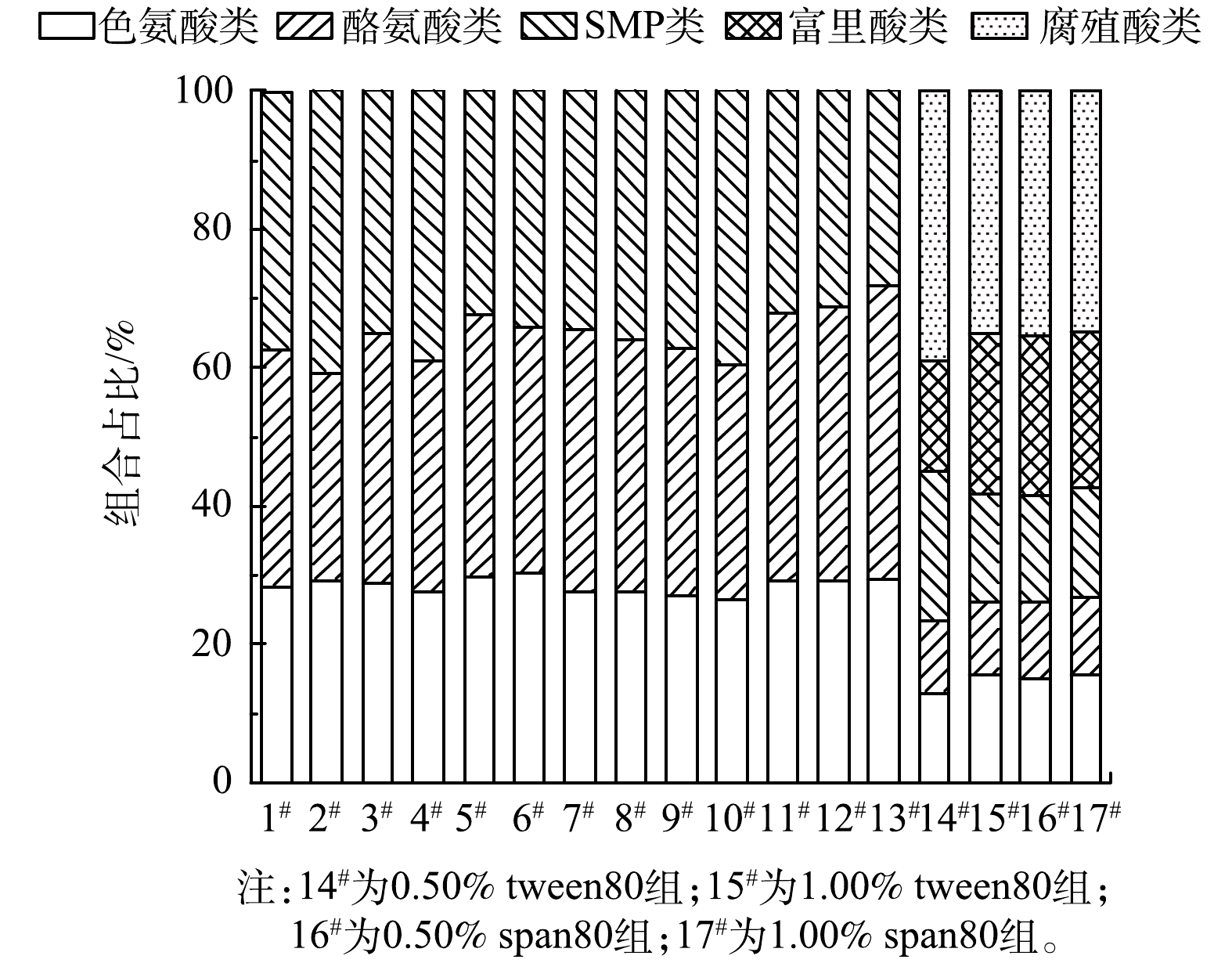
3)三维荧光特征。24 h释碳溶液三维荧光光谱(3D-EEM)结果表明,乳化剂组各种碳源释放DOM的组分一致,均为色氨酸类蛋白、酪氨酸类蛋白以及微生物代谢副产物(SMP)。由图5中可知,通过优化骨架材料和乳化剂配比,色氨酸和酪氨酸占比由59.3%提升至71.9%。根据UV-Vis检测结果,对照组(7#)的DOM分子质量和腐殖化程度最低,1.0% span80组(13#)最高。与之对应的三维荧光结果表明,对照组的蛋白类(色氨酸类和酪氨酸类总和)最低,而1.0% span80(13#)组最高,说明缓释碳源释放DOM的分子质量和腐殖化程度可能与蛋白类组分有关。综上可知,不同的制备方法对碳源释放DOM的特征官能团类型影响相对较小,对释放的各种有机物的质量浓度影响较大。
-
在制备缓释碳源的过程中,采用交联剂CaCl2对碳源进行化学交联,可能会导致缓释碳源释放Ca2+、Cl−等无机离子。如果缓释碳源释放过多无机离子,导致反硝化系统的盐度增加甚至抑制反硝化过程,因此有必要考察缓释碳源无机组分的释放情况。乳化剂优化组各缓释碳源24 h无机组分释放情况如表3所示。各组碳源释碳溶液TDS在741.63~847.07 mg·L−1,不会对微生物活性有显著抑制作用[38]。阳离子质量浓度由高到低均为Ca2+>Na+>K+>Mg2+,均以Ca2+为主。阴离子均为Cl−>SO42−,未检出F−和Br−。在交联过程中,海藻酸钠具有良好的水溶性,可与Ca2+形成稳定的凝胶[39],对于带正电的水凝胶,共存盐的阴离子可以中和其表面电荷,从而形成广泛而松散的水凝胶网络,这可以使功能性金属位点更容易吸附[40],从而吸附了大量的Cl−,因此,在碳源释放过程中主要释放的离子为Ca2+(148.3~180.5 mg·L−1)和Cl−(253.7~290.8 mg·L−1)。
-
根据2.4节研究结果,缓释碳源制备过程中加入乳化剂后,累积释碳量增加的同时释放的DOM分子质量、腐殖化程度也有所增加。因此有必要考察释放的DOM种类和特性对反硝化效果的影响。
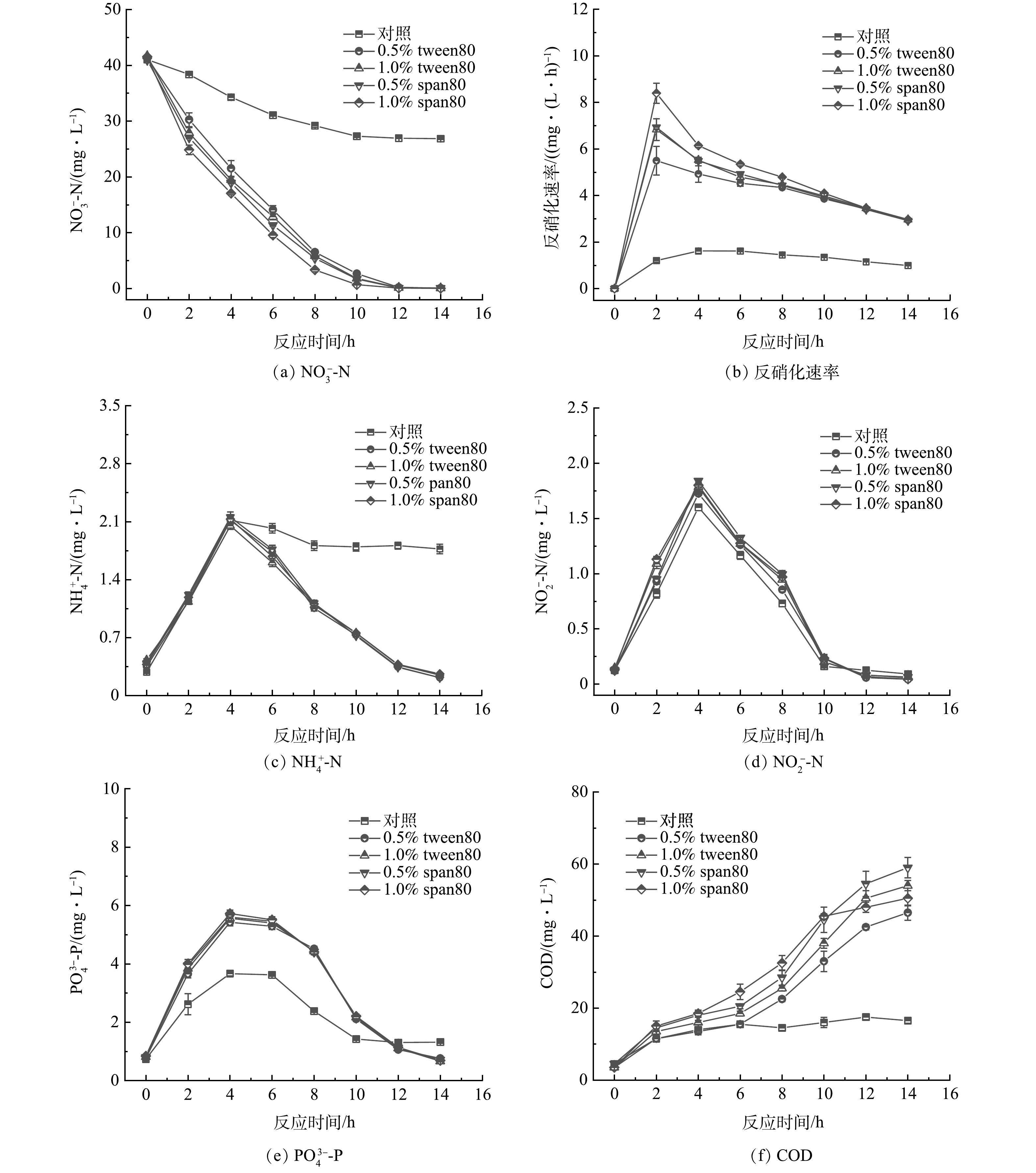
1)四种碳源反硝化效果对比。由图6(a)可知,对照组(control)在未加碳源时,硝酸盐去除率仅为34%,此时活性污泥吸附的有机物降解、活性污泥中微生物自身分解释放的有机物促进了反硝化的进行。如图6(b)所示,在缓释碳源投加量为(0.19±0.004) g·L−1时,1.0% span80组反硝化速率最快,为4.17 mg·(L·h)−1,其次是0.5% span80和1.0% tween80组反硝化速率接近,0.5% tween80组反硝化速率最慢,为3.94 mg·(L·h)−1。从图6(c)、(d)、(e)可知,NH4+-N、NO2−-N以及PO43−-P均呈现先上升后下降的趋势,去除率分别为16.3%~90.0%、88.9%~96.0%、64.0%~88.0%。各组磷酸盐均为先释放后吸收的规律。释磷的原因一方面可能是接种污泥中存在聚磷菌释磷,另一方面缓释碳源也有少量释磷,从而PO43−-P呈现上升趋势;在后续反硝化过程中的吸磷现象除了活性污泥吸附之外,还可能存在反硝化除磷过程,因而PO43−-P呈现下降趋势。从图6(f)可知,剩余COD在46~59 mg·L−1。在实际应用中,市政污水和工业园区废水的NO3−-N质量浓度常低于20 mg·L−1,碳源投加量也相应较低,可以避免出水COD超标。
XIONG等[7]投加12.5 g·L−1缓释碳源时,反硝化速率为0.178 mg·(L·h)−1;唐丹琦等[12]研究,投加66.7 g·L−1缓释碳源时,反硝化速率为1.03 mg·(L·h)−1;王润众等[10]投加68.2 g·L−1复合碳源,反硝化速率为0.182 mg·(L·h)−1。本研究仅投加0.19 g·L−1复合碳源,反硝化速率达到4.08 mg·(L·h)−1,优于以上研究结果。
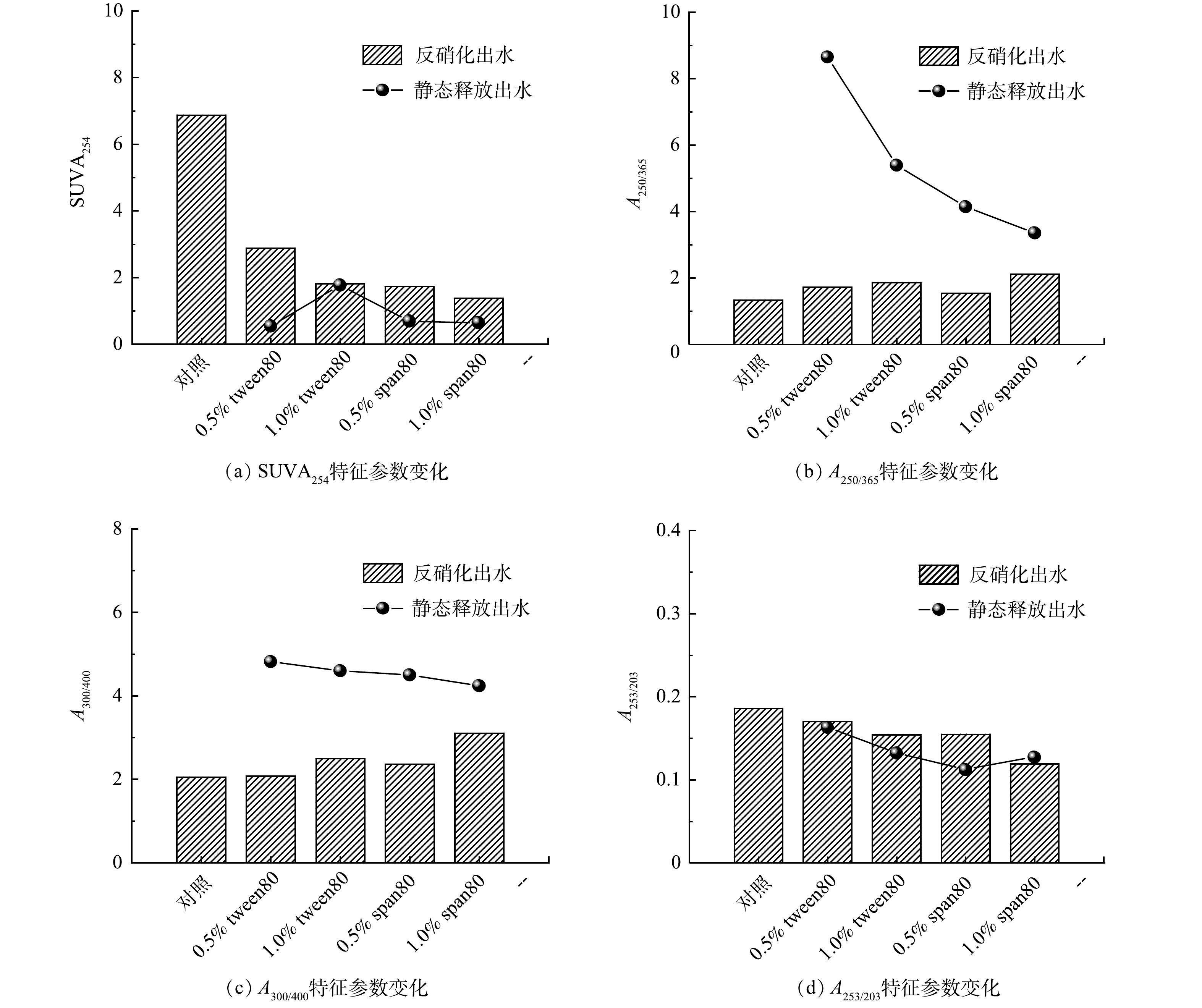
2)反硝化出水三维荧光特征。通过对比缓释碳源静态释放溶液与反硝化出水的DOM特征,可以了解缓释碳源释放的DOM中易被反硝化微生物利用的DOM组分,以及不易被利用的残余DOM组分特征。相比碳源静态释放溶液中已有的色氨酸类、酪氨酸类、以及SMP之外,反硝化出水中新增了富里酸和腐殖质类物质,这些腐殖质可能来自接种污泥携带的植物和土壤有机物降解产生的陆源腐殖质,以及微生物及藻类活动产生的自生源腐殖质。色氨酸类和酪氨酸蛋白类物质具有较高的生物降解性,容易被微生物降解和利用[41]。而富里酸和腐殖质类物质是具有较强生物惰性的芳香族聚合物,不利于微生物所利用[42]。图5中对比了静态释放DOM组分占比,各实验组反硝化出水,酪氨酸类、色氨酸类,以及SMP占比均降低,分别降低了24.9%~29.3%、11.6%~14.7%、14.3%~21.9%,而新增的富里酸和腐殖酸类物质,占比分别为16.0%~23.2%、34.9~39.1%。总之,反硝化过程中,色氨酸类蛋白和酪氨酸类蛋白为易被微生物所利用的DOM,反硝化后其占比降低,而富里酸和富里酸类物质占比上升。
3)反硝化出水UV-vis特征。反硝化出水的紫外-可见吸收光谱各参数指标随时间变化如图7所示。空白对照组SUVA254>4 L·(mg·m)−1,说明不加缓释碳源的反硝化出水DOM具有高芳香度、高分子质量以及疏水性,不易于微生物利用;1.0% tween80组和0.5% span80组以及1.0% span80组SUVA254<2 L·(mg·m)−1,表明加入缓释碳源后,反硝化出水DOM具有低芳香度、低分子质量以及亲水性(图7(a)),即缓释碳源为反硝化微生物提供了充分的易于微生物利用的优质碳源。对比静态释放出水A250/365平均值(图7(b)),反硝化出水A250/365值明显降低,表明反硝化过程中小分子有机物被微生物利用后占比减少,因此出水残余为大分子质量DOM。由图7(c)可知,相比静态释放,反硝化出水DOM的腐殖化程度有所增加。由图7(d)A253/203结果,相比静态释放,缓释碳源反硝化出水DOM芳香环取代基取代度普遍降低,且均低于对照组,表明加入缓释碳源后DOM利用效果更佳,因而反硝化出水残余DOM可生物利用性更低。总体来说,1.0% span80组反硝化出水腐殖化程度最低、DOM分子质量最小、芳香环取代基种类和取代基程度最低,反硝化速率最高,为最佳缓释碳源。
-
1)本研究采用酒糟结合PVA-SA制备复合型缓释碳源,通过骨架材料优化研究以及乳化剂优化研究,使快速释放期可延长到3 d;快速释碳过程符合骨架溶蚀和non-Fick扩散释碳机制,慢速释碳期为Fick扩散释碳机制。
2)不同骨架材料配比、乳化剂投加量对制备出的缓释碳源释放的DOM组分特征、官能团特征影响较小,对释放的有机组分的质量浓度影响较大。加入适量乳化剂可提高缓释碳源的缓释效果,增加累积释碳量,累积释碳量可达到1 089 mg·(g·L)−1;加入乳化剂后缓释碳源释放的DOM分子质量、腐殖化程度有所增加,但对反硝化效果没有负面影响。
3)复合缓释碳源为反硝化微生物提供了充足的低芳香度、低分子质量以及亲水性DOM,有利于提高反硝化速率;复合缓释碳源释放的DOM中色氨酸类蛋白、酪氨酸类蛋白为易于微生物利用有机物,反硝化出水富里酸和腐殖酸类物质占比增加;在PVA:SA=8:1,加入1.0% span80制备的缓释碳源反硝化效果最好,反硝化速率达到4.08 mg·(L·h)−1,为最佳缓释碳源。
利用工业园区酒糟固废制备复合缓释碳源及其性能评估
Preparation of composite slow-release carbon source from industrial park vinasse solid waste and its performance evaluation
-
摘要: 我国工业园区污水厂进水碳氮比(C/N)普遍较低,常需补充碳源以提高脱氮效果。酒糟富含蛋白质、碳水化合物等有机组分,可生物利用性好,但目前缺乏其作为缓释碳源的研究。本研究以工业园区酒糟固废为原材料,以聚乙烯醇(PVA)、海藻酸钠(SA)为骨架材料,利用低温冷冻化学交联法制备复合缓释碳源,并进行释碳性能和反硝化性能评估。结果表明:通过骨架材料配比以及乳化剂优化研究,缓释碳源的快速释放期可延长到3 d,此阶段释碳过程为骨架溶蚀机制,单位质量缓释碳源的累积释碳量(以化学需氧量,即COD计)可达到1 089 mg·(g·L)−1。在酒糟用量为10 g·L−1,骨架材料配比为PVA:SA=8:1,乳化剂为1.0% span80条件下制备的缓释碳源在投加量为0.19 g·L−1,初始硝态氮(NO3−-N)为(41.53±0.1) mg·L−1时,反硝化出水溶解性有机物(DOM)的腐殖化程度最低、分子质量最小、芳香环取代基种类和取代基程度最低,总氮去除率为99.2%,反硝化速率达到4.08 mg·(L·h)−1,为最佳缓释碳源。本研究结果可为工业园区的污水-固废协同治理提供参考。Abstract: The ratio of carbon to nitrogen(C/N) in influent of wastewater treatment plants of industrial parks in China is generally too low to denitrification, and supplementary carbon sources are often required to improve the nitrogen removal performance. Vinasse solid waste is rich in organic components such as proteins and carbohydrates, which have a good biodegradability. However, there is still a lack of research on its use as a slow-release carbon source. In this study, vinasse solid waste from an industrial park was used as raw material, and polyvinyl alcohol (PVA) and sodium alginate (SA) were used as hybrid scaffold to prepare a slow-release carbon source by the low-temperature freeze chemical cross-linking method. The carbon release and denitrification performance of the material were evaluated. Results showed that the optimization of hybrid scaffold ratio and emulsifier dosage could extend the rapid release period of the slow-release carbon source to 3 d. At this stage, the process of carbon release was governed by the framework dissolution mechanism. The cumulative carbon release of the slow-release carbon source (measured by chemical oxygen demand, COD) was 1 089 mg·(g·L)−1. The optimal slow-release carbon source was prepared under the conditions of 10 g·L−1 vinasse solid waste, PVA:SA ratio of 8:1, and 1.0% span80 as emulsifier. During the denitrification process, at slow-release carbon source dosage of 0.19 g·L−1 and (41.53±0.1) mg·L−1 of initial nitrate nitrogen (NO3−-N), the dissolved organic matter (DOM) of the effluent presented the highest humification degree, the lowest molecular weight, and the least aromatic ring substituent types and degree. The total nitrogen removal rate was 99.2%, and the denitrification rate reached 4.08 mg·(L·h)−1. This study could provide a reference for the collaborative management of wastewater and solid waste in industrial parks.
-
Key words:
- slow-release carbon source /
- hybrid scaffold /
- vinasse /
- carbon release mechanisms /
- denitrification
-

-
表 1 缓释碳源释碳二级反应动力学
Table 1. Second-order reaction kinetics equation for carbon release of slow-release carbon source
实验组 拟合公式 R2 Cm/(mg·g−1) K/(mg·(h·g·L)−1) T1/2/h 1# 1/c=0.035/t−0.001 1 0.96 828.72 28.46 29.12 2# 1/c=0.039/t−0.001 6 0.96 681.88 25.59 26.65 3# 1/c=0.031/t−0.001 0 0.98 890.04 32.49 27.40 4# 1/c=0.039/t−0.001 5 0.99 809.25 25.73 31.45 5# 1/c=0.035/t−0.001 7 0.97 972.85 28.84 33.74 6# 1/c=0.042/t−0.000 3 0.98 802.44 23.84 33.66 7# 1/c=0.027/t−0.003 4 0.99 858.71 37.22 23.07 8# 1/c=0.025/t−0.003 8 0.98 869.86 40.63 21.41 9# 1/c=0.026/t−0.001 7 0.97 940.21 37.95 24.87 10# 1/c=0.029/t−0.003 5 0.97 987.97 34.63 28.53 11# 1/c=0.022/t−0.001 6 0.99 941.67 45.07 20.90 12# 1/c=0.021/t−0.001 1 0.97 1021.42 48.73 20.96 13# 1/c=0.023/t−0.001 7 0.97 1089.20 43.61 24.98 表 2 Ritger-Peppas方程拟合结果
Table 2. Fitting results of Ritger-Peppas equation
实验组 0~72 h 72~168 h 拟合公式 R2 N 拟合公式 R2 N 1# Mt/M∞=0.013t1.07 0.97 1.068 Mt/M∞=0.69t0.073 0.85 0.073 2# Mt/M∞=0.026t0.90 0.96 0.900 Mt/M∞=0.71t0.069 0.84 0.069 3# Mt/M∞=0.026t0.89 0.95 0.891 Mt/M∞=0.63t0.094 0.80 0.094 4# Mt/M∞=0.021t0.92 0.97 0.925 Mt/M∞=0.55t0.12 0.80 0.120 5# Mt/M∞=0.011t1.09 0.98 1.091 Mt/M∞=0.68t0.077 0.80 0.077 6# Mt/M∞=0.023t0.90 0.91 0.899 Mt/M∞=0.58t0.11 0.82 0.110 7# Mt/M∞=0.014t1.08 0.93 1.080 Mt/M∞=0.76t0.057 0.84 0.057 8# Mt/M∞=0.014t1.09 0.92 1.093 Mt/M∞=0.79t0.048 0.85 0.048 9# Mt/M∞=0.026t0.93 0.90 0.928 Mt/M∞=0.85t0.031 0.85 0.031 10# Mt/M∞=0.015t1.07 0.89 1.068 Mt/M∞=0.84t0.036 0.84 0.036 11# Mt/M∞=0.030t0.89 0.90 0.888 Mt/M∞=0.76t0.057 0.88 0.057 12# Mt/M∞=0.035t0.84 0.92 0.843 Mt/M∞=0.83t0.038 0.88 0.038 13# Mt/M∞=0.019t0.99 0.95 0.991 Mt/M∞=0.80t0.043 0.85 0.043 表 3 释放有机、无机组分及有机组分COD当量占比
Table 3. Release component content and COD equivalent percentage
实验组释放组分质量浓度/(mg·L−1) 有机组分COD当量/% 蛋白质 多糖 Ca2+ Na+ K+ Mg2+ Cl− SO42− 蛋白质 多糖 未知组分 7# 73.4 48.5 168.9 23.5 2.6 0.63 283.2 14.2 3.3 3.1 93.6 8# 81.2 50.2 161.8 22.3 2.0 0.56 265.6 11.5 3.5 3.0 93.5 9# 79.3 51.7 155.7 19.1 2.1 0.56 257.3 10.7 3.4 3.1 93.5 10# 83.7 57.9 148.3 17.1 2.1 0.58 253.7 10.2 3.6 3.6 92.8 11# 84.8 52.4 180.5 20.0 2.4 0.70 290.8 15.7 3.3 2.9 93.8 12# 87.5 54.0 152.6 17.1 1.9 0.58 261.4 11.2 3.2 2.8 94.0 13# 96.3 65.0 159.9 16.3 2.3 0.65 279.1 12.7 3.6 3.4 93.0 -
[1] FERNANDEZ Y, MARANON E, SOONS J, et al. Denitrification of high nitrate concentration wastewater using alternative carbon sources[J]. Journal of Hazardous Materials, 2010, 173(1): 682-688. [2] NISINI E, SANTULLI C, CERUTI A, et al. High speed impact properties of carbon-basalt-flax dhec composites compared with pure carbon fibre composites[J]. Composite Structures, 2018, 192: 165-172. doi: 10.1016/j.compstruct.2018.02.058 [3] VOLOKITA M, ABEHOVICH A, SOARES M, et al. Denitrification of groundwater using cotton as energy source[J]. Water Science Technology, 1996, 34(1): 379-385. [4] 王允, 张旭, 张大奕, 等. 用于地下水原位生物脱氮的缓释碳源材料性能研究[J]. 环境科学, 2008, 29(8): 2183-2188. [5] 刘佳, 沈志强, 周岳溪, 等. 聚己内酯/淀粉共混物和砾石系统反硝化特性[J]. 环境科学研究, 2014, 27(4): 441-446. [6] 蓝梅, 许志欣, 孙文叶, 等. 缓释碳源材料的选择与制备探究[J]. 工业水处理, 2017, 37(2): 24-28. [7] XIONG R, YU X, YU L, et al. Biological denitrification using polycaprolactone-peanut shell as slow-release carbon source treating drainage of municipal wwtp[J]. Chemosphere, 2019, 235: 434-439. doi: 10.1016/j.chemosphere.2019.06.198 [8] 沈志强, 周岳溪, 王建龙, 等. 利用淀粉/PCL共混物作为反硝化固体碳源和生物膜载体的研究[J]. 环境工程技术学报, 2014, 4(2): 129-134. [9] LI C, WANG H, YAN G, et al. Initial carbon release characteristics, mechanisms and denitrification performance of a novel slow release carbon source[J]. Journal of Environmental Sciences, 2022, 118: 32-45. doi: 10.1016/j.jes.2021.08.045 [10] 王润众, 郝瑞霞, 赵文莉, 等. 新型缓释碳源的制备及其性能[J]. 环境工程学报, 2016, 10(1): 81-87. [11] 程琳, 邹琴, 邹立扣, 等. 聚乙烯醇及改性聚乙烯醇/明胶多孔支架的体外生物相容性研究[J]. 成都大学学报, 2012, 31(2): 113-116. [12] 唐丹琦, 王娟, 郑天龙, 等. 聚乳酸/淀粉固体缓释碳源生物反硝化研究[J]. 环境科学, 2014, 35(6): 2236-2240. [13] 汪江波, 王浩, 孔博, 等. 黄酒酿造技术研究进展[J]. 酿酒, 2020, 47(6): 26-30. [14] 谢广发, 彭祺, 毛青钟, 等. 《黄酒酿造技术》课程教学探索与实践研究[J]. 酿酒, 2020, 47(3): 28-30. [15] 范恩帝, 蒋梦迎, 冯敏雪, 等. 混菌发酵白酒糟生产含功能成分饲料发酵条件的优化[J]. 生物技术通报, 2021, 37(12): 91-103. [16] 柳珊, 郭春春, 何荣玉, 等. 酒糟厌氧消化产甲烷特性及微生物菌群结构分析[J]. 农业机械学报, 2023, 54: 1-21. [17] 凌宇, 闫国凯, 王海燕, 等. 6种农业废弃物初期碳源及溶解性有机物释放机制[J]. 环境科学, 2021, 42(5): 2422-2431. [18] RITGER P L, PEPPAS N A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices[J]. Journal of Controlled Release, 1987, 5(1): 37-42. doi: 10.1016/0168-3659(87)90035-6 [19] 范振兴, 赵璇, 王建龙, 等. 利用辐照预处理麦秆作为反硝化固体碳源的研究[J]. 环境科学, 2009, 30(4): 1090-1094. [20] 周蜜. 新型多碳源缓释复合材料的制备及其强化低C/N污水脱氮性能的研究[D]. 重庆: 重庆大学, 2021. [21] 国家环境环保总局. 水和废水监测分析方法(第四版)[M]. 北京: 中国环境科学出版社, 2002. [22] 宋铁红, 赵凯, 佟娟, 等. 某实际染整废水深度处理过程中无机组分与溶解性有机物的变化[J]. 环境化学, 2022, 41(11): 3482-3492. [23] 薛柯伲. 食品加工废水制备工业园区污水厂补充碳源研究[D]. 邯郸: 河北工程大学, 2022. [24] CHEN W, WESTERHOFF P, LEENHEER J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter.[J]. Environmental Science & Technology, 2003, 37(24): 5701-5710. [25] XIONG R, YU X, ZHANG Y, et al. Comparison of agricultural wastes and synthetic macromolecules as solid carbon source in treating low carbon nitrogen wastewater[J]. Science of the Total Environment, 2020, 739: 139885. doi: 10.1016/j.scitotenv.2020.139885 [26] LIMA V D O, BARROS V G D, DUDA R M, et al. Anaerobic digestion of vinasse and water treatment plant sludge increases methane production and stability of uasb reactors[J]. Journal of Environmental Management, 2023, 327: 116451. doi: 10.1016/j.jenvman.2022.116451 [27] ZHANG D, LI G, YANG Y S, et al. Bio-geological processes of nitrogen transport and transformation in the aeration zone and aquifer[J]. Hydrological Sciences Journal, 2009, 54(2): 316-326. doi: 10.1623/hysj.54.2.316 [28] 邵兵, 张立秋, 李淑更, 等. 2种缓释碳源材料的释碳特性及脱氮性能研究[J]. 水处理技术, 2020, 46(12): 34-38. [29] YU L, CHENG L, PENG Z, et al. Carbon release mechanism of synthetic and agricultural solid carbon sources[J]. Water and Environment Journal, 2020, 34(S1): 121-130. doi: 10.1111/wej.12511 [30] BRUSCHI M L. Strategies to modify the drug release from pharmaceutical systems[M]. Woodhead Publishing, 2015: 63-86. [31] PEPPAS N A, NARASIMHAN B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems[J]. Journal of Controlled Release, 2014, 190: 75-81. doi: 10.1016/j.jconrel.2014.06.041 [32] KLECH C M, SIMONELLI, A P. Examination of the moving boundaries associated with non-fickian water swelling of glassy gelatin beads: Effect of solution pH[J]. Journal of Membrane Science, 1989, 43: 87-101. doi: 10.1016/S0376-7388(00)82355-8 [33] ATES N, KITIS M, YETIS U, et al. Formation of chlorination by-products in waters with low suva—correlations with suva and differential UV spectroscopy[J]. River Research and Applications, 2007, 41(18): 4139-4148. [34] 周石磊, 孙悦, 张艺冉, 等. 雄安新区-白洋淀冬季冰封期水体溶解性有机物的空间分布、光谱特征及来源解析[J]. 环境科学, 2020, 41(1): 213-223. [35] DUARTE R M B O, PIO C A, DUARTE A C, et al. Spectroscopic study of the water-soluble organic matter isolated from atmospheric aerosols collected under different atmospheric conditions[J]. Analytica Chimica Acta, 2005, 530(1): 7-14. doi: 10.1016/j.aca.2004.08.049 [36] PEBGHUI L, JIN H. Utilization of UV-vis spectroscopy and related data analyses for dissolved organic matter (dom) studies: A review[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(3): 131-154. doi: 10.1080/10643389.2017.1309186 [37] KUMKE M U, SPECHT C H, BRINKMANN T, et al. Alkaline hydrolysis of humic substances – spectroscopic and chromatographic investigations[J]. Chemosphere, 2001, 45(6): 1023-1031. [38] CHEN Y, CHENG J J, CREAMER K S, et al. Inhibition of anaerobic digestion process: A review[J]. Bioresource Technology, 2008, 99(10): 4044-4064. doi: 10.1016/j.biortech.2007.01.057 [39] RAHA A R, WAN M F W N, RICCA R N, et al. Alginate and alginate composites for biomedical applications[J]. Asian Journal of Pharmaceutical Sciences, 2021, 16(3): 280-306. doi: 10.1016/j.ajps.2020.10.001 [40] ZHANG W, DENG Q, HE Q, et al. A facile synthesis of core-shell/bead-like poly (vinyl alcohol)/alginate@pam with good adsorption capacity, high adaptability and stability towards cu(Ⅱ) removal[J]. Chemical Engineering Journal, 2018, 351: 462-472. doi: 10.1016/j.cej.2018.06.129 [41] HE X, XI B, WEI Z, et al. Fluorescence excitation-emission matrix spectroscopy with regional integration analysis for characterizing composition and transformation of dissolved organic matter in landfill leachates[J]. Journal of Hazardous Materials, 2011, 190(1): 293-299. [42] HU X, CHEN H, ZHANG S, et al. Study on performance of carbon source released from fruit shells and the effect on biological denitrification in the advanced treatment[J]. Chemosphere, 2022, 307: 136173. doi: 10.1016/j.chemosphere.2022.136173 -



 下载:
下载: