-
病原微生物经市政污水及合流制溢流进入地表水体,对公共卫生及生态系统产生了极大威胁[1]。目前应用最广泛的消毒方法为氯消毒、臭氧消毒和紫外消毒。氯消毒应用成本低且消毒效率高,但其消毒过程中产生的卤代消毒副产物具有较强毒性;臭氧消毒效率高,接触时间短,但制备成本较高,且无持续消毒作用;紫外线主要通过破坏细胞内遗传物质 (DNA和RNA) 抑制微生物繁殖[2],消毒效率高,但在消毒过程中存在拖尾现象[3]、消毒效果易受水质影响且存在一定的光复活风险[4-7]。因此寻找绿色、高效的消毒方法意义重大。
过氧乙酸(peracetic acid,CH3C(O)OOH),简称PAA,是一种新兴氧化剂,相比于传统消毒剂具有较强的氧化性,其氧化还原电位(E0 = 1.96 eV)高于过氧化氢(E0=1.8 eV)、氯(E0=1.4 eV)及二氧化氯(E0=1.5 eV),且在消毒过程中产生的消毒副产物较少[2, 8]。PAA最早于20世纪初应用于食品工业中[9],近年来欧美国家将其用于市政污水和雨水消毒[1, 10-11],其在灭活细菌、真菌及芽孢时有较好效果[12-13]。PAA通过扩散作用从细胞膜渗透进入细胞,氧化细胞内的蛋白质和酶从而使细胞失活,而这种氧化灭活作用通常较弱[14-15],且无法消除病原菌中的抗生素的抗性基因[16]。
紫外活化PAA(UV/PAA)体系是一种新兴高级氧化体系,PAA被UV活化后可产生羟基自由基(HO·)、有机自由基(CH3C(O)O·,CH3C(O)OO·)、超氧自由基(O2•−)和单线态氧(1O2)等活性物质[17]。目前该体系广泛应用于水中微污染物去除[18],体系中的UV辐照、PAA及UV活化PAA产生的自由基均具有较好的消毒潜力[19-20]。相较于单独PAA体系,UV/PAA体系能够显著提高灭活效率,相较单独UV体系,UV/PAA体系能改善拖尾现象,快速实现高灭活率,且体系内自由基对细胞膜造成的破坏能够限制微生物的自我修复[6],降低复活风险,具有广阔的应用前景。但目前对于应用UV/PAA体系消毒时工艺参数和背景物质的影响、氧化灭活机理及反应过程中细胞特征变化的研究较少。
大肠杆菌是常见的病原微生物,也是水质分析中常用的指标菌[21]。故本研究选取大肠杆菌作为目标微生物,考察了UV/PAA体系中工艺、水质参数对大肠杆菌灭活效率的影响,并探究了其灭活机理,以期为深度理解UV/PAA体系消毒机制及其应用潜力提供理论支持。
-
主要试剂:过氧乙酸由乙酸和过氧化氢在浓硫酸催化下制备[22];过氧化氢、浓硫酸、叔丁醇(TBA)、己二烯(2,4-HD)、氯化钠、硫酸铵、磷酸一氢钠、磷酸氢二钠、牛肉浸膏、蛋白胨、琼脂粉购自国药集团化学试剂有限公司;5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)、腐殖酸购自阿拉丁试剂有限公司;Calcein-AM/PI试剂盒购自北京索莱宝科技有限公司;实验室所用试剂均采用超纯水(18.2 MΩ·cm)配置。
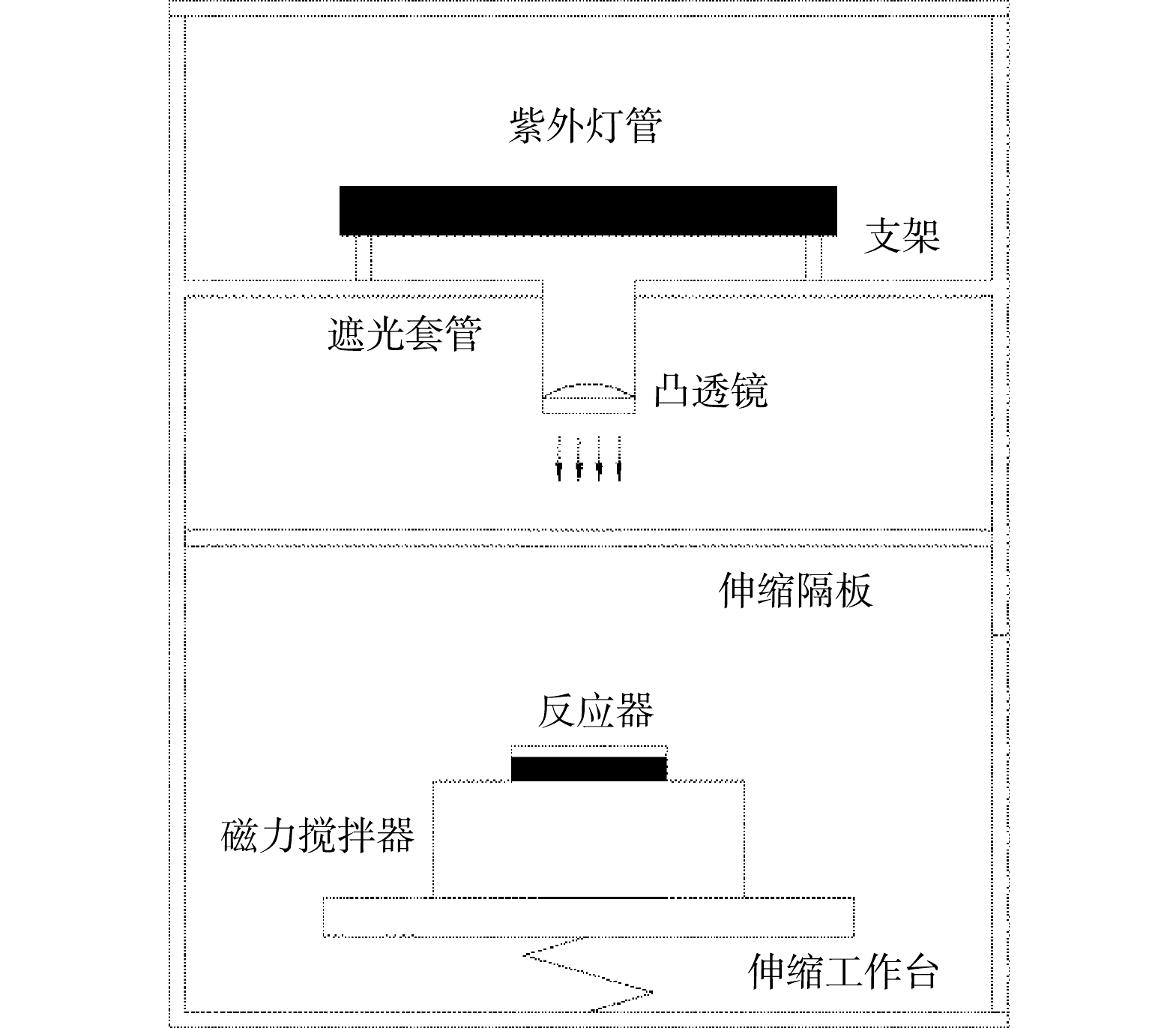
实验装置:本实验采用准平行紫外光反应装置。如图1所示,在避光箱体内安装1根波长254 nm功率为5 W的紫外灯管(GPH135T5L/4,Heraeus,德国),紫外光透过避光套管照射到反应器上,套管底部装有凸透镜产生准平行紫外光。反应器和磁力搅拌器置于伸缩工作台上,其高度可以调节,反应溶液中的紫外光强由KI/KIO3化学光度计法测定[23]。
-
1)磷酸盐缓冲液配置。称量2.864 g磷酸一氢钠和0.312 g磷酸氢二钠溶于1 000 mL超纯水中配置成浓度为0.01 mol·L−1、pH=7.5的磷酸盐缓冲液。所有灭活实验在磷酸盐缓冲液中进行,经测定反应过程中溶液中pH稳定在7.5±0.1。
2)菌液配制。配置LB(Luria-Bertani)液体培养基(10 g·L−1蛋白胨,3 g·L−1牛肉浸膏,5 g·L−1氯化钠),经高压灭菌锅灭菌20 min后冷却至室温接种大肠杆菌,放入37 ℃振荡培养箱中以150 r·min−1转速振荡培养18~20 h,随后离心分离(3 500 r·min−1,15 min),用磷酸盐缓冲液洗涤3次,洗涤后的菌体重悬于磷酸盐缓冲液中备用。
3)大肠杆菌灭活实验。在反应器内取菌液和磷酸盐缓冲液中配制成40 mL的反应溶液,溶液中大肠杆菌初始浓度为1×107~2×107 CFU·mL−1,配置完成后在磁力搅拌器转速800 r·min−1下搅拌使溶液中大肠杆菌充分分散。
进行灭活实验时,提前20 min预热紫外灯管,控制室温(25±1) ℃,反应开始时向反应器中加入PAA,拉开伸缩隔板使紫外光照射在反应器表面,分别在反应时间0、1、2、3 min时取样200 μL,取样后立刻向样品中加入5 μL 0.5 mol·L−1硫代硫酸钠溶液中止反应。采用平板计数法对于样品溶液中大肠杆菌进行计数,每组实验至少重复2次。
-
1)氧化剂浓度测定。制备的PAA原液中含有PAA和H2O2,采用碘量法测定总过氧化物的浓度。在酸性pH条件下,用高锰酸钾滴定法测定原液中H2O2的浓度。PAA浓度用总过氧化物的浓度减去H2O2的浓度计算[22]。经测定,本实验中使用的PAA原液中PAA浓度为1.14 mol·L−1,H2O2浓度为0.68 mol·L−1。
2)大肠杆菌平板计数。样品经过梯度稀释后进行涂布平板计数,取样100 μL均匀涂布在LB固体培养基(蛋白胨10 g·L−1,牛肉浸膏3 g·L−1,氯化钠5 g·L−1,琼脂20 g·L−1)上,放入37 ℃恒温培养箱中培养24 h后计数。
3)大肠杆菌灭活率。用对数去除率表示大肠杆菌灭活率,可由式(1)进行计算。
式中:n为对数去除率,N和N0为灭活后和灭活前的样品菌落数,CFU·mL−1。
4)活性物种表征。利用电子顺磁共振技术(EPR)检测体系内可能的活性物种,取1 mL反应溶液,向其中加入50 mmoL·L−1 DMPO捕获产生的自由基,利用电子顺磁共振光谱仪(EMX nano,Bruker,德国)检测DMPO加合物信号。
5)细胞膜完整性表征。使用Calcein-AM/PI试剂盒染色在激光共聚焦显微镜(TCS-SP8,Leica,德国)下观察大肠杆菌细胞膜完整性。取反应后的样品0.5 mL,加入Calcein-AM原液1 μL ,避光染色20 min,再加入PI原液4 μL避光染色5 min,染色后细胞用磷酸盐缓冲液洗涤2次,置于激光共聚焦显微镜下检测荧光。Calcein-AM染料采用488 nm激发波长,检测498~550 nm发射波长,PI染料采用的激发波长为552 nm,发射波长为562~650 nm [24]。
6)细胞表面形态表征。收集反应后溶液,经过量硫代硫酸钠中止反应并离心去除上清液,使用磷酸盐缓冲液洗涤2~3次后,收集离心管底部菌体真空冷冻干燥24 h后,在扫描电子显微镜(IT200,JSM,日本)下观察大肠杆菌表面形态。
7)荧光光谱分析。取反应后溶液,经过量硫代硫酸钠中止反应后使用0.22 μm滤头过滤,采用荧光光谱仪(F-7100,Hitachi,日本)检测反应过程中大肠杆菌悬液中溶解性成分的变化。检测的激发波长200~450 nm,发射波长280~550 nm。使用荧光体积积分的方法分析测定的荧光激发-发射光谱。将图谱分为5个区域,每个区域代表一大类物质[25],如下表1所示。对5个区域分别进行荧光体积积分,积分数值能够定量反映该区域总体荧光强度。
-
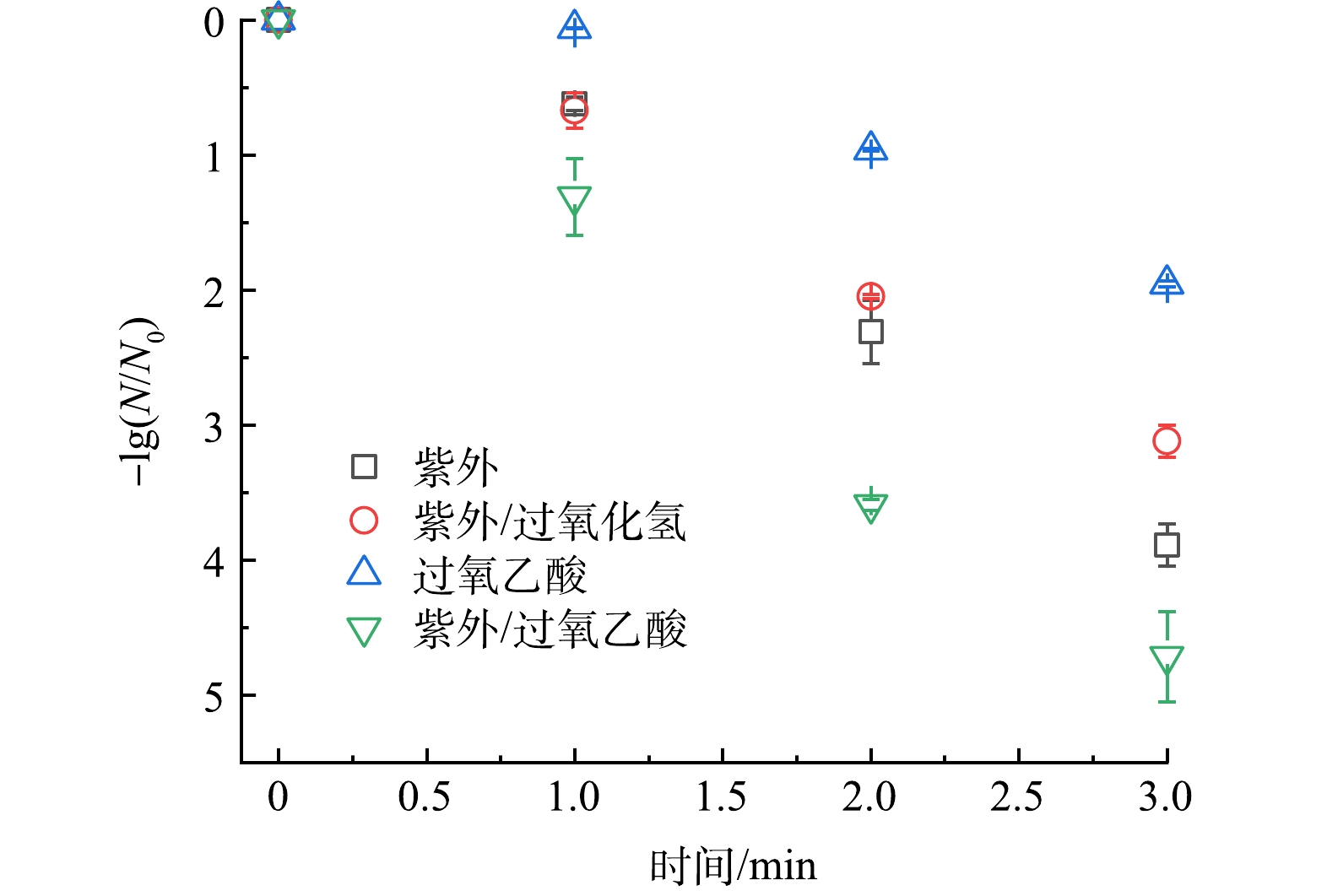
在初始大肠杆菌浓度1×107~2×107 CFU·mL−1,PAA浓度60 μmol·L−1(含36 μmol·L−1 H2O2),H2O2浓度96 μmol·L−1,紫外光强2.25×10−7 Einstein·(s·L)−1,反应时间3 min的条件下,考查了PAA、UV、UV/PAA、UV/H2O2 4种体系下大肠杆菌的灭活率。实验结果如图2所示,单独PAA对大肠杆菌的灭活率为1.95 log,说明单独PAA的对于大肠杆菌的氧化灭活能力较弱[14-15];紫外线通过破坏细胞内遗传物质能够有效地实现大肠杆菌灭活,因此单独UV对于大肠杆菌的灭活率可以达到3.89 log [2];而UV/PAA体系的大肠杆菌灭活率达到了4.71 log,相较于UV体系提升了0.82 log,说明紫外活化PAA能够有效提升大肠杆菌灭活率,这可能是由于体系中产生了大量自由基,这些自由基具有较高的氧化还原电位,对于微生物的细胞膜/壁、酶和遗传物质都有破坏作用[26]。由于本实验使用的PAA中含有一定量H2O2,为探究共存H2O2对于大肠杆菌的灭活影响,在UV体系中加入与UV/PAA体系等过氧化物浓度的H2O2(96 μmol·L−1),实验结果表明UV/H2O2体系的灭活率仅为3.12 log,低于单独UV体系的3.89 log,说明共存H2O2对于UV/PAA体系灭活效率影响可以忽略不计,这与之前的研究结果是一致的[27]。
-
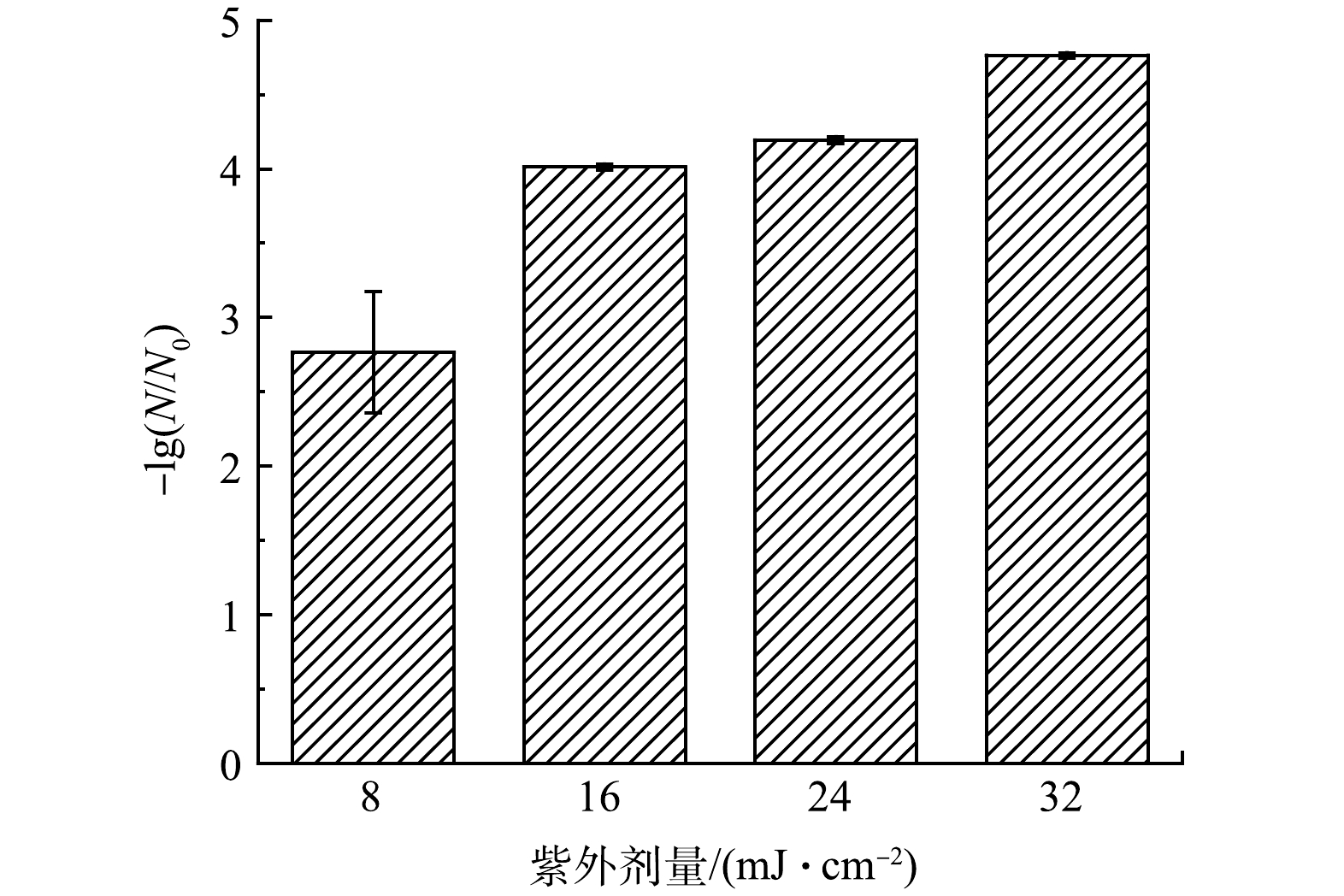
1)紫外辐射剂量影响。在大肠杆菌浓度1×107~2×107 CFU·mL−1,紫外光强2.25×10−7 Einstein·(s·L)−1的条件下改变紫外照射时间探究紫外辐射剂量对于大肠杆菌灭活的影响,2~8 min的紫外辐照时间分别对应8~32 mJ·cm−2的紫外辐射剂量,结果如图3所示。紫外辐射剂量为8 mJ·cm−2时,大肠杆菌灭活率可达到2.76 log,随着紫外辐射剂量由8 mJ·cm−2提升至16 mJ·cm−2,灭活率提升了1.25 log,达到4.01 log,而紫外辐射剂量由16 mJ·cm−2提升至32 mJ·cm−2,灭活率仅提升了0.75 log,达到4.76 log。上述结果说明紫外线照射能够实现大肠杆菌的快速灭活,但持续增加紫外线辐射剂量时灭活过程会出现一定的拖尾现象,可能与细胞聚集有关[3]。拖尾现象导致紫外消毒效率大幅受限,增加使用成本,单独紫外照射8 min的灭活率4.76 log与UV/PAA体系反应3 min的灭活率4.71 log基本相同。为避开紫外作用拖尾段,提高消毒效率,本实验选择时间为3 min,对应紫外辐射剂量为12 mJ·cm−2。
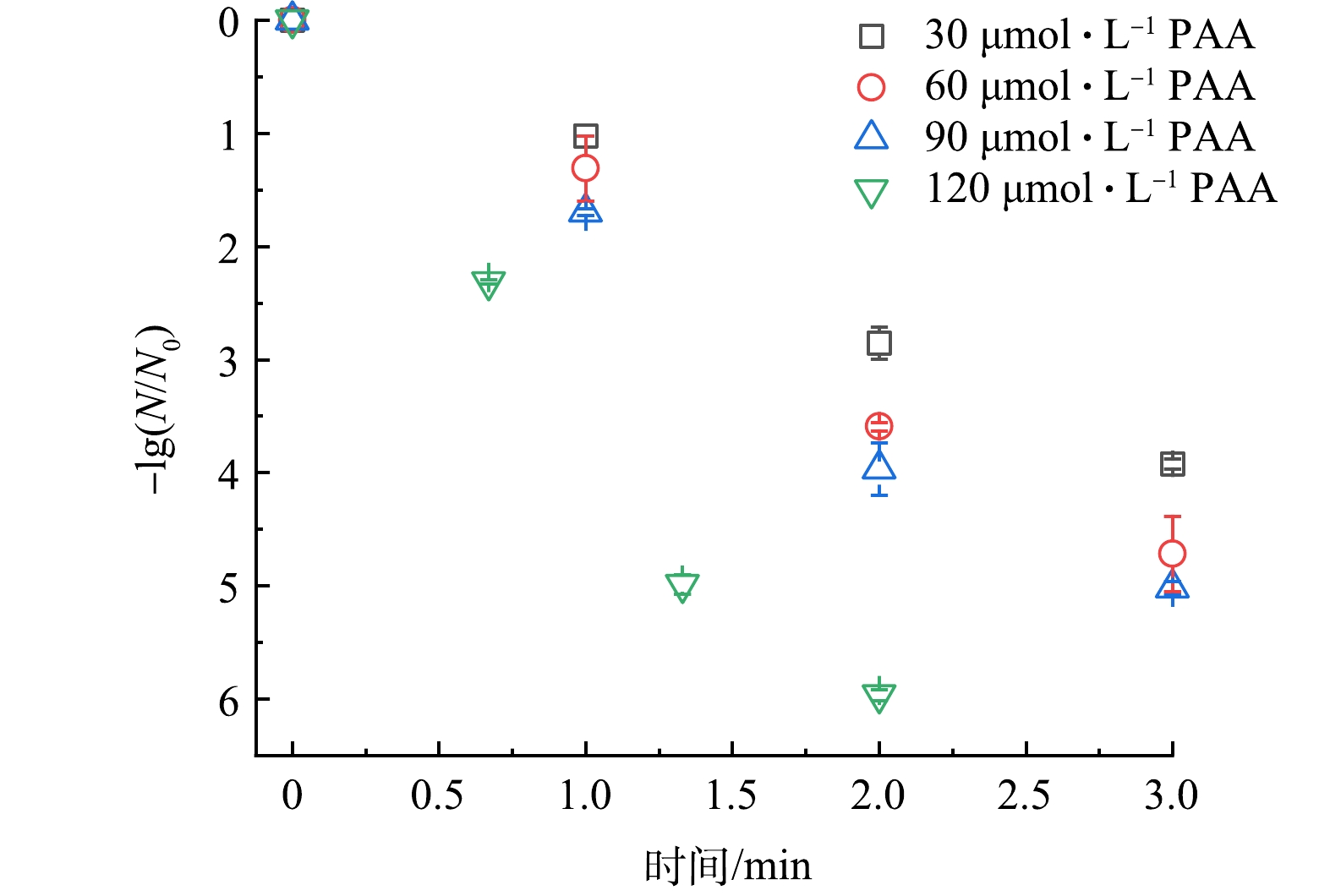
2)过氧乙酸浓度影响。在大肠杆菌浓度1×107~2×107 CFU·mL−1、紫外光强2.25×10−7 Einstein·(s·L)−1、反应时间3 min的条件下探究PAA浓度对于体系灭活率影响,结果如图4所示。当PAA浓度由30 μmol·L−1提升至90 μmol·L−1时,大肠杆菌灭活率相应由3.92 log提升至5.02 log,而当溶液中PAA浓度提升至120 μmol·L−1时仅需2 min就可以达到5.96 log的灭活率。说明提升溶液中的PAA浓度能够提高大肠杆菌灭活率,原因可能为PAA浓度增加时不仅可以增加扩散至大肠杆菌内的PAA浓度,而且可以产生更多的自由基,增强对于细胞膜结构及胞内物质的破坏[26]。综合考虑灭活效果及氧化剂使用成本,本实验采用的PAA浓度为60 μmol·L−1。
-
UV/PAA体系常用于地表污废水的消毒[28]。氨氮(NH4+)、盐度(Cl−)、及腐殖质(HA)为污废水常见水质指标,需考虑这些背景物质对于UV/PAA消毒效能的影响,其在地表水消毒中的常见质量浓度分别为[29-31]:0~15 mg·L−1 NH4+、20~100 mg·L−1 Cl−、0~10 mg·L−1 HA。为深入探究背景物质浓度影响,本实验中所选背景物质浓度均大于等于常见浓度。
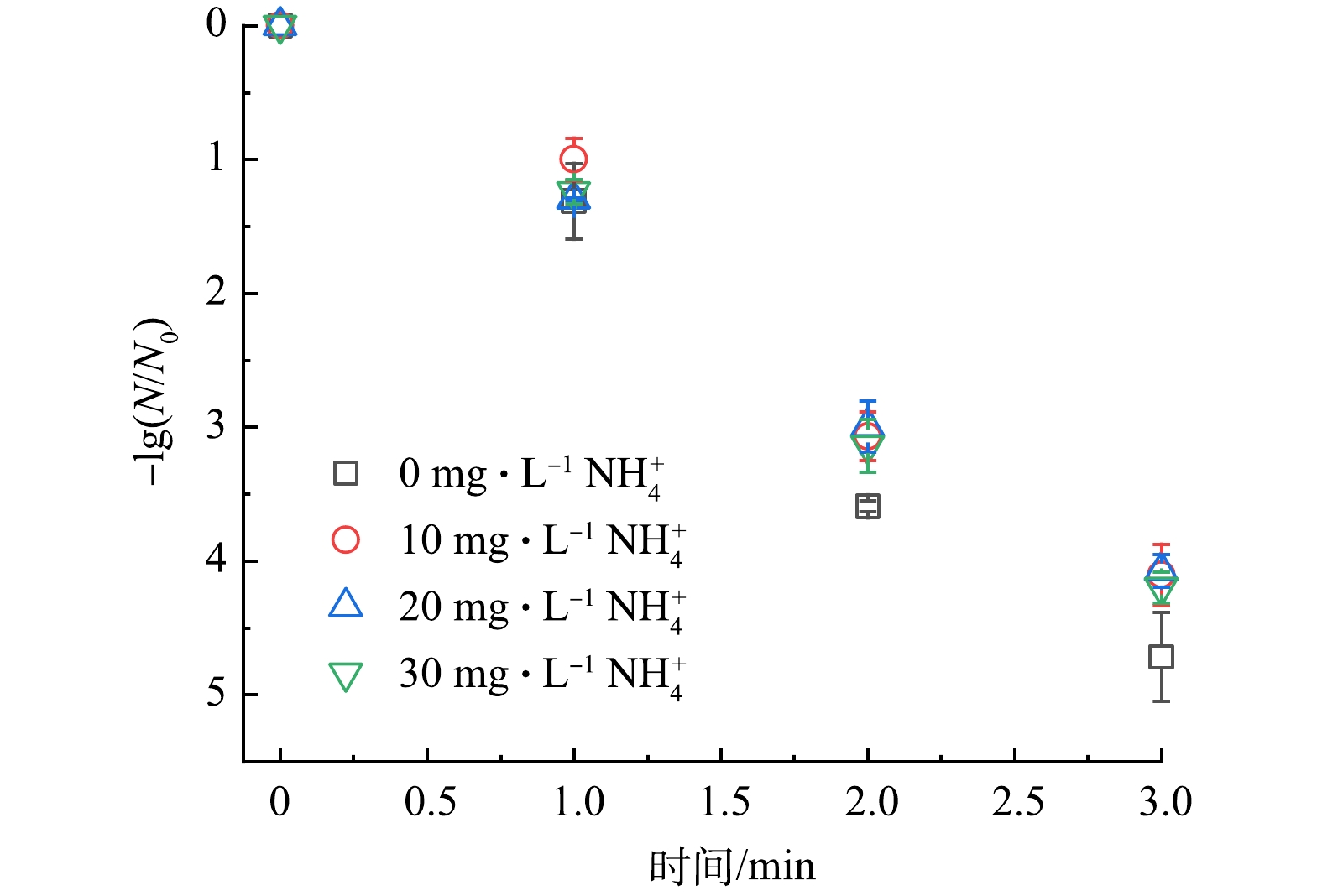
1) NH4+影响。NH4+对于UV/PAA体系的影响如图5所示,溶液中NH4+质量浓度从0 mg·L−1提升至30 mg·L−1时,大肠杆菌灭活率仅仅降低了0.52 log。由于所选的NH4+浓度范围已远大于地表水中常见的浓度,故NH4+对于UV/PAA体系灭活大肠杆菌效果无显著影响。
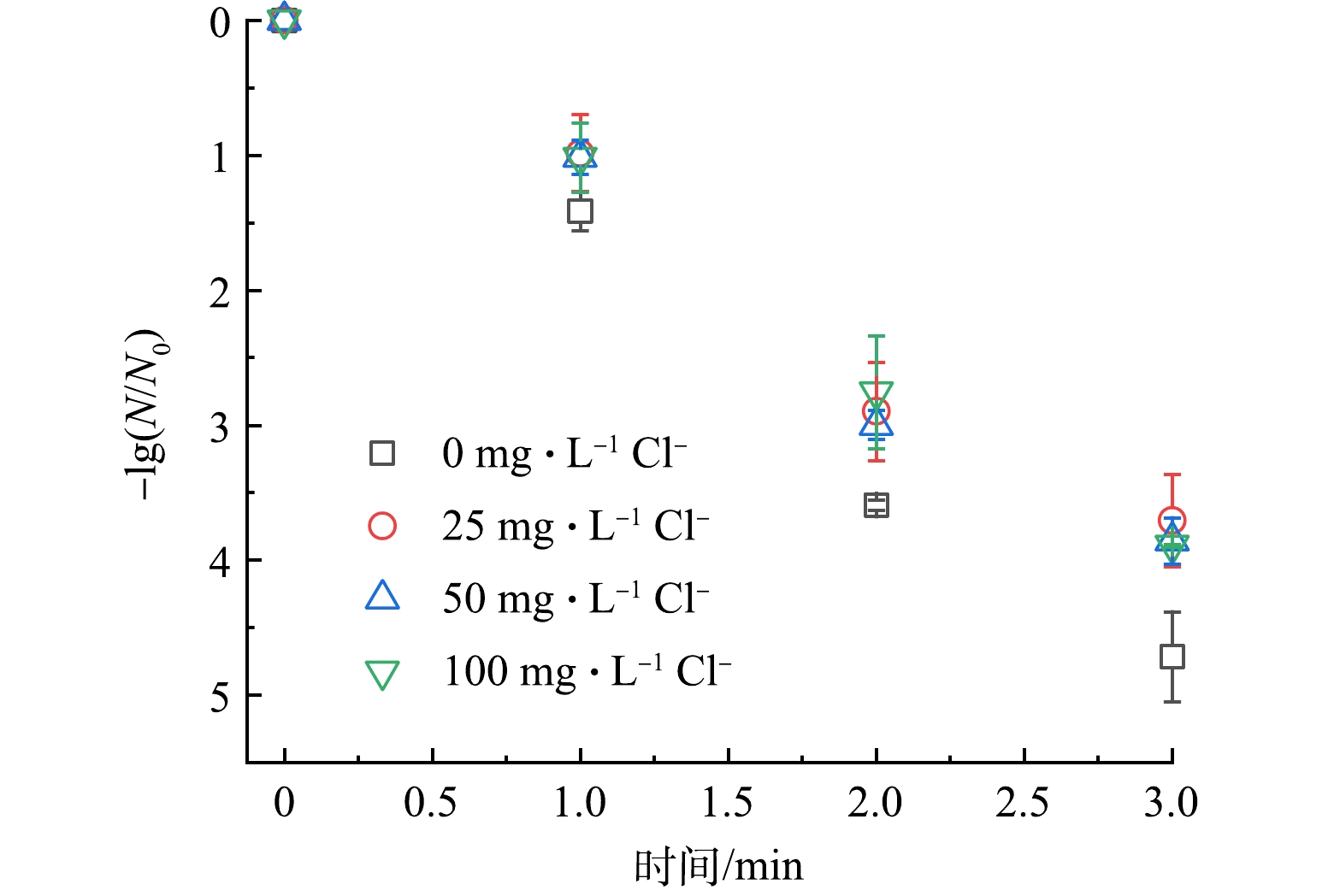
2) Cl−影响。Cl−对于UV/PAA体系的影响如图6所示。溶液中Cl−质量浓度从0 提升至100 mg·L−1时,大肠杆菌灭活率从4.71 log降至3.89 log。Cl−可以与体系中的自由基反应进而生成Cl·和Cl2•−等活性氯化物,虽然这些活性氯化物也具有一定氧化性,但其不仅会发生自分解反应[32-33],且灭活病原微生物的效率低于HO·和 RO·,因此,氯离子表现出抑制UV/PAA体系的灭活效率。
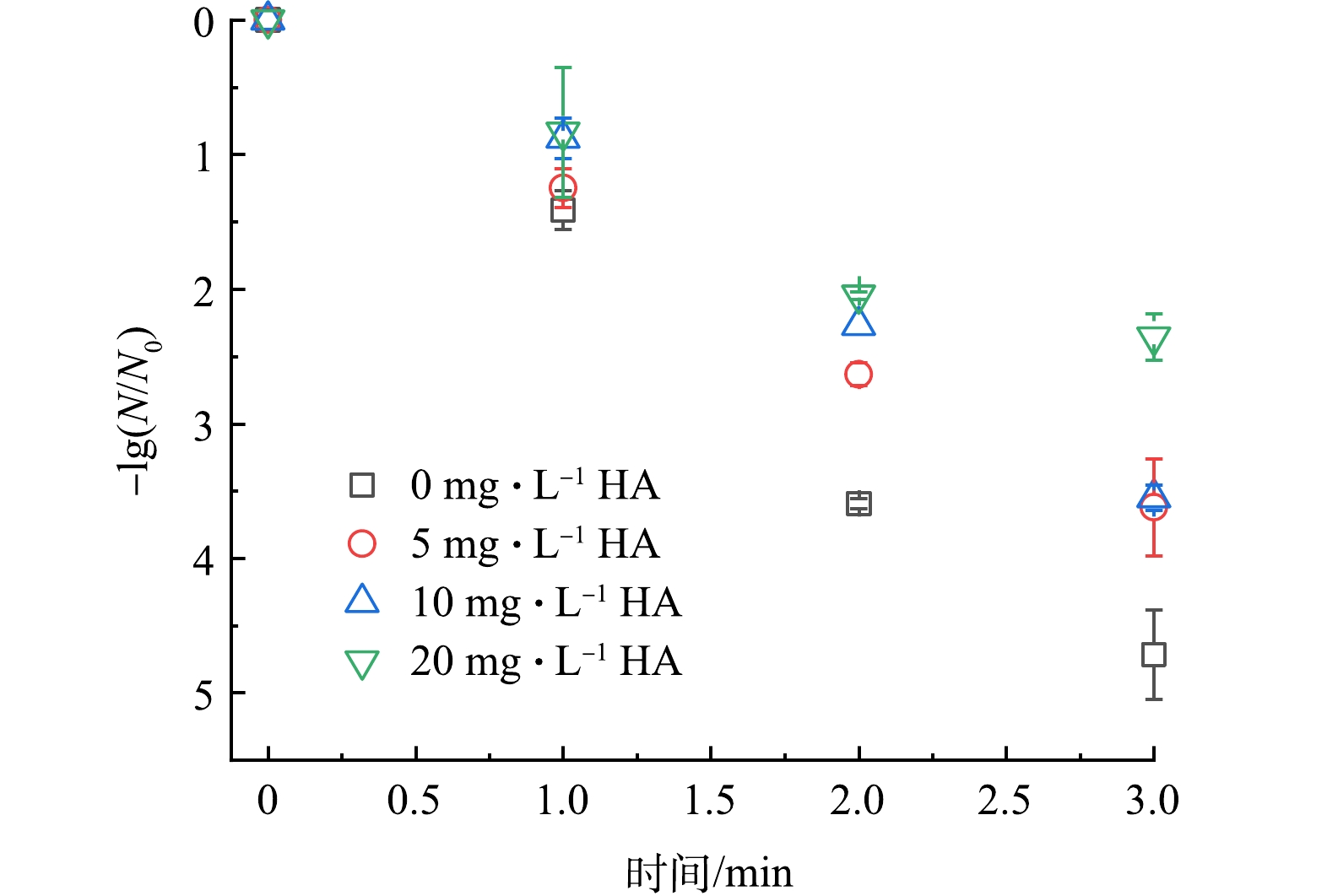
3) HA影响。HA对于UV/PAA体系灭活大肠杆菌的影响如图7所示。当溶液中HA质量浓度由0 提升至20 mg·L−1时,大肠杆菌灭活率由4.71 log降至2.36 log,说明HA对于UV/PAA体系的灭活效果产生了显著的抑制作用。主要原因是HA能够淬灭HO·和 RO· [34-35],其对应的反应速率常数分别为2.5×104 L·(mg·s)−1和5.76×104 L·(mg·s)−1,进而导致体系中与大肠杆菌作用的自由基减少,最终导致大肠杆菌灭活率下降。
-
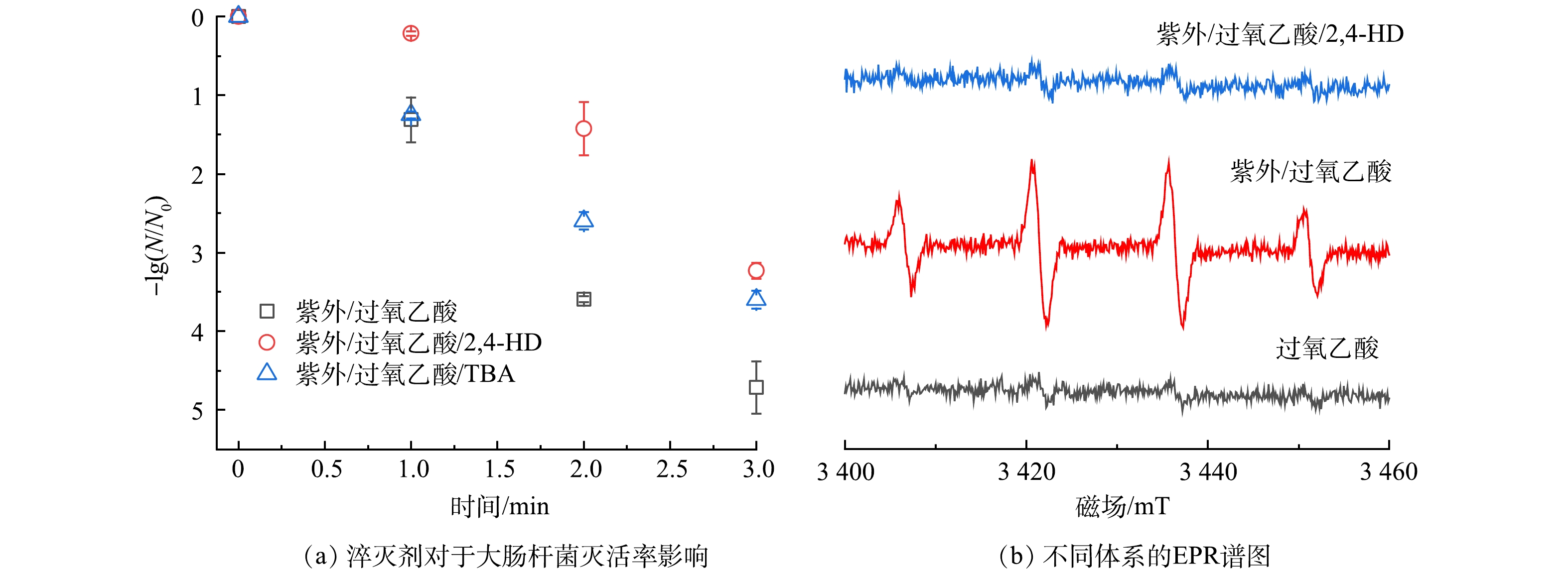
1)活性物种鉴定。现有研究表明UV/PAA体系中主要的活性物种为HO·和 RO· [17],为探究UV/PAA体系灭活大肠杆菌中主要作用的活性物种,采用了淬灭实验和EPR检测两种方式对于体系内的自由基进行了种类鉴定并分析了其贡献。
本实验采用叔丁醇(TBA)和己二烯(2,4-HD)2种物质作为淬灭剂,其中叔丁醇与HO·反应活性较高(k=6×108 L·(mol·s)−1),用作于HO·的淬灭剂,而2,4-HD与HO·(k=1×1010 L·(mol·s)−1)和RO·(k>5×108 L·(mol·s)−1)均有着很高的反应活性,用作于HO·和RO·的淬灭剂[17]。反应溶液中TBA和2,4-HD的浓度为1 mmol·L−1,远高于PAA的浓度(60 μmol·L−1),可认为反应中自由基被完全淬灭[13,36]。
如图8(a)所示,分别加入2种淬灭剂后,UV/PAA体系对大肠杆菌的灭活率均有所下降。其中加入TBA淬灭HO·后体系灭活率降至3.60 log,加入2,4-HD淬灭HO·及RO·后体系灭活率降至3.23 log,相比不加入淬灭剂时分别降低了1.11 log和1.48 log。以上结果表明体系内的HO·和RO·均对大肠杆菌灭活有贡献。
为更直观鉴定体系内自由基种类,采用DMPO作为捕获剂,利用EPR检测体系内自由基与DMPO的加合物。单独UV体系中一般不产生高浓度自由基[37],故本实验中检测了PAA、UV/PAA、UV/PAA/2,4-HD体系中产生的自由基加合物,结果如图8(b)所示。UV/PAA体系中出现了HO·和DMPO加合物的特征峰,而在PAA和UV/PAA/2,4-HD体系中无特征峰信号。说明单独PAA体系中不会产生自由基。UV活化PAA能够产生HO·,这与加入TBA淬灭HO·后体系灭活率下降的结果一致,表明HO·参与大肠杆菌的灭活。RO·无法直接检测到,可能的原因是由于体系内RO·稳态浓度较低且不稳定会进一步反应生成二级自由基,难以通过DMPO直接捕获,AO等[38]也发现类似的结果。但加入2,4-HD淬灭HO·和RO·后体系灭活率较加入TBA(淬灭HO·)后降低更明显,可从侧面说明RO·也参与了大肠杆菌灭活,且体系生成的HO·也可与PAA进一步反应生成RO•[39],体系中能直接检测到HO·表明有RO·共存。以上结果表明UV/PAA体系中HO·和 RO·对大肠杆菌灭活均有贡献。
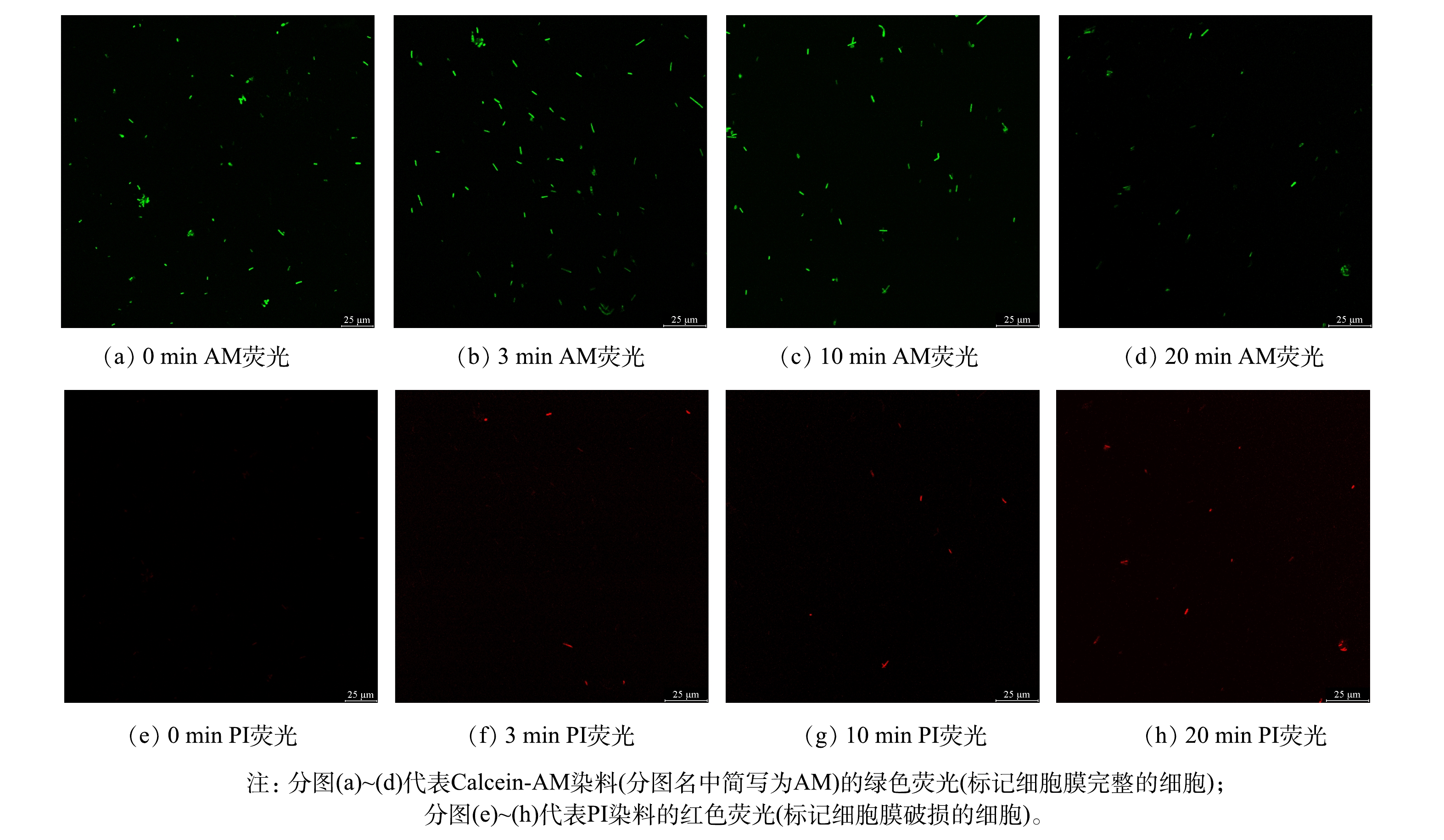
2)细胞膜完整性鉴定。为判断反应过程中大肠杆菌细胞膜完整性,采用Calcein-AM/PI两种荧光染料对处理前后的大肠杆菌进行染色后在激光共聚焦显微镜下观察荧光,其具体原理如下:Calcein-AM染料能穿透活细胞的细胞膜,与活细胞内的酯酶反应形成非渗透性分子Calcein,滞留细胞内发出绿色荧光,用于表征细胞膜完整的活细胞;PI染料能穿透细胞膜破损区域,与细胞DNA结合发出红色荧光,用于表征细胞膜破损的细胞,二者共同染色能对同时细胞膜完整和破损的细胞进行标记。激光共聚焦显微镜下观察显示绿色荧光的为细胞膜完整的细胞,显示红色荧光的为细胞膜破损的细胞,观察结果如图9所示。
结果表明,反应开始时,所有细胞均能维持其细胞膜完整性,反应时间3 min时仅有少量细胞细胞膜破损,而灭活实验结果表明此时溶液内几乎所有大肠杆菌均能被灭活,这与电化学/过氧乙酸和超声/过氧乙酸等体系中的现象不同[36,40]。为探究体系内大肠杆菌细胞灭活原因是否为细胞膜完整性被破坏,对反应时间10 min和20 min时大肠杆菌的细胞膜完整性进行了表征,此时的大肠杆菌可认为被完全灭活。结果表明即使反应时间延长至10 min也仅有少量细胞细胞膜破坏,反应时间20 min时虽然细胞膜破损的细胞数量有所增加但仍有大部分细胞能维持其细胞膜完整性。上述结果说明紫外/过氧乙酸体系灭活大肠杆菌并不以破坏其细胞完整性为主要机制,而反应过程中造成的细胞膜破坏可能是来自于自由基对于细胞膜中脂质的攻击[25]。包括大肠杆菌在内的部分微生物经灭活后不能在培养基上培养繁殖,但仍能保持一定活性和细胞膜完整性,被称为VBNC(viable but non-culturable)状态[41],结合实验结果说明本实验中部分大肠杆菌经灭活后会进入VBNC状态,虽然不能在固体培养基上培养,但仍保持一定的细胞活性和细胞膜完整性。
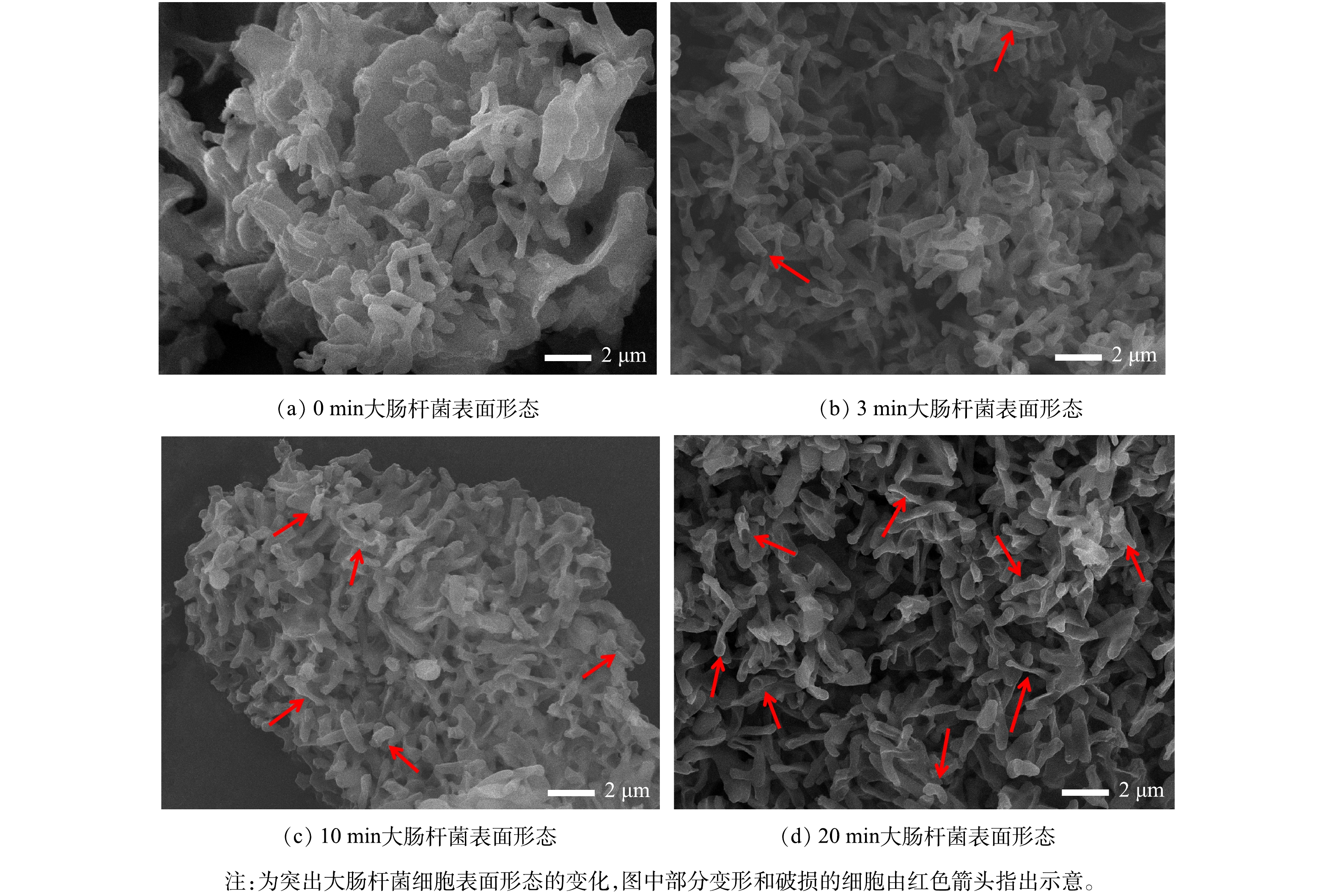
3)细胞表面形态鉴定。为进一步探究反应过程中大肠杆菌表面形态的变化,采用扫描电子显微镜拍摄了反应时间0、3、10、20 min时大肠杆菌表面形态,分别如图10(a)~(d)所示。扫描电镜图片显示,反应时间0 min时大肠杆菌细胞膜表面光滑,菌体饱满呈杆状;反应时间3 min时大部分大肠杆菌仍能保持原始饱满形态,少部分大肠杆菌细胞膜皱缩变形,个别大肠杆菌细胞膜出现破损;反应时间10 min时绝大部分大肠杆菌菌体变形,细胞膜出现皱缩,少量大肠杆菌细胞膜出现破损;反应时间20 min时细胞变形和细胞膜皱缩的程度更强,细胞膜出现破损的细胞数量继续增加。随着反应时间延长,变形破损的细胞数量逐渐增加,与激光共聚焦显微镜中观察到PI染料红色荧光随反应延长而增加的现象相对应。但破损的大肠杆菌细胞未完全裂解为碎片,说明UV/PAA体系能够对细胞膜结构造成一定损伤,但无法使大肠杆菌完全裂解,这与激光共聚焦显微镜表征得到的结论一致。
4)荧光光谱特性变化。通过荧光光谱分析溶液中大肠杆菌细胞有机物释放,如下图11所示,其中图11(a)~(e)分别为反应溶液分别在0、3、5、10、20 min的荧光光谱,图11(f)为各反应时间荧光光谱的分区荧光体积积分。由图11(a)可以看出,反应溶液的荧光光谱在Ⅳ区有较强的荧光峰,说明溶液中主要的有机物为溶解性微生物代谢产物;反应3 min后的溶液中Ⅳ区荧光强度相较于0 min降低了28.1%,说明大量溶解性微生物代谢产物被降解,原因可能为自由基与硫基团有着较强的反应活性,优先氧化降解类蛋白物质[15];反应5 min后Ⅳ区荧光强度较3 min上升了5.7%,可能为反应过程中大肠杆菌细胞破损导致胞内物质的释放;当反应时间延续至10 min和20 min时,Ⅳ区荧光强度持续增强,说明大肠杆菌细胞膜结构被破坏导致胞内有机物释放,Ⅲ区和Ⅴ区内荧光强度在反应20 min后显著增强,相较5 min时分别增加了43.3%和204.4%,说明在氧化活性物质作用下溶液中类蛋白大分子有机物向类腐殖酸和类富里酸等小分子类有机物转变[42]。反应20 min后,总荧光峰强度呈现先降后升的趋势,表明大肠杆菌释放的有机物先被迅速氧化降解,之后随大肠杆菌细胞膜结构破坏进而释放有机物,释放的大分子类蛋白有机物可被进一步氧化降解为小分子有机物。
-
1) UV/PAA体系能实现大肠杆菌高效灭活,在PAA浓度60 μmol·L−1和紫外光强2.25×10−7 Einstein·(s·L)−1的条件下反应3 min达到4.71 log灭活率,且灭活率随过氧乙酸浓度和紫外线剂量增加而提高。
2) NH4+和Cl−对于UV/PAA体系灭活大肠杆菌有轻微的抑制作用,而HA对于UV/PAA体系灭活大肠杆菌产生明显的抑制作用。
3) 在UV/PAA体系灭活大肠杆菌中起主要作用的自由基包括HO·和RO·。
4) UV/PAA体系灭活大肠杆菌过程能够对其细胞膜产生损伤,但不能使其细胞完全裂解,胞内有机物随细胞膜破损而释放,大分子类蛋白有机物最终被氧化为小分子类腐殖酸有机物。
紫外/过氧乙酸灭活大肠杆菌的效能及机理
Inactivation of Escherichia coli by peracetic acid combined with ultraviolet: Efficiency and mechanism
-
摘要: 病原微生物对公共卫生及生态系统产生了极大威胁,为研究紫外/过氧乙酸体系(UV/PAA)的消毒效能,以大肠杆菌为目标微生物,考察了PAA和UV投加剂量、不同背景物质对大肠杆菌灭活的影响,检测了体系中生成的活性物种并观察了反应过程中细胞形态变化。结果表明:在PAA浓度为60 μmol·L−1和UV光强为2.25×10−7 Einstein·(s·L)−1的条件下反应3 min后,大肠杆菌的灭活率能达到4.71 log,相较单独PAA体系和单独UV体系分别提高了2.76 log和0.82 log,且灭活率随PAA浓度和UV辐射剂量增加而提高。NH4+和Cl−对于UV/PAA体系灭活大肠杆菌有轻微的抑制作用,而腐殖酸(HA)对于体系有较强的抑制作用。自由基淬灭实验和电子顺磁共振检测结果表明,UV/PAA体系灭活大肠杆菌的主要活性物质为羟基自由基(HO·)和有机自由基(RO·)。激光共聚焦显微镜和扫描电子显微镜观察的结果表明,UV/PAA体系灭活大肠杆菌时,会引起细胞膜轻微破损,但并不会导致严重的裂解。反应溶液中溶解性成分的荧光光谱分析表明大肠杆菌灭活时释放的有机物会随着细胞膜破损而增加,但释放的类蛋白大分子物质最终可被氧化为类腐殖酸小分子有机物。Abstract: Pathogens pose great risks to public health and ecosystems. In this study, we investigated the efficiency and mechanism of the UV/peracetic acid (UV/PAA) process in inactivating Escherichia coli (E. coli) which served as the reference microorganism. The effects of PAA, UV dosages and water matrices on the inactivation rates of E. coli were systematically assessed, the generated reactive species were detected and the changes in cell morphology during inactivation were observed. Results showed that with a PAA concentration of 60 μmol·L−1 and UV intensity of 2.25×10−7 Einstein·(s·L)−1, the inactivation rate of E. coli reached 4.71 log after a 3 min reaction. An increase of 2.76 log and 0.82 log could be realized compared with that in the PAA alone and UV alone processes, respectively. Then, elevating the PAA concentrations and UV irradiation dosages would promote the inactivation efficiency. The inactivation rates of E. coli under UV/PAA were slightly inhibited by NH4+ and Cl−, while humic acid (HA) exhibited a strong inhibitory effect. Moreover, the quenching experiments and electron paramagnetic resonance detection suggested that both hydroxyl (HO·) and organic radicals (RO·) contributed to the inactivation of E. coli. The observation using a confocal laser scanning microscope and a scanning electron microscope revealed that the inactivation of E. coli under UV/PAA caused slight damage to the cell membrane but did not result in complete cell integrity loss. Although the results from the fluorescence excitation-emission matrices analysis suggested the release of bacterial organic matter increased with cell membrane damage in the UV/PAA process, the released protein-like components with high molecular weight could be subsequently oxidized into humic acids-like components with low molecular weight.
-
Key words:
- ultraviolet /
- peracetic acid /
- Escherichia coli /
- disinfection /
- radicals
-

-
表 1 荧光体积积分法各区域代表物质
Table 1. Representative components in each region by fluorescence regional integration method
荧光区域 物质种类 激发波长(Ex)和
发射波长(Em)/nm代表性物质 Ⅰ 芳香类蛋白类物质Ⅰ Ex<250,Em<330 酪氨酸 Ⅱ 芳香类蛋白类物质Ⅱ Ex<250,330<Em <380 色氨酸 Ⅲ 类富里酸物质 Ex<250,Em >380 富里酸 Ⅳ 溶解性微生物代谢产物 Ex>250,Em<380 蛋白质 Ⅴ 类腐殖酸物质 Ex>250,Em>380 腐殖酸 -
[1] CHHETRI R K, BONNERUP A, ANDERSEN H R. Combined sewer overflow pretreatment with chemical coagulation and a particle settler for improved peracetic acid disinfection[J]. Journal of Industrial and Engineering Chemistry, 2016, 37: 372-379. doi: 10.1016/j.jiec.2016.03.049 [2] HASSABALLAH A H, BHATT T, NYITRAI J, et al. Inactivation of E. coli, Enterococcus spp., somatic coliphage, and Cryptosporidium parvum in wastewater by peracetic acid (PAA) , sodium hypochlorite, and combined PAA-ultraviolet disinfection[J]. Environmental Science Water Research & Technology, 2020, 6, 197. [3] VITZILAIOU E, KURIA A M, SIEGUMFELDT H, et al. The impact of bacterial cell aggregation on UV inactivation kinetics[J]. Water Research,2021, 204: 117593. doi: 10.1016/j.watres.2021.117593 [4] LIU S, QU H, YANG D, et al. Chlorine disinfection increases both intracellular and extracellular antibiotic resistance genes in a full-scale wastewater treatment plant[J]. Water Research, 2018, 136: 131-136. doi: 10.1016/j.watres.2018.02.036 [5] RAJAB M, HEIM C, LETZEL T, et al. Electrochemical disinfection using boron-doped diamond electrode–The synergetic effects of in situ ozone and free chlorine generation[J]. Chemosphere,2015, 121: 47-53. doi: 10.1016/j.chemosphere.2014.10.075 [6] SUN P, ZHANG T, MEJIA-TICKNER B, et al. Rapid disinfection by peracetic acid combined with UV irradiation[J]. Environmental Science & Technology Letters,2018, 5(6): 400-404. [7] LI G, WANG W, HUO Z, et al. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli[J]. Water Research,2017, 126: 134-143. doi: 10.1016/j.watres.2017.09.030 [8] LUUKKONEN T, PEHKONEN S O. Peracids in water treatment: A critical review[J]. Critical Reviews in Environmental Science and Technology,2017, 47(1): 1-39. doi: 10.1080/10643389.2016.1272343 [9] FRAISE A P, MAILLARD J-Y, SATTAR S A. Russell, Hugo & Ayliffe's principles and practice of disinfection preservation and sterilization[M]. Oxford: Wiley-Blackwell, 2013. [10] MANOLI K, SARATHY S, MAFFETTONE R, et al. Detailed modeling and advanced control for chemical disinfection of secondary effluent wastewater by peracetic acid[J]. Water Research, 2019, 153: 251-262. doi: 10.1016/j.watres.2019.01.022 [11] KOIVUNEN J, HEINONEN-TANSKI H. Peracetic acid (PAA) disinfection of primary, secondary and tertiary treated municipal wastewaters[J]. Water Research, 2005, 39(18): 4445-4453. doi: 10.1016/j.watres.2005.08.016 [12] STRAUS D L, MEINELT T, FARMER B D, et al. Peracetic acid is effective for controlling fungus on channel catfish eggs[J]. Journal of Fish Diseases, 2012, 35(7): 505-511. doi: 10.1111/j.1365-2761.2012.01383.x [13] LIN W, ZUO J, LI K, et al. Pre-exposure of peracetic acid enhances its subsequent combination with ultraviolet for the inactivation of fungal spores: Efficiency, mechanisms, and implications[J]. Water Research, 2023, 229: 119404. doi: 10.1016/j.watres.2022.119404 [14] LEAPER S. Synergistic killing of spores of Bacillus subtilis by peracetic acid and alcohol[J]. International Journal of Food Science & Technology, 1984, 19(3): 355-360. [15] MCFADDEN M, LOCONSOLE J, SCHOCKLING A J, et al. Comparing peracetic acid and hypochlorite for disinfection of combined sewer overflows: Effects of suspended-solids and pH[J]. Science of the Total Environment, 2017, 599-600: 533-539. doi: 10.1016/j.scitotenv.2017.04.179 [16] TUROLLA A, SABATINO R, FONTANETO D, et al. Defence strategies and antibiotic resistance gene abundance in enterococci under stress by exposure to low doses of peracetic acid[J]. Chemosphere, 2017, 185: 480-488. doi: 10.1016/j.chemosphere.2017.07.032 [17] ZHANG T, HUANG C. Modeling the kinetics of UV/peracetic acid advanced oxidation process[J]. Environmental Science & Technology, 2020, 54(12): 7579-7590. [18] HOLLMAN J, DOMINIC J A, ACHARI G. Degradation of pharmaceutical mixtures in aqueous solutions using UV/peracetic acid process: Kinetics, degradation pathways and comparison with UV/H2O2[J]. Chemosphere, 2020, 248: 125911. doi: 10.1016/j.chemosphere.2020.125911 [19] PARK E, LEE C, BISESI M, et al. Efficiency of peracetic acid in inactivating bacteria, viruses, and spores in water determined with ATP bioluminescence, quantitative PCR, and culture-based methods[J]. Journal of Water and Health. 2014, 12(1): 13-23. doi: 10.2166/wh.2013.002 [20] BEBER DE SOUZA J, QUEIROZ V F, JERANOSKI R F, et al. Water and wastewater disinfection with peracetic acid and UV radiation and using advanced oxidative process PAA/UV[J]. International Journal of Photoenergy, 2015, 2015: 1-7. [21] VINET L, ZHEDANOV A. A‘missing’family of classical orthogonal polynomials[J]. Journal of Physics A: Mathematical and Theoretical, 2011, 44(8): 85201. [22] WANG Z, WANG J, XIONG B, et al. Application of cobalt/peracetic acid to degrade sulfamethoxazole at neutral condition: Efficiency and mechanisms[J]. Environmental Science & Technology, 2020, 54(1): 464-475. [23] RAHN R O. Potassium iodide as a chemical actinometer for 254 nm radiation: Use of iodate as an electron scavenger[J]. Photochemistry and Photobiology, 1997, 66(4): 450-455. doi: 10.1111/j.1751-1097.1997.tb03172.x [24] LIN Q S, DONG X L, XI S H, et al. Optimizing waste activated sludge disintegration by investigating multiple electrochemical pretreatment conditions: Performance, mechanism and modeling[J]. Science of the Total Environment,2023, 870: 162025. doi: 10.1016/j.scitotenv.2023.162025 [25] WAN Y, XIE P, WANG Z, et al. Comparative study on the pretreatment of algae-laden water by UV/persulfate, UV/chlorine, and UV/H2O2: Variation of characteristics and alleviation of ultrafiltration membrane fouling[J]. Water Research, 2019, 158: 213-226. doi: 10.1016/j.watres.2019.04.034 [26] BAI M, TIAN Y, YU Y, et al. Application of a hydroxyl-radical-based disinfection system for ballast water[J]. Chemosphere, 2018, 208: 541-549. doi: 10.1016/j.chemosphere.2018.06.010 [27] KOIVUNEN J, HEINONEN-TANSKI H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments[J]. Water Research,2005, 39(8): 1519-1526. doi: 10.1016/j.watres.2005.01.021 [28] LUUKKONEN T, HEYNINCK T, RÄMÖ J, et al. Comparison of organic peracids in wastewater treatment: Disinfection, oxidation and corrosion[J]. Water Research. 2015, 85: 275-285. doi: 10.1016/j.watres.2015.08.037 [29] 杨敏, 尚巍, 李鹏峰, 等. 城镇污水处理厂次氯酸钠消毒影响因素及优化研究[J]. 中国给水排水, 2022, 38(9): 76-81. doi: 10.19853/j.zgjsps.1000-4602.2022.09.012 [30] 冯璁, 林莉, 李青云. 氯离子浓度与电流密度对电解抑制铜绿微囊藻生长的影响[J]. 长江科学院院报, 2015, 32(6): 53-58. [31] 刘伟, 蔡广强, 张金松, 等. 南方某市水源水蛋白质、腐殖酸浓度变化与去除[J]. 中国给水排水, 2017, 33(17): 41-45. doi: 10.19853/j.zgjsps.1000-4602.2017.17.010 [32] SHAH A D, LIU Z, SALHI E, et al. Peracetic acid oxidation of saline waters in the absence and presence of H2O2 secondary oxidant and disinfection byproduct formation[J]. Environmental Science & Technology, 2015, 49(3): 1698-1705. [33] ZHANG L, LIU Y, FU Y. Degradation kinetics and mechanism of diclofenac by UV/peracetic acid[J]. RSC Advances, 2020, 10(17): 9907-9916. doi: 10.1039/D0RA00363H [34] CHEN S, CAI M, LIU Y, et al. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process[J]. Water Research, 2019, 150: 153-161. doi: 10.1016/j.watres.2018.11.044 [35] XIE P, MA J, LIU W, et al. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals[J]. Water Research, 2015, 69: 223-233. doi: 10.1016/j.watres.2014.11.029 [36] 蒋励铭, 卞静, 张晓晖, 等. 电化学活化过氧乙酸灭活水中大肠杆菌[J]. 中国环境科学, 2023, 43(8): 3966-3973. doi: 10.3969/j.issn.1000-6923.2023.08.012 [37] ZHANG B, LI W, ZHANG H, et al. Activation of peracetic acid by trace ferrous ion and vacuum ultraviolet for the ultrafast degradation of PPCPs[J]. ACS ES& T Water, 2022, 2(12): 2590-2601. [38] AO X W, WANG W, SUN W, et al. Degradation and transformation of norfloxacin in medium-pressure ultraviolet/peracetic acid process: An investigation of the role of pH[J]. Water Research, 2021, 203: 117458. doi: 10.1016/j.watres.2021.117458 [39] AO X W, JUSSI E, HUANG C H, et al. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: A review[J]. Water Research, 2021, 188: 116479. doi: 10.1016/j.watres.2020.116479 [40] BAI Y, SHI C, ZHOU Y, et al. Enhanced inactivation of Escherichia coli by ultrasound combined with peracetic acid during water disinfection[J]. Chemosphere,2023, 322: 138095. doi: 10.1016/j.chemosphere.2023.138095 [41] CHEN S, LI X, WANG Y H, et al. Induction of Escherichia coli into a VBNC state through chlorination/chloramination and differences in characteristics of the bacterium between states[J]. Water Research, 2018, 142: 279-288. doi: 10.1016/j.watres.2018.05.055 [42] FANG J, YANG X, MA J, et al. Characterization of algal organic matter and formation of DBPs from chlor (am) ination[J]. Water Research, 2010, 44(20): 5897-5906. doi: 10.1016/j.watres.2010.07.009 -



 下载:
下载: