-
磷超标作为水体富营养化的主要原因,对地表水质量具有重要的影响。我国V类地表水域中磷含量的限值是0.4 mg∙L−1,而I类水质的磷含量限值低至0.02 mg∙L−1,相应质量浓度限制甚至比Cu2+等部分重金属离子的质量浓度限值更低[1]。可见,我国对地表水中磷含量具有严格的要求。水体作为重要的磷汇,承载来自生活污水、农业灌溉、工业生产、矿业开发等各种行业产生的含磷废水;同时,水体底泥作为磷源,可以向水中持续释放溶解态磷[2]。上述各种途径均增大水中磷含量,进而导致水域富营养化而出现水质腐败的现象。因此,降低水体中磷的含量对于保证良好水质具有重要的意义。
已有各种技术可用于水中溶解态磷的去除,如吸附技术、膜分离技术、电化学技术、化学沉淀等。其中,吸附技术具有污染物去除效率高、操作简单、无二次污染等优点而被广泛研究与应用[3-5]。相比于其他阴离子,磷酸根具有更高的电荷密度与配位氧原子数,并且Ca2+、Mg2+、Al3+、Fe3+等金属离子及其氧化物吸附磷酸根后形成难溶性化合物[6-7]。因此,含金属元素的各种吸附材料对磷酸根通常具有高吸附容量、优异的抗干扰能力等突出的吸附性能,如Mg、Al、Fe、Ca、Zr、La等金属氧化物[8]。其中,水铁矿、针铁矿等铁(氢)氧化物因具有高磷酸根吸附容量,同时作为土壤中的天然成分而在环境中磷的迁移转化过程中显示出重要的生态环境意义[9]。MATHUR等研究表明,针铁矿吸附磷酸根PO43−后形成的内圈络合物的稳定常数logK高达30.72±0.14,且远高于SO42−、F−、CrO42−、SeO32−、SeO42−、草酸盐、邻苯二甲酸等;此外,针铁矿吸附H2PO43−形成内层络合物的稳定性比无定型铁(氢)氧化物H2PO43−形成内层络合物的稳定性更高[7,10]。可见,针铁矿等铁(氢)氧化物对磷酸根具有强的吸附亲和力,尤其适合作为水体磷的吸附净化应用。WANG等[11]发现无定型水合氧化铁改性后的硅藻土对磷的吸附容量由0.60 mg∙g−1增至25.02 mg∙g−1(以磷元素计),并且测得水合氧化铁材料对磷的吸附容量为31.25 mg∙g−1。CHEN等[12]发现在Fe-Mn二元氧化物中,尽管锰氧化物可影响材料对磷的吸附过程,但是铁氧化物是决定吸附材料对磷吸附容量的主要因素。
然而,上述各种对水体中磷具有优异吸附性能的吸附材料通常为微纳米粉体材料。虽然微纳米粉体材料对磷具有优异的吸附性能,但微纳米粉体材料同时由于粒径小、表面能大而存在回收困难且进入动植物体内造成细胞死亡、改变正常的生命代谢活动并造成严重的危害[13-16]。为此,微纳米粉体材料在环境中应用的可操作性和生态环境安全性逐渐引起了重视[17]。将上述具有优异功能的微纳米粉体材料固定化而制备整体式宏观环境功能材料已成为当前环境修复技术的研究重点之一[18-19]。近年来,玄武岩纤维因具备物理化学稳定、拉伸模量高以及原料绿色环保等特点,被作为玻璃纤维和碳纤维的替代品而引起了广泛的关注。玄武岩纤维表面能低,尤其适合作为惰性基底材而被用于制备功能性复合材料。SUN等[20]通过多巴胺-亚氨基二乙酸修饰玄武岩纤维材料制备的复合材料对水中Cu2+离子具有优异的吸附性能。XIAO等[21]则发现多巴胺修饰的玄武岩纤维可通过增强表面微生物附着性而提高玄武岩纤维材料的环境污染净化能力。可见,以玄武岩纤维作为载体而制备功能性复合材料将在环境污染净化领域发挥重要的应用价值。
鉴于此,本研究以玄武岩纤维为基底材料,通过表面活化-Fe3+原位水解技术制备了铁羟基氧化物(FeOOH)改性的玄武岩纤维复合材料。通过X-射线衍射(X-ray diffraction, XRD)和扫描电镜(scanning electron microscope, SEM)分析了玄武岩纤维表面的FeOOH晶相结构与形貌,通过傅里叶变换红外光谱(fourier transform infrared spectroscopy, FT-IR)、Zeta电位研究了FeOOH改性玄武岩纤维复合材料的表面化学键结构和表面电荷性质。然后以FeOOH改性玄武岩纤维为整体式吸附材料,研究了复合材料对水中磷的吸附去除性能。在此基础上,通过宏观吸附实验和表面光谱技术揭示了FeOOH改性玄武岩纤维对水中磷酸根的吸附机制。
-
本研究所用的化学试剂均为分析纯试剂,主要包括九水合硝酸铁、氢氧化钠、氯化钠、硝酸、硫酸、十二水磷酸钠、硫酸四水钼酸铵、酒石酸锑钾半水合物和抗坏血酸。实验用水均为超纯水(ultrapure water, UP水),电阻率为18.25 MΩ·cm;所用的玄武岩纤维单丝由四川四众玄武岩纤维有限公司提供,直径为17 μm。
-
1)玄武岩纤维的活化。取8.0 g的玄武岩纤维,用100 mL、浓度为0.10 mol∙L−1的氢氧化钠(sodium hydroxide, NaOH)溶液浸泡2.0 h,然后取出并用UP水洗涤干净后,置于80 ℃条件下烘干得到活化后的玄武岩纤维(basalt fiber, BF)。
2) FeOOH@玄武岩纤维复合材料的制备。根据已报道的Fe3+水解制备铁羟基氧化物(FeOOH)的方法制备FeOOH改性的BF复合材料[22]。具体地,取20.2 g的九水合硝酸铁溶于500 mL UP水中,得到浓度为0.10 mol∙L−1的Fe3+溶液。取8.0 g活化后的玄武岩纤维浸泡在浓度为0.10 mol∙L−1的Fe3+溶液中1.0 h,然后用保鲜膜密封并在70 ℃条件下老化6~48 h,最后取出用UP水洗涤并在80 ℃下烘干,得到FeOOH改性的玄武岩纤维复合材料,记为FeOOH@BF-x (x表示老化时间)。
-
通过XRD分析所制备材料的晶体结构与组成,XRD衍射图在X-射线衍射仪(丹东浩元,2700BH)上完成,测试条件:采用Cu靶Kα1辐射(波长为0.154 06 nm)、电压40 kV、电流30 mA、步进0.02º、扫描速度为10º∙min−1。采用扫描电镜(ZEISS Gemini 300)对空白玄武岩纤维和FeOOH包覆的玄武岩纤维测试了样品的表面形貌。利用傅里叶变换红外光谱(FT-IR)研究所制备材料的化学组成,FT-IR图谱在红外光谱仪(Thermo Fisher Scientific,Nicolet iS5)上采集,分辨率2 cm−1,扫描范围4 000~400 cm−1。通过Zeta电位研究改性前后玄武岩纤维的表面电荷,Zeta电位测试条件为:背景电解质NaCl浓度为0.01 mol∙L−1,样品浓度为5 g∙L−1,在激光粒度电位仪(Nano ZS,Malvern)上于25 ℃、自然pH条件下测试。
-
采用浓度为0.01 mol∙L−1的NaCl溶液作为背景电解质,用于配制磷酸钠吸附质溶液以及调节吸附体系的总体积。所有吸附实验均在25 ℃室温条件下完成并平行3次取平均值,同时对平行实验做误差分析。
1)等温吸附实验。取50 mL离心管若干,依次加入不同体积、质量浓度为10.0 mg∙L−1的磷酸钠溶液(实验中所有的磷酸根/磷酸钠浓度均以P元素计),并用0.01 mol∙L−1的NaCl溶液定容至20 mL,然后分别加入0.1 g制备好的BF或FeOOH@BF吸附剂材料。最终吸附体系中吸附剂剂量为5 g∙L−1,吸附质磷酸根质量浓度为0~20.0 mg∙L−1,吸附体系的pH为自然pH。在25 ℃室温条件下吸附24 h后,取出上清液利用磷钼杂多酸分光光度法在紫外可见分光光度计(普析,T600) 700 nm下测试吸附前后的磷酸根质量浓度。根据吸附前后的磷酸根浓度,按照式(1)和式(2)计算所制备材料对水中磷的吸附量和去除率。
式中:Qe表示饱和吸附量,mg∙g−1;C0和Ce分别为吸附前和吸附后溶液中磷酸根的质量浓度,mg∙L−1;η表示水中磷酸根的去除率,%。
2)动力学吸附实验。动力学吸附实验的吸附质磷酸根初始质量浓度为10.0 mg∙L−1,吸附剂FeOOH@BF剂量为5 g∙L−1,在25 ℃、自然pH条件下吸附不同时间(0~24 h)后,取出上清液用磷钼杂多酸分光光度法在700 nm下测试磷酸根质量浓度并根据式(1)、式(2)计算不同吸附时间下FeOOH@BF对水中磷的吸附量和去除率。
为分析FeOOH@BF对水中磷的动力学吸附特点,采用准一级动力学(pseudo-first order, PFO)方程和准二级动力学(pseudo-second order, PSO)方程拟合动力学吸附实验数据。PFO和PSO方程的线性表达式分别如式(3)和式(4)所示。
式中:Qt为吸附时间为t时的吸附量,mg∙g−1;k1为 PFO方程中的动力学吸附速率常数,h−1; k2为PSO方程中的动力学吸附速率常数,g∙(mg∙h)−1。
3) pH对FeOOH@BF吸附磷酸根的影响。为研究pH对FeOOH@BF吸附磷酸根的影响,设置吸附体系中吸附质磷酸根初质量始浓度为10.0 mg∙L−1并用HNO3和NaOH分别调节吸附体系的pH至2、3、5和8,然后加入FeOOH@BF吸附剂(剂量为5 g∙L−1)。在25 ℃条件下吸附24 h后,用磷钼杂多酸分光光度法在700 nm下测试磷酸根质量浓度并根据方程(1)、(2)计算不同pH条件下FeOOH@BF对水中磷的吸附量和去除率。
4) FeOOH@BF对磷酸根的循环吸附性能测试。鉴于实际应用中,吸附材料的再生性和循环吸附性能是重要的评价指标,因此研究了FeOOH@BF材料对磷酸根的循环吸附性能。吸附体系如下:吸附质磷酸根的初始质量浓度为10.0 mg∙L−1、吸附剂剂量为5 g∙L−1,自然pH条件下吸附24 h。再生操作过程:取吸附饱和的FeOOH@BF材料于室温条件下浸泡在浓度为0.05 mol∙L−1的HNO3溶液中12 h,然后用UP水洗净烘干。吸附完成后上清液的磷酸根质量浓度采用磷钼杂多酸分光光度法在700 nm下测试,利用方程(1)、(2)计算FeOOH@BF在不同再生次数下对水中磷的吸附量和去除率以评价其循环吸附性能。
-
为研究所制备FeOOH@BF整体式材料对水中磷酸根的吸附机理,将饱和吸附的FeOOH@BF材料用UP水洗净烘干,然后将其做FT-IR测试。为分析吸附后材料表面的磷酸根物种,利用Guassia函数对吸附后FeOOH@BF材料的FT-IR图谱拟合,然后根据不同处理样品的FT-IR图谱分析吸附在FeOOH@BF材料表面的磷酸根状态分析FeOOH@BF材料对水中磷的吸附机理。
-
图1为空白玄武岩纤维与典型FeOOH改性后的玄武岩纤维的宏观光学照片。可见,改性前的玄武岩纤维具有橄榄色的金属光泽,其表面经FeOOH原位生长后表现出亮黄色光泽。
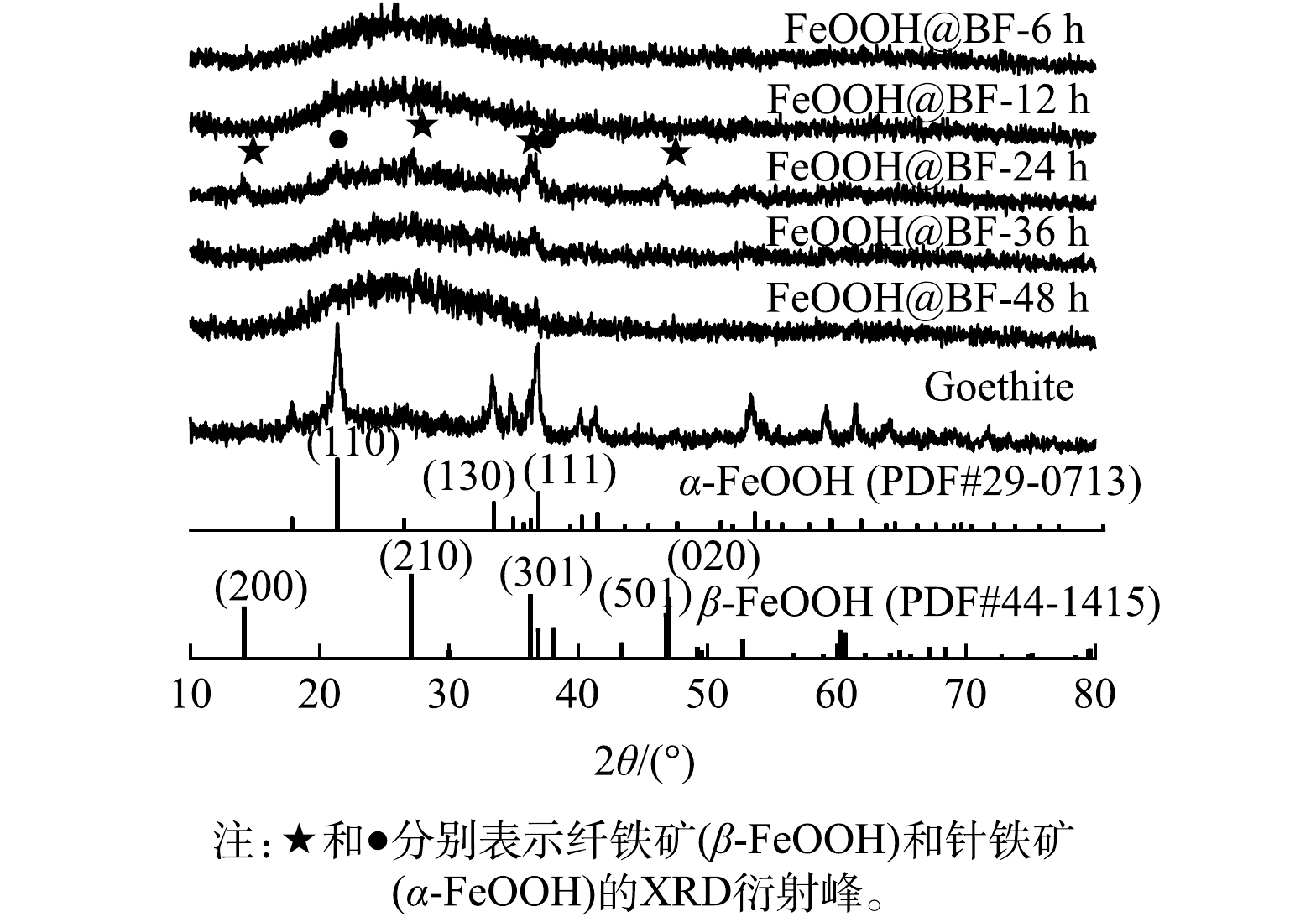
1)晶体结构组成与形貌分析。图2为在不同老化时间下所制备的样品的XRD衍射图。可见,本研究中Fe3+溶液在70 ℃制备的铁氢氧化物具有针铁矿的晶体结构(即α-FeOOH,标准PDF卡片号为29-0713)。将经NaOH刻蚀活化后的玄武岩纤维浸泡在Fe3+溶液中并在70 ℃条件老化过程中,随着老化时间的延长,玄武岩纤维表面在24 h时出现了最强的结晶铁氢氧化物的XRD衍射峰,相应的主要衍射峰可分别归属为针铁矿的(110)、(130)和(111)晶面衍射峰以及纤铁矿(β-FeOOH,标准PDF卡片号为44-1415)的(200)、(210)、(301)、(501)和(020)等晶面衍射峰。以上结果表明玄武岩纤维的浸泡改变了Fe3+水解结晶过程,导致Fe3+在玄武岩纤维表面吸附、水解并原位形成针铁矿和纤铁矿的FeOOH混合相结构。随后,对老化时间为24 h的FeOOH@BF做进一步表面性质测试与吸附性能研究。
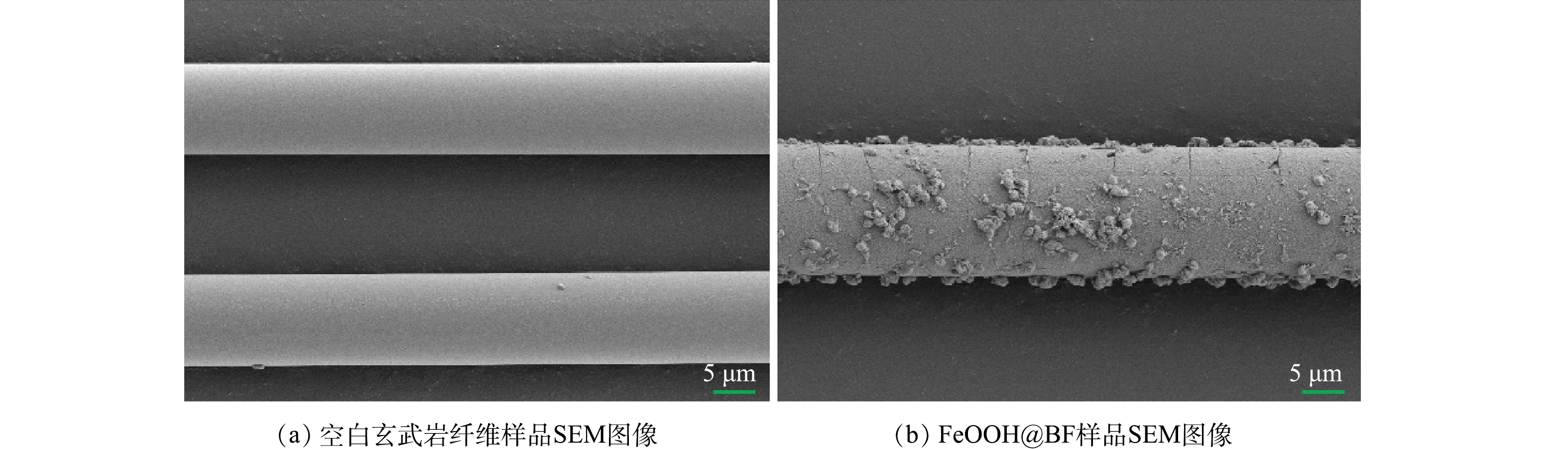
进一步,通过SEM表征空白玄武岩纤维和老化时间为24 h的FeOOH@BF样品的表面形貌。由图3(a)可见,空白玄武岩纤维具有光滑的表面结构。经FeOOH改性后,玄武岩纤维表面出现致密的FeOOH包覆层,导致玄武岩纤维的直径明显增大,且FeOOH@BF样品表面存在较多松散未形成致密包覆层的FeOOH簇状颗粒物(图3(b))。
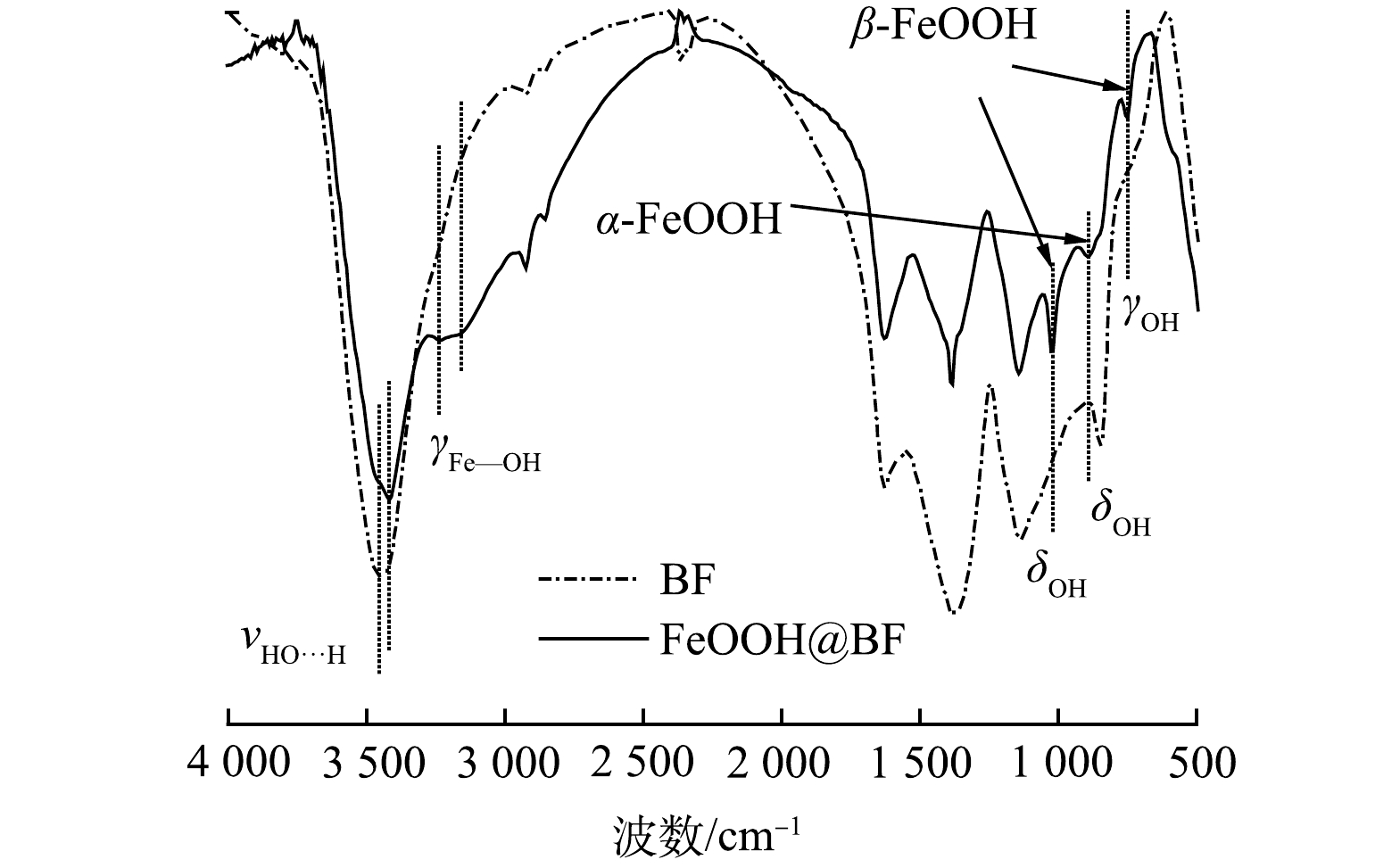
2)表面化学结构分析。图4为空白玄武岩纤维和FeOOH包覆玄武岩纤维复合材料的FT-IR图谱。FeOOH包覆玄武岩纤维复合材料的特征吸收带归属列于表1。由图4可见,空白玄武岩纤维表面仅在3 450 cm−1处出现了吸附水分子形成的氢键O-H伸缩振动吸收带[23],没有出现明显的金属端羟基振动吸收带;1 627 cm−1处的吸收带可归属为吸附水分子的变形振动。相比于空白玄武岩纤维,经Fe3+原位水解结晶后,玄武岩纤维表面出现了大量的羟基振动吸收峰。其中,FeOOH@BF材料表面吸附水分子形成的氢键伸缩振动向低波数移动至3 418 cm−1 [23]。图4中在3 237 cm−1和3 153 cm−1处的吸收峰可归属为FeOOH晶体结构中的铁羟基振动吸收峰,由于β-FeOOH和α-FeOOH中典型的结构铁羟基分别在3 160 cm−1和3 142 cm−1处存在红外吸收峰[23-24],二者波数相近,导致本研究中无法有效区分FeOOH@BF材料中β-FeOOH和α-FeOOH的铁羟基伸缩振动吸收峰。此外,FeOOH@BF材料中出现了典型的β-FeOOH晶体结构中铁羟基的面内、面外弯曲振动吸收峰 (1 022 cm−1和749 cm−1) ,以及α-FeOOH晶体结构中铁羟基的面内弯曲振动吸收峰(892 cm−1)[24]。上述FT-IR结果证实了FeOOH@BF材料的晶体结构组成,同时表明FeOOH的原位生长赋予了惰性玄武岩纤维表面丰富的铁羟基,为吸附水中污染物提供了有效吸附位点。
-
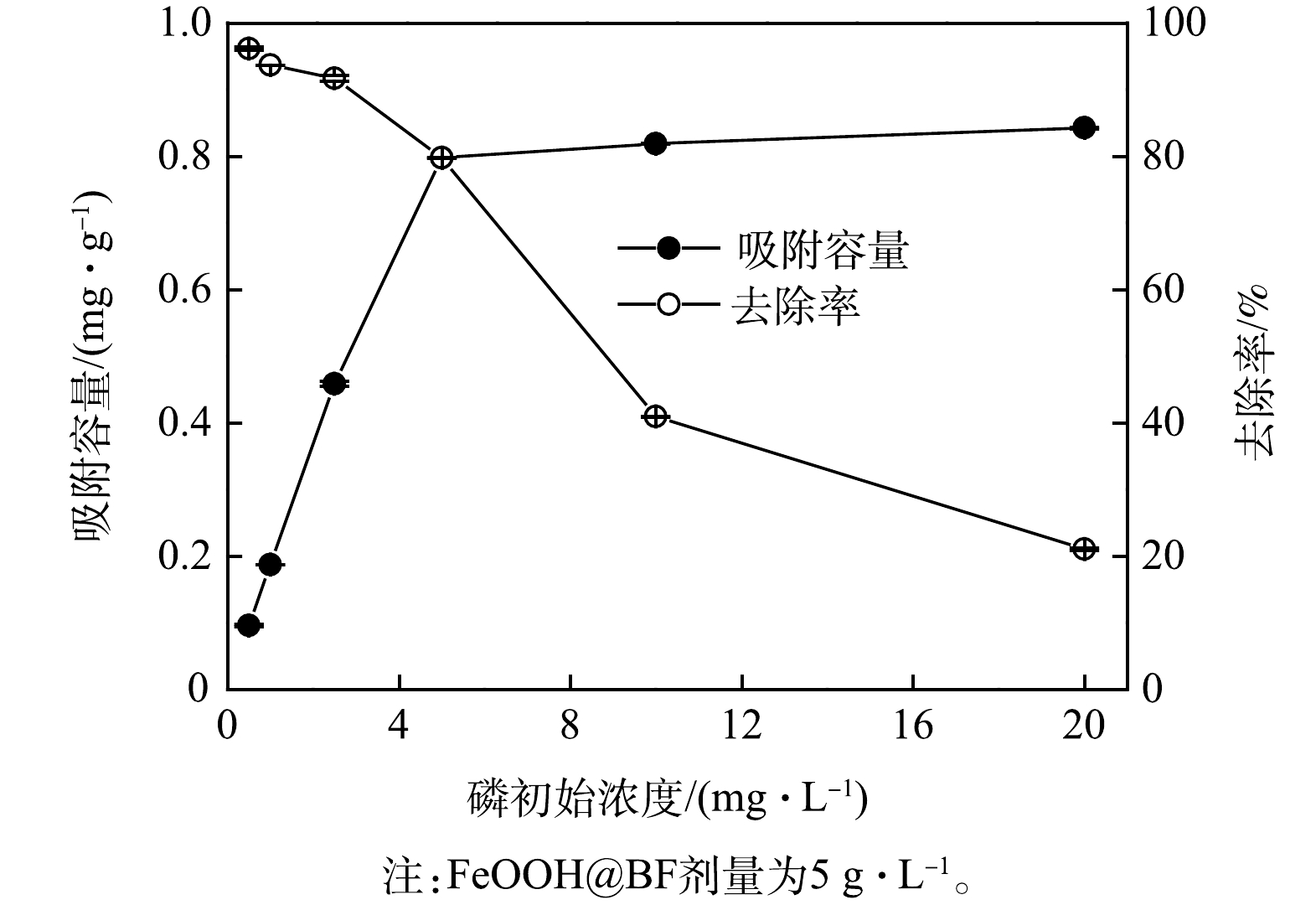
1)等温吸附分析。图5为FeOOH改性玄武岩纤维在不同初始质量浓度下对磷酸根的吸附量和去除率。由图5可见,随着磷酸根初始质量浓度的增大,FeOOH@BF整体式材料对磷酸根的吸附量快速增大,当磷酸根初始质量浓度达到5.0 mg∙L−1时,FeOOH@BF样品对磷酸根的吸附趋于饱和;在初始质量浓度为0.5~20.0 mg∙L−1,FeOOH@BF对磷酸根的最大吸附量为0.84 mg∙g−1。鉴于本研究所制备的FeOOH@BF复合材料中,α-FeOOH、β-FeOOH生长在玄武岩纤维表面,相应的负载量较低。因此,相比于纯的针铁矿或纤铁矿纳米粉末,本研究所制备的FeOOH@BF材料对水中磷的绝对吸附容量较小[25-27]。随着磷酸根初始质量浓度的增大,FeOOH@BF对水中磷酸根的去除率持续降低;在水中磷酸根初始质量浓度分别为0.5 mg∙L−1和5.0 mg∙L−1时,FeOOH@BF对磷酸根的去除率达到了96.2%和79.8%。上述结果表明,尽管FeOOH@BF对水体中磷的绝对吸附容量相较于纳米粉末材料较低,但仍能满足受污染水体中总磷有效去除的应用需求。
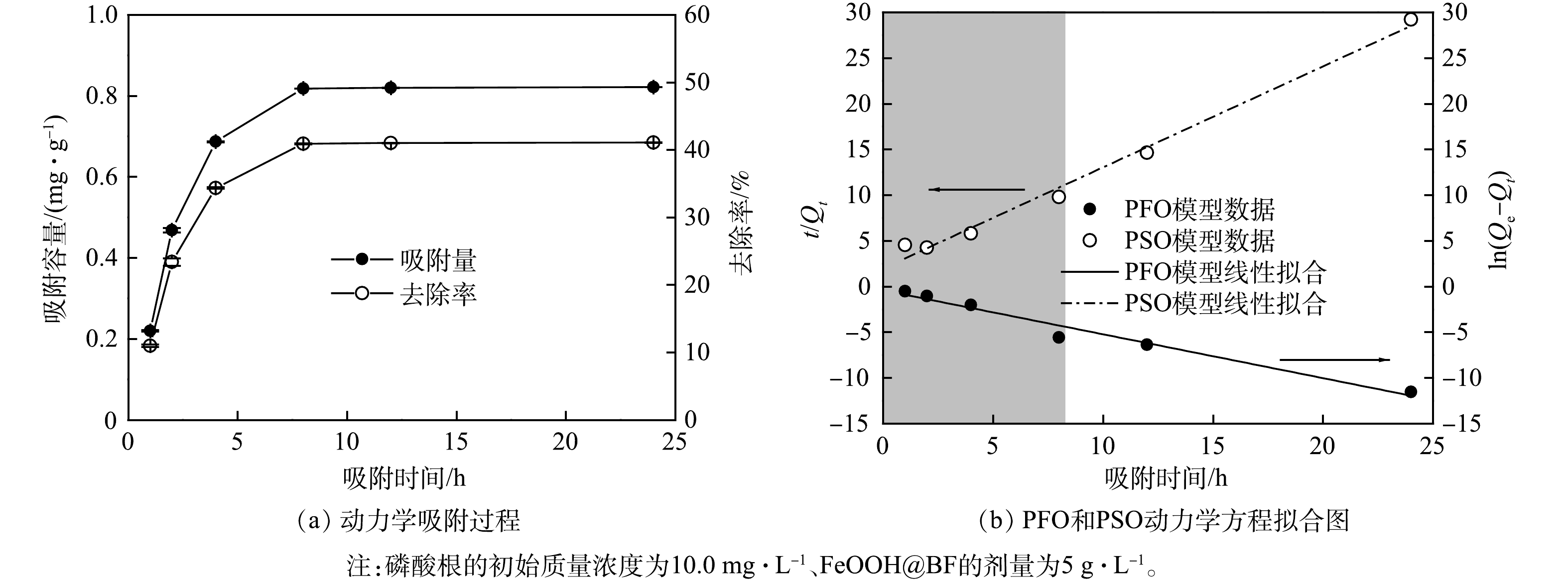
2)动力学吸附过程分析。图6为所制备FeOOH@BF吸附水中磷的动力学曲线。由图6(a)可见,在磷的初始质量浓度为10.0 mg∙L−1、吸附时间达到8 h时,FeOOH@BF对水中磷的吸附达到饱和,且饱和吸附容量为0.82 mg∙g−1。在达到吸附饱和之前,FeOOH@BF对水中磷的吸附量和去除率均随着吸附时间的延长而增大,最大去除率为41.1%。由图6(b)可见,在FeOOH@BF对水中磷的吸附达到饱和之前,FeOOH@BF对磷的吸附更适合用准一级动力学吸附过程描述。这表明FeOOH@BF对磷的吸附为扩散过程控制的物理吸附过程[27]。KIM等[25]研究表明,阴离子交换和静电作用是针铁矿等FeOOH对磷酸根的重要吸附方式,这与本研究中的动力学吸附特征相符。
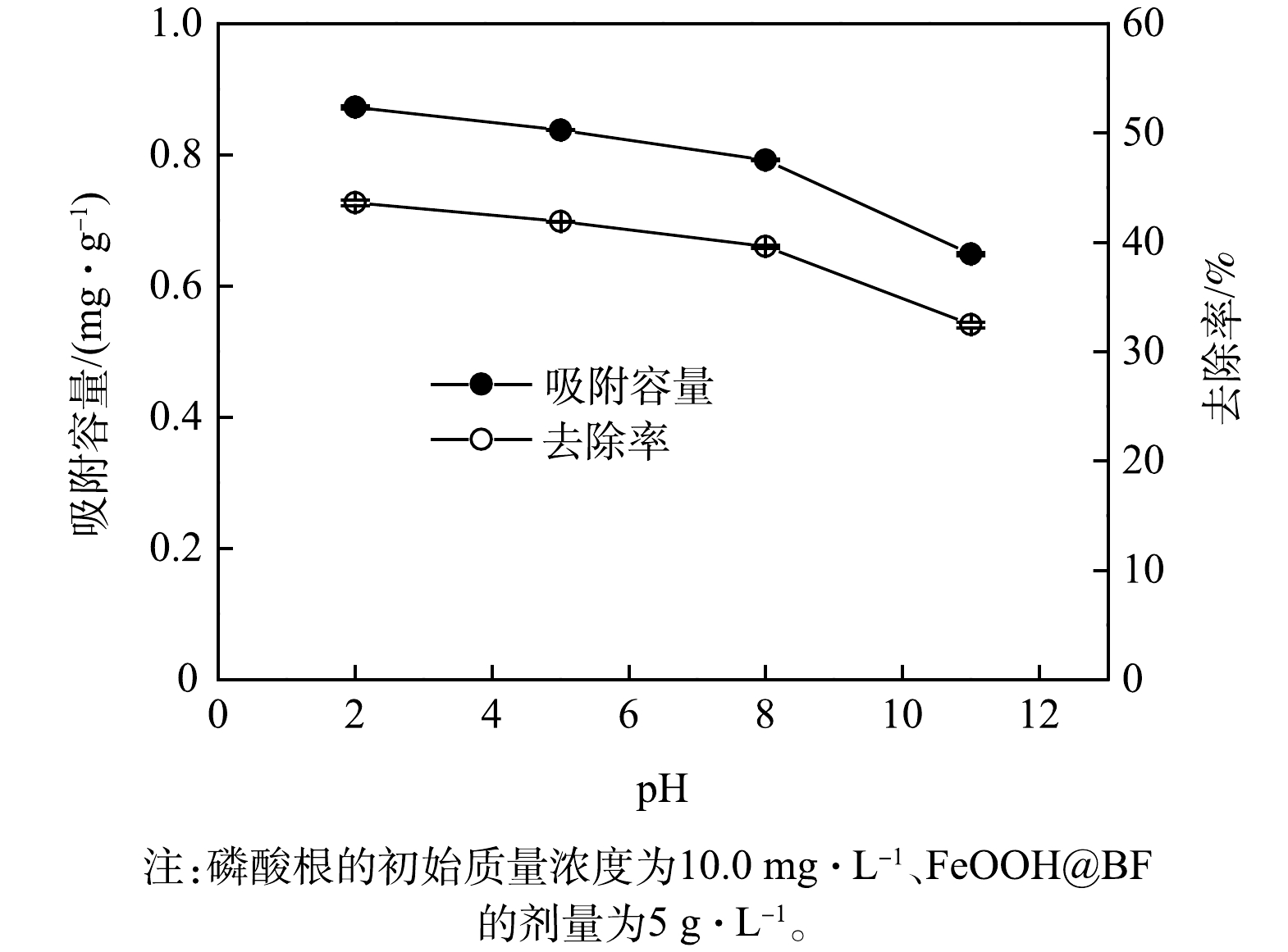
3) pH的影响。pH是影响铁氧化物表面物理化学性质和吸附质形态的关键因素,因此,对铁氧化物的吸附性能具有决定性影响[28-29]。图7为FeOOH@BF在不同pH条件下对磷酸根的吸附容量和去除率。由图7可见,随着pH的增大,FeOOH@BF对水中磷的吸附容量和去除率均降低,当pH为8.0时,FeOOH@BF对水中磷的吸附容量和去除率为0.79 mg∙g−1和39.6%;当pH进一步增大时,FeOOH@BF对水中磷的吸附去除性能出现较大程度的降低。一方面,针铁矿和纤铁矿的电荷零点pH (pHZPC)为7.5~9.5[24];当溶液pH小于pHZPC时,针铁矿和纤铁矿表面带正电荷,与带负电荷的磷酸根之间存在静电吸引,从而有利于对水中磷酸根的吸附作用。Zeta电位测试结果表明,在自然pH条件下,空白玄武岩纤维样品的Zeta电位为-6.2 mV,而FeOOH@BF样品的Zeta电位为2.7 mV。可见,FeOOH改性导致玄武岩纤维表面电荷状态由负电荷转变为正电荷,有利于与磷酸根阴离子之间通过静电吸引而发生吸附作用。随着pH的增大,针铁矿和纤铁矿的表面正电荷量降低,进而导致与磷酸根之间的静电吸附作用弱化;当溶液pH大于pHZPC时,FeOOH纳米颗粒表面将显示出负电荷而与磷酸根之间因存在静电排斥作用而不利于对磷酸根的吸附。另一方面,溶液pH的升高导致磷酸发生去质子化作用;当pH分别为5.0和8.0时,吸附质磷酸根(PO43−)发生质子化主要以H2PO4−和HPO42−形式存在[29]。此外,磷酸去质子化程度增大将增强其水化作用程度,同样可削弱其在吸附剂表面的吸附作用。上述多种因素均可导致表面FeOOH改性的玄武岩纤维随着pH的增大而削弱对磷酸根的吸附性能,且在较强碱性条件下的吸附性能损失程度显著增大。
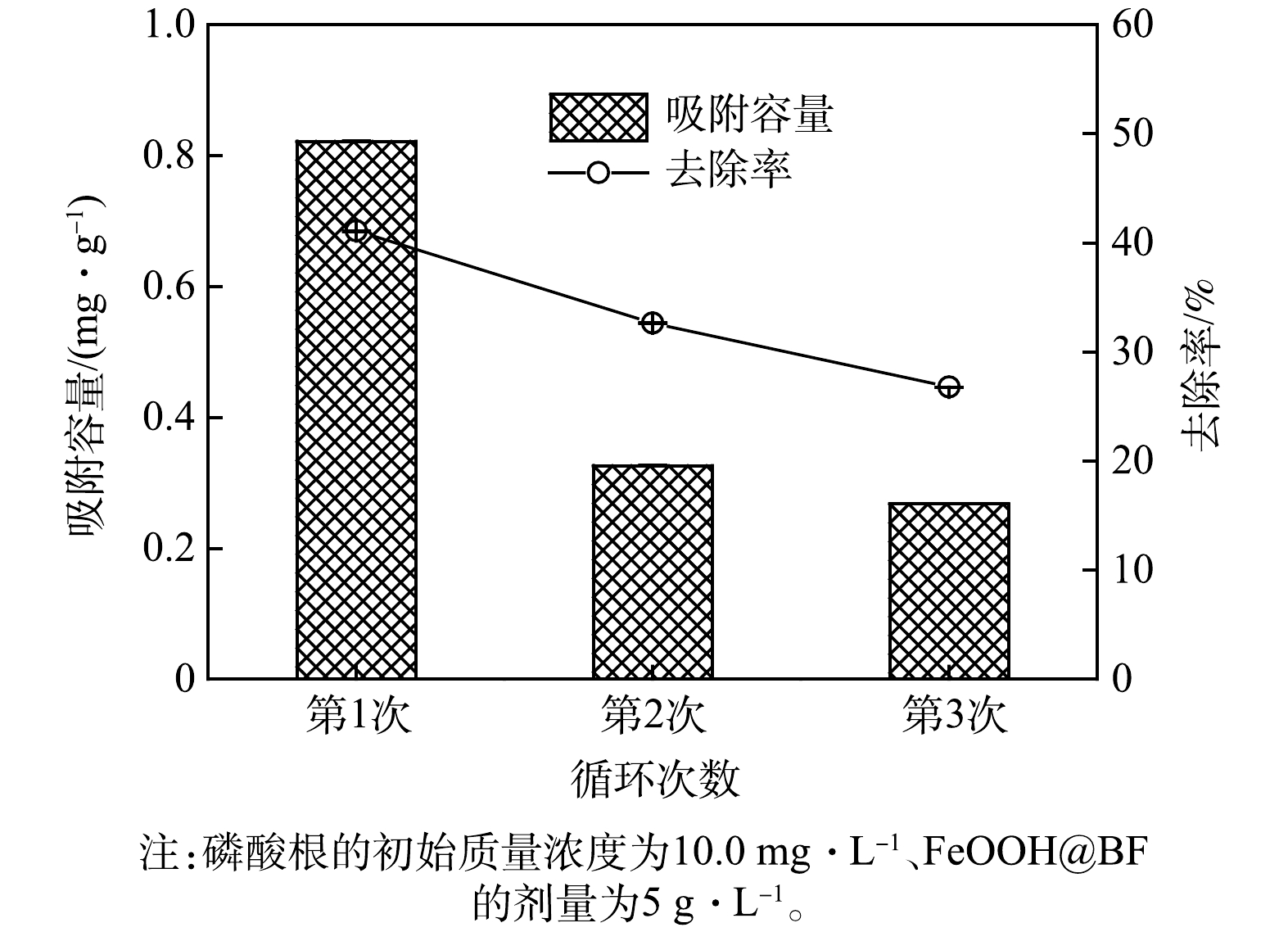
4)循环吸附性能。FeOOH@BF对磷酸根的再生吸附性能结果如图8所示。可见,在磷酸根初始质量浓度为10.0 mg∙L−1、FeOOH@BF剂量为5 g∙L−1的条件下,FeOOH@BF对磷酸根的吸附容量和去除率分别为0.82 m∙g−1和41.1%。当吸附饱和后的FeOOH@BF经0.05 mol∙L−1的HNO3溶液浸泡12 h再生后,FeOOH@BF在相同条件下对磷酸根的吸附容量和去除率均出现了较大程度的降低;浸泡24 h (即再生2次)后的FeOOH@BF对磷酸根的吸附去除率降至26.8%。此外,由图8可见,吸附饱和的FeOOH@BF经首次再生后对磷酸根的吸附性能大幅降低,但在随后的再生过程中吸附性能的降低程度较小而出现趋于稳定的现象。
-
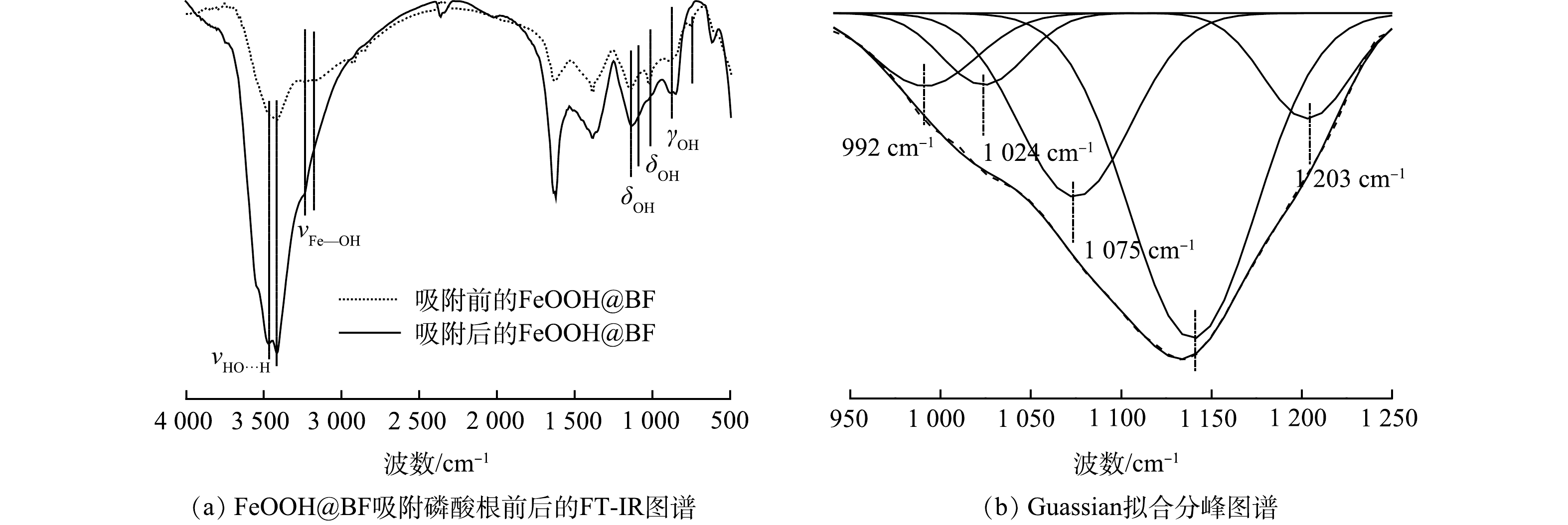
进一步通过吸附磷酸根前后FeOOH@BF材料的FT-IR光谱,研究了FeOOH@BF对磷酸根的吸附机理。图9(a)为吸附前后FeOOH@BF的FT-IR光谱图。相比于吸附前的FeOOH@BF材料,在磷酸根溶液中吸附饱和后的FeOOH@BF材料中β-FeOOH和α-FeOOH的铁羟基伸缩振动以及面外、面内弯曲振动红外吸收强度均显著减弱,而在615 cm−1处的FeO6特征红外活性吸收峰显著增强[24]。此外,吸附磷物种以后的FeOOH@BF材料在1 100~1 200 cm−1的吸收峰明显增强,鉴于磷物种的红外特征吸收峰在该区域出现,于是对吸附磷物种以后FeOOH@BF材料的FT-IR图谱(950~1 250 cm−1)做Guassian分峰拟合。图9(b)为Guassian函数拟合后的FT-IR图谱。由图9(b)可见,在磷酸根溶液中吸附饱和后的FeOOH@BF材料在992 cm−1和1 075 cm−1处出现了强的红外吸收峰,且1 075 cm−1处的红外吸收强度明显高于992 cm−1处的红外吸收强度。这与ISABEL等[30]和ELZINGA等[31]通过衰减全反射傅里叶变换红外光谱和圆柱内反射差谱傅里叶变换红外光谱研究发现的具有C3V对称性的HPO42−物种的红外光谱特征高度相符。这表明将FeOOH@BF作为吸附剂吸附磷酸根时,由于吸附剂表面的活性铁羟基发生去质子化而向溶液中释放H+,导致溶液中Td对称性的磷酸根物种(PO43−)发生质子化而形成C3V对称性的HPO42−物种;质子化后的HPO42−物种进一步可通过配体交换、离子交换和氢键相互作用等方式被玄武岩纤维表面包覆的FeOOH物种所吸附[30-32]。
-
本研究基于活化-Fe3+原位水解方法制备了铁羟基氧化物包覆的玄武岩纤维宏观整体式功能性复合材料,并将其作为脱磷材料研究了对水体中磷的吸附去除性能。主要研究结论如下。
1)铁羟基氧化物包覆的玄武岩纤维复合材料表面为α和β 2种晶相的FeOOH物种,上述结晶相羟基氧化铁为玄武岩纤维表面提供了丰富的活性铁羟基吸附位点。
2)当水中磷酸根质量浓度超过VI地表水质量标准10倍时,所制备的FeOOH@BF材料仍能有效去除水中的磷酸根(去除率高于80%),FeOOH@BF对水中磷酸根的吸附动力学过程符合物理性吸附过程,且饱和吸附容量随着水体pH的增大而降低。
3)吸附饱和后的FeOOH@BF在0.05 mol∙L−1的HNO3溶液中浸泡24 h以后仍能有效吸附水中的磷酸根而实现水体磷污染的净化。
4) FT-IR光谱分析表明,FeOOH@BF吸附剂因表面铁羟基的去质子化而导致磷酸根发生质子化作用并进一步被吸附在材料表面。
FeOOH改性玄武岩纤维整体式材料对磷的去除性能及机理
Performance and mechanism of the monolithic material of FeOOH-modified basalt fiber on phosphate removal
-
摘要: 尽管微纳米材料具有优异的环境净化功能,但直接应用微纳米材料具有潜在的生态环境威胁。本研究以惰性玄武岩纤维为基底材料,通过表面活化-Fe3+原位水解方法制备功能性整体式材料(FeOOH@BF)并研究其对水中磷的去除性能。X-射线衍射(X-ray diffraction, XRD)测试显示,在玄武岩纤维表面原位生长的羟基氧化铁(FeOOH)具有α、β两种晶体相结构。FeOOH的附着导致惰性玄武岩纤维表面具有丰富的活性铁羟基吸附位点。吸附结果表明,FeOOH改性的玄武岩纤维整体式材料可有效去除水中的磷酸根。当水中磷含量超过V类地表水质量标准10倍时,5 g∙L−1的FeOOH@BF对水中磷的去除率高于80%。FeOOH@BF对水中磷的去除率随着磷初始质量浓度和pH的增大而降低。动力学吸附结果表明,FeOOH@BF对水中磷的吸附过程在达到饱和吸附以前适合用准一级动力学吸附方程描述。吸附饱和后的FeOOH@BF在0.05 mol∙L−1的HNO3溶液中经过浸泡12 h再生后,其对磷的吸附容量出现明显的降低,但仍能有效去除水中的磷酸根;当饱和吸附后的FeOOH@BF在HNO3溶液中浸泡24 h再生后,FeOOH@BF仍可有效去除水中的磷且吸附性能趋于稳定。FeOOH@BF材料表面的铁羟基发生去质子化释放H+离子,导致磷酸根质子化形成HPO42−,并进一步与材料表面的铁羟基发生配体交换、离子交换和氢键等作用,进而被玄武岩纤维表面包覆的FeOOH所吸附。Abstract: Although the micro/nano-materials have good performance on environment purification, but their direct use will cause potential risk on safety of ecosystem. In this work, the inert basalt fiber was taken as the base material, its surface was activated by NaOH solution and was hydrolyed in Fe3+ solution, then the monolithic material (FeOOH@BF) was prepared for phosphate removal from water. The crystal covered on the surface of basalt fiber was goethite and lepidocrocite, α- and β-FeOOH identified by XRD, these FeOOH could endow the inert basalt fiber with active sites of hydroxyl (Fe-OH) for adsorption. The adsorption experiments demonstrated that FeOOH@BF could effectively remove the phosphate in water. When the concentrate of phosphate was over 10 times that of the allowable value of phosphate in the V standard of surface water, the removing ratio of phosphate by FeOOH@BF was higher than 80% at 5 g∙L−1 of FeOOH@BF dosage. And the removing ratio of phosphate on FeOOH@BF decreased along with the increase of initial concentration of phosphate and pH. The kinetic adsorption showed that the process of P adsorption on FeOOH@BF before saturation adsorption could well fitted the pseudo first-order reaction. Moreover, the FeOOH@BF could still effectively adsorb phosphate from water after regeneration by soaking in 0.05 mol∙L−1 HNO3 for 12 h though its P adsorption capactiy decreased obviously. FeOOH@BF still effectively adsorb phosphate from water with a tendency of stable P adsorption performance after regeneration for 24 h in the HNO3. Further, the adsorption mechanism was investigated by FT-IR spectrum and fitting technology over Guassian function. It was found that the Fe-OH could deprotonate in solution and release H+ from the FeOOH@BF to solution, then phosphate in solution would combine with H+ and form HPO42− species, following by the adsorption by FeOOH@BF through ligand exchange, ion exchange and hydrogen interaction with hydroxyl (Fe-OH) on its surface.
-
Key words:
- basalt fiber /
- FeOOH /
- monolithic material /
- phosphate /
- adsorption
-

-
-
[1] 中华人民共和国生态环境部. 地表水环境质量标准: GB 3838-2002[S]. 中国: 国家环境保护总局, 国家质量监督检验检疫总局, 2002. [2] ZHANG J Z, HUANG X L. Relative importance of solid-phase phosphorus and iron on the sorption behavior of sediments[J]. Environmental Science & Technology, 2007, 41: 2789-2795. [3] USMAN M O, ATURAGABA G, NTALE M, et al. A review of adsorption technique for removal of phosphates from wastewater[J]. Water Science and Technology, 2022, 86: 3113-3132. doi: 10.2166/wst.2022.382 [4] 卓亿元, 刘草葱, 姜蕾, 等. 铁/锰矿基人工湿地脱氮除磷性能及机理[J]. 环境工程学报, 2023, 17(5): 1441-1450. [5] 李迎春, 董良飞, 仝驰, 等. 稀土改性凹凸棒土对低浓度磷的吸附性能[J]. 环境工程学报, 2021, 15(10): 3214-3222. [6] HINKLE M A G, WANG Z M, GIAMMAR D E, et al. Interaction of Fe(II) with phosphate and sulfate on iron oxide surfaces[J]. Geochimica et Cosmocheimica Acta, 2015, 158: 130-146. doi: 10.1016/j.gca.2015.02.030 [7] MATHUR S S, DZOMBAK D A. Surface complexation modeling: Goethite[J]. Interface Science and Technology, 2006, 11: 443-468. [8] ZHANG P, HE M M, HUO S L, et al. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects[J]. Chemical Engineering Journal, 2022, 446: 137081. doi: 10.1016/j.cej.2022.137081 [9] FONTES M P F, WEED S B. Phosphate adsorption by clays from Brazilian Oxisols, relationships with specific surface area and mineralogy[J]. Geoderma, 1996, 72: 37-51. doi: 10.1016/0016-7061(96)00010-9 [10] DZOMBAK D A, MOREL F M M. Surface Complexation Modeling: Hydrous Ferric Oxide[M]. Wiley-Interscience, New York, 1990. [11] WANG A, LIN Y, WU D Y, et al. Hydrous iron oxide modified diatomite as an active filtration medium for phosphate capture[J]. Chemosphere, 2016, 144: 1290-1298. doi: 10.1016/j.chemosphere.2015.10.015 [12] CHEN P, ZHOU Y F, XIE Q Q, et al. Phosphate adsorption kinetics and equilibria on natural iron and manganses oxide composites[J]. Journal of Environmental Management, 2022, 323: 116222. doi: 10.1016/j.jenvman.2022.116222 [13] VON MOOS N, SLAVEYKOVA V I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae-state of the aer and knowledge gaps[J]. Nanotoxicology, 2014, 8: 605-630. doi: 10.3109/17435390.2013.809810 [14] NG E L, LWANGA E H, ELDRIDGE S M, et al. An overview of microplastic and nanoplastic pollution in agroecosytems[J]. Science of the Total Environment, 2018, 627: 1377-1388. doi: 10.1016/j.scitotenv.2018.01.341 [15] LORIGNON F, GOSSARD A, CARBONI M. Hierarchically porous monolithic MOFs: An pngoing challenge for industrial-scale effluent treatment[J]. Chemical Engineering Journal, 2020, 393: 124765. doi: 10.1016/j.cej.2020.124765 [16] GOSWAMI L, KIM K-H, DEEP A, et al. Engineered nano particles: Nature, behavior, and effect on the environment[J]. Journal of Environmental Management, 2021, 196: 297-315. [17] WANG L W, WU W M, BOLAN N S, et al. Environmental fate, toxicity and risk management strategies of nanoparticles in the ecvironment: Current status and future perspectives[J]. Journal of Hazardous Materials, 2021, 401: 123415. doi: 10.1016/j.jhazmat.2020.123415 [18] WAN W C, ZHANG R Y, MA M Z, et al. Monolithic aerogel photocatalysts: A review[J]. Journal of Materials Chemistry A, 2018, 6: 784-775. [19] 张瑞阳, 李金成, 张艾丽, 等. 整体式光催化材料的制备及应用研究进展[J]. 材料导报, 2020, 34(3): 7-22. [20] SUN Y, ZHANG P Y, ZHA Q Y, et al. Novel iminodiacetic acid functionalized basalt fiber for adsorption of Cu(II) ions in batch experiments[J]. Journal of Dispersion Science and Technology, 2021, 1947851. [21] XIAO J, CHEN M, HUANG M J, et al. Systematic evaluation of PDA-PAM-MAH-modified basalt fiber as biofilm carrier for wastewater treatment[J]. Environmental Technology, 2022, 43: 1328-1339. doi: 10.1080/09593330.2020.1829085 [22] WANG R, XU H J, LIU X, et al. In-situ growth of iron oxides with MIL-100(Fe) enhances its adsorption for selenite[J]. Surfaces and Interfaces, 2022, 34: 102325. doi: 10.1016/j.surfin.2022.102325 [23] 王锐, 方敦, 牛鹏举, 等. Mn2+掺杂对晶质氧化铁结构与红外光谱特征的影响[J]. 土壤学报, 2020, 57(4): 898-907. [24] CORNELL R M, SCHWERTMANN U. The Iron Oxides, Structure, Properties, Reactions Occurences, Uses [M]. Wiley-VCH, Weinheim, Germany, 2003. [25] KIM J, LI W, PHILIPS B L, et al. Phosphate adsorption on the iron oxyhydroxides goethite ( α-FeOOH), akaganeite ( β-FeOOH), and lepidocrocite ( γ-FeOOH): A 31P NMR study[J]. Energy & Environmental Science, 2011, 4: 4298-4305. [26] ZHANG X, YAO H, LEI X B, et al. A comparative study for phosphate adsorption on amorphous FeOOH and goethite ( α-FeOOH): An investigation of relationship between the surface chemistry and structure[J]. Environmental Research, 2021, 199: 111223. doi: 10.1016/j.envres.2021.111223 [27] 刘鑫, 吕青檐, 魏世勇. 硅/铝掺杂对针铁矿的表面性质和吸附磷的影响[J]. 湖北民族大学(自然科学版), 2022, 40(3): 282-288. [28] 王锐, 龚勇, 许海娟, 等. 不同调节剂制备MOF-Fe的性质及对Se(IV)的吸附性能[J]. 无机化学学报, 2018, 34(5): 906-916. [29] 王锐, 徐海娟, 魏世勇, 等. 磷酸盐共存对MOF-Fe吸附亚硒酸盐的影响[J]. 环境科学学报, 2019, 39(3): 737-746. [30] ISABEL TEJEDOR-TEJEDOR M, ANDERSON M A. Protonation of phosphate on the surface of goethite as studied by CIR-FTIR and electrophoretic mobility[J]. Langmuir, 1990, 6: 602-611. doi: 10.1021/la00093a015 [31] ELZINGA E J, SPARKS D L. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation[J]. Journal of Colloid and Interface Science, 2007, 308: 53-70. doi: 10.1016/j.jcis.2006.12.061 [32] 许海娟, 王锐, 魏世勇, 等. 蒙脱石-氧化铁复合体的表征及其对不同形态磷的吸附特性[J]. 土壤学报, 2019, 56(6): 1381-1389. -




 下载:
下载: