-
城市污水处理产生的污泥中富含大量有机质、无机物、重金属和病原体[1]。传统污泥处置方法如填埋、焚烧和土地利用等存在污染扩散或二次污染等缺陷,如何妥善处置污泥受到社会各界的广泛关注。将污泥制备成为功能型生物质炭 (biochar) 是实现污泥资源化的有效方法[2-3]。XIAO等[4]利用热碱预处理污泥残余固体,通过热解制备的biochar对阳离子红X-GRL最高吸附容量可达46.70 mg·g−1;ATHALATHIL等[5]通过水热法制备biochar/TiO2 纳米光催化剂,紫外光照反应60 min后可去除近36%的双酚A。
层状双氢氧化物 (layered double hydroxides,LDH) 由二价和三价金属的氧八面体构成的带正电的二维板层与层间的阴离子和H2O组成。LDH的化学式一般表示为[(M2+)1-x(M3+)x (OH)2]x+(An−)x/n·mH2O,其中,M2+为二价离子 (如Zn2+、Cu2+或Mg2+等) ,M3+为三价离子 (如Fe3+、Al3+或Cr3+等) ,An-为层间阴离子 (如Cl−、NO3−或SO42-等) ,x为M2+/(M2++M3+) 的比值,其范围为 0.17到0.33。 LDH经热处理后得到的层状双氧化物 (layered double oxide,LDO) 不仅比表面积显著增大、结构更稳定,而且其在水溶液中通过 "记忆效应"可恢复至LDH结构[6-7]。尽管LDO具有优异的阴离子交换性能和高比表面积,但在水溶液中化学稳定性仍有待提高,尤其是当溶液pH小于4时LDO因为溶解而导致吸附性能变差[8]。将生物炭与LDO复合,可以有效提高LDO的化学稳定性,同时LDO与生物炭之间的协同作用可形成更丰富的活性位点[9],提高生物炭和LDO的吸附能力[10-11]。各种LDO与生物炭复合吸附材料已经被广泛用于各种有机污染物[9]。WANG等[11]采用共沉淀和高温碳化法,以玉米芯为原料通过外加金属镍盐和铝盐制备MC1/NiAl-LDO吸附剂,50 °C下对a-啶橙的最大吸附容量为116.30 mg·g−1。MEILIA等[12]通过热解和浸渍法制备木质biochar-ZnFe2O4复合材料,锌铁摩尔比为1∶4条件下所制备的复合吸附剂对扑热息痛的吸附容量为17.66 mg·g−1。目前,有关LDO/biochar复合材料的制备过程中,往往需要额外添加金属盐作为LDO的原料,通过原位利用生物质中的金属离子制备LDO/biochar复合材料可有效降低制备成本。
铝系混凝剂在污泥废水处理和市政污泥调理过程中不断富集,使得污泥中的铝含量较高。因此,将污泥中的碳和铝元素固定并利用是提高污泥资源化效率的有效手段。本研究以含铝污泥为原料,通过外加镁盐形成共沉淀,结合热处理工艺原位制备镁铝层状双氧化物/生物炭 (MgAl LDO@biochar) 复合材料;基于对LDO@biochar的结构和组成分析,将其作为吸附剂材料应用于有机污染物的吸附去除,并阐释其吸附机理,以期为市政污泥资源化利用与高性能环境功能材料制备提供参考。
-
七水合硫酸镁 (MgSO4·7H2O) 、无水碳酸钠 (Na2CO3) 、氢氧化钠 (NaOH) 和磺胺 (C6H8N2O2S) 均为分析纯;刚果红、亚甲基蓝、四环素和酸性橙II的纯度分别为95%、82%、98%和85%。实验用水为去离子水。
所用污泥为安徽省六安市城北污水处理厂含水率60%的脱水污泥,将其置于120 ℃烘箱中干燥24 h后过筛收集粒径小于0.125 mm的粉末。采用铵根离子置换法确定污泥粉末的阳离子交换容量[13],采用X射线荧光光谱仪 (XRF, XRF-1800, 日本) 对污泥粉末中Al、Fe和重金属元素进行定量分析。污泥粉末的基本理化性质如表1所示。
-
采用比表面积分析仪 (Kubo-1108, 北京彼奥德电子技术有限责任公司) 对样品比表面积进行分析;采用X射线衍射仪 (PANalytical Empyrean, 荷兰帕纳科公司) 测试样品的晶型结构;采用X射线光电子能谱仪 (Thermo ESCALAB250Xi, 赛默飞世尔科技公司) 和傅里叶变换红外光谱仪 (Thermo Nicolet IS50 iN10, 赛默飞世尔科技公司) 分别测试样品表层原子或离子的组成或状态和表面官能团;采用扫描电子显微镜 (Nano-S450, 赛默飞世尔科技公司) 表征样品微观形貌;采用电感耦合等离子体发射光谱仪 (AGILENT 725-ES, 安捷伦科技有限公司) 检测水样中的重金属质量分数。在进行结构表征之前,固体样品在60 ℃下充分干燥,以去除自由水。
-
分别称取一定质量 (4、6、8、16和32 g) 污泥粉末加入50 mL去离子水配置成悬浮液,将其与50 mL 浓度为1.47 mol·L−1的MgSO4·7H2O溶液混合,其中铝镁摩尔比分别为1∶8、1∶4、1∶2、1∶1.5和1∶1。将2.56 g NaOH和1.71 g Na2CO3溶于100 mL去离子水配置成混合碱溶液,并缓慢滴加到上述污泥悬浮液中,保持悬浊液pH在9.00至10.00范围内,将悬浊液体系在搅拌速率为400 r·min−1,加热温度为60 ℃下反应12 h后过滤,过滤所得的固体产物在60 ℃下自然陈化12 h后经研磨得到灰白色层状双氢氧化物/污泥粉末 (LDH@S) 。将LDH@S在氮气氛围下,以5 ℃·min−1的升温速率达到480 ℃后热解碳化2 h[14],冷却至室温,得到黑色LDO@biochar粉末。将污泥粉末经相同热处理可得到生物炭作为对照。
-
取200 mL 250 mg·L−1的污染物,加入0.1 g LDO@biochar,在30 ℃和pH 7.0的溶液中进行静态吸附实验。在吸附过程中,以600 r·min−1的速度进行磁力搅拌使吸附剂与污染物充分接触。定期取样并通过0.22 μm膜过滤后测定污染物浓度。利用可见分光光度计 (VIS-723N, 北京瑞利分析仪器有限公司) 对污染物刚果红、酸性橙II、罗丹明B和四环素的浓度进行测定,其中刚果红、酸性橙II、罗丹明B的最大吸收波长分别选取500、484和553 nm,四环素溶液与0.20 mol·L−1盐酸等体积混合后的最大吸收波长为355 nm。磺胺的浓度采用Waters高效液相色谱仪测定,其流动相为水和乙腈 (体积比为40∶60) 溶液,流速1.00 mL·min−1,色谱柱为SunFireTM C18柱 (150 mm×4.6 mm,5.00 μm) 。通过式(1)和式(2)计算污染物的吸附量和去除效率。
式中:qt为t时刻的吸附量,mg·g−1;C0和Ct分别为污染物初始质量浓度和t时刻的质量浓度,mg·L−1;V为溶液体积,L;m为吸附剂质量,g。对于污染物的吸附过程分别采用准一级和准二级吸附动力学模型进行拟合,公式见式(3)和式(4)。
式中:k1为准一级吸附速率常数,min−1;k2为准二级吸附速率常数,g·(mg·min)−1;qe 为平衡吸附容量,mg·g−1。
通过等温吸附实验研究不同温度和模式污染物刚果红初始质量浓度下LDO@biochar的吸附性能。分别在20、30和40 ℃将0.1 g吸附剂加入200 mL不同初始质量浓度 (50~500 mg·L−1) 的刚果红溶液中进行静态吸附,获得不同吸附条件下的平衡吸附容量qe。每组实验重复3次,计算平均值和标准偏差。
-
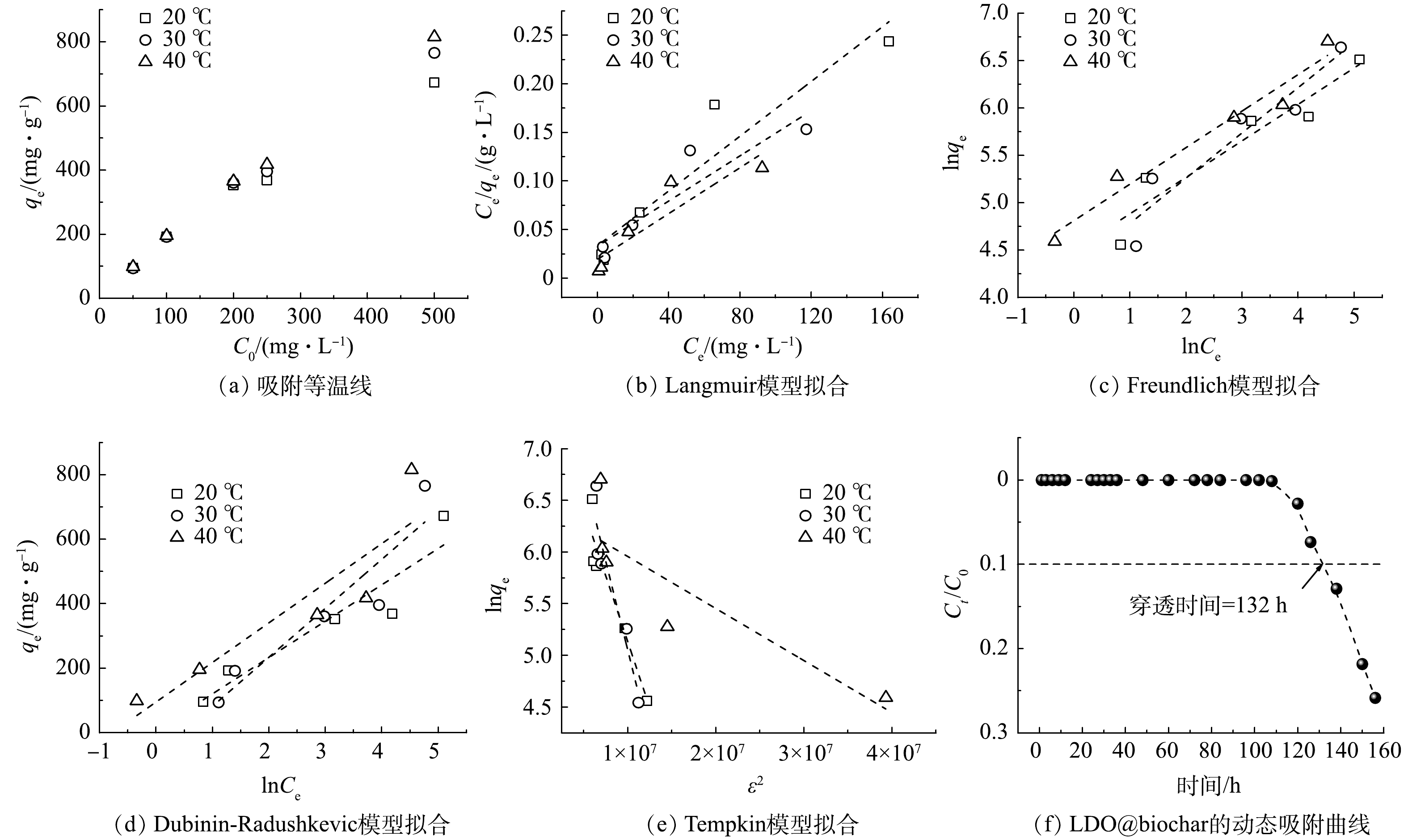
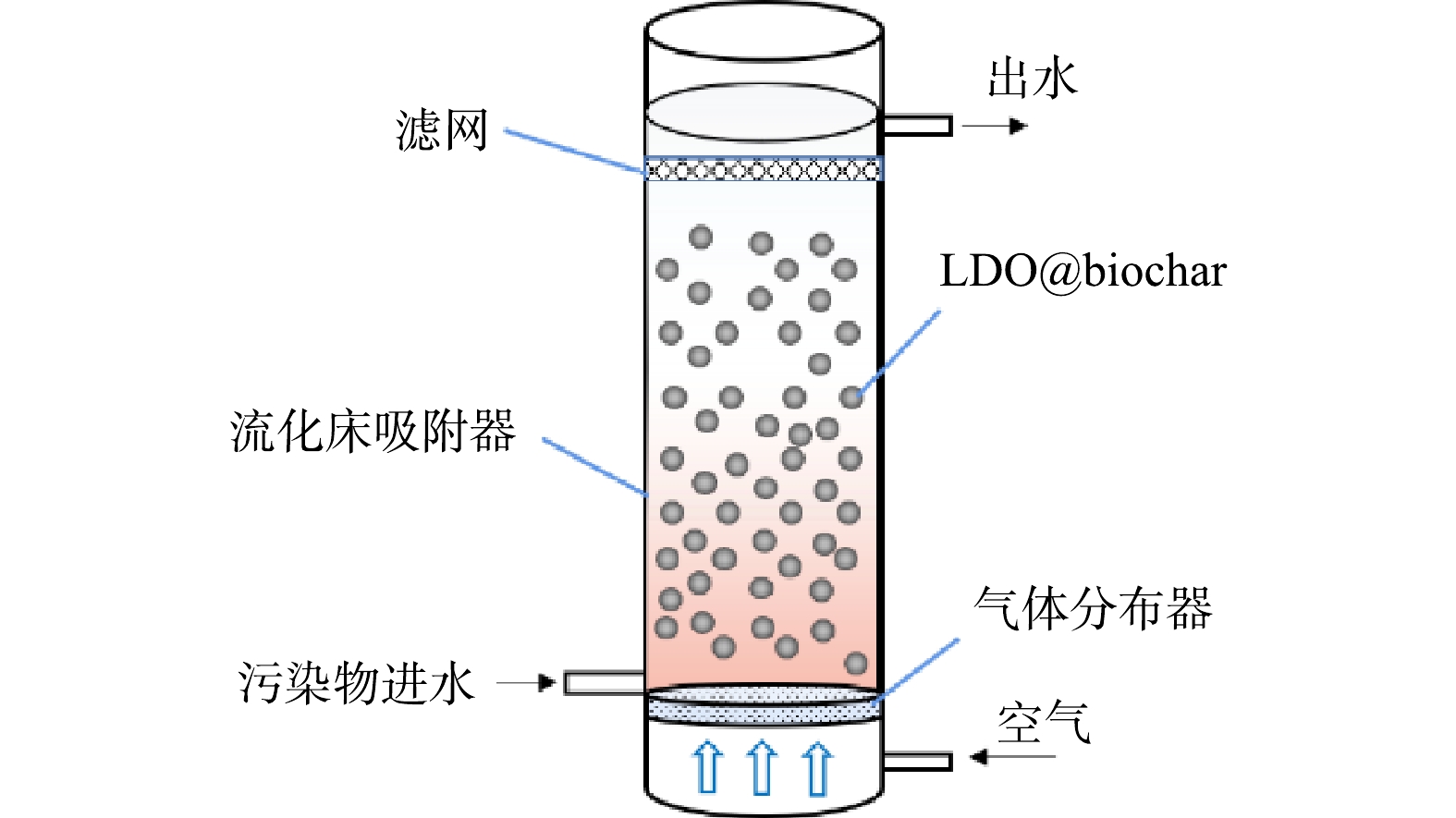
为评估LDO@biochar实际应用效果,构建流化床动态吸附装置在20 ℃恒温条件下对刚果红进行动态吸附实验,吸附装置如图1所示。在流化床反应器中加入2.00 g吸附剂,刚果红初始质量浓度 (C0) 为50 mg·L−1,流速为2.50 mL·min−1,空气流速为0.10 L·min−1。设定穿透质量浓度 (Ct) 为5 mg·L−1,即Ct/C0=0.1,所耗时间记为穿透时间。
-
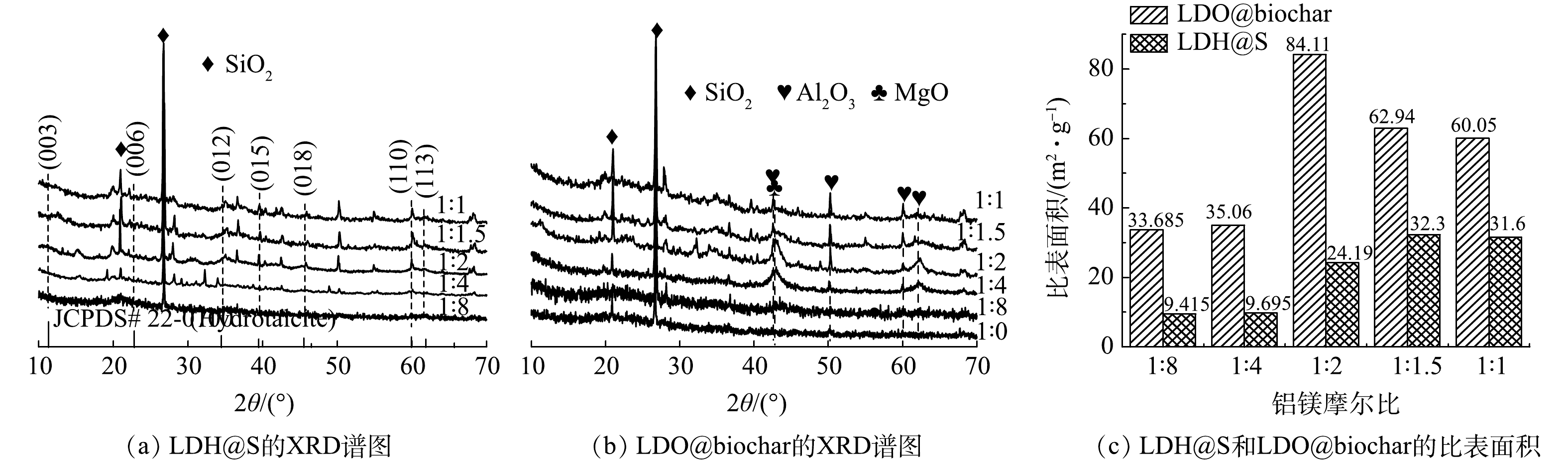
图2(a)是不同铝镁摩尔比条件下制备得到的LDH@S的XRD谱图。当铝镁摩尔比分别为1∶1、1∶1.5和1∶2时,相应XRD谱图2 θ=11.3°、 22.8°、34.5°、38.6°、 45.5°、60.0° 和 61.9°处出现的特征衍射峰分别对应于水滑石的 (003) 、 (006) 、 (012) 、 (015) 、 (018) 、 (110) 和 (113) 晶面 (JCPDS 22-0700) 。由于1∶4和1∶8的铝镁摩尔比不能满足LDH结构的形成条件,因此所制备材料的XRD谱图上没有发现水滑石特征峰。经热处理后,LDH@S转变为LDO@biochar。如图2(b)所示,LDO@biochar的XRD谱图中没有发现水滑石特征衍射峰。与此同时,MgO (JCPDS 45-0946) 和Al2O3 (JCPDS 04-0878) 特征衍射峰的出现表明LDH已经转变成为LDO。此外,2θ=21.0°~26.0°附近出现的驼峰是石墨碳 (002) 晶面的衍射峰[15],表明污泥中的有机质在热处理过程中发生碳化。随着制备过程中铝镁摩尔比的增加,MgO和Al2O3的特征衍射峰半峰宽呈现先减小后增大的趋势,当铝镁摩尔比为1∶2时,MgO和Al2O3衍射峰的半峰宽最小,其氧化物晶粒尺寸最大,可形成更多的吸附位点[16]。图2(c)是不同铝镁摩尔比条件下制备的LDH@S和LDO@biochar的比表面积对比图,可以看出,与前驱体LDH@S相比,LDO@biochar 的比表面积明显增加。
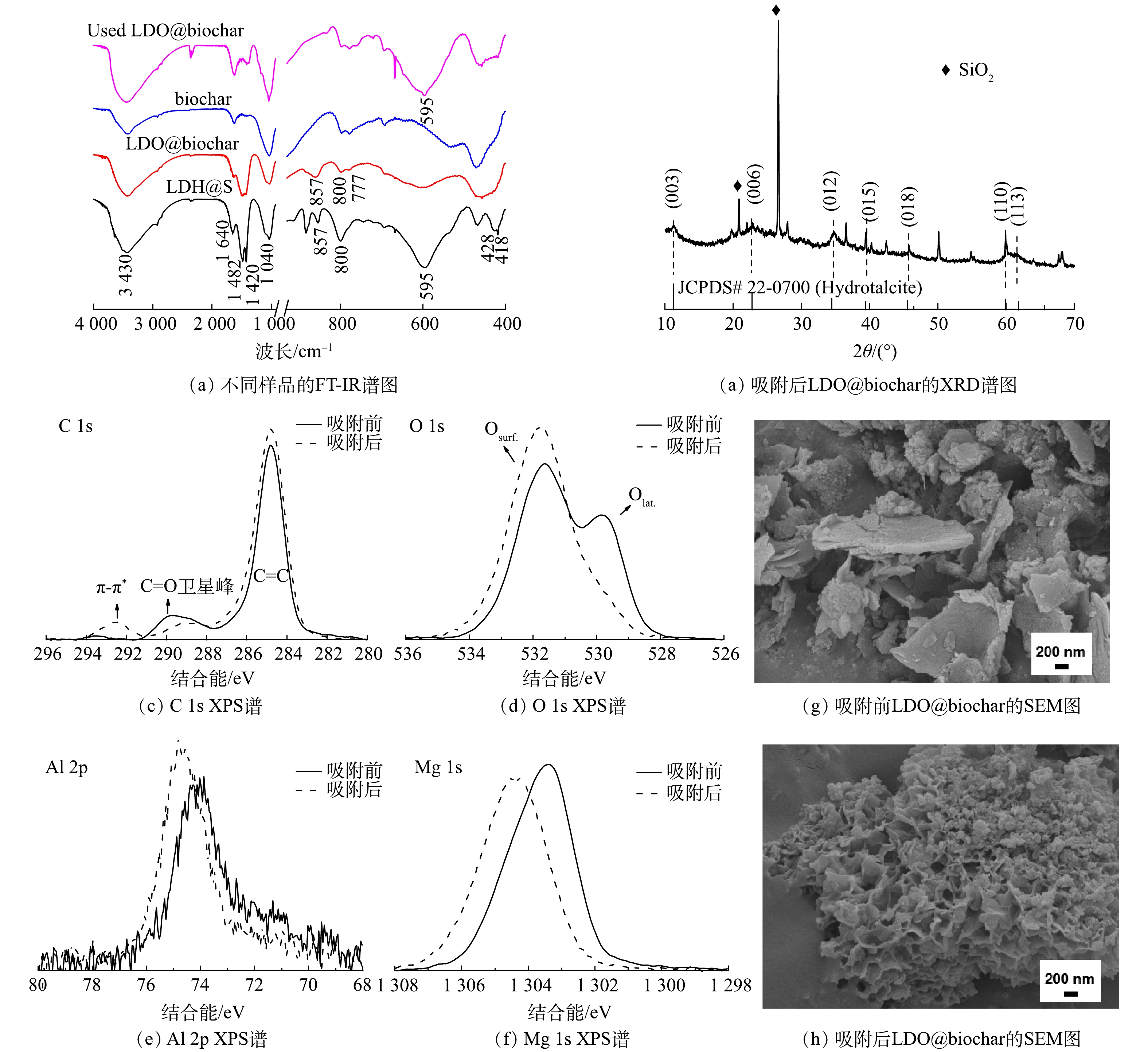
图3(a)为铝镁摩尔比为1∶2条件下所制备的LDH@S、LDO@biochar和biochar的FTIR谱图。对于biochar,其在1 640 cm−1的特征峰属于碳骨架的C=C伸缩振动,3 430和1 040 cm−1处的特征峰分别为O—H和C—O键,这些含氧基团在吸附过程中可以通过氢键作用与污染物结合[17]。对于LDH@S,其在3 430 cm−1左右的宽峰为层间水和羟基的O-H伸缩振动峰;1 482、1 420和857 cm−1处的峰归属于层间CO32-的伸缩振动,1 040 cm−1处为C—O的伸缩振动峰,800和777 cm−1处峰则归属于M—O的伸缩振动[18-19]。此外,595 cm−1处峰为水滑石的典型特征峰[20],428和418 cm−1处的双峰则是LDH板层[Mg, Al]八面体晶格O-M-O变形振动特征峰[21]。经热处理后的LDO@biochar,由于层间CO32-、水和羟基的部分脱除,其在FTIR谱图中的相应位置的特征峰强度均明显减弱;而595、428和418 cm−1处LDH特征峰强度的减弱则表明LDH结构在热处理过程中发生了改变。图3(a)同时展示了吸附刚果红后的LDO@biochar的FTIR谱图,可以发现595 cm−1、428和418 cm−1处水滑石的特征峰有所增强。如图3(b)的XRD谱图所示,对于使用后的LDO@biochar, 11.3°、22.8°、34.5°处则发现层状水滑石 (JCPDS 22-0700) (003) 、 (006) 和 (012) 晶面的特征衍射峰。结合FTIR和XRD表征结果,可知LDO@biochar在水溶液中由于“记忆效应”重构了LDH层状结构。图3(c)对比了吸附刚果红前后LDO@biochar的 C 1s XPS谱图。与未使用材料相比,吸附后材料的π-π*卫星峰的相对强度增加,表明吸附过程中吸附剂与吸附质之间发生了π-π共轭;C=O和C-O峰强度的减小则是由于其在吸附过程中作为活性位被消耗[22-23]。图3(d)中O 1s XPS谱图表明吸附刚果红过程中LDO@biochar中的晶格氧有所减少,同时 Mg 1s和Al 2p信号都转移到更高的结合能 (图3(e)和(f)) ,表明金属离子周围电子密度下降。这是由于LDO向LDH的转化造成金属氧化物解离所导致的[24]。普遍认为,具有较高比表面积的生物炭是LDO的理想载体[10]。如图3(g)所示,LDO@biochar复合材料中,biochar的表面覆盖了大量LDO纳米片。使用后 (图3(h)) , 相对分散的LDO纳米片转化成为LDH并在biochar上形成团簇结构。
-
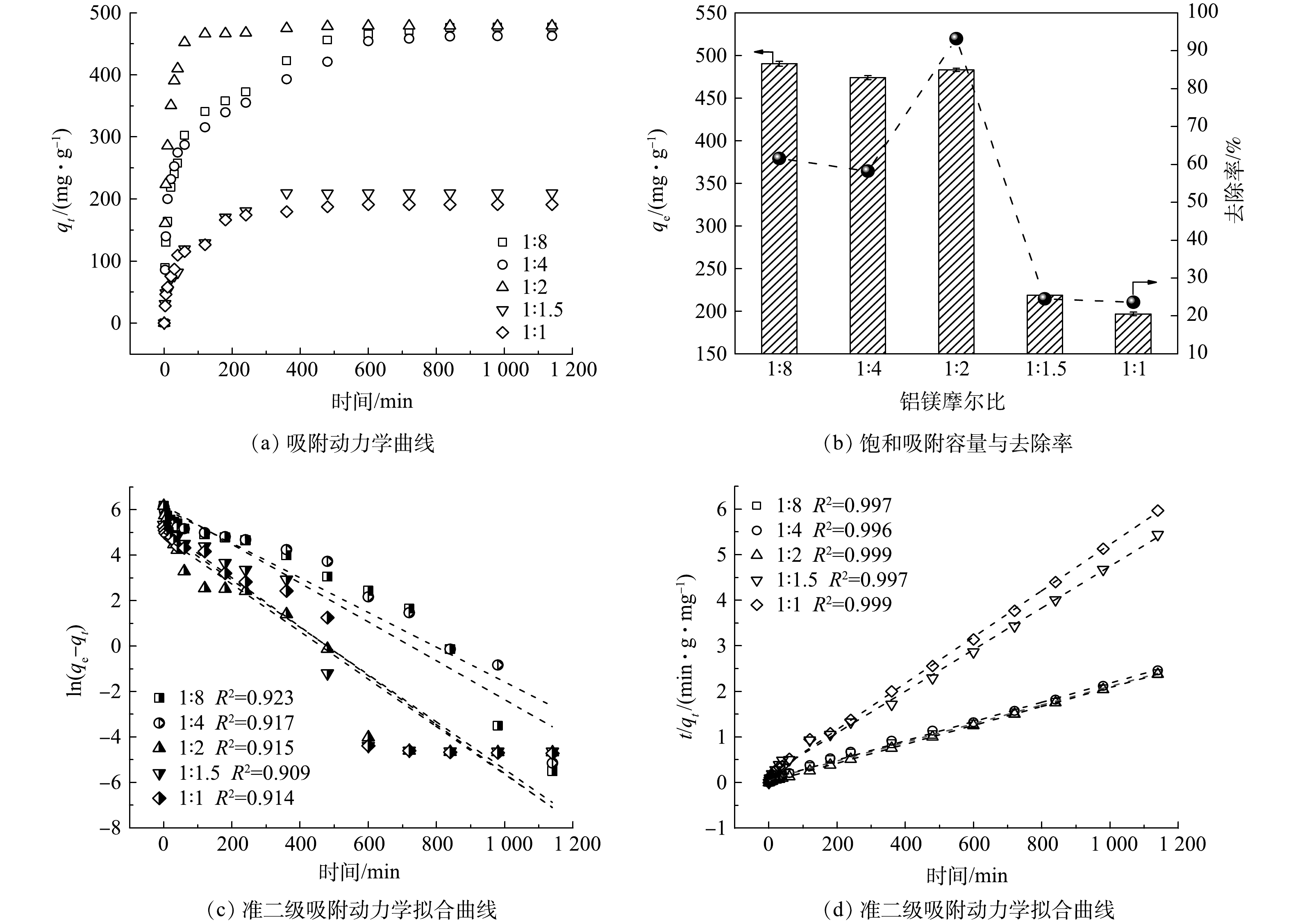
为优化LDO@biochar复合材料制备过程,对不同铝镁摩尔比条件下制备的LDO@biochar对刚果红的吸附动力学进行了研究。如图4(a)所示,随着铝镁摩尔比由1∶8增加到1∶2时,LDO@biochar对刚果红的平衡吸附容量不断增大,继续增加铝镁摩尔比,材料的平衡吸附容量反而下降。当铝镁摩尔比为1∶2时,所得材料的qe最大可达到477.46 mg·g−1。比较不同吸附剂对刚果红的去除效率发现 (图4(b)) ,铝镁摩尔比为1∶2时所制备的 LDO@biochar对刚果红的吸附速率最快,在1 h内可使其去除率达到93%。对于不同材料的吸附动力学拟合结果如图4(c)和(d)所示,准二级吸附动力学模型的相关系数 (R2) 高于准一级动力学模型,表明LDO@biochar对刚果红以化学吸附为主[25]。尽管不同铝镁摩尔比条件下所制备的材料qe值相近,但铝镁摩尔比为1∶2时所制备材料的k2 (3.87×10−4) 最高,表明其对刚果红具有最快的吸附速率。由图2可知,铝镁摩尔比为1∶2时所制备的LDO@biochar具有最大的比表面积和晶粒尺寸,可形成更多的吸附位点,因此获得最佳的吸附容量和吸附速率。
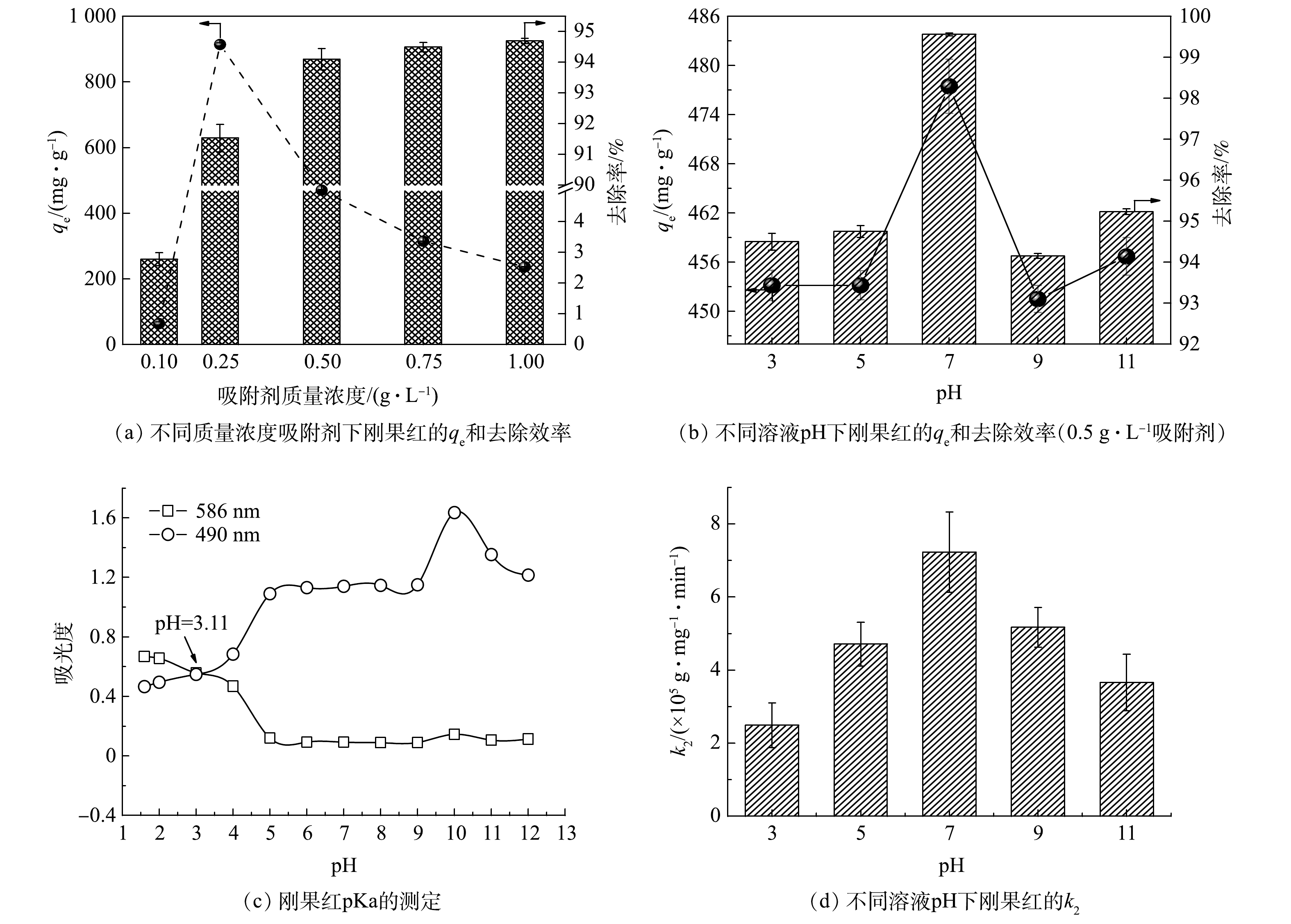
图5(a)展示了LDO@biochar质量浓度对刚果红的平衡吸附容量和去除率的影响。当吸附剂质量浓度从0.1 g·L−1增加到0.5 g·L−1时,刚果红的去除率从2.78% 增大到94.70%,平衡吸附容量先增加后减小。这是由于当LDO@biochar用量少时,参与吸附的活性位点有限,导致刚果红去除率较低[7]。继续增加吸附剂质量浓度,刚果红的去除效率改变不明显,而平衡吸附容量逐步减小。综合吸附效果和经济成本,LDO@biochar合适的质量浓度为0.5 g·L−1。溶液pH是影响吸附剂性能的关键因素[26]。如图5 (b)所示, pH 7时LDO@biochar获得最高平衡吸附容量为477.46 mg·g−1,此时刚果红去除效率最高。基于酸碱基团在不同的pH值下有不同的波长,采用双波长光谱法测量刚果红pKa [26]。由图5(c)可知, pH为3至11范围内刚果红主要以阴离子形式存在于水溶液中。酸性溶液中, H+可与吸附剂表面的阴离子结合,从而阻碍了刚果红阴离子的交换效率;而在碱性溶液中,水中的OH−与刚果红阴离子竞争吸附剂表面的阳离子吸附位点,从而导致其吸附效率降低。OH−浓度较高时,LDH板层会连接更多的羟基,可通过氢键吸附刚果红[27],因此当溶液pH由9升高到11时,吸附容量反而增加。然而,由于竞争效应的存在,碱性条件下的吸附速率较低。如图5(d)所示,随着pH的增加,k2值呈先增加后降低的趋势,溶液pH为7时获得最高吸附速率。
由于污泥中含有铬、锌、铜等重金属元素,为验证强酸碱条件是否会造成金属浸出,对溶液pH为 3和11条件下吸附实验后水体中主要金属质量浓度进行了检测,检测结果如表2所示。检出的所有金属残余质量浓度均满足生活饮用水卫生标准 (GB 5749-2022) 。
分别在20、30和40 ℃将0.1 g吸附剂加入200 mL不同初始质量浓度 (50至500 mg·L−1) 的刚果红溶液中进行等温吸附实验。如图6(a)所示, qe和C0之间几乎呈线性关系,最大吸附容量为815.10 mg·g−1 (40 ℃)。上述平衡数据通过Langmuir (5)、Freundlich (6)、Dubinin-Radushkevich (D-R) (7)和Tempkin (8)等温模型进行拟合。
式中:qe 和qm分别为刚果红的平衡吸附容量和最大吸附容量,mg·g−1;Ce为平衡吸附质量浓度,mg·L−1;KL 为Langmuir等温吸附常数,L·mg−1;n为Freundlich异质性系数;KF 为Freundlich经验常数,mg·g−1·L1/n·mg−1/n;对于D-R等温线,β 是与吸附的平均自由能有关的常数,mol2·J−2;ε 是Polanyi电位,ε=RTln(1+1/Ce),J·mol−1·K−1;R 是气体常数,J·mol−1·K−1;T 是绝对温度,K;在Tempkin模型中,B对应于吸附热;Kt 代表与最大结合能有关的平衡结合常数,L·mol−1。
各模型拟合结果如图6 (b)~图6 (e)所示,拟合参数见表3。
对比不同等温线模型的R2可以看出,Freundlich模型与实验温度下的刚果红吸附过程最匹配,表明刚果红在LDO@biochar上的吸附为不同异质表面的多层吸附,且不同温度下Freundlich模型计算的n值均大于1,表明了LDO@biochar可有效吸附刚果红[28]。D-R模型中,实验温度下的β值均小于4×10−7,根据平均吸附能E的计算公式
$\text{E}=\dfrac{\text{1}}{\sqrt{\text{2}\text{β}}}$ 可获得本吸附过程的E值均高于8 kJ·mol−1,因此该过程为化学吸附控制[29],这与动力学拟合结果相一致。Tempkin模型拟合的相关系数较小,这表明刚果红在LDO@biochar上的吸附是化学吸附和物理吸附相耦合的过程[30]。采用Langmuir等温吸附模型拟合获得20 ℃下LDO@biochar对刚果红的最大吸附容量为709.22 mg·g−1 (表4)。LDO@biochar在20 ℃下动态吸附实验结果如图6(f)所示,当水力停留时间为36 min时,吸附剂对刚果红连续吸附的穿透时间长达132 h。根据穿透时间计算可得动态吸附容量为495 mg·g−1,该值仍高于表3中同类材料对刚果红的最大吸附容量,因此体现了LDO@biochar对刚果红优异的吸附能力。
-
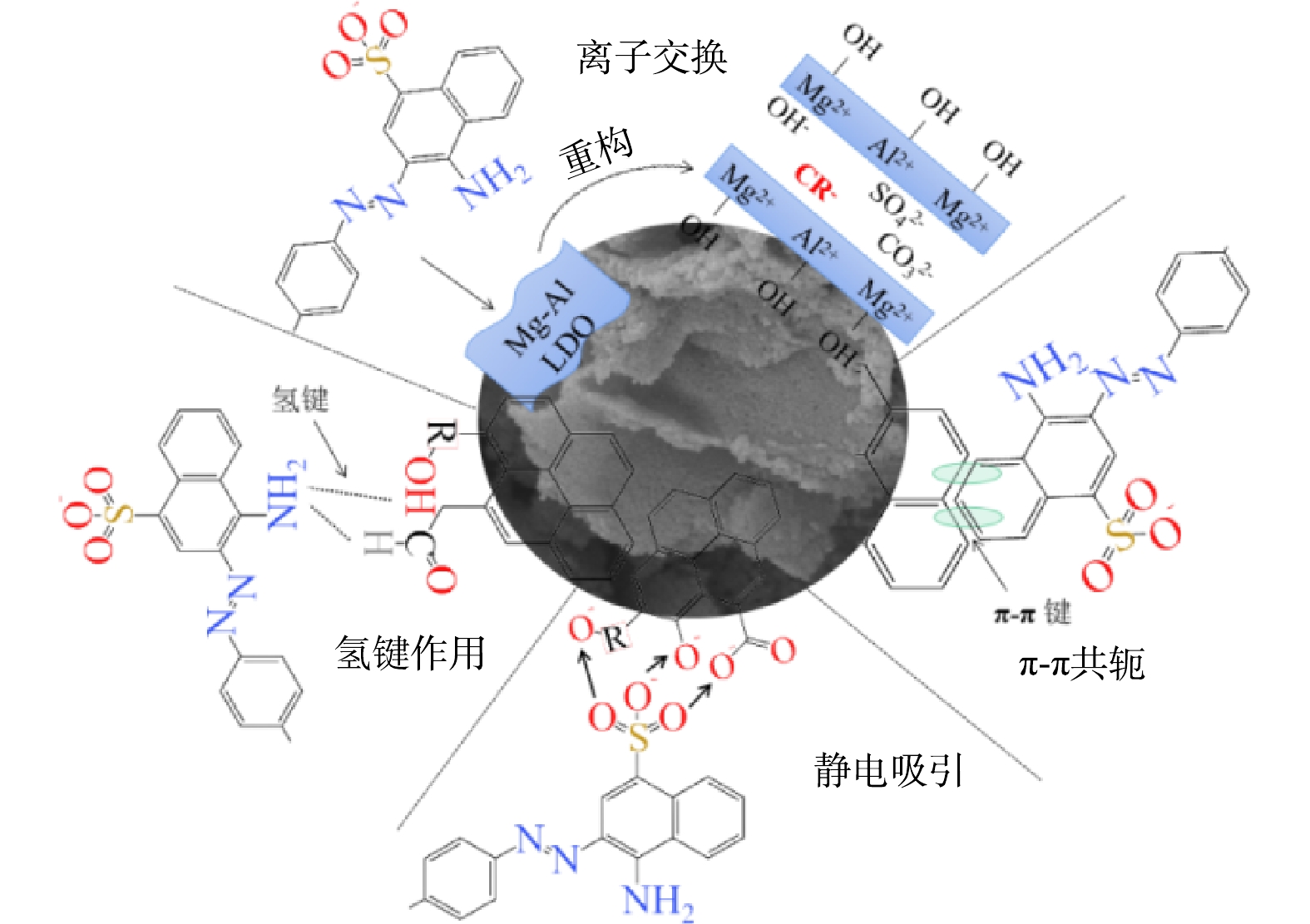
吸附动力学和吸附热力学结果表明,LDO@biochar对刚果红的吸附是物理吸附与化学吸附耦合的多层吸附过程,主要发生化学吸附。结合LDO@biochar吸附刚果红前后的结构表征结果,可知LDO在水溶液中由于“记忆效应”重构为LDH。一方面,刚果红阴离子与LDH板层间的OH−发生离子交换,通过由Al和Mg金属阳离子形成的LDH阳离子板层可以通过静电作用与刚果红阴离子结合[25]。另一方面,biochar含有石墨六元环和C=C、C=O 等含氧官能团可以充当吸附位点,石墨六元环与刚果红多环共轭结构由于π-π共轭效应结合,含氧官能团则与刚果红的磺酸基团和氨基基团由于静电吸引和氢键作用结合[20-21] (图7) 。
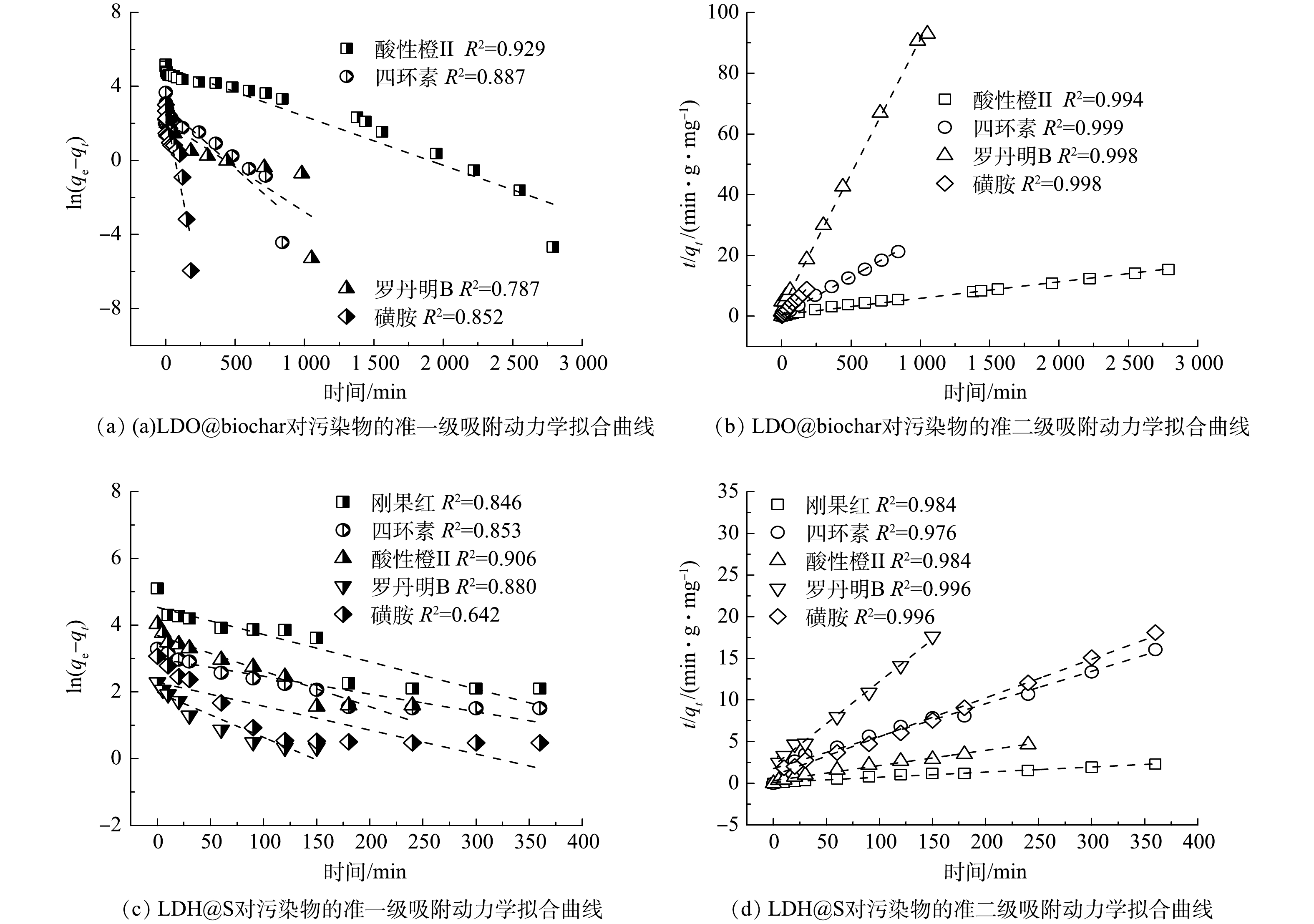
为验证LDO@biochar对刚果红的吸附机理,结合刚果红分子结构特点,筛选了阴离子污染物酸性橙II、阳离子污染物罗丹明B、具有多环共轭体系的四环素和单一苯环的磺胺进行静态吸附实验。四种污染物的准一级和准二级吸附动力学拟合曲线如图8(a)~(b)所示,与准一级动力学模型相比,准二级动力学模型的相关系数(R2)更高,表明LDO@biochar对四种污染物的吸附过程均由化学吸附所主导。与罗丹明B (11.30 mg·g−1) 和磺胺 (20.25 mg·g−1) 相比,酸性橙II (181.30 mg·g−1) 和四环素 (39.49 mg·g−1) 的吸附容量更大。如图8(c)~(d)所示,LDH@S对污染物的吸附过程也由化学吸附所主导。并且其对几种污染物的吸附容量差异与LDO@biochar相同,这进一步证实了LDH对污染物的吸附机理。与LDO@biochar相比,LDH@S对罗丹明B (8.50 mg·g−1) 和磺胺 (19.89 mg·g−1) 的吸附容量有轻微下降,而对刚果红 (155.79 mg·g−1) 、酸性橙II (51.57 mg·g−1)和四环素 (22.42 mg·g−1) 的吸附容量则显著降低。以上结果证实了与污泥相比,具有石墨六元环结构和表面丰富的含氧官能团的衍生biochar表现出更加优异的吸附性能。
-
1) XRD、FT-IR和XPS结果表明,污泥基MgAl LDO@biochar具有LDH煅烧衍生物LDO和biochar石墨炭的特征结构,当铝镁摩尔比为1∶2时,LDO晶粒尺寸和比表面积最大,对刚果红的平衡吸附容量最高。
2) LDO@biochar对刚果红的吸附符合准二级吸附动力学特征,该吸附过程为化学吸附耦合物理吸附的多层吸附。
3) 吸附过程中,LDO@biochar由于“记忆效应”重构 LDH层状结构,通过离子交换、π-π共轭、静电吸引和氢键作用吸附污染物,其对具有多环共轭结构的阴离子型污染物吸附性能显著。
污泥基层状双氧化物/生物炭复合材料的原位制备及吸附机理
In-situ fabrication of layered double oxides/biochar composites from municipal sludge and adsorption mechanism elucidation
-
摘要: 针对市政污泥中金属资源化程度低的问题,以富含铝的市政污泥为原料制备得到污泥基镁铝层状双氧化物/生物炭 (MgAl LDO@biochar) 复合材料。结合结构表征,静态吸附和动态吸附实验系统探讨LDO@biochar的吸附性能和吸附机理。结果表明,铝镁摩尔比为1∶2时,所制备LDO@biochar的比表面积和晶粒尺寸最大,其对模式污染物刚果红的吸附容量最高。在吸附过程中LDO通过“记忆效应”重构层状双氢氧化物 (LDH) 结构从而对阴离子产生吸附作用,biochar的共轭碳环和含氧官能团也可以作为污染物结合位点。污染物与吸附剂之间可通过离子交换、π-π共轭、氢键作用和静电吸引等方式结合。与阳离子型染料罗丹明B (11.30 mg·g−1) 和具有单一共轭环结构的磺胺 (20.25 mg·g−1) 相比,阴离子型染料酸性橙II (181.30 mg·g−1) 和具有多共轭环结构的四环素 (39.49 mg·g−1) 的平衡吸附容量更大,而具有多共轭环结构的阴离子型染料刚果红的平衡吸附容量高达477.46 mg·g−1。本研究结果可为综合利用市政污泥制备高附加值环境功能材料提供参考。
-
关键词:
- 市政污泥 /
- 层状双氧化物/生物炭 /
- 层状双氢氧化物 /
- 吸附机理
Abstract: Improving the metal utilization efficiency from municipal sludge is encouraged to achieve pollution mitigation and resource recovery. In this work, MgAl magnesium-aluminum layered double oxide/biochar (MgAl LDO@biochar) composite was fabricated from an Al-rich municipal sludge. The adsorption performance of LDO@biochar and the involved adsorption mechanism were investigated in terms of structural characterization, static adsorption, and dynamic adsorption experiments. The results indicated that the LDO@biochar sample obtained at an Al-Mg molar ratio of 1∶2 possessed the highest specific surface area and grain size, and as a result it exhibited the highest adsorption capacity towards the model pollutant congo red. During the adsorption process, LDO collapsed and reconstructed the layered double hydroxides (LDHs) structure due to the “memory effect”. In addition to the LDHs with specific affinity to anions, the conjugated carbon ring and oxygen-containing functional groups in biochar also served as the adsorption sites. Pollutants could be bound to the adsorbent through multiple interactions including ion exchange, π-π conjugation, hydrogen bonding and electrostatic attraction. The anionic dye Acid Orange II and tetracycline with multiple conjugated rings were adsorbed with equilibrium adsorption capacities of 181.30 and 39.49 mg·g−1, respectively, which were higher than the values for the cationic dye rhodamine B (11.30 mg·g−1) and sulfonamide with single conjugated ring (20.25 mg·g−1). The highest adsorption capacity was found on the anionic dye congo red with the largest conjugate structure, which was up to 477.46 mg·g−1. The results of this work provided a reference for the fabrication of value-added environmental functional materials from municipal sludge. -

-
表 1 污泥粉末的基本理化性质
Table 1. Basic physical and chemical properties of sludge powder
pH 阳离子交换
容量/ (mmol·g−1)金属元素质量分数/% 铝 铁 锰 锌 铬 铜 镍 6.90 0.77 11.55 20.38 0.51 0.44 0.13 0.07 0.04 表 2 吸附刚果红后水体中主要金属离子质量浓度
Table 2. Mass concentration of metal ions in water samples after adsorption of congo red
样品编号 金属离子质量浓度/(μg·L−1) 铝 铁 锰 锌 铬 铜 镍 pH=3 103.40 114.30 33.17 8.38 0.17 未检出 2.02 pH=11 76.58 50.48 22.09 40.45 1.33 4.96 0.38 标准值 200 300 100 1 000 50 1 000 20 表 3 LDO@biochar的吸附等温线模型拟合参数
Table 3. Fitting parameters for the adsorption isotherm models of LDO@biochar
T/ ℃ Langmuir Freundlich qm/ (mg·g−1) KL/ (L·mg−1) R2 KF/ (mg·g−1·L1/n·mg−1/n) n R2 20 709.22 0.042 0.876 90.01 2.599 0.875 30 862.07 0.036 0.815 74.14 2.095 0.874 40 847.46 0.061 0.789 123.049 2.595 0.947 T/ ℃ Dubinin-Radushkevic Tempkin qm/ (mg·g−1) β/ (mol2·J−2) R2 B Kt / (L·mol−1) R2 20 2 184.780 2.55×10−7 0.873 112.7 1.073 0.846 30 4 915.310 3.44×10−7 0.859 151.78 0.62 0.831 40 639.87 5.04×10−8 0.686 122.81 2.171 0.765 表 4 不同吸附剂对刚果红的吸附容量比较
Table 4. Comparison of adsorption capacities of the adsorbents towards congo red
-
[1] HU J W, ZHAO L, LUO J M, et al. A sustainable reuse strategy of converting waste activated sludge into biochar for contaminants removal from water: modifications, applications and perspectives[J]. Journal of Hazardous Materials, 2022, 438: 129437. doi: 10.1016/j.jhazmat.2022.129437 [2] YE Y Y, NGO H H, GUO W S, et al. A critical review on utilization of sewage sludge as environmental functional materials[J]. Bioresource Technology, 2023, 363: 127984. [3] 王格格, 李刚, 陆江银, 等. 热解工艺对污泥制备生物炭物理结构的影响[J]. 环境工程学报, 2016, 10(12): 7289-7293. doi: 10.12030/j.cjee.201507124 [4] XIAO B Y, DAI Q, YU X, et al. Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge[J]. Journal of Hazardous Materials, 2018, 343: 347-355. doi: 10.1016/j.jhazmat.2017.10.001 [5] ATHALATHIL S, ERJAVEC B, KAPLAN R, et al. TiO2-sludge carbon enhanced catalytic oxidative reaction in environmental wastewaters applications[J]. Journal of Hazardous Materials, 2015, 300: 406-414. doi: 10.1016/j.jhazmat.2015.07.025 [6] DAUD M, HAI A, BANAT F, et al. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)–Containing hybrids as promising adsorbents for dyes removal[J]. Journal of Molecular Liquids, 2019, 288: 110989. doi: 10.1016/j.molliq.2019.110989 [7] YE H Y, LIU S Y, YU D Y, et al. Regeneration mechanism, modification strategy, and environment application of layered double hydroxides: insights based on memory effect[J]. Coordination Chemistry Reviews, 2022, 450: 214253. doi: 10.1016/j.ccr.2021.214253 [8] LI M Z, WU G H, LIU Z H, et al. Uniformly coating Zn-Al layered double oxide nanosheets with ultra-thin carbon by ligand and phase transformation for enhanced adsorption of anionic pollutants[J]. Journal of Hazardous Materials, 2020, 397: 122766. doi: 10.1016/j.jhazmat.2020.122766 [9] ZUBAIR M, IHSANULLAH I, AZIZ H A, et al. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook[J]. Bioresource Technology, 2021, 319: 124128. doi: 10.1016/j.biortech.2020.124128 [10] PENG G, XIANG M X, WANG W Z, et al. Engineering 3D graphene-like carbon-assembled layered double oxide for efficient microplastic removal in a wide pH range[J]. Journal of Hazardous Materials, 2022, 433: 128672. doi: 10.1016/j.jhazmat.2022.128672 [11] WANG H, ZHAO W, CHEN Y N, et al. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange[J]. Bioresource Technology, 2020, 315: 123834. doi: 10.1016/j.biortech.2020.123834 [12] MEILIA D, KHUNUR M M, SETIANINGSIH T. Effect of metal cation ratio on chemical properties of ZnFe2O4/AC composite and adsorption of organic contaminant//IOP Conference Series: Materials Science and Engineering[J]. IOP Publishing, 2018, 299(1): 012036. [13] WAJIMA T. Synthesis of zeolitic material with high cation exchange capacity from paper sludge ash using EDTA[J]. Applied Sciences, 2021, 11(23): 11231. doi: 10.3390/app112311231 [14] HUANG D L, LIU C H, ZHANG C, et al. Cr (VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid[J]. Bioresource Technology, 2019, 276: 127-132. doi: 10.1016/j.biortech.2018.12.114 [15] LAIPAN M W, ZHU R L, CHEN Q Z, et al. From spent Mg/Al layered double hydroxide to porous carbon materials[J]. Journal of Hazardous Materials, 2015, 300: 572-580. doi: 10.1016/j.jhazmat.2015.07.057 [16] 郭亚祺, 杨洋, 伍新花, 等. 煅烧的水滑石同时去除水体中砷和氟[J]. 环境工程学报, 2014, 8(6): 2485-2491. [17] MA Y F, LI M, LI P, et al. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal[J]. Bioresource Technology, 2021, 319: 124199. doi: 10.1016/j.biortech.2020.124199 [18] LI S B, DONG L J, WEI Z F, et al. Adsorption and mechanistic study of the invasive plant-derived biochar functionalized with CaAl-LDH for Eu (III) in water[J]. Journal of Environmental Sciences, 2020, 96: 127-137. doi: 10.1016/j.jes.2020.05.001 [19] CHANG Z, EVANS D G, DUAN X, et al. Synthesis of [Zn-Al-CO3] layered double hydroxides by a coprecipitation method under steady-state conditions[J]. Journal of Solid State Chemistry, 2005, 178(9): 2766-2777. doi: 10.1016/j.jssc.2005.06.024 [20] LUNDEHøJ L, CELLIER J, FORANO C, et al. Atomic level understanding of orthophosphate adsorption by magnesium aluminum-layered double hydroxides-a multitechnique study[J]. The Journal of Physical Chemistry C, 2019, 123(39): 24039-24050. doi: 10.1021/acs.jpcc.9b05891 [21] CHUBAR N. EXAFS and FTIR studies of selenite and selenate sorption by alkoxide-free sol-gel generated Mg-Al-CO3 layered double hydroxide with very labile interlayer anions[J]. Journal of Materials Chemistry A, 2014, 2(38): 15995-16007. doi: 10.1039/C4TA03463E [22] AYIANIA M, SMITH M, HENSLEY A J R, et al. Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles[J]. Carbon, 2020, 162: 528-544. doi: 10.1016/j.carbon.2020.02.065 [23] SONAL S, ACHARYA S, MISHRA B K. Mesoporous carbon structure impregnated with 2D engineered zirconium: a sustainable adsorbent for the removal of dyes from the aqueous solution[J]. Journal of Environmental Management, 2022, 314: 115009. doi: 10.1016/j.jenvman.2022.115009 [24] 卢予沈, 宗莉, 于惠, 等. 混合金属氧化物/碳复合材料的制备及其对Pb (Ⅱ)的吸附性能[J]. 环境科学, 2021, 42(11): 5450-5459. [25] SHAO P H, DING L, LUO J F, et al. Lattice-defect-enhanced adsorption of arsenic on zirconia nanospheres: a combined experimental and theoretical study[J]. ACS Applied Materials & Interfaces, 2019, 11(33): 29736-29745. [26] SHAN R R, YAN L G, YANG Y M, et al. Highly efficient removal of three red dyes by adsorption onto Mg-Al-layered double hydroxide[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 561-568. doi: 10.1016/j.jiec.2014.03.019 [27] TAN X F, LIU S B, LIU Y G, et al. One-pot synthesis of carbon supported calcined-Mg/Al layered double hydroxides for antibiotic removal by slow pyrolysis of biomass waste[J]. Scientific Reports, 2016, 6(1): 39691. [27] SONG J Y, MESSELE S A, MENG L J, et al. Adsorption of metals from oil sands process water (OSPW) under natural pH by sludge-based biochar/chitosan composite[J]. Water Research, 2021, 194: 116930. [28] LIU Y, CHEN M, YONGMEI H. Study on the adsorption of Cu (II) by EDTA functionalized Fe3O4 magnetic nano-particles[J]. Chemical Engineering Journal, 2013, 218: 46-54. doi: 10.1016/j.cej.2012.12.027 [29] MA Y F, YANG L, WU L, et al. Carbon nanotube supported sludge biochar as an efficient adsorbent for low concentrations of sulfamethoxazole removal[J]. Science of the Total Environment, 2020, 718: 137299. doi: 10.1016/j.scitotenv.2020.137299 [30] ABUKHADRA M R, ADLII A, BAKRY B M. Green fabrication of bentonite/chitosan@cobalt oxide composite (BE/CH@Co) of enhanced adsorption and advanced oxidation removal of congo red dye and Cr (VI) from water[J]. International Journal of Biological Macromolecules, 2019, 126: 402-413. doi: 10.1016/j.ijbiomac.2018.12.225 [31] WANG X H, JIANG C L, HOU B X, et al. Carbon composite lignin-based adsorbents for the adsorption of dyes[J]. Chemosphere, 2018, 206: 587-596. doi: 10.1016/j.chemosphere.2018.04.183 [32] LI Z C, HANAFY H, ZHANG L, et al. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations[J]. Chemical Engineering Journal, 2020, 388: 124263. doi: 10.1016/j.cej.2020.124263 [33] TATARCHUK T, MYSLIN M, MIRONYUK I, et al. Synthesis, morphology, crystallite size and adsorption properties of nanostructured Mg-Zn ferrites with enhanced porous structure[J]. Journal of Alloys and Compounds, 2020, 819: 152945. doi: 10.1016/j.jallcom.2019.152945 [34] ZHENG Y Q, CHENG B, YOU W, et al. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to congo red, methyl orange and Cr (VI) ions[J]. Journal of Hazardous Materials, 2019, 369: 214-225. doi: 10.1016/j.jhazmat.2019.02.013 -



 下载:
下载: